Abstract
Background:
The use of antibody–drug conjugates for the treatment of advanced-stage human epidermal growth factor receptor 2 (HER2)-low expression in breast cancer (BC) has shown prominent curative effects, which has led to increased academic interest. However, the role of HER2-low expression in the prognosis of BC remains controversial.
Methods:
We conducted a systematic search of the PubMed, Embase, and Cochrane library databases and several oncology conferences until 20 September 2022. We used fixed- and random-effects models to calculate odds ratio (OR) or hazard ratio (HR) with 95% confidence interval (CI) for overall survival (OS), disease-free survival (DFS), progression-free survival (PFS), and pathological complete response (pCR) rates.
Results:
Overall, 26 studies encompassing 677,248 patients were included in the meta-analysis. Patients with HER2-low BC showed significantly better OS than those with HER2-zero BC in the overall population (HR = 0.90; 95% CI: 0.85–0.97) and hormone receptor-positive population (HR = 0.98; 95% CI: 0.96–0.99), whereas no significant difference was observed in the OS of the hormone receptor-negative population (p > 0.05). In addition, there was no significant difference in the DFS of the overall and hormone receptor-negative population (p > 0.05), but better DFS than those with HER2-zero BC in the hormone receptor-negative population (HR = 0.96; 95% CI: 0.94–0.99). There was also no significant difference in the PFS of the overall population, hormone receptor-positive, and hormone receptor-negative population (p > 0.05). Patients with HER2-low BC had a lower pCR rate after neoadjuvant treatment than those with HER2-zero BC.
Conclusions:
Compared to patients with HER2-zero BC, those with HER2-low BC had better OS in the overall population and hormone receptor-positive population, DFS in hormone receptor-positive population and lower pCR in the overall population. The biological differences between HER2-low and HER2-zero BCs, particularly in hormone receptor-positive patients, and the relationship between HER2-low expression status and prognosis need to be explored further.
Keywords: antibody–drug conjugates, breast cancer, HER2-low, overall survival, prognosis
Introduction
The prognosis of patients with human epidermal growth factor receptor 2 (HER2)-positive breast cancer (BC) is remarkably improved after anti-HER2 therapy. In clinical practice, as there are no targeted drugs, low expression of HER2 has been classified as HER2-negative to guide treatment. However, the recent development of antibody–drug conjugates (ADCs) has significantly improved the prognosis of patients with HER2-low BC.1,2 Thus, HER2-low status in BC has received increasing attention worldwide.
HER2 is a tyrosine kinase receptor that belongs to the human epidermal receptor family and is encoded by the ERBB2 gene.3 Current HER2 status assessment relies on immunohistochemistry (IHC) and in situ hybridization (ISH). If the IHC result is 0 or 1+, the cancer is considered HER2-negative. In recent years, most of the published data and ongoing clinical trials have defined HER2-low BCs as those with an IHC score of 1+ or 2+ with ISH result.4 Few studies have shown significant differences in clinical and pathological characteristics between patients with HER2-low and HER2-zero BC. For instance, patients with HER2-low BC have higher histological grading, are positive for hormone receptor, and have lower proliferation index-67 (Ki-67) expression than those with HER2-zero BC. Moreover, HER2-low tumors tend to have more mutations in PI3K–Akt signaling pathway-related genes than HER2-zero tumors.1,5
Patients with HER2-low BC account for approximately 45%–55% of all patients with BC.6 Because the biological behavior and clinical characteristics differ between patients with HER2-low and HER2-zero BC, researchers speculate that their prognosis may also be different. Many clinical studies have investigated the difference in prognostic outcome between patients with HER2-low and HER2-zero BC but have shown inconsistent and contradictory results. Therefore, we conducted a meta-analysis to explore the relationship between prognosis and HER2 expression status.
Materials and methods
Study objectives
The primary endpoint was to compare the overall survival (OS) of patients with HER2-low and HER2-zero BC. In subgroup analysis, hormone receptor-positive and hormone receptor-negative patients were compared in the HER2-low and HER2-zero cohorts, respectively. The secondary endpoint was to compare the disease-free survival (DFS) and progression-free survival (PFS) of patients with HER2-low and HER2-zero BC and conduct hormone receptor-positive and hormone receptor-negative subgroup analysis similar to that performed for OS. We also compared the pathological complete response (pCR) rates of patients with HER2-low and HER2-zero BC following drug treatment.
Literature search
A systematic search for the relationship between HER2-low expression status and survival was conducted using sources such as PubMed, Embase, the Cochrane library, American Society of Clinical Oncology (ASCO) Meeting, European Society for Medical Oncology Meeting, San Antonio Breast Cancer Symposium, and the American Association for Cancer Research Meeting. The search terms were ‘HER2-low’ or ‘ERBB2-low’ or ‘human epidermal growth factor receptor 2 low’ and ‘breast cancer’ or ‘breast neoplasm’ and ‘prognosis’ or ‘survival outcome’ and ‘overall survival’ or ‘disease-free survival’ or ‘progression-free survival’. Further details of the search strategy are shown in Supplemental eTable 1. Based on the latest definitions of HER2 from the 2018 ASCO and College of American Pathologists, HER2-low expression status was defined as an IHC score of 1+ or 2+ and an ISH result of non-amplified status (ISH−), whereas HER2-zero expression status was defined as an IHC score of 0.7 The preliminary screening was performed by carefully reading the titles and abstracts of English manuscripts, before determining whether they could be included after reading the full text. Manuscripts were searched until 20 September 2022. The Preferred Reporting Items for Systematic Reviews and Meta-analyses guidelines were used to conduct this meta-analysis.8
Inclusion and exclusion criteria
The inclusion criteria were as follows: (1) titles with HER2-low or HER2-zero in either early or advanced BC; (2) recorded odds ratios (OR) and hazard ratios (HR) with 95% confidence intervals (CI) of random survival data in the full text; and (3) either hormone receptor-positive or hormone receptor-negative data. The exclusion criteria were as follows: (1) meta-analyses, reviews, and duplicate studies and (2) only the comparative analysis of HER2 IHC score of 2+ versus 0/1+.
Data extraction
The data extracted for each study included the name of the first author, journal name, publication year of journal, type of study, sample size, median follow-up time, OS, DFS/recurrence-free survival (RFS; the DFS and RFS were combined into one category owing to their similar meanings), PFS, and pCR rates. We also extracted data from hormone receptor-positive and hormone receptor-negative populations of OS, DFS, PFS, and pCR rates. The HRs and 95% CIs of the original data were clearly written in the results section in most articles. For some articles, we extracted the HRs and 95% CIs from Kaplan–Meier curves using GetData Graph Digitizer software. To ensure that the data were not duplicated, we chose to cite the peer-reviewed full text when the same patient population appeared in both the conference and the full text. Available data were independently extracted from each study by two authors (YT and ZL) and any disagreements were resolved through negotiation.
Statistical analysis
Review Manager 5.4 software was used to generate forest and funnel plots. The HRs and 95% CIs of the original data were used to analyze the OS, DFS, and PFS. The heterogeneity of the data was determined based on p values and I2 value statistics. When p was <0.1 or I2 was >50%, the random-effects model was used; otherwise, the fixed-effects model was used (I2 < 50%, p > 0.1). A p value of <0.05 (two-side) was considered statistically significant, and I2 < 25%, I2 = 25–50%, and I2 > 50% were considered to indicate low, moderate, and high heterogeneity, respectively.9 Sensitivity analysis was used to assess the stability of the results.
Quality assessment
We used the Cochrane quality assessment tool to estimate the quality of the included studies. The risk of bias from six fields was determined, with each category comprising three levels, including ‘low risk’, ‘unclear risk’, and ‘high risk’.
Results
Identification of relevant studies
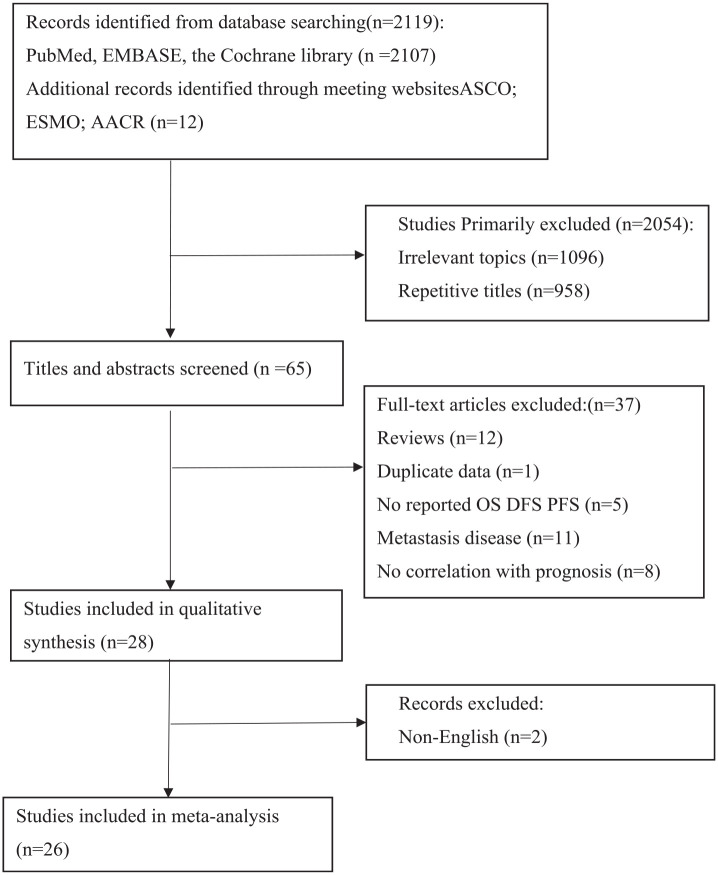
Figure 1 showed the screening process of relevant studies. We screened 2107 articles and 12 meeting abstracts, of which 26 were eventually included in the meta-analysis. All of the 26 studies were retrospective cohort studies and no prospective studies were reported up until 20 September 2022.
Figure 1.
PRISMA flow chart summarizing the study selection process.
PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-analyses.
A total of 677,248 patients were included in our meta-analysis. Among them, 446,398 patients had HER2-low BC and 230,850 patients had HER2-zero BC. The main characteristics of the included studies are summarized in Table 1.
Table 1.
Characteristics of 26 studies included in this meta-analysis.
| Study (first author, year) | Journal | Patient inclusion period | Type of study | Stage | No. of patients (N = 677,248) | HER2 low (N = 446,398) (65.9%) | HER2 zero (N = 230,850) (34.1%) | Median follow-up time (month) | No. of neoadjuvant treatment (N = 5709) | HER2-low pCR (events/total%) | HER2-zero pCR (events/total%) | Endpoints |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alexander Hein 202110 | European Journal of Cancer | 2014.7–2019.6 | Retrospective | Advance | 1022 | 525 (51%) | 497 (49%) | NA | NA | NA | NA | OS, PFS |
| Camille Domergue 202211 | Cancers | 2005–2020 | Retrospective | Early | 437 | 121 (27.7%) | 316 (72.3%) | 72.9 | 437 | 35.5 | 42.7 | pCR |
| Carsten Denkert 202112 | The Lancet Oncology | 2012.7–2019.3 | Retrospective | Early | 2310 | 41 (47.5%) | 1212 (52.5%) | 46.6 | 2310 | 29.2 | 39 | OS, DFS, pCR (ypT0/is ypN0) |
| Changchuan Jiang 202213 | 2022 ASCO | 2010–2017 | Retrospective | I–III | 553497 | 376,199 (68%) | 177,298 (32%) | NA | NA | NA | NA | OS |
| Elisa Agostinetto 202114 | Cancers | NA | Retrospective | NA | 671 | 410 (61.1%) | 261 (38.9%) | 27.7 | NA | NA | NA | OS |
| Fátima R Alves 202215 | Cureus | 2015.1–2020.12 | Retrospective | Early | 72 | 41 (56.9%) | 31 (43.1%) | 35.5 | 72 | 14.6 | 29 | OS, DFS, pCR |
| Francesco Schettini 202116 | Npj Breast Cancer | NA | Retrospective | I–III | 3689 | 2203 (59.7%) | 1486 (40.3%) | 90.3 | NA | NA | NA | OS |
| George Douganiotis 202217 | Cancer Diagnosis & Prognosis | 2007–2021 | Retrospective | Early | 949 | 632 (66.6%) | 317 (33.4%) | 33.6 | 113 | 8.8 | 9.1 | RFS, pCR |
| Guochun Zhang 20225 | BMC Medicine | NA | Retrospective | Early | 321 | 231 (72%) | 90 (28%) | NA | 321 | 15.9 | 37.5 | DFS, pCR |
| Hangcheng Xu 202218 | Frontiers in Oncology | 2013.1–2014.12 | Retrospective | Early | 777 | 598 (77%) | 179 (23%) | 78 | NA | NA | NA | OS DFS |
| Hye Sung Won 202219 | Breast Cancer Research | 2006.1–2011.12 | Retrospective | Early | 30491 | 20,985 (68.8%) | 9506 (31.2%) | 148 | NA | NA | NA | OS |
| Katrin Almstedt 202220 | European Journal of Cancer | 1985–2000 | Retrospective | Early | 351 | 198 (56.4%) | 153 (43.6%) | 200.76 | NA | NA | NA | OS, DFS |
| Kelvin K H Bao 202121 | JAMA Network | 2017.3–2020.6 | Retrospective | MBC | 106 | 82 (77.4%) | 24 (22.6%) | NA | NA | NA | NA | PFS |
| Luciana de Moura Leite 202122 | Breast Cancer Research and Treatment | 2007.1–2018.12 | Retrospective | Early | 855 | 285 (33.3%) | 570 (66.7%) | 59 | NA | NA | NA | OS, RFS |
| Michael Z Gilcrease 201123 | American Journal of Surgical Pathology | NA | Retrospective | 2–3A | 71 | 18 (25.4%) | 53 (74.6%) | 130 | NA | NA | NA | OS |
| Nanae Horisawa 202124 | Research Square | 2005.1–2015.12 | Retrospective | All | 4007 | 3169 (78.9%) | 838 (20.9%) | 5.5 | NA | NA | NA | OS, DFS |
| Ombline de Calbiac 202225 | JAMA Network | 2008–2016 | Retrospective | MBC | 15054 | 4671 (31%) | 10,383 (69%) | 49.5 | NA | NA | NA | OS, PFS1 |
| Paolo Tarantino 20224 | European Journal of Cancer | 2014.1–2020.12 | Retrospective | Advance | 232 | 122 (53%) | 110 (47%) | NA | NA | NA | NA | OS, DFS, PFS |
| Paolo Tarantino 202226 | Annals of Oncology (ESMO) | 1999.12–2021.1 | Retrospective | III–IV | 276 | 138 (50%) | 138 (50%) | 56 | 209 | 6 | 11 | OS, DFS, pCR |
| Paolo Tarantino 202227 | JAMA Oncology | 2016.1–2021.3 | Retrospective | I–III | 5235 | 2917 (55.7%) | 2318 (44.3%) | 10 | 675 | 16.6 | 26.8 | OS, DFS, pCR |
| Raz Mutai 20213 | The Breast | 2005.1–2012.3 | Retrospective | Early | 608 | 304 (50%) | 304 (50%) | 123.6 | NA | NA | NA | OS, DFS |
| Ryan Shea Ying Cong Tan 202228 | BMC Medicine | 2000.1–2015.12 | Retrospective | I–III | 28280 | 12,260 (43.4%) | 16,020 (56.6%) | 79.2 | NA | NA | NA | OS, RFS |
| Shaakir Hasan 202229 | 2022 ASCO | 2008–2015 | Retrospective | MBC | 24636 | 17,771 (72.1%) | 6865 (27.9%) | NA | NA | NA | NA | OS |
| Sora Kang 202230 | Annals of Oncology (ESMO) | 2014–2018 | Retrospective | I–III | 1572 | 754 (48%) | 818 (52%) | NA | 1572 | 10 | 16 | OS, DFS, pCR |
| William Jacot 202131 | Cancers | 2002–2012 | Retrospective | Early | 296 | 48 (16.2%) | 248 (83.8%) | 116.4 | NA | NA | NA | OS, RFS |
| Yiqun Li 202132 | Frontiers in Oncology | 2005.1–2015.12 | Retrospective | I–IV | 1433 | 618 (43.1%) | 815 (56.9%) | 62.6 | NA | NA | NA | OS |
ASCO, American Society of Clinical Oncology; DFS, disease-free survival; HER2, human epidermal growth factor receptor 2; MBC, metastatic breast cancer; OS, overall survival; pCR, pathological complete response; PFS, progression-free survival; RFS, recurrence-free survival.
Comparison of OS between patients with HER2-low and HER2-zero BC
OS of the overall population
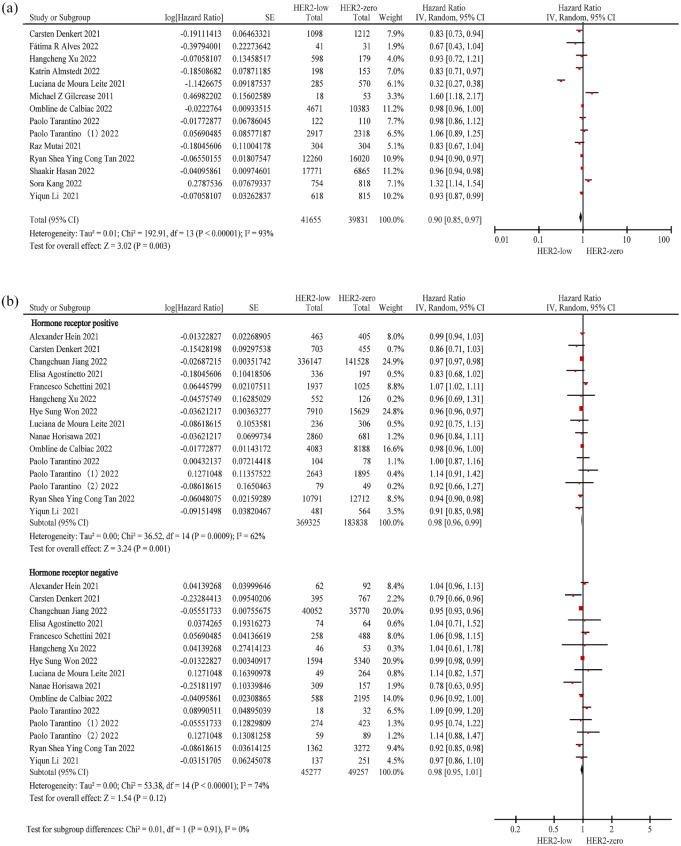
In all, 14 studies3,4,12,15,18,20,22,23,25,26,28–30,32 (n = 81,486) were included to evaluate the OS of the overall population. The results demonstrated that patients with HER2-low BC had better OS than those with HER2-zero BC (HR = 0.90; 95% CI: 0.85–0.97; p = 0.003), despite considerable heterogeneity between the studies (p < 0.01; I2 = 93%) (Figure 2(a); Table 2).
Figure 2.
Forest plot of the OS of patients with HER2-low BC and HER2-zero BC in the overall population (a), hormone receptor-positive population and hormone receptor-negative (b) population.
BC, breast cancer; HER2, human epidermal growth factor receptor 2; OS, overall survival.
Table 2.
Pooled HRs and 95% CIs for OS, DFS, PFS about HER2-low versus HER2-zero in this meta-analysis.
| Outcome | Population | No. of studies | HR (95% CI) | p | Heterogeneity | Effects model | |
|---|---|---|---|---|---|---|---|
| I2 (%) | Ph | ||||||
| OS | Overall | 14 | 0.90 (0.85–0.97) | 0.003 | 93 | <0.01 | Random |
| Hormone receptor positive | 15 | 0.98 (0.96–0.99) | 0.001 | 62 | <0.01 | Random | |
| Hormone receptor negative | 15 | 0.98 (0.95–1.01) | 0.12 | 74 | <0.01 | Random | |
| DFS | Overall | 13 | 0.97 (0.92–1.02) | 0.27 | 59 | 0.003 | Random |
| Hormone receptor positive | 9 | 0.96 (0.94–0.99) | 0.003 | 13 | 0.32 | Fixed | |
| Hormone receptor negative | 9 | 0.97 (0.94–1.00) | 0.08 | 1 | 0.42 | Fixed | |
| PFS | Overall | 3 | 1.06 (0.95–1.18) | 0.34 | 63 | 0.07 | Random |
| Hormone receptor positive | 3 | 1.00 (0.99–1.02) | 0.79 | 0 | 0.63 | Fixed | |
| Hormone receptor negative | 3 | 0.98 (0.94–1.01) | 0.15 | 49 | 0.14 | Fixed | |
CI, confidence interval; DFS, disease-free survival; HER2, human epidermal growth factor receptor 2; HR, hazard ratio; OS, overall survival; PFS, progression-free survival. Ph, p value of heterogeneity.
OS of the hormone receptor-positive and hormone receptor-negative population
We evaluated 15 studies4,10,12–14,16,18,19,22,24,25–28; 7 new studies10,13,14,16,19,24,27 were included and 6 studies3,15,20,23,29,30 were excluded from evaluation of the OS of the overall population.
The OS results for the hormone receptor-positive (n = 553,163) population were similar to those of the overall population; patients with HER2-low BC had better OS than those with HER2-zero BC (HR = 0.98; 95% CI: 0.96–0.99; p = 0.001), with high heterogeneity observed in the hormone receptor-positive population (p < 0.01; I2 = 62%). In the hormone receptor-negative population (n = 94,534), no significant difference in OS was observed between patients with HER2-low and HER2-zero BC (HR = 0.98; 95% CI: 0.95–1.01; p = 0.12), and high heterogeneity was observed in the hormone receptor-negative population (p < 0.01; I2 = 74%) (Figure 2(b); Table 2).
Comparison of DFS and PFS between patients with HER2-low and HER2-zero BC
DFS of the overall population
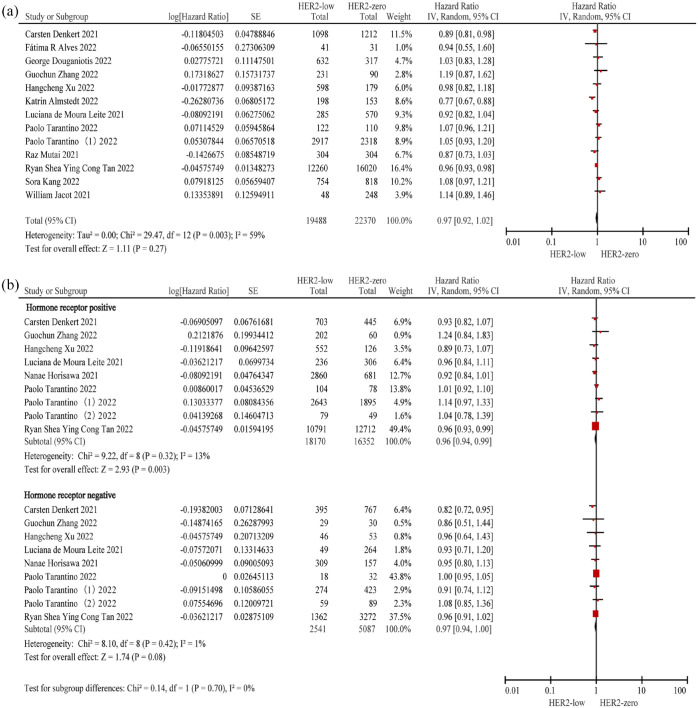
A total of 13 studies3–5,12,15,17,18,20,22,26,28,31,30 comprising 41,858 patients were included. No significant difference in DFS was observed between patients with HER2-low and HER2-zero BC (HR = 0.97; 95% CI: 0.92–1.02; p = 0.27), and high heterogeneity was observed in the overall population (p = 0.003; I2 = 59%) (Figure 3(a); Table 2).
Figure 3.
Forest plot of the DFS of patients with HER2-low BC and HER2-zero BC in the overall population (a), hormone receptor-positive population and hormone receptor-negative (b) population.
BC, breast cancer; DFS, disease-free survival; HER2, human epidermal growth factor receptor 2.
DFS of the hormone receptor-positive and hormone receptor-negative population
In this subgroup analysis, we assessed nine studies4,5,12,18,22,24,26–28 for the evaluation of DFS of the hormone receptor-positive (n = 34,522) and hormone receptor-negative (n = 7628) populations. To evaluate DFS, we included two new studies24,27 and excluded six studies3,15,17,20,31,30 from those considered for the evaluation of DFS of the overall population. Patients with HER2-low BC had better DFS than those with HER2-zero BC in the hormone receptor-positive populations (HR = 0.96; 95% CI: 0.94–0.99; p = 0.003) as well as in the hormone receptor-negative population (HR = 0.97; 95% CI: 0.94–1.00; p = 0.08). There was low heterogeneity among studies included for the evaluation of DFS in the hormone receptor-positive and hormone receptor-negative populations (hormone receptor positive: p = 0.32; I2 = 13% and hormone receptor negative: p = 0.42; I2 = 1%) (Figure 3(b); Table 2).
PFS of the overall population
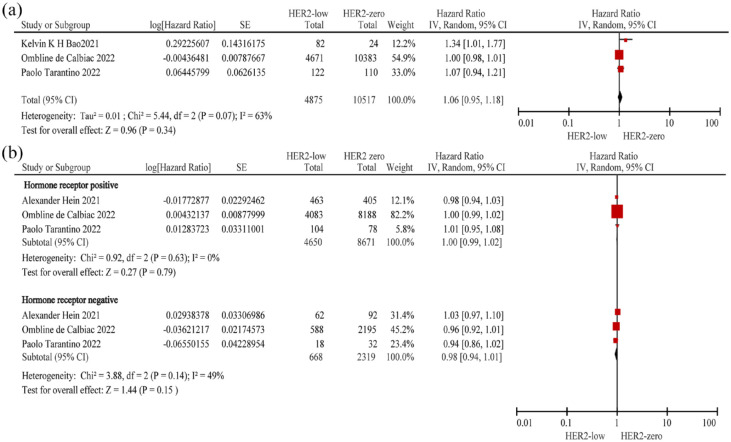
Three studies4,21,25 (n = 15,392) were included to evaluate the PFS of the overall population. No significant difference in PFS was observed between patients with HER2-low and HER2-zero BC (HR = 1.06; 95% CI: 0.95–1.18; p = 0.34), with high heterogeneity observed among the studies (p = 0.07; I2 = 63%) (Figure 4(a); Table 2).
Figure 4.
Forest plot of the PFS of patients with HER2-low BC and HER2-zero BC in the overall population (a), hormone receptor-positive population and hormone receptor-negative (b) population.
BC, breast cancer; HER2, human epidermal growth factor receptor 2; PFS, progression-free survival.
PFS of the hormone receptor-positive and hormone receptor-negative population
Three studies4,10,25 were included to determine the PFS of the hormone receptor-positive (n = 13,321) and hormone receptor-negative (n = 2987) population. We added one new study10 and excluded one study21 from those considered for the evaluation of PFS of the overall population. There was no significant difference in PFS between patients with HER2-low BC and HER2-zero BC in both the hormone receptor-positive and hormone receptor-negative populations (hormone receptor positive: HR = 1.00; 95% CI: 0.99–1.02; p = 0.79; and hormone receptor negative: HR = 0.98; 95% CI: 0.94–1.01; p = 0.15). Furthermore, there was no heterogeneity among the studies included for the evaluation of PFS in the hormone receptor-positive population (p = 0.63; I2 = 0%), but moderate heterogeneity was observed in the hormone receptor-negative population (p = 0.14; I2 = 49%) (Figure 4(b); Table 2).
Comparison of pCR rates between patients with HER2-low and HER2-zero BC
PCR rate of the overall population
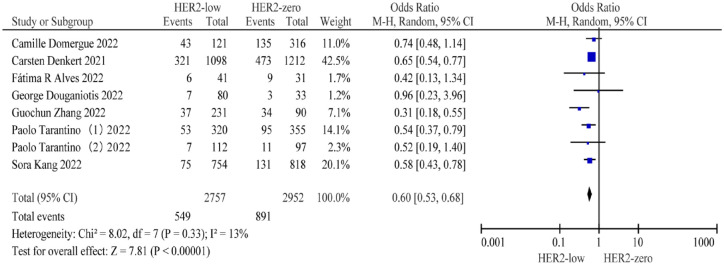
Eight studies5,11,12,15,17,26,27,30 comprising 2757 patients with HER2-low BC and 2952 patients with HER2-zero BC were included to evaluate the difference in pCR rates. Patients with HER2-low BC had a lower pCR rate than those with HER2-zero BC (19.91% versus 30.18%; OR = 0.60; 95% CI: 0.53–0.68; p < 0.001) (Figure 5).
Figure 5.
Forest plot of the pCR of patients with HER2-low BC and HER2-zero BC in the overall population.
BC, breast cancer; HER2, human epidermal growth factor receptor 2; pCR, pathological complete response.
Assessment of bias
The analysis of Cochrane risk assessment tools to estimate the risk of bias showed that the main problem we encountered in the evaluation was the high risk, as they had incomplete outcome data. Most of the studies were of moderate quality. The summary and funnel plot was used to estimate the publication bias were shown in Supplemental eFigure 2 and Supplemental eFigure 3.
Discussion
To the best of our knowledge, this is the first meta-analysis to investigate the correlation between HER2-low and HER2-zero expression status and the prognosis of patients with BC. Our results demonstrated that patients with HER2-low BC had better OS in the overall and hormone receptor-positive populations, better DFS in the hormone receptor-positive populations and lower pCR rates in the overall population than those with HER2-zero BC. Nevertheless, patients with HER2-low and HER2-zero BC had similar OS in the hormone receptor-negative population, similar DFS in the overall, and negative populations and similar PFS in the overall, hormone receptor-positive, and receptor-negative populations.
Previous prospective and retrospective studies have compared the outcomes between HER2 2+/ISH-negative patients and HER2 0/1+ patients.33–35 Novel ADCs such as trastuzumab deruxtecan have exhibited high activity in HER2-low BCs, including IHC 1+ or IHC2+/ISH-negative BC, and most recent studies also use these criteria. This meta-analysis also defined HER2-low-positive status as IHC 1+ or IHC2+/ISH-negative and HER2-zero expression status as IHC 0 according to the ASCO/College of American Pathologists guidelines. As HER2-low BC accounts for approximately 50% of all BCs6 and has significant heterogeneity,36 it is necessary to clarify the association of HER2-low and HER2-zero expression status and with prognosis.
Considering that the included studies included both early- and metastatic-stage disease over a long period (1985–2021), we used OS, DFS, and PFS as survival indices. The better OS observed for patients with HER2-low BC than for those with HER2-zero BC has several possible explanations. First, compared to patients with HER2-zero expression status, those with HER2-low expression status presented with more favorable clinical and pathological characteristics, such as a higher proportion of hormone receptor positivity rates,12,16,22,24 lesser number of grade III tumors,37 lower Ki-67 expression,37 lesser recurrences of central nervous system and visceral complications,22 and better performance status.32 Second, hormone receptor-positive patients with HER2-low expression status were less likely to be older, have basal-like subtypes according to the PAM50 intrinsic subtypes, and more likely to be progesterone receptor-positive and have the luminal A subtype compared to hormone receptor-positive patients with HER2-zero expression status; in contrast, hormone receptor-negative patients showed no such differences but had a higher proportion of molecular apocrine-like profiles.18,19,31 Third, HER2-low tumors harbor distinct clinical and molecular features, including reduced expression of TP53, increased expression of luminal-related genes,16 reduced expression of androgen receptor,31 reduced expression of proliferation-related genes and tyrosine kinase receptor genes,12,16 and increase in mutations in the PI3K-Akt signaling pathway5 compared with HER2-zero tumors. Lastly, patients with HER2-low expression status are more sensitive to some treatments, such as CDK4/6 inhibitors and PI3K–Akt signaling inhibitors, than patients with HER2-zero expression status, as they have a tendency to have HER2-enriched intrinsic subtypes and PI3K-Akt signaling mutations but less basal-like subtypes.5,6,21,38,39 Generally, these clinical, pathological, and molecular characteristics and the response to treatment are associated with hormone receptor-positive expression. This strongly suggests that hormone receptor status plays a crucial role in HER2-low BC and contributes to favorable clinical behavior and prognosis. In addition, the increased heterogeneity of PAM50 intrinsic subtypes between HER2-low and HER2-zero expression status in the hormone receptor-positive population compared with that in the hormone receptor-negative population partly accounts for the differences in prognosis of the two hormone receptor expression subgroups. Nevertheless, not all HER2-low BC characteristics are associated with better prognosis compared with HER2-zero BC. Indeed, several studies have shown that compared with HER2-zero expression status, HER2-low expression status is more common in patients who are overweight (body mass index ⩾ 25 kg/m2) and is characterized by increased axillary lymph-node involvement, a higher proportion of stage IV disease,32 and higher histological grade.16,19 To date, the exact mechanisms underlying a favorable prognosis remain poorly understood.
Though the included studies are not identical in overall and subgroup analyses for DFS, patients with HER2-low and HER2-zero expression status showed similar DFS in the overall and hormone receptor-negative populations. No difference in PFS was observed between patients with HER2-low and HER2-zero expression status in both the hormone receptor-positive and hormone receptor-negative populations. However, this result should be interpreted with caution because only three studies were included in this analysis. When combining these survival indicators, the better OS of patients with HER2-low expression status is likely because the early-stage hormone receptor-positive population comprised a large sample size, considering that this was the group of patients that showed different prognoses compared to those with HER2-zero expression status.
Moreover, patients with HER2-low expression status had a lower pCR to neoadjuvant treatment compared to those with HER2-zero expression status. This is likely related to the presence of more locally advanced tumors in patients with HER2-low expression status,16,39 a low Ki-67 index, and lower proportion of grade III tumors28,40,41 than in those with HER2-zero expression status as well as therapy resistance due to cross-talk between hormone receptor signaling and HER2 signaling.42 Patients with both early and advanced stage BC were included in this meta-analysis, and advanced stage tumors were enriched for HER2-low expression compared to early stage tumors, which was shown to be due to the fact that there is an evolution of HER2 expression from early to advanced stage, with a small percentage of HER2 low transforming to HER2 zero, while the majority still transformed from HER2 zero to HER2 low. In addition, HER2 expression is also upregulated in patients with advanced disease after multiple lines of therapy.4
The strengths of the study include the fact that this is the first available meta-analysis to investigate the association of HER2-low and HER2-zero expression status with prognosis in BC, with a large sample of patients, the adoption of new HER2 definitions, and the inclusion of multiple survival endpoints. However, this study also has several limitations. First, all of the included studies were retrospective in nature or were retrospective analyses of prospective studies that may have bias. Second, as the subgroup analyses included only few studies, the results should be interpreted with caution. Third, the included studies showed some heterogeneity considering the lack of standardized criteria for the IHC evaluation of HER2 expression status, the difference in follow-up time, systemic treatment, and the extended study period.
Conclusions
The results of this meta-analysis demonstrate that compared to patients with HER2-zero BC, those with HER2-low BC had better OS in the overall and hormone receptor-positive populations but similar OS in the hormone receptor-negative populations. HER2-low BC had better DFS in the the hormone receptor-positive populations. In addition, patients with HER2-low expression status had similar DFS in the overall and hormone receptor-negative populations, similar PFS in the overall, hormone receptor-positive, and hormone receptor-negative populations, and a lower pCR rate in the overall population than patients with HER2-zero expression status. Further studies are needed to clarify the biological differences between HER2-low and HER2-zero BCs and the association between HER2-low expression status and prognosis.
Supplemental Material
Supplemental material, sj-docx-1-tam-10.1177_17588359231156669 for The association between HER2-low expression and prognosis of breast cancer: a systematic review and meta-analysis by Yuyao Tang, Guoshuang Shen, Yuanfang Xin, Zhoujuan Li, Yonghui Zheng, Miaozhou Wang, Zhen Liu, Yi Zhao, Fuxing Zhao, Dengfeng Ren and Jiuda Zhao in Therapeutic Advances in Medical Oncology
Supplemental material, sj-svg-2-tam-10.1177_17588359231156669 for The association between HER2-low expression and prognosis of breast cancer: a systematic review and meta-analysis by Yuyao Tang, Guoshuang Shen, Yuanfang Xin, Zhoujuan Li, Yonghui Zheng, Miaozhou Wang, Zhen Liu, Yi Zhao, Fuxing Zhao, Dengfeng Ren and Jiuda Zhao in Therapeutic Advances in Medical Oncology
Supplemental material, sj-svg-3-tam-10.1177_17588359231156669 for The association between HER2-low expression and prognosis of breast cancer: a systematic review and meta-analysis by Yuyao Tang, Guoshuang Shen, Yuanfang Xin, Zhoujuan Li, Yonghui Zheng, Miaozhou Wang, Zhen Liu, Yi Zhao, Fuxing Zhao, Dengfeng Ren and Jiuda Zhao in Therapeutic Advances in Medical Oncology
Acknowledgments
Not applicable.
Footnotes
ORCID iD: Jiuda Zhao  https://orcid.org/0000-0002-1266-8943
https://orcid.org/0000-0002-1266-8943
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Yuyao Tang, Breast Disease Diagnosis and Treatment Center of Affiliated Hospital of Qinghai University & Affiliated Cancer Hospital of Qinghai University, Xining, China.
Guoshuang Shen, Breast Disease Diagnosis and Treatment Center of Affiliated Hospital of Qinghai University & Affiliated Cancer Hospital of Qinghai University, Xining, China.
Yuanfang Xin, Breast Disease Diagnosis and Treatment Center of Affiliated Hospital of Qinghai University & Affiliated Cancer Hospital of Qinghai University, Xining, China.
Zhoujuan Li, Breast Disease Diagnosis and Treatment Center of Affiliated Hospital of Qinghai University & Affiliated Cancer Hospital of Qinghai University, Xining, China.
Yonghui Zheng, Breast Disease Diagnosis and Treatment Center of Affiliated Hospital of Qinghai University & Affiliated Cancer Hospital of Qinghai University, Xining, China.
Miaozhou Wang, Breast Disease Diagnosis and Treatment Center of Affiliated Hospital of Qinghai University & Affiliated Cancer Hospital of Qinghai University, Xining, China.
Zhen Liu, Breast Disease Diagnosis and Treatment Center of Affiliated Hospital of Qinghai University & Affiliated Cancer Hospital of Qinghai University, Xining, China.
Yi Zhao, Breast Disease Diagnosis and Treatment Center of Affiliated Hospital of Qinghai University & Affiliated Cancer Hospital of Qinghai University, Xining, China.
Fuxing Zhao, Breast Disease Diagnosis and Treatment Center of Affiliated Hospital of Qinghai University & Affiliated Cancer Hospital of Qinghai University, Xining, China.
Dengfeng Ren, Breast Disease Diagnosis and Treatment Center of Affiliated Hospital of Qinghai University & Affiliated Cancer Hospital of Qinghai University, Xining, China.
Jiuda Zhao, Breast Disease Diagnosis and Treatment Center of Affiliated Hospital of Qinghai University & Affiliated Cancer Hospital of Qinghai University, Xining 810000, China; Breast Disease Diagnosis and Treatment Center of Affiliated Hospital of Qinghai University & Affiliated Cancer Hospital of Qinghai University, Xining, China.
Declarations
Ethics approval and consent to participate: Not applicable.
Consent for publication: All authors participated in this study and approved the final version.
Author contribution(s): Yuyao Tang: Data curation; Writing – original draft; Formal analysis.
Guoshuang Shen: Writing – original draft.
Yuanfang Xin: Data curation.
Zhoujuan Li: Data curation.
Yonghui Zheng: Data curation.
Miaozhou Wang: Writing – review & editing.
Zhen Liu: Writing – review & editing.
Yi Zhao: Writing – review & editing.
Fuxing Zhao: Writing – review & editing.
Dengfeng Ren: Writing – review & editing.
Jiuda Zhao: Conceptualization; Supervision; Writing – review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by Central Government Guiding Local Scientific and Technological Development Funds for Qinghai Province in China.
The authors declare that there is no conflict of interest.
Availability of data and materials: All data sets generated for this study are included in the article supplementary material.
References
- 1. Modi S, Jacot W, Yamashita T, et al. Trastuzumab deruxtecan in previously treated HER2-low advanced breast cancer. N Engl J Med 2022; 387: 9–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ueno NT, Jacot W, Yamashita T, et al. 217O patient-reported outcomes (PROs) from DESTINY-Breast04, a randomized phase III study of trastuzumab deruxtecan (T-DXd) vs treatment of physician’s choice (TPC) in patients (pts) with HER2-low metastatic breast cancer (MBC). Ann Oncol 2022; 33: S632–S633. [Google Scholar]
- 3. Mutai R, Barkan T, Moore A, et al. Prognostic impact of HER2-low expression in hormone receptor positive early breast cancer. Breast 2021; 60: 62–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tarantino P, Gandini S, Nicolò E, et al. Evolution of low HER2 expression between early and advanced-stage breast cancer. Eur J Cancer 2022; 163: 35–43. [DOI] [PubMed] [Google Scholar]
- 5. Zhang G, Ren C, Li C, et al. Distinct clinical and somatic mutational features of breast tumors with high-, low-, or non-expressing human epidermal growth factor receptor 2 status. BMC Med 2022; 20: 142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tarantino P, Hamilton E, Tolaney SM, et al. HER2-low breast cancer: pathological and clinical landscape. J Clin Oncol 2020; 38: 1951–1962. [DOI] [PubMed] [Google Scholar]
- 7. Wolff AC, Hammond MEH, Allison KH, et al. Human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. J Clin Oncol 2018; 36: 2105–2122. [DOI] [PubMed] [Google Scholar]
- 8. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 2022; 6: e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Higgins JPT, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003; 327: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hein A, Hartkopf AD, Emons J, et al. Prognostic effect of low-level HER2 expression in patients with clinically negative HER2 status. Eur J Cancer 2021; 155: 1–12. [DOI] [PubMed] [Google Scholar]
- 11. Domergue C, Martin E, Lemarié C, et al. Impact of HER2 status on pathological response after neoadjuvant chemotherapy in early triple-negative breast cancer. Cancers 2022; 14: 2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Denkert C, Seither F, Schneeweiss A, et al. Clinical and molecular characteristics of HER2-low-positive breast cancer: pooled analysis of individual patient data from four prospective, neoadjuvant clinical trials. Lancet Oncol 2021; 22: 1151–1161. [DOI] [PubMed] [Google Scholar]
- 13. Jiang C, Perimbeti S, Deng L, et al. Real-world clinical outcomes in patients with local/regional HER2-low breast cancer: an NCDB analysis. J Clin Oncol 2022; 40: 558. [Google Scholar]
- 14. Agostinetto E, Rediti M, Fimereli D, et al. HER2-low breast cancer: molecular characteristics and prognosis. Cancers 2021; 13: 2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Alves FR, Gil L, Vasconcelos de, Matos L, et al. Impact of human epidermal growth factor receptor 2 (HER2) low status in response to neoadjuvant chemotherapy in early breast cancer. Cureus 2022; 14: e22330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schettini F, Chic N, Brasó-Maristany F, et al. Clinical, pathological, and PAM50 gene expression features of HER2-low breast cancer. Npj Breast Cancer 2021; 7: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Douganiotis G, Kontovinis L, Markopoulou E, et al. Prognostic significance of low HER2 expression in patients with early hormone receptor positive breast cancer. Cancer Diagn Progn 2022; 2: 316–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Xu H, Han Y, Wu Y, et al. Clinicopathological characteristics and prognosis of HER2-low early-stage breast cancer: a single-institution experience. Front Oncol 2022; 12: 906011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Won HS, Ahn J, Kim Y, et al. Clinical significance of HER2-low expression in early breast cancer: a nationwide study from the Korean Breast Cancer Society. Breast Cancer Res 2022; 24: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Almstedt K, Heimes A-S, Kappenberg F, et al. Long-term prognostic significance of HER2-low and HER2-zero in node-negative breast cancer. Eur J Cancer 2022; 173: 10–19. [DOI] [PubMed] [Google Scholar]
- 21. Bao KKH, Sutanto L, Tse SSW, et al. The association of ERBB2-low expression with the efficacy of cyclin-dependent kinase 4/6 inhibitor in hormone receptor-positive, ERBB2-negative metastatic breast cancer. JAMA Netw Open 2021; 4: e2133132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. de Moura Leite L, Cesca MG, Tavares MC, et al. HER2-low status and response to neoadjuvant chemotherapy in HER2 negative early breast cancer. Breast Cancer Res Treat 2021; 190: 155–163. [DOI] [PubMed] [Google Scholar]
- 23. Gilcrease MZ, Woodward WA, Nicolas MM, et al. Even low-level HER2 expression may be associated with worse outcome in node-positive breast cancer. Am J Surg Pathol 2009; 33: 759–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Horisawa N, Adachi Y, Takatsuka D, et al. The frequency of low HER2 expression in breast cancer and a comparison of prognosis between patients with HER2-low and HER2-negative breast cancer by HR status. Breast Cancer 2022; 29: 234–241. [DOI] [PubMed] [Google Scholar]
- 25. de Calbiac O, Lusque A, Mailliez A, et al. Comparison of management and outcomes in ERBB2-low vs ERBB2-zero metastatic breast cancer in France. JAMA Netw Open 2022; 5: e2231170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tarantino P, Jin Q, Tayob N, et al. Prognostic and biologic significance of ERBB2-low expression in early-stage breast cancer. JAMA Oncol 2022; 8: 1177–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tarantino P, Niman SM, Erick TK, et al. HER2-low inflammatory breast cancer: clinicopathologic features and prognostic implications. Eur J Cancer 2022; 174: 277–286. [DOI] [PubMed] [Google Scholar]
- 28. Tan RSYC, Ong WS, Lee K-H, et al. HER2 expression, copy number variation and survival outcomes in HER2-low non-metastatic breast cancer: an international multicentre cohort study and TCGA-METABRIC analysis. BMC Med 2022; 20: 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hasan S, Neubauer Z, Press RH, et al. Prognostic implications of HER2Neu-low in metastatic breast cancer. J Clin Oncol 2022; 40: 1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kang S, Lee SH, Lee HJ, et al. Pathological complete response, long-term outcomes, and recurrence patterns in HER2-low versus HER2-zero breast cancer after neoadjuvant chemotherapy. Eur J Cancer 2022; 176: 30–40. [DOI] [PubMed] [Google Scholar]
- 31. Jacot W, Maran-Gonzalez A, Massol O, et al. Prognostic value of HER2-low expression in non-metastatic triple-negative breast cancer and correlation with other biomarkers. Cancers 2021; 13: 6059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Li Y, Abudureheiyimu N, Mo H, et al. In real life, low-level HER2 expression may be associated with better outcome in HER2-negative breast cancer: a study of the national cancer center, China. Front Oncol 2022; 11: 774577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Marty M, Cognetti F, Maraninchi D, et al. Randomized phase II trial of the efficacy and safety of trastuzumab combined with docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer administered as first-line treatment: the M77001 study group. J Clin Oncol 2005; 23: 4265–4274. [DOI] [PubMed] [Google Scholar]
- 34. Perez EA, Romond EH, Suman VJ, et al. Four-year follow-up of trastuzumab plus adjuvant chemotherapy for operable human epidermal growth factor receptor 2-positive breast cancer: joint analysis of data from NCCTG N9831 and NSABP B-31. J Clin Oncol 2011; 29: 3366–3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rossi V, Sarotto I, Maggiorotto F, et al. Moderate immunohistochemical expression of HER-2 (2+) without HER-2 gene amplification is a negative prognostic factor in early breast cancer. Oncologist 2012; 17: 1418–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hamilton E, Shastry M, Shiller SM, et al. Targeting HER2 heterogeneity in breast cancer. Cancer Treat Rev 2021; 100: 102286. [DOI] [PubMed] [Google Scholar]
- 37. Wang J, Xu B, Yuan P, et al. HER2 as a predictive factor for successful neoadjuvant anthracycline chemotherapy of locally advanced and early breast cancer. Int J Biol Markers 2014; 29: e187–e192. [DOI] [PubMed] [Google Scholar]
- 38. Prat A, Chaudhury A, Solovieff N, et al. Correlative biomarker analysis of intrinsic subtypes and efficacy across the MONALEESA phase III studies. J Clin Oncol 2021; 39: 1458–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Eggemann H, Ignatov T, Burger E, et al. Moderate HER2 expression as a prognostic factor in hormone receptor positive breast cancer. Endocr Relat Cancer 2015; 22: 725–733. [DOI] [PubMed] [Google Scholar]
- 40. Banerji U, van Herpen CML, Saura C, et al. Trastuzumab duocarmazine in locally advanced and metastatic solid tumours and HER2-expressing breast cancer: a phase 1 dose-escalation and dose-expansion study. Lancet Oncol 2019; 20: 1124–1135. [DOI] [PubMed] [Google Scholar]
- 41. Gampenrieder SP, Rinnerthaler G, Tinchon C, et al. Landscape of HER2-low metastatic breast cancer (MBC): results from the Austrian AGMT_MBC-Registry. Breast Cancer Res 2021; 23: 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Osborne CK, Schiff R. Mechanisms of endocrine resistance in breast cancer. Annu Rev Med 2011; 62: 233–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tam-10.1177_17588359231156669 for The association between HER2-low expression and prognosis of breast cancer: a systematic review and meta-analysis by Yuyao Tang, Guoshuang Shen, Yuanfang Xin, Zhoujuan Li, Yonghui Zheng, Miaozhou Wang, Zhen Liu, Yi Zhao, Fuxing Zhao, Dengfeng Ren and Jiuda Zhao in Therapeutic Advances in Medical Oncology
Supplemental material, sj-svg-2-tam-10.1177_17588359231156669 for The association between HER2-low expression and prognosis of breast cancer: a systematic review and meta-analysis by Yuyao Tang, Guoshuang Shen, Yuanfang Xin, Zhoujuan Li, Yonghui Zheng, Miaozhou Wang, Zhen Liu, Yi Zhao, Fuxing Zhao, Dengfeng Ren and Jiuda Zhao in Therapeutic Advances in Medical Oncology
Supplemental material, sj-svg-3-tam-10.1177_17588359231156669 for The association between HER2-low expression and prognosis of breast cancer: a systematic review and meta-analysis by Yuyao Tang, Guoshuang Shen, Yuanfang Xin, Zhoujuan Li, Yonghui Zheng, Miaozhou Wang, Zhen Liu, Yi Zhao, Fuxing Zhao, Dengfeng Ren and Jiuda Zhao in Therapeutic Advances in Medical Oncology