Abstract
Purpose
Cancer treatment–related late effects degrade survivors’ quality of life, independence, and societal integration, yet may be ameliorated, or even reversed, with effective care. Unfortunately, survivors inconsistently receive this care and the impact on their healthcare utilization is unknown. We sought to estimate differences in utilization between breast cancer (BC) survivors with and without upper extremity lymphedema; a common, remediable late effect.
Methods
We conducted a population-based, retrospective longitudinal cohort study of survivors with incident BC diagnosed from January 1, 1990, through December 31, 2010. HC utilization was characterized using the Berenson-Eggers Type of Service (BETOS) categories. Outcomes included overall healthcare utilization as well as its compartmentalization into the BETOS categories of (1) Evaluation and management, (2) Procedures, (3) Imaging, (4) Tests, (5) Durable medical equipment, (6) Physical/occupational therapy, (7) Other, and (8) Exceptions/Unclassified.
Results
The cohort included 1906 subjects of which 94% (1800) had records meeting the inclusion criteria. Mean follow-up per survivor was 12.8 years (mean, 11, range 1–25 years). Analysis revealed that (1) survivors with BC-associated lymphedema used > 30% more services annually; (2) their increased utilization lessened but persisted for at least 10 years after diagnosis; and (3) this finding of increased utilization extends across all BETOS categories, is further amplified as BMI increases, and cannot be explained solely by lymphedema-directed care.
Conclusions
BC-related lymphedema appears to be an important driver of survivors’ healthcare utilization and guideline-concordant activities to reduce its incidence and severity may be cost neutral or saving.
Implications for Cancer Survivors
Early detection and effective management of cancer-related late effects like lymphedema may reduce survivors’ healthcare needs in the decades that follow their cancer treatment.
Keywords: Late effect, Healthcare utilization, Breast cancer, Lymphedema, Cost
Introduction
Disease-free cancer survivors utilize over twice the healthcare resources consumed by their demographically and comorbidity-matched peers. [1-3] Limited understanding of the factors that drive utilization among survivors represents a problematic knowledge gap in light of their precipitously increasing numbers; by 2040 there will be over 26 million US cancer survivors [4]. Addressing preventable, low value, healthcare utilization is vital to sustainable cancer care; however, this need is particularly pressing for breast cancer survivors who comprise roughly 25% of the survivor population [5].
Adverse, chronic late effects of cancer treatment may contribute importantly to survivors’ healthcare consumption. Late effects are prevalent, affecting 2/3 of survivors, and heterogeneous, ranging from gait instability from peripheral neuropathy to chronic pain. Late effects have been causally linked to costly medical and functional morbidity [6-8]. Moreover, better late effect treatments may be a means of reducing utilization. Late effects are inconsistently detected and addressed, despite the availability of evidence-based strategies to screen for, prevent, and mitigate them [9, 10].
Breast cancer–related lymphedema (BCRL), as one of the most studied late effects, serves as a useful exemplar in estimating the contribution of late effects to cancer survivors’ overall healthcare consumption. BCRL is also of interest because it offers a pragmatic instance in which broader coverage of guideline-concordant care could potentially reduce costs. The adverse consequences of BCRL have been described; chronic pain, functional limitations, job loss, and mood disorders, as have effective strategies to prevent these poor outcomes through prevention and management practices [9, 11]. These practices are inconsistently covered by payers and therefore limitedly available to many BC survivors [12]; fee-for-service Medicare does not cover compression garments, the mainstay of BCRL treatment.
A paucity of lymphedema-related economic analyses has been previously highlighted. [13] Two reports described increased utilization during the 2 years following surgery. One noted that survivors with BCRL incurred $7300 more healthcare costs annually, in 2006 dollars, over the first 2 years following BC diagnosis [14]. The second identified a 5-fold increase in hospitalizations among survivors with complicated BCRL—defined as having been hospitalized for a diagnosis of lymphedema complications or complications thereof [15]. These reports suggest that cost shifting away from reactive measures initiated after a survivor develops BCRL toward proactive, evidence-based BCRL screening, prevention, and early treatment may be cost neutral or even saving. Anecdotal reports of models that advance early intervention approaches are promising [13]. However, determination of the duration and robustness of increased healthcare utilization among survivors with BCRL is required to support this case. This paper reports the results of a population-based, longitudinal cohort study that was designed to estimate the incremental healthcare utilization associated with BCRL over 2 decades after BC diagnosis in order to support the case for more proactive, anticipatory care. The study additionally assessed the impact of body mass index (BMI) on these differences given robust reports of its association with BCRL progression [16, 17].
Methods
Subjects
Subjects were identified through the Rochester Epidemiology Project (REP), a medical records-linkage system that has for more than 50 years identified and tracked virtually all healthcare services provided to residents of Olmsted County, MN by Olmsted County-based providers [18, 19]. The population counts obtained by the REP Census are similar to those obtained by the US Census, indicating that virtually the entire population of the county is captured by the system [20]. We used the REP Census to identify all persons who resided in Olmsted County and had granted permission to use their medical records for research, over 98%. The REP electronic indices were searched to identify BCRL-relevant ICD-9, CPT, and HCPCS codes associated with the encounter, as well as encounter date and time. Finally, the REP also tracks the vital and residential status of all Olmsted County residents, and REP data were used to censor the study population on date of death or date of last follow-up [20].
Identification of incident breast cancers in Olmsted residents
To identify incident BC cases diagnosed from 1990 to 2010, we used (1) a preexisting cohort of patients diagnosed with incident BC between 1/1/1990 and 12/31/1999 [21]; (2) the Mayo Clinic Cancer Registry (MCCR) which includes all individuals evaluated and/or treated for cancer at the Mayo Clinic (MC); and (3) the REP’s searchable index of all assigned CPT, HCPCS, and ICD codes. We then cross-referenced the entire 1990–2010 REP BC cohort with BC cases listed in MCCR and linked to Olmsted County zip codes from 1/1/1990 to 12/31/2010.
Ascertainment of BC status and abstraction of BC clinical information
The records of all identified individuals were reviewed by a trained nurse abstractor and BC clinical data were entered into computerized case report forms. BC index dates were defined as the date when a new primary breast cancer was pathologically confirmed. Individuals who did not receive a pathological diagnosis of BC, generally because workup results would not alter clinical management due to advanced age, dementia, or comorbidity burden were not included in the cohort. Abstracted clinical and treatment information included affected side, pathology type and grade, tumor size, the number of lymph nodes removed/the number that were positive as well as the type of breast, axillary, reconstructive surgery, radiation fields, and the nature of any chemo- or hormonal therapy. BC Stage was defined per the 2010 American Joint Committee on Cancer system [22] with uncertainties being resolved through review by a BC medical oncologist with over 30 years of experience (TM). If the identified BC was found to be a recurrence of BC that had been diagnosed prior to 1990, the patient was removed from the cohort. However, patients with a previous history of BC who were diagnosed with a new primary BC during the study period were included.
Ascertainment of BCRL status
Three strategies were used to identify cohort members who developed BCRL.
First, we searched for all instances, since index, in which the BCRL-specific ICD-9 code, 457.0, had been assigned to a cohort member. Other lymphedema diagnostic codes, 457.1 and 757.0, are not unique to the arm and secondary lymphedema, respectively, and were not utilized.
Second, we used the resources of the REP and the textual search tools of the MC and OMC electronic health records to review documentation from all clinical encounters for evidence of BCRL from index through December 31, 2017. Text words indicative of BCRL were identified by reviewing the charts of 100 patients with known BCRL for > 5 years and by conducting focus groups with BCRL clinical experts. Electronic search terms included lymphedema, edema, edematous, swollen, swelling, puffy, puffiness, heavy, heaviness, erythema, aching, tingling, pain, and pitting. All documents, irrespective of discipline or clinical context, describing clinical encounters, test results, or communications containing one or more of the search terms were manually reviewed for reference to arm or hand swelling, altered limb contour, generalized heaviness, or altered tissue texture or quality ipsilateral to the side of BC treatment, as outlined in Common Toxicity Criteria v.3.0 [23]. If documentation described a co-occurring condition that could produce edema or arm symptoms then the instance was coded as “possible” BCRL. Arm swelling and symptoms that occurred in the absence of an alternate etiology were considered “probable BCRL.” “Definite” instances were those documented by a lymphedema or BC specialist. A “definite” BCRL classification was also assigned to cohort members that had ≥ 3 instances of “probable” BCRL. Agreement among the four RN abstractors in reviewing a random sample of 100 documents that contained words suggestive of BCRL was excellent as reflected in a Fleiss’ Kappa of 0.85 [24].
The third BCRL ascertainment strategy consisted of a robustly validated BCRL screening questionnaire being mailed to all surviving cohort members (n = 1204). The questionnaire has a reported sensitivity > 0.86 and specificity > 0.69 for BCRL detection [25], and has been used for BCRL assessments in numerous epidemiological studies and clinical trials [26-28].
Discordances were assessed with manual review by an abstractor naive to the subject and blinded to the subject’s survey/ICD-9-based BCRL status and BC characteristics. Instances of persistent uncertainty were adjudicated by a BCRL physician specialist with 20 years of clinical experience (AC), who was similarly blinded to cohort members’ BC characteristics. Surviving cohort members whose status could not be definitively determined were telephonically interviewed.
Since BCRL is incurable [16, 29], we considered cohort members who developed BCRL to have been BCRL positive since their index date. Specifically, we did not specify a BCRL index date. Our rationale was two-fold. First, roughly 90% of BCRL cases develop during the first 24 months after treatments [30-32]. Second, incipient BCRL is not consistently detected with volume-, circumference-, or PRO-based screening [33], making the determination of a specific BCRL index date a theoretical rather than practical exercise.
Ascertainment of weight and BC recurrence status
Cohort members’ weights and heights were electronically available through the MC health record beginning in 1999; weights prior to 1999 and all OMC weights were manually abstracted from paper records. Outlying measurements, defined as a > 30% change from the next earlier or later weight, were identified and the records manually reviewed. If multiple weights were available in a single year, the average was used in analyses.
BC recurrences through December 31, 2017, among cohort members were ascertained by manually reviewing the records of those who either died during the study period or were assigned either a diagnostic code suggestive of BC recurrence or a billing code for breast or axillary surgery, radiation, or chemotherapy following completion of their primary BC treatment.
Post-index healthcare utilization
HC utilization charges from January 1, 1995, when billing codes first became available through the REP, or index date, if after 1995, through December 31, 2017, were characterized using the well validated and widely used Berenson-Eggers Type of Service (BETOS) categories [34, 35]. BETOS codes are assigned for each HCPCS procedure code. They were devised as readily understandable clinical categories for analyzing increases in Medicare expenditures. BETOS categories include (1) Evaluation and management, (2) Procedures, (3) Imaging, (4) Tests, (5) Durable medical equipment, (6) Physical/occupational therapy, (7) Other, and (8) Exceptions/Unclassified.
Statistical analysis
BETOS counts were aggregated by survivorship year of post-index follow-up. The primary analysis used all BETOS codes. Secondary analyses used specific BETOS categories. Analysis was based on Poisson regression. To account for subject-level correlation, generalized estimating equations (GEE) were used, assuming an autoregressive error pattern [36]. Model covariates included gender, age, cancer stage, breast and axillary surgery, and adjuvant hormonal therapy, chemotherapy, or radiation therapy. Splines were used to model the continuous covariates of BMI and age. Exploration of the model’s goodness of fit included use of other correlation structures (exchangeable and working independence), of zero-inflated models to account for subjects with no follow-up costs, and of over-dispersed Poisson and negative binomial models to account for possible excess between-patient variation.
Sensitivity analyses were used to evaluate the impact of BCRL ascertainment method, utilization related to BC recurrences, changes in BC care over time, and common BCRL sequelae (e.g., cellulitis). Models were constructed that (1) used BCRL status ascertained per ICD-9 code and mailed survey; (2) censored members with recurrent BC; (3) included only members with BC index dates after 2000 and 2005 when use of sentinel lymph node biopsy had become increasingly standardized; and (4) excluded years when a cohort member was assigned an ICD-9 code for cellulitis or local infection. To address the issue of potential ascertainment bias, we used the method of inverse probability weights to create causal models of utilization with BCRL. [37] First, a model was constructed with BCRL as the outcome and predictors of age, sex, Charlson score, cancer stage, and cancer treatments as predictors. From this IPW weights were constructed that balance the BCRL and non-BCRL subjects with respect to the predictors. Last a model of utilization using the IPW weights was constructed. All models were fit using the R statistical packages [38].
Results
REP BC cohort
A total of 1906 Olmsted County residents who developed incident BC from 1990 to 2010 were identified. Of these, 1800 were assessed as 25 (1.3%) had not granted permission for use of their health records and 81 (4.2%) had no recording of additional HC encounters. Table 1 describes cohort members’ characteristics at BC index, broken down by those identified as BCRL(+) and BCRL(−). An average of 86 individuals entered the cohort each calendar year (range 45 to 118). Members contributed a mean of 12.8 years of follow-up per survivor (median, 11, range 1–25 years). BC recurrence, among those who received curative treatment, was detected in 163 cohort members (9%). Prior to December 31, 2017, 483 cohort members had died and 78 had moved out of Olmsted County, MN, USA. When follow-up was completed, 1239 cohort members were alive and resided in Olmsted County.
Table 1.
Demographic and clinical characteristics of the Rochester Epidemiology Project Incident Breast Cancer cohort
| Total cohort N = 1800 |
BCRL(+) N = 253 |
BCRL(−) N = 1547 |
||
|---|---|---|---|---|
| Female | N, % | 1789 (99.4%) | 250 (98.8%) | 1539 (99.5%) |
| Age at index* | Mean (SD) | 61 (13.8) | 57 (12.9) | 62 (13.9) |
| Min, max | 26, 96 | 27, 87 | 26, 96 | |
| Age at last follow-up | Mean (SD) | 69 (14.3) | 68 (14.3) | 72 (13.8) |
| Min, max | 31.9, 106.2 | 34, 100 | 32, 106 | |
| BMI at index | ||||
| <19 | N, % | 162 (9.0%) | 15 (0.4%) | 196 (13%) |
| 19–25 | N, % | 847 (47.1%) | 103 (41%) | 694 (45%) |
| > 25–30 | N, % | 476 (26.4%) | 77 (31%) | 400 (26%) |
| > 30–35 | N, % | 218 (12.1%) | 42 (17%) | 176 (11%) |
| > 35 | N, % | 89 (4.9%) | 15 (6%) | 74 (6%) |
| Side affected by breast cancer | ||||
| Left | N, % | 925 (51.4%) | 141 (56%) | 784 (51%) |
| Right | N, % | 867 (48.2%) | 110 (43%) | 757 (49%) |
| Bilateral | N, % | 3 (0.2%) | 1 (0.4%) | 2 (0.1%) |
| Stage at diagnosis | ||||
| I | N, % | 1129 (62.7%) | 91 (36%) | 1038 (67%) |
| II | N, % | 493 (27.4%) | 102 (40%) | 391 (25%) |
| III | N, % | 111 (6.2%) | 56 (22%) | 59 (4%) |
| IV | N, % | 30 (1.7%) | 3 (1%) | 27 (2%) |
| Breast surgery | ||||
| Lumpectomy or excisional biopsy | N, % | 1027 (57.1%) | 111 (44%) | 916 (59%) |
| Modified radical | N, % | 512 (28.4%) | 96 (38%) | 416 (27%) |
| Modified radical mastectomy with simple mastectomy of uninvolved breast | N, % | 223 (12.4%) | 42 (17%) | 181 (12%) |
| Axillary surgery† | ||||
| Complete axillary dissection | N, % | 766 (42.6%) | 192 (76%) | 574 (37%) |
| Sentinel lymph node biopsy | N, % | 699 (38.8%) | 56 (22%) | 643 (42%) |
| None | N, % | 323 (17.9%) | 5 (2%) | 318 (21%) |
| Radiation | ||||
| Any radiation therapy | N, % | 990 (55.0%) | 170 (67%) | 702 (47%) |
| Tangent beams | N, % | 864 (48.0%) | 104 (41%) | 760 (49%) |
| Chest wall | N, % | 128 (7.1%) | 67 (26%) | 61 (4%) |
| Regional lymph nodes | N, % | 244 (13.6%) | 99 (39%) | 145 (9%) |
| Any chemotherapy | N, % | 1283 (71.3%) | 146 (58%) | 371 (24%) |
| Any hormonal therapy | N, % | 904 (50.2%) | 174 (69%) | 730 (47%) |
Index dates were defined as the date when a new primary breast cancer was pathologically confirmed
Categories are not mutually exclusive
BCRL
BCRL was identified in 253 (14.1%) cohort members. BCRL incidence was similar as ascertained by the screening survey. Of the 1204 surviving cohort members who were mailed the survey, 785 responded (65.2%) but 65 surveys could not be scored. Of the 720 scored survey respondents, 114 (15.8%) were BCRL(+). Among cohort members with discrepant BCRL status determination by survey versus the adjudicated chart and administrative coding, 89% had breast or truncal lymphedema which was not included in the study.
Body mass index
The majority of cohort members, 71.7%, had an annual BMI for all years following index. Among those missing at least one annual BMI measurement, only 13.1% lacked one for more than two consecutive years. On average, cohort members’ BMI increased by 3.4 (paired t test statistic = 35.2, p < .001) from index to last follow-up. However, considerable variation was noted in patterns of weight loss and gain with 17.9% experiencing BMI increases of > 5, 3.8% > 10, and 1.2% > 15. The maximal BMI at any time during follow-up for the cohort was as follows: <19 1.6%, 19–25 18.8%, 25–30 28.6%, 30–35 19.4%, 35–40 11.5%, and > 40 8.2%.
Utilization of healthcare services following index
Table 2 presents output from the multivariate model of aggregated BETOS codes and shows that patients with BCRL used an average of 31% more services, excluding the first year after index although the difference utilization between BCRL(+) and BCRL(−) survivors diminished over time.
Table 2.
Multivariate model output with estimates of the association of healthcare utilization with cohort members’ demographic, breast cancer, and treatment characteristics as well as BCRL status
| Variable | Coefficients | Robust S.E. |
Increase in utilization |
p | 95% confidence interval |
|
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| Lymphedema | 0.27 | 0.03 | 31.0% | < .001 | 0.21 | 0.33 |
| Year since index | −0.02 | 0.003 | − 2.0% | < .001 | 0.01 | 0.03 |
| Axillary surgery* | ||||||
| Sentinel lymph node biopsy | 0.06 | 0.04 | 6.2% | 0.12 | − 0.02 | 0.14 |
| Complete axillary dissection | 0.05 | 0.04 | 5.1% | 0.17 | − 0.03 | 0.13 |
| Breast surgery† | ||||||
| Mastectomy | − 0.09 | 0.04 | − 8.6% | 0.03 | − 0.17 | − 0.01 |
| Mastectomy with contralateral prophylactic simple mastectomy | − 0.11 | 0.05 | − 10.5% | .02 | − 0.21 | − 0.01 |
| Male | 0.82 | 0.25 | 127.0% | 0.001 | 0.33 | 1.31 |
| Any hormone | − 0.09 | 0.03 | − 8.6% | <.001 | − 0.15 | − 0.03 |
| Any chemotherapy | 0.11 | 0.03 | 12.0% | 0.001 | 0.05 | 0.17 |
| Any radiation‡ | − 0.1 | 0.04 | − 9.6% | 0.01 | − 0.18 | − 0.02 |
| Calendar year of service | 0.005 | 0.003 | 0.5% | 0.12 | − 0.001 | 0.01 |
| Stage II§ | 0.16 | 0.03 | 17.4% | <.001 | 0.1 | 0.22 |
| Stage III§ | 0.39 | 0.06 | 47.7% | <.001 | 0.27 | 0.51 |
| Stage IV§ | 0.43 | 0.14 | 53.8% | 0.002 | 0.16 | 0.7 |
Control was “no axillary surgery”
Control was “lumpectomy or excisional biopsy”
Control was “no radiation”
Control was “Stage B-I”
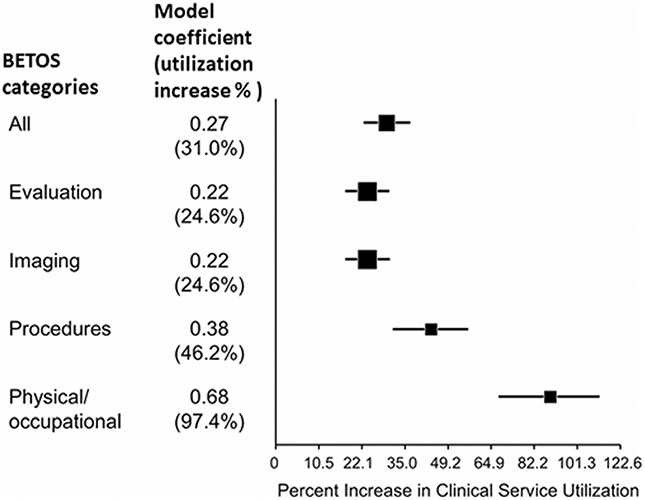
A number of BC clinical and treatment characteristics (age, BMI, gender, receipt of chemotherapy, and BC Stage) were significantly associated with increased utilization. Figure 1 presents a forest plot with estimates from multivariate models of BETOS codes for evaluation and management, imaging, procedures, and physical therapy. While the finding of increased service utilization among BCRL(+) cohort members was robust across all BETOS codes, the magnitude of the increase varied and was most marked for physical and occupational therapy.
Fig. 1.
Forest plot of the magnitude of the BCRL coefficient and related increased utilization in different BETOS categories
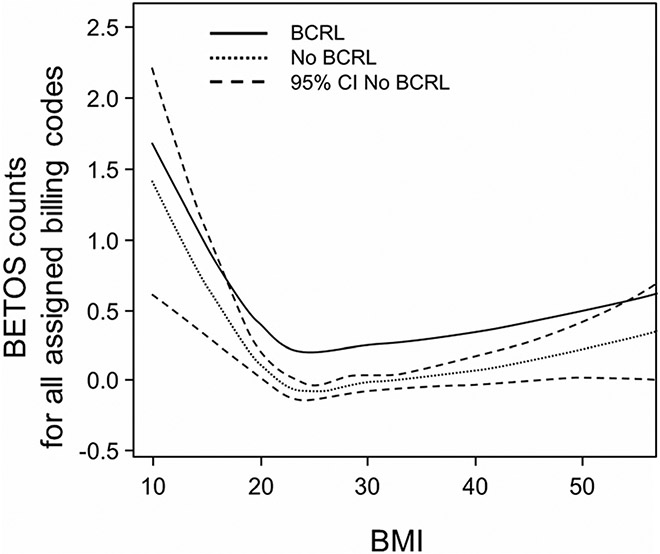
Healthcare utilization was significantly higher among cohort members as their BMIs increased. Figure 2 presents a line graph of total HC utilization, reflected by aggregated BETOS codes (procedures, imaging, DME, etc.), as a function of increasing BMI among cohort members with and without BCRL. The slope of the BRCL(+) and BCRL(−) lines are parallel as they ascend to the right and left of the nadir at BMI = 22 indicating the absence of an interaction between BMI and BCRL status. The widening of the interval for BMI > 40 and < 20 reflects the lesser representation of these BMI values in the cohort. The 95% confidence interval was identical for BCRL(+) patients, making it impossible to rule out a potential interaction between BMI and BCRL at the higher and lower BMI values. Of note, to the right of BMI = 24, the slope increased by 0.01, while to the left of BMI = 24 the slope increased by 0.10. These changes equate to a 1% and 12% increase in utilization counts per unit increase and decrease in BMI, respectively.
Fig. 2.
The line graph plots BMI against total BETOS counts per year of survivorship. The parallel slopes of the BCRL(+) and BCRL(−) lines to the right and left of BMI = 22 indicate the absences of an interaction between BMI and BCRL status. The dotted lines represent the confidence interval for the BCRL(−) line
Sensitivity analyses
The significance of the BCRL coefficient was robust in all sensitivity analyses. BCRL coefficients were 0.22 (25% increase) and 0.31 (36% increase) with survey- and code-based BCRL ascertainment, respectively; 0.24 (27% increase) with censoring after BC recurrence; 0.28 (32% increase) and 0.18 (20% increase) with restriction to BC index dates post 2000 and 2005, respectively; 0.27 (31% increase) with elimination of years when infection codes were assigned; and 0.32 (36% increase) in the models that account for Charlson index via IPW weights. All coefficient p values were < 0.001.
Discussion
This assessment of HC utilization in our population-based cohort of 1800 BC survivors yielded a number of significant findings. First, our assessment confirmed that survivors with BCRL use substantially more (> 30%) healthcare services annually. Second, the finding of increased utilization extended across all aspects of healthcare utilization and cannot be solely attributed to care delivered to address lymphedema. Third, increased utilization among BCRL(+) survivors, while it gradually lessens, persisted for at least 10 years. And fourth, increased BMI was also associated with higher utilization among BC survivors, but did not interact with BCRL in driving healthcare utilization.
The impact of chronic late effects of cancer treatment on survivors’ long-term healthcare utilization remains under-researched. Our results suggest that the increased utilization noted among survivors with BCRL in the first years after BC diagnosis [14], persists for at least a decade, and that addressable late effects may contribute importantly to the higher utilization noted among BC survivors. Obesity has also been limitedly examined as a determinant of cancer survivors’ healthcare utilization [39-41], yet our findings suggest that obesity may also be an addressable cause of increased utilization. The lack of models for testing and hypothesis generation that describe how survivors’ late effects may mediate utilization represents a problematic knowledge gap.
This study’s strengths include its use of a large (N = 1800) population-based cohort, virtually complete review of post-index healthcare encounters, rigorous adjustment for BC characteristics, and comprehensive utilization capture across all payers and providers. The study’s limitations include the fact that cohort members were not prospectively screened for BCRL; mildly affected individuals or those who died before developing BCRL may have been erroneously considered BCRL(−). However, systematic screening would not eliminate this potential bias as marked differences in BCRL incidence rates have been reported with volumetric and circumferential diagnostic criteria [42], and no screening approach has an area under the curve exceeding 0.83 [33, 42]. The relevance of our findings could be questioned in light changes in BCRL and BC treatment that have occurred during the 21-year follow-up interval. However, complex decongestive therapy has remained the BCRL treatment standard through-out this period [43], and the BCRL coefficients were limitedly changed in models that included members with BC index dates after 2000 and 2005.
Ascertainment bias is a concern since cohort members with frequent healthcare encounters had more opportunities to switch from the default BCRL(−), to BCRL(+) status. Several factors indicate that this bias did not impact our results. First, model BCRL coefficients were only slightly lower in the sensitivity analysis that used BCRL ascertained by the survey, which would be utilization independent and diminished by survivor bias. Second, the model BCRL coefficient increased with propensity score adjustment. Last, the cohort’s BCRL incidence replicates robust prior reports.
Conclusion
BCRL is associated with persistently increased utilization of all service types for at least a decade following BC diagnosis. BCRL appears to be in important driver of survivors’ healthcare utilization and guideline-concordant activities to reduce its incidence and severity may be cost neutral or saving.
Funding information
This study was funded by a NCI TREC Survivor Center grant U54-CA155850 from the National Institutes of Health. NCI TREC Survivor Center grant U54-CA155850.
Footnotes
Conflict of interest The authors declare that they have no conflict of interest.
Ethical approval All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study was approved by the Mayo Clinic Institutional Review Board. The requirement for informed consent was waived for portions of the study that involved retrospective data from clinical records. Informed consent was obtained from surviving cohort members who responded to the survey.
References
- 1.Guy GP Jr, Ekwueme DU, Yabroff KR, et al. Economic burden of cancer survivorship among adults in the United States. J Clin Oncol. 2013;31:3749–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guy GP Jr, Yabroff KR, Ekwueme DU, Rim SH, Li R, Richardson LC. Economic burden of chronic conditions among survivors of cancer in the United States. J Clin Oncol. 2017;35:2053–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ekwueme DU, Yabroff KR, Guy GP Jr, et al. Medical costs and productivity losses of cancer survivors–United States, 2008–2011. MMWR Morb Mortal Wkly Rep. 2014;63:505–10. [PMC free article] [PubMed] [Google Scholar]
- 4.Bluethmann SM, Mariotto AB, Rowland JH. Anticipating the “Silver Tsunami”: prevalence trajectories and comorbidity burden among older cancer survivors in the United States. Cancer Epidemiol Biomark Prev. 2016;25:1029–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miller KD, Siegel RL, Lin CC, Mariotto AB, Kramer JL, Rowland JH, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66:271–89. [DOI] [PubMed] [Google Scholar]
- 6.Fernandez-Pineda I, Hudson MM, Pappo AS, Bishop MW, Klosky JL, Brinkman TM, et al. Long-term functional outcomes and quality of life in adult survivors of childhood extremity sarcomas: a report from the St. Jude Lifetime Cohort Study. J Cancer Surviv. 2017;11:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hamaker ME, Prins MC, Schiphorst AH, van Tuyl SA, Pronk A, van den Bos F. Long-term changes in physical capacity after colorectal cancer treatment. J Geriatr Oncol. 2015;6:153–64. [DOI] [PubMed] [Google Scholar]
- 8.Hayes SC, Johansson K, Stout NL, Prosnitz R, Armer JM, Gabram S, et al. Upper-body morbidity after breast cancer: incidence and evidence for evaluation, prevention, and management within a prospective surveillance model of care. Cancer. 2012;118:2237–49. [DOI] [PubMed] [Google Scholar]
- 9.Soran A, Ozmen T, McGuire KP, et al. The importance of detection of subclinical lymphedema for the prevention of breast cancer-related clinical lymphedema after axillary lymph node dissection; a prospective observational study. Lymphat Res Biol. 2014;12:289–94. [DOI] [PubMed] [Google Scholar]
- 10.Nesvold IL, Fossa SD, Holm I, Naume B, Dahl AA. Arm/shoulder problems in breast cancer survivors are associated with reduced health and poorer physical quality of life. Acta Oncol. 2010;49:347–53. [DOI] [PubMed] [Google Scholar]
- 11.Qin ES, Bowen MJ, Chen WF. Diagnostic accuracy of bioimpedance spectroscopy in patients with lymphedema: a retrospective cohort analysis. J Plast Reconstr Aesthet Surg. 2018;71:1041–50. [DOI] [PubMed] [Google Scholar]
- 12.Johansson K, Branje E. Arm lymphoedema in a cohort of breast cancer survivors 10 years after diagnosis. Acta Oncol. 2010;49:166–73. [DOI] [PubMed] [Google Scholar]
- 13.Stout NL, Weiss R, Feldman JL, Stewart BR, Armer JM, Cormier JN, et al. A systematic review of care delivery models and economic analyses in lymphedema: health policy impact (2004-2011). Lymphology. 2013;46:27–41. [PubMed] [Google Scholar]
- 14.Shih YC, Xu Y, Cormier JN, Giordano S, Ridner SH, Buchholz TA, et al. Incidence, treatment costs, and complications of lymphedema after breast cancer among women of working age: a 2-year follow-up study. J Clin Oncol. 2009;27:2007–14. [DOI] [PubMed] [Google Scholar]
- 15.Basta MN, Fox JP, Kanchwala SK, et al. Complicated breast cancer-related lymphedema: evaluating health care resource utilization and associated costs of management. Am J Surg. 2016;211:133–41. [DOI] [PubMed] [Google Scholar]
- 16.Bar Ad V, Dutta PR, Solin LJ, et al. Time-course of arm lymphedema and potential risk factors for progression of lymphedema after breast conservation treatment for early stage breast cancer. Breast J. 2012;18:219–25. [DOI] [PubMed] [Google Scholar]
- 17.Specht MC, Miller CL, Russell TA, Horick N, Skolny MN, O’Toole JA, et al. Defining a threshold for intervention in breast cancer-related lymphedema: what level of arm volume increase predicts progression? Breast Cancer Res Treat. 2013;140:485–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rocca WA, Yawn BP, St Sauver JL, Grossardt BR, Melton LJ 3rd. History of the Rochester epidemiology project: half a century of medical records linkage in a US population. Mayo Clin Proc. 2012;87:1202–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.St Sauver JL, Grossardt BR, Yawn BP, et al. Data resource profile: the Rochester Epidemiology Project (REP) medical records-linkage system. Int J Epidemiol. 2012;41:1614–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.St Sauver JL, Grossardt BR, Yawn BP, Melton LJ 3rd, Rocca WA. Use of a medical records linkage system to enumerate a dynamic population over time: the Rochester Epidemiology Project. Am J Epidemiol. 2011;173:1059–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Melton LJ 3rd, Hartmann LC, Achenbach SJ, Atkinson EJ, Therneau TM, Khosla S. Fracture risk in women with breast cancer: a population-based study. J Bone Miner Res. 2012;27:1196–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cancer AJCo. Breast. AJCC Cancer Staging Manual. 7th ed. New York: Springer; 2010. p. 347–69. [Google Scholar]
- 23.Cheville AL, McGarvey CL, Petrek JA, Russo SA, Thiadens SR, Taylor ME. The grading of lymphedema in oncology clinical trials. Semin Radiat Oncol. 2003;13:214–25. [DOI] [PubMed] [Google Scholar]
- 24.Fleiss J. Measuring nominal scale agreement among many raters. Psychol Bull. 1971;76:378–82. [Google Scholar]
- 25.Norman SA, Miller LT, Erikson HB, Norman MF, McCorkle R. Development and validation of a telephone questionnaire to characterize lymphedema in women treated for breast cancer. Phys Ther. 2001;81:1192–205. [PubMed] [Google Scholar]
- 26.Sturgeon KM, Dean LT, Heroux M, Kane J, Bauer T, Palmer E, et al. Commercially available lifestyle modification program: randomized controlled trial addressing heart and bone health in BRCA1/2+ breast cancer survivors after risk-reducing salpingo-oo-phorectomy. J Cancer Surviv. 2017;11:246–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beidas RS, Paciotti B, Barg F, Branas AR, Brown JC, Glanz K, et al. A hybrid effectiveness-implementation trial of an evidence-based exercise intervention for breast cancer survivors. J Natl Cancer Inst Monogr. 2014;2014:338–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Norman SA, Localio AR, Potashnik SL, Simoes Torpey HA, Kallan MJ, Weber AL, et al. Lymphedema in breast cancer survivors: incidence, degree, time course, treatment, and symptoms. J Clin Oncol. 2009;27:390–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sagen A, Karesen R, Sandvik L, Risberg MA. Changes in arm morbidities and health-related quality of life after breast cancer surgery - a five-year follow-up study. Acta Oncol. 2009;48:1111–8. [DOI] [PubMed] [Google Scholar]
- 30.Armer JM, Radina ME, Porock D, Culbertson SD. Predicting breast cancer-related lymphedema using self-reported symptoms. Nurs Res. 2003;52:370–9. [DOI] [PubMed] [Google Scholar]
- 31.Togawa K, Ma H, Sullivan-Halley J, et al. Risk factors for self-reported arm lymphedema among female breast cancer survivors: a prospective cohort study. Breast Cancer Res. 2014;16:414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.DiSipio T, Rye S, Newman B, Hayes S. Incidence of unilateral arm lymphoedema after breast cancer: a systematic review and metaanalysis. Lancet Oncol. 2013;14:500–15. [DOI] [PubMed] [Google Scholar]
- 33.Dylke ES, Schembri GP, Bailey DL, et al. Diagnosis of upper limb lymphedema: development of an evidence-based approach. Acta Oncol. 2016;55:1477–83. [DOI] [PubMed] [Google Scholar]
- 34.Bazemore A, Petterson S, Peterson LE, Phillips RL Jr. More comprehensive care among family physicians is associated with lower costs and fewer hospitalizations. Ann Fam Med. 2015;13:206–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mahr MA, Hayes SN, Shanafelt TD, Sloan JA, Erie JC. Gender differences in physician service provision using Medicare claims data. Mayo Clin Proc. 2017;92:870–80. [DOI] [PubMed] [Google Scholar]
- 36.Zeger SL, Liang KY, Albert PS. Models for longitudinal data: a generalized estimating equation approach. Biometrics. 1988;44:1049–60. [PubMed] [Google Scholar]
- 37.Robins JM. Marginal structural models versus structural nested models as tools for causal inference. In: Halloran ME, Berry D, editors. Statistical models in epidemiology: the environment and clinical trials. New York: Springer; 1999. p. 95–134. [Google Scholar]
- 38.Team RC. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2017. [Google Scholar]
- 39.Secord AA, Hasselblad V, Von Gruenigen VE, et al. Body mass index and mortality in endometrial cancer: a systematic review and meta-analysis. Gynecol Oncol. 2016;140:184–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Playdon MC, Bracken MB, Sanft TB, Ligibel JA, Harrigan M, Irwin ML. Weight gain after breast cancer diagnosis and all-cause mortality: systematic review and meta-analysis. J Natl Cancer Inst. 2015;107:djv275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hussan H, Gray DM 2nd, Hinton A, Krishna SG, Conwell DL, Stanich PP. Morbid obesity is associated with increased mortality, surgical complications, and incremental health care utilization in the peri-operative period of colorectal cancer surgery. World J Surg. 2016;40:987–94. [DOI] [PubMed] [Google Scholar]
- 42.Smoot BJ, Wong JF, Dodd MJ. Comparison of diagnostic accuracy of clinical measures of breast cancer-related lymphedema: area under the curve. Arch Phys Med Rehabil. 2011;92:603–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rockson SG. Lymphedema after breast cancer treatment. N Engl J Med. 2018;379:1937–44. [DOI] [PubMed] [Google Scholar]