Abstract
Angiosarcoma is a rare but very aggressive tumor. It occurs in all organs of the body, and approximately 8% of all angiosarcomas arise in the breast. We reported 2 cases of primary breast angiosarcomas in young women. The 2 patients showed similar clinical features, but were quite different in dynamic contrast-enhanced MR imaging. The 2 patients were treated with mastectomy and axillary sentinel lymph node dissection and confirmed by post-operative pathological test. We suggested that dynamic contrast-enhanced MR imaging was the most helpful imaging tool in the diagnosis and pre-operative evaluation of the breast angiosarcoma.
Keywords: Breast, Angiosarcoma, MRI, Case report
Abbreviations: DCE-MRI, dynamic contrast-enhanced magnetic resonance imaging; US, Ultrasonography; ACR BI-RADS, American College of Radiology Breast Imaging Reporting and Data System; DWI, diffusion-weighted imaging; ADC, apparent diffusion coefficient; MIP, maximum intensity projection; PET/CT, positron emission tomography-computed tomography; IHC, Immunohistochemical staining
Introduction
Angiosarcoma is a rare but very aggressive tumor originating from vascular endothelial cells. It occurs in all organs of the body, and approximately 8% of all angiosarcomas arise in the breast [1]. Breast angiosarcoma can be classified into primary and secondary breast angiosarcoma. Clinical presentations and initial imaging findings in primary angiosarcoma are non-specific, resulting in delayed diagnosis and treatment. In this article, we present 2 primary breast angiosarcoma cases and discuss the typical clinical and imaging features with a literature review. The 2 patients showed similar feature in clinical presentation, but were quite different in dynamic contrast-enhanced MR imaging (DCE-MRI).
Case 1
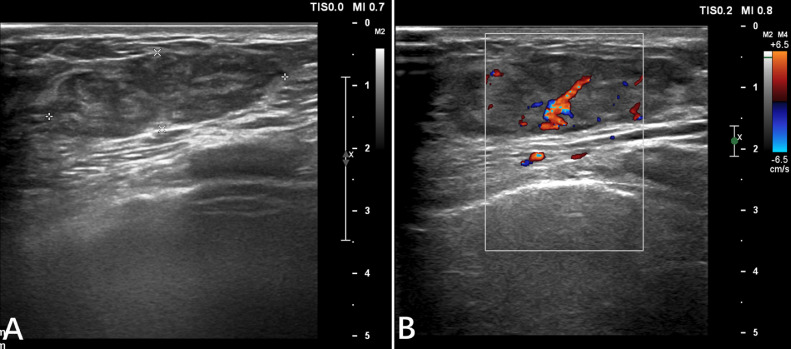
A 30-year-old woman presented to our hospital with a palpable mass in her right breast for 3 months without any specific treatments. The patient had no history of breast trauma and no breast cancer in her family. She had cesarean-section 2 years prior which was followed by successful breastfeeding. On physical examination, there was a palpable mass in the lower-inner quadrant without tenderness, skin involvement, and nipple abnormalities. There were also no palpable axillary lymph nodes. Ultrasonography (US) revealed a 3.8 × 1.2 cm hypoechogenic mass in the right breast with a well-demarcated margin and rich blood supply signal. A category of ACR BI-RADS 3 (American College of Radiology Breast Imaging Reporting and Data System) (i.e., fibroadenoma) was considered. No abnormalities were found in the left breast US image (Fig. 1). The lumpectomy was then performed in outpatient surgery. However, the pathologic tests diagnosed a well-differentiated angiosarcoma with positive CD 31, CD 34, and a high Ki-67 index of 20%. Soon afterwards, the patient was admitted to the hospital for further treatment.
Fig. 1.
The lesion was well-demarcated margin (A) and rich blood supply signal (B).
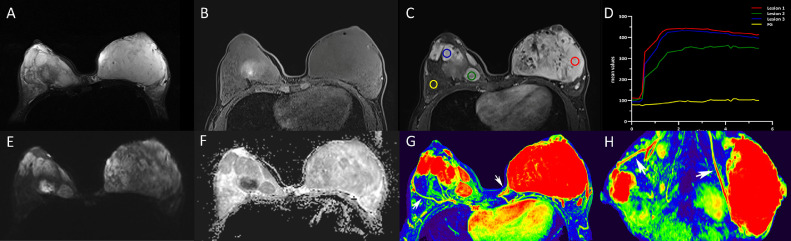
During hospitalization, Breast MRI identified a large mass (11.0 × 75.0 × 11.0 cm) occupying the entire left breast and multiple irregular mass lesions (2.0-5.0 cm) in the right breast. The lesions were characterized as isointense on T1-weighted imaging (T1WI) and high signal intensity on T2WI, with slightly restricted diffusion on diffusion-weighted imaging (DWI) and apparent diffusion coefficient maps. DCE-MRI demonstrated a rapid enhancement followed by a typical plateau pattern of the enhancement curve for the lesions of the bilateral breasts with well-demarcated margins and without the involvement of the skin and nipple. Dominant draining veins were observed on the color maps of maximum intensity projection images. No axillary lymphadenopathy was found (Fig. 2). Given the clinical and imaging information above, the diagnosis of aggressive bilateral angiosarcoma was suggested. In addition, positron emission tomography-computed tomography (PET/CT) with 18F-FDG was performed before the surgery, showing increased radioactivity uptakes with a maximum standardized uptake value (SUVmax) of 2.5 for the breast lesions. No metastases of the axillary lymph nodes and distal organs were found.
Fig. 2.
The lesions were hyperintense on fat-suppressed T2WI (A) and isointense on fat-suppressed T1-weighted imaging (T1W1) (B). DCE-MRI demonstrated a rapid enhancement followed by a typical plateau pattern of the enhancement curve for the lesions of the bilateral breasts with well-demarcated margins and without the involvement of the skin and nipple (C-D). All masses shown slightly restricted diffusion on DWI (b = 1000 s/mm2) and ADC maps (E-F). The ADC values were 1.40 × 10−3mm2/s for the left lesion. Bilateral draining vessels (white arrows) were dominant on color maps of maximum intensity projection (MIP) images in axial view (G) and sagittal view (H). ADC, apparent diffusion coefficient; DWI, diffusion-weighted imaging.
The patient was treated with bilateral mastectomy and axillary sentinel lymph node dissection followed by radiotherapy. The post-operative pathologic test confirmed high-grade angiosarcoma without axillary lymph node metastasis. The expression of the Ki-67 index was about 30%. After a 1-year follow-up, however, liver metastasis and thoracic metastasis were found.
Case 2
A 23-year-old woman was admitted to a local hospital with a palpable mass in the left breast for 2 months. The patient had no personal or family history of breast or ovarian cancer. Breast US revealed a 4.0 × 1.6 cm hypoechoic mass with poorly demarcated margins in the left breast, and granulomatous mastitis was considered. No abnormalities were found in the right breast US image. Then an imaging-guided core needle biopsy was done with a pathological result of stromal fibrosis of breast tissue.
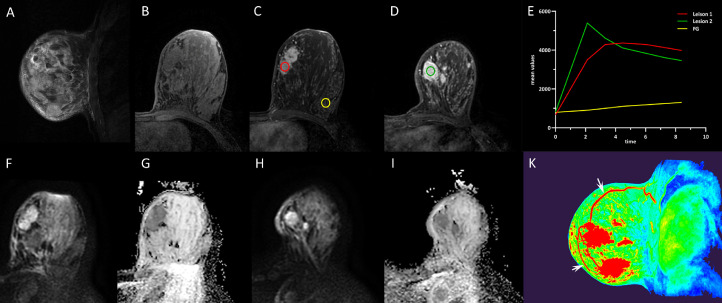
However, with breast MRI, 2 mass lesions were found in her left breast (lower-inner quadrant). The lesions presented as isointense on T1WI and mild hyperintense on T2WI, with slightly restricted diffusion on DWI. Breast DCE-MRI demonstrated an initial increase in signal intensity followed by a flattening pattern of the enhancement curve for lesion 1 (2.0 × 4.3 × 4.0 cm) and a rapid enhancement followed by a typical washout pattern of the enhancement curve for lesion 2 (2.4 × 3.7 × 2.6 cm), with irregular shape and poorly demarcated margins. Dominant draining veins around the lesions were also observed on the maximum intensity projection images. A category of ACR BI-RADS 4b was interpreted. No axillary lymphadenopathy was found (Fig. 3).
Fig. 3.
The lesions on left breast presented with high signal intensity on T2WI (A) and low-intermediate signal intensity on T1WI (B). DCE-MRI shows a rapid inhomogeneous enhancement with a plateau pattern for lesion 1 (red circle) and a rapid inhomogeneous enhancement with a washout pattern for lesion 2 (green circle) (C-E). The lesions have slightly restricted diffusion on DWI (b = 1000 s/mm2) and ADC maps (F-I). The ADC values were 1.44 × 10−3mm2/s for lesion 1 and 1.48 × 10−3 mm2/s for lesion 2. Dominant draining veins (white arrows)around the lesions were observed on the color maps of maximum intensity projection (MIP) images in sagittal view (K). ADC, apparent diffusion coefficient; DWI, diffusion-weighted imaging.
Then breast-conserving surgery was performed with a histopathological result of intermediate-grade angiosarcoma. Immunohistochemical staining (IHC) revealed the markedly increased expression of endothelial markers (i.e., CD31, CD34, and ERG) in the specimens. The Ki-67 index was about 50% in the highest proliferative area. The post-operative PET/CT with 18F-FDG found no other sites of organ metastases.
Considering the potential aggressiveness of the tumor, breast mastectomy and axillary sentinel lymph node dissection were eventually performed. The pathologic test confirmed the minimal residual tumor with negative axillary lymph nodes. The patient is still under the first 3-month follow-up, free of any evidence of recurrence and metastasis.
Discussion
Angiosarcoma is a rare but life-threatening tumor originating from vascular endothelial cells [2,3]. It is divided into primary and secondary angiosarcoma. The breast is one of the most common sites for primary angiosarcoma [4].
Primary breast angiosarcoma makes up 0.04% of breast tumors. It usually occurs in younger women in the third and fourth decades [5]. The clinical presentation is a painless mass in the breast [3]. Up to one-third of the patients have bluish skin discoloration due to the vascular nature of the tumor [5]. Meanwhile, secondary breast angiosarcoma usually occurs in older women (average age = 60 years) who usually undergo breast-conserving therapy followed by radiation therapy. Secondary breast angiosarcoma is classified into secondary cutaneous angiosarcoma associated with lymphedema and post-radiation angiosarcoma [3]. Recently, an increased incidence of secondary breast angiosarcoma has been observed due to the increasing use of whole-breast irradiation after breast-conserving surgery [3]. The primary clinical manifestations include skin discoloration (red, blue, and purple) and palpable soft lump(s) in the breast [5]. In this report, the 2 patients had primary angiosarcomas and manifested palpable lumps without skin discoloration.
Three histologic degrees of angiosarcoma have been described for breast angiosarcomas [3,6]. Low-grade angiosarcoma is composed of well-formed anastomosing vascular channels. Intermediate-grade angiosarcoma shows robust neoplastic vascular growth. Focal necrosis, hemorrhage, and infarction commonly occur in high-grade angiosarcoma. IHC test plays an important role in differentiating angiosarcoma from invasive carcinomas. The representative endothelial markers include CD 31, CD 34, and factor VIII. A high Ki-67 index usually correlates with a poor disease prognosis. Due to the well-formed vascular channels, necrotic tissue, fat, and hemorrhage within the tumor, there is a high percentage (up to 37%) of cases reported as benign diseases by fine-needle aspiration or core needle biopsy [6]. The definite diagnosis depends on the comprehensive histopathological and IHC evaluations after complete tumor resection. Case 2 in our report was initially misdiagnosed as stromal fibrosis of breast tissue by US-guided biopsy.
For imaging diagnosis, mammography is usually non-specific. The lesion usually manifests with an ill-defined mass without calcification. However, the tumor is frequently missed on mammography due to the dense breast in young women. In addition, skin thickness may be the only finding in mammography [2]. The imaging features are non-specific in breast US, including hypoechoic, hyperechoic, or heterogeneous echo region(s), with or without acoustic shadowing. Hypervascularity within the tumor may be helpful [5]. Breast angiosarcoma is often misinterpreted as granulomatous mastitis or fibromatosis with breast US.
MRI is the most sensitive imaging modality for evaluating breast disease [3,6,7]. Breast angiosarcoma usually has a round or irregular shape and well-defined margin, with low-intermediate signal intensity on T1WI and high signal intensity on T2WI. Due to the high hypervascularity within the tumor, DCE-MRI usually shows a rapid inhomogeneous enhancement with a plateau or washout pattern of the enhancement curve, according to the different differentiation grades [7]. Breast angiosarcoma has slightly restricted diffusion on DWI and apparent diffusion coefficient maps. In addition, previous studies demonstrated that FDG-PET has some advantages in detecting distal metastasis in other parts of the body [7,8]. The differential diagnoses for low-grade angiosarcoma are hemangioma, angiolipoma, and benign proliferative lesions. The differential diagnoses for higher grade angiosarcoma are mastitis, fibromatosis, and invasive mammary carcinoma [8].
Mastectomy is the first choice of treatment for primary breast angiosarcoma. Adjuvant chemotherapy could reduce the local recurrence rate, whereas some studies suggested no benefits [8], [9], [10]. A previous survey illustrated docetaxel was a promising drug for secondary breast angiosarcomas, and hyper-fractionated radiation therapy reduced cell repopulation of the rapidly growing tumor [10]. Breast angiosarcoma tends to have early metastasis through a hematogenous rather than lymphogenous pathway, which involves any body parts [10,11]. In this report, case 1 had liver and thoracic metastases only after a 1-year follow-up. The prognosis of breast angiosarcoma depends on the tumor grade (major factor), tumor size, and the resection margin status during the surgery. The probability of 5-year disease-free survival after initial treatment is 15%, and the average recurrence time of high-grade angiosarcomas is 15 months [12].
In conclusion, breast angiosarcoma is a relatively rare and highly malignant tumor. Even in the early stage, metastases to various organs of the body may occur. Breast DCE-MRI was the most helpful imaging tool in diagnosing and treating breast angiosarcoma. Therefore, it is necessary to be familiar with its clinicopathological and imaging characteristics to achieve early diagnosis and early treatment as much as possible.
Patient consent
Written informed consent for the publication of this case report was obtained from each patient.
Footnotes
Competing Interests: None.
References
- 1.West JG, Qureshi A, West JE, Chacon M, Sutherland ML, Haghighi B, et al. Risk of angiosarcoma following breast conservation: a clinical alert. Breast J. 2005;11(2):115–123. doi: 10.1111/j.1075-122X.2005.21548.x. [DOI] [PubMed] [Google Scholar]
- 2.Fraga-Guedes C, Gobbi H, Mastropasqua MG, Botteri E, Luini A, Viale G. Primary and secondary angiosarcomas of the breast: a single institution experience. Breast Cancer Res Treat. 2012;132(3):1081–1088. doi: 10.1007/s10549-011-1931-2. [DOI] [PubMed] [Google Scholar]
- 3.Chen KT, Kirkegaard DD, Bocian JJ. Angiosarcoma of the breast. Cancer. 1980;46(2):368–371. doi: 10.1002/1097-0142(19800715)46:2<368::aid-cncr2820460226>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 4.Yang WT, Hennessy BT, Dryden MJ, Valero V, Hunt KK, Krishnamurthy S. Mammary angiosarcomas: imaging findings in 24 patients. Radiology. 2007;242(3):725–734. doi: 10.1148/radiol.2423060163. [DOI] [PubMed] [Google Scholar]
- 5.Glazebrook KN, Magut MJ, Reynolds C. Angiosarcoma of the breast. AJR Am J Roentgenol. 2008;190(2):533–538. doi: 10.2214/AJR.07.2909. [DOI] [PubMed] [Google Scholar]
- 6.Wu WH, Ji QL, Li ZZ, Wang QN, Liu SY, Yu JF. Mammography and MRI manifestations of breast angiosarcoma. BMC Womens Health. 2019;19(1):73. doi: 10.1186/s12905-019-0769-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosen PP, Kimmel M, Ernsberger D. Mammary angiosarcoma. The prognostic significance of tumor differentiation. Cancer. 1988;62(10):2145–2151. doi: 10.1002/1097-0142(19881115)62:10<2145::aid-cncr2820621014>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 8.Varghese B, Deshpande P, Dixit S, Koppiker CB, Jalnapurkar N. Primary angiosarcoma of the breast: a case report. J Radiol Case Rep. 2019;13(2):15–25. doi: 10.3941/jrcr.v13i2.3449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abdelhady AM, Neamaalla S, Gittens AS, Germaine P. Primary angiosarcoma of the breast: case report of a rare vascular tumor. Radiol Case Rep. 2020;15(4):339–343. doi: 10.1016/j.radcr.2019.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maeda T, Tateishi U, Hasegawa T, Ojima H, Arai Y, Sugimura K. Primary hepatic angiosarcoma on coregistered FDG PET and CT images. AJR Am J Roentgenol. 2007;188(6):1615–1617. doi: 10.2214/AJR.05.0830. [DOI] [PubMed] [Google Scholar]
- 11.Freudenberg LS, Rosenbaum SJ, Schulte-Herbruggen J, Eising EG, Lauenstein T, Wolff A, et al. Diagnosis of a cardiac angiosarcoma by fluorine-18 fluordeoxyglucose positron emission tomography. Eur Radiol. 2002;12(Suppl. 3):S158–S161. doi: 10.1007/s00330-002-1478-z. [DOI] [PubMed] [Google Scholar]
- 12.Marchant LK, Orel SG, Perez-Jaffe LA, Reynolds C, Schnall MD. Bilateral angiosarcoma of the breast on MR imaging. AJR Am J Roentgenol. 1997;169(4):1009–1010. doi: 10.2214/ajr.169.4.9308452. [DOI] [PubMed] [Google Scholar]