Abstract
Patients with AIDS-related cytomegalovirus (CMV) retinitis receiving combined antiretroviral therapy (cART), but not specific anti-CMV therapy, consistently showed active retinitis for several months. Delayed diagnosis and treatment of CMV retinitis may have severe consequences. Patients first entering care with advanced HIV infection and vulnerability to reactivation of latent CMV infection should be screened immediately for CMV retinitis by dilated indirect ophthalmoscopy and treated with specific anti-CMV therapy without delay, in addition to cART.
INTRODUCTION
The introduction of combined antiretroviral therapy (cART) strikingly reduced the incidence of AIDS-related cytomegalovirus (CMV) retinitis in the USA and Western Europe,1 but the disease remains an important problem in many middle-income and low-income countries, particularly in resource-limited settings.2–4
Soon after cART became available, there were anecdotal reports of active CMV retinitis resolving with cART alone.5 We suspect that these reports, as well as the marked reduction of CMV retinitis that accompanied the introduction of cART in high-income countries, has led to practice patterns in resource-limited settings that embrace an incomplete understanding of the anti-CMV benefits of cART.
The phenomenon of active CMV retinitis after the start of cART is an important issue worldwide because most HIV-infected individuals in middle-income and low-income countries are first diagnosed with CMV retinitis after the start of cART, typically after substantial delay.6 7 Lesion activity is a risk factor both for vision-threatening immune recovery uveitis (IRU) among people on cART and for retinal detachment. Moreover, as active lesions expand, they can threaten structures like the fovea and optic disc. Early diagnosis of active retinitis and treatment with anti-CMV drugs are therefore critical.
We found no studies on a computerised literature search that systematically investigated the relationship between duration of cART and CMV retinitis activity at diagnosis. In Myanmar, we had the opportunity to examine a group of patients with CMV retinitis who were being treated with cART but had never been treated with specific anti-CMV therapy. With the continuing burden of CMV retinitis,2–4 6 7 this historical data set remains timely, and we undertook this study to improve clinical recognition of the relationship between CMV retinitis activity and cART, with the goal of encouraging better strategies for managing CMV retinitis. We describe a group of patients with HIV whose CMV retinitis had not become inactive after several months of cART alone in the absence of specific anti-CMV therapy.
METHODS
We performed a retrospective, cross-sectional review of medical records from a consecutive series of patients with AIDS-related CMV retinitis who were examined at five HIV disease clinics administered by Medecins Sans Frontieres/Holland in Yangon, Myanmar, in November 2006. An ophthalmologist (DH) experienced in the diagnosis of CMV retinitis had performed a single retinal examination with an indirect ophthalmoscope through a fully dilated pupil. All clinic patients with nadir CD4+ T-lymphocyte counts <50 cells/mL or visual symptoms underwent indirect ophthalmoscopy.
Visual acuity was assessed for each eye according to the following categories: counting fingers (CF) at 3 m, CF at 1 m, hand motions, light perception (LP) and no light perception (NLP). CMV retinitis was classified as active versus inactive based on standard ophthalmoscopic features. Active retinitis was characterised by border opacification (whitening), while inactive retinitis was characterised by flat chorioretinal scar with or without pigment or gliosis, and free of oedematous or granular border opacification. The extent of retinitis was assessed by estimating the total retinal surface area. The presence of posterior pole involvement (ie, zone 1), retinal detachment and IRU were noted, with the latter defined as vitritis or posterior synechiae. After ophthalmic examination, the medical record was reviewed for information on cART status and duration over the previous 6 months. The cART regimen was stavudine plus lamivudine and nevirapine, and compliance was monitored as part of routine patient care. All patients with active retinitis were treated with weekly intraocular ganciclovir, 2.5 mg in 0.05 mL volume by standard sterile technique.
The relationship between retinitis activity and duration of cART among patients with CMV retinitis was assessed using penalised maximum likelihood logistic regression.
One-year follow-up examination was attempted for the group of 19 patients with active CMV retinitis who were treated with intraocular ganciclovir.
RESULTS
Among 179 patients with HIV who were examined by indirect ophthalmoscopy, 36 patients with CMV retinitis were currently on cART and had never received specific anti-CMV therapy.
Of these 36 patients with CMV retinitis on cART, 19 had active CMV retinitis and 17 had inactive CMV retinitis. Among patients on cART 14 weeks or less, each of 16 had active retinitis. Figure 1 depicts the results of a single examination of each patient and provides the proportion of patients with active CMV retinitis stratified by increasing durations of cART. Patients with CMV retinitis who had been on shorter courses of cART were more likely to have active disease (p<0.001, test for linear trend over the time categories displayed).
Figure 1.
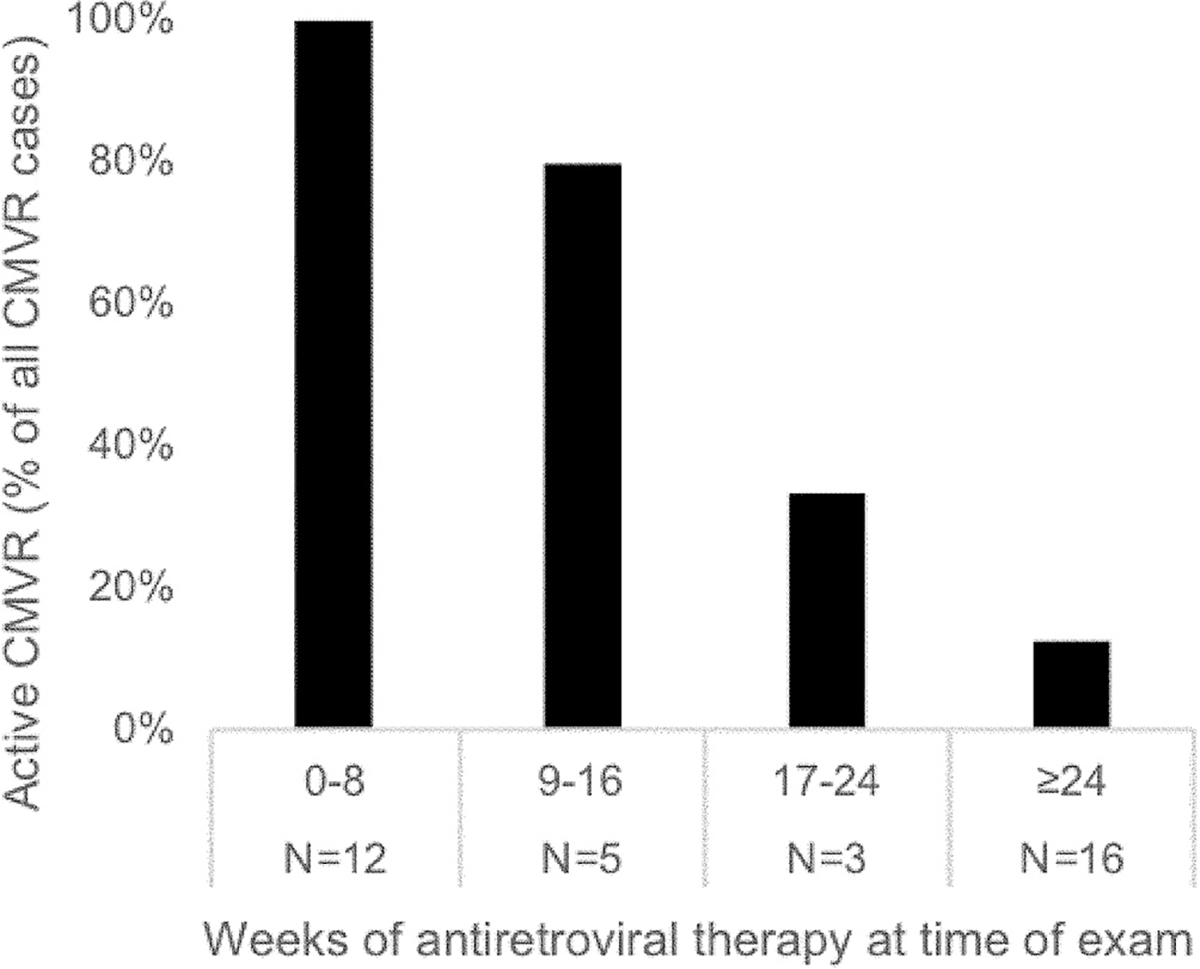
The proportion of patients with active cytomegalovirus retinitis (CMVR) stratified by increasing durations of combined antiretroviral therapy (cART). The relationship between CMVR activity and duration of cART is provided for a total of 36 patients with CMVR who were stratified into 8-week periods of cART duration; the percentage of patients in each stratum who were found to have active CMVR on a single examination is shown in black bars.
We compared the nadir CD4 count of all 19 patients with active CMV retinitis with 14 of 17 patients with inactive CMV retinitis (data missing in 3 patients with inactive CMV retinitis) and found no statistical difference between those with active retinitis (mean 16.5, 95% CI 9.7 to 23.2) and inactive retinitis (mean 19.7, 95% CI 9.1 to 30.3). In 30 of 33 (91%) patients, the CD4+ lymphocyte count was below 50 cells/μL with a range of 4–66 cells/μL.
Bilateral retinitis was present in 9 of 19 (47%) with active retinitis and 5 of 17 (29%) with inactive retinitis, a non-statistically significant difference (p=0.32). The clinical features of examined eyes are shown in table 1; there were no statistically significant differences between eyes with active and inactive CMV retinitis.
Table 1.
Clinical characteristics of eyes from a series of patients diagnosed with cytomegalovirus (CMV) retinitis who had not received prior anti-CMV treatment
| Active CMV retinitis |
Inactive CMV retinitis |
||||
|---|---|---|---|---|---|
| Characteristics | n* | n (%) or mean±SD | n* | n (%) or mean±SD | P values† |
|
| |||||
| Visual acuity | 0.30 | ||||
| CF 3 m | 28 | 18 (64) | 22 | 12 (55) | |
| CF 1 m | 28 | 1 (4) | 22 | 2 (9) | |
| HM | 28 | 0 (0) | 22 | 1 (5) | |
| LP | 28 | 4 (14) | 22 | 6 (27) | |
| NLP | 28 | 5 (18) | 22 | 1 (5) | |
| Per cent of retinal surface area | 22 | 40±33 | 17 | 33±24 | 0.83 |
| Zone 1 involvement | 22 | 9 (41) | 17 | 6 (35) | 0.49 |
| Retinal detachment | 23 | 3/23 | 17 | 5/17 | 0.25 |
| Size of detachment (%) | 3 | 81±29 | 5 | 92±14 | 0.61 |
| Immune recovery uveitis | 23 | 5 (22) | 19 | 4 (21) | 0.99 |
All patients were under treatment with cART.
Eyes with available data.
Fisher’s exact test for categorical variables and Wilcoxon rank-sum test for continuous variables, ignoring correlation between two eyes of the same patient. cART, combined antiretroviral therapy;CF, counting fingers;CMV, cytomegalovirus;HM, hand motions;LP, light perception;NLP, no light perception.
At 1 year we re-examined 9 of 19 (47%) patients who had active CMV retinitis and were treated with intraocular ganciclovir. Seven patients had been lost to follow-up and three patients had died. Among 9 patients provided with specific anti-CMV therapy, 4 patients had bilateral retinitis, giving a total of 13 eyes. Three eyes had LP or NLP vision on prior initial examination. None of 10 eyes with intact vision on initial evaluation and who had been treated with specific anti-CMV therapy had become blind at 1-year follow-up.
DISCUSSION
The observation of active retinal lesions in all patients with CMV retinitis who were receiving cART for 14 weeks or less confirms the need for early diagnosis and specific anti-CMV therapy, in addition to cART. Our clinical data also show an inverse relationship between disease activity and duration of cART, with most, but not all, patients on cART for 6 months demonstrating inactive retinitis. In order to identify patients who require specific anti-CMV therapy, thereby avoiding the potentially severe consequences of an unchecked and relentlessly progressive infectious disease,8 diagnostic eye examination must be performed on all vulnerable patients as soon as the patient first enters care.
In vitro studies suggest that functional immune reconstitution capable of control of CMV replication is distinct from the general recovery of the CD4 count. It is thought that control of CMV replication requires recovery of a specific subset of CD4 lymphocytes.9 In vivo, after a single month of cART, CD4 counts may exceed 100 cells/μL and HIV blood level may fall several log units, but CMV blood level is essentially unchanged.10 The mean time to clear CMV viraemia on cART alone has been estimated as approximately 13.5 weeks.11 Thus, productive CMV infection will continue after initiation of cART until specific CMV immune reconstitution occurs. During this window of delayed reconstitution of anti-CMV immune capacity, new cases of CMV retinitis have been recognised, even in patients with CD4 >200/μL.12
Our data and other evidence9–12 suggest that specific therapy for CMV infection must be continued until retinitis is inactive and until there has been enough time on cART for reconstitution of the capacity to contain CMV infection, typically 3–4 months. Also, surveillance of all vulnerable patients should be continued until there has been enough time for the occurrence of specific immune reconstitution to CMV.
The dramatic decline of AIDS-related CMV retinitis in high-income countries is attributable to widespread HIV screening and early initiation of cART before the immune system becomes profoundly compromised. This desired approach has been well implemented in Western and Central Europe and North America, regions that account for only 7% of the total burden of HIV-infected patients.13
While there is also a definite decline in the proportion of HIV-infected patients first presenting for care with advanced immune deficiency in middle-income and low-income countries, up to one-third still present with advanced disease.14 Success at reducing the proportion of patients who present with late disease in South-East Asia has been frustrated by the stigma associated with a diagnosis of HIV infection, as well as stigmatisation of groups at increased risk of HIV acquisition in concentrated epidemic settings (sex workers, people who use injecting drugs, men who have sex with men and transgender individuals).15
Early screening and diagnosis of CMV retinitis are desirable health policy, yet only one middle-income or low-income country (Myanmar) attempts to offer an immediate eye examination as part of routine care for new patients with a CD4 count <100 cells/μL.16 In Thailand and India, guidelines for patients with CD4 <100 cells/μL include an eye examination, but if an eye examination is performed it occurs months6 or even years7 after the patient is already on cART. In reality, an eye examination is typically only prompted by visual symptoms, but symptoms may be unrecognised until after irreversible damage occurs.17 Based on clinical experience in countries other than Myanmar, we believe that our data are generalisable to a substantial proportion of patients in resource-poor settings of South-East Asia, China and Eastern Europe.
There is persuasive clinical18 and autopsy19 evidence that AIDS-related CMV retinitis is only one part of a systemic infection and contributes to mortality, in addition to causing blindness. The powerful correlation between CMV retinitis and early AIDS mortality has long been recognised, with or without cART. Until the late 1980s, in San Francisco, with strong supportive care and medical expertise, the median survival in patients with CMV retinitis was 4 months,20 and in resource-limited settings before availability of cART, survival after a diagnosis of CMV retinitis was measured in weeks.21 At this time perhaps one-third of patients with CMV retinitis may die before this treatable opportunistic infection is diagnosed and often before initiation of cART.22 23 An oral medication, valganciclovir, is effective for both retinitis and systemic disease,24 and systemic regimens have been demonstrated to reduce all-cause mortality.25 We may have the opportunity to raise the standard of care for patients who develop CMV retinitis to closely approach the standard in high-income countries by taking two limited steps: by providing early diagnosis and by providing systemic therapy with valganciclovir.
In conclusion, routine appropriate history and physical examination provide early screening for tuberculosis, cryptococcal meningitis, pneumocystis pneumonia and penicilliosis, but appropriate physical examination is rarely provided for CMV retinitis. Moreover, a recent report of providing prophylaxis against multiple microbial pathogens to reduce mortality in patients with advanced HIV infection entirely ignores CMV.26 Until there is widespread effective early HIV diagnosis and treatment in resource-limited settings, eye examination should be performed routinely on all patients who are vulnerable to CMV retinitis at the time they first enter healthcare, just as screening is provided for other serious opportunistic infections. By neglecting to include an appropriate eye examination, the potential for use of a simple intervention (valganciclovir therapy) is forfeited. Patients with CMV retinitis must be appropriately treated with systemic anti-CMV therapy in addition to cART.
Funding
The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Footnotes
Competing interests None declared.
Patient consent Not required.
Ethics approval This study did not require ethical approval because it used retrospective de-identified data.
Provenance and peer review Not commissioned; externally peer reviewed.
REFERENCES
- 1.Mocroft A, Sabin CA, Youle M, et al. Changes in AIDS-defining illnesses in a London Clinic, 1987–1998. J Acquir Immune Defic Syndr 1999;21:401–7. [PubMed] [Google Scholar]
- 2.Heiden D, Ford N, Wilson D, et al. Cytomegalovirus retinitis: the neglected disease of the AIDS pandemic. PLoS Med 2007;4:e334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ford N, Shubber Z, Saranchuk P, et al. Burden of HIV-related cytomegalovirus retinitis in resource-limited settings: a systematic review. Clin Infect Dis 2013;57:1351–1361. [DOI] [PubMed] [Google Scholar]
- 4.Pathanapitoon K, Ausayakhun S, Kunavisarut P, et al. Blindness and low vision in a tertiary ophthalmologic center in Thailand: the importance of cytomegalovirus retinitis. Retina 2007;27:635–40. [DOI] [PubMed] [Google Scholar]
- 5.Reed JB, Schwab IR, Gordon J, et al. Regression of cytomegalovirus retinitis associated with protease-inhibitor treatment in patients with AIDS. Am J Ophthalmol 1997;124:199–205. [DOI] [PubMed] [Google Scholar]
- 6.Ausayakhun S, Keenan JD, Ausayakhun S, et al. Clinical features of newly diagnosed cytomegalovirus retinitis in northern Thailand. Am J Ophthalmol 2012;153:923–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Agarwal A, Singh R, Sharma A, et al. Ocular Manifestations in Patients with Human Immunodeficiency Virus Infection in the Pre-HAART Versus the HAART Era in the North Indian Population. Ocul Immunol Inflamm 2017;25:396–404. [DOI] [PubMed] [Google Scholar]
- 8.Palestine AG, Rodrigues MM, Macher AM, et al. Ophthalmic involvement in acquired immunodeficiency syndrome. Ophthalmology 1984;91:1092–9. [DOI] [PubMed] [Google Scholar]
- 9.Komanduri KV, Feinberg J, Hutchins RK, et al. Loss of cytomegalovirus-specific CD4+ T cell responses in human immunodeficiency virus type 1-infected patients with high CD4+ T cell counts and recurrent retinitis. J Infect Dis 2001;183:1285–9. [DOI] [PubMed] [Google Scholar]
- 10.O’Sullivan CE, Drew WL, McMullen DJ, et al. Decrease of cytomegalovirus replication in human immunodeficiency virus infected-patients after treatment with highly active antiretroviral therapy. J Infect Dis 1999;180:847–9. [DOI] [PubMed] [Google Scholar]
- 11.Deayton J, Mocroft A, Wilson P, et al. Loss of cytomegalovirus (CMV) viraemia following highly active antiretroviral therapy in the absence of specific anti-CMV therapy. AIDS 1999;13:1203–6. [DOI] [PubMed] [Google Scholar]
- 12.Jacobson MA, Zegans M, Pavan PR, et al. Cytomegalovirus retinitis after initiation of highly active antiretroviral therapy. Lancet 1997;349:1443–5. [DOI] [PubMed] [Google Scholar]
- 13.2016. World Bank and HIV/AIDS: The Facts. 2012. Fact sheet. UNAID fact sheet. Available from: http://www.unaids.org/en/resources/presscentre/factsheets/ [accessed 1 May 2017].
- 14.Ford N, Doherty M. The enduring challenge of advanced HIV infection. N Engl J Med 2017;377:283–4. [DOI] [PubMed] [Google Scholar]
- 15.Mukolo A, Villegas R, Aliyu M, et al. Predictors of late presentation for HIV diagnosis: a literature review and suggested way forward. AIDS Behav 2013;17:5–30. [DOI] [PubMed] [Google Scholar]
- 16.Tun N, London N, Kyaw M, et al. CMV retinitis screening and treatment in a resource-poor setting: three-year experience from a primary care HIV/AIDS programme in Myanmar. J Int AIDS Soc 2011;14:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu Y, Chen AS, Kamphaengkham S, et al. Diagnostic utility of ocular symptoms and vision for cytomegalovirus retinitis. PLoS One 2016;11:e0165564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yust I, Fox Z, et al. EuroSIDA. Retinal and extraocular cytomegalovirus end-organ disease in HIV-infected patients in Europe: a EuroSIDA study, 1994–2001. Eur J Clin Microbiol Infect Dis 2004;23:550–9. [DOI] [PubMed] [Google Scholar]
- 19.Brantsaeter AB, Liestøl K, Goplen AK, et al. CMV disease in AIDS patients: incidence of CMV disease and relation to survival in a population-based study from Oslo. Scand J Infect Dis 2002;34:50–5. [DOI] [PubMed] [Google Scholar]
- 20.Harb GE, Bacchetti P, Jacobson MA. Survival of patients with AIDS and cytomegalovirus disease treated with ganciclovir or foscarnet. AIDS 1991;5:959–66. [DOI] [PubMed] [Google Scholar]
- 21.Balo KP, Amoussou YP, Béchetoille A, et al. [Cytomegalovirus retinitis and ocular complications in AIDS patients in Togo]. J FrOphtalmol 1999;22:1042–6. [PubMed] [Google Scholar]
- 22.Tun N, Smithuis FM, London N, et al. Mortality in patients with AIDS-related cytomegalovirus retinitis in Myanmar. Clin Infect Dis 2014;59:1650–1. Dec 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shi Y, Lu H, He T, et al. Prevalence and clinical management of cytomegalovirus retinitis in AIDS patients in Shanghai, China. BMC Infect Dis 2011;11:326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martin DF, Sierra-Madero J, et al. A controlled trial of valganciclovir as induction therapy for cytomegalovirus retinitis. N Engl J Med 2002;346:1119–26. [DOI] [PubMed] [Google Scholar]
- 25.Jabs DA, Ahuja A, et al. Comparison of treatment regimens for cytomegalovirus retinitis in patients with AIDS in the era of highly active antiretroviral therapy. Ophthalmology 2013;120:1262–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hakim J, Musiime V, et al. Enhanced prophylaxis plus antiretroviral therapy for advanced HIV infection in Africa. N Engl J Med 2017;377:233–45. [DOI] [PMC free article] [PubMed] [Google Scholar]


