Abstract
Background:
Left ventricular hypertrophy (LVH) combined with elevations in cardiac biomarkers reflecting myocardial injury and neurohormonal stress (malignant LVH) is associated with a high risk for heart failure and death.
Objectives:
The aim of this study was to determine the impact of intensive systolic blood pressure (SBP) control on the prevention of malignant LVH and its consequences.
Methods:
8,820 participants in the Systolic Blood Pressure Intervention Trial (SPRINT) were classified into groups based on the presence or absence of LVH assessed by 12-lead ECG, and elevations in biomarker levels (high-sensitivity cardiac troponin T ≥14 ng/L or N-terminal pro-B-type natriuretic peptide ≥125 pg/mL) at baseline. The effects of intensive versus standard SBP lowering on rates of acute decompensated heart failure (ADHF) events and death and on the incidence and regression of malignant LVH were determined.
Results:
Randomization to intensive SBP lowering led to similar relative reductions in ADHF events and death across the combined LVH/biomarker groups (P for interaction =0.68). The absolute risk reduction over four years in ADHF events and death was 4.4% (95% CI: −5.2%, 13.9%) among participants with baseline malignant LVH (n=449) and 1.2% (95% CI: 0.0%, 2.5%) for those without LVH and non-elevated biomarkers (n=4361). Intensive SBP lowering also reduced the incidence of malignant LVH over two years (2.5% vs. 1.1%, odds ratio: 0.44, 95% CI: 0.30, 0.63).
Conclusions:
Intensive SBP lowering prevented malignant LVH and may provide substantial absolute risk reduction in the composite of ADHF events and death among SPRINT participants with baseline malignant LVH.
Keywords: hypertension, malignant LVH, troponin, natriuretic peptide, heart failure
Condensed abstract
Left ventricular hypertrophy (LVH) combined with elevations in cardiac biomarkers reflecting myocardial injury and neurohormonal stress (malignant LVH) is associated with a high risk for heart failure and death, but the impact of intensive systolic blood pressure (SBP) control on the prevention of malignant LVH and its consequences is unknown. In the Systolic Blood Pressure Intervention Trial (SPRINT), randomization to intensive SBP lowering versus standard SBP lowering reduced the incidence of malignant LVH and possibly led to large absolute risk reduction in the composite of acute decompensated heart failure events and death among SPRINT participants with baseline malignant LVH.
Introduction
Pathological left ventricular hypertrophy (LVH), which can be detected by 12-lead ECG or cardiac imaging, most often occurs in response to hypertension and has strong associations with incident heart failure (HF) and death.1,2 Individuals who progress from asymptomatic LVH to HF are hypothesized to have maladaptive cardiac remodeling resulting from chronic myocardial cell injury, inflammation, and fibrosis, accompanied by neurohormonal activation due to increased diastolic wall stress.3–5 Recent observational data demonstrate that LVH subphenotypes exist with markedly different risks for HF and death. LVH accompanied by biomarker evidence of chronic myocardial injury, as measured by high sensitivity cardiac troponin T (hs-cTnT) or I (hs-cTnI), and neurohormonal activation, as measured by N-terminal pro-B-type natriuretic peptide (NT-proBNP), identifies a subgroup known as malignant LVH that is at particularly high risk for HF and death.6–9
Among individuals with hypertension and at high cardiovascular disease (CVD) risk, the Systolic Blood Pressure Intervention Trial (SPRINT) demonstrated that targeting a systolic blood pressure (SBP) <120 mm Hg compared with <140 mm Hg led to significant reductions in acute decompensated heart failure (ADHF) events and death.10 SPRINT and other randomized clinical trials have also shown that intensive SBP lowering leads to the prevention and regression of LVH.11–13 However, it is unknown whether intensive SBP lowering can prevent or reverse malignant LVH, or impact the risk of ADHF events and death when malignant LVH is present.
Our objectives were to evaluate in SPRINT whether intensive versus standard SBP lowering would (1) prevent the development of malignant LVH and (2) reduce ADHF events and death among individuals with malignant LVH present at baseline.
Methods
Study design
The design and protocol of SPRINT have been reported previously.10,14 In brief, SPRINT was an NIH-funded open-label clinical trial that randomized participants with hypertension to an “intensive” SBP target (<120 mm Hg) versus a “standard” SBP target (<140 mm Hg). Inclusion criteria were age ≥50 years; systolic BP 130-180 mm Hg; and high CVD risk (defined as prior clinical or subclinical CVD other than stroke, chronic kidney disease [eGFR 20-59 ml/min/1.73m2], age ≥75 years, or 10-year CVD risk >15% based on the Framingham risk score). Key exclusion criteria included diabetes mellitus, prior stroke or transient ischemic attack, eGFR <20 ml/min/1.73m2, symptomatic heart failure, or a left ventricular ejection fraction <35%. A total of 9,361 participants were enrolled between November 2010 and March 2013 across 102 sites in the United States and Puerto Rico. The SPRINT study was approved by the Institutional Review Board at each participating study site, and all participants provided written informed consent.
This ancillary study included 8,820 (94.2%) SPRINT participants with a baseline standard 12-lead electrocardiogram (ECG) and baseline measures of hs-cTnT and NT-proBNP, and was approved by the Institutional Review Board at the University of Texas Southwestern Medical Center. In analyses of incidence and regression of malignant LVH, we excluded those without at least one follow-up ECG (n=1,059). The data that support the findings of this study are available from the National Heart, Lung, and Blood Institute (NHLBI) Biologic Specimen and Data Repositories and the corresponding author upon request.
ECG and cardiac biomarker measurements
LVH was determined using standard 12-lead ECGs obtained at baseline, year 2, year 4, and close-out visits. Digital ECG data were recorded using a GE MAC 1200 electrocardiograph (GE, Milwaukee, Wisconsin) at 10 mm/mV calibration and a speed of 25 mm/s. ECG reading was performed centrally at the Epidemiological Cardiology Research Center (EPICARE), Wake Forest School of Medicine, Winston Salem, North Carolina. All ECG tracings were initially inspected visually for technical errors and inadequate quality before being automatically processed using GE 12-SL Marquette version 2001 (GE, Milwaukee, Wisconsin).
Cardiac biomarkers were measured from blood samples obtained at the time of study entry and year 2 of the trial follow-up period. All blood samples were stored at −80°C until hs-cTnT and NT-proBNP measurements were performed at the SPRINT Central Laboratory (University of Minnesota, Minneapolis, MN). Both hs-cTnT and NT-proBNP were measured from freshly thawed serum samples using an electrochemiluminescence immunoassay on the Roche Cobas 6000 platform (Roche Diagnostics, Indianapolis, IN) as previously described.15 The hs-cTnT assay (5th Generation) has an imprecision of 3.4% at 28.3 ng/L and 2.3% at 2076 ng/L, with a lower limit of quantitation of 6 ng/L. The NT-proBNP assay has an imprecision of 2.9% at 140.3 pg/mL and 2.7% at 4563 pg/mL, with a lower limit of detection of 5 pg/mL.
Combined LVH and cardiac biomarker groups
Consistent with previous studies in SPRINT, LVH was defined using Cornell voltage criteria (RaVL amplitude + SV3 amplitude) with sex-specific thresholds of ≥2,200 microvolt (μV) in women and ≥2,800 μV in men, and elevated cardiac biomarkers was defined as hs-cTnT ≥14 ng/L and/or NT-proBNP ≥125 pg/mL.13,15,16 Participants were categorized into four groups: 1) no LVH with non-elevated cardiac biomarkers (LVH− biomarker−); 2) no LVH with elevated cardiac biomarkers (LVH− biomarker+); 3) LVH with non-elevated cardiac biomarkers (LVH+ biomarker−); and 4) LVH with elevated cardiac biomarkers (LVH+ biomarker+), which we defined as malignant LVH.6–8 In sensitivity analyses, we also defined LVH using Cornell voltage product ([RaVL amplitude + SV3 amplitude]*QRS duration) and Sokolow-Lyon (SV1 amplitude + RV5/V6 amplitude) LVH criteria.17,18
Outcomes
The primary outcome of this analysis was the composite of incident HF events and all-cause mortality. We selected this outcome because elevated cardiac biomarkers and LVH are most strongly associated with these endpoints.15 Additionally, the effect of intensive SBP lowering on the primary CVD composite outcome in SPRINT was primarily driven by reductions in these endpoints.10 The definition, ascertainment, and formal adjudication of these events have been previously described in detail.10,14,19 Incident ADHF events were defined as hospitalization or emergency department visit requiring treatment with infusion therapy (diuretic or inotropic agents) for a clinical syndrome that presented with multiple signs and symptoms consistent with ADHF. Chronic stable HF during a hospitalization, reduced left ventricular ejection fraction in the absence of symptoms, right-sided HF, volume overload due to inadequate dialysis, and new outpatient HF were not considered incident ADHF endpoints in SPRINT.19 We also evaluated all-cause mortality as a secondary clinical outcome. Secondary subclinical outcomes included: 1) incidence and regression of malignant LVH, 2) incidence and regression of LVH, and 3) annual change in Cornell voltage index.
Malignant LVH incidence and regression were restricted to the first two years of the trial follow-up period, when both cardiac biomarker and ECG measurements were available. Incident malignant LVH occurred when individuals without baseline malignant LVH subsequently met Cornell voltage criteria on ECG and had elevated cardiac biomarkers at year 2 of follow-up. Malignant LVH regression occurred when individuals with baseline malignant LVH no longer met either LVH or cardiac biomarker criteria for malignant LVH at year 2 of follow-up.
LVH incidence and regression were assessed during the total trial follow-up period. Incident LVH occurred when individuals without baseline LVH met Cornell voltage criteria for LVH on a follow-up ECG, and LVH regression occurred when individuals with baseline LVH no longer met Cornell voltage criteria for LVH on a follow-up ECG. In addition to binary LVH outcomes, we evaluated annual change in the Cornell voltage index as a continuous variable, defined as the annualized difference between the Cornell voltage index on the last available ECG and the baseline ECG.
Statistical analyses
Baseline characteristics were compared across LVH/biomarker categories using ANOVA or Kruskal-Wallis test for continuous variables, and Chi-square test for categorical variables. We replaced undetectable hs-cTnT levels (21% <6 ng/L) and NT-proBNP levels (3.4% <5 pg/mL) as the lower limit of detection divided by two.
We evaluated associations of LVH/biomarker categories with risk of the clinical outcomes in the entire SPRINT cohort using multivariable Cox proportional hazards models. There was no evidence that the proportional hazards assumptions were violated. Models were adjusted for demographics (age, sex, race, site), treatment assignment, and clinical characteristics (body mass index [BMI], smoking status, prevalent CVD, SBP, estimated glomerular filtration rate [eGFR], and low density lipoprotein [LDL] cholesterol).
Event rates were then compared between the intensive SBP and standard SBP arms among participants in each LVH/biomarker category. Heterogeneity of treatment effect across LVH/biomarker categories was tested using a likelihood ratio test for multiplicative interaction terms (treatment assignment by LVH/biomarker category) in models that included main effects. Absolute risk differences over 4 years between randomized treatment groups and corresponding 95% CIs were estimated for each endpoint using the method described by Altman and Andersen.20
Logistic regression models were used to evaluate the effect of intensive versus standard SBP lowering on incident malignant LVH and malignant LVH regression, and on incident LVH and LVH regression stratified by baseline elevated versus non-elevated cardiac biomarkers. Linear regression models were used to evaluate the treatment effect on annual changes in Cornell voltage index, and were stratified by combined LVH/biomarker category.
In sensitivity analyses, we used Cornell voltage product and Sokolow-Lyon criteria instead of Cornell voltage index to define LVH to confirm our findings were insensitive to LVH criteria.
Underlying assumptions including linearity were assessed while building multivariable models for each outcome of interest. All analyses were conducted using SAS software, version 9.4 (SAS Institute, Inc, Cary, NC).
Results
Study population and baseline characteristics
Among the 9,361 SPRINT participants, 8,820 (94.2%) had both hs-cTnT (median [IQR] 9.4 [6.4, 14.1] ng/L] and NT-proBNP (median [IQR] 86 [37, 197] pg/mL) measured at baseline. In the cardiac biomarker study sample, 4,361 (49.4%) had no LVH and non-elevated cardiac biomarkers at baseline (LVH− biomarker−), 3,761 (42.6%) had no LVH and elevated cardiac biomarkers (LVH− biomarker+), 249 (2.8%) had LVH with non-elevated cardiac biomarkers (LVH+ biomarker−), and 449 (5.1%) were categorized as malignant LVH (LVH+ biomarker+). LVH− biomarker− participants had median hs-cTnT and NT-proBNP levels of 7.3 ng/L and 44 pg/mL, respectively, and a median Cornell voltage of 1422 μV. The malignant LVH group had median hs-cTnT and NT-proBNP levels of 15.3 ng/L and 308 pg/mL, respectively, and a median Cornell voltage of 2883 μV. Older age, African American race, female sex, and a higher burden of comorbidities were also associated with baseline malignant LVH (Table 1).
Table 1.
Baseline characteristics of SPRINT participants stratified by combined LVH and cardiac biomarker categories
| Characteristica | LVH− biomarker− (n=4,361) | LVH− biomarker+ (n=3,761) | LVH+ biomarker− (n=249) | LVH+ biomarker+ (n=449) |
|---|---|---|---|---|
| Intensive BP arm | 2168 (50%) | 1918 (51%) | 121 (49%) | 211 (47%) |
| Age, years | 64 (8) | 72 (9) | 64 (8) | 70 (10) |
| Female | 1471 (34%) | 1346 (36%) | 169 (68%) | 260 (58%) |
| African Americanb | 1493 (34%) | 877 (23%) | 146 (59%) | 210 (47%) |
| Hispanicb | 581 (13%) | 290 (8%) | 32 (13%) | 41 (9%) |
| Current smoker | 670 (15%) | 377 (10%) | 43 (17%) | 57 (13%) |
| Prevalent CVD | 568 (13%) | 994 (26%) | 41 (16%) | 147 (33%) |
| Prevalent heart failureb | 67 (2%) | 187 (5%) | 5 (2%) | 41 (9%) |
| Systolic BP | ||||
| ≤132 mmHg | 573 (13%) | 448 (12%) | 20 (8%) | 28 (6%) |
| >132 mmHg to <145 mmHg | 1864 (43%) | 1579 (42%) | 72 (29%) | 147 (33%) |
| ≥145 mmHg | 1924 (44%) | 1734 (46%) | 157 (63%) | 274 (61%) |
| Diastolic BP | ||||
| ≤70 mmHg | 471 (11%) | 1130 (30%) | 25 (10%) | 122 (27%) |
| >70 mmHg to <80 mmHg | 1148 (26%) | 1090 (29%) | 61 (25%) | 120 (27%) |
| ≥80 mmHg | 2742 (63%) | 1541 (41%) | 163 (65%) | 207 (46%) |
| Total med burden ≥5 | 2070 (48%) | 2478 (66%) | 111 (45%) | 278 (62%) |
| Number of BP meds | 2 (1, 2) | 2 (1, 3) | 2 (1, 3) | 2 (2, 3) |
| BMI, kg/m2 | 30.2 (5.5) | 29.3 (5.8) | 30.4 (6.0) | 29.3 (5.7) |
| eGFR, mL/min/1.73m2 | 77 (19) | 65 (20) | 81 (20) | 65 (21) |
| Urine ACR, mg/g | 8 (5, 15) | 12 (7, 32) | 9 (6, 21) | 16 (8, 45) |
| hs-cTnT, ng/L | 7.3 (3, 9.7) | 14.4 (9.2, 19.6) | 7.1 (3, 9.9) | 15.3 (9.9, 21.1) |
| NT-proBNP, pg/mL | 44 (22, 74) | 198 (131, 352) | 55 (29, 86) | 308 (168, 569) |
| Cornell voltage index, μV | 1422 (1071, 1782) | 1409 (1043, 1797) | 2800 (2373, 3073) | 2883 (2465, 3259) |
| Sokolow-Lyon, μV | 2032 (1610, 2508) | 2034 (1570, 2580) | 2444 (2063, 3088) | 2661 (2056, 3420.5) |
Data displayed as N (%), mean (SD), or median (IQR).
LVH+ defined as Cornell voltage index on baseline ECG ≥2,200 microvolt (μV) in women and ≥2,800 μV in men. Biomarker+ defined as baseline hscTnT ≥ 14 ng/L or NTproBNP ≥ 125 pg/mL.
Abbreviations: ACR, albumin-to-creatinine ratio; BMI, body mass index; BP, blood pressure; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; hs-cTnT, high-sensitivity cardiac troponin T; LVH, left ventricular hypertrophy; NT-proBNP, N-terminal pro-B-type natriuretic peptide; SPRINT, Systolic Blood Pressure Intervention Trial.
All P values testing for differences in baseline characteristics across combined LVH and biomarker groups were <0.001 except for intensive BP arm (P =0.33).
Race, ethnicity, and history of heart failure were self-reported.
Effects of intensive versus standard SBP lowering on ADHF events and all-cause mortality
The proportion of participants who experienced the composite of ADHF events and all-cause mortality varied across LVH/biomarker categories, occurring in 13.6% of participants with baseline malignant LVH, compared with 8.2% of participants who were LVH− biomarker+, 5.6% who were LVH+ biomarker−, and 1.9% who were LVH− biomarker−. These findings persisted in multivariable-adjusted analyses, with malignant LVH participants having a 4-fold higher risk of the composite of incident ADHF events and all-cause mortality compared with LVH− biomarker− participants (adjusted hazard ratio [HR] 3.88, 95% confidence interval [CI]: 2.44, 6.18). Furthermore, LVH− biomarker+ and LVH+ biomarker− participants had a 2-fold higher risk of incident ADHF events and all-cause mortality compared with LVH− biomarker− participants (Figure 1). Similar patterns of results were noted for all-cause mortality (Figure 1).
Figure 1. Associations between LVH/biomarker categories and incident ADHF events and mortality.
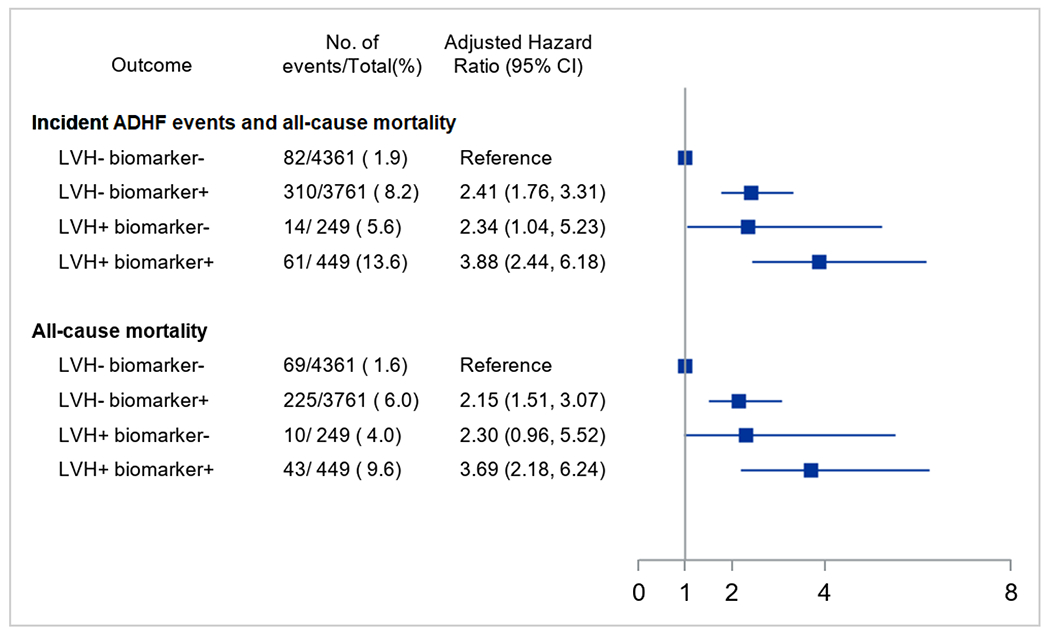
Hazard ratios with 95% confidence intervals obtained from multivariable Cox proportional hazards models that included demographics (age, sex, race, site), treatment assignment, and clinical characteristics (body mass index, smoking status, prevalent CVD, systolic blood pressure, estimated glomerular filtration rate, and low density lipoprotein cholesterol). Combined LVH and biomarker categories include: 1) no LVH and non-elevated cardiac biomarkers (LVH− biomarker−), 2) no LVH and elevated cardiac biomarkers (LVH− biomarker+), 3) LVH and non-elevated cardiac biomarkers (LVH+ biomarker−), and 4) LVH and elevated cardiac biomarkers (LVH+ biomarker+). Elevated cardiac biomarkers defined as hs-cTnT ≥ 14 ng/L or NT-proBNP ≥ 125 pg/mL. Abbreviations: ADHF, acute decompensated heart failure; CVD, cardiovascular disease; HR, hazard ratio; hs-cTnT, high-sensitivity cardiac troponin T; LVH, left ventricular hypertrophy; NT-proBNP, N-terminal pro-B-type natriuretic peptide; SPRINT, Systolic Blood Pressure Intervention Trial.
Randomization to intensive versus standard SBP lowering was associated with a similar relative risk reduction for the composite of incident ADHF events and all-cause mortality across all four LVH/biomarker categories (P for interaction = 0.68; Table 2). Relative effects in each LVH/biomarker group appeared similar among Black and White participants (data not shown). However, because of the much higher event rate in the malignant LVH group, the 4-year ARR was 4.4% (95% CI: −5.2%, 13.9%) compared with the 2.5% (95% CI: −0.3%, 5.3%) in the LVH− biomarker+ group, 2.0% (95% CI: −6.6%, 10.7%) in the LVH+ biomarker− group, and 1.2% (95% CI: 0.0%, 2.5%) in the LVH− biomarker− group, with corresponding NNTs of 23, 41, 50, and 82, respectively. A similar pattern of results was noted for all-cause mortality (Table 2).
Table 2.
Effect of intensive SBP lowering on cardiovascular outcomes stratified by combined LVH and biomarker categories
| Outcome | Standard SBP Events/Total No. (%) | Intensive SBP Events/Total No. (%) | HR (95% CI) | P a | 4-year ARR (%) (95% CI) | NNT |
|---|---|---|---|---|---|---|
| Incident ADHF events and all-cause mortality | 0.68 | |||||
| LVH − biomarker − | 54/2193 (2.5%) | 28/2168 (1.3%) | 0.52 (0.33, 0.83) | 1.2% (0.0%, 2.5%) | 82 | |
| LVH − biomarker+ | 177/1843 (9.6%) | 133/1918 (6.9%) | 0.71 (0.57, 0.89) | 2.5% (−0.3%, 5.3%) | 41 | |
| LVH+ biomarker− | 8/128 (6.3%) | 6/121 (5.0%) | 0.77 (0.27, 2.23) | 2.0% (−6.6%, 10.7%) | 50 | |
| LVH+ biomarker+ | 37/238 (15.5%) | 24/211 (11.4%) | 0.70 (0.42, 1.16) | 4.4% (−5.2%, 13.9%) | 23 | |
| All-cause mortality | 0.88 | |||||
| LVH − biomarker − | 42/2193 (1.9%) | 27/2168 (1.2%) | 0.65 (0.40, 1.06) | 0.7% (−0.5%, 1.9%) | 143 | |
| LVH − biomarker+ | 126/1843 (6.8%) | 99/1918 (5.2%) | 0.75 (0.58, 0.98) | 1.6% (−0.8%, 4.0%) | 64 | |
| LVH+ biomarker− | 6/128 (4.7%) | 4/121 (3.3%) | 0.69 (0.19, 2.43) | 2.1% (−5.3%, 9.5%) | 49 | |
| LVH+ biomarker+ | 28/238 (11.8%) | 15/211 (7.1%) | 0.58 (0.31, 1.09) | 5.9% (−2.1%, 14.0%) | 17 |
Abbreviations: ADHF, acute decompensated heart failure; CI, confidence interval; CVD, cardiovascular disease; HR, hazard ratio; LVH, left ventricular hypertrophy; SBP, systolic blood pressure.
P-value for interaction on the relative hazard scale.
Effects of intensive versus standard SBP lowering on LVH outcomes
Among 7,431 participants without malignant LVH at baseline and with one or more follow-up ECG and cardiac biomarker measurement, 133 (1.8%) developed malignant LVH. Compared with standard SBP lowering, intensive SBP lowering led to a significant reduction in the incidence of malignant LVH (2.5% vs. 1.1%, odds ratio [OR]: 0.44, 95% CI: 0.30, 0.63, Figure 2). Among the 375 participants with malignant LVH at baseline who had one or more follow-up ECG and cardiac biomarker measurement, 216 (57.6%) experienced malignant LVH regression. Randomization to intensive versus standard BP lowering was associated with numerically greater malignant LVH regression (61.8% vs. 53.4%, OR 1.41, 95% CI: 0.94, 2.13).
Figure 2. Intensive SBP lowering effects on incident LVH and malignant LVH.
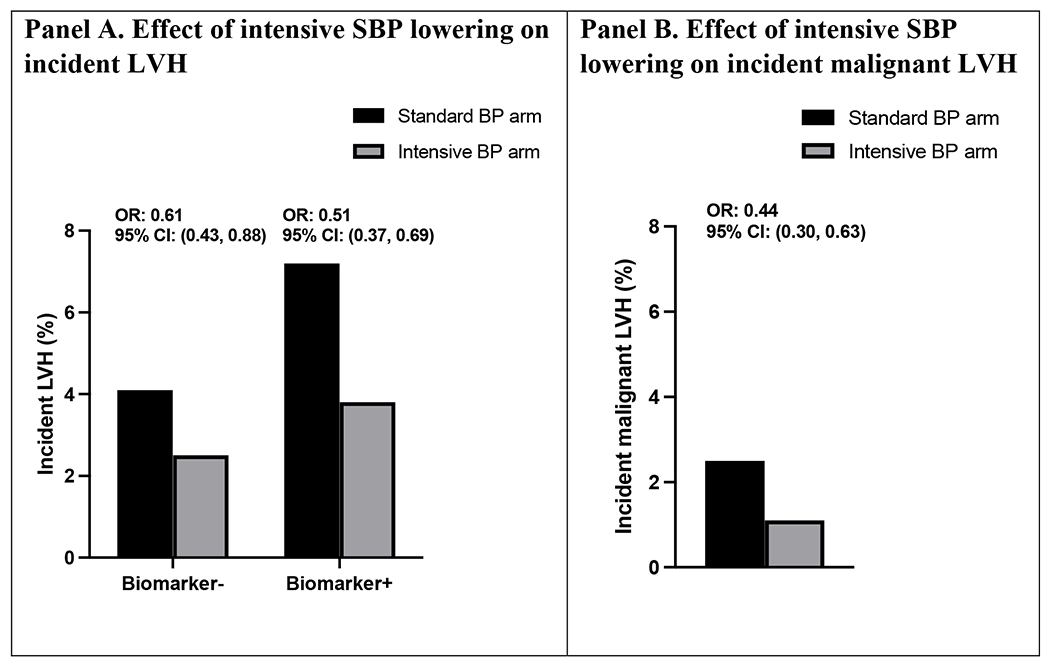
Odds ratios with 95% confidence intervals obtained from logistic regression models. Panel A displays proportion without LVH at baseline who developed LVH during follow-up stratified by randomized treatment assignment and baseline cardiac biomarker levels. Biomarker+ indicates hs-cTnT ≥14 ng/L or NT-proBNP ≥125 pg/mL. Panel B displays proportion without malignant LVH at baseline who developed malignant LVH during follow-up stratified by randomized treatment assignment. Abbreviations: hs-cTnT, high-sensitivity cardiac troponin T; LVH, left ventricular hypertrophy; NT-proBNP, N-terminal pro-B-type natriuretic peptide; OR, odds ratio; SBP, systolic blood pressure; SPRINT, Systolic Blood Pressure Intervention Trial.
Among 7,215 participants without LVH at baseline and with one or more follow-up ECGs, 310 (4.3%) developed LVH. Randomization to intensive versus standard SBP lowering reduced the risk of incident LVH similarly among biomarker− (OR: 0.51, 95% CI: 0.37, 0.69) and biomarker+ participants (OR 0.61, 95% CI: 0.43, 0.88; P for interaction =0.43, Figure 2). Among 591 participants with LVH at baseline, 366 (61.9%) experienced LVH regression. Randomization to intensive versus standard SBP lowering led to more LVH regression and was similar among biomarker− (OR 2.29, 95% CI: 1.29, 4.06) and biomarker+ participants (OR 1.71, 95% CI: 1.12, 2.59, P for interaction = 0.42).
Compared with standard SBP lowering, intensive SBP lowering led to a greater reduction in Cornell voltage index during the follow-up period across all LVH/biomarker categories (Supplementary Table S1). The effect of intensive SBP lowering on reduction in Cornell voltage index was stronger among LVH− biomarker+ participants compared with LVH− biomarker− participants (P for interaction =0.02).
In sensitivity analyses using Cornell voltage product and Sokolow-Lyon LVH criteria, the effects of intensive SBP lowering on clinical and LVH outcomes were overall similar to using Cornell voltage index in main analyses (Supplementary Tables S2–S4).
Discussion
In this ancillary analysis of SPRINT, participants with malignant LVH had markedly higher rates of ADHF events and all-cause death than those without LVH or biomarker elevation, as well as higher risk than those with either LVH or biomarker elevation. Intensive SBP lowering led to similar relative risk reductions in ADHF events and all-cause death across LVH/biomarker groups, and was compatible with large absolute risk reductions among those with baseline malignant LVH due to the high risk in this subgroup, although the finding did not reach statistical significance. We also observed that randomization to intensive versus standard SBP lowering reduced the incidence of malignant LVH. Taken together, these data highlight the importance of intensive SBP lowering in modifying the natural history of malignant LVH by preventing its development, and when present, decreasing the risk of ADHF events and death (Central Illustration).
Central Illustration: Intensive SBP lowering and the natural history of malignant LVH.
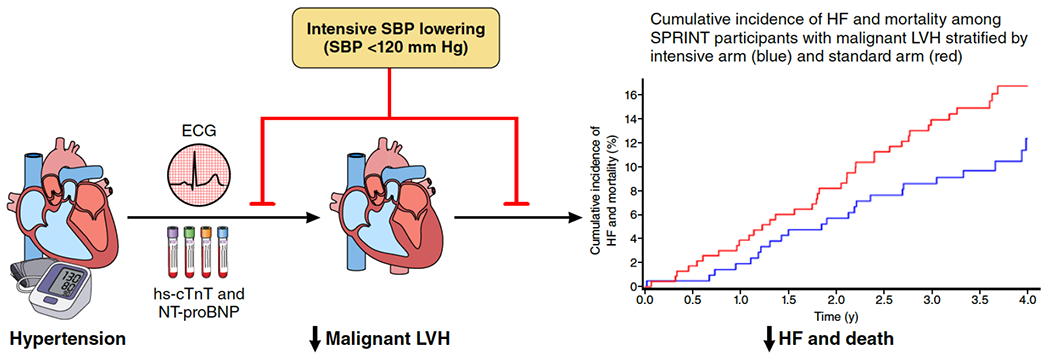
Randomization to intensive SBP lowering (SBP <120 mm Hg) versus standard SBP lowering (SBP <140 mm Hg) not only prevents the development of malignant LVH (LVH with hs-cTnT ≥14 ng/L or NT-proBNP ≥125 pg/mL), but may also lead to substantial absolute risk reductions in the composite of ADHF events and all-cause mortality when malignant LVH is present. Abbreviations: ADHF, acute decompensated heart failure; hs-cTnT, high-sensitivity cardiac troponin T; LVH, left ventricular hypertrophy; NT-proBNP, N-terminal pro-B-type natriuretic peptide; SBP, systolic blood pressure; SPRINT, Systolic Blood Pressure Intervention Trial.
To our knowledge, no therapies have been established for the specific prevention or treatment of malignant LVH. Thus, our findings have important clinical implications. First, our novel findings that intensive SBP lowering prevents malignant LVH, prevents LVH similarly among individuals with both elevated and non-elevated cardiac biomarkers, and leads to greater voltage reductions among individuals with elevated cardiac biomarkers expand upon prior work that has demonstrated achieving lower BP targets improves LVH outcomes.11–13 These results suggest that routine measurement of hs-cTnT and NT-proBNP levels among hypertensive individuals may be helpful for identifying persons for whom intensive BP control can delay the onset of structural heart disease. Second, these data provide direct trial evidence that the substantial risk associated with malignant LVH may be modifiable, suggesting that both individuals with LVH and malignant LVH appear to derive cardiovascular benefits from achieving lower BP targets.13,21 Previous work in SPRINT observed that ECG-LVH did not explain most of the reduction in the primary CVD composite from intensive SBP lowering, but the impact of BP lowering on LVH may mediate effects on specific CVD outcomes, such as heart failure (HF) and death.13
Although routine cardiac imaging or ECG to screen for LVH is not currently recommended, our findings support the concept that a multimodality screening approach among select individuals with hypertension could distinguish those with elevated yet modifiable cardiovascular risk.15,22 Universal ECG screening for LVH is not recommended owing to a lack of data on the value of ECG in assessing CVD risk to inform hypertension treatment decisions.23 However, we and others have shown that LVH is a heterogeneous phenotype with marked variation in natural history depending on the presence or absence of elevated cardiac biomarkers. Our findings suggest that ECG surveillance augmented by cardiac biomarkers to detect malignant LVH could be considered as an efficient strategy to identify those who may derive substantial absolute HF and mortality benefits from intensive SBP lowering.
Our finding that SPRINT participants with LVH and minimal elevations in cardiac biomarkers had a nearly 4-fold increased risk of ADHF events and all-cause death compared with LVH− biomarker− participants is consistent with previous observational studies.6–9 LVH is common in the setting of hypertension, and numerous studies have established that both ECG-derived and imaging-derived measures of LVH are strong risk factors for HF and mortality.1,2 While the natural history of LVH varies considerably between individuals, pathologic cardiac remodeling is thought to be the key driver of LVH progression to HF. Cardiac biomarkers may reflect several mechanisms underlying this maladaptive process, including chronic myocardial cell injury, neurohormonal activation, and myocardial fibrosis.3–5,24,25
Study limitations
Our study has several limitations. First, the relatively small proportion of SPRINT participants with baseline malignant LVH limited our ability to detect modest intensive SBP lowering effect sizes, and to evaluate effect modification by race and sex subgroups.26 Second, LVH was defined using ECG, which may have been less accurate than echocardiography or cardiac magnetic resonance imaging (MRI). However, ECGs are widely available, utilized in most patients with hypertension, and LVH by ECG is associated with CVD and mortality.27,28 Moreover, prior studies have shown similar risk of malignant LVH when defined using ECG and cardiac MRI.6 LVH misclassification would have been non-differential across randomized treatment groups and likely biased results to the null. Third, we used Cornell voltage to define LVH by ECG instead of other LVH criteria. We chose Cornell voltage because it is one of the most commonly used LVH criteria, and previous work in SPRINT demonstrated that the effect of intensive SBP lowering on LVH incidence and regression was similar using Cornell voltage or other LVH criteria.13 We also observed similar results in sensitivity analyses using two other commonly used LVH criteria (Sokolow-Lyon and Cornell voltage product). Finally, our findings may not be generalizable to individuals with malignant LVH who did not meet eligibility criteria for SPRINT, including those with diabetes, prior stroke, or at younger ages.
Despite these limitations, this analysis is the first report from a well-designed, large clinical trial describing the impact of intensive SBP lowering on outcomes in malignant LVH. As an ancillary study of SPRINT, this analysis benefited from inclusion of over 8,000 participants with baseline ECG data and hs-cTnT and NT-proBNP measurements; the use of randomized data to minimized the impact of confounding; protocol driven data collection including ECGs that were centrally read; and rigorously adjudicated HF and death outcomes.
Conclusions
This study reinforces the hypothesis that elevated cardiac biomarkers in the presence of LVH identifies a subclinical, malignant LVH phenotype, and presents the best available evidence to date that intensive SBP lowering not only prevents malignant LVH, but may also provide large absolute risk reductions for ADHF events and death when malignant LVH is present. These findings support intensive SBP lowering as an effective treatment for patients with malignant LVH, and additionally provide preliminary support that intensive SBP lowering may prevent the development of malignant LVH.
Supplementary Material
Clinical Perspectives.
Competency in Patient Care and Procedural Skills:
Compared with lowering systolic blood pressure (SBP) below 140 mmHg, more intensive lowering to <120 mmHg) reduces progression to malignant left ventricular hypertrophy (LVH), and the development of heart failure or death in those with malignant LVH.
Translational Outlook:
Larger trials with longer follow-up of individuals with malignant LVH could provide better estimates of the benefit of intensive SBP lowering.
Acknowledgements:
The authors thank the participants and staff members of the Systolic Blood Pressure Intervention Trial, which was sponsored by the National Institutes of Health (NIH), including the National Heart, Lung, and Blood Institute (NHLBI), the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), the National Institute on Aging (NIA), and the National Institute of Neurological Disorders and Stroke (NINDS), under Contract Numbers HHSN268200900040C, HHSN268200900046C, HHSN268200900047C, HHSN268200900048C, HHSN268200900049C, and Inter-Agency Agreement Number A-HL-13-002-001. It was also supported in part with resources and use of facilities through the Department of Veterans Affairs. The SPRINT investigators acknowledge the contribution of study medications (azilsartan and azilsartan combined with chlorthalidone) from Takeda Pharmaceuticals International, Inc. All components of the SPRINT study protocol were designed and implemented by the investigators. The investigative team collected, analyzed, and interpreted the data. All aspects of manuscript writing and revision were carried out by the coauthors. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH, the U.S. Department of Veterans Affairs, or the United States Government. For a full list of contributors to SPRINT, please see the supplementary acknowledgement list: https://www.sprinttrial.org/public/dspScience.cfm.
We also acknowledge the support from the following CTSAs funded by NCATS: CWRU: UL1TR000439, OSU: UL1RR025755, U Penn: UL1RR024134& UL1TR000003, Boston: UL1RR025771, Stanford: UL1TR000093, Tufts: UL1RR025752, UL1TR000073 & UL1TR001064, University of Illinois: UL1TR000050, University of Pittsburgh: UL1TR000005, UT Southwestern: 9U54TR000017-06, University of Utah: UL1TR000105-05, Vanderbilt University: UL1 TR000445, George Washington University: UL1TR000075, University of CA, Davis: UL1 TR000002, University of Florida: UL1 TR000064, University of Michigan: UL1TR000433, Tulane University: P30GM103337 COBRE Award NIGMS, Wake Forest University: UL1TR001420.
Funding:
This ancillary study was supported by the NHLBI (1R01HL144112-01 for JDB). Analytical reagents for hs-cTnT and NT-pro-BNP measurements were donated by Roche (Indianapolis, IN).
Disclosures:
JAd reports grant support from Roche Diagnostics and Abbott Diagnostics, consulting fees from Roche Diagnostics, Abbott Diagnostics, Ortho Clinical Diagnostics, Quidel Cardiovascular, Inc., and Siemen’s Health Care Diagnostics. He has been named a co-owner on a patent awarded to the University of Maryland (US Patent Application Number: 15/309,754) entitled: “Methods for Assessing Differential Risk for Developing Heart Failure.” DWK has received honoraria as a consultant outside the present study for Bayer, Merck, Pfizer, Corvia Medical, Boehringer Ingelheim, Ketyo, Rivus, NovoNordisk, AstraZeneca and Novartis, grant funding from Novartis, Bayer, Pfizer, NovoNordisk and AstraZeneca, and has stock ownership in Gilead Sciences. JDB reports grant support from the NIH, Roche Diagnostics and Abbott Diagnostics, consulting fees from Roche Diagnostics, and the Cooper Institute. The remaining authors have nothing to disclose.
Abbreviations:
- ADHF
acute decompensated heart failure
- hs-cTnT
high-sensitivity cardiac troponin T
- HF
heart failure
- LVH
left ventricular hypertrophy
- NT-proBNP
N-terminal pro-B-type natriuretic peptide
- SBP
systolic blood pressure
- SPRINT
Systolic Blood Pressure Intervention Trial
Footnotes
Tweet: Study in #SPRINT suggests intensive #bloodpressure therapy prevents malignant LVH as well as #HF and death when malignant LVH is present.
References
- 1.Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med 1990;322:1561–1566. doi: 10.1056/NEJM199005313222203. [DOI] [PubMed] [Google Scholar]
- 2.Drazner MH, Rame JE, Marino EK, Gottdiener JS, Kitzman DW, Gardin JM, Manolio TA, Dries DL, Siscovick DS. Increased left ventricular mass is a risk factor for the development of a depressed left ventricular ejection fraction within five years: the Cardiovascular Health Study. J Am Coll Cardiol 2004;43:2207–2215. doi: 10.1016/j.jacc.2003.11.064. [DOI] [PubMed] [Google Scholar]
- 3.Ahmed SH, Clark LL, Pennington WR, Webb CS, Bonnema DD, Leonardi AH, McClure CD, Spinale FG, Zile MR. Matrix metalloproteinases/tissue inhibitors of metalloproteinases: relationship between changes in proteolytic determinants of matrix composition and structural, functional, and clinical manifestations of hypertensive heart disease. Circulation 2006;113:2089–2096. doi: 10.1161/CIRCULATIONAHA.105.573865. [DOI] [PubMed] [Google Scholar]
- 4.Zile MR, Bennett TD, St John Sutton M, Cho YK, Adamson PB, Aaron MF, Aranda JM, Abraham WT, Smart FW, Stevenson LW, Kueffer FJ, Bourge RC. Transition from chronic compensated to acute decompensated heart failure: pathophysiological insights obtained from continuous monitoring of intracardiac pressures. Circulation 2008;118:1433–1441. doi: 10.1161/CIRCULATIONAHA.108.783910. [DOI] [PubMed] [Google Scholar]
- 5.Drazner MH. The progression of hypertensive heart disease. Circulation 2011;123:327–334. doi: 10.1161/CIRCULATIONAHA.108.845792. [DOI] [PubMed] [Google Scholar]
- 6.Neeland IJ, Drazner MH, Berry JD, Ayers CR, deFilippi C, Seliger SL, Nambi V, McGuire DK, Omland T, de Lemos JA. Biomarkers of chronic cardiac injury and hemodynamic stress identify a malignant phenotype of left ventricular hypertrophy in the general population. J Am Coll Cardiol 2013;61:187–195. doi: 10.1016/j.jacc.2012.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seliger SL, de Lemos J, Neeland IJ, Christenson R, Gottdiener J, Drazner MH, Berry J, Sorkin J, deFilippi C. Older Adults, “Malignant” Left Ventricular Hypertrophy, and Associated Cardiac-Specific Biomarker Phenotypes to Identify the Differential Risk of New-Onset Reduced Versus Preserved Ejection Fraction Heart Failure: CHS (Cardiovascular Health Study). JACC Heart Fail 2015;3:445–455. doi: 10.1016/j.jchf.2014.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peters MN, Seliger SL, Christenson RH, Hong-Zohlman SN, Daniels LB, Lima JAC, de Lemos JA, Neeland IJ, deFilippi CR. “Malignant” Left Ventricular Hypertrophy Identifies Subjects at High Risk for Progression to Asymptomatic Left Ventricular Dysfunction, Heart Failure, and Death: MESA (Multi-Ethnic Study of Atherosclerosis). J Am Heart Assoc 2018;7. doi: 10.1161/JAHA.117.006619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pandey A, Keshvani N, Ayers C, Correa A, Drazner MH, Lewis A, Rodriguez CJ, Hall ME, Fox ER, Mentz RJ, et al. Association of Cardiac Injury and Malignant Left Ventricular Hypertrophy With Risk of Heart Failure in African Americans: The Jackson Heart Study. JAMA Cardiol 2019;4:51–58. doi: 10.1001/jamacardio.2018.4300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.SPRINT Research Group, Wright JT, Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MV, Reboussin DM, Rahman M, Oparil S, et al. A Randomized Trial of Intensive versus Standard Blood-Pressure Control. N Engl J Med 2015;373:2103–2116. doi: 10.1056/NEJMoa1511939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Verdecchia P, Staessen JA, Angeli F, de Simone G, Achilli A, Ganau A, Mureddu G, Pede S, Maggioni AP, Lucci D, Reboldi G, Cardio-Sis investigators. Usual versus tight control of systolic blood pressure in non-diabetic patients with hypertension (Cardio-Sis): an open-label randomised trial. Lancet 2009;374:525–533. doi: 10.1016/S0140-6736(09)61340-4. [DOI] [PubMed] [Google Scholar]
- 12.Soliman EZ, Byington RP, Bigger JT, Evans G, Okin PM, Goff DC, Chen H. Effect of Intensive Blood Pressure Lowering on Left Ventricular Hypertrophy in Patients With Diabetes Mellitus: Action to Control Cardiovascular Risk in Diabetes Blood Pressure Trial. Hypertension 2015;66:1123–1129. doi: 10.1161/HYPERTENSIONAHA.115.06236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soliman EZ, Ambrosius WT, Cushman WC, Zhang Z-M, Bates JT, Neyra JA, Carson TY, Tamariz L, Ghazi L, Cho ME, et al. Effect of Intensive Blood Pressure Lowering on Left Ventricular Hypertrophy in Patients With Hypertension: SPRINT (Systolic Blood Pressure Intervention Trial). Circulation 2017;136:440–450. doi: 10.1161/CIRCULATIONAHA.117.028441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ambrosius WT, Sink KM, Foy CG, Berlowitz DR, Cheung AK, Cushman WC, Fine LJ, Goff DC, Johnson KC, Killeen AA, et al. The design and rationale of a multicenter clinical trial comparing two strategies for control of systolic blood pressure: the Systolic Blood Pressure Intervention Trial (SPRINT). Clin Trials 2014;11:532–546. doi: 10.1177/1740774514537404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berry JD, Nambi V, Ambrosius WT, Chen H, Killeen AA, Taylor A, Toto RD, Soliman EZ, McEvoy JW, Pandey A, et al. Associations of High-Sensitivity Troponin and Natriuretic Peptide Levels With Outcomes After Intensive Blood Pressure Lowering: Findings From the SPRINT Randomized Clinical Trial. JAMA Cardiol 2021;6:1397–1405. doi: 10.1001/jamacardio.2021.3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Casale PN, Devereux RB, Kligfield P, Eisenberg RR, Miller DH, Chaudhary BS, Phillips MC. Electrocardiographic detection of left ventricular hypertrophy: development and prospective validation of improved criteria. J Am Coll Cardiol 1985;6:572–580. doi: 10.1016/s0735-1097(85)80115-7. [DOI] [PubMed] [Google Scholar]
- 17.Molloy TJ, Okin PM, Devereux RB, Kligfield P. Electrocardiographic detection of left ventricular hypertrophy by the simple QRS voltage-duration product. J Am Coll Cardiol 1992;20:1180–1186. doi: 10.1016/0735-1097(92)90376-x. [DOI] [PubMed] [Google Scholar]
- 18.Sokolow M, Lyon TP. The ventricular complex in left ventricular hypertrophy as obtained by unipolar precordial and limb leads. Am Heart J 1949;37:161–186. doi: 10.1016/0002-8703(49)90562-1. [DOI] [PubMed] [Google Scholar]
- 19.Upadhya B, Rocco M, Lewis CE, Oparil S, Lovato LC, Cushman WC, Bates JT, Bello NA, Aurigemma G, Fine LJ, et al. Effect of Intensive Blood Pressure Treatment on Heart Failure Events in the Systolic Blood Pressure Reduction Intervention Trial. Circ Heart Fail 2017;10:e003613. doi: 10.1161/CIRCHEARTFAILURE.116.003613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Altman DG, Andersen PK. Calculating the number needed to treat for trials where the outcome is time to an event. BMJ 1999;319:1492–1495. doi: 10.1136/bmj.319.7223.1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee H-H, Lee H, Cho SMJ, Kim D-W, Park S, Kim HC. On-Treatment Blood Pressure and Cardiovascular Outcomes in Adults With Hypertension and Left Ventricular Hypertrophy. J Am Coll Cardiol 2021;78:1485–1495. doi: 10.1016/j.jacc.2021.08.015. [DOI] [PubMed] [Google Scholar]
- 22.de Lemos JA, Ayers CR, Levine BD, deFilippi CR, Wang TJ, Hundley WG, Berry JD, Seliger SL, McGuire DK, Ouyang P, et al. Multimodality Strategy for Cardiovascular Risk Assessment: Performance in 2 Population-Based Cohorts. Circulation 2017;135:2119–2132. doi: 10.1161/CIRCULATIONAHA.117.027272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Whelton Paul K, Carey Robert M, Aronow Wilbert S, Casey Donald E, Collins Karen J, Dennison Himmelfarb Cheryl, DePalma Sondra M., Gidding Samuel, Jamerson Kenneth A., Jones Daniel W., et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 2018;71:e13–e115. doi: 10.1161/HYP.0000000000000065. [DOI] [PubMed] [Google Scholar]
- 24.Seliger SL, Hong SN, Christenson RH, Kronmal R, Daniels LB, Lima JAC, de Lemos JA, Bertoni A, deFilippi CR. High-Sensitive Cardiac Troponin T as an Early Biochemical Signature for Clinical and Subclinical Heart Failure: MESA (Multi-Ethnic Study of Atherosclerosis). Circulation 2017;135:1494–1505. doi: 10.1161/CIRCULATIONAHA.116.025505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu C-Y, Heckbert SR, Lai S, Ambale-Venkatesh B, Ostovaneh MR, McClelland RL, Lima JAC, Bluemke DA. Association of Elevated NT-proBNP With Myocardial Fibrosis in the Multi-Ethnic Study of Atherosclerosis (MESA). J Am Coll Cardiol 2017;70:3102–3109. doi: 10.1016/j.jacc.2017.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lewis AA, Ayers CR, Selvin E, Neeland I, Ballantyne CM, Nambi V, Pandey A, Powell-Wiley TM, Drazner MH, Carnethon MR, Berry JD, Seliger SL, DeFilippi CR, de Lemos JA. Racial Differences in Malignant Left Ventricular Hypertrophy and Incidence of Heart Failure: A Multicohort Study. Circulation 2020;141:957–967. doi: 10.1161/CIRCULATIONAHA.119.043628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Okin PM, Hille DA, Kjeldsen SE, Devereux RB. Combining ECG Criteria for Left Ventricular Hypertrophy Improves Risk Prediction in Patients With Hypertension. J Am Heart Assoc 2017;6:e007564. doi: 10.1161/JAHA.117.007564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bang CN, Soliman EZ, Simpson LM, Davis BR, Devereux RB, Okin PM, ALLHAT Collaborative Research Group. Electrocardiographic Left Ventricular Hypertrophy Predicts Cardiovascular Morbidity and Mortality in Hypertensive Patients: The ALLHAT Study. Am J Hypertens 2017;30:914–922. doi: 10.1093/ajh/hpx067. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


