Abstract
Introduction
Cognitive training can potentially reduce risk of cognitive decline and dementia in older adults. To support implementation of cognitive training in the broader population of older adults, it is critical to evaluate intervention implementation and efficacy among representative samples, particularly those at highest risk of cognitive decline. Hearing and vision impairments are highly prevalent among older adults and confer increased risk of cognitive decline/dementia. Whether cognitive training interventions enroll and are designed to include this important subgroup is unknown.
Methods
A scoping review of PubMed and PsycINFO was conducted to examine the inclusion of older adults with hearing and vision impairment in cognitive training interventions. Two independent reviewers completed a full‐text review of eligible articles. Eligible articles included cognitive training and multimodal randomized controlled trials and a study population that was cognitively unimpaired, aged ≥55‐years, and community dwelling. Articles were primary outcome papers published in English.
Results
Among the 130 articles included in the review, 103 were cognitive training interventions (79%) and 27 were multimodal interventions (21%). More than half the trials systematically excluded participants with hearing and/or vision impairment (n = 60, 58%). Few studies reported hearing and vision measurement (cognitive: n = 16, 16%; multimodal: n = 3, 11%) or incorporated universal design and accessibility into intervention design (cognitive: n = 7, 7%; multimodal: n = 0, 0%).
Discussion
Older adults with hearing and vision impairment are underrepresented in cognitive training interventions. Reporting of hearing and vision measurement, proper justification of exclusions, and inclusion of accessibility and universal intervention design are also lacking. These findings raise concerns about whether current trial findings apply to those with hearing and vision impairment and generalize to the broader population of older adults. It is critical to include more diverse study populations and integrate accessibility into intervention design to include and better represent older adults with hearing and vision impairment.
Highlights
Cognitive training interventions underrepresent hearing and vision impairment.
Sensory measurement and proper justification of exclusions are rarely reported.
Interventions lack inclusion of accessibility and universal intervention design.
More diverse study populations are needed in cognitive training interventions.
Integration of accessibility into cognitive training intervention design is needed
Keywords: accessibility, cognitive training, diversity, hearing impairment, inclusion, vision impairment
1. BACKGROUND
Randomized controlled trials have demonstrated that cognitive training, that is, the systematic training of one or more cognitive domains through various tasks, can potentially improve cognitive performance and other health outcomes. The beneficial effects of training can last several years after the intervention ends and are strongest in the cognitive domain trained. 1 , 2 , 3 , 4 , 5 Effects in some studies have also extended to more distal outcomes such as functional ability and depression. 2 The National Academies of Sciences, Engineering, and Medicine cited cognitive training as one of three interventions that could potentially prevent cognitive decline and dementia. 6 Multimodal interventions that include cognitive training have also shown potential effectiveness for reducing dementia risk. 7 , 8 For example, the Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER) Study provided nutritional counseling, strength training and aerobic exercise, cognitive training, and vascular risk factor management. Participants who received the intervention showed greater improvement in performance on the neuropsychological test battery, as well as in executive function and processing speed domains, as compared to the control group after 2‐years. 7
As the evidence base for cognitive training grows, implementation and evaluation of cognitive training in representative samples of older adults is critical. It is especially important to understand the effectiveness of cognitive training among older adults at the highest risk for health outcomes that are potentially modifiable by cognitive training. These groups may derive greater benefits from cognitive training as compared to healthy or lower‐risk older adults. Despite this need, vulnerable groups of older adults have been underrepresented in cognitive training intervention studies. One recent study, for example, found that racial and ethnic minorities are largely underrepresented in cognitive training interventions. Current interventions are predominantly tested among samples of White participants. 9
Older adults with hearing or vision impairment may be underrepresented in cognitive training intervention studies. Hearing and vision impairment is highly prevalent among older adults. The prevalence of clinically significant hearing impairment increases from two‐thirds among adults aged ≥70 years to 90% among those aged ≥80 years, 10 and the prevalence of vision impairment increases from 1% among adults aged 50–54 years to 21% among those aged ≥85 years. 10 Older adults with hearing or vision impairment also have a higher risk of cognitive decline and dementia. The risk of dementia is nearly two times higher among older adults with hearing impairment and nearly 1.5 times higher among those with vision impairment. 11 , 12 Older adults with hearing and vision impairments may also have a higher risk of other poor health outcomes, including worse physical function and functional ability, 13 , 14 depression, 15 and reduced reported social well‐being. 16 , 17 Despite being a group that may derive significant benefits from cognitive training, little is known about the implementation and effectiveness of cognitive training interventions in populations with hearing and vision impairment.
The present study reviews the cognitive training intervention literature to quantify the inclusion of older adults with hearing and vision impairment in cognitive training interventions. We first address the inclusion and exclusion of participants with hearing and/or vision impairment from cognitive training intervention studies. We then review characteristics of intervention implementation related to hearing and vision impairment, including accessibility and design features for people with hearing or vision impairments. We hypothesize that most interventions exclude participants with hearing or vision impairment, and few integrate accessibility into the intervention design.
2. METHODS
2.1. Selection criteria
Articles in the present scoping review were cognitive training interventions of a randomized controlled trial study design that were published in peer‐reviewed journals prior to June 29, 2021. Participants were adults aged 55‐years or older who were living in the community without cognitive impairment. 9 Studies were excluded if they reported study populations with cognitive impairment or subjective cognitive complaints, did not report screening for cognitive impairment, or if the publication was not the primary outcome paper for the trial. 9 There were no exclusions placed on the form of cognitive training, and studies were categorized based on whether the intervention was cognitive only or multimodal. Cognitive training interventions targeted one or more cognitive domains, including attention, executive function, language, memory, and speed of processing. Multimodal interventions targeted at least one cognitive domain as well as additional interventions such as social support or physical activity. Studies were not excluded based on country of publication; however, only English‐language studies were included due to resource constraints.
Articles retrieved by the search were uploaded into Covidence systematic review software (Veritas Health Innovation, Melbourne, Australia. Available at www.covidence.org). Duplicates were manually removed by the informationist, and then remaining duplicates were automatically removed by the Covidence software and manually confirmed by a reviewer. Covidence settings were modified to require one reviewer for title and abstract screening, two for full text review, and one for data extraction. One of four reviewers screened articles for eligibility, and articles were considered for full‐text review if there was no clear reason for exclusion based on the title or abstract alone. Two independent reviewers then completed a full‐text review of the remaining articles. Both reviewers read each full‐text article and determined whether the article met the eligibility criteria. Disagreements between the two reviewers were resolved by a third independent reviewer. A third reviewer was included if the two full‐text reviewers disagreed on whether the article was eligible or what the specific reason for exclusion was. One of the four reviewers then extracted data using a Covidence extraction template, which was modified to include general information, study characteristics, and sensory‐specific characteristics. For articles missing information on the exclusion or provision of accessibility features for hearing and/or vision impairment, a member of the study team attempted to contact the lead authors via email. Authors were given approximately 6‐weeks to respond to email inquiries. Informed consent was not necessary because this was a scoping review of published studies.
2.2. Search strategy
The selection criteria and search strategy were based on a prior scoping review of the inclusion of ethnic minority participants in cognitive training interventions. 9 The two main search concepts were (a) cognitive training and (b) cognitive aging, and filters were applied to limit results to clinical trials and older adults. 9 The databases searched were PubMed and PsycINFO. The complete search strategy is available in the supplemental material (Table S1).
RESEARCH IN CONTEXT
Systematic Review: A scoping review of the literature was conducted to quantify the inclusion of older adults with hearing and vision impairments in cognitive training interventions. Search concepts included cognitive training and cognitive aging. The search strategy was systematically applied to PubMed and PsycINFO databases with the assistance of an informationist.
Interpretation: Older adults with hearing and vision impairments are excluded from the majority of cognitive training interventions. Accessibility in intervention design and measurement of hearing and vision are rarely included or reported
Future Directions: It is critical to evaluate the effectiveness of cognitive training interventions among subgroups at the highest risk of cognitive decline, including older adults with hearing and vision impairments. Future trials should enroll more diverse study populations and adopt universal design features to minimize barriers and make participation more accessible for older adults with hearing and vision impairments.
3. RESULTS
The overall screening process and the number of articles excluded at each stage is depicted in the PRISMA diagram (Figure 1). Of the 2191 articles retrieved by this search, 1439 were from PubMed, and 752 were from PsycINFO. A total of 340 duplicate articles were removed by the informationist and 19 by Covidence. During the review of article titles and abstracts, 1336 articles were excluded for failing to meet eligibility requirements. Two independent reviewers then conducted a full‐text review of the remaining 496 articles. During the full‐text review, 366 articles were excluded based on the following criteria: (a) not a randomized controlled trial (n = 68), (b) not cognitive training intervention (n = 27), (c) cognitive training, not the main intervention (n = 21), (d) study participants not cognitively normal (n = 59), (e) study participants younger than 55‐years (n = 107), (f) study participants not community‐dwelling (n = 10), (g) not a primary outcome paper (n = 57), (h) study not published in English (n = 8), and (i) other reason (n = 9; the article was retracted or full text was unavailable). After completing the screening and the full‐text review, data from 130 articles were extracted and included in this scoping review.
FIGURE 1.
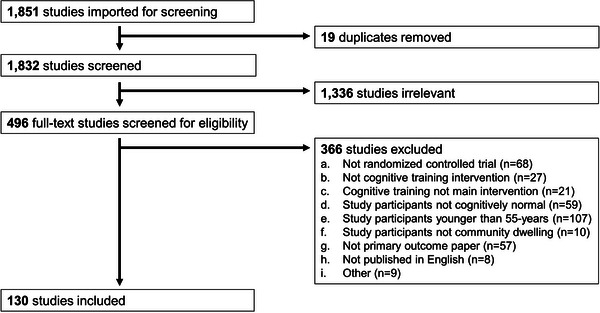
PRISMA diagram of screening process and number of articles excluded at each stage.
3.1. Description of eligible studies
Among the 130 articles reviewed, a total of 21,235 study participants were enrolled with an average of 166 participants per randomized controlled trial (range: 10–6742). The average age of study participants was 70.6 years (range: 58.8–82.5 years). Most studies were cognitive training (n = 103, 79%) versus multimodal (n = 27, 21%) interventions (Table 1, Tables S2 and S3). The cognitive domains trained included attention, executive function, language, memory, and speed of processing, and more than half the interventions trained more than one domain (n = 75, 58%). Of the multimodal interventions, the most common additional interventions included physical activity (n = 18, 62%), motor function (n = 2, 7%), and nutrition (n = 2, 7%). Overall, the format of study interventions was 68% individual versus 28% in small groups of size 4–30, 70% in‐person versus 24% at home, and 73% computerized versus 15% using pencil‐and‐paper. Some studies were flexible with 4% being both individual and group‐based, 6% in person and at home, and 12% computerized and pencil‐and‐paper. The mean study intervention duration was 10.0 weeks (SD: 11.4 weeks) (Table 1, Tables S2 and S3).
TABLE 1.
Description of eligible studies overall and by type of intervention.
| Overall (n = 130) | Cognitive training intervention (n = 103) | Multimodal intervention (n = 27) | |
|---|---|---|---|
| Sample size, mean (SD) | 165.9 (645.9) | 173.6 (715.2) | 135.8 (226.8) |
| Age, mean (SD) | 70.6 (4.3) | 70.7 (4.5) | 70.3 (3.4) |
| Individual vs. group format, n (%) | |||
| Individual | 86 (68%) | 68 (67%) | 18 (69%) |
| Group | 36 (28%) | 29 (29%) | 7 (27%) |
| Both | 5 (4%) | 4 (4%) | 1 (4%) |
| In person vs. at home, n (%) | |||
| In person | 69 (70%) | 53 (69%) | 16 (73%) |
| At home | 24 (24%) | 21 (28%) | 3 (14%) |
| Both | 6 (6%) | 3 (4%) | 3 (14%) |
| Computer vs. pencil‐and‐paper, n (%) | |||
| Computer | 30 (73%) | 29 (81%) | 1 (20%) |
| Pencil‐and‐paper | 6 (15%) | 4 (11%) | 2 (40%) |
| Both | 5 (12%) | 3 (8%) | 2 (40%) |
| Duration (weeks), mean (SD) | 10.0 (11.4) | 7.6 (5.1) | 20.1 (21.4) |
3.2. Participation of older adults with hearing and vision impairment
In this scoping review, more than half the studies excluded participants with hearing and vision impairment to some degree (n = 60, 58%). Specifically, 4% of cognitive training intervention studies excluded for only hearing impairment (n = 4), 9% for only vision impairment (n = 9), and 46% for both hearing and vision impairment (n = 47). In multimodal interventions, no studies excluded for only hearing impairment, 7% for only vision impairment (n = 2), and 52% for both hearing and vision impairment (n = 14) (Figure 2).
FIGURE 2.
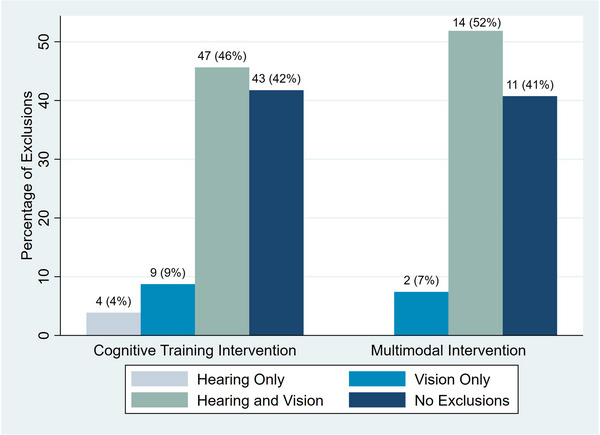
Percentage of interventions excluding participants with hearing and vision impairment by type of intervention.
Types of exclusions varied across studies. Studies defined exclusions based on “unaided hearing and vision impairment” (hearing: n = 13, 10%; vision: n = 19, 15%), “hearing and vision impairment that was perceived to interfere with cognitive training” (hearing: n = 16, 12%; vision: n = 11, 9%), “any hearing and vision impairment” (hearing: n = 26, 20%; vision: n = 26, 20%), “severe or significant hearing and vision impairment” (hearing: n = 9, 7%; vision: n = 12, 9%), or “colorblindness” (vision: n = 5, 4%). Overall, the method of assessing hearing and vision status (i.e., objectively measured, self‐reported, interviewer assessed) was not reported in 84% and 89% of cognitive training and multimodal intervention studies, respectively (n = 87 and n = 24) (Figure 3). The few studies that did report how hearing and vision status were measured used varying methods of assessment. Hearing assessments were based on pure‐tone average (n = 6, 5%), interviewer rating (n = 5, 4%), self‐report (n = 1, 1%), or portable audiometer (n = 1, 1%). Vision assessments were based on visual acuity (n = 13, 10%), self‐report (n = 2, 2%), or interviewer rating (n = 2, 2%).
FIGURE 3.
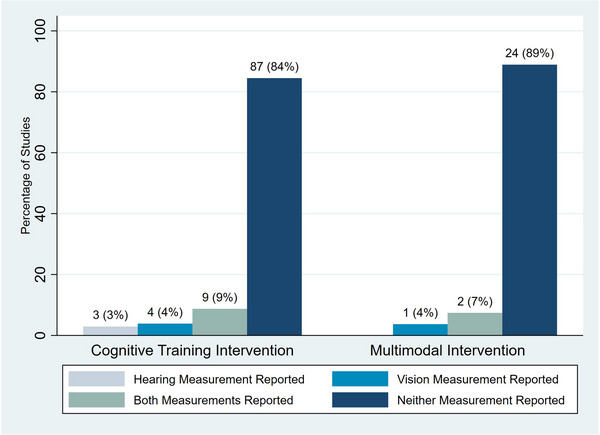
Percentage of interventions reporting method of hearing and vision measurement by type of intervention.
Only 7 (7%) of cognitive training intervention studies integrated low‐hearing and low‐vision accessibility into the intervention design. This was not reported by any of the multimodal interventions included in this review (Figure 4). Of the studies that did include accessibility features, the only types were amplification devices for hearing impairment and magnification for vision impairment. The corresponding author was contacted for every study that did not report the provision of accessibility features for hearing and vision impairment and had available contact information. Of the 125 (96%) authors contacted, 23 (18%) provided additional information about hearing and vision impairment exclusion criteria and hearing and vision measurement, and none provided additional information about accessibility.
FIGURE 4.
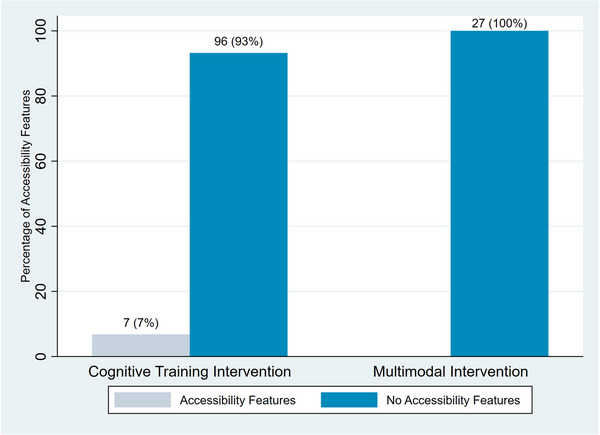
Percentage of interventions reporting inclusion of accessible design features by type of intervention.
4. DISCUSSION
Cognitive training in older adults is potentially beneficial for cognitive performance and everyday functioning. 1 , 3 , 4 In this scoping review, we found that older adults with hearing and vision impairment, particularly hearing or vision impairment that studies defined as “unaided” or “perceived to interfere with cognitive training,” were systematically excluded in nearly 60% of cognitive training intervention trials and multimodal interventions that included a cognitive training component. Almost 90% of interventions did not report how hearing and vision were measured. Furthermore, less than one‐tenth of the studies reviewed incorporated low‐hearing and low‐vision accessibility features into intervention design.
Given the high prevalence of hearing and vision impairment in older adults, 18 , 19 , 20 cognitive training interventions that specify hearing and vision impairment exclusion criteria exclude a large proportion of older adults who may benefit greatly from cognitive training. These criteria can also indirectly exclude other important groups of older adults, particularly when hearing and vision is measured subjectively. For example, when excluding participants based on self‐reported hearing and vision impairment, adults of younger age and higher education are more likely to overestimate their hearing and vision impairment. This may potentially lead to greater exclusion of these groups. 21 , 22 , 23 Additionally, interviewer assessments of eligibility and whether hearing and vision impairment would interfere with cognitive training may conflate hearing and vision impairment with cognitive impairment. This could result in the potentially greater exclusion of participants with cognitive impairment and is particularly relevant for cognitive training studies that target older adults with cognitive impairment or dementia (outside the scope of this review).
Furthermore, many of the studies included in this review specifically excluded older adults with “unaided hearing and vision impairment.” In addition to those with hearing and vision impairment, this criterion also excludes older adults who have a form of hearing and vision impairment that cannot be treated or aided through the use of hearing and vision aids, as well as older adults who lack affordable access to hearing and vision care/aids. Particularly in the United States (US), a large proportion of older adults may not have access to hearing and vision aids because hearing and vision care is not covered under Medicare health insurance. 24 For example, among older adults with hearing impairment in the United States, less than 20% use hearing aids. 25 A large and unique group of older adults are excluded and underrepresented when cognitive training intervention studies invoke hearing and vision impairment exclusion criteria.
Exclusion of participants with hearing and vision impairment is also implicit when recruitment, study documentation, and intervention design are inaccessible to those with hearing and vision impairment. 26 Inaccessible research design makes participation challenging, thereby discouraging involvement. Of the studies included in this review, only 7 (5%) interventions stated including accessible design for participants with hearing and vision impairment. Even in studies that exclude participants with severe hearing and vision impairment, it is possible that many participants have mild to moderate hearing and vision impairment and could derive benefits from accessible design. Some participants excluded for “hearing and vision impairment that is perceived to interfere with training” may have been eligible to participate if low hearing and low vision accessibility were included in the intervention design.
The underrepresentation of older adults with hearing and vision impairment in cognitive training intervention studies raises concerns about generalizability to the overall population of older adults and especially to those with hearing and vision impairment. Effects of cognitive training demonstrated in older adults without hearing and vision impairment may or may not apply to those with hearing and vision impairment. Older adults with hearing and vision impairment are more likely to experience negative health outcomes (i.e., cognitive decline, 27 , 28 , 29 reduced physical activity 13 , 30 , 31 ) that many cognitive training interventions aim to improve. The skills and strategies gained from cognitive training may benefit older adults with hearing and vision impairment more than those without. If so, the effect of cognitive training in older adults may be underestimated in current interventions that exclude those with hearing and vision impairment. The inclusion of participants with hearing and vision impairment in cognitive training interventions is imperative to fully understand the effect of cognitive training in older adults.
Furthermore, including participants with hearing and vision impairment and collecting hearing and vision‐related data allows studies to investigate differences in how older adults with hearing and vision impairment benefit from cognitive training. This information can help guide intervention design to better serve a broader population of older adults. Interventions can be tailored to maximize benefit among specific high‐risk groups, such as older adults with hearing or vision impairment, to increase overall intervention effectiveness and potentially reduce health disparities. 26 , 32 , 33 Given the high prevalence of hearing and vision impairment and its associations with higher risk of poor cognitive and health outcomes, 13 , 18 , 19 , 20 , 27 , 28 efforts to maximize cognitive training effectiveness in this group can have a meaningful impact for a large proportion of older adults. Studies have tested differences in intervention effect by certain demographic and health characteristics, but they have not yet investigated differences by hearing and vision status. 34 , 35 , 36 , 37 By including participants with hearing and vision impairment in research and collecting hearing and vision data, studies can better evaluate group‐specific differences in training effect and begin to tailor interventions to potentially maximize benefit.
4.1. Recommendations for better inclusion of older adults with hearing and vision impairment in cognitive training interventions
Support for greater inclusion of participants with hearing and vision impairment in research is necessary in both study design and research policy. First, we recommend strengthening the measurement and reporting of participant hearing and vision function in intervention studies. It is critical to understand how hearing and vision function are measured, the magnitude of hearing and vision impairment within the study sample, as well as information about intervention accessibility. This information is needed to guide interpretation of intervention results (i.e., does hearing and vision impairment impact training efficacy?). Hearing and vision function should be included as a standard measure in the description of sample characteristics, similar to how participant demographic and health characteristics are described. This information is critical, and the reporting of hearing and vision function could be improved if journals or funding sources require its reporting.
Second, integrating accessibility into intervention design (i.e., universal design) to minimize barriers to study participation makes participation more accessible and less burdensome for older adults with hearing and vision impairment. 38 For example, using assistive technologies, multisensory methods (i.e., pictures, videos, audio recordings, large text, amplification devices), or meeting with participants in familiar environments to explain study materials can improve accessibility. 32 , 38 This may also facilitate recruitment and retention of participants with hearing and vision impairment in research. 26 , 32 , 38 Investigators may also consult accessibility officers or community members with hearing and vision impairment during study design to improve intervention accessibility. 32 , 38 Greater consensus on best practices for integrating accessibility into design of interventions specifically for older adults with hearing and vision impairment is needed. Studies have detailed methods to improve study accessibility more generally, 32 , 33 , 38 , 39 but continued work is needed to tailor these recommendations to cognitive training and other interventions that include older adults with hearing and vision impairment.
Third, expansions in research policy will also facilitate the inclusion of people with disabilities, including older adults with hearing and vision impairment, in research. This includes creating sustainable funding sources and collecting and reporting hearing and vision‐related data. 33
Finally, with the support gained from the recommendations outlined above, we advocate for intentional inclusion of participants with hearing and vision impairment in cognitive training interventions. Any exclusions based on hearing and vision function should be scientifically justified and clearly reported. Unnecessary exclusion of older adults with hearing and vision impairment leads to underrepresentation of these groups in research and further marginalization of this community. As a whole, in cognitive training interventions, there is a critical need to improve measurement and reporting of hearing and vision impairment, to build accessibility into intervention design, and to increase inclusion of participants with hearing and vision impairment for study representativeness and generalizability. Although this study specifically focused on inclusion of older adults with hearing or vision impairment, these recommendations extend to many other groups underrepresented in research, such as racial and ethnic minorities, people who are pregnant, or individuals with multimorbidity. 33 , 40 , 41
4.2. Limitations and strengths
Although the search strategy was modeled from previous work, only PubMed and PsycINFO databases were formally searched. 9 These databases were determined to be most relevant for the research question. In addition, due to resource constraints, only English language studies were included. This review contained 80 (62%) studies outside the United States, and these findings are expected to apply to similar randomized cognitive training interventions from other countries. Furthermore, the study population consisted of cognitively unimpaired, community‐dwelling older adults. Results of this study may not apply to interventions targeting younger adults, non‐community dwelling individuals, or older adults with cognitive impairment, as evaluation of these study populations was beyond the scope of this project. Future reviews should be conducted to examine the exclusion of participants with hearing and vision impairment and accessibility in intervention design in these populations. Finally, there is a potential for publication bias, as only trials with results published in peer‐reviewed journals were included in this review.
Despite these potential limitations, this study has several strengths. To our knowledge, this is the first, formal review to evaluate exclusion of participants based on hearing or vision impairment, intervention accessibility, and reporting of hearing and vision measurement in randomized cognitive training interventions with older adults. The exclusion of individuals with hearing and vision impairment is a major concern, and the results of this study quantify the extent to which these exclusions occur. The composition of the study team is an additional strength of this study. The team included an informationist who translated our model search strategy to PubMed and PsycINFO and applied filters to limit results to clinical trials and older adults. The team also consisted of multiple reviewers, which allowed each article to be reviewed by at least two reviewers during the full text review stage. Finally, this study did not exclude trials conducted outside the United States, which improves the applicability of these findings.
In cognitive training interventions, the exclusion of older adults with hearing and vision impairment was common and inclusion of accessibility and universal design in intervention design was rare. Older adults with hearing and vision impairment may derive large benefits from cognitive training. Inclusion of more diverse study populations that include older adults with hearing and vision impairment and integration of accessibility into intervention design is needed to maximize the benefits of cognitive training for all older adults and particularly for those with hearing and vision impairment.
CONFLICT OF INTEREST STATEMENT
Author disclosures are available in the supporting information.
Supporting information
Supporting Information
Supporting Information
ACKNOWLEDGMENTS
We acknowledge Marian Tzuang, whose initial work indicating systematic exclusion of racial and ethnic minorities from cognitive training interventions served as the inspiration for the present study. We also acknowledge Lori Rosman for her role in translating the model search strategy to PubMed and PsycINFO and running the search. Lori is an informationist at the Welch Medical Library at Johns Hopkins University. The project described was supported by the National Heart, Lung, and Blood Institute, National Institutes of Health [Grant Number T32 HL007024] and the National Institute on Aging, National Institutes of Health [Grant Number K01 AG054693]. The funding source had no role in study design; collection, analysis, and interpretation of data; writing of the report; or decision to submit the article for publication.
Marino FR, Jiang K, Smith JR, et al. Inclusion of hearing and vision impairments in cognitive training interventions. Alzheimer's Dement. 2023;9:e12374. 10.1002/trc2.12374
REFERENCES
- 1. Butler M, McCreedy E, Nelson VA, et al. Does cognitive training prevent cognitive decline? A systematic review. Ann Intern Med. 2018;168(1):63‐68. [DOI] [PubMed] [Google Scholar]
- 2. Rebok GW, Ball K, Guey LT, et al. Ten‐year effects of the ACTIVE cognitive training trial on cognition and everyday functioning in older adults. J Am Geriatr Soc. 2014;62(1):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Coyle H, Traynor V, Solowij N. Computerized and virtual reality cognitive training for individuals at high risk of cognitive decline: systematic review of the literature. Am J Geriatr Psychiatry. 2015;23(4):335‐359. [DOI] [PubMed] [Google Scholar]
- 4. Lampit A, Hallock H, Valenzuela M. Computerized cognitive training in cognitively healthy older adults: a systematic review and meta‐analysis of effect modifiers. PLoS Med. 2014;11(11):e1001756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rebok GW, Carlson MC, Langbaum JB. Training and maintaining memory abilities in healthy older adults: traditional and novel approaches. J Gerontol B Psychol Sci Soc Sci. 2007;62(Special_Issue_1):53‐61. [DOI] [PubMed] [Google Scholar]
- 6. National Academies of Sciences, Engineering, and Medicine . Preventing cognitive decline and dementia: A way forward. Washington, DC: The National Academies Press. 2017. 10.17226/24782 [DOI] [PubMed]
- 7. Ngandu T, Lehtisalo J, Solomon A, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at‐risk elderly people (FINGER): a randomised controlled trial. Lancet. 2015;385(9984):2255‐2263. [DOI] [PubMed] [Google Scholar]
- 8. Hooper C, De Souto Barreto P, Coley N, et al. Cognitive changes with omega‐3 polyunsaturated fatty acids in non‐demented older adults with low omega‐3 index. J Nutr Health Aging. 2017;21(9):988‐993. [DOI] [PubMed] [Google Scholar]
- 9. Tzuang M, Owusu JT, Spira AP, Albert MS, Rebok GW. Cognitive training for ethnic minority older adults in the United States: a review. Gerontologist. 2018;58(5):e311‐e324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Flaxman AD, Wittenborn JS, Robalik T, et al. Prevalence of visual acuity loss or blindness in the US: a Bayesian meta‐analysis. JAMA Ophthalmol. 2021;139(7):717‐723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shang X, Zhu Z, Wang W, Ha J, He M. The association between vision impairment and incidence of dementia and cognitive impairment: a systematic review and meta‐analysis. Ophthalmology. 2021;128(8):1135‐1149. [DOI] [PubMed] [Google Scholar]
- 12. Kuo PL, Huang AR, Ehrlich JR, et al. Prevalence of concurrent functional vision and hearing impairment and association with dementia in community‐dwelling Medicare beneficiaries. JAMA Netw Open. 2021;4(3):e211558‐e211558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Martinez‐Amezcua P, Suen JJ, Lin F, Schrack JA, Deal JA. Hearing impairment and objectively measured physical activity: a systematic review. J Am Geriatr Soc. 2022;70(1):301‐304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Martinez‐Amezcua P, Kuo PL, Reed NS, et al. Association of hearing impairment with higher level physical functioning and walking endurance: results from the Baltimore Longitudinal Study of Aging. J Gerontol A Biol Sci Med Sci. 2021;76(10):e290‐e298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lawrence BJ, Jayakody DM, Bennett RJ, Eikelboom RH, Gasson N, Friedland PL. Hearing loss and depression in older adults: a systematic review and meta‐analysis. Gerontologist. 2020;60(3):e137‐e154. [DOI] [PubMed] [Google Scholar]
- 16. Shukla A, Harper M, Pedersen E, et al. Hearing loss, loneliness, and social isolation: a systematic review. Otolaryngol Head Neck Surg. 2020;162(5):622‐633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Huang AR, Deal JA, Rebok GW, Pinto JM, Waite L, Lin FR. Hearing impairment and loneliness in older adults in the United States. J Appl Gerontol. 2021;40(10):1366‐1371. [DOI] [PubMed] [Google Scholar]
- 18. Goman AM, Lin FR. Prevalence of hearing loss by severity in the United States. Am J Public Health. 2016;106(10):1820‐1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Swenor BK, Ramulu PY, Willis JR, Friedman D, Lin FR. The prevalence of concurrent hearing and vision impairment in the United States. JAMA Intern Med. 2013;173(4):312‐313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vitale S, Cotch MF, Sperduto RD. Prevalence of visual impairment in the United States. Jama. 2006;295(18):2158‐2163. [DOI] [PubMed] [Google Scholar]
- 21. Choi JS, Betz J, Deal J, et al. A comparison of self‐report and audiometric measures of hearing and their associations with functional outcomes in older adults. J Aging Health. 2016;28(5):890‐910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kim SY, Kim HJ, Kim MS, Park B, Kim JH, Choi HG. Discrepancy between self‐assessed hearing status and measured audiometric evaluation. PLoS One. 2017;12(8):e0182718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yip JL, Khawaja AP, Broadway D, et al. Visual acuity, self‐reported vision and falls in the EPIC‐Norfolk Eye study. Br J Ophthalmol. 2014;98(3):377‐382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mamo SK, Nieman CL, Lin FR. Prevalence of untreated hearing loss by income among older adults in the United States. J Health Care Poor Underserved. 2016;27(4):1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chien W, Lin FR. Prevalence of hearing aid use among older adults in the United States. Arch Intern Med. 2012;172(3):292‐293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rios D, Magasi S, Novak C, Harniss M. Conducting accessible research: including people with disabilities in public health, epidemiological, and outcomes studies. Am J Public Health. 2016;106(12):2137‐2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Loughrey DG, Kelly ME, Kelley GA, Brennan S, Lawlor BA. Association of age‐related hearing loss with cognitive function, cognitive impairment, and dementia: a systematic review and meta‐analysis. JAMA Otolaryngol Neck Surg. 2018;144(2):115‐126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ge S, McConnell ES, Wu B, Pan W, Dong X, Plassman BL. Longitudinal association between hearing loss, vision loss, dual sensory loss, and cognitive decline. J Am Geriatr Soc. 2021;69(3):644‐650. [DOI] [PubMed] [Google Scholar]
- 29. Nagarajan N, Assi L, Varadaraj V, et al. Vision impairment and cognitive decline among older adults: a systematic review. BMJ Open. 2022;12(1):e047929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gispen FE, Chen DS, Genther DJ, Lin FR. Association between hearing impairment and lower levels of physical activity in older adults. J Am Geriatr Soc. 2014;62(8):1427‐1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cai Y, Schrack JA, Wang H, et al. Visual impairment and objectively measured physical activity in middle‐aged and older adults. J Gerontol Ser A. 2021;76(12):2194‐2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Williams AS, Moore SM. Universal design of research: inclusion of persons with disabilities in mainstream biomedical studies. Sci Transl Med. 2011;3(82):82cm12‐82cm12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Swenor B, Deal JA. Disability inclusion as a key component of research study diversity. N Engl J Med. 2022;386(3):205‐207. [DOI] [PubMed] [Google Scholar]
- 34. Ball KK, Ross LA, Roth DL, Edwards JD. Speed of processing training in the ACTIVE study: how much is needed and who benefits? J Aging Health. 2013;25(8_suppl):65S‐84S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rebok GW, Langbaum JB, Jones RN, et al. Memory training in the ACTIVE study: how much is needed and who benefits? J Aging Health. 2013;25(8_suppl):21S‐42S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Willis SL, Caskie GI. Reasoning training in the ACTIVE study: how much is needed and who benefits? J Aging Health. 2013;25(8_suppl):43S‐64S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rosenberg A, Ngandu T, Rusanen M, et al. Multidomain lifestyle intervention benefits a large elderly population at risk for cognitive decline and dementia regardless of baseline characteristics: the FINGER trial. Alzheimers Dement. 2018;14(3):263‐270. [DOI] [PubMed] [Google Scholar]
- 38. Banas JR, Magasi S, The K, Victorson DE. Recruiting and retaining people with disabilities for qualitative health research: challenges and solutions. Qual Health Res. 2019;29(7):1056‐1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bell KR, Hammond F, Hart T, Bickett AK, Temkin NR, Dikmen S. Participant recruitment and retention in rehabilitation research. Am J Phys Med Rehabil. 2008;87(4):330‐338. [DOI] [PubMed] [Google Scholar]
- 40. Du Vaure CB, Dechartres A, Battin C, Ravaud P, Boutron I. Exclusion of patients with concomitant chronic conditions in ongoing randomised controlled trials targeting 10 common chronic conditions and registered at ClinicalTrials. gov: a systematic review of registration details. BMJ Open. 2016;6(9):e012265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Spong CY, Bianchi DW. Improving public health requires inclusion of underrepresented populations in research. Jama. 2018;319(4):337‐338. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Supporting Information


