Abstract
Various techniques exist to surgically treat adult spinal deformity. Traditionally, anterior-based or posterior-based procedures with or without the addition of osteotomies were utilized. More recently, lateral-based approaches to correcting deformity have become more established and widely utilized. This may include the use of large footprint interbody devices with varying degrees of lordosis. Additionally, more powerful corrective techniques via lateral approach include anterior column release of the anterior longitudinal ligament and corpectomy. These present with unique risks that are typically related to the lateral approach, however, have been shown to reduce blood loss, decrease neurologic risk, and morbidity as they can be done through a less invasive approach. This review presents the variable deformity correction techniques that are available to the spinal surgeon, as well as the evidence and evolution of lateral-based techniques.
Keywords: lateral fusion, adult spinal deformity
INTRODUCTION
Adult spinal deformity (ASD) is a clinical spectrum of disorders encompassing a range of spinal conditions which result in malalignment.1 These conditions can result in significant disability for the patient and, if progressive, can lead to neurological impairments and loss of function.1 ASD may include de novo scoliosis, adolescent idiopathic scoliosis that is progressive in adulthood, degenerative scoliosis, iatrogenic deformity, degenerative disease, and post-traumatic deformity.1–5 Prevalence of ASD has been reported to be as high as 60% in the elderly population, with the mean age of presentation at approximately 70.5 years and a 1:1 male to female ratio.2,6,7 The prevalence of ASD in North America is increasing as a result of the aging population, and the increased awareness and recognition of ASD.1
Diagnosis and management of ASD were traditionally based on the coronal plane alignment; however, the importance of sagittal plane imbalance has more recently gained significant recognition.4 The importance of sagittal alignment was demonstrated by Glassman and colleagues, where the severity of symptoms increased with progressive sagittal malalignment.8 Sagittal alignment plays a critical role in pain and disability.8–12 Research has demonstrated that the magnitude and extent of coronal deformity correction are less important and surgical correction of ASD should place greater emphasis on restoring sagittal alignment.13 This review discusses various deformity correction techniques along with the evidence and evolution of lateral-based techniques. Illustrative case examples are included.
EVALUATION OF DEFORMITIES
Classification System
The Scoliosis Research Society-Schwab classification system works well for ASD as it is a reproducible and well-validated system.14 It includes 4 types of coronal curves (thoracic only, thoracolumbar/lumbar only, double curve, or no major coronal curve) and 3 sagittal modifiers (pelvic incidence [PI]—lumbar lordosis [LL], global alignment, and pelvic tilt [PT]).(Figure 1)14 Schwab et al demonstrated an excellent interrater and intrarater reliability and agreement for curve type and each modifier, making it a consistently reproducible classification system.15 The classification scheme provides a way to describe the radiographic characteristics of the deformity. It has been demonstrated to reflect the severity of disease state, correlate with health-related quality of life measures, and aid in treatment decision-making for operative vs nonoperative management.14
Figure 1.
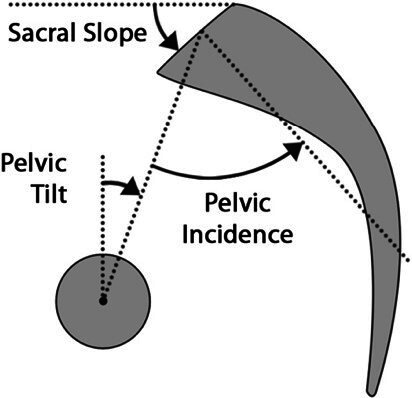
Spinopelvic parameters.16
Sagittal Alignment Parameters
Following the increased recognition of the importance of sagittal alignment, multiple studies have demonstrated the need to assess spinal alignment in the context of pelvic parameters.4,9,12,17 These parameters include PI, PT, and LL. PI describes the relationship between the spine and the femoral heads; it is specific to each individual patient and does not change with position, activity, age, or deformity and has a mean value of approximately 50°–55° in adults(Figure 2).18–20 LL is a crucial part of sagittal alignment and critical for maintaining an upright posture.18 A higher PI necessitates a high LL to maintain the spine leveled over the pelvis, and a greater mismatch between the PI and LL has been correlated with increasing disability.9,18 Sagittal vertical axis (SVA) is another important parameter which is defined as the horizontal distance from the posterior superior aspect of the S1 vertebral body and a plumb line dropped from the center of the C7 vertebral body. Multiple ranges for average SVA have been described, with different ranges based on age; however, the suggested postoperative SVA goal is <50 mm.18,21–25 Schwab et al demonstrated that a mismatch between PI and LL greater than 11°, PT greater than 22°, and SVA greater than 47 mm correlated with moderate to severe disability.9 PT is a key regulator of sagittal alignment and represents a dynamic parameter. In ASD, as the SVA becomes increasingly positive, compensatory mechanisms recruit the hamstrings and hip extensors to retrovert the pelvis, increasing PT and thereby maintaining an upright posture. These compensatory mechanisms require substantial energy expenditure and result in patients falling outside of the “cone of economy” first described by Dubousset(Figure 3).18,26
Figure 2.
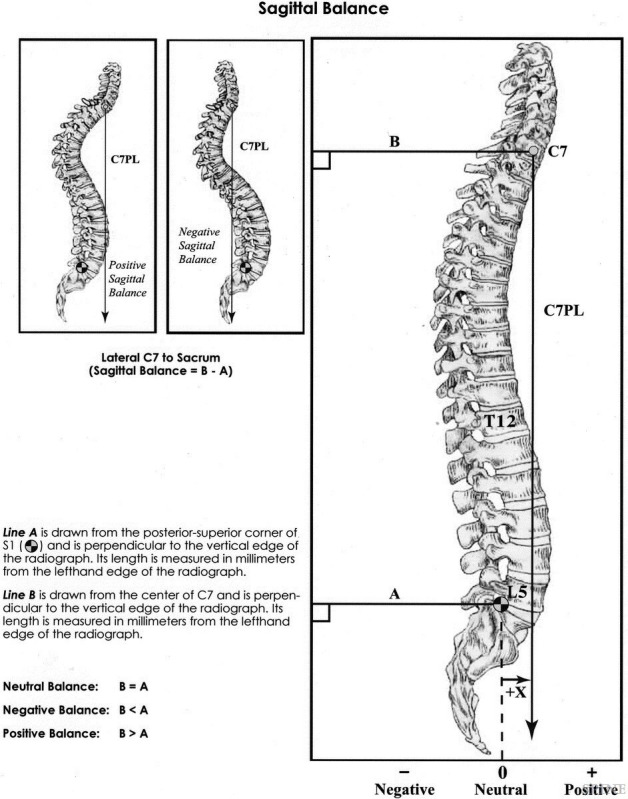
Measurement technique for global sagittal balance.8
Figure 3.
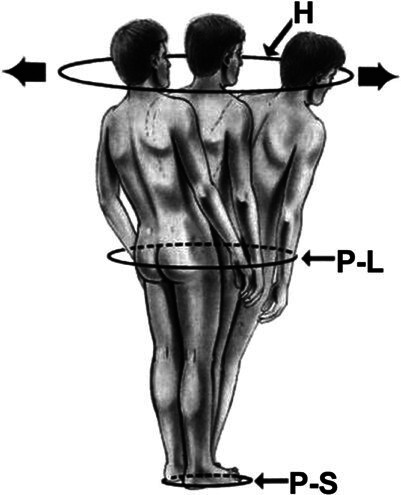
The “cone of economy” first described by Dubousset.16
Indications for Deformity Surgery
Surgical deformity correction should be considered for patients who have failed to improve with continued conservative management or patients with progressive or impending neurological deficits. Patients are evaluated clinically to asses for worsening deformity, clinical symptoms, and neurological status. Assessment is performed with full-length spine radiographs including flexion/extension views and advanced imaging with computed tomography and/or magnetic resonance imaging (MRI) scans. The decision to proceed with surgery is a shared decision-making process between the patient and the physician, with decision-making weighing the impact the deformity has had on the patient’s quality of life vs the risks associated with the proposed surgery. ASD correction has some of the highest potential perioperative risks, and detailed discussions with potential surgical patients should address these risks in detail.27–31 Mummaneni et al recently updated their minimally invasive spinal deformity surgery 2 decision-making algorithm for surgical correction of ASD to incorporate newer minimally invasive techniques.(Figure 4) The minimally invasive spinal deformity surgery-2 algorithm has demonstrated excellent interobserver and intraobserver agreement and provides decision-making guidance ranging from basic minimally invasive surgery (MIS) techniques, to advanced MIS techniques and open deformity correction.32
Figure 4.
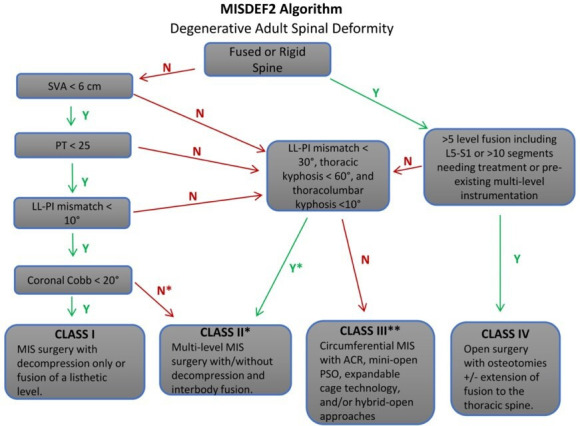
The minimally invasive spinal deformity surgery 2-deformity correction algorithm described by Mummaneni et al.32 ACR, anterior column realignment; LL, lumbar lordosis; PI, pelvic incidence; PSO, pedicle subtraction osteotomy; PT, pelvic tilt; SVA, sagittal vertical axis.
SURGICAL GOALS
Maintaining a systematic approach when addressing ASD is critically important and increases the likelihood of safe and reproducible outcomes. Iyer et al describe a systematic 6-step planning approach in addressing ASD: (1) identifying the drivers of deformity, (2) determining their effects on global alignment, (3) identifying targets for alignment, (4) assessing spinal flexibility, (5) formulating a surgical strategy, and (6) execution of the strategy.18 A typical driver of deformity is loss of LL resulting in a significant mismatch of sagittal alignment parameters. Thorough evaluation with full-length spine radiographs will help define the driver of the deformity and its effect on global alignment. Ideal goals of ASD correction should target to achieve a PI-LL mismatch of less than 10° and provide an SVA correction of within positive 5 cm.9 Spinal flexibility is easily assessed in the office with either flexion/extension views, supine or bending/bolster films, or scout images from computed tomography and/or MRI scans.18 Once the deformity has been identified, surgical planning is key to be able to execute the amount of correction desired.
SURGICAL TECHNIQUES
Various surgical strategies can be applied to meet the goals including posterior fusion, osteotomies, anterior, and lateral techniques. Posterior spinal fusion has remained a tried and true versatile approach for ASD. It is a familiar and reliable approach with a high success rate. It provides the capability of addressing a long deformity with a single surgical approach via segmental pedicle screw instrumentation, coupled with varying degrees of osteotomies as needed to achieve desired deformity correction. Posterior instrumentation and fusion works well for flexible deformities and osteotomies may not be required; however, rigid deformities require osteotomies to mobilize the spine column in order to achieve correction.33 Osteotomies are typically performed via a posterior approach and provide a greater potential for deformity correction.34 Osteotomy types range from a posterior column osteotomy such as Smith-Peterson osteotomy to more complex osteotomies including pedicle subtraction osteotomy (PSO) and vertebral column resection.33 The osteotomies are indicated based on the level of correction desired as they all have different correction potentials and degree of difficulty.
These more traditional techniques, although very powerful, are associated with high intraoperative risks including high blood loss and potential for neurological injury. Alternative anterior-based and lateral-based approaches coupled with posterior instrumentation can provide significant deformity correction with higher fusion rates than posterior-only–based procedures.35–39 Anterior procedures coupled with posterior approach provide several significant advantages when compared with posterior only surgery. Anterior approach to the lumbar spine can be either direct anterior lumbar interbody fusion (ALIF) or a lateral lumbar interbody fusion (LLIF) via a retroperitoneal approach. ALIF is a very powerful tool that is able to provide significant sagittal plane correction. LLIF used in conjunction with posterior-based approach is also very powerful in deformity correction.40–42 LLIF is performed via a retroperitoneal either anterior to the psoas or transpsoas. LLIF allows for insertion of a large interbody device into the disc space via minimally invasive technique, through minimal muscle dissection or disruption of the ligamentous structures thereby making it extremely useful for both sagittal and coronal plane correction.40–42
Ozgur et al described the extreme lateral interbody fusion which allowed access to the lumbar spine through a retroperitoneal approach, avoiding the anterior vasculature, hypogastric plexus, sympathetic chain, and the need for an approach surgeon.40–47 The approach allows easy access to a significant portion of the thoracolumbar and lumbar spine (T11-L5). In contrast to ALIF and transforaminal lumbar interbody fusion (TLIF), LLIF preserves much of the native structures including anterior longitudinal ligament (ALL), posterior longitudinal ligament, and facets joints. LLIF coupled with posterior instrumentation and osteotomies, in an open or MIS hybrid fashion, will provide greater correction than an LLIF or posterior fusion alone. There is a lower chance of injury to the nerve roots and lower durotomy risk in LLIF vs posterior-based approaches.48,49 Unlike TLIF or posterior lumbar interbody fusion, LLIF has the ability to place wider and longer interbody devices which provide the indirect foraminal decompression and restoration of the disc height and foramen.50,51 With preoperative planning of the size, lordosis, and placement of the interbody devices, LLIF allows for significant improvement of segmental lordosis in order to correct the sagittal profile.
If needed, the ALL can be released in a controlled manner to perform an anterior column realignment (ACR) which can provide much greater sagittal plane correction.52 ACR relies on the elongation of the anterior column and may be done via an ALIF or LLIF with complete release of the ALL, annulus, and insertion of hyperlordotic interbody devices.52 Saigal et al conducted a retrospective review of ACR in which 12 studies were included and demonstrated 10°–27° increase in segmental lordosis with use of hyperlordotic cages, a mean increase of 19° in intradiscal angle with ACR when combined with posterior column osteotomy, 13° more than LLIF alone.53 Mundis et al demonstrated similar radiographic results, similar complication rates, and less estimated blood loss with ACR as compared to PSO in propensity-matched patients undergoing sagittal plane deformity correction.54
Corpectomy (Figure 5) via the lateral approach is an alternative to the traditional open corpectomy and performed using minimally invasive principles. Traditional open posterior corpectomy is associated with significant morbidity and leads to prolonged surgical time and blood loss.55,56 Posterior approaches also provide less deformity correction in the sagittal plane than an anterior or lateral approach for corpectomy.57 When compared to anterior-based corpectomy, lateral corpectomy provides similar ability to directly decompress the neural elements, while avoiding the potential need for an access surgeon, as well as risks of abdominal wall or diaphragm injury and chronic incisional pain.58
Figure 5.
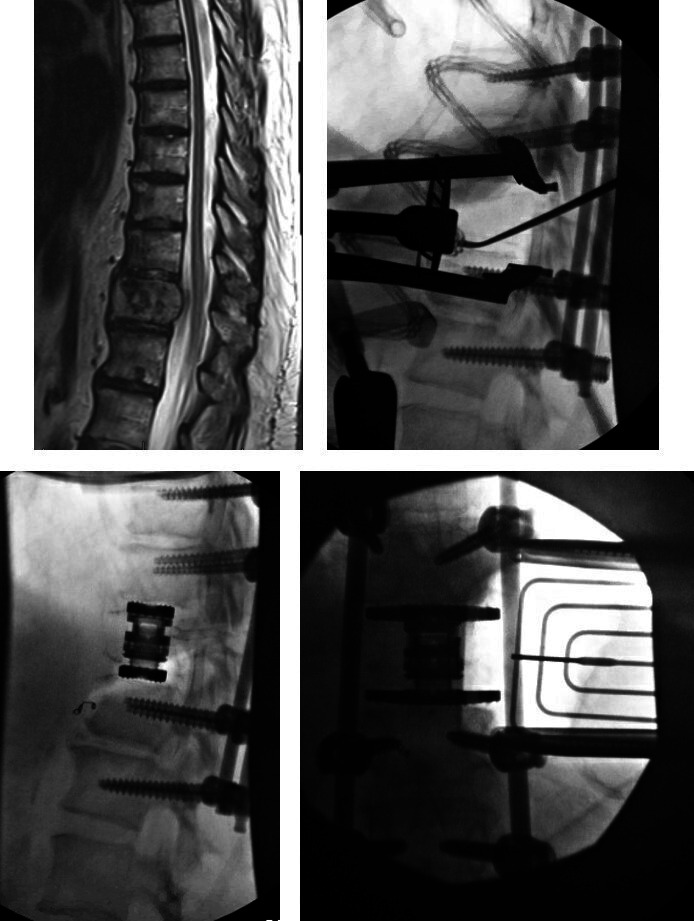
Illustration of a lateral corpectomy for a renal cell carcinoma metastasis.
LLIF can be performed in a number of different fashions depending on the training and comfort level of the surgeon. It can be performed either anterior to the psoas (oblique) or in a transpsoas fashion. The anterior to psoas (ATP) lateral interbody fusion provides access to the disc space via a retroperitoneal approach between the anterior vasculature and the psoas muscle. It avoids the lumbar plexus and does not violate the muscle fibers of the psoas muscle as a traditional transpsoas approach does.59 On the other hand, transpsoas interbody techniques access the disc space through the psoas muscle and are in close proximity to the lumbar plexus thereby can lead to sensory and motor deficits.51,60
Whether an ATP or transpsoas interbody fusion is performed, preoperative planning is vital to the successful execution of the procedure. Imaging should include plain radiographs and MRI which should be scrutinized to identify the level of interest, iliac crest level, location, and orientation of the psoas muscle, as well as surrounding structures including kidney, ureter, vasculature, and bowel. The lateral radiograph is helpful in assessing the level of the iliac crest and whether a direct lateral or oblique lateral can be performed. Intraoperative neuromonitoring of somatosensory evoked potentials, and electromyography is necessary in carrying out the procedure safely. The ATP lateral fusion procedure is carried out in the lateral decubitus position with the patient secured to the radiolucent table using tape. Access to multiple levels can be improved by breaking the table with the greater trochanter slightly inferior to the table break. This table break increases the corridor in between the ribs and the iliac crest. Surgical incision for an ATP lateral fusion is an oblique incision anterior to the disc space and dissection is carried out in between the fibers of the external oblique muscle, internal oblique fibers, and then bluntly through the transversus abdominis layer to enter the retroperitoneal space. The corridor in between the psoas muscle and anterior vasculature is identified at the level of interest. Dilators are inserted over the disc space sequentially with guidance from neuromonitoring, and the retractor is docked over the disc space. Once the retractor is secured over the disc space, disc prep can be performed carefully under fluoroscopic guidance with care taken to avoid violation anteriorly to prevent vascular injury and avoid violation posteriorly to prevent neural injury. This orthogonal working trajectory must be maintained throughout the procedure.
Surgical incision for transpsoas interbody fusion is more directly in line with the disc space. Similar dissection is performed through the abdominal muscles to enter the retroperitoneal space. Dilators are introduced through the psoas muscle at the disc space level with guidance of neuromonitoring to avoid injury to the lumbar plexus. Ideal position of the dilator is between the posterior one-third and anterior two-thirds of the disc space, parallel to the disc space. Once the retractor is docked and secured with a disc shim, the disc prep can be carried out under fluoroscopic guidance.
Pimenta et al more recently described a prone lateral interbody fusion which allows single-position lateral and posterior interbody fusion (Figure 6).61 This approach allows for simultaneous access to both the anterior and posterior spinal columns, thereby, allowing for direct decompression, instrumentation, interbody placement, and sagittal plane correction without needing to reposition the patient, therefore boasting the advantage of decreasing total anesthesia and operating room time.61 The prone position also may provide improvement in sagittal alignment due to prone patient positioning and ability to insert more lordotic implants as compared to lateral performed in the lateral decubitus position.61–64
Figure 6.
Patient positioning for prone lateral interbody fusion, allowing access to both the lateral and posterior aspect of the spine.
Following patient positioning, bolsters are used to increase the working corridor in between the ribs and the iliac crest. Fluoroscopy is used to localize the surgical incision starting from the foramen and in-line with the disc level. Once the incision is made, dissection is carried out through the abdominal muscles and into the retroperitoneal space. Dilators are introduced in a transpsoas fashion with the assistance of neuromonitoring, and the retractor is docked and opened at the disc level of interest. The disc prep is performed in a similar fashion, and an interbody cage is inserted.
When planning more significant degrees of deformity correction, ACR can provide greater degrees of deformity correction, particularly in the sagittal plane. This technique has been shown to have similar results for deformity correction when compared to open osteotomy such as PSO and is performed through a minimally invasive lateral retroperitoneal approach.65 Careful blunt dissection is performed to develop the plane between the ALL and the anterior vascular structures, and then specialized anterior retractor blades are placed for protection throughout the remainder of the procedure. Wide and thorough discectomy is then performed followed by careful sharp transection of the remaining annulus and ALL. Sequential trialing with increasingly more lordotic implants is then performed prior to implanting a hyperlordotic interbody device ranging from 20° to 30° of lordosis. Given the temporary instability created by this procedure, an attached plate with a fixation screw into the more cephalad vertebral body is utilized to prevent cage displacement. In order to maximize the power of deformity correction, ACR technique can be combined with complete facetectomy prior to final posterior spinal fixation.66
COMPLICATIONS
Despite being a very reliable procedure, lateral interbody fusion has known potential complications unique to the procedure including hip flexion or knee extension weakness, neurologic and vascular injury, subsidence, pseudohernia, vertebral body fracture, visceral injury, wound infection.67 Hijji et al conducted a systematic review evaluating the medical and surgical complications associated with LLIF and found a 36.07% incidence of transient neurologic deficits with the most common symptoms being related to the surgical approach and dissection through the psoas muscle.68 There was only 3.98% chance of persistent neurological complications.68 Tomeh et al conducted a prospective, nonrandomized clinical study in patients undergoing LLIF and found a 27.5% incidence of postoperative hip flexion weakness, upper medial thigh sensory deficits in 17.6%, and transient motor deficits in 2.9% of patients.69 Lykissas et al conducted a review of 919 levels in 451 patients and reported an incidence of 38.5% of anterior thigh pain, 38.0% of sensory, and 23.9% of motor deficits; however, motor deficits decreased to 3.2% and sensory deficits to 9.6% at 18 months of follow-up.70 Cahill et al performed a retrospective chart review of 118 patients undergoing lateral interbody fusion and reported an incidence of 4.8% of femoral nerve palsy at L4-5, and 0% incidence of femoral nerve palsy at other levels.70 Kueper et al evaluated 900 patients undergoing an LLIF and found an incidence of 0.056% incidence of vascular injury per case and 0.029% per level.71
Subsidence of the interbody device is clinically significant as it can lead to mechanical failure of the anterior column support, loss of the indirect decompression, pseudarthrosis, and sagittal imbalance.67 Le et al reviewed subsidence in patients undergoing a lateral interbody fusion and reported a 14.3% rate of radiographic subsidence, 2.1% rate of clinically significant subsidence; 14.1% rate of subsidence in cases where 18-mm cages were used and 1.9% in cases where 22-mm cages were used.72 This was also demonstrated by Marchi et al where wider interbody cages had lower subsidence rate and improved segmental lordosis.73
Vertebral body fracture can occur in patients with end plate violation, graft subsidence, osteopenia, and placement of oversize grafts.67,74 Injury to the bowel can occur in an LLIF and is a very serious and life-threatening injury, therefore, careful dissection and development of the retroperitoneal space are vital.67 Uribe et al conducted a survey study with 13 004 patients undergoing LLIF by experienced surgeons and demonstrated a rate of 0.08% visceral/bowel injury, 0.10% of vascular injury, 0.27% of superficial wound infection, and 0.14% of deep wound infection.29 Mundis et al retrospectively performed a radiographic comparison on patients undergoing ACR vs open PSO for correction of adult sagittal plane deformity. They found significant and similar improvement between preoperative and postoperative measurements of sagittal plane and spinopelvic parameters between the 2 groups. The patients who underwent ACR exhibited significantly less estimated blood loss (1.6 vs 3.6 L); however, no significant differences in overall major complication rates were demonstrated between the 2 groups (35.3% vs 41.2%). This highlights the comparative effectiveness of the more MIS ACR for the treatment of ASD. The rate of major complication regardless of technique also reinforces the importance of careful surgical decision-making and patient selection for this major undertaking.65
ILLUSTRATIVE CASES
Case 1
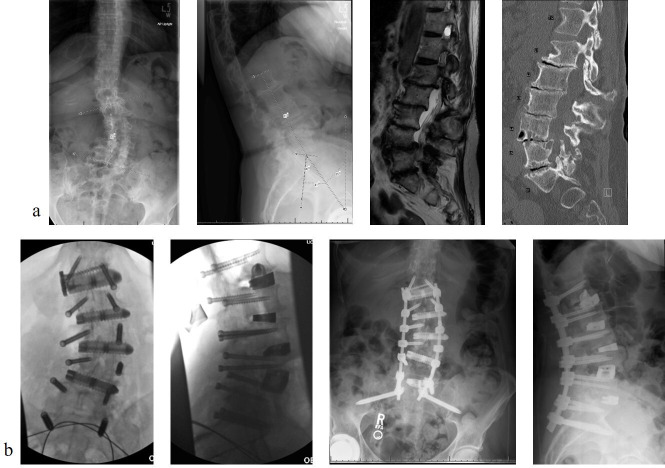
A 71-year-old woman who presented with 7 years of low back pain and left lateral thigh/calf pain. She rated the pain at an 8/10 and is now using a cane to ambulate. She failed to improve with continued conservative treatment including anti-inflammatories, analgesics, physical therapy, and 6 epidural steroid injections. She was found to have an adult degenerative scoliosis with multilevel stenosis in the lumbar spine. She elected to proceed with operative intervention and underwent a minimally invasive multilevel prone transpsoas (PTP) interbody placement from L1-5, TLIF at L5-S1 with percutaneous pedicle screw instrumentation from L1-pelvis. The PTP positioning bolsters were used to increase the working corridor between the ribs and iliac crest. PTP was first performed at L4-5 followed by L1-2, L3-4, and L2-3. Direct decompression was performed from L4-S1 followed by a TLIF at L5-S1. This allowed for correction of both the coronal and sagittal plane deformities with improvement in LL from 20° to 40° and improvement in the LL-PI mismatch from 15° to 0° (Figure 7).
Figure 7.
(a) Preoperative radiographs, magnetic resonance imaging, and computed tomographic image highlighting the coronal and sagittal plane deformity and (b) postoperative radiographs depicting the intraoperative images and postoperative correction of the coronal and sagittal plane deformity.
Case 2
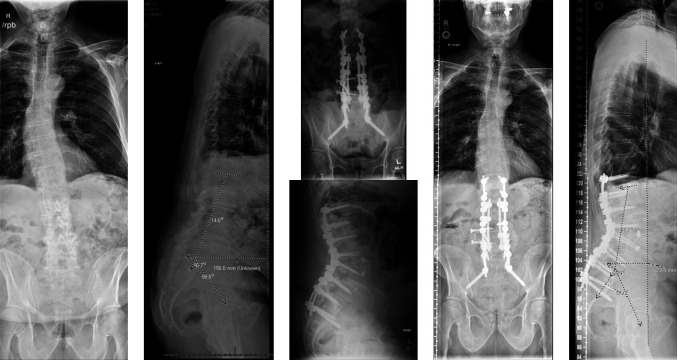
A 66-year-old woman who initially presented with severe back pain and inability to ambulate secondary to pain and bilateral leg pain/numbness. She had previously underwent single-level lateral interbody fusion at an outside hospital and was lost to follow-up. Upon presentation, she exhibited significant focal and global kyphosis and functional disability. She underwent L1-2 ACR with revision posterior T11-L4 fusion. Sagittal plane deformity was corrected, and more extensive blood loss and potential morbidity of 3-column osteotomy were avoided (Figure 8). LL improved from 11° to 40°, and global sagittal balance improved from +165 to +31 mm.
Figure 8.
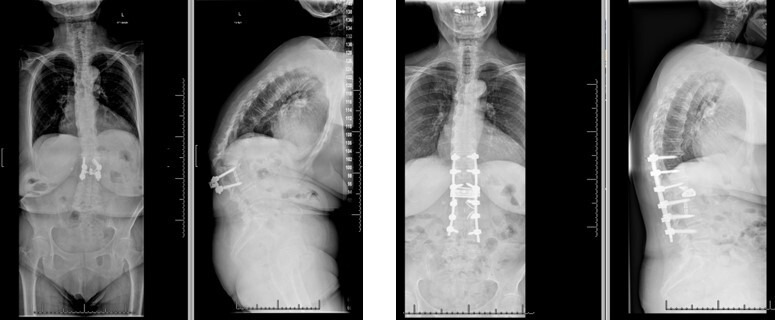
Preoperative deformity and postoperative deformity correction following an anterior column realignment.
Case 3
A 71-year-old man who presented with worsening low back pain, stooping over posture, and neurogenic claudication. He was found to have significant sagittal imbalance with +15.5 cm of SVA, 14° of LL, 58° of PI, and a PI-LL mismatch of approximately 44°. He failed to improve with conservative management and elected to undergo surgical correction. He underwent a multilevel LLIF with an ACR coupled with posterior instrumentation, providing him with significant correction of his sagittal profile, with improvement of his LL to 45°, PI to 57°, and a PI-LL mismatch of only 12°. His SVA improved to +7.9 cm (Figure 9).
Figure 9.
Figure illustrating the preoperative flatback deformity and postoperative correction following an anterior column realignment.
CONCLUSION
LLIF in deformity surgery is a very powerful tool and when applied correctly it can generate significant coronal and sagittal plane correction, in addition to indirect decompression of neural elements. Traditional open anterior and posterior approaches are very versatile and work well in deformity correction; however, lateral fusion coupled with posterior instrumentation provides a less invasive way to mitigate some of the potential complications associated with open anterior and posterior deformity correction. There are a wide variety of techniques that can be employed to perform a lateral fusion, and the performing surgeon should understand the advantages and disadvantages of each technique and be comfortable with their preferred method so as to safely and successfully carry out the desired procedure.
References
- 1.Ailon T, Smith JS, Shaffrey CI, et al. Degenerative spinal deformity. Neurosurgery. 2015;77 Suppl 4:S75-91. 10.1227/NEU.0000000000000938 [DOI] [PubMed] [Google Scholar]
- 2.Youssef JA, Orndorff DO, Patty CA, et al. Current status of adult spinal deformity. Global Spine J. 2013;3(1):51–62. 10.1055/s-0032-1326950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aebi M. The adult scoliosis. Eur Spine J. 2005;14(10):925–948. 10.1007/s00586-005-1053-9 [DOI] [PubMed] [Google Scholar]
- 4.Smith JS, Shaffrey CI, Bess S, et al. Recent and emerging advances in spinal deformity. Neurosurgery. 2017;80(3S):S70–S85. 10.1093/neuros/nyw048 [DOI] [PubMed] [Google Scholar]
- 5.Diebo BG, Shah NV, Boachie-Adjei O, et al. Adult spinal deformity. The Lancet. 2019;394(10193):160–172. 10.1016/S0140-6736(19)31125-0 [DOI] [PubMed] [Google Scholar]
- 6.Schwab F, Dubey A, Gamez L, et al. Adult scoliosis: prevalence, SF-36, and nutritional parameters in an elderly volunteer population. Spine (Phila Pa 1976). 2005;30(9):1082–1085. 10.1097/01.brs.0000160842.43482.cd [DOI] [PubMed] [Google Scholar]
- 7.Silva FE, Lenke LG. Adult degenerative scoliosis: evaluation and management. Neurosurg Focus. 2010;28(3):E1. 10.3171/2010.1.FOCUS09271 [DOI] [PubMed] [Google Scholar]
- 8.Glassman SD, Bridwell K, Dimar JR, Horton W, Berven S, Schwab F, et al. The impact of positive sagittal balance in adult spinal deformity. Spine (Phila Pa 1976). 2005;30(18):2024–2029. 10.1097/01.brs.0000179086.30449.96 [DOI] [PubMed] [Google Scholar]
- 9.Schwab FJ, Blondel B, Bess S, et al. Radiographical spinopelvic parameters and disability in the setting of adult spinal deformity: a prospective multicenter analysis. Spine (Phila Pa 1976). 2013;38(13):E803-12. 10.1097/BRS.0b013e318292b7b9 [DOI] [PubMed] [Google Scholar]
- 10.Cho K-J, Suk S-I, Park S-R, et al. Risk factors of sagittal decompensation after long posterior instrumentation and fusion for degenerative lumbar scoliosis. Spine (Phila Pa 1976). 2010;35(17):1595–1601. 10.1097/BRS.0b013e3181bdad89 [DOI] [PubMed] [Google Scholar]
- 11.Kim YJ, Bridwell KH, Lenke LG, Rhim S, Cheh G, et al. An analysis of sagittal spinal alignment following long adult lumbar instrumentation and fusion to L5 or S1: can we predict ideal lumbar lordosis? Spine (Phila Pa 1976). 2006;31(20):2343–2352. 10.1097/01.brs.0000238970.67552.f5 [DOI] [PubMed] [Google Scholar]
- 12.Smith JS, Bess S, Shaffrey CI, et al. Dynamic changes of the pelvis and spine are key to predicting postoperative sagittal alignment after pedicle subtraction osteotomy: a critical analysis of preoperative planning techniques. Spine (Phila Pa 1976). 2012;37(10):845–853. 10.1097/BRS.0b013e31823b0892 [DOI] [PubMed] [Google Scholar]
- 13.Glassman SD, Berven S, Bridwell K, Horton W, Dimar JR, et al. Correlation of radiographic parameters and clinical symptoms in adult scoliosis. Spine (Phila Pa 1976). 2005;30(6):682–688. 10.1097/01.brs.0000155425.04536.f7 [DOI] [PubMed] [Google Scholar]
- 14.Terran J, Schwab F, Shaffrey CI, et al. The SRS-Schwab adult spinal deformity classification: assessment and clinical correlations based on a prospective operative and nonoperative cohort. Neurosurgery. 2013;73(4):559–568. 10.1227/NEU.0000000000000012 [DOI] [PubMed] [Google Scholar]
- 15.Schwab F, Ungar B, Blondel B, et al. Scoliosis research society-schwab adult spinal deformity classification: a validation study. Spine (Phila Pa 1976). 2012;37(12):1077–1082. 10.1097/BRS.0b013e31823e15e2 [DOI] [PubMed] [Google Scholar]
- 16.Klineberg E, Schwab F, Smith JS, Gupta MC, Lafage V, Bess S, et al. Sagittal spinal pelvic alignment. Neurosurg Clin N Am. 2013;24(2):157–162. 10.1016/j.nec.2012.12.003 [DOI] [PubMed] [Google Scholar]
- 17.Lafage V, Schwab F, Patel A, Hawkinson N, Farcy J-P, et al. Pelvic tilt and truncal inclination: two key radiographic parameters in the setting of adults with spinal deformity. Spine (Phila Pa 1976). 2009;34(17):E599-606. 10.1097/BRS.0b013e3181aad219 [DOI] [PubMed] [Google Scholar]
- 18.Iyer S, Sheha E, MC F, et al. Sagittal spinal alignment in adult spinal deformity: an overview of current concepts and a critical analysis review. JBJS Rev. 2018;6(5):e2. 10.2106/JBJS.RVW.17.00117 [DOI] [PubMed] [Google Scholar]
- 19.Marty C, Boisaubert B, Descamps H, et al. The sagittal anatomy of the sacrum among young adults, infants, and spondylolisthesis patients. Eur Spine J. 2002;11(2):119–125. 10.1007/s00586-001-0349-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mangione P, Gomez D, Senegas J. Study of the course of the incidence angle during growth. Eur Spine J. 1997;6(3):163–167. 10.1007/BF01301430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iyer S, Lenke LG, Nemani VM, et al. Variations in sagittal alignment parameters based on age: a prospective study of asymptomatic volunteers using full-body radiographs. Spine (Phila Pa 1976). 2016;41(23):1826–1836. 10.1097/BRS.0000000000001642 [DOI] [PubMed] [Google Scholar]
- 22.Schwab F, Lafage V, Boyce R, Skalli W, Farcy J-P, et al. Gravity line analysis in adult volunteers: age-related correlation with spinal parameters, pelvic parameters, and foot position. Spine (Phila Pa 1976). 2006;31(25):E959-67. 10.1097/01.brs.0000248126.96737.0f [DOI] [PubMed] [Google Scholar]
- 23.Roussouly P, Gollogly S, Noseda O, Berthonnaud E, Dimnet J, et al. The vertical projection of the sum of the ground reactive forces of a standing patient is not the same as the C7 plumb line: a radiographic study of the sagittal alignment of 153 asymptomatic volunteers. Spine (Phila Pa 1976). 2006;31(11):E320-5. 10.1097/01.brs.0000218263.58642.ff [DOI] [PubMed] [Google Scholar]
- 24.Mendoza-Lattes S, Ries Z, Gao Y, Weinstein SL, et al. Natural history of spinopelvic alignment differs from symptomatic deformity of the spine. Spine (Phila Pa 1976). 2010;35(16):E792-8. 10.1097/BRS.0b013e3181d35ca9 [DOI] [PubMed] [Google Scholar]
- 25.Schwab F, Patel A, Ungar B, Farcy J-P, Lafage V, et al. Adult spinal deformity-postoperative standing imbalance: how much can you tolerate? An overview of key parameters in assessing alignment and planning corrective surgery. Spine (Phila Pa 1976). 2010;35(25):2224–2231. 10.1097/BRS.0b013e3181ee6bd4 [DOI] [PubMed] [Google Scholar]
- 26.Dubousset . Importance de La Vertebre Pelvienne Dans l’equilibre Rachidien. Application a La Chirurgie de La Colonne Vertebrale Chez l’enfant et l’adolescent. Pied Equilibre et Rachis; 1998:141–149. [Google Scholar]
- 27.Soroceanu A, Burton DC, Oren JH, et al. Medical complications after adult spinal deformity surgery: incidence, risk factors, and clinical impact. Spine (Phila Pa 1976). 2016;41(22):1718–1723. 10.1097/BRS.0000000000001636 [DOI] [PubMed] [Google Scholar]
- 28.Uribe JS, Deukmedjian AR, Mummaneni PV, et al. Complications in adult spinal deformity surgery: an analysis of minimally invasive, hybrid, and open surgical techniques. Neurosurg Focus. 2014;36(5):E15. 10.3171/2014.3.FOCUS13534 [DOI] [PubMed] [Google Scholar]
- 29.Uribe JS, Deukmedjian AR. Visceral, vascular, and wound complications following over 13,000 lateral interbody fusions: a survey study and literature review. Eur Spine J. 2015;24 Suppl 3:386–396. 10.1007/s00586-015-3806-4 [DOI] [PubMed] [Google Scholar]
- 30.Patel SA, McDonald CL, Reid DBC, DiSilvestro KJ, Daniels AH, Rihn JA, et al. Complications of thoracolumbar adult spinal deformity surgery. JBJS Rev. 2020;8(5):e0214. 10.2106/JBJS.RVW.19.00214 [DOI] [PubMed] [Google Scholar]
- 31.Lee NJ, Kothari P, Kim JS, et al. Early complications and outcomes in adult spinal deformity surgery: an NSQIP study based on 5803 patients. Global Spine J. 2017;7(5):432–440. 10.1177/2192568217699384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mummaneni PV, Park P, Shaffrey CI, et al. The MISDEF2 algorithm: an updated algorithm for patient selection in minimally invasive deformity surgery. J Neurosurg Spine. 2019;32(2):221–228. 10.3171/2019.7.SPINE181104 [DOI] [PubMed] [Google Scholar]
- 33.Enercan M, Ozturk C, Kahraman S, Sarıer M, Hamzaoglu A, Alanay A, et al. Osteotomies/spinal column resections in adult deformity. Eur Spine J. 2013;22 Suppl 2:S254-64. 10.1007/s00586-012-2313-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schwab F, Blondel B, Chay E, et al. The comprehensive anatomical spinal osteotomy classification. Neurosurgery. 2014;74(1):112–120. 10.1227/NEU.0000000000000182o [DOI] [PubMed] [Google Scholar]
- 35.Iorio JA, Reid P, Kim HJ. Neurological complications in adult spinal deformity surgery. Curr Rev Musculoskelet Med. 2016;9(3):290–298. 10.1007/s12178-016-9350-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bridwell KH, Lewis SJ, Lenke LG, Baldus C, Blanke K, et al. Pedicle subtraction osteotomy for the treatment of fixed sagittal imbalance. J Bone Joint Surg Am. 2003;85(3):454–463. 10.2106/00004623-200303000-00009 [DOI] [PubMed] [Google Scholar]
- 37.Cho K-J, Bridwell KH, Lenke LG, Berra A, Baldus C, et al. Comparison of smith-petersen versus pedicle subtraction osteotomy for the correction of fixed sagittal imbalance. Spine (Phila Pa 1976). 2005;30(18):2030–2037. 10.1097/01.brs.0000179085.92998.ee [DOI] [PubMed] [Google Scholar]
- 38.Kim K-T, Lee S-H, Suk K-S, Lee J-H, Jeong B-O, et al. Outcome of pedicle subtraction osteotomies for fixed sagittal imbalance of multiple etiologies: a retrospective review of 140 patients. Spine (Phila Pa 1976). 2012;37(19):1667–1675. 10.1097/BRS.0b013e3182552fd0 [DOI] [PubMed] [Google Scholar]
- 39.Lenke LG, Sides BA, Koester LA, Hensley M, Blanke KM, et al. Vertebral column resection for the treatment of severe spinal deformity. Clin Orthop Relat Res. 2010;468(3):687–699. 10.1007/s11999-009-1037-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ozgur BM, Aryan HE, Pimenta L, Taylor WR, et al. Extreme lateral interbody fusion (XLIF): a novel surgical technique for anterior lumbar interbody fusion. Spine J. 2006;6(4):435–443. 10.1016/j.spinee.2005.08.012 [DOI] [PubMed] [Google Scholar]
- 41.McAfee PC, Regan JJ, Geis WP, Fedder IL, et al. Minimally invasive anterior retroperitoneal approach to the lumbar spine. Emphasis on the lateral BAK. Spine (Phila Pa 1976). 1998;23(13):1476–1484. 10.1097/00007632-199807010-00009 [DOI] [PubMed] [Google Scholar]
- 42.Phan K, Rao PJ, Scherman DB, Dandie G, Mobbs RJ, et al. Lateral lumbar interbody fusion for sagittal balance correction and spinal deformity. J Clin Neurosci. 2015;22(11):1714–1721. 10.1016/j.jocn.2015.03.050 [DOI] [PubMed] [Google Scholar]
- 43.Youssef JA, McAfee PC, Patty CA, et al. Minimally invasive surgery: lateral approach interbody fusion: results and review. Spine (Phila Pa 1976). 2010;35(26 Suppl):S302-11. 10.1097/BRS.0b013e3182023438 [DOI] [PubMed] [Google Scholar]
- 44.Isaacs RE, Hyde J, Goodrich JA, Rodgers WB, Phillips FM, et al. A prospective, nonrandomized, multicenter evaluation of extreme lateral interbody fusion for the treatment of adult degenerative scoliosis: perioperative outcomes and complications. Spine (Phila Pa 1976). 2010;35(26 Suppl):S322-30. 10.1097/BRS.0b013e3182022e04 [DOI] [PubMed] [Google Scholar]
- 45.Flynn JC, Price CT. Sexual complications of anterior fusion of the lumbar spine. Spine (Phila Pa 1976). 1984;9(5):489–492. 10.1097/00007632-198407000-00013 [DOI] [PubMed] [Google Scholar]
- 46.Regan JJ, McAfee PC, Guyer RD, Aronoff RJ, et al. Laparoscopic fusion of the lumbar spine in a multicenter series of the first 34 consecutive patients. Surg Laparosc Endosc. 1996;6(6):459–468. [PubMed] [Google Scholar]
- 47.Baker JK, Reardon PR, Reardon MJ, Heggeness MH, et al. Vascular injury in anterior lumbar surgery. Spine (Phila Pa 1976). 1993;18(15):2227–2230. 10.1097/00007632-199311000-00014 [DOI] [PubMed] [Google Scholar]
- 48.Blumenthal SL, Ohnmeiss DD, NASS . Intervertebral cages for degenerative spinal diseases. Spine J. 2003;3(4):301–309. 10.1016/s1529-9430(03)00004-4 [DOI] [PubMed] [Google Scholar]
- 49.DiPaola CP, Molinari RW. Posterior lumbar interbody fusion. J Am Acad Orthop Surg. 2008;16(3):130–139. 10.5435/00124635-200803000-00004 [DOI] [PubMed] [Google Scholar]
- 50.Rao PJ, Maharaj MM, Phan K, Lakshan Abeygunasekara M, Mobbs RJ, et al. Indirect foraminal decompression after anterior lumbar interbody fusion: a prospective radiographic study using a new pedicle-to-pedicle technique. Spine J. 2015;15(5):817–824. 10.1016/j.spinee.2014.12.019 [DOI] [PubMed] [Google Scholar]
- 51.Sharma AK, Kepler CK, Girardi FP, Cammisa FP, Huang RC, Sama AA, et al. Lateral lumbar interbody fusion: clinical and radiographic outcomes at 1 year: a preliminary report. J Spinal Disord Tech. 2011;24(4):242–250. 10.1097/BSD.0b013e3181ecf995 [DOI] [PubMed] [Google Scholar]
- 52.Pimenta L, Fortti F, Oliveira L, et al. Anterior column realignment following lateral interbody fusion for sagittal deformity correction. Eur J Orthop Surg Traumatol. 2015;25 Suppl 1:S29-33. 10.1007/s00590-015-1642-1 [DOI] [PubMed] [Google Scholar]
- 53.Saigal R, Mundis GM, Eastlack R, Uribe J, Phillips F, Akbarnia BA, et al. Anterior column realignment (ACR) in adult sagittal deformity correction. SPINE. 2016:1. 10.1097/BRS.0000000000001483 [DOI] [PubMed] [Google Scholar]
- 54.Mundis GM, Turner JD, Kabirian N, et al. Anterior column realignment has similar results to pedicle subtraction osteotomy in treating adults with sagittal plane deformity. World Neurosurg. 2017;105:249–256. 10.1016/j.wneu.2017.05.122 [DOI] [PubMed] [Google Scholar]
- 55.Smith WD, Dakwar E, Le TV, Christian G, Serrano S, Uribe JS, et al. Minimally invasive surgery for traumatic spinal pathologies: a mini-open, lateral approach in the thoracic and lumbar spine. Spine (Phila Pa 1976). 2010;35(26 Suppl):S338-46. 10.1097/BRS.0b013e3182023113 [DOI] [PubMed] [Google Scholar]
- 56.Podet AG, Morrow KD, Robichaux JM, Shields JA, DiGiorgio AM, Tender GC, et al. Minimally invasive lateral corpectomy for thoracolumbar traumatic burst fractures. Neurosurg Focus. 2020;49(3):2020.6.FOCUS20366. 10.3171/2020.6.FOCUS20366 [DOI] [PubMed] [Google Scholar]
- 57.Sasso RC, Cotler HB. Posterior instrumentation and fusion for unstable fractures and fracture-dislocations of the thoracic and lumbar spine. A comparative study of three fixation devices in 70 patients. Spine (Phila Pa 1976). 1993;18(4):450–460. [PubMed] [Google Scholar]
- 58.Amaral R, Marchi L, Oliveira L, Coutinho T, Pimenta L, et al. Acute lumbar burst fracture treated by minimally invasive lateral corpectomy. Case Rep Orthop. 2013;2013:953897. 10.1155/2013/953897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim K-T, Jo D-J, Lee S-H, Seo E-M, et al. Oblique retroperitoneal approach for lumbar interbody fusion from L1 to S1 in adult spinal deformity. Neurosurg Rev. 2018;41(1):355–363. 10.1007/s10143-017-0927-8 [DOI] [PubMed] [Google Scholar]
- 60.Caputo AM, Michael KW, Chapman TM, et al. Extreme lateral interbody fusion for the treatment of adult degenerative scoliosis. J Clin Neurosci. 2013;20(11):1558–1563. 10.1016/j.jocn.2012.12.024 [DOI] [PubMed] [Google Scholar]
- 61.Pimenta L, Taylor WR, Stone LE, Wali AR, Santiago-Dieppa DR, et al. Prone transpsoas technique for simultaneous single-position access to the anterior and posterior lumbar spine. Oper Neurosurg (Hagerstown). 2020;20(1):E5–E12. 10.1093/ons/opaa328 [DOI] [PubMed] [Google Scholar]
- 62.Yson SC, Sembrano JN, Santos ERG, Luna JTP, Polly DW. Does prone repositioning before posterior fixation produce greater lordosis in lateral lumbar interbody fusion (LLIF)? J Spinal Disord Tech. 2014;27(7):364–369. 10.1097/BSD.0b013e318268007b [DOI] [PubMed] [Google Scholar]
- 63.Harimaya K, Lenke LG, Mishiro T, Bridwell KH, Koester LA, Sides BA, et al. Increasing lumbar lordosis of adult spinal deformity patients via intraoperative prone positioning. Spine (Phila Pa 1976). 2009;34(22):2406–2412. 10.1097/BRS.0b013e3181bab13b [DOI] [PubMed] [Google Scholar]
- 64.Benfanti PL, Geissele AE. The effect of intraoperative hip position on maintenance of lumbar lordosis: a radiographic study of anesthetized patients and unanesthetized volunteers on the Wilson frame. Spine (Phila Pa 1976). 1997;22(19):2299–2303. 10.1097/00007632-199710010-00021 [DOI] [PubMed] [Google Scholar]
- 65.Mundis GM, Turner JD, Kabirian N, et al. Anterior column realignment has similar results to pedicle subtraction osteotomy in treating adults with sagittal plane deformity. World Neurosurg. 2017;105:249–256. 10.1016/j.wneu.2017.05.122 [DOI] [PubMed] [Google Scholar]
- 66.Akbarnia BA, Mundis GM, Moazzaz P, et al. Anterior column realignment (ACR) for focal kyphotic spinal deformity using a lateral transpsoas approach and ALL release. J Spinal Disord Tech. 2014;27(1):29–39. 10.1097/BSD.0b013e318287bdc1 [DOI] [PubMed] [Google Scholar]
- 67.Salzmann SN, Shue J, Hughes AP. Lateral lumbar interbody fusion-outcomes and complications. Curr Rev Musculoskelet Med. 2017;10(4):539–546. 10.1007/s12178-017-9444-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hijji FY, Narain AS, Bohl DD, et al. Lateral lumbar interbody fusion: a systematic review of complication rates. Spine J. 2017;17(10):1412–1419. 10.1016/j.spinee.2017.04.022 [DOI] [PubMed] [Google Scholar]
- 69.Tohmeh AG, Rodgers WB, Peterson MD. Dynamically evoked, discrete-threshold electromyography in the extreme lateral interbody fusion approach. J Neurosurg Spine. 2011;14(1):31–37. 10.3171/2010.9.SPINE09871 [DOI] [PubMed] [Google Scholar]
- 70.Cahill KS, Martinez JL, Wang MY, Vanni S, Levi AD, et al. Motor nerve injuries following the minimally invasive lateral transpsoas approach. J Neurosurg Spine. 2012;17(3):227–231. 10.3171/2012.5.SPINE1288 [DOI] [PubMed] [Google Scholar]
- 71.Kueper J, Fantini GA, Walker BR, Aichmair A, Hughes AP, et al. Incidence of vascular complications during lateral lumbar interbody fusion: an examination of the mini-open access technique. Eur Spine J. 2015;24(4):800–809. 10.1007/s00586-015-3796-2 [DOI] [PubMed] [Google Scholar]
- 72.Le TV, Baaj AA, Dakwar E, et al. Subsidence of polyetheretherketone intervertebral cages in minimally invasive lateral retroperitoneal transpsoas lumbar interbody fusion. Spine (Phila Pa 1976). 2012;37(14):1268–1273. 10.1097/BRS.0b013e3182458b2f [DOI] [PubMed] [Google Scholar]
- 73.Marchi L, Abdala N, Oliveira L, Amaral R, Coutinho E, Pimenta L, et al. Radiographic and clinical evaluation of cage subsidence after stand-alone lateral interbody fusion. J Neurosurg Spine. 2013;19(1):110–118. 10.3171/2013.4.SPINE12319 [DOI] [PubMed] [Google Scholar]
- 74.Tempel ZJ, Gandhoke GS, Bolinger BD, Okonkwo DO, Kanter AS, et al. Vertebral body fracture following stand-alone lateral lumbar interbody fusion (LLIF): report of two events out of 712 levels. Eur Spine J. 2015;24 Suppl 3:409–413. 10.1007/s00586-015-3845-x [DOI] [PubMed] [Google Scholar]