Abstract
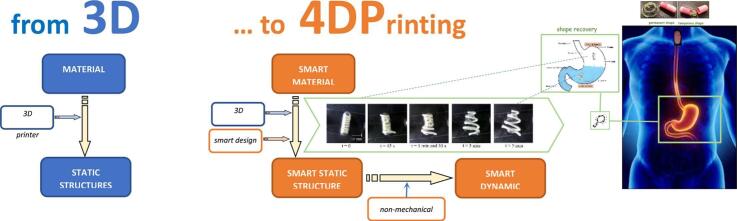
Four-dimensional printing (4DP) is emerging as an innovative research topic. It involves the use of smart materials for three-dimensional printing (3DP) of items that change their shape after production, in a programmed way over time, when exposed to appropriate external non-mechanical stimuli (moisture, electric or magnetic fields, UV, temperature, pH or ion composition). In the performance of 4D printed devices, time is involved as the 4th dimension. 4D smart structures have been known for many years in the scientific literature, well before the advent of 3D printing, and the concepts of shape evolution as well as self-assembly have been applied to drug delivery at the nano-, micro- and macro-scale levels. The neologism “4DP” was coined by Tibbits, Massachusetts Institute of Technology, in 2013, who also showed the earliest examples of 4D printed objects. Since then, smart materials have often been combined with additive manufacturing, which makes production of complex shapes easy to achieve: going beyond 3DP, 4D printed items are no static objects. Two main categories of raw materials have been employed for 4DP: shape memory polymers (SMPs) and shape morphing hydrogels (SMHs). In principle, all types of 3D printers could be used for 4DP. In this article, examples of systems for use in the biomedical field, such as stents and scaffolds, and in drug delivery are reviewed, with special emphasis on indwelling devices for retention in the urinary bladder and in the stomach.
Keywords: 4D printing, 3D printing, Smart materials, Shape memory polymers, Hydrogels, Drug release, Delivery systems
Graphical abstract
1. Introduction
Research in the field of drug delivery always strives to meet new challenges and hard-to-reach therapeutic objectives, for instance through improvement of pharmacokinetic profiles, enhanced patient compliance and possible adaption to the needs of a specific subject. This approach entails the identification of new therapeutic agents, including biological compounds, novel formulation strategies and, recently, has been broadened to manufacturing approaches unusual for the pharmaceutical area. Such a scenario laid the basis for scientists to start investigating the application potential of new fabrication techniques. The growing interest in 3D printing (3DP) would be one of the main examples in this respect.
4D printing (4DP) is also emerging as an innovative and challenging research topic. As an advancement of 3D printing, it involves the use of special raw materials, often referred to as smart or intelligent, to print items that can change their shape after production when exposed to an appropriate external non-mechanical stimulus. This peculiar behavior could be attained because smart materials are characterized by one or more properties that undergo major modifications in response to environmental variations, such as changes in moisture, electric and magnetic fields, light, temperature, pH or ion composition. Items made of smart materials and fabricated through 3DP processes are often considered as smart devices, since they can change their morphology in a programmed way over time. This is the reason why they are categorized as 4D printed systems, being time - the 4th dimension- involved in their definition.
2. When “4DP” was coined?
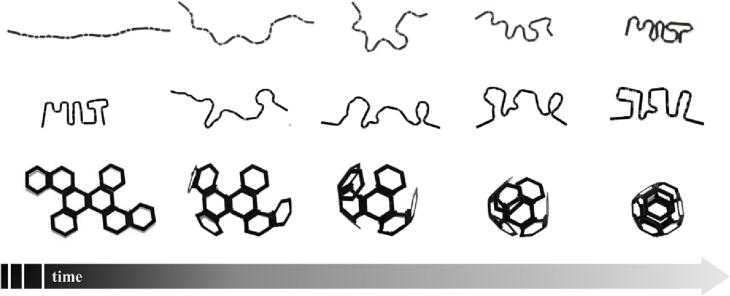
The neologism “4DP” was born on stage during a Technology, Entertainment, Design (TED) Talk, which was held in February 2013. TED Talks are public debates, which are posted online for free distribution under the slogan “ideas worth spreading” by an homonymous US-based media organization. In that occasion, the founder and director of the Self-Assembly Lab (SAL) at the Massachusetts Institute of Technology (MIT), Skylar Tibbits, showed what can be defined as the earliest examples of 4D printed objects capable of self-transformation over time (Tibbits, 2013). These structures were printed as a single strand and were composed of a sequence of rigid parts and flexible hinges. The latter were made of smart materials that, when immersed in water, underwent asymmetric swelling, thus resulting into folding of the strands to take on the desired shape (e.g. MIT and the SAL acronyms and a closed polygon) (Fig. 1).
Fig. 1.
Sequential images of the printed structures developed in the Self-Assembly Lab at MIT, showing self-modification of shape over time. Adapted from (Raviv et al., 2014).
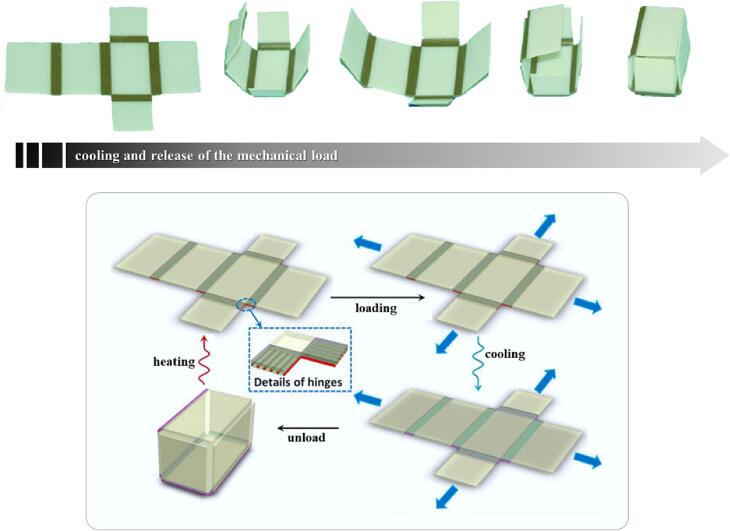
Almost at the same time, other research groups came up with 4DP self-moving systems. By way of example, Ge and colleagues designed and printed devices including hinges made of an “active” composite material and “inactive” plates based on rigid plastics (Ge et al., 2013). The resulting devices were then thermo-mechanically programmed to take on complex 3D configurations, and shape modifications were triggered in response to change in temperature (Fig. 2). Subsequently, 3DP self-folding origami started to be described in the scientific literature. In this respect, most applications were aimed at the fabrication of innovative actuators to be used in robots (De Marco et al., 2019; Hann et al., 2020; Shiblee et al., 2019). Opportunities in the biomedical field were also explored. This is the case with Jamal and coworkers, who proposed printed scaffolds composed of differently cured hydrogel bilayers able to transform into curved micrometer-scale geometries encapsulating insulin-secreting cells (Jamal et al., 2013).
Fig. 2.
Photographs of the self-folding printed structure developed by Ge and colleagues (top image) and outline of the thermomechanical protocol followed to attain the desired shape modifications (bottom image). Adapted from (Ge et al., 2013).
3. Where does 4DP find its roots?
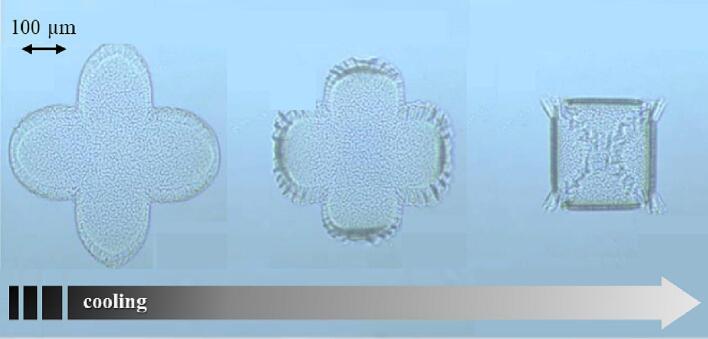
So-called smart devices have been known in the biomedical and pharmaceutical fields for many years. 4D smart structures, intended as items capable to transform over time and adapt to the surrounding environment, have been fabricated taking advantage of a range of manufacturing processes and may be found in the scientific literature well before the advent of 3D printers. Actually, the concepts of shape evolution and self-assembly have been applied to drug delivery systems at the nano-, micro- and macro (i.e. human)-scale levels. Self-assembly of molecules of different chemical nature (e.g. lipidic, peptidic, polymeric) resulting in supramolecular structures such as micelles, liposomes and nanoparticles are known for their ability to encapsulate active compounds, thus being examples of shape modifications at the nano-level (Jing et al., 2022). Microstructures based on self-folding/unfolding thin films are also described. In this respect, self-folding units were developed by Stoychev and colleagues for controlled capture and release of yeast cells in response to a temperature signal (Stoychev et al., 2011). The systems comprised two layers, one of which was made of a thermoresponsive hydrogel able to swell and shrink upon temperature variation. The other one was composed of a biodegradable hydrophobic polymer responsible for contrasting the change in volume of the hydrogel layer in one direction. This peculiar composition resulted in a programmed change in geometry of the entire structure, as highlighted in Fig. 3.
Fig. 3.
Optical microscope photographs of the self-folding device developed by Stoychev et al. displaying shape modifications triggered by cooling. Adapted from (Stoychev et al., 2011).
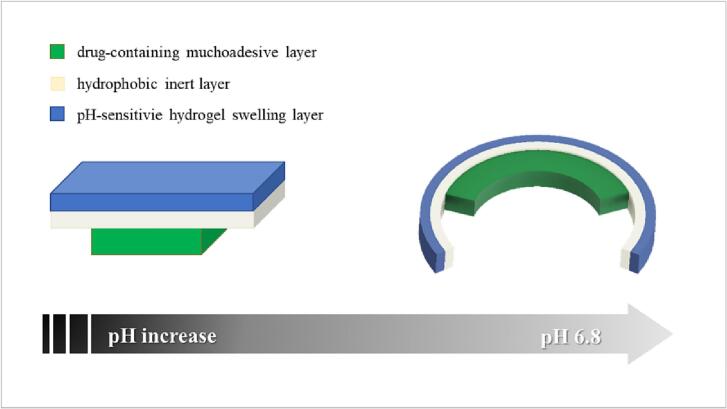
Moving to smart structures performing at the macro/human-scale, a noteworthy application of the self-folding concept is represented by the device designed by He and colleagues to be retained in the intestine (He et al., 2006). In this case, a drug-containing mucoadhesive layer was coupled with bilayer laminates made of soft smart “active” materials and inert “passive” ones, respectively (Fig. 4). In more detail, a layer based on a pH-sensitive hydrophilic polymer, able to swell at pH 6.5, was superimposed on a film composed of a hydrophobic material. Once in the intestinal environment, the drug-containing layer allowed mucoadhesion and, due to the different swelling capabilities of the layers the self-folding laminate was composed of, the overall structure tended to curl into the mucus, thus resulting in a prolonged residence at porcine small intestinal wall.
Fig. 4.
Outline of the self-folding device developed by He et al. displaying shape modifications triggered by the pH of the intestinal environment. Adapted from (He et al., 2006).
4. 3DP makes its way in the landscape of smart materials
The development of 3DP over the last decades has led to a huge number of applications of additive manufacturing (AM) in many fields, including the biomedical and, more recently, the phamaceutical ones. In this respect, among all the 3D printing techniques avaialable, binder jetting, fused depositon modeling, stereolithography and selective laser sintering were those having the greatest application potential for the fabrication of DDS, at least at the research level (Melocchi et al., 2020, Melocchi et al., 2021a).
Since 4DP was first mentioned in 2013, many attempts to combine smart materials and AM have been described. The main advantage for resorting to 3DP is represented by the possibility of easily obtaining complex shapes that would not be feasible with other methods in a single step. In this respect, 4DP would involve printing of single- and/or multi-material objects capable of changing their configuration over time, thus going beyond the concept of 3DP, 4D printed items being no longer static objects.
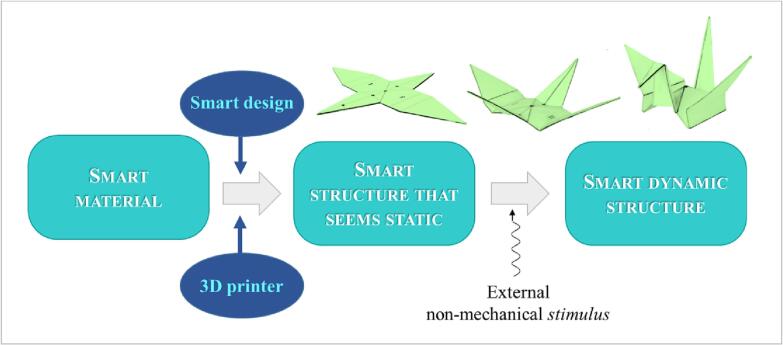
As several research groups have been working on this topic, the significance of 4DP has expanded: items showing self-transformation ability after production, in response to a desired stimulus, have been fabricated via different 3DP techniques using smart materials as feedstocks (Fig. 5). Although modifications would mostly occur in the size and shape of the object, these may reflect in improvement of its performance thus possibly adding new functionalities. Notably, when dealing with applications relevant to the biomedical/pharmaceutical field, shape changes are mainly intended to take place after application/administration into the human body. This behavior can even be more advantageous when a physiological condition (e.g. body temperature, biological fluids and their pH or ion composition in a specific body district) would trigger the above-mentioned evolution of the system.
Fig. 5.
Schematic of the 4DP concept.
The overall shape shifting process of 3D printed items would necessarily involve i) use of smart materials, as such and/or in combination, ii) fine-tuning of the item design and iii) appropriate setup of the printing process (e.g. printing orientation and composition of layers). Accordingly, 4DP also takes into account how x, y and z coordinates change after the production process and during transformation of the product. Due to the complexity of such a fabrication approach, the conceptualization step is often referred to as smart design phase. In such a phase, various aspects have to be carefully considered: i) the peculiar initial and transformed shapes the object would present, ii) the transformations to shift from one to another and iii) the relevant mechanisms. The basic principle of 4DP is to create precisely localized stress within a printed item that, upon release of this stress, would undergo further 3D shape changes in a predictable manner. This can be attained by exploiting shape memory effect or deformation mismatch. In the former case, materials capable of recovering the original shape in which they were fabricated are involved, after being fixed in a temporary shape and following a specific non-mechanical triggering stimulus. The mechanism based on deformation mismatch, which is typically used in multi-material printing, relies on material differences in terms of physical properties, such as thermal expansion coefficient and swelling ratio. In this case, 4D printed items either involve rigid structures coupled with soft ones or materials having different degrees of crosslinking. By using materials with different chemical structures and physical properties, endless combinations can therefore be imagined and created.
5. Shape memory polymers and shape morphing hydrogels: cornerstone of 4DP applications
In the last two decades, much effort has been devoted to smart materials or to identifying smart properties of those already available for use in 4DP. The previous knowledge of self-transforming drug delivery structures along with the diffusion of 3DP has certainly given a strong boost to 4DP. This, in turn, has further prompted the research to develop novel smart materials, often designed for specific 3DP applications - especially in stereolithography and gel extrusion - as well as to in depth characterize the existing ones. Based on the mechanisms discussed above for 4D printing, shape memory polymers (SMPs) and shape morphing hydrogels (SMHs) are the two main categories of raw materials employed, demonstrating potential for addressing special needs related to 4DP in terms of both processability and functionality (Champeau et al., 2020; Melocchi et al., 2021b; Zhang et al., 2019). Feedstocks of both categories are mostly synthesized on an ad hoc basis and would reasonably have the regulatory status of new chemical entities. In Table 1, a selection of printable materials of potential interest for biomedical and/or pharmaceutical 4DP applications, being already employed in the fields or of proven biocompatibility, is presented.
Table 1.
Selection of printable materials of potential interest for biomedical and/or pharmaceutical 4DP applications.
| Stimulus- responsive materials | Printing technique and post-printing treatment | Printed object | Stimulus-induced change | Application | Reference |
|---|---|---|---|---|---|
| gelatin/EC | direct ink writing (gel extrusion) | casted gelatin films with printed EC patterns | swelling mismatch upon hydration | edible composites | (Pulatsu et al., 2022) |
| AA | digital light processing | items of different shape | pH-responsive swelling | modulated drug release | (Larush et al., 2017) |
| PLA/PCL | direct ink writing + temporary shape programming by heating/cooling | tubular scaffolds | temperature-induced shape recovery | tracheal scaffold | (Pandey et al., 2022) |
| PVA | FDM 3DP + temporary shape programming by heating/cooling | items of different shape | temperature- and water-induced shape recovery | gastro-retentive and intravesical drug delivery systems | (Melocchi et al., 2019b, Melocchi et al., 2019a)(Inverardi et al., 2021) |
| PNIPAM | gel extrusion and UV cross-linking | cylindrical hydrogel shell | temperature-induced modification of pore size | modulated drug release | (Zu et al., 2022) |
| PDA | gel extrusion of hydrogel core + coating with PDA and PCL by soaking in CHCl3 solution | core/shell fiber scaffolds: core (alginate + gelatine), shell (PCL and PDA) |
NIR irradiation triggered drug release | localized cancer therapy and tissue regeneration | (Liu et al., 2021) |
| s-PCL-MA | direct ink printing + UV photocuring with polythiol as initiator | vascular stent | temperature-induced shape recovery | drug-releasing deployable devices | (He et al., 2022) |
| βCD-g-PCL | direct ink printing + UV photocuring | vascular stent | temperature-induced shape recovery | stent for 1-month drug release | (Zhou et al., 2021) |
| crosslinked alginate and F127DA hydrogels | direct ink printing + subsequent UV light photocuring + temporary shape programming (immersion in CaCl2 solution) | hydrogel with an internal mesh structure | unfolding by Ca2+ removal upon immersion in Na2CO3 solution | actuators, tissue engineering, drug delivery | (Wang et al., 2018) |
AA Acrylic Acid; βCD-g-PCL star polymer having βCD core and 21 PCL arms with acrylate end group; EC ethylcellulose F127DA Pluronic F127 diacrylate macromer; PCL poly-ε-caprolactone; PDA polydopamine; PLA poly-l-lactide; PNIPAM poly(N-isopropylacrylamide); PVA polyvinyl alcohol; s-PCL-MA star poly-caprolactone with methacrylated end-group.
In the molecular network of a typical SMP, permanent netpoints, involved in memorizing the original shape, coexist interconnected with chain segments responsible for the material deformability. Such netpoints can result from either chemical bonding or physical interactions, and are not affected in the programming step. The most common type of chemical netpoint is crosslinking, which can be introduced directly during the synthesis of the SMP or in a post-processing/printing step (e.g. UV curing) (Liang et al., 2018; Song et al., 2020).
Physical netpoints are typical of semicrystalline polymers, where domains with different transition temperatures can be found. Specifically, domains that are responsible for shape retainment are generally named as permanent and characterized by the highest thermal transition temperature (Tperm). Conversely, chain segments having lower thermal transition temperature (Ttrans) behave as switching domains and are essential for programming the temporary shape.
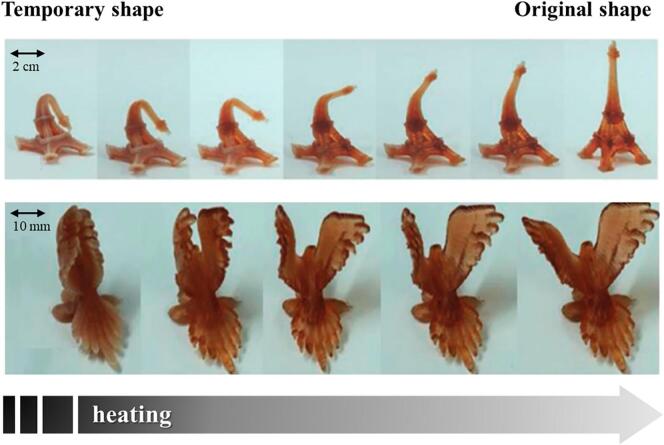
Focusing on the mechanisms triggering the shape change, thermo- and chemo-responsive SMPs are those mainly described. In thermo-responsive SMPs, the temporary shape is generally programmed by heating the printed item at a temperature above Ttrans (but below Tperm) while applying an external stress. The temporary shape is then set by cooling the item below Ttrans. Subsequently, the recovery of the original shape occurs when the temperature is raised above Ttrans. In this respect, Fig. 6 shows the behavior, upon heating, of 4D printed objects fabricated by Zarek et al. starting from temperature-sensitive SMPs.
Fig. 6.
Photographs of 4D printed objects fabricated by stereolithography during relevant shape recovery triggered by heating. Adapted from (Zarek et al., 2016).
In the case of chemo-responsive SMPs, the programming step is similar although the shape recovery process is activated by a chemical triggering stimulus able to alter the polymer mobility through softening, swelling or dissolution. In particular, in softening-induced shape memory effect, solvent molecules absorbed by the polymer would act as a plasticizer, thus promoting the mobility of its chains. The switching temperature is thus reduced, allowing the recovery of the original shape to take place even at body or room temperature. This represents the basis for the water-induced shape memory effect, which has started to be tested in the medical field. As regards the swelling-induced shape memory behavior, the polymer network, upon immersion into the appropriate medium, progressively hydrates and swells, thus resulting in the formation of a gel. The latter is able to undergo a relatively large deformation, which can be translated in a volume expansion until an equilibrium state for a given environment is reached. At the same time, a reduction in the transition temperature of the polymer could be attained activating the shape recovery process.
Hydrogels are three-dimensional networks of hydrophilic, cross-linked polymers that can adsorb a large amount of water without dissolving. Shape and/or functionality change of hydrogel-based items could occur, as already observed for SMPs, in response to a range of triggering stimuli, such as changes in temperature, pH, ion concentration or upon the application of electric as well as magnetic fields (Champeau et al., 2020; Dong et al., 2020; Larush et al., 2017). More commonly, shape modifications can be obtained by making use of materials that respond to simple solvent absorption/desorption phenomena, thus leading to the so-called shape morphing or smart hydrogels. Indeed, diffusion of water into the network would cause controlled swelling to a different extent, depending on the specific characteristics of the area directly involved. In any case, to manage the modifications of the object in shape and size, a swelling mismatch needs to be induced in the structure, for instance by defining the material composition and its spatial arrangement during printing. To this aim, hydrogels having diverse hydration ability can be combined into multi-material structures. Alternatively, a single hydrogel type capable of different swelling degree along its structure (anisotropic swelling), can be developed. This can be pursued by incorporating anisotropic particles to the hydrogel or by creating regions with a different degree of crosslinking and density in the material (Huang et al., 2017; Jamal et al., 2011; Mulakkal et al., 2018; Zhao et al., 2017).
Notably, for some hydrogels shape programming of the 3D printed object is also possible so that they can be referred to as shape memory hydrogels, being included into the SMP category (Korde and Kandasubramanian, 2020; Wang et al., 2018).
6. Insights on 4DP in the biomedical field
In the last few decades, the exploration of smart materials for application in the medical field has been strongly prompted by the progress of 3DP techniques and the continuous improvement in the understanding of biological systems. The benefits associated with 4DP have been recognized in many different areas, such as tissue regeneration, soft robotics, medical devices, diagnosis and delivery of biologics (Javaid and Haleem, 2019; Moroni et al., 2022; Zhou et al., 2021). In case of tissue regeneration, for example, functional scaffolds purposely engineered to assist in remodeling and replacing diseased or injured areas represent an ongoing topic of research. In this respect, new materials showing bio-mimicking features and bio-responsiveness can greatly help the development of such technologies (Free, 2021; Naniza et al., 2022; Senatov et al., 2016; Tamay et al., 2019; Zhang et al., 2018).
Personalized medical devices, provided with a dynamic behavior in a desired timescale and designed around the needs of a specific patient, may represent an advantageous alternative to otherwise static implants. By way of example, they can be positioned in the body in a minimally invasive way or can adapt themselves to physiological growth conditions. The use of printing processes in this specific context can be considered particularly suitable for obtaining the desired complex geometries, and potentially applicable to the fabrication of customized products in a rapid and cost-efficient manner. Multiple studies have reported proof-of-concept 4DP applications for thermally induced SMP-based devices, such as catheters, microgrippers, vascular stents, aneurysmal foams, orthodontic wires and surgical sutures (Delaey et al., 2020; Javaid and Haleem, 2019; Yakacki and Gall, 2010; Zhao et al., 2019). Nevertheless, despite the strong academic interest generated, engineering into clinically useful devices has demonstrated to be rather challenging.
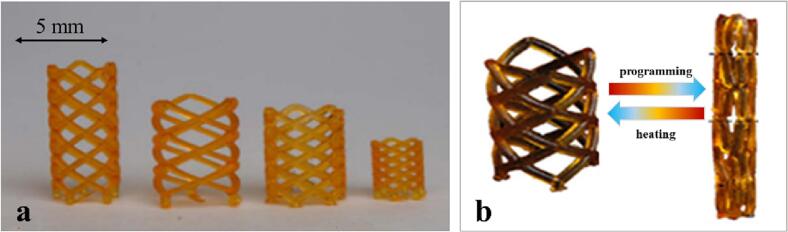
The most advanced examples in this respect are represented by stents to be used in cardiovascular applications. They are conceived to have a small size to enable minimally invasive insertion, and to expand once positioned into the body. Having as a reference the traditionally used shape memory alloys (e.g. nitinol), researchers started to test photo-curable methacrylate copolymers when dealing with 3DP. Through high resolution projection microstereolithography (μSLA), polymeric stents were successfully printed in the desired original shape and, afterwards, were thermally programmed in the temporary - generally shrunk - shape, which eased insertion into the blood vessel. Restoration to the larger original 3D-printed shape was generally triggered when the temperature of the stent reached that of the body (Fig. 7). A paclitaxel-loaded vascular stent was also successfully prepared making use of a purposely designed photocurable star polymer composed of a β-cyclodextrin core and polycaprolactone arms with acrylate end groups (Zhou et al., 2021). The stent showed biocompatibility and shape memory effect, along with suitable mechanical properties and the ability to provide sustained release of the loaded drug.
Fig. 7.
Photographs of 3D printed stents with different geometric parameters (height, diameter, number of joints, diameter of ligaments and angle between ligaments) (a); 3D printed stent programmed into the temporary shape with a smaller diameter for minimally invasive surgery: after heating, the stent recovers the original shape with a larger diameter (b). Adapted from (Ge et al., 2016).
Such a peculiar behavior was also exploited in non-cardiovascular applications. By way of example, pediatric bronchial growing stents, conceived to adapt themselves to child's growth over time, have been proposed (Freitag et al., 2017). In this case, a different 4DP approach was followed, entailing the combination of degradable and stable elastic polymers. Hybrid stents were printed by means of a self-developed 3D multimode-multimaterial printer using polyurethane for the elastic tubular structure of the stent and polylactic acid to create a biodegradable restricting element. In vivo degradation of the latter polymer enabled slow expansion of the device over an adequate time frame.
4DP was also proposed to address the need for personalized endoluminal stents intended to maintain the respiratory tract patency in a variety of disorders affecting the trachea. In this case, engineering fully conformable devices, able to ensure a precise fit and anchor-in-place of the stent represents a potential approach to prevent stent migration and related complications. Zarek et al. printed by SLA a prototype of tracheal stent based on semicrystalline methacrylated polycaprolactone, having peculiar C-shaped protuberances of the trachea (Zarek et al., 2017). Personalization of the printed object based on anatomical dimensions was pursued by obtaining a digital model of the trachea from an online repository of human anatomy, which was acquired by whole body MRI. The printed constructs, obtained after a proper selection of molecular weight fractions of the polycaprolactone precursor, displayed mechanical properties suitable to prevent obstruction of the trachea by counteracting external compression, while allowing a certain degree of bending freedom. When shape memory recovery was thermally triggered the airway stent proved able to promptly restore its original expanded shape, from the temporary programmed abridged one, thus matching the target anatomy (Fig. 8).
Fig. 8.
Series of photographs of a 3D printed airway tracheal stent, showing the transition between the temporary open configuration state (for deployment) to the permanent shape (for performance): dorsal view (a) on-end view of the stent (b). Reprinted with permission from (Zarek et al., 2017).
7. 4DP in drug delivery
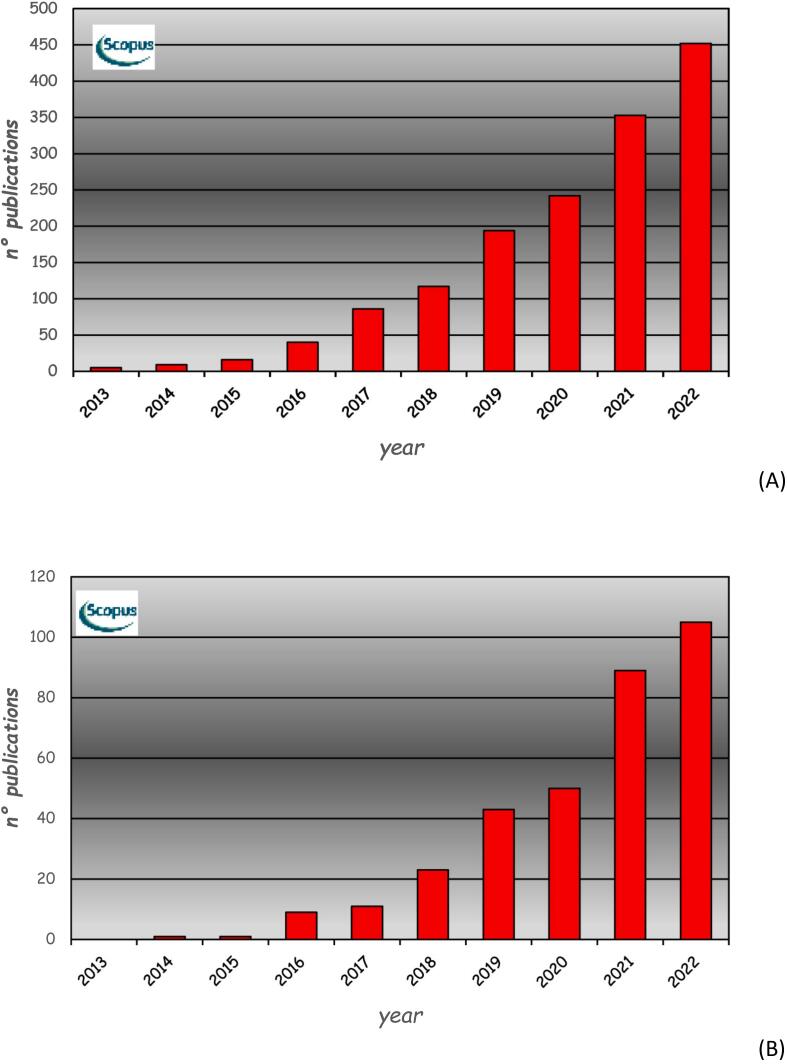
Despite the huge interest in the application of 4DP, few projects have been pursued so far in the pharmaceutical field, and a limited number of papers have been published on this topic. Indeed, although many articles have been published on 4D printing since 2013 (Fig. 9A), only a relatively small number involved drug, pharmaceutical or medical applications (Fig. 9B).This is also highlighted in recent reviews on 3DP, which also covered the specific domain of 4DP (Firth et al., 2018; Trenfield et al., 2019). Within the specific area of drug delivery, efforts have mainly been directed towards the development of novel responsive materials, suitable for being used in the design of DDSs with desired kinetics and/or providing localized release of drugs (Ahmed, 2018; Larush et al., 2017; Li et al., 2016; Wang et al., 2021).
Fig. 9.
Articles published in the last decade in the scientific literature on 4D printing (A), and specifically involving drug, pharmaceutical or medical applications (B) (source: Scopus).
From reactive materials to reactive devices, the inventive step has been relatively short, with the advent of new ideas to conceive more acceptable ways to deliver drugs to the patient. In some cases, inspiration for new proposals originated from the observation of elements present in nature. This was for example the case with the microneedles proposed by Li et al. for painless administration of drugs through the skin, which have a hierarchical, limpet tooth-inspired architectures (Li et al., 2021). The proposed devices are reinforced composite structures obtained by magnetic field-assisted 3D printing able to reach high mechanical strength upon shape change, thus potentially being more suitable for harmless penetration into the skin. Moreover, a certain number of preliminary 4D printed products were proposed as drug-loaded scaffolds or implantable devices. They would take advantage of the in vivo changes occurring in their shape and size to enable minimal surgical intervention for insertion into the body (Vehse et al., 2014; Wang et al., 2018). These systems could be considered at the intersection of medical devices, scaffolds and actual DDSs, because they were generally proposed in response to the strong clinical trend towards minimally invasive surgery without identifying a specific administration site or therapeutic goal.
With a similar intent of reducing the discomfort associated with medication, and more specifically to enhance the patient adherence to a complex therapy, DDSs designed to enable a less frequent regimen of administration certainly play a prominent role. In this respect, 4DP has been proposed to prepare different organ-retentive devices intended to be held within hollow organs and to release drugs meanwhile in a controlled manner. Theragrippes for example are gastrointestinal-resident mini- or microdevices, generally consisting of multilayer structures provided with sharp microtips, which can latch (self-close) onto the mucosal tissue following different kind of stimuli (Ghosh et al., 2020; Malachowski et al., 2014). These systems were proved able to remain within the GI tract of live animals for 24 h after rectal administration.
More recently, 4DP has been applied to other administration routes for the development of novel DDSs designed to be retained in the target organ following a shape change and to release drug in a controlled manner. The urinary bladder and stomach were particularly appealing for such 4DP devices because of the number of still pending issues associated with the delivery of drugs to these anatomical sites (Maroni et al., 2020; Melocchi et al., 2019a, Melocchi et al., 2019b; Palugan et al., 2021; Uboldi et al., 2022). As already observed with stents, key points in the design and formulation of such systems are that sizes differing from one another would be required for safe entry into and effective retention within the hollow organs, respectively. On the one hand, a sufficiently small-sized conformation would be needed for administration and positioning of the drug-loaded device inside the body cavity. On the other hand, a larger spatial encumbrance would be mandatory for preventing untimely emptying of the system. Evolution from a smaller to a larger size can effectively arise from unfolding processes triggered by stimuli, such as the uptake of physiological fluids and/or the achievement of body temperature. In these cases, SMPs are necessarily involved.
Basic requirements need to be fulfilled in the development of these DDSs, namely i) prompt achievement of the retentive configuration after dosing, ii) its maintenance over the desired time, also in spite of the mechanical stresses associated to smooth muscle contraction undergone by the device during its residence in the organ, iii) release of the drug in a rate-controlled way and iv) spontaneous removal from the retaining organ after exhaustion.
Polyvinyl alcohol of pharmaceutical grade having diverse molecular weight was selected as the main polymeric component to fabricate both bladder and stomach-retentive devices by fused deposition modeling 3DP, by virtue of its swelling/erosion properties, good hot-processability and shape memory ability. PVA-based items were either printed or extruded in the desired original shape and then programmed into a temporary one by heating up the prototypes and shaping them manually or with the aid of templates. The temporary shape was then fixed by cooling the samples in the deformed shape at low temperatures.
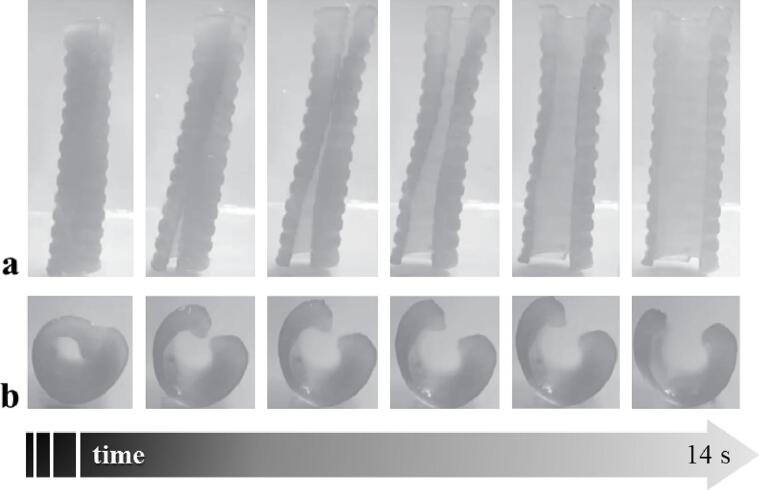
In more detail, the retentive system for intravesical drug delivery was designed to be administered into the bladder in a temporary rod-like form, suitable for insertion by urethral catheterization, and to be retained after recovery of the original bent or curled shape not compatible with physiological bladder emptying (Fig. 10). Elimination of the device would therefore be possible only after its dissolution/erosion. Prototypes were successfully obtained and characterized by differential scanning calorimetry and dynamic-mechanical thermal analysis as well as in terms of fluid uptake, mass loss, shape recovery and release behavior. The samples exhibited the desired ability to recover the original shape, consistent in kinetics with the relevant thermo-mechanical properties, and concomitant prolonged release of a drug tracer. Although preliminary in scope, this study pointed out the viability of the proposed approach to the design of retentive intravesical DDSs.
Fig. 10.
4DP vesical-retentive DDS: virtual model of U and helix-shaped devices (a), photographs of printed prototypes in their original (b) and programmed temporary rod shape (c), photographs acquired during shape recovery experiments (37 °C) of prototypes having original helix shape and programmed to take on a temporary I-shape (d). Adapted from (Melocchi et al., 2019a).
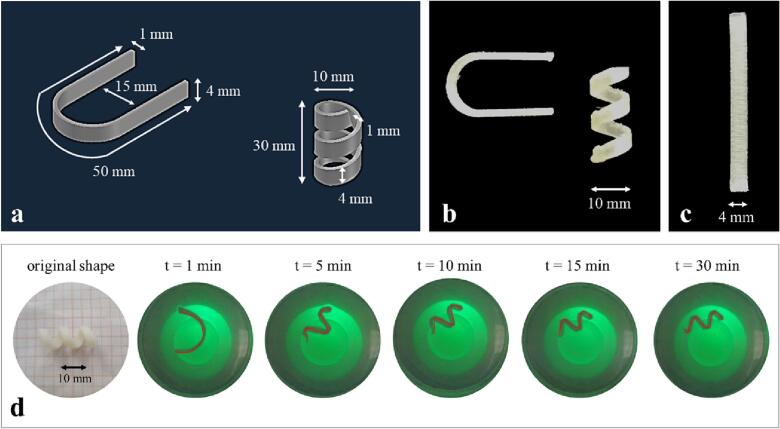
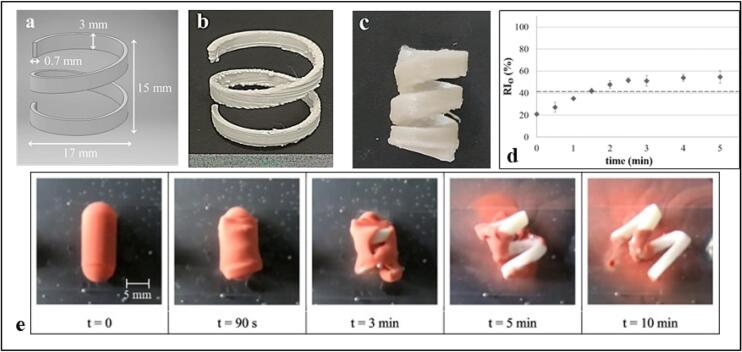
The gastroretentive system, based on the same approach described for the vesical one, was devised as a step forward with respect to expandable devices already described in the scientific literature, which generally relied on a mechanically-driven working mechanism. The shape was selected to enable retention in the stomach during the interdigestive phase. For this reason, it showed 2 dimensions ≥15 mm, which represent a commonly reported size of open pylorus. On the other hand, the system temporary shape was conceived to be in principle compatible with swallowing, i.e. suitable to be inserted into commercially available hard-gelatin capsules. Once in the stomach, following dissolution of the capsule, the device was expected to recover its original cumbersome configuration upon contact with gastric fluids at body temperature. Allopurinol-containing cylindrical helices were manufactured by FDM and then manually fixed into supercoiled configurations (Fig. 11b-c). When immersed in 0.1 N HCl solution at 37 °C, the prototypes showed the expected behavior, reaching the desired spatial encumbrance within few minutes (Fig. 11d-e) and releasing the tracer in a controlled manner. Additionally, the application of mixed Eudragit® RS/RL or of Eudragit® NE polymeric films by means of a purposely assembled small-scale coating equipment, turned out to be a suitable strategy to control the release rate of the delivery systems without affecting their smart performance (Uboldi et al., 2021). Furthermore, by means of an engineering approach, relying on the comparison between computational and experimental mechanical and thermo-mechanical characterization data, a validated model was developed to investigate - and potentially predict - the shape changes undergone by a specific PVA-based geometry, allowing to choose an optimized shape without the need for fabricating a range of different prototypes (Inverardi et al., 2021).
Fig. 11.
4DP gastroretentive helix-shaped device: virtual model of the device (a), photographs of a printed prototype in its original (b) and programmed temporary shape (c), shape recovery profile (d), photographic sequence acquired during shape recovery of the device (e). Adapted from (Melocchi et al., 2019b).
8. Conclusion
4D printing is an emerging technology and represents an evolution of 3DP, encompassing the use of smart materials to design structures with the ability to transform over time, in a predictable manner, in response to external stimuli of non-mechanical nature.
Since its introduction in 2013, this new concept has been attracting more and more interest in different areas of research. This prompted major advances in material science, to make printable ones available, and researchers to develop increasingly complex mathematical models to boost the shift from proof of concept to real-life 4DP applications. This would also require the identification of processes that could be suitable for mass production, enabling the scaling up of 4D printing.
As far as the pharmaceutical field is concerned, the opportunity to attain complex, purposely designed structures starting from materials either newly synthesized or already in use and showing a potential for shape memory, is deemed of particular interest. The implementation of 4D printing in drug delivery would allow to address unmet needs, for instance to develop less invasive and more compliant ways to administer medications and attain therapeutic objectives that are currently unfeasible. In this respect, a few original ideas have already been put forward, mainly relating to the fabrication of scaffolds/implants and systems intended for retention into hollow muscular organs, able to transform themselves upon administration and to release the drug conveyed according to the desired kinetics. Although the exploitation of 4DP concept to pharmaceutics is still in its infancy, its application potential seems huge, allowing the researchers to focus on very innovative devices and purse delivery approaches that have not yet been explored. In such a sense, significant progress will be possible taking advantage of creativity and especially if the horizons of research will be widened towards new targets in terms of temporal and spatial control of release.
CRediT authorship contribution statement
Andrea Gazzaniga: Conceptualization, Methodology, Validation, Formal analysis, Project administration, Writing – original draft, Supervision. Anastasia Foppoli: Conceptualization, Methodology, Investigation, Data curation, Writing – original draft, Writing – review & editing. Matteo Cerea: Methodology, Validation, Writing – original draft, Supervision. Luca Palugan: Validation, Resources, Writing – original draft, Supervision. Micol Cirilli: Validation, Investigation, Visualization. Saliha Moutaharrik: Validation, Investigation, Visualization. Alice Melocchi: Validation, Investigation, Visualization, Writing – review & editing. Alessandra Maroni: Conceptualization, Methodology, Formal analysis, Resources, Writing – review & editing, Supervision.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Partially presented by AG at APGI Hot Topic Day: 3D Printing in Pharmaceutics, Université de Lille (F), November 30, 2020.
Data availability
No data was used for the research described in the article.
References
- Ahmed J. In: 3D and 4D Printing in Biomedical Applications: Process Engineering and Additive Manufacturing. Maniruzzaman Mohammed., editor. Wiley-VCH Verlag GmbH & Co; KGaA: 2018. Recent advances of novel materials for 3D/4D printing in biomedical applications; pp. 239–271. [DOI] [Google Scholar]
- Champeau M., Heinze D.A., Viana T.N., de Souza E.R., Chinellato A.C., Titotto S. 4D printing of hydrogels: a review. Adv. Funct. Mater. 2020;30:1–22. doi: 10.1002/adfm.201910606. [DOI] [Google Scholar]
- De Marco C., Alcântara C.C.J., Kim S., Briatico F., Kadioglu A., de Bernardis G., Chen X., Marano C., Nelson B.J., Pané S. Indirect 3D and 4D printing of soft robotic microstructures. Adv. Mater. Technol. 2019;4:1900332. doi: 10.1002/admt.201900332. [DOI] [Google Scholar]
- Delaey J., Dubruel P., Van Vlierberghe S. Shape-memory polymers for biomedical applications. Adv. Funct. Mater. 2020;30:1909047. doi: 10.1002/adfm.201909047. [DOI] [Google Scholar]
- Dong Y., Wang S., Ke Y., Ding L., Zeng X., Magdassi S., Long Y. 4D printed hydrogels: fabrication, materials, and applications. Adv. Mater. Technol. 2020;5:1–19. doi: 10.1002/admt.202000034. [DOI] [Google Scholar]
- Firth J., Gaisford S., Basit A.W. In: 3D Printing of Pharmaceuticals. Basit A.W., Gaisford S., editors. Springer International Publishing; Cham: 2018. A new dimension: 4D printing opportunities in pharmaceutics; pp. 153–162. [DOI] [Google Scholar]
- Free T. Stay smart: It’s all about the materials for 4D printing in biomedicine. Biotechniques. 2021;70:191–194. doi: 10.2144/BTN-2021-0016. [DOI] [PubMed] [Google Scholar]
- Freitag L., Gördes M., Zarogoulidis P., Darwiche K., Franzen D., Funke F., Hohenforst-Schmidt W., Dutau H. Towards individualized tracheobronchial stents: technical, practical and legal considerations. Respiration. 2017;94:442–456. doi: 10.1159/000479164. [DOI] [PubMed] [Google Scholar]
- Ge Q., Qi H.J., Dunn M.L. Active materials by four-dimension printing. Appl. Phys. Lett. 2013;103:1–5. doi: 10.1063/1.4819837. [DOI] [Google Scholar]
- Ge Q., Sakhaei A.H., Lee H., Dunn C.K., Fang N.X., Dunn M.L. Multimaterial 4D printing with tailorable shape memory polymers. Sci. Rep. 2016;6:1–11. doi: 10.1038/srep31110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A., Li L., Xu L., Dash R.P., Gupta N., Lam J., Jin Q., Akshintala V., Pahapale G., Liu W., Sarkar A., Rais R., Gracias D.H., Selaru F.M. Gastrointestinal-resident, shape-changing microdevices extend drug release in vivo. Sci. Adv. 2020;6 doi: 10.1126/sciadv.abb4133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hann S.Y., Cui H., Nowicki M., Zhang L.G. 4D printing soft robotics for biomedical applications. Addit. Manuf. 2020;36 doi: 10.1016/j.addma.2020.101567. [DOI] [Google Scholar]
- He H., Guan J., Lee J.L. An oral delivery device based on self-folding hydrogels. J. Control. Release. 2006;110:339–346. doi: 10.1016/j.jconrel.2005.10.017. [DOI] [PubMed] [Google Scholar]
- He W., Zhou D., Gu H., Qu R., Cui C., Zhou Y., Wang Y., Zhang X., Wang Q., Wang T., Zhang Y. A biocompatible 4D printing shape memory polymer as emerging strategy for fabrication of deployable medical devices. Macromol. Rapid Commun. 2022;2200553:1–12. doi: 10.1002/marc.202200553. [DOI] [PubMed] [Google Scholar]
- Huang L., Jiang R., Wu J., Song J., Bai H., Li B., Zhao Q., Xie T. Ultrafast digital printing toward 4D shape changing materials. Adv. Mater. 2017;29:1605390. doi: 10.1002/adma.201605390. [DOI] [PubMed] [Google Scholar]
- Inverardi N., Scalet G., Melocchi A., Uboldi M., Maroni A., Zema L., Gazzaniga A., Auricchio F., Briatico-Vangosa F., Baldi F., Pandini S. Experimental and computational analysis of a pharmaceutical-grade shape memory polymer applied to the development of gastroretentive drug delivery systems. J. Mech. Behav. Biomed. Mater. 2021;124 doi: 10.1016/j.jmbbm.2021.104814. [DOI] [PubMed] [Google Scholar]
- Jamal M., Zarafshar A.M., Gracias D.H. Differentially photo-crosslinked polymers enable self-assembling microfluidics. Nat. Commun. 2011;2 doi: 10.1038/ncomms1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamal M., Kadam S.S., Xiao R., Jivan F., Onn T.M., Fernandes R., Nguyen T.D., Gracias D.H. Bio-origami hydrogel scaffolds composed of photocrosslinked PEG bilayers. Adv. Healthc. Mater. 2013;2:1142–1150. doi: 10.1002/adhm.201200458. [DOI] [PubMed] [Google Scholar]
- Javaid M., Haleem A. 4D printing applications in medical field: a brief review. Clin. Epidemiol. Glob. Heal. 2019;7:317–321. doi: 10.1016/j.cegh.2018.09.007. [DOI] [Google Scholar]
- Jing Z., Ma Y., Zhu J. Application of a novel electrostatic dry powder coating technology on capsules for enteric release. J. Drug Deliv. Sci. Technol. 2022;68 doi: 10.1016/j.jddst.2021.103058. [DOI] [Google Scholar]
- Korde J.M., Kandasubramanian B. Naturally biomimicked smart shape memory hydrogels for biomedical functions. Chem. Eng. J. 2020;379 doi: 10.1016/j.cej.2019.122430. [DOI] [Google Scholar]
- Larush L., Kaner I., Fluksman A., Tamsut A., Pawar A.A., Lesnovski P., Benny O., Magdassi S. 3D printing of responsive hydrogels for drug-delivery systems. J. 3D Print. Med. 2017;1:219–229. doi: 10.2217/3dp-2017-0009. [DOI] [Google Scholar]
- Li H., Go G., Ko S.Y., Park J.O., Park S. Magnetic actuated pH-responsive hydrogel-based soft micro-robot for targeted drug delivery. Smart Mater. Struct. 2016;25 doi: 10.1088/0964-1726/25/2/027001. [DOI] [Google Scholar]
- Li X., Shan W., Yang Y., Joralmon D., Zhu Y., Chen Yiyu, Yuan Y., Xu H., Rong J., Dai R., Nian Q., Chai Y., Chen Yong. Limpet tooth-inspired painless microneedles fabricated by magnetic field-assisted 3D printing. Adv. Funct. Mater. 2021;31 doi: 10.1002/adfm.202003725. [DOI] [Google Scholar]
- Liang Z., Liu Y., Zhang F., Ai Y., Liang Q. Dehydration-triggered shape morphing based on asymmetric bubble hydrogel microfibers. Soft Matter. 2018;14:6623–6626. doi: 10.1039/c8sm00984h. [DOI] [PubMed] [Google Scholar]
- Liu C., Wang Z., Wei X., Chen B., Luo Y. 3D printed hydrogel/PCL core/shell fiber scaffolds with NIR-triggered drug release for cancer therapy and wound healing. Acta Biomater. 2021;131:314–325. doi: 10.1016/j.actbio.2021.07.011. [DOI] [PubMed] [Google Scholar]
- Malachowski K., Breger J., Kwag H.R., Wang M.O., Fisher J.P., Selaru F.M., Gracias D.H. Stimuli-responsive theragrippers for chemomechanical controlled release. Angew. Chem. Int. Ed. 2014;53:8045–8049. doi: 10.1002/anie.201311047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroni A., Melocchi A., Zema L., Foppoli A.A., Gazzaniga A. Retentive drug delivery systems based on shape memory materials. J. Appl. Polym. Sci. 2020;137:1–10. doi: 10.1002/app.48798. [DOI] [Google Scholar]
- Melocchi A., Inverardi N., Uboldi M., Baldi F., Maroni A., Pandini S., Briatico-Vangosa F., Zema L., Gazzaniga A. Retentive device for intravesical drug delivery based on water-induced shape memory response of poly(vinyl alcohol): design concept and 4D printing feasibility. Int. J. Pharm. 2019;559:299–311. doi: 10.1016/j.ijpharm.2019.01.045. [DOI] [PubMed] [Google Scholar]
- Melocchi A., Uboldi M., Inverardi N., Briatico-Vangosa F., Baldi F., Pandini S., Scalet G., Auricchio F., Cerea M., Foppoli A., Maroni A., Zema L., Gazzaniga A. Expandable drug delivery system for gastric retention based on shape memory polymers: development via 4D printing and extrusion. Int. J. Pharm. 2019;571 doi: 10.1016/j.ijpharm.2019.118700. [DOI] [PubMed] [Google Scholar]
- Melocchi A., Uboldi M., Cerea M., Foppoli A., Maroni A., Moutaharrik S., Palugan L., Zema L., Gazzaniga A. A graphical review on the escalation of fused deposition modeling (FDM) 3D printing in the pharmaceutical field. J. Pharm. Sci. 2020;109:2943–2957. doi: 10.1016/j.xphs.2020.07.011. [DOI] [PubMed] [Google Scholar]
- Melocchi A., Briatico-Vangosa F., Uboldi M., Parietti F., Turchi M., von Zeppelin D., Maroni A., Zema L., Gazzaniga A., Zidan A. Quality considerations on the pharmaceutical applications of fused deposition modeling 3D printing. Int. J. Pharm. 2021;592 doi: 10.1016/J.IJPHARM.2020.119901. [DOI] [PubMed] [Google Scholar]
- Melocchi A., Uboldi M., Cerea M., Foppoli A., Maroni A., Moutaharrik S., Palugan L., Zema L., Gazzaniga A. Shape memory materials and 4D printing in pharmaceutics. Adv. Drug Deliv. Rev. 2021;173:216–237. doi: 10.1016/J.ADDR.2021.03.013. [DOI] [PubMed] [Google Scholar]
- Moroni S., Casettari L., Lamprou D.A. 3D and 4D printing in the fight against breast cancer. Biosensors. 2022;12 doi: 10.3390/bios12080568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulakkal M.C., Trask R.S., Ting V.P., Seddon A.M. Responsive cellulose-hydrogel composite ink for 4D printing. Mater. Des. 2018;160:108–118. doi: 10.1016/j.matdes.2018.09.009. [DOI] [Google Scholar]
- Naniza M.A., Askarib M., Zolfaghariand A., Nanize M.A., Bodaghi M. 4D printing: a cutting-edge platform for biomedical applications. ACS Appl. Mater. Interfaces. 2022;10:22408–22418. doi: 10.1088/1748-605X/ac8e42. [DOI] [PubMed] [Google Scholar]
- Palugan L., Cerea M., Cirilli M., Moutaharrik S., Maroni A., Zema L., Melocchi A., Uboldi M., Filippin I., Foppoli A., Gazzaniga A. Intravesical drug delivery approaches for improved therapy of urinary bladder diseases. Int. J. Pharm. X. 2021;3 doi: 10.1016/j.ijpx.2021.100100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey H., Mohol S.S., Kandi R. 4D printing of tracheal scaffold using shape-memory polymer composite. Mater. Lett. 2022;329 doi: 10.1016/j.matlet.2022.133238. [DOI] [Google Scholar]
- Pulatsu E., Su J.W., Lin J., Lin M. Utilization of ethyl cellulose in the osmotically-driven and anisotropically-actuated 4D printing concept of edible food composites. Carbohydr. Polym. Technol. Appl. 2022;3 doi: 10.1016/j.carpta.2022.100183. [DOI] [Google Scholar]
- Raviv D., Zhao W., McKnelly C., Papadopoulou A., Kadambi A., Shi B., Hirsch S., Dikovsky D., Zyracki M., Olguin C., Raskar R., Tibbits S. Active printed materials for complex self-evolving deformations. Sci. Rep. 2014;4:1–9. doi: 10.1038/srep07422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senatov F.S., Niaza K.V., Zadorozhnyy M.Y., Maksimkin A.V., Kaloshkin S.D., Estrin Y.Z. Mechanical properties and shape memory effect of 3D-printed PLA-based porous scaffolds. J. Mech. Behav. Biomed. Mater. 2016;57:139–148. doi: 10.1016/j.jmbbm.2015.11.036. [DOI] [PubMed] [Google Scholar]
- Shiblee M.D.N.I., Ahmed K., Kawakami M., Furukawa H. 4D printing of shape-memory hydrogels for soft-robotic functions. Adv. Mater. Technol. 2019;4:1900071. doi: 10.1002/admt.201900071. [DOI] [Google Scholar]
- Song Z., Ren Luquan, Zhao C., Liu H., Yu Z., Liu Q., Ren Lei. Biomimetic nonuniform, dual-stimuli self-morphing enabled by gradient four-dimensional printing. ACS Appl. Mater. Interfaces. 2020;12:6351–6361. doi: 10.1021/acsami.9b17577. [DOI] [PubMed] [Google Scholar]
- Stoychev G., Puretskiy N., Ionov L. Self-folding all-polymer thermoresponsive microcapsules. Soft Matter. 2011;7:3277–3279. doi: 10.1039/c1sm05109a. [DOI] [Google Scholar]
- Tamay D.G., Usal T.D., Alagoz A.S., Yucel D., Hasirci N., Hasirci V. 3D and 4D printing of polymers for tissue engineering applications. Front. Bioeng. Biotechnol. 2019 doi: 10.3389/fbioe.2019.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibbits S. The Emergence of “4D printing” [WWW Document] 2013. https://www.ted.com/talks/skylar_tibbits_the_emergence_of_4d_printing URL.
- Trenfield S.J., Awad A., Madla C.M., Hatton G.B., Firth J., Goyanes A., Gaisford S., Basit A.W. Shaping the future: recent advances of 3D printing in drug delivery and healthcare. Expert Opin. Drug Deliv. 2019;16:1081–1094. doi: 10.1080/17425247.2019.1660318. [DOI] [PubMed] [Google Scholar]
- Uboldi M., Melocchi A., Moutaharrik S., Cerea M., Gazzaniga A., Zema L. Dataset on a small-scale film-coating process developed for self-expanding 4D printed drug delivery devices. Coatings. 2021 doi: 10.3390/coatings11101252. [DOI] [Google Scholar]
- Uboldi M., Melocchi A., Moutaharrik S., Palugan L., Cerea M., Foppoli A., Maroni A., Gazzaniga A., Zema L. Administration strategies and smart devices for drug release in specific sites of the upper GI tract. J. Control. Release. 2022;348:537–552. doi: 10.1016/j.jconrel.2022.06.005. [DOI] [PubMed] [Google Scholar]
- Vehse M., Petersen S., Sternberg K., Schmitz K.P., Seitz H. Drug delivery from poly(ethylene glycol) diacrylate scaffolds produced by DLC based micro-stereolithography. Macromol. Symp. 2014;346:43–47. doi: 10.1002/masy.201400060. [DOI] [Google Scholar]
- Wang Y., Miao Y., Zhang J., Wu J.P., Kirk T.B., Xu J., Ma D., Xue W. Three-dimensional printing of shape memory hydrogels with internal structure for drug delivery. Mater. Sci. Eng. C. 2018;84:44–51. doi: 10.1016/j.msec.2017.11.025. [DOI] [PubMed] [Google Scholar]
- Wang J., Zhang Y., Aghda N.H., Pillai A.R., Thakkar R., Nokhodchi A., Maniruzzaman M. Emerging 3D printing technologies for drug delivery devices: current status and future perspective. Adv. Drug Deliv. Rev. 2021;174:294–316. doi: 10.1016/j.addr.2021.04.019. [DOI] [PubMed] [Google Scholar]
- Yakacki C.M., Gall K. In: Shape-Memory Polymers for Biomedical Applications BT - Shape-Memory Polymers. Lendlein A., editor. Springer; Berlin Heidelberg, Berlin, Heidelberg: 2010. pp. 147–175. [DOI] [Google Scholar]
- Zarek M., Layani M., Cooperstein I., Sachyani E., Cohn D., Magdassi S. 3D printing of shape memory polymers for flexible electronic devices. Adv. Mater. 2016;28:4449–4454. doi: 10.1002/adma.201503132. [DOI] [PubMed] [Google Scholar]
- Zarek M., Mansour N., Shapira S., Cohn D. 4D printing of shape memory-based personalized endoluminal medical devices. Macromol. Rapid Commun. 2017;38:1–6. doi: 10.1002/marc.201600628. [DOI] [PubMed] [Google Scholar]
- Zhang K., Wang S., Zhou C., Cheng L., Gao X., Xie X., Sun J., Wang H., Weir M.D., Reynolds M.A., Zhang N., Bai Y., Xu H.H.K. Advanced smart biomaterials and constructs for hard tissue engineering and regeneration. Bone Res. 2018;6:31. doi: 10.1038/s41413-018-0032-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z., Demir K.G., Gu G.X. Developments in 4D-printing: a review on current smart materials, technologies, and applications. Int. J. Smart Nano Mater. 2019;10:205–224. doi: 10.1080/19475411.2019.1591541. [DOI] [Google Scholar]
- Zhao Z., Wu J., Mu X., Chen H., Qi H.J., Fang D. Desolvation induced origami of photocurable polymers by digit light processing. Macromol. Rapid Commun. 2017;38:1–6. doi: 10.1002/marc.201600625. [DOI] [PubMed] [Google Scholar]
- Zhao W., Liu L., Zhang F., Leng J., Liu Y. Shape memory polymers and their composites in biomedical applications. Mater. Sci. Eng. C. 2019;97:864–883. doi: 10.1016/j.msec.2018.12.054. [DOI] [PubMed] [Google Scholar]
- Zhou Y., Zhou D., Cao P., Zhang X., Wang Q., Wang T., Li Z., He W., Ju J., Zhang Y. 4D printing of shape memory vascular stent based on βCD-g-polycaprolactone. Macromol. Rapid Commun. 2021;2100176:1–9. doi: 10.1002/marc.202100176. [DOI] [PubMed] [Google Scholar]
- Zu S., Zhang Z., Liu Q., Wang Z., Song Z., Guo Y., Xin Y., Zhang S. 4D printing of core–shell hydrogel capsules for smart controlled drug release. Bio-Design Manuf. 2022;5:294–304. doi: 10.1007/s42242-021-00175-y. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.