Abstract
During the treatment of a patient on hemodialysis with severe coronavirus disease 2019 (COVID-19), the patient was weaned from extracorporeal membrane oxygenation, which was used to treat severe COVID-19 pneumonia. However, the patient’s condition worsened after the peak infection phase of COVID-19 because of acute respiratory distress syndrome with suspected hemophagocytic lymphohistiocytosis (HLH). After a bone marrow biopsy confirmed the diagnosis, methylprednisolone pulse therapy, followed by combination therapy (including oral prednisolone and cyclosporine) was immediately administered, and the patient survived. Because HLH can occur a month or more after the onset of COVID-19, even if the viral load is reduced to the point of being undetectable by reverse transcriptase-polymerase chain reaction, it can be considered to correspond to the “post-acute COVID-19 syndrome,” which has recently been proposed. Early intervention is necessary, because HLH can be fatal. Therefore, it is important to know that HLH can occur at any stage of COVID-19 and to pay attention to the patient’s progress over time, including checking the HScore.
Keywords: Hemophagocytic lymphohistiocytosis, COVID-19, Hemophagocytic syndrome, HScore
Introduction
Hemophagocytic lymphohistiocytosis (HLH), or hemophagocytic syndrome (HPS), is a disease characterized by histiocyte- and macrophage-mediated hemophagocytosis. It can be divided into primary and secondary forms, with viral infection being one of the leading causes [1]. Severe coronavirus disease 2019 (COVID-19)-associated pneumonia and acute respiratory distress syndrome (ARDS) may also lead to cytokine storm/release syndrome and, less commonly, fulfill the criteria for the diagnosis of secondary HLH [2]. We report the case of a hemodialysis patient with COVID-19 in whom HLH became obvious after the COVID-19 infection showed an improving trend, and the patient was weaned from extracorporeal membrane oxygenation (ECMO). Immunosuppressive therapy from an early stage after diagnosis saved the patient’s life.
Case report
A 45-year-old Japanese male hemodialysis patient was admitted to our hospital in August 2020 with fever and a positive test result for severe acute respiratory syndrome-associated coronavirus-2 (SARS-CoV-2) by reverse transcription-polymerase chain reaction (RT-PCR) using a nasopharyngeal swab specimen. The patient had end-stage renal disease due to idiopathic chronic interstitial nephritis, started peritoneal dialysis 20 years prior to treatment at our hospital, and had been on hemodialysis for 15 years. The patient completed treatment for interstitial nephritis upon beginning dialysis. At the time of COVID-19 disease onset, the patient was not taking immunosuppressive medications and had received lansoprazole 15 mg/day and oral phosphorus adsorbents (lanthanum carbonate 1500 mg/day and ferric citrate 750 mg). No malignant or collagen-related complications were observed in this study. The patient had no history of drug allergies, alcohol consumption, or smoking.
At the time of admission, the patient had normal consciousness, a blood pressure of 157/79 mmHg, pulse rate of 98 beats/min, respiratory rate of 21 breaths/min, body temperature of 36.8 °C, and percutaneous oxygen (SpO2) saturation of 82% (94% with 4 L/min of oxygen inhaled by mask). Chest radiography and computed tomography (CT) revealed frosted shadows in both lung fields, suggestive of respiratory failure. The results of blood tests are shown in Table 1. The patient was treated with dexamethasone, favipiravir, and other antimicrobials. However, owing to respiratory failure progression, the patient was placed on invasive mechanical ventilation on day 4 of hospitalization (Fig. 1). On day 13, the ratio of the partial pressure of arterial oxygen/inspired oxygen fraction (P/F) was 89. Hence, venovenous (VV) ECMO and methylprednisolone pulse therapy (1000 mg/day for 3 days) were initiated on day 14 to treat ARDS. Bedside transthoracic echocardiography revealed no valvular disease, abnormal left ventricular wall motion, or impaired cardiac function.
Table 1.
Results of blood tests on admission
| Complete blood count | C-reactive protein | 27.1 mg/dL | |
| White blood cell | 6340/μL | Procalcitonin | 17.44 ng/mL |
| Neutrophil | 89.0% | Sialylated carbohydrate antigen KL-6 | 414 U/mL |
| Lymphocyte | 5.2% | Surfactant Protein D | 95.5 ng/mL |
| *Count for lymphocyte | 330/μL | β-d-glucan | < 6.0 pg/mL |
| Red blood cell | 3.67 × 106/μL | Brain natriuretic peptide | 133.6 pg/mL |
| Hemoglobin | 11.8 g/dL | ||
| Hematocrit | 34.7% | Prothrombin time-international normalized ratio | 0.89 |
| Platelet | 5.5 × 104/μL | Activated-partial thromboplastin time | 42.9 s |
| Total protein | 7.5 g/dL | D-dimer | 2.47 μg/mL |
| Albumin | 3.5 g/dL | Arterial blood gas analysis | |
| Aspartate aminotransferase | 21 U/L | ||
| Alanine aminotransferase | 18 U/L | pH | 7.262 |
| Lactate dehydrogenase | 479 U/L | pCO2 | 33.8 mmHg |
| Blood urea nitrogen | 101.9 mg/dL | pO2 | 75 mmHg |
| Creatinine | 21.74 mg/dL | HCO3− | 14.7 mEq/L |
| Uric acid | 12.2 mg/dL | Lactate | 1.2 mmol/L |
| Triglyceride | 320 mg/dL | ||
| Total cholesterol | 202 mg/dL | ||
| High-density lipoprotein | 37 mg/dL | ||
| Low-density lipoprotein | 101 mg/dL | ||
| Sodium | 132 mEq/L | ||
| Chloride | 87 mEq/L | ||
| Potassium | 7.3 mEq/L |
Fig. 1.
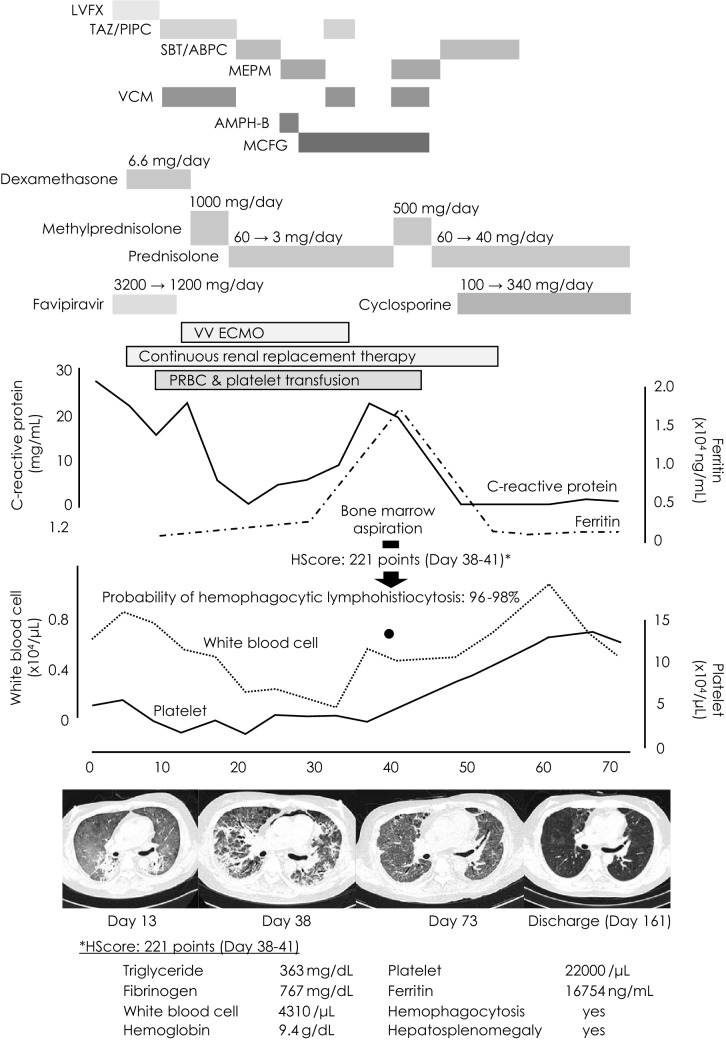
Clinical course and chest X-ray computed tomography findings. Although the C-reactive protein level and respiratory status improved with recovery from COVID-19, the patient developed hemophagocytic lymphohistiocytosis. After methylprednisolone pulse therapy, ferritin and C-reactive protein levels and bone marrow suppression improved. The extreme HScore was 221, which was high in this case. LVFX levofloxacin, TAZ/PIPC tazobactam/piperacillin, SBT/ABPC sulbactam/ampicillin, MEPM meropenem, VCM vancomycin, AMPH-B amphotericin B, MCFG micafungin, VV ECMO venovenous extracorporeal membrane oxygenation, PRBC packed red blood cells
The peak infection phase had passed, but the patient developed myelosuppression that required frequent blood transfusions. Since the patient had renal anemia as an underlying disease, 46 units of packed red blood cells were needed to maintain the hemoglobin level at approximately 8–10 g/dL. Moreover, a total of 450-unit platelet transfusions were performed (Fig. 1). The patient was weaned from ECMO on day 34 and subsequently became tachypneic with more than 40 breaths/min and a worsening P/F ratio. Although the SARS-CoV-2 RT-PCR results were negative on day 26, chest and abdominal CT scans on day 35 showed worsening lung lesions and marked hepatosplenomegaly with elevated inflammatory marker levels. HLH was suspected and confirmed using a bone marrow biopsy performed on day 38 (Fig. 2). The patient’s HScore for reactive HLH was 221 points and the probability of HLH was 96–98%. The laboratory visually checked the peripheral blood smear multiple times, and no schizocytes were noted. However, the DIC score did not meet the diagnostic criteria. The patient had a slight fever that sometimes exceeded 37 °C beginning on day 33, and from day 36, the patient’s fever exceeded 37.5 °C. Since the prednisolone dose did not change during this period, the fever may have been due to worsening of HLH.
Fig. 2.
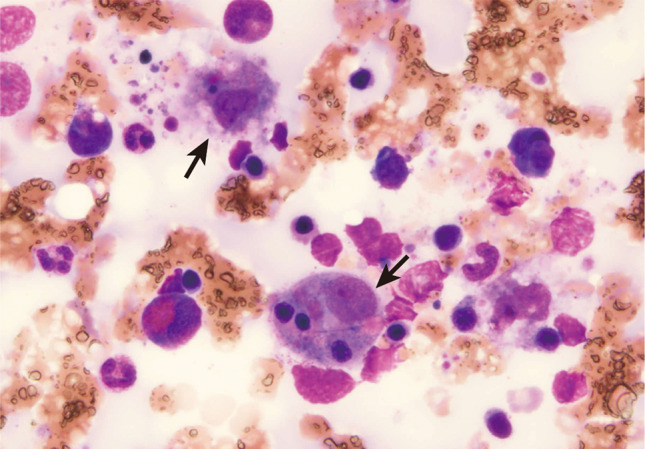
Hemophagocytic findings of monocytes seen on a smear specimen upon bone marrow examination (arrow, objective lens 40×)
Thus, HLH was diagnosed, and methylprednisolone pulse therapy (500 mg/day for 3 days) was initiated on day 39. Thereafter, respiratory status and myelosuppression improved, and blood transfusions were no longer required. During the clinical course, no pathogenic bacteria of pneumonia were isolated from sputum or blood specimens. When Candida glabrata was detected in the blood culture, antifungal drugs were initiated; however, Candida glabrata was detected only once, the effect of antifungal medications was not apparent, and β-d-glucan was never positive during the course. The antifungal medication was discontinued when the C-reactive protein (CRP) level decreased with increased steroid dosage, but there was no worsening of the patient's condition. Cyclosporine was initiated for early steroid reduction and up-titrated to a trough level of 100–150 ng/mL. The patient was removed from the intensive care unit on day 52 and discharged on day 169.
Discussion
Our patient on dialysis developed COVID-19 with ARDS, which resolved with the introduction of ECMO. However, the patient’s ARDS worsened again after discontinuation of ECMO. Based on pancytopenia and hepatosplenomegaly, we suspected HLH as a complication and subsequently performed a bone marrow biopsy.
COVID-19 presents with cytokine release syndrome during the course of severe disease [2]. Complications of HLH have also been reported. The HScore is a proposed method to assist in the diagnosis of secondary HLH. The following parameters were weighed and then added together [3]: known underlying immunosuppression [0 (no) or 18 (yes)], temperature (°C) [0 (< 38.4), 33 (38.4–39.4), or 49 (> 39.4)], organomegaly [0 (no), 23 (hepatomegaly or splenomegaly), or 38 (hepatomegaly and splenomegaly)], no. of cytopenias [0 (1 lineage), 24 (2 lineages), or 34 (3 lineages)], ferritin (ng/ml) [0 (< 2000), 35 (2000–6000), or 50 (> 6000)], triglyceride (mmol/L) [0 (< 1.5), 44 (1.5–4), or 64 (> 4)], fibrinogen (g/L) [0 (> 2.5) or 30 (≤ 2.5)], serum glutamic oxaloacetic transaminase (IU/L) [0 (< 30) or 19 (≥ 30)], and hemophagocytosis features on bone marrow aspirate [0 (no) or 35 (yes)]. According to the same study, the median HScore was 230 (interquartile range [IQR] 203–257) for patients with a positive diagnosis of reactive hemophagocytic syndrome and 125 (IQR 91–150) for patients with a negative diagnosis. In our case, there were 64 points for triglycerides, 34 points for three-lineage cytopenia, 50 points for ferritin, 35 points for bone marrow findings, and 38 points for organomegaly, for a total of 221 points. According to previous reports on the HScore of HLH with COVID-19, the percentage of HScores > 169 in severe cases is approximately 10% [4], whereas HLH was found in a much higher percentage of autopsies [5]. Postmortem biopsies of patients who died with a diagnosis of COVID-19 were performed. Bone marrow biopsy samples were obtained from 17 patients. There was a relative increase in the overall cellularity in the bone marrow in all cases; the most conspicuous finding was the presence of scattered macrophages that engulfed erythrocytes and erythroblasts. Although the relative proportions of these cells differed from case to case, hemophagocytosis was present in 16/17 cases [5]. The reason for the discrepancy between HScore and autopsy findings of HLH may be that pathological evaluation is complex under intensive care; it may also be due to the different severities of the cases investigated. Furthermore, cytokine release syndromes associated with COVID-19 have been reported to have low HScores and lack hepatosplenomegaly, hypofibrinemia, and pancytopenia, suggesting the need to validate the use of conventional HScores for the diagnosis of secondary (reactive) HLH associated with COVID-19 [6].
Most cases of COVID-19-associated HLH have been reported in the acute phase, with only a few cases reported after day 38 [6]. In the present case, pancytopenia occurred after day 14 of the disease; however, we did not evaluate the longitudinal HScore because ferritin was not elevated. However, the patient later developed delayed hyperferritinemia, hypertriglyceridemia, and hepatosplenomegaly. Since HLH occurred when the COVID-19 virus was undetectable, we assumed that it was caused by persistent immune abnormalities associated with the COVID-19 infection. Recently, as COVID-19-related case reports have accumulated, the concept of “post-acute COVID-19 syndrome” has been proposed for similar presentations [7]. Most sequelae are mild, and typical symptoms include fatigue (58%), headache (44%), and attention deficit disorder (27%) [8], which were reported early during the pandemic. Nevertheless, fatal outcomes have been reported not only from HLH, but also from various severe organ failure cases, which can also be considered post-acute COVID-19 syndrome. It is a well-known fact that the readmission rate after hospital discharge is high after completing the acute phase of COVID-19 treatment. It is common for patients to die of heart failure or other causes after readmission [9]. There have been reports of HLH occurring 8 weeks after mild COVID-19 infection [10]. More recently, HLH occurred after injection of the RNA vaccine [11, 12], suggesting that the immune abnormalities associated with COVID-19 are unlikely to be related to the viral load in the host.
Approximately, 1 month after disease onset, prednisolone was tapered off to 3 mg, ferritin was 3210 ng/mL, as shown in Fig. 1, and sIL-2R and triglyceride levels were 6671 U/mL and 256 mg/dL, respectively, indicating hypercytokinemia. It is possible that the onset of HLH occurred somewhat earlier than the time of diagnosis and the gradual decrease in prednisolone may have resulted in hypercytokinemia. However, SARS-CoV RT-PCR was negative at this point, and ECMO was weaned off afterward. Nevertheless, the patient continued to have decreased blood counts and elevated CRP levels, which were inconsistent with the course of COVID-19 infection. Based on the above, we judged that the course of the disease was different from that of HLH caused by cytokine storms that occur during the acute phase of COVID-19 infection, and we consider the disease to be in the category of post-acute COVID-19 syndrome. The two reported cases of HLH as post-acute COVID-19 syndrome were diagnosed 2 weeks and 4 weeks after the onset of COVID-19 infection [7, 13]. Therefore, the diagnosis of post-acute COVID-19 syndrome is based on the onset of HLH that occurs during recovery from the acute phase of COVID-19, as in our case.
The mortality rate of COVID-19 patients with HLH has been reported to be 46.6% [14], and early therapeutic intervention is required in such cases. Conversely, minimal immunosuppressive therapy is recommended for the treatment of COVID-19 because of delayed viral shedding and secondary infection. In this case, we initially did not suspect HLH, because the only laboratory abnormality suggestive of HLH was hematopenia, and glucocorticoids were continued as treatment for the COVID-19 sequelae. Consequently, we were reluctant to perform a bone marrow biopsy and intensify immunosuppressive therapy until the HScore was sufficiently high and until after the onset of ARDS, which may have been caused by HLH. As a one-time assessment of the HScore likely underestimates the possibility of HLH, repeated assessments are important to better understand its changes. In retrospect, bone marrow biopsy could have been performed earlier in our case. A protocol for immunosuppressive therapy in HLH associated with COVID-19 has not yet been fully established and further studies are needed.
Although patients with end-stage kidney disease are regarded as compromised hosts in routine clinical practice, the impact of reduced kidney function on the immune system is diverse [15]. Several cases of secondary HLH due to infection in patients with end-stage kidney disease have been reported, with tuberculosis being the most common cause [16–21]. In addition, viral infections have been reported with BK [22, 23] and cytomegalovirus viruses [24] in patients who underwent renal transplant. However, it is not known how impaired kidney function affects the development and course of HLH.
Cyclosporine is a calcineurin inhibitor that modulates cytokine production and is a therapeutic option for HLH associated with collagen diseases [25]. Furthermore, it has been postulated that cyclosporine may be effective as a therapeutic agent for COVID-19 by inhibiting COVID-19 proliferation and hypercytokinemia [26, 27]. Recently, a comparison of prednisolone alone versus a reduced-dose prednisolone plus cyclosporine protocol in patients with moderate to severe COVID-19 infection was reported [28]; as expected, the cyclosporine group had a significantly better prognosis. However, there was no significant difference in the complication rates of secondary infections between the two groups. In terms of prevention of secondary infections, which was our intention at the time, conclusions await further accumulation of cases and clinical studies.
Based on the course of this case, COVID-19 may cause cytokine release syndrome and, in rare cases, secondary HLH with potentially fatal consequences. Given the discrepancy between the HScore and HLH frequencies observed in autopsies, the possibility of secondary HLH during COVID-19 treatment should always be considered. Our experience, in this case, was useful for the treatment of other patients with COVID-19 and may be important not only for COVID-19, but also for acute viral infections that may cause future pandemics.
Acknowledgements
We thank Editage (https://www.editage.jp/) for editing the manuscript.
Funding
None.
Declarations
Conflict of interest
The authors have declared that no conflict of interest exists.
Human and animal rights
This article does not contain any studies involving human participants or animals performed by any of the authors.
Informed consent
Case reports using existing data were included in the comprehensive written informed consent form obtained at the time of admission. All procedures performed in this study involving human participants were in accordance with the 1964 Declaration of Helsinki and its later amendments or with comparable ethical standards. IRB approval was not required for the single case study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ramos-Casals M, Brito-Zerón P, López-Guillermo A, Khamashta MA, Bosch X. Adult haemophagocytic syndrome. Lancet. 2014;383:1503–1516. doi: 10.1016/S0140-6736(13)61048-X. [DOI] [PubMed] [Google Scholar]
- 2.Liu JM, Chi J. Is COVID-19-associated cytokine storm distinct from non-COVID-19 secondary hemophagocytic lymphohistiocytosis? Exp Biol Med. 2022;247:330–337. doi: 10.1177/15353702211068840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fardet L, Galicier L, Lambotte O, Marzac C, Aumont C, Chahwan D, et al. Development and validation of the HScore, a score for the diagnosis of reactive hemophagocytic syndrome. Arthritis Rheumatol. 2014;66:2613–2620. doi: 10.1002/art.38690. [DOI] [PubMed] [Google Scholar]
- 4.Wood H, Jones JR, Hui K, Mare T, Pirani T, Galloway J, et al. Secondary HLH is uncommon in severe COVID-19. Br J Haematol. 2020;190:e283–e285. doi: 10.1111/bjh.16934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prieto-Pérez L, Fortes J, Soto C, Vidal-González Á, Alonso-Riaño M, Lafarga M, et al. Histiocytic hyperplasia with hemophagocytosis and acute alveolar damage in COVID-19 infection. Mod Pathol. 2020;33:2139–2146. doi: 10.1038/s41379-020-0613-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hakim NN, Chi J, Olazagasti C, Liu JM. Secondary hemophagocytic lymphohistiocytosis versus cytokine release syndrome in severe COVID-19 patients. Exp Biol Med. 2021;246:5–9. doi: 10.1177/1535370220962043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Naous E, Nassani B-M, Yaghi C, Nasr F, Medlej R. Hemophagocytic lymphohistiocytosis, a new cause of death during “post-acute COVID-19 syndrome?”. A case report J Hematop. 2021;14:229–233. doi: 10.1007/s12308-021-00452-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lopez-Leon S, Wegman-Ostrosky T, Perelman C, Sepulveda R, Rebolledo PA, Cuapio A, et al. More than 50 long-term effects of COVID-19: a systematic review and meta-analysis. Sci Rep. 2021;11:16144. doi: 10.1038/s41598-021-95565-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Donnelly JP, Wang XQ, Iwashyna TJ, Prescott HC. Readmission and death after initial hospital discharge among patients with COVID-19 in a large multihospital system. JAMA. 2021;325:304–306. doi: 10.1001/jama.2020.21465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bandaru SS, Capace A, Busa V, Williams A. Secondary hemophagocytic lymphohistiocytosis in a post-COVID-19 patient. Cureus. 2022;14:e22620. doi: 10.7759/cureus.22620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nasir S, Khan SR, Iqbal R, Hashmi AP, Moosajee M, Nasir N. Inactivated COVID-19 vaccine triggering hemophagocytic lymphohistiocytosis in an immunocompetent adult - a case report. Transl Res. 2022;8:152–155. [PMC free article] [PubMed] [Google Scholar]
- 12.Lin T-Y, Yeh Y-H, Chen L-W, Cheng C-N, Chang C, Roan J-N, et al. Hemophagocytic lymphohistiocytosis following BNT162b2 mRNA COVID-19 vaccination. Vaccines (Basel). 2022 doi: 10.3390/vaccines10040573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gautam S, Sharma G, Singla S, Garg S. Case report: secondary hemophagocytic lymphohistiocytosis (sHLH) and candida auris fungemia in post-acute covid-19 syndrome: a clinical challenge. Front Med. 2022;9:835421. doi: 10.3389/fmed.2022.835421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Retamozo S, Brito-Zerón P, Sisó-Almirall A, Flores-Chávez A, Soto-Cárdenas M-J, Ramos-Casals M. Haemophagocytic syndrome and COVID-19. Clin Rheumatol. 2021;40:1233–1244. doi: 10.1007/s10067-020-05569-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Campo S, Lacquaniti A, Trombetta D, Smeriglio A, Monardo P. Immune system dysfunction and inflammation in hemodialysis patients: two sides of the same coin. J Clin Med Res. 2022 doi: 10.3390/jcm11133759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koulmane Laxminarayana SL, Nagaraju SP, Prabhu Attur R, Manohar C, Parthasarathy R, Chari B. Hemophagocytic lymphohistiocytosis: an unusual presentation of tuberculosis in hemodialysis patients. Hemodial Int. 2015;19:E16–E19. doi: 10.1111/hdi.12232. [DOI] [PubMed] [Google Scholar]
- 17.Castellano I, Gómez-Martino JR, Hernández T, Mateos L, Argüello C. Hemophagocytic syndrome as an unusual form of presentation of tuberculosis in a hemodialysis patient: case report and review of the literature. Am J Nephrol. 2000;20:214–216. doi: 10.1159/000013590. [DOI] [PubMed] [Google Scholar]
- 18.Su N-W, Chen C-K, Chen G-S, Hsieh R-K, Chang M-C. A case of tuberculosis-induced hemophagocytic lymphohistiocytosis in a patient under hemodialysis. Int J Hematol. 2009;89:298–301. doi: 10.1007/s12185-009-0265-x. [DOI] [PubMed] [Google Scholar]
- 19.Chien C-C, Chiou T-J, Lee M-Y, Hsiao L-T, Kwang W-K. Tuberculosis-associated hemophagocytic syndrome in a hemodialysis patient with protracted fever. Int J Hematol. 2004;79:334–336. doi: 10.1532/IJH97.A10315. [DOI] [PubMed] [Google Scholar]
- 20.Seminari E, Contardi G, Rubert L, Fronti E, Comoli P, Minoli L, et al. Tuberculosis-induced haemophagocytic syndrome in a patient on haemodialysis treated with anti-thymocyte globulin. Int J Tuberc Lung Dis. 2014;18:248–249. doi: 10.5588/ijtld.13.0533. [DOI] [PubMed] [Google Scholar]
- 21.Yang CW, Lee JH, Kim YG, Kim YO, Lee SH, Kim BK, et al. Tuberculosis-associated hemophagocytic syndrome in a hemodialysis patient: case report and review of the literature. Nephron. 1996;72:690–692. doi: 10.1159/000188963. [DOI] [PubMed] [Google Scholar]
- 22.Esposito L, Hirsch H, Basse G, Fillola G, Kamar N, Rostaing L. BK virus-related hemophagocytic syndrome in a renal transplant patient. Transplantation. 2007;83:365. doi: 10.1097/01.tp.0000248807.63325.cc. [DOI] [PubMed] [Google Scholar]
- 23.Yaich S, Charfeddine K, Hsairi D, Zaghdane S, Kammoun K, Makni S, et al. BK virus-associated hemophagocytic syndrome in a renal transplant recipient. Saudi J Kidney Dis Transpl. 2014;25:610–614. doi: 10.4103/1319-2442.132205. [DOI] [PubMed] [Google Scholar]
- 24.Ben Salem M, Bchir S, Taieb SK, Hamouda M, Mezrigui R, Ben Saleh M, et al. Hemophagocytic syndrome in a pregnant renal transplant recipient associated with cytomegalovirus infection. Exp Clin Transplant. 2021;19:739–743. doi: 10.6002/ect.2021.0041. [DOI] [PubMed] [Google Scholar]
- 25.Griffin G, Shenoi S, Hughes GC. Hemophagocytic lymphohistiocytosis: an update on pathogenesis, diagnosis, and therapy. Best Pract Res Clin Rheumatol. 2020;34:101515. doi: 10.1016/j.berh.2020.101515. [DOI] [PubMed] [Google Scholar]
- 26.Blumberg EA, Noll JH, Tebas P, Fraietta JA, Frank I, Marshall A, et al. A phase I trial of cyclosporine for hospitalized patients with COVID-19. JCI Insight. 2022 doi: 10.1172/jci.insight.155682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Poulsen NN, von Brunn A, Hornum M, Blomberg JM. Cyclosporine and COVID-19: risk or favorable? Am J Transplant. 2020;20:2975–2982. doi: 10.1111/ajt.16250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gálvez-Romero JL, Palmeros-Rojas O, Real-Ramírez FA, Sánchez-Romero S, Tome-Maxil R, Ramírez-Sandoval MP, et al. Cyclosporine A plus low-dose steroid treatment in COVID-19 improves clinical outcomes in patients with moderate to severe disease: a pilot study. J Intern Med. 2021;289:906–920. doi: 10.1111/joim.13223. [DOI] [PMC free article] [PubMed] [Google Scholar]