Abstract
We present the first ancient DNA data from the Pre-Pottery Neolithic of Mesopotamia (Southeastern Turkey and Northern Iraq), Cyprus, and the Northwest Zagros, along with the first data from Neolithic Armenia. We show that these and neighboring populations were formed through admixture of pre-Neolithic sources related to Anatolian, Caucasus, and Levantine hunter-gatherers, forming a Neolithic continuum of ancestry mirroring the geography of West Asia. By analyzing Pre-Pottery and Pottery Neolithic populations of Anatolia we show that the former were derived from admixture between Mesopotamian-related and local Epipaleolithicrelated sources, but the latter experienced additional Levantine-related gene flow, thus documenting at least two pulses of migration from the Fertile Crescent heartland to the early farmers of Anatolia.
One-Sentence Summary:
Ancient DNA from Mesopotamia documents a West Asian Neolithic continuum and proves two pulses of migration contributing to the early farmers of Anatolia.
Previous work has documented the existence of highly differentiated Neolithic populations in ancient West Asia(1–9) and some of their pre-Neolithic antecessors in the Caucasus(10), Iran(1, 11), Anatolia(6), and the Levant(1). To anchor our integrative genomic history of the Southern Arc, a region we define as including Anatolia and its neighbors in Southeastern Europe and West Asia(12), we sought to understand how the earliest Neolithic populations were formed, with a particular focus on the Pre-Pottery period of northern (or Upper) Mesopotamia, the area between the Tigris and Euphrates rivers of Southeast Turkey, Northwest Iraq and Northeast Syria, within the Pre-Pottery Neolithic interaction sphere (13). Despite the centrality of Mesopotamia in the archaeological record of the origin of farming(14), no genome-wide ancient DNA data from early Mesopotamian farmers has been published. We used in-solution enrichment for approximately 1.2 million single nucleotide polymorphisms (SNPs) to study Pre-Pottery Neolithic farmers from the Tigris side of northern Mesopotamia: one from Boncuklu Tarla near Mardin in southeastern Turkey and two from Nemrik 9 in northern Iraq. We also report the first Pre-Pottery Neolithic data from Cyprus, an island to the south of the Anatolian peninsula and west of the Levant, which witnessed the earliest maritime expansion of Pre-Pottery farmers from the eastern Mediterranean; our data come from three individuals whose fragmentary remains were found in a disused water well at Kissonerga-Mylouthkia(15). We furthermore report the first ancient DNA data from the Neolithic of Armenia, from two individuals buried at the sites of Masis Blur and Aknashen in the 6th millennium BCE. These individuals represent an inland Pottery Neolithic population, which we could compare to the Pre-Pottery one from northern Mesopotamia to its south, the Pottery Neolithic one of Azerbaijan to its east(7), and later Chalcolithic individuals from Armenia (1). Finally, we sampled three PrePottery Neolithic farmers from the northern Zagros at Bestansur and the Zawi Chemi component of Shanidar Cave in Iraq, who fill a gap between the more western and northern individuals and published data from the central Zagros in Iran(1).
Details of the newly sampled individuals can be found in (12), and their geographical and temporal distributions can be seen in Fig. 1. To improve the statistical power of our analyses, we also increased data quality for a number of individuals with previously reported data, making and sequencing additional ancient DNA libraries from 4 Epipaleolithic Natufians from Israel and 6 Pre-Pottery Neolithic individuals from Jordan(1), and 9 Neolithic individuals from the Eastern Marmara region (Northwest Anatolia, sites of Barcın and Menteşe)(2). From Eastern Marmara, we also sampled an individual from Barcın and two from the previously unsampled site of Ilıpınar. Individuals from the three sites were genetically similar, and we analyze them, together with later Chalcolithic individuals from the same site, in a study of later periods of Anatolia (12).
Fig. 1. Studied individuals.
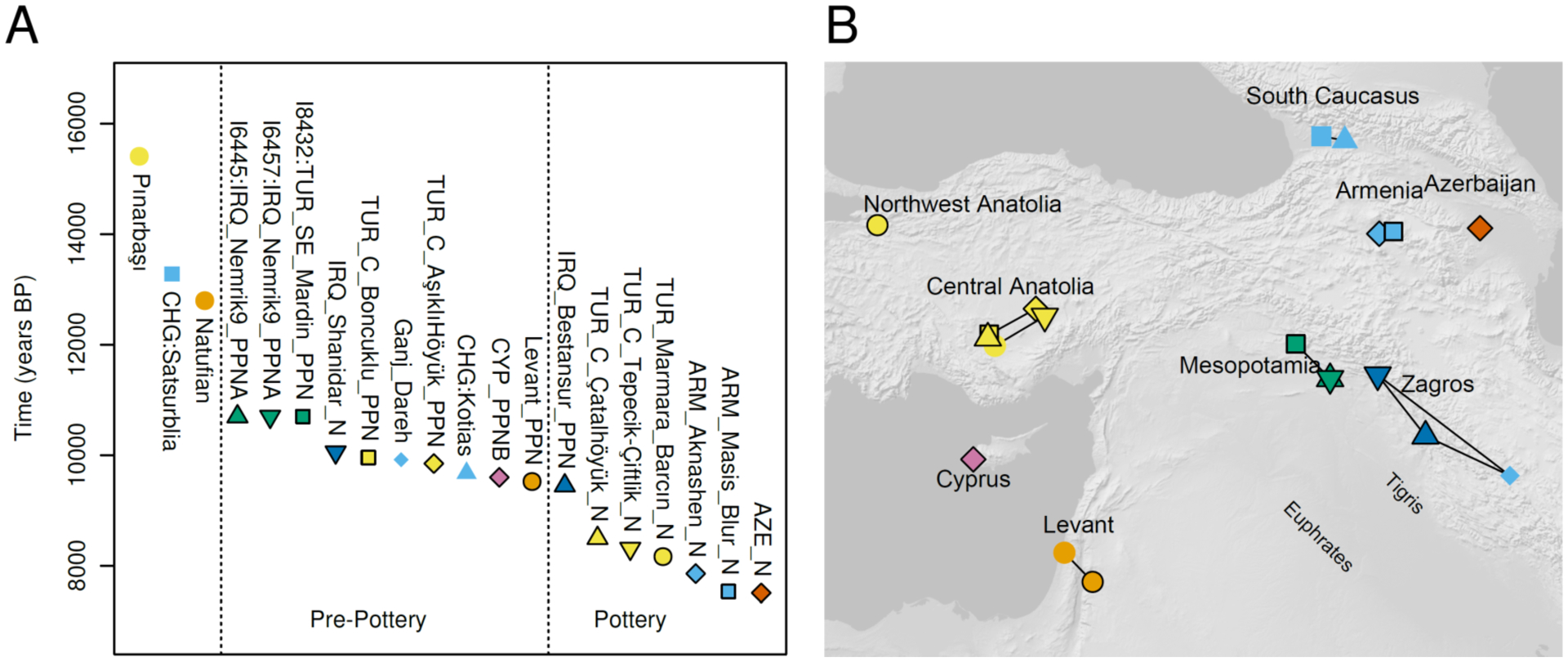
(A) Timeframe of Pre-Neolithic, Pre-Pottery Neolithic, and Pottery Neolithic populations in West Asia. (B) Geographical location of populations from panel (A) on the map of West Asia.
We carried out principal component analysis (PCA) (16) (Fig. 2A), projecting the ancient individuals onto the variation of present-day West Eurasians(17). Two main clusters emerge: an “Eastern Mediterranean” Anatolian/Levantine one that also includes the geographically intermediate individuals from Cyprus, and an “Inland” Zagros-Caucasus-Mesopotamia-ArmeniaAzerbaijan one. There is structure within these groupings. Anatolian individuals group with each other and with the ones from Cyprus, separately from Levantine individuals. Within the “Inland” cluster, individuals that are more geographically distant from the Mediterranean, such as those from the South Caucasus (Caucasus hunter-gatherers from Georgia(10) and Ganj Dareh from central Zagros), are also genetically more distant as compared to the geographically and genetically intermediate individuals from Mesopotamia and Armenia/Azerbaijan. The “Eastern Mediterranean” and “Inland” clusters are separated by a gap in Fig. 2A which may correspond to geographically intermediate areas between sampling locations, for example the Euphrates region of North Mesopotamia. The totality of Neolithic West Asia is enclosed within the range of variation of the quadrangle formed by Caucasus hunter-gatherers, Ganj Dareh, Levantine Natufians(1) from Israel, and Epipaleolithic Pınarbaşı(6) from Central Anatolia.
Fig. 2. Overview of Neolithic variation.
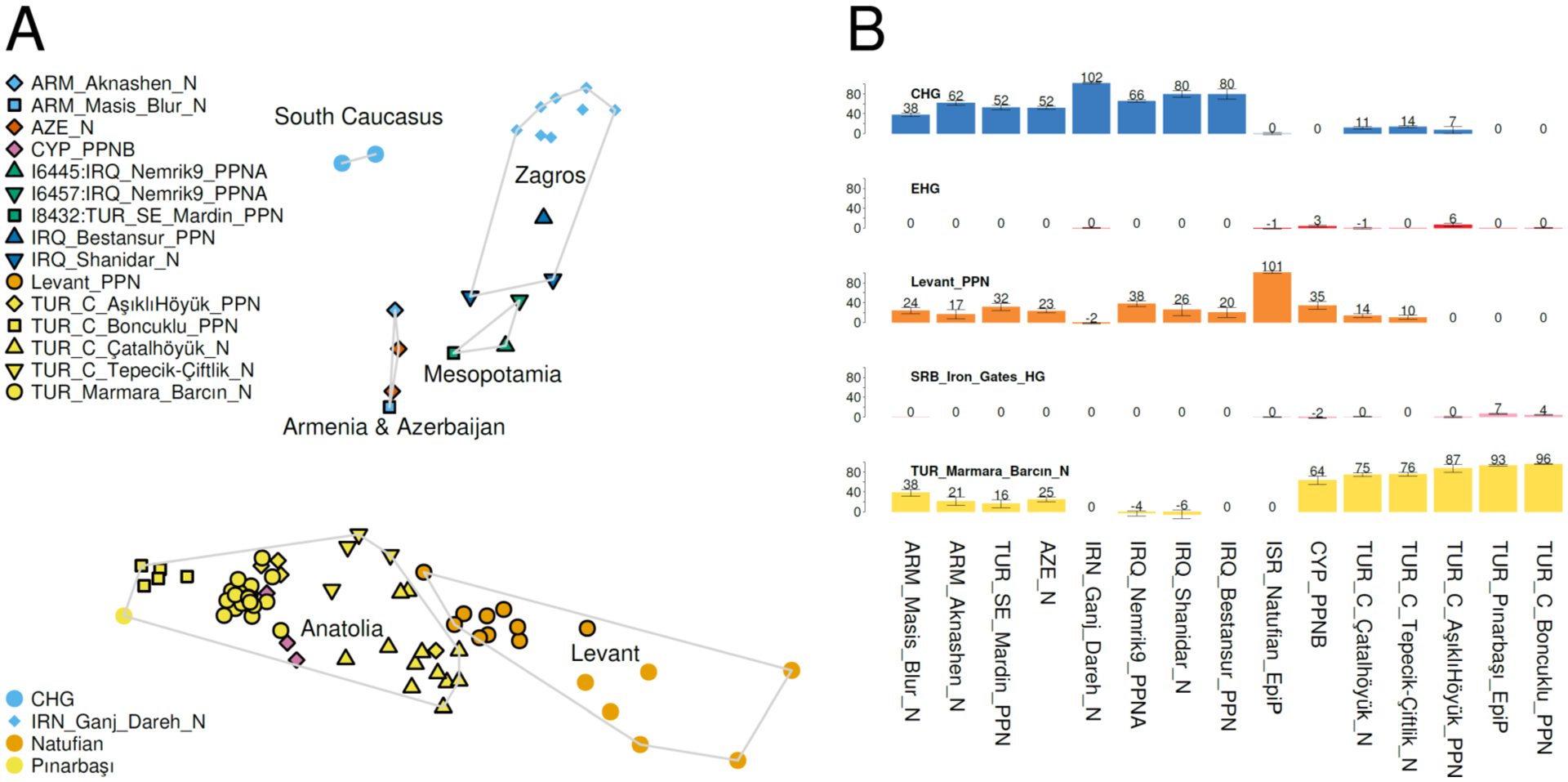
(A) Principal component analysis of ancient individuals projected onto West Eurasian variation. (B) Application of 5-way model of (12) on Neolithic populations with Caucasus hunter-gatherer (CHG), Eastern European hunter-gatherer (EHG), Levant Pre-Pottery Neolithic (PPN), Serbian Iron Gates hunter-gatherer, and NW Anatolian Neolithic from Barcın sources.
In a parallel study we develop a mathematical framework for estimating the ancestry proportions of individuals of the entire Southern Arc across space and time with a common metric (12), and here we discuss the results of applying this model to the Neolithic period (Fig. 2B). This model includes Caucasus hunter-gatherers (10), Eastern European hunter-gatherers (2, 18), Levantine Pre-Pottery Neolithic(1), Balkan hunter-gatherers from the Iron Gates in Serbia(19) and Anatolian Neolithic (from Barcın in the Marmara region of NW Anatolia(2)) as surrogates for five ancestry sources. Within this framework, the highest proportion of Anatolian Neolithicrelated ancestry is observed in Neolithic Anatolian populations as well as the early farmers of Cyprus. The Balkan hunter-gatherer-related affinity in the Pre-Pottery population at Boncuklu and the Epipaleolithic one from Pınarbaşı—both of which predate the Pottery Neolithic from Barcın by thousands of years—does not indicate that these older individuals were admixed with European hunter-gatherers. Rather, it means that in comparison to the Barcın population, both Pınarbaşı and Boncuklu were “less Levantine” (Fig. 2A), a finding that is consistent with the Levantine influx into the Pottery Neolithic populations that is revealed by the analysis that follows. A contrasting case is that of the Natufians who are inferred to be “more Levantine” (along the Anatolian/Levantine cline) and are unsurprisingly inferred to derive all of their ancestry from the Levant Pre-Pottery Neolithic source; this of course does not mean that the earlier Natufians are descended from the Pre-Pottery Neolithic farmers that followed them, but that both share ancestry (in reality from the Natufians to the Pre-Pottery Neolithic farmers), which is thus modeled within the limitations of the 5-way model. Similarly, the Ganj Dareh population (most extreme) of the “Inland” group derives all its ancestry from the Caucasus hunter-gatherer source used in the 5-way model, and Caucasus hunter-gatherer-related ancestry levels are high in all “Inland” populations, i.e., of the North Zagros, Armenia, and Azerbaijan, as well as those of North Mesopotamia.
The high Anatolian-related ancestry in Cyprus revealed by this model (Fig. 2) and subsequent analyses (Fig. 3) sheds light on debates about the origins of the people who spread Pre-Pottery Neolithic culture to Cyprus. Parallels in subsistence, technology, settlement organization, and ideological indicators(15) suggest close contacts between Pre-Pottery Neolithic B people in Cyprus and on the mainland(13), but the geographic source of the Cypriot Pre-Pottery Neolithic populations has been unclear with many possible points of origin(20). An inland Middle Euphrates source has been suggested on the basis of architectural, artefactual and ideological similarities (14, 21). However, the faunal record at Cypriot Pre-Pottery Neolithic B sites, and the use of Anatolian obsidian as raw material, suggest linkages with central and southern Anatolia (15, 22), and the genetic data increase the weight of evidence in favor of this scenario of a primary source in Anatolia.
Fig. 3. The Neolithic continuum.
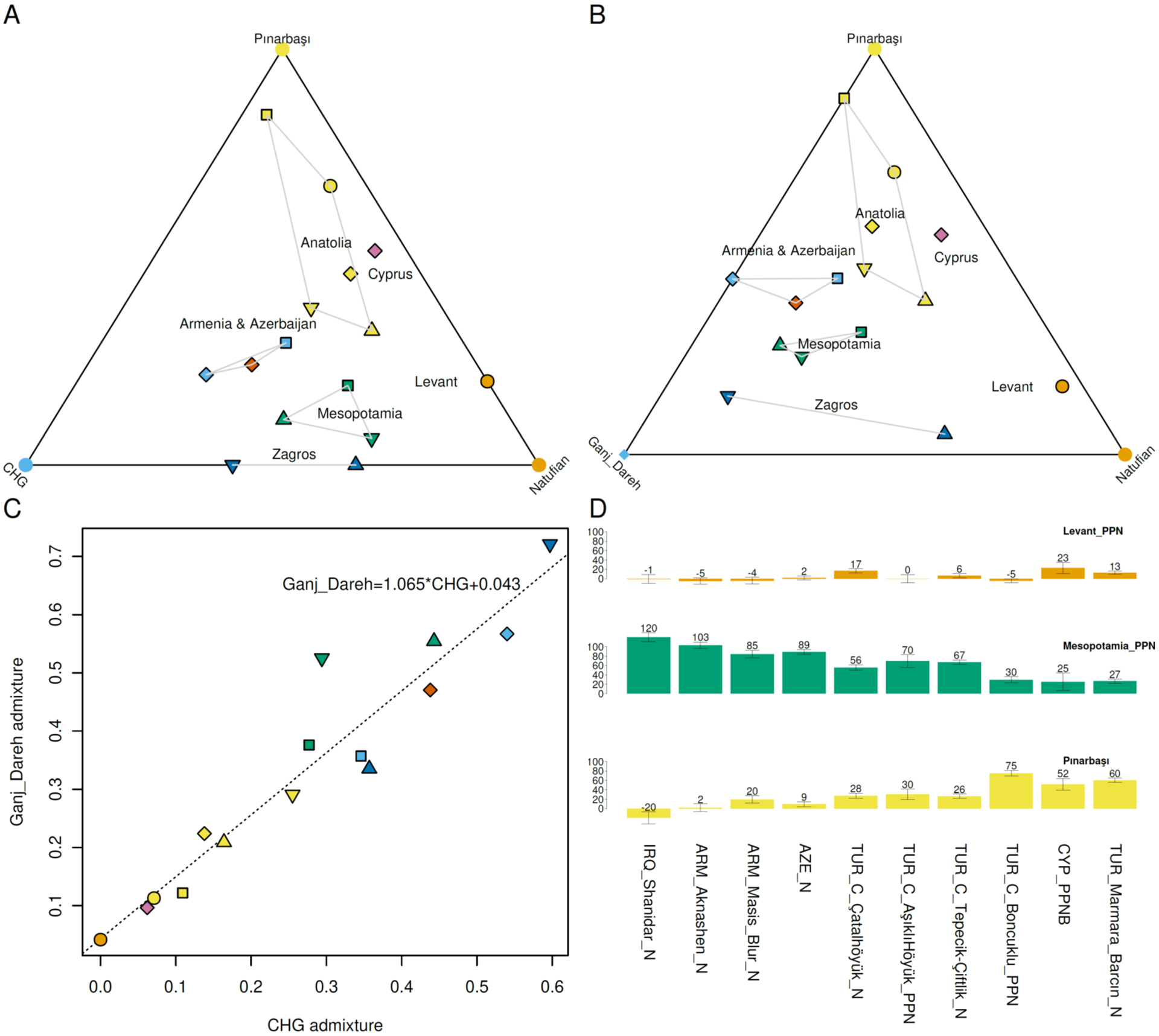
(A) 3-way model of Neolithic admixture with Caucasus hunter-gatherer (CHG) (10) as a source. (B) 3-way model of Neolithic admixture with Ganj Dareh(1) as a source. (C) Caucasus hunter-gatherer and Ganj Dareh admixture proportions from panels A, B are strongly correlated (R2=0.91; p<1e-7). (D) We also modeled Neolithic populations with local, Anatolian (Pınarbaşı(6)) and eastern, Mesopotamian Pre-Pottery Neolithic (PPN), proximal sources. Both Pre-Pottery Neolithic populations from Anatolia (from Boncuklu(6) and Aşıklı Höyük(8)) have no significant evidence for extra Levantine ancestry. However, all three Pottery Neolithic ones (from Barcın in NW Anatolia and Tepecik-Çiftlik(5) and Çatalhöyük(8) in Central Anatolia) have significant additional Levantine ancestry.
The two individuals from Armenia, from the sites of Aknashen (~5900BCE) and Masis Blur (~5600BCE) differ in being more Caucasus- and Anatolia/Levant-like respectively despite being buried just ~200km and a few centuries apart; thus, Neolithic people of Armenia were not homogeneous but instead exhibited variation which also encompassed two ~5700–5400BCE individuals buried in neighboring Azerbaijan(7), who are intermediate between the two from Armenia in both PCA and the 5-way model. But, in comparison to the individuals from Mesopotamia to the south, the individuals from Armenia and Azerbaijan had more Anatolian Neolithic admixture (visible in both PCA and the 5-way model). Conversely, some Neolithic Anatolian populations from Central Anatolia had Caucasus hunter-gatherer-related admixture, more than Pınarbaşı and the NW Anatolian source population where such ancestry is not evident, but less than the proportion inferred for the individual from Mardin from SE Anatolia which belonged (together with its neighbors at Nemrik9 in North Iraq) to the “Inland” group characterized by high Caucasus hunter-gatherer-related ancestry. These observations form a consistent picture of a Neolithic continuum characterized by the Anatolian/Levantine cline on one end and “Inland” influence related to the Zagros-Caucasus set of populations, with the geographically intermediate individuals from Mesopotamia, Armenia, and Azerbaijan occupying genetically intermediate positions.
To avoid “publication order bias”, that is, the tendency to update published models to accommodate new data rather than always inferring models taking all samples equally into account, we co-analyzed new data from the Neolithic together with previously published data to arrive at a model of Neolithic origins that can account for patterns of genetic variation in Neolithic West Asia as a whole (23). The Neolithic continuum emerges from this analysis too, as all Neolithic populations under study can be modeled as mixtures of three pre-Neolithic sources representing Anatolian (Pınarbaşı), Levantine (Natufian) and “Inland” sources (either Caucasus hunter-gatherer as in Fig. 3A or Ganj Dareh as in Fig. 3B); the two Inland sources are not independent, but to a first degree of approximation represent the same source of ancestry (Fig. 3C). When we attempt to model Neolithic populations using either Caucasus hunter-gatherers or Ganj Dareh as a source population and the other one as an outgroup, we obtain good model fits for most populations (further suggesting that neither one is a better source than the other), except (i) for the high Caucasus hunter-gatherer ancestry individual from Aknashen where the Caucasus hunter-gatherer model is not rejected (p=0.46) while the Ganj Dareh one is (p<0.001), (ii) the Azerbaijan and Mesopotamian Neolithic for which both models are rejected (p<0.01), and (iii) the Barcın Neolithic for which the Ganj Dareh model is narrowly not rejected at the p=0.01 level (p=0.0142) while the Caucasus hunter-gatherer one is rejected (p=0.001). These results tentatively suggest that Caucasus hunter-gatherer and Ganj Dareh Neolithic are interchangeable for the purposes of quantifying the amount of “Inland” admixture, although some populations may have a clearer connection with one or the other (e.g., the Neolithic of Armenia with the hunter-gatherers of the South Caucasus rather than Iran, and the geographically intermediate Azerbaijan and Mesopotamia with both).
The fact that regardless of the chosen sources, none of the Neolithic populations of West Asia were simple descendants of their pre-Neolithic antecedents when we had the data to test this (in which case some of them would occupy the corner positions of Fig. 3A, B) suggests that some history of admixture may have led to their appearance; the details of this process could be elucidated by examining even older populations from across West Asia. When pre-Neolithic antecedents are not available, as is the case for North Mesopotamia, it remains an open question whether the local hunter-gatherers were genetically continuous with the first farmers of the region, or, if there was a history of admixture across the Neolithic transition there as well. Importantly, this highlights that intermediate populations of the ternary plots of Fig. 3 need not have come about by admixture from the corner populations used to model them; alternatively, they could be drawn towards the middle by unsampled pre-Neolithic populations of West Asia, e.g., hunter-gatherers of the Tigris and Euphrates regions predating the Pre-Pottery Neolithic farmers studied here.
When we attempt to model Neolithic populations as mixtures of each other, we observed that at least in Anatolia (Fig. 3D) where most of the data is from and from which both Pre-Pottery and Pottery Neolithic populations have been published, an interesting distinction is clear. Pre-Pottery Neolithic populations from Central Anatolia can be modeled as mixtures of the local Pınarbaşı Epipaleolithic with variable (~30–70%) Mesopotamian admixture, suggesting that Pre-Pottery cultures of Anatolia may have been formed with the contribution of both local hunter-gatherers and migrants from the east where agriculture first appeared. Conversely, we cannot model the Pottery Neolithic Anatolians with just these two sources but only with an extra ~6–23% Levantine Neolithic admixture. The source of this admixture is unclear; it need not have come from the southern Levant (Jordan) from which the Levantine Neolithic individuals were sampled, and may instead represent a geographically closer source for which there is no available genome-wide data, for example from Syria in which early Pottery Neolithic cultures such as the Halafian flourished, and for which the available PCR-based mitochondrial DNA cannot distinguish alternative scenarios(24). We caution that while our results point to migration from, and admixture with, Mesopotamian and Levantine populations, when we use the term “migration” we are not claiming that we have detected a “migratory movement,” that is, a planned translocation of a large number of people over a long distance within the space of years (for discussion of nuances in the use of the term migration see(25)).
Migration in the sense we use it may either be intentional or not; it may involve few or many individuals; and it may either be rapid or continue across many generations. Some such migration and admixture must have taken place on the basis of the genetic data, but its causes, routes, and fine-grained temporality remain to be clarified. We caution that the Levantine influence detected in Anatolian Pottery Neolithic populations need not have been the result of unidirectional migration into Anatolia but may also have come about if Anatolia and the Levant became part of a mating network spanning both regions. Data from Pottery Neolithic cultures of the Levant are needed to test this hypothesis and to determine whether there was movement of mating partners in both directions.
Levantine ancestry may have flourished during the Neolithic, and yet its later trajectory in the Levant itself (including individuals from Jordan, Israel, Syria, and Lebanon) exhibits a decrease of ~8% per millennium from the Pre-Pottery Neolithic down to the Medieval period, largely replaced by Caucasus- and Anatolian-related ancestry from the north and west (Fig. 4). This persistent and sustained trend following the formation of the Neolithic West Asian populations studied here reminds us that large-scale admixture continued in ensuing millennia. Despite the major decline in the contribution of Levantine Neolithic farmers to peoples in the region where they originated, this key ancestry source made an important contribution to peoples of later periods continuing until the present, weaving, through migrations and mixtures within and beyond the Southern Arc (12, 26), the tapestry of ancestry of all those that followed them.
Fig. 4: The dilution of Neolithic ancestry in the Levant.
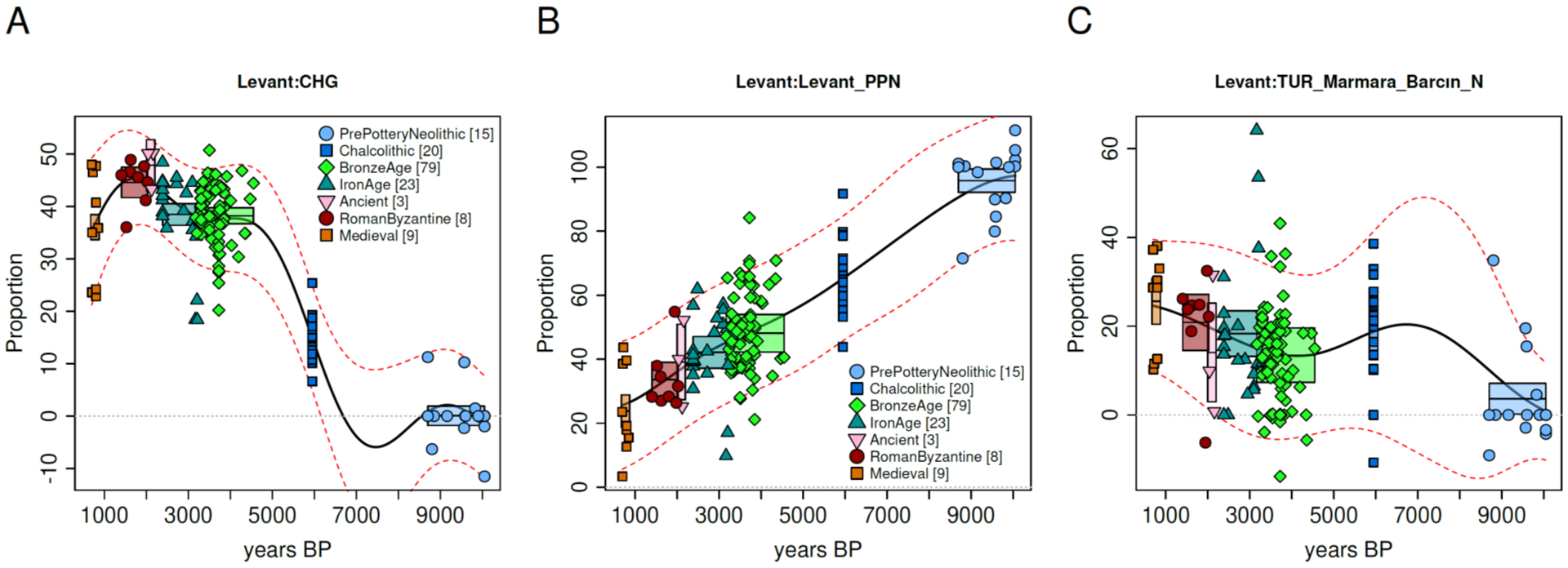
The trajectory of West Asian components of ancestry in the Levant. (A) Caucasus hunter-gatherer ancestry increased over time, first, by the Chalcolithic period, and furthermore by the Bronze Age, while the local Levantine ancestry (B) was diluted during the last 10,000 years; (C) Anatolian ancestry, like Caucasus hunter-gatherer ancestry, also increased by the Chalcolithic period(28), undergoing fluctuations thereafter.
Supplementary Material
Ethics Statement and Acknowledgments:
This study was carried following the principles for ethical DNA research on human remains laid out in (27). We are grateful to the authorities and sample stewards including museums, museum curators, and archaeologists, for providing written permission to sample each human remain. We acknowledge the ancient individuals whose genetic data we analyzed and whose permission we could not directly ask. We aimed to write a manuscript that was respectful of the ancient individuals, treating samples from them as derived from real people whose memories must be respected. We sought to reflect the perspectives of people from the diverse geographic regions and cultural contexts from which the sampled individuals came by having each sample be represented by at least one co-author who was a sample steward and was part of a network engaged with local communities. We thank J. Bennett, V. Narasimhan, H. Ringbauer; J. Sedig, A. Shaus, L. Vokotopoulos, M. Wiener, and several anonymous reviewers for critical comments.
Funding:
The newly reported dataset is described in detail in an accompanying manuscript where we also acknowledge the funders who supported dataset generation (12). Analysis of data was supported by the National Institutes of Health (GM100233 and HG012287), the John Templeton Foundation (grant 61220), by a private gift from Jean-Francois Clin, by the Allen Discovery Center program, a Paul G. Allen Frontiers Group advised program of the Paul G. Allen Family Foundation, and by the Howard Hughes Medical Institute (DR).
Footnotes
Competing interests: The authors declare that they have no competing interests.
Supplementary Materials:
Data and Materials availability:
Genotype data for individuals included in this study can be obtained from the Harvard Dataverse repository through the following link (doi to be added upon publication). BAM files of aligned reads can be obtained from the European Nucleotide Archive (Accession number PRJEB54831).
References and Notes
- 1.Lazaridis I et al. , Genomic insights into the origin of farming in the ancient Near East. Nature 536, 419–424 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mathieson I et al. , Genome-wide patterns of selection in 230 ancient Eurasians. Nature 528, 499–503 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Broushaki F et al. , Early Neolithic genomes from the eastern Fertile Crescent. Science 353, 499 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hofmanová Z et al. , Early farmers from across Europe directly descended from Neolithic Aegeans. Proceedings of the National Academy of Sciences 113, 6886 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kilinc GM et al. , The Demographic Development of the First Farmers in Anatolia. Curr Biol 26, 2659–2666 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feldman M et al. , Late Pleistocene human genome suggests a local origin for the first farmers of central Anatolia. Nature Communications 10, 1218 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Skourtanioti E et al. , Genomic History of Neolithic to Bronze Age Anatolia, Northern Levant, and Southern Caucasus. Cell 181, 1158–1175.e1128 (2020). [DOI] [PubMed] [Google Scholar]
- 8.Yaka R et al. , Variable kinship patterns in Neolithic Anatolia revealed by ancient genomes. Current Biology, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gallego-Llorente M et al. , The genetics of an early Neolithic pastoralist from the Zagros, Iran. Scientific Reports 6, 31326 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jones ER et al. , Upper Palaeolithic genomes reveal deep roots of modern Eurasians. Nature Communications 6, 8912 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Narasimhan Vagheesh M et al. , The formation of human populations in South and Central Asia. Science 365, eaat7487 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lazaridis I, Alpaslan-Roodenberg S et al. , The genetic history of the Southern Arc: a bridge between West Asia and Europe (in submission), (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Asouti E, Beyond the Pre-Pottery Neolithic B interaction sphere. Journal of World Prehistory 20, 87–126 (2006). [Google Scholar]
- 14.Cauvin J, Naissance des divinités, naissance de l’agriculture: la révolution des symboles au néolithique. Empreintes de l’homme (CNRS Éditions, Paris, 1997). [Google Scholar]
- 15.Peltenburg E, The colonisation and settlement of Cyprus. Investigations at Kissonerga-Mylouthkia, 1976–1996, (2003). [Google Scholar]
- 16.Patterson N, Price AL, Reich D, Population Structure and Eigenanalysis. PLOS Genetics 2, e190 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lazaridis I et al. , Ancient human genomes suggest three ancestral populations for present-day Europeans. Nature 513, 409–413 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haak W et al. , Massive migration from the steppe was a source for Indo-European languages in Europe. Nature 522, 207–211 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mathieson I et al. , The genomic history of southeastern Europe. Nature 555, 197–203 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Simmons Alan H, Mandel Rolfe D, How Old Is the Human Presence on Cyprus? Science 317, 1679–1679 (2007). [DOI] [PubMed] [Google Scholar]
- 21.Stordeur D, in Bulletin de Correspondance Hellenique. (2003), pp. 353–371. [Google Scholar]
- 22.Peltenburg E, The Colonisation and Settlement of Cyprus: Investigations at Kissonerga-Mylouthkia, 1976–1996. Peltenburg E, Ed., LAP; (2003), vol. III. [Google Scholar]
- 23.Detailed information is provided in the supplementary materials.
- 24.Fernández E et al. , Ancient DNA Analysis of 8000 B.C. Near Eastern Farmers Supports an Early Neolithic Pioneer Maritime Colonization of Mainland Europe through Cyprus and the Aegean Islands. PLOS Genetics 10, e1004401 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patterson N et al. , Large-scale migration into Britain during the Middle to Late Bronze Age. Nature 601, 588–594 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lazaridis I, Alpaslan-Roodenberg S et al. , A genetic probe into the ancient and medieval history of Southern Europe and West Asia. (in submission), (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alpaslan-Roodenberg S et al. , Ethics of DNA research on human remains: five globally applicable guidelines. Nature 599, 41–46 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harney É et al. , Ancient DNA from Chalcolithic Israel reveals the role of population mixture in cultural transformation. Nature Communications 9, 3336 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haak W et al. , Massive migration from the steppe was a source for Indo-European languages in Europe. Nature 522, 207–211 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mallick S et al. , The Simons Genome Diversity Project: 300 genomes from 142 diverse populations. Nature 538, 201–206 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jones ER et al. , Upper Palaeolithic genomes reveal deep roots of modern Eurasians. Nat Commun 6, 8912 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van de Loosdrecht M et al. , Pleistocene North African genomes link Near Eastern and sub-Saharan African human populations. Science, (2018). [DOI] [PubMed] [Google Scholar]
- 33.Fu Q et al. , The genetic history of Ice Age Europe. Nature 534, 200–205 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Olalde I et al. , Derived immune and ancestral pigmentation alleles in a 7,000-year-old Mesolithic European. Nature 507, 225–228 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van de Loosdrecht M et al. , Pleistocene North African genomes link Near Eastern and sub-Saharan African human populations. Science 360, 548 (2018). [DOI] [PubMed] [Google Scholar]
- 36.Raghavan M et al. , Upper Palaeolithic Siberian genome reveals dual ancestry of Native Americans. Nature 505, 87–91 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Genotype data for individuals included in this study can be obtained from the Harvard Dataverse repository through the following link (doi to be added upon publication). BAM files of aligned reads can be obtained from the European Nucleotide Archive (Accession number PRJEB54831).


