Abstract
Background:
Emergency laparotomy for abdominal trauma is associated with high rates of surgical site infection (SSI). A protocol for antimicrobial prophylaxis (AMP) for trauma laparotomy was implemented to determine whether SSI could be reduced by adhering to established principles of AMP.
Patients and Methods:
A protocol utilizing ertapenem administered immediately before initiation of trauma laparotomy was adopted. Compliance with measures of adequate AMP were determined before and after protocol implementation, as were rates of SSI and other infections related to abdominal trauma. Univariable and multivariable analyses were performed to determine risk factors for development of infection related to trauma laparotomy.
Results:
Over a four-year period, 320 patient operations were reviewed. Ertapenem use for prophylaxis increased to 54% in the post-intervention cohort. Compliance with individual measures of appropriate AMP improved modestly. Overall, infections related to trauma laparotomy decreased by 46% (absolute decrease of 13%) in the post-intervention cohort. Multivariable analysis confirmed that treatment during the post-intervention phase was associated with this decrease, with a separate analysis suggesting that ertapenem use was an important factor in this decrease.
Conclusions:
Development of a standardized protocol for AMP in trauma laparotomy led to decreases in infectious complications after that procedure.
Keywords: abdominal trauma, antimicrobial prophylaxis, emergency laparotomy, intra-abdominal infection, surgical site infection
Surgical site infection (SSI) is a common complication after laparotomy for abdominal trauma.1–5 Antimicrobial prophylaxis (AMP) is an effective practice for reducing SSI rates in patients undergoing high-risk abdominal surgery,6–8 and is recommended for patients undergoing trauma laparotomy, especially for penetrating trauma, even though prospective randomized controlled trials (PRCT) have not been performed to definitively demonstrate its efficacy.9,10
Fundamental principles of AMP for elective surgery include appropriate timing of initial antibiotic administration, appropriate antimicrobial spectrum for the specific procedure, appropriate dosing of agents particularly in obese patients, timely redosing of antibiotic agents during prolonged procedures, and limiting the duration of antibiotic use post-operatively.6–8 Adherence to these principles may be difficult in patients undergoing trauma laparotomy. The surgical site is frequently already contaminated because of skin and soft tissue or intra-abdominal injuries. Antimicrobial prophylaxis may not be timed ideally because of the exigencies of emergency laparotomy. Intra-operatively, prolonged operations or large blood losses may result in sub-therapeutic concentrations of prophylactic agents. Finally, an appropriate agent may not have been administered if a bowel injury was not suspected prior to laparotomy.
Use of protocols or bundles to optimize AMP has been associated with reduction of SSI rates in elective general surgery.12–14 However, there are sparse data regarding use of such protocols to prevent SSI in patients undergoing trauma laparotomy.15 We therefore developed a protocol for AMP in these patients designed to facilitate adherence with the principles of AMP. The protocol promoted use of ertapenem as the preferred antibiotic for trauma laparotomy, to be given immediately prior to the start of laparotomy, with redosing only if massive blood loss occurred during the procedure. Ertapenem was chosen because of its spectrum of activity, its prolonged half-life, and its demonstrated efficacy for prophylaxis in elective colorectal procedures.16 We hypothesized that use of this protocol would lead to decreased rates of SSI directly related to exploratory laparotomy in abdominal trauma patients compared to those rates in a patient cohort treated immediately prior to protocol implementation.
Patients and Methods
Consent
The study design and procedures were approved by the Washington University Institutional Review Board. Informed consent was not required for most patients in the study. Patients contacted by telephone after discharge for purposes of this study provided oral consent for interview according to a script approved by the Institutional Review Board.
Inclusion and exclusion criteria
All patients undergoing exploratory laparotomy for trauma at this level one trauma center from November 2009 to October 2013 were evaluated for inclusion in the study. Patients who underwent laparotomy between November 2009 and November 2011 were included in the pre-intervention cohort, and those who underwent laparotomy between December 2011 and October 2013 were included in the post-intervention cohort. Starting in November 2010, all patients were identified prospectively though screening by the research coordinators; prior to that, patients were identified through retrospective review of the trauma database.
Patients were excluded from any analysis if a resuscitative thoracotomy or cesarean delivery was performed in the emergency department, if the initial laparotomy was performed by a non-trauma service, or if the anesthesia record containing details of prophylactic antibiotic administration was missing.
Patients were excluded from analyses of infectious endpoints, but were included in analyses of process endpoints if they died within five days of admission, if laparotomy was delayed more than 24 hours from the time of injury or more than 12 hours from a traumatic bowel perforation, or if they underwent only laparoscopy or wound exploration in the operating room, without subsequent laparotomy
Patients who underwent other operations by the trauma service during the laparotomy, such as thoracotomy or groin exploration for a vascular injury, or who underwent a subsequent procedure by a non-trauma service, such as stabilization of a fracture, were included in all analyses. In the latter patients, the laparotomy procedure was considered finished when the skin was either closed or packed, or, in patients having delayed fascial closure, when a temporary abdominal closure had been fashioned. Patients who underwent minor procedures prior to laparotomy, such as tube thoracostomy or wound explorations were included in all analyses, even if antibiotic agents were administered for these procedures.
Intervention
Prior to December 2011, all AMP for patients undergoing trauma laparotomy was given according to attending surgeon preference. In December 2011, a standardized protocol for AMP agreed to by the trauma surgery and trauma anesthesia services was implemented for these patients. The protocol included use of 1 gm of ertapenem as the antibiotic of choice for all trauma laparotomies, to be delivered upon arrival in the operating room prior to incision. This antibiotic was to be given regardless of whether or not the patient received an antibiotic while in the emergency department; administration of antibiotic agents in the emergency department was strongly discouraged. Patients who had a documented carbapenem allergy, or who had had an anaphylactic reaction to a β-lactam antibiotic could receive alternative antibiotic agents considered appropriate by the attending surgeon. The protocol also called for re-dosing of ertapenem after 10 units of red blood cells had been transfused since the previous dose. It was recommended that no post-operative antibiotic agents be administered, but if given, they were limited to 24 hours after the operation except for patients undergoing delayed fascial closure, in whom antibiotic duration was left to surgeon discretion.
Data collection
Basic demographic, injury severity, and clinical data were collected on all patients, including age, gender, race, medical comorbidities, prior abdominal operations, mechanism of injury, lactic acid values, abdominal and extra-abdominal injuries, Injury Severity Score (ISS), abdominal ISS, calculated probability of survival, duration of laparotomy, number of units of blood transfused, whether or not delayed fascial closure was used, and whether or not the skin was closed at the time of the final abdominal procedure.
Post-operative data were obtained regarding temperature and leukocyte counts, administration of antimicrobial agents, culture results, imaging studies to investigate for infection, and all other operative or other procedures. All available records were utilized, including initial and subsequent hospital admissions, outpatient encounters, emergency department visits, and ancillary home health visits. Interactions were evaluated in detail up to 30 days after initial laparotomy, and additional information from 30 to 90 days after laparotomy was reviewed, if available.
In selected cohorts of patients during the pre-intervention and post-intervention phases of the study, an attempt was made to contact them by phone after 30 days if the final encounter was less than 30 days after laparotomy. This was done between November 2010 and June 2011 for the pre-intervention cohort and between November 2012 and October 2013 for the post-intervention cohort. Patients contacted by phone were asked whether or not they had had any further medical interventions, complications related to trauma, or antibiotic prescriptions since their last encounter.
Process endpoints
Antimicrobial prophylaxis was characterized according to timing of initial administration, dosage, spectrum of activity, redosing for length of operation or blood loss, duration of prophylaxis, and whether additional antibiotic agents were administered pre-operatively. Definitions of endpoints are shown in Table 1.
Table 1.
Definitions of Process Endpoints
| AMP use | Administration of any prophylactic antibiotic prior to the end time for laparotomy was noted; if no AMP had been administered, these patients were not evaluated for any other process endpoint. |
| AMP timing | Timing was considered compliant if given within one hour prior to up to 15 minutes after the surgical start time. |
| AMP dosing | Dosing was considered compliant if the recommended dose was administered; for patients whose estimated weight was >100 kg the recommended dosage of cefazolin, cefoxitin, or cefotetan was 2 gm rather than 1 gm. |
| AMP spectrum | AMP had to include an agent or agents with antianaerobic activity for all patients with penetrating abdominal trauma unless pre-operative imaging ruled out a bowel injury, and for any patients with blunt abdominal trauma in whom a bowel injury was suspected. |
| AMP re-dosing for duration of procedure | Re-dosing of antibiotic agents was considered necessary if the duration of the laparotomy exceeded two half-lives of the antibiotic; recommended re-dosing intervals were two hours for ampicillin/sulbactam, cefoxitin, or piperacillin/tazobactam, four hours for cefazolin, and six hours for cefotetan. |
| AMP re-dosing for blood loss | Redosing for blood loss was scored as compliant if it was given within 15 minutes after transfusion of more than 10 units of blood from the time of previous administration of AMP. |
| Pre-operative antibiotic use | Any extra pre-operative antibiotic use prior to laparotomy other than for AMP was noted. |
| Post-operative antibiotic use | All post-operative use of AMP was noted. Compliance was scored both for discontinuation of all AMP use after the end of laparotomy, and for discontinuation of all AMP use 24 ± 6 h after the end of laparotomy |
AMP = antimicrobial prophylaxis.
Process endpoints were evaluated in all patients included in the study, including those excluded from infection endpoints. The primary process endpoint was the proportion of patients who were compliant with all standards related to prophylaxis during the first trauma laparotomy, including initial timing, dosage, antibiotic selection, and re-dosing for length or operation and blood loss. Secondary endpoints were the proportion of patients compliant with each individual standard. Additional secondary endpoints were the proportion of patients who received extra pre-operative antibiotic therapy, and the proportion compliant with discontinuation of prophylactic antibiotic agents at the end of laparotomy or within 24 hours of laparotomy.
Infection endpoints
Definitions for infections were developed prior to study initiation and are shown in Table 2. The primary endpoint for the study was the number of patients who sustained an infection directly related to the abdominal trauma within 30 days of laparotomy. This expanded endpoint was primarily related to incisional and intra-abdominal infections, but also included other infections that were directly associated with the abdominal trauma. Thus, infections in a thoracic cavity directly contaminated because of a thoraco-abdominal gunshot injury, or a soft tissue infection in the operative field related to an abdominal injury were included in this expanded endpoint. Subsequent pneumonias, urinary tract infections (UTIs), soft tissue infections away from the abdominal region, or bacteremias were not considered related infections. Secondary endpoints included the number of patients with an SSI, intra-abdominal infection (IAI), or any other type of infection at 30 days. Similar endpoints were assessed up to 90 days after laparotomy. Patients who lacked follow-up data at 30 or 90 days were assumed to have not had an infection if none had been documented at the time of the last available encounter. Data at 90 days were excluded in two patients because of a late death in one and a new trauma laparotomy in the other. All infections were identified through the medical records; no additional infections were uncovered in patients contacted via telephone.
Table 2.
Definitions of Infection Endpoints
| SSI | SSI was defined according to NHSN criteria: Purulent drainage from the incision; organism(s) identified from culture of an aseptically obtained specimen from the superficial incision or subcutaneous tissue; incision deliberately opened by a physician or physician designee without culture testing in a patient with localized pain or tenderness, localized swelling, erythema, or heat; or diagnosis of an SSI by a physician or physician designee. If documentation was inadequate, but the patient had the wound re-opened and was prescribed antibiotic therapy directed against likely wound pathogens, it was considered an SSI. Minor skin dehiscences or serous drainage documented as such were not counted as SSI. SSIs were characterized as a superficial SSI if it involved the skin and subcutaneous tissue only, or as a deep SSI if the infection extended into the fascia or deeper layers of the abdominal wall. SSI involving the laparotomy incision was counted as a related SSI; SSI at a surgical site for an operation unrelated to trauma laparotomy (such as open reduction and internal fixation of a fracture) was counted as an unrelated SSI. |
| IAI | IAI was diagnosed based on findings at re-laparotomy, percutaneous drainage, or clinical and radiographic findings if no source control procedure was performed. Purulent fluid identified within the abdominal cavity at the time of reoperation was considered diagnostic of IAI. Patients undergoing percutaneous drainage of postoperative fluid collections were counted as having IAI if abdominal cavity fluid cultures were positive, or in the presence of a negative culture if a gram stain revealed leukocytes and micro-organisms and the patient was receiving antimicrobials that may have inhibited growth of organisms. Fluid collections characterized as urinomas or bilomas were not considered IAI. Patients who had clinical symptoms and signs of infection and had CT findings consistent with IAI but did not undergo any procedure for drainage were counted as having IAI if they were treated with an antibiotic regimen directed against presumed IAI pathogens. Patients who had positive laparotomy findings or a positive abdominal fluid culture were counted as having a confirmed IAI, and those who had negative cultures or did not have cultures obtained were counted as having an unconfirmed IAI. |
| Empyema | Empyema was identified if the patient had purulent pleural fluid found at the time of an operation or formal drainage procedure and was treated with antimicrobials for the infection. Patients whose pleural fluid cultures were positive were considered to have had a confirmed empyema. Those who had negative cultures but had leukocytes and organisms identified on gram stain were considered to have an unconfirmed empyema, as were patients who did not have cultures sent but who had clinical symptoms and signs consistent with an empyema and had pleural fluid described as purulent. Empyema was considered related to abdominal trauma if the patient had a thoraco-abdominal injury, and had a direct communication between the abdominal and the affected thoracic cavity. Empyema was considered unrelated to abdominal trauma if the thoracic cavity had not been contaminated as a result of the abdominal trauma. For instance, a patient who underwent a laparotomy for blunt trauma but developed an empyema after tube thoracostomy for a hemo- or pneumothorax was counted as having an unrelated infection. |
| SSTI | SSTI was identified by purulent drainage from a traumatic wound, or by cellulitis associated with a traumatic wound that was treated with anti-infective therapy. Culture confirmation was not required. An SSTI treated with operative drainage, or that resulted in prolongation of the hospital stay or re-admission to the hospital was considered a major SSTI; if managed non-operatively without a new or prolonged hospital admission, it was counted as a minor SSTI. The SSTI was considered a related infection if the infected traumatic wound was in direct continuity with the abdominal cavity or was directly related to the abdominal trauma. An SSTI occurring elsewhere was counted as an unrelated SSTI. |
| Pneumonia | Pneumonia was diagnosed in patients with a new or worsening chest infiltrate on a routine chest radiograph or CT examination, clinical findings consistent with pneumonia (such as cough, dyspnea, production of purulent sputum, new or worsening fever, or leukocytosis) and a decision by the treating clinicians to prescribe an antibiotic regimen appropriate for treatment of a pneumonia. Patients who had a positive tracheal aspirate or bronchoalveolar lavage culture were considered to have a confirmed pneumonia. Patients who met the criteria for pneumonia but did not have respiratory cultures obtained were considered to have an unconfirmed pneumonia. |
| UTI | UTI was diagnosed by a urinary culture with >50,000 organisms per milliliter (the criteria reported by the hospital microbiology laboratory at the time of the study). Patients whose cultures revealed coagulase-negative staphylococci or Candida were only diagnosed with a UTI if a subsequent culture confirmed the same organism. |
| Blood stream infection | Patients who had a positive blood culture were counted as having a blood stream infection; however, if the culture was positive for coagulase-negative staphylococci or other skin potential contaminants, these were only counted as a bloodstream infection if the same organism was obtained from two separate cultures obtained at different times. Blood stream infections were characterized as primary if no other source for the organism(s) was identified. A secondary bloodstream infection was diagnosed if the same organism or organisms were cultured from another source within 72 h before or after the positive blood culture. |
| Clostridiodes difficile colitis | Clostridiodes difficile colitis was diagnosed by a positive Clostridiodes difficile toxin assay in a patient who had new-onset diarrhea. |
| Other infections | Patients who had positive cultures from the eye, bone specimens, or cerebrospinal fluid were counted as having an ocular infection, osteomyelitis, or meningitis, all of which were considered as unrelated to the trauma laparotomy. |
| Uncounted infections | Infections diagnosed within 24 h of admission to the hospital were considered pre-existing, and not included in the infection endpoints. Similarly, patients diagnosed with Helicobacter pylori or a sexually transmitted disease subsequently during hospitalization were considered to also have had a pre-existing illness and were not included in infection endpoints. |
SSI = surgical site infection; NHSN = National Healthcare Safety Network; IAI = intra-abdominal infection; SSTI = skin and soft tissue infection; CT = computed tomography; UTI = urinary tract infection.
Statistical analysis
Prior to initiation of the study, a power analysis indicated that approximately 106 patients would be needed in each group to detect a 25% decrease in the primary infection endpoint, based on an estimated 20% incidence of these infections in control patients. Based on usual numbers of laparotomies and potential exclusion of 10% of patients, it was estimated that 18 to 24 months would be required for each phase of the study.
The primary analyses were process and infection infectious endpoints in the pre- and post-intervention cohorts. Secondary analyses compared these outcomes in patients who received or did not receive ertapenem as their peri-operative antibiotic, regardless of cohort.
Data were presented as mean with standard deviation or median with interquartile ranges for quantitative variables, as appropriate, and frequency with percentage for qualitative variables. Two-sample t-test or Wilcoxon rank sum test was used to assess the differences of numeric factors (i.e., age, weight) between pre- and post-intervention cohorts, as appropriate. Either χ2 test or Fisher exact test was used to assess the differences of primary/secondary outcomes between pre- and post-intervention cohorts, as appropriate. Univariable and multivariable logistic regression analyses were performed to detect the effects of demographic and clinical factors on primary outcomes. Odds ratios with 95% confidence intervals were provided to describe the magnitudes of the effects. Data were analyzed by SAS 9.4 (SAS Institute Inc, Cary, NC). A p value <0.05 was considered statistically significant.
Results
Patients and exclusions
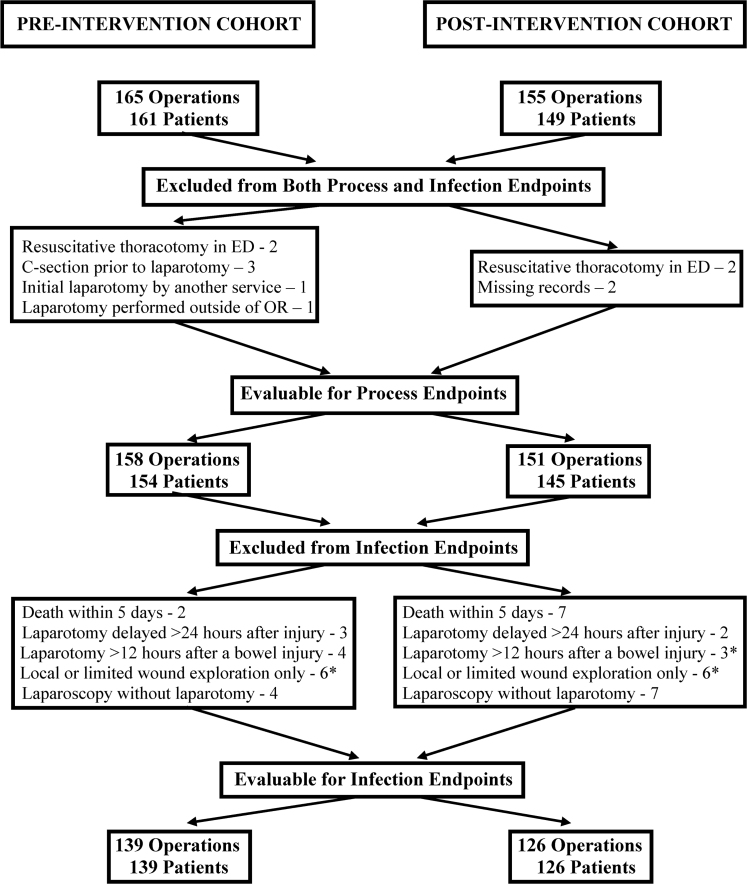
A total of 320 operations for abdominal trauma in 309 patients were performed over the four-year study period. Except for one patient who underwent 12 separate procedures for recurrent self-inflicted stab wounds, no patient was entered into the database more than once. After exclusions, there were 158 patient operations in the pre-intervention cohort and 151 patient operations in the post-intervention cohort that were analyzed for process endpoints. After further exclusions, there were 139 patients in the pre-intervention cohort and 126 patients in the post-intervention cohort who were evaluable for infection endpoints. The reasons for exclusions are detailed in Figure 1.
FIG. 1.
Patients excluded from analysis of process endpoints and both process and infection endpoints. *One patient was excluded five times in both the pre- and post-intervention cohorts for undergoing a local or limited wound exploration only, and twice in the post-intervention cohort for undergoing laparotomy more than 12 hours after a bowel injury. ED = emergency department; OR = operating room; C-section = cesarean delivery.
Compliance with the protocol
Ertapenem use for AMP increased from 2.5% of laparotomies during the pre-intervention phase to 54.3% of laparotomies during the post-intervention phase. This was associated with a concomitant decrease in the use of cefoxitin, cefazolin, or any cephalosporin in the post-intervention cohort (Table 3). During the first five months of the post-intervention phase, only 10% of patients (3/30) received ertapenem. After re-education of anesthesia and surgical personnel regarding the AMP protocol, ertapenem was used for AMP in 65% of patients (79/121; p < 0.001) during the final 18 months of the post-intervention phase. Nonetheless, use of ertapenem never exceeded 80% in any one quarter of the post-intervention phase, primarily because of a decision of the surgical or anesthesia provider to use an alternative regimen. This decision was because of a reported β-lactam allergy in only three of the 69 patients in whom ertapenem was not used.
Table 3.
Antibiotic Agents Used for Prophyaxis
| |
Pre-intervention cohort (n = 158) |
Post-intervention cohort (n = 151) |
|
||
|---|---|---|---|---|---|
| Antibiotic | Number of patient operations | % | Number of patient operations | % | p |
| Cefoxitin | 82 | 51.9 | 26 | 17.2 | <0.001 |
| Cefotetan | 9 | 5.7 | 3 | 2.0 | 0.139 |
| Cefazolin ± metronidazole | 41 | 25.9 | 24 | 15.9 | 0.030 |
| Any cephalosporin | 132 | 83.5 | 53 | 35.1 | <0.001 |
| Ertapenem | 4 | 2.5 | 82 | 54.3 | <0.001 |
| Other antibiotic agents | 10a | 6.3 | 4b | 2.6 | 0.120 |
| No antibiotic given before completion of laparotomy | 12 | 7.6 | 12 | 7.9 | 0.908 |
Ampicillin/sulbactam, 1; piperacillin/tazobactam, 4; ciprofloxacin ± metronidazole or clindamycin, 4; vancomycin + metronidazole, 1.
Piperacillin/tazobactam, 1; ciprofloxacin ± metronidazole or clindamycin, 2; vancomycin, 1.
Antimicrobial prophylaxis usage was assessed for timely administration of the initial dose, antibiotic selection, correct initial dosage, and timely redosing for duration of laparotomy or large blood loss (Table 4). Overall compliance with these measures improved from 49% to 62% between the pre- and post-intervention cohorts. However, there were no statistically significant differences in any of the individual measures. Increased compliance in the post-intervention cohort was primarily because of fewer patients being at risk for non-compliance because of incorrect dosing, inadequate spectrum of activity, and failure to re-dose AMP for a prolonged procedure.
Table 4.
Compliance with Measures of Appropriate AMP Use
| |
Pre-intervention cohort (n = 158) |
Post-intervention cohort (n = 151) |
|
||||
|---|---|---|---|---|---|---|---|
| Specific measure | Number at risk | Number compliant | % compliance | Number at risk | Number compliant | % compliance | p |
| Timely delivery of initial antibiotic dose: Any antibiotic administered peri-operatively for laparotomy. Initial dose correctly timed. |
158 158 146 |
129 146 129 |
81.6 92.4 88.4 |
151 151 139 |
120 139 120 |
79.5 92.1 86.3 |
0.629 0.908 0.607 |
| Correct dosage given | 25a | 21 | 84.0 | 12a | 8 | 66.7 | 0.395 |
| Adequate antibiotic spectrum | 46b | 29 | 63.0 | 27b | 11 | 40.7 | 0.065 |
| Re-dosing for length of procedure | 38c | 1 | 2.6 | 9c | 2 | 22.2 | 0.173 |
| Re-dosing for large blood loss | 5 | 2 | 40.0 | 9 | 2 | 22.2 | 0.580 |
| Compliant with all measures | 158 | 77 | 48.7 | 151 | 94 | 62.3 | 0.017 |
AMP = antimicrobial prophylaxis.
p = 0.033 comparing number of patients at risk in the pre- versus the post-intervention cohorts.
p = 0.019 comparing number of patients at risk in the pre- versus the post-intervention cohorts.
p = 0.001 comparing number of patients at risk in the pre- versus the post-intervention cohorts.
Pre-operative and post-operative use of antibiotic agents also declined with implementation of the protocol. Extra pre-operative administration of antibiotic agents (primarily in the emergency department), declined in the post-intervention cohorts, as did administration of any post-operative AMP or any AMP given for greater than 24 hours after laparotomy (Table 5).
Table 5.
Pre-Operative and Post-Operative Antibiotic Use
| |
Pre-intervention cohort (n = 158) |
Post-intervention cohort (n = 151) |
|
||||
|---|---|---|---|---|---|---|---|
| Specific Measure | Number at risk | Number compliant | % compliance | Number at risk | Number compliant | % compliance | p |
| No extra pre-operative antibiotic administered | 158 | 113 | 71.5 | 151 | 131 | 86.8 | 0.001 |
| Post-operative antibiotic agents discontinued within 24 h | 152 | 117 | 77.0 | 146 | 131 | 89.7 | 0.002 |
| No post-operative antibiotic agents given after completion of laparotomy | 152 | 51 | 33.6 | 146 | 90 | 61.6 | < 0.001 |
Ertapenem was used for AMP in this protocol to obviate necessity for dose adjustment for patient weight, addition of a second agent to the regimen to provide anti-anaerobic activity, or redosing for a prolonged procedure. When analyzed according to the actual antibiotic used, 75 of 86 patients who received ertapenem (87%) were compliant with all measures, compared with 100 of 199 patients who received any other antibiotic agent (50%; p < 0.001). Use of cefazolin or any cephalosporin was negatively associated with compliance (data not shown). A multivariable logistic regression analysis confirmed that ertapenem use was positively associated with improved compliance (data not shown).
Patient characteristics
The demographic and injury patterns of patients in the pre-intervention and post-intervention cohorts assessed for infection endpoints are shown in Table 6. Patients were well-matched for most characteristics. The only significant difference identified was a greater number of patients in the post-intervention cohort who had colorectal injuries. Follow-up was similar for patients in the two cohorts (Table 6). Overall, more than 70% of patients had follow-up 30 days or more after injury and 30% of patients had follow-up 90 days or more after injury.
Table 6.
Comparisons of Pre- and Post-Intervention Cohorts
| |
Pre-intervention cohort |
Post-intervention cohort |
|
||
|---|---|---|---|---|---|
| |
Value (n = 139) |
% |
Value (n = 126) |
% |
p
|
| Demographics | |||||
| Age (Mean ± SD) | 33.1 ± 13.8 | 32.9 ± 13.1 | 0.883 | ||
| Gender (male) | 118 | 84.9 | 113 | 89.7 | 0.244 |
| Race (African American) | 99 | 71.2 | 87 | 69.0 | 0.699 |
| Weight (kg) [mean ± SD] | 80.1 ± 17.4 | 82.8 ± 20.0 | 0.244 | ||
| Median income of home zip code: <50% 50%–100% >100% |
30 70 39 |
21.6 50.4 28.1 |
25 64 37 |
19.8 50.8 29.4 |
0.933 |
| Transfer patient | 35 | 25.2 | 28 | 22.2 | 0.572 |
| Medical comorbidities | |||||
|---|---|---|---|---|---|
| Any significant medical comorbidity | 82 | 59.0 | 81 | 64.3 | 0.376 |
| More than one medical comorbidity | 35 | 25.2 | 35 | 27.8 | 0.632 |
| Blood alcohol level >80 mg/dL | 26 (n = 113) | 23.0 | 29 (n = 112) | 25.9 | 0.615 |
| Urine drug screen positive for amphetamines, barbiturates, cocaine, or non-medicinal opioids | 9 (n = 67) | 13.4 | 14 (n = 59) | 23.7 | 0.136 |
| Prior abdominal surgery | 18 | 12.9 | 20 | 15.9 | 0.498 |
| Mechanism of injury | |||||
|---|---|---|---|---|---|
| Penetrating injury | 105 | 75.5 | 97 | 77.0 | 0.783 |
| Mechanism of injury: Gunshot injury Stabbing or other penetrating injury Injury secondary to motor vehicle Other blunt injury |
72 33 28 6 |
51.8 23.7 20.1 4.3 |
77 20 24 5 |
61.1 15.9 19.0 4.0 |
0.447 |
| Severity of injury | |||||
|---|---|---|---|---|---|
| Overall ISS (median, IQR) | 16 (9–25) (n = 137) | 17 (9–26) (n = 123) | 0.106 | ||
| Abdominal abbreviated ISS (median, IQR) | 3 (2–4) (n = 137) | 3 (3–4) (n = 123) | 0.293 | ||
| Probability of survival (mean ± SD) | 0.913 ± 0.203 (n = 135) | 0.886 ± 0.249 (n = 120) | 0.355 | ||
| Initial lactate concentration (mmol/L) [mean ± SD] | 4.7 ± 2.0 (n = 13) | 4.8 ± 3.3 (n = 90) | 0.901 | ||
| Initial lactate concentration ≥2.2 mmol/L | 12 (n = 13) | 92.3 | 72 (n = 90) | 80.0 | 0.453 |
| Concomitant injuries | |||||
|---|---|---|---|---|---|
| Any chest injury | 53 | 38.1 | 53 | 42.1 | 0.514 |
| Any pelvic fracture | 26 | 18.7 | 32 | 25.4 | 0.188 |
| Major spine or spinal cord injury (excluding spinous/transverse process fractures) | 15 | 10.8 | 22 | 17.5 | 0.118 |
| Fracture or major soft tissue extremity injury | 36 | 25.9 | 39 | 31.0 | 0.362 |
| Extra-abdominal vascular injury | 5 | 3.6 | 11 | 8.7 | 0.080 |
| Any significant extra-abdominal injury | 93 | 66.9 | 96 | 76.2 | 0.095 |
| Timing of laparotomy | |||||
|---|---|---|---|---|---|
| Time in ED (min) [median, IQR] | 36 (22-89) (n = 138) | 44.5 (24-79) | 0.593 | ||
| Time from injury to operation (min) [median, IQR] | 118 (68-231) | 118.5 (77-217) | 0.780 | ||
| Laparotomy findings/details | |||||
|---|---|---|---|---|---|
| Negative laparotomy (no abdominal injury encountered) | 8 | 5.8 | 4 | 3.2 | 0.313 |
| Non-therapeutic laparotomy (no abdominal injury treated during laparotomy) | 13 | 9.4 | 14 | 11.1 | 0.637 |
| Any gastrointestinal tract injury | 66 | 47.5 | 70 | 55.6 | 0.189 |
| Any colorectal injury | 35 | 25.2 | 46 | 36.5 | 0.046 |
| Number of units of PRBCs transfused (median, IQR) | 0 (0–4) | 0 (0–4) | 0.947 | ||
| Any preoperative or intraoperative PRBC Transfusion | 68 | 48.9 | 61 | 48.4 | 0.934 |
| Transfusion of ≥10 units of PRBCs (pre- operative and intra-operative) | 13 | 9.4 | 16 | 12.7 | 0.384 |
| Duration of laparotomy (min) [mean ± SD] | 123.6 ± 57.6 | 133.5 ± 74.6 | 0.234 | ||
| Concomitant extra-abdominal procedure | 17 | 12.2 | 10 | 7.9 | 0.249 |
| Abdominal fascia left open at end of initial laparotomy | 29 | 20.9 | 29 | 23.0 | 0.672 |
| Subsequent management | |||||
|---|---|---|---|---|---|
| Postoperative ICU destination | 77 | 55.4 | 77 | 61.1 | 0.346 |
| Subsequent abdominal procedure | 36 | 25.9 | 34 | 27.0 | 0.841 |
| Subsequent extra-abdominal procedure | 36 | 25.9 | 36 | 28.6 | 0.625 |
| Days abdominal fascia left open (median, IQR) | 3 (2–8) (n = 29) | 2 (1–4) (n = 29) | 0.214 | ||
| Skin left open after final abdominal procedure | 55 | 39.6 | 62 | 49.2 | 0.115 |
| Postoperative use of vasopressors for >6 Hours | 16 | 11.5 | 9 | 7.1 | 0.224 |
| Follow-up | |||||
|---|---|---|---|---|---|
| Days of follow-up (median, IQR) | 45 (20 to >90) | 58.5 (21 to >90) | 0.446 | ||
| Any contact made after hospital discharge | 128 | 92.1 | 119 | 93.7 | 0.622 |
| Contact made ≥30 d after laparotomy | 100 | 71.9 | 93 | 73.8 | 0.733 |
| Contact made ≥90 d after laparotomy | 44 | 31.9 | 48 | 38.4 | 0.269 |
| Patients whose only contact at ≥30 d was by telephone | 7 (n = 11) | 63.6 | 10 (n = 17) | 58.8 | 0.799 |
SD = standard deviation; ISS = Injury Severity Score ; IQR = interquartile range; ED = emergency department; PRBCs = packed red blood cells; ICU = intensive care unit.
Infections
The primary endoint of the study (percentage of patients having an infection directly related to abdominal trauma at 30 days) declined from 27% (37/139) in the pre-intervention cohort to 14% (18/126) in the post-intervention cohort (Table 7). This represented an absolute decrease of 13% and a relative decrease of 46% in infections directly related to trauma laparotomy. This resulted in an overall relative decrease of 26% in the percentage of patients sustaining any infection, although this change just missed statistical significance. Results at 90 days were similar to those at 30 days, with the exception that the decrease in overall infections reached statistical significance in this analysis.
Table 7.
Infections in Pre- and Post-Intervention Cohorts
| |
Pre-intervention cohort (n = 139) |
Post-intervention cohort (n = 126) |
Absolute change |
Relative change |
|
||
|---|---|---|---|---|---|---|---|
| Endpoint |
n
|
% |
n
|
% |
% |
% |
p
|
| Number of patients with infections at 30 daysa | |||||||
| Any related infection | 37 | 26.6 | 18 | 14.3 | −12.3 | −46.3 | 0.013 |
| Any SSI | 19 | 13.7 | 6 | 4.8 | −8.9 | −65.2 | 0.013 |
| Superficial SSI | 14 | 10.1 | 3 | 2.4 | −7.7 | -76.2 | 0.011 |
| Deep SSI | 5 | 3.6 | 3 | 2.4 | −1.2 | −33.3 | 0.725 |
| Intra-abdominal infection | 17 | 12.2 | 12 | 9.5 | −2.7 | −22.1 | 0.481 |
| Related empyema | 4 | 2.9 | 2 | 1.6 | −1.3 | −44.8 | 0.686 |
| Related SSTI | 4 | 2.9 | 1 | 0.8 | -2.1 | −72.4 | 0.373 |
| Secondary blood stream infection | 2 | 1.4 | 5 | 4.0 | +2.6 | +186 | 0.263 |
| Any related infection other than superficial SSI and minor SSTI | 26 | 18.7 | 14 | 11.1 | −7.6 | −40.6 | 0.085 |
| Any unrelated infection | 38 | 27.3 | 30 | 23.8 | −3.5 | −12.9 | 0.511 |
| Pneumonia | 23 | 16.6 | 16 | 12.7 | −3.9 | −23.3 | 0.377 |
| UTI | 15 | 10.8 | 7 | 5.6 | −5.2 | −48.5 | 0.123 |
| Primary bloodstream infection | 7 | 5.0 | 1 | 0.8 | −4.2 | −84.2 | 0.069 |
| Unrelated SSI or SSTI | 4 | 2.9 | 7 | 5.6 | +2.7 | +93.1 | 0.275 |
| Otherb | 1 | 0.7 | 3 | 2.4 | +1.7 | +231 | 0.349 |
| Clostridiodes difficile-related disease | 5 | 3.6 | 0 | 0.0 | −3.6 | −100 | 0.062 |
| Any related or unrelated infection | 60 | 43.2 | 40 | 31.8 | −11.4 | −26.5 | 0.056 |
| Number of patients with infections at 90 daysa | |||||||
|---|---|---|---|---|---|---|---|
| Any related infection | 42 | 30.2 | 22 | 17.5 | −12.8 | −42.2 | 0.015 |
| Any SSI | 25 | 18.0 | 9 | 7.1 | −10.8 | −60.3 | 0.008 |
| Superficial SSI | 20 | 14.4 | 5 | 4.0 | −10.4 | −72.4 | 0.004 |
| Deep SSI | 6 | 4.3 | 4 | 3.2 | −1.1 | −26.5 | 0.752 |
| Intra-abdominal infection | 18 | 13.0 | 14 | 11.1 | −1.8 | −14.2 | 0.631 |
| Related empyema | 4 | 2.9 | 2 | 1.6 | −1.3 | −44.8 | 0.686 |
| Related SSTI | 5 | 3.6 | 3 | 2.4 | −1.2 | −33.8 | 0.725 |
| Secondary blood stream infection | 2 | 1.4 | 5 | 4.0 | +2.5 | +176 | 0.263 |
| Any related infection other than superficial SSI and minor SSTI | 27 | 19.4 | 16 | 12.7 | −6.7 | −34.6 | 0.139 |
| Any unrelated infection | 45 | 32.4 | 34 | 27.0 | −5.4 | −16.7 | 0.338 |
| Pneumonia | 24 | 17.3 | 16 | 12.7 | −4.6 | −26.5 | 0.300 |
| UTI | 19 | 13.7 | 13 | 10.3 | −3.4 | −24.5 | 0.403 |
| Primary blood stream infection | 10 | 7.2 | 2 | 1.6 | −5.6 | −77.9 | 0.037 |
| Unrelated SSI or SSTI | 5 | 3.6 | 9 | 7.1 | +3.6 | +97.2 | 0.198 |
| Otherc | 2 | 1.4 | 3 | 2.4 | +0.9 | +71.4 | 0.671 |
| Clostridiodes difficile-related disease | 5 | 3.6 | 0 | 0 | −3.6 | −100 | 0.062 |
| Any related or unrelated infection | 67 | 48.2 | 43 | 34.1 | −14.1 | −29.2 | 0.020 |
SSI = surgical site infection; SSTI = skin and soft tissue infection; UTI = urinary tract infection.
Patients in either cohort may have had more than one type of infection.
Pre-intervention cohort: ocular infection, 1; post-intervention cohort: osteomyelitis, 2; Meningitis, 1.
Pre-intervention cohort: ocular infection, 1; osteomyelitis, 1; post-intervention cohort: osteomyelitis, 2; meningitis, 1.
The types of infections patients in both cohorts sustained are shown in Table 7. There was a statistically significant decrease in the incidence of SSI in the post-intervention cohort, primarily due to a decrease in superficial SSI at both 30 and 90 days. Deep SSI, organ/space infections, and most other related infections declined among patients in the post-intervention cohort, although these decreases did not reach statistical significance. There were fewer patients with unrelated infections in the post-intervention cohort, but this did not reach significance except for primary blood stream infections at 90 days; this difference may have reflected misclassification, since the overall number of patients with blood stream infections (both primary and secondary) was not significantly different at 90 days (12 in the pre-intervention cohort, 7 in the post-intervention cohort; p = 0.332). Five patients developed Clostridiodes difficile-associated disease (CDAD) in the pre-intervention cohort whereas none did in the post-intervention cohort, although this difference did not reach statistical significance. Among the patients who developed CDAD, four of the five received a cephalosporin for AMP.
Univariable and multivariable analyses were performed to determine factors that increased the risk of a related infection. In univariable analysis, multiple risk factors were identified, which tended to segregate into two groups: those related to the severity of injury, such as a penetrating mechanism of injury, a higher ISS or abdominal ISS score, a colorectal or any gastrointestinal tract injury, a larger number of units of blood transfused, and fascia being left open at the end of the initial procedure; and those related to prophylactic antibiotic administration, including an operation prior to protocol implementation, non-compliance with measures of appropriate antibiotic use, longer post-operative use of prophylactic antibiotic agents, and use of an antibiotic other than ertapenem for prophylaxis (Table 8). Multivariable logistic regression of these risk factors revealed that laparotomy prior to protocol implementation was strongly associated with the risk of a related infection; additional risk factors included a penetrating mechanism of injury, ISS score, a colorectal injury, and the number of units of PRBCs transfused (Table 9).
Table 8.
Univariable Analysis of Risk Factors for Related Infection at Thirty Days
| |
Patients without a related infection (n = 210) |
Patients with a related infection (n = 55) |
|
|
||
|---|---|---|---|---|---|---|
| Factor |
Number or value |
% |
Value |
% |
p
a
|
Odds ratiob |
| Demographic factors | ||||||
| Age (mean ± SD) | 33.8 ± 14.1 | 29.8 ± 10.2 | 0.042 | 0.97 (0.95–0.99) | ||
| Male Gender | 182 | 86.7 | 49 | 89.1 | 0.632 | 1.26 (0.52–3.51) |
| Race (African American) | 140 | 66.7 | 46 | 83.6 | 0.014 | 2.56 (1.23–5.85) |
| Weight (kg) [mean ± SD] | 80.7 ± 18.0 | 84.6 ± 21.5 | 0.171 | 1.01 (0.99–1.03) | ||
| Median Income of home zip code: | ||||||
| <50% | 41 | 19.5 | 14 | 25.5 | 0.234 | Reference |
| 50%–100% | 104 | 49.5 | 30 | 54.6 | 0.551 | 0.85 (0.41–1.79) |
| >100% | 65 | 31.0 | 11 | 20.0 | 0.100 | 0.50 (0.20–1.03) |
| Transfer patient | 55 | 26.2 | 8 | 14.6 | 0.071 | 0.48 (0.20–1.03) |
| Medical comorbidities | ||||||
|---|---|---|---|---|---|---|
| Any significant medical comorbidity | 130 | 61.9 | 33 | 60.0 | 0.796 | 0.92 (0.51–1.71) |
| More than one medical comorbidity | 58 | 27.6 | 12 | 21.8 | 0.385 | 0.73 (0.35–6.29) |
| Blood alcohol level >80 mg/dL | 45 (n = 177) | 25.4 | 10 (n = 48) | 20.8 | 0.512 | 0.77 (0.34–1.63) |
| Urine drug screen positive for amphetamines, barbiturates, cocaine, or non-medicinal opioids | 15 (n = 99) | 15.2 | 8 (n = 27) | 29.6 | 0.084 | 2.36 (0.85–6.29) |
| Prior abdominal surgery | 30 | 14.3 | 8 | 14.6 | 0.961 | 1.02 (0.41–2.28) |
| Mechanism of injury | ||||||
|---|---|---|---|---|---|---|
| Penetrating versus blunt | 154 | 73.3 | 48 | 87.3 | 0.031 | 2.49 (1.13–6.32) |
| Mechanism of Injury: | ||||||
| Gunshot injury | 111 | 52.9 | 38 | 69.1 | 0.049 | Reference |
| Stabbing or other penetrating injury | 43 | 20.5 | 10 | 18.2 | 0.754 | 0.68 (0.30–1.44) |
| Motor vehicle injury | 47 | 22.4 | 5 | 9.1 | 0.027 | 0.31 (0.10–0.77) |
| Other blunt injury | 9 | 4.3 | 2 | 3.6 | 0.915 | 0.65 (0.10–2.66) |
| Severity of injury | ||||||
|---|---|---|---|---|---|---|
| Median overall ISS [IQR] | 16 (n = 205) | 24.5 (n = 54) | 0.041 | 1.03 (1.001–1.05) | ||
| [9 – 25] | [10–26] | |||||
| Median abdominal ISS [IQR] | 3.0 (n = 205) | 3.5 (n = 54) | 0.001 | 1.52 (1.17–2.03) | ||
| [2 – 4] | [3 – 4] | |||||
| Probability of survival (mean ± SD) | 0.902 ± 0.231 (n = 202) | 0.895 ± 0.205 (n = 53) | 0.841 | 0.87 (0.26–3.69) | ||
| Initial lactate concentration (mmol/L) [mean ± SD] | 4.6 ± 3.0 (n = 86) | 5.7 ± 4.2 (n = 17) | 0.201 | 1.10 (0.94–1.27) | ||
| Initial lactate concentration ≥2.2 mmol/L | 71 (n = 86) | 82.6 | 13 (n = 17) | 76.5 | 0.554 | 1.46 (0.37–4.8) |
| Concomitant injuries | ||||||
|---|---|---|---|---|---|---|
| Any chest injury | 86 | 41.0 | 20 | 36.4 | 0.536 | 0.82 (0.44–1.51) |
| Any pelvic fracture | 49 | 23.3 | 9 | 16.4 | 0.266 | 0.64 (0.28–1.35) |
| Major spine or spinal cord Injury (excluding spinous/transverse process fractures) | 27 | 12.9 | 10 | 18.2 | 0.311 | 1.51 (0.65–3.26) |
| Fracture or major soft tissue extremity injury | 60 | 28.6 | 15 | 27.3 | 0.849 | 0.94 (047–1.79) |
| Extra-abdominal vascular injury | 9 | 4.3 | 7 | 12.7 | 0.019 | 3.26 (1.11–9.18) |
| Any extra-abdominal injury | 148 | 70.5 | 41 | 74.6 | 0.553 | 1.23 (0.64–2.48) |
| Timing of laparotomy | ||||||
|---|---|---|---|---|---|---|
| Median time in ED (min) [IQR] | 46 | 29 | 0.379 | 0.99 (0.98–1.02) | ||
| [27–93] | [18–55] | |||||
| Median time from injury to operation (min) [IQR] | 130 | 72 | 0.117 | 0.99 (0.99–1.00) | ||
| [81–249] | [59–125] | |||||
| Details of initial laparotomy | ||||||
|---|---|---|---|---|---|---|
| Negative laparotomy (no abdominal injury encountered) | 10 | 4.8 | 2 | 3.6 | 0.721 | 0.76 (0.11–2.97) |
| Non-therapeutic laparotomy (no abdominal injury treated during laparotomy) | 24 | 11.4 | 3 | 5.5 | 0.192 | 0.45 (0.10–1.35) |
| Any gastrointestinal tract injury | 94 | 44.8 | 42 | 76.4 | <0.001 | 3.99 (2.07–8.13) |
| Any colon and/or rectal injury | 50 | 23.8 | 31 | 56.4 | <0.001 | 4.13 (2.23–7.76) |
| Median number of units of PRBCs transfused [IQR] | 0 | 1 | <0.001 | 1.07 (1.03–1.12) | ||
| [0–4] | [0–9] | |||||
| Any pre-operative or intra-operative PRBC transfusion | 97 | 46.2 | 32 | 58.1 | 0.113 | 1.62 (0.89–2.98) |
| Transfusion of ≥10 units of PRBCs (pre-operative and intra-operative) | 16 | 7.6 | 13 | 23.6 | 0.001 | 3.75 (1.66–8.39) |
| Duration of laparotomy (min) (mean ± SD) | 123.6 ± 63.5 | 146.2 ± 67.0 | 0.028 | 1.005 (1.001–1.009) | ||
| Concomitant extra-abdominal procedure | 20 | 9.5 | 7 | 12.7 | 0.496 | 1.39 (0.52–3.33) |
| Abdominal fascia left open at end of initial laparotomy | 37 | 17.6 | 21 | 38.2 | 0.001 | 2.89 (1.50–5.52) |
| Subsequent management | ||||||
|---|---|---|---|---|---|---|
| Post-operative ICU destination | 117 | 55.7 | 37 | 67.3 | 0.122 | 1.63 (0.88–3.11) |
| Subsequent abdominal procedure | 44 | 21.0 | 26 | 47.3 | <0.001 | 3.38 (1.81–6.34) |
| Subsequent extra-abdominal procedure | 48 | 22.9 | 24 | 43.6 | 0.003 | 2.61 (1.40–4.87) |
| Median days abdominal fascia left open [IQR) | 2 (n = 37) | 3 (n = 21) | 0.004 | 1.23 (1.08–1.44) | ||
| [1-4] | [2-13] | |||||
| Skin left open after final abdominal procedure | 82 | 39.1 | 35 | 63.6 | 0.001 | 2.73 (1.49–5.13) |
| Post-operative use of vasopressors for >6 h | 18 | 8.6 | 7 | 12.7 | 0.348 | 1.56 (0.58–3.98) |
| Compliance with measures of appropriate antibiotic use | ||||||
|---|---|---|---|---|---|---|
| Pre- versus post-intervention cohort: | ||||||
| Pre-intervention | 102 | 48.6 | 37 | 67.3 | 0.013 | 0.46 (0.24–0.85) |
| Post-intervention | 108 | 51.4 | 18 | 32.7 | ||
| Compliant with all measures | 123 | 58.6 | 22 | 40.0 | 0.014 | 0.47 (0.26–0.86) |
| Timely delivery of initial antibiotic dose | 174 | 82.8 | 41 | 74.6 | 0.161 | 0.61 (0.30–1.26) |
| Any antibiotic administered for laparotomy | 195 | 92.9 | 50 | 0–0.9 | 0.626 | 1.30 (0.41–3.54) |
| Initial dose correctly timed | 174 (n = 195) | 89.2 | 41 (n = 50) | 82.0 | 0.164 | 0.55 (0.24–1.34) |
| Correct dosage given | 17 (n = 25) | 68.0 | 9 (n = 9) | 100 | 0.077 | Not calculable |
| Adequate antibiotic spectrum | 28 (n = 46) | 60.9 | 4 (n = 12) | 33.3 | 0.088 | 0.32 (0.08–1.18) |
| Re-dosing for length of procedure | 3 (n = 35) | 8.6 | 0 (n = 15) | 0 | 0.136 | Not calculable |
| Re-dosing for large blood loss | 3 (n = 5) | 60.0 | 1 (n = 7) | 14.3 | 0.222 | 0.11 (0.004–1.43) |
| No extra pre-operative antibiotic agents administered | 170 | 80.9 | 41 | 74.5 | 0.294 | 0.69 (0.35–1.42) |
| Post-operative antibiotic agents discontinued within 24 h | 179 | 85.2 | 40 | 72.7 | 0.029 | 0.46 (0.23–0.95) |
| No post-operative antibiotic agents given | 103 | 49.0 | 14 | 25.5 | 0.002 | 0.36 (0.18–0.68) |
| Prophylactic antibiotic administered | ||||||
|---|---|---|---|---|---|---|
| Cefoxitin | 73 | 34.8 | 24 | 43.6 | 0.224 | 1.45 (0.79–2.65) |
| Cefotetan | 8 | 3.8 | 3 | 5.5 | 0.598 | 1.46 (0.31–5.24) |
| Cefazolin ± metronidazole | 43 | 20.5 | 12 | 21.8 | 0.827 | 1.08 (0.51–2.18) |
| Any cephalosporin | 124 | 59.1 | 39 | 70.9 | 0.108 | 1.69 (0.90–3.29) |
| Ertapenem | 65 | 31.0 | 9 | 16.4 | 0.032 | 0.44 (0.19–0.91) |
| Other antibiotics | 6c | 2.9 | 2d | 3.6 | 0.764 | 1.28 (0.18–5.75) |
SD = standard deviation; ISS = Injury Severity Score ; IQR = interquartile range; ED = emergency department; PRBCs = packed red blood cells; ICU = intensive care unit.
For categorical factors with more than two subgroups, a global p value was presented for the relation between the factor and outcome; individual p values are presented for each odds ratio.
Odds ratio with 95% confidence interval was presented to measure the odds of having infection of the subgroup compared with the reference group, or the odds of having infection per one unit increase of the numeric factor.
Ampicillin/sulbactam, 1; piperacillin/tazobactam, 2; ciprofloxacin + metronidazole or clindamycin, 3.
Piperacillin/tazobactam, 2.
Table 9.
Multivariable Logistic Regression for Any Related Infection at Thirty Days
| Risk factor | Estimate | Standard error | OR (95% CI) | p |
|---|---|---|---|---|
| Pre-intervention cohort | 0.6308 | 0.1899 | 3.53 (1.72–7.66) | 0.0009 |
| Penetrating mechanism of injury | 0.6139 | 0.2640 | 3.41 (1.28–10.36) | 0.0200 |
| ISS | 0.0416 | 0.0189 | 1.04 (1.01–1.08) | 0.0276 |
| Any colorectal injury | 0.8119 | 0.1842 | 5.07 (2.50–10.65) | <0.0001 |
| Units of PRBCs transfused | 0.0542 | 0.0227 | 1.06 (1.01–1.11) | 0.0168 |
OR = odds ratio; CI = confidence interval; ISS = Injury Severity Score; PRBCs = packed red blood cells.
Secondary analyses were performed to explore whether ertapenem use contributed to the decreased risk of related infection after protocol implementation. Ertapenem use was associated with significant relative decreases of 49% and 46% in the number of patients who sustained a related infection at 30 and 90 days (Table 10). Although trends toward a decrease in overall infections were seen at both 30 and 90 days, these numbers did not reach statistical significance. When patient cohort was excluded, a multivariable logistic regression analysis showed that ertapenem use was strongly associated with a statistically significant decline in the number of patients with a related infection at 30 days (Table 11).
Table 10.
Infection Outcomes with Use of Ertapenem
| |
Ertapenem not used (n = 191) |
Ertapenem used (n = 74) |
Absolute change |
Relative change |
|
||
|---|---|---|---|---|---|---|---|
| Endpoint |
n
|
% |
n
|
% |
% |
% |
p
|
| Patients with infection at 30 da | |||||||
| Any related infection | 46 | 24.1 | 9 | 12.2 | −11.9 | −49.5 | 0.032 |
| Any unrelated infection | 51 | 26.7 | 17 | 23.0 | −3.7 | −14.0 | 0.533 |
| Any related or unrelated infection | 77 | 40.3 | 23 | 31.1 | −9.2 | −22.9 | 0.164 |
| Patients with infection at 90 da | |||||||
|---|---|---|---|---|---|---|---|
| Any related infection | 53 | 27.8 | 11 | 14.9 | −12.9% | −46.4 | 0.028 |
| Any unrelated infection | 60 | 31.4 | 19 | 25.7 | -5.8 | −18.3 | 0.360 |
| Any related or unrelated infection | 85 | 44.5 | 25 | 33.8 | −10.7 | −24.1 | 0.112 |
Some patients in each group had both a related and an unrelated infection.
Table 11.
Multivariate Logistic Regression for Any Related Infection at Thirty Days, Excluding Cohort
| | ||||
|---|---|---|---|---|
| Risk factor | Estimate | Standard error | OR (95% CI) | p |
| Ertapenem as peri-operative antibiotic | −0.5606 | 0.2145 | 0.33 (0.13–0.73) | 0.0089 |
| Penetrating mechanism of injury | 0.4236 | 0.2395 | 2.33 (1.02–6.45) | 0.0469 |
| Any colorectal injury | 0.7427 | 0.1747 | 4.42 (2.25–8.88) | <0.0001 |
| Units of PRBCs transfused | 0.0784 | 0.0211 | 1.08 (1.04–1.13) | 0.0105 |
OR = odds ratio; CI = confidence interval; PRBCs = packed red blood cells.
Other outcomes
Nine patients died from their injuries, all of whom were excluded from analysis of infection endpoints. There were no other patient deaths in either cohort at 30 days, although one elderly patient in the post-intervention cohort died more than 30 days after laparotomy at a long-term acute care facility from non-infectious causes. The number of hospital days, intensive care unit (ICU) days, and ventilator days did not differ significantly between the two cohorts, but the number of patients discharged to a long-term or skilled nursing facility was higher in the pre-intervention cohort, nearly reaching statistical significance (p = 0.0504). Overall days of anti-infective agent use were decreased in the post-intervention cohort, as were days of inappropriate antimicrobial use (Table 12).
Table 12.
Other Outcomes
| |
Pre-intervention cohort (n = 139) |
Post-intervention cohort (n = 126) |
|
||
|---|---|---|---|---|---|
| Endpoint | n | % | n | % | p |
| Patient with non-infectious complications at 30 d | 64 | 46.0 | 51 | 40.5 | 0.361 |
| Re-hospitalization within 30 d | 23 | 16.6 | 14 | 11.1 | 0.202 |
| Discharged to a long-term or another facility | 37 | 26.7 | 21 | 16.7 | 0.050 |
| Median | IQR | Median | IQR | p | |
|---|---|---|---|---|---|
| Hospital days |
8 |
5–16 |
8 |
5–13 |
0.428 |
| ICU days |
1 |
0–4 |
1 |
0–4 |
0.571 |
| Ventilator days |
0 |
0–2 |
0 |
0–2 |
0.966 |
| Days receiving anti-Infective agents |
5 |
2–14 |
3 |
1–11 |
0.009 |
| Days receiving prophylactic anti-infective agents |
2 |
1–4 |
2 |
1–3 |
0.107 |
| Days receiving empiric anti-infective agents |
0 |
0–0 |
0 |
0–0 |
0.128 |
| Days receiving anti-infective agents for treatment of an infection |
0 |
0–10 |
0 |
0–8 |
0.035 |
| Days receiving anti-infective agents for treatment of a related infection |
0 |
0–0 |
0 |
0–0 |
0.275 |
| Days in which all anti-infective agents administered were considered inappropriate |
0 |
0–1 |
0 |
0–1 |
0.227 |
| Days in which any anti-infective agent administered was considered inappropriate | 1 | 0–5 | 0 | 0–3 | 0.046 |
IQR = interquartile range; ICU = intensive care unit
Discussion
Various series of infection after trauma laparotomy have documented rates of infection of 7.1% to 28.4% for superficial and deep SSI and 7.9% to 25.2% for IAI after trauma laparotomy.1–5 In a review of PRCTs on AMP for trauma laparotomy, rates of SSI and IAI were 18.4% and 8.6%, respectively.5 In a previous analysis, we found that 31% of patients had infections directly related to laparotomy.17
Given the high incidence of these infections, this study was undertaken to determine if a protocol designed to facilitate optimal use of AMP for trauma laparotomy, based on established principles for AMP for elective surgical procedures, could decrease this incidence. Important considerations were that tissue levels of the antibiotic should be maintained throughout trauma laparotomy, and that the agent selected have coverage of the organisms expected to contaminate the abdomen in these patients. Prior to protocol development, cefazolin and cefoxitin were the most common agents utilized for prophylaxis in patients undergoing trauma laparotomy. These were frequently administered in the emergency department prior to transport to the operating room. This resulted in a number of deviations from recommended processes used for AMP. Occasionally, the prophylactic agent was administered more than one hour before the operative incision was made. There were also failures to utilize larger dosages in obese patients, to re-dose the agent when the operative time exceeded two half-lives of the agent administered, and to include anaerobic coverage when a bowel injury was present.
To simplify the process of antibiotic selection, the protocol specified use of ertapenem as the preferred agent because of its prolonged half-life and spectrum of activity including anaerobic organisms. To ensure adequate drug levels, the protocol also specified that the drug was to be delivered in the operating room by the anesthesia team immediately prior to the start of the operation. The antibiotic was to be re-dosed in the event of blood loss exceeding one blood volume, based on use of blood products for patients placed on the massive transfusion protocol. Ertapenem re-dosing was otherwise recommended only if the duration of the laparotomy exceeded six hours. Unless there had been an anaphylactic reaction to a penicillin or cephalosporin, ertapenem could be used in patients with a reported history of a β-lactam allergy. No recommendation was made regarding antibiotic prophylaxis for a subsequent extra-abdominal procedure carried out by another service after trauma laparotomy. Antibiotic agents given for trauma laparotomy were to be discontinued within 24 hours after the end of the operation, with a preference for no antibiotic agents to be given post-operatively; however, no recommendation was made regarding duration of prophylaxis for patients whose abdominal fascia was left open after damage control laparotomy.
The primary endpoint for the study was the proportion of patients who developed a post-operative infection directly related to the abdominal trauma within 30 days of injury. A decrease of 46% in the percentage of patients sustaining these infections was noted. This was statistically significant and in excess of the predicted 25% decrease that had been hypothesized as part of the study design and sample size calculations. The multivariable analysis identified being part of the post-intervention cohort as a highly significant factor in the reduced rates of infections. With regard to specific infection type, the decrease was significant for superficial SSI or the combination of superficial and deep SSI. However, decreases were also seen in the incidence of more serious infections, including organ/space infections, although these did not reach statistical significance, in part because of their lower frequency.
Another goal of this study was to determine if compliance with measures thought important for optimal antimicrobial prophylaxis would be improved through use of the protocol. Although a statistically significant improvement in compliance with all measures was observed in the post-intervention cohort, no improvement was noted for compliance for any individual measure. Overall compliance was improved because fewer patients in the post-intervention cohort were at risk for failure to adjust dosing for obesity, to use a supplemental anti-anaerobic agent, or to re-dose for the length of the procedure. Implementation of the protocol also was associated with some improvements in antimicrobial stewardship. There was decreased utilization of extra pre-operative antibiotic agents in the emergency department prior to trauma laparotomy, and decreased use of prophylactic agents in the post-operative phase, with only 10% of patients, primarily those undergoing delayed fascial closure, receiving prophylactic agents for more than 24 hours.
Failure to use ertapenem as the preferred antibiotic in patients in the post-intervention cohort was quite frequent. The study called for roll-out of the protocol in November 2011, which ultimately had to be delayed for one month due to logistical issues. Prior to implementation, the protocol was not disseminated to avoid premature use of ertapenem in the pre-intervention cohort. However, this resulted in poor compliance with ertapenem administration during the first several months the protocol was in effect, because many anesthesiologists were unaware that ertapenem was the preferred agent. Compliance improved with further educational efforts, but there remained a reluctance on the part of some to use ertapenem for all trauma laparotomy, making compliance with ertapenem administration only 65% after renewed educational efforts.
Nonetheless, ertapenem use appeared to be a factor in reducing the number of patients with SSI and other related infections in the post-intervention cohort. A secondary analysis comparing patients receiving ertapenem with those who did not demonstrated virtually the same results as the comparison of patients in the pre-intervention and post-intervention cohorts. Moreover, the multivariable analysis of infection risk regardless of cohort demonstrated that ertapenem use was highly associated with a decreased risk of infection.
Studies of prophylaxis of elective colorectal surgery have suggested ertapenem use is more efficacious than some other agents. In a PRCT, ertapenem appeared to be superior to cefotetan for prevention of SSI.16 In a large population-based analysis, ertapenem use was associated with fewer SSI than cefoxitin and cefotetan.18
Alternatively, however, the development of the protocol and its implementation could have led to the decreased rate of infections in the post-intervention cohort. Apropos to this, Dellinger19 has suggested that fostering teamwork and collaboration among the surgical team may be more important than specific measures in reducing rates of SSI.
There are a number of limitations to this study. The principal one relates to study design, being a before/after trial and not a randomized trial. No important changes were made in treatment approaches during the years of the study to our knowledge. Although there may have been a modest increase in the use of minimally invasive approaches in the post-intervention cohort, these patients were excluded from infection endpoints, and would not favorably influence infection rates in the post-intervention cohort. The risk factors identified in the multivariable analysis were fairly evenly distributed between the two cohorts; the only statistically significant difference was a higher number of patients with colorectal injuries in the post-intervention cohort, which would have favored the pre-intervention cohort. Ascertainment of infection could introduce a bias into the study because the investigators were not blinded to patient assignment. We attempted to avoid bias by having all patient records reviewed independently by a study coordinator and principal investigator to determine if an infection had occurred, utilizing criteria developed prior to the start of the study. To the extent possible, the study coordinators concurrently reviewed all study patients for any diagnosis of infection while they were in the hospital. In the event of ambiguity or disagreement between the principal investigator and the study coordinator with regard to the diagnosis of an infection, a second clinician reviewed the information. If there was disagreement between the clinicians, a third clinician reviewed the clinical information to make the final decision. The second and third reviewers were kept blinded to treatment assignment.
Conclusions
In this study, adoption of a protocol for standardized AMP in patients undergoing laparotomy for abdominal trauma was associated with a decrease in the risk of infectious complications directly related to that procedure. This result was obtained despite incomplete compliance with the protocol. The protocol had been designed specifically to facilitate adherence with established principles of AMP. For this study, ertapenem appeared to be a good choice for the prophylactic agent, although other agents might have produced similar results if included in the protocol. Overall, the development of this coordinated process to optimize AMP in this group of patients at high risk for SSI led to declines in this complication. This reinforces the concept that development of specific protocols emphasizing team-based approaches to optimize patient care can lead to improvements in outcomes.
Acknowledgments
The authors would like to express their appreciation to the other members of the Section of Acute and Critical Care Surgery who have contributed their clinical and investigational expertise to this research project.
We also acknowledge the contributions of Hongjie Gu from the Division of Biostatistics in analyzing the data.
Authors' Contributions
Conceptualization: Mazuski. Methodology: Mazuski. Validation: Mazuski, Symons. Formal analysis: Mazuski, Symons. Investigation: Mazuski, Symons, Jarman, Sato, Carroll, Bochicchio, Kirby, Schuerer. Data curation: Mazuski, Symons, Jarman, Sato. Writing–original draft: Mazuski, Symons. Writing–review and editing: Mazuski, Symons, Kirby, Schuerer. Supervision: Mazuski.
Project administration: Mazuski. Funding acquisition: Mazuski. Resources: Bochicchio.
Funding Information
Supported in part by an Investigator Initiated Studies Program Grant #37280 from Merck and Co.
Author Disclosure Statement
No financial conflicts of interest have been reported by the authors other than grant support provided by Merck and Co.
References
- 1. Morales CH, Escobar RM, Villegas MI, et al. Surgical site infection in abdominal trauma patients: Risk prediction and performance of the NNIS and SENIC indexes. Can J Surg 2011;54(1):17–24; doi: 10.1503/cjs.022109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Seamon MJ, Smith BP, Capano-Wehrle L, et al. Skin closure after trauma laparotomy in high-risk patients: Opening opportunities for improvement. J Trauma Acute Care Surg 2013;74(2):433–440; doi: 10.1097/TA.0b013e31827e2589 [DOI] [PubMed] [Google Scholar]
- 3. Acker A, Leonard J, Seamon MJ, et al. Leaving contaminated trauma laparotomy wounds open reduces wound infections but does not add value. J Surg Res 2018;232:450–455; doi: 10.1016/j.jss.2018.05.083 [DOI] [PubMed] [Google Scholar]
- 4. Bozzay JD, Walker PF, Schechtman DW, et al. Outcomes of exploratory laparotomy and abdominal infections among combat casualties. J Surg Res 2021;257:285–293; doi: 10.1016/j.jss.2020.07.075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hanna K, Asmar S, Ditillo M, et al. Readmission with major abdominal complications after penetrating abdominal trauma. J Surg Res 2021;257:69–78; doi: 10.1016/j.jss.2020.07.060 [DOI] [PubMed] [Google Scholar]
- 6. Bratzler DW, Dellinger, EP, Olsen, KM, et al. Clinical practice guidelines for antimicrobial prophylaxis in surgery. Surg Infect 2013;14(1):73–156; doi: 10.1089/sur.2013.9999 [DOI] [PubMed] [Google Scholar]
- 7. Ban KA, Minei JP, Laronga C, et al. American College of Surgeons and Surgical Infection Society: Surgical Site Infection Guidelines, 2016 Update. J Am Coll Surg 2017; 224(1):59–74; doi: 10.1016/j.jamcollsurg.2016.10.029 [DOI] [PubMed] [Google Scholar]
- 8. Berríos-Torres SI, Umscheid CA, Bratzler DW, et al. Centers for Disease Control and Prevention guideline for the Prevention of Surgical Site Infection, 2017. JAMA Surg 2017;152(8):784–791; doi: 10.1001/jamasurg.2017.0904 [DOI] [PubMed] [Google Scholar]
- 9. Brand M, Grieve A. Prophylactic antibiotics for penetrating abdominal trauma. Cochrane Database Syst Rev 2019;12(12):CD007370; doi: 10.1002/14651858.CD007370.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Goldberg SR, Anand RJ, Como JJ, et al. Prophylactic antibiotic use in penetrating abdominal trauma: An Eastern Association for the Surgery of Trauma practice management guideline. J Trauma Acute Care Surg 2012;73(5 Suppl 4):S321–S325; doi: 10.1097/TA.0b013e3182701902 [DOI] [PubMed] [Google Scholar]
- 11. Herrod PJ, Boyd-Carson H, Doleman Bet al. Prophylactic antibiotics for penetrating abdominal trauma: Duration of use and antibiotic choice. Cochrane Database Syst Rev 2019;12;12(12):CD010808; doi: 10.1002/14651858.CD010808.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. McGee MF, Kreutzer L, Quinn CM, et al. Leveraging a comprehensive program to implement a colorectal surgical site infection reduction bundle in a statewide quality improvement collaborative. Ann Surg 2019;270(4):701–711; doi: 10.1097/SLA.0000000000003524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Weiser MR, Gonen M, Usiak S, et al. Effectiveness of a multidisciplinary patient care bundle for reducing surgical-site infections. Br J Surg 2018;105(12):1680–1687; doi: 10.1002/bjs.10896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zywot A, Lau CSM, Fletcher H, et al. Bundles prevent surgical site infections after colorectal surgery: Meta-analysis and systematic review. J Gastrointest Surg 2017;21(11):1915–1930; doi: 10.1007/s11605-017-3465-3 [DOI] [PubMed] [Google Scholar]
- 15. Mc Geehan G, Edelduok IM, Bucholc M, et al. Systematic review and meta-analysis of wound bundles in emergency midline laparotomy identifies that it is time for improvement. Life (Basel) 2021;11(2):138; doi: 10.3390/life11020138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Itani KM, Wilson SE, Awad SS, et al. Ertapenem versus cefotetan prophylaxis in elective colorectal surgery. N Engl J Med 2006;355(25):2640–2651; doi: 10.1056/NEJMoa054408 [DOI] [PubMed] [Google Scholar]
- 17. Husain KD, Keune J, Sato B, et al. Infectious complications following exploratory laparotomy for trauma: The case for improved antibiotic prophylaxis. Surg Infect 2012;13(Suppl 1):S9. [Google Scholar]
- 18. Hendren S, Fritze D, Banerjee M, et al. Antibiotic choice is independently associated with risk of surgical site infection after colectomy: A population-based cohort study. Ann Surg 2013;257(3):469–475; doi: 10.1097/SLA.0b013e31826c4009 [DOI] [PubMed] [Google Scholar]
- 19. Dellinger EP. Teamwork and collaboration for prevention of surgical site infections. Surg Infect 2016;17(2):198–202; doi: 10.1089/sur.2015.260 [DOI] [PubMed] [Google Scholar]