Abstract
Background
Relapse is a frequent occurrence in autologous hematopoietic stem cell transplantation (AHSCT), and early relapse after AHSCT results in poor survival and low quality of life. Predictive marker determination for AHSCT outcomes could be helpful in the prevention of relapse through personalized medicine. Here the predictive value of circulatory microRNAs (miRs) expression for AHSCT outcomes was studied.
Methods
50 MM and lymphoma candidates for AHSCT participated in this study. Two plasma samples were obtained before AHSCT from each candidate; one before mobilization and the other after conditioning. Extracellular vesicles (EVs) were isolated by ultracentrifugation. miR-125b, miR-126, miR-150, and miR-155 expression were analyzed in both plasma and EVs using real time polymerase chain reaction analysis. Other data related to AHSCT and its outcomes were also collected. The predictive value of miRs and other factors for outcomes was assessed by multi-variant analysis.
Results
By 90 weeks follow up after AHSCT, multi-variant and ROC analysis showed miR-125b as a predictive marker for relapse, high lactate dehydrogenase (LDH), and high erythrocyte sedimentation rate (ESR). The cumulative incidence of relapse, high LDH, and high ESR increased with an increase in circulatory miR-125b expression.
Conclusion
miR-125b could be applicable in prognosis evaluation and also create a possible new targeted therapy opportunity for enhanced outcomes and survival after AHSCT.
Trial registration
The study was retrospectively registered. Ethic code No: IR.UMSHA.REC.1400.541.
Keywords: Autologous hematopoietic stem cell transplantation, Relapse, Lymphoma, Multiple myeloma, miR-125b
Background
Many years have passed since autologous hematopoietic stem cell transplantation (AHSCT) or rescue therapy was considered the primary and effective therapeutic approach to multiple myeloma (MM), lymphoma, and other neoplasms [1, 2]. Relapse is one of the leading causes of treatment failure after stem cell transplantation [3]. While some patients get long-term remissions and rarely, even cure, the prognosis for high-risk MM patients remains poor. Treatment response and early relapse after AHSCT can be affected by characteristics associated with patients or diseases, such as weakness, clinical stage, age, cytogenetic abnormalities, and comorbidities [4]. Early relapse will decrease survival in standard and high-risk MM patients [4]. The relapsed cases have so heterogeneous pattern of clinical expression. Therefore, a personalized approach should be taken for treatment in transplantation time [5]. Also, recurrence of Hodgkin lymphoma after AHSCT occurs in 50% of cases in a year [6]. Relapse after AHSCT in non-Hodgkin lymphoma like follicular lymphoma is common and treatment line, age, graft purging, and myeloablative regimens are considered effective factors in it [7].
MicroRNAs (miRNAs or miRs) as small non-coding RNAs have critical and wide roles in the expression of genes and also cellular function including cell cycle, growth, differentiation, apoptosis, and so on, either in normal or in the malignant form [8]. Many of miRNAs have been used as biomarkers for early detection, target therapy, and follow-up in various diseases like lymphoma [9]. For instance, up-regulation of miR-125b results in down-regulation of its target i.e., SRY-Box Transcription Factor 30 (SOX30) in malignant lymphomas that can be regarded as a useful biomarker, diagnostically and therapeutically [10]. Poor clinical outcomes are associated with elevated expression of mir-126 in angioimmunoblastic T-cell lymphoma [11]. miR-150 increases the sensitivity of natural killer/ T (NK/T) cell lymphoma to ionizing radiation through suppression of phosphoinositide 3-kinases/ serine-threonine protein kinas/ mechanistic target of rapamycin kinase (PI3K/AKT/mTOR) pathway [12] and in MM it has seen that less expression of miR-155 related to resistance to bortezomib [13]. miRNAs can be packaged in membranous enclosed structures, known as extracellular vesicles (EVs), carried to definite targets via plasma, and protected from environmental factors [14].
Here, we were going to find a predictive marker for outcomes after AHSCT like relapse or signs of relapse before being evident. In our center, many variables related to patients and microRNA expression are considered in this regard. Given to importance of miRNAs, especially those in EVs, in this study the predictive value of miR-125b, miR-126, miR-150, and miR-155 expression in plasma and EVs, along with many outcome-related data from AHSCT candidates has been evaluated.
Methods
Patients and sample collection
50 MM and lymphoma candidates for AHSCT in the department of bone marrow transplantation center, Taleghani hospital (Tehran, Iran) were selected between September 2021 and August 2022. All protocols and blood sampling were conducted after filling out the informed consent form by patients and confirmation in ethics committee of Hamadan university of medical sciences (Ethic code No: IR.UMSHA.REC.1400.541). Mobilization was done by granulocyte colony-stimulating factor (G-CSF) administration in a steady state. After mobilization and leukocyte count reaches to the appropriate number, for HSCs collection from peripheral blood, Spectra Optia Apheresis System was applied. Lomustine, Etoposide, Cytarabine, Melphalan (CEAM) or Carmustine, Etoposide, Cytarabine, Melphalan (BEAM), and Melphalan/Velcade were conditioning regimens for lymphoma and MM patients, respectively. Patients’ available data was collected from diagnosis to transplantation phase and almost 90 weeks (as the median) follow-up from medical records and new laboratory tests that were done on the patients. 2–4 milliliters of peripheral blood samples were obtained in EDTA containing tube at two times. The first sample was 1–2 days before mobilization and the second sample was 1–2 days after conditioning and before HSCs re-infusion (post conditioning sample).
EVs isolation and confirmation
Based on our previous study [15], EV isolation was performed by ultracentrifugation. Plasma was isolated by centrifugation at 1000 g for 10 min. 1/1 dilution of plasma with phosphate buffer solution (PBS) was prepared and then centrifugation was performed for 10 min at 2000 g to precipitate dead cells. Subsequently, the supernatant was re-centrifuged for 30 min at 10,000 g to discard cell debris. The obtained supernatant was ultracentrifuged at 100,000 g for 80 min and after the discard of the supernatant, precipitated EVs were diluted by filtered PBS. Then precipitated EVs were filtered through a 0.2 μm filter to remove apoptotic bodies. Finally, ultracentrifugation was done again like before on it. For confirmation of isolated EVs, CD9 and CD63 markers were evaluated by flow cytometry (Dako antibodies, Denmark, and Attune™ NXT Flow Cytometer). Their size was measured by dynamic light scatter (DLS) (Zeta-sizer, Malvern, UK) and the transmission electron microscopy (TEM) technique was done for morphology visualization of them.
Circulatory microRNAs extraction and expression analysis
Expression of miR-125b, miR-126, miR-150, and miR155 in plasma and EVs, before mobilization and post conditioning were analyzed by real time polymerase chain reaction (PCR). The miR extraction was done on MVs/plasma by GeneAll RiboEx™ LS Kit (GeneAll Biotech, south Korea) based on the manufacturer’s instruction. cDNA was synthesized using ExcelRT ™ Reverse Transcriptase kit (SMOBio, South Korea) according to the manufacturer’s instruction and stem-loop primer (Metabion, Germany). In the expression analysis step, real time PCR reaction was performed in the total volume of 15 µl including 7.5 µl of Master mix 2× (Real Q Plus Master Mix, Denmark), 0.5 µl of each primer, 3 µl of the synthesized cDNA, and 3.5 µl of nuclease-free water in Corbett Rotor-Gene 600 thermocycler (Germany). The reactions were done in this condition: initial 95 °C for 15 min, 40 cycles of denaturation at 95 °C for 10s, annealing at 53–54 °C for 15s, extension at 72 °C for the 20s, and 10 min for the melting curve. miR-16 and SNORD47 geometric mean cycle threshold(CT)was used to calculate basal expression or 2−ΔCT. Sequences of a housekeeping gene, stem-loop, and miRNAs primers were shown in Table 1.
Table 1.
Primers for expression of microRNAs and stem loop for cDNA synthesis
| miRs | Sequences |
|---|---|
| miR-125b (5P) | 5-GATGTCCCTGAGACCCTAA-3 |
| miR-126 (3P) | 5-GACAGTCGTACCGTGAGTA-3 |
| miR-150 (5P) | 5-GTATGTCTCCCAACCCTTG-3 |
| miR-155 (5P) | 5-GCTCAGTTAATGCTAATCGTG-3 |
| miR-16 (5P) | 5-GACAGTAGCAGCACGTAAAT-3 |
| SNORD47 | 5-CCAATGATGTAATGATTCTGCC-3 |
| Reverse | 5-GAGGAAGAAGACGGAAGAAT-3 |
| Stem loop | 5GAAAGAAGGCGAGGAGCAGATCGAGGAAGAAGACGGAAGAATGTGCGTCTCGCCTTCTTTCHVMHNN-3 |
Patients follow up
After 16–130 weeks, almost 90 weeks (as the median), all of the patients were followed about their outcomes. Factors including complete blood count (CBC) parameters, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), urea, creatinine, lactate dehydrogenase (LDH), calcium, alkaline phosphatase, total protein, gamma globin, M protein spike, β2 macroglobulin, lytic lesion in bone, plasma cell percent in bone marrow, and clinical features like lymphadenopathy, bone pain, weakness, headache, and fever as outcomes were evaluated and recorded from medical reports and new laboratory tests that were done on the patients. These factors are related to remission and relapse.
Statistical analysis
All of the collected data from diagnosis to transplantation and follow-up time were analyzed in Poisson multivariate analysis to find predictive markers for all outcome factors using South Texas Art Therapy Association STATA software (College Station, Texas, USA, V14). Significant hazard ratio (HR) and p value show how often relapse happen over time in a dysregulated parameter. Evaluation of the predictive value for variables in the prediction of outcomes was analyzed by receiver-operating characteristic (ROC) analysis. In addition, Kaplan-Meier was used to estimate the cumulative incidence (CI) of outcomes and relapse free survival (RFS) analysis by effective variables. p value less than 0.05 was considered statistically significant.
Results
Patients’ data
50 candidates of AHSCT, with a mean and standard deviation of age 42.4 ± 14.6 years and 50% of them were female, were incorporated into the study. The median time for the engraftment of neutrophils and platelet was 11 days after AHSCT. Demographic data, CD34+ cell count/kg harvested by mobilization and apheresis, mobilization day number, the total dose of G-CSF, neutrophil and platelet count at apheresis day, and other AHSCT-related data were recorded (Table 2). The median follow-up was 90 weeks (range 16–130 weeks) and nine patients relapsed. In Table 2, other recorded variables as outcomes were shown.
Table 2.
Participants criteria (N = 50)
| Variables at the AHSCT time | Outcome variables | ||
|---|---|---|---|
| Features | Median (Rang) / Frequency (%) | Features | Median (Rang) / Frequency (%) |
| Age (year) | 40.5 (18–70) | Hemoglobin (g/dl) | 12.3 (8.8–16.2) |
| Gender | WBC (×10 3 /µl) | 5.03 (2.8–10.2) | |
| Male | 25 (50%) | PLT (×10 3 /µl) | 178 (133–295) |
| Female | 25 (50%) | ESR (mm/hours) | 13.5 (2–82) |
| Diagnosis | LDH (U/L) | 287 (148–450) | |
| Multiple myeloma | 24 (48%) | Ca (mg/dl) | 9.3 (7.8–13) |
| Hodgkin lymphoma | 15 (30%) | Cr (mg/dl) | 1.03 (0.6-2.0) |
| Non-Hodgkin lymphoma | 11(22%) | ALP (IU/L) | 102 (51–373) |
| BMI after transplantation | 27.1 (14.8–42.1) | Urea (mg/dl) | 18 (11–23) |
| Relapse | 9 (18%) | ||
| Mobilization day number | 6 (5–9) |
Positive lytic lesion (In MM patients) |
2 (8%) |
| Total dose of infused G-CSF for mobilization (µg) | 3750 (1500–7200) | Positive M protein spike | 4 (16%) |
| WBC count at start day of mobilization (×10 3 /µl) | 6.05 (2.4–22.1) | Positive radiologic signs in lymphoma patients | 6 (23%) |
| Last injection of G-CSF to apheresis (hour) | 6 (1–22) | ||
| Neutrophil count on apheresis day (×10 3 /µl) | 35.13 (10.7–94.5) | ||
| Apheresis duration (min) | 377.5 (270–520) | ||
| Total processed blood in apheresis (Littr) | 17.97 (10.4–25.5) | ||
| WBC count in apheresis product (×10 8 /kg) | 10.9 (4.2–26.2) | ||
| MNC count in apheresis product (×10 8 /kg) | 6.20 (3.3–17.5) | ||
| CD34 + cell count/kg (×10 6 /kg) | 3.5 (1-15.4) | ||
| CD34 + in PAPBS (/µl) | 35 (4.1–116) | ||
| Platelet engraftment day | 11 (9–19) | ||
| Platelet count at engraftment day (×10 3 /µl) | 28 (20–110) | ||
BMI: Body mass index, WBC: White blood cell, MNC: Mono nuclear cell, PAPBS: Pre-apheresis peripheral blood sample, ESR: Erythrocyte sedimentation rate, LDH: Lactate dehydrogenase, Ca: Calcium, Cr: Creatinine, ALP: Alkaline phosphatase
Isolated EV’s characterization
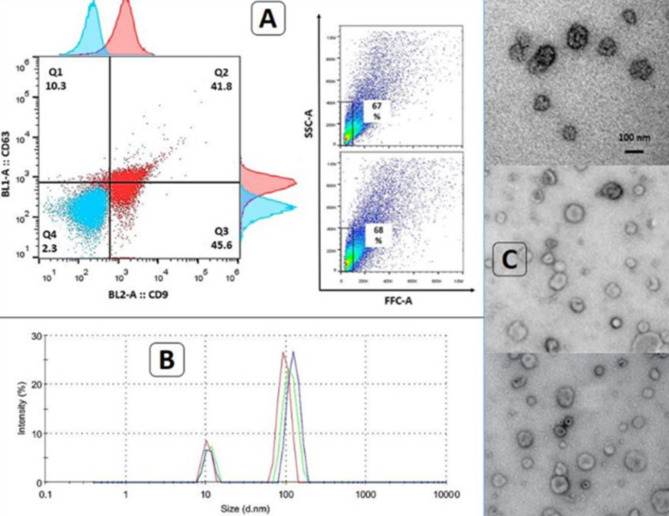
Expression of CD9 and CD63 on EVs were verified by flow cytometry (Fig. 1A). DLS analysis of isolated EVs indicated that the majority of EVs were ~ 100 nm (Fig. 1B).TEM images confirmed the morphological criteria of EVs (Fig. 1C).
Fig. 1.
Isolated EVs confirmation. A: Flow cytometry for CD9 and CD63 with 87.4% and 52.1% positivity, respectively. B: DLS results indicated majority of EVs were ~ 100 nm. C: TEM images were in accordance with DLS results in term of size. EVs: Extracellular vesicles, DLS: Dynamic light scatter, TEM: Transmission electron microscopy
The microRNAs expression
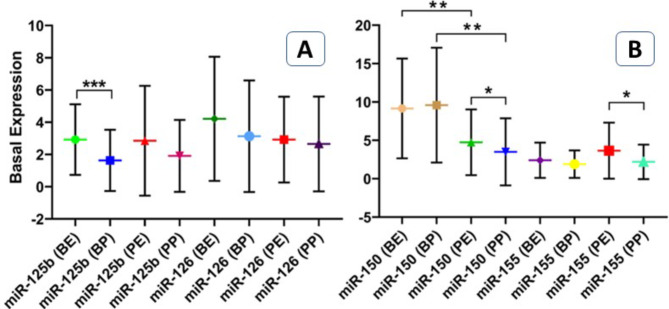
Basal expression of four circulatory miRNAs including miR-125b, miR-126, miR-150, and miR155 in plasma and EVs, before mobilization and post conditioning were analyzed. Significantly higher expression of miR-125b was detected in EVs before mobilization in comparison with plasma (p value < 0.001) (Fig. 2A). Both miR-150 and miR-155 in EVs were significantly higher than plasma post conditioning (p value < 0.05) (Fig. 2B). There was no significant difference between before mobilization and post conditioning miRNAs, except miR-150 which was higher before than post conditioning (p value < 0.01) (Fig. 2B).
Fig. 2.
Expression of miRNAs in EVs and plasma. Expression of miR-125b, miR-126, miR-150, and miR155 in plasma and EVs, before mobilization and post conditioning were analyzed by real time polymerase chain reaction. *, ** and *** p value < 0.05, < 0.01 and < 0.001. EVs: Extracellular vesicles, BE: Before mobilization EVs, BP: Before mobilization plasma, PE: Post conditioning EVs, PP: Post conditioning plasma
Multi-variate and ROC analysis results
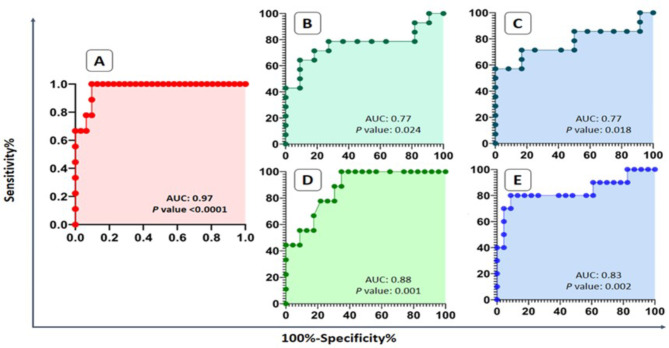
COX and Poisson regression (multi-variate) analysis were done on all variables and all outcomes that were shown in Table 3. These covariates which could be related to outcomes like relapse, LDH, and ESR, were obtained from available medical records and tests and we wanted to evaluate these. There were valuable predictive markers for relapse, high LDH (> 280 U/L), and high ESR (> 25 mm/hour) as outcome variables (Table 3). miR-125b expression level before mobilization associated with relapse, high LDH, and high ESR level. Based on the results, miR-125b before mobilization was associated with all three outcome parameters. miR-125b before mobilization in EVs can predict relapse with HR: 2.23 and p value: 0.01, and in plasma with HR: 6.7 and p value < 0.001 (Table 3). As shown in Table 3, some other variables were significantly associated with relapse, high LDH, and high ESR through multivariate analysis. We have analyzed the predictive value of miR-125b in the prediction of the three outcomes by ROC that was shown in Fig. 3A-E. miR-125b level before mobilization in both EVs and plasma was a convenient predictor for relapse, high ESR, and high LDH after AHSCT (Fig. 3A-E) (p value: 0.0001, 0.024, 0.001, 0.018, and 0.002).
Table 3.
Predictive variable for relapse, LDH, and ESR as AHSCT outcomes by multivariate analysis. COX regression for relapse and Poisson regression for LDH and ESR
| Relapse | LDH | ESR | ||||||
|---|---|---|---|---|---|---|---|---|
| Variables | HR (95% CI) | P value | Variables | Beta | P value | Variables | Beta | P value |
| miR-125b BE | 2.23 (2.12–2.78) | 0.01 | Intercept | 6.65 | < 0.0001 | Intercept | 2.01 | 0.044 |
| miR-125b BP | 6.7 (6.41–7.02) | < 0.001 | miR-125b BE | 4.79 | < 0.0001 | miR-125b BE | 5.65 | < 0.0001 |
| Age | 0.24 (0.20–1.02) | 0.426 | miR-125b BP | 8.52 | < 0.0001 | miR-125b BP | 2.41 | 0.015 |
| Engraftment day | 1.04 (0.74–1.91) | 0.091 | Age | 0.35 | 0.72 | Age | 1.11 | 0.267 |
| Diagnosis to transplantation | 0.72 (0.57–1.22) | 0.372 | Weight | 2.45 | 0.064 | Weight | 1.65 | 0.098 |
| G-CSF dose for mobilization | 0.8 (0.07–1.8) | 0.263 | Diagnosis to transplantation | 1.83 | 0.067 | Chemotherapy Courses | 1.48 | 0.139 |
| CD34+ cell count/kg | 1.01 (0.04–1.43) | 0.117 | Mobilization Days | 4.46 | < 0.1 | WBC count in apheresis | 0.92 | 0.356 |
| G-CSF dose for mobilization | 6.28 | 0.1 | MNC count in apheresis | 1.74 | 0.081 | |||
| Pre-apheresis CD34+cell count | 1.78 | 0.074 | CD34+ cell count/kg | 2.42 | 0.15 | |||
| Total processed blood in Apheresis | 3.64 | 0.12 | Conditioning days | 1.35 | 0.177 | |||
| MNC count in apheresis | 4.01 | 0.08 | Engraftment day WBC count | 1.97 | 0.068 | |||
| CD34 + cell count/kg | 3.02 | 0.0026 | Engraftment day PLT count | 1.63 | 0.103 | |||
| Conditioning days | 2.07 | 0.38 | Engraftment Day | 2.34 | 0.19 | |||
| Engraftment day | 4.50 | < 0.0001 | miR-155 PE | 0.69 | 0.489 | |||
| miR-155 BP | 1.26 | 0.207 | ||||||
| miR-126 BP | 1.17 | 0.07 | ||||||
| miR-126 PP | 0.09 | 0.926 | ||||||
ESR: Erythrocyte sedimentation rate, LDH: Lactate dehydrogenase, EVs: Extracellular vesicles, HR: Hazard ratio, BE: Before mobilization EVs, BP: Before mobilization plasma, PE: Post conditioning EVs, PP: Post conditioning plasma, WBC: White blood cell, MNC: Mono nuclear cell, G-CSF: Granulocyte colony stimulating factor
Fig. 3.
Receiver-operating characteristic (ROC) analysis. ROC curves indicated powerful predictive value of miR-125b in EVs and plasma for relapse (A), in EVs for high ESR (B) in plasma for high ESR (C), in EVs for high LDH (D), and in plasma for high LDH (E). EVs: Extracellular vesicles, ESR: Erythrocyte sedimentation rate, LDH: Lactate dehydrogenase
RFS and CI of outcomes
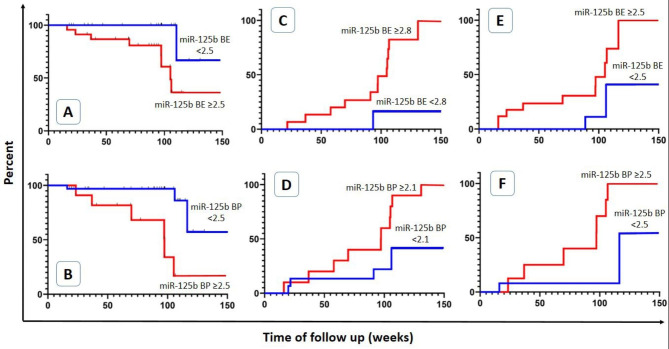
RFS and CI of high LDH (> 280 U/L), and high ESR (> 25 mm/hour) based on miR-125b expression before mobilization was evaluated by the Kaplan-Meier test (Fig. 4A-F). RFS analysis show increased incidence of relapse (decrease in RFS) after AHSCT increases in miR-125b expression ≥ 2.5 in plasma and EVs, significantly (p value: 0.006 and 0.0003) (Fig. 4A and B). Based on CI, with an increase of miR-125b expression, high LDH, and high ESR will be increased. More LDH after AHSCT was significantly associated with miR-125b expression of more than 2.1 in plasma (p value: 0.04 and 0.03) (Fig. 4C and D). Also, increasing miR-125b expression by more than 2.5 in plasma and EVs, resulted in a significantly increasing in ESR after AHSCT (p value: 0.04 and 0.0003) (Fig. 4E and F).
Fig. 4.
Relapse free survival (RFS) analysis and cumulative incidence by Kaplan-Meier test. Decreasing RFS is associated with increased expression of miR-125b (A & B), and high LDH (C & D) and high ESR (E & F) associated with increased expression of miR-125b (more than cut off). BE: Before mobilization EVs, BP: Before mobilization Plasma. p values A: 0.006, B: 0.0003, C: 0.04, D: 0.03, E: 0.04 and F: 0.0003
Discussion
Therapeutic options for relapsed Hodgkin and non-Hodgkin lymphoma are so limited, except allogeneic HSCT with some challenges like graft versus host disease [16]. There is no standard treatment for relapsed MM after AHSCT [17]. So, the detection of predicting factors for relapse can be applicable to the prevention of it and therapy modification at AHSCT time [18]. Also, there are some strategies after AHSCT for improving the outcomes, if physicians are notified in time by some markers [18]. In our study, circulatory miRNAs and many variables were evaluated to their probable predictive potential of AHSCT and relapse-related outcomes. We found that circulatory miR-125b expression level in EVs and plasma is a strong predictor for relapse after AHSCT and also, LDH and ESR are as informative factors of risk, prognosis, and relapse of MM and lymphoma. In MM patients with upregulated miR-125b, event-free survival is shorter significantly [19]. miR-125b reduces the metastasis-associated lung adenocarcinoma transcript 1 (MALAT1) gene expression via neurogenic locus notch homolog protein 1 (NOTCH1) signaling pathway in MM cells, results in more progressiveness of MM and it can be a therapeutic target [20]. Indeed miR-125b, as an onco-miR, targets tumor suppressors including long non-coding RNA named insulin-like growth factor 1 receptor antisense imprinted non-protein coding RNA (IRAIN) and PH domain and leucine-rich repeat protein phosphatase 2 (PHLPP2) that finally elevates AKT signaling pathway and myeloma cell progression [20, 21]. However, miR-125b is a multifunctional miRNA [22] with a positive effect on dexamethasone [23] and stress adaptation induced myeloma cell death [24]. Due to the high expression of miR-125b in EVs [25], its up-regulation in serum associated with Rituximab, Cyclophosphamide, Hydroxy daunorubicin, Vincristine, and Prednisone (R-CHOP) resistance, further relapse, and diminished survival in diffuse large B cell lymphoma patients [26]. Nuclear factor kappa-light-chain-enhancer of activated B cells (NFkB) activity in B cell lymphoma increases by miR-125b that targets the tumor necrosis factor alpha-induced protein 3, thereby facilitating the cancer progression and cell proliferation [27, 28]. Totally, silencing of miR-125b is required for normal hematopoietic stem cell development toward B lymphocyte and neoplasia suppression [29]. These published studies indicate that mir-125b induces the progression and relapse of MM and lymphoma. Based on our hypothesis, circulatory miR-125b expression level might be able to the prediction of relapse after AHSCT. We introduce miR-125b as a potent predictor of relapse after AHSCT, and also a good predictor of LDH and ESR. Although high LDH is known as a bad prognosis marker in newly diagnosed MM patients [30], it is a valuable informative parameter about outcomes and survival after allogeneic HSCT for MM [31]. LDH concentration should be considered as an applicable variable for risk stratification of MM [32], as a high probability of relapse after two years of AHSCT in patients with a high level of LDH is reported [33]. In addition, high concentrations of calcium and LDH (> 240 U/L) are criteria for stage III of the international staging system with elevated relapse risk of MM [33]. In the study, we considered high LDH as more than 280 U/L. Relapse and mortality after AHSCT is predicted by increased LDH (over a period of three months) in patients with lymphoma [34, 35].Through LDH along with ESR, survival analysis and monitoring of therapy in MM and Hodgkin lymphoma will be reliable, respectively [36, 37]. Elevated ESR after treatment like radiotherapy, chemotherapy, and HSCT is associated with relapse of Hodgkin lymphoma [38, 39]. So, evaluation of LDH and ESR can be valuable for the prediction of relapse of MM and lymphoma after AHSCT. Not only the evaluation of these parameters by themselves, weeks after AHSCT but also the measurement of associated parameters like miRNAs at the time of AHSCT could be informative about relapse and its predictive markers (LDH and ESR). Indeed miR-125b is able to prediction of relapse much earlier than LDH and ESR [40]. According to the effect of miR-125b on MM and lymphoma, administration of miR-125b antagomir inhibits cell proliferation, tumor growth, and neoplasm relapse [40].
Conclusion
As a conclusion circulatory miR-125b is associated with the risk of relapse and relapse-related parameters in MM and lymphoma patients. Monitoring of the circulatory level of miR-125b at the AHSCT time predicts the treatment response and helps to do additional strategies for the prevention of relapse.
Acknowledgements
This work was founded by Hamadan University of Medical Sciences. Thank Iranian biophysics and biochemistry center for TEM technical support.
Abbreviations
- AHSCT
Autologous hematopoietic stem cell transplantation
- MM
Multiple myeloma
- miRNAs or miR
MicroRNAs
- SOX30
SRY-box transcription factor 30
- NK/T
Natural killer/ T cell
- PI3K/AKT/mTOR
Phosphoinositide 3-kinases/ Serine-threonine protein kinas/ Mechanistic target of rapamycin kinase
- G-CSF
Granulocyte colony stimulating factor
- CEAM
Lomustine, Etoposide, Cytarabine, Melphalan
- BEAM
Carmustine, Etoposide, Cytarabine, Melphalan
- PBS
Phosphate buffer solution
- DLS
Dynamic light scatter
- TEM
Transmission electron microscopy
- PCR
Polymerase chain reaction
- CT
Cycle threshold
- CBC
Complete blood count
- ESR
Erythrocyte sedimentation rate
- CRP
C-reactive protein
- LDH
Lactate dehydrogenase
- STATA
South texas art therapy association
- HR
Hazard ratio
- ROC
Receiver-operating characteristic
- CI
Cumulative incidence
- BMI
Body mass index
- WBC
White blood cell
- MNC
Mono nuclear cell
- CPK
Count per kilogram
- PAPBS
Pre-apheresis peripheral blood sample
- Ca
Calcium
- Cr
Creatinine
- ALP
Alkaline phosphatase
- BE
Before mobilization EVs
- BP
Before mobilization plasma
- PE
Post conditioning EVs
- PP
Post conditioning plasma
- MALAT1
Metastasis associated lung adenocarcinoma transcript 1
- NOTCH1
Neurogenic locus notch homolog protein 1
- CHOP
Rituximab, Cyclophosphamide, Hydroxy daunorubicin, Vincristine, and Prednisone
- NFkB
Nuclear factor kappa-light-chain-enhancer of activated B cells
- IRAIN
Insulin like growth factor 1 receptor antisense imprinted non-protein coding RNA
- PHLPP2
PH domain and leucine rich repeat protein phosphatase 2
- AKT
Serine-threonine protein kinas
Authors’ contributions
M.R. and F.A. wrote the first main manuscript text. MH.M. prepared figures. A.H. collected and analyzed the data. All authors reviewed the manuscript.
Funding
This study was founded by Hamadan University of Medical Sciences, Ethic Code: Ethic code No: IR.UMSHA.REC.1400.541, grant No: 140008186756.
Availability of data and materials
The data have included in the text and documented/reserved with the corresponding author.
Code availability
Not applicable.
Declarations
Competing interests
All authors state that there is no conflict of interest.
Ethics approval
All procedures were in accordance with the ethical standards of the institutional and/or national research committee. This study was confirmed in ethic committee of Hamadan university of medical sciences (Ethic code No: IR.UMSHA.REC.1400.541).
Consent to participate
All protocols and blood sampling were conducted after filling out the informed consent form by patients.
Consent for publication
Not applicable.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Vaxman I, Visram A, Kumar S, Dispenzieri A, Buadi F, Dingli D, et al. Autologous stem cell transplantation for multiple myeloma patients aged ≥ 75 treated with novel agents. Bone Marrow Transplant. 2021;56(5):1144–50. doi: 10.1038/s41409-020-01159-9. [DOI] [PubMed] [Google Scholar]
- 2.Wudhikarn K, Johnson BK, Villasboas JC, Paludo J, Inwards DJ, Porrata LF, et al. Improvement in outcomes of autologous stem cell transplant in patients with Lymphoma Older Than 70 years: the significance of age in 2020s? Blood. 2021;138:2908. doi: 10.1182/blood-2021-144507. [DOI] [Google Scholar]
- 3.Chen Y-B, McCarthy PL, Hahn T, Holstein SA, Ueda M, Kröger N, et al. Methods to prevent and treat relapse after hematopoietic stem cell transplantation with tyrosine kinase inhibitors, immunomodulating drugs, deacetylase inhibitors, and hypomethylating agents. Bone Marrow Transplant. 2019;54(4):497–507. doi: 10.1038/s41409-018-0269-3. [DOI] [PubMed] [Google Scholar]
- 4.Corre J, Montes L, Martin E, Perrot A, Caillot D, Leleu X, et al. Early relapse after autologous transplant for myeloma is associated with poor survival regardless of cytogenetic risk. Haematologica. 2020;105(9):e480. doi: 10.3324/haematol.2019.236588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alegre A, Granda A, Martínez-Chamorro C, Díaz-Mediavilla J, Martínez R, García-Laraña J, et al. Different patterns of relapse after autologous peripheral blood stem cell transplantation in multiple myeloma: clinical results of 280 cases from the Spanish Registry. Haematologica. 2002;87(6):609–14. [PubMed] [Google Scholar]
- 6.Crump M. Management of Hodgkin lymphoma in relapse after autologous stem cell transplant. ASH Educ Program Book. 2008;2008(1):326–33. doi: 10.1182/asheducation-2008.1.326. [DOI] [PubMed] [Google Scholar]
- 7.Kornacker M, Stumm J, Pott C, Dietrich S, Süssmilch S, Hensel M, et al. Characteristics of relapse after autologous stem-cell transplantation for follicular lymphoma: a long-term follow-up. Ann Oncol. 2009;20(4):722–8. doi: 10.1093/annonc/mdn691. [DOI] [PubMed] [Google Scholar]
- 8.Asgarpour K, Shojaei Z, Amiri F, Ai J, Mahjoubin-Tehran M, Ghasemi F, et al. Exosomal microRNAs derived from mesenchymal stem cells: cell-to-cell messages. Cell Commun Signal. 2020;18(1):1–16. doi: 10.1186/s12964-020-00650-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Han B, Wang S, Zhao H. MicroRNA-21 and microRNA-155 promote the progression of Burkitt’s lymphoma by the PI3K/AKT signaling pathway. IJ Clin Exp Pathol. 2020;13(1):89. [PMC free article] [PubMed] [Google Scholar]
- 10.Zhan C, Wang T, You H, Si C. Different expressions of miR-125b and SOX30 in malignant lymphomas and their significance. J BUON. 2018;23(4):1179–84. [PubMed] [Google Scholar]
- 11.Lone W, Bouska A, Sharma S, Amador C, Saumyaranjan M, Herek TA, et al. Genome-wide miRNA expression profiling of molecular subgroups of peripheral T-cell lymphoma. Clin Cancer Res. 2021;27(21):6039–53. doi: 10.1158/1078-0432.CCR-21-0573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu SJ, Chen J, Wu B, Wang YJ, Guo KY. MicroRNA-150 enhances radiosensitivity by inhibiting the AKT pathway in NK/T cell lymphoma. J Exp Clin Cancer Res. 2018;37(1):1–10. doi: 10.1186/s13046-017-0639-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amodio N, Gallo Cantafio ME, Botta C, Agosti V, Federico C, Caracciolo D, et al. Replacement of miR-155 elicits tumor suppressive activity and antagonizes bortezomib resistance in multiple myeloma. Cancer. 2019;11(2):236. doi: 10.3390/cancers11020236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma Y, Li C, Huang Y, Wang Y, Xia X, Zheng CJ. Exosomes released from neural progenitor cells and induced neural progenitor cells regulate neurogenesis through miR-21a. Cell Commun Signal. 2019;17:96. doi: 10.1186/s12964-019-0418-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rafiee M, Allahbakhshian FM, Amiri V, Hajifathali A, Gharehbaghian A, Mohammadi MH. Circulatory miR–155 correlation with platelet and neutrophil recovery after autologous hematopoietic stem cell transplantation a multivariate analysis. Int J Hematol. 2021;114(2):235–45. doi: 10.1007/s12185-021-03154-2. [DOI] [PubMed] [Google Scholar]
- 16.Smith SM, van Besien K, Carreras J, Bashey A, Cairo MS, Freytes CO, et al. Second autologous stem cell transplantation for relapsed lymphoma after a prior autologous transplant. Biol blood Marrow Transplant. 2008;14(8):904–12. doi: 10.1016/j.bbmt.2008.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Malard F, Harousseau J, Mohty M. Multiple myeloma treatment at relapse after autologous stem cell transplantation: a practical analysis. Cancer Treat Rev. 2017;52:41–7. doi: 10.1016/j.ctrv.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 18.Sureda A, André M, Borchmann P, da Silva MG, Gisselbrecht C, Vassilakopoulos TP, et al. Improving outcomes after autologous transplantation in relapsed/refractory Hodgkin lymphoma: a european expert perspective. BMC Cancer. 2020;20(1):1–14. doi: 10.1186/s12885-020-07561-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jiang Y, Luan Y, Chang H, Chen G. The diagnostic and prognostic value of plasma microRNA–125b–5p in patients with multiple myeloma. OncolLett. 2018;16(3):4001–7. doi: 10.3892/ol.2018.9128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gao D, Xiao Z, Li HP, Han DH, Zhang YP. The mechanism study of miR-125b in occurrence and progression of multiple myeloma. Cancer Med. 2018;7(1):134–45. doi: 10.1002/cam4.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang Y, Ding J, Li J, Chen G. Effects of microRNA–125b on multiple myeloma cell growth in vitro and in vivo. Oncol Rep. 2018;40(5):2864–75. doi: 10.3892/or.2018.6668. [DOI] [PubMed] [Google Scholar]
- 22.Wang Y, Wei Y, Fan X, Zhang P, Wang P, Cheng S, et al. MicroRNA-125b as a tumor suppressor by targeting MMP11 in breast cancer. ThoracCancer. 2020;11(6):1613–20. doi: 10.1111/1759-7714.13441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murray M, Rushworth SA, Zaitseva L, Bowles KM, MacEwan DJ. Attenuation of dexamethasone-induced cell death in multiple myeloma is mediated by miR-125b expression. Cell Cycle. 2013;12(13):2144–53. doi: 10.4161/cc.25251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Misso G, Zarone MR, Lombardi A, Grimaldi A, Cossu AM, Ferri C, et al. miR-125b upregulates miR-34a and sequentially activates stress adaption and cell death mechanisms in multiple myeloma. Mol Ther Nucleic Acids. 2019;16:391–406. doi: 10.1016/j.omtn.2019.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feng Y, Zhong M, Zeng S, Wang L, Liu P, Xiao X, et al. Exosome-derived miRNAs as predictive biomarkers for diffuse large B-cell lymphoma chemotherapy resistance. Epigenomics. 2019;11(1):35–51. doi: 10.2217/epi-2018-0123. [DOI] [PubMed] [Google Scholar]
- 26.Yuan WX, Gui YX, Na WN, Chao J, Yang X. Circulating microRNA-125b and microRNA-130a expression profiles predict chemoresistance to R-CHOP in diffuse large B-cell lymphoma patients. Oncol Lett. 2016;11(1):423–32. doi: 10.3892/ol.2015.3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim S-W, Ramasamy K, Bouamar H, Lin A-P, Jiang D, Aguiar RC. MicroRNAs miR-125a and miR-125b constitutively activate the NF-κB pathway by targeting the tumor necrosis factor alpha-induced protein 3 (TNFAIP3, A20) PNAS. 2012;109(20):7865–70. doi: 10.1073/pnas.1200081109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peng B, Theng PY, Le MT. Essential functions of miR-125b in cancer. Cell Prolif. 2021;54(2):e12913. doi: 10.1111/cpr.12913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li G, So AY-L, Sookram R, Wong S, Wang JK, Ouyang Y, et al. Epigenetic silencing of miR-125b is required for normal B-cell development. Blood. 2018;131(17):1920–30. doi: 10.1182/blood-2018-01-824540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gu Y, Yuan Y-H, Xu J, Shi Q-L, Qu X-Y, Guo R, et al. High serum lactate dehydrogenase predicts an unfavorable outcome in chinese elderly patients with multiple myeloma. Oncotarget. 2017;8(29):48350. doi: 10.18632/oncotarget.16237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shouval R, Teper O, Fein JA, Danylesko I, Shem Tov N, Yerushalmi R, et al. LDH and renal function are prognostic factors for long-term outcomes of multiple myeloma patients undergoing allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2020;55(9):1736–43. doi: 10.1038/s41409-020-0829-1. [DOI] [PubMed] [Google Scholar]
- 32.Dimopoulos MA, Kastritis E, Roussou M, Gkotzamanidou M, Migkou M, Gavriatopoulou M, et al. Elevated serum lactate dehydrogenase (LDH) should be included among the variables which define high risk multiple myeloma. Am Society Hematol; 2010.
- 33.Huang B, Li J, Lu J, Xiao Y, Zhao Y, Huang H. Scoring system for predicting risk of relapse in patients with multiple myeloma within two years after stem cell transplantation. Am Society Hematol Washington, DC; 2017.
- 34.Sorror M, Storer B, Gopal A, Holmberg L, Sandmaier B, Bensinger W, et al. Comorbidity, Lactate dehydrogenase (LDH), and Chemosensitivity are independent predictors of mortality after autologous hematopoietic cell transplantation (HCT) for patients (pts) with lymphoma. Blood. 2007;110(11):616. doi: 10.1182/blood.V110.11.616.616. [DOI] [Google Scholar]
- 35.William BM, Bongu NR, Bast M, Bociek RG, Bierman PJ, Vose JM, et al. The utility of lactate dehydrogenase in the follow up of patients with diffuse large B-cell lymphoma. Rev bras hematol hemoter. 2013;35:189–91. doi: 10.5581/1516-8484.20130055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alexandrakis M, Passam F, Ganotakis E, Sfiridaki K, Xilouri I, Perisinakis K, et al. The clinical and prognostic significance of erythrocyte sedimentation rate (ESR), serum interleukin-6 (IL‐6) and acute phase protein levels in multiple myeloma. Clin Lab Haematol. 2003;25(1):41–6. doi: 10.1046/j.1365-2257.2003.00492.x. [DOI] [PubMed] [Google Scholar]
- 37.Bien E, Balcerska A. Serum Soluble Interleukin-2 receptor, Beta2‐Microglobulin, Lactate dehydrogenase and erythrocyte sedimentation rate in children with Hodgkin’s lymphoma. Scand J immunol. 2009;70(5):490–500. doi: 10.1111/j.1365-3083.2009.02313.x. [DOI] [PubMed] [Google Scholar]
- 38.Friedman S, Henry-Amar M, Cosset JM, Carde P, Hayat M, Dupouy N, et al. Therapeutic implications and sites of relapse predicted by elevated posttherapy erythrocyte sedimentation rate in early stage Hodgkin disease. Am J hematol. 1991;37(4):253–7. doi: 10.1002/ajh.2830370408. [DOI] [PubMed] [Google Scholar]
- 39.Henry-Amar M, Friedman S, Hayat M, Somers R, Meerwaldt JH, Carde P, et al. Erythrocyte sedimentation rate predicts early relapse and survival in early-stage Hodgkin disease. Ann Inter Med. 1991;114(5):361–5. doi: 10.7326/0003-4819-114-5-361. [DOI] [PubMed] [Google Scholar]
- 40.Zheng Z, Qu J-Q, Yi H-M, Ye X, Huang W, Xiao T, et al. MiR-125b regulates proliferation and apoptosis of nasopharyngeal carcinoma by targeting A20/NF-κB signaling pathway. Cell Death Disease. 2017;8(6):e2855–e. doi: 10.1038/cddis.2017.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data have included in the text and documented/reserved with the corresponding author.
Not applicable.