Abstract
Background
Neonates may undergo surgery because of malformations such as diaphragmatic hernia, gastroschisis, congenital heart disease, and hypertrophic pyloric stenosis, or complications of prematurity, such as necrotizing enterocolitis, spontaneous intestinal perforation, and retinopathy of prematurity that require surgical treatment. Options for treatment of postoperative pain include opioids, non‐pharmacological interventions, and other drugs. Morphine, fentanyl, and remifentanil are the opioids most often used in neonates. However, negative impact of opioids on the structure and function of the developing brain has been reported. The assessment of the effects of opioids is of utmost importance, especially for neonates in substantial pain during the postoperative period.
Objectives
To evaluate the benefits and harms of systemic opioid analgesics in neonates who underwent surgery on all‐cause mortality, pain, and significant neurodevelopmental disability compared to no intervention, placebo, non‐pharmacological interventions, different types of opioids, or other drugs.
Search methods
We searched Cochrane CENTRAL, MEDLINE via PubMed and CINAHL in May 2021. We searched the WHO ICTRP, clinicaltrials.gov, and ICTRP trial registries. We searched conference proceedings, and the reference lists of retrieved articles for RCTs and quasi‐RCTs.
Selection criteria
We included randomized controlled trials (RCTs) conducted in preterm and term infants of a postmenstrual age up to 46 weeks and 0 days with postoperative pain where systemic opioids were compared to 1) placebo or no intervention; 2) non‐pharmacological interventions; 3) different types of opioids; or 4) other drugs.
Data collection and analysis
We used standard Cochrane methods. Our primary outcomes were pain assessed with validated methods, all‐cause mortality during initial hospitalization, major neurodevelopmental disability, and cognitive and educational outcomes in children more than five years old. We used the fixed‐effect model with risk ratio (RR) and risk difference (RD) for dichotomous data and mean difference (MD) for continuous data. We used GRADE to assess the certainty of evidence for each outcome.
Main results
We included four RCTs enrolling 331 infants in four countries across different continents. Most studies considered patients undergoing large or medium surgical procedures (including major thoracic or abdominal surgery), who potentially required pain control through opioid administration after surgery. The randomized trials did not consider patients undergoing minor surgery (including inguinal hernia repair) and those individuals exposed to opioids before the beginning of the trial. Two RCTs compared opioids with placebo; one fentanyl with tramadol; and one morphine with paracetamol. No meta‐analyses could be performed because the included RCTs reported no more than three outcomes within the prespecified comparisons. Certainty of the evidence was very low for all outcomes due to imprecision of the estimates (downgrade by two levels) and study limitations (downgrade by one level).
Comparison 1: opioids versus no treatment or placebo
Two trials were included in this comparison, comparing either tramadol or tapentadol with placebo. No data were reported on the following critical outcomes: pain; major neurodevelopmental disability; or cognitive and educational outcomes in children more than five years old. The evidence is very uncertain about the effect of tramadol compared with placebo on all‐cause mortality during initial hospitalization (RR 0.32, 95% Confidence Interval (CI) 0.01 to 7.70; RD ‐0.03, 95% CI ‐0.10 to 0.05, 71 participants, 1 study; I² = not applicable). No data were reported on: retinopathy of prematurity; or intraventricular hemorrhage.
Comparison 2: opioids versus non‐pharmacological interventions
No trials were included in this comparison.
Comparison 3: head‐to‐head comparisons of different opioids
One trial comparing fentanyl with tramadol was included in this comparison. No data were reported on the following critical outcomes: pain; major neurodevelopmental disability; or cognitive and educational outcomes in children more than five years old. The evidence is very uncertain about the effect of fentanyl compared with tramadol on all‐cause mortality during initial hospitalization (RR 0.99, 95% CI 0.59 to 1.64; RD 0.00, 95% CI ‐0.13 to 0.13, 171 participants, 1 study; I² = not applicable). No data were reported on: retinopathy of prematurity; or intraventricular hemorrhage.
Comparison 4: opioids versus other analgesics and sedatives
One trial comparing morphine with paracetamol was included in this comparison. The evidence is very uncertain about the effect of morphine compared with paracetamol on COMFORT pain scores (MD 0.10, 95% CI ‐0.85 to 1.05; 71 participants, 1 study; I² = not applicable). No data were reported on the other critical outcomes, i.e. major neurodevelopmental disability; cognitive and educational outcomes in children more than five years old, all‐cause mortality during initial hospitalization; retinopathy of prematurity; or intraventricular hemorrhage.
Authors' conclusions
Limited evidence is available on opioid administration for postoperative pain in newborn infants compared to either placebo, other opioids, or paracetamol.
We are uncertain whether tramadol reduces mortality compared to placebo; none of the studies reported pain scores, major neurodevelopmental disability, cognitive and educational outcomes in children older than five years old, retinopathy of prematurity, or intraventricular hemorrhage. We are uncertain whether fentanyl reduces mortality compared to tramadol; none of the studies reported pain scores, major neurodevelopmental disability, cognitive and educational outcomes in children older than five years old, retinopathy of prematurity, or intraventricular hemorrhage. We are uncertain whether morphine reduces pain compared to paracetamol; none of the studies reported major neurodevelopmental disability, cognitive and educational outcomes in children more than five years old, all‐cause mortality during initial hospitalization, retinopathy of prematurity, or intraventricular hemorrhage. We identified no studies comparing opioids versus non‐pharmacological interventions.
Plain language summary
Are opioids the best choice for managing pain in babies after surgery?
Key messages
• We did not find enough good‐quality evidence about the benefits and risks of opioids (a group of pain‐relieving medicines) to manage pain after surgery in babies. We found only four studies and they had not enrolled enough babies to give reliable results.
• Larger, well‐designed studies are needed to give better estimates of the benefits and potential harms of opioids, other medicines and non‐medicine‐based treatments.
Why are opioids given to manage pain after surgery in babies?
Babies (particularly in the first four weeks after birth) often have to have surgeries. Similar to adults, they need constant pain management after these operations and opioids are commonly used for post‐surgery pain relief in babies.
What did we want to find out?
We wanted to find out the impact of giving opioids to babies having surgery, compared to:
1) no treatment or placebo (a 'dummy' treatment, or sham treatment, that does not contain any medicine but looks or tastes identical to the medicine being tested);
2) non‐medicine‐based treatments (such as sweet solutions);
3) other medicines; or
4) different types of opioids.
What did we do?
We searched for studies that compared opioids with the four treatments described above. We compared and summarized their results, and rated our confidence in the evidence, based on factors such as study methods and sizes.
What did we find?
We included four studies that involved 331 babies. The biggest study was in 171 babies and the smallest study was in 15 babies.
• Two studies compared opioids with placebo: it is unclear if opioids have an effect on mortality; no studies reported pain, long‐term development, vision problems (retinopathy of prematurity) or bleeding to the brain (intraventricular hemorrhage).
• One study compared one type of opioid to another type of opioid: it is unclear if fentanyl has an effect on mortality compared to tramadol; no studies reported pain, long‐term development, vision problems or bleeding to the brain.
• One study compared an opioid to a different type of pain‐relieving medicine: it is unclear if the opioid morphine has an effect on pain compared with paracetamol; no studies reported long‐term development, mortality, vision problems or bleeding to the brain.
What are the limitations of the evidence?
We are not confident in the evidence because there were not enough studies to be certain about the results of our outcomes. Also, it is possible that people in the studies were aware of what treatment they were given. Not all the studies provided data about everything that we were interested in.
How up‐to‐date is this review? We searched for studies that were available up to May 2021.
Summary of findings
Summary of findings 1. Tramadol compared to no treatment or placebo for postoperative pain in neonates.
| Tramadol compared to no treatment or placebo for postoperative pain in neonates | ||||||
| Patient or population: postoperative pain in preterm and term infants Setting: neonatal intensive care unit Intervention: tramadol Comparison: no treatment or placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with no treatment or placebo | Risk with tramadol | |||||
| Pain assessed with validated methods during the administration of selected drugs | ‐ | ‐ | ‐ | ‐ | This outcome was not reported. | |
| Major neurodevelopmental disability in children aged 18 to 24 months | ‐ | ‐ | ‐ | ‐ | This outcome was not reported. | |
| Major neurodevelopmental disability in children aged three to five years | ‐ | ‐ | ‐ | ‐ | This outcome was not reported. | |
| Cognitive and educational outcomes in children more than five years old | ‐ | ‐ | ‐ | ‐ | This outcome was not reported. | |
| All‐cause mortality during initial hospitalization | Study population | RR 0.32
(0.01 to 7.70) RD ‐0.03, (‐0.10 to 0.05) |
71 (1 RCT) | ⊕⊝⊝⊝ Very low 1 | The evidence is very uncertain about the effect of tramadol on this outcome compared to placebo. | |
| 29 per 1000 | 9 per 1000 (0 to 220) | |||||
| Severe retinopathy of prematurity (defined as stage 3 or greater) | ‐ | ‐ | ‐ | ‐ | This outcome was not reported. | |
| Severe intraventricular hemorrhage (grade 3 or greater) on cranial ultrasound, as per Papile classification | ‐ | ‐ | ‐ | ‐ | This outcome was not reported. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OR: odds ratio; RCT: randomized controlled trial; RD: risk difference; RR: risk ratio; | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Downgraded one level for study limitations: unclear selection and reporting bias; downgraded two levels for imprecision: one small trial with wide confidence of interval
Summary of findings 2. Fentanyl compared to tramadol for postoperative pain in neonates.
| Fentanyl compared to tramadol for postoperative pain in neonates | ||||||
| Patient or population: postoperative pain in preterm and term infants Setting: neonatal intensive care unit Intervention: fentanyl Comparison: tramadol | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with tramadol | Risk with fentanyl | |||||
| Pain assessed with validated methods during the administration of selected drugs | ‐ | ‐ | ‐ | ‐ | This outcome was not reported. | |
| Major neurodevelopmental disability in children aged 18 to 24 months | ‐ | ‐ | ‐ | ‐ | This outcome was not reported. | |
| Major neurodevelopmental disability in children aged three to five years | ‐ | ‐ | ‐ | ‐ | This outcome was not reported. | |
| Cognitive and educational outcomes in children more than five years old | ‐ | ‐ | ‐ | ‐ | This outcome was not reported. | |
| All‐cause mortality during initial hospitalization | Study population | RR 0.99
(0.59 to 1.64) RD 0.00, (‐0.13 to 0.13) |
171 (1 RCT) | ⊕⊝⊝⊝ Very low 1 | The evidence is very uncertain about the effect of fentanyl on this outcome compared to tramadol. | |
| 259 per 1000 | 256 per 1000 (153 to 424) | |||||
| Severe retinopathy of prematurity (defined as stage 3 or greater) | ‐ | ‐ | ‐ | ‐ | This outcome was not reported. | |
| Severe intraventricular hemorrhage (grade 3 or greater) on cranial ultrasound, as per Papile classification | ‐ | ‐ | ‐ | ‐ | This outcome was not reported. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OR: odds ratio; RCT: randomized controlled trial; RD: risk difference; RR: risk ratio; | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Downgraded one level for study limitations: unclear risk of bias for selection and other biases; downgraded two levels for imprecision: one small trial with wide confidence of interval
Summary of findings 3. Morphine compared to paracetamol for postoperative pain in neonates.
| Morphine compared to paracetamol for postoperative pain in neonates | ||||||
| Patient or population: postoperative pain in preterm and term infants Setting: neonatal intensive care unit Intervention: morphine Comparison: paracetamol | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with paracetamol | Risk with morphine | |||||
| Pain assessed with COMFORT scale | The mean pain assessed with COMFORT scale was 0. | MD 0.10 higher (0.85 lower to 1.05 higher) | ‐ | 71 (1 RCT) | ⊕⊝⊝⊝ Very low1 | The evidence is very uncertain about the effect of morphine on this outcome compared to paracetamol. |
| Major neurodevelopmental disability in children aged 18 to 24 months | ‐ | ‐ | ‐ | ‐ | This outcome was not reported. | |
| Major neurodevelopmental disability in children aged three to five years | ‐ | ‐ | ‐ | ‐ | This outcome was not reported. | |
| Cognitive and educational outcomes in children more than five years old | ‐ | ‐ | ‐ | ‐ | This outcome was not reported. | |
| All‐cause mortality during initial hospitalization | ‐ | ‐ | ‐ | ‐ | This outcome was not reported. | |
| Severe retinopathy of prematurity (defined as stage 3 or greater) | ‐ | ‐ | ‐ | ‐ | This outcome was not reported. | |
| Severe intraventricular hemorrhage (grade 3 or greater) on cranial ultrasound, as per Papile classification | ‐ | ‐ | ‐ | ‐ | This outcome was not reported. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; OR: odds ratio; RCT: randomized controlled trial; RR: risk ratio; | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Downgraded one level for study limitations: unclear selection and reporting risk of bias; downgraded two levels for imprecision: one small trial with wide confidence of interval
Background
Description of the condition
According to the United States' National Surgical Quality Improvement Program‐Pediatric (NSQIP‐P), during 2012 to 2017, 19,312 neonates received inpatient surgery (Mpody 2020). NSQIP‐P was designed to prospectively and nationally collect the perioperative data of children from across hospitals (Mpody 2020). Newborn infants undergo surgeries for treatment of congenital abnormalities and neonatal morbidities, and are managed in the neonatal intensive care unit (NICU) thereafter. Malformations range from conditions such as diaphragmatic hernia and gastroschisis that require surgical repair immediately or relatively early after birth, to conditions such as congenital heart disease and hypertrophic pyloric stenosis that can wait several weeks during the neonatal period. Neonatal morbidities include complications often due to prematurity, such as necrotizing enterocolitis (NEC), spontaneous intestinal perforation, and retinopathy of prematurity (ROP) that requires surgical treatment. Such surgical interventions result in acute pain during and after surgery, and also easily can cause chronic pain, due to hyperalgesia, during a vital period of complex brain development (Fitzgerald 1989). Major surgeries involving larger incisions (e.g. thoracotomy, laparotomy) are considered to be more painful than minor surgeries limited to a local area (e.g. circumcision). The plasticity of the neonatal brain might increase its vulnerability to these early adverse events, thereby leading to abnormal neurodevelopmental, behavioral, and cognitive outcomes (Anand 1998; Anand 2000; Duerden 2014; Ranger 2014; Vinall 2014). Moreover, preterm infants with even more immature brains are already predisposed to developing such sequelae from inadequately treated pain, while being more likely to be exposed to more pain during their longer NICU hospitalization. The unique character of the neonatal population strengthens the rationale to establish the best therapeutic approach for adequate analgesia.
Neonatal pain might have a negative impact not only on neonates' clinical recovery in the NICU, but also on their neuropsychological long‐term development. Therefore, it is of utmost importance to accurately identify and appropriately manage pain, for which reviews and guidelines have been continuously updated (Carter 2017; Derieg 2016; Maitra 2014; Maxwell 2019). However, major gaps in knowledge exist regarding the objective assessment of pain, the most effective way to prevent and relieve pain as well as the long‐term effects of drug therapy. Systematic evaluation of pain has increased the awareness of treating pain, but pain assessment continues to pose a challenge (Olsson 2021). Pain assessment tools like NIPS (Neonatal Infant Pain Scale), and CRIES (Crying, Requires oxygen saturation, Increased vital signs, Expression, Sleeplessness) have been developed and their use in postoperative neonates has been validated (Maitra 2014). In the Poppi study, a randomized controlled trial (RCT) investigating the analgesic efficacy of oral morphine for retinopathy of prematurity (ROP) screening, investigators revised an existing pain measure specifically for the study (Monk 2019). Nonetheless, a fully reliable and objective assessment method is still lacking (Eriksson 2019; Olsson 2021).
Investigators have made various attempts to find treatment strategies to prevent or minimize neonates' pain, stress and discomfort to improve outcomes. Currently, healthcare providers routinely adopt an approach that uses both non‐pharmacological and pharmacological interventions in the NICU (Allegaert 2013; Allegaert 2016; Lim 2017). However, a significant portion of the drugs administered is used 'off‐label' and according to clinical experience extrapolated from adults and older children, thus administered on the basis of experience rather than evidence. This practice highlights the reality that the pharmacokinetics (PK) and pharmacodynamics (PD) are not known for the neonatal population. In the daily NICU setting, healthcare providers constantly weigh the potential and actual benefits against harms in choosing the right intervention based on available evidence, taking extra caution when considering medications for which neonatal data is sparse. Such a balanced approach is to be recommended (Lim 2017). To better meet the needs of newborn sick infants, we need more thorough knowledge of the pharmacokinetics and pharmacodynamics, as well as the pharmacogenetics, in this specific immature population, which is in all respects very different from older children (Allegaert 2013; Allegaert 2016).
A recent review of pediatric perioperative controlled trials published between 2008 and 2018 reported that outcomes related to patient comfort, including pain management, were the most frequent domain across age groups beyond infancy, while clinical variables such as cardiorespiratory or medication‐related adverse events were the most common outcome for neonates and infants under 60 weeks of age (Muhly 2020). The review also pointed out that the youngest age group of neonates and infants under 60 weeks of age were significantly under‐represented in perioperative trials (Muhly 2020). This could be due to the higher perioperative risk of morbidity and mortality in neonates compared to older children (Kuan 2020), as well as to neonatal pharmacokinetics, which is not yet well characterized (Euteneuer 2020). The present reality is that optimal pain management in newborns is yet to be achieved, with further primary studies and updated systematic reviews needed for this unique age group.
Description of the intervention
For mild‐to‐moderate pain, the use of non‐pharmacological strategies (e.g. non‐nutritive sucking, swaddling, facilitated tucking, kangaroo care, music therapy, multi‐sensorial stimulation, acupuncture) with or without oral sucrose should always be considered (Bucsea 2019). For moderate‐to‐severe pain, as in the postoperative setting, opioids have traditionally been used, but they have several side effects such as respiratory depression, hypotension, constipation, as well as development of tachyphylaxis and abstinence (Kinoshita 2020).
Morphine, fentanyl, and remifentanil are the opioids most often used during neonatal intensive care, whereas the fentanyl derivatives, alfentanil and sufentanil, are less frequently used. These opioids have varying pharmacokinetic and pharmacodynamic profiles and should optimally be administered in an individualized way according to the need, clinical state, and expected course of hospitalization. Fentanyl and remifentanil are administered intravenously in very sick infants, whereas morphine can be administered by both intravenous and oral routes.
Morphine has the longest duration of onset, half‐life, and elimination time, followed by fentanyl and remifentanil (Thigpen 2019; Van Gonge 2018; Ziesenitz 2018). Remifentanil is a short‐acting opioid with ultra‐rapid onset and very fast elimination profile, thus very suitable for rapid painful procedures such as tracheal intubations (McPherson 2018). Pharmacodynamic studies on opioids report hypotension as the most common adverse effect (Thigpen 2019). Several larger studies have questioned the effects of opioids and reported on negative outcomes (Anand 2004; Hall 2005; Simons 2003). There are accumulating data on the negative impact of opioids on the structure and function of the developing brain, including neuronal apoptosis (McPherson 2015; Sanders 2013; Zwicker 2016).
How the intervention might work
After major surgery (e.g. cardiothoracic or brain surgery), opioids are indicated due to the associated rapid onset of action (typically less than five minutes), and a moderate duration of action (four to five hours). However, drugs such as methadone (preferably given intravenously) are more likely to exhibit an accentuated duration of action, particularly due to their slow elimination. The decision to initiate or replace opioids in neonates should rely cautiously on parameters of age, body weight, and both hepatic and renal function, as neonates tend to have immature metabolism during the first two to four weeks of life compared to older infants and children (Hong 2010; Van der Marel 2007). Morphine is unusual among opioids in that it requires an age‐adjusted dose regimen. In neonates, morphine is administered in a starting lower dose of 50 mcg/kg per hour for a two‐hour loading period, followed by 10 mcg/kg per hour, with regular neonate assessment to examine clinical progression and response (Anand 2004). Taking into account the limited literature on the other opioid‐class representatives (fentanyl, sufentanil and alfentanil), fewer problems regarding their pharmacodynamic and pharmacokinetic features have been observed, as these drugs undergo expedited renal clearance in comparison to morphine. When neonates have been on continuous or intermittent use of any opioid‐class drug for fewer than three days, and in the absence of severe pain, a complete and abrupt cessation is usually recommended (Balda 2019). However, for treatment over longer periods, a gradual withdrawal is advised, in order to minimize potential effects from abstinence syndrome. Besides the analgesic effects of opioids, euphoria and systemic effects (respiratory or cardiovascular) may also be correlated with their use. Additionally, it is noteworthy that the use of opioids in neonates might be linked to adverse effects ‐ including hypotension, bradycardia, and chest wall rigidity ‐ and can create tolerance over time (Anand 2006; Mitchell 2000).
In addition to opiate painkillers, other pharmacological interventions (such as traditional non‐opioid analgesics and sedative medications) play an important role in post‐surgical pain control among neonates (Silva 2007). It has been suggested that opioids can be combined with other drugs to achieve a balanced analgesic status among neonates suffering from postoperative pain. Most commonly used for control of mild pain or as co‐adjuvants in inflammatory processes, nonsteroidal anti‐inflammatory drugs (NSAIDs) act by inhibiting circulating cyclo‐oxygenase enzymes (I and II), thereupon diminishing inflammatory biomarkers throughout peripheral targets (Antonucci 2009). For instance, intermittent and intravenous acetaminophen (up to 48 hours after surgery) appears to intensify pain relief when used in combination with morphine or fentanyl for most major surgeries, and impact positively on decreasing opioid‐related side effects, such as abstinence syndrome (Hong 2010). Wong and colleagues have referred to this as the ‘opioid‐sparing effect’ of co‐adjuvants (Wong 2013). Their research has shown that neonates who received continuous acetaminophen as the primary choice of analgesia required less morphine and, significantly, had fewer adverse effects (Wong 2013). Furthermore, a growing literature describes potential synergic action from the use of ketorolac in combination with opioids, mainly because of ketorolac's prominent safety and adequate pain control outcomes (Dawkins 2009; Moffett 2006). Several advantages associated with the use of NSAIDs have been described, but the most important benefits are regarding their safety (low hepatotoxicity and nephrotoxicity), reduction of gastrointestinal disorders, as well as improvement in ventilation parameters (Mather 1992). Along with acetaminophen and NSAIDs, ketamine has also been suggested to decrease postoperative pain and opioid consumption (Zhu 2017). Ketamine has anxiolytic, analgesic, and amnestic effects, with few cardiovascular and respiratory effects (Carter 2017; Saarenmaa 2001).
In addition to pharmacological interventions, the establishment of an adequate environment, including reducing noise and light, has been suggested to reduce neonatal pain in a holistic way (Anand 2007).
Why it is important to do this review
Based on previous systematic reviews (Cochrane Reviews and non‐Cochrane reviews), the American Academy of Pediatrics has highlighted both the conflicting findings and lack of findings published in recent years about the use of opioids for analgesia in neonates (American Academy of Pediatrics 2016). Some particular populations have been widely evaluated for the use of opioids, such as mechanically ventilated neonates (Bellù 2021), and those requiring non‐emergency intubation (Ayed 2017). It has become evident that inadequate pain management in early human life, besides causing neuropsychological impairment, can be related to neuronal apoptosis, which directly impacts human neurodevelopment (Pacifici 2014; Schiller 2018). Therefore, the assessment of the contemporary practice of analgesic and sedative procedures is of utmost importance, especially for infants in substantial pain during the postoperative period. A systematic review of opioids for postoperative pain in neonates is called for to summarize concrete evidence from existing literature, provide updated guidance for clinical practice, as well as to determine current gaps that entail additional clinical research. The use of different regimens to administer systemic opioids for postoperative pain in neonates is assessed in a separate ongoing Cochrane Review (Kinoshita 2021a).
Objectives
To evaluate the benefits and harms of systemic opioid analgesics in neonates who underwent surgery on all‐cause mortality, pain, and significant neurodevelopmental disability compared to no intervention, placebo, non‐pharmacological interventions, different types of opioids, or other drugs.
Methods
Criteria for considering studies for this review
Types of studies
We included prospective randomized controlled trials (RCTs), quasi‐RCTs, cluster‐RCTs and cross‐over RCTs.
Types of participants
We included preterm and term infants of a postmenstrual age (PMA) up to 46 weeks and 0 days, irrespective of their gestational age at birth, receiving opioids following neonatal surgery where the surgery was performed in the operating room under general anesthesia (e.g. hernia repair surgery) or in the neonatal ward for minor surgery (e.g. patent ductus arteriosus ligation, surgery for retinopathy of prematurity, positioning of surgical drainage for air leak, thoracocentesis, placement of reservoir, or peritoneal dialysis for acute kidney failure).
We excluded:
infants receiving opioids during mechanical ventilation for respiratory morbidity;
infants receiving opioids pre‐intubation;
infants receiving opioids for procedural pain;
infants treated for neonatal abstinence syndrome; and
infants undergoing hemodialysis.
Types of interventions
We included studies on any opioids (e.g. morphine, diamorphine, fentanyl, alfentanil, sufentanil, pethidine, meperidine, codeine) following neonatal surgery. The following acceptable comparisons were included.
Comparison 1: opioids versus no treatment or placebo.
Comparison 2: opioids versus non‐pharmacological intervention (oral sugar solution, skin‐to‐skin contact, music exposure, non‐nutritive sucking, swaddling, etc.).
Comparison 3: head‐to‐head comparisons of different opioids (e.g. morphine versus fentanyl).
Comparison 4: opioids versus other analgesics (e.g. acetaminophen, N‐methyl‐D‐aspartate (NMDA) receptor antagonists (e.g. ketamine), and sedatives (e.g. benzodiazepines such as midazolam)).
We included any systemic route of administration (e.g. enteral, rectal, and intravenous).
We excluded spinal administration (i.e. intrathecal, epidural, caudal), intraosseous infusion, nerve blocks or wound infusions.
We included studies where the interventions were started during surgery, if their administration was continued postoperatively.
Studies comparing different regimens of the same opioid are included in the ongoing Cochrane Review, 'Systemic opioids regimens for postoperative pain in neonates' (Kinoshita 2021a).
Types of outcome measures
Outcome measures do not form part of the eligibility criteria.
Primary outcomes
Pain assessed with validated methods during the administration of selected drugs. The following scales were developed to assess pain, fulfill validity and reliability criteria for newborn infants (term and preterm on mechanical ventilation for any respiratory disease) when critically reviewed (Giordano 2019): NIPS (Lawrence 1983); Premature Infant Pain Profile (PIPP) (Stevens 1996); COMFORTneo (Van Dijk 2009); and Neonatal Pain, Agitation and Sedation Scale (N‐PASS) (Hummel 2008).
Major neurodevelopmental disability: cerebral palsy; developmental delay (Bayley Scales of Infant Development ‐ Mental Development Index Edition II (BSID‐MDI‐II; Bayley 1993), Bayley Scales of Infant and Toddler Development ‐ Edition III Cognitive Scale (BSITD‐III) (Bayley 2005), or Griffiths Mental Development Scale ‐ General Cognitive Index (GCI) (Griffiths 1954; Griffiths 1970), assessment greater than two standard deviations (SDs) below the mean); intellectual impairment (intelligence quotient (IQ) greater than two SDs below the mean); blindness (vision less than 6/60 in both eyes); or sensorineural deafness requiring amplification (Jacobs 2013). We assessed data on children aged 18 to 24 months and aged three to five years separately.
Cognitive and educational outcomes in children more than five years old.
All‐cause mortality during initial hospitalization.
Secondary outcomes
Retinopathy of prematurity (ROP) in infants examined (all stages (stage 1 or greater) and severe (defined as stage 3 or greater)) (ICCROP 2005).
Intraventricular hemorrhage (IVH; all (grade 1 or 2) or severe (grade 3 or greater) on cranial ultrasound, as per Papile classification) (Papile 1978).
All‐cause neonatal mortality (death until postnatal day 28).
Episodes of bradycardia defined as a fall in heart rate of more than 30% below the baseline or less than 100 beats per minute for 10 seconds or longer.
Hypotension requiring medical therapy (vasopressors or fluid boluses).
Periventricular leukomalacia (PVL) (any grade (grade 1 or greater), on the basis of ultrasound or magnetic resonance imaging) (De Vries 1992).
Necrotizing enterocolitis (NEC) (modified Bell stage 2/3; Walsh 1986).
-
Bronchopulmonary dysplasia/chronic lung disease:
28 days (NIH 1979);
36 weeks' postmenstrual age (Jobe 2001);
physiological definition (Walsh 2004).
Constipation defined as a delay in defecation sufficient to cause significant distress to the infant.
Focal gastrointestinal perforation.
Duration of mechanical ventilation (days).
Duration of oxygen supplementation (days).
Hospital stay (days).
Time to full enteral feeding (days).
Cost of neonatal care.
Search methods for identification of studies
We used the criteria and standard methods of Cochrane and Cochrane Neonatal (see the Cochrane Neonatal search strategy for specialized register). We searched for errata or retractions for included studies published in full text on PubMed (www.ncbi.nlm.nih.gov/pubmed).
Electronic searches
The timeline for this publication was disrupted by the COVID‐19 pandemic and staffing issues at the Cochrane Neonatal editorial base. As a result, publication of this review has been delayed, and the literature search is more than one year old. We will endeavor to undertake an updated search within the next calendar year.
We conducted a comprehensive search including: the Cochrane Central Register of Controlled Trials (CENTRAL 2021, Issue 5) in the Cochrane Library; MEDLINE via PubMed (1966 to 14 May 2021); and CINAHL (Cumulative Index to Nursing and Allied Health Literature; 1982 to 14 May 2021). We searched clinical trials databases, conference proceedings, and the reference lists of retrieved articles for RCTs and quasi‐RCTs (searched to 14 May 2021). We used Cochrane Neonatal's search strategy for neonates and RCTs (see Appendix 1 for the full search strategies for each database). We did not apply any language restrictions.
We searched clinical trials registries for ongoing or recently completed trials. We searched the World Health Organization’s International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/search/en/, searched to 14 May 2021), and the United States' National Library of Medicine’s ClinicalTrials.gov (clinicaltrials.gov, searched to 14 May 2021), via Cochrane CENTRAL. Additionally, we searched the ISRCTN Registry for any unique trials not found through the Cochrane CENTRAL search (searched to 14 May 2021).
Searching other resources
We also reviewed the reference lists of all identified articles for relevant articles not located in the primary search.
Data collection and analysis
We collected information regarding the method of randomization, blinding, intervention, stratification, and whether the trial was single or multicenter for each included study. We noted information regarding trial participants including birth weight, gestational age, number of participants, modality of administration and dose of opioids. We analyzed the clinical outcomes noted above in Types of outcome measures.
Selection of studies
We used Cochrane’s Screen4Me workflow to help assess the search results. Screen4Me comprises three components: known assessments – a service that matches records in the search results to records that have already been screened in Cochrane Crowd and labeled as an 'RCT' or as 'Not an RCT'; the RCT classifier – a machine learning model that distinguishes RCTs from non‐RCTs; and, if appropriate, Cochrane Crowd (https://crowd.cochrane.org) – Cochrane’s citizen science platform where the Crowd help to identify and describe health evidence.
For more information about Screen4Me, please visit: https://community.cochrane.org/organizational-info/resources/resources-groups/information-specialists-portal/crs-videos-and-quick-reference-guides#Screen4Me. Detailed information regarding evaluations of the Screen4Me components can be found in the following publications: Marshall 2018; Noel‐Storr 2020; Noel‐Storr 2021; Thomas 2020.
We included all randomized, quasi‐randomized, cluster‐randomized and cross‐over controlled trials fulfilling our inclusion criteria. Two review authors (IJBN; KS) reviewed the results of the search and independently selected studies for inclusion. We resolved any disagreements through discussion or, when necessary, by involving a third review author.
We recorded the selection process in sufficient detail to complete a PRISMA flow diagram and 'Characteristics of excluded studies' table (Moher 2009).
Data extraction and management
Two review authors (MK, KS) independently extracted data using a data extraction form integrated with a modified version of the Cochrane Effective Practice and Organisation of Care Group data collection checklist (Cochrane EPOC Group 2017). We piloted the form within the review team using a sample of included studies.
We extracted these characteristics from each included study:
administrative details: study author(s); published or unpublished; year of publication; year in which study was conducted; presence of vested interest; details of other relevant papers cited;
study: study design; type, duration, and completeness of follow‐up (e.g. greater than 80%); country and location of study; informed consent; ethics approval;
participants: sex, birth weight, gestational age, number of participants;
interventions: initiation, dose, and duration of administration; and
outcomes as mentioned above under Types of outcome measures.
We resolved any disagreements through discussion. We described ongoing studies identified by our search detailing the primary author, research question(s), methods, and outcome measures, together with an estimate of the reporting date and reported them in the 'Characteristics of ongoing studies' table.
We planned to contact study investigators or authors for clarification should any queries arise (e.g. discrepancies in the definitions of the outcomes in the trials and under Types of outcome measures), or in cases for which additional data were required. Two review authors (MK, IJBN) used the Cochrane statistical tool for data entry (Review Manager 2020). We planned to replace any standard error of the mean (SEM) with the corresponding SD; however, this was not necessary.
Assessment of risk of bias in included studies
Two review authors (MK, KS) independently assessed the risk of bias (low, high, or unclear) of all included trials using the Cochrane Risk of bias tool for the following domains (Higgins 2011).
Sequence generation (selection bias).
Allocation concealment (selection bias).
Blinding of participants and personnel (performance bias).
Blinding of outcome assessment (detection bias).
Incomplete outcome data (attrition bias).
Selective reporting (reporting bias).
Any other bias.
We resolved any disagreements through discussion or, if necessary, by consulting a third review author (IJBN). See Appendix 2 for a more detailed description of risk of bias for each domain.
Measures of treatment effect
We performed the statistical analyses using Review Manager 5 software (Review Manager 2020). We planned to summarize the data in a meta‐analysis; however, this was not conducted because no more than one study reported the same outcome within the same comparison.
Dichotomous data
For dichotomous data, we presented results using risk ratios (RR) and risk differences (RD) with 95% confidence intervals (CIs). We planned to calculate the number needed to treat for an additional beneficial outcome (NNTB), or number needed to treat for an additional harmful outcome (NNTH) with 95% CIs, however, there was not a statistically significant reduction (or increase) in RD.
Continuous data
For continuous data, we used the mean difference (MD) when outcomes were measured in the same way between trials. We planned to use the standardized mean difference (SMD) to combine trials that measured the same outcome but used different methods, however, this was not the case. Where trials reported continuous data as median and interquartile range (IQR) and data passed the test of skewness, we planned to convert the median to a mean and estimate the standard deviation as IQR/1.35.
Unit of analysis issues
The unit of analysis was the participating infant in individually randomized trials, and an infant was considered only once in the analysis. The participating neonatal unit or section of a neonatal unit or hospital was the unit of analysis in cluster‐randomized trials. We planned to analyze them using an estimate of the intracluster correlation coefficient (ICC) derived from the trial (if possible), or from a similar trial, or from a study with a similar population, as described in Section 16.3.6 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2020), however no cluster‐randomized trials were included. If we had used ICCs from a similar trial or from a study with a similar population, we planned to report this and conduct a sensitivity analysis to investigate the effect of variation in the ICC.
If we had identified both cluster‐randomized trials and individually randomized trials, we would only combine the results from both if there was little heterogeneity between the study designs, and the interaction between the effect of the intervention and the choice of randomization unit was considered to be unlikely.
In the event that we had identified cross‐over trials, in which the reporting of continuous outcome data precludes paired analysis, we would not include these data in a meta‐analysis, in order to avoid a unit of analysis error. If carry‐over effects were thought to exist, and where sufficient data existed, we would only include data from the first period in the analysis (Higgins 2021).
We planned to acknowledge any possible heterogeneity in the randomization unit and perform a sensitivity analysis to investigate possible effects of the randomization unit.
Dealing with missing data
Where feasible, we intended to carry out analysis on an intention‐to‐treat basis for all outcomes. Whenever possible, we analyzed all participants in the treatment group to which they were randomized, regardless of the actual treatment received. If we identified important missing data (in the outcomes) or unclear data, we would request the missing data by contacting the original investigators. We would make explicit the assumptions of any methods used to deal with missing data. We would perform sensitivity analyses to assess how sensitive results were to reasonable changes in the undertaken assumptions. We would address the potential impact of missing data on the findings of the review in the Discussion section.
Assessment of heterogeneity
We planned to estimate the treatment effects of individual trials and examine heterogeneity among trials by inspecting the forest plots and quantifying the impact of heterogeneity using the I2 statistic (Deeks 2020), however, no meta‐analysis was conducted. We planned to grade the degree of heterogeneity as:
0% to 40% might not represent important heterogeneity;
30% to 60% may represent moderate heterogeneity;
50% to 90% may represent substantial heterogeneity;
more than 75% may represent considerable heterogeneity.
If we had noted statistical heterogeneity (I2 > 50%), we would explore the possible causes (e.g. differences in study quality, participants, intervention regimens, or outcome assessments).
Assessment of reporting biases
We intended to conduct a comprehensive search for eligible studies and be alert for duplication of data. If we identified 10 or more trials for meta‐analysis, we would assess possible publication bias by inspection of a funnel plot. If we uncovered reporting bias that could, in the opinion of the review authors, introduce serious bias, we would conduct a sensitivity analysis to determine the effect of including and excluding these studies in the analysis.
Data synthesis
We planned to perform meta‐analysis using Review Manager 5 if we identified multiple studies that we considered to be sufficiently similar (Review Manager 2020). For categorical outcomes, we would calculate the typical estimates of RR and RD, each with its 95% CI. For continuous outcomes, we would calculate the MD or the SMD, each with its 95% CI. We would use a fixed‐effect model to combine data where it was reasonable to assume that studies were estimating the same underlying treatment effect. If we judged meta‐analysis to be inappropriate, we would analyze and interpret individual trials separately. If there was evidence of clinical heterogeneity, we would try to explain this based on the different study characteristics and subgroup analyses. In the end, meta‐analysis could not be done, because the studies were grouped in separate comparisons, or reported different outcomes.
Subgroup analysis and investigation of heterogeneity
Tests for subgroup differences in effects should be interpreted with caution given the potential for confounding with other study characteristics and the observational nature of the comparisons (see Section 10.11.2 Cochrane handbook version six). In particular, subgroup analyses with fewer than five studies per category are unlikely to be adequate to ascertain valid differences in effects and we planned to not highlight these in our results. We planned to conduct stratified meta‐analysis and a formal statistical test for interaction to examine subgroup differences that could account for effect heterogeneity (e.g. Cochran’s Q test, meta‐regression) (Borenstein 2013; Higgins 2020), however no meta‐analysis was conducted.
Given the potential differences in the intervention effectiveness related to gestational age (extremely preterm infants are more vulnerable), duration and timing of opioids administration (which might affect the outcomes), type of surgery (more invasive surgery is likely to require additional pharmacological management) and presence of co‐interventions (which might interact with opioids), we planned to conduct subgroup comparisons to see if the intervention was more effective for the following groups for subgroup analysis where data were available.
Gestational age (GA): term; moderately preterm (32 to 36 weeks' GA); very preterm (less than 32 weeks' GA).
Duration of opioids administration: up to 72 hours after surgery; beyond 72 hours.
Studies where the administration was started during the surgery; after the surgery.
Surgery performed in the operating room under general anesthesia; surgery in the neonatal ward for minor surgery such as patent ductus arteriosus ligation, surgery for retinopathy of prematurity, positioning of surgical drainage for air leak, thoracocentesis or peritoneal dialysis for acute kidney failure.
Within studies that accepted the use of co‐interventions: studies where investigators allowed co‐interventions for pain management; and studies that obligated its use, as well as by the type of co‐interventions (corticosteroids or nonsteroidal anti‐inflammatory drugs).
We planned to restrict these analyses to the primary outcomes. However, we did not do so because no meta‐analysis was conducted.
Sensitivity analysis
Should we identify substantial heterogeneity, we would conduct sensitivity analysis to determine if the findings are affected by inclusion of only those trials considered to have used adequate methodology with a low risk of bias (selection and performance bias). We would report results of sensitivity analyses for primary outcomes only. However, we were unable to because no meta‐analysis was conducted.
Summary of findings and assessment of the certainty of the evidence
We used the GRADE approach, as outlined in the GRADE Handbook (Schünemann 2013), to assess the certainty of evidence for the following (clinically relevant) outcomes.
Pain assessed with validated methods during the administration of selected drugs.
Major neurodevelopmental disability in children aged 18 to 24 months: cerebral palsy, developmental delay assessment (greater than two standard deviations (SDs) below the mean), intellectual impairment (intelligence quotient (IQ) greater than two SDs below the mean), blindness (vision less than 6/60 in both eyes), or sensorineural deafness requiring amplification (Jacobs 2013).
Major neurodevelopmental disability (see above) in children aged three to five years.
Cognitive and educational outcomes in children more than five years old.
All‐cause mortality during initial hospitalization.
Severe (defined as stage 3 or greater) retinopathy of prematurity in infants examined.
Severe (grade 3 or greater) intraventricular hemorrhage (IVH) on cranial ultrasound.
Two review authors (MK, MB) independently assessed the certainty of the evidence for each of the outcomes above. We planned to include a Summary of Findings table for each of the specified comparison in Types of interventions, however we could include only three (Table 1; Table 2; Table 3), because no studies were included in the comparison opioids versus non‐pharmacological interventions. We considered evidence from RCTs as high certainty, downgrading the evidence one level for serious (or two levels for very serious) limitations based upon the following: design (risk of bias), consistency across studies, directness of the evidence, precision of estimates, and presence of publication bias.
We used the GRADEpro GDT Guideline Development Tool to create Summary of findings tables to report the certainty of the evidence.
The GRADE approach results in an assessment of the certainty of a body of evidence in one of the following four grades.
High: we are very confident that the true effect lies close to that of the estimate of the effect;
Moderate: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different;
Low: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect;
Very low: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect.
Results
Description of studies
See Characteristics of included studies; Characteristics of excluded studies; Characteristics of studies awaiting classification; Characteristics of ongoing studies.
Results of the search
The literature search that was run in May 2021 identified a total of 2457 potential studies. In assessing the studies, we used Cochrane’s Screen4Me workflow to help identify potential reports of randomized trials. The results of the Screen4Me assessment process can be seen in Figure 1. We then assessed the remaining 1516 records.
1.
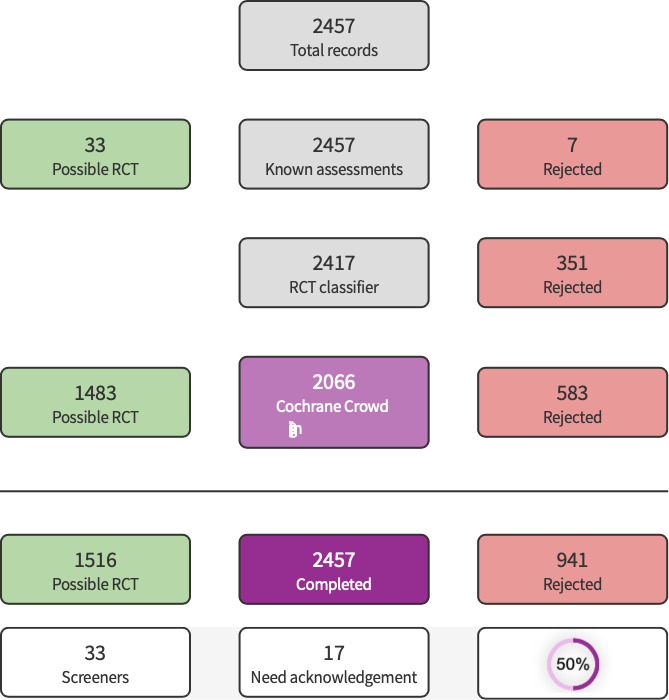
Screen4Me Summary Diagram
After screening, we assessed 33 full‐text articles (corresponding to 30 studies) for eligibility and included four trials (Figure 2). We excluded 19 studies, classified five studies as awaiting classification and classified two as ongoing studies.
2.

Flow diagram, after Screen4Me (Figure 1)
Included studies
Four studies were included in this review, enrolling a total of 331 neonates. Most studies considered patients undergoing large or medium surgical procedures (including major thoracic or abdominal surgery), who potentially required pain control through opioid administration after surgery. Most included trials did not enroll patients who were likely to need an additional surgical procedure 72 hours after the initial surgery, or had a history of neurological, pulmonary, hepatic, or renal dysfunction. Moreover, all randomized trials did not consider patients undergoing minor surgery (including inguinal hernia repair) and those individuals exposed to opioids before the beginning of the trial.
Of the four studies, one study was executed in Brazil (Alencar 2012), one in Australia (Olischar 2014), one in the United States of America (Eissa 2021), and one was a multicentric study carried out in The Netherlands (Ceelie 2013). The publication years of the primary studies ranged from 2012 to 2021. Information associated with financial sources were noted in all studies and, essentially, the studies' investigators did not have any relevant active role in influencing the design and conduct of the studies.
Baseline characteristics and types of interventions are shown in Table 4.
1. Primary characteristics of included trials.
| Study ID | N*** | Included age | Intervention (s) | Comparator (s) |
| Alencar 2012 | 171 | 0 to 28 days | Tramadol | Fentanyl |
| Ceelie 2013 | 74 | Post‐conceptual age of 36 1/7 week or older to 1 year of age | Continuous morphine | Intermittent paracetamol |
| Eissa 2021* | 15 | Patients under 2 years old | Tapentadol | Placebo |
| Olischar 2014** | 71 | Neonates born ≥ 32 weeks postmenstrual age | Tramadol | Placebo |
*This trial was composed of three different trials, of which only the third trial was a randomized controlled trial: Trial 1 included neonates from birth to < 2 years and considered only pharmacokinetic, safety and tolerability, and exploratory efficacy analyses; trial 2 enrolled preterm neonates (≥ 24 weeks gestational age) to < 2 years; and trial 3 enrolled neonates from birth to < 2 years old and considered efficacy and safety analyses, immediate rescue design with an alternative efficacy endpoint, and subgrouped patients for < 2 years' assessments. For additional information regarding the trial's differences, please, check the full text.
**This trial aimed to assess whether tramadol’s addition to standard analgesia resulted in earlier extubation or reduced analgesic/sedative requirements in postsurgical neonates. All neonates received morphine and 6‐hourly IV acetaminophen.
***The number of patients for each study is the number of infants that were randomized in the trial. In Alencar 2012 and Ceelie 2013, some infants were excluded after the randomization and thus not included in the analyses; therefore, the N used in our analyses may be different from that in the table above.
Alencar 2012 enrolled infants admitted to a neonatal intensive care unit for up to 28 days of life requiring major or minor surgeries. Patients were distributed into two groups of comparison, either to receive analgesia with fentanyl (1 to 2 μg/kg/h intravenously) or tramadol (0.1 to 0.2 mg/kg/h intravenously) in the first 72 hours of the postoperative period, stratified by surgical size and by patient’s gender. Ceelie 2013 included patients treated in a level 3 pediatric intensive care unit in The Netherlands, who were children younger than one year undergoing major thoracic or abdominal surgery. Remarkably, all patients received a loading dose of morphine 30 minutes before the end of the surgery, followed by continuous morphine or intermittent intravenous paracetamol up to 48 hours post‐surgery. On the other hand, Eissa 2021, who investigated the efficacy, safety, profile, and tolerability of tapentadol (either oral or by intravenous infusion), enrolled children from birth to less than two years of age, in three different trials. Lastly, Olischar 2014 included neonates under 32 weeks of post‐menstrual age that received either tramadol (2 mg/kg) or a placebo, six‐hourly for up to five days post‐surgery in addition to morphine and intravenous acetaminophen.
Regarding outcomes, included trials considered a wide variety of primary and secondary outcomes. For instance, Alencar 2012 and Ceelie 2013 have focused on the disclosure of baseline data and the number of adverse effects associated with each administered drug, but also have displayed hormonal and metabolic concentrations within the comparison groups (including cumulative doses). Furthermore, Alencar 2012 also presented the all‐cause mortality rate, the number of patients developing sepsis/necrotizing enterocolitis, and supplemental intra‐ and post‐operative data (including the number of infusions of vasoactive, arterial blood gas analysis, use of concomitant anesthetic agents, duration of procedures). Ceelie 2013 has shown outcomes associated with pain assessment (using two validated scores ‐ numeric rating scale and the COMFORT Behavior scale). Likewise, Eissa 2021 and Olischar 2014 showed baseline data and the number of adverse events experienced by each comparison group, but also presented data regarding pain assessment (Face, Legs, Activity, Cry, and Consolability (FLACC) scale and Pain Assessment Tool (PAT) score, respectively).
Based on our search, we observed two records published in registries webpages (ISRCTN99206122; Zeilmaker‐Roest 2018; see Characteristics of ongoing studies). ISRCTN99206122 aims to compare the effect of morphine and ketamine infusions in infants undergoing major surgery. Zeilmaker‐Roest 2018 outlined a protocol in which intravenous morphine is compared to intravenous paracetamol after cardiac surgery in neonates and infants.
Excluded studies
Excluded studies following full‐text screening are listed in Characteristics of excluded studies.
After the full‐text screening phase, we excluded 19 studies mainly because the studies were not primarily focused on neonates (wrong patient population [n = 15]), had a wrong intervention comparison (n = 3), or had a wrong study design (n = 1). Furthermore, five studies are currently awaiting classification.
Risk of bias in included studies
The overall risk of bias assessment for each study, including all domain evaluations and justifications for judgment, is displayed in the risk of bias section (Characteristics of included studies), on the right side of all forest plots and in Figure 3 and Figure 4. The overall quality of studies was good (Figure 3), as none of the studies had any high risk of bias for any of the items in the Cochrane Risk of bias tool.
3.

Risk of bias graph
4.

Risk of bias summary
Allocation
All four studies did not provide any details on allocation concealment. Randomization was judged to be adequate in two studies where the method of randomization was described: in both Ceelie 2013 and Olischar 2014, computer‐generated block randomization was used. Alencar 2012 and Eissa 2021 stated that patients were randomized but without further details regarding each specific method.
Blinding
Blinding of caregivers and assessors to the intervention was stated in all studies, except for Eissa 2021. In Eissa 2021, it was mentioned that the trial was double‐blind and the patients received either tapentadol or a matching placebo, but blinding of the assessors was not described. All other studies stated that the pharmacy had access to group allocation and prepared the drugs, thereby ensuring the blinding of other study participants.
Incomplete outcome data
In general, follow‐up was complete for all studies. In Alencar 2012, 171 patients were randomized to receive either intravenous continuous tramadol or fentanyl, and outcomes for 160 infants were reported after eight deaths and three re‐operations during the first 72 postoperative hours. In Ceelie 2013, 74 patients were randomized to receive intravenous intermittent paracetamol or continuous morphine, of which two infants in the paracetamol group and one infant in the morphine group ended up not receiving the drugs due to various reasons (i.e. withdrawal of informed consent, abnormal liver function, no surgery). In Eissa 2021, 23 patients were enrolled in the study, but eight were not randomized due to inclusion/exclusion criteria or consent withdrawal, leaving 15 patients to be randomized to receive either oral tapentadol or placebo. In Olischar 2014, 71 patients were randomized to receive either intravenous intermittent tramadol or placebo.
Selective reporting
Since most of the studies failed to clearly present that there were no relevant differences between outcomes in the study protocol and those reported in the published article, only one study was judged to be at low risk of bias (Eissa 2021).
Other potential sources of bias
In Alencar 2012, surgical anesthesia protocols as well as the decision‐making process to extubate and increase enteral feeding of patients were not standardized.
Effects of interventions
See: Table 1; Table 2; Table 3
Comparison 1: Opioids versus no treatment or placebo
Two studies are included in this comparison, comparing either tramadol with placebo (Olischar 2014), or tapentadol with placebo (Eissa 2021). See Table 1.
Primary outcomes
Pain assessed with validated methods during the administration of selected drugs
The included studies did not report this outcome.
Major neurodevelopmental disability
The included studies did not report this outcome.
Cognitive and educational outcomes in children more than five years old
The included studies did not report this outcome.
All‐cause mortality during initial hospitalization
One trial comparing tramadol with placebo (Olischar 2014), reported this outcome (RR 0.32, 95% CI 0.01 to 7.70; RD ‐0.03, 95% CI ‐0.10 to 0.05, 71 participants, 1 study; I² = not applicable, Analysis 1.1). The certainty of the evidence is very low because of imprecision of the estimate (downgraded by two levels) and limitations in study design (downgraded by one level). See Table 1.
1.1. Analysis.

Comparison 1: Tramadol versus no treatment or placebo, Outcome 1: All‐cause mortality during initial hospitalization
Secondary outcomes
Constipation
One trial comparing tapentadol with placebo (Eissa 2021), reported this outcome (RR 1.25, 95% CI 0.06 to 25.76; RD 0.09, 95% CI ‐0.23 to 0.41, 15 participants, 1 study; I² = not applicable, Analysis 2.1). the certainty of the evidence is very low because of imprecision of the estimate (downgraded by two levels) and limitations in study design (downgraded by one level).
2.1. Analysis.

Comparison 2: Tapentadol versus no treatment or placebo, Outcome 1: Constipation
The studies included within this comparison (Eissa 2021; Olischar 2014), did not report on: all‐cause neonatal mortality; episodes of bradycardia; hypotension requiring medical therapy; retinopathy of prematurity; intraventricular hemorrhage; periventricular leukomalacia; necrotizing enterocolitis; bronchopulmonary dysplasia; focal gastrointestinal perforation; duration of mechanical ventilation; duration of oxygen supplementation; hospital stay; time to full enteral feeding; cost of neonatal care.
Comparison 2: Opioids versus non‐pharmacological intervention (oral sugar solution, skin‐to‐skin contact, music exposure, non‐nutritive sucking, swaddling, etc.)
None of the studies were included in this comparison.
Comparison 3: Head‐to‐head comparisons of different opioids (e.g. morphine versus fentanyl)
One study comparing fentanyl with tramadol is included in this comparison (Alencar 2012). See Table 2.
Primary outcomes
Pain assessed with validated methods during the administration of selected drugs
The included study did not report this outcome.
Major neurodevelopmental disability
The included study did not report this outcome.
Cognitive and educational outcomes in children more than five years old
The included study did not report this outcome.
All‐cause mortality during initial hospitalization
One study comparing fentanyl with tramadol (Alencar 2012), reported this outcome (RR 0.99, 95% CI 0.59 to 1.64; RD 0.00, 95% CI ‐0.13 to 0.13, 171 participants, 1 study; I² = not applicable, Analysis 3.1). the certainty of the evidence is very low because of imprecision of the estimate (downgraded by two levels) and limitations in study design (downgraded by one level). See Table 2.
3.1. Analysis.

Comparison 3: Fentanyl versus tramadol, Outcome 1: All‐cause mortality during initial hospitalization
Secondary outcomes
Episodes of bradycardia
One study comparing fentanyl with tramadol (Alencar 2012), reported this outcome (RR 2.17, 95% CI 0.87 to 5.42; RD 0.09, 95% CI ‐0.01 to 0.19, 160 participants, 1 study; I² = not applicable, Analysis 3.2). the certainty of the evidence is very low because of imprecision of the estimate (downgraded by two levels) and limitations in study design (downgraded by one level).
3.2. Analysis.

Comparison 3: Fentanyl versus tramadol, Outcome 2: Episodes of bradycardia
The study included within this comparison (Alencar 2012), did not report on: all‐cause neonatal mortality; constipation; hypotension requiring medical therapy; retinopathy of prematurity; intraventricular hemorrhage; periventricular leukomalacia; necrotizing enterocolitis; bronchopulmonary dysplasia; focal gastrointestinal perforation; duration of mechanical ventilation; duration of oxygen supplementation; hospital stay; time to full enteral feeding; cost of neonatal care.
Comparison 4: Opioids versus other analgesics (e.g. acetaminophen), N‐methyl‐D‐aspartate (NMDA) receptor antagonists (e.g. ketamine), and sedatives (e.g. benzodiazepines such as midazolam)
One study comparing morphine with paracetamol is included in this comparison (Ceelie 2013). See Table 3.
Primary outcomes
Pain assessed with validated methods during the administration of selected drugs
One study comparing morphine with paracetamol (Ceelie 2013), reported this outcome (MD 0.10, 95% CI ‐0.85 to 1.05; 71 participants, 1 study; I² = not applicable, Analysis 4.1). the certainty of the evidence is very low because of imprecision of the estimate (downgraded by two levels) and limitations in study design (downgraded by one level). See Table 3.
4.1. Analysis.

Comparison 4: Morphine versus paracetamol, Outcome 1: Pain assessed with COMFORT
Major neurodevelopmental disability
The included study did not report this outcome.
Cognitive and educational outcomes in children more than five years old
The included study did not report this outcome.
All‐cause mortality during initial hospitalization
The included study did not report this outcome
Secondary outcomes
Episodes of bradycardia
One study comparing morphine with paracetamol (Ceelie 2013), reported this outcome (RR 1.01, 95% CI 0.38 to 2.71; RD 0.00, 95% CI ‐0.18 to 0.18, 71 participants, 1 study; I² = not applicable, Analysis 4.2). the certainty of the evidence is very low because of imprecision of the estimate (downgraded by two levels) and limitations in study design (downgraded by one level).
4.2. Analysis.

Comparison 4: Morphine versus paracetamol, Outcome 2: Episodes of bradycardia
Hypotension requiring medical therapy
One study comparing morphine with paracetamol (Ceelie 2013), reported no events for this outcome (RR not estimable; RD 0.00, 95% CI ‐0.05 to 0.05, 71 participants, 1 study; I² = not applicable, Analysis 4.3). the certainty of the evidence is very low because of imprecision of the estimate (downgraded by two levels) and limitations in study design (downgraded by one level).
4.3. Analysis.

Comparison 4: Morphine versus paracetamol, Outcome 3: Hypotension requiring medical therapy
The study included within this comparison (Ceelie 2013), did not report on: all‐cause neonatal mortality; constipation; retinopathy of prematurity; intraventricular hemorrhage; periventricular leukomalacia; necrotizing enterocolitis; bronchopulmonary dysplasia; focal gastrointestinal perforation; duration of mechanical ventilation; duration of oxygen supplementation; hospital stay; time to full enteral feeding; cost of neonatal care.
Discussion
Summary of main results
In this review, we included four studies with a total of 331 newborn infants: two studies compared opioids with placebo, either tramadol (Olischar 2014), or tapentadol (Eissa 2021); one study fentanyl with tramadol (Alencar 2012); and one study morphine with paracetamol (Ceelie 2013). No more than three outcomes were reported in these comparisons. No meta‐analyses could be performed. Amongst the primary outcomes of this review, mortality during initial hospitalization and pain scales were reported by two and one study, respectively. We identified no studies comparing opioids versus non‐pharmacological interventions. We are uncertain whether opioids reduce pain or mortality compared with placebo, other opioids or other drugs. No studies reported on major neurodevelopmental disability.
Overall completeness and applicability of evidence
We identified four studies that reported comparisons in 331 infants of systemic opioid regimens versus placebo, other opioid, or other analgesic, but no two studies assessed the effectiveness of opioids after surgery for a same comparison. Moreover, the majority of the outcomes of the review were not reported by the included studies. Therefore, we could not summarize the available evidence in a comprehensive manner due to the paucity of outcome data among the limited number of included studies. Evidence is insufficient to support or refute the effectiveness of opioids for postoperative pain management in neonates.
The objective of our review was to determine the effects of systemic opioid analgesics in neonates (term or preterm) undergoing surgery. In regard to addressing all relevant types of participants, the majority of the recruited infants were term neonates receiving surgery under general anesthesia that was considered to produce at least moderate pain requiring postoperative pain management (Alencar 2012; Ceelie 2013; Olischar 2014). Only one study (Olischar 2014), included more than a few preterm infants, but the majority of infants in each study were term. Thus, evidence is even more scarce concerning the use of opioids to manage postoperative pain in preterm neonates.
Quality of the evidence
Following the GRADE approach, the certainty of evidence for the few reported outcomes on postoperative systemic opioid administration was very low (See Table 1; Table 2; Table 3). Reasons for the downgrade were: limitations in study design (by one level) owing to the unclear risk of selection and reporting bias; imprecision (by two levels) owing to the small sample size, only one included study, and width of the confidence interval in each comparison. We did not use funnel plots to evaluate publication bias because there were fewer than 10 studies that met the inclusion criteria of this Cochrane Review.
Potential biases in the review process
Since this systematic review was conducted under the standard methodology of Cochrane Neonatal, we are confident that the literature search allowed inclusion of all relevant studies to summarize the currently available evidence on opioids versus non‐opioids (or another opioid) in postoperative infants. We did not apply any language restrictions and had one Korean study classified as awaiting classification (Hwang 1999).
Two studies (Ceelie 2013; Eissa 2021), included patients older than our criteria of preterm and term infants of postmenstrual age up to 46 weeks and 0 days, but we were unable to obtain study data specific to our review criteria.
Agreements and disagreements with other studies or reviews
We could not conduct a meta‐analysis, so our results are basically consistent with that of the included studies.
Two Cochrane Reviews published in 2020 (Ohlsson 2020; Romantsik 2020), have addressed opioid use for neonatal pain management after surgery, but only as comparison to another drug that was the main focus of each review: paracetamol in Ohlsson 2020 and clonidine in Romantsik 2020. Similar to our review, both reviews did not perform meta‐analysis due to limited data. A Cochrane Review assessing whether clonidine administered to newborn infants receiving mechanical ventilation included only one trial (Romantsik 2017). A recent Cochrane Review (Bellù 2021), compared the use of opioids with placebo or no intervention and another analgesic or sedative (including other opioids) in ventilated infants. Although the review targeted a different neonatal condition from our review, Bellù and colleagues similarly reported that they were unable to reach conclusions about the effect of opioids on pain and neurodevelopmental disability, which we have in common as primary outcomes.
Furthermore, several Cochrane Reviews and one non‐Cochrane review on opioids for neonatal pain management in various settings are under preparation (Ayed 2017; Kinoshita 2020; Kinoshita 2021a; Kinoshita 2021b; Pirlotte 2019). In these reviews, opioids are compared to placebo or no intervention, pharmacological interventions, and non‐pharmacological interventions to prevent or to treat procedural and postoperative pain.
Authors' conclusions
Implications for practice.
Limited evidence is available on opioid administration for postoperative pain in newborn infants compared to either placebo, other opioids, or paracetamol.
We are uncertain whether tramadol reduces mortality compared to placebo; none of the studies reported pain scores, major neurodevelopmental disability, cognitive and educational outcomes in children older than five years old, retinopathy of prematurity, or intraventricular hemorrhage. We are uncertain whether fentanyl reduces mortality compared to tramadol; none of the studies reported pain scores, major neurodevelopmental disability, cognitive and educational outcomes in children older than five years old, retinopathy of prematurity, or intraventricular hemorrhage. We are uncertain whether morphine reduces pain compared to paracetamol; none of the studies reported major neurodevelopmental disability, cognitive and educational outcomes in children more than five years old, all‐cause mortality during initial hospitalization, retinopathy of prematurity, or intraventricular hemorrhage. We identified no studies comparing opioids versus non‐pharmacological interventions.
Implications for research.
This systematic review highlights the need for large randomized controlled trials to evaluate the effectiveness of systemic opioid analgesics compared to placebo or no drug, non‐pharmacological intervention, other opioids or analgesics or sedatives in neonates undergoing surgery. Future trials should also enroll preterm infants as well as focus on specific comparisons to allow assessment of the intervention in the target population. There are various types of opioids used in the clinical setting, but it is probably most reasonable to first focus on the most commonly used morphine and fentanyl (Bellù 2021), to clarify their active role in postoperative pain management. If they are indeed effective in reducing postoperative pain and beneficial for critical outcomes, further comparisons of opioids with placebo would be deemed unnecessary. Neither beneficial nor harmful effects of postoperative use of opioids have been adequately addressed to date, and routine collection of critical outcomes such as pain, mortality, and neurodevelopmental disability is strongly called for. As neurodevelopmental consequences of neonatal management would take time to be recognized, recruited infants would need to be efficiently followed to obtain valuable data.
History
Protocol first published: Issue 5, 2021
Notes
Editorial note:
The timeline for this publication was disrupted by the COVID‐19 pandemic and staffing issues at the Cochrane Neonatal editorial base. As a result, publication of this review has been delayed, and the literature search is more than one year old (May 2021). We will endeavor to undertake an updated search within the next calendar year.
Acknowledgements
The methods section of this review is based on a standard template used by Cochrane Neonatal.
We would like to thank Cochrane Neonatal: Fiona Russell, Jane Cracknell and Michelle Fiander, Managing Editors; and Roger Soll and Bill McGuire, Co‐coordinating Editors, who provided editorial and administrative support. We thank Georg Schmölzer, Associate Editor, for feedback on the protocol.
Matthias Bank (Library and ICT services, Lund University) designed and ran the literature searches, and Carol Friesen, former Cochrane Neonatal Information Specialist, peer reviewed the searches.
We would like to thank the following two peer reviewers:
Dr Lauren Young, Consultant Neonatologist, Department of Neonatal Medicine, Birmingham Women's and Children's NHS Foundation Trust, Birmingham UK.
Elaine M Boyle, Professor of Neonatal Medicine, University of Leicester, Leicester, UK.
We would like to thank Malinee Laopaiboon and Pisake Lumbiganon (Cochrane Thailand) for their help in providing the full text of a trial published in a Thai journal.
We would like to acknowledge and thank the following people for their help in assessing the search results for this review via Cochrane’s Screen4Me workflow: Akhilanand Chaurasia, Anna Noel‐Storr, Shammas Mohammed, Ciara Gleeson, Mohammad Aloulou, Ana‐Marija Ljubenković, Eleanor McKean, Vighnesh Devulapalli, Fatima Assad Alagelli, Ashutosh Kumar Singh, Neetu Bhadra, Carmen La Cerra, Devesh Srivastava, Raluca Radu, Olivia Canie, Alejandro Ceballos Sandoval, Rubyath Binte Hasan.
We thank Anne Lethaby for copy editing this review.
Appendices
Appendix 1. Search strategy
Date of search: 14 May 2021
Pubmed
#1 (((infant, newborn[MeSH] OR newborn*[TIAB] OR "new born"[TIAB] OR "new borns"[TIAB] OR "newly born"[TIAB] OR baby*[TIAB] OR babies*[TIAB] OR premature[TIAB] OR prematurity[TIAB] OR preterm[TIAB] OR "pre term"[TIAB] OR “low birth weight”[TIAB] OR "low birthweight"[TIAB] OR VLBW[TIAB] OR LBW[TIAB] OR infan*[TIAB] OR neonat*[TIAB])))
#2 (((((morphine OR diamorphine OR fentanyl OR alfentanil OR sufentanil OR pethidine OR meperidine OR codeine OR methadone))) OR ("Narcotics"[Majr] OR "Analgesia"[Majr] OR sedation[Title/Abstract] OR opioid*[Title/Abstract] OR remifentanil)) OR (((((((("Morphine"[Mesh]) OR "Heroin"[Mesh]) OR "Fentanyl"[Mesh]) OR "Alfentanil"[Mesh]) OR "Sufentanil"[Mesh]) OR "Meperidine"[Mesh]) OR "Codeine"[Mesh]) OR "Methadone"[Mesh] OR “Remifentanil”[Mesh]))
#3 ("Surgical Procedures, Operative"[Mesh] OR surgery[TIAB] OR surgical[TIAB] OR "postoperat*"[TIAB] OR "post operat*"[TIAB] OR "postsurg*"[TIAB] OR "post surg*"[TIAB] OR operative[TIAB] OR operation*[TIAB] OR ligation*[TIAB] OR repair[TIAB])
#4 ((((randomized controlled trial [pt] OR controlled clinical trial [pt] OR randomized [tiab] OR placebo [tiab] OR drug therapy [sh] OR randomly [tiab] OR trial [tiab] OR groups [tiab])) NOT (animals[MH] NOT humans[MH])))
#5 #1 AND #2 AND #3 AND #4
Cochrane Library/CENTRAL via Wiley
#1 MeSH descriptor: [Infant, Newborn] explode all trees
#2 (infan* or newborn* or "new born" or "new borns" or "newly born" or neonat* or baby* or babies or premature or prematures or prematurity or preterm* or "pre term" or premies or "low birth weight" or "low birthweight" or VLBW or LBW or ELBW or NICU):ti,ab,kw (Word variations have been searched)
#3 (morphine OR diamorphine OR fentanyl OR alfentanil OR sufentanil OR pethidine OR meperidine OR codeine OR methadone OR remifentanil):ti,ab,kw (Word variations have been searched)
#4 (surgery OR surgical OR postoperat* OR "post operat*" OR postsurg* OR "post surg*" OR operative OR operation*):ti,ab,kw (Word variations have been searched)
#5 MeSH descriptor: [Surgical Procedures, Operative] explode all trees
#6 #1 OR #2
#7 #4 OR #5
#8 #3 AND #6 AND #7
CINAHL via EBSCOHost
#1 (infant or infants or infant’s or infantile or infancy or newborn* or "new born" or "new borns" or "newly born" or neonat* or baby* or babies or premature or prematures or prematurity or preterm or preterms or "pre term" or premies or "low birth weight" or "low birthweight" or VLBW or LBW)
#2 (morphine OR diamorphine OR fentanyl OR alfentanil OR sufentanil OR pethidine OR meperidine OR codeine OR methadone OR MH morphine OR MH diamorphine OR MH fentanyl OR MH alfentanil OR MH sufentanil OR MH pethidine OR MH meperidine OR MH codeine OR MH methadone OR MH remifentanil OR MJ narcotics OR MJ sedation OR MJ analgesia OR TI opioid* OR AB opioid*)
#3 (MH "Surgery, Operative+")
#4 surgery OR surgical OR postoperat* OR "post operat*" OR postsurg* OR "post surg*" OR operative OR operation*
#5 #3 OR #4
#6 (randomized controlled trial OR controlled clinical trial OR randomized OR randomised OR placebo OR clinical trials as topic OR randomly OR trial OR PT clinical trial)
#7 #1 AND #2 AND #5 AND #6
Appendix 2. 'Risk of bias' tool
We used the standard methods of Cochrane and Cochrane Neonatal to assess the methodological quality of the trials. For each trial, we sought information regarding the method of randomization, blinding, and reporting of all outcomes of all the infants enrolled in the trial. We assessed each criterion as being at a low, high, or unclear risk of bias. Two review authors separately assessed each study. We resolved any disagreements by discussion. We added this information to the 'Characteristics of included studies' table. We evaluated the following issues and entered the findings into the Risk of bias table.
1. Sequence generation (checking for possible selection bias). Was the allocation sequence adequately generated?
For each included study, we categorized the method used to generate the allocation sequence as:
low risk (any truly random process, e.g. random number table; computer random number generator);
high risk (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number); or
unclear risk.
2. Allocation concealment (checking for possible selection bias). Was allocation adequately concealed?
For each included study, we categorized the method used to conceal the allocation sequence as:
low risk (e.g. telephone or central randomization; consecutively numbered, sealed, opaque envelopes);
high risk (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth); or
unclear risk
3. Blinding of participants and personnel (checking for possible performance bias). Was knowledge of the allocated intervention adequately prevented during the study?
For each included study, we categorized the methods used to blind study participants and personnel from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or class of outcomes. We categorized the methods as:
low risk, high risk, or unclear risk for participants; and
low risk, high risk, or unclear risk for personnel.
4. Blinding of outcome assessment (checking for possible detection bias). Was knowledge of the allocated intervention adequately prevented at the time of outcome assessment?
For each included study, we categorized the methods used to blind outcome assessment. We assessed blinding separately for different outcomes or class of outcomes. We categorized the methods as:
low risk for outcome assessors;
high risk for outcome assessors; or
unclear risk for outcome assessors.
5. Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations). Were incomplete outcome data adequately addressed?
For each included study and for each outcome, we described the completeness of data including attrition and exclusions from the analysis. We noted whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomized participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported or supplied by the trial authors, we re‐included missing data in the analyses. We categorized the methods as:
low risk (< 20% missing data);
high risk (≥ 20% missing data); or
unclear risk.
6. Selective reporting bias. Are reports of the study free of the suggestion of selective outcome reporting?
For each included study, we described how we investigated the possibility of selective outcome reporting bias and what we found. For studies in which study protocols were published in advance, we compared prespecified outcomes versus outcomes eventually reported in the published results. If the study protocol was not published in advance, we contacted study authors to gain access to the study protocol. We assessed the methods as:
low risk (where it is clear that all of the study's prespecified outcomes and all expected outcomes of interest to the review have been reported);
high risk (where not all the study's prespecified outcomes have been reported; one or more reported primary outcomes were not prespecified outcomes of interest and are reported incompletely and so cannot be used; the study fails to include results of a key outcome that would have been expected to have been reported); or
unclear risk.
7. Other sources of bias. Was the study apparently free of other problems that could put it at high risk of bias?
For each included study, we described any important concerns we had about other possible sources of bias (e.g. whether there was a potential source of bias related to the specific study design or whether the trial was stopped early due to some data‐dependent process). We assessed whether each study was free of other problems that could put it at risk of bias as:
low risk;
high risk;
unclear risk.
If needed, we planned to explore the impact of the level of bias by undertaking sensitivity analyses.
Data and analyses
Comparison 1. Tramadol versus no treatment or placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 All‐cause mortality during initial hospitalization | 1 | 71 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.01, 7.70] |
Comparison 2. Tapentadol versus no treatment or placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Constipation | 1 | 15 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.06, 25.76] |
Comparison 3. Fentanyl versus tramadol.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 All‐cause mortality during initial hospitalization | 1 | 171 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.59, 1.64] |
| 3.2 Episodes of bradycardia | 1 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.17 [0.87, 5.42] |
Comparison 4. Morphine versus paracetamol.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Pain assessed with COMFORT | 1 | 71 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.85, 1.05] |
| 4.2 Episodes of bradycardia | 1 | 71 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.38, 2.71] |
| 4.3 Hypotension requiring medical therapy | 1 | 71 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Alencar 2012.
| Study characteristics | ||
| Methods | RCT, parallel groups | |
| Participants | 171 infants admitted to a referral pediatric hospital in Brazil
|
|
| Interventions |
|
|
| Outcomes | Primary: time from the end of the surgical procedure until extubation (hours) Secondary: time to reach 100 ml/kg of enteral feeding (hours); pain evaluation during the first 72 hours after surgery (two pain scales: CRIES and NFCS). Pain scales were applied every 2 hours for the first 24 postoperative hours and every 4 hours for the following 48 hours. |
|
| Notes | Authors had nothing to declare. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation not reported Quote: "For randomisation, four opaque envelopes were prepared (male/female infants with major/minor surgeries). Each envelope contained 10 blocks of four patients randomly ordered as ‘fentanyl’ or ‘tramadol’. The central pharmacy performed the randomisation". |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported Quote: "For randomisation, four opaque envelopes were prepared (male/female infants with major/minor surgeries). Each envelope contained 10 blocks of four patients randomly ordered as ‘fentanyl’ or ‘tramadol’. The central pharmacy performed the randomisation". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Though it was not mentioned that the two preparations were indistinguishable, it was plausible (because of the color, opacity etc. of tramadol and fentanyl). Quote: "The phials of fentanyl (50 μg/mL) and tramadol (50 mg/mL) were diluted in 9 mL of normal saline. Tramadol solution was further diluted in 9 mL of normal saline. Therefore, 0.2 mL/h of the solution was equivalent to 1 μg/h of fentanyl and 0.1 mg/h of tramadol." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The decisions regarding extubation and management of enteral feeding were managed by attending neonatologists who were blind to group allocation of the patients. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients seem accounted for. |
| Selective reporting (reporting bias) | Unclear risk | All planned outcomes reported, however, primary and secondary outcomes were switched (protocol versus manuscript) |
| Other bias | Unclear risk | Lack of standardization of surgical anesthesia; the study design left to the attending neonatologists the decision to extubate and increase enteral feeding of randomized patients. |
Ceelie 2013.
| Study characteristics | ||
| Methods | RCT, parallel groups | |
| Participants | 71 infants admitted to a level 3 pediatric intensive care unit in Netherlands
|
|
| Interventions |
|
|
| Outcomes | Primary: cumulative morphine dose (i.e. the sum of the intraoperative loading dose, the morphine study dose, and the rescue morphine doses) Secondary: morphine rescue dose (microgram/kg) in the first 48 postoperative hours; number of extra rescue morphine doses and infusions; number of patients receiving rescue doses; pain scores (NRS‐11, COMFORT‐B); morphine‐related adverse effects (need for mechanical ventilation or/and reintubation, apnea, naloxone administration, bradycardia, hypotension, seizures, gastrointestinal adverse effects, urinary retention) |
|
| Notes | Authors had nothing to declare. Funding source: ZonMw Priority Medicines for Children grant |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients had an equal probability of assignment to study groups. Stratified randomization was used in combination with random permuted blocks." |
| Allocation concealment (selection bias) | Unclear risk | Quote: "A hospital pharmacist carried out computer randomization in advance, and codes were safely stored. (...) A new randomization schedule was computer generated by the same pharmacist. Only the pharmacist had access to group allocation during the study period, for preparation of study medication." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "When patients were randomized to receive paracetamol (30 mg/kg per day in 4 doses), a placebo infusion of normal saline was administered continuously at the same rate as an equivalent morphine infusion. When randomized to receive morphine (...), normal saline was administered 4 times daily as placebo in a volume similar to the intravenous paracetamol dose. Placebos could not be distinguished from the active study drug in color, odor, or viscosity." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The clinical personnel (blinded as per the previous point) were outcome assessors. Also, "The pharmacist and the statistician performed this interim evaluation after inclusion of 20 patients; the pharmacist, statistician, and investigators remained blinded." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data reported for all assessed infants |
| Selective reporting (reporting bias) | Unclear risk | Some of the secondary outcomes planned in the trial registry (www.trialregister.nl/trial/1378) were not reported, for example, saliva cortisol levels |
| Other bias | Low risk | None |
Eissa 2021.
| Study characteristics | ||
| Methods | RCT, parallel groups | |
| Participants | 15 infants enrolled at 7 trial sites in a global setting
|
|
| Interventions |
|
|
| Outcomes | Efficacy: total amount of supplemental opioid analgesic medication administered via nurse‐controlled analgesia pump within the first 12 to 24 hours after the first dose of trial medication; time to first administration of supplemental opioid analgesic medication; changes from baseline in pain intensity (FLACC scale) over the treatment period; ratings regarding the patients' overall improvement Safety/tolerability: adverse events |
|
| Notes | Authors were paid or employed by Grünenthal GmbH. Funding source: Grünenthal GmbH |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported Quote: "Patients were randomly allocated (2:1) to receive either tapentadol OS or a matching placebo OS". |
| Allocation concealment (selection bias) | Unclear risk | Not reported Quote: "Patients were randomly allocated (2:1) to receive either tapentadol OS or a matching placebo OS". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Reported as double‐blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Reported as double‐blinded; unclear if outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All included patients accounted for in the results |
| Selective reporting (reporting bias) | Low risk | All planned outcomes/endpoints were reported. |
| Other bias | Low risk | None |
Olischar 2014.
| Study characteristics | ||
| Methods | RCT, parallel groups | |
| Participants | 71 infants admitted to a primary newborn surgical unit in Australia
|
|
| Interventions |
|
|
| Outcomes | Primary: time to extubation (hours) Secondary: analgesic and sedative medications (morphine, midazolam) received during the five days measured as duration of administration, number of boluses, total mg/kg; hourly pain scores; adverse events |
|
| Notes | Authors had nothing to declare regarding the performance of the study. Funding source: Murdoch Childrens Research Institute |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "An independent statistician performed computer‐generated (STATA 10; 2007, Stata Statistical Software, StataCorp, TX, USA) block randomization with variable block sizes, stratified by PMA: 32–36 weeks and > 36 weeks." |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "An independent pharmacist prepared study drug in 5‐mL Terumo [Philippines] syringes labeled with a study number. Each syringe contained 5 mL volume of either 50 mg tramadol in saline or saline alone (placebo) [the two being indistinguishable]". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessment seemed to be blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data appeared to be reported. |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | Low risk | None |
CRIES: Crying; Requires increased oxygen administration; Increased vital signs; Expression; Sleeplessness ECG: electrocardiogram FLACC: Face, Legs, Activity, Cry, Consolability IV: intravenous NFCS: Neonatal Facial Coding System NRS‐11: numeric rating scale‐11 PMA: post‐menstrual age RCT: randomized controlled trial
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Academy of Neonatal Nursing 2013 | Wrong study design |
| Chhabra 2005 | Wrong patient population |
| Chiaretti 1997 | Wrong patient population |
| Chiaretti 2000 | Wrong patient population |
| ChiCTR‐TRC‐13002993 | Wrong intervention |
| Dake 1997 | Wrong intervention |
| Fenlon 2007 | Wrong patient population |
| IRCT20180726040601N | Wrong intervention |
| ISRCTN86816150 | Wrong patient population |
| Jo 2011 | Wrong patient population |
| Karl 2012 | Wrong patient population |
| Kururattapun 1986 | Wrong patient population |
| NCT00386269 | Wrong patient population |
| Pappas 2003 | Wrong patient population |
| Pestieau 2011 | Wrong patient population |
| Tree‐Trakarn 1985 | Wrong patient population |
| VanStraaten 1994 | Wrong patient population |
| Waterworth 1974 | Wrong patient population |
| Xiang 2014 | Wrong patient population |
Characteristics of studies awaiting classification [ordered by study ID]
CTRI/2020/03/023882.
| Methods | Unclear |
| Participants | Weight more than two kilograms, hemodynamically stable neonates with tracheoesophageal fistula |
| Interventions | Central neuraxial block‐caudal epidural block: Caudal epidural in neonates posted for tracheoesophageal fistula surgeries. 1 mL/kg bolus 0.2 percentage ropivacaine followed by 0.1 mL/kg/hr infusion for 72 hrs One more group received standard intravenous fentanyl analgesia during surgery and after surgery for 72 hrs, dose of 1 ug/kg |
| Outcomes | Primary outcome: Time for extubation; postoperative pain score Secondary outcome: Pain score (NIPS); recovery profile postoperatively |
| Notes |
De Alencar 2009.
| Methods | abstract not available |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Hwang 1999.
| Methods | Randomized trial |
| Participants | Neonates admitted to the NICU of Kyungpook University Hospital, requiring surgery (sample size = 12) or mechanical ventilation |
| Interventions | After operation, one group received fentanyl and the other was given saline. |
| Outcomes | Behavioral distress using postoperative comfort scores, heart rate, blood pressure and blood glucose were evaluated before and after operation. Cortisol concentration and beta endorphin were measured before and at the end of operation and at 60 minutes after fentanyl infusion. In neonates undergoing surgery, fentanyl infusion diminished the elevation of postoperation heart rate and blood glucose (P < 0.05) and induced the improvement of postoperation comfort scores (P < 0.05). |
| Notes | Translation from Korean not obtained |
IRCT20171218037936N2.
| Methods | Unclear |
| Participants | Neonates with gestational age of 36 weeks and more with thoracic surgery; neonates with gestational age of 36 weeks and more with abdominal surgery |
| Interventions | IV acetaminophen 10 mg/kg every 6 hours up to 48 hours |
| Outcomes | Primary outcome: Pain score, NIPS (Neonatal Infant Pain Scale) |
| Notes |
NCT01094522.
| Methods | Unclear |
| Participants | Neonates, infants and children after cardiac surgery |
| Interventions | Fentanyl will be administered for intraoperative analgesia by the treating anesthesiologist in a dose range of 25‐50 mcg/kg. No other intraoperative opioids will be given. Subjects will receive intravenous methadone or morphine ("study drug") delivered by an initial IV "bolus" injection followed by a nurse‐administered patient controlled analgesia (PCA) device for postoperative pain for a period of 24 hours. The initial dose of study drug will be 0.2 mg/kg IV administered following admission to the ICU after surgery. The study drug will then be given at a dose of 0.035 mg/kg IV as needed q30 min via PCA. The study drug may be increased or decreased in increments of 20‐25% according to the discretion of the investigator as needed to maintain a FLACC pain assessment tool < 4. Subjects will also receive lorazepam 0.025 mg/kg IV q2 hr as needed for agitation as indicated by specific criteria. The study drug will be discontinued after 24 hours to facilitate "washout" sampling and determination of elimination half‐life. Beginning at 24 hours, fentanyl will be used for analgesia at an equianalgesic dose to be determined by the investigator based upon the current PCA "study drug" dose. |
| Outcomes | Primary endpoints: Pharmacokinetics of methadone and morphine, including its metabolites (morphine‐3‐glucuronide and morphine‐6‐glucuronide). Secondary endpoints: Pain scores (FLACC) during the 24 hours study period; amount of study drug administered during the 24‐hour dosing period; changes in heart rate, systemic arterial blood pressure and laboratory test values |
| Notes |
FLACC: Face, Legs, Activity, Cry, Consolability ICU: intensive care unit| IV: intravenously NICU: neonate intensive care unit NIPS: Neonatal Infant Pain Scale PCA: patient controlled analgesia
Characteristics of ongoing studies [ordered by study ID]
ISRCTN99206122.
| Study name | Randomized, blinded, comparison of the respiratory depressant effects of morphine and S(+) ketamine infusions when used to provide postoperative analgesia in infants undergoing major surgery |
| Methods | Controlled study (unclear if randomized) |
| Participants | 70 infants aged less than 60 weeks post‐conceptual age undergoing elective or urgent abdominal surgery who would not be expected to require postoperative artificial ventilation |
| Interventions | Ketamine or morphine by direct continuous infusion |
| Outcomes | Primary outcome: Total number of respiratory depression episodes measured over the first 24 hours after return to the ward following surgery as primary clinical relevant variable Secondary outcome: Not provided at time of registration |
| Starting date | 11 October 2004 |
| Contact information | Richmond House, dhmail@doh.gsi.org.uk |
| Notes |
Zeilmaker‐Roest 2018.
| Study name | Intravenous morphine versus intravenous paracetamol after cardiac surgery in neonates and infants: a study protocol for a randomized controlled trial |
| Methods | Multicenter, randomized controlled trial at four level‐3 pediatric intensive care units (ICUs) in the Netherlands and Belgium |
| Participants | Children who are 0‐36 months old; sample size: n = 208 |
| Interventions | Either intermittent intravenous paracetamol or continuous intravenous morphine up to 48 h postoperatively. Morphine will be available as rescue medication for both groups. |
| Outcomes | Validated pain and sedation assessment tools |
| Starting date | Not available |
| Contact information | Gerdien A Zeilmaker‐Roest, g.zeilmaker@erasmusmc.nl |
| Notes |
ICU: intensive care unit
Differences between protocol and review
We made the following changes to the protocol (Kinoshita 2021)
Following editorial feedback, we edited the order of the outcomes Types of outcome measures to follow the order in the section "Summary of findings and assessment of the certainty of the evidence".
Contributions of authors
Conceiving the protocol: MK, KS, MB
Designing the review: MK, KS, MB
Coordinating the review: MB
Data collection for the review: MK, KS, IJBN
Screening search results: MK, KS, IJBN
Organizing retrieval of papers: MK, KS, IJBN
Screening retrieved papers against eligibility criteria: MK, KS, IJBN
Appraising quality of papers: MK, KS, IJBN
Extracting data from papers: MK, KS, IJBN
Writing to authors of papers for additional information: MK, KS, IJBN
Data management for the review: MK, MB
Entering data into RevMan: MK, KS
Analysis of data: MK, KS, MB
Interpretation of data: MK, KS, MB
Providing a methodological and a clinical perspective: MB
Writing the protocol: MK, KS, IJBN, MB
Sources of support
Internal sources
-
Institute for Clinical Sciences, Lund University, Lund, Sweden
MB is employed by this organization.
External sources
-
Vermont Oxford Network, USA
Cochrane Neonatal Reviews are produced with support from Vermont Oxford Network, a worldwide collaboration of health professionals dedicated to providing evidence‐based care of the highest quality for newborn infants and their families.
-
Region Skåne, Skåne University Hospital, Lund University and Region Västra Götaland, Sweden, Sweden
Cochrane Sweden is supported from Region Skåne, Skåne University Hospital Lund University and Region Västra Götaland.
-
Erasmus + Programme of the European Union, Other
PhD scholarship of MK was funded by the Erasmus + Programme of the European Union (Framework Agreement number: 2013‐0040).
-
Fundació Clínic per a la Recerca Biomèdica, Spain
MK was employed by this non‐profit organization for research projects not related to the review.
Declarations of interest
MK has no interests to declare.
KS has no interests to declare.
IJBN has no interests to declare.
MB is an Associate Editor for the Cochrane Neonatal Group. However, he had no involvement in the editorial processing of this protocol.
New
References
References to studies included in this review
Alencar 2012 {published data only}
- Alencar AJ, Sanudo A, Sampaio VM, Gois RP, Benevides FA, Guinsburg R. Efficacy of tramadol versus fentanyl for postoperative analgesia in neonates. Archives of Disease in Childhood. Fetal and Neonatal Edition 2012;97(1):F24-9. [DOI: 10.1136/adc.2010.203851] [PMID: ] [DOI] [PubMed] [Google Scholar]
- NCT00713726. Tramadol versus fentanyl for post-operative analgesia in newborn infants. clinicaltrials.gov/show/NCT00713726 (first received 11 July 2008).
Ceelie 2013 {published data only}
- Ceelie I, De Wildt SN, Van Dijk M, Van den Berg MM, Van den Bosch GE, Duivenvoorden HJ, et al. Effect of intravenous paracetamol on postoperative morphine requirements in neonates and infants undergoing major noncardiac surgery: a randomized controlled trial. JAMA 2013;309(2):149-54. [DOI: 10.1001/jama.2012.148050] [PMID: ] [DOI] [PubMed] [Google Scholar]
Eissa 2021 {published data only}
- Eissa A, Tarau E, Beuter C, Radic T, Watson E, Sohns M, et al. Tapentadol for the treatment of moderate-to-severe acute pain in children under the age of two years. Journal of Pain Research 2021;14:229-48. [DOI: 10.2147/JPR.S269530] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Olischar 2014 {published data only}
- Olischar M, Palmer GM, Orsini F, Davidson AJ, Perkins EJ, Lee KJ, et al. The addition of tramadol to the standard of i.v. acetaminophen and morphine infusion for postoperative analgesia in neonates offers no clinical benefit: a randomized placebo-controlled trial. Paediatric Anaesthesia 2014;24(11):1149-57. [DOI: 10.1111/pan.12477] [PMID: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Academy of Neonatal Nursing 2013 {published data only}
- Anonymous. Effect of intravenous paracetamol on postoperative morphine requirements for major noncardiac surgery. Academy of Neonatal Nursing 2013;32(3):224-5. [DOI: 10.1891/0730-0832.323.221] [DOI] [PubMed] [Google Scholar]
Chhabra 2005 {published data only}
- Chhabra A, Pandey R, Khandelwal M, Subramaniam R, Gupta S. Anesthetic techniques and postoperative emesis in pediatric strabismus surgery. Regional Anesthesia and Pain Medicine 2005;30(1):43-7. [DOI: 10.1016/j.rapm.2004.08.023] [DOI] [PubMed] [Google Scholar]
Chiaretti 1997 {published data only}
- Chiaretti A, Simeone E, Langer A, Butera G, Piastra M, Tortorolo L, et al. Analgesic efficacy of ketorolac and fentanyl in pediatric intensive care. Pediatria Medica e Chirurgica [Medical and Surgical Pediatrics] 1997;19(6):419-24. [PubMed] [Google Scholar]
Chiaretti 2000 {published data only}
- Chiaretti A, Viola L, Pietrini D, Piastra M, Savioli A, Tortorolo L, et al. Preemptive analgesia with tramadol and fentanyl in pediatric neurosurgery. Child's Nervous System 2000;16(2):93-9; discussion 100. [DOI: 10.1007/s003810050019] [DOI] [PubMed] [Google Scholar]
ChiCTR‐TRC‐13002993 {published data only}
- ChiCTR-TRC-13002993. Effect of caudal levobupivacaine mixed with ketamine and fentanyl on MAC-BAR of sevoflurane and postoperative analgesia in Chinese neonates. who.int/trialsearch/Trial2.aspx?TrialID=ChiCTR-TRC-13002993 [currently link not active] (first received: date not available).
Dake 1997 {published data only}
- Dake P, French L. Analgesia during circumcision. Journal of Family Practice 1997;45(2):100-1. [PubMed] [Google Scholar]
Fenlon 2007 {published data only}
- Fenlon S, Somerville N. Comparison of codeine phosphate and morphine sulphate in infants undergoing cleft palate repair. Cleft Palate-Craniofacial Journal 2007;44(5):528-31. [DOI: 10.1597/06-206.1] [DOI] [PubMed] [Google Scholar]
IRCT20180726040601N {published data only}
- IRCT20180726040601N1. Comparing the time of extubation, postoperative apnea incidence, and the quality of perioperative analgesia in infants scheduled for pyloromyotomy - intravenous bolus fentanyl vs rectal acetaminophen. www.who.int/trialsearch/Trial2.aspx?TrialID=IRCT20180726040601N1 [currently link not active] (first received: date not available).
ISRCTN86816150 {published data only}
- ISRCTN86816150. Double-blind randomised study into the efficacy of codeine phosphate analgesia after cleft palate repair in infants. www.who.int/trialsearch/Trial2.aspx?TrialID=ISRCTN86816150 [currently link not active] (first received: date not available).
Jo 2011 {published data only}
- Jo YY, Hong JY, Choi EK, Kil HK. Ketorolac or fentanyl continuous infusion for post-operative analgesia in children undergoing ureteroneocystostomy. Acta Anaesthesiologica Scandinavica 2011;55(1):54-9. [DOI: 10.1111/j.1399-6576.2010.02354.x] [DOI] [PubMed] [Google Scholar]
Karl 2012 {published data only}
- Karl HW, Tyler DC, Miser AW. Controlled trial of morphine vs hydromorphone for patient-controlled analgesia in children with postoperative pain. Pain Medicine 2012;13(12):1658-9. [DOI: 10.1111/j.1526-4637.2012.01496.x] [DOI] [PubMed] [Google Scholar]
Kururattapun 1986 {published data only}
- Kururattapun S, Prakanrattana U. Nalbuphine versus morphine for postoperative analgesia in critically ill patients. Chotmaihet Thangphaet [Journal of the Medical Association of Thailand] 1986;69(4):210-5. [PubMed] [Google Scholar]
NCT00386269 {published data only}
- NCT00386269. Double blind randomized study Into the efficacy of codeine phosphate analgesia after cleft palate repair in infants. clinicaltrials.gov/show/NCT00386269 (first received 11 October 2006).
Pappas 2003 {published data only}
- Pappas AL, Fluder EM, Creech S, Hotaling A, Park A. Postoperative analgesia in children undergoing myringotomy and placement equalization tubes in ambulatory surgery. Anesthesia and Analgesia 2003;96(6):1621-4. [DOI: 10.1213/01.ane.0000064206.51296.1d] [DOI] [PubMed] [Google Scholar]
Pestieau 2011 {published data only}
- Pestieau SR, Quezado ZM, Johnson YJ, Anderson JL, Cheng YI, McCarter RJ, et al. The effect of dexmedetomidine during myringotomy and pressure-equalizing tube placement in children. Paediatric Anaesthesia 2011;21(11):1128-35. [DOI: 10.1111/j.1460-9592.2011.03615.x] [DOI] [PubMed] [Google Scholar]
Tree‐Trakarn 1985 {published data only}
- Tree-Trakarn T, Pirayavaraporn S. Postoperative pain relief for circumcision in children: comparison among morphine, nerve block, and topical analgesia. Anesthesiology 1985;62(4):519-22. [DOI: 10.1097/00000542-198504000-00027] [DOI] [PubMed] [Google Scholar]
VanStraaten 1994 {published data only}
- Van Straaten V, Neidecker J, Brule P, Caillet JB, Laroux MC, Assoune P, et al. Haemodynamic responses to fentanyl or sufentanil anaesthesia in infants undergoing open heart surgery. Journal of Cardiothoracic and Vascular Anesthesia 1994;8(3 SUPPL. 2):192. [DOI: 10.1016/1053-0770(94)90594-0] [DOI] [Google Scholar]
Waterworth 1974 {published data only}
- Waterworth TA. Pentazocine (Fortral) as postoperative analgesic in children. Archives of Disease in Childhood 1974;49(6):488-90. [DOI: 10.1136/adc.49.6.488] [DOI] [PMC free article] [PubMed] [Google Scholar]
Xiang 2014 {published data only}
- Xiang K, Cai H, Song Z. Comparison of analgesic effects of remifentanil and fentanyl NCA after pediatric cardiac surgery. Journal of Investigative Surgery 2014;27(4):214-8. [DOI: 10.3109/08941939.2013.879968] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
CTRI/2020/03/023882 {published data only}
- CTRI/2020/03/023882. Providing analgesia for neonates during surgery and after surgery, for better outcome. trialsearch.who.int/Trial2.aspx?TrialID=CTRI/2020/03/023882 (first received 11 March 2020).
De Alencar 2009 {published data only}
- De Alencar AJ, Guinsburg R, De Almeida PC, Do Carmo FLM, Sampaio VMR. Post-operative analgesia in newborn infants: a randomized controlled clinical trial of tramadol versus fentanyl. In: Pediatric Academic Societies Annual Meeting; 2009 May 2-5; Baltimore MD, United States. 2009.
Hwang 1999 {published data only}
- Hwang WK, Kim HM. Pain reduction effects of continuous fentanyl infusion to intensive care neonates. Journal of the Korean Pediatric Society, 1999;42(5): 657-65. [LINK: https://www.e-cep.org/journal/view.php?number=1999420508] [Google Scholar]
IRCT20171218037936N2 {published data only}
- IRCT20171218037936N2. Comparison of the analgesic effect of intravenous acetaminophen and fentanyl (opiate analgesic) in thoracic and abdominal surgery of neonates. trialsearch.who.int/Trial2.aspx?TrialID=IRCT20171218037936N2 (first received 11 March 2019).
NCT01094522 {published data only}
- NCT01094522. Measuring the amount of methadone or morphine in the blood of neonates, infants & children after cardiac surgery. clinicaltrials.gov/show/NCT01094522 (first received 29 March 2010).
References to ongoing studies
ISRCTN99206122 {published data only}
- ISRCTN99206122. Randomised, blinded, comparison of the respiratory depressant effects of morphine and S(+) ketamin infusions when used to provide postoperative analgesia in infants undergoing major surgery. www.who.int/trialsearch/Trial2.aspx?TrialID=ISRCTN99206122 (first received 30 September 2005).
Zeilmaker‐Roest 2018 {published data only}
- NTR5448. Morphine intravenous vs. paracetamol intravenous after cardiac surgery in neonates and infants. https://trialsearch.who.int/Trial2.aspx?TrialID=NTR5448 (first received 1 September 2015).
- Zeilmaker-Roest GA, Van Rosmalen J, Van Dijk M, Koomen E, Jansen NJ, Kneyber MC, et al. Intravenous morphine versus intravenous paracetamol after cardiac surgery in neonates and infants: a study protocol for a randomized controlled trial. Trials 2018;19(1):318. [DOI: 10.1186/s13063-018-2705-5] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Allegaert 2013
- Allegaert K, Tibboel D, Van den Anker J. Pharmacological treatment of neonatal pain: in search of a new equipoise. Seminars in Fetal & Neonatal Medicine 2013;18(1):42-7. [DOI: 10.1016/j.siny.2012.10.001] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Allegaert 2016
- Allegaert K, Van den Anker JN. Neonatal pain management: still in search for the Holy Grail. International Journal of Clinical Pharmacology and Therapeutics 2016;54(7):514-23. [DOI: 10.5414/CP202561] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
American Academy of Pediatrics 2016
- Keels E, Sethna N, Watterberg KL, Cummings JJ, Benitz WE, Eichenwald EC, et al, Committee on Fetus and Newborn and Section on Anesthesiology and Pain Medicine. Prevention and management of procedural pain in the neonate: an update . Pediatrics 2016;137(2):e20154271. [DOI: 10.1542/peds.2015-4271] [pediatrics.aappublications.org/content/pediatrics/137/2/e20154271.full.pdf] [DOI] [PubMed] [Google Scholar]
Anand 1998
- Anand KJ. Clinical importance of pain and stress in preterm neonates. Biology of the Neonate 1998;73(1):1-9. [DOI: 10.1159/000013953] [PMID: ] [DOI] [PubMed] [Google Scholar]
Anand 2000
- Anand KJ, Scalzo FM. Can adverse neonatal experiences alter brain development and subsequent behavior? Biology of the Neonate 2000;77(2):69-82. [DOI: 10.1159/000014197] [PMID: ] [DOI] [PubMed] [Google Scholar]
Anand 2004
- Anand KJ, Hall RW, Desai N, Shephard B, Bergqvist LL, Young TE, et al, NEOPAIN Trial Investigators Group. Effects of morphine analgesia in ventilated preterm neonates: primary outcomes from the NEOPAIN randomised trial. Lancet 2004;363(9422):1673-82. [DOI: 10.1016/S0140-6736(04)16251-X] [PMID: ] [DOI] [PubMed] [Google Scholar]
Anand 2006
- Anand KJ, Hall RW. Pharmacological therapy for analgesia and sedation in the newborn. Archives of Disease in Childhood. Fetal and Neonatal edition 2006;91:F448-53 [Erratum in Archives of Disease in Childhood 2007 Mar;92(2):F156. Dosage error in article text]. [DOI: 10.1136/adc.2005.082263] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Anand 2007
- Anand KJ. Pharmacological approaches to the management of pain in the neonatal intensive care unit. Journal of Perinatology 2007;27(Suppl 1):S4-11. [DOI: 10.1038/sj.jp.7211712] [PMID: ] [DOI] [PubMed] [Google Scholar]
Antonucci 2009
- Antonucci R, Fanos V. NSAIDs, prostaglandins and the neonatal kidney. Journal of Maternal-Fetal & Neonatal Medicine 2009;22(Suppl 3):23-6. [DOI: 10.1080/14767050903184447] [PMID: ] [DOI] [PubMed] [Google Scholar]
Ayed 2017
- Ayed M, Shah VS, Taddio A. Premedication for non-urgent endotracheal intubation for preventing pain in neonates. Cochrane Database of Systematic Reviews 2017, Issue 2. Art. No: CD012562. [DOI: 10.1002/14651858.CD012562] [DOI] [Google Scholar]
Balda 2019
- Balda RC, Guinsburg R. Evaluation and treatment of pain in the neonatal period [Avaliação e tratamento da dor no período neonatal]. Revista Pediátrica 2019;9(1):43-52. [DOI: 10.25060/residpediatr-2019.v9n1-13] [DOI] [Google Scholar]
Bayley 1993
- Bayley N. Bayley Scales of Infant Development–II. San Antonio, Texas: Psychological Corporation, 1993. [Google Scholar]
Bayley 2005
- Bayley N. Bayley Scales of Infant and Toddler Development. 3rd edition. San Antonio, TX: Harcourt Assessment, 2005. [Google Scholar]
Bellù 2021
- Bellù R, Romantsik O, Nava C, De Waal KA, Zanini R, Bruschettini M. Opioids for newborn infants receiving mechanical ventilation. Cochrane Database of Systematic Reviews 2021, Issue 3. Art. No: CD013732. [DOI: 10.1002/14651858.CD013732.pub2] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Borenstein 2013
- Borenstein M, Higgins JPT. Meta-analysis and subgroups. Prevention Science 2013;14:134-43. [DOI: 10.1007/s11121-013-0377-7] [PMID: ] [DOI] [PubMed] [Google Scholar]
Bucsea 2019
- Bucsea O, Pillai RR. Non-pharmacological pain management in the neonatal intensive care unit: managing neonatal pain without drugs. Seminars in Fetal & Neonatal Medicine 2019;24(4):101017. [DOI: 10.1016/j.siny.2019.05.009] [PMID: ] [DOI] [PubMed] [Google Scholar]
Carter 2017
- Carter BS, Brunkhorst J. Neonatal pain management. Seminars in Perinatology 2017;41(2):111-6. [DOI: 10.1053/j.semperi.2016.11.001] [PMID: ] [DOI] [PubMed] [Google Scholar]
Cochrane EPOC Group 2017
- Cochrane Effective Practice and Organisation of Care (EPOC) Group. Data extraction and management. EPOC resources for review authors, 2017. epoc.cochrane.org/resources/epoc-resources-review-authors (accessed prior to 20 April 2021).
Dawkins 2009
- Dawkins TN, Barclay CA, Gardiner RL, Krawczeski CD. Safety of intravenous use of ketorolac in infants following cardiothoracic surgery. Cardiology in the Young 2009;19(1):105-8. [DOI: 10.1017/S1047951109003527] [PMID: ] [DOI] [PubMed] [Google Scholar]
Deeks 2020
- Deeks JJ, Higgins JP, Altman DG. Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.1 (updated September 2020). Cochrane, 2020. Available from www.training.cochrane.org/handbook.
Derieg 2016
- Derieg S. An overview of perioperative care for pediatric patients. Association of Operating Room Nurses Journal 2016;104(1):4-10. [DOI: 10.1016/j.aorn.2016.05.001] [PMID: ] [DOI] [PubMed] [Google Scholar]
De Vries 1992
- De Vries LS, Eken P, Dubowitz LM. The spectrum of leukomalacia using cranial ultrasound. Behavioural Brain Research 1992;49(1):1-6. [DOI: 10.1016/s0166-4328(05)80189-5] [PMID: ] [DOI] [PubMed] [Google Scholar]
Duerden 2014
- Duerden EG, Grunau RE, Guo T, Foong J, Pearson A, Au-Young S, et al. Early procedural pain is associated with regionally-specific alterations in thalamic development in preterm neonates. The Journal of Neuroscience 2014;38(4):878-86. [DOI: 10.1523/JNEUROSCI.0867-17.2017] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Eriksson 2019
- Eriksson M, Campbell-Yeo M. Assessment of pain in newborn infants. Seminars in Fetal and Neonatal Medicine 2019;24(4):101003. [DOI: 10.1016/j.siny.2019.04.003] [PMID: ] [DOI] [PubMed] [Google Scholar]
Euteneuer 2020
- Euteneuer JC, Mizuno T, Fukuda T, Zhao J, Setchell KD, Muglia LJ, et al. Model-informed Bayesian estimation improves the prediction of morphine exposure in neonates and infants. Therapeutic Drug Monitoring 2020;42(5):778-86. [DOI: 10.1097/FTD.0000000000000763.] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fitzgerald 1989
- Fitzgerald M, Millard C, McIntosh N. Cutaneous hypersensitivity following peripheral tissue damage in newborn infants and its reversal with topical anaesthesia. Pain 1989;39(1):31-6. [DOI: 10.1016/0304-3959(89)90172-3] [PMID: ] [DOI] [PubMed] [Google Scholar]
Giordano 2019
- Giordano V, Edobor J, Deindl P, Wildner B, Goeral K, Steinbauer P, et al. Pain and sedation scales for neonatal and pediatric patients in a preverbal stage of development: a systematic review. JAMA Pediatrics 2019;173(12):1186-97. [DOI: 10.1001/jamapediatrics.2019.3351] [PMID: ] [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADEpro GDT. Version accessed 11 September 2020. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015. Available at gradepro.org.
Griffiths 1954
- Griffiths R. The Abilities of Babies: A Study of Mental Measurement. London: University of London Press, 1954. [Google Scholar]
Griffiths 1970
- Griffiths R. The Abilities of Young Children: A Comprehensive System of Mental Measurement For The First Eight Years. London: Child Development Research Center, 1970. [Google Scholar]
Hall 2005
- Hall RW, Kronsberg SS, Barton BA, Kaiser JR, Anand KJ, NEOPAIN Trial Investigators Group. Morphine, hypotension, and adverse outcomes among preterm neonates: who's to blame? Secondary results from the NEOPAIN trial. Pediatrics 2005;115(5):1351-9. [DOI: 10.1542/peds.2004-1398] [PMID: ] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Altman DG, Sterne JA, Cochrane Statistical Methods Group and the Cochrane Bias Methods Group. Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1.
Higgins 2020
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane, 2020. Available from www.training.cochrane.org/handbook.
Higgins 2021
- Higgins JP, Eldridge S, Li T (editors). Chapter 23: Including variants on randomized trials. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
Hong 2010
- Hong JY, Kim WO, Koo BN, Cho JS, Suk EH, Kil HK. Fentanyl-sparing effect of acetaminophen as a mixture of fentanyl in intravenous parent-/nurse-controlled analgesia after pediatric ureteroneocystostomy. Anesthesiology 2010;113(3):672-7. [DOI: 10.1097/ALN.0b013e3181e2c34b] [PMID: ] [DOI] [PubMed] [Google Scholar]
Hummel 2008
- Hummel P, Puchalski M, Creech SD, Weiss MG. Clinical reliability and validity of the N-PASS: neonatal pain, agitation and sedation scale with prolonged pain. Journal of Perinatology 2008;28(1):55-60. [DOI: 10.1038/sj.jp.7211861] [PMID: ] [DOI] [PubMed] [Google Scholar]
ICCROP 2005
- International Committee for the Classification of Retinopathy of Prematurity. The international classification of retinopathy of prematurity revisited. Archives of Ophthalmology 2005;123(7):991-9. [DOI: 10.1001/archopht.123.7.991] [PMID: ] [DOI] [PubMed] [Google Scholar]
Jacobs 2013
- Jacobs SE, Berg M, Hunt R, Tarnow-Mordi WO, Inder TE, Davis PG. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database of Systematic Reviews 2013, Issue 1. Art. No: CD003311. [DOI: 10.1002/14651858.CD003311.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jobe 2001
- Jobe AH, Bancalari E. Bronchopulmonary dysplasia. American Journal of Respiratory and Critical Care Medicine 2001;163(7):1723-9. [DOI: 10.1164/ajrccm.163.7.2011060] [PMID: ] [DOI] [PubMed] [Google Scholar]
Kinoshita 2020
- Kinoshita M, Stempel K, Borges do Nascimento IJ, Vejayaram DN, Norman E, Bruschettini M. Opioids and alpha-2-agonists for analgesia and sedation in newborn infants: protocol of a systematic review. Systematic Reviews 2020;9(1):183. [DOI: 10.1186/s13643-020-01436-0] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kinoshita 2021a
- Kinoshita M, Styrmisdóttir L, Borges do Nascimento IJ, Bruschettini M. Systemic opioid regimens for postoperative pain in neonates. Cochrane Database of Systematic Reviews 2021, Issue 5. Art. No: CD015016. [DOI: 10.1002/14651858.CD015016] [DOI] [Google Scholar]
Kinoshita 2021b
- Kinoshita M, Olsson E, Borys F, Bruschettini M. Opioids for procedural pain in neonates. Cochrane Database of Systematic Reviews 2021, Issue 12. Art. No: CD015056. [DOI: 10.1002/14651858.CD015056] [DOI] [Google Scholar]
Kuan 2020
- Kuan CC, Shaw SJ. Anesthesia for major surgery in the neonate. Anesthesiology Clinics 2020;38(1):1-18. [DOI: 10.1016/j.anclin.2019.10.001] [PMID: ] [DOI] [PubMed] [Google Scholar]
Lawrence 1983
- Lawrence J, Alcock D, McGrath P, Kay J, MacMurray SB, Dulberg C. The development of a tool to assess neonatal pain. Neonatal Network 1993;12:59-66. [PMID: ] [PubMed] [Google Scholar]
Lim 2017
- Lim Y, Godambe S. Prevention and management of procedural pain in the neonate: an update, American Academy of Pediatrics, 2016. Archives of Disease in Childhood. Education and Practice Edition 2017;102(5):254-6. [DOI: 10.1136/archdischild-2016-311066] [PMID: ] [DOI] [PubMed] [Google Scholar]
Maitra 2014
- Maitra S, Baidya DK, Khanna P, Ray BR, Panda SS, Bajpai M. Acute perioperative pain in neonates: an evidence-based review of neurophysiology and management. Acta Anaesthesiologica Taiwanica 2014;52(1):30-7. [DOI: 10.1016/j.aat.2014.02.004] [PMID: ] [DOI] [PubMed] [Google Scholar]
Marshall 2018
- Marshall IJ, Noel-Storr AH, Kuiper J, Thomas J, Wallace BC. Machine learning for identifying randomized controlled trials: an evaluation and practitioner’s guide. Research Synthesis Methods 2018;9(4):602-14. [DOI: 10.1002/jrsm.1287] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mather 1992
- Mather LE. Do the pharmacodynamics of the nonsteroidal anti-inflammatory drugs suggest a role in the management of postoperative pain? Drugs 1992;44(Suppl 5):1-13. [DOI: 10.2165/00003495-199200445-00003] [PMID: ] [DOI] [PubMed] [Google Scholar]
Maxwell 2019
- Maxwell LG, Fraga MV, Malavolta CP. Assessment of pain in the newborn: an update. Clinics in Perinatology 2019;46(4):693-707. [DOI: 10.1016/j.clp.2019.08.005] [PMID: ] [DOI] [PubMed] [Google Scholar]
McPherson 2015
- McPherson C, Haslam M, Pineda R, Rogers C, Neil JJ, Inder TE. Brain injury and development in preterm infants exposed to fentanyl. Annals of Pharmacotherapy 2015;49(12):1291-7. [DOI: 10.1177/1060028015606732] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
McPherson 2018
- McPherson C. Premedication for endotracheal intubation in the neonate. Neonatal Network 2018;37(4):238-47. [DOI: 10.1891/0730-0832.37.4.238] [PMID: ] [DOI] [PubMed] [Google Scholar]
Mitchell 2000
- Mitchell A, Brooks S, Roane D. The premature infant and painful procedures. Pain Management Nursing 2000;1(2):58-65. [DOI: 10.1053/jpmn.2000.7781] [PMID: ] [DOI] [PubMed] [Google Scholar]
Moffett 2006
- Moffett BS, Wann TI, Carberry KE, Mott AR. Safety of ketorolac in neonates and infants after cardiac surgery. Paediatric Anaesthesia 2006;16(4):424-8. [DOI: 10.1111/j.1460-9592.2005.01806.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Journal of Clinical Epidemiology 2009;62(10):1006-12. [DOI: 10.1016/j.jclinepi.2009.06.005] [PMID: ] [DOI] [PubMed] [Google Scholar]
Monk 2019
- Monk V, Moultrie F, Hartley C, Hoskin A, Green G, Bell JL, et al. Oral morphine analgesia for preventing pain during invasive procedures in non-ventilated premature infants in hospital: the Poppi RCT. Efficacy and Mechanism Evaluation 2019;6(9):no pagination. [DOI: 10.3310/eme06090] [PMID: ] [DOI] [PubMed] [Google Scholar]
Mpody 2020
- Mpody C, Shepherd EG, Thakkar RK, Dairo OO, Tobias JD, Nafiu OO. Synergistic effects of sepsis and prematurity on neonatal postoperative mortality. British Journal of Anaesthesia 2020;125(6):1056-63. [DOI: 10.1016/j.bja.2020.07.026] [PMID: ] [DOI] [PubMed] [Google Scholar]
Muhly 2020
- Muhly WT, Taylor E, Razavi C, Walker SM, Yang L, De Graaff JC, et al, Pediatric Perioperative Outcomes Group. A systematic review of outcomes reported in pediatric perioperative research: a report from the Pediatric Perioperative Outcomes Group. Paediatric Anaesthesia 2020 [Epub ahead of print]. [DOI: 10.1111/pan.13981] [PMID: ] [DOI] [PubMed] [Google Scholar]
NIH 1979
- National Institutes of Health. Report of workshop on bronchopulmonary dysplasia. Journal of Pediatrics 1979;95(5 Pt 2, 1-9):815-920. [PubMed]
Noel‐Storr 2020
- Noel-Storr AH, Dooley G, Wisniewski S, Glanville J, Thomas J, Cox S, et al. Cochrane Centralised Search Service showed high sensitivity identifying randomised controlled trials: a retrospective analysis. Journal of Clinical Epidemiology 2020;127:142-50. [DOI: 10.1016/j.jclinepi.2020.08.008] [PMID: ] [DOI] [PubMed] [Google Scholar]
Noel‐Storr 2021
- Noel-Storr AH, Dooley G, Elliott J, Steele E, Shemilt I, Mavergames C, et al. An evaluation of Cochrane Crowd found that crowdsourcing produced accurate results in identifying randomised trials. Journal of Clinical Epidemiology 2021;4356(21):00008-1. [DOI: 10.1016/j.jclinepi.2021.01.006] [PMID: ] [DOI] [PubMed] [Google Scholar]
Ohlsson 2020
- Ohlsson A, Shah PS. Paracetamol (acetaminophen) for prevention or treatment of pain in newborns. Cochrane Database of Systematic Reviews 2020, Issue 1. Art. No: CD011219. [DOI: 10.1002/14651858.CD011219.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Olsson 2021
- Olsson E, Ahl H, Bengtsson K, Vejayaram DN, Norman E, Bruschettini M, et al. The use and reporting of neonatal pain scales: a systematic review of randomized trials. Pain 2021;162(2):353-60. [DOI: 10.1097/j.pain.0000000000002046] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pacifici 2014
- Pacifici GM. Clinical pharmacology of analgesics in infants and the pharmacologic management of pain in neonates. MedicalExpress (São Paulo, online) 2014;1(3):105-15. [DOI: 10.5935/MedicalExpress.2014.03.03] [DOI] [Google Scholar]
Papile 1978
- Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. Journal of Pediatrics 1978;92(4):529-34. [DOI: 10.1016/s0022-3476(78)80282-0] [PMID: ] [DOI] [PubMed] [Google Scholar]
Pirlotte 2019
- Pirlotte S, Beeckman K, Ooms I, Van Rompaey B, Cools F. Pharmacological interventions for the prevention of pain during endotracheal suctioning in ventilated neonates. Cochrane Database of Systematic Reviews 2019, Issue 6. Art. No: CD013355. [DOI: 10.1002/14651858.CD013355] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ranger 2014
- Ranger M, Grunau RE. Early repetitive pain in preterm infants in relation to the developing brain. Pain Management 2014;4(1):57-67. [DOI: 10.2217/pmt.13.61] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 2020 [Computer program]
- Review Manager 5 (RevMan 5). Version 5.4. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2020.
Romantsik 2017
- Romantsik O, Calevo MG, Norman E, Bruschettini M. Clonidine for sedation and analgesia for neonates receiving mechanical ventilation. Cochrane Database of Systematic Reviews 2017, Issue 5. Art. No: CD012468. [DOI: 10.1002/14651858.CD012468.pub2] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Romantsik 2020
- Romantsik O, Calevo MG, Norman E, Bruschettini M. Clonidine for pain in non-ventilated infants. Cochrane Database of Systematic Reviews 2020, Issue 4. Art. No: CD013104. [DOI: 10.1002/14651858.CD013104.pub2] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Saarenmaa 2001
- Saarenmaa E, Neuvonen PJ, Huttunen P, Fellman V. Ketamine for procedural pain relief in newborn infants. Archives of Disease in Childhood. Fetal and Neonatal Edition 2001;85(1):F53-6. [DOI: 10.1136/fn.85.1.f53] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sanders 2013
- Sanders RD, Hassell J, Davidson AJ, Robertson NJ, Ma D. Impact of anaesthetics and surgery on neurodevelopment: an update. British Journal of Anaesthesiology 2013;110(Suppl 1):i53-72. [DOI: 10.1093/bja/aet054] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schiller 2018
- Schiller RM, Allegaert K, Hunfeld M, Van den Bosch GE, Van den Anker J, Tibboel D. Analgesics and sedatives in critically ill newborns and infants: the impact on long-term neurodevelopment. Journal of Clinical Pharmacology 2018;58(Suppl 10):S140-50. [DOI: 10.1002/jcph.1139] [PMID: ] [DOI] [PubMed] [Google Scholar]
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
Silva 2007
- Silva YP, Gomez RS, Máximo TA, Silva AC. Sedation and analgesia in neonatology [Sedação e analgesia em neonatologia]. Revista Brasileira de Anestesiologia 2007;57(5):575-87. [DOI: 10.1590/s0034-70942007000500013] [PMID: ] [DOI] [PubMed] [Google Scholar]
Simons 2003
- Simons SH, Van Dijk M, Van Lingen RA, Roofthooft D, Duivenvoorden HJ, Jongeneel N, et al. Routine morphine infusion in preterm newborns who received ventilatory support: a randomized controlled trial. JAMA 2003;290(18):2419-27. [DOI: 10.1001/jama.290.18.2419] [PMID: ] [DOI] [PubMed] [Google Scholar]
Stevens 1996
- Stevens B, Johnstone C, Petryshen P, Taddio A. Premature infant pain profile: development and initial validation. Clinical Journal of Pain 1996;12:13-22. [DOI: 10.1097/00002508-199603000-00004] [PMID: ] [DOI] [PubMed] [Google Scholar]
Thigpen 2019
- Thigpen JC, Odle BL, Harirforoosh S. Opioids: a review of pharmacokinetics and pharmacodynamics in neonates, infants, and children. European Journal of Drug Metabolism and Pharmacokinetics 2019;44(5):591-609. [DOI: 10.1007/s13318-019-00552-0] [PMID: ] [DOI] [PubMed] [Google Scholar]
Thomas 2020
- Thomas J, McDonald S, Noel-Storr AH, Shemilt I, Elliott J, Mavergames C, et al. Machine learning reduces workload with minimal risk of missing studies: development and evaluation of an RCT classifier for Cochrane Reviews. Journal of Clinical Epidemiology 2020;S0895-4356(20):31172-0. [DOI: 10.1016/j.jclinepi.2020.11.003vcvc] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Van der Marel 2007
- Van der Marel CD, Peters JW, Bouwmeester NJ, Jacqz-Aigrain E, Van den Anker JN, Tibboel D. Rectal acetaminophen does not reduce morphine consumption after major surgery in young infants. British Journal of Anaesthesiology 2007;98(3):372-9. [DOI: 10.1093/bja/ael371] [PMID: ] [DOI] [PubMed] [Google Scholar]
Van Dijk 2009
- Van Dijk M, Roofthooft DW, Anand KJ, Guldemond F, De Graaf J, Simons S, et al. Taking up the challenge of measuring prolonged pain in (premature) neonates: the COMFORTneo scale seems promising. Clinical Journal of Pain 2009;25(7):607-16. [DOI: 10.1097/AJP.0b013e3181a5b52a] [PMID: ] [DOI] [PubMed] [Google Scholar]
Van Gonge 2018
- Van Donge T, Mian P, Tibboel D, Van Den Anker J, Allegaert K. Drug metabolism in early infancy: opioids as an illustration. Expert Opinion on Drug Metabolism & Toxicology 2018;14(3):287-301. [DOI: 10.1080/17425255.2018.1432595] [PMID: ] [DOI] [PubMed] [Google Scholar]
Vinall 2014
- Vinall J, Grunau RE. Impact of repeated procedural pain-related stress in infants born very preterm. Pediatric Research 2014;75(5):584-7. [DOI: 10.1038/pr.2014.16. Epub 2014 Feb 5.] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Walsh 1986
- Walsh MC, Kliegman RM. Necrotizing enterocolitis: treatment based on staging criteria. Pediatric Clinics of North America 1986;33(1):179-201. [DOI: 10.1016/s0031-3955(16)34975-6] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Walsh 2004
- Walsh MC, Yao Q, Gettner P, Hale E, Collins M, Hensman A, et al. Impact of a physiologic definition on bronchopulmonary dysplasia rates. Pediatrics 2004;114(5):1305-11. [DOI: 10.1542/peds.2004-0204] [PMID: ] [DOI] [PubMed] [Google Scholar]
Wong 2013
- Wong I, St John-Green C, Walker SM. Opioid-sparing effects of perioperative paracetamol and nonsteroidal anti-inflammatory drugs (NSAIDs) in children. Paediatric Anaesthesia 2013;23(6):475-95. [DOI: 10.1111/pan.12163] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhu 2017
- Zhu A, Benzon HA, Anderson TA. Evidence for the efficacy of systemic opioid-sparing analgesics in pediatric surgical populations: a systematic review. Anesthesia and Analgesia 2017;125(5):1569-87. [DOI: 10.1213/ANE.0000000000002434] [PMID: ] [DOI] [PubMed] [Google Scholar]
Ziesenitz 2018
- Ziesenitz VC, Vaughns JD, Koch G, Mikus G, Van den Anker JN. Correction to: Pharmacokinetics of fentanyl and Its derivatives in children: a comprehensive review. Clinical Pharmacokinetics 2018;57(3):393-417. [DOI: 10.1007/s40262-017-0609-2] [PMID: ] [DOI] [PubMed] [Google Scholar]
Zwicker 2016
- Zwicker JG, Miller SP, Grunau RE, Chau V, Brant R, Studholme C, et al. Smaller cerebellar growth and poorer neurodevelopmental outcomes in very preterm infants exposed to neonatal morphine. Journal of Pediatrics 2016;172:81-7.e2. [DOI: 10.1016/j.jpeds.2015.12.024] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Kinoshita 2021
- Kinoshita M, Stempel KS, Borges do Nascimento IJ, Bruschettini M. Systemic opioids versus other analgesics and sedatives for postoperative pain in neonates. Cochrane Database of Systematic Reviews 2021, Issue 5. Art. No: CD014876. [DOI: 10.1002/14651858.CD014876] [DOI] [PMC free article] [PubMed] [Google Scholar]


