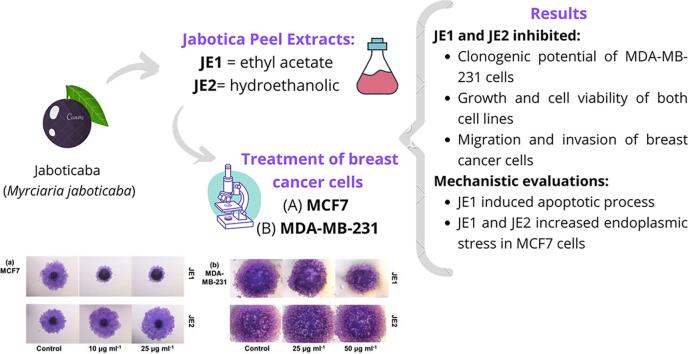
Graphical abstract
Keywords: Antitumor, Bioactive compounds, Breast cancer, Myrciaria jaboticaba
Highlights
-
•
Ethyl acetate and water–ethanol extracts from Jaboticaba peel were assessed.
-
•
Both extracts inhibited the clonogenic potential of MDA-MB-231 breast cancer cells.
-
•
Ethyl acetate extract was more effective against MCF7 cells.
-
•
Extracts’ action mechanisms can be the induction of endoplasmic stress and apoptosis.
Abstract
Jaboticaba peel (Myrciaria jaboticaba) is a source of bioactive compounds. We investigated the anticancer activity of ethyl acetate extract (JE1) and hydroethanolic extract (JE2) of Jaboticaba peel against breast cancer. Both JE1 and JE2 inhibited clonogenic potential of MDA-MB-231 cells while JE1 was particularly effective in MCF7 cells. Anchorage-independent growth and cell viability was also inhibited by JE1 and JE2. In addition to growth inhibition, JE1 and JE2 could also inhibit migration and invasion of cells. Interestingly, JE1 and JE2 show selective inhibition towards certain breast cancer cells and biological processes. Mechanistic evaluations showed that JE1 induced PARP cleavage, BAX and BIP indicating apoptotic induction. An elevation of phosphorylated ERK was observed in MCF7 cells in response to JE1 and JE2 along with increased IRE-α and CHOP expression indicating increased endoplasmic stress. Therefore, Jaboticaba peel extracts could be potentially considered for further development for breast cancer inhibition.
1. Introduction
Cancer has high mortality rates worldwide. Breast cancer is one the most important health problems around the world (Sharma, 2019). Breast cancer became the most common cancer as of 2021, accounting for nearly 12% of all new annual cancer cases worldwide, overcoming lung cancer, as reported in Global Cancer Statistics 2020 (GLOBOCAN) according to Sung et al (2021). By January 2021, there were more than 3.8 million women with a history of breast cancer in the U.S. Approximately, 281,550 new cases are expected this year and 43,600 women are predicted to succumb to breast cancer-related mortality in the U.S (American Cancer Society, 2021). In Brazil, the estimated number of new cases of breast cancer in 2021 is 66,280, which represents an incidence rate of 43.73 cases by 100,000 women, and the mortality rate was 14.63 deaths by 100,000 women in 2019 (INCA, 2021). Despite advances in screening programs and therapeutic approaches, the mortality remains at a high level with 1 in 35 women succumbing to breast cancer. Problems associated with available therapies include high toxicity, limited efficacy, therapeutic resistance, and therapy-related morbidity (Nagalingam, Arbiser, Bonner, Saxena, & Sharma, 2012). Therefore, new therapeutic strategies have been investigated to reduce morbidity-mortality by breast cancer.
Malignant tumors can be formed by cells with different phenotypes, metastatic potential and malignancy degree. Two common cell lines of breast cancer are MCF-7 and MDA-MB-231. The MCF-7 cells are non-invasive and estrogen epidermal growth factor dependent for growth, while MDA-MB-231 cells are more aggressive and hormone-independent. These characteristics can affect the response to different treatments. A challenge in breast oncology is the lack of homogeneity of the lesions where different cell components respond differently to treatments. Triple negative breast cancers are insensitive to treatments with antiestrogens, such as the selective estrogen receptor modulator tamoxifen, which is widely used in breast cancer, due the lack of estrogen receptors (Theodossiou et al., 2019). Additionally, the development of acquired resistance leading to recurrent disease and metastasis, and non-compliance in patients owing to poor tolerance of drug-related side effects are few additional factors that further complicate the breast cancer management (Muniraj et al., 2020). Various studies have supported the efficacy of several bioactive compounds against cancer and many successful drugs such as vincristine, vinblastine, doxorubicin, etoposide and paclitaxel have natural product origins. Mechanistic studies have suggested that these compounds act via multiple pathways. In addition, bioactive compounds in combination with chemotherapy possibly increase the efficacy and decreases the toxicity of chemotherapeutic agents (Muniraj, Siddharth, & Sharma, 2019).
The interest in natural agents as anticancer regimens has increased over the years, owing to their preventive and therapeutic properties. Jaboticaba (Myrciaria jaboticaba) is a native Brazilian berry. The dark violet and thick epicarp is not usually consumed in nature as it has a bitter and astringent taste, this peel can be used as flour or infusion in preparations adding color and bioactive compounds (Freitas-Sá et al., 2018) (da Silva et al., 2017) (Gurak et al, 2014). Jaboticaba peel concentrates possess copious amount of phenolic and other compounds (Albuquerque et al., 2020). Although jaboticaba peel has been highlighted for the possession of large concentration of anthocyanins, a class of flavonoid compound with polar affinity, other compounds can also be found in this food matrix such as other flavonoids, tannins, depsides, volatiles, tocopherols, and organic acids (Albuquerque, et al., 2020). Previous research related to jaboticaba functionality has mainly focused on antioxidants, anti-inflammatory and metabolic effects (Batista et al., 2018, Plaza et al., 2016), however, some studies showing the anticarcinogenic action of jaboticaba peel in vitro and in vivo have also been reported (Leite-Legatti et al., 2012) (Paludo et al., 2022) (Longo et al., 2023). Other berries have been shown antitumor potential such as strawberry, raspberry, blueberry, blackberry, and the Indian gooseberry (Baby, Antony, Vijayin, 2018). A polyphenol-rich berry fruit (camu-camu) demonstrated antitumor activity in breast cancer tumor mouse model (Messaoudene, et al., 2022). Euterpe oleracea (açaí), an anthocyanin source, has been investigated as a chemoprotective agent against cancer development (Alessandra-Perini et al., 2018). Free radical scavenging, protection from DNA damage, induction of apoptosis, and inhibition of growth and proliferation of cancer cells are mechanisms attributed to berries (Baby et al., 2018).
In the present study, we extracted jaboticaba peels using ethyl acetate (less polar solvent) and hydroethanol (high polar solvent) and investigated the effect of these novel extracts on the growth, migration and invasion of breast cancer cells and examined the underlying molecular mechanisms.
2. Materials and methods
2.1. Jaboticaba peels’ extracts
Myrciaria jaboticaba peel powder was obtained by oven-drying (96 h with air circulation at 40 °C), milling (Marconi, model MA 630/1, Piracicaba, SP, Brazil) and freezing at – 20 °C. This work was registered in the National System of Genetic Resource Management and Associated Traditional Knowledge (SISGEN-Brazil) under the number A240544. Ethyl acetate extract of jaboticaba peel (JE1) – 10 g of jaboticaba peel powder was mixed with 100 ml of ethyl acetate, agitated for 1 h in room temperature (22° C) and filtered through a paper filter (18 mm). The residue was re-extracted twice with 50 ml of ethyl acetate for 1 h in room temperature (22° C) and filtered through a paper filter (18 mm). Finally, the extracts were combined and subjected to rotary evaporation at 40° C. Hydroethanolic extract of jaboticaba peel (JE2) – The residue from previous extraction to obtain the JE1 extract, was used for polar extraction. The residue was mixed with 100 ml of ethanol: water solution (8:2), agitated for 1 h in room temperature (22° C) and filtered through a paper filter (18 mm). The residue was re-extracted twice with 50 ml of ethyl acetate for 1 h in room temperature (22° C) and filtered through a paper filter (18 mm). Finally, the extracts were combined and subjected to rotary evaporation at 40° C. For in vitro experiments, the extracts were individually resuspended in dimethyl sulfoxide (DMSO) at 250 mg ml−1 concentration and diluted in appropriate culture medium according to the experiment.
2.2. Cell culture
The human breast cancer cell lines, MCF7 and MDA-MB-231 were obtained from the American Type Culture Collection (ATCC, Manassas, VA) and maintained in DMEM supplemented with 10% fetal bovine serum (FBS) (Gemini Bioproducts, Woodland, CA, USA) and 2 μM L-glutamine (Invitrogen, Carlsbad, CA, USA). For treatments, cells were seeded at a density of 1 × 106/ 100-mm tissue culture dish. The treatment doses were established as JE1 extract (10, 25, 50 and 100 μg ml−1) and JE2 extract (50, 100 and 250 μg ml−1) according to a pilot experiment testing optimum effective concentration. Honokiol (HNK) was used as a positive control at 5 or 10 μM dose based on our previous studies. Honokiol is a bioactive polyphenol extracted from seed cones of Magnolia grandiflora as previously described (Nagalingam, Arbiser, Bonner, Saxena, & Sharma, 2012).
2.3. Cell-viability, colony-formation and anchorage-independent assays
Cell-viability assay was conducted using a commercial kit (Roche) to assess the reduction of MTT radical (3-(4, 5-dimethylthiazol2-yl)-2,5-diphenylformazan bromide) (Nagalingam, Arbiser, Bonner, Saxena, & Sharma, 2012), the cells were treated with JE1 (50 μg ml−1) or JE2 (50 μg ml−1) or HNK (5 μM). For colony-formation assay, MCF7 and MDA-MB-231 cells were treated with JE1 (10 and 25 μg ml−1), JE2 (50 and 100 μg ml−1) or HNK (5 and 10 μM) for 7–10 days. Colonies of MCF7 and MDA-MB-231 cells containing greater than 50 normal-appearing cells were counted (Nagalingam, Arbiser, Bonner, Saxena, & Sharma, 2012). Anchorage-independent growth of both cell lines treated with JE1 (50 μg ml−1), JE2 (50 μg ml−1) or HNK (5 μM) was determined by colony formation in soft-agar (Nagalingam, Arbiser, Bonner, Saxena, & Sharma, 2012).
2.4. Scratch-migration assay, spheroid migration assay and Matrigel invasion assay
Scratch-migration assay was performed according to the published protocol (Saxena et al., 2010). Cells were treated with JE1 (100 μg ml−1), JE2 (250 μg ml−1) or HNK (5 μM). Scratched plates were photographed after 24 and 48 h at the identical location of the initial image. The gap of the scratch was measured using the Image J 1.38 software (US National Institutes of Health, Bethesda, Maryland, USA). For spheroid migration assay, MCF7 and MDA-MB-231 cells were seeded in 0.5% agar-coated plates and cultured on an orbital shaker (100 rpm) for 48 h in a humidified atmosphere containing 5% CO2 at 37 °C. Intact tumor spheroids were selected, transferred to six-well plates and treated with JE1 (10 and 25 μg ml−1), JE2 (25 and 50 μg ml−1) or HNK (5 μM). Spheroids were fixed with 10 % buffered formalin in PBS and stained with crystal violet after 48 h of incubation. The migration of cells from spheroids was observed and photographed under a light microscope. Matrigel invasion assay was performed as an in vitro model system of metastasis (Saxena et al., 2007, Saxena et al., 2007) using a Matrigel invasion chamber from BD Biocoat Cellware (San Jose, CA, USA). The number of invaded cells on representative sections of each membrane were counted under light microscope and the result expressed by average of triplicate wells.
2.5. Western blotting and RT-PCR
The western blot analysis (Saxena, Vertino, Anania, & Sharma, 2007) was performed for the evaluation of protein expression of PARP, cleaved-PARP, BAX, Cyclin D1, ERK 1/2, phosphorylated-ERK 1/2, IRE 1-α, and BIP using antibodies from Cell Signaling Technology (Danvers, MA, USA). MCF7 and MDA-MB-231 cell lysates were prepared using 250 μl of ice-cold modified RIPA buffer. Protein extracts were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), transferred to nitrocellulose membrane, and immuno blot analysis was performed. Immunodetection was performed using enhanced chemiluminescence (ECL system; Amersham Pharmacia Biotech, Arlington Heights, IL, USA) according to manufacturer’s instructions. Endogenous control (β-actin) was used to normalize the results. For RNA isolation and qPCR, total cellular RNA was extracted using TRIzol Reagent (Life Technologies, Inc. Rockville, MD). PCR were performed using send and antisense PCR primer to CHOP and VEGF proteins.
2.6. Statistical analysis
Significant differences were analyzed by using the Student t test and two-tailed distribution. Data were considered to be statistically significant if p < 0.05. Data were expressed as mean ± standard deviation (SD). The GraphPad Prism 5.0 (GraphPad Software, Inc., La Jolla, CA, USA) software was used to analyze the data.
3. Results and discussion
Jaboticaba is a rich source of phytochemicals, mainly phenolic compounds. It has been reported to contain flavonoids, gallotannins, ellagitannins, depsides and prominently anthocyanins. However, carotenoids, tocopherols, terpenes, alcohols as well as organic acids have also been identified in this fruit (Albuquerque, et al., 2020) (Paludo, et al., 2022) (Rufino, Alves, de Brito, Pérez-Jiménez, Saura-Calixto, & Mancini-Filho, 2010). The total polyphenols, anthocyanins and carotenoids in 100 g fresh jaboticaba were found to be approximately 440, 58 and 0.32 mg, respectively (Rufino et al., 2010), and 1.7 mg tocopherol in 100 g of dried jaboticaba peel (Albuquerque, et al., 2020). The tocopherol concentration in jaboticaba skin greatly exceeds (100 times) the concentration in grape skin and grape pomace (Albuquerque, et al., 2020). The jaboticaba peel has shown a higher concentration of the above-mentioned compounds, however, its palatability is limited due to its hardness and astringent taste hence wasting a great resource of bioactive compounds (Albuquerque, et al., 2020) (da Silva et al., 2017). Alternative of use and consuming jaboticaba peel is the incorporation in preparations such as flour or infusion, beverages, and processed products development, which can aggregate color and bioactive compounds (Freitas-Sá et al., 2018) (da Silva et al., 2017) (Gurak et al, 2014). Many health benefits have been associated to the effect of bioactive compounds from jaboticaba peel, highlighting, antioxidant, anti-inflammatory and also antitumor properties (Batista et al., 2018) (Plaza et al., 2016) (Leite-Legatti et al., 2012) (Paludo et al., 2022) (Longo et al., 2023).
The anti-proliferative activity of the extracts of jaboticaba peel against non-small lung cancer cells was previously described (Leite-Legatti, et al., 2012). The authors found the optimum concentration of the extract that inhibited 50% of cell proliferation as 28 and 209 μg ml−1 for dichloromethane extract and ethanol extract, respectively. This result suggested that less-polar extract has stronger action in cancer inhibition, which is consonant with found in the present work. In this study, the authors affirm that volatiles compounds may have a synergistic cytostatic action with polyphenols against the tumor cell lines (Leite-Legatti, et al., 2012). Indeed, some terpenes have demonstrated the chemopreventive action against cancer cells lines; for example, the anti-tumorigenic activity of d-limonene is well-established (Cho, Lim, Lee, Lee, Lee, & Lee, 2017). Limonene and other terpenes have also been identified in jaboticaba fruit (Plagemann et al., 2012).
In the present study, two breast cancer cell lines, MCF7 and MDA-MB-231, were treated with ethyl acetate extract (JE1) or hydroethanolic extract (JE2) of jaboticaba peel, or with honokiol (HNK). HNK is an example of a bioactive compound with anticancer potential, it is a small phenolic compound purified from the seed cones and bark extracts of Magnolia grandiflora, and effectively inhibits growth and induce cell cycle arrest and apoptosis according to previously studies (Ong, Lee, Tang, & Yap, 2019) (Nagalingam et al., 2012). In this sense it was used was positive control in this experiment. The lower dose of HNK can be used as this is a purified compound extracted from the seed cones of Magnolia species (Ong, Lee, Tang, & Yap, 2019), different from jaboticaba extracts used in this work which are crude extracts. The selected concentration of JE1 extract was lower than JE2 owing to the results from the pilot experiments, as well as previous studies (Leite-Legatti et al., 2012).
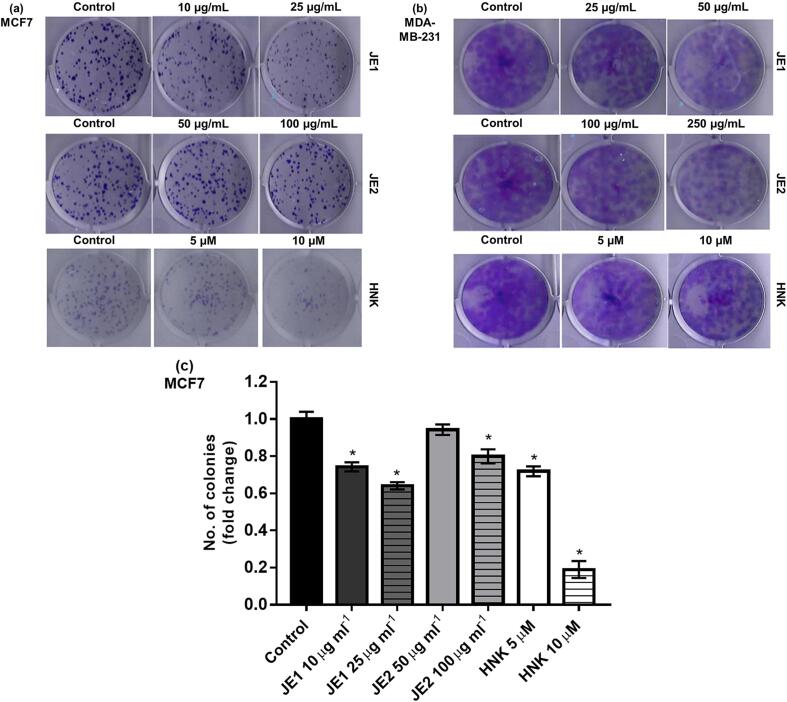
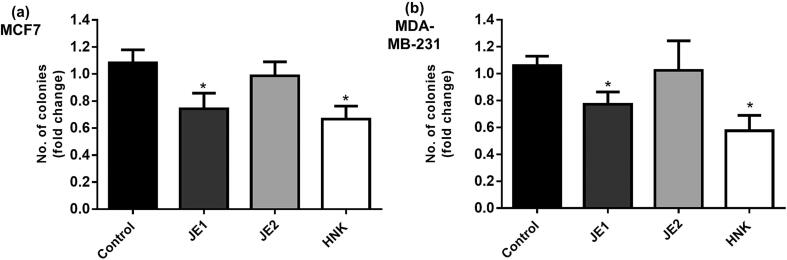
Cell division process is generally unlimited in tumor cells; two mechanisms that are involved in tumor reduction are growth-inhibition and apoptosis-induction (Martin, 2006). Colony-formation assay was performed to assess the clonogenic potential. The results showed that jaboticaba extracts were able to reduce the clonogenicity of breast cancer cells. JE1 (10 and 25 μg ml−1) and JE2 (100 μg m1-1) extracts inhibited the MCF-7 colonies leading to a 20–40% reduction compared with untreated cells (Fig. 1a-c). An inhibitory action of the extracts was also observed in MDA-MB-231, but it was not possible to count the colonies (Fig. 1b). The anchorage-independent growth was evaluated by soft agar assay. JEI extract inhibited the anchorage-independent growth at 50 μg ml−1 dose. HNK inhibited soft-agar colonies in both cell lines. No significant reduction of soft-agar colonies was found in JE2-treated cells (Fig. 2). The ability to exhibit anchorage independent cell growth has been connected to tumorigenic potential (Mori et al., 2009).
Fig. 1.
Jaboticaba peel extracts reduced the clonogenicity of breast cancer cells. MCF-7 (a, c) and MDA-MB-231(b) cells were treated with 10 and 25 μg ml−1 of ethyl acetate extract of jaboticaba peel (JE1), 50 and 100 μg ml−1 of hydroethanolic extract of jaboticaba peel (JE2), 5 and 10 μM of Honokiol (HNK) and were subjected to colony formation assay. Results are expressed as the mean ± standard deviation (n = 3). * Indicates significant difference from Control group by t test (P < 0.05).
Fig. 2.
JE1 inhibited the anchorage-independent cell growth of breast cancer cells. MCF-7 (a) and MDA-MB-231(b) cells were treated with 50 μg ml−1 of ethyl acetate extract of jaboticaba peel (JE1), 50 μg ml−1 of hydroethanolic extract of jaboticaba peel (JE2), 5 μM of Honokiol (HNK) and subjected to soft-agar assay. Results are expressed as the mean ± standard deviation (n = 3). * Indicates significant difference from Control group by t test (P < 0.05).
Accordingly, MTT assay showed 20% and 50% lower cell viability of MDA-MB-231 and MCF-7 cells, respectively, after 24 h-treatment with JE1 extract (50 μg ml−1) compared to untreated cells (Control) (Fig. 3). On the other hand, the more polar extract (JE2) increased the cell viability of both cell lines in this test. A study with Lycium barbarum (Goji berry) has also found the antineoplastic activity against breast cancer cells, MDA-MB-231 and MCF-7, according to the MTT dye reduction assay (Georgiev, Slavov, & Iliev, 2019).
Fig. 3.
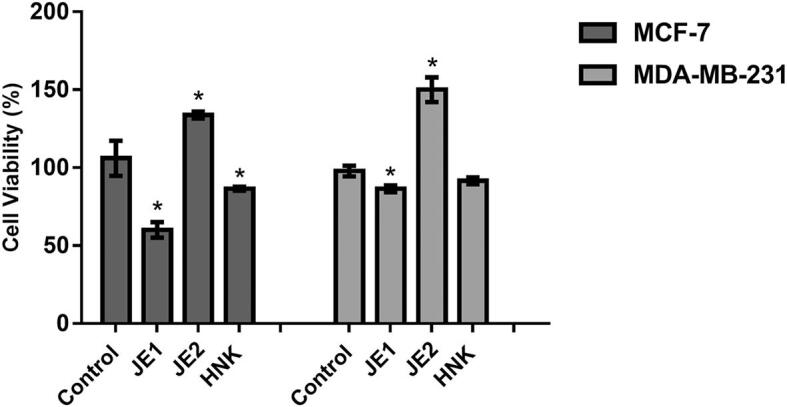
JE1 reduced the cell viability of breast cancer cells. MCF-7 and MDA-MB-231 were treated with 50 μg ml−1 of ethyl acetate extract of jaboticaba peel (JE1), 50 μg ml−1 of hydroethanolic extract of jaboticaba peel (JE2), 5 μM of Honokiol (HNK) and were subjected to MTT assay. Results are expressed as the mean ± standard deviation (n = 3). * Indicates significant difference from Control group by t test (P < 0.05).
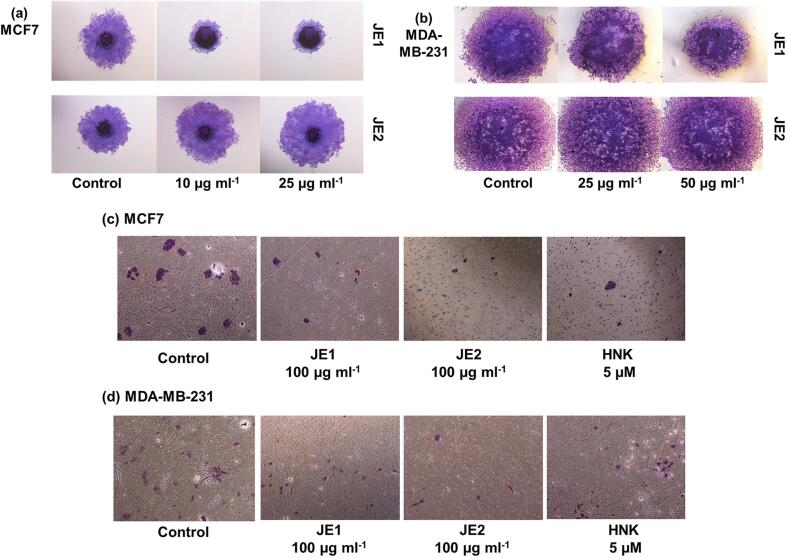
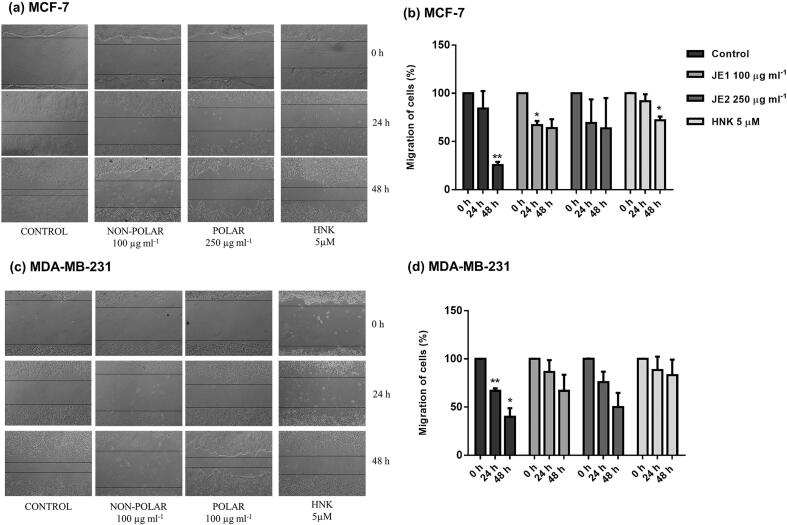
Invasion of basement membrane by tumor cell is an essential step towards cancer progression and metastasis process (Steeg & Theodorescu, 2008). The action of the jaboticaba extracts on breast cancer cells migration and invasion was examined using spheroid migration, scratch migration, and Matrigel invasion assay. The spheroid assay illustrated the action of JE1 extract in MCF-7 cells as it resulted in the reduction of spheroid migration. On the other hand, MDA-MB-231 cells were only slightly affected (Fig. 4a-b). In addition, Matrigel invasion suggested the inhibition of invasion potential in all treated cell lines (Fig. 4c-d). Migration assessment by scratch-assay showed that the treatments were effective in mitigating the migration of more invasive cell line (MDA-MB-231). The migration rate was similar in 24 h and 48 h post-treatment without statistical differences from initial assessment (time 0 h) in JE1 treated-cells line (MDA-MB-231), indicating a lower migration potential compared to untreated cells. In addition, the JE1 (24 h) and HNK (48 h) showed statistically significant reduction in the migration of MCF-7 cells compared to initial time, however, these treatments had higher scratch gap when compared to control group (untreated) at the same time (Fig. 5). These data conclude that jaboticaba peel can inhibit the migration and invasion of breast cancer cells, specifically the low polar extract (ethyl acetate extract). Similar to our findings, Al-Zharani et al. (2019) has shown that a vegetal extract (Rhazya stricta) exerted a potent anti-migration activity against the invasive MDA-MB-231 cell line.
Fig. 4.
Images of spheroid assay and matrigel assay indicate that JE1 mitigated the invasion potential and migration of breast cancer cells. MCF-7 (a, c) and MDA-MB-231 (b, d) cells were treated with 25 μg ml−1 ethyl acetate extract of jaboticaba peel (JE1), 50 μg ml−1 of hydroethanolic extract of jaboticaba peel (JE2), and 5 μM of Honokiol (HNK) and were subject to spheroid assay (a-b) and matrigel assay (c-d).
Fig. 5.
Jaboticaba peel extracts inhibited cell migration of breast cancer cells. –MCF7 (a, b) and MDA-MB-231 cells (c, d) were treated with 50 μg ml−1 of ethyl acetate extract of jaboticaba peel (JE1), 50 μg ml−1 of hydroethanolic extract of jaboticaba peel (JE2), 5 μM of Honokiol (HNK) and were subjected to scratch-migration assay. Bar graphs show quantitation of migration of cells. Results are expressed as the mean ± standard deviation (n = 2). * Indicates significant difference from Control group by t test (P < 0.05).
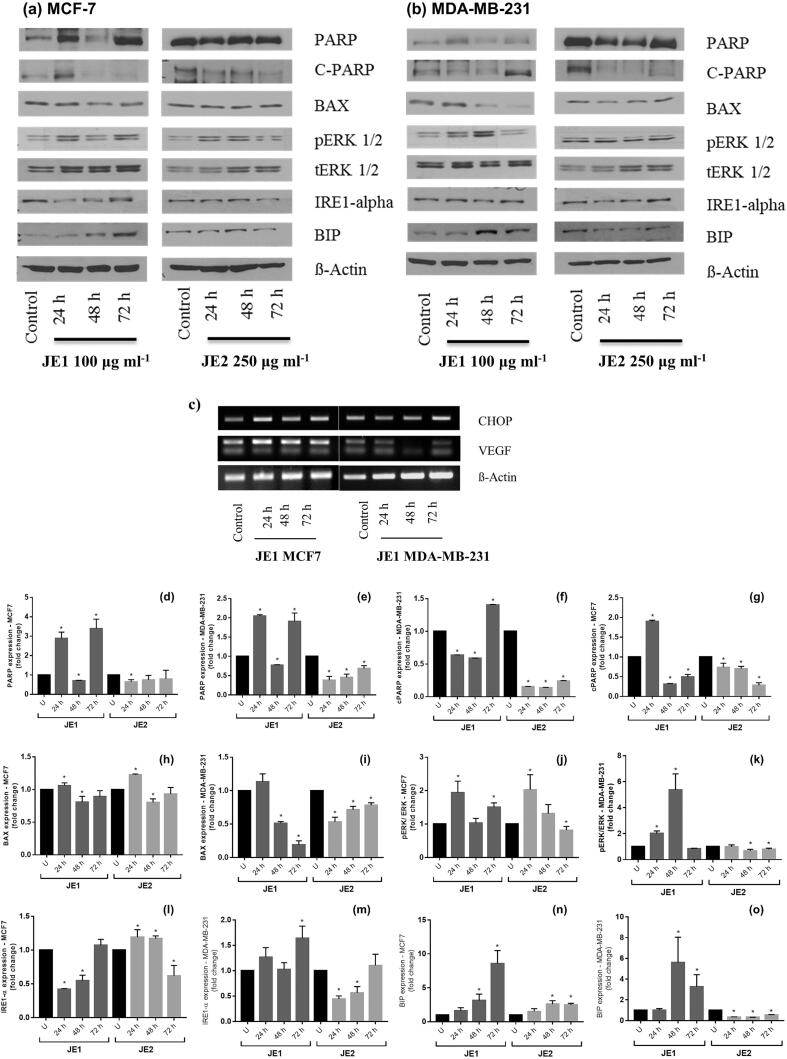
The chemopreventive properties attributed to anthocyanin rich matrices against several types of cancer cells have been reported. The underlying molecular mechanisms appear to involve several signaling pathways and biological processes from modulation of cell cycle, induction of apoptosis to alteration of autophagy (Li, Wang, Luo, Zhao, & Chen, 2017).In order to uncover the possible mechanisms involved in the action of jaboticaba extracts on breast cancer cells, the expression of proteins involved in the apoptotic pathways and endoplasmic reticulum stress were assessed by western-blotting assays. Firstly, JE1-treated MCF7 (Fig. 6 a and d) and MDA-MB-231 (Fig. 6 b and e) cells showed higher level of total poly (ADP-ribose) polymerase (PARP) after 24 and 72 h of treatment. PARPs are enzymes that catalyze the transfer of ADP-ribose to target proteins; they have an important role in the modulation of chromatin structure, transcription, replication, recombination, and DNA repair (Morales et al., 2014). More importantly, JE1 extract increased the level of cleaved poly (ADP-ribose) polymerase (cPARP) in MCF-7 at 24 h (Fig. 6 a and g) and in MDA-MB-231 (Fig. 6 b and f) at 72 h compared to untreated controls. The hydroethanolic extract showed a lower impact on the expression of PARP and cPARP in both cell lines (Fig. 6 a, b, d-g). PARP proteolysis produces a C-terminal fragment with a reduced catalytic activity, and a N-terminal peptide which retains the DNA binding domains (Soldani & Scovassi, 2002). Inactive-PARP could prevent the depletion of NAD (a PARP substrate) and ATP, which are thought to be required for the programmed cell death (Chaitanya, Alexander, & Babu, 2010); consequently, cPARP is a marker of cell apoptosis. A terpene found in jaboticaba fruit, the limonene, could mediate the cell death pathway via activated PARP cleavage (Cho, Lim, Lee, Lee, Lee, & Lee, 2017). There was an increased expression of Bax protein post-24 h treatment with both extracts in MCF-7 cells while in MDA-MB-231 cells Bax protein remained at a lower level than untreated cells (time 0 h) (Fig. 6 a,b, h and i). Bax is associated with mitochondrial stress and higher membrane permeability. Bax is a pro-apoptotic member of the Bcl-2 family that controls apoptosis in normal and cancer cells (Liu, Ding, Ye, Wild, Chen, & Zhou, 2016). Extracellular signal–regulated kinase ERK1 and ERK2 (ERK1/2) cascade regulates a variety of cellular processes by phosphorylating multiple target proteins and mediates cell proliferation as well as cell death (Deschênes-Simard, Kottakis, Meloche, & Ferbeyre, 2014). Studies have discussed the role of ERKs in enhancing tumorigenesis as well as in tumor suppression (Deschênes-Simard et al., 2014, Lavoie et al., 2020). In line with the previous results, the ratio between the activated form (phosphorylated) of ERK 1/2 and total ERK 1/2 was greater after 24 h and 72 h JE1-treatment, and after 24 h of JE2-treatment in MCF-7 breast cancer cells. JE1 also increased the phosphorylated ERK 1/2 (pERK1/2) in MDA-MB-231 after 24 h and 48 h treatment (Fig. 6 a, b, j and k). To confirm that pERK 1/2 was boosting the apoptotic pathway, the CCAAT-enhancer-binding protein homologous protein (CHOP) expression was examined with qPCR in cells treated with JE1. CHOP is known to be induced by endoplasmic reticulum stress and it mediates apoptosis (Nishitoh, 2011). Consistent with apoptosis induction, images of PCR analysis suggest that JE1 boosted the level of CHOP in MCF-7 at 24 and 72 h post-treatment interval, and in MDA-MB-231 at 72 h post-treatment interval without the alteration of vascular endothelial growth factor (VEFG), a mediator of cell proliferation (Fig. 6c) (Wang et al., 2008). These results show that the less-polar extracts inhibit breast cancer cell proliferation by apoptosis induction. Differences in the response of MCF7 and MDA-MB-231 cells indicate a subtype specificity to these extracts with luminal cells showing greater response.
Fig. 6.
Possible mechanisms of action of jaboticaba peel extracts on breast cancer cells. MCF-7 and MDA-MB-231 cells were treated with 100 μg ml−1 of ethyl acetate extract of jaboticaba peel (JE1), 250 μg ml−1 of hydroethanolic extract of jaboticaba peel (JE2) and cell lysates were subjected to western-blotting (a-b) and qPCR (c).. The blots are representative of multiple experiments. The bar graphs show expression of proteins involved in apoptotic pathways and reticulum stress, assed by western-blotting. PARP (d-e); cPARP (f-g); BAX (h-i); pERK/ERK (j-k), IRE (l-m); BIP (n-o). MCF-7 (d,f,h,j,l,n); MDA-MB-231 (e,g,i,k,m,o). Results are expressed as the mean ± standard deviation (n = 3). * Indicates significant difference from Control group by t test (P < 0.05).
The endoplasmic reticulum (ER) is the major site of cellular homeostasis regulation, particularly in the unfolded protein response, which has been implicated to play a role in cancer and other diseases (Moenner, Pluquet, Bouchecareilh, & Chevet, 2007). Endoplasmic reticulum stress is responsible for the “recycling process”, identifying protein alterations and avoiding tumor cell growth (Yadav, Chae, Kim, & Chae, 2014). Many studies have asserted the suppressive impact of natural products against cancers via endoplasmic reticulum stress (Kim & Kim, 2018). Reinforcement of endoplasmic reticulum stress, as demonstrated by increment in proteins such as IRE-1α and BIP, is a signal of reduction of tumor cells proliferation. Treatment with JE1 increased the endoplasmic reticulum stress in MCF-7 and MDA-MB-231 at 48 h and 72 h post-treatment intervals according to BIP expression (Fig. 6 a, b, n and o). However, the IRE-1α findings only corroborated the endoplasmic reticulum stress induction by JE1 treatment in MDA-MB-231 cells at 72 h (Fig. 6, b, and m). Unexpectedly, JE2 extract showed high level of IRE-1α in MCF-7 after 24 and 48 h treatment (Fig. 6 a, l)., and a greater BIP concentration in MCF-7 at 48 and 72 h (Fig. 6 a, b, and l-o). It is unclear whether the induction of endoplasmic reticulum stress is also a mechanism by which jaboticaba extracts exert their anti-cancer potential in breast cancer cells.
4. Conclusions
In conclusion, the jaboticaba extracts have demonstrated an anticancer potential in breast cancer. The ethyl acetate extract- JE1, which is less polar than hydroethanolic extract, reduced the growth, invasion, and migration of breast cancer cells. The ethyl acetate extract exhibited stronger biological action as compared to the hydroethanolic extract of jaboticaba peel. So far only anthocyanins, hydrophilic and highly polar bioactive compounds from jaboticaba peel have been explored for their biological activities. Our findings have highlighted the less-polar fractions from this fruit for their anti-cancer potential. Future studies can well-characterize the ethyl acetate extract and examine its efficacy in multiple breast cancer cell lines and in vivo models in order to establish the potential of this extract in breast cancer treatment. This study showed, for the first time, the anti-breast cancer action of jaboticaba peel less-polar extract and will contribute to expand the benefits attributed to this berry.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgments
Red Iberomericana de Alimentos Autoctonos Subutilizados (ALSUB-CYTED, 118RT0543).
Funding
This study was financed in part by the Coordination for the Improvement of Higher Education Personnel - Brazil (CAPES) - Finance Code 001; The National Council for Scientific and Technological Development – CNPq (grant number 142493/2013-9; 204088/2014-3; 403328/2016-0; 301496/2019-6) and The São Paulo Research Foundation - FAPESP (grant number 2015/50333-1; 2018/11069-5; 2015/13320-9, 2019/13465-8).
Contributor Information
Juliana Kelly da Silva-Maia, Email: juliana.maia@ufrn.br.
Arumugam Nagalingam, Email: anagali1@jhmi.edu.
Cinthia Baú Betim Cazarin, Email: cbetim@unicamp.br.
Mário Roberto Marostica Junior, Email: mmarosti@unicamp.br.
Dipali Sharma, Email: dsharma7@jhmi.edu.
Data availability
The authors are unable or have chosen not to specify which data has been used.
References
- Alessandra-Perini J., Rodrigues-Baptista K.C., Machado D.E., Nasciutti L.E., Perini J.A. Anticancer potential, molecular mechanisms and toxicity of Euterpe oleracea extract (acaí): A systematic review. PLoS ONE. 2018;13(7):e0200101. doi: 10.1371/journal.pone.0200101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Zharani M., Nasr F.A., Abutaha N., Alqahtani A.S., Noman O.M., Mubarak M., et al. Apoptotic induction and anti-migratory effects of Rhazya stricta fruit extracts on a human breast cancer cell line. Molecules. 2019;24(21) doi: 10.3390/molecules24213968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albuquerque B.R., Pereira C., Calhelha R.C., José Alves M., Abreu R.M.V., Barros L., et al. Jabuticaba residues (Myrciaria jaboticaba (Vell.) Berg) are rich sources of valuable compounds with bioactive properties. Food Chemistry. 2020;309 doi: 10.1016/j.foodchem.2019.125735. [DOI] [PubMed] [Google Scholar]
- American Cancer Society (2021). Cancer Statistic Center. Retrieved from https://cancerstatisticscenter.cancer.org/#!/cancer-site/Breast. Accessed by April 15, 2021.
- Batista Â.G., da Silva-Maia J.K., Mendonça M.C.P., Soares E.S., Lima G.C., Bogusz Junior S., et al. Jaboticaba berry peel intake increases short chain fatty acids production and prevent hepatic steatosis in mice fed high-fat diet. Journal of Functional Foods. 2018;48:266–274. [Google Scholar]
- Baby B., Antony P., Vijayan R. Antioxidant and anticancer properties of berries. Critical Reviews in Food Science and Nutrition. 2018;58(15):2491–2507. doi: 10.1080/10408398.2017.1329198. [DOI] [PubMed] [Google Scholar]
- Chaitanya G.V., Alexander J.S., Babu P.P. PARP-1 cleavage fragments: Signatures of cell-death proteases in neurodegeneration. Cell Communication and Signaling. 2010;8(1):31. doi: 10.1186/1478-811X-8-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho K.S., Lim Y.-R., Lee K., Lee J., Lee J.H., Lee I.-S. Terpenes from forests and human health. Toxicological research. 2017;33(2):97–106. doi: 10.5487/TR.2017.33.2.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva J.K., Batista Â.G., Cazarin C.B.B., Dionísio A.P., de Brito E.S., Marques A.T.B., et al. Functional tea from a Brazilian berry: Overview of the bioactives compounds. LWT – Food Science and Technology. 2017;76:292–298. [Google Scholar]
- Deschênes-Simard X., Kottakis F., Meloche S., Ferbeyre G. ERKs in cancer: Friends or foes? Cancer Research. 2014;74(2):412–419. doi: 10.1158/0008-5472.CAN-13-2381. [DOI] [PubMed] [Google Scholar]
- Freitas-Sá D.D.G.C., de Souza R.C., de Araujo M.C.P., Borguini R.G., de Mattos L.D.S., Pacheco S., et al. Effect of jabuticaba (Myrciaria jaboticaba (Vell) O. Berg) and jamelão (Syzygium cumini (L.) Skeels) peel powders as colorants on color-flavor congruence and acceptability of yogurts. LWT – Food Science and Technology. 2018;96:215–221. [Google Scholar]
- Georgiev K.D., Slavov I.J., Iliev I.A. Antioxidant Activity and Antiproliferative Effects of Lycium barbarum's (Goji berry) Fractions on Breast Cancer Cell Lines. Folia Medica (Plovdiv) 2019;61(1):104–112. doi: 10.2478/folmed-2018-0053. [DOI] [PubMed] [Google Scholar]
- Gurak P.D., De Bona G.S., Tessaro I.C., Marczak L.D.F. Jaboticaba pomace powder obtained as a co-product of juice extraction: A comparative study of powder obtained from peel and whole fruit. Food Research International. 2014;62:786–792. [Google Scholar]
- Instituto Nacional de Câncer José Alencar Gomes da Silva – INCA (2021). Atlas da mortalidade. Retrieved from https://www.inca.gov.br/aplicativos/atlas-de-mortalidade-por-cancer/. Accessed March 6, 2021.
- Kim C., Kim B. Anti-cancer natural products and their bioactive compounds inducing ER stress-mediated apoptosis: A review. Nutrients. 2018;10(8):1021. doi: 10.3390/nu10081021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavoie H., Gagnon J., Therrien M. ERK signalling: A master regulator of cell behaviour, life and fate. Nature Reviews: Molecular Cell Biology. 2020;21(10):607–632. doi: 10.1038/s41580-020-0255-7. [DOI] [PubMed] [Google Scholar]
- Leite-Legatti A.V., Batista A.G., Dragano N.R.V., Marques A.C., Malta L.G., Riccio M.F., et al. Jaboticaba peel: Antioxidant compounds, antiproliferative and antimutagenic activities. Food Research International. 2012;49:596–603. [Google Scholar]
- Li D., Wang P., Luo Y., Zhao M., Chen F. Health benefits of anthocyanins and molecular mechanisms: Update from recent decade. Critical Reviews in Food Science and Nutrition. 2017;57(8):1729–1741. doi: 10.1080/10408398.2015.1030064. [DOI] [PubMed] [Google Scholar]
- Liu Z., Ding Y., Ye N., Wild C., Chen H., Zhou J. Direct activation of bax protein for cancer therapy. Medicinal Research Reviews. 2016;36(2):313–341. doi: 10.1002/med.21379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo M.B., et al. Brazilian berry extract chemopreventive action: Hormone receptors as a target to mitigate aging prostatic disorders. Brazilian Archives of Biology and Technology [online] 2023;66:e23220075. [Google Scholar]
- Martin K.R. Targeting apoptosis with dietary bioactive agents. Experimental Biology and Medicine. 2006;231(2):117–129. doi: 10.1177/153537020623100201. [DOI] [PubMed] [Google Scholar]
- Messaoudene M., et al. A natural polyphenol exerts antitumor activity and circumvents anti–PD-1 resistance through effects on the gut microbiota. Cancer Discovery. 2022;12(4):170–1087. doi: 10.1158/2159-8290.CD-21-0808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moenner M., Pluquet O., Bouchecareilh M., Chevet E. Integrated endoplasmic reticulum stress responses in cancer. Cancer Research. 2007;67(22):10631. doi: 10.1158/0008-5472.CAN-07-1705. [DOI] [PubMed] [Google Scholar]
- Morales J., Li L., Fattah F.J., Dong Y., Bey E.A., Patel M., et al. Review of poly (ADP-ribose) polymerase (PARP) mechanisms of action and rationale for targeting in cancer and other diseases. Critical Reviews in Eukaryotic Gene Expression. 2014;24(1):15–28. doi: 10.1615/critreveukaryotgeneexpr.2013006875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S., Chang J.T., Andrechek E.R., Matsumura N., Baba T., Yao G., et al. Anchorage-independent cell growth signature identifies tumors with metastatic potential. Oncogene. 2009;28(31):2796–2805. doi: 10.1038/onc.2009.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muniraj N., Siddharth S., Sharma D. Bioactive compounds: Multi-targeting silver bullets for preventing and treating breast cancer. Cancers. 2019;11(10):1163. doi: 10.3390/cancers11101563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muniraj N., Siddharth S., Shriver M., Nagalingam A., Parida S., Woo J., et al. Induction of STK11-dependent cytoprotective autophagy in breast cancer cells upon honokiol treatment. Cell Death Discovery. 2020;6(1):81. doi: 10.1038/s41420-020-00315-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagalingam A., Arbiser J.L., Bonner M.Y., Saxena N.K., Sharma D. Honokiol activates AMP-activated protein kinase in breast cancer cells via an LKB1-dependent pathway and inhibits breast carcinogenesis. Breast Cancer Research. 2012;14(1):R35. doi: 10.1186/bcr3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishitoh H. CHOP is a multifunctional transcription factor in the ER stress response. The Journal of Biochemistry. 2011;151(3):217–219. doi: 10.1093/jb/mvr143. [DOI] [PubMed] [Google Scholar]
- Ong C.P., Lee W.L., Tang Y.Q., Yap W.H. Honokiol: A review of its anticancer potential and mechanisms. Cancers. 2019;12(1):48. doi: 10.3390/cancers12010048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paludo M.C., de Oliveira S.B.P., de Oliveira L.F., Colombo R.C., Gómez-Alonso S., Hermosín-Gutiérrez I., et al. Phenolic composition of peels from different Jaboticaba species determined by HPLC-DAD-ESI/MSn and antiproliferative activity in tumor cell lines. Current Plant Biology. 2022;29 [Google Scholar]
- Plagemann I., Krings I., Berger R.G., Maróstica Júnior M.R. Volatile constituents of jabuticaba (Myrciaria jaboticaba (Vell.) O. Berg) fruits. Journal of Essential Oil Research. 2012;24(1):45–51. [Google Scholar]
- Plaza M., Batista Â.G., Cazarin C.B.B., Sandahl M., Turner C., Östman E., et al. Characterization of antioxidant polyphenols from Myrciaria jaboticaba peel and their effects on glucose metabolism and antioxidant status: A pilot clinical study. Food Chemistry. 2016;211:185–197. doi: 10.1016/j.foodchem.2016.04.142. [DOI] [PubMed] [Google Scholar]
- Rufino M.d.S.M., Alves R.E., de Brito E.S., Pérez-Jiménez J., Saura-Calixto F., Mancini-Filho J. Bioactive compounds and antioxidant capacities of 18 non-traditional tropical fruits from Brazil. Food Chemistry. 2010;121(4):996–1002. [Google Scholar]
- Saxena N.K., Fu P.P., Nagalingam A., Wang J., Handy J., Cohen C., et al. Adiponectin modulates C-Jun N-terminal kinase and mammalian target of rapamycin and inhibits hepatocellular carcinoma. Gastroenterology. 2010;139(5):1762–1773.e1765. doi: 10.1053/j.gastro.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena N.K., Sharma D., Ding X., Lin S., Marra F., Merlin D., et al. Concomitant activation of the JAK/STAT, PI3K/AKT, and ERK signaling is involved in leptin-mediated promotion of invasion and migration of hepatocellular carcinoma cells. Cancer Research. 2007;67(6):2497–2507. doi: 10.1158/0008-5472.CAN-06-3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena N.K., Vertino P.M., Anania F.A., Sharma D. Leptin-induced growth stimulation of breast cancer cells involves recruitment of histone acetyltransferases and mediator complex to <em>CYCLIN D1</em> promoter via activation of Stat3 *. Journal of Biological Chemistry. 2007;282(18):13316–13325. doi: 10.1074/jbc.M609798200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma R. Breast cancer incidence, mortality and mortality-to-incidence ratio (MIR) are associated with human development, 1990–2016: Evidence from Global Burden of Disease Study 2016. Breast cancer (Tokyo, Japan) 2019;26(4):428–445. doi: 10.1007/s12282-018-00941-4. [DOI] [PubMed] [Google Scholar]
- Soldani C., Scovassi A.I. Poly(ADP-ribose) polymerase-1 cleavage during apoptosis: An update. Apoptosis. 2002;7(4):321–328. doi: 10.1023/a:1016119328968. [DOI] [PubMed] [Google Scholar]
- Steeg P.S., Theodorescu D. Metastasis: A therapeutic target for cancer. Nature clinical practice. Oncology. 2008;5(4):206–219. doi: 10.1038/ncponc1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung, Hyuna et al. (2021) “Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries.” CA: A Cancer Journal for Clinicians vol. 71(3): 209-249. [DOI] [PubMed]
- Theodossiou T.A., Ali M., Grigalavicius M., Grallert B., Dillard P., Schink K.O., et al. Simultaneous defeat of MCF7 and MDA-MB-231 resistances by a hypericin PDT–tamoxifen hybrid therapy. npj Breast Cancer. 2019;5(1):13. doi: 10.1038/s41523-019-0108-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, S., Li, X., Parra, M., Verdin, E., Bassel-Duby, R., & Olson, E. N. (2008). Control of endothelial cell proliferation and migration by VEGF signaling to histone deacetylase 7. Proceedings of the National Academy of Sciences of the United States of America, 105(22), 7738-7743. [DOI] [PMC free article] [PubMed]
- Yadav R.K., Chae S.-W., Kim H.-R., Chae H.J. Endoplasmic reticulum stress and cancer. Journal of Cancer Prevention. 2014;19(2):75–88. doi: 10.15430/JCP.2014.19.2.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors are unable or have chosen not to specify which data has been used.