Abstract
Purpose
Treatment options for pancreatic ductal adenocarcinoma (PDAC) are commonly limited for patients with advanced age due to medical comorbidities and/or poor performance status. These patients may not be candidates for more aggressive chemotherapy regimens and/or surgical resection leaving few, if any, other effective treatments. Ablative stereotactic MRI-guided adaptive radiation therapy (A-SMART) is both efficacious and safe for PDAC and can achieve excellent long-term local control, however, the appropriateness of A-SMART for elderly patients with inoperable PDAC is not well understood.
Methods
A retrospective analysis was performed of inoperable non-metastatic PDAC patients aged 75 years or older treated on the MRIdian Linac at 2 institutions. Clinical outcomes of interest included overall survival (OS), progression-free survival (PFS), distant metastasis-free survival (DMFS), and locoregional (LRC). Toxicity was graded according to Common Terminology Criteria for Adverse Events (CTCAE, v5).
Results
A total of 49 patients were evaluated with a median age of 81 years (range, 75-91) and a median follow-up of 14 months from diagnosis. PDAC was classified as locally advanced (46.9%), borderline resectable (36.7%), or medically inoperable (16.3%). Neoadjuvant chemotherapy was delivered to 84% of patients and all received A-SMART to a median 50 Gy (range, 40-50 Gy) in 5 fractions. 1 Year LRC, PFS, and OS were 88.9%, 53.8%, and 78.9%, respectively. Nine patients (18%) had resection after A-SMART and benefited from PFS improvement (26 vs 6 months, P = .01). ECOG PS <2 was the only predictor of improved OS on multivariate analysis. Acute and late grade 3 + toxicity rates were 8.2% and 4.1%, respectively.
Conclusions
A-SMART is associated with encouraging LRC and OS in elderly patients with initially inoperable PDAC. This novel non-invasive treatment strategy appears to be well-tolerated in patients with advanced age and should be considered in this population that has limited treatment options.
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is the third leading cause of cancer mortality in the United States and is responsible for over 49,000 deaths and 62,000 new cases annually.1 PDAC projects to become the second leading cause of cancer mortality by 2030.2 PDAC mortality is driven by a variety of factors including the lack of an effective screening method that leads to advanced disease at presentation and relatively slow progress in therapeutic advances. Moreover, as the median age of diagnosis of PDAC is approximately 70 years, a significant portion of this patient population suffers from age-related comorbidities and as a result may have limited treatment options. PDAC patients are frequently inoperable due to disease extent or medical comorbidities, and therefore will be offered chemotherapy and potentially radiation therapy (RT).
Elderly patients often have limited treatment options regardless of the extent of their disease due to multiple medical comorbidities and increased baseline fragility3,4 and are underrepresented in clinical PDAC trials.5 Even in the absence of metastatic disease, overall survival (OS) for these patients can be expected in the range of 12-15 months for both resectable and borderline resectable pancreatic cancer (BRPC).4,6 Only 10-15% of patients at presentation have surgery as a treatment option and these patients have an increased risk of early mortality with upfront resection.4 Other patients are only offered either single-agent chemotherapy or supportive care.7 Historically, concurrent chemoradiotherapy (CRT) has been associated with no difference in OS but a modest benefit in local control.8 However, CRT is generally well tolerated and has been associated with an OS of 11.3 months for patients with unresectable PDAC.9 However, definitive CRT is usually delivered over 5 to 6 weeks, which is a burden for patients with short life expectancies. Stereotactic body radiotherapy (SBRT) has inherent improved patient convenience and can be easily sequenced with minimal interruptions to systemic therapy; thus, making it an attractive therapy for elderly patients with PDAC.10,11
While the radiation dose prescribed for inoperable PDAC has historically been in a non-ablative range to prioritize patient safety because of nearby gastrointestinal (GI) luminal structures, advances in motion management and image guidance have permitted significant dose escalation, which may be associated with improved OS.12,13 Several recent studies have demonstrated favorable long-term efficacy and safety using ablative RT delivered in a moderately hypofractionated or ultra-hypofractionated manner, typically following induction chemotherapy.12-14 The recent advent of magnetic resonance guided radiotherapy (MRgRT) has allowed for dose escalation via ablative dose stereotactic magnetic resonance image-guided adaptive radiation therapy (A-SMART) due to its superior soft tissue visualization, daily on-table adaptive re-planning, and automatic beam gating based on target position.15-17 It is hypothesized that the ability to account for interfraction anatomic changes and intrafraction motion management with real-time gating will facilitate safe isotoxic dose escalation with ultra-hypofractionation. An additional benefit of A-SMART is that fiducial markers are not required and thus complications regarding their placement will not impede a patient’s ability to receive RT.18 However, there is concern within the community of oncology specialists that dose escalated SBRT poses a greater risk of toxicity when compared to more conventional forms of radiotherapy for elderly patients with inoperable PDAC due to their generally higher baseline fragility compared to younger cohorts.
Due to improvements in treatment delivery afforded by MRgRT and concerns regarding increased toxicity, we sought to assess our early collective experience of A-SMART for elderly patients with inoperable and non-metastatic PDAC.
Materials and Methods
After obtaining institutional review board (IRB) approval, a retrospective analysis was performed at 2 institutions of medically inoperable, locally advanced PDAC (LAPC), and BRPC patients aged 75 years or older at time of diagnosis and treated on the .35 T (T) MRIdian Linac (ViewRay, Oakwood Village, OH) from September 2018 to May 2019. This study did not include any patients with metastatic disease at any time prior to radiotherapy. Due to the retrospective nature of this study, written consent was not needed and both institutions proceeded under IRB-approved protocols. All patients were deidentified. Staging was performed with endoscopic ultrasound and computerized tomography scans of the chest, abdomen/pancreas protocol, and pelvis (CT CAP) with an optional diagnostic magnetic resonance (MR) scan of the abdomen or positron emission tomography (PET/CT) scan. National Comprehensive Cancer Network (NCCN) guidelines were used to define resectability. After completion of A-SMART, patient tumors were reassessed for resectability. Patients who were found to have tumors that converted to a resectable subtype were then taken to surgery. Induction chemotherapy was considered for all patients before A-SMART, although was not offered to some based on suboptimal performance status.
RT simulation included both MR and CT scans acquired in the treatment position, which typically was supine with both arms at the sides for patient comfort and setup reproducibility. The primary scan for treatment planning was the true fast imaging with steady-state free precession (TRUFI) sequence obtained on the MRIdian Linac in breath hold. A TRUFI scan with contours is demonstrated in Figure 1. A CT scan was obtained immediately after in the same treatment position and used for electron density measurements. Contrast enhanced CT was optional and used to help with target volume delineation. Fiducial markers are not required for MRgRT and were not used. Patients were instructed to refrain from having any oral intake for at least 2 – 3 hours prior to both simulation and treatments. An isotoxicity approach, ie, maximizing the dose delivered to the target above the prescription dose as far as OAR restrictions would allow for, was used where the plan was normalized to the closest GI organ at risk (OAR), which routinely required sacrificing target volume coverage. Diagnostic CT and MR imaging were used to guide gross tumor volume (GTV) delineation. Elective volume coverage was routinely used at only 1 institution during the time of this study, usually including a 5-10 mm margin around at least the proximal 2-3 cm of the celiac artery (CA) and superior mesenteric artery (SMA). A 3 mm planning target volume (PTV) margin was standard at both institutions.
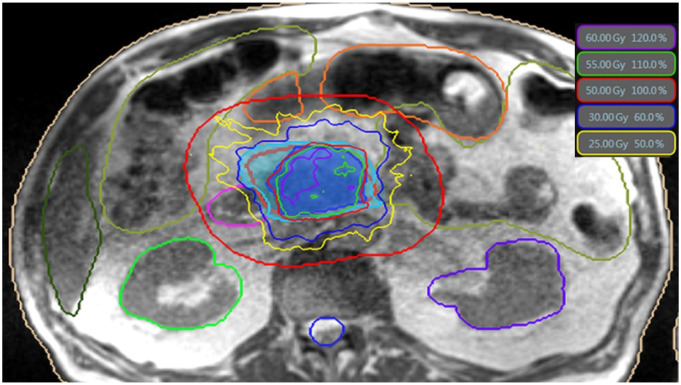
Figure 1.
Example daily online adaptive treatment plan on ViewRay planning software for LAPC. This is an axial slice through the abdomen on the TRUFI MR sequence with isodose lines color-coded (isodose legend in top right of figure). Prescription isodose line is 50 Gy/100% in red. PTV is isotropically expanded by 3 cm to create an OAR eval structure, represented by larger red ring surrounding the target, within which the OAR is recontoured daily. Contoured OAR from left to right are liver (hunter green), bowel (lizard green), right kidney (lime green), duodenum (pink), stomach (orange), spinal cord (blue), left kidney (purple).
Details regarding the daily online adaptive replanning technique and OAR constraints at both institutions have been previously published.19,20 Briefly, on-table adaptive replanning was performed for each fraction if the current day’s anatomy would lead to OAR constraint violations using the original plan and/or if plan reoptimization could significantly increase target volume coverage while meeting OAR constraints. OAR were recontoured daily and a planning OAR volume (PRV) was generated by a 3-5 mm expansion. This avoidance structure was subtracted from the PTV to generate a PTVopti structure. Density structures were then added to account for any changes in density. Max point dose constraints for bowel, stomach, and duodenum were <39.5 Gy, <38 Gy, and <38 Gy at 1 center, respectively, and ≤39.5 for these structures in the other. Mean dose constraints for bowel and kidneys were <25 Gy and <10 Gy, respectively, for both centers. The stomach, duodenum, and bowel all had V32 Gy ≤ 2 cc and V35 Gy ≤ .5 cc for both centers. Similar dose constraints and plan adaptation triggers have been reported for A-SMART by other institutions.21-23
All patients were treated in 5 fractions. Continuous intrafraction cine-MRI scans acquired in the sagittal plane were performed throughout treatment. Patients were treated using soft tissue tracking of the primary pancreatic tumor using a manually delineated “tracking structure” in the sagittal plane. Treatment delivery was automatically held by the treatment machine when >5% of the tracking structure exceeded a 3 mm boundary threshold outside of the intended treatment position. Treatment was typically given using a breath hold technique and otherwise with free breathing respiratory gating.
Treatment response was defined using RECIST 1.1 criteria. Common Terminology Criteria for Adverse Events version 5 was used to assess toxicity with acute toxicity being defined as occurring during or within 90 days after completion of RT. Follow-up was performed every 3 months post-treatment with restaging imaging studies.
Multi-disciplinary evaluation of resectability after A-SMART was typically done at least once every 2-3 months upon restaging imaging studies being completed. Resected tumor specimens were examined at the time of grossing to confirm the anatomic tumor site. If there was no residual grossly visible tumor, then the residual area with fibrosis was submitted for further evaluation. All the sections derived from the tumor bed are assessed by a board-certified pathologist. Tumor response was evaluated according to the College of American Pathologists tumor regression grading criteria (CAP-TRG), ranging from CAP grade 0, indicating pathologic complete response (pCR), CAP grade 1, indicating marked response (minimal residual cancer with single cells or small groups of cancer cells), CAP grade 2, indicating moderate response (residual cancer outgrown by fibrosis), and CAP grade 3, indicating no response (extensive residual cancer).24
Statistical Analyses
Clinical outcomes included OS calculated from the first date of A-SMART and censored at the last documented clinical follow-up. Progression-free survival (PFS), distant metastasis-free survival (DMFS), and locoregional control (LRC) were calculated from the first date of A-SMART and censored at last PET/CT, MRI, or CT CAP. Progression was coded at the date of biopsy-proven pathological confirmation if available, otherwise, date of radiographical progression with the elevation of tumor markers or treating oncologist clinical suspicion to initiate or alter systemic therapy was sufficient. The Kaplan-Meier method and log-rank test were used to assess the significance between groups. Univariate Cox analysis (UVA) and multivariable Cox analysis (MVA) was performed to evaluate associations between the clinical variables with clinical outcomes.
Results
Patient, tumor, and treatment characteristics are described in Table 1. Briefly, 49 patients were evaluated with a median age of 81years (range, 75 - 91). Most had favorable Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0 to 1 (90%), and most commonly, locally advanced disease (46.9%). All patients were either medically inoperable or did not have resectable disease prior to the initiation of A-SMART. The median CA19-9 at diagnosis was 235.8 U/mL (range, 3 - 9204). Most patients received induction chemotherapy (84%), which most commonly consisted of a gemcitabine-based regimen (71%) for a median 3.3 months (range, .2 - 8.1). All patients completed 5 fractions with a median prescribed dose of 50 Gy (range, 40 - 50) and median biologically effective dose (BED10) was 100 Gy (range, 72 - 100). Adjuvant chemotherapy was given to 43% (n = 21) of patients.
Table 1.
Patient, Tumor, and Treatment Characteristics.
| Median/N (range/%) | |
|---|---|
| Age at diagnosis, year | 81 (75–91) |
| Gender | |
| Female | 21 (43%) |
| Male | 28 (57%) |
| ECOG PS | |
| 0 | 21 (43%) |
| 1 | 23 (47%) |
| 2 | 5 (10%) |
| Histology | |
| Adenocarcinoma | 49 (100%) |
| Tumor location (on pancreas) | |
| Head/neck | 42 (86%) |
| Body/tail | 7 (14%) |
| Tumor cT stage | |
| 1 | 3 (6%) |
| 2 | 18 (37%) |
| 3 | 4 (8%) |
| 4 | 24 (49%) |
| Tumor cN stage | |
| 0 | 42 (86%) |
| 1 | 6 (12%) |
| 2 | 1 (2%) |
| Resectability | |
| Medically inoperable | 8 (16%) |
| Borderline | 18 (37%) |
| Locally advanced | 23 (47%) |
| Initial CA19-9 Level (U/mL) | 235.8 (3–9204) |
| Neoadjuvant chemotherapy | |
| None | 8 (16%) |
| FOLFIRINOX | 6 (12%) |
| Gemcitabine | 4 (8%) |
| Gemcitabine/paclitaxel | 30 (61%) |
| Gemcitabine/paclitaxel/capecitabine | 1 (2%) |
| Duration, days | 98 (7–244) |
| RT details | |
| Total dose, Gy | 50 (40–50) |
| Number of fractions | 5 |
| BED α/β = 10, Gy | 100 (72–100) |
| BED α/β = 3, Gy | 216.7 (146.7–216.7) |
| EQD210, Gy | 83.3 (60–83.3) |
| EQD23, Gy | 130 (88–130) |
| Elective nodal irradiation | 17 (35%) |
| Post RT resection | |
| None | 40 (82%) |
| Pancreaticoduodenectomy | 8 (16%) |
| Distal pancreatectomy | 1 (2%) |
| Time from RT, days | 72 (48–309) |
| R0 | 9 (100%) |
| 90-day mortality | 0 |
| Adjuvant chemotherapy | 21 (43%) |
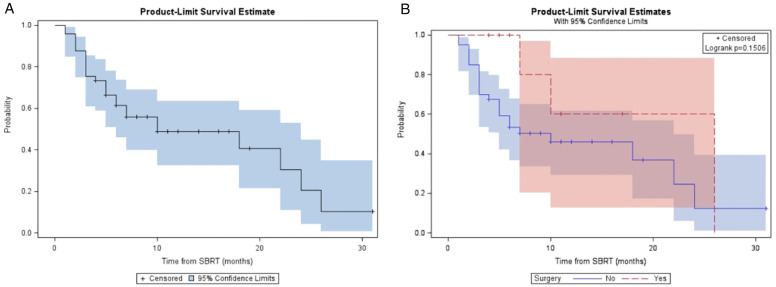
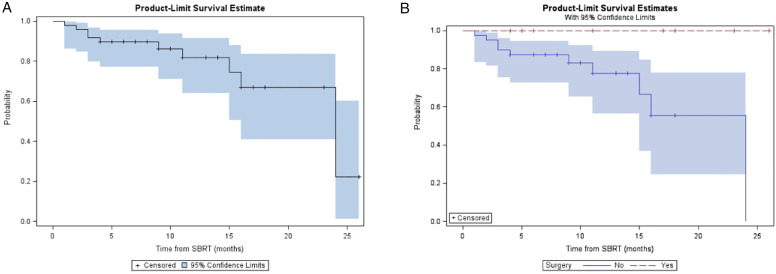
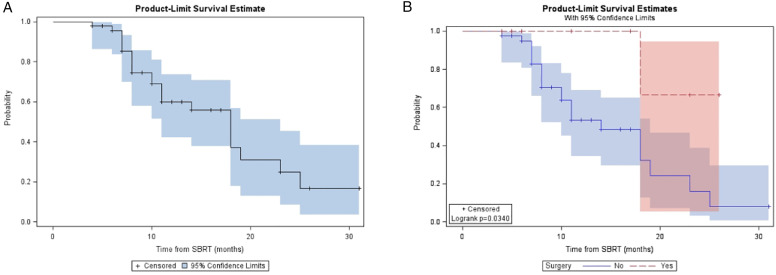
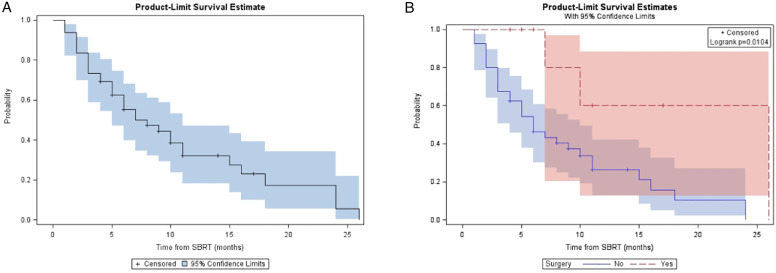
For the entire cohort, the median follow-up was 14 (range, 5 - 36) months from diagnosis and 10 (range, 4-31) months from A-SMART. LRC, DMFS, PFS, and OS at one year for the overall cohort were 81.9%, 48.9%, 32.2%, and 59.9% respectively (Table 2). Median LRC, DMFS, PFS, and OS for the overall cohort were 24, 10, 7, and 18 months, respectively. Figure 2, Figure 3, Figure 4 and Figure 5 demonstrate the Kaplan-Meier curves for LRC, DMFS, PFS, and OS, respectively, of the overall cohort and resected vs non-resected cohorts. Three patients (3/9; 33%) who underwent surgery developed distant metastatic progression but had no local recurrences. In the unresected cohort, twelve (12/40; 30%) patients developed distant metastatic progression, and seven (7/40; 18%) patients developed a locoregional recurrence.
Table 2.
Clinical Outcomes of LRC, DMFS, PFS, and OS from A-SMART.
| Combined Cohort | Resected Cohort | Non-resected Cohort | P Value* | |
|---|---|---|---|---|
| N | 49 | 9 | 40 | |
| LRC | ||||
| Median (months) | 24 (16 – NR) | 24 (15–24) | NR | NR |
| 1-year (%) | 81.9% | |||
| DMFS | ||||
| Median (months) | 10 (6 – 24) | 26 (7–26) | 10 (5–22) | .15 |
| 1-year (%) | 48.9% | |||
| PFS | ||||
| Median (months) | 7 (5-11) | 26 (7–26) | 6 (4–10) | .01 |
| 1-year (%) | 32.2% | |||
| OS | ||||
| Median (months) | 18 (11 – 23) | NR (18–NR) | 14 (10–19) | .03 |
| 1-year (%) | 59.9% | |||
NR: Not reached; *log-rank test between resected vs non-resected cohorts; Bold text indicates P-value <.05.
Figure 2.
(A) Kaplan-Meier plot of LRC measured from time of SMART for entire cohort. (B) Kaplan-Meier plot of LRC measured from time of SMART based on resection status.
Figure 3.
(A) Kaplan-Meier plot of DMFS measured from time of SMART for entire cohort. (B) Kaplan-Meier plot of DMFS measured from time of SMART based on resection status.
Figure 4.
(A) Kaplan-Meier plot of PFS measured from time of SMART for entire cohort. (B) Kaplan-Meier plot of PFS measured from time of SMART based on resection status.
Figure 5.
(A) Kaplan-Meier plot of OS measured from time of SMART for entire cohort. (B) Kaplan-Meier plot of OS measured from time of SMART based on resection status.
Surgery was performed in nine patients (18%) initially diagnosed with BRPC after a median of 72 (range, 48-309) days from A-SMART with all having negative margins and a 90-day postoperative mortality rate of 0%. Pancreaticoduodenectomy was performed in 89% (8/9) of patients and one patient had a distal pancreatectomy. Surgical patients had an ECOG PS of 0 to 1, with a majority (56%) having an ECOG PS of 0. Non-surgical patients had an ECOG PS of 0 to 2, with a minority (40%) having an ECOG PS of 0. Six of the surgical patients had neoadjuvant chemotherapy of with two receiving FOLRININOX and then switching to gemcitabine and paclitaxel, and four receiving gemcitabine and paclitaxel initially. Median neoadjuvant chemotherapy duration was 3.3 (range, 2.3 – 8.1) months. Five patients had TRG score of one (5/9; 56%), three had TRG score of 2 (3/9; 33%), and one patient had TRG score of 3 (1/9; 11%).
Five patients (5/49; 10%) had an ECOG status of 2. Three (3/5; 60%) received neoadjuvant chemotherapy that consisted of gemcitabine and paclitaxel (2/3; 66%) or gemcitabine monotherapy (1/3; 33%) for a median of 4.1 (range, 2.4 – 4.5) months. Two (2/5; 40%) patients had distant metastatic disease progression while none had locoregional progression. Three patients (3/5) died, two of them from disease progression and one from causes unrelated to A-SMART or chemotherapy-related toxicities.
ECOG PS <2 was the only significant predictor of improved OS on MVA with a trend towards significance for induction chemotherapy ≥3 months (Table 3). Surgical resection was the only significant predictor of improved PFS on MVA with a trend towards significance for RT dose of 50 Gy (Table 4). Female sex was the only significant predictor of improved LRC on MVA.
Table 3.
Survival Univariate and Multivariate Analysis.
| Variables | Univariate | Multivariate | ||
|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Age | 1.01 (.91–1.11) | .855 | — | |
| Sex | .708 | |||
| Male | Reference | — | ||
| Female | .85 (.35–2.03) | |||
| Performance status | .015 | — | .037 | |
| <2 | Reference | — | Reference | — |
| ≥2 | 4.87 (1.35–17.62) | — | 4.02 (1.0.8–14.88) | — |
| T Stage | .667 | — | ||
| 1 and 2 | Reference | — | ||
| 3 and 4 | .83 (.36–1.93) | |||
| N stage | .978 | — | ||
| 0 | Reference | — | ||
| 1 and 2 | .99 (.33–2.93) | |||
| Tumor location | .694 | — | ||
| Body/tail | Reference | — | ||
| Head | 1.34 (.31–5.77) | |||
| Induction chemotherapy | .953 | — | ||
| No | Reference | — | ||
| Yes | .96 (.28–3.28) | |||
| Induction chemotherapy duration | .044 | — | ||
| <3 months | Reference | — | Reference | .086 |
| ≥3 months | .4 (.16–.98) | — | .45 (.18–1.11) | — |
| Chemotherapy type | .262 | — | .149 | |
| Other | Reference | — | Reference | — |
| FOLFIRINOX | .32 (.04–2.37) | — | .22 (.02–1.71) | — |
| Surgery | .065 | — | ||
| No | Reference | — | ||
| Yes | .15 (.02–1.13) | |||
| RT dose | .109 | — | ||
| <50 Gy | Reference | — | ||
| 50 Gy | 2.81 (.79–9.98) | |||
| Elective coverage | .422 | — | ||
| No | Reference | — | ||
| Yes | .7 (.29–1.67) | |||
CI: confidence interval; HR: Hazard ratio.
Table 4.
Disease Progression Univariate and Multivariate Analysis.
| Variables | Univariate | Multivariate | ||
|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Age | .97 (.89–1.05) | .414 | — | |
| Sex | .792 | |||
| Male | Reference | — | ||
| Female | .91 (.45–1.83) | |||
| Performance status | .173 | — | ||
| <2 | Reference | — | ||
| ≥2 | 2.4 (.68–8.46) | |||
| T Stage | .185 | — | ||
| 1 and 2 | Reference | — | ||
| 3 and 4 | 1.63 (.79-3.38) | |||
| N stage | .109 | — | ||
| 0 | Reference | — | ||
| 1 and 2 | 2.09 (.85–5.13) | |||
| Tumor location | .430 | — | ||
| Body/tail | Reference | — | ||
| Head | 1.62 (.49–5.36) | |||
| Induction chemotherapy | .461 | — | ||
| No | Reference | — | ||
| Yes | 1.57 (.47–5.19) | |||
| Induction chemotherapy duration | .164 | — | ||
| <3 months | Reference | — | ||
| ≥3 months | .59 (.28-1.24) | |||
| Chemotherapy type | .862 | — | ||
| Other | Reference | — | ||
| FOLFIRINOX | .91 (.32–2.61) | |||
| Surgery | .025 | — | .045 | |
| No | Reference | — | Reference | — |
| Yes | .19 (.05–.82) | — | .22 (.05–.97) | — |
| Radiotherapy dose | .056 | — | .135 | |
| <50 Gy | Reference | — | Reference | — |
| 50 Gy | 3.30 (.97–11.23) | — | 2.54 (.74–8.64) | — |
| Elective coverage | .215 | — | ||
| No | Reference | — | ||
| Yes | .63 (.31–1.30) | |||
CI: confidence interval; HR: Hazard ratio.
Acute grade 3 or higher toxicity was experienced in 4 patients (8.2%). Acute grade 3 toxicity consisted of diarrhea (2%; 1/49), abdominal pain (2%; 1/49), and weakness (2%; 1/49). A single patient experienced an acute grade 4 gastric outlet obstruction that required gastrojejunostomy at 26 days after A-SMART. Late grade 3 toxicity was 4.1% and consisted of gastrointestinal bleeding (2%; 1/49) and duodenal ulceration (2%; 1/49) that occurred at 6 and 5 months after end of A-SMART, respectively. There were no late grade 4 or 5 toxicities. The rate of late grade 3 or higher toxicity in patients with at least one year of follow up is 4.9%.
Discussion
To our best knowledge, this is the first description of A-SMART outcomes in a cohort of initially inoperable PDAC patients with an age of at least 75 years. This cohort experienced 1-year OS, PFS, LRC, and DMFS rates of 59.9%, 32.2%, 81.9%, and 48.9% from A-SMART, respectively. In addition, there were low rates of clinically significant toxicity. Nearly 20% of our cohort were able to proceed to tumor resection and benefited from improvements in OS, PFS, and LRC (Table 2). These patients were fitter at baseline, with ECOG PS rates of 0 and 1 being 56% and 44%, respectively. The patients unable to undergo resection were able to maintain adequate locoregional control with a 1-year locoregional control rate of 77%. These patients tended to progress distantly. Our data suggest that treatment intensification with A-SMART for the elderly is safe and efficacious; thus, A-SMART should be considered as an option for these patients. These data also encourage enrollment of this traditionally underrepresented patient population5 on prospective trials for better assessment of A-SMART in elderly patients with inoperable PDAC.
SBRT has been shown to be well-tolerated and possibly more effective for PDAC than conventionally fractionated treatment.25 A recent meta-analysis demonstrated improved 2-year OS from 14% to 27% associated with SBRT compared to conventionally fractionated CRT, as well as a greater than 30% reduction of grade 3 and 4 toxicities.26 Multiple studies have shown SBRT to be safe and effective for elderly patients with PDAC.27-29 Given the limited options for elderly patients with PDAC and the efficacy of SBRT, the International Society of Geriatric Oncology (SIOG) has recommended SBRT for elderly patients that are medically inoperable.30 The American Society of Clinical Oncology (ASCO) has also included a recommendation of SBRT for patients with LAPC who have a decline in performance status in their clinical practice guidelines.31 These recommendations are due to the shortened course required for SBRT delivery and the favorable toxicity profile of SBRT vs conventional CRT.26 In addition, SBRT is an effective and efficient method of dose-escalation for PDAC.
There is a growing body of literature that suggests dose-escalation may improve LC and OS for inoperable PDAC patients.12,13,32-35 This is particularly important in an elderly population where successful resection may be limited by the degree of local tumoral involvement of critical surrounding structures and medical inoperability. LRC in PDAC patients is an important clinical outcome due to the significant impact on quality of life (QoL) with local progression. Local progression of these tumors often leads to worsening pain, pancreatic insufficiency, biliary obstruction, and early satiety/gastric outlet obstruction. Improvements in QoL with non-ablative SBRT are often due to better pain control, reduced biliary complications, better nutritional status, and reduced nausea.36 Ryan et al37 reported that SBRT (median dose of 28 Gy in 5 fractions) for an inoperable elderly population resulted in significant QoL improvements. SBRT provided symptom palliation of pain, fatigue, anorexia, weight loss, and nausea in 73%, 20%, 58%, 80%, and 100%, respectively, within 3 months. Most importantly, patients with the worst baseline performance statuses had the greatest benefit, thus emphasizing the importance of an effective radiotherapy modality for these patients. It is believed that all these QoL benefits are directly due to the local tumor control provided by radiotherapy. However, despite evidence for improvements in local control with increased radiotherapy dose for PDAC,13 there is concern for radiotherapy dose escalation in the frailer elderly populations with PDAC due to the higher rates of toxicities this population seen with other cytotoxic therapies, often requiring dose reductions to improve tolerability.38
Dose escalation for PDAC has traditionally been limited due to poor visualization of gross disease and surrounding radiosensitive GI OAR.39 SMART is a new modality that allows for the safe delivery of ablative (median BED10 of 100 Gy in our study) doses with minimal toxicities for PDAC due to its superior soft tissues visualization, daily on-table adaptive re-planning, and automatic beam gating based on target position.15-17 Furthermore, the benefits of SMART make it an ideal treatment option for a population with high morbidity rates, such as the elderly, especially if they are not candidates for other modalities, including surgery, irreversible electroporation, or chemotherapy. SMART can deliver high ablative doses, referred to as ablative-SMART (A-SMART), to the tumor within a 5-fraction regimen delivered daily, typically over 1 to 2 weeks, while working well within a chemotherapy and surgical resection paradigm. SMART also does not require fiducial marker placement, thereby decreasing the need for invasive procedures in a population that is at higher risk of complications and eliminates the need for internal target volumes (ITV) due to its real-time target tracking. A-SMART for PDAC has been shown to be safe and efficacious in initial retrospective reports.12,19-21 The first prospective data of A-SMART for inoperable PDAC were recently presented and was in agreement with prior reported outcomes.40 These initial data of this multi-center single-arm phase II trial (ClinicalTrials.gov Identifier: NCT03621644) of 136 patients met the primary objective of minimal acute toxicity and demonstrated a 1-year OS of 65% from A-SMART.40 In addition, nine patients within our cohort were successfully converted to surgical candidates after neoadjuvant chemotherapy and A-SMART and had excellent surgical outcomes with R0 resection rate of 100% and no post-surgical 90-day mortalities. These favorable surgical outcomes are in agreement with prior data for resected PDAC patients after A-SMART.20
Our early efficacy results compare favorably with prior studies that evaluated pancreatic SBRT in an elderly patient population (Table 5).28,29,41-43 There appears to be a significant improvement in LRC and OS with A-SMART over other historical cohorts for unresected patients. Specifically, A-SMART compares favorably to the Reddy et al unresected cohort, which delivered a non-ablative SBRT (BED10 = 54.8 Gy). There appears to be an improvement in OS of 14 months vs 7 months in favor of A-SMART despite the Reddy et al cohort being younger overall and having a significantly higher rate (52% vs 12%) of patients who underwent the more potent induction FOLFIRNOX chemotherapy regimen. While an indirect comparison, these data suggest that ablative dose radiotherapy contributed to the improved clinical outcomes within our cohort. Kim et al and Ryan et al both reported median and 1-year overall survival rates of 10 months, 8 months, 36%, and 28%, respectively, for inoperable patients.37,42 These rates are lower than our unresected cohort, who had a median and 1-year OS rate of 14 months and 53%, respectively. The improved survival benefit of our resected cohort is unsurprising considering that resection remains the only potential cure for these patients and is congruent with the rates of prior studies.20,29
Table 5.
Selected Studies of Stereotactic Body Radiation Therapy for Elderly Patients with Pancreas Adenocarcinoma.
| Study | Median Age at Dx (range) | RT Modality | Image Guidance | Gating | Stage | N | Median BED10 (range) Fx range | Median FU, m | Treatment | Overall survival | Local control | Acute toxicity Grading Scale | Late toxicity |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yechieli et al. 20171 | 83.2 y (77-90 y) | SBRT | Orthogonal portal films | NR | IA-IIIB | 20 | 59.5 Gy (48-79.2 Gy) | 5.3 | RT: 90% Surg+RT: 10% |
Median: 6.4 m 2-year: 7.7% |
LC:NR | G3+: 0% CTCAE v4.0 |
G2+: 15% |
| 3-5 Fx | |||||||||||||
| Zhu et al. 20172 |
73 y (65-90 y) | SBRT | CBCT | Respiratory tracking system | II-IV | 417 | NR (48-74.2 Gy) | 11 | CTX+RT: 24.3% CTX+Surg+RT: 3.4% Surg+RT: 9.4% RT: 58.4% Other+RT: 7.9% |
Median: 10 m 1-year: 35.5% |
Median: 10 m 1-year: 26.6% |
G3+: .5% RTOG |
G3+: 0% |
| 5-8 Fx | |||||||||||||
| Kim et al. 20133 | 86 y (80-91 y) | SBRT | CBCT | Respiratory tracking system | I-IV | 26 | 81.6 Gy (70.4-87.5 Gy) | 11.6 | CTX+RT: 15% RT+CTX: 23% RT: 62% |
Median: 7.6 m 2-year: 6.6% |
Median LC: 11.5 m | G3+: .0% NR |
G2+: .0% |
| 1-3 Fx | |||||||||||||
| Sutera et al. 20184 | 79 y (70-90 y) | SBRT | CBCT | NR | I-III | 145 | Single Fx (37%) 81.6 Gy (50.4 – 87.5 Gy) 68 Gy (42-72.9 Gy) |
12.3 | CTX+RT: 42.1% CRT: 3.4% RT+CTX: 37% RT: 17.5% Surg: 33.8%* |
Median: 14 m 1-year: 60% 2-year: 27% |
1-year LC: 72% 2-year LC: 63% |
G3+: .7% CTCAE v4.0 |
G2+: 2% |
| Multiple Fx (63%) 79.2 Gy (5m1.3-79.2 Gy) 66 Gy (42.8-66 Gy) | |||||||||||||
| Ryan et al. 20185 | 74 y (IQR, 68-79) | SBRT | Orthogonal portal films | Active breathing control | NR | 29 | 43.7 Gy (IQR, 37.5-54.8 Gy) | 15 | CTX+RT: 52% RT+CTX: 7% CTX+RT+CTX: 24% RT: 17% |
Median: 8 m 1-year: 28% |
Median: Not reached 1-year LC: 78% |
G2+: 41 CTCAE |
G2and3: 0% G4: 4% |
| 5 Fx | |||||||||||||
| Reddy et al. 20226 | 74 y (70-84 y) | SBRT | Pretreatment and intrafractional CBCT | Respiratory tracking system | II-III | 57 | 54.8 Gy (48 – 61.9 Gy) | 13.5 | CTX+RT: 33% CTX+RT+Surg: 67% |
Median: 19.6 m 1-year: 66.5% 2-year: 42.4% |
Median: 18.7 m 1-year: 73.3% 2-year: 44.6% |
G3+: 0% CTCAE |
G3+: 5% |
| 5 Fx | |||||||||||||
| Current study | 81 y (75-91 y) | A-SMART | MRI | Real time MR gating | I-III | 49 | 100 Gy (72-100 Gy) | 14 | RT: 6% CTX RT: 76% CTX+RT+Surg: 8% RT+Surg: 10% |
Median: 18 m 1-year: 59.9% |
Median: 24 m 1-year: 81.9% |
G3+: 8.2% CTCAE v5.0 |
G3+: 4.1% |
| 5 Fx |
BED10: biologically effect dose with alpha/beta ratio of 10; CBCT: cone-beam computerized tomography; CCRT: chemoradiotherapy; CTX: chemotherapy; CTCAE: Common Terminology Criteria for adverse Events; 3D-CRT: three dimensional conformal radiotherapy; Dx: Diagnosis; FU: follow-up; Fx: Fraction(s); IMRT: intensity modulated radiotherapy; IQR: interquartile range; LC: local control; LRC: locoregional control; m: month(s); NR: not reported; OS: overall survival; RT: radiotherapy; RTOG: Radiation Therapy Oncology Group; SBRT: stereotactic body radiotherapy; A-SMART: ablative stereotactic magnetic resonance image-guided adaptive radiation therapy; Surg: surgery; y: year(s)
aCombination of surgery with either SBRT or CTX is not reported.
Our acute grade three or higher toxicity rate of 8.2% is similar to prior non-ablative SBRT studies for elderly patients with PDAC. However, the vast majority of these were self-limited grade three toxicities. The late grade three or higher toxicity profile is consistent with historical outcomes for pancreatic SBRT in an elderly cohort. These favorable toxicity rates highlight the safety of A-SMART for isotoxic dose escalation in elderly patients with PDAC, which mirrors prior studies that utilized A-SMART for the treatment of LAPC.19,44-46 Neoadjuvant chemotherapy was given to most patients in our study (86%), which is expected to have influenced clinical outcomes when compared to other studies with lower rates of chemotherapy administration (Table 5).43 However, most patients within our study received a gemcitabine-based chemotherapy regimen that is often used in elderly patients due to increased toxicity concerns of the more potent FOLFIRINOX regimen. These toxicity data suggest that A-SMART does not appear to be more toxic than other standard radiotherapy regimens routinely used for unresectable PDAC; thus, A-SMART should be considered a safe treatment option for these vulnerable patients with limited options.
Study limitations include that this is a retrospective analysis and is thus subject to underreporting of toxicities even though toxicities were evaluated and recorded prospectively at time of each patient encounter. The study cohort is relatively small, and a sample size analysis was not performed prior to data collection. There is a limited median follow-up time of only 14 months from diagnosis and 10 months from A-SMART. Important clinical outcomes of our resected cohort did not reach statistical significance due to the low number of events within our follow-up time. Our results would benefit from further follow-up time; however, we believe that our follow-up time in this elderly patient population with PDAC is meaningful given the poor prognosis of this disease. The elderly population within this study had favorable baseline ECOG PS, thus elderly patients with worse baseline ECOG PS may not tolerate this therapy as well as our study cohort. In addition, all patients in this trial were treated at large tertiary cancer centers, which were both early adopters of A-SMART for PDAC, and thus these safety data may not translate to centers with less experience in A-SMART for PDAC. Lastly, we acknowledge that we intentionally selected patients who did not progress after initial chemotherapy as it is this subset that likely achieves the greatest benefit from A-SMART. Whether A-SMART meaningfully improves OS over chemotherapy has not yet been studied prospectively although a multi-center phase III randomized study for LAPC (LAP-ABLATE; NCT05585554) may answer this question.
Conclusions
A-SMART for elderly PDAC patients appears to be an effective and safe therapy associated with excellent LRC with minimal acute and late toxicities in this vulnerable patient population that often has few treatment options. The soft tissue visualization, real time gating, and online adaptive planning of MRgRT increase the safety of isotoxic dose escalation and underpins the feasibility and tolerability of A-SMART for elderly patients. Centers with access to and experience with pancreatic A-SMART should consider this modality as a treatment option for their elderly PDAC patients.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Data Sharing: The data related to this study are available upon request.
Ethics Statement: This study received ethical approval from the H. Lee Moffitt Cancer Center and Research Institute IRB scientific review committees (Approval #3382) on June 18th, 2019, and from the Miami Cancer Center IRB (Approval #1468012) on April 3rd, 2020. This is an IRB-approved retrospective study, all patient information was deidentified and patient consent was not required.
ORCID iDs
JM Bryant https://orcid.org/0000-0002-0207-1991
Russell F Palm https://orcid.org/0000-0002-0448-8715
Muni Rubens https://orcid.org/0000-0003-1812-9753
References
- 1.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics. CA Cancer J Clin. 2022;72(1):7-33. doi: 10.3322/caac.21708 [DOI] [PubMed] [Google Scholar]
- 2.Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74(11):2913-2921. doi: 10.1158/0008-5472.CAN-14-0155 [DOI] [PubMed] [Google Scholar]
- 3.Higuera O, Ghanem I, Nasimi R, Prieto I, Koren L, Feliu J. Management of pancreatic cancer in the elderly. World J Gastroenterol. 2016;22(2):764-775. doi: 10.3748/wjg.v22.i2.764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hsu CC, Wolfgang CL, Laheru DA, et al. Early mortality risk score: Identification of poor outcomes following upfront surgery for resectable pancreatic cancer. J Gastrointest Surg. 2012;16(4):753-761. doi: 10.1007/s11605-011-1811-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Talarico L, Chen G, Pazdur R. Enrollment of elderly patients in clinical trials for cancer drug registration: A 7-year experience by the US Food and Drug Administration. J Clin Oncol. 2004;22(22):4626-4631. doi: 10.1200/JCO.2004.02.175 [DOI] [PubMed] [Google Scholar]
- 6.Hodul P, Tansey J, Golts E, Oh D, Pickleman J, Aranha GV. Age is not a contraindication to pancreaticoduodenectomy. Am Surg. 2001;67(3):270-275. doi: 10.1016/s0016-5085(00)81967-8 [DOI] [PubMed] [Google Scholar]
- 7.Matsumoto K, Miyake Y, Kato H, et al. Effect of low-dose gemcitabine on unresectable pancreatic cancer in elderly patients. Digestion. 2011;84(3):230-235. doi: 10.1159/000330384 [DOI] [PubMed] [Google Scholar]
- 8.Hammel P, Huguet F, van Laethem JL, et al. Effect of chemoradiotherapy vs chemotherapy on survival in patients with locally advanced pancreatic cancer controlled after 4 months of gemcitabine with or without Erlotinib: The LAP07 randomized clinical trial. JAMA. 2016;315(17):1844-1853. doi: 10.1001/jama.2016.4324 [DOI] [PubMed] [Google Scholar]
- 9.Morizane C, Okusaka T, Ito Y, et al. Chemoradiotherapy for locally advanced pancreatic carcinoma in elderly patients. Oncology. 2005;68(4-6):432-437. doi: 10.1159/000086985 [DOI] [PubMed] [Google Scholar]
- 10.Herman JM, Chang DT, Goodman KA, et al. Phase 2 multi-institutional trial evaluating gemcitabine and stereotactic body radiotherapy for patients with locally advanced unresectable pancreatic adenocarcinoma. Cancer. 2015;121(7):1128-1137. doi: 10.1002/cncr.29161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herman JM, Koong AC. Stereotactic body radiation therapy: A new standard option for pancreatic cancer? J Natl Compr Canc Netw. 2014;12(10):1489-1493. doi: 10.6004/jnccn.2014.0143 [DOI] [PubMed] [Google Scholar]
- 12.Rudra S, Jiang N, Rosenberg SA, et al. Using adaptive magnetic resonance image-guided radiation therapy for treatment of inoperable pancreatic cancer. Cancer Med. 2019;8(5):2123-2132. doi: 10.1002/cam4.2100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krishnan S, Chadha AS, Suh Y, et al. Focal radiation therapy dose escalation improves overall survival in locally advanced pancreatic cancer patients receiving induction chemotherapy and consolidative chemoradiation. Int J Radiat Oncol Biol Phys. 2016;94(4):755-765. doi: 10.1016/j.ijrobp.2015.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reyngold M, O'Reilly EM, Varghese AM, et al. Association of ablative radiation therapy with survival among patients with inoperable pancreatic cancer. JAMA Oncol. 2021;7(5):735-738. doi: 10.1001/jamaoncol.2021.0057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Noel CE, Parikh PJ, Spencer CR, et al. Comparison of onboard low-field magnetic resonance imaging versus onboard computed tomography for anatomy visualization in radiotherapy. Acta Oncol. 2015;54(9):1474-1482. doi: 10.3109/0284186X.2015.1062541 [DOI] [PubMed] [Google Scholar]
- 16.Bohoudi O, Bruynzeel AME, Senan S, et al. Fast and robust online adaptive planning in stereotactic MR-guided adaptive radiation therapy (SMART) for pancreatic cancer. Radiother Oncol. 2017;125(3):439-444. doi: 10.1016/j.radonc.2017.07.028 [DOI] [PubMed] [Google Scholar]
- 17.Boldrini L, Cusumano D, Cellini F, Azario L, Mattiucci GC, Valentini V. Online adaptive magnetic resonance guided radiotherapy for pancreatic cancer: state of the art, pearls and pitfalls. Radiat Oncol. 2019;14(1):71. doi: 10.1186/s13014-019-1275-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bouchart C, Engelholm JL, Closset J, et al. Isotoxic high-dose stereotactic body radiotherapy integrated in a total multimodal neoadjuvant strategy for the treatment of localized pancreatic ductal adenocarcinoma. Ther Adv Med Oncol. 2021;13:17588359211045860. doi: 10.1177/17588359211045860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chuong MD, Bryant J, Mittauer KE, et al. Ablative 5-Fraction stereotactic magnetic resonance-guided radiation therapy with on-table adaptive replanning and elective nodal irradiation for inoperable pancreas cancer. Pract Radiat Oncol. 2021;11(2):134-147. doi: 10.1016/j.prro.2020.09.005 [DOI] [PubMed] [Google Scholar]
- 20.Bryant JM, Palm RF, Liveringhouse C, et al. Surgical and pathologic outcomes of pancreatic adenocarcinoma (PA) after preoperative ablative stereotactic magnetic resonance image guided adaptive radiation therapy (A-SMART). Advances in Radiation Oncology. 2022;7(6):101045. doi: 10.1016/j.adro.2022.101045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hassanzadeh C, Rudra S, Bommireddy A, et al. Ablative Five-Fraction Stereotactic Body Radiation Therapy for Inoperable Pancreatic Cancer Using Online MR-Guided Adaptation. Adv Radiat Oncol. 2021;6(1):100506. doi: 10.1016/j.adro.2020.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosenberg SA, Henke LE, Shaverdian N, Mittauer K, Wojcieszynski AP, Hullett CR, et al. A Multi-Institutional Experience of MR-Guided Liver Stereotactic Body Radiation Therapy. Adv Radiat Oncol. 2019;4(1):142-149. doi: 10.1016/j.adro.2018.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Henke L, Kashani R, Robinson C, et al. Phase I trial of stereotactic MR-guided online adaptive radiation therapy (SMART) for the treatment of oligometastatic or unresectable primary malignancies of the abdomen. Radiother Oncol. 2018;126(3):519-526. doi: 10.1016/j.radonc.2017.11.032. [DOI] [PubMed] [Google Scholar]
- 24.Ryan R, Gibbons D, Hyland JM, et al. Pathological response following long-course neoadjuvant chemoradiotherapy for locally advanced rectal cancer. Histopathology. 2005;47(2):141-146. doi: 10.1111/j.1365-2559.2005.02176.x [DOI] [PubMed] [Google Scholar]
- 25.Abi Jaoude J, Thunshelle CP, Kouzy R, et al. Stereotactic Versus Conventional Radiation Therapy for Patients With Pancreatic Cancer in the Modern Era. Adv Radiat Oncol. 2021;6(6):100763. doi: 10.1016/j.adro.2021.100763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tchelebi LT, Lehrer EJ, Trifiletti DM, et al. Conventionally fractionated radiation therapy versus stereotactic body radiation therapy for locally advanced pancreatic cancer (CRiSP): An international systematic review and meta-analysis. Cancer. 2020;126(10):2120-2131. doi: 10.1002/cncr.32756 [DOI] [PubMed] [Google Scholar]
- 27.Ciabatti S, Cammelli S, Frakulli R, et al. Radiotherapy of pancreatic cancer in older patients: A systematic review. J Geriatr Oncol. 2019;10(4):534-539. doi: 10.1016/j.jgo.2018.09.007 [DOI] [PubMed] [Google Scholar]
- 28.Zhu X, Li F, Ju X, et al. Prognostic role of stereotactic body radiation therapy for elderly patients with advanced and medically inoperable pancreatic cancer. Cancer Med. 2017;6(10):2263-2270. doi: 10.1002/cam4.1164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sutera PA, Bernard ME, Wang H, Heron DE. Prognostic factors for elderly patients treated with stereotactic body radiation therapy for pancreatic adenocarcinoma. Front Oncol. 2018;8:282. doi: 10.3389/fonc.2018.00282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kunkler IH, Audisio R, Belkacemi Y, et al. Review of current best practice and priorities for research in radiation oncology for elderly patients with cancer: The International Society of Geriatric Oncology (SIOG) task force. Ann Oncol. 2014;25(11):2134-2146. doi: 10.1093/annonc/mdu104. [DOI] [PubMed] [Google Scholar]
- 31.Balaban EP, Mangu PB, Khorana AA, et al. Locally Advanced, Unresectable Pancreatic Cancer: American society of clinical oncology clinical practice guideline. J Clin Oncol. 2016;34(22):2654-2668. doi: 10.1200/JCO.2016.67.5561. [DOI] [PubMed] [Google Scholar]
- 32.Zhu X, Ju X, Cao Y, et al. Patterns of local failure after stereotactic body radiation therapy and sequential chemotherapy as initial treatment for pancreatic Cancer: Implications of target volume design. Int J Radiat Oncol Biol Phys. 2019;104(1):101-110. doi: 10.1016/j.ijrobp.2019.01.075 [DOI] [PubMed] [Google Scholar]
- 33.Reyngold M, Parikh P, Crane CH. Ablative radiation therapy for locally advanced pancreatic cancer: techniques and results. Radiat Oncol. 2019;14(1):95. doi: 10.1186/s13014-019-1309-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ma SJ, Prezzano KM, Hermann GM, Singh AK. Dose escalation of radiation therapy with or without induction chemotherapy for unresectable locally advanced pancreatic cancer. Radiat Oncol. 2018;13(1):214. doi: 10.1186/s13014-018-1158-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arcelli A, Guido A, Buwenge M, et al. Higher Biologically Effective Dose Predicts Survival in SBRT of Pancreatic Cancer: A Multicentric Analysis (PAULA-1). Anticancer Res. 2020;40(1):465-472. doi: 10.21873/anticanres.13975 [DOI] [PubMed] [Google Scholar]
- 36.Vornhulz M, Anton S, Eross B, et al. Role of stereotactic body radiation in the enhancement of the quality of life in locally advanced pancreatic adenocarcinoma: A systematic review. Radiat Oncol. 2022;17(1):108. doi: 10.1186/s13014-022-02076-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ryan JF, Rosati LM, Groot VP, et al. Stereotactic body radiation therapy for palliative management of pancreatic adenocarcinoma in elderly and medically inoperable patients. Oncotarget. 2018;279(23):16427-16436. doi: 10.18632/oncotarget.24713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Garcia G, Odaimi M. Systemic combination chemotherapy in elderly pancreatic cancer: A review. J Gastrointest Cancer. 2017;48(2):121-128. doi: 10.1007/s12029-017-9930-0 [DOI] [PubMed] [Google Scholar]
- 39.Murphy JD, Christman-Skieller C, Kim J, Dieterich S, Chang DT, Koong AC. A dosimetric model of duodenal toxicity after stereotactic body radiotherapy for pancreatic cancer. Int J Radiat Oncol Biol Phys. 2010;78(5):1420-1426. doi: 10.1016/j.ijrobp.2009.09.075. [DOI] [PubMed] [Google Scholar]
- 40.Parikh PJ, Lee P, Low D, et al. Stereotactic MR-Guided On-Table Adaptive Radiation Therapy (SMART) for Patients with Borderline or Locally Advanced Pancreatic Cancer: Primary Endpoint Outcomes of a Prospective Phase II Multi-Center International Trial. San Antonio, TX. 2022: American Society for Radiation Oncology 2022 annual meeting. [Google Scholar]
- 41.Yechieli RL, Robbins JR, Mahan M, Siddiqui F, Ajlouni M. Stereotactic Body Radiotherapy for Elderly Patients With Medically Inoperable Pancreatic Cancer. Am J Clin Oncol. 2017;40(1):22-26. doi: 10.1097/COC.0000000000000090 [DOI] [PubMed] [Google Scholar]
- 42.Kim CH, Ling DC, Wegner RE, et al. Stereotactic body radiotherapy in the treatment of pancreatic adenocarcinoma in elderly patients. Radiat Oncol. 2013;8:240. doi: 10.1186/1748-717X-8-240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reddy AV, Sehgal S, Hill CS, et al. upfront chemotherapy followed by stereotactic body radiation therapy with or without surgery in older patients with localized pancreatic cancer: A single institution experience and review of the literature. Curr Oncol. 2022;29(1):308-320. doi: 10.3390/curroncol29010028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tetar SU, Bruynzeel AME, Oei SS, et al. Magnetic resonance-guided stereotactic radiotherapy for localized prostate cancer: Final results on patient-reported outcomes of a prospective phase 2 study. Eur Urol Oncol. 2021;4(4):628-634. doi: 10.1016/j.euo.2020.05.007 [DOI] [PubMed] [Google Scholar]
- 45.Finazzi T, Haasbeek CJA, Spoelstra FOB, et al. Clinical Outcomes of Stereotactic MR-Guided adaptive radiation therapy for high-risk lung tumors. Int J Radiat Oncol Biol Phys. 2020;107(2):270-278. doi: 10.1016/j.ijrobp.2020.02.025 [DOI] [PubMed] [Google Scholar]
- 46.Boldrini L, Corradini S, Gani C, et al. MR-Guided radiotherapy for liver malignancies. Front Oncol. 2021;11:616027. doi: 10.3389/fonc.2021.616027 [DOI] [PMC free article] [PubMed] [Google Scholar]