Abstract
Purpose
The aim of the study was to investigate the effects of body mass index (BMI) on the response to neoadjuvant chemotherapy (NACT) in Turkish patients with local and locally advanced breast cancer.
Methods
The pathological responses for the breast and axilla were assessed according to the Miller-Payne grading (MPG) system. Tumors were grouped into molecular phenotypes and classified as response rates according to the MPG system after the completion of NACT. A 90% or greater reduction in tumor cellularity was considered a good response to treatment. Additionally, patients were grouped according to BMI into <25 (group A) and ≥25 (group B).
Results
In total, 647 Turkish women with breast cancer were included in the study. In the univariate analysis, age, menopause status, tumor diameter, stage, histological grade, Ki-67, estrogen receptor (ER) status, progesterone receptor (PR) status, human epidermal growth factor receptor 2 (HER2) status, and BMI were assessed to determine which of these factors were associated with a ≥90% response rate. Stage, HER2 positivity, triple-negative breast cancer (TNBC; ER-negative, PR-negative, and HER2-negative breast cancer), grade, Ki-67 levels, and BMI were found to be the statistically significant factors for a ≥90% response rate. In the multivariate analysis, grade III disease, HER2 positivity, and TNBC were found to be the factors associated with a high pathological response. Meanwhile, hormone receptor (HR) positivity and a higher BMI were associated with a decreased pathological response in patients receiving NACT for breast cancer.
Conclusion
Our results show that a high BMI and HR positivity are associated with a poor response to NACT in Turkish patients with breast cancer. The findings presented in this study may guide novel studies to examine the NACT response in obese patients with and without insulin resistance.
Keywords: Body mass index, Breast neoplasms, Neoadjuvant therapy, Pathological response
Introduction
Breast cancer is the most frequently diagnosed malignancy worldwide, and it is the leading cause of cancer death in women [1]. Most patients with human epidermal growth factor receptor 2 (HER2)-positive and triple-negative breast cancer (TNBC) or locally advanced hormone receptor (HR)-positive breast cancer receive neoadjuvant chemotherapy (NACT) (mostly anthracycline-based regimens) to obtain a tumor response before surgery and enable breast conservation. Patients treated with NACT commonly undergo surgery for resection of primary tumors. In a meta-analysis, Mieog et al. [2] demonstrated some outcomes of NACT. According to their report, compared with ACT, there was a reduced risk of radical mastectomy, an increased risk of locoregional recurrence, and an equivalent chance of overall survival and disease-free survival with NACT. Pathological complete response (pCR) is one of the most important markers for disease-free survival [3, 4].
The Miller-Payne grading system (MPG) is commonly used to assess pathological response by comparing cancer cellularity in core biopsy samples obtained before treatment with the resected tumor after treatment. pCR entails a >90% reduction in tumor cellularity and no residual invasive cancer [5]. Previous studies have examined markers that predict pCR, such as tumor subtype, grade, or other conditions [3, 6, 7].
Overweight and obesity are major public health problems worldwide. Overweight (defined by the World Health Organization as a body mass index [BMI] ≥25 kg/m2) and obesity (BMI ≥30 kg/m2) have been associated with an increased risk of developing breast cancer. It is also associated with worse outcomes in both pre- and postmenopausal breast cancer patients [8, 9, 10].
Many studies have found that obesity at the time of breast cancer diagnosis is associated with poorer outcomes and with an increased risk of recurrence and mortality [11, 12, 13]. BMI, a standard method for identifying obese or overweight people, is widely accepted as a contributing factor in the development and prognosis of breast cancer. A high BMI is associated with a higher risk of breast cancer in multiple studies [14]. Some studies in different ethnicities focused on a potential correlation between BMI and NACT efficacy. Although the results remain controversial, some showed a distinct correlation between overweight and obesity and significantly lower pCR rates, whereas others found no association between increased BMI and pCR in BC patients [15, 16, 17, 18, 19]. In this study, we aimed to investigate the effects of BMI on the NACT response in patients with local and locally advanced breast cancer.
Materials and Methods
Study Population and Treatment
This trial was planned as a retrospective multicenter study. Medical details were obtained from archived files of patients who had been treated with NACT between 2014 and 2019 for invasive ductal breast cancer in the medical oncology clinics of Prof. Dr. Cemil Tascioglu City Hospital, Istanbul University, Medipol University, Uludag University, Medeniyet University, and University of Health Sciences. Disease staging was performed according to the tumor node metastasis staging system 7. Age and pathological results such as tumor size, histological type, lymph node status, grade, hormonal status, and HER2 receptor status were obtained from the archived files of patients. Patients without a pathology report and archived files were excluded (Fig. 1). The histological responses of the breast and axilla were assessed according to the MPG system. Tumors were grouped into molecular phenotypes and classified as response rates according to the MPG system after completion of NACT. BMI was calculated for each patient before NACT, and patients were grouped according to whether their BMI was <25 kg/m2 (group A) or ≥25 kg/m2 (group B).
Fig. 1.
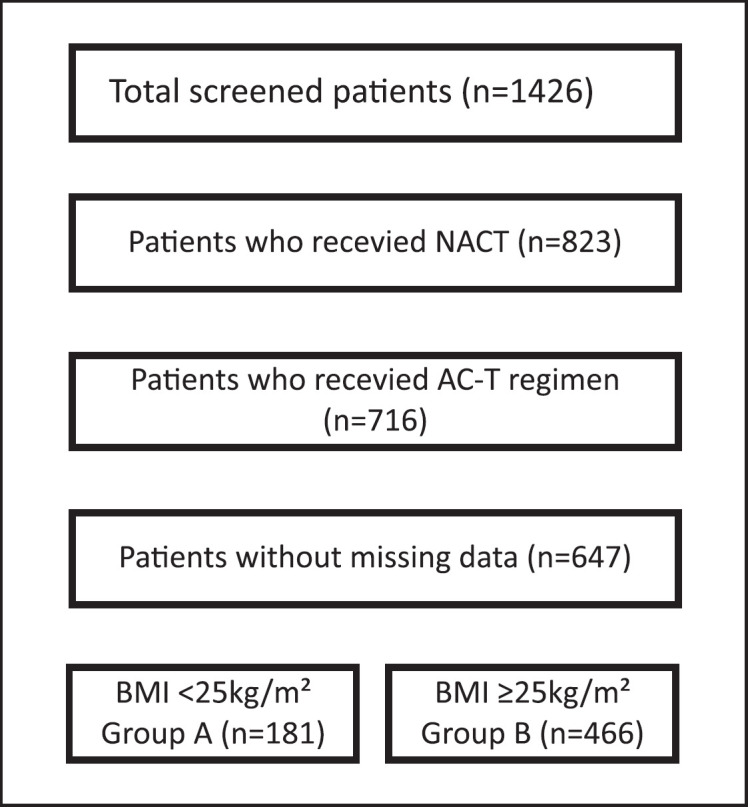
Consort diagram.
Patients with stage III disease irrespective of the subtype received NACT. Patients with stage II disease received NACT if either breast-conserving surgery was not possible due to a high tumor to breast ratio, if the anticipated cosmetic outcome would be suboptimal due to the tumor location, or if triple-negative or HER2-positive subtype was diagnosed.
In the neoadjuvant setting, patients received intravenous doxorubicin 60 mg/m2 plus intravenous cyclophosphamide 600 mg/m2 every 3 weeks for four cycles followed by paclitaxel 80 mg/m2 weekly for 12 cycles. Patients with HER2-positive breast cancer received trastuzumab in addition to paclitaxel (4 mg/kg loading dose and 2 mg/kg weekly) for 12 weeks.
Immunohistochemistry and Pathological Evaluation
The following antibodies were used: rabbit monoclonal antibody against human ERa (ER; SP1, Bio-Care, USA); rabbit monoclonal antibody against human progesterone (PgR) (SP2, Bio-Care); rabbit monoclonal antibody against human HER2 (SP3, Cell Marque, USA); and rabbit monoclonal antibody against Ki-67 (SP6, Bio-Care). ER and PgR immunohistochemistry was scored positive if at least 10% of tumor cell nuclei showed a staining signal. HR positivity was defined as ER and/or PgR positive [20]. HER2 overexpression required either an immunohistochemical staining of 3 + or positivity by fluorescence in situ hybridization technique in case of a 2 + immunohistochemistry score. In the evaluation of Ki-67, nuclear staining was considered positive. The proportion of Ki-67-positive cells in the total number of evaluated cells was calculated [21].
Pathological response was determined using the MPG system, in which reduction in tumor cellularity was graded from 1 to 5 (grade 1 = 0% decrease, grade 2 = 0–30% decrease, grade 3 = 30–90% decrease, grade 4 = 90% decrease, grade 5 = 100% decrease). Good response was defined as ≥90% decrease in tumor cellularity or the total absence of invasive tumor cells in the breast and axillary tissues [5].
Statistical Methods
SPSS 15.0 for Windows was used for statistical analysis. Categorical variables are presented as numbers and percentages, while numerical variables are presented as average, standard deviation, and minimum and maximum. Numerical variables that did not meet the normal distribution condition and comparisons of more than two independent groups were analyzed using the Kruskal-Wallis test, while comparisons of two independent groups were made using the Mann-Whitney U test. Comparisons of the ratios in the groups were made using the χ2 test. The determinant factors were examined by logistic regression analysis, and a statistical significance level of alpha was accepted as p < 0.05.
Results
In total, 647 women with breast cancer were included in the study. All patients had invasive ductal carcinoma, and the mean age was 49.2 years. The most common comorbidities were hypertension (9.9%) and diabetes mellitus (4.5%). Overall, 317 (49.0%) patients were postmenopausal. The numbers of patients with stage II and III disease were 324 (50.1%) and 323 (49.9%), respectively. The numbers of HR-positive and negative patients were 436 (67.4%) and 211 (32.6%), respectively. HER2 positivity was observed in 170 (26.3%) patients. The percentages of patients with histological grades I, II, and III were 12.8%, 54.3%, and 32.9%, respectively. The mean tumor diameter was 31.4 mm, and the mean Ki-67 level was 36.6%. According to the MPG system, 76 (11.7%) patients showed 0% response, 128 (19.8%) patients showed 1–30% response, 178 (27.5%) patients showed 31–90% response, 105 (16.2%) patients showed 90–99% response, and 160 (24.7%) showed 100% response. In total, 181 (28%) patients were in group A and 466 (72.0%) patients were in group B (Table 1).
Table 1.
Patient's characteristics and BMI status
| All patients |
BMI, kg/m2 |
|||||
|---|---|---|---|---|---|---|
| Variables | mean ± SD | <25 (n = 181) |
≥25 (n = 466) |
|||
| mean ± SD | mean ± SD | p value | ||||
| Age, years | 49.2±10.9 | 43.5±10.8 (43) | 51.3±10.3 (51.9) | <0.001 | ||
| BMI, kg/m2 | 28.4±5.5 | |||||
| Tumor size, cm | 31.4±20.3 | 31.5±16.8 (30) | 31.5±21.5 (25) | 0.175 | ||
| Ki-67 (%) | 36.6±25.3 | 37.3±27.2 (28.5) | 36.3±24.5 (30) | 0.911 | ||
| Variables | n (%) | n | % | n | % | p value |
|---|---|---|---|---|---|---|
| Menopause status | 647 (100.0) | |||||
| Pre | 330 (51.0) | 128 | 75.7 | 202 | 42.3 | <0.001 |
| Post | 317 (49.0) | 41 | 24.3 | 276 | 57.7 | |
| Comorbidities | 84 (12.5) | 3 | 3 | 78 | 34.4 | <0.001 |
| HT | 64 (9.9) | 3 | 3 | 61 | 26.9 | <0.001 |
| DM | 29 (4.5) | 0 | 0 | 29 | 12.8 | <0.001 |
| Tumor sidedness | 647 (100.0) | |||||
| Right | 338 (52.2) | 89 | 52.4 | 245 | 52.6 | 0.624 |
| Left | 309 (47.8) | 78 | 45.9 | 217 | 46.6 | |
| Stage | 647 (100.0) | |||||
| 2 | 324 (50.1) | 89 | 52 | 230 | 49.4 | 0.547 |
| 3 | 323 (49.9) | 82 | 48 | 236 | 50.6 | |
| Grade | 462 (71.4) | |||||
| 1 | 59 (12.8) | 18 | 13.7 | 39 | 12.1 | 0.849 |
| 2 | 251 (54.3) | 71 | 54.2 | 172 | 53.6 | |
| 3 | 152 (32.9) | 42 | 32.1 | 110 | 34.3 | |
| Receptor status | 647 (100.0) | |||||
| HR+ | 436 (67.4) | 107 | 63.3 | 325 | 72.5 | 0.026 |
| HER2+ | 170 (26.3) | 41 | 25.3 | 126 | 29.2 | 0.344 |
| TNBC | 95 (14.7) | 43 | 26.4 | 52 | 12.1 | <0.001 |
BMI, body mass index; HT, hypertension; DM, diabetes mellitus; HR, hormone receptor; HER2, human epidermal growth factor receptor 2; TNBC, triple-negative breast cancer.
According to BMI status, the median age was 43.0 years and 51.9 years in groups A and B, respectively (p < 0.001). Both hypertension and diabetes mellitus were more common in group B (both p < 0.001). The median tumor diameters were 30 mm and 25 mm in groups A and B, respectively (p = 0.175). The numbers of patients with stage II and III disease were 89 and 82, and 230 and 236, in groups A and B, respectively (p = 0.547). The median Ki-67 levels and histological grades were similar in both groups (p = 0.911 and p = 0.849, respectively). There were more patients with HR-positive breast cancer in group B (n = 325) (p = 0.026). The rate of HER2 positivity was similar in both groups (p = 0.344). The numbers of patients with TNBC were 43 (26.4%) and 52 (12.1%) in groups A and B, respectively (p < 0.001). The number of patients with >90% response was statistically higher in group A than in group B (p = 0.026; Table 1).
In the univariate analysis, age, menopause status, tumor diameter, stage, histological grade, Ki-67, ER, PR, and HER2 status, and BMI were assessed to determine which of these factors were associated with a ≥90% response. Stage, HER2 positivity, TNBC, grade, Ki-67 levels, and lower BMI were found to be the statistically significant factors (p = 0.034, p < 0.001, p = 0.002, p < 0.001, p < 0.001, and p = 0.026, respectively; Table 2). HR positivity and higher BMI were associated with poor pathological response (p < 0.001, and p = 0.026, respectively; Table 2).
Table 2.
Univariate analysis for pathological response
| Variables | Miller response |
||||
|---|---|---|---|---|---|
| <90% (n = 382) mean ± SD |
≥90% (n = 265) mean ± SD |
p value | |||
| Age, years | 49.6±10.7 (49) | 48.5±11.3 (49) | 0.299 | ||
| BMI, kg/m2 | 28.3±5.1 (28.2) | 28.6±5.9 (28.5) | 0.874 | ||
| Tumor size, cm | 31.3±19.0 (28) | 31.7±22.3 (28.5) | 0.938 | ||
| Ki-67, % | 31.5±23.7 (25) | 42.9±25.8 (35) | <0.001 | ||
| Variables | n | % | N | % | p value |
|---|---|---|---|---|---|
| Menopause status | |||||
| Pre | 189 | 53.4 | 118 | 49 | 0.289 |
| Post | 165 | 46.6 | 123 | 51 | |
| Comorbidities | 54 | 26.3 | 30 | 23.3 | 0.605 |
| HT | 42 | 20.5 | 25 | 19.4 | 0.806 |
| DM | 21 | 10.2 | 8 | 6.2 | 0.201 |
| Tumor sidedness | |||||
| Right | 190 | 49.7 | 147 | 55.9 | 0.156 |
| Left | 189 | 49.5 | 112 | 42.6 | |
| Stage | |||||
| 2 | 178 | 46.6 | 146 | 55.1 | 0.034 |
| 3 | 204 | 53.4 | 119 | 44.9 | |
| Grade | |||||
| 1 | 52 | 16.2 | 7 | 5 | <0.001 |
| 2 | 159 | 60.5 | 92 | 46.2 | |
| 3 | 56 | 21.3 | 96 | 48.2 | |
| Receptor status | |||||
| HR+ | 292 | 77 | 144 | 58.1 | <0.001 |
| HER2+ | 53 | 14.8 | 117 | 47.6 | <0.001 |
| TNBC | 46 | 12.9 | 55 | 22.3 | 0.002 |
| BMI, kg/m2 | |||||
| <25 | 94 | 23.6 | 87 | 31.5 | 0.026 |
| ≥25 | 288 | 76.4 | 178 | 68.5 |
BMI, body mass index; HT, hypertension; DM, diabetes mellitus; HR, hormone receptor; HER2, human epidermal growth factor receptor 2; TNBC, triple-negative breast cancer.
In the multivariate analysis that included statistically significant variables in the univariate analysis, grade III disease, HER2 positivity, and TNBC were found to be the factors associated with increased pathological response, and HR positivity and higher BMI were associated with decreased pathological response in patients receiving NACT for breast cancer (Table 3).
Table 3.
Multivariate analysis for ≥90% pathological response
| p value | OR | 95% CI | |||
|---|---|---|---|---|---|
| Grade (ref: 1) | 0.001 | ||||
| 2 | 0.1 | 4.079 | 0.765 | 21.76 | |
| 3 | 0.007 | 10.638 | 1.93 | 58.631 | |
|
| |||||
| Ki-67 | 0.715 | 1.002 | 0.99 | 1.015 | |
|
| |||||
| Receptor status (ref: TNBC) | <0.001 | ||||
| HR (+). HER2 (+) | 0.061 | 2.157 | 0.965 | 4.821 | |
| HR (+). HER2 (−) | 0.009 | 0.32 | 0.136 | 0.753 | |
| HR (−). HER2 (+) | <0.001 | 6.999 | 2.663 | 18.396 | |
|
| |||||
| BMI (ref: <25) | ≥25 | 0.006 | 0.401 | 0.209 | 0.768 |
TNBC, triple-negative breast cancer; HR, hormone receptor; HER2, human epidermal growth factor receptor 2; BMI, body mass index.
Discussion
This retrospective study was performed to determine the relationship between BMI and pathological response in patients receiving NACT for stage II/III breast cancer. We found that a higher BMI was a negative predictor for pCR regardless of the molecular subtype in patients with breast cancer. Also, we found that the prevalence of HR-positive breast cancer and TNBC were higher in overweight or obese patients, but the HER2 status, Ki-67 levels, tumor diameters, grades, and stages were similar in both groups.
Molecular mechanisms between high BMI and poor outcomes in breast cancer are not fully understood. Obese/overweight women have higher circulating estrogen levels as compared to those with normal weight [22]. Obese patients also have higher levels of circulating insulin, which is associated with insulin resistance and high insulin-like growth factor levels that could potentially stimulate mechanisms of tumor cell survival [23, 24]. Hyperinsulinemia accompanying insulin resistance is associated with increased incidence and mortality of breast cancer [25]. In addition, chronic activation of nuclear factor kappa B in adipose tissue could stimulate breast cancer cell proliferation, invasion, angiogenesis, and metastasis [26]. Studies have also shown that obesity can induce immune dysfunction, specifically programed cell death protein 1-mediated T-cell dysfunction [27].
Some studies have focused on the relationship between obesity and pCR in patients with breast cancer. Obese and overweight patients are reportedly more likely to present with larger tumors and more advanced clinical stages at diagnosis than normal or underweight patients [17, 28]. However, this association was not observed in other studies [29, 30]. Two previous Turkish studies found no association between BMI and tumor stage and size [18, 31]. A few studies reported that HR-positive tumors are more prevalent in overweight or obese patients, whereas others found that HR positivity was more prevalent in normal and underweight patients [17, 30]. In fact, no difference was observed in some studies [32, 33]. Given that patient characteristics are similar between our study and previous studies, these conflicting reports may be explained by differences in ethnicities.
In the NACT setting, some trials have evaluated the effects of BMI on the pathological response to NACT in patients with breast cancer. In a Korean study, 438 stage II and III breast cancer patients who had received NACT were evaluated to assess the predictive effects of BMI on pCR, which showed that BMI was not a predictor for pCR [19]. Similarly, in a study of 324 patients who had received NACT for nonmetastatic breast cancer, BMI was not a predictive marker of pCR [33]. Another study of 110 patients who were younger than 35 years at diagnosis and treated with NACT found no association between BMI and pCR [18]. Furthermore, in a pooled analysis of 1,797 women enrolled in four NACT trials (CALGB 40601, 40603; ACOSOG Z1041, Z1071), overweight and obese women had lower pCR rates in ER+/HER2+ cancers but higher pCR rates in ER/HER2+ cancers; overall, there was no major difference in pCR rates by BMI [34]. Inversely, some trials have shown that higher BMI was associated with poorer response to NACT in patients with breast cancer. In a study of 235 Turkish patients who received NACT for breast cancer, obesity was an important independent prognostic factor that has an adverse effect on pCR [31]. A 2019 study of 243 patients found that pCR was related to lower BMI [35]. In a pooled analysis of 8,872 patients from eight prospective studies, the pCR rate was higher in normal-weight patients compared with all other BMI groups. In addition, it was shown that the pCR rate decreased with increasing BMI [36]. A Chinese study found that a higher BMI was associated with a worse response of breast cancer to NACT [15]. Similar results have been observed in older studies [17, 37]. These conflicting results may be caused by differences in treatment regimens. In our study, we enrolled only patients who received anthracycline- and taxane-based chemotherapy (plus trastusumab for HER2-positive patients). However, a more likely explanation for this difference may be insulin resistance. It is well known that abdominal obesity is a more important indicator of insulin resistance than a high BMI [38]. Some studies have shown that insulin resistance, higher fasting plasma glucose, and visceral adiposity were related to poorer response to NACT in breast cancer [32, 39, 40]. This might also explain the higher rate of hypertension and diabetes mellitus in group B in our study.
Our trial has a few limitations. This study was retrospective in nature, which might lead to several biases. BMI could change during the course of NACT. Hence, the importance of this change requires a prospective study design. The absence of other parameters to indicate abdominal obesity may cause conflicting results. In our study, the mean BMI value of the patients was 28.4 ± 5.5; therefore, we divided the patients into two groups according to their BMI. In addition, although the low mean age of our patients and the high rate of HER2 positivity may seem as biases, these can be explained by the fact that the number of patients who are suitable for NACT is higher in these subgroups. Nevertheless, in the studies summarized above, the effects of BMI on predicting the response to NACT have been shown to be different in different ethnicities. Thus, our study is important because it is the first multicenter study to show the predictive role of BMI in the response to NACT for each subtype of breast cancer in Turkish patients.
In conclusion, our results show that a higher BMI is associated with a poor response to NACT in breast cancer patients. The findings presented in this study may guide novel studies to examine the NACT response in obese patients with and without insulin resistance.
Statement of Ethics
The study was performed in accordance with the Declaration of Helsinki. The patients give a written informed consent before the study. Both patient consent and the approval from the Health Sciences University and Okmeydani Training and Research Hospital Ethics Committee approval were received (IRB approval number: 50200903-903.07.02).
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
There is no grant funding for this study.
Author Contributions
Levent Emirzeoglu and Serdar Arici designed and managed the study, performed the analyses, and wrote the manuscript. Levent Emirzeoglu, Serdar Arici, Ahmet Bilgehan Sahin, Birol Ocak, Naziye Ak, Seval Ay, Elkhan Mammadov, Hande Turna, and Ahmet Bilici extracted the data. Ahmet Bilgehan Sahin, Birol Ocak, and Naziye Ak provided comments on drafts of the manuscript. Ahmet Bilici revised the article. All the authors agreed to be accountable for the work.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.
Funding Statement
There is no grant funding for this study.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics 2018. CA, Cancer J Clin. 2018 Jan;68((1)):7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 2.Mieog JSD, van der Hage JA, van de Velde CJH, Charehbili A. Preoperative chemotherapy for women with operable breast cancer. Cochrane Database Syst Rev. 2007;2007((2)):CD005002. doi: 10.1002/14651858.CD005002.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Von Minckwitz G, Untch M, Blohmer JU, Costa SD, Eidtmann H, Fasching PA, et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol. 2012;30((15)):1796–1804. doi: 10.1200/JCO.2011.38.8595. [DOI] [PubMed] [Google Scholar]
- 4.Liedtke C, Mazouni C, Hess KR, André F, Tordai A, Mejia JA, et al. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol. 2008 Mar;26((8)):1275–1281. doi: 10.1200/JCO.2007.14.4147. [DOI] [PubMed] [Google Scholar]
- 5.Ogston KN, Miller ID, Payne S, Hutcheon AW, Sarkar TK, Smith I, et al. A new histological grading system to assess response of breast cancers to primary chemotherapy prognostic significance and survival. Breast. 2003;12((5)):320–327. doi: 10.1016/s0960-9776(03)00106-1. [DOI] [PubMed] [Google Scholar]
- 6.Cortazar P, Zhang L, Untch M, Mehta K, Costantino JP, Wolmark N, et al. Pathological complete response and long-term clinical benefit in breast cancer the CTNeoBC pooled analysis. Lancet. 2014;384((9938)):164–172. doi: 10.1016/S0140-6736(13)62422-8. [DOI] [PubMed] [Google Scholar]
- 7.Arici S, Karyagar SS, Karyagar S, Geredeli C, Cekin R, Seçmeler Ş, et al. The predictive role of metabolic tumor volume on no response to neoadjuvant chemotherapy in patients with breast cancer. J Oncol Pharm Pract. 2020;26((6)):1415–1420. doi: 10.1177/1078155219898504. [DOI] [PubMed] [Google Scholar]
- 8.Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL. Trends in obesity among adults in the United States 2005 to 2014. JAMA. 2016;315((21)):2284–2291. doi: 10.1001/jama.2016.6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kroenke CH, Chen WY, Rosner B, Holmes MD. Weight weight gain and survival after breast cancer diagnosis. J Clin Oncol. 2005;23((7)):1370–1378. doi: 10.1200/JCO.2005.01.079. [DOI] [PubMed] [Google Scholar]
- 10.Protani M, Coory M, Martin JH. Effect of obesity on survival of women with breast cancer systematic review and meta-analysis. Breast Cancer Res Treat. 2010;123((3)):627–635. doi: 10.1007/s10549-010-0990-0. [DOI] [PubMed] [Google Scholar]
- 11.Yung RL, Ligibel JA. Obesity and breast cancer risk, outcomes, and future considerations. Clin Adv Hematol Oncol. 2016;14((10)):790–797. [PubMed] [Google Scholar]
- 12.Caan BJ, Kwan ML, Hartzell G, Castillo A, Slattery ML, Sternfeld B, et al. Pre-diagnosis body mass index post-diagnosis weight change and prognosis among women with early stage breast cancer. Cancer Causes Control. 2008;19((10)):1319–1328. doi: 10.1007/s10552-008-9203-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Majed B, Moreau T, Senouci K, Salmon RJ, Fourquet A, Asselain B. Is obesity an independent prognosis factor in woman breast cancer? Breast Cancer Res Treat. 2008;111((2)):329–342. doi: 10.1007/s10549-007-9785-3. [DOI] [PubMed] [Google Scholar]
- 14.van den Brandt PA, Spiegelman D, Yaun SS, Adami HO, Beeson L, Folsom AR, et al. Pooled analysis of prospective cohort studies on height and breast cancer risk. Am J Epidemiol. 2000;152((6)):514–527. doi: 10.1093/aje/152.6.514. [DOI] [PubMed] [Google Scholar]
- 15.Chen S, Chen CM, Zhou Y, Zhou RJ, Yu KD, Shao ZM. Obesity or overweight is associated with worse pathological response to neoadjuvant chemotherapy among Chinese women with breast cancer. PLoS One. 2012;7((7)):e41380. doi: 10.1371/journal.pone.0041380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ewertz M, Jensen MB, Gunnarsdóttir KÁ, Højris I, Jakobsen EH, Nielsen D, et al. Effect of obesity on prognosis after early-stage breast cancer. J Clin Oncol. 2011;29((1)):25–31. doi: 10.1200/JCO.2010.29.7614. [DOI] [PubMed] [Google Scholar]
- 17.Litton JK, Gonzalez-Angulo AM, Warneke CL, Buzdar AU, Kau SW, Bondy M, et al. Relationship between obesity and pathologic response to neoadjuvant chemotherapy among women with operable breast cancer. J Clin Oncol. 2008;26((25)):4072–4077. doi: 10.1200/JCO.2007.14.4527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eralp Y, Smith TL, Altundağ K, Kau SW, Litton J, Valero V, et al. Clinical features associated with a favorable outcome following neoadjuvant chemotherapy in women with localized breast cancer aged 35 years or younger. J Cancer Res Clin Oncol. 2009;135((1)):141–148. doi: 10.1007/s00432-008-0428-9. [DOI] [PubMed] [Google Scholar]
- 19.Lee KH, Keam B, Im SA, Kim TY, Han SW, Oh DY, et al. Body mass index is not associated with treatment outcomes of breast cancer patients receiving neoadjuvant chemotherapy Korean data. J Breast Cancer. 2012;15((4)):427–433. doi: 10.4048/jbc.2012.15.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huober J, Von Minckwitz G, Denkert C, Tesch H, Weiss E, Zahm DM, et al. Effect of neoadjuvant anthracycline–taxane-based chemotherapy in different biological breast cancer phenotypes overall results from the GeparTrio study. Breast Cancer Res Treat. 2010;124((1)):133–140. doi: 10.1007/s10549-010-1103-9. [DOI] [PubMed] [Google Scholar]
- 21.Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, et al. American Society of clinical oncology/college of American pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer (unabridged version) Arch Pathol Lab Med. 2010;134((7)):e48–e72. doi: 10.5858/134.7.e48. [DOI] [PubMed] [Google Scholar]
- 22.Ligibel JA, Strickler HD. Obesity and its impact on breast cancer tumor incidence, recurrence, survival, and possible interventions. Am Soc Clin Oncol Educ Book. 2013:52–59. doi: 10.14694/EdBook_AM.2013.33.52. [DOI] [PubMed] [Google Scholar]
- 23.Goodwin PJ, Ennis M, Bahl M, Fantus IG, Pritchard KI, Trudeau ME, et al. High insulin levels in newly diagnosed breast cancer patients reflect underlying insulin resistance and are associated with components of the insulin resistance syndrome. Breast Cancer Res Treat. 2009;114((3)):517–525. doi: 10.1007/s10549-008-0019-0. [DOI] [PubMed] [Google Scholar]
- 24.Kang C, LeRoith D, Gallagher EJ. Diabetes and breast cancer. Endocrinology. 2018;159((11)):3801–3812. doi: 10.1210/en.2018-00574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Irwin ML, Duggan C, Wang CY, Smith AW, McTiernan A, Baumgartner RN, et al. Fasting C-peptide levels and death resulting from all causes and breast cancer the health, eating, activity, and lifestyle study. J Clin Oncol. 2011;29((1)):47–53. doi: 10.1200/JCO.2010.28.4752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prasad S, Ravindran J, Aggarwal BB. NF-κB and cancer how intimate is this relationship. Mol Cell Biochem. 2010;336((1–2)):25–37. doi: 10.1007/s11010-009-0267-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Z, Aguilar EG, Luna JI, Dunai C, Khuat LT, Le CT, et al. Paradoxical effects of obesity on T cell function during tumor progression and PD-1 checkpoint blockade. Nat Med. 2019;25((1)):141–151. doi: 10.1038/s41591-018-0221-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moorman PG, Jones BA, Millikan RC, Hall IJ, Newman B. Race anthropometric factors and stage at diagnosis of breast cancer. Am J Epidemiol. 2001;153((3)):284–291. doi: 10.1093/aje/153.3.284. [DOI] [PubMed] [Google Scholar]
- 29.Wasserman L, Flatt SW, Natarajan L, Laughlin G, Matusalem M, Faerber S, et al. Correlates of obesity in postmenopausal women with breast cancer comparison of genetic, demographic, disease-related, life history and dietary factors. Int J Obes Relat Metab Disord. 2004;28((1)):49–56. doi: 10.1038/sj.ijo.0802481. [DOI] [PubMed] [Google Scholar]
- 30.Chagpar AB, McMasters KM, Saul J, Nurko J, Martin RC, Scoggins CR, et al. Body mass index influences palpability but not stage of breast cancer at diagnosis. Am Surgeon. 2007;73((6)):555–560. [PubMed] [Google Scholar]
- 31.Karatas F, Erdem GU, Sahin S, Aytekin A, Yuce D, Sever AR, et al. Obesity is an independent prognostic factor of decreased pathological complete response to neoadjuvant chemotherapy in breast cancer patients. Breast. 2017;32:237–244. doi: 10.1016/j.breast.2016.05.013. [DOI] [PubMed] [Google Scholar]
- 32.Omarini C, Palumbo P, Pecchi A, Draisci S, Balduzzi S, Nasso C, et al. Predictive role of body composition parameters in operable breast cancer patients treated with neoadjuvant chemotherapy. Cancer Manag Res. 2019;11:9563–9569. doi: 10.2147/CMAR.S216034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Erbes T, Stickeler E, Rücker G, Buroh S, Asberger J, Dany N, et al. BMI and pathologic complete response to neoadjuvant chemotherapy in breast cancer a study and meta-analysis. Clin Breast Cancer. 2016;16((4)):e119–e132. doi: 10.1016/j.clbc.2016.02.018. [DOI] [PubMed] [Google Scholar]
- 34.Warner ET, Ballman KV, Strand C, Boughey JC, Buzdar AU, Carey LA, et al. Impact of race and BMI on achievement of pathologic complete response following neoadjuvant chemotherapy for breast cancer a pooled analysis of four prospective alliance clinical trials (A151426) Breast Cancer Res Treat. 2016;159((1)):109–118. doi: 10.1007/s10549-016-3918-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Usiskin I, Li F, Irwin ML, Cartmel B, Sanft T. Association between pre-diagnosis BMI physical activity pathologic complete response and chemotherapy completion in women treated with neoadjuvant chemotherapy for breast cancer. Breast Cancer. 2019;26((6)):719–728. doi: 10.1007/s12282-019-00974-3. [DOI] [PubMed] [Google Scholar]
- 36.Fontanella C, Lederer B, Gade S, Vanoppen M, Blohmer JU, Costa SD, et al. Impact of body mass index on neoadjuvant treatment outcome a pooled analysis of eight prospective neoadjuvant breast cancer trials. Breast Cancer Res Treat. 2015;150((1)):127–139. doi: 10.1007/s10549-015-3287-5. [DOI] [PubMed] [Google Scholar]
- 37.Del Fabbro E, Parsons H, Warneke CL, Pulivarthi K, Litton JK, Dev R, et al. The relationship between body composition and response to neoadjuvant chemotherapy in women with operable breast cancer. Oncologist. 2012;17((10)):1240–1245. doi: 10.1634/theoncologist.2012-0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the metabolic syndrome a joint interim statement of the international diabetes federation task force on epidemiology and prevention; national heart, lung, and blood institute; American heart association; world heart federation; international atherosclerosis society; and international association for the study of obesity. Circulation. 2009;120((16)):1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 39.Alan O, Akin Telli T, Aktas B, Koca S, Ökten IN, Hasanov R, et al. Is insulin resistance a predictor for complete response in breast cancer patients who underwent neoadjuvant treatment? World J Surg Oncol. 2020;18((1)):242. doi: 10.1186/s12957-020-02019-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arici S, Geredeli C, Secmeler S, Cekin R, Sakin A, Cihan S. The effects of diabetes and fasting plasma glucose on treatment of breast cancer with neoadjuvant chemotherapy. Curr Probl Cancer. 2020;44((1)):100485. doi: 10.1016/j.currproblcancer.2019.05.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.


