Abstract
Background
The triglyceride-glucose (TyG) index, a reliable surrogate indicator of insulin resistance, is independently associated with coronary artery disease of various clinical manifestations. This study aimed to investigate the prognostic value of the TyG index in predicting repeat revascularization and in-stent restenosis (ISR) in chronic coronary syndrome (CCS) patients undergoing percutaneous coronary intervention (PCI).
Methods
A total of 1414 participants were enrolled and divided into groups according to the tertiles of the TyG index. The primary endpoint was a composite of PCI complications, including repeat revascularization and ISR. The associations between the TyG index and the primary endpoint were assessed by multivariable Cox proportional hazards regression analysis with restricted cubic splines (RCS). The TyG index was calculated as Ln (fasting triglycerides (mg/dL) × fasting plasma glucose (mg/dL)/2).
Results
Over a median follow-up of 60 months, 548 (38.76%) patients had experienced at least one primary endpoint event. The follow-up incidence of the primary endpoint increased with the TyG index tertiles. After adjusting for potential confounders, the TyG index was independently associated with the primary endpoint in CCS patients (HR, 1.191; 95% CI 1.038–1.367; P = 0.013). Additionally, the highest tertile of the TyG group was correlated with a 1.319-fold risk of the primary endpoint compared with the lowest tertile of the TyG group (HR, 1.319; 95% CI 1.063–1.637; P = 0.012). Furthermore, a linear and dose–response relationship was observed between the TyG index and the primary endpoint (non-linear P = 0.373, P overall = 0.035).
Conclusions
An increased TyG index was associated with elevated risk for long-term PCI complications, including repeat revascularization and ISR. Our study suggested that the TyG index could be a potent predictor in evaluating the prognosis of CCS patients undergoing PCI.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12933-023-01779-7.
Keywords: Triglyceride-glucose index, Coronary artery disease, Percutaneous coronary intervention, Repeat revascularization
Background
Coronary artery disease (CAD) has affected 244.11 million individuals worldwide and is the leading cause of death [1]. Chronic coronary syndrome (CCS), previously referred to as stable CAD, contributes to the major population of CAD and encompasses patients with or without history of acute coronary syndrome (ACS) or coronary revascularization [2].
Percutaneous coronary intervention (PCI) with drug-eluting stent (DES) is the most common revascularization strategy utilized in CCS patients. During the past decades, although great advances have been made in the management of CCS patients undergoing PCI-DES, the incidence of repeat revascularization driven by in-stent restenosis (ISR) or progression of non-target lesions persisted over 25% during a 5-year follow-up [3, 4]. Moreover, repeat revascularization was demonstrated to be an independent predictor of myocardial infarction, stroke, and cardiovascular mortality [5]. Of note, despite a reduced risk of ISR observed with DES, ISR remained another major complication associated with adverse cardiac events in patients undergoing DES-PCI [6]. Given that more than 1,000,000 PCIs are performed in China each year, it is of clinical significance to identify risk factors for repeat revascularization and ISR [7].
Insulin resistance (IR) is a clinical state of impaired insulin sensitivity which could result in cardiometabolic alterations, including hyperglycemia, dyslipidemia, and hypertension [8]. Studies discovered that insulin resistance was a strong predictor of CAD and adverse cardiac events in diabetic and nondiabetic subjects [9, 10]. The triglyceride-glucose (TyG) index is a novel surrogate indicator of IR [11]. Growing evidence suggested that the TyG index could independently predict adverse cardiovascular outcomes among CAD cohorts with different clinical manifestations [12–14]. Furthermore, a recent study on the ACS population found that an increased TyG index was positively correlated to ISR [15]. Therefore, the TyG index is now proposed as a potential indicator in identifying patients at high risk of poor prognosis.
In this study, we aimed to address the gap that there is limited evidence regarding the associations of the TyG index for long-term PCI complications, including repeat revascularization and ISR, which might provide clinical benefits for risk stratification and management in CCS patients after DES implantation.
Methods
Study population
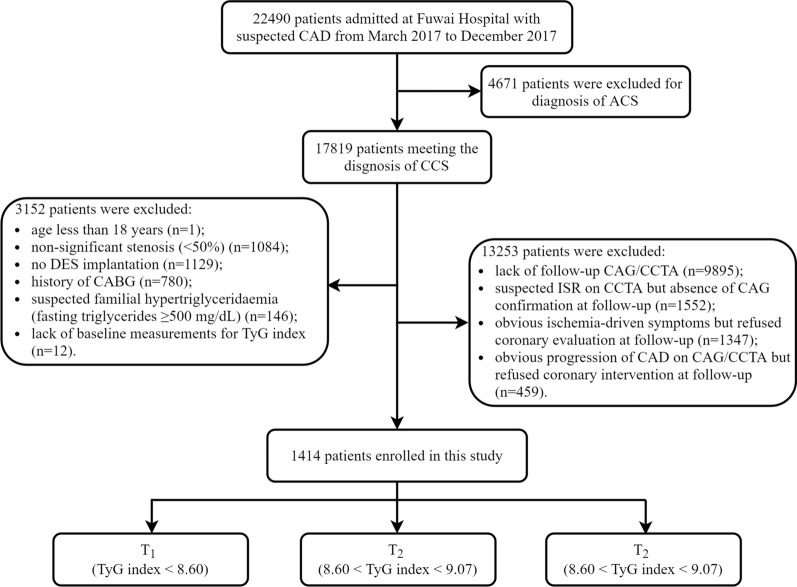
This was a retrospective observational study. A total of 1414 consecutive patients diagnosed with CCS in Fuwai Hospital, Chinese National Center for Cardiovascular Diseases, were enrolled from March 2017 to December 2017. The exclusion criteria were as follows: (1) age less than 18 years; (2) non-significant stenosis (< 50%); (3) no DES implantation; (4) history of coronary artery bypass grafting (CABG); (5) lack of follow-up coronary angiography (CAG)/coronary CT angiography (CCTA); (6) lack of baseline measurements for TyG index; (7) suspected familial hypertriglyceridemia (plasma triglycerides ≥ 500 mg/dL); (8) suspected ISR on CCTA but the absence of CAG confirmation at follow-up; (9) obvious ischemia-driven symptoms but refused coronary evaluation at follow-up; (10) obvious progression of CAD on CAG/CCTA but refused coronary intervention at follow-up. The detailed screening process is shown in Fig. 1. This study was performed in line with the Declaration of Helsinki and was authorized by the Ethics Committee of Fuwai Hospital. All participants provided written/oral informed consent.
Fig. 1.
Flowchart of study participants. ACS acute coronary syndrome, CAD coronary artery disease, CCS chronic coronary syndrome, CABG coronary artery bypass graft, CAG coronary angiography, CCTA coronary computed tomography angiography, DES drug-eluting stent, TyG triglyceride-glucose
Data collection and definitions
Clinical data, including demographics, medical history, and laboratory tests, were collected from medical records. Laboratory tests consisting of fasting blood glucose (FBG), triglyceride (TG), total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), plasma creatinine (Pcr), high-sensitive C-reactive protein (hs-CRP) and N-terminal pro-B-type natriuretic peptide (NT-proBNP) were performed under standardized instructions and assaying system (LABOSPECT 008, Hitachi, Tokyo, Japan). Glycated hemoglobin (HbA1c) was determined by high-performance liquid chromatography (G8, TOSOH, Tokyo, Japan). All blood samples were collected after overnight fasting before CAG. Therefore, all indicators were at the same temporal window for each participant. The left ventricular ejection fraction (LVEF) was measured by echocardiography using the biplane Simpson method. The angiographic data was obtained from the cardiac catheterization laboratory records.
The definition of CCS complied with the current guideline of the European Society of Cardiology [2]. Body mass index (BMI) was calculated as weight (kg)/height’s square (m2). Diabetes mellitus was defined as a past diagnosis with diabetes, FBG ≥ 7.0 mmol/L, HbA1c ≥ 6.5%, or use of antidiabetic drugs. Hypertension was defined as a history of hypertension with anti-hypertensive drugs or repeated blood pressure ≥ 140/90 mmHg. Dyslipidemia was diagnosed as LDL-C ≥ 3.4 mmol/L, HDL-C < 1.0 mmol/L, TG ≥ 1.7 mmol/L, or use of lipid-lowering treatment. As previously reported, the TyG index was calculated as Ln (fasting triglycerides (mg/dL) × fasting plasma glucose (mg/dL)/2) [11]. The estimated glomerular filtration rate (eGFR) was estimated according to the modified Modification of Diet in Renal Disease equations that are based on the Chinese population: 186 × Pcr−1.154 × age−0.203 × 0.742 (if female) × 1.233 (if Chinese) [16].
Endpoints and follow-up
The primary endpoint was a composite of PCI complications, including repeat revascularization and ISR. The secondary endpoints were repeat revascularization and ISR. Strategies for repeat revascularization at follow-up comprise DES, bare metal stenting, plain balloon angioplasty, drug-coated balloon angioplasty, and CABG. The repeat revascularization was determined by experienced interventional cardiologists regarding individual risk and patients’ decisions. To avoid counting endpoints in patients with staged PCI, we excluded the records of staged PCI and collected the endpoints under the judgment of experienced cardiologists. The ISR was defined as the presence of significant diameter stenosis (≥ 50%) at the segment inside the stent or involving its 5-mm edges.
All patients underwent follow-up CAG or CCTA in Fuwai Hospital after the baseline successful PCI. The follow-up period lasted until October 2022. Considering the high specificity of CCTA, we mainly included patients diagnosed with the absence of ISR by CCTA. For the patients with suspected ISR, a CAG confirmation was required. Importantly, the follow-up CAG and CCTA were interpreted by a panel of dependent experienced radiologists and cardiologists. The coronary revascularization and periprocedural management were performed according to current guidelines and regulations of our center. All patients have received standard secondary prevention recommended by current guidelines [2].
Statistical analysis
Continuous variables were described as mean ± standard deviation if consistent with a normal distribution, otherwise as median and interquartile range (IQR). Categorical variables were presented as numbers and percentages (%). All participants were stratified into three groups: T1 (TyG index < 8.60), T2 (8.60 ≤ TyG index < 9.07), and T3 (TyG index ≥ 9.07) in accordance with the TyG index tertiles. The Analysis of variance was applied to analyze the difference in continuous variables between groups, while the Kruskal–Wallis test was used to analyze the difference in categorical variables.
Multiple imputation was used in the imputation of the missing data by Gibbs sampling [17, 18]. The target variables containing missing data were predicted based on the whole dataset. The process was iterated until all the missing values converged, and five imputed datasets with chained equations were created using R multivariate imputation by chained equation software package [19].
Log-rank tests and Kaplan–Meier methods were performed to explore differences in event rates between TyG tertiles and plot time-to-event curves. P values for pairwise comparisons were corrected for multiple testing by the Benjamin-Hochberg algorithm. The Cox proportional hazards regression analysis was used to estimate the hazard ratio (HR) and the 95% confidence interval (CI) of the TyG index in developing the primary and secondary endpoints. In the multivariable model, the following confounders were chosen considering the clinical importance: age (continuous), sex, BMI, previous PCI, presence of peripheral artery disease (PAD), presence of multivessel CAD, eGFR (continuous), hs-CRP (continuous), presence of lesion’s length ≥ 20 mm, stent length (continuous). Trend analyses were conducted by entering the tertiles of the TyG index as a continuous variable and rerunning the corresponding regression models. Moreover, the RCS were used to examine the possible nonlinear relationships of the TyG index with the primary and secondary endpoints. To balance best fit and overfitting in the RCS, the Akaike information criterion was used [20]. The potential non-linearity of the RCS was tested through a likelihood ratio test that compares the model presenting only a linear term with the model presenting linear and cubic spline terms [18, 21, 22]. The median of the TyG index was assigned as the reference value.
All statistical analyses were performed in R software (version 4.1.2). A two‐sided P value < 0.05 was considered statistically significant.
Results
Baseline characteristics
The average age of the 1414 CCS participants undergoing DES-PCI was 58.04 ± 0.25 years, and 1103 (78.00%) were men. Compared with the T1 group, T2 and T3 group participants tended to have higher BMI (P < 0.001). The proportions of cardiometabolic risk factors, including diabetes, hypertension, family history of CAD, and the use of insulin and oral hypoglycemic drugs, were higher in the T3 group than in the other two groups (all P < 0.05). Moreover, the participants of the T3 group had higher TC, LDL-C, triglycerides, FPG, HbA1c, and Pcr (all P < 0.001) (Table 1).
Table 1.
Baseline characteristics according to tertiles of the TyG index
| Variable | T1 (n = 467) | T2 (n = 466) | T3 (n = 481) | P-value |
|---|---|---|---|---|
| Demographics | ||||
| Age (years) | 58.94 ± 9.55 | 58.34 ± 9.80 | 56.86 ± 8.52 | 0.002 |
| Male sex, n (%) | 371 (79.44) | 365 (78.33) | 367 (76.30) | 0.495 |
| BMI (kg/m2) | 24.80 (23.04, 26.73) | 26.12 (24.22, 28.37) | 26.08 (24.54, 28.01) | < 0.001 |
| Risk factors | ||||
| Cigarette smoking, n (%) | 281 (60.17) | 280 (60.09) | 297 (61.75) | 0.840 |
| Diabetes, n (%) | 125 (26.77) | 169 (36.27) | 303 (62.99) | < 0.001 |
| Hypertension, n (%) | 272 (58.24) | 301 (64.59) | 333 (69.23) | 0.002 |
| Dyslipidemia, n (%) | 464 (99.36) | 465 (99.79) | 481 (100.00) | 0.167 |
| Previous MI, n (%) | 66 (14.13) | 69 (14.81) | 72 (14.97) | 0.929 |
| Previous stroke, n (%) | 46 (9.85) | 52 (11.16) | 63 (13.10) | 0.285 |
| Previous PCI, n (%) | 133 (28.48) | 132 (28.33) | 131 (27.23) | 0.897 |
| PAD, n (%) | 75 (16.06) | 62 (13.30) | 57 (11.85) | 0.161 |
| Family history of CAD, n (%) | 80 (17.13) | 72 (15.45) | 108 (22.45) | 0.015 |
| Clinical presentations | ||||
| Multi-vessel CAD, n (%) | 364 (77.94) | 380 (81.55) | 396 (82.33) | 0.1926 |
| LVEF (%) | 64 (61, 66) | 64 (60, 66) | 64 (60, 66) | 0.852 |
| Laboratory measurements | ||||
| TC (mmol/L) | 3.65 ± 0.84 | 4.06 ± 0.96 | 4.45 ± 1.14 | < 0.001 |
| LDL-C (mmol/L) | 2.16 ± 0.70 | 2.50 ± 0.85 | 2.58 ± 0.92 | < 0.001 |
| HDL-C (mmol/L) | 1.18 ± 0.30 | 1.10 ± 0.30 | 1.01 ± 0.28 | < 0.001 |
| Triglycerides (mmol/L) | 0.98 ± 0.22 | 1.51 ± 0.32 | 2.74 ± 1.60 | < 0.001 |
| FPG (mmol/L) | 5.28 ± 0.88 | 5.94 ± 1.39 | 7.57 ± 2.83 | < 0.001 |
| HbA1c (%) | 5.92 ± 0.78 | 6.24 ± 0.98 | 6.95 ± 1.47 | < 0.001 |
| Pcr (μmol/L) | 79.38 ± 14.46 | 81.71 ± 16.40 | 83.59 ± 21.41 | 0.001 |
| eGFR (mL/min per 1.73 m2) | 112.44 (100.22, 127.78) | 110.84 (98.58, 124.76) | 108.33 (93.36, 125.47) | 0.005 |
| NT-proBNP (pg/mL) | 194.31 ± 428.14 | 163.92 ± 296.40 | 140.80 ± 256.45 | 0.048 |
| hs-CRP (mg/L) | 3.58 ± 6.36 | 4.30 ± 6.57 | 4.27 ± 4.74 | 0.112 |
| Medications at discharge | ||||
| DAPT, n (%) | 464 (99.36) | 464 (99.57) | 476 (98.96) | 0.523 |
| Statins, n (%) | 454 (97.22) | 458 (98.28) | 474 (98.54) | 0.301 |
| Dual-lipid lowering therapy, n (%) | 15 (3.21) | 19 (4.08) | 28 (5.82) | 0.135 |
| ACEI/ARBs, n (%) | 199 (42.61) | 244 (52.36) | 264 (54.89) | < 0.001 |
| β-Blockers | 372 (79.66) | 393 (84.33) | 418 (86.90) | 0.009 |
| Insulin, n (%) | 36 (7.71) | 32 (6.87) | 87 (18.09) | < 0.001 |
| Oral hypoglycemic drugs, n (%) | 74 (15.85) | 100 (21.46) | 193 (40.12) | < 0.001 |
| Angiographic findings | ||||
| Restenotic lesions, n (%) | 20 (4.28) | 26 (5.58) | 27 (5.61) | 0.576 |
| Chronic total occlusions, n (%) | 53 (11.35) | 74 (15.88) | 64 (13.31) | 0.1272 |
| Lesions > 20 mm long, n (%) | 321 (68.74) | 335 (71.89) | 344 (71.52) | 0.511 |
| Number of stents | 2 (1, 2) | 2 (1, 2.75) | 2 (1, 2) | 0.076 |
| Length of stent (mm) | 30.00 (20.00, 45.50) | 33.00 (21.00, 51.00) | 30.00 (22.00, 46.00) | 0.231 |
ACEI angiotensin-converting enzyme inhibitor, ARBs angiotensin receptor blockers, BMI body mass index, CAD coronary artery disease, DAPT dual antiplatelet therapy, eGFR estimated glomerular filtration rate, FPG fasting plasma glucose, HDL-C high-density lipoprotein-cholesterol, HbA1c glycated hemoglobin A1c, hs-CRP hypersensitive C-reactive protein, LVEF left ventricular ejection fraction, LDL-C low-density lipoprotein-cholesterol, MI myocardial infarction, NT-proBNP N-terminal pro-B-type natriuretic peptide, PCI percutaneous coronary intervention, PAD peripheral arterial disease, Pcr plasma creatine, TC total cholesterol, TyG triglyceride-glucose
Table 2 presents the baseline characteristics according to the primary endpoint. A total of 548 participants were observed experiencing repeat revascularization and/or ISR during the follow-up. Compared with participants without endpoints, participants with an endpoint event had higher BMI and greater proportions of diabetes, previous MI, previous PCI, multi-vessel CAD, and use of insulin and oral hypoglycemic drugs (all P < 0.05). Besides. participants with an endpoint event showed elevated concentrations of LDL-C, FPG, HbA1c, and statistically significant different LVEF (all P < 0.05). Furthermore, participants experiencing a primary endpoint had higher levels of TyG, and the participants with an endpoint event tended to be in the T3 group (both P < 0.05). Compared to those without an endpoint event, patients with the primary endpoint had higher proportions of restenotic lesions, chronic total occlusions, lesions > 20 mm long, number of stents, and smaller diameter of stent (all P < 0.05).
Table 2.
Baseline characteristics according to the primary endpoint
| Variable | No such events (n = 866) | Primary endpoint (n = 548) | P-value |
|---|---|---|---|
| Demographics | |||
| Age (years) | 58.21 ± 9.35 | 57.76 ± 9.31 | 0.374 |
| Male sex, n (%) | 668 (77.14) | 435 (79.38) | 0.354 |
| BMI (kg/m2) | 25.64 (23.80, 27.64) | 25.95 (24.22, 28.29) | 0.004 |
| Risk factors | |||
| Cigarette smoking, n (%) | 521 (60.16) | 337 (61.50) | 0.657 |
| Diabetes, n (%) | 329 (37.99) | 268 (48.91) | < 0.001 |
| Hypertension, n (%) | 550 (63.51) | 356 (64.96) | 0.619 |
| Dyslipidemia, n (%) | 864 (99.77) | 546 (99.64) | 1 |
| Previous MI, n (%) | 104 (12.01) | 103 (18.80) | 0.001 |
| Previous stroke, n (%) | 96 (11.09) | 65 (11.86) | 0.718 |
| Previous PCI, n (%) | 198 (22.86) | 198 (36.13) | < 0.001 |
| PAD, n (%) | 120 (13.86) | 74 (13.50) | 0.913 |
| Family history of CAD, n (%) | 168 (19.40) | 92 (16.79) | 0.244 |
| Clinical presentations | |||
| Multi-vessel CAD, n (%) | 652 (75.29) | 488 (89.05) | < 0.001 |
| LVEF (%) | 64 (61, 66) | 64 (60, 66) | 0.010 |
| Laboratory measurements | |||
| TC (mmol/L) | 4.02 ± 1.03 | 4.10 ± 1.06 | 0.159 |
| LDL-C (mmol/L) | 2.38 ± 0.83 | 2.47 ± 0.88 | 0.049 |
| HDL-C (mmol/L) | 1.11 ± 0.30 | 1.07 ± 0.29 | 0.003 |
| Triglycerides (mmol/L) | 1.71 ± 1.22 | 1.82 ± 1.20 | 0.129 |
| FPG (mmol/L) | 6.09 ± 1.93 | 6.57 ± 2.39 | < 0.001 |
| HbA1c (%) | 6.24 ± 1.06 | 6.60 ± 1.36 | < 0.001 |
| Pcr (μmol/L) | 81.24 ± 15.59 | 82.11 ± 20.79 | 0.370 |
| eGFR (mL/min per 1.73 m2) | 110.43 (97.32, 123.70) | 111.88 (95.43, 128.43) | 0.309 |
| NT-proBNP (pg/mL) | 156.03 ± 329.01 | 181.99 ± 343.80 | 0.156 |
| hs-CRP (mg/L) | 3.83 ± 5.01 | 4.40 ± 7.15 | 0.079 |
| Medications at discharge | |||
| DAPT, n (%) | 859 (99.19) | 545 (99.45) | 0.807 |
| Statins, n (%) | 849 (98.04) | 537 (97.99) | 1.000 |
| Dual-lipid lowering therapy, n (%) | 36 (4.16) | 26 (4.74) | 0.695 |
| ACEI/ARBs, n (%) | 414 (47.81) | 293 (53.47) | 0.043 |
| β-Blockers | 697 (80.48) | 486 (88.69) | 0.0001 |
| Insulin, n (%) | 69 (7.97) | 86 (15.69) | < 0.001 |
| Oral hypoglycemic drugs, n (%) | 202 (23.33) | 165 (30.11) | 0.006 |
| Angiographic findings | |||
| Restenotic lesions, n (%) | 29 (3.35) | 44 (8.03) | < 0.001 |
| Chronic total occlusions, n (%) | 94 (10.85) | 97 (17.70) | < 0.001 |
| Lesions > 20 mm long, n (%) | 588 (67.90) | 412 (75.18) | 0.004 |
| Number of stents | 2 (1, 2) | 2 (1, 3) | < 0.001 |
| Length of stent (mm) | 26.50 (16.00, 40.00) | 30.00 (20.00, 50.00) | < 0.001 |
| TyG index | 8.80 (8.45, 9.20) | 8.90 (8.55, 9.34) | < 0.001 |
| TyG tertiles | 0.001 | ||
| T1, n (%) | 316 (67.67) | 151 (32.33) | |
| T2, n (%) | 279 (59.87) | 187 (40.13) | |
| T3, n (%) | 271 (56.34) | 210 (43.66) | |
ACEI angiotensin-converting enzyme inhibitor, ARBs angiotensin receptor blockers, BMI body mass index, CAD coronary artery disease, DAPT dual antiplatelet therapy, eGFR estimated glomerular filtration rate, FPG fasting plasma glucose, HDL-C high-density lipoprotein-cholesterol, HbA1c glycated hemoglobin A1c, hs-CRP hypersensitive C-reactive protein, LVEF left ventricular ejection fraction, LDL-C low-density lipoprotein-cholesterol, MI myocardial infarction, NT-proBNP N-terminal pro-B-type natriuretic peptide, PCI percutaneous coronary intervention, PAD peripheral arterial disease, Pcr plasma creatine, TC total cholesterol, TyG triglyceride-glucose
The respective baseline information according to the secondary endpoints is shown in Additional file 1: Tables S1–S2. More details about the angiographic findings and medications at charge among the TyG index tertiles are shown in Additional file 1: Table S3.
Association between the TyG index and the primary endpoint
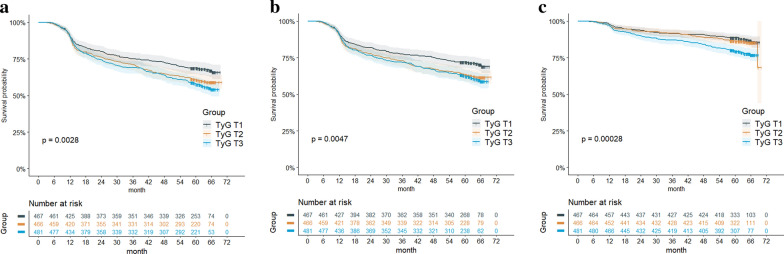
The median follow-up duration of the patients for the primary endpoint was 60 months (IQR 56–64 months). During the follow-up period, 548 (38.76%) patients had the primary endpoint. Results of the Kaplan–Meier survival analyses are presented in Fig. 2. It’s indicated that the incidence of the primary endpoint in T3 was significantly higher than that in the T1 (log-rank test, overall P = 0.0028, adjusted pairwise P values between T1 and T3 = 0.0021), which was driven by the increase in repeat revascularization (log-rank test, overall P = 0.0047, adjusted pairwise P values between T1 and T3 = 0.0058) as well as ISR (log-rank test, overall P < 0.001, adjusted pairwise P values between T1 and T3 = 0.0007). Table 3 describes the results of the multivariable Cox proportional hazards regression analysis. The unadjusted model 1 indicated that the TyG index was statistically significantly associated with the primary endpoint, and the T3 was at elevated risk for an endpoint event. After adjusting for age, sex, BMI, previous PCI, presence of PAD, presence of multivessel CAD, hs-CRP, eGFR, and presence of lesion’s length ≥ 20 mm in model 2, the TyG index as a continuous variable was an independent predictor for the primary endpoint (HR, 1.201; 95% CI 1.047–1.377; P = 0.009). Taking the T1 as a reference, the risk of the primary endpoint was 1.338-fold higher (HR, 1.338; 95% CI 1.078–1.659; P = 0.008) in the T3. After further adjusting for stent length in model 3, the TyG index as a continuous variable remained independently associated with the primary endpoint (HR, 1.191; 95% CI 1.038–1.367; P = 0.013). Using T1 as the reference, the risk of participants experiencing the primary endpoint increased by 31.9% (HR, 1.319; 95% CI 1.063–1.637; P = 0.012) in the T3. The trend analyses for the three models were all statistically significant (all P for trend < 0.05).
Fig. 2.
Kaplan–Meier analyses for the incidences of the primary endpoint and secondary endpoints among the TyG index tertiles. a The primary endpoint including repeat revascularization and/or ISR; b repeat revascularization; c ISR. ISR in-stent restenosis, TyG triglyceride-glucose
Table 3.
Associations between the TyG index and the primary endpoint
| Model 1 | Model 2 | Model 3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95%CI | P-value | HR | 95% CI | P-value | |
| TyG index | 1.274 | 1.114–1.456 | < 0.001 | 1.201 | 1.047–1.377 | 0.009 | 1.191 | 1.038–1.367 | 0.013 |
| TyG tertiles | |||||||||
| T1 | Reference | Reference | Reference | ||||||
| T2 | 1.301 | 1.050–1.612 | 0.016 | 1.212 | 0.974–1.509 | 0.085 | 1.194 | 0.959–1.487 | 0.112 |
| T3 | 1.432 | 1.162–1.765 | < 0.001 | 1.338 | 1.078–1.659 | 0.008 | 1.319 | 1.063–1.637 | 0.012 |
| P for trend | < 0.001 | 0.009 | 0.013 | ||||||
Model 1: unadjusted
Model 2: adjusted for age, sex, BMI, previous PCI, presence of PAD, presence of multivessel CAD, high-sensitivity CRP, eGFR, presence of lesion’s length ≥ 20 mm
Model 3: adjusted for age, sex, BMI, previous PCI, presence of PAD, presence of multivessel CAD, high-sensitivity CRP, eGFR, presence of lesion’s length ≥ 20 mm, stent length
BMI body mass index, CI confidence interval, CAD coronary artery disease, CRP C-reactive protein, eGFR estimated glomerular filtration rate, HR hazard ratio, PCI percutaneous coronary intervention, PAD peripheral artery disease, TyG triglyceride-glucose
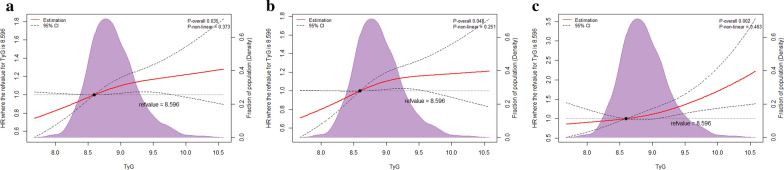
The association of the TyG index with the primary endpoint in model 3 was visualized in Fig. 3a using RCS. A linear and dose–response relationship between the TyG index and the primary endpoint was observed (non-linear P = 0.373, P overall = 0.035).
Fig. 3.
Restricted cubic splines for the adjusted dose–response association of the TyG index for the primary and secondary endpoints. Data of the TyG index for repeat revascularization was fitted with a linear regression model using restricted cubic spines with three knots at the 5th, 50th, and 95th percentiles of the TyG index. Y-axis represents the odds ratio, and the dashed lines are 95% confidence intervals. a The primary endpoint including repeat revascularization and/or ISR; b repeat revascularization; c ISR. CI confidence interval, ISR in-stent restenosis, OR odds ratio, TyG triglyceride-glucose
Association between the TyG index and the secondary endpoint
A sum of 499 participants experienced repeat revascularization. The TyG index, as a continuous variable, was found to be significantly associated with repeat revascularization in model 1 and independently predicted repeat revascularization in model 2 and model 3 (all P < 0.05). Using the T1 as the reference, each additional unit increase in the TyG index was found to increase the risk for repeat revascularization by 29.2% (HR, 1.292; 95% CI 1.030–1.622; P = 0.027) and 27.4% (HR, 1.274; 95% CI 1.015–1.600; P = 0.037) in model 2 and model 3, respectively. Besides, there was statistical significance in the trend analyses (all P for trend < 0.05). The relation of the TyG index with repeat revascularization in model 3 was fitted by RCS (Fig. 3b). Similarly, the TyG index was related to the risk of repeat revascularization in a linear and dose–response relationship (non-linear P = 0.251, P overall = 0.048).
There were 230 participants having ISR. The overall association between the TyG index and the risk of ISR was statistically significant (P overall = 0.002). After adjusting for potential confounders in model 3, the result of the RCS revealed a linear association between the TyG index and ISR (non-linear P = 0.463) (Fig. 3c). Moreover, the TyG index at 8.596 was indicated to be the reference value for the dose–response relationship that participants with a TyG index higher than 8.596 were associated with higher risk for ISR (HR, 1.738; 95% CI 1.250–2.417; P = 0.001). Detailed results of Cox regression analyses for the secondary endpoints are shown in Additional file 1: Tables S4, S5.
Discussion
This is the first study to investigate the prognostic value of the TyG index in the CCS population. In the present study, we observed a significant correlation between the TyG index and the incidence of the primary endpoint, including repeat revascularization and ISR, in the CCS patients undergoing DES-PCI. After adjusting for potential confounding risk factors, an independent association remained. Additionally, the highest tertile of the TyG group was correlated with a 1.319-fold risk of the primary endpoint compared with the lowest tertile of the TyG group. Moreover, a linear and dose–response relationship was detected between the TyG index and the risk of the primary endpoint. These findings suggested that the TyG index could be a promising predictor in evaluating the prognosis of CCS patients undergoing DES-PCI, thus benefiting risk stratification and management.
Although repeat revascularization was a controversial outcome in clinical trials for its subjective and biased nature, previous studies demonstrated that it was significantly associated with elevated risk for mortality and morbidity in the short term and composite safety events in the long term [23]. Moreover, reductions in repeat revascularization were shown to translate into improved prognosis [4]. Therefore, it’s of great significance in discussing repeat revascularization, which might provide insights into risk modification. The TyG index was found positively associated with ischemia-driven revascularization and target vessel revascularization in ACS patients [24, 25]. Based on this, this study was designed to focus on the overall repeat revascularization in the CCS population, which reflects not only the target lesion failure but also the progression of non-target lesions. We have confirmed a significant association between the TyG index and repeat revascularization. Additionally, the TyG index was correlated with the risk of repeat revascularization in a linear and dose–response way.
ISR is a delayed complication of stenting [26]. Observational studies discovered that patients with ISR were more prone to develop ACS and adverse cardiac events at follow-up [27]. Recently, the TyG index was identified as an independent predictor of ISR in ACS patients, indicating a prospect for the TyG index in ISR evaluation [15]. Consistently, our study discovered that the TyG index was significantly associated with ISR in a linear relationship after adjusting for all potential cofounders, in which a TyG index of 8.596 may serve as a cut-off value.
Putative mechanisms underlying the associations of the TyG index with repeat revascularization and ISR are not fully elucidated, although several underlying mechanisms have been proposed. Firstly, given that diagnostic studies suggested an outstanding accuracy of the TyG index in predicting IR, it’s speculated that the TyG index is a reliable surrogate indicator of IR which could reflect the pro-atherogenic roles of IR [28]. Previous experimental data established that IR could promote atherosclerosis and neointimal hyperplasia by activating the mitogen-activated protein kinase signaling pathway, resulting in vascular smooth muscle cell proliferation, inflammatory aggravation, and lipogenesis stimulation [29, 30]. Besides, IR could induce plaque development by affecting nitric oxide production (endothelial function) [31]. Observational studies verified that IR was independently associated with CAD incidence and ISR among patients with or without diabetes [10, 32]. Secondly, the chronic inflammation, disturbed lipid, and glucose homeostasis following IR may participate in the pathophysiology of repeat revascularization and ISR [33]. Still, the roles of the TyG index in repeat revascularization and ISR warrant further research.
Currently, the prognostic value of the TyG index is widely investigated in CAD. In a meta-analysis of eight cohorts involving 5,731,294 participants, the TyG index was independently associated with an increased incidence of CAD [34]. Another population-based study with a sample size of 5,593,134 demonstrated an elevated risk for MI in participants with the highest TyG index quartile [35]. Clinical investigations further supported that the TyG index was a reliable predictor of adverse coronary events and all-cause death in ACS cohorts [13]. Moreover, the TyG index was explored in various vascular diseases such as subclinical atherosclerosis, hypertension, coronary calcification, and arterial stiffness [36–38]. In this context, the present study powered by repeat revascularization and ISR among the CCS population has extended the association between the TyG index and CAD, indicating that the TyG index could serve as a potent prognostic indicator for risk stratification in CCS patients undergoing DES-PCI.
Our study had several limitations. First, the study excluded CCS patients with CABG, which might affect the generalizability of the findings. However, given that different incentives for repeat revascularization have been reported in patients with PCI or CABG, the population in our study was selected to avoid bias and emphasize the prognosis of DES-PCI. Second, there might be collection bias as we excluded patients without follow-up CAG or CCTA. Finally, the TyG index in our study was measured only once at the baseline hence the change in the TyG index during follow-up could not be taken into consideration. Therefore, a longitudinal study that monitors long-term levels of the TyG index after discharge might need to validate the finding.
Conclusion
Our study indicated that an increased TyG index was independently and significantly associated with a higher risk of long-term PCI complications, including repeat revascularization and ISR among CCS patients undergoing DES-PCI. Additionally, a linear and dose–response relationship was observed between the TyG index and the risk of developing repeat revascularization and ISR. Therefore, our study suggested that the assessment of the TyG index might help identify patients with high risk in the early stages, thus benefiting CCS management. Further prospective cohort studies are needed to confirm our findings.
Supplementary Information
Acknowledgements
The authors would like to thank all the participants.
Abbreviations
- ACS
Acute coronary syndrome
- BMI
Body mass index
- CAD
Coronary artery disease
- CCS
Chronic coronary syndrome
- CABG
Coronary artery bypass grafting
- CAG
Coronary angiography
- CCTA
Coronary CT angiography
- Cis
Confidence intervals
- DES
Drug-eluting stent
- eGFR
Estimated glomerular filtration rate
- FBG
Fasting blood glucose
- HDL-C
High-density lipoprotein cholesterol
- hs-CRP
High-sensitive C-reactive protein
- HbA1c
Glycated hemoglobin
- HR
Hazard ratio
- ISR
In-stent restenosis
- IQR
Interquartile range
- IR
Insulin resistance
- LDL-C
Low-density lipoprotein cholesterol
- LVEF
Left ventricular ejection fraction
- NT-proBNP
N-terminal pro-B-type natriuretic peptide
- OR
Odd ratio
- PCI
Percutaneous coronary intervention
- Pcr
Plasma creatine
- PAD
Peripheral artery disease
- RCS
Restricted cubic splines
- TG
Triglyceride
- TC
Total cholesterol
Author contributions
Study concept and design: LM and XG; acquisition, analysis, and interpretation of the data: XG; drafting of the manuscript: RS; revision: all authors. Each author contributed important intellectual content during the process of manuscript writing. All authors read and approved the final manuscript.
Funding
This work was supported by National Key R&D Program of China (2021ZD0111003), Capital’s Funds for Health Improvement and Research from Beijing Municipal Health Commission (Grant number: SF 2022-2-4035) and the Capital Health Development Project of China grant (SHF-2016-2-4032).
Declarations
Ethics approval and consent to participate
This study was performed in line with the Declaration of Helsinki and was authorized by the Ethics Committee of Fuwai Hospital (Reference Number: 2016-786). All participants provided written/oral informed consent for participating.
Consent for publication
All participants provided written/oral informed consent for publication which has been approved by the Ethics Committee of Fuwai Hospital.
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Xuantong Guo and Ruihuan Shen contributed equally and are regarded as co-first authors
References
- 1.Tsao CW, Aday AW, Almarzooq ZI, Alonso A, Beaton AZ, Bittencourt MS, Boehme AK, Buxton AE, Carson AP, Commodore-Mensah Y, Elkind MSV, Evenson KR, Eze-Nliam C, Ferguson JF, Generoso G, Ho JE, Kalani R, Khan SS, Kissela BM, Knutson KL, Levine DA, Lewis TT, Liu J, Loop MS, Ma J, Mussolino ME, Navaneethan SD, Perak AM, Poudel R, Rezk-Hanna M, Roth GA, Schroeder EB, Shah SH, Thacker EL, VanWagner LB, Virani SS, Voecks JH, Wang NY, Yaffe K, Martin SS. Heart disease and stroke statistics-2022 update: a report from the American heart association. Circulation. 2022;145(8):e153–e639. doi: 10.1161/CIR.0000000000001052. [DOI] [PubMed] [Google Scholar]
- 2.Knuuti J, Wijns W, Saraste A, Capodanno D, Barbato E, Funck-Brentano C, Prescott E, Storey RF, Deaton C, Cuisset T, Agewall S, Dickstein K, Edvardsen T, Escaned J, Gersh BJ, Svitil P, Gilard M, Hasdai D, Hatala R, Mahfoud F, Masip J, Muneretto C, Valgimigli M, Achenbach S, Bax JJ, ESC Scientific Document Group 2019 ESC guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020;41(3):407–477. doi: 10.1093/eurheartj/ehz425. [DOI] [PubMed] [Google Scholar]
- 3.Tam DY, Dharma C, Rocha R, Farkouh ME, Abdel-Qadir H, Sun LY, Wijeysundera HC, Austin PC, Udell JA, Gaudino M, Fremes SE, Lee DS. Long-term survival after surgical or percutaneous revascularization in patients with diabetes and multivessel coronary disease. J Am Coll Cardiol. 2020;76(10):1153–1164. doi: 10.1016/j.jacc.2020.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parasca CA, Head SJ, Milojevic M, Mack MJ, Serruys PW, Morice MC, Mohr FW, Feldman TE, Colombo A, Dawkins KD, Holmes DR, Jr, Kappetein PA, SYNTAX Investigators Incidence, characteristics, predictors, and outcomes of repeat revascularization after percutaneous coronary intervention and coronary artery bypass grafting: the SYNTAX trial at 5 years. JACC Cardiovasc Interv. 2016;9(24):2493–2507. doi: 10.1016/j.jcin.2016.09.044. [DOI] [PubMed] [Google Scholar]
- 5.Giustino G, Serruys PW, Sabik JF, 3rd, Mehran R, Maehara A, Puskas JD, Simonton CA, Lembo NJ, Kandzari DE, Morice MC, Taggart DP, Gershlick AH, Ragosta M, 3rd, Kron IL, Liu Y, Zhang Z, McAndrew T, Dressler O, Généreux P, Ben-Yehuda O, Pocock SJ, Kappetein AP, Stone GW. Mortality after repeat revascularization following PCI or CABG for left main disease: the EXCEL trial. JACC Cardiovasc Interv. 2020;13(3):375–387. doi: 10.1016/j.jcin.2019.09.019. [DOI] [PubMed] [Google Scholar]
- 6.Giustino G, Colombo A, Camaj A, Yasumura K, Mehran R, Stone GW, Kini A, Sharma SK. Coronary in-stent restenosis: JACC state-of-the-art review. J Am Coll Cardiol. 2022;80(4):348–372. doi: 10.1016/j.jacc.2022.05.017. [DOI] [PubMed] [Google Scholar]
- 7.The Writing Committee of the Report on Cardiovascular Health and Diseases in China Report on cardiovascular health and diseases in China 2021: an updated summary. Chin Circ J. 2022;37:553. doi: 10.26599/1671-5411.2023.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Di Pino A, DeFronzo RA. Insulin resistance and atherosclerosis: implications for insulin-sensitizing agents. Endocr Rev. 2019;40(6):1447–1467. doi: 10.1210/er.2018-00141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bonora E, Formentini G, Calcaterra F, Lombardi S, Marini F, Zenari L, Saggiani F, Poli M, Perbellini S, Raffaelli A, Cacciatori V, Santi L, Targher G, Bonadonna R, Muggeo M. HOMA-estimated insulin resistance is an independent predictor of cardiovascular disease in type 2 diabetic subjects: prospective data from the Verona diabetes complications study. Diabetes Care. 2002;25(7):1135–1141. doi: 10.2337/diacare.25.7.1135. [DOI] [PubMed] [Google Scholar]
- 10.Farhan S, Redfors B, Maehara A, McAndrew T, Ben-Yehuda O, De Bruyne B, Mehran R, Vogel B, Giustino G, Serruys PW, Mintz GS, Stone GW. Relationship between insulin resistance, coronary plaque, and clinical outcomes in patients with acute coronary syndromes: an analysis from the PROSPECT study. Cardiovasc Diabetol. 2021;20(1):10. doi: 10.1186/s12933-020-01207-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guerrero-Romero F, Villalobos-Molina R, Jiménez-Flores JR, Simental-Mendia LE, Méndez-Cruz R, Murguía-Romero M, Rodríguez-Morán M. Fasting triglycerides and glucose index as a diagnostic test for insulin resistance in young adults. Arch Med Res. 2016;47(5):382–387. doi: 10.1016/j.arcmed.2016.08.012. [DOI] [PubMed] [Google Scholar]
- 12.Barzegar N, Tohidi M, Hasheminia M, Azizi F, Hadaegh F. The impact of triglyceride-glucose index on incident cardiovascular events during 16 years of follow-up: Tehran lipid and glucose study. Cardiovasc Diabetol. 2020;19(1):155. doi: 10.1186/s12933-020-01121-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luo E, Wang D, Yan G, Qiao Y, Liu B, Hou J, Tang C. High triglyceride-glucose index is associated with poor prognosis in patients with acute ST-elevation myocardial infarction after percutaneous coronary intervention. Cardiovasc Diabetol. 2019;18(1):150. doi: 10.1186/s12933-019-0957-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma X, Dong L, Shao Q, Cheng Y, Lv S, Sun Y, Shen H, Wang Z, Zhou Y, Liu X. Triglyceride glucose index for predicting cardiovascular outcomes after percutaneous coronary intervention in patients with type 2 diabetes mellitus and acute coronary syndrome. Cardiovasc Diabetol. 2020;19(1):31. doi: 10.1186/s12933-020-01006-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu Y, Liu K, Chen M, Liu Y, Gao A, Hu C, Li H, Zhu H, Han H, Zhang J, Zhao Y. Triglyceride-glucose index is associated with in-stent restenosis in patients with acute coronary syndrome after percutaneous coronary intervention with drug-eluting stents. Cardiovasc Diabetol. 2021;20(1):137. doi: 10.1186/s12933-021-01332-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ma YC, Zuo L, Chen JH, Luo Q, Yu XQ, Li Y, Xu JS, Huang SM, Wang LN, Huang W, Wang M, Xu GB, Wang HY. Modified glomerular filtration rate estimating equation for Chinese patients with chronic kidney disease. J Am Soc Nephrol. 2006;17(10):2937–2944. doi: 10.1681/ASN.2006040368. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Z. Multiple imputation with multivariate imputation by chained equation (MICE) package. Ann T ransl Med. 2016;4(2):30. doi: 10.3978/j.issn.2305-5839.2015.12.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Govindarajulu U, Spiegelman D, Thurston S, Eisen E. Comparing smoothing techniques for modeling exposure-response curves in Cox models. Stat Med. 2007;26:52. doi: 10.1002/sim.2848. [DOI] [PubMed] [Google Scholar]
- 19.Bernhardt PW. Model validation and influence diagnostics for regression models with missing covariates. Stat Med. 2018;37(8):1325–1342. doi: 10.1002/sim.7584. [DOI] [PubMed] [Google Scholar]
- 20.Johannesen CDL, Langsted A, Mortensen MB, Nordestgaard BG. Association between low density lipoprotein and all cause and cause specific mortality in Denmark: prospective cohort study. BMJ. 2020;371:m4266. doi: 10.1136/bmj.m4266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med. 1989;8:551–561. doi: 10.1002/sim.4780080504. [DOI] [PubMed] [Google Scholar]
- 22.Smith PL. Splines as a useful and convenient statistical tool. Am Stat. 1979;33:57–62. [Google Scholar]
- 23.Palmerini T, Della Riva D, Biondi-Zoccai G, Leon MB, Serruys PW, Smits PC, von Birgelen C, Ben-Yehuda O, Généreux P, Bruno AG, Jenkins P, Stone GW. Mortality following nonemergent, uncomplicated target lesion revascularization after percutaneous coronary intervention: an individual patient data pooled analysis of 21 randomized trials and 32,524 patients. JACC Cardiovasc Interv. 2018;11(9):892–902. doi: 10.1016/j.jcin.2018.01.277. [DOI] [PubMed] [Google Scholar]
- 24.Zhao Q, Zhang TY, Cheng YJ, Ma Y, Xu YK, Yang JQ, Zhou YJ. Impacts of triglyceride-glucose index on prognosis of patients with type 2 diabetes mellitus and non-ST-segment elevation acute coronary syndrome: results from an observational cohort study in China. Cardiovasc Diabetol. 2020;19(1):108. doi: 10.1186/s12933-020-01086-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mao Q, Zhou D, Li Y, Wang Y, Xu SC, Zhao XH. The triglyceride-glucose index predicts coronary artery disease severity and cardiovascular outcomes in patients with non-ST-segment elevation acute coronary syndrome. Dis Markers. 2019;2019:6891537. doi: 10.1155/2019/6891537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moussa ID, Mohananey D, Saucedo J, Stone GW, Yeh RW, Kennedy KF, Waksman R, Teirstein P, Moses JW, Simonton C. Trends and outcomes of restenosis after coronary stent implantation in the United States. J Am Coll Cardiol. 2020;76(13):1521–1531. doi: 10.1016/j.jacc.2020.08.002. [DOI] [PubMed] [Google Scholar]
- 27.Assali AR, Moustapha A, Sdringola S, Denktas AE, Willerson JT, Holmes DR, Jr, Smalling RW. Acute coronary syndrome may occur with in-stent restenosis and is associated with adverse outcomes (the PRESTO trial) Am J Cardiol. 2006;98(6):729–733. doi: 10.1016/j.amjcard.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 28.Brito ADM, Hermsdorff HHM, Filgueiras MS, Suhett LG, Vieira-Ribeiro SA, Franceschini SDCC, Novaes JF. Predictive capacity of triglyceride-glucose (TyG) index for insulin resistance and cardiometabolic risk in children and adolescents: a systematic review. Crit Rev Food Sci Nutr. 2021;61(16):2783–2792. doi: 10.1080/10408398.2020.1788501. [DOI] [PubMed] [Google Scholar]
- 29.Zeng G, Nystrom FH, Ravichandran LV, Cong LN, Kirby M, Mostowski H, Quon MJ. Roles for insulin receptor, PI3-kinase, and Akt in insulin-signaling pathways related to production of nitric oxide in human vascular endothelial cells. Circulation. 2000;101(13):1539–1545. doi: 10.1161/01.CIR.101.13.1539. [DOI] [PubMed] [Google Scholar]
- 30.Cusi K, Maezono K, Osman A, Pendergrass M, Patti ME, Pratipanawatr T, DeFronzo RA, Kahn CR, Mandarino LJ. Insulin resistance differentially affects the PI 3-kinase- and MAP kinase-mediated signaling in human muscle. J Clin Invest. 2000;105(3):311–320. doi: 10.1172/JCI7535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Piatti P, Di Mario C, Monti LD, Fragasso G, Sgura F, Caumo A, Setola E, Lucotti P, Galluccio E, Ronchi C, Origgi A, Zavaroni I, Margonato A, Colombo A. Association of insulin resistance, hyperleptinemia, and impaired nitric oxide release with in-stent restenosis in patients undergoing coronary stenting. Circulation. 2003;108(17):2074–2081. doi: 10.1161/01.CIR.0000095272.67948.17. [DOI] [PubMed] [Google Scholar]
- 32.Cho YR, Ann SH, Won KB, Park GM, Kim YG, Yang DH, Kang JW, Lim TH, Kim HK, Choe J, Lee SW, Kim YH, Kim SJ, Lee SG. Association between insulin resistance, hyperglycemia, and coronary artery disease according to the presence of diabetes. Sci Rep. 2019;9(1):6129. doi: 10.1038/s41598-019-42700-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tao LC, Xu JN, Wang TT, Hua F, Li JJ. Triglyceride-glucose index as a marker in cardiovascular diseases: landscape and limitations. Cardiovasc Diabetol. 2022;21(1):68. doi: 10.1186/s12933-022-01511-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ding X, Wang X, Wu J, Zhang M, Cui M. Triglyceride-glucose index and the incidence of atherosclerotic cardiovascular diseases: a meta-analysis of cohort studies. Cardiovasc Diabetol. 2021;20(1):76. doi: 10.1186/s12933-021-01268-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hong S, Han K, Park CY. The triglyceride glucose index is a simple and low-cost marker associated with atherosclerotic cardiovascular disease: a population-based study. BMC Med. 2020;18(1):361. doi: 10.1186/s12916-020-01824-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang X, Xu W, Song Q, Zhao Z, Meng X, Xia C, Xie Y, Yang C, Jin P, Wang F. Association between the triglyceride-glucose index and severity of coronary artery disease. Cardiovasc Diabetol. 2022;21(1):168. doi: 10.1186/s12933-022-01606-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yan Y, Wang D, Sun Y, Ma Q, Wang K, Liao Y, Chen C, Jia H, Chu C, Zheng W, Hu J, Yuan Y, Wang Y, Wu Y, Mu J. Triglyceride-glucose index trajectory and arterial stiffness: results from Hanzhong adolescent hypertension cohort study. Cardiovasc Diabetol. 2022;21(1):33. doi: 10.1186/s12933-022-01453-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Park K, Ahn CW, Lee SB, Kang S, Nam JS, Lee BK, Kim JH, Park JS. Elevated TyG index predicts progression of coronary artery calcification. Diabetes Care. 2019;42(8):1569–1573. doi: 10.2337/dc18-1920. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request.