Abstract
Introduction
This study investigated the feasibility of ultrasound (US)-guided microwave ablation (MWA) as a treatment for nonpuerperal mastitis (NPM).
Methods
Fifty-three patients with NPM diagnosed by biopsy and treated with US-guided MWA at the Affiliated Hospital of Nantong University between September 2020 and February 2022 were classified according to whether they underwent MWA alone (n = 29) or MWA with incision and drainage (n = 24). Patients were followed up by interviews, physical and US examinations, and evaluation of breast skin at 1 week and 1, 2, and 3 months after treatment. Data from these patients were prospectively collected and retrospectively analyzed.
Results
The overall mean patient age was 34.42 ± 9.20 years. The groups differed significantly by age, involved quadrants, and the initial maximum diameter of lesions. In the MWA group, the cure rate was 34.48%, and the apparent efficiency rate was 65.52%. In the MWA with incision and drainage, the apparent efficiency rate was 91.66%, and the effective rate was 4.17%. The excellent rate for breast aesthetics in the MWA group was 79.31%, and the good rate was 20.69%. The excellent rate in the MWA with incision and drainage group was 45.83%, the good rate was 41.67%, and the qualified rate was 12.5%. The mean maximum diameter of lesions in the two groups decreased significantly.
Conclusion
For NPM with small lesions in a single quadrant, MWA therapy is a direct and effective method. For larger lesions involving two or more quadrants, the combined treatment of MWA with incision and drainage showed significant improvement in a short period. MWA treatment of NPM has importance for further research and clinical applications.
Keywords: Ultrasound-guided microwave ablation, Microwave ablation, Incision and drainage, Nonpuerperal mastitis, Thermal ablation
Introduction
Nonpuerperal mastitis (NPM) is a benign disease that causes an inflammatory response in the breast and nipple in the absence of lactation [1]. The latest data show that NPM accounts for 2–5% of all breast diseases in China and 0.3–1.9% globally [2]. However, cases of NPM, particularly periductal mastitis and granulomatous mastitis, are thought to be more common in clinical practice [3].
Most patients with NPM have local breast discomfort, swelling, pain, or erythema in the initial stages and then progress to the formation of a secondary abscess [4, 5]. In severe cases, the skin becomes transparent, ulcerates, and discharges purulent material. NPM can progress to involve the entire breast with widespread erythema and extensive ulceration of the skin [6], causing immense physical and mental distress to patients [7].
The typical ultrasonographic manifestations of NPM are an irregular hypoechoic mixed mass, in which there are anechoic areas, dense punctate echoes, pressure that can cause movement, and peripheral breast echo thickening [8, 9]. NPM is generally treated with pharmacological agents or by surgery. Drug treatments include traditional Chinese medicine, antibiotics, or corticosteroids [10], and surgical treatment consists of incision and drainage or resection [11]. However, whether used alone or in combination, these treatments have not been shown to achieve ideal outcomes [12]. Antibiotics are often used to control the inflammatory response in the acute stage, but their therapeutic effect on local masses and abscesses is unclear [13]. High-dose prednisolone has high success and low recurrence rates in patients with idiopathic granulomatous mastitis; however, long-term use of high-dose corticosteroids is generally not recommended because it is often accompanied by side effects [14]. Surgical resection may be direct or vacuum-assisted. However, the focus has no obvious boundary, so it is not easy to remove completely; the recurrence rate is high; there are many complications, including persistent infection, hematoma, delayed healing, and scarring [3]. Incision and drainage is conventional procedure that allows continuous discharge of thick liquid and accelerate recovery [15].
At present, thermal ablation therapy is mainly used for tumors and includes radiofrequency ablation, microwave ablation (MWA), and laser ablation [16]. Ultrasound (US)-guided MWA is a minimally invasive technique that can be used to treat lesions without the need for open surgery. This technique allows accurate positioning and planning of the ablation range, causes less trauma, takes less time to perform, has a wide application range, results in fewer postoperative complications, is repeatable, and can treat multiple lesions simultaneously [17, 18]. MWA is now widely used in the treatment of a variety of solid tumors in various parts of the body [19, 20, 21, 22].
The etiology of NPM is unknown [3]. However, several studies have indicated a close relationship between NPM and immune or aseptic inflammation [8, 23, 24, 25]. MWA can denature the protein in inflammatory cells in the breast and cause them to lose their biological function, thereby eliminating their toxicity and antigenicity and making it possible to completely remove the focus and avoid repeated inflammatory attacks [26, 27, 28]. Therefore, there is a theoretical basis for the use of MWA in the treatment of NPM. In this study, we investigated the feasibility of US-guided MWA in the treatment of patients with NPM.
Materials and Methods
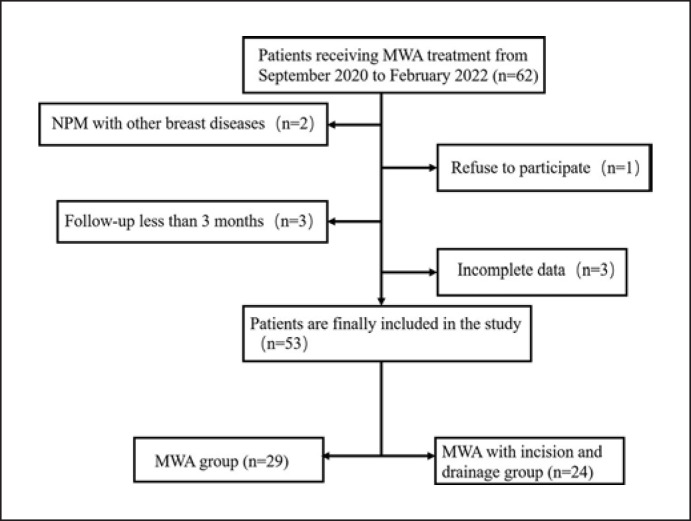
The study was approved by the Institutional Review Committee of the Affiliated Hospital of Nantong University (2021-k114-01). Written informed consent was obtained from all study participants. Fifty-three patients treated in the Ultrasound Department of the Affiliated Hospital of Nantong University from September 2020 to February 2022 were included in our study and followed up closely. A retrospective analysis of the collected data was performed. Depending on the postoperative treatment received, the patients were classified as those who received MWA alone (n = 29) and those who received MWA combined with incision and drainage (n = 24). The flowchart of this study is shown in Figure 1.
Fig. 1.
Research flowchart. MWA, microwave ablation; NPM, nonpuerperal mastitis.
A metronidazole wash was performed in each patient under US guidance at least once before MWA with observation for 1 week. Patients whose clinical symptoms and US images were significantly improved continued drug treatment. Patients with no improvement in their condition or expansion of the lesion were treated by MWA.
At least one of the following criteria needed to be met for inclusion in the study: (1) inflammation diagnosed by US-guided biopsy; (2) persistence of disease for more than 2 months; and (3) relapse after drug treatment or surgery. All patients signed an informed consent form before puncture biopsy and US-guided MWA. The exclusion criteria were as follows: (1) significant improvement after puncture and irrigation; (2) presence of inflammatory breast cancer; (3) mastitis during lactation or pregnancy; (4) a severe coagulation disorder or heart, lung, liver, or kidney dysfunction; and (5) history of underlying or chronic disease.
Preoperative Evaluation
A physical examination was performed, and the patient's medical history was reviewed before MWA. Relevant laboratory findings, imaging reports, and information on previous treatment protocols were noted. Each patient underwent a US examination, US-guided puncture biopsy, and laboratory examination before MWA. The maximum diameter, location, and ultrasonographic characteristics of the lesions were measured and recorded by routine US. NPM was diagnosed by pathological section. Laboratory tests included a complete blood cell count and coagulation function. The patients were evaluated, and the needle entry point, ablation path, ablation time, and ablation effect were preset according to clinical circumstances.
Ablation Procedure
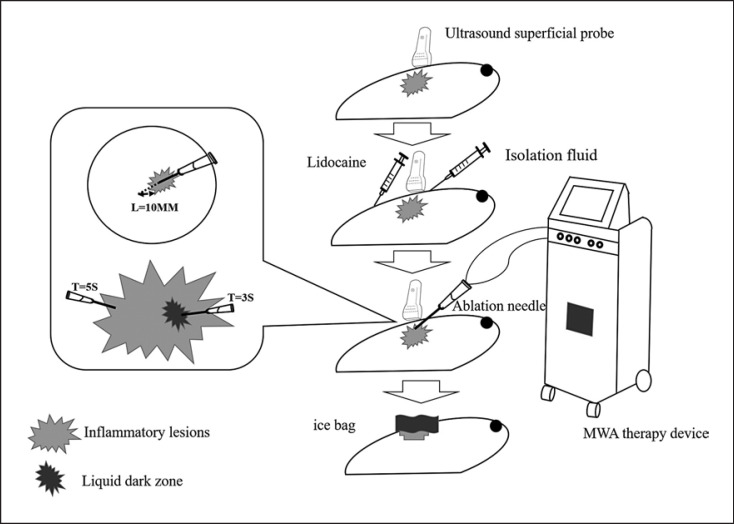
MWA was performed using an ECO-100C system (Nanjing ECO Microwave System Co., Ltd., Nanjing, China), which typically operates at a frequency of 2450 MHz with an output power in the range of 0–150 W (continuously adjustable) and is supplemented by a circulating water cooling system to reduce the surface temperature and improve the patient's ability to tolerate ablation. The effective length of the MWA needle is 15 mm, and the outer diameter is 16 G. A MyLab Twice US system (Esaote [Shenzhen] Medical Equipment Co., Ltd., Genova, Italy) was used for US guidance intraoperatively, and a Resona 6 US system (Shenzhen Mindray Biomedical Electronics Co., Ltd., Shenzhen, China) was used during postoperative follow-up.
US-guided MWA was performed with the patient in the supine position and both upper limbs abducted to fully expose the breast. The mark of the needle entry position was preset. If the lesion was large, the needle entry position was set as far as possible below the lesion to allow for incision and drainage later if necessary. For small lesions, the needle position was placed as close to the areola as possible for cosmetic purposes. Disinfection, local anesthesia (infiltration anesthesia under the skin and in the retromammary space), and peripheral injection of isolation solution (a mixture of normal saline + 1% lidocaine + 2% ropivacaine) were then performed to protect the surrounding skin and other anatomically important tissues. The MWA instrument automatically selected and set the power (15–35 W) according to the distance between the focus and the skin. The duration of ablation at sites in the parenchymal area was 5 s, and that in the liquid area was 3 s. The area ablated was monitored in real time. Under US guidance, the tip of the ablation needle was placed in the focus, and the ablation was continuously expanded from deep to shallow and from one edge to the other edge. The ablation target was moved as necessary to achieve the best area of overlapping ablation and to ensure an adequate safety margin around the focus being ablated [29]. The ablation range was extended to the surrounding tissue 10 mm outside the focus, and the pathway of the needle was observed as an area with strong echoes.
The above steps were repeated as necessary until the strong echoes were seen in the needle pathway covering the focus and surrounding tissues. When each part of the inflammatory area was completely covered, the ablation was terminated, and a local elevated echo could be detected. If a patient was found to have a fistula, the entire lesion was treated with microwaves, and each sinus was individually intensified.
Incision and drainage were performed in the following two cases: (1) when the original sinus tract was enlarged, incision and drainage were performed at the sinus tract; and (2) when the abnormal echo area increased, the low fluidity echo appeared, and a new sinus was generated. At the lowest point, incision and drainage were performed, and the patient was instructed to change his or her dressing for 4–5 days.
The ablation method, ablation time, and immediate postoperative visual analog scale (VAS) pain score were recorded during the procedure. The patient's condition was closely monitored postoperatively, and the patient was asked to apply ice for 12–24 h. The ablation procedure is demonstrated in Figure 2.
Fig. 2.
US-guided MWA as a treatment for NPM.
Postoperative Follow-Up
All patients were followed up regularly in the US clinic after ablation, and the echo characteristics, size, and postoperative skin changes were recorded. Recovery criteria were as follows: follow-up for 3 months, postoperative efficacy − (1) cure: the lesions disappeared under color Doppler US, and the patients had no redness, swelling, and tenderness; (2) apparent effect: the lesions did not completely disappear under color Doppler US, significantly reduced by 50% or more, and the patient's symptoms disappeared; (3) effective: the lesions did not completely disappear under color Doppler US, reduced by 1–50%, and the main complaint and symptoms of the last visit had positive changes; (4) ineffective: under color Doppler US, the focus did not shrink significantly; on the contrary, it increased, the patient's pain worsened, or new focus occurred during follow-up. The score of breast aesthetic effect is 0–3, with 4 grades: 0 is bad; 1 point: acceptable; 2 points: good; 3 points: excellent.
Statistical Analyses
Numerical data are reported as the mean ± standard deviation, percentage. The statistical analyses were performed using SPSS statistical software (version 26.0; IBM Corp., Armonk, NY, USA). Mapping was performed using GraphPad Prism 9 (GraphPad Software, San Diego, CA, USA). Statistical analysis was conducted using unpaired two-tailed Student's t test (*p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001), independent sample t test to compare continuous variables, and Pearson χ2 test to compare the statistical differences of classified variables. p ≤ 0.05 was statistically significant.
Results
A total of 53 patients (mean age 34.42 ± 9.20 years) were enrolled in this project: follow-up time 3–12 months (median 6 months). The MWA group comprised 29 patients (1 man; mean age, 36.62 ± 11.60 years) with a mean maximum lesion diameter of 51.59 ± 35.02 mm. In this group, preoperative interventions had been implemented for a mean of 88.14 ± 66.67 days. One patient (3.45%) had no obvious clinical symptoms, and the lesion was detected incidentally by ultrasonography. Twenty-one patients (72.41%) had breast pain and/or swelling, and seven (24.14%) had a single fistula. There were 24 patients (mean age, 31.75 ± 3.72 years) in the group that underwent MWA combined with incision and drainage. The mean maximum lesion diameter was 120.04 ± 60.67 mm. Preoperative interventions had been implemented for a mean of 102.38 ± 193.37 days. The lesions in this group involved more than two quadrants, and in five cases (20.83%), the entire breast was red and inflamed with damage to the skin. Compared with the MWA combined with incision and drainage group, the MWA group had younger patients and a smaller maximum diameter of lesions (all p < 0.05). Most of the lesions in the MWA group were limited to a single quadrant. The MWA combined with incision and drainage group had larger lesions in two or more quadrants. Demographic characteristics of the study sample are shown in Table 1.
Table 1.
Basic characteristics of the study population
| Variable | MWA (n = 29) | MWA combined with incision and drainage (n = 24) |
p value |
|---|---|---|---|
| Age, years* | 36.62±11.60 | 31.75±3.72 | 0.000 |
| Patients | 29 | 24 | |
| Sex | |||
| Men | 1 | 0 | 0.358 |
| Women | 28 | 24 | |
| Location of lesions | |||
| Left side | 16 | 13 | 0.942 |
| Right side | 13 | 11 | |
| Involving quadrant(s) | |||
| Single quadrant | 27 | 0 | 0.000 |
| Two or more quadrants | 2 | 24 | |
| Congenital nipple depression (cases) | |||
| Yes | 9 | 7 | 0.883 |
| No | 20 | 17 | |
| Swollen axillary lymph node | |||
| Yes | 17 | 20 | 0.051 |
| No | 12 | 4 | |
| Pathological results | |||
| Granulomatous mastitis | 16 | 16 | 0.394 |
| Periductal mastitis | 13 | 8 | |
| The course of preoperative intervention (D)* | 88.14±66.67 | 102.38±193.37 | 0.058 |
| Maximum diameter of lesion, mm* | 51.59±35.02 | 120.04±60.67 | 0.049 |
MWA, microwave ablation. A total of 53 participants were involved.
Data are mean ± standard deviation.
Treatment was completed successfully in all patients. Some patients experienced pain of varying severity during treatment. There was no significant difference in VAS pain score between the two groups (p > 0.05). In the MWA group, the ablation power was 26.03 ± 7.72 w and ablation time was 197.21 ± 117.14 s. The ablation power of the MWA combined with incision and drainage group was 25.83 ± 6.20 w and ablation time was 346.10 ± 218.53 s (Table 2). Typical changes seen on imaging during ablation are shown in Figure 3.
Table 2.
MWA general information
| MWA general information | MWA (n = 29) | MWA combined with incision and drainage (n = 24) | p value |
|---|---|---|---|
| Power, W* | 26.03±7.72 | 25.83±6.20 | 0.030 |
| MWA time, s* | 197.21±117.141 | 346.10±218.53 | 0.001 |
| VAS score* | 0.62±1.347 | 0.79±1.35 | 0.549 |
MWA, microwave ablation. A total of 53 participants were involved.
Data are mean ± standard deviation.
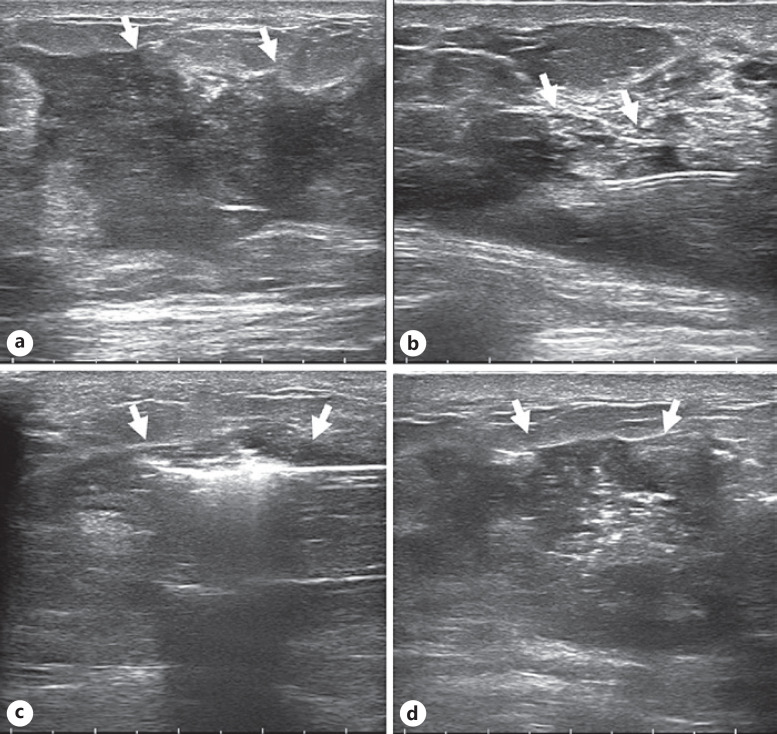
Fig. 3.
Image obtained for a 34-year-old woman who underwent US-guided MWA for NPM in the right breast. Intraoperative parameters: power, 20 W, and ablation time, 299 s. a Ultrasonographic image showing the lesion (arrow). b Water isolation technology (arrow) was used to protect important surrounding anatomical tissues. c Hyperechoic areas in lesions (arrows) seen during ablation. d Ultrasonographic image after ablation shows the lesion area (arrow).
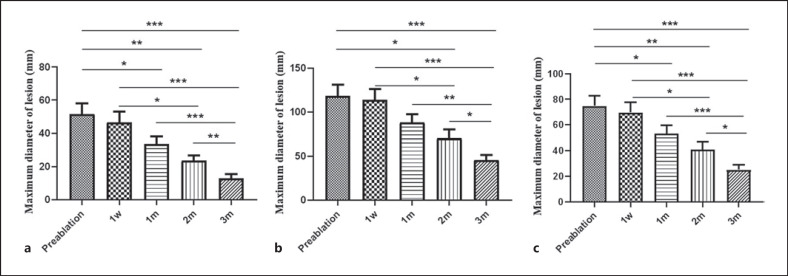
During the follow-up after treatment, the maximum diameter of lesions decreased significantly in both groups (p < 0.001) (Fig. 4). The cure rate of the MWA group was 34.48%, and the apparent effective rate was 65.52%. The apparent effective rate of the MWA combined with incision and drainage group was 91.66%, the effective rate was 4.17%, and the ineffective rate was 4.17%. There was a significant difference in the treatment effect between the two groups (p < 0.05). The excellent rate for the MWA group was 79.31%, and the good rate was 20.69%. The excellent rate for the MWA combined with incision and drainage group was 45.83%, the good rate was 41.67%, and the acceptable rate was 12.5%. There was a significant difference in breast aesthetic effect between the two groups (p < 0.05), and the patients were satisfied with the cosmetic effect (Table 3). No recurrence events were observed during follow-up. Typical image changes in the MWA group are shown in Figures 5 and 6 for the MWA combined with incision and drainage group.
Fig. 4.
a Mean maximum lesion diameter in the group that underwent US-guided MWA before ablation and at 1 week, 1 month, 2 months, and 3 months after ablation. b Mean maximum lesion diameter in the group that underwent ablation combined with incision and drainage before ablation and at 1 week, 1 month, 2 months, and 3 months after ablation. c Mean maximum lesion diameter in all patients before ablation and 1 week, 1 month, 2 months, and 3 months after surgery. *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001.
Table 3.
General situation of the postoperative curative effect
| Variable | MWA (n = 29) | MWA combined with incision and drainage (n = 24) | p value |
|---|---|---|---|
| Postoperative efficacy | |||
| Cure | 10 (34.48%) | 0 | 0.008 |
| Apparent effective | 19 (65.52%) | 22 (91.66%) | |
| Effective | 0 | 1 (4.17%) | |
| Ineffective | 0 | 1 (4.17%) | |
| Breast aesthetic effect | |||
| Bad | 0 | 0 | 0.020 |
| Acceptable | 0 | 3 (12.5%) | |
| Good | 6 (20.69%) | 10 (41.67%) | |
| Excellent | 23 (79.31%) | 11 (45.83%) |
MWA, microwave ablation. A total of 53 participants were involved.
Fig. 5.
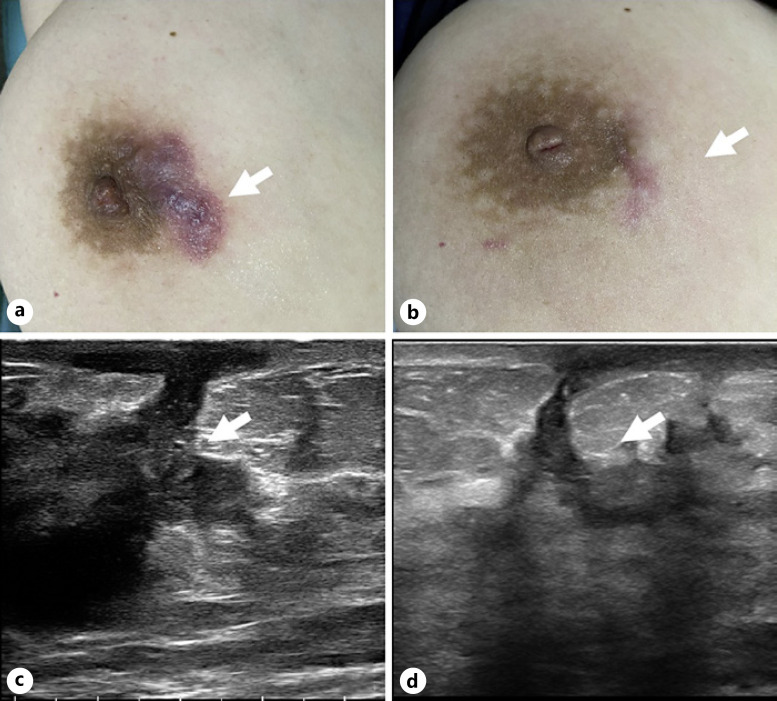
Skin and ultrasonographic changes in a 56-year-old woman who underwent US-guided MWA for NPM in the right breast. a Preoperative skin condition. b Skin changes observed 45 days after MWA. c Ultrasonographic image showing the preoperative focus (arrow). d Ultrasonographic image of the lesion area 45 days after ablation (arrow).
Fig. 6.
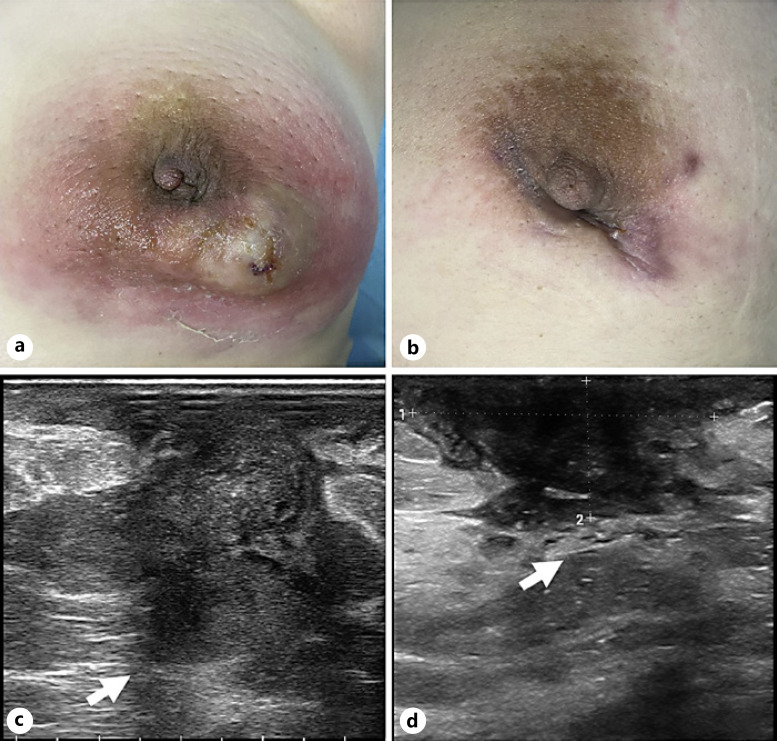
Skin and ultrasonographic changes in a 30-year-old woman who underwent US-guided MWA for NPM in the left breast. a Skin condition before ablation. b Skin changes 7 days after incision and drainage. c Ultrasonographic image showing the preoperative focus (arrow). d Ultrasonographic image showing the lesion area 7 days after incision and drainage (arrow).
One patient (3.45%) in the MWA group reported fat liquefaction. Since the liquefaction range was limited to 1–2 mm, it could be absorbed without additional treatment. Eight patients (27.59%) developed 2–3 mm new sinus and excreted a small amount of light-yellow liquid or bean curd residue-like necrotic substances. After a routine dressing change, this sinus was able to heal in about 10 days without an obvious scar. The MWA combined with incision and drainage group reported adverse events such as fat liquefaction, local skin pain 1 week after the operation, and new sinus. Local skin pain could be recovered without special treatment. In a large range of fat liquefaction, US-guided puncture and fluid extraction were used. For the new sinus, fluid extraction and drainage were performed (Table 4).
Table 4.
Clinical complications
| Variable | MWA (n = 29) | MWA combined with incision and drainage (n = 24) | p value | ||
|---|---|---|---|---|---|
| Complications | |||||
| No surgical complications | 20 | 10 | 0.003 | ||
| Skin thermal injury | 0 | 0 | |||
| Fat liquefaction | 1 | 4 | |||
| Local pain 1 week after operation | 0 | 8 | |||
| New sinus | 8 | 5 | |||
MWA, microwave ablation. A total of 53 participants were involved.
Discussion
Currently, the etiology of NPM remains unknown, and there is no gold standard for diagnosis or any standardized treatment plan [30]. The high recurrence rate, need for long-term drug treatment, associated trauma, and incompleteness of surgical treatment cause considerable emotional and financial stress for patients [15]. Moreover, single or combined treatment is ineffective in some patients, which results in considerable distress [23]. Anxiety aggravates NPM and increases the likelihood of recurrence, creating a vicious cycle and having marked adverse effects on the physical and mental health of patients [31].
Treatment of NPM by US-guided MWA directly inactivates the protein in inflammatory lesions by applying a high temperature and coagulates necrotic tissue. It has the advantages of a short treatment time and a rapid effect [32, 33]. US guidance is used to estimate the ablation range. Combined with water isolation technology, this technique can minimize damage to normal breast tissue. Furthermore, US-guided MWA causes little trauma and has a good cosmetic outcome. Therefore, this technique has unique advantages over traditional open surgery and allows more accurate monitoring for changes on internal images of lesions during follow-up. If there is a possibility of recurrence, puncture treatment can be carried out in a timely manner.
The US manifestations of NPM include a mixed mass with a predominance of areas with irregular low echogenicity. As the disease progresses, the focus expands to the surrounding breast tissue, internal areas of abnormal echogenicity gradually expand, and its internal point-like hyperechoic pressurization is movable and has a sense of flow [10, 34]. After MWA, with increasing duration of follow-up, the US manifestations of NPM lesions include gradual narrowing of the hypoechoic area, an increase in internal echoes, and no continuous increase in anechoic or flowing punctate hyperechogenic foci. These observations confirm the effectiveness and feasibility of ablation in NPM.
In our study, the patients treated with US-guided MWA alone needed only one treatment session and can be discharged after the operation on the same day. There is no need for drugs and other treatment after the operation, and the obvious improvement rate can reach 100%. Similar to the previous research reported by Zhou Y et al. [35], the total effective rate is 98.1%. The focus range was small, all of which were mass-like and local catheter dilation. During postoperative follow-up, the maximum diameter of the focus area became smaller over time, the echo increased, and the skin returned to normal from redness and swelling. Microscopic abscesses in some breast tissues disappeared completely within about 15 days. In patients with NPM, the scope is not large, the treatment time is short, the trauma is small, the medical cost is low, damage to the normal breast tissue is avoided to the greatest extent, and the cosmetic effect is very good. The excellent rate and good rate can reach 100%. The complications in this group were mild and could be recovered without special treatment. For example, Lin L et al. [33] showed a very good therapeutic effect when using MWA combined with glucocorticoids to treat granulomatous mastitis, and the recurrence rate was 2%. In this study, fully one-quarter of the patients in this group has been followed up for 8 months, and no obvious recurrence was found. We will continue to keep tracking on all the patients involved in this study to observe their final recurrence rate.
Patients who underwent MWA combined with incision and drainage generally had extensive inflammation, often affecting two or more quadrants, multiple abscesses and fistulas, and large areas of skin redness and ulceration. Due to the large scope used, there is pus and exudation after high-temperature treatment, which can be discharged over time by incision and drainage. After full preoperative evaluation, the treatment time is relatively long. According to the amount of exudate, incision and drainage can be performed during the procedure or after a week or two. In this study, the pus and exudation after high-temperature treatment did not affect the healing of the drainage incision. With the absorption of necrotic tissue and the rapid discharge of pus and inflammatory exudation, the recovery of the disease becomes accelerated, and some breast surfaces have slight depressions. However, with the extension of follow-up time, the appearance of the breast will gradually recover. Three months after the operation, most patients (91.66%) showed significant improvement, and ultrasonography showed that the focus was reduced by more than 50%. Only two cases had stable or enlarged lesions. Through continuous observation of US, the team speculated that the 2 patients had a thicker fat layer and more fat liquefaction. The patient gradually recovered through puncture, fluid extraction, and drainage. The incidence of complications in this group was higher than that in the MWA group, but the patients recovered well through treatment.
During follow-up, we found that a small number of patients with large lesions did not need incision and drainage after ablation. After exploration and treatment, the area in the mass with abnormal low echoes increased rapidly, but the red and swollen skin slowly returned to normal without exudate or pain. No increase in the nonechogenic area or fistula formation was seen on US, and the low echo area gradually decreased later. We speculate that pus and exudate diffused into the surrounding breast tissue after high-temperature treatment and were gradually absorbed without causing infection in normal breast tissue.
The combination of MWA technology and surgical incision and drainage solves the problems of frequent recurrence, extensive trauma, and difficult healing after treatment of NPM and affords the possibility of a short-term, highly efficient, minimally traumatic, and highly curative treatment. In this study, there were no serious complications during follow-up, which indicates that US-guided MWA is a safe treatment for NPM.
This study has several limitations. First, MWA technology is fairly new and not fully understood by clinicians or patients. Therefore, the number of study participants was relatively small. Second, considering the high recurrence rate of NPM, the follow-up duration was short, and a longer follow-up period is needed to evaluate the overall efficacy of MWA in the treatment of NPM. Furthermore, the mechanism of MWA needs further molecular and genetic research. Our team is continuing to gather data on the use of US-guided MWA in patients with NPM, with an extension of follow-up and strengthening of the skills of the team delivering this treatment.
In conclusion, the findings of this study show that US-guided MWA leads to the disappearance of inflammatory symptoms of NPM, resolution of erythema and ulceration, and improvement in US images with no evidence of recurrence on clinical and US examinations for at least 3 months after the procedure. US-guided MWA has a positive role in the treatment of NPM and is potentially curative.
Statement of Ethics
The study was approved by the Institutional Review Committee of the Affiliated Hospital of Nantong University (2021-k114-01). Written informed consent was obtained from all study participants.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
This study was not supported by any funding.
Author Contributions
Shengluan Zhou, Chenyi Sheng, Ping Hu, Hui Zhao, and Xiaoyang Chen designed the study and drafted the initial manuscript. Xuejun Ni, Xiaoping Xu, Qian Song, and Xiaoxiao Jiang collected all data. All the authors reviewed and approved the final manuscript version.
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
The authors wish to thank the Affiliated Hospital of Nantong University and the patients who agreed to participate in the study.
Funding Statement
This study was not supported by any funding.
References
- 1.Tan H, Li R, Peng W, Liu H, Gu Y, Shen X. Radiological and clinical features of adult non-puerperal mastitis. Br J Radiol. 2013;86((1024)):20120657. doi: 10.1259/bjr.20120657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang L, Hu J, Guys N, Meng J, Chu J, Zhang W, et al. Diffusion-weighted imaging in relation to morphology on dynamic contrast enhancement MRI the diagnostic value of characterizing non-puerperal mastitis. Eur Radiol. 2018;28((3)):992–999. doi: 10.1007/s00330-017-5051-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou F, Shang XC, Tian XS, Yu ZG. Chinese Society of Breast Surgery. Clinical practice guidelines for diagnosis and treatment of patients with non-puerperal mastitis Chinese Society of Breast Surgery (CSBrS) practice guideline 2021. Chin Med J. 2021;134((15)):1765–1767. doi: 10.1097/CM9.0000000000001532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang YM, Lo C, Cheng CF, Lu CH, Hsieh SC, Li KJ. Serum C-reactive protein and interleukin-6 levels as biomarkers for disease severity and clinical outcomes in patients with idiopathic granulomatous mastitis. J Clin Med. 2021;10((10)):2077. doi: 10.3390/jcm10102077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.An JK, Woo JJ, Lee SA. Non-puerperal mastitis masking pre-existing breast malignancy importance of follow-up imaging. Ultrasonography. 2016;35((2)):159–163. doi: 10.14366/usg.15024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang Y, Zhou Y, Mao F, Guan J, Sun Q. Clinical characteristics classification and surgical treatment of periductal mastitis. J Thorac Dis. 2018;10((4)):2420–2427. doi: 10.21037/jtd.2018.04.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goulabchand R, Hafidi A, van de Perre P, Millet I, Maria ATJ, Morel J, et al. Mastitis in autoimmune diseases review of the literature, diagnostic pathway, and pathophysiological key players. J Clin Med. 2020;9((4)):958. doi: 10.3390/jcm9040958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fazzio RT, Shah SS, Sandhu NP, Glazebrook KN. Idiopathic granulomatous mastitis imaging update and review. Insights Imaging. 2016;7((4)):531–539. doi: 10.1007/s13244-016-0499-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hu J, Huang X. Combining ultrasonography and mammography to improve diagnostic accuracy of plasma cell mastitis. J Xray Sci Technol. 2020;28((3)):555–561. doi: 10.3233/XST-190607. [DOI] [PubMed] [Google Scholar]
- 10.Co M, Cheng VCC, Wei J, Wong SCY, Chan SMS, Shek T, et al. Idiopathic granulomatous mastitis a 10-year study from a multicentre clinical database. Pathology. 2018;50((7)):742–747. doi: 10.1016/j.pathol.2018.08.010. [DOI] [PubMed] [Google Scholar]
- 11.Taffurelli M, Pellegrini A, Santini D, Zanotti S, Di Simone D, Serra M. Recurrent periductal mastitis surgical treatment. Surgery. 2016;160((6)):1689–1692. doi: 10.1016/j.surg.2016.06.048. [DOI] [PubMed] [Google Scholar]
- 12.Barreto DS, Sedgwick EL, Nagi CS, Benveniste AP. Granulomatous mastitis etiology, imaging, pathology, treatment, and clinical findings. Breast Cancer Res Treat. 2018;171((3)):527–534. doi: 10.1007/s10549-018-4870-3. [DOI] [PubMed] [Google Scholar]
- 13.Zhu Q, Wang L, Wang PJ. The identification of gene expression profiles associated with granulomatous mastitis. Breast Care. 2021;16((4)):319–327. doi: 10.1159/000507474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Postolova A, Troxell ML, Wapnir IL, Genovese MC. Methotrexate in the treatment of idiopathic granulomatous mastitis. J Rheumatol. 2020;47((6)):924–927. doi: 10.3899/jrheum.181205. [DOI] [PubMed] [Google Scholar]
- 15.Patani N, MacAskill F, Eshelby S, Omar A, Kaura A, Contractor K, et al. Best-practice care pathway for improving management of mastitis and breast abscess. Br J Surg. 2018;105((12)):1615–1622. doi: 10.1002/bjs.10919. [DOI] [PubMed] [Google Scholar]
- 16.Liu F, Zang L, Liu Y, Yu X, Cheng Z, Han Z, et al. Risk factors influencing cure of ultrasound-guided microwave ablation for primary hyperparathyroidism. Int J Hyperthermia. 2022;39((1)):258–264. doi: 10.1080/02656736.2022.2029957. [DOI] [PubMed] [Google Scholar]
- 17.Yang Q, Li H, Chen BH, He GZ, Wu XP, Wang LX, et al. Ultrasound-guided percutaneous microwave ablation for 755 benign breast lesions a prospective multicenter study. Eur Radiol. 2020;30((9)):5029–5038. doi: 10.1007/s00330-020-06868-9. [DOI] [PubMed] [Google Scholar]
- 18.Li C, Li C, Ge H, Liang M, Ma G, Ling L, et al. Technical analysis of US imaging for precise microwave ablation for benign breast tumours. Int J Hyperthermia. 2018;34((8)):1179–1185. doi: 10.1080/02656736.2018.1442589. [DOI] [PubMed] [Google Scholar]
- 19.Yao J, Liu B, Wang X, Yu J, Cheng Z, Han Z, et al. Long-term efficacy of microwave ablation in the treatment of subcapsular hepatocellular carcinomas of ≤3 cm in diameter a multicenter, propensity score-matched study. Int J Hyperthermia. 2022;39((1)):209–216. doi: 10.1080/02656736.2021.2023228. [DOI] [PubMed] [Google Scholar]
- 20.Liu LH, Yang BB, Liu Y, Wang JL, Wang DD, Ding HY, et al. Factors related to the absorption rate of benign thyroid nodules after image-guided microwave ablation a 3-year follow-up. Int J Hyperthermia. 2022;39((1)):8–14. doi: 10.1080/02656736.2021.1995632. [DOI] [PubMed] [Google Scholar]
- 21.Xu S, Qi J, Bie ZX, Li YM, Li B, Guo RQ, et al. Local progression after computed tomography-guided microwave ablation in non-small cell lung cancer patients prediction using a nomogram model. Int J Hyperthermia. 2021;38((1)):1366–1374. doi: 10.1080/02656736.2021.1976852. [DOI] [PubMed] [Google Scholar]
- 22.Yu J, Yu XL, Cheng ZG, Hu B, Han ZY, Liu FY, et al. Percutaneous microwave ablation of renal cell carcinoma practice guidelines of the ultrasound committee of Chinese medical association, interventional oncology committee of Chinese research hospital association. Int J Hyperthermia. 2020;37((1)):827–835. doi: 10.1080/02656736.2020.1779356. [DOI] [PubMed] [Google Scholar]
- 23.Toktas O, Konca C, Trabulus DC, Soyder A, Koksal H, Karanlik H, et al. A novel first-line treatment alternative for noncomplicated idiopathic granulomatous mastitis combined intralesional steroid injection with topical steroid administration. Breast Care. 2021;16((2)):181–187. doi: 10.1159/000507951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kafadar MT, Bahadır MV, Girgin S. Low-dose methotrexate use in idiopathic granulomatous mastitis an alternative treatment method. Breast Care. 2021;16((4)):402–407. doi: 10.1159/000513879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu H, Jiang Y, Liao M, Li D, Zhu C. Continuous postoperative negative pressure irrigation assisted mammaplasty in treating chronic refractory plasma cell mastitis. Gland Surg. 2020;9((6)):2071–2078. doi: 10.21037/gs-20-795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.He X, Wolkers WF, Crowe JH, Swanlund DJ, Bischof JC. In situ thermal denaturation of proteins in dunning AT-1 prostate cancer cells implication for hyperthermic cell injury. Ann Biomed Eng. 2004;32((10)):1384–1398. doi: 10.1114/b:abme.0000042226.97347.de. [DOI] [PubMed] [Google Scholar]
- 27.Liu D, Brace CL. Evaluation of tissue deformation during radiofrequency and microwave ablation procedures influence of output energy delivery. Med Phys. 2019;46((9)):4127–4134. doi: 10.1002/mp.13688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roknsharifi S, Wattamwar K, Fishman MDC, Ward RC, Ford K, Faintuch S, et al. Image-guided microinvasive percutaneous treatment of breast lesions where do we stand? RadioGraphics. 2021;41((4)):945–966. doi: 10.1148/rg.2021200156. [DOI] [PubMed] [Google Scholar]
- 29.Cao XJ, Wei Y, Zhao ZL, Peng LL, Li Y, Yu MA. Efficacy and safety of microwave ablation for cervical metastatic lymph nodes arising post resection of papillary thyroid carcinoma a retrospective study. Int J Hyperthermia. 2020;37((1)):450–455. doi: 10.1080/02656736.2020.1759829. [DOI] [PubMed] [Google Scholar]
- 30.Zuo X, Shi X, Gao X, Lai R, Liu P, Zhao Z. Treatment effect of mammary duct exploration combined with focal resection on granulomatous lobular mastitis. J Inflamm Res. 2021;14:2641–2646. doi: 10.2147/JIR.S309101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koh A, Parks RM, Courtney A, Leff DR, MAMMA Steering Committee Mastitis and mammary abscess management audit (MAMMA) Br J Surg. 2021;108((9)):e286–e287. doi: 10.1093/bjs/znab155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Neira LM, Mays RO, Sawicki JF, Schulman A, Harter J, Wilke LG, et al. A pilot study of the impact of microwave ablation on the dielectric properties of breast tissue. Sensors (Basel) 2020;20((19)):5698. doi: 10.3390/s20195698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin L, Zheng Z, Zhang J, Liu X, Chen DR, Wang H. Treatment of idiopathic granulomatous mastitis using ultrasound-guided microwave ablation a report of 50 cases. Int J Hyperthermia. 2021;38((1)):1242–1250. doi: 10.1080/02656736.2021.1965225. [DOI] [PubMed] [Google Scholar]
- 34.Pluguez-Turull CW, Nanyes JE, Quintero CJ, Alizai H, Mais DD, Kist KA, et al. Idiopathic granulomatous mastitis manifestations at multimodality imaging and pitfalls. RadioGraphics. 2018;38((2)):330–356. doi: 10.1148/rg.2018170095. [DOI] [PubMed] [Google Scholar]
- 35.Zhou Y, Wang SR, Xu D. Clinical study of ultrasound-guided microwave ablation in the treatment of non-lactating mastitis. Research Square Preprint. 2021 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.