This cohort study uses data from the Intelligent Research in Sight (IRIS) Registry to assess factors associated with repeated nasolacrimal duct probing among US children.
Key Points
Question
What factors are associated with reoperation after nasolacrimal duct probing in young children?
Findings
In this cohort study of 19 357 children younger than 4 years in the Intelligent Research in Sight Registry who underwent nasolacrimal duct probing, 7.2% had a reoperation within 2 years. Higher risk of reoperation was associated with bilateral obstruction and probing performed in an office, whereas lower risk was associated with primary balloon catheter dilation and procedures performed by high-volume surgeons.
Meaning
In this study, most children undergoing nasolacrimal duct probing did not require additional interventions, but risk of reoperation was associated with procedure location, surgeon experience, and use of adjuvant techniques.
Abstract
Importance
Understanding the factors associated with nasolacrimal duct probing failure in young children may help inform practice patterns.
Objective
To identify factors associated with repeated nasolacrimal duct probing in young children.
Design, Setting, and Participants
This retrospective cohort study analyzed data from the Intelligent Research in Sight (IRIS) Registry for all children who underwent nasolacrimal duct probing before 4 years of age between January 1, 2013, and December 31, 2020.
Main Outcomes and Measures
The Kaplan-Meier estimator was used to assess the cumulative incidence of a repeated procedure within 2 years of the initial procedure. Hazard ratios (HRs) derived from multivariable Cox proportional hazards regression models were used to evaluate the association between repeated probing and patient age, sex, race and ethnicity, geographic region, operative side, laterality of obstruction, type of initial procedure, and surgeon volume.
Results
This study included 19 357 children (9823 [50.7%] male; mean [SD] age, 1.40 [0.74] years) undergoing nasolacrimal duct probing. The cumulative incidence of repeated nasolacrimal duct probing was 7.2% (95% CI, 6.8%-7.5%) within 2 years of the initial procedure. Among 1333 repeated procedures, the second procedure involved silicone intubation in 669 (50.2%) and balloon catheter dilation in 256 (19.2%). Among 12 008 children aged 1 year or younger, office-based simple probing was associated with a slightly higher probability of reoperation compared with facility-based simple probing (9.5% [95% CI, 8.2%-10.8%] vs 7.1% [95% CI, 6.5%-7.7%]; P < .001). In the multivariable model, a greater risk of repeated probing was associated with bilateral obstruction (HR, 1.48; 95% CI, 1.32-1.65; P < .001) and office-based simple probing (HR, 1.33; 95% CI, 1.13-1.55; P < .001), and a lower risk was associated with primary balloon catheter dilation (HR, 0.69; 95% CI, 0.56-0.85; P < .001) and procedures performed by high-volume surgeons (HR, 0.84; 95% CI, 0.73-0.97; P = .02). Age, sex, race and ethnicity, geographic region, and operative side were not associated with reoperation risk in the multivariable model.
Conclusions and Relevance
In this cohort study, most children in the IRIS Registry undergoing nasolacrimal duct probing before 4 years of age did not require any additional intervention. Factors associated with lower risk of reoperation include surgeon experience, probing performed under anesthesia, and primary balloon catheter dilation.
Introduction
Congenital nasolacrimal duct obstruction (NLDO) is the most common cause of tearing in young children.1 Although often resolving spontaneously, NLDO causing persistent symptoms is typically managed through probing of the nasolacrimal duct system.2 It is not uncommon for symptoms to recur following initial probing, in which case a repeated procedure with or without the use of additional techniques, such as silicone intubation or balloon catheter dilation, may be indicated.3 Treatment failure is associated not only with increased morbidity but also with elevated risk of general anesthesia–associated complications and increased overall cost to the health care system.4 A greater understanding of patient and surgeon factors associated with treatment failure on the population level may help guide treatment guidelines. In this study, we assessed the risk of a repeated procedure following initial nasolacrimal duct probing among young children in the US using data from the Intelligent Research in Sight (IRIS) Registry.
Methods
This was a retrospective cohort study performed using electronic health record data of patients followed up at practices in the US that have participated in the IRIS Registry. The version of the database that this study used was frozen on December 24, 2021, and accessed on March 30, 2022. The data collection methods of the IRIS Registry have been described previously.5 This study was approved by the Massachusetts General Brigham institutional review board with an exemption of informed consent due to the use of deidentified data. The research adhered to the Declaration of Helsinki6 and the US Health Insurance Portability and Accountability Act and followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
The records of all children in the IRIS Registry who underwent nasolacrimal duct probing before 4 years of age between January 1, 2013, and December 31, 2020, were included in this study. Nasolacrimal probing procedures were identified using Current Procedural Terminology (CPT) codes for simple probing (office based and facility based), silicone intubation, and balloon catheter dilation (CPT codes 68810, 68811, 68815, and 68816). Children undergoing canalicular repair (CPT code 68700) on the same date or prior to the initial nasolacrimal duct procedure were excluded from this study. Any procedures missing a CPT code specification for laterality were also excluded. The first nasolacrimal duct procedure performed for each patient in each eye within the study period was considered the initial procedure for that eye. Treatment failure was defined as the identification of a subsequent procedure, including any of the nasolacrimal duct probing codes (CPT codes 68810, 68811, 68815, or 68816), performed on the same eye within the study interval.
Outcome Measures and Variables
The main outcome measure was the cumulative incidence of repeated probing within 2 years of initial nasolacrimal duct probing obtained using the Kaplan-Meier estimator. Multivariable Cox proportional hazards regression models were used to quantify the association between risk of repeated nasolacrimal duct probing and patient age, sex, race and ethnicity, geographic location, operative side, laterality of obstruction, type of procedure, and surgeon volume. Age was treated as a continuous variable. Sex, race, and ethnicity data were extracted from the electronic health records contributing to the IRIS Registry. Race and ethnicity were treated as a categorical covariate with 4 groups: Hispanic, non-Hispanic Black, and non-Hispanic White individuals and those with other race and ethnicity (Asian, Native American or Alaska Native, Native Hawaiian or Other Pacific Islander, and other [“other” reported in the registry]). Geographic location was identified based on the 3-digit zip code of residence for each individual and categorized into 1 of 4 US census regions: Northeast, Midwest, South, and West. The laterality of obstruction was considered unilateral if the patient had a procedure performed in a single eye and bilateral if the patient had procedures performed in both eyes. The type of initial procedure was categorized as simple probing performed in the clinic (office-based simple probing), simple probing performed under general anesthesia (facility-based simple probing), probing involving placement of a nasolacrimal tube (silicone intubation), or probing involving balloon dacryoplasty (balloon catheter dilation). Silicone intubation and balloon catheter dilation procedures were assumed to be performed in a facility but were not included in the analyses comparing office-based simple probing with facility-based simple probing. Since the distribution of initial procedure type may differ between younger and older children, we performed a secondary stratified analysis comparing office-based simple probing with facility-based simple probing in the younger subcohort (age ≤1 year) and primary silicone intubation with primary balloon catheter dilation in the older subcohort (age ≥2 years). High-volume surgeons were defined as those performing at least 20 nasolacrimal probing procedures per year on average during the study interval.
Statistical Analysis
For each child undergoing bilateral nasolacrimal duct probing, a single eye was selected at random to be included in the analysis using a function in the statistical software with a seed set for reproducibility. We report medians and IQRs for continuous variables and frequencies and percentages for categorical variables. χ2 Tests were used in the complete case univariate analysis to compare the patient demographic and surgical factors based on the type of procedure. Log-rank tests were used to compare Kaplan-Meier estimates, and P values reported from pairwise tests were adjusted by the Holm-Bonferroni method. Hazard ratios (HRs) with 95% CIs are reported from the multivariable Cox proportional hazards regression model. Multivariate imputation by chained equations7 was performed for any covariates with more than 5% missing data included in the regression model. All analyses were performed using R, version 4.2.0 (R Project for Statistical Computing) with the multivariate imputation by chained equations package in R, version 3.13.0.8 Two-sided P < .05 was considered significant.
Results
Nasolacrimal duct probing was performed in 24 869 children, of whom 5512 (22%) were excluded for missing CPT laterality specification. A total of 19 357 children were included in the analysis (9823 [50.7%] male; mean [SD] age, 1.40 [0.74] years). Of these, 1730 (8.9%) were Hispanic, 788 (4.1%) were non-Hispanic Black, and 11 862 (61.3%) were non-Hispanic White; 368 (1.9%) had other and 4609 (23.8%) had unknown race and ethnicity reported. The types of initial procedures included 13 688 simple probing (70.7%), of which 11 338 (58.6%) were facility based and 2350 (12.1%) were office based; 3613 primary silicone intubation (18.7%); and 2056 primary balloon catheter dilation (10.6%) (Table).
Table. Baseline Patient Demographics and Surgical Factors Stratified by Initial Procedure Performed.
| Characteristic | Patients, No. (%) | |||
|---|---|---|---|---|
| Facility-based simple probing (n = 11 338) | Office-based simple probing (n = 2350) | Silicone intubation (n = 3613) | Balloon dilation (n = 2056) | |
| Age, mean (SD), y | 1.36 (0.69) | 0.92 (0.59) | 1.71 (0.79) | 1.67 (0.75) |
| Sexa | ||||
| Female | 5501 (49) | 1181 (50) | 1778 (49) | 1037 (50) |
| Male | 5814 (51) | 1163 (49) | 1831 (51) | 1015 (49) |
| Unknown | 23 (<1) | 6 (<1) | 4 (<1) | 4 (<1) |
| Laterality | ||||
| Unilateral | 7941 (70) | 1788 (76) | 2444 (68) | 1230 (60) |
| Bilateral | 3397 (30) | 562 (24) | 1169 (32) | 826 (40) |
| Operative eye | ||||
| Left | 5950 (52) | 1182 (50) | 1877 (52) | 1006 (49) |
| Right | 5388 (48) | 1168 (50) | 1736 (48) | 1050 (51) |
| Surgeon volumeb | ||||
| Low | 9116 (80) | 1641 (70) | 2884 (80) | 1438 (70) |
| High | 2001 (18) | 667 (28) | 602 (17) | 590 (29) |
| Unknown | 221 (2) | 42 (2) | 127 (4) | 28 (1) |
| Race and ethnicitya | ||||
| Hispanic | 924 (8) | 241 (10) | 395 (11) | 170 (8) |
| Non-Hispanic Black | 413 (4) | 67 (3) | 178 (5) | 130 (6) |
| Non-Hispanic White | 7337 (65) | 1333 (57) | 2103 (58) | 1089 (53) |
| Otherc | 187 (2) | 21 (1) | 75 (2) | 85 (4) |
| Unknown | 2477 (22) | 688 (29) | 862 (24) | 582 (28) |
| US census region | ||||
| South | 3957 (35) | 1074 (46) | 1348 (37) | 1186 (58) |
| Midwest | 3645 (32) | 775 (33) | 978 (27) | 439 (21) |
| Northeast | 1735 (15) | 151 (6) | 448 (12) | 139 (7) |
| West | 1864 (16) | 302 (13) | 753 (21) | 273 (13) |
| Unknown | 137 (1) | 48 (2) | 86 (2) | 19 (1) |
Sex, race, and ethnicity data were extracted from the electronic health records contributing to the Intelligent Research in Sight Registry.
High-volume surgeons were defined as those performing at least 20 nasolacrimal probing procedures per year.
Other race and ethnicity included Asian, Native American or Alaska Native, Native Hawaiian or Other Pacific Islander, and other (“other” reported in the registry).
Repeated procedures were performed on the same side in 1333 eyes (6.9%). The second procedure was most commonly silicone intubation (669 [50.2%]), followed by repeated simple probing (408 [30.6%]; 318 [23.9%] facility based and 90 [6.8%] office based), and balloon catheter dilation (256 [19.2%]). The resulting Kaplan-Meier estimated cumulative incidence of reoperation within 2 years of the initial procedure was 7.2% (95% CI, 6.8%-7.5%) for the entire cohort and 9.7% (95% CI, 8.0%-10.8%) for the subgroup younger than 1 year (n = 1273). The unadjusted cumulative incidence of repeated probing within 2 years of initial surgery was higher for office-based vs facility-based simple probing (9.2% [95% CI, 8.1%-10.4%] vs 7.3% [95% CI, 6.8%-7.8%]; P < .001), with consistent findings in the subcohort of 12 008 children aged 1 year or younger (9.5% [95% CI, 8.2%-10.8%] vs 7.1% [95% CI, 6.5%-7.7%]; P < .001). The incidence of repeated probing was also greater after primary silicone intubation vs balloon catheter dilation (6.5% [95% CI, 5.7%-7.4%] vs 5.1% [95% CI, 4.2%-6.1%]; P = .02) but not in the subcohort of children aged 2 years or older (n = 7349; 6.4% [95% CI, 5.3%-7.5%] vs 5.1% [3.8%-6.2%]; P = .21). There was a slightly lower probability of repeated probing among patients with unilateral vs bilateral NLDO (6.3% [95% CI, 5.9%-6.7%] vs 9.1% [8.4%-9.9%]; P < .001) and those treated by high-volume vs low-volume surgeons (6.3% vs 7.2%; P = .03). There was no significant difference in the probability of repeated probing based on patient sex, race and ethnicity, geographic region, or operative side (Figure 1).
Figure 1. Kaplan-Meier Curves Comparing the Cumulative Incidence of Repeated Nasolacrimal Duct Probing Based on Patient Age, Type of Initial Procedure, Laterality of Obstruction, and Surgeon Volume.
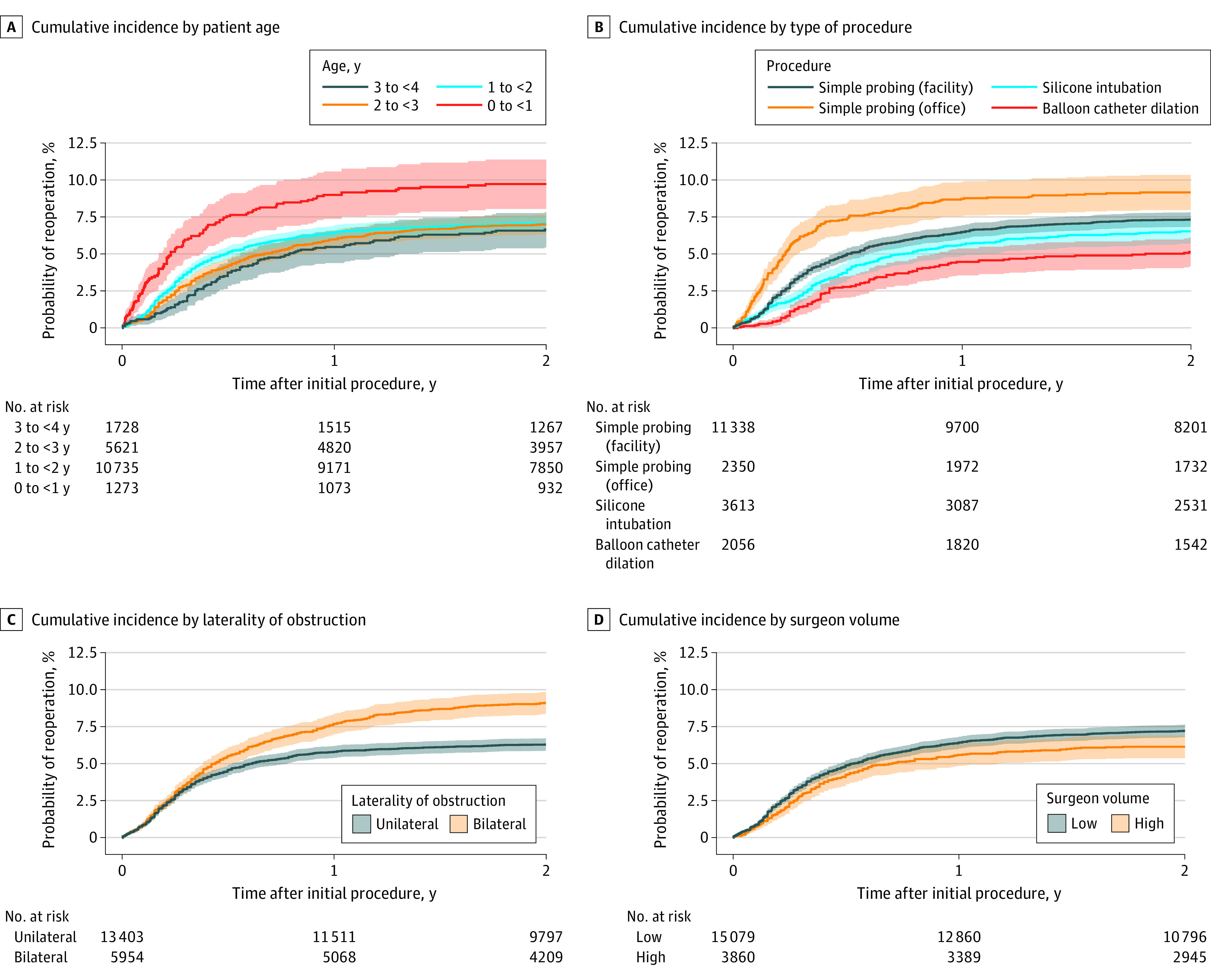
Surgeon volume was considered high for those performing at least 20 nasolacrimal probing procedures per year on average during the study interval.
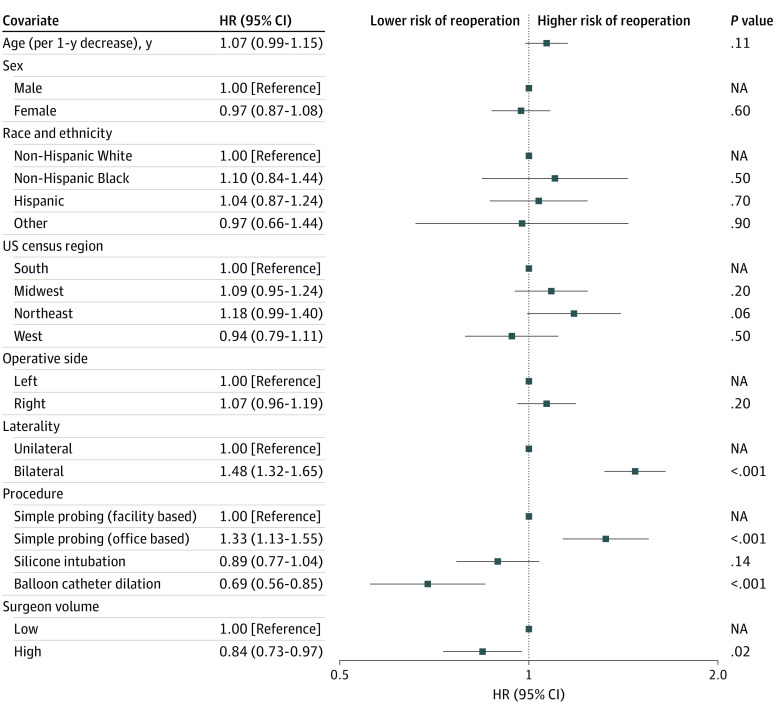
After adjusting for all other covariates, the Cox proportional hazards regression model revealed a higher risk of reoperation associated with bilateral compared with unilateral NLDO (HR, 1.48; 95% CI, 1.32-1.65; P < .001) and office-based compared with facility-based simple probing (HR, 1.33; 95% CI, 1.13-1.55; P < .001). A lower risk of reoperation was associated with primary balloon catheter dilation compared with facility-based simple probing (HR, 0.69; 95% CI, 0.56-0.85; P < .001) and procedures performed by high-volume surgeons compared with low-volume surgeons (HR, 0.84; 95% CI, 0.73-0.97; P = .02). Age, sex, race and ethnicity, geographic region, and operative side were not associated with risk of reoperation in the multivariable model (Figure 2).
Figure 2. Forest Plot of the Association Between All Covariates and Repeated Nasolacrimal Duct Probing.
Hazard ratios (HRs) were determined using a multivariable Cox proportional hazards regression model. Sex, race, and ethnicity data were extracted from the electronic health records contributing to the Intelligent Research in Sight Registry. Other race and ethnicity included Asian, Native American or Alaska Native, Native Hawaiian or Other Pacific Islander, and other (“other” reported in the registry). High-volume surgeons were defined as those performing at least 20 nasolacrimal probing procedures per year. Markers indicate HRs, with horizontal lines indicating 95% CIs. NA indicates not applicable.
Discussion
In this cohort study, approximately 1 in 14 children younger than 4 years in the IRIS Registry required a reoperation within 2 years of initial nasolacrimal duct probing. The most frequent primary procedure performed was facility-based simple probing, and repeated procedures most often involved silicone intubation. The reoperation rate of office-based simple probing was greater than that of facility-based simple probing, and the reoperation rate of primary silicone intubation was greater than that of primary balloon catheter dilation. Other factors associated with reoperation risk included the laterality of obstruction and surgeon experience with nasolacrimal duct probing. Procedures performed by high-volume surgeons (≥20 procedures per year on average) were associated with a lower risk of reoperation, which supports the importance of development and maintenance of expertise in treating this patient population.
Using the Optum insurance claims database to identify a cohort of 16 538 children undergoing nasolacrimal duct probing between 2003 and 2016, Muminovic and colleagues9 found a higher probability of repeated procedures for both office-based simple probing (23.0% vs 9.2% in the present study) and facility-based simple probing (19.5% vs 7.3% in the present study). We suspect that the difference in estimates may be explained by the lack of laterality data in the Optum dataset; therefore, procedures performed on the contralateral eye may have been counted as repeated procedures. The results of our study also reflect a shorter follow-up interval, with repeated probing within 2 years instead of at any subsequent time point as defined in the Optum study.9 Among children undergoing office-based simple probing between 6 and 10 months of age, the Pediatric Eye Disease Investigator Group (PEDIG) reported a similar rate of repeated probing (9% for unilateral and 13% for bilateral NLDO) by 18 months of age.10,11
There is evidence to support the efficacy and safety of both office-based and facility-based simple probing for congenital NLDO in young children.12 In a prospective, observational, multicenter study, PEDIG reported a lower probability of success when simple probing was performed in an office compared with in a facility (72% vs 80%).13 However, other retrospective studies have described success rates of up to 94% for office-based simple probing,14,15 and furthermore, the PEDIG randomized clinical trial demonstrated a similar success rate of office-based and deferred facility-based simple probing.10,11 The criteria for success in the PEDIG studies required the absence of any clinical signs of NLDO in both eyes, which may be related to but is not directly comparable with the reoperation rates reported in this study. Office-based simple probing has also been found to be less successful for bilateral NLDO compared with unilateral NLDO, and this finding seems to be unrelated to age.16 Although we found a slight reduction in the reoperation risk for facility-based compared with office-based simple probing after adjusting for laterality and age, these results should be interpreted with the risks and benefits of each approach in consideration. Some ophthalmologists prefer to proceed directly to facility-based simple probing given that the office-based approach may be more painful or otherwise traumatizing, particularly for older infants.12 Procedures performed in a facility with the child under sedation may also facilitate the management of complex obstructions with the opportunity for multiple attempts at cannulating the nasolacrimal duct system. However, the majority of NLDO cases self-resolve, and there is benefit to avoiding the potential risks associated with general anesthesia.17,18 High parental satisfaction has been reported for office-based simple probing, with many families, when given a choice, preferring an office-based to a facility-based approach.19 Both office-based and facility-based simple probing play an important role in the management of congenital NLDO in infants, and the options merit discussion between the physician and family.
Silicone intubation and balloon catheter dilation have been described as successful secondary procedures to address recurrent or persistent NLDO.3,20 Both approaches are also used in the primary treatment of NLDO, with similar success rates.21,22 In this study, silicone intubation and balloon catheter dilation were performed more frequently in the older subcohort (age ≥2 years). Children with congenital NLDO undergoing probing at an older age may be more likely to have unusual pathology that may put them at greater risk of recurrence,23,24 thus justifying starting with a more complex procedure that may have a higher success rate. We found a slightly lower risk of a repeated procedure following primary balloon catheter dilation compared with simple probing. A disadvantage of balloon catheter dilation is the additional cost associated with this disposable medical equipment compared with nasolacrimal tubes.22 An important benefit of balloon catheter dilation over silicone intubation is the avoidance of a second procedure to remove the silicone tube, although tube removal can generally be performed in the office without sedation or discomfort.
Limitations
There are several limitations of the data, study design, and analytic approach to consider in the interpretation of our findings. First, although our study sample represents a large cohort of children undergoing nasolacrimal duct probing in the US, certain geographic regions and racial and ethnic groups may be underrepresented in the IRIS Registry, thus limiting the generalizability of our results. In addition, since we were able to include only patients with clinical encounters involving practices participating in the IRIS Registry, there was potential for selection bias based on the systematic inclusion or exclusion of certain types of practices. For instance, academic medical centers are underrepresented in the IRIS Registry yet may receive referrals for repeated nasolacrimal duct probing, thus potentially resulting in an underestimation of our failure rate. Similarly, an important assumption of our study design was that the first procedure performed within the study interval was the primary procedure performed. While many surgeons prefer to perform primary silicone intubation or balloon catheter dilation for congenital NLDO beyond a certain age threshold, it is possible that some of these procedures represented repeated procedures. If the initial procedure was performed outside a practice in the IRIS Registry, we may have overestimated the reduction in failure rate associated with these procedures. The clinical examination elements currently available in the IRIS Registry limited our ability to account for all potential sources of confounding. In particular, there may have been underlying differences in the indications for the type of procedure performed that were not accounted for in this analysis. Another potential source of selection bias involves the exclusion of procedures without specified laterality, which was necessary to track repeated procedures on the same eye but may not have been consistently used by certain groups of surgeons. Since CPT codes are not collected for the primary purpose of investigation, there is potential for miscoding in the registry. In addition, our criterion for failure was a repeated nasolacrimal duct probing procedure; however, this may have underestimated clinical failure in cases in which a decision was made not to perform any additional intervention (or when the patient left the IRIS Registry–participating practice).
Conclusions
By leveraging electronic health record data from practices across the US from the IRIS Registry, this cohort study found that 7.2% of children younger than 4 years who underwent NLDO had a reoperation within 2 years. Higher risk of reoperation was associated with bilateral obstruction and probing performed in an office, whereas lower risk was associated with primary balloon catheter dilation and procedures performed by high-volume surgeons. Many factors may contribute to the choice of the initial procedure, and surgeons should be aware of the differences in reoperation rates and the potential association with experience in performing procedures in this population. Although office-based simple probing had a slightly higher probability of reoperation compared with facility-based simple probing, most children did not require additional intervention, which should be considered when counseling patients about treatment options. The IRIS Registry may have utility for assessing practice patterns and further exploring factors associated with nasolacrimal duct probing failure to better inform practice guidelines that may improve the care of children with congenital NLDO.
IRIS Registry Analytic Center Consortium
Data Sharing Statement
References
- 1.MacEwen CJ, Young JD. Epiphora during the first year of life. Eye (Lond). 1991;5:596-600. doi: 10.1038/eye.1991.103 [DOI] [PubMed] [Google Scholar]
- 2.Petersen RA, Robb RM. The natural course of congenital obstruction of the nasolacrimal duct. J Pediatr Ophthalmol Strabismus. 1978;15(4):246-250. doi: 10.3928/0191-3913-19780701-14 [DOI] [PubMed] [Google Scholar]
- 3.Repka MX, Chandler DL, Holmes JM, et al. ; Pediatric Eye Disease Investigator Group . Balloon catheter dilation and nasolacrimal duct intubation for treatment of nasolacrimal duct obstruction after failed probing. Arch Ophthalmol. 2009;127(5):633-639. doi: 10.1001/archophthalmol.2009.66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Petris C, Liu D. Probing for congenital nasolacrimal duct obstruction. Cochrane Database Syst Rev. 2017;7(7):CD011109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chiang MF, Sommer A, Rich WL, Lum F, Parke DW II. The 2016 American Academy of Ophthalmology IRIS® Registry (Intelligent Research in Sight) database: characteristics and methods. Ophthalmology. 2018;125(8):1143-1148. doi: 10.1016/j.ophtha.2017.12.001 [DOI] [PubMed] [Google Scholar]
- 6.World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 7.van Buuren S, Boshuizen HC, Knook DL. Multiple imputation of missing blood pressure covariates in survival analysis. Stat Med. 1999;18(6):681-694. doi: [DOI] [PubMed] [Google Scholar]
- 8.van Buuren S, Groothuis-Oudshoorn K. mice: Multivariate Imputation by Chained Equations in R. J Stat Softw. 2011;45(3):1-67. https://www.jstatsoft.org/v45/i03/
- 9.Muminovic I, Ryu WY, Lambert SR. Trends in congenital nasolacrimal duct obstruction surgical procedures in the United States from 2003 to 2016. J AAPOS. 2021;25(6):354-356. doi: 10.1016/j.jaapos.2021.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee KA, Chandler DL, Repka MX, et al. ; PEDIG . A comparison of treatment approaches for bilateral congenital nasolacrimal duct obstruction. Am J Ophthalmol. 2013;156(5):1045-1050. doi: 10.1016/j.ajo.2013.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pediatric Eye Disease Investigator Group . A randomized trial comparing the cost-effectiveness of 2 approaches for treating unilateral nasolacrimal duct obstruction. Arch Ophthalmol. 2012;130(12):1525-1533. doi: 10.1001/archophthalmol.2012.2853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morrison DG, Binenbaum G, Chang MY, et al. Office- or facility-based probing for congenital nasolacrimal duct obstruction: a report by the American Academy of Ophthalmology. Ophthalmology. 2021;128(6):920-927. doi: 10.1016/j.ophtha.2020.10.028 [DOI] [PubMed] [Google Scholar]
- 13.Repka MX, Chandler DL, Beck RW, et al. ; Pediatric Eye Disease Investigator Group . Primary treatment of nasolacrimal duct obstruction with probing in children younger than 4 years. Ophthalmology. 2008;115(3):577-584.e3. doi: 10.1016/j.ophtha.2007.07.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stager D, Baker JD, Frey T, Weakley DR Jr, Birch EE. Office probing of congenital nasolacrimal duct obstruction. Ophthalmic Surg. 1992;23(7):482-484. doi: 10.3928/1542-8877-19920701-11 [DOI] [PubMed] [Google Scholar]
- 15.Baker JD. Treatment of congenital nasolacrimal system obstruction. J Pediatr Ophthalmol Strabismus. 1985;22(1):34-36. doi: 10.3928/0191-3913-19850101-12 [DOI] [PubMed] [Google Scholar]
- 16.Miller AM, Chandler DL, Repka MX, et al. ; Pediatric Eye Disease Investigator Group . Office probing for treatment of nasolacrimal duct obstruction in infants. J AAPOS. 2014;18(1):26-30. doi: 10.1016/j.jaapos.2013.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilder RT, Flick RP, Sprung J, et al. Early exposure to anesthesia and learning disabilities in a population-based birth cohort. Anesthesiology. 2009;110(4):796-804. doi: 10.1097/01.anes.0000344728.34332.5d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Flick RP, Sprung J, Harrison TE, et al. Perioperative cardiac arrests in children between 1988 and 2005 at a tertiary referral center: a study of 92,881 patients. Anesthesiology. 2007;106(2):226-237. doi: 10.1097/00000542-200702000-00009 [DOI] [PubMed] [Google Scholar]
- 19.Goldblum TA, Summers CG, Egbert JE, Letson RD. Office probing for congenital nasolacrimal duct obstruction: a study of parental satisfaction. J Pediatr Ophthalmol Strabismus. 1996;33(4):244-247. doi: 10.3928/0191-3913-19960701-09 [DOI] [PubMed] [Google Scholar]
- 20.Goldstein SM, Goldstein JB, Katowitz JA. Comparison of monocanalicular stenting and balloon dacryoplasty in secondary treatment of congenital nasolacrimal duct obstruction after failed primary probing. Ophthalmic Plast Reconstr Surg. 2004;20(5):352-357. doi: 10.1097/01.IOP.0000134271.25794.96 [DOI] [PubMed] [Google Scholar]
- 21.Repka MX, Melia BM, Beck RW, et al. ; Pediatric Eye Disease Investigator Group . Primary treatment of nasolacrimal duct obstruction with nasolacrimal duct intubation in children younger than 4 years of age. J AAPOS. 2008;12(5):445-450. doi: 10.1016/j.jaapos.2008.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Repka MX, Melia BM, Beck RW, et al. ; Pediatric Eye Disease Investigator Group . Primary treatment of nasolacrimal duct obstruction with balloon catheter dilation in children younger than 4 years of age. J AAPOS. 2008;12(5):451-455. doi: 10.1016/j.jaapos.2008.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paul TO, Shepherd R. Congenital nasolacrimal duct obstruction: natural history and the timing of optimal intervention. J Pediatr Ophthalmol Strabismus. 1994;31(6):362-367. doi: 10.3928/0191-3913-19941101-04 [DOI] [PubMed] [Google Scholar]
- 24.Kushner BJ. The management of nasolacrimal duct obstruction in children between 18 months and 4 years old. J AAPOS. 1998;2(1):57-60. doi: 10.1016/S1091-8531(98)90112-4 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
IRIS Registry Analytic Center Consortium
Data Sharing Statement