Key Points
Question
How can the most effective systemic treatment for heterogeneous metastatic castration-sensitive prostate cancer (mCSPC) be chosen?
Findings
In this living systematic review and network meta-analysis of 10 clinical trials with 11 043 patients with mCSPC, abiraterone triplet therapy was ranked potentially as the most efficacious treatment for high-volume disease and was associated with significant improvement in survival compared with doublet regimens. For low-volume and metachronous disease, androgen pathway inhibitor (API) doublet therapies were ranked potentially as the most efficacious treatment options and were not significantly different from triplet regimen.
Meaning
The results of this systematic review and meta-analysis found that triplet therapy may be preferred for synchronous (de novo) high-volume disease; API doublet therapies may be preferred for metachronous (recurrent) low-volume disease.
Abstract
Importance
The effectiveness of triplet therapy compared with androgen pathway inhibitor (API) doublets in a heterogeneous patient population with metastatic castration-sensitive prostate cancer (mCSPC) is unknown.
Objective
To assess the comparative effectiveness of contemporary systemic treatment options for patients with mCSPC across clinically relevant subgroups.
Data Sources
For this systematic review and meta-analysis, Ovid MEDLINE and Embase were searched from each database’s inception (MEDLINE, 1946; Embase, 1974) through June 16, 2021. Subsequently, a “living” auto search was created with weekly updates to identify new evidence as it became available.
Study Selection
Phase 3 randomized clinical trials (RCTs) assessing first-line treatment options for mCSPC.
Data Extraction and Synthesis
Two independent reviewers extracted data from eligible RCTs. The comparative effectiveness of different treatment options was assessed with a fixed-effect network meta-analysis. Data were analyzed on July 10, 2022.
Main Outcomes and Measures
Outcomes of interest included overall survival (OS), progression-free survival (PFS), grade 3 or higher adverse events, and health-related quality of life.
Results
This report included 10 RCTs with 11 043 patients and 9 unique treatment groups. Median ages of the included population ranged from 63 to 70 years. Current evidence for the overall population suggests that the darolutamide (DARO) triplet (DARO + docetaxel [D] + androgen deprivation therapy [ADT]; hazard ratio [HR], 0.68; 95% CI, 0.57-0.81), as well as the abiraterone (AAP) triplet (AAP + D + ADT; HR, 0.75; 95% CI, 0.59-0.95), are associated with improved OS compared with D doublet (D + ADT) but not compared with API doublets. Among patients with high-volume disease, AAP + D + ADT may improve OS compared with D + ADT (HR, 0.72; 95% CI, 0.55-0.95) but not compared with AAP + ADT, enzalutamide (E) + ADT, and apalutamide (APA) + ADT. For patients with low-volume disease, AAP + D + ADT may not improve OS compared with APA + ADT, AAP + ADT, E + ADT, and D + ADT.
Conclusions and Relevance
The potential benefit observed with triplet therapy must be interpreted with careful accounting for the volume of disease and the choice of doublet comparisons used in the clinical trials. These findings suggest an equipoise to how triplet regimens compare with API doublet combinations and provide direction for future clinical trials.
This systematic review and meta-analysis investigates the comparative effectiveness of triplet therapy vs androgen pathway inhibitors doublet therapy for patients with heterogeneous metastatic castration-sensitive prostate cancer.
Introduction
Intensification of androgen deprivation therapy (ADT) with androgen pathway inhibitor (API) agents (abiraterone [AAP], apalutamide [APA], and enzalutamide [E])1,2,3,4,5 or chemotherapy (docetaxel [D])6,7 for patients with metastatic castration-sensitive prostate cancer (mCSPC) has been demonstrated to delay disease progression and prolong patient survival. Recently, the PEACE-1 trial8 and the ARASENS trial9 showed incremental benefit among patients with mCSPC with the further intensification of treatment using triplet therapy (API + D + ADT). The PEACE-1 trial found that the combination of AAP acetate and D caused a substantial delay in disease progression and a remarkable improvement in overall survival (OS; 25% risk reduction in death).8 Similarly, the ARASENS trial showed that adding darolutamide (DARO) to D doublet resulted in a consistent delay in disease progression and survival benefit (32.5% risk reduction of death).9 Both PEACE-1 (100%) and ARASENS (86%) primarily included patients at high risk with synchronous (de novo) presentation.
The availability of multiple effective therapies and the clinical heterogeneity of patients with mCSPC make the choice of optimal treatment complicated. First, available evidence indicates that the D treatment effect for patients with mCSPC may vary by volume of disease and timing of metastatic presentation.10,11,12 Second, it is unknown how triplet therapy compares with API doublets. Thus, the absence of evidence comparing triplet therapy with API doublets together with the prognostic variability by volume of disease provides a compelling rationale to conduct a systematic review and assess the comparative effectiveness of current treatment options in the overall patient population with mCSPC and across clinically relevant subgroups.
Here, we reported the contemporary findings from a network meta-analysis, with a particular emphasis on triplet vs API doublet comparisons in the context of clinically relevant subgroups defined by volume of disease and timing of metastatic presentation. We also maintained a living evidence profile for systemic treatment options for patients with mCSPC patients, using the “living” interactive evidence synthesis framework.13
Methods
This systematic review and meta-analysis is reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline extension statement for systematic reviews, incorporating network meta-analyses for health care interventions.14 This study is registered at the open science framework (OSF) registries.15
Literature Search and Study Selection
Embase and Ovid MEDLINE were searched from each database’s inception (MEDLINE, 1946; Embase, 1974) through July 10, 2022, to retrieve published reports of mCSPC clinical trials. The detailed living search strategy and eligibility criteria are provided in the eAppendix in Supplement 1.
Data Extraction and Quality Assessment
Patient-important outcomes included OS, radiographic or clinical progression-free survival (PFS), grade 3 or higher adverse events, and health-related quality of life. These outcomes were defined in accordance with definitions in the included clinical trials (eTable 1 in Supplement 1). Clinically relevant subgroups were mainly defined by timing of metastatic presentation (synchronous [de novo] and metachronous [recurrent]) and volume of disease (high and low). In instances in which an eligible trial had multiple reports, data from most updated or longest follow-up were included in the analysis. The quality of included trials was assessed with the Cochrane Risk of Bias Tool, version 2.16 This process of data extraction and quality assessment was carried out by 2 independent reviewers (I.B.R. and S.A.A.N.). Discrepancies in the process were resolved by consensus and input from a senior reviewer (A.H.B.).
Data Analysis
Pairwise Meta-analysis
A DerSimonian and Laird random-effects meta-analysis was conducted to make direct (pairwise) comparisons. The Cochran Q test was used to assess statistically significant heterogeneity not explained by chance, whereas I2 was used to quantify the total observed variability due to between-study heterogeneity. Statistical significance was established with a 2-sided α level of .05 and .1 for primary and subgroup analyses, respectively. Details for pairwise meta-analysis are described in the eAppendix in Supplement 1. Data were analyzed on July 10, 2022.
Network Meta-analysis
Direct evidence and indirect evidence were used to compute mixed treatment comparisons using a multivariate metaregression within the frequentist framework.17,18 Both fixed-effect and random-effects models were fitted; however, the final choice of model was made according to a priori criteria, and the fixed-effect model was used if the network was open and sparse, given that the common between-study heterogeneity cannot be estimated reliably in such networks.19 Relative treatment rankings for each outcome were assessed with a P score and were evaluated according to their congruence with pairwise estimates.20 A higher relative treatment rank indicated potentially better efficacy and safety. Details are described in the eAppendix in Supplement 1.
Secondary Analyses
Secondary analyses were performed for prespecified subgroups of interest, which are described in the eAppendix in Supplement 1. All statistical analyses were conducted in R, version 4.1.1 (R Foundation for Statistical Computing). Pairwise and network meta-analyses were conducted with meta version 5.1-1 and netmeta version 2.0-1, respectively.
Certainty of Evidence
The Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach was used to assess the certainty of evidence.21,22 Details are described in the eAppendix in Supplement 1.
Results
This present report from a living systematic review includes data from 10 clinical trials (28 references) published as of July 10, 2022.1,2,3,4,5,6,7,8,9,11,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40 The process of study selection is shown in the PRISMA flowchart (eFigure 1 in Supplement 1). All results of this systematic review are available on the Living Interactive Systematic Reviews website.13
Baseline Trial and Population Characteristics
A total of 11 043 patients and 9 unique treatment options were assessed. Median ages of the included population ranged from 63 to 70 years. Data for race and ethnicity were not collected because the published trials included in this meta-analysis did not consistently report outcomes by race and ethnicity. All included clinical trials followed a randomized phase 3 design; most were open-label trials, and only 4 were double-blinded trials. All trials included only patients with mCSPC except STAMPEDE, which included a small subset of patients with high-risk localized prostate cancer.4,6 The PEACE-1 trial8 reported data for both the overall population and patients who received D; however, only data for patients who received D were used for analysis. The overall risk of bias for OS outcome was low for all trials. Some concerns due to the open-label nature of the trials were raised for the assessment of PFS and grade 3 or higher adverse events. A detailed summary of the risk of bias is provided in eFigure 2 in Supplement 1. Additional baseline trial and population characteristics are outlined in Table 11,2,3,4,5,6,7,8,9,24,27 and eTables 5 and 6 in Supplement 1. Proportions of patients and clinical outcomes by volume of disease and timing of metastatic presentation are outlined in eTable 3 and eTable 4 in Supplement 1, respectively.
Table 1. Baseline Trial and Population Characteristics of Included Clinical Trials.
| Source | Treatment groups | Median age, y | Median follow-up, mo | Volume of disease, % | Metastatic presentation, % | Docetaxel (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Experimental | Control | Total participants | Experimental treatment group | Control | High | Low | Synchronous | Metachronous | |||
| GETUG-AFU1,27 2013 | Docetaxel + ADT | ADT | 385 | 63 | 64 | 83 | 48 | 52 | 71 | 29 | Yes (100) |
| CHAARTED,7 2015 | Docetaxel + ADT | ADT | 790 | 64 | 63 | 54 | 65 | 35 | 73 | 27 | Yes (100) |
| STAMPEDE,4,6 2016, 2017a | Docetaxel + ADT | ADT | 1086b | 65 | 65 | 79 | 43c | 33c | ≈95 | ≈5 | Yes (100) |
| Abiraterone + ADT | ADT | 1002b | 67 | 67 | 42 | 56 | 45 | 97 | 3 | No | |
| LATITUDE,5 2017 | Abiraterone + ADT | ADT | 1199 | 68 | 67 | 52 | 80 | 20 | 100 | 0 | No |
| ENZAMET,3 2019 | Enzalutamide + ADT | NSAA + ADT | 1125 | 69 | 69 | 34 | 53 | 47 | 67 | 33 | Yes (concurrent, 45) |
| ARCHES,1 2019 | Enzalutamide + ADT | ADT | 1150 | 70 | 70 | 45 | 63 | 37 | 67d | 15d | Yes (prior, 18) |
| TITAN,2 2019 | Apalutamide + ADT | ADT | 1052 | 69 | 68 | 44 | 63 | 37 | 76d | 19d | Yes (prior, 11) |
| SWOG 1216,24 2022 | TAK + ADT | NSAA + ADT | 1279 | 68 | 68 | 59 | NA | NA | NA | NA | No |
| PEACE-1,8 2022 | Abiraterone + docetaxel + ADT | Docetaxel + ADT | 1172e | 67 | 66 | 46 | 57 | 43 | 100 | 0 | Yes (concurrent, 61) |
| ARASENS,9 2022 | Darolutamide + docetaxel + ADT | Docetaxel + ADT | 1305 | 67 | 67 | ≈43 | NA | NA | 86f | 13f | Yes (concurrent, 100) |
Abbreviations: ADT, androgen deprivation therapy; CHAARTED, Chemohormonal Therapy vs Androgen Ablation Randomized Trial for Extensive Disease in Prostate Cancer; ENZAMET, Enzalutamide in First Line Androgen Deprivation Therapy for Metastatic Prostate Cancer; GETUG-AFU, Groupe d’Etude des Tumeurs Urogénitales-Association Française d’Urologie; NA, not available; NSAA, nonsteroidal antiandrogen; PEACE, Prostate Cancer Consortium in Europe; STAMPEDE, Systemic Therapy in Advancing or Metastatic Prostate Cancer, Evaluation of Drug Efficacy; SWOG, Southwest Cancer Chemotherapy Study Group; TAK, orteronel; TITAN, Targeted Investigational Treatment Analysis of Novel Anti-androgen.
The STAMPEDE trial followed an adaptive multigroup and multistage randomized design in which active experimental treatment groups were assessed with a shared control group. The trial compared among more than 10 groups; however, groups relevant to our question of interest (group C, docetaxel; and group G, abiraterone acetate) were included in this analysis.
Number represents combined number of participants with metastatic disease in the experimental treatment group and control group. The number of participants in the control group is overlapping, considering an adaptive shared design; however, in the calculation of total number of participants included in this analysis, only 1 control group with the largest number of participants was used. The STAMPEDE trial was the only study that also included a cohort of high-risk localized (nonmetastatic) prostate cancer (990 in docetaxel group C comparisons and 690 in abiraterone acetate group G comparisons). These numbers are not presented here because we focused only on patients with metastatic castration-sensitive prostate cancer.
The relative percentages of patients with high and low volume of disease in STAMPEDE group C do not add up to 100 because 55 patients did not have a required bone scan or underwent unconventional bone imaging.
The relative percentages of patients with synchronous and metachronous metastases in ARCHES and TITAN do not add up to 100. A total of 213 patients in ARCHES and 56 in TITAN had unknown or undetermined status of metastatic presentation at initial diagnosis.
The PEACE-1 trial had protocol modification to incorporate the addition of docetaxel to the combination. The numbers presented in this table reflect the overall population. A total of 463 participants did not receive docetaxel and were not included in the statistical analysis.
The relative percentages of patients with synchronous and metachronous metastases in the ARASENS trial do not add up to 100. A total of 13 patients (≈1% of the total population) had unknown status of metastases at initial diagnosis.
Direct Comparisons
Direct comparative meta-analysis was derived from 8 trials,1,2,3,4,5,6,7,11,23,24,26,27,28,29,31,39,40 some with more than 1 published report, that assessed doublet regimens (either API or D as add-on treatments to ADT) vs ADT alone. The relative efficacy of the control group—nonsteroidal antiandrogen (including bicalutamide, flutamide, or nilutamide) and ADT—in 2 trials3,24 was considered equivalent to ADT for the purpose of pooling studies. Doublet therapy was associated with statistically significant OS benefit compared with ADT alone in the overall population. Detailed results are shown in eFigures 3 to 7 in Supplement 1.
Results by choice of doublet therapy, volume of disease, and timing of metastatic presentation are outlined in Table 23,4,6,8,9 (for OS, see eTables 7 and 8 in Supplement 1; for PFS outcome, see eTable 9 in Supplement 1). For patients with low-volume disease, API doublet was significantly associated with OS improvement compared with ADT alone (hazard ratio [HR], 0.58; 95% CI, 0.49-0.68; I2 = 0%); however, no statistically significant association was observed with D doublet compared with ADT (HR, 0.91; 95% CI, 0.73-1.13; I2 = 0%). There was a statistically significant effect modification by the choice of doublet therapy for patients with low-volume disease (P < .01 for heterogeneity). In contrast, there was no statistically significant effect modification by choice of doublet therapy for patients with high-volume disease in regard to OS benefit. The detailed results of additional analyses are shown in eFigures 8 to 49 and eTable 10 in Supplement 1.
Table 2. Overall Survival With Doublet Therapy (API or Docetaxel) Compared With ADT by Volume of Disease.
| Population | Total participantsa | Hazard ratio (95% CI)b | P value of interactionc | Interpretation |
|---|---|---|---|---|
| Overall patient populationb | ||||
| API doublet | 6808 | 0.69 (0.62-0.76) | .06 | API doublet therapy derived greater OS benefit than docetaxel doublet therapy compared with ADT alone. There was statistically significant effect modification by choice of doublet therapy in the overall population. |
| Docetaxel doublet | 2261 | 0.79 (0.71-0.89) | ||
| High volume | ||||
| API doublet | 3443 | 0.66 (0.60-0.73) | .26 | In patients with high-volume disease, there was consistent OS benefit with API and docetaxel doublets compared with ADT alone. There was no effect modification by choice of doublet therapy in high-volume disease. |
| Docetaxel doublet | 1164 | 0.73 (0.62-0.86) | ||
| Low volume | ||||
| API doublet | 1983 | 0.58 (0.49-0.68) | <.01 | In patients with low-volume disease, API doublet therapy derived significantly greater OS benefit than docetaxel doublet therapy compared with ADT alone. There was statistically significant effect modification by choice of doublet therapy in low-volume disease. |
| Docetaxel doublet | 841 | 0.91 (0.73-1.13) |
Abbreviations: ADT, androgen deprivation therapy; API, androgen pathway inhibitors (including abiraterone acetate, apalutamide, and enzalutamide); ENZAMET, Enzalutamide in First Line Androgen Deprivation Therapy for Metastatic Prostate Cancer; OS, overall survival; STAMPEDE, Systemic Therapy in Advancing or Metastatic Prostate Cancer, Evaluation of Drug Efficacy.
“Total participants” represents the total number of participants who were included in each comparison, which includes the number of participants in the treatment group and the number in the control for a comparison. The STAMPEDE trial4,6 followed an adaptive multigroup and multistage randomized design in which active experimental treatments groups were assessed with a shared control group. Hence, there may be overlap of patients included for abiraterone or docetaxel comparisons from STAMPEDE.
All effect estimates (hazard ratios) are for doublet regimens compared with standard ADT. These comparisons include only trials that assessed the efficacy of addition of API or docetaxel to standard ADT relative to ADT only. We assumed the relative efficacy of ADT to be similar to ADT plus nonsteroidal antiandrogen, which was the comparator in the ENZAMET trial3 for the purpose of pooling studies for direct comparisons. These comparisons do not include evidence from trials assessing triplet therapy relative to docetaxel and ADT (ARASENS9 and PEACE-18).
Statistical significance was established at a prespecified 2-sided α threshold of .10.
Mixed Treatment Comparisons
A total of 10 trials1,2,3,4,5,6,7,8,9,11,23,24,25,26,27,28,29,31,39,40 contributed to the network for OS outcome, 9 trials1,2,3,4,5,6,7,8,11,23,24,25,26,27,28,29,31 for PFS, and 8 trials1,2,3,4,5,6,8,24 for grade 3 or higher adverse events (some trials had more than 1 published report). Network plots are shown in eFigure 50 in Supplement 1. Results from the fixed-effect model are reported here considering the open network geometry with sparse direct evidence. Mixed treatment comparisons were also made with a random-effects model that indicated consistent direction of the results but wider 95% CIs.
In the overall population, mixed treatment comparisons showed a statistically significant improvement in OS with DARO + D + ADT (HR, 0.68; 95% CI, 0.57-0.81; rank 1) and AAP + D + ADT (HR, 0.75; 95% CI, 0.59-0.95; rank 2) compared with D + ADT (rank 6). However, no statistically significant association was observed with triplet regimens compared with APA + ADT (rank 3), E + ADT (rank 4), and AAP + ADT (rank 5) regarding OS improvement. The AAP + D + ADT treatment (rank 1) was associated with statistically significant PFS improvement compared with AAP + ADT (HR, 0.61; 95% CI, 0.41-0.91; rank 4) and D + ADT (HR, 0.50; 95% CI, 0.35-0.72; rank 6). However, no statistically significant association was observed with AAP + D + ADT compared with E + ADT (rank 2) and APA + ADT (rank 3). The PFS data for DARO + D + ADT are not available.9 The results of mixed treatment comparisons for OS and PFS were consistent when patients who received D in ENZAMET,3 ARCHES,1,25 and TITAN2,31 were excluded (eTable 2 in Supplement 1). In terms of safety, AAP + D + ADT (rank 8) was associated with an increased risk of grade 3 or higher adverse events compared with D + ADT (relative risk [RR], 1.22; 95% CI, 1.07-1.39; rank 6), AAP + ADT (RR, 1.23; 95% CI, 1.04-1.47; rank 5), APA + ADT (RR, 1.45; 95% CI, 1.18-1.78; rank 4), and E + ADT (RR, 1.80; 95% CI, 1.39-2.34; rank 2). The DARO + D + ADT treatment (rank 7) was also associated with an increased risk of grade 3 or higher adverse events compared with APA + ADT (RR, 1.23; 95% CI, 1.04-1.47) and E + ADT (RR, 1.55; 95% CI, 1.22-1.96) but not compared with D + ADT (RR, 1.04; 95% CI, 0.97-1.12). Detailed results of these analyses are shown in eFigures 51 to 55 in Supplement 1.
Data were not available by volume of disease for DARO + D + ADT. For patients with high-volume disease, AAP + D + ADT (rank 1) was associated with a significant improvement in OS compared with D + ADT (HR, 0.72; 95% CI, 0.55-0.95; rank 5) but not compared with AAP + ADT (rank 2), E + ADT (rank 3), or APA + ADT (rank 4). The AAP + D + ADT treatment (rank 1) was associated with significant PFS improvement in patients with high-volume disease compared with APA + ADT (HR, 0.54; 95% CI, 0.32-0.90; rank 4) and D + ADT (HR, 0.47; 95% CI, 0.30-0.73; rank 5). However, no significant improvement was observed with AAP + D + ADT compared with E + ADT (HR, 0.66; 95% CI, 0.39-1.13; rank 2) and AAP + ADT (HR, 0.62; 95% CI, 0.38-1.00; rank 3).
For patients with low-volume disease, AAP + D + ADT (rank 4) was not associated with statistically significant OS improvement compared with APA + ADT (HR, 1.45; 95% CI, 0.73-2.89; rank 1), AAP + ADT (HR, 1.27; 95% CI, 0.68-2.33; rank 2), E + ADT (HR, 1.14; 95% CI, 0.56-2.32; rank 3), or D + ADT (HR, 0.83; 0.50-1.38; rank 5). Similar results were observed regarding PFS improvement. Efficacy by volume of disease was not available for DARO + D + ADT. A summary of clinically important comparisons by volume of disease and timing of metastatic presentation is shown in Figure 1. The detailed results of these analyses are available in eFigures 56 to 80 in Supplement 1.
Figure 1. Forest Plot Summarizing Evidence for Overall Survival Across Clinically Relevant Subgroups: Volume of Disease and Timing of Metastatic Presentation.
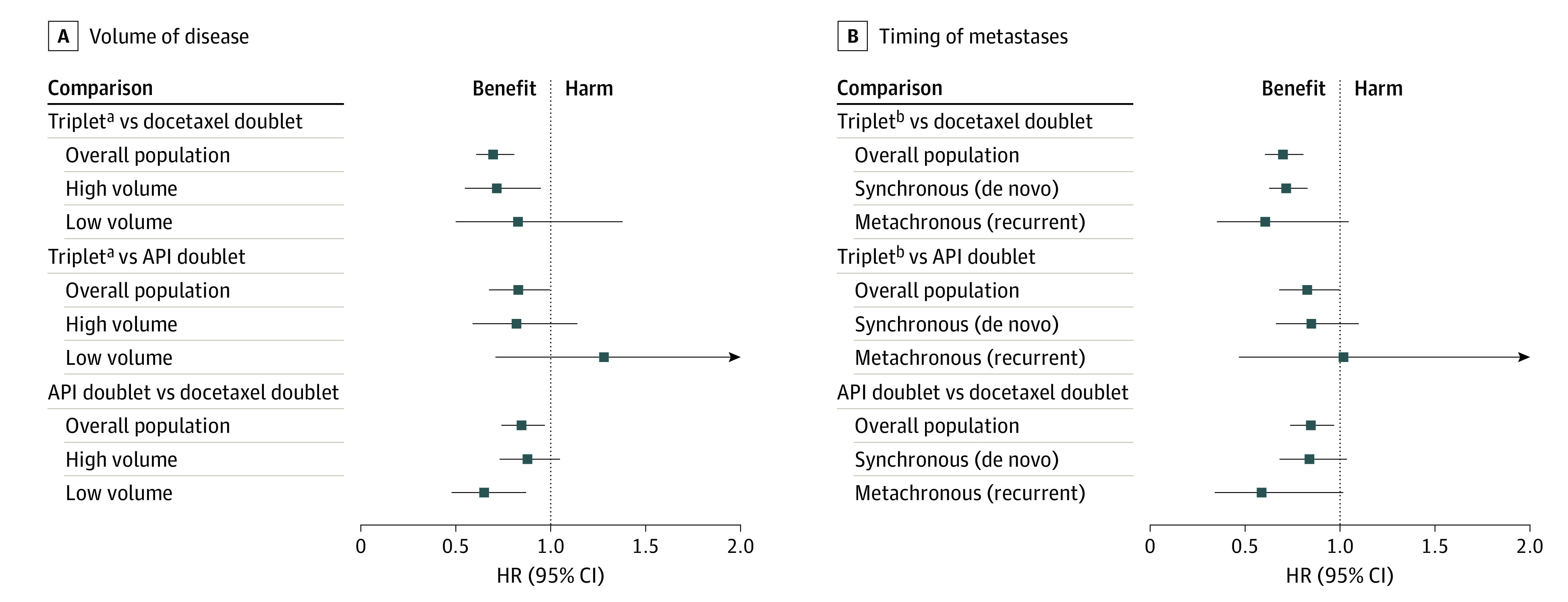
The forest plot summarizes relative effect (hazard ratios [HRs]) of different comparisons by volume of disease and timing of metastatic presentation derived from mixed treatment comparisons. An HR greater than 1 indicates potential clinical harm with the treatment; an HR less than 1 indicates potential clinical benefit with the treatment. 95% CIs crossing 1 indicate statistically nonsignificant results. API indicates androgen pathway inhibitors.
aTriplet therapy is abiraterone or darolutamide plus docetaxel and androgen deprivation therapy (ADT) in overall patient population. Triplet therapy is abiraterone plus docetaxel and ADT in high and low volume of disease.
bTriplet therapy is abiraterone or darolutamide plus docetaxel and ADT in overall patient population and in synchronous (de novo) metastases. Triplet therapy is darolutamide plus docetaxel and ADT in metachronous (recurrent) metastases.
Certainty of Evidence
Summaries of findings for pairwise comparisons between doublet therapy and ADT are provided in eTables 11 and 12 in Supplement 1. A summary of findings for mixed treatment comparisons in the overall patient population is outlined in Figure 2. Absolute estimates by volume of disease and timing of metastatic presentation were derived from subgroup data from trials and had low to moderate certainty of evidence (Figure 3; eTable 13 in Supplement 1).
Figure 2. Grading of Recommendations Assessment, Development and Evaluation Summary of Findings Table Outlining Certainty of Evidence and Absolute Risks With Triplet Therapy (Columns) Compared With Other Treatments (Rows) in Overall Patient Population.
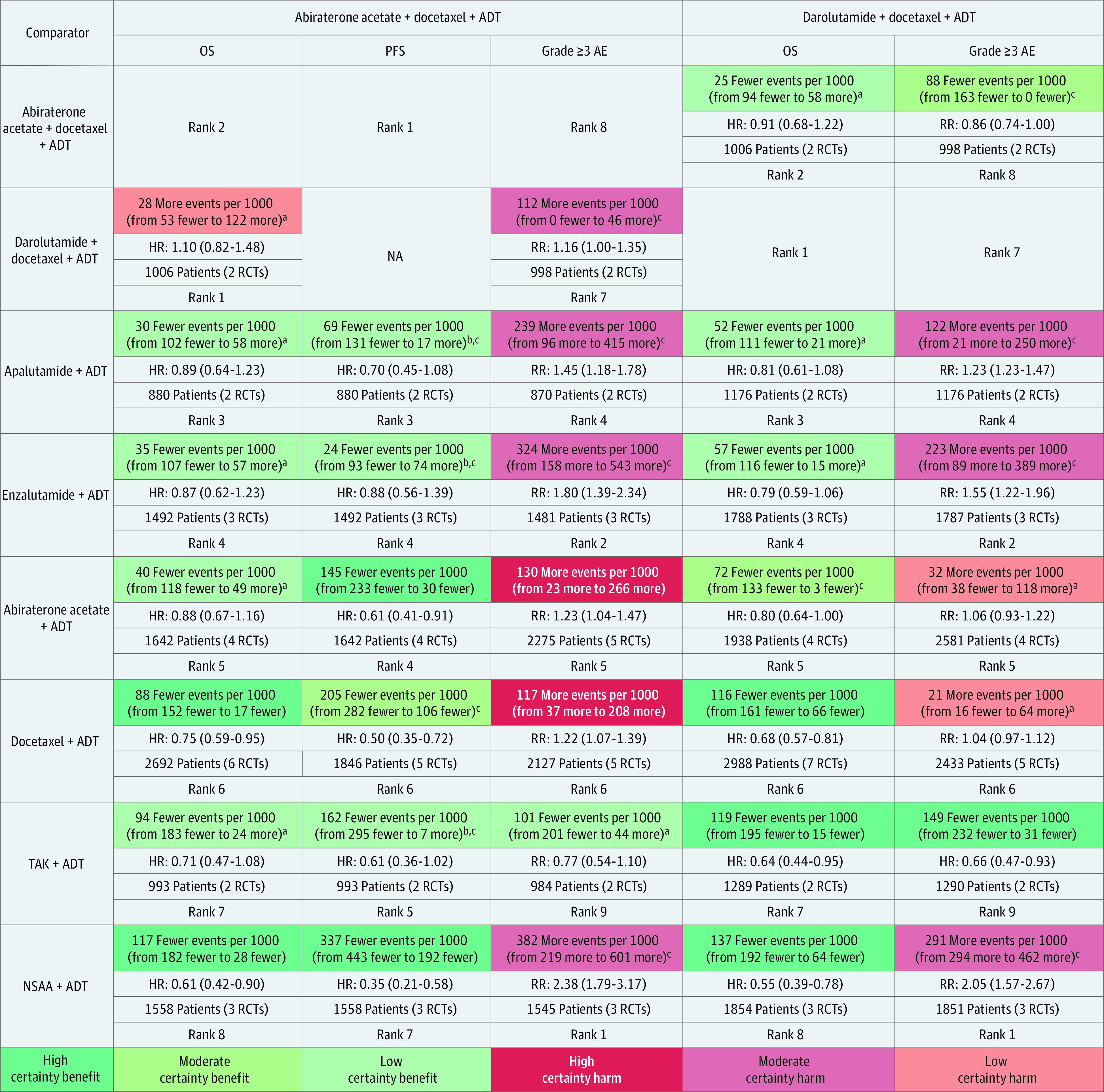
Summary of relative and absolute risks for mixed treatment comparisons derived from frequentist network meta-analysis using 4 levels of certainty: high (further research very unlikely to change confidence in estimate of effect), moderate (further research likely to have important association with confidence in estimate of effect and may change the estimate), low (further research very likely to have important association with confidence in estimate of effect and likely to change the estimate), and very low (very uncertain about the estimate). Values in cells indicate relative and absolute effect estimates for comparisons between triplet therapies (abiraterone and darolutamide triplet therapies) and other treatment options (androgen pathway inhibitor [API] doublet, docetaxel doublet, and androgen deprivation therapy [ADT]). Each comparison consists of absolute reduction in risk of events (upper cell) with triplet therapies compared with other treatments, relative effect hazard ratios (HRs; upper middle cell), sample size contributive to evidence (lower middle cell), and relative rank of treatment in a row (lower cell). AE indicates adverse event; NSAA, nonsteroidal antiandrogen; OS, overall survival; PFS, progression-free survival; RCTs, randomized clinical trials; RR, relative risk; and TAK, orteronel.
aRated down 2 levels owing to very serious imprecision, considering null effect and wide 95% CIs indicating both potential benefit and harm.
bRated down 1 level owing to serious imprecision, considering wide 95% CIs.
cRated down 1 level owing to serious indirectness in definition of PFS used across trials; PFS may be an advantageous end point for APIs owing to fixed dosing schedule of docetaxel compared with most API trials that used an indefinite dosing until disease progression.
Figure 3. Grading of Recommendations Assessment, Development and Evaluation Summary of Findings Table Outlining Certainty of Evidence and Absolute Risks With Triplet Therapy Compared With Other Treatments by Volume of Disease.
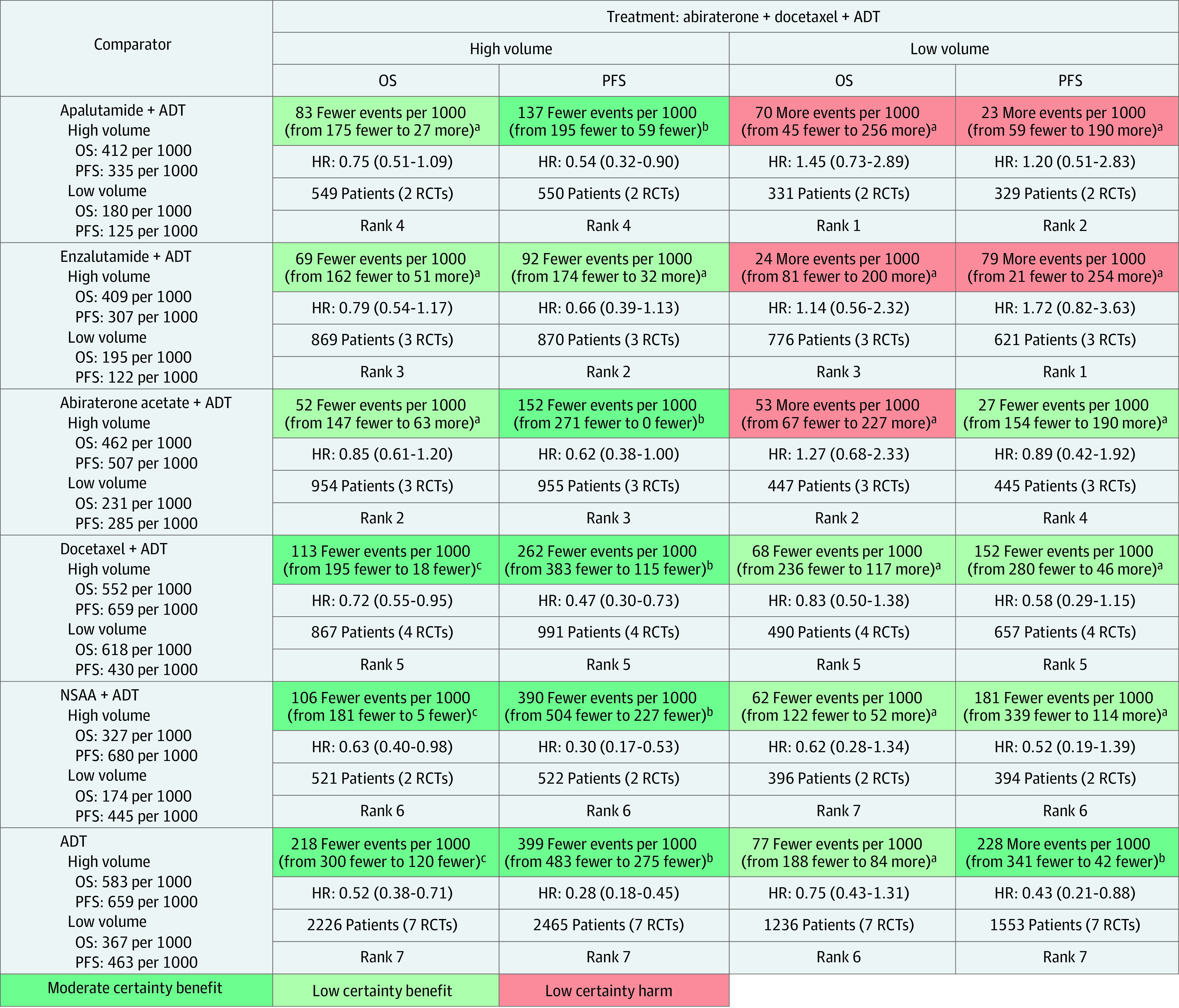
Summary of relative and absolute risks for mixed treatment comparisons derived from frequentist network meta-analysis using 4 levels of certainty: high (further research very unlikely to change confidence in the estimate of effect), moderate (further research likely to have important association with confidence in estimate of effect and may change the estimate), low (further research very likely to have important association with confidence in estimate of effect and likely to change the estimate), and very low (very uncertain about the estimate). Values in cells indicate relative and absolute effect estimates for comparisons between triplet therapy (abiraterone triplet) and other treatment options (androgen pathway inhibitor [API] doublet, docetaxel doublet, and androgen deprivation therapy [ADT]) by volume of disease. Each comparison consists of absolute reduction in risk of events (upper cell) with triplet therapy compared with other treatments, relative effect hazard ratios (HRs; upper middle cell), sample size contributive to evidence (lower middle cell), and relative rank of treatment in a row (lower cell). NSAA indicates nonsteroidal antiandrogen; OS, overall survival; PFS, progression-free survival; and RCTs, randomized clinical trials.
aRated down 2 levels owing to very serious imprecision, considering null effect, wide 95% CIs indicating both potential benefit and harm, and low sample size of less than 1000.
bRated down 1 level owing to serious indirectness in definition of PFS used across trials; PFS may be an advantageous end point for APIs owing to fixed dosing schedule of docetaxel compared with most API trials that used an indefinite dosing until disease progression.
cRated down 1 level owing to wide 95% CI.
Discussion
This report from a “living systematic” review presents the benefits and harms of contemporary treatment options with relative and absolute effect estimates across patient-important outcomes by volume of disease for patients with mCSPC. Using mixed treatment comparison methods, we provided the most up-to-date evidence for performance of triplet therapy compared with API-based doublets, a question not answered in clinical trials but paramount to clinical practice and design of future clinical trials. In the overall population, intensified treatment with triplet therapies improved OS compared with D + ADT but not compared with API doublets. These results remained consistent even after exclusion of patients who received D in the ENZAMET,3 ARCHES,1,25 and TITAN2,31 trials. Although triplet regimens offer promising efficacy, they do so at the expense of increased toxicity. The API doublets were ranked the safest treatment options in terms of the risk of grade 3 or higher adverse events. Our analysis also suggests that volume of disease and timing of metastatic presentation (synchronous vs metachronous) may offer insights into treatment selection for patients with mCSPC until more sophisticated and validated biomarkers become available (Figure 1).
The available evidence suggests that patients with high-volume disease appear to derive the greatest benefit from triplet therapy (AAP + D + ADT). Although data by volume of disease were not available from the ARASENS trial,9 the present analysis showed that triplet therapy with AAP + D + ADT may delay disease progression compared with D + ADT and API doublets, such as APA + ADT. For patients with low-volume disease, triplet therapy with AAP + D + ADT did not show a survival benefit compared with D + ADT and may perform worse compared with API-based doublets. Patients with low-volume disease derived greater treatment benefit with API doublets compared with D + ADT (Table 2; eFigure 34 in Supplement 1). The API-based doublets appear to be the most efficacious and least toxic treatment options in this patient population. Lack of benefit with D intensification in patients with low-volume disease is consistent with the combined analysis of the CHAARTED and GETUG-AFU15 trials, which showed that the addition of D to ADT had a consistent effect of improving survival among patients with high volume of disease but not among patients with low-volume disease.10 It is possible that volume is an effective surrogate for the underlying disease biology, with high-volume disease containing a higher proportion of patients from androgen receptor–independent populations, thus explaining the heterogeneity of the treatment effect. Alternatively, the small sample size in the D subgroup may have precluded statistical significance and the potential OS benefit for patients with low volume of disease owing to insufficient power.
Our analysis also suggests that the timing of metastatic presentation may be particularly important for patients presenting with low-volume disease. Patients with low-volume metachronous (recurrent) presentation are less likely to benefit from D doublets (eTable 8 and eFigures 42 and 43 in Supplement 1). Similarly, the lack of D benefit observed in patients with low-volume disease from the combined CHAARTED and GETUG-AFU15 trials’ analysis may have been associated with metachronous (recurrent) metastases in one-fourth of patients. In contrast, a post hoc analysis of group C of the STAMPEDE trial,28 which included 95% of the patients who had synchronous (de novo) metastases, found no evidence of a difference in survival between patients with high and patients with low metastatic volume. Taken together, these findings, along with the STOPCaP individual patient data meta-analysis,41 suggest that although D has a modest benefit in synchronous (de novo) low-volume metastases, the lack of any PFS or OS benefit indicates that the risks outweigh the benefits in low-volume metachronous (recurrent) presentation.
It remains important to critically analyze toxicity when considering treatment escalation. Sixteen of 1774 treatment-related deaths (0.90%) were observed in trials assessing the addition of D to ADT,6,7,27 and 34 of 1039 treatment-related deaths (3.27%) were observed in trials assessing the addition of D to API + ADT.8,9 These findings suggest caution with the use of D for patients with a low volume of disease who tend to live naturally longer, especially those with metachronous presentation with a median OS of 8 years with ADT alone.12 Our analysis showed that DARO + D + ADT, AAP + D + ADT, and D + ADT were ranked the least in terms of grade 3 or higher adverse events. Hence, it would be plausible to favor API doublets for older and less fit patients. However, the choice of an optimal API doublet may be guided by unique toxic effects and patient-level consideration (eTable 14 in Supplement 1). The reporting of health-related quality of life across the included trials is summarized in eTables 15 and 16 in Supplement 1. Health-related quality-of-life data are still emerging and will offer further guidance on treatment selection for patients with mCSPC. As treatment moves toward intensification with triplet therapy, even the use of doublet agents remains suboptimal in actual clinical practice. More than two-thirds of patients are being prescribed ADT only. In fact, the use of API doublets in clinical practice has been decreasing.42,43 This may be due to the cost and access to the intensified treatments. In terms of the US-based health care perspective, intensification with D is likely to be the most cost-effective treatment for the overall population with mCSPC. However, future cost-effectiveness analysis adjusting for volume of disease and timing of metastatic presentation is required and may offer additional insights.
Strengths and Limitations
Our approach to living systematic reviews represents the next generation of systematic reviews, enhanced by a semiautomated approach through a framework supported by advanced programming and artificial intelligence. Our website (not peer reviewed) is updated every Monday with the most contemporary evidence. The strengths of this study are highlighted in eTable 18 in Supplement 1. The present study is limited by a small number of trials with sparse direct comparative evidence and an open network that did not allow us to assess incoherence for most comparisons and precluded formal assessment of publication bias. Patients in the trials assessing D doublet therapies were less likely to receive subsequent life-prolonging API therapies (eTable 6 in Supplement 1). Moreover, there was variability in PFS definitions and inconsistent reporting of other patient-important end points across the included trials (eTable 17 in Supplement 1), different follow-up durations for treatments, limited efficacy data by volume of disease for triplet therapy, use of post hoc subgroup analyses in the included trials, and a lack of patient-level data that could potentially offer more granular estimates. Detailed discussion on limitations is provided in eTable 19 in Supplement 1.
Conclusions
The findings of this systematic review and meta-analysis indicate that the decision of treatment intensification with triplet therapy for patients with mCSPC must be considered carefully by accounting for the volume of disease, the timing of metastatic presentation, and API doublet options with significant survival benefit and fitness for chemotherapy. These findings provide direction for future clinical trials and suggest an equipoise to the question of how triplet regimens compare with API doublet combinations. In summary, triplet therapy may be preferred for fit patients with synchronous (de novo) high-volume disease. The API doublet combinations may be preferred for patients with metachronous (recurrent) low-volume disease (Figure 1). The choice of treatment with metachronous (recurrent) high-volume disease and synchronous (de novo) low-volume disease requires an individualized risk-based approach, including consideration of patient comorbidities. Evidence in this regard is rapidly increasing, and the results of this living meta-analysis will be updated as new data are published.
eAppendix. Detailed Search Strategy and Methodology
eFigure 1. PRISMA Flowchart Outlining the Process of Study Selection
eFigure 2. Risk of Bias for Included Trials Assessing Patient-Important Outcomes
eFigure 3. Forest Plot Showing Overall Survival in the Overall Patient Population
eFigure 4. Forest Plot Showing Progression-Free Survival in the Overall Patient Population
eFigure 5. Forest Plot Showing Adverse Events (Grade 3 or Higher)
eFigure 6. Forest Plot Showing Overall Survival in the Overall Patient Population (Excluding Patients Who Received Docetaxel in 3 Trials)
eFigure 7. Forest Plot Showing Progression-Free Survival in the Overall Patient Population (Excluding Patients Who Received Docetaxel in 3 Trials)
eFigure 8. Forest Plot Showing Overall Survival in Low-Volume Disease
eFigure 9. Forest Plot Showing Overall Survival in High-Volume Disease
eFigure 10. Forest Plot Showing Progression-Free Survival in Low-Volume Disease
eFigure 11. Forest Plot Showing Progression-Free Survival in High-Volume Disease
eFigure 12. Forest Plot Showing Overall Survival in Synchronous Disease
eFigure 13. Forest Plot Showing Overall Survival in Metachronous Disease
eFigure 14. Forest Plot Showing Progression-Free Survival in Synchronous Disease
eFigure 15. Forest Plot Showing Progression-Free Survival in Metachronous Disease
eFigure 16. Forest Plot Showing Overall Survival in Younger Patients
eFigure 17. Forest Plot Showing Overall Survival in Older Patients
eFigure 18. Forest Plot Showing Progression-Free Survival in Younger Patients
eFigure 19. Forest Plot Showing Progression-Free Survival in Older Patients
eFigure 20. Forest Plot Showing Overall Survival With Gleason Score 8 or Higher
eFigure 21. Forest Plot Showing Overall Survival With Gleason Score 8 or Lower
eFigure 22. Forest Plot Showing Progression-Free Survival With Gleason Score 8 or Higher
eFigure 23. Forest Plot Showing Progression-Free Survival With Gleason Score 8 or Lower
eFigure 24. Forest Plot Showing Overall Survival With Performance Status Score 0
eFigure 25. Forest Plot Showing Overall Survival With Performance Status Score ½
eFigure 26. Forest Plot Showing Progression-Free Survival With Performance Status Score 0
eFigure 27. Forest Plot Showing Progression-Free Survival With Performance Status Score ½
eFigure 28. Forest Plot Showing Sensitivity Analysis for Overall Survival Excluding GETUG Trial
eFigure 29. Forest Plot Showing Sensitivity Analysis for Progression-Free Survival Excluding GETUG Trial
eFigure 30. Subgroup Analysis for Overall Survival by Choice of Doublet Therapy
eFigure 31. Subgroup Analysis for Progression-Free Survival by Choice of Doublet Therapy
eFigure 32. Subgroup Analysis for Overall Survival by Volume of Disease
eFigure 33. Subgroup Analysis for Progression-Free Survival by Volume of Disease
eFigure 34. Subgroup Analysis for Overall Survival in Low Volume by Choice of Doublet Therapy
eFigure 35. Subgroup Analysis for Overall Survival in High Volume by Choice of Doublet Therapy
eFigure 36. Subgroup Analysis for Progression-Free Survival in Low Volume by Choice of Doublet Therapy
eFigure 37. Subgroup Analysis for Progression-Free Survival in High Volume by Choice of Doublet Therapy
eFigure 38. Subgroup Analysis for Overall Survival by Mode of Metastatic Presentation
eFigure 39. Subgroup Analysis for Overall Survival in Synchronous Metastases by Choice of Doublet Therapy
eFigure 40. Subgroup Analysis for Overall Survival in Metachronous Metastases by Choice of Doublet Therapy
eFigure 41. Subgroup Analysis for Progression-Free Survival by Mode of Metastatic Presentation
eFigure 42. Subgroup Analysis for Overall Survival With Docetaxel Doublet Between High-Volume and Low-Volume Synchronous Disease
eFigure 43. Subgroup Analysis for Overall Survival With Docetaxel Doublet Between High-Volume and Low-Volume Metachronous Disease
eFigure 44. Subgroup Analysis for Overall Survival by Gleason Score
eFigure 45. Subgroup Analysis for Progression-Free Survival by Gleason Score
eFigure 46. Subgroup Analysis for Overall Survival by Performance Status Score
eFigure 47. Subgroup Analysis for Progression-Free Survival by Performance Status Score
eFigure 48. Subgroup Analyses for Overall Survival Excluding GETUG Trial
eFigure 49. Subgroup Analyses for Progression-Free Survival Excluding GETUG Trial
eFigure 50. Network Plots for Patient-Important Outcomes in Overall Population and Contemporary Subgroups
eFigure 51. Mixed Treatment Comparisons for Overall Survival in the Overall Patient Population
eFigure 52. Mixed Treatment Comparisons for Progression-Free Survival in the Overall Patient Population
eFigure 53. Mixed Treatment Comparisons for Adverse Events (Grade 3 or Higher) in the Overall Patient Population
eFigure 54. Mixed Treatment Comparisons for Overall Survival in the Overall Patient Population (Excluding Patients Who Received Docetaxel in 3 Trials)
eFigure 55. Mixed Treatment Comparisons for Progression-Free Survival in the Overall Patient Population (Excluding Patients Who Received Docetaxel in 3 Trials)
eFigure 56. Mixed Treatment Comparisons for Overall Survival in Low-Volume Disease
eFigure 57. Mixed Treatment Comparisons for Overall Survival in High-Volume Disease
eFigure 58. Mixed Treatment Comparisons for Progression-Free Survival in Low-Volume Disease
eFigure 59. Mixed Treatment Comparisons for Progression-Free Survival in High-Volume Disease
eFigure 60. Mixed Treatment Comparisons for Overall Survival in Synchronous Disease
eFigure 61. Mixed Treatment Comparisons for Overall Survival in Metachronous Disease
eFigure 62. Mixed Treatment Comparisons for Progression-Free Survival in Synchronous Disease
eFigure 63. Mixed Treatment Comparisons for Progression-Free Survival in Metachronous Disease
eFigure 64. Mixed Treatment Comparisons for Overall Survival in Younger Patients
eFigure 65. Mixed Treatment Comparisons for Overall Survival in Older Patients
eFigure 66. Mixed Treatment Comparisons for Progression-Free Survival in Younger Patients
eFigure 67. Mixed Treatment Comparisons for Progression-Free Survival in Older Patients
eFigure 68. Mixed Treatment Comparisons for Overall Survival With Gleason Score 8 or Higher
eFigure 69. Mixed Treatment Comparisons for Overall Survival With Gleason Score 8 or Lower
eFigure 70. Mixed Treatment Comparisons for Progression-Free Survival With Gleason Score 8 or Higher
eFigure 71. Mixed Treatment Comparisons for Progression-Free Survival With Gleason Score 8 or Lower
eFigure 72. Mixed Treatment Comparisons for Overall Survival With Performance Status Score 0
eFigure 73. Mixed Treatment Comparisons for Overall Survival With Performance Status Score ½
eFigure 74. Mixed Treatment Comparisons for Progression-Free Survival With Performance Status Score 0
eFigure 75. Mixed Treatment Comparisons for Progression-Free Survival With Performance Status Score ½
eFigure 76. Mixed Treatment Comparisons for Overall Survival in the Overall Population and High and Low Volume of Disease Excluding the GETUG Trial
eFigure 77. Mixed Treatment Comparisons for Overall Survival in Older and Younger Patients and Gleason Score 8 or Higher and Lower Than 8 Excluding the GETUG Trial
eFigure 78. Mixed Treatment Comparisons for Progression-Free Survival in the Overall Population and High and Low Volume of Disease Excluding the GETUG Trial
eFigure 79. Mixed Treatment Comparisons for Overall Survival Using Subgroup Data (Docetaxel and Nondocetaxel) From the PEACE-1 and ENZAMET Trials
eFigure 80. Mixed Treatment Comparisons for Progression-Free Survival Using Subgroup Data (Docetaxel and Nondocetaxel) From the PEACE-1 and ENZAMET Trials
eTable 1. Outcome Definitions in Included Clinical Trials
eTable 2. Overall Survival and Progression-Free Survival by Receipt of Docetaxel in the ENZAMET, ARCHES, and TITAN Trials
eTable 3. Proportions of Patients by Volume of Disease and Timing of Metastatic Presentation in Included Trials
eTable 4. Overall Survival Rate by Volume of Disease and Timing of Metastatic Presentation in Included Trials
eTable 5. Summary of Additional Trial and Population Characteristics
eTable 6. Summary of Subsequent Therapy Across the Included Trials
eTable 7. Overall Survival in Patients Receiving Doublet Therapy (API or Docetaxel) Stratified by Volume of Disease and Timing of Metastatic Presentation
eTable 8. Overall Survival With Docetaxel Doublet Therapy in Patients With High-Volume Disease and Low-Volume Synchronous and Metachronous Presentation
eTable 9. Progression-Free Survival With Doublet Therapy (API or Docetaxel) Compared With ADT by Clinically Relevant Subgroups
eTable 10. Survival Outcomes With Doublet Therapy (API or Docetaxel) Compared With ADT by Additional Subgroups of Interest
eTable 11. GRADE Summary of Findings Table Outlining Certainty of Evidence and Absolute Risks With Doublet Therapy Compared With ADT Alone in the Overall Patient Population
eTable 12. GRADE Summary of Findings Table Outlining Certainty of Evidence and Absolute Risks With Doublet Therapy Compared With ADT Alone in Clinically Relevant Prognostic Subgroups
eTable 13. GRADE Summary of Findings Table Outlining Certainty of Evidence and Absolute Risks With Triplet Therapy Compared With Other Treatments by Timing of Metastatic Presentation
eTable 14. Adverse Events and Patient-Level Considerations for Androgen Pathway Inhibitors (API) in Patients With mCSPC
eTable 15. Reporting Matrix Outlining the Heterogeneity in Health-Related Quality-of-Life Assessment in Included Trials
eTable 16. Summary of the Quality of Life With Contemporary Systemic Therapies in Patients With mCSPC
eTable 17. Reporting Matrix for Outcomes Assessed in Included Trials
eTable 18. Strengths
eTable 19. Limitations
Data Sharing Statement
References
- 1.Armstrong AJ, Szmulewitz RZ, Petrylak DP, et al. ARCHES: a randomized, phase III study of androgen deprivation therapy with enzalutamide or placebo in men with metastatic hormone-sensitive prostate cancer. J Clin Oncol. 2019;37(32):2974-2986. doi: 10.1200/JCO.19.00799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chi KN, Agarwal N, Bjartell A, et al. ; TITAN Investigators . Apalutamide for metastatic, castration-sensitive prostate cancer. N Engl J Med. 2019;381(1):13-24. doi: 10.1056/NEJMoa1903307 [DOI] [PubMed] [Google Scholar]
- 3.Davis ID, Martin AJ, Stockler MR, et al. ; ENZAMET Trial Investigators and the Australian and New Zealand Urogenital and Prostate Cancer Trials Group . Enzalutamide with standard first-line therapy in metastatic prostate cancer. N Engl J Med. 2019;381(2):121-131. doi: 10.1056/NEJMoa1903835 [DOI] [PubMed] [Google Scholar]
- 4.James ND, de Bono JS, Spears MR, et al. ; STAMPEDE Investigators . Abiraterone for prostate cancer not previously treated with hormone therapy. N Engl J Med. 2017;377(4):338-351. doi: 10.1056/NEJMoa1702900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fizazi K, Tran N, Fein L, et al. ; LATITUDE Investigators . Abiraterone plus prednisone in metastatic, castration-sensitive prostate cancer. N Engl J Med. 2017;377(4):352-360. doi: 10.1056/NEJMoa1704174 [DOI] [PubMed] [Google Scholar]
- 6.James ND, Sydes MR, Clarke NW, et al. ; STAMPEDE Investigators . Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet. 2016;387(10024):1163-1177. doi: 10.1016/S0140-6736(15)01037-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sweeney CJ, Chen YH, Carducci M, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N Engl J Med. 2015;373(8):737-746. doi: 10.1056/NEJMoa1503747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fizazi K, Foulon S, Carles J, et al. ; PEACE-1 Investigators . Abiraterone plus prednisone added to androgen deprivation therapy and docetaxel in de novo metastatic castration-sensitive prostate cancer (PEACE-1): a multicentre, open-label, randomised, phase 3 study with a 2 × 2 factorial design. Lancet. 2022;399(10336):1695-1707. doi: 10.1016/S0140-6736(22)00367-1 [DOI] [PubMed] [Google Scholar]
- 9.Smith MR, Hussain M, Saad F, et al. ; ARASENS Trial Investigators . Darolutamide and survival in metastatic, hormone-sensitive prostate cancer. N Engl J Med. 2022;386(12):1132-1142. doi: 10.1056/NEJMoa2119115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gravis G, Boher JM, Chen YH, et al. Burden of metastatic castrate naive prostate cancer patients, to identify men more likely to benefit from early docetaxel: further analyses of CHAARTED and GETUG-AFU15 studies. Eur Urol. 2018;73(6):847-855. doi: 10.1016/j.eururo.2018.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kyriakopoulos CE, Chen YH, Carducci MA, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer: long-term survival analysis of the randomized phase III E3805 CHAARTED trial. J Clin Oncol. 2018;36(11):1080-1087. doi: 10.1200/JCO.2017.75.3657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Riaz IB, Sweeney CJ. The role of chemotherapy in metastatic prostate cancer. Curr Opin Urol. 2022;32(3):292-301. doi: 10.1097/MOU.0000000000000985 [DOI] [PubMed] [Google Scholar]
- 13.Riaz IBHH, He H, Naqvi SAA, Murad MH, Liu H. A living interactive systematic review and network meta-analysis on first-line treatment options in metastatic castration sensitive prostate cancer. Living Interactive Systematic Reviews. Accessed July 18, 2022. https://mcspc.living-evidence.com/
- 14.Hutton B, Salanti G, Caldwell DM, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162(11):777-784. doi: 10.7326/M14-2385 [DOI] [PubMed] [Google Scholar]
- 15.Naqvi SAA. A living interactive systematic review and network meta-analysis for first-line systemic treatment options for metastatic castration sensitive prostate cancer (mCSPC). OSF Registries. Accessed July 18, 2022. https://osf.io/e2q3w [DOI] [PMC free article] [PubMed]
- 16.Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898 [DOI] [PubMed] [Google Scholar]
- 17.Chaimani A, Caldwell DM, Li T, Higgins JP, Salanti G. Undertaking network meta-analyses. In: Higgins JPT, Thomas J, Chandler J, et al, eds. Cochrane Handbook for Systematic Reviews of Interventions. Wiley; 2019:285-320. [Google Scholar]
- 18.White IR, Barrett JK, Jackson D, Higgins JP. Consistency and inconsistency in network meta-analysis: model estimation using multivariate meta-regression. Res Synth Methods. 2012;3(2):111-125. doi: 10.1002/jrsm.1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brignardello-Petersen R, Murad MH, Walter SD, et al. ; GRADE Working Group . GRADE approach to rate the certainty from a network meta-analysis: avoiding spurious judgments of imprecision in sparse networks. J Clin Epidemiol. 2019;105:60-67. doi: 10.1016/j.jclinepi.2018.08.022 [DOI] [PubMed] [Google Scholar]
- 20.Rücker G, Schwarzer G. Ranking treatments in frequentist network meta-analysis works without resampling methods. BMC Med Res Methodol. 2015;15(1):58. doi: 10.1186/s12874-015-0060-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Balshem H, Helfand M, Schünemann HJ, et al. GRADE guidelines: 3, rating the quality of evidence. J Clin Epidemiol. 2011;64(4):401-406. doi: 10.1016/j.jclinepi.2010.07.015 [DOI] [PubMed] [Google Scholar]
- 22.Brignardello-Petersen R, Bonner A, Alexander PE, et al. ; GRADE Working Group . Advances in the GRADE approach to rate the certainty in estimates from a network meta-analysis. J Clin Epidemiol. 2018;93:36-44. doi: 10.1016/j.jclinepi.2017.10.005 [DOI] [PubMed] [Google Scholar]
- 23.Fizazi K, Tran N, Fein L, et al. Abiraterone acetate plus prednisone in patients with newly diagnosed high-risk metastatic castration-sensitive prostate cancer (LATITUDE): final overall survival analysis of a randomised, double-blind, phase 3 trial. Lancet Oncol. 2019;20(5):686-700. doi: 10.1016/S1470-2045(19)30082-8 [DOI] [PubMed] [Google Scholar]
- 24.Agarwal N, Tangen CM, Hussain MHA, et al. Orteronel for metastatic hormone-sensitive prostate cancer: a multicenter, randomized, open-label phase III trial (SWOG-1216). J Clin Oncol. 2022;40(28):3301-3309. doi: 10.1200/JCO.21.02517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Armstrong AJ, Azad AA, Iguchi T, et al. Improved survival with enzalutamide in patients with metastatic hormone-sensitive prostate cancer. J Clin Oncol. 2022;40(15):1616-1622. doi: 10.1200/JCO.22.00193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gravis G, Boher JM, Joly F, et al. ; GETUG . Androgen deprivation therapy (ADT) plus docetaxel versus ADT alone in metastatic non castrate prostate cancer: impact of metastatic burden and long-term survival analysis of the randomized phase 3 GETUG-AFU15 trial. Eur Urol. 2016;70(2):256-262. doi: 10.1016/j.eururo.2015.11.005 [DOI] [PubMed] [Google Scholar]
- 27.Gravis G, Fizazi K, Joly F, et al. Androgen-deprivation therapy alone or with docetaxel in non-castrate metastatic prostate cancer (GETUG-AFU 15): a randomised, open-label, phase 3 trial. Lancet Oncol. 2013;14(2):149-158. doi: 10.1016/S1470-2045(12)70560-0 [DOI] [PubMed] [Google Scholar]
- 28.Clarke NW, Ali A, Ingleby FC, et al. Addition of docetaxel to hormonal therapy in low- and high-burden metastatic hormone sensitive prostate cancer: long-term survival results from the STAMPEDE trial. Ann Oncol. 2019;30(12):1992-2003. doi: 10.1093/annonc/mdz396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoyle AP, Ali A, James ND, et al. ; STAMPEDE Investigators . Abiraterone in “high-” and “low-risk” metastatic hormone-sensitive prostate cancer. Eur Urol. 2019;76(6):719-728. doi: 10.1016/j.eururo.2019.08.006 [DOI] [PubMed] [Google Scholar]
- 30.Sydes MR, Spears MR, Mason MD, et al. ; STAMPEDE Investigators . Adding abiraterone or docetaxel to long-term hormone therapy for prostate cancer: directly randomised data from the STAMPEDE multi-arm, multi-stage platform protocol. Ann Oncol. 2018;29(5):1235-1248. doi: 10.1093/annonc/mdy072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chi KN, Chowdhury S, Bjartell A, et al. Final analysis results from TITAN: a phase III study of apalutamide (APA) versus placebo (PBO) in patients (pts) with metastatic castration-sensitive prostate cancer (mCSPC) receiving androgen deprivation therapy (ADT). J Clin Oncol. 2021;39(6)(suppl):11. doi: 10.1200/JCO.2021.39.6_suppl.11 [DOI] [Google Scholar]
- 32.Rush HL, Murphy L, Morgans AK, et al. Quality of life in men with prostate cancer randomly allocated to receive docetaxel or abiraterone in the STAMPEDE trial. J Clin Oncol. 2022;40(8):825-836. doi: 10.1200/JCO.21.00728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morgans AK, Chen YH, Sweeney CJ, et al. Quality of life during treatment with chemohormonal therapy: analysis of E3805 chemohormonal androgen ablation randomized trial in prostate cancer. J Clin Oncol. 2018;36(11):1088-1095. doi: 10.1200/JCO.2017.75.3335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Patrick-Miller LJ, Chen YH, Carducci MA, et al. Quality of life (QOL) analysis from CHAARTED: Chemohormonal androgen ablation randomized trial in prostate cancer (E3805). J Clin Oncol. 2016;34(15)(suppl):5004. doi: 10.1200/JCO.2016.34.15_suppl.5004 [DOI] [Google Scholar]
- 35.Chi KN, Protheroe A, Rodríguez-Antolín A, et al. Patient-reported outcomes following abiraterone acetate plus prednisone added to androgen deprivation therapy in patients with newly diagnosed metastatic castration-naive prostate cancer (LATITUDE): an international, randomised phase 3 trial. Lancet Oncol. 2018;19(2):194-206. doi: 10.1016/S1470-2045(17)30911-7 [DOI] [PubMed] [Google Scholar]
- 36.Stockler MR, Martin AJ, Dhillon H, et al. Health-related quality of life (HRQL) in a randomized phase III trial of enzalutamide with standard first-line therapy for metastatic, hormone-sensitive prostate cancer (mHSPC): ENZAMET (ANZUP 1304), an ANZUP-led, international, co-operative group trial. Ann Oncol. 2019;30:v886-v887. doi: 10.1093/annonc/mdz394.046 [DOI] [Google Scholar]
- 37.Agarwal N, McQuarrie K, Bjartell A, et al. ; TITAN Investigators . Health-related quality of life after apalutamide treatment in patients with metastatic castration-sensitive prostate cancer (TITAN): a randomised, placebo-controlled, phase 3 study. Lancet Oncol. 2019;20(11):1518-1530. doi: 10.1016/S1470-2045(19)30620-5 [DOI] [PubMed] [Google Scholar]
- 38.Stenzl A, Dunshee C, De Giorgi U, et al. Effect of enzalutamide plus androgen deprivation therapy on health-related quality of life in patients with metastatic hormone-sensitive prostate cancer: an analysis of the ARCHES randomised, placebo-controlled, phase 3 study. Eur Urol. 2020;78(4):603-614. doi: 10.1016/j.eururo.2020.03.019 [DOI] [PubMed] [Google Scholar]
- 39.James ND, Clarke NW, Cook A, et al. ; STAMPEDE Trials Collaborative Group . Abiraterone acetate plus prednisolone for metastatic patients starting hormone therapy: 5-year follow-up results from the STAMPEDE randomised trial (NCT00268476). Int J Cancer. 2022;151(3):422-434. doi: 10.1002/ijc.34018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Davis ID, Martin AJ, Zielinski RR, et al. Updated overall survival outcomes in ENZAMET (ANZUP 1304), an international, cooperative group trial of enzalutamide in metastatic hormone-sensitive prostate cancer (mHSPC). J Clin Oncol. 2022;40(17)(suppl):LBA5004. doi: 10.1200/JCO.2022.40.17_suppl.LBA5004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vale CL, Fisher D, Godolphin P, et al. Defining more precisely the effects of docetaxel plus ADT for men with mHSPC: meta-analysis of individual participant data from randomized trials. J Clin Oncol. 2022;40(16)(suppl):5070. doi: 10.1200/JCO.2022.40.16_suppl.5070 [DOI] [Google Scholar]
- 42.Freedland SJ, Agarwal N, Ramaswamy K, et al. Real-world utilization of advanced therapies and racial disparity among patients with metastatic castration-sensitive prostate cancer (mCSPC): a Medicare database analysis. J Clin Oncol. 2021;39(15)(suppl):5073. doi: 10.1200/JCO.2021.39.15_suppl.5073 [DOI] [Google Scholar]
- 43.Swami U, Hong A, El-Chaar NN, et al. Real-world first-line (1L) treatment patterns in patients (pts) with metastatic castration-sensitive prostate cancer (mCSPC) in a US health insurance database. J Clin Oncol. 2021;39(15)(suppl):5072. doi: 10.1200/JCO.2021.39.15_suppl.5072 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix. Detailed Search Strategy and Methodology
eFigure 1. PRISMA Flowchart Outlining the Process of Study Selection
eFigure 2. Risk of Bias for Included Trials Assessing Patient-Important Outcomes
eFigure 3. Forest Plot Showing Overall Survival in the Overall Patient Population
eFigure 4. Forest Plot Showing Progression-Free Survival in the Overall Patient Population
eFigure 5. Forest Plot Showing Adverse Events (Grade 3 or Higher)
eFigure 6. Forest Plot Showing Overall Survival in the Overall Patient Population (Excluding Patients Who Received Docetaxel in 3 Trials)
eFigure 7. Forest Plot Showing Progression-Free Survival in the Overall Patient Population (Excluding Patients Who Received Docetaxel in 3 Trials)
eFigure 8. Forest Plot Showing Overall Survival in Low-Volume Disease
eFigure 9. Forest Plot Showing Overall Survival in High-Volume Disease
eFigure 10. Forest Plot Showing Progression-Free Survival in Low-Volume Disease
eFigure 11. Forest Plot Showing Progression-Free Survival in High-Volume Disease
eFigure 12. Forest Plot Showing Overall Survival in Synchronous Disease
eFigure 13. Forest Plot Showing Overall Survival in Metachronous Disease
eFigure 14. Forest Plot Showing Progression-Free Survival in Synchronous Disease
eFigure 15. Forest Plot Showing Progression-Free Survival in Metachronous Disease
eFigure 16. Forest Plot Showing Overall Survival in Younger Patients
eFigure 17. Forest Plot Showing Overall Survival in Older Patients
eFigure 18. Forest Plot Showing Progression-Free Survival in Younger Patients
eFigure 19. Forest Plot Showing Progression-Free Survival in Older Patients
eFigure 20. Forest Plot Showing Overall Survival With Gleason Score 8 or Higher
eFigure 21. Forest Plot Showing Overall Survival With Gleason Score 8 or Lower
eFigure 22. Forest Plot Showing Progression-Free Survival With Gleason Score 8 or Higher
eFigure 23. Forest Plot Showing Progression-Free Survival With Gleason Score 8 or Lower
eFigure 24. Forest Plot Showing Overall Survival With Performance Status Score 0
eFigure 25. Forest Plot Showing Overall Survival With Performance Status Score ½
eFigure 26. Forest Plot Showing Progression-Free Survival With Performance Status Score 0
eFigure 27. Forest Plot Showing Progression-Free Survival With Performance Status Score ½
eFigure 28. Forest Plot Showing Sensitivity Analysis for Overall Survival Excluding GETUG Trial
eFigure 29. Forest Plot Showing Sensitivity Analysis for Progression-Free Survival Excluding GETUG Trial
eFigure 30. Subgroup Analysis for Overall Survival by Choice of Doublet Therapy
eFigure 31. Subgroup Analysis for Progression-Free Survival by Choice of Doublet Therapy
eFigure 32. Subgroup Analysis for Overall Survival by Volume of Disease
eFigure 33. Subgroup Analysis for Progression-Free Survival by Volume of Disease
eFigure 34. Subgroup Analysis for Overall Survival in Low Volume by Choice of Doublet Therapy
eFigure 35. Subgroup Analysis for Overall Survival in High Volume by Choice of Doublet Therapy
eFigure 36. Subgroup Analysis for Progression-Free Survival in Low Volume by Choice of Doublet Therapy
eFigure 37. Subgroup Analysis for Progression-Free Survival in High Volume by Choice of Doublet Therapy
eFigure 38. Subgroup Analysis for Overall Survival by Mode of Metastatic Presentation
eFigure 39. Subgroup Analysis for Overall Survival in Synchronous Metastases by Choice of Doublet Therapy
eFigure 40. Subgroup Analysis for Overall Survival in Metachronous Metastases by Choice of Doublet Therapy
eFigure 41. Subgroup Analysis for Progression-Free Survival by Mode of Metastatic Presentation
eFigure 42. Subgroup Analysis for Overall Survival With Docetaxel Doublet Between High-Volume and Low-Volume Synchronous Disease
eFigure 43. Subgroup Analysis for Overall Survival With Docetaxel Doublet Between High-Volume and Low-Volume Metachronous Disease
eFigure 44. Subgroup Analysis for Overall Survival by Gleason Score
eFigure 45. Subgroup Analysis for Progression-Free Survival by Gleason Score
eFigure 46. Subgroup Analysis for Overall Survival by Performance Status Score
eFigure 47. Subgroup Analysis for Progression-Free Survival by Performance Status Score
eFigure 48. Subgroup Analyses for Overall Survival Excluding GETUG Trial
eFigure 49. Subgroup Analyses for Progression-Free Survival Excluding GETUG Trial
eFigure 50. Network Plots for Patient-Important Outcomes in Overall Population and Contemporary Subgroups
eFigure 51. Mixed Treatment Comparisons for Overall Survival in the Overall Patient Population
eFigure 52. Mixed Treatment Comparisons for Progression-Free Survival in the Overall Patient Population
eFigure 53. Mixed Treatment Comparisons for Adverse Events (Grade 3 or Higher) in the Overall Patient Population
eFigure 54. Mixed Treatment Comparisons for Overall Survival in the Overall Patient Population (Excluding Patients Who Received Docetaxel in 3 Trials)
eFigure 55. Mixed Treatment Comparisons for Progression-Free Survival in the Overall Patient Population (Excluding Patients Who Received Docetaxel in 3 Trials)
eFigure 56. Mixed Treatment Comparisons for Overall Survival in Low-Volume Disease
eFigure 57. Mixed Treatment Comparisons for Overall Survival in High-Volume Disease
eFigure 58. Mixed Treatment Comparisons for Progression-Free Survival in Low-Volume Disease
eFigure 59. Mixed Treatment Comparisons for Progression-Free Survival in High-Volume Disease
eFigure 60. Mixed Treatment Comparisons for Overall Survival in Synchronous Disease
eFigure 61. Mixed Treatment Comparisons for Overall Survival in Metachronous Disease
eFigure 62. Mixed Treatment Comparisons for Progression-Free Survival in Synchronous Disease
eFigure 63. Mixed Treatment Comparisons for Progression-Free Survival in Metachronous Disease
eFigure 64. Mixed Treatment Comparisons for Overall Survival in Younger Patients
eFigure 65. Mixed Treatment Comparisons for Overall Survival in Older Patients
eFigure 66. Mixed Treatment Comparisons for Progression-Free Survival in Younger Patients
eFigure 67. Mixed Treatment Comparisons for Progression-Free Survival in Older Patients
eFigure 68. Mixed Treatment Comparisons for Overall Survival With Gleason Score 8 or Higher
eFigure 69. Mixed Treatment Comparisons for Overall Survival With Gleason Score 8 or Lower
eFigure 70. Mixed Treatment Comparisons for Progression-Free Survival With Gleason Score 8 or Higher
eFigure 71. Mixed Treatment Comparisons for Progression-Free Survival With Gleason Score 8 or Lower
eFigure 72. Mixed Treatment Comparisons for Overall Survival With Performance Status Score 0
eFigure 73. Mixed Treatment Comparisons for Overall Survival With Performance Status Score ½
eFigure 74. Mixed Treatment Comparisons for Progression-Free Survival With Performance Status Score 0
eFigure 75. Mixed Treatment Comparisons for Progression-Free Survival With Performance Status Score ½
eFigure 76. Mixed Treatment Comparisons for Overall Survival in the Overall Population and High and Low Volume of Disease Excluding the GETUG Trial
eFigure 77. Mixed Treatment Comparisons for Overall Survival in Older and Younger Patients and Gleason Score 8 or Higher and Lower Than 8 Excluding the GETUG Trial
eFigure 78. Mixed Treatment Comparisons for Progression-Free Survival in the Overall Population and High and Low Volume of Disease Excluding the GETUG Trial
eFigure 79. Mixed Treatment Comparisons for Overall Survival Using Subgroup Data (Docetaxel and Nondocetaxel) From the PEACE-1 and ENZAMET Trials
eFigure 80. Mixed Treatment Comparisons for Progression-Free Survival Using Subgroup Data (Docetaxel and Nondocetaxel) From the PEACE-1 and ENZAMET Trials
eTable 1. Outcome Definitions in Included Clinical Trials
eTable 2. Overall Survival and Progression-Free Survival by Receipt of Docetaxel in the ENZAMET, ARCHES, and TITAN Trials
eTable 3. Proportions of Patients by Volume of Disease and Timing of Metastatic Presentation in Included Trials
eTable 4. Overall Survival Rate by Volume of Disease and Timing of Metastatic Presentation in Included Trials
eTable 5. Summary of Additional Trial and Population Characteristics
eTable 6. Summary of Subsequent Therapy Across the Included Trials
eTable 7. Overall Survival in Patients Receiving Doublet Therapy (API or Docetaxel) Stratified by Volume of Disease and Timing of Metastatic Presentation
eTable 8. Overall Survival With Docetaxel Doublet Therapy in Patients With High-Volume Disease and Low-Volume Synchronous and Metachronous Presentation
eTable 9. Progression-Free Survival With Doublet Therapy (API or Docetaxel) Compared With ADT by Clinically Relevant Subgroups
eTable 10. Survival Outcomes With Doublet Therapy (API or Docetaxel) Compared With ADT by Additional Subgroups of Interest
eTable 11. GRADE Summary of Findings Table Outlining Certainty of Evidence and Absolute Risks With Doublet Therapy Compared With ADT Alone in the Overall Patient Population
eTable 12. GRADE Summary of Findings Table Outlining Certainty of Evidence and Absolute Risks With Doublet Therapy Compared With ADT Alone in Clinically Relevant Prognostic Subgroups
eTable 13. GRADE Summary of Findings Table Outlining Certainty of Evidence and Absolute Risks With Triplet Therapy Compared With Other Treatments by Timing of Metastatic Presentation
eTable 14. Adverse Events and Patient-Level Considerations for Androgen Pathway Inhibitors (API) in Patients With mCSPC
eTable 15. Reporting Matrix Outlining the Heterogeneity in Health-Related Quality-of-Life Assessment in Included Trials
eTable 16. Summary of the Quality of Life With Contemporary Systemic Therapies in Patients With mCSPC
eTable 17. Reporting Matrix for Outcomes Assessed in Included Trials
eTable 18. Strengths
eTable 19. Limitations
Data Sharing Statement


