Abstract
Background
Trimethylamine N-oxide (TMAO) and its precursors have an association with type 2 diabetes mellitus (T2DM); however, the evidence is unclear. The current study examined the association of serial measures of serum TMAO and related metabolite concentrations with the risk of T2DM.
Methods
Our study was designed as a community case-control study with 300 participants (150 T2DM and 150 non-T2DM). We examined the association of serum concentrations of TMAO and its related metabolites [trimethylamine, choline, betaine, and L-carnitine] using UPLC-MS/MS. The association between these metabolites and the risk of T2DM was analyzed using a restricted cubic spline and binary logistic regression.
Results
A higher serum choline concentration was significantly associated with an increased risk of T2DM. Serum choline > 22.62 μmol/L was independently associated with an increased risk of T2DM, and the odds ratio was 3.615 [95% CI: (1.453,8.993), P = 0.006]. Similarly, serum betaine and L-carnitine concentrations had a markedly decreased risk of T2DM even after adjusting for the traditional risk factors for T2DM and betaine (0.978 [95% CI:0.964–0.992], P = 0.002) and L-carnitine (0.949 [95% CI: 0.9222–0.978], P = 0.001), respectively.
Conclusion
Choline, betaine, and L-carnitine are associated with the risk of T2DM and may be appropriate risk markers to protect high-risk individuals from T2DM.
Keywords: type 2 diabetes, T2DM, trimethylamine N-oxide, TMAO, betaine, choline, L-carnitine, trimethylamine N-oxide related metabolites
Introduction
A high prevalence of type 2 diabetes mellitus (T2DM) has been observed in China and worldwide, representing a major public health challenge in the 21st century.1–3 According to the 10th edition of the International Diabetes Federation (IDF) Diabetes Atlas, approximately 790 million people worldwide have T2DM, with a steady increase in global incidence and prevalence over the past several decades, 90% of whom have T2DM.4 Moreover, these patients experience microvascular and macrovascular complications from their condition, including diabetic kidney disease, retinopathy, and cardiovascular disease, with a direct cost increasing from USD 232 billion in 2007 to USD 966 billion in 2021.4–6 Therefore, it is critical to reducing the direct and indirect losses caused by T2DM and its complications.
Given the long-term harm of T2DM and the cost of implementing an intervention, there is a pressing need to accurately identify high-risk individuals early and better understand the etiology of T2DM for the design of new and effective interventions. A variety of factors influence the risk of type 2 diabetes, including lifestyle, environmental, and genetic factors.7 They consistently increase the risk of developing T2DM. Early detection and treatment can prevent or delay the occurrence and development of diabetes. However, our understanding of the risk of diabetes remains incomplete.
Recently, researchers focused on metabolites that can allow the identification of individuals at high risk for T2DM by monitoring their concentrations.8 Trimethylamine N-oxide (TMAO) and its precursors, including trimethylamine (TMA), carnitine, choline, and betaine, play a significant role in glucose levels.9–11 The results of a cohort study show that betaine was associated with a lower incidence of T2DM and a risk ratio of 0.50 (95% CI:0.32–0.80).12 A meta-analysis showed a dose-dependent positive association between circulating TMAO levels, and increased risk of diabetes, with an odds ratio of 1.54.13 In contrast, the atherosclerosis risk in communities (ARIC) study showed an association between T2DM and an increase in choline and betaine levels (HR:1.01, [95% CI 0.87–1.16] for choline; HR:1.01, [95% CI 0.94–1.10] for betaine)14 were not significant. In the cardiovascular health study (CHS), TMAO and related metabolites did not show a statistically significant association with the risk of type 2 diabetes (HR: 1.20, [95% CI, 0.94–1.55] for TMAO; HR: 0.96, [95% CI, 0.74–1.24] for choline; HR: 0.88, [95% CI, 0.67–1.15] for betaine; HR: 1.07, [95% CI, 0.83–1.37] for carnitine; HR: 0.79, [95% CI, 0.60–1.04]).11 Specifically, because epidemiological evidence on the association of TMAO and its precursors with mortality and/or incident T2DM remains inconsistent, exploring the possible influence of TMAO and related precursors on the risk of T2DM remains essential.
For this purpose, the objective of the present study was to assess the association between TMAO and related metabolite levels and the risk of T2DM in a Chinese population in Ningxia, China.
Materials and Methods
Subject Recruitment
This study was conducted using a community case-control design. In 2018, participants in our study were recruited from four of twenty-two counties/districts after using a multistage sampling method in the Ningxia Hui Autonomous Region. According to the economic development level and location distribution, Xingqing District (plain), Litong District (plain), Pengyang County (mountainous area), and Xiji County (mountainous area) were selected as the survey sites. The detailed sampling process is described elsewhere (15). A total of 3985 eligible participants were selected. These participants underwent face-to-face interviews and physical examinations, and serum samples were collected by local physicians at once. (Supplementary Figure 1).
Based on previous studies (16), our study was designed to have more than 80% power at a type I error of 5% on two sides to detect a difference between the T2DM and non-T2DM groups in the primary outcome (TMAO concentration). Ultimately, 300 participants were recruited in this study; 150 randomly selected patients with T2DM and 150 patients without T2DM. The inclusion criteria for patients with T2DM patients are as follows: age ≥ 18, FPG ≥ 7.0 mmol/L, FPG < 7.0 mmol/L, and a history of diabetes medication. The patients whose physical examination was uncompleted and serum not collected were excluded. Concomitantly, each case was individually matched with a non-diabetic individual according to age (±3 years), sex, FPG < 7.0 mmol / L, and no medications or with a history of medication.
Variable Definitions
Participants who participated in moderate or vigorous activity for at least 30 min per day were defined as having physical activity. Diabetes within three generations (having a parent, grandparent, or sibling) was defined as having a family history of diabetes. Dietary habits were assessed using a food frequency questionnaire.15 For each category of food (refined grains and their products, whole grains and related products, vegetables, and fruits, meat, milk and its products, aquatic products, beans, and products), participants were asked about their consumption (weekly, monthly, or annual) and converted to daily consumption. Standardized procedures were used to measure participants’ height, weight, and blood pressure (BP). Body mass index (BMI) was calculated as body weight in kilograms divided by height in meters squared.
Laboratory Measurements
Previously, plasma concentrations of low-density lipoprotein (LDL), high-density lipoprotein (HDL), total cholesterol (TC), and triglycerides (TG) were examined.15
Our group measured TMAO and related metabolites (TMA, choline, betaine, L-carnitine) in 1.5mL cryogenic tubes stored at −80 °C. These metabolites are stable during long-term storage at −80°C.8,16 A Waters Acquity UPLC (Waters Corp., Milford, USA)-AB Sciex 5500+ triple quadrupole mass spectrometer (AB Sciex, Singapore) was used to quantify trimethylamine oxide and its precursors. A (1.7μm, 2.1×100 mm) Acquity Amide HILIC column (Waters) was used to separate the different components. Separation was achieved using a controlled gradient of mobile phase A, which consisted of 0.1% (v/v) formic acid and 10 mM ammonium formate in 10% (v/v) water and 90% (v/v) acetonitrile, and mobile phase B was composed of 0.1% formic acid and 10 mM ammonium formate in 50% (v/v) water and 50% (v/v) acetonitrile. The chromatographic gradient was increased from 100% weak elution to 30% over 5 min and the flow rate was set at 0.3 mL / min. Using TurboVTM heat for electrospray ionization, the metabolites were ionized and detected by selected reaction monitoring (SRM). The injection volume of the sample was 2 μL.
The main parameters were optimized as follows. Ion gas 1 (GS1), 50 psi, ion gas 2 (GS2) 50 psi, curtain gas (CUR) 35 psi, heater temperature 550 °C, and floating ion spray voltage as 5.5kV in positive mode.
The coefficients of variation of the laboratory assay during the study period varied in batches: 2.88% for TMAO, 7.58% for TMA, 2.12% for choline, 4.38% for betaine, and 5.86% for L-carnitine.
Statistical Analysis
All statistical analyzes were performed with SPSS, version 26.0 (IBM Corp., Armonk, New York) and R version 4.1.0. At baseline, continuous variables are presented as means with standard deviations or medians (interquartile ranges), and categorical variables are presented as counts and proportions (n, %). The baseline characteristics of the participants according to the study regimen were compared using Student’s t-test, the Wilcoxon-Mann–Whitney rank sum test, or the chi-square tests.
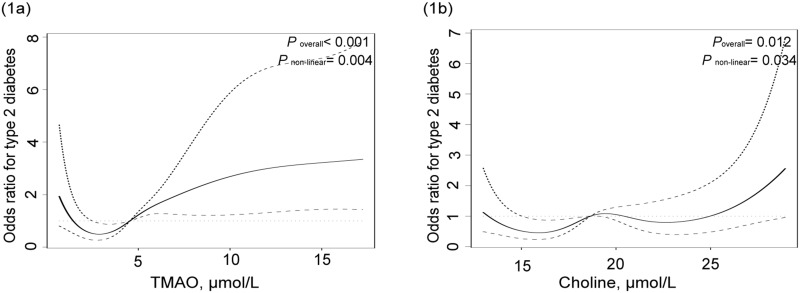
We found that TMAO and choline levels were nonlinearly associated with the risk of T2DM. Therefore, we used restricted cubic splines (RCS) nested in univariate and multivariate logistic regression analyzes to flexibly model the association of TMAO and choline with the risk of T2DM separately, and to define the cut-off points of these metabolites for T2DM. Then, the specific value at 2.85umol/L of TMAO, and 15.31μmol/L,19.40μmol/L, 22.62μmol/L of choline were chosen as the cut-off values because they are the knee of RCS curve (Figure 1). In Model 1, we performed an analysis to obtain adjusted ORs for TMAO and related metabolites. In Model 2, we explored the association between T2DM and traditional risk factors of T2DM, including education, physical activity, family history of diabetes, BMI, SBP, TG, vegetables and fruits, milk and its products, aquatic products, beans, and their products. Furthermore, TMAO, its precursors, and traditional risk factors were simultaneously included in model 3. We performed multivariate logistic regression analysis to identify significant TMAO and its precursors with predictive values, in addition to traditional risk factors (P=0.05, entry and P=0.10, respectively).
Figure 1.
(a) Association between TMAO and the risk of T2DM from restricted cubic splines nested in logistic regression analyses. (b) Association between choline and the risk of T2DM from restricted cubic splines nested in logistic regression analyses.
Finally, to assess whether the inclusion of TMAO and its precursors in the traditional risk factors model improved the discrimination of T2DM, we tested the effect sizes of these metabolites as continuous variables and compared the areas under the receiver operating characteristic curves (AUC) for the traditional risk factors model, the TMAO and related metabolites model, TMAO and related metabolites and traditional risk factors. Statistical significance was set at P < 0.05.
Results
Characteristics of the Study Population
The baseline characteristics of the study population are shown in Table 1. There were no significant differences in basic characteristics (age, sex, DBP, TC, LDL-C, refined grains and their products, whole grains and related products, meats, vegetables and fruits, and TMA), while there were significant differences in other basic characteristics. Compared to participants without T2DM, those who developed T2DM had a higher family history of diabetes, BMI, SBP, TG, HDL-C, TMAO, and choline. Meanwhile, education, physical activity, HDL-C, consumption of some dietary (milk and its products, aquatic products, beans and its products), betaine and L-carnitine were lower in patients with T2DM than in patients without T2DM.
Table 1.
The Basic Characteristics of the Study
| Variable | Non-T2DM (n=150) | T2DM (n=150) | P |
|---|---|---|---|
| Agea, year | 61.82±9.28 | 61.88±9.10 | 0.955 |
| Maleb, n (%) | 73(48.67) | 73(48.67) | 1.000 |
| Educationb, n (%) | <0.001 | ||
| Primary school or below | 54(36.0) | 85(56.7) | |
| Junior high school | 46(30.7) | 36(24.0) | |
| Senior high school or above | 50(33.3) | 29(19.3) | |
| Physical activityb, n (%) | 133(88.67) | 116(77.33) | 0.013 |
| Family history of diabetesb, n (%) | 1(0.67) | 13(8.67) | 0.001 |
| Dietc | |||
| Refined grains and their products, g/d | 262.5(200.0,400.0) | 353.4(257.1,444.7) | 0.988 |
| Whole grains and related products, g/d | 64.3(32.5,100.0) | 57.1(25.4,101.0) | 0.353 |
| Vegetables and fruit, g/d | 250.0(150.0,400.0) | 200.0(150.0,292.9) | 0.139 |
| Meats, g/d | 48.2(18.5,80.4) | 42.9(19.3,74.8) | 0.554 |
| Milk and its products, g/d | 60.7(8.8,155.0) | 24.6(0.0,152.3) | 0.026 |
| Aquatic products, g/d | 4.2(1.0,14.3) | 3.3(0.5,6.7) | 0.011 |
| Bean and its products, g/d | 55.8(20.0,100.0) | 27.6(7.1,54.4) | <0.001 |
| BMIa, kg/m² | 24.72±3.28 | 26.71±4.56 | <0.001 |
| SBPa, mmHg | 129.53±18.39 | 136.90±21.76 | 0.002 |
| DBPa, mmHg | 83.55±10.83 | 85.49±11.67 | 0.136 |
| TGa, mmol/L | 1.87±1.53 | 2.41±2.06 | 0.010 |
| TCa, mmol/L, | 3.97±0.72 | 3.94±0.75 | 0.734 |
| HDLa, mmol/L | 1.07±0.25 | 1.00±0.24 | 0.014 |
| LDLa, mmol/L | 2.83±0.69 | 2.76±0.68 | 0.345 |
| TMAOc, μmol/L | 3.93(2.41,5.78) | 5.79(3.08,8.93) | <0.001 |
| Betainec, μmol/L | 65.57(52.84,80.37) | 55.52(48.37,69.37) | <0.001 |
| L-carnitinec, μmol/L | 45.58(38.80,52.77) | 42.09(35.41,50.41) | 0.005 |
| Cholinec, μmol/L | 18.08(15.84,21.55) | 19.20(16.54,23.01) | 0.032 |
| TMAc, μmol/L | 0.16(0.15,0.17) | 0.16(0.15,0.18) | 0.524 |
Notes: Quantitative variables are shown as mean ± SD and median (IQR), and qualitative variables are presented as numbers with the percentage. aThe significant difference were compared by using the Student ‘s t-test; bThe significant difference were compared by using the chi-square tests; cThe significant difference were compared by using the Wilcoxon-Mann–Whitney rank sum test.
Abbreviations: T2DM, type 2 diabetes mellitus; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; TG, triglyceride; TC, total cholesterol; LDL low-density lipoprotein; HDL, high-density lipoprotein; TMAO, Trimethylamine N-Oxide; TMA, trimethylamine.
Associations Between T2DM and the Traditional Risk Factors, TMAO and Its Precursors
We investigated the traditional risk factors and TMAO and its precursors (betaine, L-carnitine, TMA, and choline) to determine the risk of T2DM in northwest China (Table 2). TMAO and choline were associated with the risk of T2DM in a nonlinear manner (Table 2 and Figure 1). Choline had a clear threshold effect and increased levels (choline > 22.62 μmol/L), which was associated with a rapid increase in the risk of T2DM. On the contrary, the precursors of TMAO (betaine and L-carnitine) were linearly inversely associated with the risk of T2DM (Table 2 and Supplementary Figure 2). Participants with betaine and L-carnitine had a markedly reduced risk of T2DM in model 1 (OR:0.983, 95% CI: 0.972–0.995, P = 0.005; OR:0.966, 95% CI: 0.943–0.988, P = 0.003, respectively) and multivariable analysis (OR:0.979, 95% CI: 0.966–0.993, P = 0.003; OR:0.952, 95% CI: 0.925–0.979, P = 0.001, respectively). However, TMAO and TMA, in addition to other TMAO precursors, were not selected for the model using stepwise procedures (Table 2). Diabetes history, education, BMI, and bean and its product were significantly associated with the risk of T2DM in model 2, while model 3 found that no history of diabetes (OR:0.072, 95% CI:0.007–0.714, P = 0.025), BMI (OR:1.152, 95% CI:1.052–1.261, P = 0.002), SBP (OR:1.019, 95% CI:1.004–1.033, P = 0.011), beans and their products (OR:0.992, 95% CI:0.987–0.997, P = 0.001) were significantly associated with the risk of T2DM.
Table 2.
The Basic Characteristics of the Study and the Risk of T2DM
| Variable | Model 1 OR (95% CI) | P | Model 2 OR (95% CI) | P | Model 3 OR (95% CI) | P |
|---|---|---|---|---|---|---|
| TMAO and its precursors | ||||||
| TMAO (ref.=TMAO≤2.85μmol/L) | ||||||
| TMAO1 (> 2.85μmol/L) | 1.706(1.023,2.844) | 0.041 | – | – | 1.718(0.938,3.149) | 0.080 |
| Choline (ref.=Choline≤15.31μmol/L) | ||||||
| Choline1 (15.32μmol/L ~) | 0.887(0.465,1.691) | 0.716 | – | – | 0.708(0.327,1.534) | 0.382 |
| Choline2 (19.41μmol/L ~) | 1.284(0.613,2.691) | 0.507 | – | – | 1.504(0.611,3.703) | 0.374 |
| Choline3 (> 22.62μmol/L) | 1.949(0.940,4.039) | 0.073 | – | – | 3.511(1.395,8.834) | 0.008 |
| Betaine | 0.983(0.972,0.995) | 0.005 | – | – | 0.978(0.964,0.992) | 0.002 |
| L-carnitine | 0.966(0.943,0.988) | 0.003 | - | - | 0.949(0.922,0.978) | 0.001 |
| TMA | 5.507(0.052,580.627) | 0.473 | - | - | - | - |
| Traditional risk factors | ||||||
| Without diabetes history | - | - | 0.062(0.007,0.544) | 0.011 | 0.072(0.007,0.714) | 0.025 |
| Education (ref.=primary school and below) | ||||||
| Junior high school | - | - | 0.584(0.316,1.078) | 0.085 | 0.508(0.266,0.971) | 0.040 |
| Senior high school or above | - | - | 0.399(0.213,0.747) | 0.004 | 0.386(0.196,0.761) | 0.006 |
| Physical activity | - | - | 0.503(0.252,1.004) | 0.051 | - | - |
| BMI | - | - | 1.161(1.067,1.263) | 0.001 | 1.152(1.052,1.261) | 0.002 |
| SBP | - | - | 1.015(1.001,1.028) | 0.029 | 1.019(1.004,1.033) | 0.011 |
| Bean and its product | - | - | 0.993(0.988,0.997) | 0.001 | 0.992(0.987,0.997) | 0.001 |
Notes: Model 1, model of TMAO and its precursors on the risk of T2DM. Model 2, model of traditional risk factors on the risk of T2DM (adjusted for triglyceride, high-density lipoprotein, milk and its product, aquatic products, vegetables and fruit). Model 3, model of TMAO and its precursors and traditional risk factors on the risk of T2DM. (adjusted for triglyceride, high-density lipoprotein, milk and its product, aquatic products, vegetables and fruit).
Potential Increase in Predictive Values of TMAO and Its Precursors for T2DM
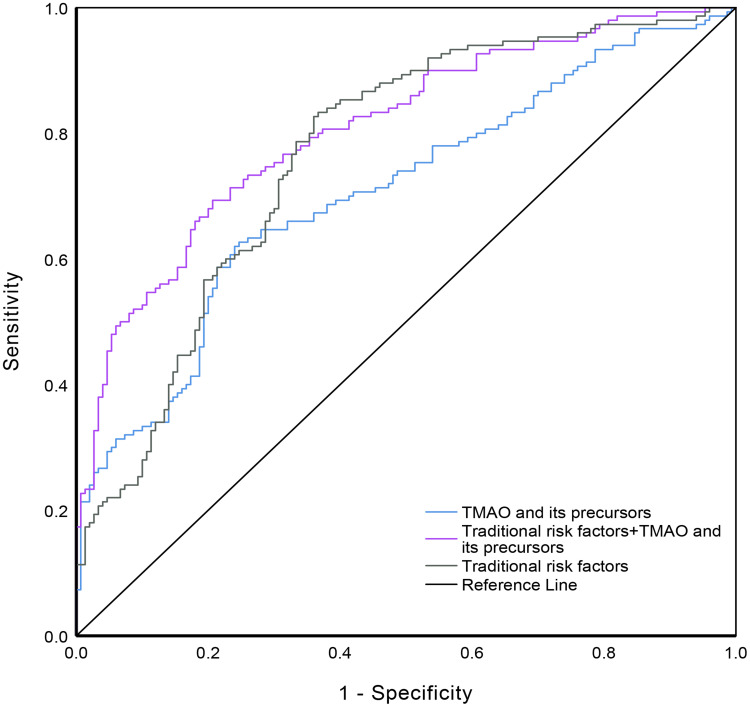
Both the TMAO and its precursor model (L-carnitine, betaine, choline) (AUC:0.706, 95% CI:0.647–0.765) and the traditional risk factors model (AUC:0.761, 95% CI:0.707–0.816) had a similar AUC (P = 0.136). The AUC was significantly increased to 0.816 (95% CI:0.768–0.863, P<0.05) by including TMAO and its precursors (L-carnitine, betaine, choline) in the traditional risk factor model (Figure 2).
Figure 2.
Receiver operating characteristic curves of TMAO and its precursors (blue), traditional risk factors (grey), TMAO and precursors plus traditional risk factors (purple) for type 2 diabetes mellitus. The area under the operating characteristic curve (AUC) was 0.706 (95% CI: 0.647–0.765) for TMAO and its precursors model; 0.761 (95% CI: 0.707–0.816) for the traditional risk factors model; 0.816 (95% CI: 0.768–0.863) for the traditional risk factors plus TMAO and its precursors model. (P<0.05 for comparison of TMAO and its precursors plus traditional risk factors model with the other two models).
Discussion
In this case-control study among Chinese adult in the northwest, we sought to evaluate whether serum concentrations of TMAO, choline, TMA, betaine and L-carnitine were associated with the risk of T2DM. Evidence from cross-sectional and case-control studies is consistent.12–14,17–20 The association of these metabolites (choline, betaine, and L-carnitine) with the risk of T2DM was significant. Higher levels of betaine and l-carnitine were associated with a decreased risk of T2DM, even after adjusting for traditional risk factors for T2DM. Meanwhile, choline had a threshold effect on T2DM, and a level > 22.62μmol/L was associated with a markedly higher risk of T2DM. A nested case-control study showed that choline was associated with diabetes with a significant threshold effect, and the threshold ranges were 110–270 nmol/mL.21
Although previous studies have revealed that TMAO is linked with the risk of T2DM,13,17 our results showed that TMAO is not associated with T2DM. This result may be attributed to the analysis method, which may be affected by the type of TMAO. Karaghi’s study showed that using the quartile, the highest quartile in TMAO was associated with diabetes risk.22 In this study, we considered the link between TMAO and T2DM as nonlinear and then used RCS to transform the type of TMAO from a continuous variable to a qualitative variable before regression analysis.
In baseline characteristics, daily consumption of aquatic products, beans and related products, and milk and its products in the non-T2DM group was higher than that in the T2DM group, whereas betaine and choline were higher in the non-T2DM group, and L-carnitine was higher in the T2DM group. Dietary sources of metabolic precursors are inconsistent with their distribution of metabolic precursors. Choline, betaine, and L-carnitine are dietary precursors of TMAO. Choline is derived primarily from beef, chicken, milk, cruciferous vegetables, and beans, which through the action of choline TMA lyase directly contributes to the formation of TMA or indirectly through conversion to betaine indirectly.23,24 Betaine comes primarily from spinach, beets, wheat, and related products, and is then transformed into dimethylglycine by betaine reductase, which is metabolized into TMA.9,23 Similarly, L-carnitine, present in red meat, contributes to the formation of TMA directly through the action of a Rieske-type carnitine reductase/oxidase (cntAB) or indirectly through the conversion to betaine.25,26 Thereafter, trimethylamine is converted to TMAO by flavin monooxygenase 3 (FMO3) in the liver.27 The conversion was bilateral between TMAO and TMA, which was conducted by the action of TMA monooxygenase and reductase. Furthermore, this conversion relationship also exists for other precursors. This could explain why food intake and measured levels of metabolic precursors were not evenly distributed between the two groups.
Furthermore, our study showed that the inclusion of TMAO, choline, L-carnitine, and betaine in the prediction model consisting of traditional risk factors of T2DM significantly increased the predictive accuracy of traditional risk factors. In the Dutch Diabetes Cohort study, TMAO was considered an independent risk marker and a possible treatment target in individuals with T2DM, with coefficients of variation for intra- and inter-assay precision ranging from 4.3% to 10.3% and 9.8% to 14.5%, respectively.28 In the PREVEND study, betaine was associated with a lower risk of incident diabetes. Furthermore, the coefficient variation of the betaine level for the precision of the intra- and inter-assay ranged from 1.5% to 4.3% and from 2.5% to 5.5%, respectively, which showed stability in assessing the risk of T2DM.12 Another study based on the diabetes excess weight loss trial (DEWL) showed that the coefficients of variation throughout the study were generally < 7% for choline and carnitine. It is more reliable than osmolytes, GPC, or taurine.8 However, the action to control cardiovascular risk in Diabetes trial showed that TMAO’s prognostic value of TMAO for incident ASCVD events may be diminished when applied to individuals with T2DM with poor glycemic control and high risk of atherosclerotic cardiovascular disease at baseline.29 Therefore, TMAO and its precursors (choline, l-carnitine, and betaine) can be appropriate risk markers for individuals who only have a high risk of T2DM.
Our study has several strengths. First, the participants were derived from mountainous rural (MR), mountainous urban (MU), plain rural (PR), and plain urban (PU) areas,15 which made the research population more representative. Second, restricted cubic splines were used to transform nonlinear data, such as TMAO and choline, into linear data. This more directly and accurately depicted the association between these metabolites and T2DM, making the results more reliable. Third, previous studies were mostly cross-sectional and hospital-based case-control studies. However, our study adopted community-based case-control studies, which further enhanced the representativeness of the research results. Despite the robust study protocols, the current study had several limitations. First, since this study was designed as an observational study, we were unable to infer causal inferences or elucidate the cause-effect relationship. More replication studies are required in cohorts and randomized trials in other populations. Second, although we adjusted for multiple potential confounders, residual confounding factors could not be ruled out. However, in our study, the traditional risk covariates for T2DM were carefully adjusted in the current analysis; therefore, the unadjusted bias was minimal. Third, we did not consider the use of nondiabetic drugs, which may have affected the results of the analysis.
Conclusion
Our findings suggest that precursors related to TMAO (choline, betaine, and L-carnitine) were associated with the risk of type 2 diabetes, even though adjustments were made. The traditional risk factors in combination with choline, L-carnitine, and betaine can significantly increase the accuracy of predicting the risk of T2DM. Given the close link between T2DM and TMAO precursors, randomized controlled trials are warranted to test whether changing the concentration of these metabolites can prevent high-risk individuals from developing type 2 diabetes.
Acknowledgments
We express our sincere gratitude to all participants for their efforts in this investigation.
Funding Statement
The Natural Science Foundation of Ningxia (no. 2020AAC03159) supported this study.
Data Sharing Statement
The datasets used and/or analyzed in the current study are available upon reasonable request from the corresponding authors (Xian Sun: sunxvic@126.com).
Ethics Approval and Consent to Participate
This study was approved by the Ethics Committee of Ningxia Medical University (No. 2020-089). All participants were provided written and oral information about the study and provided written informed consent before participating. And our study complies with the Declaration of Helsinki.
Author Contributions
Methodology: LL. Software: JL. Formal analysis: SQ, LW, and XS, SH. Data curation: JL. Writing-original draft preparation: SQ. Writing—review and editing: SQ, XS, and LL. All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Disease GBD, Injury I, Prevalence C. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1545–1602. doi: 10.1016/S0140-6736(16)31678-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chatterjee S, Khunti K, Davies MJ. Type 2 diabetes. Lancet. 2017;389(10085):2239–2251. doi: 10.1016/S0140-6736(17)30058-2 [DOI] [PubMed] [Google Scholar]
- 3.Ke P, Wu X, Xu M, et al. Comparison of obesity indices and triglyceride glucose-related parameters to predict type 2 diabetes mellitus among normal-weight elderly in China. Eat Weight Disord. 2022;27(3):1181–1191. doi: 10.1007/s40519-021-01238-w [DOI] [PubMed] [Google Scholar]
- 4.Federation ID. IDF Diabetes Atlas. 10th ed. International Diabetes Federation Diabetes Atlas; 2021:1–135. [Google Scholar]
- 5.Rahman S, Rahman T, Ismail AA, Rashid AR. Diabetes-associated macrovasculopathy: pathophysiology and pathogenesis. Diabetes Obes Metab. 2007;9(6):767–780. doi: 10.1111/j.1463-1326.2006.00655.x [DOI] [PubMed] [Google Scholar]
- 6.Orasanu G, Plutzky J. The pathologic continuum of diabetic vascular disease. J Am Coll Cardiol. 2009;53(5 Suppl):S35–42. doi: 10.1016/j.jacc.2008.09.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lascar N, Brown J, Pattison H, Barnett AH, Bailey CJ, Bellary S. Type 2 diabetes in adolescents and young adults. Lancet Diabetes Endocrinol. 2018;6(1):69–80. doi: 10.1016/S2213-8587(17)30186-9 [DOI] [PubMed] [Google Scholar]
- 8.McEntyre CJ, Lever M, Chambers ST, et al. Variation of betaine, N,N-dimethylglycine, choline, glycerophosphorylcholine, taurine and trimethylamine-N-oxide in the plasma and urine of overweight people with type 2 diabetes over a two-year period. Ann Clin Biochem. 2015;52(Pt 3):352–360. doi: 10.1177/0004563214545346 [DOI] [PubMed] [Google Scholar]
- 9.Fennema D, Phillips IR, Shephard EA. Trimethylamine and Trimethylamine N-Oxide, a flavin-containing monooxygenase 3 (FMO3)-mediated host-microbiome metabolic axis implicated in health and disease. Drug Metab Dispos. 2016;44(11):1839–1850. doi: 10.1124/dmd.116.070615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen S, Henderson A, Petriello MC, et al. Trimethylamine N-Oxide binds and activates PERK to promote metabolic dysfunction. Cell Metab. 2019;30(6):1141–1151 e5. doi: 10.1016/j.cmet.2019.08.021 [DOI] [PubMed] [Google Scholar]
- 11.Lemaitre RN, Jensen PN, Wang Z, et al. Association of trimethylamine N -Oxide and related metabolites in plasma and incident type 2 diabetes. JAMA Netw Open. 2021;4(8):e2122844. doi: 10.1001/jamanetworkopen.2021.22844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garcia E, Oste MCJ, Bennett DW, et al. High betaine, a Trimethylamine N-Oxide related metabolite, is prospectively associated with low future risk of type 2 diabetes mellitus in the PREVEND study. J Clin Med Nov. 2019;8(11). doi: 10.3390/jcm8111813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhuang R, Ge X, Han L, et al. Gut microbe–generated metabolite trimethylamine N -oxide and the risk of diabetes: a systematic review and dose-response meta-analysis. Obes Rev. 2019;20(6):883–894. doi: 10.1111/obr.12843 [DOI] [PubMed] [Google Scholar]
- 14.Dibaba DT, Johnson KC, Kucharska-Newton AM, Meyer K, Zeisel SH, Bidulescu A. The association of dietary choline and betaine with the risk of type 2 diabetes: the Atherosclerosis Risk in Communities (ARIC) Study. Diabetes Care. 2020;43(11):2840–2846. doi: 10.2337/dc20-0733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Du Y, Ding L, Na L, et al. Prevalence of chronic diseases and alterations of gut microbiome in people of Ningxia china during urbanization: an epidemiological survey. Front Cell Infect Microbiol. 2021;11:707402. doi: 10.3389/fcimb.2021.707402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Z, Levison BS, Hazen JE, Donahue L, Li X-M, Hazen SL. Measurement of trimethylamine-N-oxide by stable isotope dilution liquid chromatography tandem mass spectrometry. Anal Biochem. 2014;455:35–40. doi: 10.1016/j.ab.2014.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shan Z, Sun T, Huang H, et al. Association between microbiota-dependent metabolite trimethylamine-N-oxide and type 2 diabetes. Am J Clin Nutr. 2017;106(3):888–894. doi: 10.3945/ajcn.117.157107 [DOI] [PubMed] [Google Scholar]
- 18.Tang WH, Wang Z, Li XS, et al. Increased Trimethylamine N-Oxide portends high mortality risk independent of glycemic control in patients with type 2 diabetes mellitus. Clin Chem. 2017;63(1):297–306. doi: 10.1373/clinchem.2016.263640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heianza Y, Sun D, Smith SR, Bray GA, Sacks FM, Qi L. Changes in gut Microbiota–related metabolites and long-term successful weight loss in response to weight-loss diets: the POUNDS lost trial. Diabetes Care. 2018;41(3):413–419. doi: 10.2337/dc17-2108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Papandreou C, Bullo M, Zheng Y, et al. Plasma trimethylamine-N-oxide and related metabolites are associated with type 2 diabetes risk in the Prevención con Dieta Mediterránea (PREDIMED) trial. Am J Clin Nutr. 2018;108(1):163–173. doi: 10.1093/ajcn/nqy058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huo X, Li J, Cao Y-F, et al. Trimethylamine N-Oxide metabolites in early pregnancy and risk of gestational diabetes: a nested case-control study. J Clin Endocrinol Metab. 2019;104(11):5529–5539. doi: 10.1210/jc.2019-00710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kalagi NA, Thota RN, Stojanovski E, Alburikan KA, Garg ML. Association between Plasma Trimethylamine N-Oxide Levels and Type 2 Diabetes: a Case Control Study. Nutrients. 2022;14(10):2093. doi: 10.3390/nu14102093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zeisel SH, Mar MH, Howe JC, Holden JM. Concentrations of choline-containing compounds and betaine in common foods. J Nutr. 2003;133(5):1302–1307. doi: 10.1093/jn/133.5.1302 [DOI] [PubMed] [Google Scholar]
- 24.Zeisel SH, Klatt KC, Caudill MA. Choline. Adv Nutr. 2018;9(1):58–60. doi: 10.1093/advances/nmx004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feller AG, Rudman D. Role of carnitine in human nutrition. J Nutr. 1988;118(5):541–547. doi: 10.1093/jn/118.5.541 [DOI] [PubMed] [Google Scholar]
- 26.Zhu Y, Jameson E, Crosatti M, et al. Carnitine metabolism to trimethylamine by an unusual Rieske-type oxygenase from human microbiota. Proc Natl Acad Sci U S A. 2014;111(11):4268–4273. doi: 10.1073/pnas.1316569111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lang DH, Yeung CK, Peter RM, et al. Isoform specificity of trimethylamine N-oxygenation by human flavin-containing monooxygenase (FMO) and P450 enzymes: selective catalysis by FMO3. Biochem Pharmacol. 1998;56(8):1005–1012. doi: 10.1016/s0006-2952(98)00218-4 [DOI] [PubMed] [Google Scholar]
- 28.Flores-Guerrero JL, van Dijk PR, Connelly MA, et al. Circulating Trimethylamine N-Oxide is associated with increased risk of cardiovascular mortality in type-2 diabetes: results from a Dutch Diabetes Cohort (ZODIAC-59). J Clin Med. 2021;10(11). doi: 10.3390/jcm10112269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cardona A, O’Brien A, Bernier MC, et al. Trimethylamine N-oxide and incident atherosclerotic events in high-risk individuals with diabetes: an ACCORD trial post hoc analysis. BMJ Open Diabetes Res Care. 2019;7(1):e000718. doi: 10.1136/bmjdrc-2019-000718 [DOI] [PMC free article] [PubMed] [Google Scholar]