Abstract
Psoriatic arthritis is a chronic systemic inflammatory disease that presents with a variable clinical course and is typically associated with joint inflammation, together with cutaneous psoriasis. In recent decades, knowledge of the pathogenesis of psoriatic arthritis has advanced considerably and has allowed for development of new highly effective therapies, transforming the treatment landscape. Upadacitinib is a Janus kinase inhibitor (JAK) that is orally reversible with high selectivity for JAK1 and its signal transduction molecules. The results obtained in the phase III clinical trials (SELECT-PsA 1 and SELEC-PsA 2) demonstrated that upadacitinib was highly effective over placebo and non-inferior to adalimumab in several important domains of the disease. Improvements were observed in dactylitis, enthesitis and spondylitis as well as in physical function, pain, fatigue and overall quality of life. The safety profile of these results resembled that of adalimumab, apart from a slightly higher rate of herpes zoster infection, an increase of creatine kinase and an incidence of lymphopenia. However, none of these events was considered a serious adverse advent. Additionally, another analysis demonstrated that combining upadacitinib with methotrexate was associated with a similar efficacy to upadacitinib in monotherapy, both for patients that are naive to biologics treatment and for those previously treated with biologics. Therefore, upadacitinib is a new option for the treatment of psoriatic arthritis, presenting a series of beneficial characteristics. At this stage, it is important to collect long-term data to confirm the efficacy and safety profiles shown in clinical trials.
Keywords: antirheumatic agents, arthritis, Janus kinases, psoriatic drug development, rheumatology
Introduction
Psoriatic arthritis (PsA) is an inflammatory chronic systemic disease that presents a variable clinical course. Features of PsA include dactylitis, peripheral arthritis, spondylitis and enthesitis1 and might also be associated with psoriasis (skin or nail lesions).1 The pathogenesis of PsA remains incompletely understood. Innate and adaptive cells and pro-inflammatory cytokines seem to play a major role.1 In recent decades, knowledge of the pathogenesis of PsA has advanced considerably and allowed for the development of highly effective therapies, reshaping the treatment landscape.2
Current guidelines advocate an approach whereby the goal of treatment is to achieve remission or low disease activity across various clinical domains.3–5 Non-steroidal anti-inflammatory drugs, conventional synthetic disease-modifying anti-rheumatic drugs (DMARDs), namely methotrexate (MTX) and leflunomide, biologic DMARDs, including TNF inhibitors, IL-12/23 inhibitors and IL-17 inhibitors, and targeted synthetic DMARDs, such as PDE4 inhibitors or Janus kinase inhibitors (JAKi), are amongst the available treatment options.3–5
There is still no definite consensus on whether conventional DMARDs should be used as concomitant therapy with biologic agents or JAKi. Despite the limited evidence to support the decision of concomitant treatment as demonstrated with a clinical trial with etanercept with or without MTX,6 the EULAR guidelines5 recommend combining biologics with conventional DMARDs in particular cases. On the other hand, biologic monotherapy is favoured by the American College of Rheumatology (ACR) guidelines.4 Moreover, a number of patients have been unable to tolerate high doses of or present contraindications to MTX.7 Agents that contain new action mechanisms that are as effective as monotherapy could also be useful treatment options for PsA.
Despite the recent emergence of treatment options with distinct mechanisms of action, the majority of patients (>70%) do not experience a 70% improvement compared with the baseline as defined by the ACR criteria (ACR70).8–12 In most placebo-controlled trials, only one-third of patients achieves minimal disease activity (MDA).13–18 Therefore, there is a clear and unmet need for effective control of disease activity, using additional therapeutic agents. For patients with PsA that have experienced primary or secondary inefficacy, this becomes especially important as well as for patients who are intolerant to biologic therapy.
This article intends to review the more recently published results on the JAKi upadacitinib (UPA) and its use in the treatment of PsA.
Review
The role of JAK–STAT pathway in PsA pathogenesis
JAK belongs to the protein tyrosine kinases (PTKs) family, which bind the cytoplasmic part of transmembrane cytokine receptors and act as signalling mediators through type I and II cytokine receptors.19 Following interaction between the receptor and the ligand, a range of JAKs are activated, leading to a tyrosine phosphorylation of the receptor and, subsequently, to an activation of STAT proteins, which act as factors for transcription.19 Activated STATs then enter the cell nucleus and connect with specific enhancer sequences in specific target genes, ultimately impacting gene transcription.20
JAK1, JAK2, JAK3 and tyrosine kinase 2 (TYK2) are the four types of JAK proteins. STAT1, STAT2, STAT3, STAT4, STAT5a, STAT5b and STAT6 are the seven types of STAT proteins. Each STAT protein can bind to different JAK proteins.21
JAK–STAT signalling acts as a mediator of cellular response to multiple cytokines and growth factors. Depending on the context of the signal and the cell, these responses can be, amongst others, differentiation, migration, proliferation, apoptosis and cell survival.22
In PsA, the transcriptional JAK1–STAT3–STAT5 pathway associated with specific joint T cell populations has increased expression.23 Studies have demonstrated that a JAK inhibitor can regulate the frequency of CD4+CD11a+CD45RO+IL-17+ T cells. Additionally, JAKi can inhibit monocyte-derived dendritic cell differentiation through the production of reactive oxygen species and NOX5, and decrease dendritic cell T cell-stimulatory capacity by suppressing type I interferon signalling as observed by the induction of enthesitis in an A20meIKO animal model.24–27
Moreover, PsA synovial biopsies have shown an enhanced expression of STAT signalling components. A spontaneous release of JAKi inhibiting pro-inflammatory cytokines from ex-vivo PsA synovial explant cultures was observed in addition to the inhibition of the invasive and migratory capacity of PsA synovial fibroblasts.27,28
Mechanism of action and pharmacology of UPA
UPA is an orally administered reversible JAKi with increased JAK1 selectivity over JAK2, JAK3 and TYK2.29 UPA 15 mg once daily is approved and recommended by the European Medicines Agency (EMA) and the Food and Drugs Administration (FDA) for the treatment of moderate-to-severe cases of rheumatoid arthritis (RA), especially in patients with intolerance to MTX or who have had an inadequate response to the substance.30,31 More recently, UPA was also approved by the EMA for the treatment of ankylosing spondylitis, atopic dermatitis and active PsA in patients intolerant to DMARDs or who have had an inadequate response to one or more DMARDs or conventional therapy.30,31
Five clinical trials in RA,32–36 one in ankylosing spondylitis,37 and four in atopic dermatitis38 have established the efficacy and safety of UPA. UPA has been evaluated for the treatment of PsA in two phase III clinical trials, namely SELECT-PsA 1 (ref.39) and SELECT-PsA 2.40 UPA 15 mg and 30 mg once daily was significantly more effective than placebo in both trials with regards to improvements in key clinical manifestations of PsA. Improvements were also demonstrated through a 56-week extension period of these clinical trials.41,42
Clinical efficacy in PsA
UPA has demonstrated very promising results for the treatment of PsA. Herein, we describe the recent clinical data from phase III randomized placebo-controlled study of UPA in PsA.39,40 These phase III results are summarized in Table 1 and important endpoints are highlighted in Figures 1 and 2.
Table 1.
Results of phase III clinical trials of upadacitinib in the treatment of psoriatic arthritis.
| Study | Study design | Endpoints | Results | Adverse effects |
|---|---|---|---|---|
| SELECT-PsA 1 | 24 weeks 1704 patients: UPA 15 mg (n=429) UPA 30 mg (n=423) PBO (n=423) ADA (n=429) |
Primary ACR20 response at week 12 Secondary SF-36 PCS score change at week 12 FACIT-Fatigue score change at week 12 HAQ-DI score change at week 12 ACR20 response at week 12: non-inferiority of UPA to ADA ACR20 response at week 12: superiority of UPA to ADA Mean change in patient’s assessment of pain at week 12: superiority of UPA to ADA Mean change in HAQ-DI score at week 12: superiority of UPA to ADA PASI75 response at week 16 Mean change in mTSS at week 24 Resolution of dactylitis (LDI=0) at week 24 Resolution of enthesitis (LEI=0) at week 24 MDA at week 24 |
Week 12 ACR20versus placebo: UPA15 (70.6%) (p<0.001) UPA30 (78.5%) (p<0.001) PBO (36.3%) ADA (65%) ACR20 non-inferiority of UPA to ADA: demonstrated with both doses ACR20 superiority of UPA to ADA: demonstrated with 30mg dose SF-36 PCS score change: UPA15 7.9 (7.1 to 8.6) (p<0.001) UPA30 8.9 (8.1 to 9.7) (p<0.001) PBO 3.2 (2.4 to 4.0) ADA 6.8 (6.1 to 7.6) FACIT-Fatigue score change: UPA15 6.3 (5.4 to 7.2) (p<0.001) UPA30 7.1 (6.2 to 8.0) (p<0.001) PBO 2.8 (1.9 to 3.7) ADA 5.7 (4.8 to 6.6) HAQ-DI score change: UPA15 −0.42 (−0.47 to −0.37) (p<0.001) UPA30 −0.47 (−0.52 to −0.42) (p<0.001) PBO −0.14 (−0.18 to −0.09) ADA −0.34 (−0.38 to −0.29) Week 16 PASI75: UPA15 (62.6%) (p<0.001) UPA30 (62.4%) (p<0.001) PBO (21.3%) ADA (53.1%) Week 24 Mean change in mTSS: UPA15 −0.04 (−0.16 to 0.007) (p<0.001) UPA30 0.03 (−0.08 to 0.15) (p=0.007) PBO 0.25 (0.13 to 0.36) ADA 0.01 (−0.11 to 0.13) LDI=0: UPA15 (76.5%) UPA30 (79.5%) PBO (39.7%) ADA (74.0%) LEI=0: UPA15 (53.7%) (p<0.0001) UPA30 (57.7%) (p<0.001) PBO (32.4%) ADA (47.2%) MDA: UPA15 (36.6%) (p<0.001) UPA30 (45.4%) (p<0.001) PBO (12.3%) ADA (33.3%) |
Any AE UPA15 (66.9%) UPA30 (72.3%) PBO (59.6%) ADA (64.8%) SAEs UPA15 (3.3%) UPA30 (6.1%) PBO (3.1%) ADA (3.7%) Death UPA15 (0.0%) UPA30 (0.0%) PBO (0.2%) ADA (0.0%) Infection UPA15 (39.4%) UPA30 (43.3%) PBO (33.1%) ADA (34.0%) HZ infection UPA15 (0.9%) UPA30 (1.2%) PBO (0.7%) ADA (0.0%) Malignancies UPA15 (0.2%) UPA30 (0.7%) PBO (0.2%) ADA (0.7%) MACEs UPA15 (0.0%) UPA30 (0.0%) PBO (0.2%) ADA (0.5%) VTE UPA15 (0.0%) UPA30 (0.2%) PBO (0.2%) ADA (0.5%) Hepatic disorder UPA15 (9.1%) UPA30 (12.3%) PBO (3.8%) ADA (15.6%) Anaemia UPA15 (0.7%) UPA30 (4.7%) PBO (0.9%) ADA (0.2%) Neutropenia UPA15 (0.9%) UPA30 (5.0%) PBO (0.2%) ADA (2.3%) Lymphopenia UPA15 (1.4%) UPA30 (3.5%) PBO (1.2%) ADA (0.2%) Elevated CK UPA15 (8.9%) UPA30 (9.7%) PBO (1.4%) ADA (5.6%) |
| SELECT-PsA 2 | 24 weeks 641 patients: UPA 15 mg (n=211) UPA 30 mg (n=218) PBO (n=212) |
Primary ACR20 response at week 12 Secondary ACR20 response at week 2 ACR50 response at week 12 ACR70 response at week 12 SF-36 PCS score change at week 12 FACIT-Fatigue score change at week 12 HAQ-DI score change at week 12 PASI75 response at week 16 Resolution of dactylitis (LDI=0) at week 12 Resolution of enthesitis (LEI=0) at week 12 MDA at week 24 |
Week 2 ACR20: UPA15 (32.7%) (p<0.001) UPA30 (33.5%) (p<0.001) PBO (10.8%) Week 12 ACR20: UPA15 (56.9%) (p<0.001) UPA30 (63.8%) (p<0.001) PBO (24.1%) ACR50: UPA15 (31.8%) (p<0.001) UPA30 (37.6%) (p<0.001) PBO (4.7%) ACR70: UPA15 (8.5%) (p<0.001) UPA30 (16.5%) (p<0.001) PBO (0.5%) SF-36 PCS score change: UPA15 5.2 (4.1 to 6.2) (p<0.001) UPA30 7.1 (6.1 to 8.1) (p<0.001) PBO 1.6 (0.6 to 2.7) FACIT-Fatigue score change: UPA15 5.0 (3.8 to 6.1) (p<0.001) UPA30 6.1 (4.9 to 7.2) (p<0.001) PBO 1.3 (0.1 to 2.5) HAQ-DI score change: UPA15 −0.30 (−0.37 to −0.24) (p<0.001) UPA30 −0.41 (−0.47 to −0.35) (p<0.001) PBO −0.10 (−0.16 to −0.03) Week 16 PASI75: UPA15 (52.3%) (p<0.001) UPA30 (56.5%) (p<0.001) PBO (16.0%) LDI=0: UPA15 (63.6%) (p<0.001) UPA30 (76.0%) (p<0.001) PBO (35.9%) LEI=0: UPA15 (39.1%) (p<0.001) UPA30 (48.0%) (p<0.001) PBO (20.1%) Week 24 MDA: UPA15 (25.1%) (p<0.001) UPA30 (28.9%) (p<0.001) PBO (2.8%) |
Any AE UPA15 (64.0%) UPA30 (78.0%) PBO (65.6%) SAEs UPA15 (5.7%) UPA30 (8.3%) PBO (1.9%) Death UPA15 (0.0%) UPA30 (0.0%) PBO (0.5%) Infection UPA15 (33.6%) UPA30 (49.5%) PBO (34.4%) HZ infection UPA15 (1.4%) UPA30 (3.7 %) PBO (0.9%) Malignancies UPA15 (1.4%) UPA30 (1.4%) PBO (0.0%) MACEs UPA15 (0.5%) UPA30 (0.0%) PBO (0.0%) VTE UPA15 (0.5%) UPA30 (0.0%) PBO (0.0%) Hepatic disorder UPA15 (1.9%) UPA30 (8.3%) PBO (1.4%) Anaemia UPA15 (1.9%) UPA30 (6.4%) PBO (0.9%) Neutropenia UPA15 (0.9%) UPA30 (2.8%) PBO (0.5%) Lymphopenia UPA15 (0.9%) UPA30 (0.9%) PBO (0.0%) Elevated CK UPA15 (1.9%) UPA30 (5.5%) PBO (1.9%) |
ACR20 (50, 70) response, a disease improvement of at least 20% (50%, 70%) in the American College Criteria; ADA, adalimumab; AE, adverse event; CK, creatine kinase; FACIT, Functional Assessment of Chronic Illness Therapy; HAQ-DI, Health Assessment Questionnaire-Disability Index; HZ, herpes zoster; LDI, Leeds Dactylitis Index; LEI, Leeds Enthesitis Index; MACEs, major adverse cardiovascular events; MDA, minimal disease; mTSS, modified total Sharp-van der Heijde Score; PASI, Psoriasis Area and Severity Index; PBO, placebo; SAEs, serious adverse events; SF-36 PCS, 36-Item Short Form Health Survey Physical Component Summary; UPA15(30), upadacitinib 15 mg or 30 mg; VTE, venous thromboembolism.
Figure 1. Highlighted endpoints in SELECT PsA-1.
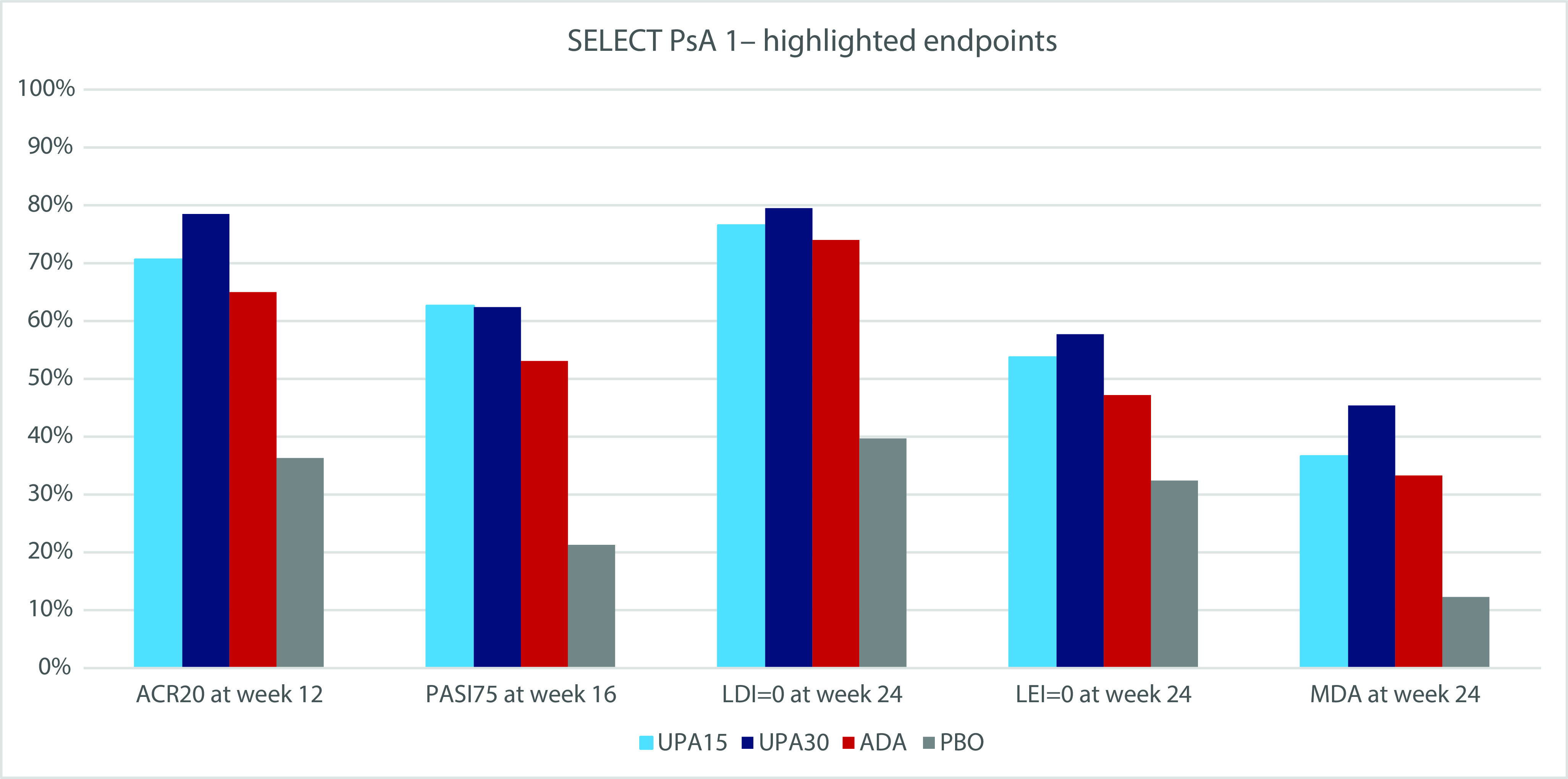
ACR20 response, a disease improvement of at least 20% in the American College Criteria; ADA, adalimumab; LDI, Leeds Dactylitis Index; LEI, Leeds Enthesitis Index; MDA, minimal disease; PASI, Psoriasis Area and Severity Index; PBO, placebo; UPA15(30), upadacitinib 15 mg or 30 mg.
Figure 2. Highlighted endpoints in SELECT PsA-2.
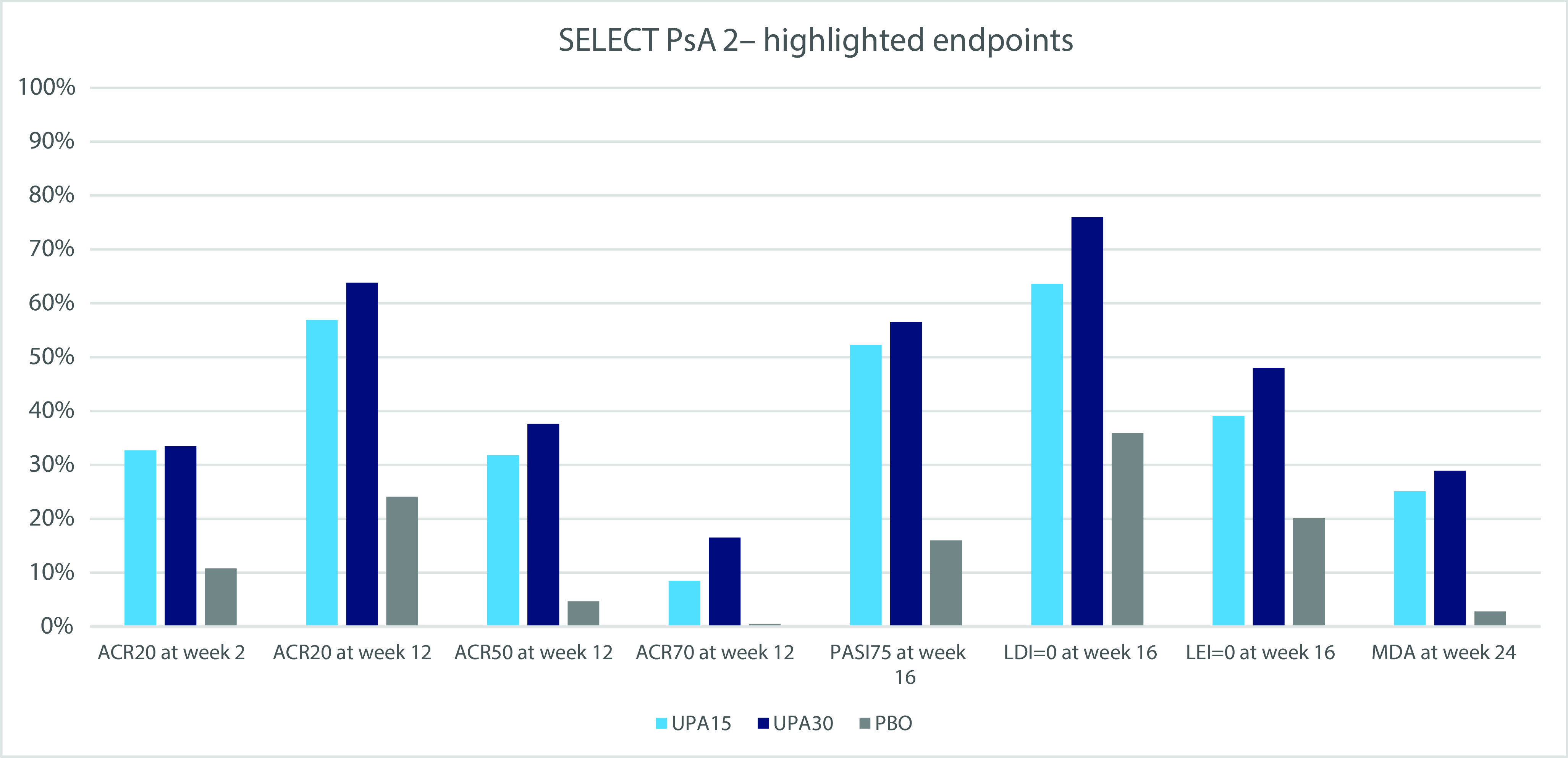
ACR20 (50, 70) response, a disease improvement of at least 20% (50%, 70%) in the American College Criteria; LDI, Leeds Dactylitis Index; LEI, Leeds Enthesitis Index; MDA, minimal disease; PASI, Psoriasis Area and Severity Index; PBO, placebo; UPA15(30), upadacitinib 15 mg or 30 mg.
SELECT-PsA 1
This was a phase III, 24-week active comparator and placebo-controlled randomized study.39 The study enrolled adult patients with active PsA, meeting the Classification Criteria for Psoriatic Arthritis (CASPAR) and presenting a history of or currently having plaque psoriasis, at least three swollen and three tender joints (out of 68) at baseline, one or more erosions on the feet or the hands on radiography, a high-sensitivity C-reactive protein level, and an intolerance or inadequate response to at least one non-biologic DMARD. Stable non-steroidal anti-inflammatory drug-based treatment was allowed but not considered a requirement. The same applied for treatment with glucocorticoids and no more than two non-biologic DMARDs. Patients were ruled out if they had previously been exposed to JAKi or biologic DMARDs.
Patients (n=1704) were randomized (1:1:1:1) to receive UPA 15 mg, UPA 30 mg, adalimumab (ADA) or placebo.
The main end point was the production of an ACR20 response using UPA as compared with placebo at week 12. ACR20 represents an improvement of 20% of the baseline swollen joint count (SJC) and tender joint count (TJC), and at least three of five other parameters (the patient and physician global assessment of disease activity, the patient assessment of pain, a functional capacity questionnaire and the level of high-sensitivity C-reactive protein).
There was a range of multiple secondary end points, with the objective to evaluate all variables of the clinical course and establish a potential comparison with ADA.
At week 12, changes from baseline in scores on the 36-Item Short Form Health Survey Physical Component Summary (SF-36 PCS), Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-Fatigue) and Health Assessment Questionnaire-Disability Index (HAQ-DI) were evaluated. Additionally, at week 12 was also evaluated the non-inferiority of UPA to ADA in ACR20 response and the superiority of UPA to ADA in relation to ACR20 response, change from baseline in the patient assessment of pain and change from baseline in the HAQ-DI score.
At week 16, an assessment of the percentage of patients with a reduction from baseline of at least 75% in the Psoriasis Area and Severity Index (PASI) (PASI75 response) score was conducted. Only those who had an affected body-surface area (BSA) of at least 3% at baseline were considered in this analysis.
At week 24, the change from baseline in the modified total Sharp-van der Heijde score, the percentage of patients with resolution of dactylitis (score of 0 on the Leeds Dactylitis Index (LDI)) and with resolution of enthesitis (score of 0 on the Leeds Enthesitis Index (LEI)) were evaluated. Both LDI and LEI were only assessed in patients with a baseline score greater than 0. Finally, the percentage of patients with MDA at week 24 was measured. MDA is determined by fulfilling five of seven criteria: a SJC of ≤1; a TJC of ≤1; an affected BSA of ≤3% or a PASI score of ≤1; a score on patient assessment of pain of ≤1.5; a score on patient global assessment of disease activity of ≤2; a score on the LEI of ≤1; and a HAQ-DI score of ≤0.5.
ACR20 response was the primary end point. At week 12, it had been achieved by 70.6% (n=303) receiving UPA 15 mg and 78.5% (n=332) receiving UPA 30 mg, compared with 36.2% (n=153) receiving placebo (p<0.001) and 65.0% (n=279) receiving ADA. The 30 mg dose presented considerable improvement as compared with ADA (p<0.001) and both were non-inferior to it.
Patient-reported outcomes (PROs), including FACIT-Fatigue, SF-36 PCS and HAQ-DI score, were better in patients on both of the UPA doses than in patients on placebo or ADA.
Regarding other domains of disease activity, such as skin improvement, UPA 15 mg showed the highest improvement in PASI75 but both doses performed better than ADA. In terms of resolution of enthesitis and dactylitis, both doses of UPA outperformed the other treatment groups.
UPA 15 mg showed the smallest radiographic progression by modified total Sharp-van der Heijde score. Both UPA doses had a greater share of patients reaching MDA, which was higher in the 30 mg dose (45.4%).
Extension of SELECT-PsA 1
The SELECT-PsA 1 trial was extended up to week 56.41 At week 24, all patients in the placebo group switched to UPA 15 mg or 30 mg.
The share of patients with an ACR20/50/70 response was maintained from week 24 through week 56, presenting a nominal p≤0.05 for UPA 15 mg as opposed to ADA for ACR50/70 and for UPA 30 mg versus ADA for ACR20/50/70. Patients originally allocated to placebo that switched to UPA at week 24 showed a similar response to those that started with UPA since day 1.
The improvement in skin outcomes was continuous over time in PASI75/90/100 responses. Improvements were also sustained through week 56 in FACIT-Fatigue, HAQ-DI and SF-36 PCS scores. Additionally, considerable improvement was observed in the UPA 15 mg and 30 mg groups when compared with the ADA group and with regards to the change from baseline in SF-36 PCS and HAQ-DI.
Patients at week 56 also improved in terms of Disease Activity in Psoriatic Arthritis. Patients who had baseline evidence of psoriatic spondylitis also registered improvements in Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) and Ankylosing Spondylitis Disease Activity Score (ASDAS) scores.
The overall share of patients achieving MDA was sustained through week 56, and a higher number of patients treated with UPA 30 mg was able to reach MDA versus ADA at week 56.
SELECT-PsA 2
This clinical trial was a phase III, 24-week, placebo-controlled, randomized study.40 It used the same criteria for the selection of patients as SELECT-PsA 1, with the exception of not being mandatory to have one or more erosions nor a high-sensitivity C-reactive protein level. On the other hand, in this clinical trial, patients were required to be intolerant to at least one biologic DMARD or have at least an inadequate response to it.
A total of 641 patients were randomized (1:1:1) to receive UPA 15 mg, UPA 30 mg or placebo. The primary end point was achievement of an ACR20 response with UPA as compared with the response to placebo at week 12. There were multiple relevant secondary end points: (i) ACR20 response at week 2; (ii) change from baseline in FACIT-Fatigue, SF-36 PCS and HAQ-DI scores at week 12; (iii) ACR50/70 response at week 12; (iv) enthesitis (LEI=0) and dactylitis (LDI=0) resolution at week 12 (both LDI and LEI were only evaluated in patients that had a baseline score >0); (v) share of patients with PASI75 response at week 16 (only assessed in patients whose affected BSA was at least 3% at baseline); and (vi) percentage of patients achieving MDA at week 24.
The primary end point (ACR20 response at week 12) was achieved by 56.9% (n=120) of patients receiving UPA 15 mg dose and 63.8% (n=139) of those receiving UPA 30 mg, both significantly higher versus the placebo arm (24.1%, n=51; p<0.001).
ACR20 response had already been achieved at week 2 by more patients treated with UPA 15 mg and UPA 30 mg (p<0.001) when compared with placebo. Additionally, a greater number of patients in both UPA groups achieved ACR70 and ACR50 responses versus placebo at week 12.
The 15 mg and 30 mg doses of UPA also showed greater improvement on all key secondary endpoints compared to placebo, including all other domains of disease activity: plaque psoriasis, enthesitis, and dactylitis. Both doses of UPA had a greater share of patients reaching MDA, which was highest in the 30 mg dose group (28.9%).
Extension of SELECT-PsA 2
This study was an extension of the SELECT-PsA 2 clinical trial up to week 56.42 As in the extension of SELECT-PsA 1, at week 24, all patients in the placebo group switched to UPA 15 mg or 30 mg.
The proportion of patients attaining ACR20/50/70 responses was sustained from week 24 to week 56. Patients that were originally on placebo and switched to UPA showed response rates similar to those receiving UPA since day 1. Of note, a numerically greater proportion of patients who received UPA since baseline achieved MDA through week 56 in comparison with the group that was randomized to placebo at baseline. An improvement in skin outcomes was also sustained over time in PASI75/90/100 responses. Additionally, patients with axial PsA showed an improvement in the BASDAI and ASDAS scores from baseline up to week 56 using observation data. The improvement in FACIT-Fatigue, HAQ-DI and SF-36 PCS scores was also sustained through week 56, with patients who switched from placebo to UPA achieving similar results at week 56 compared with those who received UPA since the beginning of SELECT-PsA 2 trial.
Pooled subgroup analysis of the two SELECT-PsA studies
Nash et al. executed a pooled subgroup analysis of both SELECT-PsA studies. Safety and efficacy outcomes of patients treated with UPA as monotherapy or combined with non-biologic DMARDs were assessed.43 The primary end point in both studies was ACR20 response at week 12; this was achieved by 33.7% and 34.0% of patients in the UPA 15 mg monotherapy and combined therapy groups, respectively, and by 45.7% and 39.6% of patients in the UPA 30 mg monotherapy and combined therapy groups, respectively. MDA at week 24 was achieved by 24.9% and 23.1% of patients in the UPA 15 mg monotherapy and combination therapy groups, respectively, and by 35.0% and 28.9% of patients in the UPA 30 mg monotherapy and combination therapy groups, respectively. The efficacy outcomes were consistent between mono and combination therapy. This consistency extended to every other secondary endpoints evaluated.43
Safety
SELECT-PsA 1 and extension study
The SELECT-PsA 1 study showed that the incidence of both adverse events (AEs) and serious adverse events (SAEs), including serious infections, were more prevalent with the 30 mg dose of UPA when compared to the 15 mg dose. The most common AE was found to be an infection of the upper respiratory tract. Herpes zoster (HZ), an AE historically associated with inhibition of the JAK–STAT pathway, was diagnosed in four (0.9%) patients who had received UPA 15 mg, five (1.2%) patients receiving UPA 30 mg, three (0.7%) patients receiving placebo, and none receiving ADA. Malignancies were reported in one (0.2%) patient each in the placebo and 15-mg UPA groups and in three (0.7%) patients each in the 30-mg UPA and ADA groups. No major adverse cardiovascular events (MACEs) were reported with UPA. Venous thromboembolism (VTE) was not more common in the UPA groups compared with the other groups. Neutropenia was more common with UPA than with placebo but increments of grade 3 or higher were not observed in more than 2.1% of patients in both UPA groups. Hepatic disorders were lower in the UPA and placebo groups than in the ADA group (9.1% in UPA 15 mg; 12.3% in UPA 30 mg; 15.6% in ADA; 3.8% in placebo). Not a single patient met criteria (according to Hy’s law) indicative of drug-induced liver injury. Two other parameters should be highlighted. First, grade 3 or 4 increments in creatine kinase (CK) levels were more common with UPA (versus both ADA and placebo) but no patients had symptomatic rhabdomyolysis. Second, the levels of high-density lipoprotein (HDL) cholesterol and low-density lipoprotein (LDL) cholesterol increased more often with UPA than with placebo; however, the LDL-to-HDL cholesterol ratio and HDL-to-total cholesterol ratio did not present any significant changes.39
The extension of the SELECT-PsA 1 study confirmed that the incidence of AEs and SAEs was more frequent with the 30 mg dose of UPA versus the 15 mg dose or ADA. Blood CK evaluation was also amongst the most reported AEs. The rate of HZ was higher in the UPA groups (3.9% in the 15 mg dose, 6.4% in the 30 mg dose) versus the ADA group (0.5%). However, the majority of events were mild/moderate in severity. A maximum of two dermatomes were detected and these did not lead to the discontinuation of the study drug. In terms of cytopenias, each group registered grade 3 decreases in blood cell counts (platelet, lymphocytes, haemoglobin and neutrophils) in ≤4% of patients in each group; grade 4 decreases were observed in ≤1% of patients in each group. With UPA 15 mg, UPA 30 mg and ADA, the hepatic disorder rate was 19.1%, 22.2% and 24.9%, respectively. As previously mentioned in the SELECT-PsA 1 study, CK increments of grade 3 or 4 were more prevalent with UPA and were reported in <5.7% of patients, who were generally asymptomatic.41
SELECT-PsA 2 and extension study
The SELECT-PsA 2 study demonstrated that the incidence of SAEs was higher with UPA 30 mg (8.3%) than with UPA 15 mg (5.7%) and placebo (1.9%). HZ was reported in two (0.9%) patients receiving placebo, three (1.4%) patients receiving UPA 15 mg and eight (3.7%) patients receiving UPA 30 mg. None of these cases were serious. Malignancies were diagnosed in three (1.4%) patients in each UPA group and in none in the placebo group. One case (0.5%) of MACE and one case (0.5%) of VTE were reported in the UPA 15 mg group, both with at least one factor that increased risk for MACE or VTE (obesity, hypertension or hypercholesterolaemia). Hepatic disorders were described in a higher proportion of patients (8.3%) administered UPA 30 mg than in those administered UPA 15 mg (1.9%) or placebo (1.4%). Most were asymptomatic liver enzymes elevations. Grade 3 decreases in neutrophils were also more common (1.8%) in patients administered UPA 30 mg than in those administered UPA 15 mg (1.0%) or placebo (0.5%). None developed a grade 4 decrease in any cytopenia. CK elevations of grade 3 or higher were described in three (1.4%) patients on placebo, two (0.9%) on UPA 15 mg and five (2.3%) on UPA 30 mg. There was no need to discontinue and not a single event of rhabdomyolysis was reported. Slight mean elevations of HDL cholesterol and LDL cholesterol were described in the UPA arms; however, the ratios of LDL-to-HDL cholesterol and of HDL-to-total cholesterol remained constant.40
The extension of the SELECT-PsA 2 study confirmed that the incidence of SAEs was more frequent with UPA 30 mg than with UPA 15 mg (6.1% versus 2.6%). Nasopharyngitis and upper respiratory tract infection were the most commonly reported AEs in both groups. The rate of HZ was higher in UPA 30 mg (8.5% versus 3.8%), with one case of serious infection reported. Out of both groups, only one patient reported MACEs and one other patient reported VTE. Hepatic disorders were less prevalent in the UPA 15 mg group than in the UPA 30 mg group (4.8% versus 17.7%). The majority were transient, non-serious and did not lead to the discontinuation of the study drug. No grade 4 increases were observed in either group. Similarly, CK elevation rates were slightly lower with UPA 15 mg than with UPA 30 mg, and two patients in the latter group had to suspend the study drug due to CK elevation. Grade 3 cytopenias were relatively uncommon, occurring in ≤2% of patients in both groups. None developed a grade 4 decrease in any cytopenia.42
Pooled subgroup analysis of the two SELECT-PsA studies
In the previously mentioned subgroup analysis, Nash et al. found that the frequency of SAEs and AEs was similar in patients receiving UPA in monotherapy and in combination therapy. Amongst the AEs, non-serious CK and transaminase elevations were more prevalent in the combination therapy group.43
Discussion
JAKi are an interesting class of drugs that has showcased significant safety and efficacy levels for the treatment of PsA by inhibiting the action of several important inflammatory cytokines. UPA is an oral selective JAK1 inhibitor that has recently been tested on patients with PsA.
The SELECT-PsA1 results show considerable improvement with UPA in patients with PsA that were naive to biologic treatment. Both UPA groups were non-inferior to ADA, a biologic considered a first-line therapy for the biologic treatment of PsA and superior to placebo. The 30 mg dose of UPA was even superior to ADA with respect to the proportion of patients achieving ACR20 response at week 12. The efficacy of UPA was extended to other important aspects of PsA, including resolution of enthesitis, improvement of physical function, reduction of fatigue, improvement of quality of life, inhibition of radiographic progression of the disease and achievement of MDA. Additionally, UPA demonstrated satisfactory improvement in cutaneous psoriasis.
On the other hand, SELECT-PsA 2 demonstrated the benefits of UPA in PsA even in biologic-experienced patients. Both doses of UPA were superior to placebo in several aspects of PsA, similar to what was observed in SELECT-PsA 1 (improvements in dactylitis, enthesitis, spondylitis, physical function, pain, fatigue and quality of life). It is important to mention that the population of this last study was particularly refractory to treatment, wherein approximately 31% of the patients had failed to see improvement on two or more biologic DMARDs. UPA also demonstrated a fast onset of action given that an improvement in the proportion of patients achieving ACR20 response was registered as early as week 2.
The safety profile of UPA was generally similar to that previously reported in trials of patients with RA; however, these should not be directly compared given that they are different disease entities with different underlying pathogeneses.32–36 The proportion of AEs and SAEs of UPA 15 mg was similar to that of ADA or placebo.
MACEs and VTE events were not more frequent with UPA than with placebo in SELECT-PsA 1 and were only developed in one patient receiving UPA in SELECT-PsA 2, demonstrating that this drug seems to be free of the association with thromboembolic episodes that was observed with other JAKi.44
The rate of HZ infection was slightly higher with UPA than with placebo or ADA, despite being quite rare. Other noteworthy AEs that were more common with UPA than with ADA were the incidence of lymphopenia and CK elevations; however, neither is considered an SAE. There were no different safety signals compared with what has been observed with UPA in RA.
In the extension studies, the efficacy of UPA for PsA was sustained or improved through 56 weeks. Patients who switched to UPA after placebo achieved similar efficacy at week 24 than those originally randomized to UPA. Additionally, the safety profile was similar to SELECT-PsA 1 and SELECT-PsA 2.
In the extension study of SELECT-PsA 1 spondylitis was also evaluated, however the diagnosis was not necessarily confirmed by magnetic resonance or radiographic image. This is potentially a major limitation to interpret results regarding axial symptoms.
In the subgroup analysis on monotherapy with UPA or combination therapy with non-biologic DMARDs, a similar efficacy was observed between groups and monotherapy reduced the risk of mild laboratory abnormalities. This further substantiates the use of UPA (monotherapy or combination therapy with non-biologic DMARDs) in PsA and suggests that UPA monotherapy could be an effective treatment option for patients who are not suitable for treatment with MTX.
Conclusion
Upadacitinib, a potent JAK1 inhibitor, has demonstrated a favourable safety profile and high efficacy in the treatment of PsA. The results reported in the phase III studies showed that UPA 15 mg was non-inferior to ADA in efficacy, with a similar rate of AEs. A higher dose (30 mg) of UPA proved superior to ADA. However, this increase in dose seems to be associated with a significantly higher risk of AEs; this is likely the reason why this dose was not approved by EMA for PsA.
UPA 15 mg is therefore a new option in the treatment of PsA, with the advantages of being an oral formulation, having a rapid onset of action (ideal for patients who require rapid control of inflammation to prevent additional joint damage) and having equal efficacy in mono or combined therapy.
However, long-term data are required to reassure the safety and efficacy demonstrated in these clinical trials.
Acknowledgements
None.
Footnotes
Contributions: All authors contributed equally to the preparation of this manuscript. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosure and potential conflicts of interest: DF has no conflict of interest. MN has received consultancy and/or speaker’s honoraria from AbbVie and Leo Pharma. TT has received consultancy and/or speaker’s honoraria from and/or participated in clinical trials sponsored by AbbVie, Amgen, Almirall, Amgen, Arena Pharmaceuticals, Biocad, Biogen, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Fresenius-Kabi, Janssen, LEO Pharma, Eli Lilly, MSD, Mylan, Novartis, Pfizer, Samsung-Bioepis, Sanofi-Genzyme, Sandoz and UCB. He is also an Associate Editor for Drugs in Context. The International Committee of Medical Journal Editors (ICMJE) Potential Conflicts of Interests form for the authors is available for download at: https://www.drugsincontext.com/wp-content/uploads/2023/01/dic.2022-11-6-COI.pdf
Funding declaration: There was no funding associated with the preparation of this article.
Correct attribution: Copyright © 2023 Fonseca D, Nogueira M, Torres T. https://doi.org/10.7573/dic.2022-11-6. Published by Drugs in Context under Creative Commons License Deed CC BY NC ND 4.0.
Provenance: Invited; externally peer reviewed.
Drugs in Context is published by BioExcel Publishing Ltd. Registered office: 6 Green Lane Business Park, 238 Green Lane, New Eltham, London, SE9 3TL, UK.
BioExcel Publishing Limited is registered in England Number 10038393. VAT GB 252 7720 07.
For all manuscript and submissions enquiries, contact the Editorial office editorial@drugsincontext.com
For all permissions, rights and reprints, contact David Hughes david.hughes@bioexcelpublishing.com
References
- 1.Gladman DD. Psoriatic arthritis: epidemiology, clinical features, course, and outcome. Ann Rheum Dis. 2005;64(Suppl 2):ii14–ii17. doi: 10.1136/ard.2004.032482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vivekanantham A, McGagh D, Coates LC. Current treatments and recommendations for psoriatic arthritis. Best Pract Res Clin Rheumatol. 2021;35(2):101680. doi: 10.1016/j.berh.2021.101680. [DOI] [PubMed] [Google Scholar]
- 3.Coates LC, Kavanaugh A, Mease PJ, et al. Group for research and assessment of psoriasis and psoriatic arthritis 2015 treatment recommendations for psoriatic arthritis. Arthritis Rheumatol. 2016;68(5):1060–1071. doi: 10.1002/art.39573. [DOI] [PubMed] [Google Scholar]
- 4.Singh JA, Guyatt G, Ogdie A, et al. 2018 American College of Rheumatology/National Psoriasis Foundation guideline for the treatment of psoriatic arthritis. Arthritis Rheumatol. 2019;71(1):5–32. doi: 10.1002/art.40726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gossec L, Baraliakos X, Kerschbaumer A, et al. EULAR recommendations for the management of psoriatic arthritis with pharmacological therapies: 2019 update. Ann Rheum Dis. 2020;79(6):700.1–712. doi: 10.1136/annrheumdis-2020-217159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mease PJ, Gladman DD, Collier DH, et al. Etanercept and methotrexate as monotherapy or in combination for psoriatic arthritis: primary results from a randomized, controlled phase III trial. Arthritis Rheumatol. 2019;71(7):1112–1124. doi: 10.1002/art.40851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ye W, Coates LC. Should methotrexate have any place in the treatment of psoriatic arthritis? Rheum Dis Clin North Am. 2019;45(3):325–339. doi: 10.1016/j.rdc.2019.04.007. [DOI] [PubMed] [Google Scholar]
- 8.Nash P, Ohson K, Walsh J, et al. Early and sustained efficacy with apremilast monotherapy in biological-naïve patients with psoriatic arthritis: a phase IIIB, randomised controlled trial (ACTIVE) Ann Rheum Dis. 2018;77(5):690–698. doi: 10.1136/annrheumdis-2017-211568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kavanaugh A, McInnes IB, Mease PJ, et al. Efficacy of subcutaneous Secukinumab in patients with active psoriatic arthritis stratified by prior Tumor necrosis factor inhibitor use: results from the randomized placebo-controlled future 2 study. J Rheumatol. 2016;43(9):1713–1717. doi: 10.3899/jrheum.160275. [DOI] [PubMed] [Google Scholar]
- 10.Gladman D, Rigby W, Azevedo VF, et al. Tofacitinib for psoriatic arthritis in patients with an inadequate response to TNF inhibitors. N Engl J Med. 2017;377(16):1525–1536. doi: 10.1056/NEJMoa1615977. [DOI] [PubMed] [Google Scholar]
- 11.Ritchlin C, Rahman P, Kavanaugh A, et al. Efficacy and safety of the anti-IL-12/23 p40 monoclonal antibody, ustekinumab, in patients with active psoriatic arthritis despite conventional non-biological and biological anti-tumour necrosis factor therapy: 6-month and 1-year results of the phase 3, multicentre, double-blind, placebo-controlled, randomised PSUMMIT 2 trial. Ann Rheum Dis. 2014;73(6):990–999. doi: 10.1136/annrheumdis-2013-204655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mease PJ, Fleischmann R, Deodhar AA, et al. Effect of certolizumab pegol on signs and symptoms in patients with psoriatic arthritis: 24-week results of a phase 3 double-blind randomised placebo-controlled study (RAPID-PsA) Ann Rheum Dis. 2014;73(1):48–55. doi: 10.1136/annrheumdis-2013-203696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mease PJ, van der Heijde D, Ritchlin CT, et al. Ixekizumab, an interleukin-17A specific monoclonal antibody, for the treatment of biologic-naive patients with active psoriatic arthritis: results from the 24-week randomised, double-blind, placebo-controlled and active (adalimumab)-controlled period of the phase III trial SPIRIT-P1. Ann Rheum Dis. 2017;76(1):79–87. doi: 10.1136/annrheumdis-2016-209709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mease P, van der Heijde D, Landewé R, et al. Secukinumab improves active psoriatic arthritis symptoms and inhibits radiographic progression: primary results from the randomised, double-blind, phase III FUTURE 5 study. Ann Rheum Dis. 2018;77:890–897. doi: 10.1136/annrheumdis-2017-212687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mease P, Hall S, FitzGerald O, et al. Tofacitinib or adalimumab versus placebo for psoriatic arthritis. N Engl J Med. 2017;377(16):1537–1550. doi: 10.1056/NEJMoa1615975. [DOI] [PubMed] [Google Scholar]
- 16.Mease P, Coates LC, Helliwell PS, et al. Efficacy and safety of filgotinib, a selective Janus kinase 1 inhibitor, in patients with active psoriatic arthritis (EQUATOR): results from a randomised, placebo-controlled, phase 2 trial. Lancet. 2018;392(10162):2367–2377. doi: 10.1016/S0140-6736(18)32483-8. [DOI] [PubMed] [Google Scholar]
- 17.McInnes IB, Kavanaugh A, Gottlieb AB, et al. Efficacy and safety of ustekinumab in patients with active psoriatic arthritis: 1 year results of the phase 3, multicentre, double-blind, placebo-controlled PSUMMIT 1 trial. Lancet. 2013;382(9894):780–789. doi: 10.1016/S0140-6736(13)60594-2. [DOI] [PubMed] [Google Scholar]
- 18.Deodhar A, Gottlieb AB, Boehncke WH, et al. Efficacy and safety of guselkumab in patients with active psoriatic arthritis: a randomised, double-blind, placebo-controlled, phase 2 study. Lancet. 2018;391(10136):2213–2224. doi: 10.1016/S0140-6736(18)30952-8. [DOI] [PubMed] [Google Scholar]
- 19.Ghoreschi K, Laurence A, O’Shea JJ. Janus kinases in immune cell signaling. Immunol Rev. 2009;228(1):273–287. doi: 10.1111/j.1600-065X.2008.00754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Darnell JE, Kerr lan M, Stark GR. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994;264(5164):1415–1421. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- 21.Harrison DA. The JAK/STAT pathway. Cold Spring Harb Perspect Biol. 2012;4(3):a011205. doi: 10.1101/cshperspect.a011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O’Shea JJ, Schwartz DM, Villarino AV, Gadina M, McInnes IB, Laurence A. The JAK-STAT pathway: impact on human disease and therapeutic intervention. Annu Rev Med. 2015;66(1):311–328. doi: 10.1146/annurev-med-051113-024537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fiocco U, Accordi B, Martini V, et al. JAK/STAT/PKCδ molecular pathways in synovial fluid T lymphocytes reflect the in vivo T helper-17 expansion in psoriatic arthritis. Immunol Res. 2014;58(1):61–69. doi: 10.1007/s12026-013-8481-0. [DOI] [PubMed] [Google Scholar]
- 24.Raychaudhuri SK, Abria C, Raychaudhuri SP. Regulatory role of the JAK STAT kinase signalling system on the IL-23/IL-17 cytokine axis in psoriatic arthritis. Ann Rheum Dis. 2017;76(10):e36. doi: 10.1136/annrheumdis-2016-211046. [DOI] [PubMed] [Google Scholar]
- 25.Marzaioli V, Canavan M, Floudas A, et al. Monocyte-derived dendritic cell differentiation in inflammatory arthritis is regulated by the JAK/STAT axis via NADPH oxidase regulation. Front Immunol. 2020;11:1406. doi: 10.3389/fimmu.2020.01406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kubo S, Yamaoka K, Kondo M, et al. The JAK inhibitor, tofacitinib, reduces the T cell stimulatory capacity of human monocyte-derived dendritic cells. Ann Rheum Dis. 2014;73(12):2192–2198. doi: 10.1136/annrheumdis-2013-203756. [DOI] [PubMed] [Google Scholar]
- 27.de Wilde K, Martens A, Lambrecht S, et al. A20 inhibition of STAT1 expression in myeloid cells: a novel endogenous regulatory mechanism preventing development of enthesitis. Ann Rheum Dis. 2017;76(3):585–592. doi: 10.1136/annrheumdis-2016-209454. [DOI] [PubMed] [Google Scholar]
- 28.Gao W, McGarry T, Orr C, McCormick J, Veale DJ, Fearon U. Tofacitinib regulates synovial inflammation in psoriatic arthritis, inhibiting STAT activation and induction of negative feedback inhibitors. Ann Rheum Dis. 2016;75(1):311–315. doi: 10.1136/annrheumdis-2014-207201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parmentier JM, Voss J, Graff C, et al. In vitro and in vivo characterization of the JAK1 selectivity of upadacitinib (ABT-494) BMC Rheumatol. 2018;2(1):23. doi: 10.1186/s41927-018-0031-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.European Medicines Agency. Upadacitinib summary of product characteristics. 2021. [Accessed October 1, 2022]. https://www.ema.europa.eu/en/documents/product-information/rinvoq-epar-product-information_en.pdf .
- 31.US Food and Drug Administration. Upadacitinib prescribing information. 2019. [Accessed October 1, 2022]. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/211675s000lbl.pdf .
- 32.Genovese MC, Fleischmann R, Combe B, et al. Safety and efficacy of upadacitinib in patients with active rheumatoid arthritis refractory to biologic disease-modifying anti-rheumatic drugs (SELECT-BEYOND): a double-blind, randomised controlled phase 3 trial. Lancet. 2018;391(10139):2513–2524. doi: 10.1016/S0140-6736(18)31116-4. [DOI] [PubMed] [Google Scholar]
- 33.Smolen JS, Pangan AL, Emery P, et al. Upadacitinib as monotherapy in patients with active rheumatoid arthritis and inadequate response to methotrexate (SELECT-MONOTHERAPY): a randomised, placebo-controlled, double-blind phase 3 study. Lancet. 2019;393(10188):2303–2311. doi: 10.1016/S0140-6736(19)30419-2. [DOI] [PubMed] [Google Scholar]
- 34.Fleischmann R, Pangan AL, Song I, et al. Upadacitinib versus placebo or Adalimumab in patients with rheumatoid arthritis and an inadequate response to methotrexate: results of a phase III, double-blind, randomized controlled trial. Arthritis Rheumatol. 2019;71(11):1788–1800. doi: 10.1002/art.41032. [DOI] [PubMed] [Google Scholar]
- 35.Vollenhoven R, Takeuchi T, Pangan AL, et al. Efficacy and safety of Upadacitinib Monotherapy in methotrexate-naive patients with moderately-to-severely active rheumatoid arthritis (SELECT-EARLY): a multicenter, multi-country, randomized, double-blind, active comparator–controlled trial. Arthritis Rheumatol. 2020;72(10):1607–1620. doi: 10.1002/art.41384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Burmester GR, Kremer JM, van den Bosch F, et al. Safety and efficacy of upadacitinib in patients with rheumatoid arthritis and inadequate response to conventional synthetic disease-modifying anti-rheumatic drugs (SELECT-NEXT): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2018;391(10139):2503–2512. doi: 10.1016/S0140-6736(18)31115-2. [DOI] [PubMed] [Google Scholar]
- 37.van der Heijde D, Song IH, Pangan AL, et al. Efficacy and safety of upadacitinib in patients with active ankylosing spondylitis (SELECT-AXIS 1): a multicentre, randomised, double-blind, placebo-controlled, phase 2/3 trial. Lancet. 2019;394(10214):2108–2117. doi: 10.1016/S0140-6736(19)32534-6. [DOI] [PubMed] [Google Scholar]
- 38.Nogueira M, Torres T. Janus Kinase inhibitors for the treatment of atopic dermatitis: focus on abrocitinib, baricitinib, and upadacitinib. Dermatol Pract Concept. 2021;11(4):e2021145. doi: 10.5826/dpc.1104a145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McInnes IB, Anderson JK, Magrey M, et al. Trial of upadacitinib and adalimumab for psoriatic arthritis. N Engl J Med. 2021;384(13):1227–1239. doi: 10.1056/NEJMoa2022516. [DOI] [PubMed] [Google Scholar]
- 40.Mease PJ, Lertratanakul A, Anderson JK, et al. Upadacitinib for psoriatic arthritis refractory to biologics: SELECT-PsA 2. Ann Rheum Dis. 2021;80(3):312–320. doi: 10.1136/annrheumdis-2020-218870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McInnes IB, Kato K, Magrey M, et al. Upadacitinib in patients with psoriatic arthritis and an inadequate response to non-biological therapy: 56-week data from the phase 3 SELECT-PsA 1 study. RMD Open. 2021;7(3):e001838. doi: 10.1136/rmdopen-2021-001838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mease PJ, Lertratanakul A, Papp KA, et al. Upadacitinib in patients with psoriatic arthritis and inadequate response to biologics: 56-week data from the randomized controlled phase 3 SELECT-PsA 2 study. Rheumatol Ther. 2021;8(2):903–919. doi: 10.1007/s40744-021-00305-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nash P, Richette P, Gossec L, et al. Upadacitinib as monotherapy and in combination with non-biologic disease-modifying antirheumatic drugs for psoriatic arthritis. Rheumatology. 2022;61(8):3257–3268. doi: 10.1093/rheumatology/keab905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yates M, Mootoo A, Adas M, et al. Venous thromboembolism risk with JAK inhibitors: a meta-analysis. Arthritis Rheumatol. 2021;73(5):779–788. doi: 10.1002/art.41580. [DOI] [PubMed] [Google Scholar]

