Abstract
Using a lacZ plasmid transgenic mouse model, spectra of spontaneous point mutations were determined in brain, heart, liver, spleen and small intestine in young and old mice. While similar at a young age, the mutation spectra among these organs were significantly different in old age. In brain and heart G:C→A:T transitions at CpG sites were the predominant mutation, suggesting that oxidative damage is not a major mutagenic event in these tissues. Other base changes, especially those affecting A:T base pairs, positively correlated with increasing proliferative activity of the different tissues. A relatively high percentage of base changes at A:T base pairs and compound mutants were found in both spleen and spontaneous lymphoma, suggesting a possible role of the hypermutation process in splenocytes in carcinogenesis. The similar mutant spectra observed at a young age may reflect a common mutation mechanism for all tissues that could be driven by the rapid cell division that takes place during development. However, the spectra of the young tissues did not resemble that of the most proliferative aged tissue, implying that replicative history per se is not the underlying causal factor of age-related organ-specific differences in mutation spectra. Rather, differences in organ function, possibly in association with replicative history, may explain the divergence in mutation spectra during aging.
INTRODUCTION
Somatic mutations are thought to play a major causal role in cancer and, possibly, aging (1,2). To monitor tissue-specific patterns of somatic mutation accumulation during aging, a plasmid transgenic mouse model sensitive to a broad range of mutational events has been developed (3). These mice harbor chromosomally integrated plasmids that can be efficiently recovered from genomic DNA and transferred into a suitable Escherichia coli host for mutant selection, quantitation and characterization. The advantages of this system include an extensive choice of tissue types suitable for mutation examination and the absence of any selection pressure in vivo of a mutation in the neutral reporter. On the other hand, mutagenic events coupled to transcription might be under-represented or not detected at all in the silent reporter gene.
Using the plasmid transgenic mouse model, we have previously reported organ-specific differences in mutation accumulation with age (4,5). Further characterization of the mutational spectra as they unfold in old age could provide molecular fingerprints to obtain an insight into the possible sources of molecular damage, which has been implicated as the ultimate cause of aging and its associated diseases (6,7). Here we specifically compare the point mutational spectra in five organs, with different proliferative histories, in young and old mice and in lymphomas, the most frequent neoplastic lesion in old age in these mice. The results indicate that the mutation spectra in the different organs diverge during the aging process. It is suggested that the organ-specific mutation spectra emerging in old age reflect a combination of the proliferative history and unique function of each organ.
MATERIALS AND METHODS
Plasmid rescue
Aging cohorts of male C57Bl/6 pUR288-lacZ mice of line 60 were maintained in the animal facilities of the Beth Israel Deaconess Medical Center (Boston, MA) as described previously (4). The animals were killed by decapitation following asphyxiation with CO2. Organs and tissues were removed, rinsed in PBS, placed in 1.5 ml microcentrifuge tubes and frozen on dry ice. Any macroscopic lesions observed during tissue collection were excised and stored separately. The tissues were maintained at –80°C until used. DNA was extracted by routine phenol/chloroform extraction. Complete protocols for plasmid rescue and mutant frequency determinations with this model are given elsewhere (8). Briefly, between 10 and 20 µg genomic DNA was digested with HindIII for 1 h in the presence of magnetic beads (Dynal) pre-coated with lacI–lacZ fusion protein. The beads were washed three times to remove the unbound mouse genomic DNA. Plasmids were subsequently eluted from the beads with IPTG. After circularization of the plasmids with T4 DNA ligase they were ethanol precipitated and used to electrotransform E.coli C (ΔlacZ, galE–) cells. One-thousandth of the transformed cells were plated on a titer plate (with X-gal) and the remainder on a selective plate (with p-gal). The plates were incubated for 15 h at 37°C. Mutant frequencies were determined as the number of colonies on the selective plates versus the number of colonies on the titer plate (times the dilution factor of 1000).
Mock-recovery
To check for a possible E.coli contribution to the spontaneous mutation spectra as observed in the lacZ plasmids obtained from the mouse, the same plasmids were grown in E.coli. For these experiments, E.coli C cells harboring the wild-type pUR288 plasmid were obtained in the form of a dark blue staining colony on a titer plate from a regular mutant frequency determination. These cells were grown in 3 ml of LB medium containing 75 µg/ml ampicillin and 25 µg/ml kanamycin for 8 h at 37°C at 225 r.p.m. Cells were harvested by centrifugation for 10 min at 1000 g. DNA was extracted by routine phenol/chloroform extraction. The plasmid preparations were mixed with non-transgenic liver DNA, after which mutant plasmids were recovered as described above for genomic DNA isolation.
Mutant classification
Mutant colonies were taken from the selective plates and grown overnight in 3 ml of LB medium. Then, 1 µl was directly plated onto X-gal to screen for galactose-insensitive host cells (9). The remainder of the cell culture was used for plasmid mini preparation (Wizard 9600; Promega). The purified plasmids were digested with PstI and AvaI and size separated on 1% agarose gels. Mutant plasmids with restriction patterns resembling and deviating from the wild-type restriction pattern were classified as ‘no-change’ and ‘size-change’ mutants, respectively.
Sequencing
Sequencing reactions were performed with the CEQ dye terminator cycle sequencing kit (Beckman, Fullerton, CA), according to the manufacturer’s standard protocol, and analyzed with a CEQ 2000 DNA analysis system (Beckman). The primers used were as described earlier (9).
RESULTS AND DISCUSSION
Mutant lacZ plasmids were recovered from brain, heart, liver, spleen and small intestine of young (3–4 months) and old (30–33 months) pUR288 C57Bl/6 mice and subdivided, based on size, into no-change and size-change mutants. From the no-change mutants, which were presumed to be point mutations, 20–22 were randomly selected per age/organ group from three to four animals and completely sequenced (Table 1). The point mutational spectra, limited to base changes and single base deletions, of the five organs in the two age groups are expressed as mutant frequencies in Figure 1. Statistical analyses of the mutational frequencies and spectra were conducted using a Bayesian approach (10). In testing for differences in the mutational spectra between groups, the mean square error was used as the measure of discrepancy.
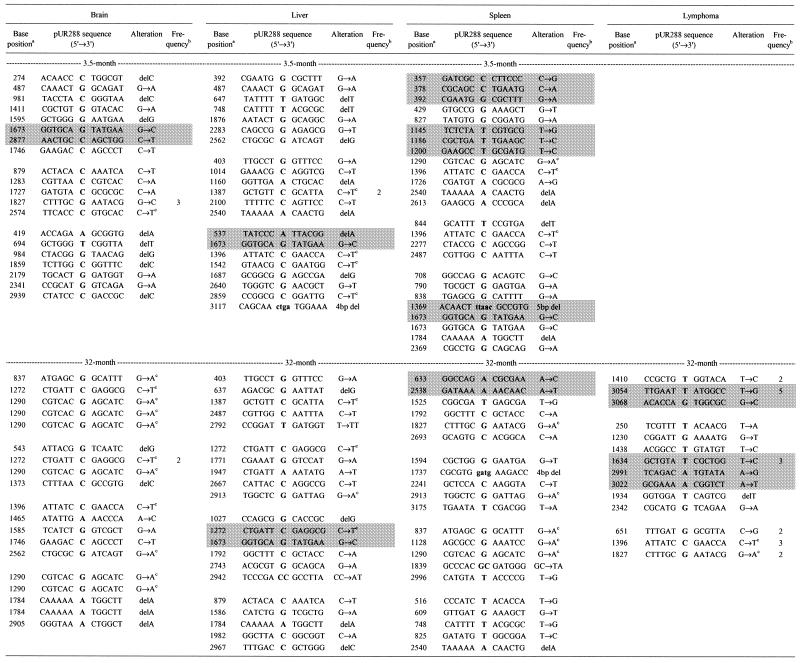
Table 1. Sequenced lacZ no-change mutations recovered from brain, liver, spleen and lymphoma of 3.5- and 32-month-old mice.
Empty lines separate mutants obtained from individual mice. The cross-hatched areas indicate compound mutants; all other mutants contained single mutations. Sequence data on point mutations for heart and small intestine have been published elsewhere (5).
aNucleotide numbering according to SYNPUR288V (GenBank accession no. L09147).
bFrequency of recurrent mutations, i.e. identical mutants recovered from the same tissue sample. Other seemingly recurrent mutations in this table were unique, based on the presence of different polymorphic markers among the mutated plasmids. (These single nucleotide polymorphisms were shown to be present among the integrated wild-type plasmid copies of this transgenic mouse model; 26.)
cG:C→A:T base change at a CpG site.
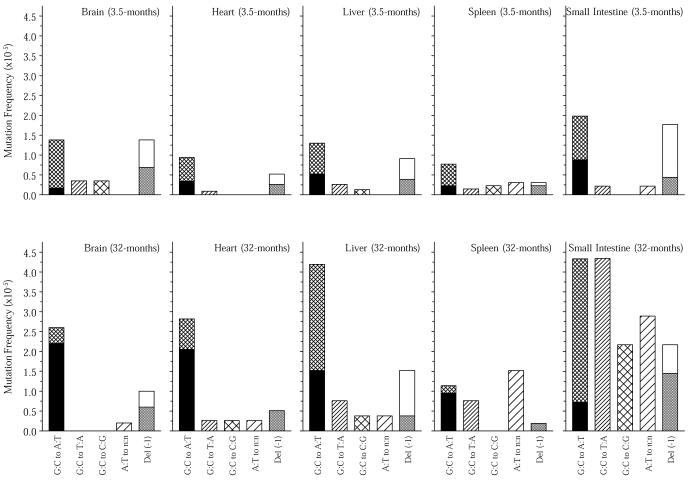
Figure 1.
Point mutational spectra of the lacZ reporter gene in brain, heart, liver, spleen and small intestine from young (3.5 months) and old (32 months) mice. Bars represent the frequency of each type of point mutation as indicated. The black areas in the G:C→A:T bars indicate the fraction of such mutations that had occurred at CpG sites and the gray areas in the Del (–1) bars indicate the fraction of such mutations that had occurred at reiterated sites, i.e. a sequence of three or more of the same nucleotide. Corrections for recurrent mutations were made.
The total and categorized point mutation frequencies were higher on average for the older mice (a posteriori P value < 0.01). An increase in the frequency of all point mutations was observed in the heart (P < 0.01), liver (P < 0.01), spleen (P = 0.01) and small intestine (P < 0.01), but not in the brain (P = 0.41). The magnitude of the difference between young and old mice was higher in the small intestine than in the other organs (P = 0.05). There were also clear differences between age groups in the proportions of mutations falling into the different subclasses (P < 0.01). This difference was evident in the brain (P < 0.01), spleen (P = 0.03) and small intestine (P = 0.03), but not in the heart (P = 0.13) or liver (P = 0.88). In addition to these effects, the old mice exhibited clear differences between organs in both the mutation frequencies (P < 0.01) and subclass proportions (P < 0.01). However, such differences were not apparent among the younger mice (P = 0.09 and P = 0.81, respectively).
The point mutational spectra in the five organs, which are similar at a young age, diverge in a way that, at least in part, seems to reflect their proliferative history over the lifespan of the mouse. The organs in Figure 1 have been arranged from left to right by increasing proliferative activity. Studies comparing DNA-incorporated radioactivity per organ after administration of [3H]thymidine clearly set the small intestine apart from the other four organs in terms of proliferative activity (11,12). Spleen is the second most proliferative organ among the five organs studied. Both Kuppfer and parenchymal cells contributed to some remaining proliferative activity in the liver (11). Based on the cardiomyocytes, the heart is virtually a post-mitotic organ, but some proliferative activity, possibly due to other cell types, has been found (12). Brain appears to be virtually devoid of proliferative activity (11,12). From our present studies it appears that G:C→A:T transitions at CpG sites correlate strongly with a lack of proliferative activity over a lifetime (Fig. 1). Other base changes than G:C→A:T transitions emerge with increasing frequency from brain to small intestine, the latter organ being dramatically different, with relatively high frequencies of G:C→A:T at non-CpG sites, G:C→T:A, G:C→G:T and base changes at A:T base pairs. In general, while G:C→A:T transitions at CpG sites are predominantly found in post-mitotic organs, changes at A:T base pairs correlate positively with proliferative activity (Fig. 1).
The increase in G:C→A:T transitions at CpG sites in the brain and heart indicates that the predominant mutational mechanism in post-mitotic tissue during aging is spontaneous deamination of 5-methylcytosine. As proposed by MacPhee (13), mismatch repair would have a 50% chance of reverting a C:T mismatch to the original sequence and a 50% chance of creating a stable G:C→A:T transition in the absence of proper strand recognition signals. However, most spontaneous deaminations of 5-methylcytosine are repaired correctly, for instance by specific glycosylases (14).
Oxidative stress has been suggested to play an important role in aging, damaging DNA and other macromolecules alike (15). Because our data indicate that age-related mutation accumulation in the brain and heart is mainly due to spontaneous deamination of 5-methylcytosine, oxidative damage does not seem to be a major mutagenic event in these tissues. In this respect, it is conceivable that the relatively high rate of oxidative metabolism in brain and heart mainly causes mutations in the mitochondrial genome (16), while oxidative damage to the nuclear genome of these post-mitotic tissues might be repaired without a mutagenic consequence.
A question of major importance is the source of the mutations in the more proliferative organs, most notably the small intestine, which are likely to be caused by misreplication at damaged sites. In this respect, at least two possibilities come to mind. First, oxidative damage might play a role, since predominantly G:C base pairs were affected (Fig. 1). G or C bases have been identified as the main target for oxidative damage in vitro (17). Second, replication errors may also arise as a consequence of DNA lesions induced by environmental mutagens, taken up with food by the small intestine or detoxified in the liver.
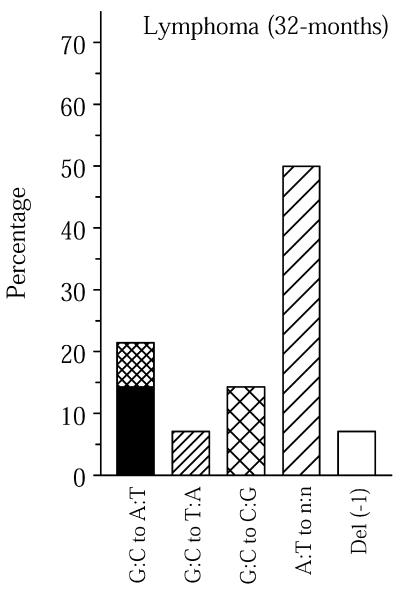
In old spleen a relatively high percentage of base changes was found to involve A:T base pairs (Fig. 1). This is in keeping with the observed point mutational spectra of both human and mouse lymphocytes at the Hprt locus (18,19). In this respect, it appears that Hprt mutation spectra in blood lymphocytes are not representative of other cell and tissue types. Interestingly, a high frequency of base changes at A:T was also found in the mutation spectrum of spontaneous lymphomas isolated from old mice (Fig. 2). Two of the fourteen unique lymphoma mutants sequenced contained multiple mutations, i.e. 14%. In spleen, the main target organ for lymphomas in aging mice, the frequency of lacZ mutants containing two or more mutations was 10%, while averaging only 2% in the other four organs (Table 1). We interpret the high percentage of such compound mutants as evidence for a temporal burst of mutational activity at some point in the history of these tissues. As such, this finding is in keeping with the mutator phenotype postulated to underlie the initiation and progression of tumors (20). It is tempting to speculate that somatic hypermutation, a predominantly point mutational process improving the affinity of Ig molecules in germinal centers in lymph nodes and spleen, causes such compound mutants, which may increase cancer risk. Recently, somatic hypermutation has been associated with DNA polymerase η as an A:T mutator (21,22), which could explain the relatively large fraction of mutations found at A:T base pairs in old spleen and lymphoma (Figs 1 and 2).
Figure 2.
Point mutational spectra of the lacZ reporter gene in spontaneous lymphomas found in old mice. See legend to Figure 1 for an explanation of the bars. Mutational spectra are expressed as percentages to omit inaccuracies in correcting mutant frequencies for frequent recurrent mutations leading to a small number of unique plasmids analyzed for some lymphomas (Table 1).
While the observed mutation spectrum in the spleen could be causally related to the etiology of lymphomas, the same does not apply to the small intestine. Indeed, while small intestine has the highest spontaneous mutation rate of all tissues tested (Fig. 1) (5), tumors in this tissue occur at very low frequencies (23). Possibly, other factors than somatic mutation rate alone play a role in determining susceptibility of an organ to tumor formation. In this respect, it should be noted that mice deficient for the mismatch repair genes Mlh1 and Pms2 show 18- and 13-fold increases in point mutations in the small intestine at a lacI transgene (24), respectively, which did not dramatically increase the frequency of small intestinal tumors (25).
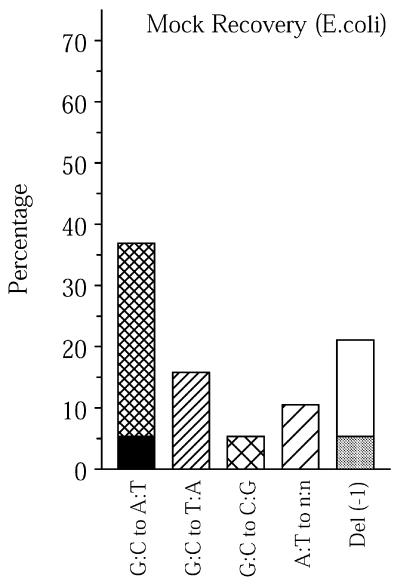
At a young age the mutation spectra in brain, heart, liver, spleen and small intestine are remarkably similar (Fig. 1). To exclude the possibility that this similarity is due to a background level of mutations due to the rescue process, a point mutational spectrum from mock-recovered plasmids grown in E.coli was determined (Fig. 3). Although the mock-recovered spectrum resembled the spectra of the young mouse tissues (Figs 1 and 3), the mock-recovered point mutant frequency was only ∼0.6 × 10–5, as compared with 2.8 × 10–5 on average in tissues from young animals. Furthermore, the mock-recovered mutant frequency is an overestimate due to the inability to obtain mutation-free starting material, i.e. wild-type plasmid preparations. Based on our previous results (26), many of the mutations occur during growth in E.coli prior to plasmid preparation and not during mock recovery. Indeed, when the sites of the base changes were taken into account, many of the mock-recovered mutants turned out to be unique, i.e. only 17% of the point mutations were found among 140 different point mutations recovered from the mouse. The young mouse tissues shared on average 39% of their point mutations with this mouse mutation database (Table 2).
Figure 3.
Point mutational spectra of mock-recovered plasmids grown in E.coli C. See legend to Figure 1 for an explanation of the bars. Mutational spectra are expressed as percentages, since thus far there is no evidence that they represent a real background, i.e. find their origin in the rescue process (see text).
Table 2. Number of identical point mutations found in the lacZ transgene of other mouse tissues.
| Source | Compared | Matching | Percentage |
|---|---|---|---|
| Young brain | 20 | 5 | 25 |
| Young heart | 20 | 10 | 50 |
| Young liver | 20 | 11 | 55 |
| Young spleen | 23 | 8 | 35 |
| Young small intestine | 19 | 6 | 32 |
| Average young mouse tissues | 39 | ||
| Mock-recovery (E.coli) | 18 | 3 | 17 |
The mouse database comprised 140 unique point mutations.
Hence, we believe that the mutation spectra in the young somatic tissues (Fig. 1) are genuinely similar, which suggests that, in contrast to aging, development is associated with a mutation mechanism common to all cells and tissues. In this respect, one would suspect that these early mutation spectra are associated with replication errors and, hence, resemble the spectra in actively proliferating tissue, such as spleen and small intestine, at a greater age. However, this is not the case, which suggests that replicative history per se is not the underlying causal factor of age-related organ-specific mutation spectra. Rather, differences in organ function, possibly in association with replicative history, may explain the divergence in mutation spectra during aging. Such in vivo mutational fingerprints are likely to provide clues as to the various sources of somatic damage thought to underlie age-related cellular degeneration and death under various environmental conditions.
Acknowledgments
ACKNOWLEDGEMENTS
This work was supported by grants 1PO1AG17242-01, AG13319-05 and 1RO1CA75653-03.
REFERENCES
- 1.Vijg J. (2000) Somatic mutations and aging: a re-evaluation. Mutat. Res., 447, 117–135. [DOI] [PubMed] [Google Scholar]
- 2.DePinho R.A. (2000) The age of cancer. Nature, 408, 248–254. [DOI] [PubMed] [Google Scholar]
- 3.Boerrigter M.E.T.I., Dollé,M.E.T., Martus,H.-J., Gossen,J.A. and Vijg,J. (1995) Plasmid-based transgenic mouse model for studying in vivo mutations. Nature, 377, 657–659. [DOI] [PubMed] [Google Scholar]
- 4.Dollé M.E.T., Giese,H., Hopkins,C.L., Martus,H.-J., Hausdorff,J.M. and Vijg,J. (1997) Rapid accumulation of genome rearrangements in liver but not in brain of old mice. Nature Genet., 17, 431–434. [DOI] [PubMed] [Google Scholar]
- 5.Dollé M.E.T., Snyder,W.K., Gossen,J.A., Lohman,P.H. and Vijg,J. (2000) Distinct spectra of somatic mutations accumulated with age in mouse heart and small intestine. Proc. Natl Acad. Sci. USA, 97, 8403–8408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kirkwood T.B. and Austad,S.N. (2000) Why do we age? Nature, 408, 233–238. [DOI] [PubMed] [Google Scholar]
- 7.Vijg J. and Dollé,M.E.T. (2001) Instability of the nuclear genome and the role of DNA repair. In Masoro,E.J. and Austad,S.N. (eds), Handbook of the Biology of Aging, 5th Edn. Academic Press, San Diego, CA, pp. 84–113.
- 8.Vijg J., Boerrigter,M.E.T.I. and Dollé,M.E.T. (1999) A transgenic mouse model for studying mutations in vivo. In Yu,B.P. (ed.), Methods in Aging Research: Section D. CRC Press, Boca Raton, FL, pp. 621–635.
- 9.Dollé M.E.T., Martus,H.-J., Novak,M., van Orsouw,N.J. and Vijg,J. (1999) Characterization of color mutants in lacZ plasmid-based transgenic mice, as detected by positive selection. Mutagenesis, 14, 287–293. [DOI] [PubMed] [Google Scholar]
- 10.Dunson D.B. and Tindall,K.R. (2000) Bayesian analysis of mutational spectra. Genetics, 156, 1411–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Edwards J.L. and Klein,R.E. (1961) Cell renewal in adult mouse tissues. Am. J. Pathol., 38, 437–453. [PMC free article] [PubMed] [Google Scholar]
- 12.Hinrichs H.R., Petersen,R.O. and Baserga,R. (1964) Incorporation of thymidine into DNA of mouse organs. Arch. Pathol., 78, 245–253. [PubMed] [Google Scholar]
- 13.MacPhee D.G. (1995) Mismatch repair, somatic mutations and the origins of cancer. Cancer Res., 55, 5489–5492. [PubMed] [Google Scholar]
- 14.Hendrich B., Hardeland,U., Ng,H.-H., Jiricny,J. and Bird,A. (1999) The thymine glycosylase MBD4 can bind to the product of deamination at methylated CpG sites. Nature, 401, 301–304. [DOI] [PubMed] [Google Scholar]
- 15.Sohal R.S. and Weindruch,R. (1996) Oxidative stress, caloric restriction and aging. Science, 273, 59–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee C.M., Weindruch,R. and Aiken,J.M. (1997) Age-associated alterations of the mitochondrial genome. Free Radic. Biol. Med., 22, 1259–1269. [DOI] [PubMed] [Google Scholar]
- 17.Reid T.M., Feig,D.I. and Loeb,L.A. (1994) Mutagenesis by metal-induced oxygen radicals. Environ. Health Perspect., 102 (suppl. 3), 57–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cariello N.F., Douglas,G.R. and Soussi,T. (1996) Databases and software for the analysis of mutations in the human p53 gene, the human hprt gene and the lacZ gene in transgenic rodents. Nucleic Acids Res., 24, 119–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meng Q., Singh,N., Heflich,R.H., Bauer,M.J. and Walker,V.E. (2000) Comparison of the mutations at Hprt exon 3 of T-lymphocytes from B6C3F1 mice and F344 rats exposed by inhalation to 1,3-butadiene or the racemic mixture of 1,2:3,4-diepoxybutane. Mutat. Res. 464, 169–184. [DOI] [PubMed] [Google Scholar]
- 20.Loeb L.A. (2001) A mutator phenotype in cancer. Cancer Res., 61, 3230–3239. [PubMed] [Google Scholar]
- 21.Rogozin I.B., Pavlov,Y.I., Bebenek,K., Matsuda,T. and Kunkel,T.A. (2001) Somatic mutation hotspots correlate with DNA polymerase eta error spectrum. Nature Immunol., 2, 530–536. [DOI] [PubMed] [Google Scholar]
- 22.Zeng X., Winter,D.B., Kasmer,C., Kraemer,K.H., Lehmann,A.R. and Gearhart,P.J. (2001) DNA polymerase η is an A-T mutator in somatic hypermutation of immunoglobulin variable genes. Nature Immunol., 2, 537–541. [DOI] [PubMed] [Google Scholar]
- 23.Bult C.J., Krupke,D.M., Naf,D., Sundberg,J.P. and Eppig,J.T. (2001) Web-based access to mouse models of human cancers: the Mouse Tumor Biology (MTB) Database. Nucleic Acids Res., 29, 95–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baross-Francis A., Makhani,N., Liskay,R.M. and Jirik,F.R. (2001) Elevated mutant frequencies and increased C:G→T:A transitions in Mlh1–/– versus Pms2–/– murine small intestinal epithelial cells. Oncogene, 20, 619–625. [DOI] [PubMed] [Google Scholar]
- 25.Prolla T.A., Baker,S.M., Harris,A.C., Tsao,J.L., Yao,X., Bronner,C.E., Zheng,B., Gordon,M., Reneker,J., Arnheim,N., Shibata,D., Bradley,A. and Liskay,R.M. (1998) Tumour susceptibility and spontaneous mutation in mice deficient in Mlh1, Pms1 and Pms2 DNA mismatch repair. Nature Genet., 18, 276–279. [DOI] [PubMed] [Google Scholar]
- 26.Dollé M.E.T., Snyder,W.K., van Orsouw,N.J. and Vijg,J. (1999) Background mutations and polymorphisms in lacZ-plasmid transgenic mice. Environ. Mol. Mutagen., 34, 112–120. [PubMed] [Google Scholar]