Abstract
Background:
Some studies have suggested that running increases the risk of knee osteoarthritis (OA), while others believe it serves a protective function.
Purpose:
To perform an updated systematic review of the literature to determine the effects of running on the development of knee OA.
Study Design:
Systematic review; Level of evidence, 4.
Methods:
A systematic review was performed by searching the PubMed, Cochrane Library, and Embase databases to identify studies evaluating the effect of cumulative running on the development of knee OA or chondral damage based on imaging and/or patient-reported outcomes (PROs). The search terms used were “knee AND osteoarthritis AND (run OR running OR runner).” Patients were evaluated based on plain radiographs, magnetic resonance imaging (MRI), and PROs (presence of knee pain, Health Assessment Questionnaire-Disability Index, and the Knee injury and Osteoarthritis Outcome Score).
Results:
Seventeen studies (6 level 2 studies, 9 level 3 studies, and 2 level 4 studies), with 7194 runners and 6947 nonrunners, met the inclusion criteria. The mean follow-up time was 55.8 months in the runner group and 99.7 months in the nonrunner group. The mean age was 56.2 years in the runner group and 61.6 years in the nonrunner group. The overall percentage of men was 58.5%. There was a significantly higher prevalence of knee pain in the nonrunner group (P < .0001). Although 1 study found a significantly higher prevalence of osteophytes in the tibiofemoral (TF) and patellofemoral (PF) joints within the runner group, multiple studies found no significant differences in the prevalence of radiographic knee OA (based on TF/PF joint-space narrowing or Kellgren-Lawrence grade) or cartilage thickness on MRI between runners and nonrunners (P > .05). One study found a significantly higher risk of knee OA progressing to total knee replacement among nonrunners (4.6% vs 2.6%; P = .014).
Conclusion:
In the short term, running is not associated with worsening PROs or radiological signs of knee OA and may be protective against generalized knee pain.
Keywords: knee joint, osteoarthritis, running
Osteoarthritis (OA) is the most common joint disorder in the United States and a leading cause of disability in the elderly population. 8,28 This chronic condition commonly affects load-bearing joints such as the knee and is characterized by pain, impaired physical function, and other adverse effects that may have a profound effect on the quality of life. 8,29 Knee OA is seen radiographically in 33% of the population >60 years, although there is considerable discordance between joint symptoms and radiographic findings. 2 The prevalence of symptomatic knee OA (SOA) in adults >60 years is approximately 10% in men and 13% in women. 8,31
Knee OA is not a localized, degenerative disease of cartilage alone but is regarded as a chronic disease of the entire joint, including articular cartilage, meniscus, ligament, and periarticular muscle. 8 Age, obesity, occupation, and trauma to the joint because of repetitive movements such as kneeling or squatting have been identified as several risk factors for knee OA. Other factors, including cytokines, leptin, and mechanical forces, are pathogenic components of knee OA. 8 However, the association between physical activity such as running and the development of knee OA is less transparent, as some believe running increases the risk of knee OA, while others believe it is protective. 16,29
Several studies have investigated the role of physical activity, particularly running, in the development of knee OA and have been inconclusive or contradictory. 9,29 The purpose of the present study was to perform an updated systematic review of the literature to determine the effects of running on the development of knee OA. We hypothesized that there would be no significant differences in the development of knee OA between high-volume runners and nonrunners.
Methods
This systematic review was conducted according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines using a PRISMA checklist. Two independent reviewers (J.D., J.W.B.) searched the PubMed, Embase, and Cochrane Library databases up to October 3, 2021. The following electronic search strategy was used: knee AND osteoarthritis AND (run OR running OR runner). A total of 1485 studies were reviewed by title and/or abstract to determine study eligibility based on inclusion criteria, and 13 additional studies were identified through a gray literature search. In cases of disagreement, a third reviewer (M.J.K.) made the final decision. The inclusion criteria were as follows: studies evaluating the effect of cumulative running on the development of knee OA or chondral damage based on imaging or patient-reported outcomes (PROs). The exclusion criteria were as follows: studies unrelated to the knee, nonhuman studies, and non-English studies. The full text of 1 study was not available in online databases and therefore was excluded. Data extraction from each study was performed independently and then reviewed by a second author (M.J.K.).
Reporting Outcomes
Outcomes assessed included PROs and radiological outcomes. Six studies 12,14,15,17,18,24 assessed the presence of knee pain, 2 studies 6,9 used the Knee injury and Osteoarthritis Outcome Score (KOOS), 25 and 1 study 3 used the Health Assessment Questionnaire Disability Index (HAQ–DI).
Six studies 3,5,14,17,18,27 assessed the presence of radiographic knee OA (ROA) using the Kellgren-Lawrence 11 (KL) scale, 1 study 21 used magnetic resonance imaging (MRI) T2 mapping, 1 study 9 used the MRI Osteoarthritis Knee Score 10 (MOAKS), 3 studies 3,14,17 assessed the presence of ROA according to the Ahlbäck criteria, 1 and 3 studies 5,18,27 assessed joint-space narrowing using the Osteoarthritis Research Society International Atlas. 1 Two studies 14,27 assessed the presence of osteophytes at the tibiofemoral (TF) and patellofemoral (PF) joints using the Osteoarthritis Research Society International Atlas. 1
Study Methodology Assessment
The Modified Coleman Methodology Score (MCMS) 4 was used to evaluate the methodology quality of studies. The MCMS has a scaled potential score ranging from 0 to 100. Scores ranging from 85 to 100 are excellent, 70 to 84 are good, 55 to 69 are fair, and <55 are poor. The primary outcomes assessed by the MCMS are study size and type, follow-up time, attrition rates, number of interventions per group, and proper description of study methodology.
Statistical Analysis
A weighted average was calculated for numerical demographics (age, sex, body mass index [BMI], and follow-up). The chi-square test was used to compare the presence of knee pain and total knee replacement (TKR) between the runner and nonrunner groups.
Results
A total of 17 studies met the inclusion and exclusion criteria (Figure 1), with 14,141 patients (7194 runners and 6947 nonrunners) (Table 1). The mean age among runners was 56.2 years (range, 26-81 years) and 61.6 years among nonrunners (range, 25-73 years). The mean BMI was 26.7 kg/m2 in the runner group and 28 kg/m2 in the nonrunner group. The mean follow-up time was 55.8 months in the runner group and 99.7 months in the nonrunner group. The overall percentage of men was 58.5%.
Figure 1.
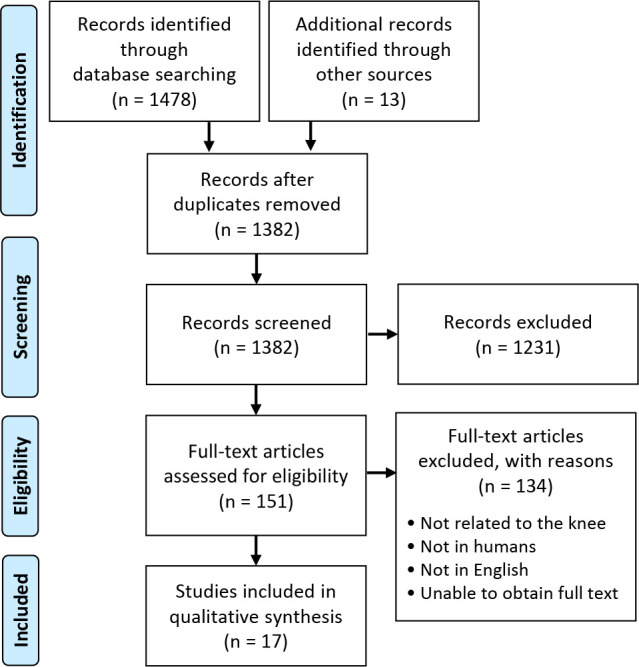
A PRISMA flowchart. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Table 1.
Studies Included a
| Study, Lead Author (year) | LOE | n (Run; Non) | Patient Age (Run; Non), y (range) | Follow-up (Run; Non), mo | BMI (Run; Non), kg/m2 | Sex, % male |
|---|---|---|---|---|---|---|
| Chakravarty 3 (2008) | 3 | 45; 53 | 59.8 ± 1; 60.2 ± 1 (50-72) | 216; 216 | 22.3 ± 0.3; 24 ± 0.5 | 67.3 |
| Felson (2007) | 2 | 1279; 0 | 53.2 (26.0-81) | 8.8 ± 1.04 | 27.4 | 44 |
| Greaves 6 (2021) | 4 | 16; 0 | 30.8 ± 6.3 | 1.5 | 22.9 ± 1.6 | 56.3 |
| Horga 9 (2019) | 2 | 71; 11 | 44.0 ± 8.5; 44 ± 7 (25-73) | 7.5; 7.5 | 25.2 ± 3.6; 24.2 ± 2.2 | 50.2 |
| Kujala 13 (1994) | 2 | 2049; 1403 | NR | NR | NR | 100 |
| Kujala 14 (1995) | 2 | 117; 0 | 59.1 (45-68) | NR | 22.8, NR | 100 |
| Kujala 15 (1999) | 2 | 269; 179 | 58.5; 60.3 (47-71) | 132; 132 | 23.2; 25.5 | 100 |
| Konradsen 12 (1990) | 3 | 27; 27 | 58; 57; (50-68) | NR | 22.9; 23.9 | 100 |
| Lo 17 (2017) | 3 | 778; 1859 | 62 ± 8.4; 65.3 ± 9 | 96; 96 | 27.9 ± 4.7; 28.8 ± 5 | 44.2 |
| Lo 18 (2018) | 3 | 138; 1065 | 62.9 ± 7.3, 63.2 ± 8 | 96; 96 | 28.4 ± 4; 29.6 ± 4.7 | 45.3 |
| Manninen 19 (2001) | 3 | 281, 524 | NR | NR | NR | NR |
| Miller 20 (2014) | 4 | 14; 0 | 25 ± 11 | NR | 24.2 | 50 |
| Mosher 21 (2010) | 2 | 22; 15 | 40; 37 | NR | 23.7, 25.4 | 47.8 |
| Mühlbauer 22 (2000) | 3 | 9; 9 | 27.4 ± 3.3; 22.2 ± 1.9 | NR | 22.4 ± 1.1; 23.1 ± 3.1 | 100 |
| Sandmark and Vingård 26 (1999) | 3 | 1173; 0 | NR | NR | NR | 50.2 |
| Spector 27 (1996) | 3 | 81; 977 | 52.3 ± 6.1; 54.2 ± 6 (40-67) | NR | 22.1 ± 2.8; 25.6 ± 4.3 | 0.0 |
| Thelin 28 (2006) | 3 | 825; 825 | NR (51-70) | NR | NR | 43.2 |
| Total | — | 7194; 6947 | 56.2; 61.6 | 55.8; 99.7 | 26.7; 28 | 58.5 |
a Patient age and follow-up are reported as mean ± SD (range, when reported), with the “Total” row reported as a weighted mean. “n” refers to the number of runners/nonrunners who were included in each study. BMI, body mass index; LOE, level of evidence; Non, nonrunners; NR, not reported; Run, runners.
Modified Coleman Methodology Score
Table 2 shows the MCMS scores from the 17 included studies. Two studies 15,28 received an excellent score, 4 studies 6,12,18,26 received good scores, 6 studies 3,5,13,14,17,27 received fair scores, and 5 studies 9,19 –22 received poor scores.
Table 2.
Modified Coleman Methodology Score a
| Study (year) | MCMS |
|---|---|
| Thelin et al 28 (2006) | 89 |
| Kujala et al 15 (1999) | 88 |
| Konradsen et al 12 (1990) | 79 |
| Sandmark and Vingård 26 (1999) | 77 |
| Greaves et al 6 (2021) | 73 |
| Lo et al 18 (2018) | 70 |
| Felson et al 5 (2007) | 64 |
| Spector et al 27 (1996) | 64 |
| Lo et al 17 (2017) | 57 |
| Chakravarty et al 3 (2008) | 55 |
| Kujala et al 13 (1994) | 55 |
| Kujala et al 14 (1995) | 55 |
| Manninen et al 19 (2001) | 54 |
| Mühlbauer et al 22 (2000) | 54 |
| Miller et al 20 (2014) | 49 |
| Horga et al 9 (2019) | 48 |
| Mosher et al 21 (2010) | 44 |
| Total | 63.2 ± 13.9 |
a MCMS, Modified Coleman Methodology Score.
Patient Characteristics/Study Methodology
Eight studies 5,12,14,17,18,26 –28 evaluated the association of running on OA symptom and structure progression in patients with baseline knee OA. Two studies 3,22 evaluated the differences in progression to knee OA in runners compared with healthy nonrunners with radiographic observation. Horga et al 9 evaluated the short-term impact of long-distance running using MRI and the KOOS subscores, while Miller et al 20 compared peak and per-unit distance knee joint loads between human walking and running. Mosher et al 21 evaluated the effects of age and physical activity level on cartilage thickness and MRI T2 response immediately after running. Kujala et al 15 investigated whether men participating in competitive endurance sports in middle and old age are at increased risk of lower-limb OA and disability. Greaves et al 6 analyzed the effects of a rehabilitative exercise program on pain, function, kinesiophobia, running biomechanics, quadriceps strength, and quadriceps muscle inhibition in individuals with PF pain. Manninen et al 19 examined the association between physical exercise and the risk of severe knee OA requiring arthroplasty. Kujala et al 13 compared the incidence of hospital admission for OA of the hip, knee, or ankle between former elite athletes and control participants.
Seven studies 3,12,14,18,21,22,27 reported physical activity in the runner group. In 1 study, 18 74.6% (103/138) of runners ran for 6 or more years and 92.7% (128/138) ran for 5 to 12 months per year, 88.4% (122/138) of runners ran more than 4 times per month, and 13% (18/138) participated in competitive running. In a study by Chakravarty et al, 3 runners ran for a mean of 213.9 ± 18.7 minutes per week. In a study by Mosher et al, 21 runners ran for a mean of 23.1 miles per week. In a study by Konradsen et al, 12 it was found that 90% of runners (27/30) ran for a range of 12 to 24 miles per week for 40 years. Three runners had stopped running because of OA of both lower and upper extremity joints. In a study by Mühlbauer et al, 22 the runners trained for 10 hours per week for at least 3 years. In a study by Spector, 27 the runner group averaged 14.6 miles per week. The runners in the study of Kujala et al 14 ran for a mean of 9408 ± 4213 hours over a mean of 31.7 ± 16.6 years. In 10 studies, 5,6,9,13,15,17,19,20,26,28 the authors did not report on the running distance within the running groups. In all 17 studies, 3,5,6,9,12 –15,17 –22,26 –28 the runners were running before the initiation of the study.
Four studies 3,21,22,27 reported physical activity in the nonrunner group. In the study 21 by Mosher et al, 2 patients in the nonrunner group reported occasionally running 5 miles per week over the past 2 years, and the rest of the patients reported no history of running exercise. In a study 27 by Spector et al, 81.3% (811/977) of nonrunners participated in little to no physical activity during the study. In a study by Mühlbauer et al, 22 participants in the nonrunner group reported <1 hour of physical activity per week throughout life. In a study by Chakravarty et al, 3 nonrunners ran for a mean of 0.9 ± 0.7 minutes per week. In 9 studies, 9,12,13,15,17 –19,26,28 the authors did not report the level of physical activity in the nonrunner group.
Patient-Reported Outcomes
Six studies 12,14,15,17,18,27 assessed the presence of knee pain at the final follow-up (Table 3). Konradsen et al 12 did not report their findings for knee pain. Overall, the presence of knee pain ranged from 10.2% to 35.4% in the runner group and 13.4% to 58.8% in the nonrunner group. Lo et al 17 found a significantly higher prevalence of knee pain in the nonrunner group.
Table 3.
Knee Pain a
| Study | Runners | Nonrunners | P |
|---|---|---|---|
| Lo et al 18 (2018) | 33/123 (26.8) | 293/1009 (29) | .61 |
| Lo et al 17 (2017) | 274/775 (35.4) | 1093/1859 (58.8) | <.0001 |
| Kujala et al 14 (1995) | 23/117 (19.7) | — | NA |
| Kujala et al 15 (1999) | 27/264 (10.2) | 24/179 (13.4) | .30 |
| Spector et al 27 (1996) | 27/81 (33.3) | 248/994 (24.9) | .096 |
| Total | 384/1360 (28.2) | 1658/4041 (41) | <.0001 |
a Data are reported as knee pain/total No. of patients within each group at the latest follow-up (%). Bold P values indicate statistically significant differences between runners and nonrunners (P < .05). NA, not applicable.
Chakravarty et al 3 reported results for the HAQ–DI and found no significant difference between runners and the control group (P > .05). Horga et al 9 reported results for the KOOS and found no significant changes between premarathon and postmarathon KOOS scores in the runner group in the subscales of symptoms (P = .981), pain (P = .121), activities of daily living (P = .303), sports and recreational activities (P = 0.133), and quality of life (P = .096). These authors also found no changes between baseline and final follow-up KOOS subscale scores in the nonrunner group—symptoms (P = .375), pain (P = .250), activities of daily living (P > .999), sports and recreational activities (P > .999), and quality of life (P = .250). Greaves et al 6 reported results for the KOOS subscales for pain and function and found that both subscores significantly improved after a 6-week exercise program was implemented (P = .0001, for both).
Radiological Outcomes
Three studies 5,18,27 assessed knee joint-space narrowing on a plain radiograph (Table 4). Spector et al 27 found a significantly higher prevalence of PF joint-space narrowing in the runner group at the final follow-up (P < .05). Two studies 14,27 assessed the presence of osteophytes at the TF and PF joints, of which Spector et al 27 found a significantly higher prevalence of osteophytes about both joints in the runner group at the final follow-up (P < .05). Three studies 3,14,17 assessed ROA. The 2 studies 3,17 that compared ROA between runners and nonrunners found no differences between groups. Chakravarty et al 3 assessed the progression of ROA by using a modification of the KL method. The study by Lo et al 17 defined ROA as a KL grade ≥2 in at least 1 knee and also assessed SOA, which was defined as having at least 1 knee with both ROA and frequent knee pain present. No difference was found in the prevalence of SOA between groups.
Table 4.
Radiological Outcomes a
| Results | ||||
|---|---|---|---|---|
| Study | Outcome Measure | Runners | Nonrunners | P |
| Felson et al 5 (2007) | TF JSN | 222/2259 (9.8) | — | NA |
| Lo et al 18 (2018) | Medial JSN | 40/205 (19.5) | 378/1063 (23.6) | >.05 |
| Spector et al 27 (1996) | TF JSN | 28/81 (34.6) | 359/911 (36.7) | >.05 |
| Spector et al 27 (1996) | PF JSN | 11/81 (13.6) | 27/215 (12.6) | <.05 |
| Spector et al 27 (1996) | Osteophytes (TF joint) | 18/81 (22.2) | 145/977 (14.8) | <.05 |
| Spector et al 27 (1996) | Osteophytes (PF joint) | 34/81 (42) | 60/215 (28) | <.05 |
| Lo et al 17 (2017) | ROA | 416/778 (53.5) | 1093/1859 (58.8) | >.05 |
| Lo et al 17 (2017) | SOA | 177/778 (22.8) | 547/1859 (29.4) | >.05 |
| Chakravarty et al 3 (2008) | ROA | 9/45 (20) | 17/53 (32.1) | >.05 |
| Kujala et al 14 (1995) | ROA | 31/117 (26.5) | — | NA |
| Kujala et al 14 (1995) | Osteophytes (TF joint) | 11/117 (9.4) | — | NA |
| Kujala et al 14 (1995) | Osteophytes (PF joint) | 22/117 (18.8) | — | NA |
a Results are reported as patients affected/total No. of patients within each group at the latest follow-up (%). Bold P values indicate statistically significant differences between runners and nonrunners (P < .05). JSN, joint-space narrowing; NA, not applicable; PF, patellofemoral; ROA, radiographic knee osteoarthritis; SOA, symptomatic knee osteoarthritis; TF, tibiofemoral.
Chakravarty et al 3 assessed the total knee score and joint-space width. No significant differences were found between runners and the community control group regarding the total knee score or joint-space width (P > .05, for both).
Mosher et al 21 assessed cartilage MRI T2 values and cartilage thickness from the central femoral and tibial cartilage and found no significant differences between the runners and nonrunners at the final follow-up (P > .05). A study by Mühlbauer et al 22 assessed cartilage thickness in the patella, trochlea, lateral femoral condyle, medial femoral condyle, and the medial and lateral tibial plateau and found no significant differences between the runner and control groups (P > .05). Konradsen et al 12 assessed cartilage thickness, grade of degeneration, and osteophytosis of the knee and found no significant differences between runners and nonrunners (P > .05).
Horga et al 9 assessed findings of the knee joint using the MOAKS score and found a significant improvement in the following structures within the runner group at the final follow-up: cartilage of the lateral patella (P = .0005); semimembranosus tendon (P = .016); iliotibial band (P < .0001); and the prepatellar bursa (P = .016). Furthermore, the authors assessed the presence of meniscal tears and found no significant differences in the prevalence of meniscal lesions between runners and nonrunners (P > .05).
Progression to Surgery
Three studies 17 –19 reported on patients undergoing TKR due to OA. In the Lo et al (2018) study, 18 TKR was performed in 3.9% (8/205) of runners and 4.3% (69/1603) of nonrunners (P = .79). Lo et al 17 (2017) reported performing TKR in 2.6% (20/778) of runners and 4.6% (86/1859) of nonrunners (P = .014). Manninen et al 19 assessed physical exercise as it related to the risk of knee arthroplasty. In both men and women, the risk was significantly less in those with a high number of cumulative hours of exercise. This was defined by at least 8654 total exercise hours in men and at least 6862 total exercise hours in women by the age of 49 years, compared with those who had no regular physical exercise (P < .05).
Discussion
Based on the findings of this systematic review, we found a significantly higher prevalence of knee pain in the nonrunner group. Although a single study 27 found a significantly higher prevalence of osteophytes in the TF and PF joints among runners, multiple studies 3,17,18,27 found no significant differences in the prevalence of ROA (based on TF/PF joint-space narrowing or KL grade) between runners and nonrunners. Additional studies 12,21,22 found no significant differences in cartilage thickness on MRI between runners and nonrunners. Finally, 2 studies 17,18 assessed the risk of progression of knee OA to TKR, with 1 study 17 finding a significantly lower risk of TKR among runners compared with nonrunners.
A 2017 meta-analysis by Timmins et al, 29 with a total of 6197 patients, found no significant differences in PROs between runners and nonrunners. While the authors reported mixed outcomes with regard to the presence of knee OA, there did appear to be a protective effect of running against surgery due to OA. The present systematic review builds upon this previous review with 7944 additional patients included for a more robust set of clinical findings.
Animal studies have shown that immobilization of the knee joint 30 as well as prolonged activity 24 can lead to osteoarthritic degeneration and that beneficial effects are determined by a dose-dependent relationship. 12,23 Furthermore, runners typically have a lower BMI, which could protect against knee OA. 17 How the distinct knee structures respond to dynamic, cyclical loading patterns during running—especially over prolonged periods—remains unclear. 29 Various intrinsic and extrinsic factors affect a joint’s ability to withstand destructive forces. Intrinsic factors include the thickness and composition of the articular cartilage as well as the strength of the adjacent bone, muscle, and periarticular ligaments. Extrinsic factors consist of nutrition; training technique; and the magnitude, direction, and duration of the applied force. 7 Running may have a protective effect if the resulting mechanical loading stimuli help elicit beneficial adaptation to the joints and surrounding structures. On the contrary, if a joint’s tolerance to loading is surpassed as a consequence of running, it could be a risk factor for knee OA. This relationship is further complicated because running is both directly and indirectly associated with other risk factors such as joint injury and BMI. 29 More recently, barefoot running has been proposed as a potential strategy to decrease the risk of acquiring running injuries because of its effects on the biomechanics of running and joint loading. 7
Limitations
The limitations of this study should be noted. First, the included studies demonstrated heterogeneity in the amount of running, age of the patients included, outcomes reported, and study designs. It also should be noted that some studies included a small number of patients and that 5 studies 5,6,14,20,26 lacked a control group. There is also the potential for confounders and selection bias (eg, runners may have a better baseline joint health compared with nonrunners). Furthermore, there was a difference in the follow-up duration and age of the runners versus nonrunners included in the studies. Finally, most studies were retrospective in study design and could not assess changes in radiological or PROs based on running.
Conclusion
In the short term, running is not associated with worsening PROs or radiological signs of knee OA and may be protective against generalized knee pain.
Footnotes
Final revision submitted September 10, 2022; accepted November 8, 2022.
One or more of the authors has declared the following potential conflict of interest or source of funding: A.J.S. has received consulting fees from DePuy/Medical Device Business Services; and hospitality payments from Globus Medical. E.C.M. has received education payments from Gemini Mountain Medical; consulting fees from Medical Device Business Services, Flexion Therapeutics, and Zimmer Biomet; and nonconsulting fees from Arthrex. P.C.M. has received education payments from MedInc and Smith & Nephew; consulting fees from Smith & Nephew; and speaking fees and honoraria from Vericel. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
References
- 1. Ahlbäck S. Osteoarthrosis of the knee. A radiographic investigation. Acta Radiol Diagn (Stockh).1968; 277 (Suppl):7–72. [PubMed] [Google Scholar]
- 2. Bosomworth NJ. Exercise and knee osteoarthritis: benefit or hazard? Can Fam Physician. 2009;55(9):871–878. [PMC free article] [PubMed] [Google Scholar]
- 3. Chakravarty EF, Hubert HB, Lingala VB, Zatarain E, Fries JF. Long distance running and knee osteoarthritis. A prospective study. Am J Prev Med. 2008;35(2):133–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Coleman BD, Khan HM, Maffulli N, Cook JL, Wark JD. Studies of surgical outcome after patellar tendinopathy: clinical significance of methodological deficiencies and guidelines for future studies. Victorian Institute of Sport Tendon Study Group. Scand J Med Sci Sports. 2000;10(1):2–11. [DOI] [PubMed] [Google Scholar]
- 5. Felson DT, Niu J, Clancy M, Sack B, Aliabadi P, Zhang Y. Effect of recreational physical activities on the development of knee osteoarthritis in older adults of different weights: the Framingham Study. Arthritis Rheum. 2007;57(1):6–12. [DOI] [PubMed] [Google Scholar]
- 6. Greaves H, Comfort P, Liu A, Lee H, Richard J. How effective is an evidence-based exercise intervention in individuals with patellofemoral pain? Phys Ther Sport. 2021;51(9):92–101. [DOI] [PubMed] [Google Scholar]
- 7. Hansen P, English M, Willick SE. Does running cause osteoarthritis in the hip or knee? PM R. 2012;4(Suppl 5):117S–121S. [DOI] [PubMed] [Google Scholar]
- 8. Heidari B. Knee osteoarthritis prevalence, risk factors, pathogenesis and features: part I. Caspian J Intern Med. 2011;2(2):205–212. [PMC free article] [PubMed] [Google Scholar]
- 9. Horga LM, Henckel J, Fotiadou A, et al. Can marathon running improve knee damage of middle-aged adults? A prospective cohort study. BMJ Open Sport Exerc Med. 2019;5(1): e000586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hunter DJ, Guermazi A, Lo GH, et al. Evolution of semi-quantitative whole joint assessment of knee OA: MOAKS (MRI Osteoarthritis Knee Score). Osteoarthritis Cartilage. 2011;19(8):990–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16(4):494–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Konradsen L, Hansen EM, Søndergaard L. Long distance running and osteoarthrosis. Am J Sports Med. 1990;18(4):379–381. [DOI] [PubMed] [Google Scholar]
- 13. Kujala UM, Kaprio J, Sarna S. Osteoarthritis of weight bearing joints of lower limbs in former élite male athletes. BMJ. 1994;308(6923):231–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kujala UM, Kettunen J, Paananen H, et al. Knee osteoarthritis in former runners, soccer players, weight lifters, and shooters. Arthritis Rheum. 1995;38(4):539–546. [DOI] [PubMed] [Google Scholar]
- 15. Kujala UM, Sarna S, Kaprio J, Koskenvuo M, Karjalainen J. Heart attacks and lower-limb function in master endurance athletes. Med Sci Sports Exerc. 1999;31(7):1041–1046. [DOI] [PubMed] [Google Scholar]
- 16. Lo G, Driban J, Kriska A, et al. Habitual running any time in life is not detrimental and may be protective of symptomatic knee osteoarthritis: data from the osteoarthritis initiative [Abstract]. Arthritis Rheumatol. 2014;66:1265–1266. [Google Scholar]
- 17. Lo GH, Driban JB, Kriska AM, et al. Is there an association between a history of running and symptomatic knee osteoarthritis? A cross-sectional study from the osteoarthritis initiative. Arthritis Care Res (Hoboken). 2017;69(2):183–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lo GH, Musa SM, Driban JB, et al. Running does not increase symptoms or structural progression in people with knee osteoarthritis: data from the osteoarthritis initiative. Clin Rheumatol. 2018;37(9):2497–2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Manninen P, Riihimaki H, Heliovaara M, Suomalainen O. Physical exercise and risk of severe knee osteoarthritis requiring arthroplasty. Rheumatology (Oxford). 2001;40(4):432–437. [DOI] [PubMed] [Google Scholar]
- 20. Miller RH, Edwards WB, Brandon SC, Morton AM, Deluzio KJ. Why don’t most runners get knee osteoarthritis? A case for per-unit-distance loads. Med Sci Sports Exerc. 2014;46(3):572–579. [DOI] [PubMed] [Google Scholar]
- 21. Mosher TJ, Liu Y, Torok CM. Functional cartilage MRI T2 mapping: evaluating the effect of age and training on knee cartilage response to running. Osteoarthritis Cartilage. 2010;18(3):358–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mühlbauer R, Lukasz TS, Faber TS, Stammberger T, Eckstein F. Comparison of knee joint cartilage thickness in triathletes and physically inactive volunteers based on magnetic resonance imaging and three-dimensional analysis. Am J Sports Med. 2000;28(4):541–546. [DOI] [PubMed] [Google Scholar]
- 23. Peyron JG. Epidemiologic and etiologic approach of osteoarthritis. Semin Arthritis Rheum. 1979;8(4):288–306. [DOI] [PubMed] [Google Scholar]
- 24. Radin EL, Orr RB, Kelman JL, Paul IL, Rose RM. Effect of prolonged walking on concrete on the knees of sheep. J Biomech. 1982;15(7):487–492. [DOI] [PubMed] [Google Scholar]
- 25. Roos EM, Lohmander LS. The Knee injury and Osteoarthritis Outcome Score (KOOS): from joint injury to osteoarthritis. Health Qual Life Outcomes. 2003;1(11):64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sandmark H, Vingård E. Sports and risk for severe osteoarthrosis of the knee. Scand J Med Sci Sports. 1999;9(5):279–284. [DOI] [PubMed] [Google Scholar]
- 27. Spector TD, Harris PA, Hart DJ, et al. Risk of osteoarthritis associated with long-term weight-bearing sports: a radiologic survey of the hips and knees in female ex-athletes and population controls. Arthritis Rheum. 1996;39(6):988–995. [DOI] [PubMed] [Google Scholar]
- 28. Thelin N, Holmberg S, Thelin A. Knee injuries account for the sports-related increased risk of knee osteoarthritis. Scand J Med Sci Sports. 2006;16(5):329–333. [DOI] [PubMed] [Google Scholar]
- 29. Timmins KA, Leech RD, Batt ME, Edwards KL. Running and knee osteoarthritis: a systematic review and meta-analysis. Am J Sports Med. 2017;45(6):1447–1457. [DOI] [PubMed] [Google Scholar]
- 30. Troyer H. The effect of short-term immobilization on the rabbit knee joint cartilage. A histochemical study. Clin Orthop Relat Res. 1975;(107):249–257. [DOI] [PubMed] [Google Scholar]
- 31. Zhang Y, Jordan JM. Epidemiology of osteoarthritis. Clin Geriatr Med. 2010;26(3):355–369. [DOI] [PMC free article] [PubMed] [Google Scholar]


