Abstract
Background
Noninvasive ventilation (NIV) is an established first-line treatment of acute respiratory failure both in emergency departments (ED) and intensive care unit (ICU) settings. It is however not always successful.
Materials and methods
Prospective, observational study was done among patients above 18 years presenting with acute respiratory failure initiated on NIV. Patients were placed in one of two groups covering successful NIV treatment and NIV failure. Two groups were compared on four variables: initial respiratory rate (RR), initial high-sensitivity C-reactive protein (hs-CRP), PaO2/FiO2 ratio (p/f ratio), and heart rate, acidosis, consciousness, oxygenation, and respiratory rate (HACOR) score at the end of 1 hour of initiation of NIV.
Results
A total of 104 patients fulfilling the inclusion criteria were included in the study, of which 55 (52.88%) were exclusively treated with NIV (NIV success group), and 49 (47.11%) required endotracheal intubation and mechanical ventilation (NIV failure group). Noninvasive ventilation failure group had a higher mean initial RR compared with NIV success group (40.65 ± 3.88 vs 31.98 ± 3.15, p <0.001). Mean initial PaO2/FiO2 ratio was also significantly lower in the NIV failure group (184.57 ± 50.33 vs 277.29 ± 34.70, p <0.001). Odds ratio for successful NIV treatment with a high initial RR was 0.503 (95% confidence interval (CI), 0.390–0.649) and with a higher initial PaO2/FiO2 ratio was 1.053 (95% CI: 1.032–1.071 and with a HACOR score of >5 at the end of 1 hour of initiation of NIV was highly associated with NIV failure (p <0.001). A high initial level of hs-CRP was 0.949 (95% CI: 0.927–0.970).
Conclusion
Noninvasive ventilation failure could be predicted with information available at presentation in ED, and unnecessary delay in endotracheal intubation could possibly be prevented.
How to cite this article
Mathen PG, Kumar KPG, Mohan N, Sreekrishnan TP, Nair SB, Krishnan AK, et al. Prediction of Noninvasive Ventilation Failure in a Mixed Population Visiting the Emergency Department in a Tertiary Care Center in India. Indian J Crit Care Med 2022;26(10):1115–1119.
Keywords: HACOR score, High-sensitivity C-reactive protein, Noninvasive ventilation failure, Noninvasive ventilation, PaO2/FiO2 ratio, Prediction, Respiratory rate
Highlights
Noninvasive ventilation failure among patients with acute respiratory failure could be easily predicted based on four bedside variables available at presentation in ED, viz., high initial RR, low initial PaO2/FiO2 ratio, a HACOR score of >5 at the end of 1 hour of trial of NIV, and high initial levels of hs-CRP.
Introduction
Acute respiratory failure is a commonly encountered presentation to the ED and ICU. It remains to be one of the major causes of mortality among all age-groups and all sexes.
Acute respiratory failure is classically defined by an arterial oxygen tension (PaO2) of less than 8.0 kPa (60 mm Hg), an arterial carbon dioxide tension (PaCO2) of more than 6.0 kPa (45 mm Hg), or both.1–3
Treatment of respiratory failure (both acute and chronic) both in ED and ICU for management of type-I and type-II respiratory failure has met new horizons with the advent of NIV.2,4,5 It has considerably reduced the need for mechanical ventilation among patients presenting with both acute respiratory failure and acute on chronic respiratory failure to the ED and ICU, thereby appreciably reducing the hassles of mechanical ventilation such as ventilator-associated pneumonia (VAP) and ventilator-associated lung injury (VALI).5,6
Noninvasive ventilation is most beneficial in hypercapnic respiratory failure commonly encountered in acute exacerbation of chronic obstructive pulmonary disease (COPD).2,6 It has also been tried successfully in acute exacerbation of bronchial asthma (BA) and interstitial lung disease (ILD). It has drastically decreased the need for mechanical ventilation.7 Noninvasive ventilation is also widely used in cardiogenic and noncardiogenic pulmonary edema.8,9 It has also been tried in patients with mild-to-moderate acute respiratory distress syndrome (ARDS).8–10,11
Noninvasive ventilation failure is associated with the urgent requirement of endotracheal intubation and mechanical ventilation.12 There are several reasons for NIV failure, including absent respiratory drive (such as in a central nervous insult causing central apnea), severe hypoxemia (seen in severe ARDS), severe hypercapnic respiratory failure (severe enough to cause a fall in the level of consciousness), severe claustrophobia, profound immunosuppression, hemodynamic instability, multiple organ failure, etc. Noninvasive ventilation is not preferred in such scenarios. Like any positive-pressure ventilation device, NIV must not be used in proven or suspected cases of pneumothorax or bronchopleural fistula.12,13 Noninvasive ventilation can itself cause a fall in blood pressure, so, therefore, must be used with caution in hypotensive patients.13–15
Predictors of NIV failure have been extensively researched and include several variables that may be used in the ED or ICU for predicting NIV failure and preventing delay in mechanical ventilation.11,12,16,17 Studies have shown higher C-reactive protein among patients who failed the NIV trial.5
The present study aims to determine if NIV failure could be predicted based on information available during the presentation to the ED.
Objectives
Primary: To assess the relationship between initial clinical parameters and NIV failure and to predict NIV failure based on those parameters.
Secondary: To compare the C-reactive protein levels between NIV and the success group.
Materials and Methods
Study Design
Prospective, observational study.
Study Setting
Tertiary care setting – Department of Emergency Medicine and Critical Care, Amrita Institute of Medical Sciences, Kochi, India
Study Patients
Patients presenting to the ED with acute respiratory failure initiated on NIV. Strict inclusion and exclusion criteria were followed.
Inclusion Criteria
Patients with acute respiratory failure – both type I and II.
Age more than 18 years.
Patients with preserved respiratory effort and good cough reflex.
Patients with a normal sensorium.
Exclusion Criteria
Pregnant women.
Patients with facial trauma.
Patients without preserved respiratory effort and poor cough reflex.
Patients with a decreased sensorium, comatose patients.
Patients in cardiac arrest or impending cardiac arrest.
Patients with DNI/DNR status.
Method of Study
All patients presenting to the ED initiated on NIV for acute respiratory failure (type I, type II, and mixed), meeting the inclusion criteria, were included in the study. Patients in the study were placed in one of two groups covering successful NIV treatment and NIV failure. The two groups were compared on four variables, viz., initial RR, initial PaO2/FiO2 ratio, HACOR score at the end of 1 hour of initiation of NIV, and initial hs-CRP. There parameters were used to determine whether NIV failure could be predicted. Data collection was done with help of pretyped proforma after obtaining written consent.
The research work was reviewed and granted approval by the Institutional Review Board (IRB) and Institutional Ethics Committee (IEC), AIMS, Kochi.
Study Duration
August 2017 to August 2019 (2 years).
Study Interventions
Nil.
Study Tools
Respiratory rate was measured by the standard method of counting the number of chest rises in 1 whole minute by the investigator. PaO2/FiO2 ratio was calculated from arterial blood gas (ABG) analysis, done with the help of Radiometer ABL800 FLEX. Heart rate, Acidosis, Consciousness, Oxygenation, and Respiratory rate score was measured for all patients in the study group at the end of 1 hour of initiation of NIV. Heart rate was calculated by standard palpatory method (number of beats in 1 whole minute), acidosis (in the form of pH) noted from the ABG, consciousness evaluated by Glasgow Coma Scale (GCS range 3–15), and oxygenation measured by PaO2/FiO2 ratio measured from ABG. High-sensitivity C-reactive protein was assayed by a standardized turbidimetric immunoassay method. A comparison of all these four variables was made between the NIV success and NIV failure group.
Sample Size
Based on the result of mean and standard deviation (SD) of C-reactive protein level in NIV success group (79 ± 91) and NIV failure group (138 ± 113) observed in an earlier publication (Anders Bastiansen, Predicting failure of noninvasive ventilation in a mixed population. Journal of Anesthesia & Clinical Research 2014;5:378) and with 80% power and 95% confidence, the minimum sample size comes to 100 (50 in each group).
Statistical Analysis
Statistical analysis was performed using IBM SPSS version 21.0 software. Categorical variables are expressed using frequency and percentage. Numerical variables are represented using mean ± SD. To test the statistically significant difference in hs-CRP, initial RR, initial PaO2/FiO2 ratio between NIV success and failure group, Student's t-test was applied. Chi-square analysis was done to find the association between HACOR score at the end of 1 hour of initiation of NIV and NIV failure. Pearson's correlation coefficient (r) was used to find the correlation between initial RR and initial PaO2/FiO2 ratio and also hs-CRP. p-value of less than 0.05 was considered as statistically significant. Univariate analysis was done to calculate odds ratio (OR) for successful NIV treatment with three variables, viz., initial RR, initial PaO2/FiO2 ratio, and initial hs-CRP levels.
Results
A total of 104 patients who fulfilled the inclusion criteria were included in the study. Out of the 104, 55 (52.88%) were exclusively treated with NIV (NIV success group), and 49 (47.11%) required endotracheal intubation and mechanical ventilation (NIV failure group). The most common indication to initiate NIV was type-II respiratory failure. On comparing means of age in both the groups it was noticed that the success group had a lower mean age (54.41 ± 17.48 vs 63.76 ± 13. 756, p = 0.003). There was no statistically significant association of sex and NIV failure.
Comparison of the mean initial RR was made among the two groups, viz., NIV treatment success and failure groups. It was noticed that mean initial RR of the success of the group was 31.98 ± 3.159 and of the failure group was 40.65 ± 3.881, with a p-value of <0.001, which was statistically significant (Table 1, Fig. 1).
Comparison of the mean initial PaO2/FiO2 ratio was made among the two groups, viz., NIV treatment success and failure groups. It was noticed that mean initial PaO2/FiO2 ratio of the success of the group was 277.29 ± 34.702 and of the failure group was 184.57 ± 50.338, with a p-value of <0.001, which was statistically significant (Table 2, Fig. 2).
Heart Rate, Acidosis, Consciousness, Oxygenation, and Respiratory Rate score is abbreviated to HACOR score. In the study population, all patients were treated with NIV, HACOR score was calculated. It was noticed that 98.2% of patients successfully treated with NIV had a HACOR score of less than 5 at the end of 1 hour of initiation of NIV (p-value <0.001). All patients who failed NIV had a HACOR score of more than 5 at the end of 1 hour (p-value <0.001). A score of more than 5 at the end of 1 hour of initiation of NIV was having a statistically significant association with NIV failure. It can be used as a bedside tool to predict NIV failure (Table 3, Fig. 3).
Comparison of mean initial hs-CRP was made among the two groups, viz., NIV treatment success and failure groups. It was noticed that the mean initial PaO2/FiO2 ratio of the success of the group was 45.88 ± 31.494 and of the failure group was 226.74 ± 97.651, with a p-value of <0.001, which was statistically significant (Table 4, Fig. 4).
Table 1.
Initial respiratory rate and outcome of treatment with noninvasive ventilation
| NIV treatment group | n | Initial RR* (breaths/min) (mean ± SD) | p-value |
|---|---|---|---|
| Success | 55 | 31.98 ± 3.159 | <0.001 |
| Failure | 49 | 40.65 ± 3.881 |
*RR, respiratory rate
Fig. 1.
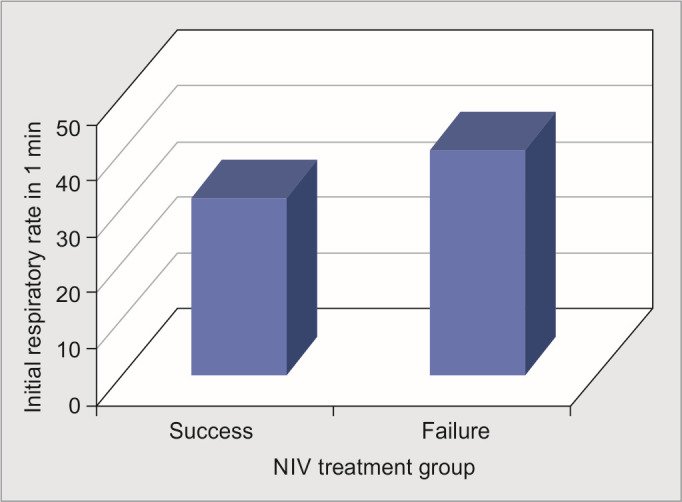
Chart showing comparison of mean initial respiratory rate in noninvasive ventilation success and failure groups
Table 2.
Initial PaO2/FiO2 ratio and outcome of treatment with noninvasive ventilation
| NIV treatment group | n | Initial PaO2/FiO2 ratio (mean ± SD) | p-value |
|---|---|---|---|
| Success | 55 | 277.29 ± 34.702 | <0.001 |
| Failure | 49 | 184.57 ± 50.338 |
Fig. 2.
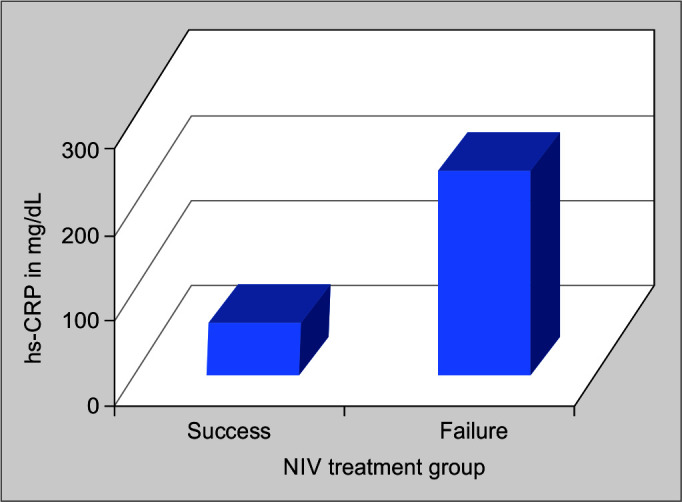
Comparison of mean initial hs-CRP in noninvasive ventilation success and failure groups
Table 3.
Association of HACOR score at the end of 1 hour of initiation of NIV and NIV failure
| HACOR* score at the end of 1 hour of initiation of NIV | NIV treatment group | Total | p-value | |||
|---|---|---|---|---|---|---|
| Success | Failure | |||||
| n | % | n | % | |||
| <5 | 55 | 98.2 | 1 | 1.8 | 56 | <0.001 |
| >5 | 0 | 0 | 48 | 100 | 48 | |
*HACOR, heart rate, acidosis, confusion, oxygenation, respiratory rate
Fig. 3.
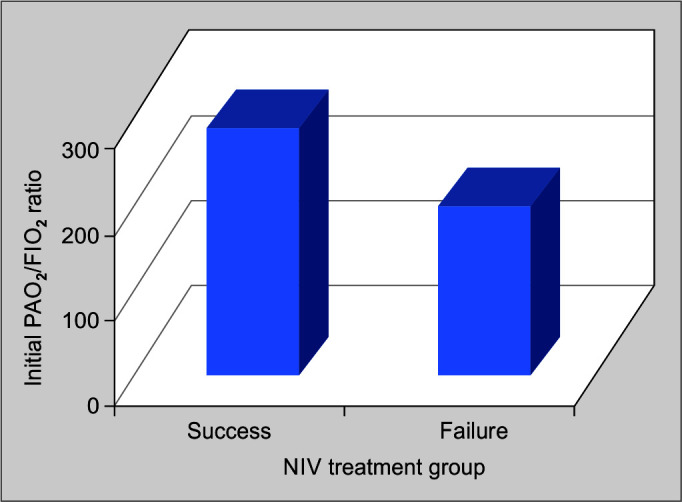
Comparison of mean initial PaO2/FiO (p/f) ratio and noninvasive ventilation (NIV) treatment outcome
Table 4.
Initial hs-CRP and outcome of treatment with NIV
| NIV treatment group | n | Initial hs-CRP (mean ± SD) | p-value |
|---|---|---|---|
| Success | 55 | 45.88 ± 31.494 | <0.001 |
| Failure | 49 | 226.74 ± 97.651 |
Fig. 4.
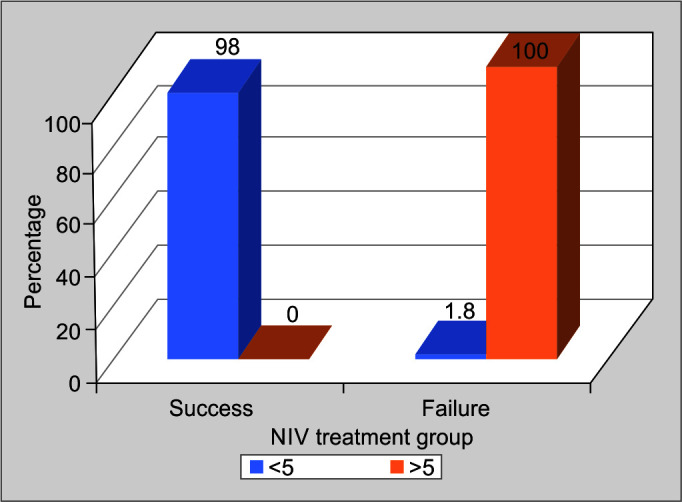
Association of HACOR* score at the end of 1 hour of initiation of noninvasive ventilation (NIV) and NIV failure. (*Heart rate, acidosis, oxygenation, and respiratory rate)
Discussion
In the present study, patients presenting with acute respiratory failure to the ED treated with NIV who met the inclusion criteria were carefully studied for a period of 2 years (August 2017–August 2019). The study population was divided into two groups covering successful NIV treatment and NIV failure respectfully. No distinction was made based on co-existing illnesses. The two groups were compared with variables at presentation, viz., initial RR, PaO2/FiO2 ratio, HACOR score at the end of 1 hour of initiation of NIV, and initial hs-CRP level.
Demographics
A total of 104 patients who fulfilled the inclusion criteria were included in the study. Majority of patients belonged to the age-group 51–75 years (57.69%). Mean age of the study population was 59.36 ± 16.23 years. Gender distribution of the study population showed a male preponderance (60.6% vs 39.4%).
Out of the 104 patients in the study, 52.9% were exclusively treated with NIV (success group) and 47.1% required endotracheal intubation and mechanical ventilation (failure group).
Mean age of patients who were successfully treated with NIV was 63.76 ± 13.75 years and in the failure group 54.41 ± 17.48 years. These results were similar to the study done by Corrêa et al., which showed OR of 0.96 (95% CI: 0.93–0.99) for age predicting NIV failure.9 Although this was in contrast to the review article by Mehta et al., which stated young age as a predictor of success during acute applications of noninvasive positive-pressure ventilation (NPPV).13
Out of the 63 males in the study group, 52.4% failed NIV treatment, and out of 41 females in the study group, 39% failed NIV treatment. There was no statistically significant association of sex with NIV failure (p-value = 0.182).
Initial RR and NIV Failure
In the present study, it was observed that patients in the NIV failure group had a significantly higher initial RR (40.65 ± 3.88, p-value <0.001) than the success group (31.98 ± 3.15, p-value <0.001). Univariate logistic regression showed an OR of 0.503 (95% CI: 0.390–0.649) for successful NIV treatment with a high initial RR. This result was similar to the multicenter study by Antonelli et al., which showed that a RR ≥38 showed an OR for NIV failure of 1.89 (95% CI: 1.06–5.74). Similarly, a retrospective study done on ICU patients treated with NIV for acute respiratory failure by Bastiansen showed OR for NIV failure with increased RR (OR 1.13, 95% CI: 1.02–1.25). The review article by Ozyilmaz et al. says an average RR >25 breaths/min on NIV is a predictor of failure, because it is a surrogate of increased work of breathing.8 The rapid shallow breathing index (i.e., the ratio between RR/ tidal volume) of more than 105 among patients treated with NIV has also been demonstrated to be independently associated with NIV failure.8
Initial PaO2/FiO2 Ratio and NIV Failure
PaO2/FiO2 (p/f) ratio is a marker of oxygenation. It was calculated for all patients in the study group from ABG analysis. On comparing the mean initial PaO2/FiO2 ratio among NIV success and failure group, it was observed that the NIV failure group had significantly lower p/f ratio (184.57 ± 50.33, p-value <0.001) compared with NIV success group (277.29 ± 34.70, p-value <0.001). Univariate regression analysis revealed an OR of 1.052 (95% CI: 1.032–1.071) for a higher initial p/f ratio for successful NIV treatment. A study by Carrillo et al. on NIV in community-acquired pneumonia (CAP) showed that a lower PaO2/FiO2 ratio after 1 hour of NIV treatment in acute respiratory failure (among previously normal individuals) independently predicted NIV failure. The review article by Ozyilmaz et al. states that NIV should be performed very cautiously among patients with PaO2/FiO2 ratio <150 because of high risk of NIV failure.8,11
HACOR Score at the End of 1 Hour of Initiation of NIV Treatment and NIV Failure
Heart rate, Acidosis, Consciousness, Oxygenation, and Respiratory rate score was calculated for all patients in the study group. Heart rate, Acidosis, Consciousness, Oxygenation, and Respiratory rate score at the end of 1 hour of initiation of NIV treatment >5 had a highly significant association with NIV failure.11,15 In the study group, all patients having HACOR score >5 at the end of 1 hour of initiation of NIV failed NIV treatment (p-value <0.001), and it was noticed only one patient who had a HACOR score of <5 at the end of 1 hour of initiation of NIV failed NIV treatment (p-value <0.001). A cohort study by Duan et al. revealed that HACOR score at the end of 1 hour had good predictive power for NIV failure.12 Using five points as the cutoff value, the sensitivity, specificity, positive predictive value, negative predictive value, and diagnostic accuracy for NIV failure were 72.6, 90.2, 87.2, 78.1, and 81.8%, respectively.
A correlation analysis between initial RR and initial PaO2/FiO2 ratio and hs-CRP was done. Pearson's correlation coefficient (r) for initial RR and PaO2/FiO2 ratio was –0.670 (p-value <0.001) showing a very statistically significant negative correlation, i.e., the higher the initial RR, the lower the initial PaO2/FiO2. Initial RR and initial hs-CRP had a positive Pearson correlation coefficient of + 0.644 (p-value <0.001) showing a significant positive correlation i.e., higher the initial RR, the higher the hs-CRP.
Initial High-sensitivity C-reactive Protein (hs-CRP) and NIV Failure
On comparing the mean initial hs-CRP among the two NIV treatment groups, i.e. NIV success group and NIV failure, it was noticed that NIV failure group had a significantly higher hs-CRP value (226.74 ± 97.65, p-value <0.001) compared with NIV success group (45.88 ± 31.49, p-value <0.001). Univariate logistic regression showed an OR of 0.949 (95% CI: 0.927–0.970) for successful NIV treatment with high initial hs-CRP levels. These results were similar to the retrospective study published by Bastiansen, which also showed a higher mean CRP value in the NIV failure group (138 ± 113, p-value 0.02) compared with NIV success group (79 ± 91, p-value 0.02).10 Multivariate regression analysis in the same study showed an OR of 1.01 (95% CI: 1.00–1.01) for NIV failure among patients with high levels of C-reactive protein.10
The most common indication to initiate NIV in ED was type-II respiratory failure (41.34%), closely followed by type-I respiratory failure (40.38%). Rarely a combination of both type-I and type-II respiratory failure co-existed in the same patient (18.26%). One of the most common indications to initiate patients on NIV was pulmonary edema. Pulmonary edema may be associated with type-I or type-II respiratory failure or both.
The most commonly encountered comorbidity among patients who required NIV was COPD (47.1%). It was also noticed that individuals with more number of comorbidities (calculated by Charlson comorbidity index) were at higher risk for NIV failure.
Risk assessment of NIV failure with above-discussed variables at presentation is, therefore, necessary to predict NIV failure and prevent unnecessary delays in invasive ventilation.
Limitations of the Study
Patient characteristics such as comorbidities, severity of illness, agitation, and severe claustrophobia could have resulted in NIV failure and skewed the results.
Variables such as GCS and RR can show interobserver variation.
Single-center observation.
Conclusion
Acute respiratory failure is a major cause of ED and ICU admissions. Noninvasive ventilation is an excellent treatment modality for acute respiratory failure. It is without doubt that a huge proportion of patients treated with NIV will fail it and inevitably require intubation and mechanical ventilation. Initial patient characteristics such as a high initial RR, a very low PaO2/FiO2 ratio, HACOR score at the end of 1 hour more than 5, and highly elevated hs-CRP, all predict high risk for NIV failure. These parameters may be used as early as possible to prevent unnecessary delay in intubation and mechanical ventilation among patients who need them.
Orcid
Prannoy George Mathen https://orcid.org/0000-0002-7291-6160
Gireesh Kumar KP https://orcid.org/0000-0002-9856-1193
Naveen Mohan https://orcid.org/0000-0001-8006-9427
Sreekrishnan TP https://orcid.org/0000-0002-3741-064X
Sabarish B Nair https://orcid.org/0000-0001-6333-4479
Arun Kumar Krishnan https://orcid.org/0000-0002-9367-5714
Bharath Prasad S https://orcid.org/0000-0002-4175-0102
Riaz Ahamed D https://orcid.org/0000-0002-3553-5211
Manna Maria Theresa https://orcid.org/0000-0002-6759-0549
Kathyayini VR https://orcid.org/0000-0003-1327-7693
Footnotes
Source of support: Nil
Conflict of interest: None
References
- 1.Ambrosino N, Foglio K, Rubini F, Clini E, Nava S, Vitacca M. Non-invasive mechanical ventilation in acute respiratory failure due to chronic obstructive airways disease: Correlates for success. Thorax. 1995;50(7):755–757. doi: 10.1136/thx.50.7.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brochard L. Non-invasive ventilation for acute exacerbations of COPD: A new standard of care. Thorax. 2000;55(10):817–818. doi: 10.1136/thorax.55.10.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nava S, Ceriana P. Causes of failure of noninvasive mechanical ventilation. Respir Care. 2004;49(3):295–303. 14982651 [PubMed] [Google Scholar]
- 4.Yoshida Y, Takeda S, Akada S, Hongo T, Tanaka K, Sakamoto A. Factors predicting successful noninvasive ventilation in acute lung injury. J Anesth. 2008;22(3):201–206. doi: 10.1007/s00540-008-0637-z. [DOI] [PubMed] [Google Scholar]
- 5.Rana S, Jenad H, Gay PC, Buck CF, Hubmayr RD, Gajic O. Failure of non-invasive ventilation in patients with acute lung injury: Observational cohort study. Crit Care. 2006;10(3):R79. doi: 10.1186/cc4923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hill NS. Where should noninvasive ventilation be delivered? Respir Care. 2009;54(1):62–70. 19111107 [PubMed] [Google Scholar]
- 7.Maheshwari V, Paioli D, Rothaar R, Hill NS. Utilization of noninvasive ventilation in acute care hospitals: A regional survey. Chest. 2006;129(5):1226–1233. doi: 10.1378/chest.129.5.1226. [DOI] [PubMed] [Google Scholar]
- 8.Ozyilmaz E, Ugurlu AO, Nava S. Timing of noninvasive ventilation failure: Causes, risk factors, and potential remedies. BMC Pulm Med. 2014;14:19. doi: 10.1186/1471-2466-14-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Corrêa TD, Sanches PR, de Morais LC, Scarin FC, Silva E, Valente Barbas CS, et al. Performance of noninvasive ventilation in acute respiratory failure in critically ill patients: A prospective, observational, cohort study. BMC Pulm Med. 2015;15:144. doi: 10.1186/s12890-015-0139-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bastiansen A. Predicting failure of non-invasive ventilation in a mixed population. J Anesth Clin Res. 2014;5:378. doi: 10.4172/2155-6148.1000378. [DOI] [Google Scholar]
- 11.Sehgal IS, Chaudhuri S, Dhooria S, Agarwal R, Chaudhry D. A study on the role of noninvasive ventilation in mild-to-moderate acute respiratory distress syndrome. Indian J Crit Care Med. 2015;19(10):593–599. doi: 10.4103/0972-5229.167037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duan J, Han X, Bai L, Zhou L, Huang S. Assessment of heart rate, acidosis, consciousness, oxygenation, and respiratory rate to predict noninvasive ventilation failure in hypoxemic patients. Intensive Care Med. 2017;43(2):192–199. doi: 10.1007/s00134-016-4601-3. [DOI] [PubMed] [Google Scholar]
- 13.Mehta S, Hill NS. Noninvasive ventilation. Am J Respir Crit Care Med. 2001;163(2):540–577. doi: 10.1164/ajrccm.163.2.9906116. [DOI] [PubMed] [Google Scholar]
- 14.Wood KA, Lewis L, Von Harz B, Kollef MH. The use of non-invasive positive pressure ventilation in the emergency department. Chest. 1998;113(5):1339–1346. doi: 10.1378/chest.113.5.1339. [DOI] [PubMed] [Google Scholar]
- 15.Nair A, Esquinas A. HACOR score to predict failure of non-invasive ventilation in patients with acute hypoxemic respiratory failure: When simplicity is best. Saudi J Anaesth. 2022;16(2):267–268. doi: 10.4103/sja.sja_88_22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hadda V, Chawla G, Tiwari P, Madan K, Khan MA, Mohan A, et al. Noninvasive ventilation for acute respiratory failure due to noncystic fibrosis bronchiectasis. Indian J Crit Care Med. 2018;22(5):326–331. doi: 10.4103/ijccm.IJCCM_474_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saxena P, Mani RK. Noninvasive ventilation success: Combining knowledge and experience. Indian J Crit Care Med. 2014;18(8):492–493. doi: 10.4103/0972-5229.138138. [DOI] [PMC free article] [PubMed] [Google Scholar]


