Abstract
The discovery of ketamine as a rapid-acting antidepressant spurred significant research to understand its underlying mechanisms of action and to identify other novel compounds that may act similarly. Serotonergic psychedelics (SPs) have shown initial promise in treating depression, though the challenge of conducting randomized controlled trials with SPs and the necessity of long-term clinical observation are important limitations. This review summarizes the similarities and differences between the psychoactive effects associated with both ketamine and SPs and the mechanisms of action of these compounds, with a focus on the monoaminergic, glutamatergic, gamma-aminobutyric acid (GABA)-ergic, opioid, and inflammatory systems. Both molecular and neuroimaging aspects are considered. While their main mechanisms of action differ—SPs increase serotonergic signaling while ketamine is a glutamatergic modulator—evidence suggests that the downstream mechanisms of action of both ketamine and SPs include mechanistic target of rapamycin complex 1 (mTORC1) signaling and downstream GABAA receptor activity. The similarities in downstream mechanisms may explain why ketamine, and potentially SPs, exert rapid-acting antidepressant effects. However, research on SPs is still in its infancy compared to the ongoing research that has been conducted with ketamine. For both therapeutics, issues with regulation and proper controls should be addressed before more widespread implementation.
Keywords: Ketamine, serotonergic psychedelics, depression, rapid-acting therapeutics, biosignatures
1.0. Introduction
Pharmacotherapies developed to treat major depressive disorder (MDD) have historically been unsuccessful for roughly a third of patients (Rush et al., 2006; Trivedi et al., 2006). Furthermore, even when successful at reducing depressive symptoms, these drugs—typically monoaminergic-based antidepressants—have a substantial therapeutic time lag of weeks to months, limited remission rates, and undesirable side effects (Drewiany et al., 2015).
In this context, the recent discovery that sub-anesthetic doses of racemic (R,S)-ketamine (hereafter referred to as ketamine), an N-methyl-D-aspartate receptor (NMDAR) antagonist and glutamatergic modulator, have rapid antidepressant effects that occur in a matter of hours dramatically altered the therapeutic landscape (Kishimoto et al., 2016). Ketamine was initially approved by the US Food and Drug Administration (FDA) in 1970 as a rapid-acting general anesthetic. In recent years, multiple randomized, placebo-controlled trials have validated its robust antidepressant effects in individuals with MDD (Alnefeesi et al., 2022; McIntyre et al., 2020), those with treatment-resistant depression (TRD) who had not previously responded to conventional therapeutics (Dai et al., 2022; Zarate et al., 2006), and those with treatment-resistant bipolar depression (Diazgranados et al., 2010; Zarate et al., 2012). These antidepressant effects were found to be sustained well beyond the half-life of the drug and its peak pharmacokinetic exposure in the body, suggesting that its effects are maintained via a timely activation of signaling cascades in the brain. One meta-analysis demonstrated that ketamine’s antidepressant effects peaked at 24 hours post-infusion and faded after 10 to 12 days (Kishimoto et al., 2016). These findings led the FDA to approve intranasal esketamine (Spravato)—the (S)-enantiomer of ketamine—for adults with TRD in 2019, and for adults with MDD and acute suicidal ideation or behavior in 2020 (U.S. Food & Drug Administration, 2019) under a Risk Evaluation and Management Schedule (REMS). This agent has also been approved by the European Union for the same indications.
Despite its encouraging antidepressant profile, concerns regarding the use of ketamine as an antidepressant persist, particularly given its potential for abuse and the unknown effects of its long-term use in clinical settings (Kokane et al., 2020; Liu et al., 2016). Ketamine also generates a transient psychoactive state that peaks around 40 minutes post-infusion, including changes in perception, mood, thought, and self-awareness, which necessitate its administration under medical supervision (Acevedo-Diaz et al., 2019; Kraus et al., 2017; Sassano-Higgins et al., 2016). Nevertheless, the re-purposing of ketamine from an anesthetic to an antidepressant created a paradigm shift, sparking a surge in research to develop and/or repurpose other therapeutic compounds with rapid and robust actions and similar behavioral and biological effects (Kadriu et al., 2020).
The most prominent example is research into the effects of so-called “classic” psychedelics, including lysergic acid diethylamide-25 (LSD), psilocybin and its active metabolite psilocin, 2,5-Dimethoxy-4-iodoamphetamine (DOI), N,N-dimethyltryptamine (DMT), 5-methoxy-DMT (5-MeO-DMT), and the related empathogen 3,4-methylenedioxy-methamphetamine (MDMA) (Carhart-Harris and Goodwin, 2017). Coined by Osmond in 1957, the word “psychedelic” is often used interchangeably with the terms “hallucinogen” and “psychotomimetic” in the literature. In this manuscript, we refer to this class of drugs as serotonergic psychedelics (SPs), which reflects both their primary mechanism of action as 5-HT2A receptor agonists and their unique effects on the ego and consciousness (Carhart-Harris et al., 2012; Johnson et al., 2019; Mason et al., 2020; Rucker et al., 2018). Depending on the agent, psychoactive states will alternatively be referred to as psychedelic, psychotomimetic, or dissociative (Nichols, 2016). The term psychedelic refers to diverse agents that alter a person’s thoughts, feelings, and awareness of their own surroundings; in contrast, the term psychotomimetic reflects an alteration of behavior or personality that mimics psychosis, and the term dissociative references a state in which a disconnection or lack of continuity exists between a person’s thoughts, memories, or feelings. SPs produce both psychedelic and psychotomimetic states, whereas ketamine appears to induce primarily transient psychotomimetic and dissociative states.
In the 1950s and 1960s, over 1000 clinical research papers explored the therapeutic effects of SPs, particularly with regard to treating addiction, but they often lacked scientific rigor, including failure to incorporate control groups or blinding, report adverse effects, and validate outcome measures (Carhart-Harris and Goodwin, 2017). In 1967, research into SPs was brought to an abrupt halt when they were classified as Schedule I drugs (indicating that they had no medical value and were highly addictive), a decision that stemmed from concerns regarding their non-clinical use (Rucker et al., 2018) and the potential harm associated with their ability to engender life-altering experiences. However, there has been a recent resurgence of interest in clinical and preclinical SP research, especially with psilocybin; indeed, recent findings suggest that just one or two doses of SPs may persistently mitigate the symptoms of MDD and anxiety, as well as alcohol and tobacco addiction, more effectively than conventional treatments (Agin-Liebes et al., 2020; Carhart-Harris et al., 2018; Griffiths et al., 2016; Muttoni et al., 2019; Nichols et al., 2017; Ross et al., 2016). This work led the FDA to designate psilocybin as a “breakthrough therapy” in 2018 for TRD and again in 2019 for MDD.
Despite proposed parallels, much remains unknown about the convergent and divergent mechanisms of ketamine and SPs, particularly given the comparative dearth of properly controlled clinical trials and experiments investigating the therapeutic effects of SPs. While clinical trials of ketamine have consistently proven that it effectively targets TRD, properly controlled studies of SPs are still in their infancy, and many more unanswered clinical and mechanistic questions surround their use. Nevertheless, the rapid recent pace of new studies focusing on the effects of SPs requires that the literature in this area be regularly updated. This paper, which updates recent findings in this area (Kadriu et al., 2021), will review the molecular, imaging, and behavioral data believed to underlie the distinct and overlapping mechanisms of both therapeutics with the goal of informing future research into rapid-acting antidepressants. Unravelling the mechanism of antidepressant action that underlies both ketamine and SPs would dramatically expand our understanding of psychiatric disorders and have important implications for drug development. Towards this end, the manuscript explores the potential role of psychotomimetic or psychedelic effects in antidepressant efficacy, the divergent main mechanisms of these two therapeutic compounds, their potential common downstream targets, and regulatory issues that must be considered for both.
2.0. The Convergent and Divergent Mechanisms of Ketamine and SPs
2.1. An overview of the major mechanisms of action of ketamine and SPs
While initially thought to primarily exert its antidepressant effects through NMDAR antagonism, research suggests that ketamine has several potentially relevant mechanisms of antidepressant action (Yang et al., 2019; Zanos et al., 2018a). Currently, two main NMDAR-related hypotheses exist for ketamine’s effects: the disinhibition hypothesis and the direct inhibition hypothesis (Miller et al., 2016). The disinhibition hypothesis states that, at subanesthetic doses, ketamine preferentially antagonizes NMDARs on gamma-aminobutyric acid (GABA)-ergic interneurons. Blockade of these inhibitory interneurons increases firing of excitatory pyramidal neurons, which increases glutamate release and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR) transmission; this, in turn, activates integral synaptic signaling pathways that contribute to ketamine’s therapeutic effects (Gerhard et al., 2020). The direct inhibition hypothesis posits that ketamine’s antagonism of NMDARs on excitatory pyramidal neurons lessens tonic NMDAR activation by circulating glutamate, which subsequently increases protein synthesis by decreasing suppression of eukaryotic elongation factor 2 (eEF2) (Autry et al., 2011; Nosyreva et al., 2013). Interestingly, the preliminary efficacy of (R)-ketamine—which is a less potent NMDAR antagonist—has spurred research into NMDAR-independent mechanisms of ketamine. This NMDAR-independent mechanism, which is thought to be mediated via AMPAR activation, activates a downstream BDNF-TrkB cascade to promote excitatory signaling in the cortex and hippocampus (Yang et al., 2019; Zanos et al., 2018a). Although the mechanisms underlying ketamine’s NMDAR-independent effects on AMPAR transmission remain unclear, ketamine and (2R,6R)-HNK have been shown to upregulate the expression and phosphorylation of GluA1 independent of NMDAR antagonism (Li et al., 2022b; Yao et al., 2018; Zanos et al., 2016). Recent research also found increased cyclic adenosine monophosphate (cAMP)-induced phosphorylation of cAMP response element-binding protein (CREB) after ketamine administration, even after NMDAR knockout. This CREB phosphorylation increased BDNF release, which can activate the downstream BDNF-TrkB cascade to increase excitatory signaling (Wray et al., 2019). In addition to its glutamatergic mechanisms, evidence suggests that ketamine is also a monoamine reuptake inhibitor, a modulator of opioid receptor (mu, delta, kappa) activity, a dopamine receptor agonist, and a muscarinic receptor antagonist, among other mechanisms of action (Matveychuk et al., 2020).
Broadly, SPs interact with a variety of serotonergic receptors (5-HT1A, 5-HT1B, 5-HT1D, 5-HT1E, 5-HT1F, 5-HT2A, 5-HT2B, 5-HT2C, 5-HT4, 5-HT5, and 5-HT6) and, with less affinity, some non-serotonergic receptors (e.g., dopamine receptors, certain trace amine receptors, kappa opioid receptors, sigma receptors, etc) (reviewed in (McClure-Begley and Roth, 2022; Slocum et al., 2022)). Their psychedelic activity is believed to be primarily mediated by 5-HT2A receptor agonism (López-Giménez and González-Maeso, 2018; Rolland et al., 2014). The overlapping pharmacology of SPs (i.e., 5-HT2A binding) was also demonstrated in a recent clinical trial that found that participants could not differentiate between the psychedelic experiences produced by LSD or psilocybin, except for their duration (Holze et al., 2022). Mechanistic and genetic rodent studies have shown that abolishing 5-HT2A receptor activity prevents the antidepressant-like effects of SPs on behavior and structural plasticity (Ly et al., 2018; Pędzich et al., 2022). On the other hand, antidepressant-like behavioral and electrophysiological responses to psilocybin were not blocked by the 5-HT2A/2C receptor antagonist ketanserin, though this experiment should be repeated with an antagonist that is more selective for the 5-HT2A receptor, such as volinanserin (Hesselgrave et al., 2021). In addition, the selective 5-HT2A receptor agonist DOI had no antidepressant-like effects in an LPS-induced mouse model of depression, but it did increase dendritic spine density (Qu et al., 2022). Interestingly, a non-psychedelic analog studied with the same LPS mouse model had antidepressant-like effects that were not associated with 5-HT2AR agonism (Qu et al., 2022). For a summary of hypothesized mechanisms underlying the antidepressant effects of ketamine and SPs, please see Table 1.
Table 1.
Hypothesized mechanisms underlying the antidepressant actions of ketamine and psychedelics.
| Mechanism | Area of Effect | Ketamine | Psychedelics |
|---|---|---|---|
| Serotonergic Signaling | Expression | Ketamine increased extracellular levels of 5-HT in the PFC (Ago et al., 2019; López-Gil et al., 2019) | |
| 5-HT depletion blocked the effects of (S)-ketamine (du Jardin et al., 2018) | |||
| 5-HT depletion did not block the effects of (R)-ketamine (Zhang et al., 2018) | |||
| 5-HT Receptors | Ketamine increased 5-HT1B receptor binding (Spies et al., 2018; Tiger et al., 2020; Yamanaka et al., 2014) | Non-hallucinogenic analogues of psychedelics (Cao et al., 2022) and novel monoamine transporter ligands (Rudin et al., 2022) exerted antidepressant effects via 5-HT receptors | |
| Antidepressant and antidepressant-like effects were reportedly primarily mediated through 5-HT2A receptor activation (Cao et al., 2022; López-Giménez and González-Maeso, 2018; Ly et al., 2018; Pędzich et al., 2022; Rolland et al., 2014) | |||
| Behavioral response to psilocybin was not blocked by ketanserin (a 5-HT2A/2C receptor antagonist) | |||
| DOI (a selective 5-HT2A receptor antagonist) did not have antidepressant-like effects | |||
| SERT binding | Ketamine increased SERT binding ((Spies et al., 2018; Tiger et al., 2020; Yamanaka et al., 2014) | Increased occupancy with LSD and 5-MeO-DMT administration (Kyzar and Kalueff, 2016; Rickli et al., 2015), but no interactions with LSD (Blough et al., 2014; Rickli et al., 2015) | |
| Dopaminergic Signaling | Expression | Chemogenetic inhibition of dopamine signaling blocked ketamine’s antidepressant-like effects (Wu et al., 2021a; Wu et al., 2021b) | DMT, psilocybin, and mescaline may convert to dopamine after ingestion (Fitzgerald, 2021) |
| Dopaminergic Receptors | Drd1 activation mediated the antidepressant-like behavioral effects of ketamine and increased cortical spinogenesis (Hare et al., 2019; Wu et al., 2021a). Drd1 expression also increased after ketamine administration (Li et al., 2022a) | ||
| (R)-ketamine had Drd1-activation independent effects (Chang et al., 2020) | |||
| Firing activity | Ketamine increased the firing activity of dopaminergic neurons (Iro et al., 2021) | In high doses, LSD increased dopaminergic firing activity (De Gregorio et al., 2016) | |
| Glutamatergic Signaling | Glutamate surge | Glutamate “surge” (reviewed in (Kadriu et al., 2021)) | Glutamate “surge” (reviewed in (Kadriu et al., 2021)) |
| NMDAR-related effects | An extensive literature describes the role of NMDAR antagonism in ketamine’s antidepressant effects, particularly on GABA-ergic interneurons (reviewed in (Miller et al., 2016; Zanos and Gould, 2018)) | Psilocybin increased AMPAR/NMDAR ratios in hippocampal slices (Hesselgrave et al., 2021) | |
| (R)-ketamine and (2R,6R)-HNK appeared to have NMDAR-independent effects (Dravid et al., 2007; Lumsden et al., 2019; Zhao et al., 2012) | Ibogaine may antagonize NMDARs (Underwood et al., 2021) | ||
| Psilocybin increased NR2A expression but was not associated with an antidepressant response (Wotjas et al., 2022) | |||
| AMPAR-related effects | Ketamine upregulated mTORC1 signaling via increased AMPAR activation (Aguilar-Valles et al., 2021; Li et al., 2010; Rafało-Ulińska and Pałucha-Poniewiera, 2022; Zanos et al., 2016; Zhou et al., 2014) | Psychedelics upregulated mTORC1 signaling via increased AMPAR activation (Ly et al., 2020; Madrid-Gambin et al., 2022; Ornelas et al., 2022; Vollenweider and Preller, 2020; Vollenweider and Smallridge, 2022) | |
| (2R,6R)-HNK had mGluR2-dependent antidepressant-like effects (Zanos et al., 2019) | |||
| (R)-ketamine increased ERK signaling, particularly on microglia, which mediated its antidepressant-like effects (Yang et al., 2018b; Yao et al., 2022) | |||
| mGluR-related effects | Co-administration of ketamine and an mGluR2/3 antagonist sustained antidepressant-like response (Pałucha-Poniewiera et al., 2021; Rafało-Ulińska et al., 2022) | mGluR2/3 agonists inhibited the effects of DOI in mice (Benvenga et al., 2018) | |
| GABAergic signaling | Expression | Ketamine increased hippocampal GABA turnover (Silberbauer et al., 2020) and GABA release (Pham et al., 2020) | Psychedelics increased GABA expression in the mPFC (Carhart-Harris and Nutt, 2017; Mason et al., 2020) |
| Receptors | GABAA receptor activity was upregulated by ketamine (Wang et al., 2017) | LSD did not affect EEG response in GABAA receptor delta subunit knockout mice (Grotell et al., 2021) | |
| Benzodiazepines (which also increase GABAA receptor activity) decreased ketamine’s antidepressant effects (Andrashko et al., 2020; Fuchikami et al., 2015) | |||
| Signaling | Ketamine rescued deficits in synaptic GABA-ergic markers and the frequency of inhibitory post-synaptic currents in the mPFC (Ghosal et al., 2020) | Stress-induced alterations in GABA-ergic circuitry were reversed by 5-HT2aR agonists in the VTA (Kimmey et al., 2019) | |
| Opioid system | Receptors | Ketamine had a strong affinity for the mu-opioid receptor and weak affinity for the kappa opioid receptor (Bonaventura et al., 2021) | Psychedelic binding to mu- and kappa-opioid receptors correlated with “therapeutic component scores” (Zamberlan et al., 2018) |
| Opioid receptor antagonists abolished ketamine’s (and its metabolites’) rapid-acting antidepressant effects in clinical and preclinical models (Klein et al., 2020; Williams et al., 2019; Williams et al., 2018; Wulf et al., 2022; Zhang et al., 2021a) | Mu-opioid receptor binding after psychedelic administration correlated with self-report dependence measures (Zamberlan et al., 2018) | ||
| Inflammation | Cytokines | (R)-, but not (S)-ketamine reduced blood IL-6 levels in a model of ulcerative colitis (Fujita et al., 2021) | Psilocybin, LSD, and DOI reduced levels of cytokines and TNF-α (Kozłowska et al., 2021; Nardai et al., 2020; Nkadimeng et al., 2021; Smedfors et al., 2022; Yu et al., 2008) |
| (R)-ketamine reduced central and peripheral levels of pro-inflammatory cytokines in mice administered LPS (Zhang et al., 2021b) | |||
| Ketamine decreased levels of pro-inflammatory cytokines in a sex-dependent manner after maternal deprivation (Abelaira et al., 2022) | |||
| Baseline IL-8 levels predicted treatment response to ketamine in females but not males (Kruse et al., 2021) | |||
| Ketamine had prophylactic effects against upregulation of inflammatory markers after stress exposure (Brachman et al., 2016; Camargo et al., 2021; Costi et al., 2022) | DOI had prophylactic effects against TNF-alpha administration, preventing the upregulation of pro-inflammatory cytokines (Nau et al., 2013) | ||
| HPA-axis signaling | Ketamine restored glucocorticoid receptor expression in the hippocampus (Wang et al., 2019) | Short-term increases in cortisol and ACTH were observed during the peak hallucinogenic effects of psilocybin (Hasler et al., 2004) | |
| Corticosterone and ACTH levels were reduced by ketamine after LPS injection (Besnier et al., 2017) | |||
| Kynurenic signaling | Ketamine restored the KYN:tryptophan ratio (Moaddel et al., 2018; Wang et al., 2015) | ||
| Increased kynurenic acid post-ketamine correlated with treatment response (Zhou et al., 2018) |
5-HT: 5-hydroxytryptamine; 5-Meo-DMT: 5-methoxy-N,N-dimethyltryptamine; ACTH: adrenocorticotropic hormone; AMPAR: α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor; DOI: 2,5-Dimethoxy-4-iodoamphetamine; Drd1: dopamine receptor D1; ERK: extracellular signal-related kinase; GABA: gamma aminobutyric acid; HNK: hydroxynorketamine; IL: interleukin; KYN: kynurenine; LPS: lipopolysaccharide; LSD: lysergic acid diethylamide; mGluR: metabotropic glutamate receptor; mPFC: medial prefrontal cortex; mTORC1: mechanistic target of rapamycin complex 1; NMDAR: N-methyl-D-aspartate receptor; PFC: prefrontal cortex; SERT: serotonin transporter; TNF-α: tumor necrosis factor alpha; VTA: ventral tegmental area
2.2. Psychoactive Effects
In the development of novel, rapid-acting antidepressant agents, one key question of interest is how pharmacotherapies that appear to work through diverse mechanisms nevertheless exert similar—and perhaps equally rapid-acting—effects. Broadly, ketamine’s antidepressant effects peak at 24 hours; depressive symptom reduction has been reported within one hour and can last upwards of two weeks (Hashimoto, 2019). Initial trials exploring the ability of SPs to reduce depressive symptoms found an effect within one day (Palhano-Fontes et al., 2019; Sanches et al., 2016), but this result has not always been replicated (Grob et al., 2011). However, this may be due to differences in participant population (e.g., individuals with recurrent depression or TRD vs. those with late-stage cancer) or the SP used (e.g., ayahuasca vs. psilocybin). A recent Phase 2 trial found reduced Montgomery-Asberg Depression Rating Scale (MADRS) scores in participants with TRD three weeks after an acute dose of psilocybin (Goodwin et al., 2022a). While not analyzed, MADRS scores were also reduced at Day 2 after psilocybin administration. Overall, however, most research exploring the effects of SPs on depressive symptoms has focused on longer time-points (Romeo et al., 2020), and further research is necessary to determine the extent to which SPs can be classified as a rapid-acting antidepressant.
Although they target different neurotransmitter systems, both ketamine and SPs similarly induce psychoactive states. These psychoactive experiences, however, appear to be mediated via different mechanisms: NMDAR antagonism for ketamine and 5-HTR agonism for SPs. It is important to note at the outset that most research conducted in SPs is classified as qualitative or quasi-experimental, in sharp contrast to ketamine, whose therapeutic effects have often been assessed via randomized controlled trials.
Initial research for ketamine focused on its underlying mechanisms of action, not the role of its psychoactive effects, particularly because it induces only mild and fleeting psychoactive effects at antidepressant doses (around 0.5 mg/kg). At these sub-anesthetic doses, approximately 80% of individuals with TRD report dissociative symptoms in response to ketamine, including “feeling strange, weird, or bizarre”, and 50% report stronger psychoactive effects such as a sensation of “floating” (Acevedo-Diaz et al., 2020b); however, these effects are transient, peaking around 40 minutes post-infusion and dissipating shortly thereafter (Acevedo-Diaz et al., 2020b). Interestingly, initial reports regarding subjective experiences after (R)-ketamine administration have differentiated its effects from those of (S)-ketamine, with very few psychotomimetic effects reported in early clinical trials (Zhang et al., 2022).
In contrast, SPs are often administered at much higher doses than the minimum needed to produce a psychedelic effect (e.g., ~0.3 mg/kg for psilocybin, ~1.67 mg/kg for DMT) (Knudsen, 2023; Sanches et al., 2016). Although ketamine and certain SPs (such as psilocin) have similar half-lives (~2.5 hours), the higher doses needed for SPs to exert their therapeutic effects typically lead to much stronger and longer-lasting psychoactive and psychotomimetic effects. While the timing of these effects varies widely between SPs due to differences in plasma half-lives, the psychoactive effects of most SPs can last for hours—for example, approximately six hours for psilocybin (Passie et al., 2002) and up to 12 hours for LSD (Dolder et al., 2015); in contrast, some SPs have extremely strong but very transient effects—for example, the effects of DMT only last from five to 30 minutes (Strassman and Qualls, 1994).
Across cultures and contexts, “mystical-type experiences” are commonly reported after SP administration, including reports of bliss, connection, acceptance, transcendence of space and time, and sacredness (Johnson et al., 2019). For example, 4.5 years after a single treatment, 71–100% of patients with life-threatening cancer treated with psilocybin attributed reductions in depressive and anxiety symptoms to the treatment (Agin-Liebes et al., 2020), though these measures were self-reported, and 39% of the patients had also received psychotherapy in the interim period. It should also be noted that SPs are not always significantly more effective than traditional antidepressants such as escitalopram (Carhart-Harris et al., 2021), and the varied results warrant further exploration.
The degree to which psychoactive effects and therapeutic efficacy are linked for each of these compounds remains unclear. The association between ketamine’s efficacy and its psychoactive effects appears to be weak, and most data indicate that its psychotomimetic effects are not related to its antidepressant outcomes (Acevedo-Diaz et al., 2020a; Ballard and Zarate, 2020). Furthermore, one recent study found no interaction between reported adverse events post-ketamine and later MADRS scores (Greenwald et al., 2021). This lack of association between ketamine’s dissociative and antidepressant effects, as well as the effects of other NMDAR antagonists, suggests that some of ketamine’s antidepressant effects may be NMDAR-independent. In contrast, the psychoactive effects induced by SPs appear to be dose-dependent and to correlate with self-reported positive persistent effects in healthy volunteers, as assessed via a non-validated scale (the Persistent Effects Questionnaire) (Barrett and Griffiths, 2018; McCulloch et al., 2022). In addition, the “quality” of psychedelic experience after psilocybin administration, as assessed through the Altered States of Consciousness Questionnaire, predicted future reduction of depressive symptoms measured by the QIDS-SR (Self-Reported Quick Inventory of Depressive Symptoms) (QIDS-SR) at five weeks (Roseman et al., 2018). A recent systematic review across disorders that included TRD and substance use disorder found parallel results—namely, that the intensity of the acute psychedelic experience (measured through various scales) was the most significant predictor of future treatment response (Romeo et al., 2021). In contrast, recent literature reviews that included non-psychedelic analogs such as 6-MeO-isoDMT have suggested that the psychedelic experience is not necessary for antidepressant effects (Olson, 2020). Nevertheless, further validation is needed of both the scales used to assess psychedelic experience as well as the precise nature of this association in participants diagnosed with depression.
Another key point is that therapeutic milieu may play a confounding role in the psychoactive differences between ketamine and SPs. First introduced in 1965, the concepts of set (referring to mindset) and setting (the location in which the SPs are administered) (Hartogsohn, 2017) became heavily intertwined in SP research. It should be noted from the outset that the effects of set and setting on ketamine administration have rarely been studied or considered. Ketamine is usually offered in stringent clinical settings, though recent qualitative studies suggest that treatment environment may affect participant experience, particularly a strong therapeutic alliance with the prescribing clinician (Griffiths et al., 2021). In contrast, SPs are often administered after extensive preparation, often involving long discussions between the participant and practitioner of what to expect after administration of the SP, in a carefully orchestrated space designed to be soothing (Johnson et al., 2008; Schatzberg, 2020). It is important to note that the more stringent administration of ketamine has led to more conclusive evidence of its antidepressant effects, given that set and setting vary so widely in trials of the therapeutic effects of SPs (Golden et al., 2022).
Given the psychoactive effects of both ketamine and SPs, considerable research has sought to identify similarly effective compounds that lack these psychoactive effects. However, efforts to develop ketamine-like drugs that mirror its antidepressant effects but lack its dissociative effects have, to date, proven largely unsuccessful (Kadriu et al., 2020). With regard to SPs, microdosing—the act of taking sub-psychedelic doses—has generated significant interest. Nevertheless, the COMPASS Pathways Phase 2b trial found that a 25mg dose of psilocybin (considered a “macrodose”) was significantly more effective than a 1mg “microdose” in reducing depressive symptoms three weeks later, though the higher dose was also associated with more adverse events on the first day (61% vs. 38%) (Goodwin et al., 2022b). Other studies found that non-psychedelic levels of psilocybin/LSD lowered levels of self-reported depression and anxiety symptoms as assessed via the Depression, Anxiety, Stress Scale-21 (DASS-21), but this was in the general population and not in those diagnosed with depression or anxiety (Rootman et al., 2022). A placebo-controlled crossover trial in the general population found similar results, with reduced anxiety and stress symptoms in response to non-psychedelic doses of psilocybin versus placebo as assessed through the DASS-21 (Marschall et al., 2022); however, that study excluded those previously diagnosed with any psychiatric disorder.
As noted previously, research into the efficacy of SPs in TRD is still in its infancy. In comparison, a meta-analysis that reviewed a decade of ketamine trials found clinical response rates (defined as a 50% reduction in depressive scale scores) of 36.5% within 24 hours, a percentage that rose to 47.6% over two to seven days. Remission, defined as the absence of depressive symptoms, was found in 18% of the TRD participants within 24 hours and 28.2% within two to seven days (Marcantoni et al., 2020). Although there is less clinical research to draw on when determining the efficacy of SPs in TRD, one small, open-label trial of psilocybin in conjunction with psychological support found that 67% of patients (8/12 participants) met criteria for remission at one week (Carhart-Harris et al., 2016a); results were not provided for earlier timepoints. In a larger trial, the response rate at Week 3 was between 18–37% depending on dose (1–25 mg). Remission rates at Week 3 ranged from 8–29%, again in a dose-dependent manner (Goodwin et al., 2022a), suggesting the importance of proper dosing in SP research. Although trials to determine the effects of SPs in TRD are growing, it is important to note that much previous research has focused primarily on the impact of SPs on depressive symptoms, and efficacy rates could change when SPs are used in a TRD population.
2.3. The Monoaminergic System
Traditional antidepressants are based on the monoaminergic hypothesis of depression, which suggests that a depletion of serotonin, norepinephrine, and/or dopamine underlies depressive symptomatology. While the depletion of these monoamines is a consistent finding in MDD (Delgado, 2000; Hamon and Blier, 2013), the upregulation of monoamines after treatment does not consistently parallel the alleviation of depressive symptoms (Hirschfeld, 2000; Liu et al., 2017). mGlu2/3 antagonists, whose antidepressant-like effects are similar to those of ketamine, appear to mediate their actions by increasing the firing of 5-HT neurons as well as via 5-HT release (Chaki and Fukumoto, 2019).
In addition to its glutamatergic effects, ketamine appears to indirectly modulate monoaminergic neurotransmission. In the prefrontal cortex (PFC) of mice, ketamine, its enantiomers, and/or its metabolites increased extracellular levels of serotonin, dopamine, and norepinephrine (Ago et al., 2019). This transient increase in monoamine levels appeared to be mediated through glutamatergic projections from the medial PFC (mPFC) into the dorsal raphe and locus coeruleus (López-Gil et al., 2019). Serotonin depletion through a tryptophan hydroxylase inhibitor also blocked the antidepressant-like effects of (S)-ketamine in an animal model (du Jardin et al., 2018). In preclinical and clinical PET studies, 5-HT1B receptor binding and serotonin transporter (SERT) occupancy were found to be increased in most, but not all, studies (Spies et al., 2018; Tiger et al., 2020; Yamanaka et al., 2014). However, the role of the serotonergic system in the antidepressant actions of (R)-ketamine are less clear; for instance, at least one study found that 5-HT depletion had no effect on (R)-ketamine’s behavioral antidepressant-like effects (Zhang et al., 2018).
Behaviorally, ketamine effectively targets the motivational dysfunction believed to be related to dopamine reward circuits (Abdallah et al., 2017b; Mkrtchian et al., 2021); clinical trials found that it was particularly successful in ameliorating anhedonia, a symptom rarely improved by traditional antidepressants (Ballard et al., 2017; Lally et al., 2014; Nogo et al., 2022). Briefly, anhedonia is considered a core symptom of depression and a predictor of worse course of illness and treatment response (McMakin et al., 2012; Morris et al., 2009; Uher et al., 2012). Historically, traditional antidepressants have more effectively treated increased negative affect than decreased positive affect (Uher et al., 2012). Thus, while traditional antidepressants can improve symptoms such as a depressed mood, which can improve depressive scale scores and classify patients as “responders” or “remitters”, anhedonia often remains a residual issue (Landen et al., 2005; McCabe et al., 2010; Nierenberg et al., 1999; Price et al., 2009), a distinction that underscores ketamine’s unique effects on anhedonic symptoms. With regard to dopaminergic pathways in particular, preclinical studies found that the Drd1 antagonist SCH39166 blocked the behavioral effects of repeated ketamine administration (Hare et al., 2019).
More recently, ketamine was found to restore multiple behaviors dampened by stress in mice, an effect blocked by inhibiting dopaminergic signaling (Wu et al., 2021a; Wu et al., 2021b). Activation of Drd1 after ketamine administration also increased cortical spinogenesis in parallel timing to ketamine’s behavioral effects (Wu et al., 2021a). High-dose ketamine was also found to dose-dependently increase Drd1 expression in the PFC and hippocampus in a mouse model of schizophrenia by inhibiting related microRNAs (Li et al., 2022a). Lastly, repeated administration of ketamine increased the firing activity of dopaminergic and norepinephrinergic—but not serotonergic—neurons (Iro et al., 2021). Interestingly, the actions of (R)-ketamine were not blocked by the Drd1 antagonist SCH-23390, suggesting that (R)-ketamine may have dopaminergic-independent effects (Chang et al., 2020). Collectively, these results suggest that monoaminergic signaling contributes to ketamine’s antidepressant effects, though it is probably not the main mechanism underlying its rapid-acting impact, especially in light of (R)-ketamine’s 5-HT- and dopaminergic-independent antidepressant-like effects.
In contrast, SPs have clear direct actions on monoaminergic neurotransmission and bind directly to serotonin, dopamine, and norepinephrine receptors. The pharmacology of these various compounds is quite complex and has recently been reviewed in great detail (Inserra et al., 2021). As mentioned above, the main mechanism of action of SPs is agonism of various 5-HT receptors. LSD, DMT, and 5-MeO-DMT may also affect the SERT (Kyzar and Kalueff, 2016; Rickli et al., 2015), though in vitro work reported no interaction between LSD and the SERT (Blough et al., 2014; Rickli et al., 2015). Novel research that seeks to develop non-psychedelic analogues is focusing on β-arrestin-biased ligands, which have lower transduction efficacy for the 5-HT2A receptor, the primary mediator of psychedelic activity (Cao et al., 2022). Further supporting the role of transporters in antidepressant response to SPs, novel monoamine transporter ligands ((2-Aminopropyl)benzo[β]thiophenes) also stimulate a behavioral head-twitch response that mimics psychedelic activity (Rudin et al., 2022).
The degree to which SPs mediate dopaminergic transmission is less clear. In larger amounts, LSD can decrease dopaminergic firing activity, which may account for the psychotic symptoms associated with high doses (De Gregorio et al., 2016). Nevertheless, the reinforcing effects of dopamine may also be associated with greater abuse liability (Berke and Hyman, 2000). Some research even suggests that DMT, psilocybin, and mescaline may convert into dopamine after ingestion, adding another layer of complexity to the impact of SPs on monoaminergic transmission (Fitzgerald, 2021).
2.4. The Glutamatergic System
Both ketamine and SPs are believed to increase neurite outgrowth, synapse formation, and synaptic connections by facilitating changes in neuroplasticity (Aleksandrova and Phillips, 2021). These neuroplastic changes are driven by glutamatergic signaling (primarily in the PFC), with increases in AMPAR activation, a burst of glutamate release, and activation of the mechanistic target of rapamycin (mTOR) signaling pathway contributing to the antidepressant effects of both ketamine and SPs (Aleksandrova and Phillips, 2021). While ketamine and SPs have different primary mechanisms of action, both NMDAR antagonism and 5-HTR agonism activate mTOR downstream, increasing protein synthesis and excitatory activity. Another potential overlapping downstream target is the dephosphorylation of eukaryotic elongation factor 2 (eEF2), which can directly upregulate protein synthesis that contributes to synaptogenesis. This increase in dendritic outgrowth and synaptogenesis from both ketamine and SPs could lay the foundation for their longer-lasting antidepressant effects by reversing both structural and functional synaptic deficits associated with stress and depression. For a more in-depth overview of the impact of ketamine and SPs on indicators of neuroplasticity, see (Aleksandrova and Phillips, 2021; Kadriu et al., 2021).
As an anesthetic, ketamine functions primarily as a non-competitive NMDAR antagonist through an open channel block mechanism (MacDonald et al., 1987). Recent cryo-electron microscope studies of human NMDAR structures found that esketamine appears to bind between the channel gate and the selectivity filter and to move between two locations in the binding pocket (Zhang et al., 2021c). As noted above, the two main mechanistic hypotheses of ketamine (disinhibition and direct inhibition) both center on ketamine’s effects on the glutamatergic system. The disinhibition hypothesis postulates that ketamine acts by favorably binding to NMDARs that express the GluN1/GluN2C, which are preferentially expressed on GABAergic interneurons, therefore blocking the inhibitory activity on excitatory pyramidal neurons in the cortex. Updated research supports this theory, demonstrating that ketamine’s antidepressant effects stem from its ability to preferentially bind to NMDARs on GABAergic interneurons, an effect that is blocked by NMDAR-GluN2B knockdown on Gad1-expressing neurons (Pothula et al., 2021).
The premise of the direct inhibition hypothesis is that ketamine inhibits NMDARs located on post-synaptic terminals, altering cellular signaling pathways that influence protein expression (Miller et al., 2016). Interestingly, nitrous oxide (a non-competitive NMDAR inhibitor) was recently shown to have electrophysiological antidepressant effects similar to those of ketamine in rodents (Izumi et al., 2022). NMDAR antagonism has also been associated with ketamine’s dissociative effects (Lodge and Mercier, 2015; Moghaddam et al., 1997).
Research into the effects of SPs on NMDARs is more mixed. Changes in NMDAR function are known to impact 5-HT2AR expression, which could contribute to the action of SPs. In a mouse model of schizophrenia, NMDAR blockade increased 5-HT2A excitability in the cortex, an effect potentially mediated through the NMDAR-subunit Grin1 and the Gαq protein pathway (Nakao et al., 2022). In a chronic stress animal model, psilocybin greatly increased AMPA/NMDA ratios in hippocampal slices compared to those treated with vehicle or ketanserin (an anti-hypertensive agent used to study the serotonergic system), an effect that was significantly correlated with anhedonic behavior in the sucrose preference test (Hesselgrave et al., 2021). Ibogaine, a psychedelic alkaloid, also appears to be a potential NMDAR antagonist, and this mechanism plays a primary role in its use as a treatment for substance use disorders (Underwood et al., 2021). Another study also found increased NR2A levels after psilocybin administration, though that study also noted that psilocybin was not associated with behavioral antidepressant-like effects (Wotjas et al., 2022). In contrast, repeated administration of LSD did not potentiate synaptic response to NMDAR agonists or increase NMDA currents (De Gregorio et al., 2018).
Despite evidence that both ketamine and SPs may work through NMDARs, the low concentrations needed for ketamine to exert antidepressant effects has cast doubt on how necessary NMDAR inhibition is in this regard (Zanos et al., 2016). It is likely that ketamine inhibits only a fraction (less than half at physiological conditions) of NMDARs, even at peak drug concentrations (Dravid et al., 2007; Zhao et al., 2012), and therapeutic concentrations of the ketamine metabolite (2R,6R)-hydroxynorketamine (HNK) did not block NMDAR function (Lumsden et al., 2019). In addition, other NMDAR antagonists have not been able to fully mimic ketamine’s antidepressant effects; as one example, MK-801 (a structural analog that has been heavily researched) had non-sustainable antidepressant effects in rodents (Autry et al., 2011; Piva et al., 2021; Yang et al., 2016; Zanos et al., 2016). NMDAR antagonists such as memantine, lanicemine, and many others have had little clinical success despite early preclinical evidence (Kadriu et al., 2020; Pochwat et al., 2019). Nevertheless, NMDAR antagonists may still be clinically useful. For instance, a recent meta-analysis found that memantine, despite failing in clinical trials, effectively treated depressive symptoms in those with mood disorders (Hsu et al., 2022). Rapastinel also showed promise in Phase 2 clinical trials for use in TRD, although Phase 3 clinical trials were negative, potentially due to problems with study design (Kato and Duman, 2020). In addition, a recent proof-of-concept study found that the novel intravenous NR2B antagonist MIJ821 had antidepressant effects in individuals with TRD and caused few psychotomimetic side effects (Ghaemi et al., 2021). Finally, dextromethorphan in combination with bupropion was recently FDA-approved to treat MDD (Majeed et al., 2021).
More recently, attention has turned to the AMPAR-mediated mechanisms that may underlie the actions of both ketamine and SPs. The role of AMPAR transmission in the effects of rapid-acting antidepressants has been extensively described (Abdallah et al., 2016; Alt et al., 2006; Kadriu et al., 2019; Zanos and Gould, 2018; Zanos et al., 2018b). Briefly, inhibition of GABA signaling disinhibits glutamate release from excitatory pyramidal neurons. The increased glutamate binds to post-synaptic AMPARs, which increase brain-derived neurotrophic factor (BDNF) release through a rise in Ca2+ influx. BDNF then binds to tropomyosin-related kinase B (TrkB) which, through downstream signaling molecules, activates the mTOR complex 1 (mTORC1). This transient activation upregulates proteins related to increased excitatory transmission, such as post-synaptic density-95, Synapsin I, and increased membrane insertion of GluA1.
Many preclinical studies support this mechanism of action for ketamine (Li et al., 2010; Zanos et al., 2016; Zhou et al., 2014). There has also been a drive to classify the actions of ketamine’s enantiomers and metabolites, particularly (R)-ketamine and (2R,6R)-HNK (Hess et al., 2022). Notably, the actions of (R)-ketamine, which induced longer-lasting antidepressant effects in a chronic stress model, appeared to depend on AMPA and TrkB activation. (S)-ketamine, in turn, activated mTOR independently of TrkB activation (Rafało-Ulińska and Pałucha-Poniewiera, 2022). The inactivation of eukaryotic initiation factor 4E-binding proteins (4E-BPs) through mTOR is essential for the antidepressant-like effects of both ketamine and (2R,6R)-HNK (Aguilar-Valles et al., 2021). Recent research into the role of mTORC1 on ketamine’s effects have produced somewhat unexpected results. Specifically, rapamycin (which inhibits the actions of mTOR) prolonged ketamine’s antidepressant effects in participants with TRD (Abdallah et al., 2020). A recent clinical trial similarly found that pre-treatment with rapamycin prolonged ketamine’s antidepressant, but not anti-suicidal, effects (Averill et al., 2022). This may be due, in part, to rapamycin’s anti-inflammatory effects in the periphery, which could contribute to ketamine’s antidepressant effects (Attur et al., 2000; Chen et al., 2013). Preclinical studies also found that (R)-ketamine had mTOR-independent antidepressant-like effects in a chronic stress model, where administration of (R)-ketamine, but not (S)-ketamine, significantly attenuated phosphorylation of extracellular signal-regulated kinase (ERK) (Yang et al., 2018b). Further research also identified the ERK-NRBP1-CREB-BDNF signaling pathway in microglia as important to (R)-ketamine’s sustained effects (Yao et al., 2022).
Evidence similarly suggests that the therapeutic effects of SPs are mediated through mTORC1 activation (Aleksandrova and Phillips, 2021; Kadriu et al., 2021; Vollenweider and Preller, 2020; Vollenweider and Smallridge, 2022). For instance, sustained AMPAR and mTOR activation was found to be necessary for SP-induced neuronal growth in cortical cultures (Ly et al., 2020). Proteomic analyses in human brain organoids also found that LSD increased mTOR activation (Ornelas et al., 2022), further supporting the importance of mTOR in neural plasticity in humans. Finally, a metabolomics study also found increased mTOR-related signaling after ayahuasca consumption (Madrid-Gambin et al., 2022). In contrast, elevated plasma levels of BDNF, which are generally associated with increased mTOR-related signaling, were not observed in healthy volunteers following administration of LSD or psilocybin (Holze et al., 2022). Nevertheless, there is a paucity of clinical research regarding how necessary mTORC1 is to the therapeutic effects of SPs, and no registered clinical trials are presently investigating this important question.
Finally, while ionotropic glutamatergic transmission is clearly essential for the antidepressant effects of both ketamine and SPs, metabotropic glutamate receptors (mGluRs) may also play an important role (Chaki, 2021; Musazzi, 2021). mGluR levels have consistently been linked to stress vulnerability (Peterlik et al., 2016), and mGluR2/3 antagonism has been an area of particular interest in drug discovery. mGluR2/3 antagonists were found to exert similar antidepressant-like effects to ketamine, potentially through their interactions with the serotonergic system (Chaki and Fukumoto, 2019), and have shown early promise as rapid-acting antidepressants (Pilc et al., 2022). Interestingly, (2R,6R)-HNK was found to exert mGlu2R-dependent antidepressant effects (Zanos et al., 2019). Another recent study found that while ketamine did not alter mRNA or the protein expression of ionotropic glutamate receptors, both chronic stress and ketamine administration altered mGluR2 expression (Elhussiny et al., 2021). Co-administration of ketamine and an mGluR2/3 antagonist also sustained antidepressant response in animal models (Pałucha-Poniewiera et al., 2021; Rafało-Ulińska et al., 2022). In contrast, few studies have examined the effects of SPs on mGluRs. mGluR2/3 agonists inhibited the effects of DOI in mice, as it appears that mGluR2s and 5-HT2ARs form a G-protein coupled receptor complex particularly important for the head-twitch response (Benvenga et al., 2018; Delille et al., 2012; Gewirtz et al., 2002; Kłodzinska et al., 2002). However, these findings are preliminary, and more research is needed to determine whether mGluRs play a role in the antidepressant response associated with SPs.
2.5. The GABAergic system
A large body of evidence links depression with GABAergic signaling deficiencies (Duman et al., 2019), and the disinhibition hypothesis of ketamine’s mechanism of action relatedly posits that GABAergic neurotransmission dysfunction is involved in the pathophysiology of depression (Zanos and Gould, 2018). Overall, MDD is associated with GABAergic deficits that coincide with homeostatic-like reductions in glutamatergic neurotransmission and can be rescued by rapid-acting antidepressants (Ren et al., 2016). A similar mechanism may underlie, at least to some extent, the mechanism of action of SPs. Indeed, excitatory pyramidal cells are regulated by GABAergic interneurons that express serotonin receptors (Nichols, 2016). Cortical GABAergic interneurons are abundant, but only 13–46% of them express the 5-HT2AR compared to 86–100% of excitatory cells in layers II-IV of the human and monkey cortex (De Almeida and Mengod, 2007).
Considerable research has examined how ketamine may influence GABAergic signaling. Studies suggest that ketamine upregulates GABAA receptor activity in the cortex and hippocampus (Wang et al., 2017), increases turnover of hippocampal GABA (Silberbauer et al., 2020), and increases GABA release in the mPFC (Pham et al., 2020). Other compounds that upregulate GABAA receptor activity by preferentially binding to the delta subunit also have antidepressant properties, including the progesterone metabolite allopregnanolone (brexanolone) (Lüscher and Möhler, 2019), which was FDA-approved to treat postpartum depression in 2019 (Pinna et al., 2022). Negative GABAA receptor allosteric modulators also exert antidepressant effects by strengthening excitatory synapses without ketamine’s adverse effects (Fischell et al., 2015). Muscimol and high doses of benzodiazepines, both of which increase GABAA receptor transmission, were found to dampen ketamine’s antidepressant effects (Andrashko et al., 2020; Fuchikami et al., 2015), a finding paralleled by results showing that increased activation of GABAA receptors decreased antidepressant response to ketamine (Pham et al., 2020). Regardless of the implicated mechanisms, ketamine rapidly alleviates deficits in synaptic GABAergic markers as well as the frequency of inhibitory post-synaptic currents in the mPFC induced by stress, perhaps by transiently releasing large amounts of glutamate and/or directly facilitating the plasticity of GABAergic interneurons (Ghosal et al., 2020).
With regard to SPs, psilocybin has been found to raise both GABA and glutamate levels in the mPFC and to inhibit glutamate release in the hippocampus by acting on serotonin receptors located on GABAergic interneurons (Carhart-Harris and Nutt, 2017; Mason et al., 2020). Stress-induced alterations in GABAergic circuitry in the ventral tegmental area, a dopaminergic region strongly associated with reward and addiction, were normalized by 5-HT2AR agonists (Kimmey et al., 2019), suggesting that SPs would have similar actions. However, the therapeutic effects of SPs are more likely to be related to their direct action on excitatory pyramidal neurons than on their ability to mediate GABAergic interneurons. In support of this notion, the pharmaco-electroencephalogram response to LSD was found to be similar between GABAA receptor δ subunit knockout mice and wildtype controls (Grotell et al., 2021).
Given that the balance between inhibitory and excitatory neurotransmission needs to be appropriately maintained to prevent psychiatric disturbances, additional research is needed to elucidate how GABAergic signaling contributes to the rapid and sustained antidepressant effects of ketamine and—even more so—to that of SPs. For instance, it would be interesting to evaluate whether ketamine and SPs recover levels of the enzyme glutamate decarboxylase (GAD) in cortical and limbic brain centers, like the hippocampus, which is sensitive to excitotoxicity (Gruenbaum et al., 2022). GAD converts glutamate into GABA in a single step, quickly distinguishing excitatory from inhibitory signals, and low GAD levels are associated with chronic stress and depression (Miyata et al., 2021). GABAB receptors are another pharmacological target of interest; generally, GABAB receptor agonists are pro-depressive whereas antagonists are anti-depressive (Jacobson et al., 2018). It is unknown whether SPs work via GABAB receptors, but administration of the GABAB receptor agonist baclofen prevented the antidepressant-like effects of ketamine in a mouse model, further suggesting that GABAB receptor inhibition may have beneficial effects (Rosa et al., 2016).
2.6. Opioid System
There has been a resurgence of interest regarding the role of the opioid system in MDD (Perez-Caballero et al., 2020), in part due to observations that ketamine’s antidepressant effects may require the activation of certain opioid receptors (Williams et al., 2019; Williams et al., 2018). The opioid system is highly dysregulated in depression; for instance, a reduction in mu-opioid receptor availability was associated with treatment-resistance and with the improper processing of social cues (Peciña et al., 2019). The efficacy of low-dose buprenorphine, an opioid partial agonist, further implicates the opioid system in antidepressant treatment (Peciña et al., 2019). Self-medication with opioid agonists has also been repeatedly observed in individuals with depression, and associations are frequently found between depressive symptoms and opioid misuse (Rogers et al., 2021).
Recent research suggests that ketamine interacts significantly with the mu-opioid receptor and has weak affinity for the kappa-opioid receptor (Bonaventura et al., 2021). This affinity to opioid receptors is only a five- to 20-fold difference less than ketamine and its enantiomers’ affinity for the NMDAR. Some preclinical research found that opioid antagonists abolish ketamine’s rapid-acting antidepressant effects, though an opiate agonist alone is not sufficient to induce a parallel antidepressant effect (Klein et al., 2020; Zhang et al., 2021a). However, other preclinical studies found that naltrexone had no effect on ketamine’s antidepressant-like effects in a chronic social defeat stress model (Zhang and Hashimoto, 2019). Despite ketamine’s weak affinity for kappa-opioid receptors, blockade of these with a high-affinity and selective short-acting kappa-opioid receptor antagonist (LY2444296) abolished the antidepressant effects of both ketamine and its metabolite (2R,6R)-HNK (Wulf et al., 2022). In addition, administration of an opioid antagonist (naltrexone) blocked ketamine’s antidepressant effects in participants with TRD (Williams et al., 2019; Williams et al., 2018). A current clinical trial is building on this work to correlate naltrexone’s blockade of antidepressant effects with neuroimaging data (NCT04977674), and other clinical trials are investigating adjunctive ketamine use in combination with buprenorphine or methadone for the treatment of opioid use disorder and comorbid depression (NCT04177706, NCT05051449).
Very little is known about the link between SPs and the opioid system. The plant alkaloid ibogaine has strong psychedelic properties and affinity for kappa-opioid receptors (Wasko et al., 2018). SP binding to mu-opioid and kappa-opioid receptors has also been found to correlate positively with subjective “therapeutic component scores”, though it is important to note that mu-opioid receptor binding also correlated positively with self-reported measures of dependence (Zamberlan et al., 2018). SPs may also inadvertently modulate the addiction circuitry related to opioid use disorders, with preliminary research showing early success (reviewed in (Lee et al., 2022)). As an example, a single dose of ibogaine was effective against opioid dependence 12 months later (Noller et al., 2018). Nevertheless, significantly more research on the effects of SPs on the opioid system is needed to corroborate these findings.
2.7. Inflammation
Evidence suggests that stress-induced pro-inflammatory states in the central nervous system may contribute to the pathology of TRD (Yang et al., 2018a). Greater inflammatory responses have typically been associated with a more severe course of illness, higher chance of recurrence, and worse treatment outcomes (Suneson et al., 2021), though not always (Kofod et al., 2022). These inflammatory findings have been paralleled in preclinical research, which found that administration of the endotoxin lipopolysaccaharide (LPS) triggers “depressive-like” behaviors in rodents (Zhao et al., 2020). Peripheral blood mononuclear cells from individuals with MDD stimulated with LPS ex vivo had greater reactivity than cells of healthy volunteers, a response that also correlated with greater amygdala activation (Boukezzi et al., 2022).
Inflammatory markers such as tumor necrosis factor alpha (TNF-α), C-reactive protein (CRP), interleukin 1β (IL-1β), interleukin 6 (IL-6), and interleukin 8 (IL-8) have consistently been found to be upregulated in both animal models of depression and individuals diagnosed with MDD (Haapakoski et al., 2015; Köhler et al., 2017; Kubera et al., 2011; Wang and Miller, 2018). CRP appears to play a particularly important role, as it has been able to predict subsequent depressive symptoms (Valkanova et al., 2013) and distinguish TRD participants from treatment-responsive, unmedicated participants as well as healthy volunteers (Chamberlain et al., 2019). A positron emission tomography (PET) imaging study also reported increased binding of translocator protein 18kDa (TSPO), a biomarker of inflammation, in the PFC and anterior cingulate cortex of individuals currently experiencing a major depressive episode (Meyer et al., 2020).
Two potential mediators of these inflammatory responses are the hypothalamic-pituitary-adrenal (HPA) axis and the kynurenine pathway. Overactivation of the HPA axis is characteristic of depression and chronic stress (Stetler and Miller, 2011), and hypersecretion of corticotropin-releasing hormone (CRH) can cause hypercortisolism and decreased reward-system responsivity of the dopaminergic system (Gold and Chrousos, 2002). Indicators of HPA axis hyperactivity are also strongly associated with somatic symptoms, which are reported more often in TRD than MDD (Iob et al., 2020).
The kynurenine pathway, a major modulator of glutamatergic and serotonergic signaling, is also heavily impacted by inflammation (Kowalczyk et al., 2019). Tryptophan-2,3-dioxygenase (TDO) is the kynurenine pathway’s main enzyme and catabolizes tryptophan, the precursor for serotonin synthesis. TDO is induced in pro-inflammatory states (Chen and Guillemin, 2009), leading to a tryptophan depletion that can cause depressive symptoms in vulnerable persons (Wichers et al., 2005). The products of the kynurenine pathway are also biologically active, with kynurenic acid (KA) acting as a neuroprotective agent (Foster et al., 1984) and quinolinic acid (QA) as an endogenous neurotoxin (Lugo-Huitrón et al., 2013). Altered ratios of KA and QA have repeatedly been found in participants with depression (Allen et al., 2018; Doolin et al., 2018; Moaddel et al., 2018; Zhou et al., 2018). Considerable research has examined the role of the kynurenine pathway in depression and inflammation; we refer the interested to reader to (Kowalczyk et al., 2019) for a more in-depth discussion.
Ketamine appears to have mixed results on markers of inflammation (see (Johnston et al., submitted) for a recent review). In a mouse model of ulcerative colitis, (R)-ketamine, but not (S)-ketamine, decreased blood IL-6 levels, an anti-inflammatory effect blocked by administration of a TrkB antagonist, suggesting that ketamine might have anti-inflammatory effects (Fujita et al., 2021). Preclinically, (R)-ketamine also significantly attenuated levels of splenomegaly, central and peripheral measures of pro-inflammatory cytokines, and cognitive impairment in mice administered LPS (Zhang et al., 2021b). Interestingly, Wistar rats subjected to maternal deprivation had increased levels of pro-inflammatory cytokines that were reversed in a sex-dependent manner by ketamine or ketamine in combination with either/both electroconvulsive stimulation (ECS) and the selective serotonin reuptake inhibitor (SSRI) escitalopram (Abelaira et al., 2022). However, certain measures of inflammation did not respond to ketamine, including catalase activity and carbonyl levels in the PFC and hippocampus of female rats (Abelaira et al., 2022). Clinical studies have echoed these sex-dependent preclinical results, finding that lower baseline levels of IL-8 predicted treatment response to ketamine in females but not males with depression (Kruse et al., 2021).
Ketamine also affects both the HPA axis and kynurenine pathway. In an animal model of depression, ketamine restored hippocampal glucocorticoid receptor expression, thus helping to restore the HPA axis’s negative feedback loop (Wang et al., 2019). Ketamine also reduced levels of corticosterone and adrenocorticotropic hormone (ACTH) after LPS injection (Besnier et al., 2017). Clinically, it seems that ketamine promotes an early burst of cortisol in healthy volunteers (Hergovich et al., 2001; Khalili-Mahani et al., 2015), though exploration of this phenomenon in individuals with TRD is needed. Ketamine may also mediate the effects of QA, the pro-inflammatory byproduct of the kynurenine pathway that binds to NMDARs via NMDAR blockade (Miller, 2013; Walker et al., 2013). Ketamine restored the decreased KYN:tryptophan ratio both clinically (Moaddel et al., 2018) and in a chronic unpredictable mild stress model (Wang et al., 2015). Increased KA levels after ketamine administration also correlated with MADRS score reductions at Days 1, 13, and 26 post-ketamine (Zhou et al., 2018).
Some studies suggest that ketamine acts on the reactivity of the immune system to aversive stimuli such as stressors. For example, ketamine demonstrated prophylactic effects against chronic stressors, preventing stress-induced behavioral changes (Brachman et al., 2016) as well as resilience against LPS- or TNF-α-induced depressive-like behaviors associated with the NLRP3 inflammasome pathway (Camargo et al., 2021). A recent proof-of-concept study found that a pre-stress ketamine infusion in healthy volunteers significantly decreased levels of salivary cortisol and alpha amylase (Costi et al., 2022). It should be noted that chronic, high-frequency ketamine use may increase neurogenic and IgE inflammation to contribute to urological toxicity (Ng et al., 2021), but this has only been observed in ketamine abusers and not at antidepressant doses.
Compared to their antidepressant (or antidepressant-like) effects, research on the anti-inflammatory effects of SPs is still in its infancy, but some information can be extrapolated from other disorders (see (Saeger and Olson, 2022) for a recent review of the anti-inflammatory effects of SPs on various neurodegenerative disorders). SPs appear to produce strong anti-inflammatory effects by binding to 5-HT2A receptors on immune cells (Flanagan and Nichols, 2018), where activation was found to lessen airway inflammation in a mouse model of asthma (Flanagan et al., 2019a) and vascular inflammation in a high fat diet model (Flanagan et al., 2019b). Microglia also express receptors targeted by SPs, including 5-HT2A, 5-HT2B, 5-HT7, and sigma-1 receptors (Jia et al., 2018; Turkin et al., 2021). Recent studies have also found associations between lifetime self-reported SP use and lower rates of heart disease, diabetes, body mass index, and hypertension (Simonsson et al., 2021a; Simonsson et al., 2021b; Simonsson et al., 2021c). Further research is needed to explore the potential mechanisms underlying these broad anti-inflammatory outcomes, including potential lifestyle confounders not listed in these studies, such as diet, socioeconomic status, and age.
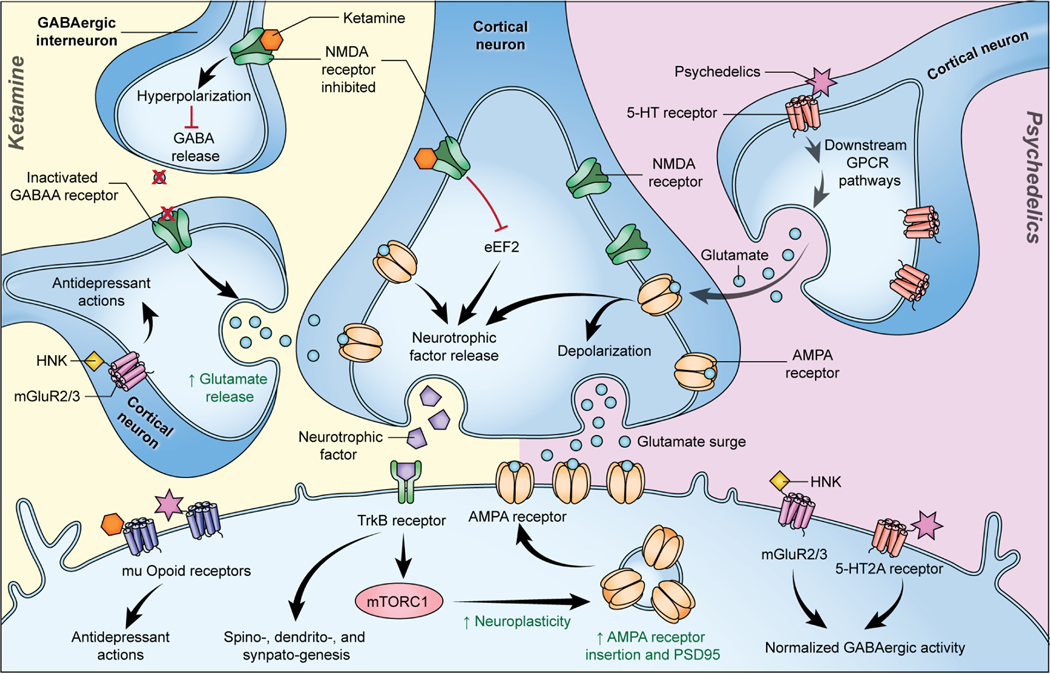
With regard to the link between SPs, depression, and inflammation in particular, very little recent research has been published (we refer the interested reader to (Flanagan and Nichols, 2018; Flanagan and Nichols, 2022; Kadriu et al., 2021) for an overview of previous work). Briefly, the 5-HT2A receptor is expressed in many immune tissues, including the spleen and circulating lymphocytes. SPs appear to have strong anti-inflammatory effects due their action on the 5-HT2A receptor, decreasing levels of cytokines and TNF-α within hours of administration (Pelletier & Segel, 2009). DOI, in particular, was found to provide a prophylactic effect against TNF-α administration, preventing cytokine and chemokine upregulation (Martin & Nichols, 2013). Interestingly, activation of the 5-HT2A receptor by serotonin is primarily pro-inflammatory, leading Flanagan and Nichols to propose that the effects of SPs may be explained by functional selectivity or by the ability of the receptor to use different conformations to activate differing effector pathways (Flanagan and Nichols, 2018). In recent pre-print reports, researchers found that psilocybin decreased TNF-α secretion (Smedfors et al., 2022) and that both psilocin and DMT decreased levels of TLR4, p65, and CD80 after LPS challenge in microglial cell lines (Kozłowska et al., 2021). In LPS-treated macrophage cells, psilocybin treatment decreased levels of TNF-α, IL-1β, IL-6, and cyclooxygenase-2 (COX-2) (Nkadimeng et al., 2021). In a chronic stress mouse model of PTSD, both DMT and “pharmahuasca” (DMT+harmaline) decreased reactive oxygen species (ROS) and inflammatory gene expression (Kelley et al., 2022). DMT also decreased TNF-α and IL1-β levels and increased IL-10 levels in an in vivo model of ischemic brain injury (Nardai et al., 2020). While SPs play a large role in the serotonergic system, to the best of our knowledge, no research has explored their impact on the kynurenine pathway. As a precursor of serotonin itself, tryptophan is uniquely placed to affect the impact of SPs and could be an area of future interest. Preliminary research has also investigated the role of the HPA axis in healthy volunteers, finding increased cortisol and ACTH in plasma during the peak psychedelic effects of psilocybin (Hasler et al., 2004). However, long-term measures in participants with depression are needed to determine the role of HPA axis function in the effect of SPs, and further research is needed more broadly to determine the role of SPs in treating inflammation-associated depression. The hypothesized convergent and divergent mechanisms of ketamine and SPs can be viewed in Figure 1.
Figure 1. Hypothesized convergent and divergent mechanisms of ketamine and serotonergic psychedelics (SPs).
Ketamine increases glutamate release into the synapse through preferential blockade of NMDARs on GABAergic interneurons (disinhibition hypothesis) or other methods. This glutamate “surge” leads to downstream signaling that transiently activates mTORC1, increasing synaptic protein translation of PSD-95, AMPARs, and others. SPs trigger this glutamate surge through downstream GPCR pathways after 5-HT receptor activation, which leads to parallel mTORC1 activation and subsequent increases in synaptic protein translation. Other proposed mechanisms of antidepressant effects include binding to mu-opioid receptors and normalizing GABAergic activity through either mGluR2/3 (ketamine/(2R,6R)-HNK) or post-synaptic 5-HT2A receptors (SPs). Figure is approximate for illustrative purposes. Abbreviations: 5-HT: 5-hydroxytryptamine; AMPAR: α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor; GPCR: G protein-coupled receptors; HNK: hydroxynorketamine; mGluR: metabotropic glutamate receptor; mTORC1: mechanistic target of rapamycin complex 1; PSD-95: postsynaptic density protein 95.
3.0. Imaging and pharmacodynamic biosignatures of ketamine and SPs
Developing sensitive and reliable neurophysiological and neuroimaging biomarkers for MDD—and TRD in particular—is critical to improving diagnosis and treatment selection, especially in the context of novel therapeutics such as ketamine and SPs. Conversely, understanding the mechanisms by which novel, rapid-acting therapeutics impact macroscopic brain activity (as recorded by neurophysiological techniques such as electroencephalography (EEG) or magnetoencephalography (MEG) or by hemodynamic response using functional magnetic resonance imaging (fMRI)) can help develop the next generation of rapid-acting antidepressants that lack dissociative or psychotomimetic effects, improving treatment tolerability and effectiveness. Dysregulated neuroplasticity in vulnerable brain regions, including the PFC and hippocampus, is implicated in the pathophysiology of MDD (Liu et al., 2017; Price and Duman, 2020), and facilitation of adaptive neuroplasticity might be a convergent mechanism for the antidepressant efficacy of both ketamine and SPs (Aleksandrova and Phillips, 2021). This section reviews the evidence for convergent neuroplasticity mechanisms from MEG and EEG studies of long-term potentiation (LTP) and gamma power, as well as fMRI studies examining resting-state connectivity.
Neurophysiologically, synaptic efficacy has been examined using tasks that measure LTP, such as amplitude changes of sensory evoked potentials to visual, auditory, and somatosensory stimulation. In a double-blind, placebo-controlled trial, ketamine enhanced visual sensory evoked potentials three to four hours post-ketamine administration compared to an active placebo (remifentanil) in individuals with MDD, potentially reflecting increased synaptic efficacy concomitant with the drug’s antidepressant effects (though there was no association between the evoked potential changes and treatment response) (Sumner et al., 2020a). In addition to LTP, shorter-term plasticity has been measured using event-related response EEG tasks that take advantage of deviance detection in the context of repeated visual or auditory stimuli, for example using a mismatch negativity (MMN) task. In a second double-blind, placebo-controlled trial, ketamine significantly increased the MMN event-related response three to four hours after infusion in individuals with MDD (Sumner et al., 2020b), though this increase was not associated with treatment response and contradicts a host of studies suggesting that ketamine attenuates MMN response during drug administration in healthy volunteers (Rosburg and Kreitschmann-Andermahr, 2016; Rosch et al., 2019; Schmidt et al., 2012). Ketamine has also been found to alter the amplitude and latency of a number of additional ERP components in healthy volunteers during the MMN task (for a review, see (Schwertner et al., 2018)).
In contrast to ketamine, few studies have been conducted to assess the effects of SPs on the MMN response, and existing studies indicate mixed findings in healthy volunteers. For example, while a few studies found that psilocybin did not acutely attenuate the auditory or visual MMN response in healthy volunteers (Schmidt et al., 2012; Schmidt et al., 2013; Umbricht et al., 2003), at least one study reported that psilocybin reduced the MMN response to tactile oddball stimuli (Duerler et al., 2021). In contrast to psilocybin, DMT was found to blunt the auditory MMN response in healthy volunteers (Heekeren et al., 2008), and LSD was found to blunt several event-related potential responses to oddball neutral and angry faces in healthy volunteers (Murray et al., 2022). Further research is needed to elucidate the effects of SPs on MMN response, particularly in individuals with MDD, in order to clarify whether SPs upregulate short- and long-term plasticity mechanisms that mirror ketamine’s proposed mechanisms of action.
Another emerging area of neurophysiological interest is gamma rhythms, particularly gamma power, as a biomarker or endophenotype for MDD. Findings suggest that gamma can distinguish individuals with MDD from healthy volunteers, as well as distinguish subtypes of depression (e.g., bipolar disorder from unipolar depression) (Fitzgerald and Watson, 2018). Gamma rhythms have been found to correlate with action potential generation (Watson et al., 2018), emerge from the coordinated interaction of glutamatergic excitation and GABAergic inhibition (Buzsáki and Wang, 2012), and robustly increase following ketamine administration (Gilbert and Zarate, 2020). In randomized, double-blind, crossover trials in individuals with TRD, ketamine significantly increased gamma (30–50 Hz) power immediately and at two hours post-infusion compared to active placebo (midazolam) (de la Salle et al., 2022), and at six to nine hours following ketamine administration compared to saline placebo (Nugent et al., 2019a; Nugent et al., 2019b); gamma power changes were consistently associated with antidepressant response (de la Salle et al., 2022; Nugent et al., 2019a; Nugent et al., 2019b).
To date, few studies have investigated the effects of SPs on oscillatory power, particularly gamma. In animal work, psilocybin increased local field potential power in the anterior cingulate cortex (ACC) in low (35–55 Hz) and high (65–120 Hz) gamma (at trend levels) while decreasing power in the lower frequency bands including delta, theta, and alpha (Golden and Chadderton, 2022). In healthy volunteers, LSD significantly reduced gamma power during resting-state recordings (Murray et al., 2022) while also reducing power in the lower frequency bands including theta, alpha, and beta (Carhart-Harris et al., 2016b; Murray et al., 2022). As with LTP changes, more work is needed to determine whether SPs have shared downstream mechanisms with ketamine that increase cortical excitability and gamma power.
Resting-state functional connectivity changes measured with fMRI are another method to examine changes in synaptic plasticity following ketamine and SP administration. In particular, systems-level dysregulation in the default mode network (DMN), including regions such as the ACC, posterior cingulate cortex (PCC), and mPFC is implicated in MDD (Marchetti et al., 2012; Sheline et al., 2010), with findings suggesting reduced connectivity in MDD participants relative to healthy volunteers within these regions (Abdallah et al., 2017a; Kraus et al., 2020). In an open-label study of individuals with TRD, ketamine increased global brain connectivity in the PFC at one day post-infusion (Abdallah et al., 2017a). Another randomized, double-blind, crossover trial found that ketamine increased DMN and insula connectivity in individuals with TRD at two days post-infusion, normalizing connectivity between these regions to levels seen in healthy volunteers at baseline (Evans et al., 2018). A separate randomized, double-blind, crossover study found that ketamine increased PFC and striatal connectivity in individuals with TRD at two days post-infusion, normalizing connectivity levels to those seen in healthy volunteers; in addition, this increase was associated with sustained improvements in anhedonia—defined as loss of interest or pleasure—in those with TRD (Mkrtchian et al., 2021). Interestingly, an open-label study of repeated ketamine dosing in individuals with MDD found that ketamine also modulated connectivity between limbic regions and other resting-state network nodes of the salience and central executive networks at one day after the first and fourth ketamine infusions (Vasavada et al., 2021).
Studies of resting-state functional connectivity in SPs also indicate that these drugs increase network connectivity in regions of the DMN and modulate connectivity between the DMN and other regions implicated in the pathophysiology of depression. For example, increased functional connectivity within the DMN was observed one day following open-label psilocybin administration in individuals with TRD, accompanied by decreased cerebral blood flow in the temporal cortex, including the amygdala (Carhart-Harris et al., 2017). In addition, increased ACC and PCC connectivity was found post-psilocybin administration, though this did not correlate with change in depression scores; in that study, increased ventromedial PFC and bilateral inferior lateral parietal cortex connectivity and decreased parahippocampal and PFC connectivity predicted treatment response at five weeks (Carhart-Harris et al., 2017). A double-blind, randomized, placebo-controlled trial of psilocybin found reduced brain network modularity at three weeks following drug administration in individuals with MDD, indicating greater brain network integration that was associated with treatment response (Daws et al., 2022). Finally, an open-label study of ayahuasca also found increased ACC and PCC connectivity, as well as increased ACC and right mesial temporal lobe limbic structure connectivity, one day after ayahuasca ingestion in healthy volunteers (Sampedro et al., 2017).
4.0. Limitations, Considerations, and Regulatory Issues
Regulatory issues are associated with the use of both ketamine and SPs for the treatment of MDD. However, given that ketamine has been approved at high doses for anesthetic use with few associated harms for nearly 60 years (Peltoniemi et al., 2016), such issues are an order of magnitude larger for SPs.
As noted above, in light of concerns about ketamine’s abuse potential, FDA approval for ketamine only extends to the specific use of intranasal esketamine under a REMS monitoring agreement (U.S. Food & Drug Administration, 2019). However, despite the precautions around ketamine/esketamine’s potential abuse liability and misuse outside of clinical supervision (Liu et al., 2016), it remains unclear whether the subanesthetic doses of ketamine used to exert antidepressant effects are enough to spur abuse (Simmler et al., 2022). In high—not antidepressant—doses, ketamine has been associated with disruptions in learning and memory as well as urinary dysfunction (Liu et al., 2016; Schifano et al., 2021), and can also mimic and induce symptoms of psychosis, particularly in those at high risk for psychosis or individuals diagnosed with schizophrenia (Lahti et al., 1995). A recent meta-analysis found that even antidepressant-dose ketamine was associated with psychosis-like symptoms in healthy volunteers and participants with schizophrenia (Beck et al., 2020), suggesting that it is not an ideal candidate for use in some neuropsychiatric disorders.
Although SPs have been used for centuries, particularly in traditional medicine practices, concerns about their therapeutic use persist, as evidenced by their classification as Schedule I substances. In one survey of individuals who had ever used SPs, 13% endorsed at least one harm associated with SP use (cigarette smoking and problematic marijuana use were the two most frequently-reported problems), and these participants benefited less from the experience than those who reported no harm (Raison et al., 2022); nevertheless, it is important to specify that this study examined naturalistic use of SPs and may thus not reflect harms associated with SP administration in a clinical setting. SP dose can also impact their adverse event profile; specifically, high doses associated with antidepressant effects appear to cause more adverse events than low doses (Goodwin et al., 2022a). Other survey-based studies found that psychedelic experiences were associated with long-term improvements in mental well-being in the general population, even following a single lifetime use (Mans et al., 2021). Adverse effects linked to therapeutic SP use include psychological distress and prolonged psychosis, both of which can carry significant risks for participants (Carbonaro et al., 2016; Martinotti et al., 2018); nevertheless, in clinical settings, these effects often resolve after drug effects have dissipated (Johnson et al., 2019; Johnson et al., 2008) and are thought to fall within acceptable limits (Reiff et al., 2020). Given the serotonergic mediation of neural plasticity, the higher serotonin levels associated with SP use can also bring about mixed effects (e.g., improved recovery capacity but increased vulnerability to depression) (Branchi, 2011).
Administration of SPs may also contribute to serotonin toxicity. While an increase in serotonin is characteristic of psychedelic action, SPs alone pose a low risk for serotonin toxicity. However, combining SPs with therapeutics such as monoamine oxidase inhibitors (MAOIs) may increase risk, particularly for ayahuasca, the brew containing the MAO-A isozyme inhibitor harmine, which prolongs the effects of DMT (Malcolm and Thomas, 2022). Symptoms of serotonin toxicity include fluctuating vital signs, agitation, muscle rigidity, and seizure activity. Use of certain SPs has also been linked to neurological and cognitive impairments through cytotoxicity and oxidative stress (Malcolm and Thomas, 2022).
Participant inclusion issues may also bias the results of SP research, given that a high proportion of research participants have previously used SPs (36–56% compared to 11.5% in the US general population) (McClure-Begley and Roth, 2022), though use of SPs as self-medication may play a role in this higher proportion. Researchers also found that mystical-type experiences were more likely in those who were ranked higher in the undefined personality traits of “openness” and “acceptance” (Aday et al., 2021), which suggests that including individuals who have previously used SPs and have positive expectations surrounding their use may confound study results. Another key point is that most trials of SPs are still open-label, long-term follow-up, or retrospective analyses (Berkovitch et al., 2021; Carhart-Harris et al., 2018). Placebo-controlled studies are necessary, though many challenges are associated with conducting a proper randomized, controlled clinical trial for SPs; these include resolving the role of psychoactive effects in the mechanism of action behind the antidepressant effects of SPs and the need for a comparator psychedelic compound that works through different mechanisms (Aday et al., 2022; Muthukumaraswamy, 2021). For instance, in early ketamine randomized clinical trials, midazolam (McGirr et al., 2015), a benzodiazepine that produces similar side effects to ketamine, provided adequate blinding. However, it should be noted that while ketamine produces psychotomimetic effects such as dissociation, these are mild compared to those of SPs and can thus be more easily controlled for with an appropriate placebo. In this context, an interesting future solution could be the use of SP microdosing (defined as doses that have no psychedelic effects), which could be placebo-controlled. Early microdose randomized clinical trials have begun for psilocybin (NCT05227742).
5.0. Future Research Directions
Rapid-acting antidepressants have led to a paradigm shift in neuropsychiatry and reinvigorated the search for novel pharmacological approaches to disorders such as MDD. However, research into how rapid-acting antidepressants mechanistically affect the underlying psychopathology of neuropsychiatric disorders is in its earliest stages. Novel innovations and approaches, such as PsychLight—a biosensor capable of detecting psychedelic potential in novel compounds by measuring endogenous 5-HT activity in awake mice—should help shed light on the underlying mechanisms of SPs and other potential rapid-acting antidepressants (Cameron et al., 2021; Dong et al., 2021). In addition, identifying compounds that have SP- and ketamine-like attributes (i.e., upregulation of neurotrophic signaling) but lack hallucinogenic properties may be an effective strategy for developing therapies that allow widespread clinical use. Non-psychedelic analogues, such as tabernanthalog (Cameron et al., 2021), could also help mitigate some of the negative psychoactive effects associated with SP and ketamine administration. In addition, the further development of (R)-ketamine as an antidepressant has shown promise, both in terms of its potentially reduced side effect profile and preclinical and early clinical therapeutic efficacy versus (S)-ketamine (Wei et al., 2022; Zhang et al., 2022); nevertheless, it should be noted that the evidence for (R)-ketamine, though promising, is relatively limited compared to (S)-ketamine, and additional clinical work is needed to fully ascertain (R)-ketamine’s precise therapeutic profile. Most recently, results from a Phase 2a double-blind, randomized, placebo-controlled, multicenter clinical trial of (R)-ketamine (PCN-101) (NCT05414422) for TRD found that this agent failed to meet its primary endpoint, showing no significant change from baseline MADRS scores at 24 hours, despite an overall positive safety profile (Atai Life Sciences, 2022).
Further research is also still needed to clarify whether SPs can truly be classified as rapid-acting antidepressants, given that most research in this area has focused on the impact of acute SP administration on longer time courses (Romeo et al., 2020). In addition, the ability of SPs to ameliorate symptoms in TRD patients needs further investigation; though several studies showed initial promise (Carhart-Harris et al., 2016a; Goodwin et al., 2022a), others found no difference between response rates to SPs and monoaminergic-based antidepressants (Carhart-Harris et al., 2021). With the recent surge in interest in SPs, future studies should also be able to examine the varying impacts of different SPs.
Another area for future research is sex differences. Although prevalence rates of MDD are two- to three-fold higher in females than males, few studies have addressed potential sex differences with regard to ketamine, and almost none have done so for SPs. Notably, no apparent sex differences have emerged in clinical studies of ketamine for depression (Freeman et al., 2019) though both clinical and preclinical studies suggest that there may be some mechanistic differences (reviewed in (Ponton et al., 2022)). One systematic review found no apparent sex differences in clinical studies of SPs for depression (Aday et al., 2021), though preliminary preclinical work suggests that there may be sex differences in amygdala reactivity (Effinger et al., 2022) and prepulse inhibition of startle reactions after SP administration (Vohra et al., 2022). In addition, lack of inclusion of certain racial and ethnic minorities in research regarding both ketamine and SPs limits conclusions that can be made about therapeutic benefits. For example, recent research found that while psilocybin use conferred lower reports of distress amongst white participants, there were significantly lower associations for participants from different racial and ethnic minorities (Grant and Nock, 2022).
Both ketamine and SPs are being investigated for disorders other than depression, and we expect that future work in this area will expand. Specifically, randomized clinical trials found that ketamine and its enantiomers can be successfully used to target alcohol and other substance use disorders (Worrell and Gould, 2021), anxiety spectrum disorders (such as PTSD and social anxiety disorder) (Whittaker et al., 2021), and suicidal ideation (Wilkinson et al., 2018), among others. In contrast to the more robust evidence available for ketamine, most of the findings for SPs are anecdotal. These promising preliminary results nevertheless suggest that SPs may be useful in treating disorders characterized by maladaptive cognitive distortions, like OCD and anorexia nervosa (Foldi et al., 2020; Moreno et al., 2006; Spriggs et al., 2021). Given that SPs also preliminarily appear to mitigate tobacco, alcohol, cannabis, stimulant, and opioid use (Davis et al., 2018; Garcia-Romeu et al., 2019; Johnson et al., 2014; Jones and Nock, 2022; Noller et al., 2018), the anti-addictive potential of SPs also warrants additional investigation.
Despite the disparity in the number of rigorous studies conducted, comparing the mechanistic differences between ketamine and SPs can provide valuable insight into the mechanism of action underlying rapid-acting antidepressants. The most promising convergent mechanism between ketamine and SPs is activation of the mTORC1 pathway and the resultant changes in neuroplasticity. In addition, agonism of the mu-opioid and kappa-opioid receptors may contribute to some of the similarities observed between ketamine and SPs. Differences between the psychedelic and psychotomimetic effects of these compounds, as well as the success of some preliminary non-psychedelic analogues, suggests that the therapeutic experience itself does not fully mediate antidepressant effects. Disparities also exist with regard to how these compounds affect the monoaminergic system, and the evidence is quite mixed regarding how both ketamine and SPs mediate this system, leading to doubts that the monoaminergic system is necessary for rapid-acting antidepressant effects.
Elucidating the mechanisms responsible for the antidepressant effects of both ketamine and SPs is necessary for developing effective treatment approaches, including mechanistically novel pharmacotherapeutics. As the evidence reviewed above makes clear, significantly more research has been conducted with regard to the mechanisms of action that underlie ketamine’s antidepressant effects compared to SPs. Nevertheless, we expect that further research into SPs will be forthcoming.
Highlights.
Ketamine and psychedelics have different initial mechanisms of action
Parallel downstream mechanisms may account for therapeutic similarities
Monoamines, opioid and sigma receptors, and inflammation are all promising targets
More research on psychedelics is needed compared to ketamine
Consideration of regulatory issues is crucial to implementation
Acknowledgements
The authors thank the 7SE research unit and staff for their support.
Funding and Role of Funding Source
Funding for this work was provided by the Intramural Research Program at the National Institute of Mental Health, National Institutes of Health (IRP-NIMH-NIH; ZIAMH002857). The NIMH had no further role in study design; in the collection, analysis, or interpretation of data; in the writing of the report; or in the decision to submit the paper for publication. The work was completed as part of the authors’ official duties as Government employees. The views expressed do not necessarily reflect the views of the NIH, the Department of Health and Human Services, or the United States Government.
Dr. Zarate is listed as a co-inventor on a patent for the use of ketamine in major depression and suicidal ideation; as a co-inventor on a patent for the use of (2R,6R)-hydroxynorketamine, (S)-dehydronorketamine, and other stereoisomeric dehydroxylated and hydroxylated metabolites of (R,S)-ketamine metabolites in the treatment of depression and neuropathic pain; and as a co-inventor on a patent application for the use of (2R,6R)-hydroxynorketamine and (2S,6S)-hydroxynorketamine in the treatment of depression, anxiety, anhedonia, suicidal ideation, and post-traumatic stress disorders. He has assigned his patent rights to the U.S. government but will share a percentage of any royalties that may be received by the government. Dr. Kadriu is now a full-time employee of Jazz Pharmaceuticals.
Footnotes
Declaration of Interest
All other authors have no conflict of interest to disclose, financial or otherwise.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdallah CG, Adams TG, Kelmendi B, Esterlis I, Sanacora G, Krystal JH, 2016. Ketamine’s mechanism of action: a path to rapid-acting antidepressants. Depress Anxiety 33, 689–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdallah CG, Averill LA, Collins KA, Geha P, Schwartz J, Averill C, DeWilde KE, Wong E, Anticevic A, Tang CY, Iosifescu DV, Charney DS, Murrough JW, 2017a. Ketamine treatment and global brain connectivity in major depression. Neuropsychopharmacology 42, 1210–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdallah CG, Averill LA, Gueorguieva R, Goktas S, Purohit P, Ranganathan M, Sherif M., Ahn K-H., D’Souza DC., Formica R., Southwick SM., Duman RS., Sanacora G., Krystal JH., 2020. Modulation of the antidepressant effects of ketamine by the mTORC1 inhibitor rapamycin. Neuropsychopharmacology 45, 990–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdallah CG, Jackowski A, Salas R, Gupta S, Sato JR, Mao X, Coplan JD, Shungu DC, Mathew SJ, 2017b. The nucleus accumbens and ketamine treatment in major depressive disorder. Neuropsychopharmacology 42, 1739–1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abelaira HM, Rosa T, de Moura AB, Andrade NM, Martinello NS, Maciel LR, Botelho MEM, Borba LA, Chede BC, Arent CO, Joaquim L, Bonfante S, Danielski LG, Tuon T, Petronilho F, Quevedo J, Réus GZ, 2022. Combination of electroconvulsive stimulation with ketamine or escitalopram protects the brain against inflammation and oxidative stress induced by maternal deprivation and is critical for associated behaviors in male and female rats. Mol Neurobiol 59, 1452–1475. [DOI] [PubMed] [Google Scholar]
- Acevedo-Diaz EE, Cavanaugh GW, Greenstein D, Kraus C, Kadriu B, Park L, Zarate CA, 2020a. Can ‘floating’predict treatment response to ketamine? Data from three randomized trials of individuals with treatment-resistant depression. J Psychiatr Res 130, 280–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acevedo-Diaz EE, Cavanaugh GW, Greenstein D, Kraus C, Kadriu B, Zarate CA, Park LT, 2019. Comprehensive assessment of side effects associated with a single dose of ketamine in treatment-resistant depression. J Affect Disord. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acevedo-Diaz EE., Cavanaugh GW., Greenstein D., Kraus C., Kadriu B., Zarate CAJ., Park LT., 2020b. Comprehensive assessment of side effects associated with a single dose of ketamine in treatment-resistant depression. J Affect Disord 263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aday JS, Davis AK, Mitzkovitz CM, Bloesch EK, Davoli CC, 2021. Predicting reactions to psychedelic drugs: A systematic review of states and traits related to acute drug effects. ACS Pharmacol Transl Sci 4, 424–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aday JS, Heifets BD, Pratscher SD, Bradley E, Rosen R, Woolley JD, 2022. Great expectations: Recommendations for improving the methodological rigor of psychedelic clinical trials. Psychopharmacology (Berl) 239, 1989–2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agin-Liebes GI, Malone T, Yalch MM, Mennenga SE, Ponté KL, Guss J, Bossis AP, Grigsby J, Fischer S, Ross S, 2020. Long-term follow-up of psilocybin-assisted psychotherapy for psychiatric and existential distress in patients with life-threatening cancer. J Psychopharmacol 34, 155–166. [DOI] [PubMed] [Google Scholar]
- Ago Y, Tanabe W, Higuchi M, Tsukada S, Tanaka T, Yamaguchi T, Igarashi H, Yokoyama R, Seiriki K, Kasai A, Nakazawa T, Nakagawa S, Hashimoto K, Hashimoto H, 2019. (R)-ketamine induces a greater increase in prefrontal 5-HT release than (S)-ketamine and ketamine metabolites via an AMPA receptor-independent mechanism. Int J Neuropsychopharmacol 22, 665–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilar-Valles A, De Gregorio D, Matta-Camacho E, Eslamizade MJ, Khlaifia A, Skaleka A, Lopez-Canul M, Torres-Berrio A, Bermudez S, Rurak GM, Simard S, Salmaso N, Gobbi G, Lacaille JC, Sonenberg N, 2021. Antidepressant actions of ketamine engage cell-specific translation via eIF4E. Nature 590, 315–319. [DOI] [PubMed] [Google Scholar]
- Aleksandrova LR, Phillips AG, 2021. Neuroplasticity as a convergent mechanism of ketamine and classical psychedelics. Trends Pharmacol Sci 42, 929–942. [DOI] [PubMed] [Google Scholar]
- Allen AP., Naughton M., Dowling J., Walsh A., O’Shea R., Shorten G., Scott L., McLoughlin DM., Cryan JF., Clarke G., Dinan TG., 2018. Kynurenine pathway metabolism and the neurobiology of treatment-resistant depression: Comparison of multiple ketamine infusions and electroconvulsive therapy. J Psychiatr Res 100, 24–32. [DOI] [PubMed] [Google Scholar]
- Alnefeesi Y, Chen-Li D, Krane E, Jawad MY, Rodgrigues NB, Ceban F, Di Vincenzo JD, Meshkat S, Ho RCM, Gill H, Teopiz KM, Cao B, Lee Y, McIntyre RS, Rosenblat JD, 2022. Real-world effectiveness of ketamine in treatment-resistant depression: A systematic review & meta-analysis. J Psychiatr Res 151, 693–709. [DOI] [PubMed] [Google Scholar]
- Alt A, Nisenbaum ES, Bleakman D, Witkin JM, 2006. A role for AMPA receptors in mood disorders. Biochem Pharmacol 71, 1273–1288. [DOI] [PubMed] [Google Scholar]
- Andrashko V, Novak T, Brunovsky M, Klirova M, Sos P, Horacek J, 2020. The antidepressant effect of ketamine is dampened by concomitant benzodiazepine medication. Front Psychiatry 11, 844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atai Life Sciences, 2023. atai Life Sciences announces results from Phase 2a trial of PCN-101 (R-ketamine) for treatment-resistant depression. January 6, 2023. Available at: https://www.globenewswire.com/news-release/2023/01/06/2584334/0/en/atai-Life-SciencesAnnounces-Results-from-Phase-2a-Trial-of-PCN-101-R-ketamine-for-Treatment-ResistantDepression.html.
- Attur MG, Patel R, Thakker G, Vyas P, Levartovsky D, Patel P, Naqvi S, Raza R, Patel K, Abramson D, Bruno G, Abramson SB, Amin AR, 2000. Differential anti-inflammatory effects of immunosuppressive drugs: cyclosporin, rapamycin and FK-506 on inducible nitric oxide synthase, nitric oxide, cyclooxygenase-2 and PGE2 production. Inflamm Res 49, 20–26. [DOI] [PubMed] [Google Scholar]
- Autry AE., Adachi M., Nosyreva E., Na ES., Los MF., Cheng PF., Kavalali ET., Monteggia LM., 2011. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature 475, 91–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Averill LA, Averill CL, Gueorguieva R, Fouda S, Sherif M, Ahn K-H, Ranganathan M, D’Souza DC, Southwick SM, Sanacora G, Duman RS, Krystal JH, Abdallah CG, 2022. mTORC1 inhibitor effects on rapid ketamine-induced reductions in suicidal ideation in patients with treatment-resistant depression. J Affect Disord 303, 91–97. [DOI] [PubMed] [Google Scholar]
- Ballard ED, Wills K, Lally N, Richards EM, Luckenbaugh DA, Walls T, Ameli R, Niciu MJ, Brutsche NE, Park L, Zarate CA Jr., 2017. Anhedonia as a clinical correlate of suicidal thoughts in clinical ketamine trials. J Affect Disord 218, 195–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard ED, Zarate CAJ, 2020. The role of dissociation in ketamine’s antidepressant effects. Nat Commun 11, 6431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett FS, Griffiths RR, 2018. Classic hallucinogens and mystical experiences: phenomenology and neural correlates. In: Halberstadt AL, Vollenweider FX, Nichols DE, (Eds), Behavioral Neurobiology of Psychedelic Drugs. Springer Berlin Heidelberg, Berlin, Heidelberg, pp. 393–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck K, Hindley G, Borgan F, Ginestet C, McCutcheon R, Brugger S, Driesen N, Ranganathan M, D’Souza DC, Taylor M, Krystal JH, Howes OD, 2020. Association of ketamine with psychiatric symptoms and implications for its therapeutic use and for understanding schizophrenia: a systematic review and meta-analysis. JAMA Netw Open 3, e204693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benvenga MJ., Chaney SF., Baez M., Britton TC., Hornback WJ., Monn JA., Marek GJ., 2018. Metabotropic glutamate2 receptors play a key role in modulating head twitches induced by a serotonergic hallucinogen in mice. Front Pharmacol 9, 208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berke JD, Hyman SE, 2000. Addiction, dopamine, and the molecular mechanisms of memory. Neuron 25, 515–532. [DOI] [PubMed] [Google Scholar]
- Berkovitch L, Roméo B, Karila L, Gaillard R, Benyamina A, 2021. [Efficacy of psychedelics in psychiatry, a systematic review of the literature] [French]. Encephale 47, 376–387. [DOI] [PubMed] [Google Scholar]
- Besnier E, Clavier T, Tonon M-C, Selim J, Lefevre-Scelles A, Morin F, Tamion F, Dureuil B, Castel H, Compere V, 2017. Ketamine and etomidate down-regulate the hypothalamic-pituitary-adrenal axis in an endotoxemic mouse model. Anesthesiology 127, 347–354. [DOI] [PubMed] [Google Scholar]
- Blough BE, Landavazo A, Decker AM, Partilla J, Saumann MH, Rothman RB, 2014. Interaction of psychoactive tryptamines with biogenic amine transporters and serotonin receptor subtypes. Psychopharmacology 231, 4135–4144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaventura J, Lam S, Carlton M, Boehm MA, Gomez JL, Solís O, Sánchez-Soto M, Morris PJ, Fredriksson I, Thomas CJ, 2021. Pharmacological and behavioral divergence of ketamine enantiomers: implications for abuse liability. Mol Psychiatry 26, 6704–6722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boukezzi S, Costi S, Shin LM, Kim-Schulze S, Cathomas F, Collins A, Russo SJ, Morris LS, Murrough JW, 2022. Exaggerated amygdala response to threat and association with immune hyperactivity in depression. Brain Behav Immun 104, 205–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brachman RA., McGowan JC., Perusini JN., Lim SC., Pham TH., Faye C., Gardier AM., Mendez-David I., David DJ., Hen R., Denny CA., 2016. Ketamine as a prophylactic against stress-induced depressive-like behavior. Biol Psychiatry 79, 776–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branchi I, 2011. The double edged sword of neural plasticity: increasing serotonin levels leads to both greater vulnerability to depression and improved capacity to recover. Psychoneuroendocrinology 36, 339–351. [DOI] [PubMed] [Google Scholar]
- Buzsáki G, Wang X-J, 2012. Mechanisms of gamma oscillations. Ann Rev Neurosci 35, 203–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camargo A, Dalmagro AP, Wolin IAV, Kaster MP, Rodrigues ALS, 2021. The resilient phenotype elicited by ketamine against inflammatory stressors-induced depressive-like behavior is associated with NLRP3-driven signaling pathway. J Psychiatr Res 144, 118–128. [DOI] [PubMed] [Google Scholar]
- Cameron LP, Tombari RJ, Lu J, Pell AJ, Hurley ZQ, Ehinger Y, Vargas MV, McCarroll MN, Taylor JC, Myers-Turnbull D, Liu T, Yaghoobi B, Laskowski LJ, Anderson EI, Zhang G, Viswanathan J, Brown BM, Tjia M, Dunlap LE, Rabow ZT, Fiehn O, Wulff H, McCorvy JD, Lein PJ, Kokel D, Ron D, Peters J, Zuo Y, Olson DE, 2021. A non-hallucinogenic psychedelic analogue with therapeutic potential. Nature 589, 474–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao D, Yu J, Wang H, Luo Z, Liu X, He L, Qi J, Fan L, Tang L, Chen Z, Li J, Cheng J, Wang S, 2022. Structure-based discovery of nonhallucinogenic psychedelic analogs. Science 375, 403–411. [DOI] [PubMed] [Google Scholar]
- Carbonaro TM, Bradstreet MP, Barrett FS, MacLean KA, Jesse R, Johnson MW, Griffiths RR, 2016. Survey study of challenging experiences after ingesting psilocybin mushrooms: Acute and enduring positive and negative consequences. J Psychopharmacol 30, 1268–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carhart-Harris R., Giribaldi B., Watts R., Baker-Jones M., Murphy-Beiner A., Martell J., Blemings A., Erritzoe D., Nutt DJ., 2021. Trial of psilocybin versus escitalopram for depression. N Engl J Med 384, 1402–1411. [DOI] [PubMed] [Google Scholar]
- Carhart-Harris RL, Bolstridge M, Day CMJ, Rucker JJH, Watts R, Erritzoe DE, Kaelen M, Giribaldi B, Bloomfield M, Pilling S, Rickard JA, Forbes B, Feilding A, Taylor D, Curran HV, Nutt DJ, 2018. Psilocybin with psychological support for treatment-resistant depression: six-month follow-up. Psychopharmacology 235, 399–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carhart-Harris RL, Bolstridge M, Rucker J, Day CMJ, Erritzoe D, Kaelen M, Bloomfield M, Rickard JA, Forbes B, Feilding A, Taylor D, Pilling S, Curran VH, Nutt DJ, 2016a. Psilocybin with psychological support for treatment-resistant depression: an open-label feasibility study. Lancet Psychiatry 3, 619–627. [DOI] [PubMed] [Google Scholar]
- Carhart-Harris RL, Erritzoe D, Williams T, Stone JM, Reed LJ, Colasanti A, Tyacke RJ, Leech R, Malizia AL, Murphy K, Hobden P, Evans J, Feilding A, Wise RG, Nutt DJ, 2012. Neural correlates of the psychedelic state as determined by fMRI studies with psilocybin. Proc Natl Acad Sci USA 109, 2138–2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carhart-Harris RL, Goodwin GM, 2017. The therapeutic potential of psychedelic drugs: past, present, and future. Neuropsychopharmacology 42, 2105–2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carhart-Harris RL, Muthukumaraswamy S, Roseman L, Kaelen M, Droog W, Murphy K, Tagliazucchi E, Schenberg EE, Nest T, Orban C, Leech R, Williams LT, Williams TM, Bolstridge M, Sessa B, McGonigle J, Sereno MI, Nichols D, Hellyer PJ, Hobden P, Evans J, Singh KD, Wise RG, Curran HV, Feilding A, Nutt DJ, 2016b. Neural correlates of the LSD experience revealed by multimodal neuroimaging. Proc Natl Acad Sci U S A 113, 4853–4858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carhart-Harris RL, Nutt DJ, 2017. Serotonin and brain function: a tale of two receptors. J Psychopharmacol 31, 1091–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carhart-Harris RL, Roseman L, Bolstridge M, Demetriou L, Pannekoek JN, Wall MB., Tanner M., Kaelen M., McGonigle J., Murphy K., Leech R., Curran HV., Nutt DJ., 2017. Psilocybin for treatment-resistant depression: fMRI-measured brain mechanisms. Sci Rep 7, 13187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaki S, 2021. mGlu2/3 receptor antagonists as rapid-acting antidepressants. In: Hashimoto K, Manto M, (Eds), New Rapid-Acting Antidepressants. Springer, Cham, Switzerland, pp. 111–126. [Google Scholar]
- Chaki S, Fukumoto K, 2019. Role of serotonergic system in the antidepressant actions of mGlu2/3 receptor antagonists: Similarity to ketamine. Int J Mol Sci 20, 1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain SR, Cavanagh J, de Boer P, Mondelli V, Jones DNC, Drevets WC, Cowen PJ, Harrison NA, Pointon L, Pariante CM, Bullmore ET, 2019. Treatment-resistant depression and peripheral C-reactive protein. Br J Psychiatry 214, 11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Zhang K, Pu Y, Qu Y, Wang S-M, Xiong Z, Shirayama Y, Hashimoto K, 2020. Lack of dopamine D1 receptors in the antidepressant actions of (R)-ketamine in a chronic social defeat stress model. Eur Arch Psychiatry Clin Neurosci 270, 271–275. [DOI] [PubMed] [Google Scholar]
- Chen H-C, Fong T-H, Hsu P-W, Chiu W-T, 2013. Multifaceted effects of rapamycin on functional recovery after spinal cord injury in rats through autophagy promotion, anti-inflammation, and neuroprotection. J Surg Res 179, e203–210. [DOI] [PubMed] [Google Scholar]
- Chen Y, Guillemin GJ, 2009. Kynurenine pathway metabolites in humans: disease and healthy states. Int J Tryptophan Res 2, 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costi S., Evers AG., Jha MK., Overbey J., Goosens KA., Alvarez K., Collins KA., Klein M., Feder A., Charney DS., Murrough JW., 2022. P310. Effect of a sub-anesthetic infusion of ketamine on laboratory-induced stress in healthy volunteers: A proof-of-concept translational study. Biol Psychiatry 91 (Issue 9, Supplement), S213. [Google Scholar]
- Dai D, Miller C, Valdivia V, Boyle B, Bolton P, Li S, Seiner S, Meisner R, 2022. Neurocognitive effects of repeated ketamine infusion treatments in patients with treatment resistant depression: a retrospective chart review. BMC Psychiatry 22, 140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis AK, Barsuglia JP, Lancelotta R, Grant RM, Renn E, 2018. The epidemiology of 5-methoxy-N, N-dimethyltryptamine (5-MeO-DMT) use: Benefits, consequences, patterns of use, subjective effects, and reasons for consumption. J Psychopharmacol 32, 779–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daws RE, Timmermann C, Giribaldi B, Sexton JD, Wall MB, Erritzoe D, Roseman L, Nutt D, Carhart-Harris R, 2022. Increased global integration in the brain after psilocybin therapy for depression. Nat Med 28, 844–851. [DOI] [PubMed] [Google Scholar]
- De Almeida J, Mengod G, 2007. Quantitative analysis of glutamatergic and GABAergic neurons expressing 5-HT2A receptors in human and monkey prefrontal cortex. J Neurochem 103, 475–486. [DOI] [PubMed] [Google Scholar]
- De Gregorio D, Enns JP, Nuñez NA, Posa L, Gobbi G, 2018. d-Lysergic acid diethylamide, psilocybin, and other classic hallucinogens: mechanism of action and potential therapeutic applications in mood disorders. In: Calvey T, (Ed), Progress in Brain Research. Elsevier, pp. 69–96. [DOI] [PubMed] [Google Scholar]
- De Gregorio D., Posa L., Ochoa-Sanchez R., McLaughlin R., Maione S., Comai S., Gobbi G., 2016. The hallucinogen d-lysergic diethylamide (LSD) decreases dopamine firing activity through 5-HT1A, D2 and TAAR1 receptors. Pharmacol Res 113, 81–91. [DOI] [PubMed] [Google Scholar]
- de la Salle S, Phillips JL, Blier P, Knott V, 2022. Electrophysiological correlates and predictors of the antidepressant response to repeated ketamine infusions in treatment-resistant depression. Prog Neuropsychopharmacol Biol Psychiatry 115, 110507. [DOI] [PubMed] [Google Scholar]
- Delgado PL, 2000. Depression: the case for a monoamine deficiency. J Clin Psychiatry 61, 7–11. [PubMed] [Google Scholar]
- Delille HK, Becker JM, Burkhardt S, Bleher B, Terstappen GC, Schmidt M, Meyer AH, Unger L, Marek GJ, Mezler M, 2012. Heterocomplex formation of 5-HT2A-mGlu2 and its relevance for cellular signaling cascades. Neuropharmacology 62, 2184–2191. [DOI] [PubMed] [Google Scholar]
- Diazgranados N, Ibrahim L, Brutsche NE, Newberg A, Kronstein P, Khalife S, Kammerer WA, Quezado Z, Luckenbaugh DA, Salvadore G, Machado-Vieira R, Manji HK, Zarate CA Jr., 2010. A randomized add-on trial of an N-methyl-D-aspartate antagonist in treatment-resistant bipolar depression. Arch Gen Psychiatry 67, 793–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolder PC, Schmid Y, Haschke M, Rentsch KM, Liechti ME, 2015. Pharmacokinetics and concentration-effect relationship of oral LSD in humans. Int J Neuropsychopharmacol 19, pyv072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong C, Ly C, Dunlap LE, Vargas MV, Sun J, Hwang I-W, Azinfar A, Oh WC, Wetsel WC, Olson DE, Tian L, 2021. Psychedelic-inspired drug discovery using an engineered biosensor. Cell 184, 2779–2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doolin K, Allers KA, Pleiner S, Liesener A, Farrell C, Tozzi L, O’Hanlon E, Roddy D, Frodl T, Harkin A, O’Keane V, 2018. Altered tryptophan catabolite concentrations in major depressive disorder and associated changes in hippocampal subfield volumes. Psychoneuroendocrinology 95, 8–17. [DOI] [PubMed] [Google Scholar]
- Dravid S., Erreger K., Yuan H., Nicholson K., Le P., Lyuboslavsky P., Almonte A., Murray E., Mosely C., Barber J., French A., Balster R., Murray TF., Traynelis SF., 2007. Subunit-specific mechanisms and proton sensitivity of NMDA receptor channel block. J Physiol 581, 107–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drewiany E, Han J, Hancock C, Jones RL, Lim J, Nemat Gorgani N, Sperry J. K. r., Yu HJ, Raffa RB, 2015. Rapid-onset antidepressant action of ketamine: potential revolution in understanding and future pharmacologic treatment of depression. J Clin Pharm Ther 40, 125–130. [DOI] [PubMed] [Google Scholar]
- du Jardin KG, Liebenberg N, Cajina M, Müller HK, Elfving B, Sanchez C, Wegener G, 2018. S-ketamine mediates its acute and sustained antidepressant-like activity through a 5-HT1B receptor dependent mechanism in a genetic rat model of depression. Front Pharmacol 8, 978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duerler P, Brem S, Fraga-González G, Neef T, Allen M, Zeidman P, Stämpfli P, Vollenweider FX, Preller KH, 2021. Psilocybin induces aberrant prediction error processing of tactile mismatch responses—A simultaneous EEG–FMRI study. Cereb Cortex 32, 186–196. [DOI] [PubMed] [Google Scholar]
- Duman RS, Sanacora G, Krystal JH, 2019. Altered connectivity in depression: GABA and glutamate neurotransmitter deficits and reversal by novel treatments. Neuron 102, 75–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Effinger DP, Quadir SG, Ramage MC, Cone MG, Herman MA, 2022. Sex differences in central amygdala reactivity following psychedelic drug exposure. bioRxiv 2022.04.28.489882. [Google Scholar]
- Elhussiny MEA., Carini G., Mingardi J., Tornese P., Sala N., Bono F., Fiorentini C., La Via L., Popoli M., Musazzi L., Barbon A., 2021. Modulation by chronic stress and ketamine of ionotropic AMPA/NMDA and metabotropic glutamate receptors in the rat hippocampus. Prog Neuropsychopharmacol Biol Psychiatry 104, 110033. [DOI] [PubMed] [Google Scholar]
- Evans JW, Szczepanik J, Brutsché N, Park LT, Nugent AC, Zarate CA, 2018. Default mode connectivity in major depressive disorder measured up to 10 days after ketamine administration. Biol Psychiatry 84, 582–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischell J, Van Dyke AM, Kvarta MD, LeGates TA, Thompson SM, 2015. Rapid antidepressant action and restoration of excitatory synaptic strength after chronic stress by negative modulators of alpha5-containing GABAA receptors. Neuropsychopharmacology 40, 2499–2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald PJ, 2021. Many drugs of abuse may be acutely transformed to dopamine, norepinephrine and epinephrine in vivo. Int J Mol Sci 22, 10706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald PJ, Watson BO, 2018. Gamma oscillations as a biomarker for major depression: an emerging topic. Transl Psychiatry 8, 177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan TW, Nichols CD, 2018. Psychedelics as anti-inflammatory agents. International Review of Psychiatry 30, 363–375. [DOI] [PubMed] [Google Scholar]
- Flanagan TW, Nichols CD, 2022. Psychedelics and anti-inflammatory activity in animal models. Curr Top Behav Neurosci 56, 229–245. [DOI] [PubMed] [Google Scholar]
- Flanagan TW, Sebastian MN, Battaglia DM, Foster TP, Cormier SA, Nichols CD, 2019a. 5-HT2 receptor activation alleviates airway inflammation and structural remodeling in a chronic mouse asthma model. Life Sci 236, 116790. [DOI] [PubMed] [Google Scholar]
- Flanagan TW., Sebastian MN., Battaglia DM., Foster TP., Maillet EL., Nichols CD., 2019b. Activation of 5-HT 2 receptors reduces inflammation in vascular tissue and cholesterol levels in high-fat diet-fed apolipoprotein E knockout mice. Sci Rep 9, 13444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foldi CJ, Liknaitzky P, Williams M, Oldfield BJ, 2020. Rethinking therapeutic strategies for anorexia nervosa: insights from psychedelic medicine and animal models. Front Neurosci 14, 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster AC, Vezzani A, French ED, Schwarcz R, 1984. Kynurenic acid blocks neurotoxicity and seizures induced in rats by the related brain metabolite quinolinic acid. Neuroscience Letters 48, 273–278. [DOI] [PubMed] [Google Scholar]
- Freeman MP, Papakostas GI, Hoeppner B, Mazzone E, Judge H, Cusin C, Mathew SJ, Sanacora G, Iosifescu D, DeBattista C, Trivedi MH, Fava M, 2019. Sex differences in response to ketamine as a rapidly acting intervention for treatment resistant depression. J Psychiatr Res 110, 166–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchikami M, Thomas A, Liu R, Wohleb ES, Land BB, DiLeone RJ, Aghajanian GK, Duman RS, 2015. Optogenetic stimulation of infralimbic PFC reproduces ketamine’s rapid and sustained antidepressant actions. Proc Natl Acad Sci U S A 112, 8106–8111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita Y, Hashimoto Y, Hashimoto H, Chang L, Hashimoto K, 2021. Dextran sulfate sodium-induced inflammation and colitis in mice are ameliorated by (R)-ketamine, but not (S)-ketamine: A role of TrkB signaling. Eur J Pharmacol 897, 173954. [DOI] [PubMed] [Google Scholar]
- Garcia-Romeu A, Davis AK, Erowid F, Erowid E, Griffiths RR, Johnson MW, 2019. Cessation and reduction in alcohol consumption and misuse after psychedelic use. J Psychopharmacol 33, 1088–1101. [DOI] [PubMed] [Google Scholar]
- Gerhard DM, Pothula S, Liu R-J, Wu M, Li X-Y, Girgenti MJ, Taylor SR, Duman CH, Delpire E, Picciotto M, Wohleb ES, Duman RS, 2020. GABA interneurons are the cellular trigger for ketamine’s rapid antidepressant actions. J Clin Invest 130, 1336–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gewirtz JC, Chen AC, Terwilliger R, Duman RC, Marek GJ, 2002. Modulation of DOI-induced increases in cortical BDNF expression by group II mGlu receptors. Pharmacol Biochem Behav 73, 317–326. [DOI] [PubMed] [Google Scholar]
- Ghaem N., Sverdlo A., Shelto R., Litma R., 2021. Efficacy and safety of mij821 in patients with treatment-resistant depression: Results from a randomized, placebo-controlled, proof-of-concept study. Eur Psychiatry 64 (Special Issue S1: Abstracts of the 29th European Congress of Psychiatry), S334–S335. [Google Scholar]
- Ghosal S, Duman CH, Liu R-J, Wu M, Terwilliger R, Girgenti MJ, Wohleb E, Fogaca MV, Teichman EM, Hare B, Duman RS, 2020. Ketamine rapidly reverses stress-induced impairments in GABAergic transmission in the prefrontal cortex in male rodents. Neurobiol Dis 134, 104669. [DOI] [PubMed] [Google Scholar]
- Gilbert JR, Zarate CA, 2020. Electrophysiological biomarkers of antidepressant response to ketamine in treatment-resistant depression: Gamma power and long-term potentiation. Pharmacol Biochem Behav 189, 172856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold PW, Chrousos GP, 2002. Organization of the stress system and its dysregulation in melancholic and atypical depression: high vs low CRH/NE states. Mol Psychiatry 7, 254–275. [DOI] [PubMed] [Google Scholar]
- Golden CT, Chadderton P, 2022. Psilocybin reduces low frequency oscillatory power and neuronal phase-locking in the anterior cingulate cortex of awake rodents. Sci Rep 12, 12702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden TL, Magsamen S, Sandu CC, Lin S, Roebuck GM, Shi KM, Barrett FS, 2022. Effects of setting on psychedelic experiences, therapies, and outcomes: A rapid scoping review of the literature. Curr Top Behav Neurosci 56, 35–70. [DOI] [PubMed] [Google Scholar]
- Goodwin GM, Aaronson ST, Alvarez O, Arden PC, Baker A, Bennett JC, Bird C, Blom RE, Brennan C, Brusch D, Burke L, CampbellCoker K, Carhart-Harris R, Cattell J, Daniel A, Debattista C, Dunlop BW, Eisen K, Feifel D, Forbes M, Haumann HM, Hellerstein DJ, Hoppe AI, Husain MI, Jelen LA, Kamphuis J, Kawasaki J, Kelly JR, Key RE, Kishon R, Maples-Keller JL, Mars J, Marwood L, McElhiney MC, Miller TL, Mirow A, Mistry S, Mletzko-Crowe T, Modlin LN, Nielsen RE, Nielson EM, Offerhaus SR, O’Keane V, Páleníček T, Printz D, Rademaker MC, van Reemst A., Reinholdt F., Repantis D., Rucker J., Rudow S., Ruffell S., Rush AJ., Schoevers RA., Seynaeve M., Shao S., Soares JC., Somers M., Stansfield SC., Sterling D., Strockis A., Tsai J., Visser L., Wahba M., Williams S., Young AH., Ywema P., Zisook S., Malievskaia E., 2022a. Single-dose psilocybin for a treatment-resistant episode of major depression. N Engl J Med 387, 1637–1648. [DOI] [PubMed] [Google Scholar]
- Goodwin GM, Stansfield SC, Hellerstein DJ, Young AH, Malievskaia E, 2022b. The safety and efficacy of COMP360 psilocybin therapy in treatment-resistant depression: Results from a phase IIb randomized controlled trial. APA: Abstract Number: 5301 Session Title: Poster Session 6. American Psychiatric Association, New Orleans. [Google Scholar]
- Grant RM, Nock MK, 2022. Race and ethnicity moderate the associations between lifetime psychedelic use (MDMA and psilocybin) and psychological distress and suicidality. Sci Rep 12, 16976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwald M, Greenstein D, Park LT, Zarate C, 2021. Acute experiences during infusions cause extra-pharmacological antidepressant effects in ketamine treatment: evidence from randomized, placebo-controlled trials. Biol Psychiatry 89, S322–S323. [Google Scholar]
- Griffiths C, Walker K, Reid I, Maravic da Silva K, O’Neill-Kerr A, 2021. A qualitative study of patients’ experience of ketamine treatment for depression: The ‘Ketamine and me’ project. J Affect Disord Rep 4, 100079. [Google Scholar]
- Griffiths RR., Johnson MW., Carducci MA., Umbricht A., Richards WA., Richards BD., Cosimano MP., Klinedinst MA., 2016. Psilocybin produces substantial and sustained decreases in depression and anxiety in patients with life-threatening cancer: A randomized double-blind trial. J Psychopharmacol 30, 1181–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grob CS, Danforth AL, Chopra GS, Hagerty M, McKay CR, Halberstadt AL, Greer GR, 2011. Pilot study of psilocybin treatment for anxiety in patients with advanced-stage cancer. Arch Gen Psychiatry 68, 71–78. [DOI] [PubMed] [Google Scholar]
- Grotell M, Abdurakhmanova S, Elsilä LV, Korpi ER, 2021. Mice lacking GABAA receptor δ subunit have altered pharmaco-EEG responses to multiple drugs. Front Pharmacol 12, 706894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruenbaum BF, Zlotnik A, Frenkel A, Fleidervish I, Boyko M, 2022. Glutamate efflux across the blood–brain barrier: new perspectives on the relationship between depression and the glutamatergic system. Metabolites 12, 459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haapakoski R, Mathieu J, Ebmeier KP, Alenius H, Kivimäki M, 2015. Cumulative meta-analysis of interleukins 6 and 1β, tumour necrosis factor α and C-reactive protein in patients with major depressive disorder. Brain Behav Immun 49, 206–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamon M, Blier P, 2013. Monoamine neurocircuitry in depression and strategies for new treatments. Prog Neuropsychopharmacol Biol Psychiatry 45, 54–63. [DOI] [PubMed] [Google Scholar]
- Hare BD, Shinohara R, Liu R-J, Pothula S, DiLeone RJ, Duman RS, 2019. Optogenetic stimulation of medial prefrontal cortex Drd1 neurons produces rapid and long-lasting antidepressant effects. Nat Commun 10, 223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartogsohn I, 2017. Constructing drug effects: A history of set and setting. Drug Sci Policy Law 3. [Google Scholar]
- Hashimoto K, 2019. Rapid-acting antidepressant ketamine, its metabolites and other candidates: A historical overview and future perspective. Psychiatry Clin Neurosci 73, 613–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler F, Grimberg U, Benz MA, Huber T, Vollenweider FX, 2004. Acute psychological and physiological effects of psilocybin in healthy humans: a double-blind, placebo-controlled dose–effect study. Psychopharmacology 172, 145–156. [DOI] [PubMed] [Google Scholar]
- Heekeren K, Daumann J, Neukirch A, Stock C, Kawohl W, Norra C, Waberski TD, Gouzoulis-Mayfrank E, 2008. Mismatch negativity generation in the human 5HT2A agonist and NMDA antagonist model of psychosis. Psychopharmacology 199, 77–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hergovich N, Singer E, Agneter E, Eichler HG, Graselli U, Simhandl C, Jilma B, 2001. Comparison of the effects of ketamine and memantine on prolactin and cortisol release in men. a randomized, double-blind, placebo-controlled trial. Neuropsychopharmacology 24, 590–593. [DOI] [PubMed] [Google Scholar]
- Hess EM, Riggs LM, Michaelides M, Gould TD, 2022. Mechanisms of ketamine and its metabolites as antidepressants. Biochem Pharmacol 197, 114892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesselgrave N, Troppoli TA, Wulff AB, Cole AB, Thompson SM, 2021. Harnessing psilocybin: antidepressant-like behavioral and synaptic actions of psilocybin are independent of 5-HT2R activation in mice. Proc Natl Acad Sci U S A 118, e2022489118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschfeld RM, 2000. History and evolution of the monoamine hypothesis of depression. J Clin Psychiatry 61 (Suppl 6), 4–6. [PubMed] [Google Scholar]
- Holze F., Ley L., Müller F., Becker AM., Straumann I., Vizeli P., Kuehne SS., Roder MA., Duthaler U., Kolaczynska KE., Varghese N., Eckert A., Liechti ME., 2022. Direct comparison of the acute effects of lysergic acid diethylamide and psilocybin in a double-blind placebo-controlled study in healthy subjects. Neuropsychopharmacology 47, 1180–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu TW, Chu C-S, Ching P-Y, Chen G-W, Pan C-C, 2022. The efficacy and tolerability of memantine for depressive symptoms in major mental diseases: A systematic review and updated meta-analysis of double-blind randomized controlled trials. J Affect Disord 306, 182–189. [DOI] [PubMed] [Google Scholar]
- Inserra A, De Gregorio D, Gobbi G, 2021. Psychedelics in psychiatry: neuroplastic, immunomodulatory, and neurotransmitter mechanisms. Pharmacol Rev 73, 202–277. [DOI] [PubMed] [Google Scholar]
- Iob E, Kirschbaum C, Steptoe A, 2020. Persistent depressive symptoms, HPA-axis hyperactivity, and inflammation: the role of cognitive-affective and somatic symptoms. Mol Psychiatry 25, 1130–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iro CM, Hamati R, El Mansari M, Blier P, 2021. Repeated but not single administration of ketamine prolongs increases of the firing activity of norepinephrine and dopamine neurons. Int J Neuropsychopharmacol 24, 570–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi Y, Hsu F-F, Conway CR, Nagele P, Mennerick SJ, Zorumski CF, 2022. Nitrous oxide, a rapid antidepressant, has ketamine-like effects on excitatory transmission in adult hippocampus. Biol Psychiatry Jun 22 [online ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson LH, Vlachou S, Slattery DA, Li X, Cryan JF, 2018. The gamma-aminobutyric acid B receptor in depression and reward. Biol Psychiatry 83, 963–976. [DOI] [PubMed] [Google Scholar]
- Jia J, Cheng J, Wang C, Zhen X, 2018. Sigma-1 receptor-modulated neuroinflammation in neurological diseases. Front Cell Neurosci 12, 314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MW., Garcia-Romeu A., Cosimano MP., Griffiths RR., 2014. Pilot study of the 5-HT2AR agonist psilocybin in the treatment of tobacco addiction. J Psychopharmacol 28, 983–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MW, Hendricks PS, Barrett FS, Griffiths RR, 2019. Classic psychedelics: an integrative review of epidemiology, therapeutics, mystical experience, and brain network function. Pharmacol Ther 197, 83–102. [DOI] [PubMed] [Google Scholar]
- Johnson MW, Richards WA, Griffiths RR, 2008. Human hallucinogen research: guidelines for safety. J Psychopharmacol 22, 603–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston JN, Greenwald M, Henter ID, Kraus C, Mkrtchian A, Clark NG, Park LT, Gold P, Zarate CAJ, Kadriu B, submitted. Inflammation, stress, and depression: an exploration of ketamine’s therapeutic profile. Drug Discov Today. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones GM, Nock MK, 2022. Exploring protective associations between the use of classic psychedelics and cocaine use disorder: A population-based survey study. Sci Rep 12, 2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadriu B, Deng ZD, Kraus C, Henter ID, Lisanby SH, Zarate CA, 2020. Not So Fast: Recent Successes and Failures in Treating Depression. J Clin Psychiatry 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadriu B, Greenwald M, Henter ID, Gilbert JR, Kraus C, Park LT, Zarate CAJ, 2021. Ketamine and serotonergic psychedelics: common mechanisms underlying the effects of rapid-acting antidepressants. . Int J Neuropsychopharmacol 24, 8–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadriu B, Musazzi L, Henter ID, Graves M, Popoli M, Zarate CA Jr., 2019. Glutamatergic neurotransmission: pathway to developing novel rapid-acting antidepressant treatments. Int J Neuropsychopharmacol 22, 119–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato T, Duman RS, 2020. Rapastinel, a novel glutamatergic agent with ketamine-like antidepressant actions: convergent mechanisms. Pharmacol Biochem Behav 188, 172827. [DOI] [PubMed] [Google Scholar]
- Kelley DP, Venable K, Destouni A, Billac G, Ebenezer P, Stadler K, Nichols C, Barker S, Francis J, 2022. Pharmahuasca and DMT rescue ROS production and differentially expressed genes observed after predator and psychosocial stress: relevance to human PTSD. ACS Chem Neurosci 13, 257–274. [DOI] [PubMed] [Google Scholar]
- Khalili-Mahani N., Martini CH., Olofsen E., Dahan A., Niesters M., 2015. Effect of subanaesthetic ketamine on plasma and saliva cortisol secretion. Br J Anaesth 115, 68–75. [DOI] [PubMed] [Google Scholar]
- Kimmey BA, Ostroumov A, Dani JA, 2019. 5-HT2A receptor activation normalizes stress-induced dysregulation of GABAergic signaling in the ventral tegmental area. Proc Natl Acad Sci U S A 116, 27028–27034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto T, Chawla JM, Hagi K, Zarate CA, Kane JM, Bauer M, Correll CU, 2016. Single-dose infusion ketamine and non-ketamine N-methyl-d-aspartate receptor antagonists for unipolar and bipolar depression: a meta-analysis of efficacy, safety and time trajectories. Psychol Med 46, 1459–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein ME, Chandra J, Sheriff S, Malinow R, 2020. Opioid system is necessary but not sufficient for antidepressive actions of ketamine in rodents. Proc Natl Acad Sci USA 117, 2656–2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kłodzinska A, Bijak M, Tokarski K, Pilc A, 2002. Group II mGlu receptor agonists inhibit behavioural and electrophysiological effects of DOI in mice. Pharmacol Biochem Behav 73, 327–332. [DOI] [PubMed] [Google Scholar]
- Knudsen GM, 2023. Sustained effects of single doses of classical psychedelics in humans. Neuropsychopharmacology 48, 145–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kofod J., Elfving B., Nielsen EH., Mors O., Köhler-Forsberg O., 2022. Depression and inflammation: Correlation between changes in inflammatory markers with antidepressant response and long-term prognosis. Eur Neuropsychopharmacol 54, 116–125. [DOI] [PubMed] [Google Scholar]
- Köhler CA, Freitas TH, Maes M, Andrade N. Q. d., Liu CS, Fernandes BS, Stubbs B, Solmi M, Veronese N, Herrmann N, Raison CL, Miller BJ, Lanctôt KL, Carvalho AF, 2017. Peripheral cytokine and chemokine alterations in depression: a meta-analysis of 82 studies. Acta Psychiatr Scand 135, 373–387. [DOI] [PubMed] [Google Scholar]
- Kokane SS, Armant RJ, Bolaños-Guzmán CA, Perrotti LI, 2020. Overlap in the neural circuitry and molecular mechanisms underlying ketamine abuse and its use as an antidepressant. Behav Brain Res 384, 112548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalczyk M, Szemraj J, Bliźniewska K, Maes M, Berk M, Su K-P, Gałecki P, 2019. An immune gate of depression – Early neuroimmune development in the formation of the underlying depressive disorder. Pharmacol Rep 71, 1299–1307. [DOI] [PubMed] [Google Scholar]
- Kozłowska U, Klimczak A, Wiatr K, Figiel M, 2021. The DMT and psilocin treatment changes CD11b+ activated microglia immunological phenotype. bioRxiv 2021.03.07.434103. [Google Scholar]
- Kraus C, Mkrtchian A, Kadriu B, Nugent AC, Zarate CA, Evans JW, 2020. Evaluating global brain connectivity as an imaging marker for depression: influence of preprocessing strategies and placebo-controlled ketamine treatment. Neuropsychopharmacology 45, 982–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus C, Rabl U, Vanicek T, Carlberg L, Popovic A, Spies M, Bartova L, Gryglewski G, Papageorgiou K, Lanzenberger R, Willeit M, Winkler D, Rybakowski JK, Kasper S, 2017. Administration of ketamine for unipolar and bipolar depression. Int J Psychiatry Clin Pract 21, 2–12. [DOI] [PubMed] [Google Scholar]
- Kruse JL, Vasavada MM, Olmstead R, Helleman G, Wade B, Breen EC, Brooks JO, Congdon E, Espinoza R, Narr KL, Irwin MR, 2021. Depression treatment response to ketamine: sex-specific role of interleukin-8, but not other inflammatory markers. Transl Psychiatry 11, 167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubera M., Obuchowicz E., Goehler L., Brzeszcz J., Maes M., 2011. In animal models, psychosocial stress-induced (neuro)inflammation, apoptosis and reduced neurogenesis are associated to the onset of depression. Prog Neuropsychopharmacol Biol Psychiatry 35, 744–759. [DOI] [PubMed] [Google Scholar]
- Kyzar EJ, Kalueff AV, 2016. Exploring hallucinogen pharmacology and psychedelic medicine with zebrafish models. Zebrafish 13, 379–390. [DOI] [PubMed] [Google Scholar]
- Lahti AC, Koffel B, LaPorte D, Tamminga CA, 1995. Subanesthetic doses of ketamine stimulate psychosis in schizophrenia. Neuropsychopharmacology 13, 9–19. [DOI] [PubMed] [Google Scholar]
- Lally N, Nugent AC, Luckenbaugh DA, Ameli R, Roiser JP, Zarate CA, 2014. Anti-anhedonic effect of ketamine and its neural correlates in treatment-resistant bipolar depression. Transl Psychiatry 4, e469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landen M, Hogberg P, Thase ME, 2005. Incidence of sexual side effects in refractory depression during treatment with citalopram or paroxetine. J Clin Psychiatry 66, 100–106. [DOI] [PubMed] [Google Scholar]
- Lee YK, Gold MS, Fuehrlein BS, 2022. Looking beyond the opioid receptor: A desperate need for new treatments for opioid use disorder. J Neurol Sci 432, 120094. [DOI] [PubMed] [Google Scholar]
- Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, Li XY, Aghajanian G, Duman RS, 2010. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science 329, 959–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X-J, Yu J-H, Wu X, Zhu X-M, Lv P, Du Z, Lu Y, Wu X, Yao J, 2022a. Ketamine enhances dopamine D1 receptor expression by modulating microRNAs in a ketamine-induced schizophrenia-like mouse model. Neurotoxicol Teratol 91, 107079. [DOI] [PubMed] [Google Scholar]
- Li Y., Du Y., Wang C., Lu G., Sun H., Kong Y., Wang W., Lian B., Li C., Wang L., Zhang X., Sun L., 2022b. (2R, 6R)-hydroxynorketamine acts through GluA1-induced synaptic plasticity to alleviate PTSD-like effects in rat models. Neurobiol Stress 21, 100503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Liu J, Wang M, Zhang Y, Li L, 2017. From serotonin to neuroplasticity: evolvement of theories for major depressive disorder. Front Cell Neurosci 11, 305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Lin D, Wu B, Zhou W, 2016. Ketamine abuse potential and use disorder. Brain Res Bull 126, 68–73. [DOI] [PubMed] [Google Scholar]
- Lodge DA, Mercier MS, 2015. Ketamine and phencyclidine: the good, the bad and the unexpected. Br J Pharmacol 172, 4254–4276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Gil X, Jiménez-Sánchez L, Campa L, Castro E, Frago C, Adell A, 2019. Role of serotonin and noradrenaline in the rapid antidepressant action of ketamine. ACS Chem Neurosci 10, 3318–3326. [DOI] [PubMed] [Google Scholar]
- López-Giménez JF, González-Maeso J, 2018. Hallucinogens and serotonin 5-HT2A receptor-mediated signaling pathways. Curr Top Behav Neurosci 36, 45–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugo-Huitrón R, Ugalde Muñiz P, Pineda B, Pedraza-Chaverrí J, Ríos C, Pérez-de la Cruz V, 2013. Quinolinic acid: an endogenous neurotoxin with multiple targets. Oxid Med Cell Longev 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumsden EW, Troppoli TA, Myers SJ, Zanos P, Aracava Y, Kehr J, Lovett J, Kim S, Wang FH, Schmidt S, Jenne CE, Yuan P, Morris PJ, Thomas CJ, Zarate CA Jr., Moaddel R., Traynelis SF., Pereira EFR., Thompson SM., Albuquerque EX., Gould TD., 2019. Antidepressant-relevant concentrations of the ketamine metabolite (2R,6R)-hydroxynorketamine do not block NMDA receptor function. Proc Natl Acad Sci U S A 116, 5160–5169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüscher B, Möhler H, 2019. Brexanolone, a neurosteroid antidepressant, vindicates the GABAergic deficit hypothesis of depression and may foster resilience. F1000Res 8, F1000 Faculty Rev-1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ly C, Greb AC, Cameron LP, Wong JM, Barragan EV, Wilson PC, Burbach KF, Zarandi SS, Sood A, Paddy MR, Duim WC, Dennis MY, McAllister AK, Ori-McKenney KM, Gray JA, Olson DE, 2018. Psychedelics promote structural and functional neural plasticity. Cell Rep 23, 3170–3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ly C, Greb AC, Vargas MV, Duim WC, Grodzki ACG, Lein PJ, Olson DE, 2020. Transient stimulation with psychoplastogens is sufficient to initiate neuronal growth. ACS Pharmacol Transl Sci 4, 452–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald JF, Miljkovic Z, Pennefather P, 1987. Use-dependent block of excitatory amino acid currents in cultured neurons by ketamine. J Neurophysiol 58, 251–266. [DOI] [PubMed] [Google Scholar]
- Madrid-Gambin F, Gomez-Gomez A, Busquets-Garcia A, Haro N, Marco S, Mason NL, Reckweg JT, Mallaroni P, Kloft L, van Oorsouw K, Toennes SW, de la Torre R, Ramaekers JG, Pozo OJ, 2022. Metabolomics and integrated network analysis reveal roles of endocannabinoids and large neutral amino acid balance in the ayahuasca experience. Biomed Pharmacother 149, 112845. [DOI] [PubMed] [Google Scholar]
- Majeed A, Xiong J, Teopiz KM, Ng J, Ho R, Rosenblat JD, Phan L, Cao B, McIntyre RS, 2021. Efficacy of dextromethorphan for the treatment of depression: A systematic review of preclinical and clinical trials. Expert Opin Emerg Drugs 26, 63–74. [DOI] [PubMed] [Google Scholar]
- Malcolm B, Thomas K, 2022. Serotonin toxicity of serotonergic psychedelics. Psychopharmacology (Berl) 239, 1881–1891. [DOI] [PubMed] [Google Scholar]
- Mans K, Kettner H, Erritzoe D, Haijen ECHM, Kaelen M, Carhart-Harris RL, 2021. Sustained, multifaceted improvements in mental well-being following psychedelic experiences in a prospective opportunity sample. Front Psychiatry 12, 647909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcantoni WS., Akoumba BS., Wassef M., Mayrand J., Lai H., Richard-Devantoy S., Beauchamp S., 2020. A systematic review and meta-analysis of the efficacy of intravenous ketamine infusion for treatment resistant depression: January 2009–January 2019. J Affect Disord 277, 831–841. [DOI] [PubMed] [Google Scholar]
- Marchetti I, Koster EHW, Sonuga-Barke EJ, De Raedt R, 2012. The default mode network and recurrent depression: A neurobiological model of cognitive risk factors. Neuropsychol Rev 22, 229–251. [DOI] [PubMed] [Google Scholar]
- Marschall J, Fejer G, Lempe P, Prochazkova L, Kuchar M, Hajkova K, van Elk M, 2022. Psilocybin microdosing does not affect emotion-related symptoms and processing: A preregistered field and lab-based study. J Psychopharmacol 36, 97–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinotti G, Santacroce R, Pettorruso M, Montemitro C, Spano MC, Lorusso M, di Giannantonio M, Lerner AG, 2018. Hallucinogen persisting perception disorder: etiology, clinical features, and therapeutic perspectives. Brain Sciences 8, 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason NL, Kuypers KPC, Müller F, Reckweg J, Tse DHY, Toennes SW, Hutten NRPW, Jansen JFA, Stiers P, Feilding A, Ramaekers JG, 2020. Me, myself, bye: regional alterations in glutamate and the experience of ego dissolution with psilocybin. Neuropsychopharmacology May 23 [epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matveychuk D., Thomas RK., Swainson J., Khullar A., MacKay M-A., Baker GB., Dursun SM., 2020. Ketamine as an antidepressant: overview of its mechanisms of action and potential predictive biomarkers. Ther Adv Psychopharmacol 10, 2045125320916657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe C, Mishor Z, Cowen PJ, Harmer CJ, 2010. Diminished neural processing of aversive and rewarding stimuli during selective serotonin reuptake inhibitor treatment. Biol Psychiatry 67, 439–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure-Begley TD, Roth BL, 2022. The promises and perils of psychedelic pharmacology for psychiatry. Nat Rev Drug Discov 21, 463–473. [DOI] [PubMed] [Google Scholar]
- McCulloch DE, Grzywacz MZ, Madsen MK, Jensen PS, Ozenne B, Armand S, Knudsen GM, Fisher PM, Stenbaek DS, 2022. Psilocybin-induced mystical-type experiences are related to persisting positive effects: a quantitative and qualitative report. Front Pharmacol 13, 841648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGirr A, Berlim MT, Bond DJ, Fleck MP, Yatham LN, Lam RW, 2015. A systematic review and meta-analysis of randomized, double-blind, placebo-controlled trials of ketamine in the rapid treatment of major depressive episodes. Psychol Med 45, 693–704. [DOI] [PubMed] [Google Scholar]
- McIntyre RS, Carvalho IP, Lui LMW, Majeed A, Masand PS, Gill H, Rodrigues NB, Lipsitz O, Coles AC, Lee Y, Tamura JK, Iacobucci M, Phan L, Nasri F, Singhal N, Wong ER, Subramaniapillai M, Mansur RB, Ho R, Lam RW, Rosenblat JD, 2020. The effect of intravenous, intranasal, and oral ketamine in mood disorders: A meta-analysis. J Affect Disord 276, 576–584. [DOI] [PubMed] [Google Scholar]
- McMakin DL, Olino TM, Porta G, Dietz LJ, Emslie G, Clarke G, Wagner KD, Asarnow JR., Ryan ND., Birmaher B., Shamseddeen W., Mayes T., Kennard B., Spirito A., Keller M., Lynch FL., Dickerson JF., Brent DA., 2012. Anhedonia predicts poorer recovery among youth with selective serotonin reuptake inhibitor treatment-resistant depression. J Am Acad Child Adolesc Psychiatry 51, 404–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer JH, Cervenka S, Kim M-J, Kreisl WC, Henter ID, Innis RB, 2020. Neuroinflammation in psychiatric disorders: PET imaging and promising new targets. Lancet Psychiatry 7, 1064–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AH, 2013. Conceptual confluence: the kynurenine pathway as a common target for ketamine and the convergence of the inflammation and glutamate hypotheses of depression. Neuropsychopharmacology 38, 1607–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller OH, Moran JT, Hall BJ, 2016. Two cellular hypotheses explaining the initiation of ketamine’s antidepressant actions: Direct inhibition and disinhibition. Neuropharmacology 100, 17–26. [DOI] [PubMed] [Google Scholar]
- Miyata S, Kakizaki T, Fujihara K, Obinata H, Hirano T, Nakai J, Tanaka M, Itohara S, Watanabe M, Tanaka KF, Abe M, Sakimura K, Yanagawa Y, 2021. Global knockdown of glutamate decarboxylase 67 elicits emotional abnormality in mice. Mol Brain 14, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mkrtchian A, Evans JW, Kraus C, Yuan P, Kadriu B, Nugent AC, Roiser JP, Zarate CA Jr., 2021. Ketamine modulates fronto-striatal circuitry in depressed and healthy individuals. Mol Psychiatry 26, 3292–3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moaddel R, Shardell M, Khadeer M, Lovett J, Kadriu B, Ravichandran S, Morris PJ, Yuan P, Thomas CJ, Gould TD, Ferrucci L, Zarate CA, 2018. Plasma metabolomic profiling of a ketamine and placebo crossover trial of major depressive disorder and healthy control subjects. Psychopharmacology (Berl) 235, 3017–3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghaddam B., Adams B., Verma A., Daly D., 1997. Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci 17, 2921–2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno FA, Wiegand CB, Taitano EK, Delgado PL, 2006. Safety, tolerability, and efficacy of psilocybin in 9 patients with obsessive-compulsive disorder. J Clin Psychiatry 67, 1735–1740. [DOI] [PubMed] [Google Scholar]
- Morris BH, Bylsma LM, Rottenberg J, 2009. Does emotion predict the course of major depressive disorder? A review of prospective studies. Br J Clin Psychol 48, 255–273. [DOI] [PubMed] [Google Scholar]
- Murray CH, Tare I, Perry CM, Malina M, Lee R, de Wit H, 2022. Low doses of LSD reduce broadband oscillatory power and modulate event-related potentials in healthy adults. Psychopharmacology 239, 1735–1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musazzi L, 2021. Targeting metabotropic glutamate receptors for rapid-acting antidepressant drug discovery. Expert Opin Drug Discov 16, 147–157. [DOI] [PubMed] [Google Scholar]
- Muthukumaraswamy SD, 2021. Blinding and expectancy confounds in psychedelic randomized controlled trials. Expert Rev Clin Pharmacol 14, 1133–1152. [DOI] [PubMed] [Google Scholar]
- Muttoni S, Ardissino M, John C, 2019. Classical psychedelics for the treatment of depression and anxiety: A systematic review. J Affect Disord 258, 11–24. [DOI] [PubMed] [Google Scholar]
- Nakao K, Singh M, Sapkota K, Fitzgerald A, Hablitz JJ, Nakazawa K, 2022. 5-HT2A receptor dysregulation in a schizophrenia relevant mouse model of NMDA receptor hypofunction. Transl Psychiatry 12, 168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardai S, László M, Szabó A, Alpár A, Hanics J, Zahola P, Merkely B, Frecska E, Nagy Z, 2020. N, N-dimethyltryptamine reduces infarct size and improves functional recovery following transient focal brain ischemia in rats. Exp Neurol 327, 113245. [DOI] [PubMed] [Google Scholar]
- Nau FJ, Yu B, Martin D, Nichols CD, 2013. Serotonin 5-HT2A receptor activation blocks TNF-alpha mediated inflammation in vivo. PLoS One 8, e75426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng J, Lui LMW, Rosenblat JD, Teopiz KM, Lipsitz O, Cha DS, Xiong J, Nasri F., Lee Y., Kratiuk K., Rodrigues NB., Gill H., Subramaniapillai M., Mansur RB., Ho R., Cao B., McIntyre RS., 2021. Ketamine-induced urological toxicity: potential mechanisms and translation for adults with mood disorders receiving ketamine treatment. Psychopharmacology (Berl) 238, 917–926. [DOI] [PubMed] [Google Scholar]
- Nichols DE, 2016. Psychedelics. Pharmacol Rev 68, 264–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols DE, Johnson MW, Nichols CD, 2017. Psychedelics as medicines: an emerging new paradigm. Clin Pharmacol Ther 101, 209–219. [DOI] [PubMed] [Google Scholar]
- Nierenberg AA, Keefe BR, Leslie VC, Alpert JE, Pava JA, Worthington JJ 3rd, Rosenbaum JF, Fava M, 1999. Residual symptoms in depressed patients who respond acutely to fluoxetine. J Clin Psychiatry 60, 221–225. [DOI] [PubMed] [Google Scholar]
- Nkadimeng SM, Steinmann CML, Eloff JN, 2021. Anti-inflammatory effects of four psilocybin-containing magic mushroom water extracts in vitro on 15-lipoxygenase activity and on lipopolysaccharide-induced cyclooxygenase-2 and inflammatory cytokines in human U937 macrophage cells. J Inflamm Res 14, 3729–3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogo D, Jasrai AK, Kim H, Nasri F, Ceban F, Lui LMW, Rosenblat JD, Vinberg M, Ho R, McIntyre RS, 2022. The effect of ketamine on anhedonia: improvements in dimensions of anticipatory, consummatory, and motivation-related reward deficits. Psychopharmacology (Berl) 239, 2011–2039. [DOI] [PubMed] [Google Scholar]
- Noller GE, Frampton CM, Yazar-Klosinski B, 2018. Ibogaine treatment outcomes for opioid dependence from a twelve-month follow-up observational study. The American Journal of Drug and Alcohol Abuse 44, 37–46. [DOI] [PubMed] [Google Scholar]
- Nosyreva E, Szabla K, Autry AE, Ryazanov AG, Monteggia LM, Kavalali ET, 2013. Acute suppression of spontaneous neurotransmission drives synaptic potentiation. J Neurosci 33, 6990–7002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugent AC., Ballard ED., Gould TD., Park LT., Moaddel R., Brutsche NE., Zarate CA., 2019a. Ketamine has distinct electrophysiological and behavioral effects in depressed and healthy subjects. Mol Psychiatry 24, 1040–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugent AC, Wills KE, Gilbert JR, Zarate CA, 2019b. Synaptic potentiation and rapid antidepressant response to ketamine in treatment-resistant major depression: A replication study. Psychiatry Res Neuroimaging 283, 64–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson DE, 2020. The subjective effects of psychedelics may not be necessary for their enduring therapeutic effects. ACS Pharmacol Transl Sci 4, 563–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornelas IM, Cini FA, Wießner I, Marcos E, Araújo DB, Goto-Silva L, Nascimento J, Silva SRB, Costa MN, Falchi M, Olivieri R, Palhano-Fontes F, Sequerra E, Martins-de-Souza D, Fielding A, Rennó-Costa C, Tófoli LF, Rehen SK, Ribeiro S, 2022. Nootropic effects of LSD: Behavioral, molecular and computational evidence. Exp Neurol 356, 114148. [DOI] [PubMed] [Google Scholar]
- Palhano-Fontes F, Barreto D, Onias H, Andrade KC, Novaes MM, Pessoa JA, MotaRolim SA, Osório FL, Sanches RF, Dos Santos RG, Tófoli LF, de Oliviera Silveira G, Yonamine M, Riba J, Santos FR, Silva-Junior AA, Alchieri JC, Galvão-Coelho NL, Lobão-Soares B, Hallak JEC, Arcoverde E, Maia-de-Oliveira JP, Araújo DB, 2019. Rapid antidepressant effects of the psychedelic ayahuasca in treatment-resistant depression: A randomized placebo-controlled trial. Psychol Med 49, 655–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pałucha-Poniewiera A., Podkowa K., Rafało-Ulińska A., 2021. The group II mGlu receptor antagonist LY341495 induces a rapid antidepressant-like effect and enhances the effect of ketamine in the chronic unpredictable mild stress model of depression in C57BL/6J mice. Prog Neuropsychopharmacol Biol Psychiatry 109, 110239. [DOI] [PubMed] [Google Scholar]
- Passie T, Seifert J, Schneider U, Emrich HM, 2002. The pharmacology of psilocybin. Addict Biol 7, 357–364. [DOI] [PubMed] [Google Scholar]
- Peciña M, Karp JF, Mathew S, Todtenkopf MS, Ehrich EW, Zubieta J-K, 2019. Endogenous opioid system dysregulation in depression: implications for new therapeutic approaches. Mol Psychiatry 24, 576–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pędzich BD, Rubens S, Sekssaoui M, Pierre A, Van Schuerbeek A, Marin P, Bockaert J, Valjent E, Bécamel C, De Bundel D, 2022. Effects of a psychedelic 5-HT2A receptor agonist on anxiety-related behavior and fear processing in mice. Neuropsychopharmacology 47, 1304–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltoniemi MA, Hagelberg NM, Olkkola KT, Saari TI, 2016. Ketamine: a review of clinical pharmacokinetics and pharmacodynamics in anesthesia and pain therapy. Clin Pharmacokinet 55, 1059–1077. [DOI] [PubMed] [Google Scholar]
- Perez-Caballero L, Perez V, Berrocoso E, 2020. What ketamine can teach us about the opioid system in depression. Expert Opin Drug Discov 15, 1369–1372. [DOI] [PubMed] [Google Scholar]
- Peterlik D, Flor PJ, Uschold-Schmidt N, 2016. The emerging role of metabotropic glutamate receptors in the pathophysiology of chronic stress-related disorders. Curr Neuropharmacol 14, 514–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham TH., Defaix C., Nguyen TML., Mendez-David I., Tritschler L., David DJ., Gardier AM., 2020. Cortical and raphe GABAA, AMPA receptors and glial GLT-1 glutamate transporter contribute to the sustained antidepressant activity of ketamine. Pharmacol Biochem Behav 192, 172913. [DOI] [PubMed] [Google Scholar]
- Pilc A, Machaczka A, Kwalec P, Smith JL, Witkin JM, 2022. Where do we go next in antidepressant drug discovery? A new generation of antidepressants: a pivotal role of AMPA receptor potentiation and mGlu2/3 receptor antagonism. Expert Opin Drug Discov 17, 1131–1146. [DOI] [PubMed] [Google Scholar]
- Pinna G, Almeida FB, Davis JM, 2022. Allopregnanolone in postpartum depression. Front Glob Womens Health 3, 823616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piva A, Caffino L, Mottarlini F, Pintori N, Castillo Díaz F, Fumagalli F, Chiamulera C, 2021. Metaplastic effects of ketamine and MK-801 on glutamate receptors expression in rat medial prefrontal cortex and hippocampus. . Mol Neurobiol 58, 3443–3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pochwat B, Nowak G, Szewczyk B, 2019. An update on NMDA antagonists in depression. Expert Rev Neurother 19, 1055–1067. [DOI] [PubMed] [Google Scholar]
- Ponton E, Turecki G, Nagy C, 2022. Sex differences in the behavioral, molecular, and structural effects of ketamine treatment in depression. Int J Neuropsychopharmacol 25, 75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pothula S, Kato T, Liu R-J, Wu M, Gerhard D, Shinohara R, Sliby A-N, Chowdhury GMI, Behar KL, Sanacora G, Banerjee P, Duman RS, 2021. Cell-type specific modulation of NMDA receptors triggers antidepressant actions. Mol Psychiatry 26, 5097–5111. [DOI] [PubMed] [Google Scholar]
- Price J, Cole V, Goodwin GM, 2009. Emotional side-effects of selective serotonin reuptake inhibitors: qualitative study. Br J Psychiatry 195, 211–217. [DOI] [PubMed] [Google Scholar]
- Price RB, Duman R, 2020. Neuroplasticity in cognitive and psychological mechanisms of depression: an integrative model. Mol Psychiatry 25, 530–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu Y, Chang L, Ma L, Wan X, Hashimoto H, 2022. Rapid antidepressant-like effect of non-hallucinogenic psychedelic analog lisuride, but not hallucinogenic psychedelic DOI, in lipopolysaccharide-treated mice. Pharmacol Biochem Behav 222, 173500. [DOI] [PubMed] [Google Scholar]
- Rafało-Ulińska A., Brański P., Pałucha-Poniewiera A., 2022. Combined administration of (R)-ketamine and the mGlu2/3 receptor antagonist LY341495 induces rapid and sustained effects in the CUMS model of depression via a TrkB/BDNF-dependent mechanism. Pharmaceuticals (Basel) 15, 125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafało-Ulińska A, Pałucha-Poniewiera A, 2022. The effectiveness of (R)-ketamine and its mechanism of action differ from those of (S)-ketamine in a chronic unpredictable mild stress model of depression in C57BL/6J mice. Behav Brain Res 418, 113633. [DOI] [PubMed] [Google Scholar]
- Raison CL, Jain R, Penn AD, Cole SP, Jain S, 2022. Effects of naturalistic psychedelic use on depression, anxiety, and well-being: associations with patterns of use, reported harms, and transformative mental states. Front Psychiatry 13, 831092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiff CM, Richman EE, Nemerof CB, Carpenter LL, Widge AS, Rodriguez CI, Kalin NH, McDonald WM, Work Group on Biomarkers and Novel Treatments, a. D. o. t. A. P. A. C. o. R., 2020. Psychedelics and psychedelic-assisted psychotherapy. Am J Psychiatry 177, 391–410. [DOI] [PubMed] [Google Scholar]
- Ren Z, Pribiag H, Jefferson SJ, Shorey M, Fuchs T, Stellwagen D, Luscher B, 2016. Bidirectional homeostatic regulation of a depression-related brain state by GABAergic deficits and ketamine treatment. Biol Psychiatry 80, 457–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickli A, Luethi D, Reinisch J, Buchy D, Hoener MC, Liechti ME, 2015. Receptor interaction profiles of novel N-2-methoxybenzyl (NBOMe) derivatives of 2, 5-dimethoxy-substituted phenethylamines (2C drugs). Neuropharmacology 99, 546–553. [DOI] [PubMed] [Google Scholar]
- Rogers AH, Zvolensky MJ, Ditre JW, Buckner JD, Asmundson GJ, 2021. Association of opioid misuse with anxiety and depression: A systematic review of the literature. Clin Psychol Rev 84, 101978. [DOI] [PubMed] [Google Scholar]
- Rolland B., Jardri R., Amad A., Thomas P., Cottencin O., Bordet R., 2014. Pharmacology of hallucinations: Several mechanisms for one single symptom. Biomed Res Int 2014, 307106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeo B, Hermand M, Pétillon A, Karila L, Benyamina A, 2021. Clinical and biological predictors of psychedelic response in the treatment of psychiatric and addictive disorders: A systematic review. J Psychiatr Res 137, 273–282. [DOI] [PubMed] [Google Scholar]
- Romeo B, Karila L, Martelli C, Benyamina A, 2020. Efficacy of psychedelic treatments on depressive symptoms: A meta-analysis. J Psychopharmacol 34, 1079–1085. [DOI] [PubMed] [Google Scholar]
- Rootman JM, Kiraga M, Kryskow P, Harvey K, Stamets P, Santos-Brault E, Kuypers KPC, Walsh Z, 2022. Psilocybin microdosers demonstrate greater observed improvements in mood and mental health at one month relative to non-microdosing controls. Sci Rep 12, 11091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa PB, Neis VB, Ribeiro CM, Moretti M, Rodrigues ALS, 2016. Antidepressant-like effects of ascorbic acid and ketamine involve modulation of GABAA and GABAB receptors. Pharmacol Rep 68, 996–1001. [DOI] [PubMed] [Google Scholar]
- Rosburg T, Kreitschmann-Andermahr I, 2016. The effects of ketamine on the mismatch negativity (MMN) in humans – A meta-analysis. Clin Neurophysiol 127, 1387–1394. [DOI] [PubMed] [Google Scholar]
- Rosch RE, Auksztulewicz R, Leung PD, Friston KJ, Baldeweg T, 2019. Selective prefrontal disinhibition in a roving auditory oddball paradigm under N-methyl-D-aspartate receptor blockade. Biol Psychiatry Cogn Neurosci Neuroimaging 4, 140–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roseman L, Nutt DJ, Carhart-Harris RL, 2018. Quality of acute psychedelic experience predicts therapeutic efficacy of psilocybin for treatment-resistant depression. Front Pharmacol 8, 974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross S., Bossis A., Guss J., Agin-Liebes G., Malone T., Cohen B., Mennenga SE., Belser A., Kalliontzi K., Babb J., Su Z., Corby P., Schmidt BL., 2016. Rapid and sustained symptom reduction following psilocybin treatment for anxiety and depression in patients with life-threatening cancer: a randomized controlled trial. J Psychopharmacol 30, 1165–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rucker JJH, Iliff J, Nutt DJ, 2018. Psychiatry & the psychedelic drugs. Past, present & future. Neuropharmacology 142, 200–218. [DOI] [PubMed] [Google Scholar]
- Rudin D, McCorvy JD, Glatfelter GC, Luethi D, Szöllósi D, Ljubišić T, Kavanagh PV, Dowling G, Holy M, Jaentsch K, Walther D, Brandt SD, Stockner T, Baumann MH, Halberstadt AL, Sitte HH, 2022. (2-Aminopropyl) benzo [β] thiophenes (APBTs) are novel monoamine transporter ligands that lack stimulant effects but display psychedelic-like activity in mice. Neuropsychopharmacology 47, 914–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, Niederehe G, Thase ME, Lavori PW, Lebowitz BD, McGrath PJ, Rosenbaum JF, Sackeim HA, Kupfer DJ, Luther J, Fava M, 2006. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry 163, 1905–1917. [DOI] [PubMed] [Google Scholar]
- Saeger HN, Olson DE, 2022. Psychedelic-inspired approaches for treating neurodegenerative disorders. J Neurochem 162, 109–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampedro F, de la Fuente Revenga M, Valle M, Roberto N, Domínguez-Clavé E, Elices M, Luna LE, Crippa JAS, Hallak JEC, de Araujo DB, Friedlander P, Barker SA, Álvarez E, Soler J, Pascual JC, Feilding A, Riba J, 2017. Assessing the Psychedelic “After-Glow” in Ayahuasca Users: Post-Acute Neurometabolic and Functional Connectivity Changes Are Associated with Enhanced Mindfulness Capacities. International Journal of Neuropsychopharmacology 20, 698–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanches RF., de Lima Osório F., Dos Santos RG., Macedo LR., Maia-de-Oliveira JP., Wichert-Ana L., de Araujo DB., Riba J., Crippa JA., Hallak JE., 2016. Antidepressant effects of a single dose of ayahuasca in patients with recurrent depression: a SPECT study. J Clin Psychopharmacol 36, 77–81. [DOI] [PubMed] [Google Scholar]
- Sassano-Higgins S, Baron D, Juarez G, Esmaili N, Gold M, 2016. A review of ketamine abuse and diversion. Depress Anxiety 33, 718–727. [DOI] [PubMed] [Google Scholar]
- Schatzberg AF, 2020. Some comments on psychedelic research. Am J Psychiatry 177, 368–369. [DOI] [PubMed] [Google Scholar]
- Schifano N, Chiappini S, Castiglione F, Salonia A, Schifano F, 2021. Is medicinal ketamine associated with urinary dysfunction issues? Assessment of both the European Medicines Agency (EMA) and the UK Yellow Card Scheme pharmacovigilance database-related reports. . Low Urin Tract Symptoms 13, 230–237. [DOI] [PubMed] [Google Scholar]
- Schmidt A, Bachmann R, Kometer M, Csomor PA, Stephan KE, Seifritz E, Vollenweider FX, 2012. Mismatch negativity encoding of prediction errors predicts S-ketamine-induced cognitive impairments. Neuropsychopharmacology 37, 865–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt A, Kometer M, Bachmann R, Seifritz E, Vollenweider F, 2013. The NMDA antagonist ketamine and the 5-HT agonist psilocybin produce dissociable effects on structural encoding of emotional face expressions. Psychopharmacology 225, 227–239. [DOI] [PubMed] [Google Scholar]
- Schwertner A., Zortea M., Torres FV., Caumo W., 2018. Effects of subanesthetic ketamine administration on visual and auditory event-related potentials (ERP) in humans: A systematic review. Front Behav Neurosci 12, 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Price JL, Yan Z, Mintun MA, 2010. Resting-state functional MRI in depression unmasks increased connectivity between networks via the dorsal nexus. Proc Natl Acad Sci U S A 107, 11020–11025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberbauer LR, Spurny B, Handschuh P, Klöbl M, Bednarik P, Reiter B, Ritter V, Trost P, Konadu ME, Windpassinger M, Stimpfl T, Bogner W, Lanzenberger R, Spies M, 2020. Effect of ketamine on limbic GABA and glutamate: a human in vivo multivoxel magnetic resonance spectroscopy study. Front Psychiatry 11, 549903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmler LD, Li Y, Hadjas LC, Hiver A, van Zessen R, Lüscher C, 2022. Dual action of ketamine confines addiction liability. Nature 608, 368–373. [DOI] [PubMed] [Google Scholar]
- Simonsson O, Hendricks PS, Carhart-Harris R, Kettner H, Osika W, 2021a. Association between lifetime classic psychedelic use and hypertension in the past year. Hypertension 77, 1510–1516. [DOI] [PubMed] [Google Scholar]
- Simonsson O, Osika W, Carhart-Harris R, Hendricks PS, 2021b. Associations between lifetime classic psychedelic use and cardiometabolic diseases. Sci Rep 11, 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonsson O, Sexton JD, Hendricks PS, 2021c. Associations between lifetime classic psychedelic use and markers of physical health. J Psychopharmacol 35, 447–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slocum ST, DiBerto JF, Roth BL, 2022. Molecular insights into psychedelic drug action. J Neurochem 162, 24–38. [DOI] [PubMed] [Google Scholar]
- Smedfors G., Glotfelty E., Kalani N., Hjelle CP., Horntvedt O., Wellfelt K., Brodin A., von Kieseritzky F., Olson L., Karlsson T., 2022. Psilocybin combines rapid synaptogenic and anti-inflammatory effects in vitro. Research Square 08 Mar 2022. DOI: 10.21203/rs.3.rs-1321542/v1. [DOI] [Google Scholar]
- Spies M, James GM, Berroteran-Infante N, Ibeschitz H, Kranz GS, Unterholzner J, Godbersen M, Gryglewski G, Hienert M, Jungwirth J, Pichler V, Reiter B, Silberbauer L, Winkler D, Mitterhauser M, Stimpfl T, Hacker M, Kasper S, Lanzenberger R, 2018. Assessment of Ketamine Binding of the Serotonin Transporter in Humans with Positron Emission Tomography. The international journal of neuropsychopharmacology 21, 145–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spriggs MJ, Douglass HM, Park RJ, Read T, Danby JL, de Magalhães FJC, Alderton KL, Williams TM, Blemings A, Lafrance A, Nicholls DE, Erritzoe D, Nutt DJ, Carhart-Harris RL, 2021. Study protocol for “Psilocybin as a Treatment for Anorexia Nervosa: A Pilot Study”. Front Psychiatry 12, 735523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stetler C, Miller GE, 2011. Depression and hypothalamic-pituitary-adrenal activation: a quantitative summary of four decades of research. Psychosom Med 73, 114–126. [DOI] [PubMed] [Google Scholar]
- Strassman RJ, Qualls CR, 1994. Dose-response study of N, N-dimethyltryptamine in humans. I. Neuroendocrine, autonomic, and cardiovascular effects. Arch Gen Psychiatry 51, 85–97. [DOI] [PubMed] [Google Scholar]
- Sumner RL, McMillan R, Spriggs MJ, Campbell D, Malpas G, Maxwell E, Deng C, Hay J, Ponton R, Kirk IJ, Sundram F, Muthukumaraswamy SD, 2020a. Ketamine enhances visual sensory evoked potential long-term potentiation in patients with major depressive disorder. Biol Psychiatry Cogn Neurosci Neuroimaging 5, 45–55. [DOI] [PubMed] [Google Scholar]
- Sumner RL, McMillan R, Spriggs MJ, Campbell D, Malpas G, Maxwell E, Deng C, Hay J, Ponton R, Sundram F, Muthukumaraswamy SD, 2020b. Ketamine improves short-term plasticity in depression by enhancing sensitivity to prediction errors. Eur Neuropsychopharmacol 38, 73–85. [DOI] [PubMed] [Google Scholar]
- Suneson K., Lindahl J., Chamli Hårsmar S., Söderberg G., Lindqvist D., 2021. Inflammatory depression—mechanisms and non-pharmacological intervention. Int J Mol Sci 22, 1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiger M, Veldman ER, Ekman C-J, Halldin C, Svenningsson P, Lundberg J, 2020. A randomized placebo-controlled PET study of ketaminé s effect on serotonin1B receptor binding in patients with SSRI-resistant depression. Transl Psychiatry 10, 159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L, Norquist G, Howland RH, Lebowitz B, McGrath PJ, Shores-Wilson K, Biggs MM, Balasubramani GK, Fava M, Team SDS, 2006. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry 163, 28–40. [DOI] [PubMed] [Google Scholar]
- Turkin A, Tuchina O, Klempin F, 2021. Microglia function on precursor cells in the adult hippocampus and their responsiveness to serotonin signaling. Front Cell Dev Biol 9, 665739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Food & Drug Administration, 2019. FDA approves new nasal spray medication for treatment-resistant depression. Available at: https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm632761.htm.
- Uher R, Perlis RH, Henigsberg N, Zobel A, Rietschel M, Mors O, Hauser J, Dernovsek MZ, Souery D, Bajs M, Maier W, Aitchison KJ, Farmer A, McGuffin P, 2012. Depression symptom dimensions as predictors of antidepressant treatment outcome: replicable evidence for interest-activity symptoms. Psychol Med 42, 967–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umbricht D, Vollenweider FX, Schmid L, Grübel C, Skrabo A, Huber T, Koller R, 2003. Effects of the 5-HT2A agonist psilocybin on mismatch negativity generation and AX-continuous performance task: Implications for the neuropharmacology of cognitive deficits in schizophrenia. Neuropsychopharmacology 28, 170–181. [DOI] [PubMed] [Google Scholar]
- Underwood MS., Bright SJ., Lancaster BL., 2021. A narrative review of the pharmacological, cultural and psychological literature on ibogaine. J Psychedelic Stud 5, 44–54. [Google Scholar]
- Valkanova V, Ebmeier KP, Allan CL, 2013. CRP, IL-6 and depression: A systematic review and meta-analysis of longitudinal studies. J Affect Disord 150, 736–744. [DOI] [PubMed] [Google Scholar]
- Vasavada MM, Loureiro J, Kubicki A, Sahib A, Wade B, Hellemann G, Espinoza RT, Congdon E, Narr KL, Leaver AM, 2021. Effects of serial ketamine infusions on corticolimbic functional connectivity in major depression. Biol Psychiatry Cogn Neurosci Neuroimaging 6, 735–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vohra HZ, Saunders JM, Jaster AM, Fuente Revenga MD, Jimenez J, Fernández-Teruel A, Wolstenholme JT, Beardsley PM, González-Maeso J, 2022. Sex-specific effects of psychedelics on prepulse inhibition of startle in 129S6/SvEv mice. Psychopharmacology (Berl) 239, 1649–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollenweider FX, Preller KH, 2020. Psychedelic drugs: neurobiology and potential for treatment of psychiatric disorders. Nat Rev Neurosci 21, 611–624. [DOI] [PubMed] [Google Scholar]
- Vollenweider FX, Smallridge JW, 2022. Classic psychedelic drugs: update on biological mechanisms. Pharmacopsychiatry 55, 121–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker AK, Budac DP, Bisulco S, Lee AW, Smith RA, Beenders B, Kelley KW, Dantzer R, 2013. NMDA receptor blockade by ketamine abrogates lipopolysaccharide-induced depressive-like behavior in C57BL/6J mice. Neuropsychopharmacology 38, 1609–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang AK, Miller BJ, 2018. Meta-analysis of cerebrospinal fluid cytokine and tryptophan catabolite alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder, and depression. Schizophr Bull 44, 75–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D-S., Penna A., Orser BA., 2017. Ketamine increases the function of γ-aminobutyric acid type A receptors in hippocampal and cortical neurons. Anesthesiology 126, 666–677. [DOI] [PubMed] [Google Scholar]
- Wang N, Yu H-Y, Shen X-F, Gao Z-Q, Yang C, Yang J-J, Zhang G-F, 2015. The rapid antidepressant effect of ketamine in rats is associated with down-regulation of pro-inflammatory cytokines in the hippocampus. Ups J Med Sci 120, 241–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Liu L, Yang X, Gao H, Tang Q-K, Yin L-Y, Yin X-Y, Hao J-R, Geng D-Q, Gao C, 2019. Ketamine improved depressive-like behaviors via hippocampal glucocorticoid receptor in chronic stress induced- susceptible mice. Behav Brain Res 364, 75–84. [DOI] [PubMed] [Google Scholar]
- Wasko MJ, Witt-Enderby PA, Surratt CK, 2018. DARK classics in chemical neuroscience: ibogaine. ACS Chem Neurosci 9, 2475–2483. [DOI] [PubMed] [Google Scholar]
- Watson BO, Ding M, Buzsáki G, 2018. Temporal coupling of field potentials and action potentials in the neocortex. Eur J Neurosci 48, 2482–2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y, Chang L, Hashimoto K, 2022. Molecular mechanisms underlying the antidepressant actions of arketamine: beyond the NMDA receptor. Mol Psychiatry 27, 559–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittaker E, Dadabayev AR, Joshi SA, Glue P, 2021. Systematic review and meta-analysis of randomized controlled trials of ketamine in the treatment of refractory anxiety spectrum disorders. Ther Adv Psychopharmacol 11, 20451253211056743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichers MC, Koek GH, Robaeys G, Verkerk R, Scharpe S, Maes MJMP, 2005. IDO and interferon-alpha-induced depressive symptoms: a shift in hypothesis from tryptophan depletion to neurotoxicity. Mol Psychiatry 10, 538–544. [DOI] [PubMed] [Google Scholar]
- Wilkinson ST, Ballard ED, Bloch MH, Mathew SJ, Murrough JW, Feder A, Sos P, Wang G, Zarate CA Jr., Sanacora G, 2018. The effect of a single dose of intravenous ketamine on suicidal ideation: a systematic review and individual participant data meta-analysis. Am J Psychiatry 175, 150–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams NR, Heifets BD, Bentzley BS, Blasey C, Sudheimer KD, Hawkins J, Lyons DM, Schatzberg AF, 2019. Attenuation of antidepressant and antisuicidal effects of ketamine by opioid receptor antagonism. Mol Psychiatry 24, 1779–1786. [DOI] [PubMed] [Google Scholar]
- Williams NR., Heifets BD., Blasey C., Sudheimer K., Pannu J., Pankow H., Hawkins J., Birnbaum J., Lyons DM., Rodriguez CI., Schatzberg AF., 2018. Attenuation of antidepressant effects of ketamine by opioid receptor antagonism. Am J Psychiatry 175, 1205–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worrell SD, Gould TJ, 2021. Therapeutic potential of ketamine for alcohol use disorder. Neurosci Biobehav Rev 126, 573–589. [DOI] [PubMed] [Google Scholar]
- Wotjas A, Bysiek A, Wawrzczak-Bargiela A, Szych Z, Majcher-Maślanka I, Herian M, Maćkowiak M, Gołembiowska K, 2022. Effect of psilocybin and ketamine on brain neurotransmitters, glutamate receptors, DNA and rat behavior. Int J Mol Sci 23, 6713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray NH, Schappi JM, Singh H, Senese NB, Rasenick MM, 2019. NMDAR-independent, cAMP-dependent antidepressant actions of ketamine. Mol Psychiatry 24, 1833–1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M, Minkowicz S, Dumrongprechachan V, Hamilton P, Kozorovitskiy Y, 2021a. Ketamine rapidly enhances glutamate-evoked dendritic spinogenesis in medial prefrontal cortex through dopaminergic mechanisms. Biol Psychiatry 89, 1096–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M, Minkowicz S, Dumrongprechachan V, Hamilton P, Xiao L, Kozorovitskiy Y, 2021b. Attenuated dopamine signaling after aversive learning is restored by ketamine to rescue escape actions. Elife 10, e64041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wulf HA, Browne CA, Zarate CA, Lucki I, 2022. Mediation of the behavioral effects of ketamine and (2R, 6R)-hydroxynorketamine in mice by kappa opioid receptors. Psychopharmacology (Berl) 239, 2309–2316. [DOI] [PubMed] [Google Scholar]
- Yamanaka H., Yokoyama C., Mizuma H., Kurai S., Finnema SJ., Halldin C., Doi H., Onoe H., 2014. A possible mechanism of the nucleus accumbens and ventral pallidum 5-HT1B receptors underlying the antidepressant action of ketamine: a PET study with macaques. Transl Psychiatry 4, e342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B, Ren Q, Ma M, Chen Q-X, Hashimoto K, 2016. Antidepressant effects of (+)-MK-801 and (−)-MK-801 in the social defeat stress model. Int J Neuropsychopharmacol 19, pyw080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Bosker FJ, Li J, Schoevers RA, 2018a. N-acetylcysteine as add-on to antidepressant medication in therapy refractory major depressive disorder patients with increased inflammatory activity: study protocol of a double-blind randomized placebo-controlled trial. BMC Psychiatry 18, 279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Ren Q, Qu Y, Zhang J-C, Ma M, Dong C, Hashimoto K, 2018b. Mechanistic target of rapamycin–independent antidepressant effects of (R)-ketamine in a social defeat stress model. Biol Psychiatry 83, 18–28. [DOI] [PubMed] [Google Scholar]
- Yang C, Yang J, Luo A, Hashimoto K, 2019. Molecular and cellular mechanisms underlying the antidepressant effects of ketamine enantiomers and its metabolites. Transl Psychiatry 9, 280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao N., Skiteva O., Zhang X., Svenningsson P., Chergui K., 2018. Ketamine and its metabolite (2R, 6R)-hydroxynorketamine induce lasting alterations in glutamatergic synaptic plasticity in the mesolimbic circuit. Mol Psychiatry 23, 2066–2077. [DOI] [PubMed] [Google Scholar]
- Yao W, Cao Q, Luo S, He L, Yang C, Chen J, Qi Q, Hashimoto K, Zhang J-C, 2022. Microglial ERK-NRBP1-CREB-BDNF signaling in sustained antidepressant actions of (R)-ketamine. Mol Psychiatry 27, 1618–1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu B, Becnel J, Zerfaoui M, Rohatgi R, Boulares AH, Nichols CD, 2008. Serotonin 5-hydroxytryptamine2A receptor activation suppresses tumor necrosis factor-α-induced inflammation with extraordinary potency. J Pharmacol Exp Ther 327, 316–323. [DOI] [PubMed] [Google Scholar]
- Zamberlan F, Sanz C, Martínez Vivot R, Pallavacini C, Erowid F, Erowid E, Taglazucchi E, 2018. The varieties of the psychedelic experience: a preliminary study of the association between the reported subjective effects and the binding affinity profiles of substituted phenethylamines and tryptamines. Front Integr Neurosci 12, 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanos P, Gould TD, 2018. Mechanisms of ketamine action as an antidepressant. Mol Psychiatry 23, 801–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanos P, Highland JN, Stewart BW, Georgiou P, Jenne CE, Lovett J, Morris PJ, Thomas CJ, Moaddel R, Zarate CA Jr., Gould TD, 2019. (2R,6R)-hydroxynorketamine exerts mGlu2 receptor-dependent antidepressant actions. Proc Natl Acad Sci U S A 116, 6441–6450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanos P, Moaddel R, Morris PJ, Georgiou P, Fischell J, Elmer GI, Alkondon M, Yuan P, Pribut HJ, Singh NS, Dossou KS, Fang Y, Huang XP, Mayo CL, Wainer IW, Albuquerque EX, Thompson SM, Thomas CJ, Zarate CA Jr., Gould TD, 2016. NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature 533, 481–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanos P., Moaddel R., Morris PJ., Riggs LM., Highland JN., Georgiou P., Pereira EFR., Albuquerque EX., Thomas CJ., Zarate CA Jr., Gould TD., 2018a. Ketamine and ketamine metabolite pharmacology: Insights into therapeutic mechanisms. Pharmacol Rev 70, 621–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanos P, Thompson SM, Duman RS, Zarate CA Jr., Gould TD, 2018b. Convergent mechanisms underlying rapid antidepressant action. CNS Drugs 32, 197–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate CA Jr., Brutsche NE, Ibrahim L, Franco-Chaves J, Diazgranados N, Cravchik A, Selter J, Marquardt CA, Liberty V, Luckenbaugh DA, 2012. Replication of ketamine’s antidepressant efficacy in bipolar depression: a randomized controlled add-on trial. Biol Psychiatry 71, 939–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate CA Jr., Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, Charney DS, Manji HK, 2006. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry 63, 856–864. [DOI] [PubMed] [Google Scholar]
- Zhang F, Hillhouse TM, Anderson PM, Koppenhaver PO, Kegen TN, Manicka SG, Lane JT, Pottanat E, Van Fossen M, Rice R, 2021a. Opioid receptor system contributes to the acute and sustained antidepressant-like effects, but not the hyperactivity motor effects of ketamine in mice. Pharmacol Biochem Behav 208, 173228. [DOI] [PubMed] [Google Scholar]
- Zhang J, Ma L, Wan X, Shan J, Qu Y, Hashimoto K, 2021b. (R)-Ketamine attenuates LPS-induced endotoxin-derived delirium through inhibition of neuroinflammation. Psychopharmacology 238, 2743–2753. [DOI] [PubMed] [Google Scholar]
- Zhang J-C, Yao W, Hashimoto K, 2022. Arketamine, a new rapid-acting antidepressant: a historical review and future directions. Neuropharmacology 218, 109219. [DOI] [PubMed] [Google Scholar]
- Zhang K, Dong C, Fujita Y, Fujita A, Hashimoto K, 2018. 5-Hydroxytryptamine-independent antidepressant actions of (R)-ketamine in a chronic social defeat stress model. Int J Neuropsychopharmacol 21, 157–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Hashimoto K, 2019. Lack of opioid system in the antidepressant actions of ketamine. Biol Psychiatry 85, e25–e27. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Ye F, Zhang T, Lv S, Zhou L, Du D, Lin H, Guo F, Luo C, Zhu S, 2021c. Structural basis of ketamine action on human NMDA receptors. Nature 596, 301–305. [DOI] [PubMed] [Google Scholar]
- Zhao J, Liu X, Chang D, Zhang X, Lian H, Du X, Gao L, 2020. Low-dose ketamine improves LPS-induced depression-like behavior in rats by activating cholinergic anti-inflammatory pathways. ACS Chem Neurosci 11, 752–762. [DOI] [PubMed] [Google Scholar]
- Zhao X, Venkata SL, Moaddel R, Luckenbaugh DA, Brutsche NE, Ibrahim L, Zarate CA Jr., Mager DE, Wainer IW, 2012. Simultaneous population pharmacokinetic modelling of ketamine and three major metabolites in patients with treatment-resistant bipolar depression. Br J Clin Pharmacol 74, 304–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Wang N, Yang C, Li XM, Zhou ZQ, Yang JJ, 2014. Ketamine-induced antidepressant effects are associated with AMPA receptors-mediated upregulation of mTOR and BDNF in rat hippocampus and prefrontal cortex. Eur Psychiatry 29, 419–423. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Zheng W, Liu W, Wang C, Zhan Y, Li H, Chen L, Li M, Ning Y, 2018. Antidepressant effect of repeated ketamine administration on kynurenine pathway metabolites in patients with unipolar and bipolar depression. Brain Behav Immun 74, 205–212. [DOI] [PubMed] [Google Scholar]