Abstract
Background:
This study explored the longitudinal relationship of lipoprotein(a) and hypertension to cardiovascular outcomes in a large multiethnic cohort free of baseline cardiovascular disease.
Methods:
Individuals from the Multi-Ethnic Study of Atherosclerosis (MESA) (n=6,674) were grouped as follows: Group 1: lipoprotein(a) <50 mg/dL and no hypertension; Group 2: lipoprotein(a) ≥50 mg/dL and no hypertension; Group 3: lipoprotein(a) <50 mg/dL and hypertension; and Group 4: lipoprotein(a) ≥50 mg/dL and hypertension. Kaplan-Meier curves and multivariable Cox proportional hazard models were used to assess the relationship of lipoprotein(a) and hypertension with time to cardiovascular disease events.
Results:
Mean follow-up time was 13.9 (5.0) years and 809 participants experienced a cardiovascular disease event. A statistically significant interaction was found between Log[lipoprotein(a)] and hypertension status (p=0.091). Compared to the reference group (lipoprotein(a) <50 mg/dL and no hypertension), those with lipoprotein(a) ≥50 mg/dL and no hypertension had no increased risk for cardiovascular disease events (HR: 1.09; 95% CI: 0.79, 1.50). However, those with lipoprotein(a) <50 mg/dL and hypertension or lipoprotein(a) ≥50 mg/dL and hypertension demonstrated a statistically significant increase in risk compared to the reference group (HR: 1.66; 95% CI: 1.39, 1.98) and (HR: 2.07; 95% CI: 1.63, 2.62), respectively. Among those with hypertension, lipoprotein(a) was associated with a significant increase in cardiovascular disease risk (HR: 1.24; 95% CI: 1.01, 1.53).
Conclusions:
Although the major contribution to cardiovascular risk was hypertension, elevated lipoprotein(a) significantly modified the association of hypertension with cardiovascular disease. More research is needed to understand mechanistic links among lipoprotein(a), hypertension, and cardiovascular disease.
Keywords: Hypertension, Lipoprotein(a), Apolipoprotein B, Atherosclerotic cardiovascular disease, Cardiovascular risk, Primary Prevention
Graphical Abstract
Introduction
Over the last century, a preponderance of evidence has supported a causal link between hypertension and atherosclerotic cardiovascular disease (ASCVD).1,2 In fact, the population attributable risk for ASCVD due to hypertension is estimated at 25%.1,3 This high population attributable risk highlights the importance of blood pressure control, as a 20 mm Hg increase in systolic blood pressure (SBP) or a 10 mm Hg increase in diastolic blood pressure (DBP) is associated with twice the CVD risk.1,2 Additionally, hypertension leads to more CVD deaths in the United States compared to other risk factors, including smoking, physical inactivity, diabetes mellitus, or dyslipidemia.2 Of these modifiable risk factors, dyslipidemia is of particular interest because the coexistence of hypertension and dyslipidemia approximately triples CVD risk.4 In particular, a paucity of evidence exists regarding elevated lipoprotein(a) [Lp(a)] and its significance in individuals with hypertension.5
Lp(a) is an apolipoprotein B (apoB)-containing lipoprotein independently and causally associated with ASCVD.6 The molecular structure of Lp(a) is similar to that of low-density lipoprotein (LDL), with an additional apolipoprotein(a) moiety bound to apoB found on the LDL-like particle.5 Plasma levels of Lp(a) are almost exclusively genetically determined and median values vary across race and ethnicity, highest in African Americans.6–8 There is a linear increase in ASCVD risk with increasing Lp(a) levels, which becomes clinically significant above 50 mg/dL.6,9 However, limited data exist regarding the significance of Lp(a) in individuals with hypertension.10 Some evidence suggests that elevated Lp(a) is independently associated with the severity of target organ damage in patients with hypertension.11 Moreover, a recent study in patients with established coronary artery disease found that ASCVD risk associated with Lp(a) was present only in individuals with hypertension and elevated Lp(a).10 These data suggest a shared mechanistic link or effect modification between Lp(a) and hypertension.
Given these findings, individuals with hypertension and elevated Lp(a) may have a considerable increase in ASCVD risk. However, the longitudinal relationship of Lp(a) and hypertension to cardiovascular outcomes in primary prevention has not been described. To investigate this issue, we compared incident ASCVD events stratified by hypertension and Lp(a) in a large multi-ethnic cohort free from CVD at baseline.
Methods
Data availability
All data used in this study were obtained from the Multi-Ethnic Study of Atherosclerosis (MESA) (https://www.mesa-nhlbi.org) in accordance with their published data access procedures. Dr. Rikhi had full access to all the data in the study and takes responsibility for its integrity and data analysis.
Study population
The methods and study design for the MESA have been previously described.12 In short, MESA is an ongoing community-based cohort study aimed at better understanding subclinical ASCVD.12 The study enrolled a multi-ethnic cohort of 6,814 men and women, 45-84 years of age, all free of clinical ASCVD at baseline.12 Participants were recruited from July 2000 to 2002 from 6 communities in the United States.12 The present study included 6,674 participants with measured baseline Lp(a), blood pressure, and documented CVD event data over follow-up. Institutional Review Board approval was obtained at all participating institutions, and written informed consent was obtained from all participants.
Demographic and baseline characteristics
All demographic and covariate data were obtained from the baseline MESA exam in 2000-2002.12 Demographic data (age, sex, race/ethnicity) were obtained through standardized questionnaires.12 Body mass index (BMI) was calculated by dividing weight (kg) by height squared (m2) and treated continuously. Cigarette use was categorized as never, former, and current.12 For this study, current smoking was defined as any cigarette use in the last 30 days.12 Blood pressure was measured in a seated position using an automated oscillometric sphygmomanometer (Dinamap model Pro 100, Critikon, Tampa, Florida).12 Participants had three different measurements, at two-minute intervals, and the mean of the second and third readings were used to determine hypertension status.12 Hypertension was defined according to JNC VI (1997) guidelines as a systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥ 90 mmHg, or blood pressure medication use. Diabetes mellitus was defined as fasting plasma glucose ≥126 mg/dL or medication use for diabetes mellitus.
Laboratory measurements
Blood samples obtained from all participants were stored in EDTA-anticoagulant tubes at −70° C (University of Vermont and University of Minnesota).12 Total cholesterol, high-density lipoprotein (HDL)-cholesterol (HDL-C), and triglycerides were measured from fasting samples. The Friedewald equation was used to calculate LDL-C when triglycerides were < 400 mg/dL.13 Individuals with triglycerides ≥ 400 mg/dL were excluded in the fully adjusted model when LDL-C was added (n=85). A latex-enhanced turbidimetric immunoassay (Denka Seiken, Tokyo, Japan) by Health Diagnostics Laboratory (Richmond, Virginia) was used to measure plasma Lp(a) in mg/dL.7
Clinical endpoints
Participants were followed for an average of 13.9 (5.0) years from 2000 to 2018. Time to CVD events, defined as centrally adjudicated myocardial infarction, resuscitated cardiac arrest, stroke (not including transient ischemic attack), stroke death, and coronary heart disease death were documented. Telephone interviews were completed every 9 to 12 months to gather interim data on new diagnoses, procedures, hospitalizations, and deaths. Additionally, medical information was obtained throughout the calendar year from clinic visits, call-ins from participants, and obituaries. Self-reported diagnoses were confirmed with medical records.
Statistical analysis
Our study population was grouped as follows:
Group 1: Lp(a) < 50 mg/dL and no hypertension
Group 2: Lp(a) ≥ 50mg/dL and no hypertension
Group 3: Lp(a) < 50mg/dL and hypertension
Group 4: Lp(a) ≥ 50mg/dL and hypertension
We compared baseline characteristics among these four groups using chi-square tests for proportions, analysis of variance for normally distributed continuous variables, and Kruskal-Wallis tests for non-normally distributed continuous variables. Lp(a) was log-transformed when treated as continuous to normalize its skewed distribution. A Student’s t-test was used to compare Log[Lp(a)] values between those with and without hypertension. Lp(a) was also treated as a categorical variable using a threshold of 50 mg/dL, which is the threshold recommended by the 2018 American College of Cardiology (ACC) / American Heart Association (AHA) cholesterol guideline for consideration as an ASCVD risk-enhancing factor.14 Formal interaction testing between Log[Lp(a)] and hypertension status was performed.
Differences in time-to-CVD between individuals with and without hypertension, elevated and normal Lp(a), and among the four groups were assessed with a Kaplan-Meier plot and log-rank test. Incident rates per 1,000 person years were calculated for each group. Multivariable Cox proportional hazards models were used to assess differences in time-to-CVD among the four groups, with Group 1 as the reference group (Lp(a) < 50 mg/dL and no hypertension). Three models were constructed: model 1 was unadjusted; model 2 adjusted for age, sex, race/ethnicity; and model 3 adjusted for model 2 + total cholesterol, HDL-C, LDL-C, triglycerides, diabetes, cigarette use, baseline statin use, and BMI. Stratified analyses were performed to compare females and males, as well as White, Chinese American, and Hispanic participants and African American participants. Hypothesis testing was conducted with a two-sided [alpha] of 0.05 for main effects and 0.10 for interactions. SAS version 9.4 and GraphPad Prism version 9.3.1 were used to perform all statistical analyses.
Results
Baseline characteristics of study population
Baseline characteristics of our study population are shown in Table 1. The sample was 52.8% female (n=3,523), 38.6% White (n=2,575), 11.9% Chinese American (n=791), 27.5% African American (n=1,832), and 22.1% Hispanic (n=1,476). The mean (SD) age of the cohort was 62.1 (10.2) years. Those with hypertension were significantly older and had higher BMI. Diabetes, former cigarette use, and statin use were more prevalent in the groups with hypertension. Mean total cholesterol, HDL-C, and LDL-C were higher in the groups with elevated Lp(a). Mean triglycerides were lower in the groups with elevated Lp(a). Participants with hypertension had higher average Log[Lp(a)] levels than those without hypertension (2.9 U versus 2.8 U, respectively) (p<0.001).
Table 1.
Baseline Characteristics of Lp(a) and Hypertension Groups
| No Hypertension | Hypertension | Total | P-value | |||
|---|---|---|---|---|---|---|
| Baseline Characteristics | Lp(a) < 50 mg/dL Group 1 (n= 2,837) |
Lp(a) ≥ 50 mg/dL Group 2 (n= 615) |
Lp(a) < 50 mg/dL Group 3 (n= 2502) |
Lp(a) ≥ 50 mg/dL Group 4 (n= 720) |
n= 6,674 |
|
| Age, yrs (SD) | 58.7 (9.8) | 58.9 (9.7) | 65.8 (9.5) | 65.4 (9.3) | 62.1 (10.2) | <0.001 |
| Female (%) | 1,426 (50.3) | 361 (58.7) | 1,307 (52.2) | 429 (59.6) | 3,523 (52.8) | <0.001 |
| Race/ethnicity (%) | <0.001 | |||||
| White | 1216 (42.9) | 236 (38.4) | 948 (37.9) | 175 (24.3) | 2,575 (38.6) | |
| Chinese American | 436 (15.4) | 38 (6.2) | 281 (11.2) | 36 (5.0) | 791 (11.9) | |
| African American | 479 (16.9) | 221 (35.9) | 719 (28.7) | 413 (57.4) | 1,832 (27.5) | |
| Hispanic | 706 (24.9) | 120 (19.5) | 554 (22.1) | 96 (13.3) | 1,476 (22.1) | |
| Lipids | ||||||
| Total Cholesterol (SD) | 194.0 (35.6) | 205.7 (35.4) | 189.2 (34.2) | 201.0 (37.5) | 194.0 (35.7) | <0.001 |
| HDL-C (SD) | 50.5 (14.7) | 53.9 (15.3) | 50.0 (14.5) | 53.4 (15.3) | 51.0 (14.8) | <0.001 |
| LDL-C, mg/dL (SD) | 117.6 (31.1) | 128.6 (31.5) | 111.7 (30.2) | 124.2 (32.5) | 117.1 (31.4) | <0.001 |
| Lp(a), mg/dL (IQR) | 12.2 (5.9-22.9) | 76.5 (61.3-98.3) | 12.6 (5.9-25.0) | 77.4 (61.7-101.5) | 17.3 (7.5-40.5) | <0.001 |
| LogLp(a), U (SD) | 2.4 (0.9) | 4.4 (0.3) | 2.4 (0.9) | 4.4 (0.4) | 2.8 (1.1) | <0.001 |
| Triglycerides, mg/dL (IQR) | 109 (75.5-159) | 100 (73-140) | 119 (85-172) | 99 (71-146) | 111 (78-161) | <0.001 |
| Diabetes (%) | 186 (6.6) | 42 (6.8) | 459 (18.4) | 147 (20.4) | 834 (12.5) | <0.001 |
| Cigarette Use (%) | Former: 980 (34.6) Current: 426 (15.0) |
Former: 203 (33.2) Current: 101 (16.5) |
Former: 994 (39.9) Current: 248 (10.0) |
Former: 266 (37.1) Current: 92 (12.8) |
Former: 2,443 (36.7) Current: 867 (13.0) |
<0.001 |
| Statin Therapy (%) | 226 (8.0) | 84 (13.7) | 502 (20.1) | 183 (25.5) | 995 (15.0) | <0.001 |
| BMI, kg/m2 (SD) | 27.3 (5.1) | 27.6 (5.1) | 29.2 (5.6) | 29.9 (5.9) | 28.3 (5.5) | <0.001 |
Continuous variables reported as mean (standard deviation) if normal distribution and median (interquartile range) if skewed distribution. Categorical variables displayed as total count (%).
Lp(a) = lipoprotein(a); yrs = years; SD = standard deviation, IQR = interquartile rang, BMI = body mass index.
Association of Lp(a) and Hypertension with CVD
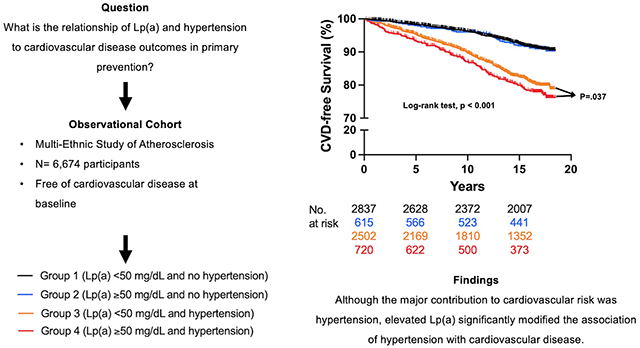
Kaplan-Meier curves displaying time to CVD events for Lp(a) ≥ 50 mg/dL and Lp(a) < 50 mg/dL groups (log-rank p-value <0.001) and hypertension and no hypertension groups (log-rank p-value =0.026) over the mean 13.9 years are displayed in Figure 1.
Figure 1.
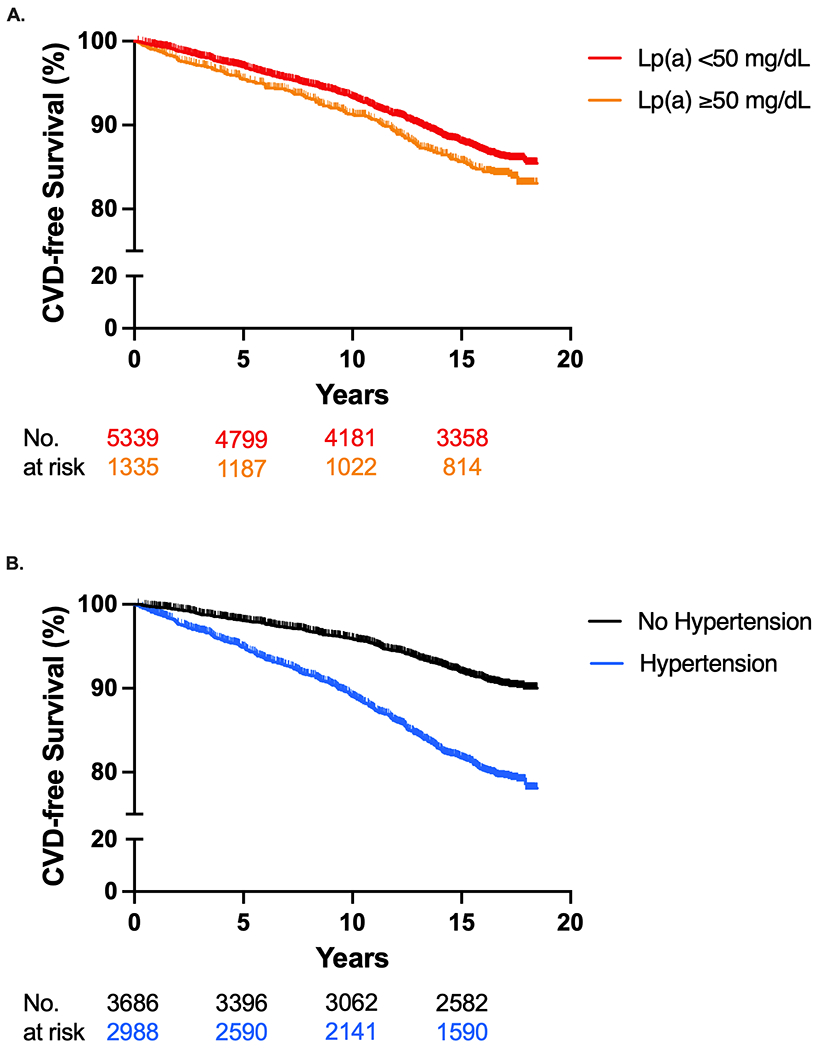
Incident Cardiovascular Disease Events for Lp(a) and Hypertension
Kaplan-Meier curves of incident CVD events for (A) Lp(a) ≥ 50 mg/dL and Lp(a) < 50 mg/dL groups (log-rank p-value <0.001) and (B) hypertension and no hypertension groups (log-rank p-value =0.026). Lp(a) = lipoprotein(a); CVD = cardiovascular disease.
CVD incident rate per 1,000 person-years for Group 1 was 5.2, Group 2 was 5.4, Group 3 was 12.5, and Group 4 was 14.7. Kaplan-Meier curves displaying time to CVD events for the four groups over the mean 13.9 years are displayed in Figure 2. Time to CVD events differed significantly among the four groups (log-rank test, p <0.001). A statistically significant interaction was found between Log[Lp(a)] and hypertension status (p = 0.091). Hazard ratios from multivariable Cox proportional hazards models are displayed in Table 2. In the fully adjusted model, compared to the reference group (Lp(a) < 50 mg/dL and no hypertension), those with Lp(a) ≥ 50 mg/dL and no hypertension (Group 2) had no increase in risk for CVD events (HR: 1.09; 95% CI: 0.79, 1.50). However, those with Lp(a) < 50 mg/dL and hypertension (Group 3) or Lp(a) ≥ 50 mg/dL and hypertension (Group 4) demonstrated a statistically significant increase in CVD risk compared to the reference group (HR: 1.66; 95% CI: 1.39, 1.98) and (HR: 2.07; 95% CI: 1.63, 2.62), respectively. Among those with hypertension (Groups 3 and 4), Lp(a) was associated with a significant increase in CVD risk (HR: 1.24; 95% CI: 1.01, 1.53).
Figure 2.
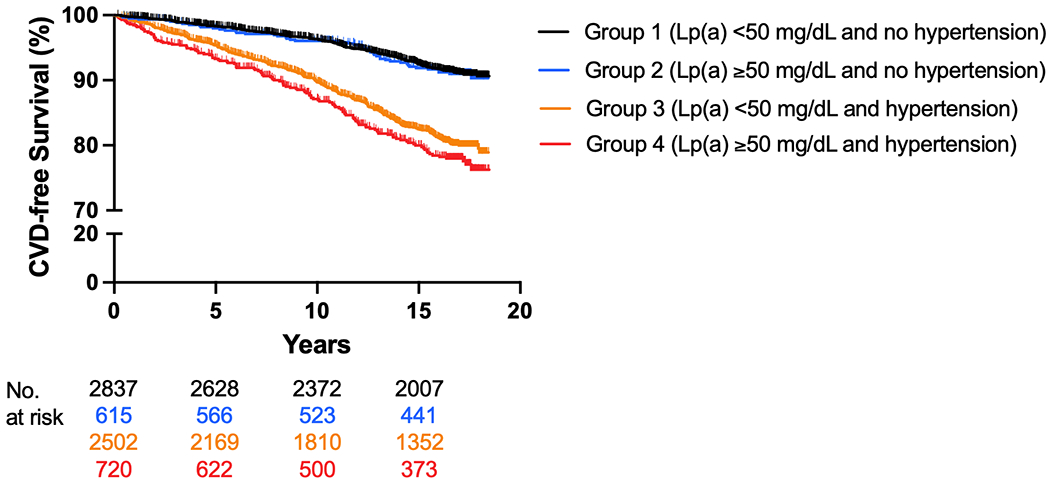
Incident Cardiovascular Disease Events According to Lp(a) and Hypertension Groups
Kaplan-Meier curves of incident CVD events (log-rank p-value <0.001). The group with Lp(a) < 50 mg/dL without hypertension served as the reference. Lp(a) = lipoprotein(a); CVD = cardiovascular disease.
Table 2.
Risk for Cardiovascular Disease Events According to Lp(a) and Hypertension Groups
| Risk Groups | Events/Total (%) | Incidence Rate (per 1,000 p-yrs) | HR (95% CI), P value (Model 1) | HR (95% CI), P value (Model 2) | HR (95% CI), P value (Model 3) |
|---|---|---|---|---|---|
| Group 1 (Lp(a) < 50 mg/dL and No Hypertension) | 219/2,837 (7.7) | 5.2 | 1.00 (reference), NA | 1.00 (reference), NA | 1.00 (reference), NA |
| Group 2 (Lp(a) ≥ 50 mg/dL and No Hypertension) | 49/615 (8.0) | 5.4 | 1.03 (0.76, 1.41), 0.840 | 1.05 (0.77, 1.44), 0.743 | 1.09 (0.79, 1.50), 0.793 |
| Group 3 (Lp(a) < 50 mg/dL and Hypertension) | 406/2,502 (16.2) | 12.5 | 2.43 (2.06, 2.87), <0.001 | 1.74 (1.47, 2.06), <0.001 | 1.66 (1.39, 1.98), <0.001 |
| Group 4 (Lp(a) ≥ 50 mg/dL and Hypertension) | 135/720 (18.8) | 14.7 | 2.89 (2.33, 3.58), <0.001 | 2.22 (1.76, 2.78), <0.001 | 2.07 (1.63, 2.62), <0.001 |
Multivariable Cox proportional hazards model 1 unadjusted; model 2 adjusted for age, sex, race/ethnicity; and model 3 adjusted for model 2 + total cholesterol, HDL-C, LDL-C, triglycerides, diabetes, cigarette use, baseline statin use, and BMI.
P-yrs= person-years; HR = hazard ratio; Lp(a) = lipoprotein(a).
Association of Lp(a) and Hypertension with CVD by Sex
In women, CVD incident rate per 1,000 person-years for Group 1 was 3.4, Group 2 was 4.6, Group 3 was 10.3, and Group 4 was 11.8. In males, CVD incident rate per 1,000 person-years for Group 1 was 7.1, Group 2 was 6.6, Group 3 was 15.0, and Group 4 was 19.5. Kaplan-Meier curves displaying time to CVD events for the four groups over the mean 13.9 years are displayed in Figure 3A. For both females and males, differences in time to CVD events were statistically significant (log-rank test, p <0.001), with a shorter time to CVD events in the groups with hypertension. Interaction testing between Log[Lp(a)] and hypertension was not significant in females (p = 0.860), but was significant in males (p = 0.052). Hazard ratios from the multivariable Cox proportional hazards model for both females and males are displayed in Table 3. In a fully adjusted model, compared to the reference group (Lp(a) <50 mg/dL and no hypertension), those with Lp(a) ≥50 mg/dL and no hypertension (Group 2) had no increase in risk for CVD events in females (HR: 1.20; 95% CI: 0.75, 1.92) or in males (HR: 1.02; 95% CI: 0.66, 1.58), respectively. However, those with Lp(a) <50 mg/dL and hypertension (Group 3) or Lp(a) ≥50 mg/dL and hypertension (Group 4) demonstrated a statistically significant increase in CVD risk compared to the reference group (HR: 1.90; 95% CI: 1.42, 2.54) and (HR: 2.29; 95% CI: 1.59, 3.31), respectively in females, and (HR: 1.52; 95% CI: 1.21, 1.91) and (HR: 1.94; 95% CI: 1.42, 2.66), respectively in males. Lp(a) was not associated with an increase in CVD risk in those with hypertension (HR: 1.20; 95% CI: 0.89, 1.63) and (HR: 1.28; 95% CI: 0.96, 1.69), in females and males respectively.
Figure 3.
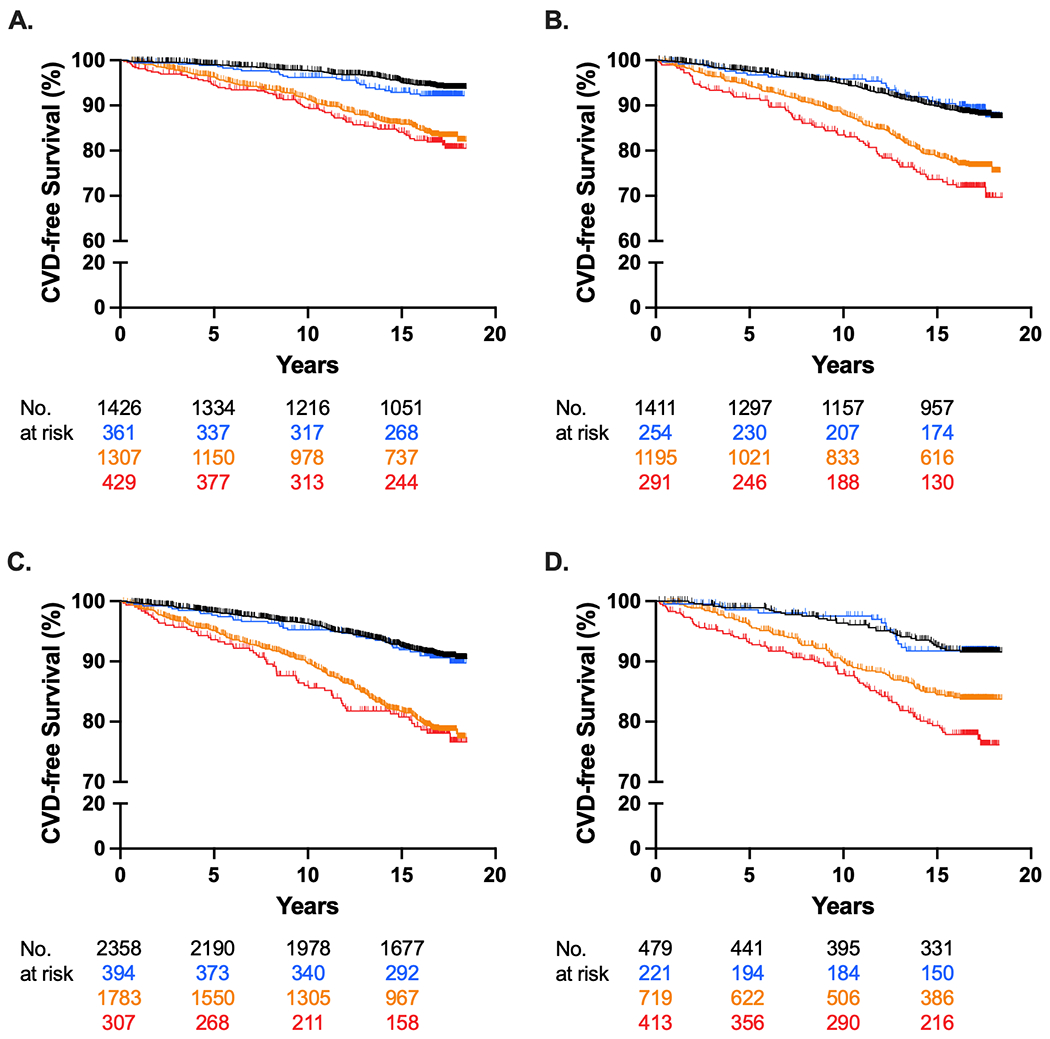
Incident Cardiovascular Disease Events According to Lp(a) and Hypertension by Sex and Race/Ethnicity
Kaplan-Meier curves of incident CVD events for (A) females (log-rank p-value <0.001) and (B) males (log-rank p-value <0.001) and (C) White, Chinese American, and Hispanic participants (log-rank p-value <0.001) and (D) African American participants (log-rank p-value <0.001). The group with Lp(a) < 50 mg/dL without hypertension served as the reference. Lp(a) = lipoprotein(a); CVD = cardiovascular disease.
Table 3.
Risk for Cardiovascular Disease Events According to Lp(a) and Hypertension Groups by Sex and Race/Ethnicity
| Female | Male | |||||
|---|---|---|---|---|---|---|
| Events/Total (%) | Incidence Rate (per 1000 p-years) | HR (95% CI), P value | Events/Total (%) | Incidence Rate (per 1000 p-years) | HR (95% CI), P value | |
| Group 1 (Lp(a) < 50 mg/dL and No Hypertension) | 74/1,426 (5.2) | 3.4 | Reference | 145/1,411 (10.3) | 7.1 | Reference |
| Group 2 (Lp(a) ≥ 50 mg/dL and No Hypertension) | 25/361 (6.9) | 4.6 | 1.20 (0.75, 1.92), 0.439 | 24/254 (9.4) | 6.6 | 1.02 (0.66, 1.58), 0.938 |
| Group 3 (Lp(a) < 50 mg/dL and Hypertension) | 179/1307 (13.7) | 10.3 | 1.90 (1.42, 2.54), <0.001 | 227/1195 (19.0) | 15 | 1.52 (1.21, 1.91), <0.001 |
| Group 4 (Lp(a) ≥ 50 mg/dL and Hypertension) | 67/429 (15.6) | 11.8 | 2.29 (1.59, 3.31), <0.001 | 68/291 (23.4) | 19.5 | 1.94 (1.42, 2.66), <0.001 |
| White, Chinese American, Hispanic | African American | |||||
| Events/Total (%) | Incidence Rate (per 1000 p-years) | HR (95% CI), P value | Events/Total (%) | Incidence Rate (per 1000 p-years) | HR (95% CI), P value | |
| Group 1 (Lp(a) < 50 mg/dL and No Hypertension) | 185/2358 (7.8) | 5.3 | Reference | 34/479 (7.1) | 4.9 | Reference |
| Group 2 (Lp(a) ≥ 50 mg/dL and No Hypertension) | 34/394 (8.6) | 5.7 | 1.18 (0.81, 1.72), 0.375 | 15/221 (6.8) | 4.8 | 0.93 (0.50, 1.72), 0.822 |
| Group 3 (Lp(a) < 50 mg/dL and Hypertension) | 311/1783 (17.4) | 13.3 | 1.71 (1.40, 2.08), <0.001 | 95/719 (13.2) | 10.3 | 1.40 (0.92, 2.12), 0.120 |
| Group 4 (Lp(a) ≥ 50 mg/dL and Hypertension) | 57/307 (18.6) | 14.6 | 1.95 (1.44, 2.65), <0.001 | 78/413 (18.9) | 14.8 | 2.07 (1.34, 3.21), 0.001 |
Multivariable Cox proportional hazards adjusted for age, race/ethnicity, total cholesterol, HDL-C, LDL-C, triglycerides, diabetes, cigarette use, baseline statin use, and BMI.
P-yrs= person-years; HR = hazard ratio; Lp(a) = lipoprotein(a).
Association of Lp(a) and Hypertension with CVD by White, Chinese American, and Hispanic participants and African American participants
In White, Chinese American, and Hispanic participants, CVD incident rate per 1,000 person-years for Group 1 was 5.3, Group 2 was 5.7, Group 3 was 13.3, and Group 4 was 14.6. In African Americans, CVD incident rate per 1,000 person-years for Group 1 was 4.9, Group 2 was 4.8, Group 3 was 10.3, and Group 4 was 14.8. Kaplan-Meier curves displaying time to CVD events for the four groups over the mean 13.9 years are displayed in Figure 3B. For both White, Chinese American, and Hispanic participants and African American participants, differences in the time to CVD events were statistically significant (log-rank test, p < 0.001). Interaction testing between Log[Lp(a)] and hypertension was not significant in White, Chinese American, and Hispanic participants (p = 0.260), but was significant in African American participants (p = 0.079). Hazard ratios from the multivariable Cox proportional hazards model for both White, Chinese American, and Hispanic participants and African American participants are displayed in Table 3. In a fully adjusted model, compared to the reference group (Lp(a) < 50 mg/dL and no hypertension), those with Lp(a) ≥ 50 mg/dL and no hypertension (Group 2) had no increase in risk for CVD events (HR: 1.18; 95% CI: 0.81, 1.72) and (HR: 0.93; 95% CI: 0.50, 1.72) in both White, Chinese American, and Hispanic participants and African American participants, respectively. In those with Lp(a) < 50 mg/dL and hypertension (Group 3) there was a significant increase in CVD risk in White, Chinese American, and Hispanic participants (HR: 1.71; 95% CI: 1.40, 2.08). There was an increase in CVD risk in African Americans from Group 3; however, the 95% CI overlapped with the null (HR: 1.40; 95% CI: 0.92, 2.12). Both White, Chinese American, and Hispanic participants and African American participants with Lp(a) ≥ 50 mg/dL and hypertension (Group 4) had a significant increase in CVD risk compared to the reference group (HR: 1.95; 95% CI: 1.44, 2.65) and (HR: 2.07; 95% CI: 1.34, 3.21), respectively. Among White, Chinese American, and Hispanic participants with hypertension, Lp(a) was not associated with an increase in CVD risk (HR: 1.14; 95% CI: 0.86, 1.52). However, among African Americans with hypertension, Lp(a) was associated with an increase in CVD risk (HR: 1.48; 95% CI: 1.09, 2.02).
Discussion
Epidemiologic and genetic studies suggest an independent and causal association between Lp(a) and CVD.6 However, controversy exists regarding the relationship between Lp(a) and hypertension, as well the association of Lp(a) with CVD risk in hypertensive and non-hypertensive patients.5,10 In this analysis from a large, multiethnic cohort free of clinical ASCVD at baseline, we evaluated the joint association between Lp(a) and hypertension on incident ASCVD events. Our study highlights the already known strong relationship between hypertension and ASCVD, as survival probabilities were significantly lower in participants with hypertension compared to no hypertension by year 1 (Table S1). Survival probabilities among participants with Lp(a) ≥ 50 mg/dL compared to Lp(a) < 50 mg/dL appear to diverge more slowly, but are significantly lower by year 2 (Table S1). The main finding from our study of 6,674 participants followed for a mean 13.9 years was that elevated Lp(a) significantly modified the association of hypertension with incident CVD, such that individuals with hypertension and Lp(a) > 50mg/dL had a significantly higher risk for incident CVD than individuals with hypertension and normal Lp(a) levels.
As seen in Figure 2, ASCVD risk appears to be largely driven by hypertension, with clear divergence between groups with hypertension (3 and 4) and groups without hypertension (1 and 2). However, we can see that groups 1 and 2 (without hypertension) overlap each other, and there is separation in risk between groups 3 and 4 (with hypertension), indicating that Lp(a) was not associated with CVD risk in those without hypertension, but was significantly associated with CVD risk in those with hypertension. When stratified by sex, we found that Lp(a) was not associated with CVD risk regardless of hypertension status. The same result was also seen in White, Chinese American, and Hispanic participants. However, in African Americans, greater CVD risk was seen when both risk factors (Lp(a) ≥ 50 mg/dL and hypertension) were present.
CVD risk associated with both Lp(a) and hypertension has not been well described in populations free of clinical CVD at baseline. Liu et at. investigated this association in a prospective study following 8,668 individuals with stable coronary artery disease over a mean of 55 months.10 The authors stratified Lp(a) (< 10 mg/dL, ≥ 10 mg/dL and < 30 mg/dL, and ≥ 30 mg/dL) and found that elevated CVD risk was significant only in the group with Lp(a) ≥ 30 mg/dL and hypertension (HR: 1.88; 95% CI: 1.10, 3.22).10 However, the external validity of this study is limited as it included only Chinese participants.10 Additionally, this population had established coronary artery disease and thus is not applicable to primary prevention.10 In our large, multi-ethnic study of participants free of clinical CVD at baseline, we found a statistically significant interaction between blood pressure and Lp(a). Specifically, results from this analysis suggest that Lp(a) modifies the relationship between hypertension and ASCVD.
It is unclear why cardiovascular disease risk associated with Lp(a) would not be significant in those without hypertension; however, there may be other potential mechanisms at play. It is known that hypertension results in hemodynamic changes that promote endothelial dysfunction and heightened inflammation.5 Similarly, Lp(a) attenuates endothelial-dependent relaxation and the apo(a) component of Lp(a) is hydrophilic, which allows for Lp(a) to interact with the endothelial surface, resulting in endothelial dysfunction, proliferation of vascular smooth muscle cells, and cytokine production.6,15 Further, in the setting of endothelial dysfunction, Lp(a) can bind to exposed lysine via lysine binding sites and become more atherogenic.16 Thus, Lp(a) may require a specific substrate to exert its inflammatory and atherogenic potential, such as endothelial damage caused by hypertension. Of course, this is a hypothesis that needs formal testing.
Limitations
There are several limitations that should be considered when interpreting the results of our study. First, given the longitudinal nature of this study, differential loss to follow up could have resulted in selection bias. Second, there were relatively few CVD events in Group 2 (Lp(a) ≥ 50 mg/dL and no hypertension), which may have limited our ability to detect differences in the groups without hypertension, particularly in the subgroup analyses. Third, participants may have developed hypertension during follow up, resulting in misclassification bias. Lastly, given the observational nature of this study, residual confounding could occur, and causal inferences between Lp(a), hypertension and CVD cannot be made.
Perspectives
This study illustrates a significant interaction between Lp(a) and hypertension. Further research is needed to better understand the mechanistic link between Lp(a), hypertension, and CVD.
Supplementary Material
Novelty and Relevance.
What is new?
Hypertension is a known strong causal risk factor for the development of cardiovascular disease, yet little is known regarding its relationship with lipoprotein(a). In our large, multi-ethnic, primary prevention study, we found a significant interaction between lipoprotein(a) and hypertension.
What is Relevant?
Our study is the first to illustrate that lipoprotein(a) modifies the relationship between hypertension and cardiovascular disease.
Clinical/Pathophysiological Implications?
These novel findings suggest a causal link between hypertension, lipoprotein(a) and the development of cardiovascular disease in primary prevention. Further research is needed to better understand the mechanistic link between lipoprotein(a) and hypertension.
Acknowledgements:
The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Sources of Funding:
This research was supported by contracts 75N92020D00001, HHSN268201500003I, N01-HC-95159, 75N92020D00005, N01-HC-95160, 75N92020D00002, N01-HC-95161, 75N92020D00003, N01-HC-95162, 75N92020D00006, N01-HC-95163, 75N92020D00004, N01-HC-95164, 75N92020D00007, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168 and N01-HC-95169 from the National Heart, Lung, and Blood Institute, and by grants UL1-TR-000040, UL1-TR-001079, and UL1-TR-001420 from the National Center for Advancing Translational Sciences (NCATS).
Disclosures:
Dr. Rikhi and Dr. Ashburn are supported by the National Heart, Lung, And Blood Institute of the National Institutes of Health under Award Number T32HL076132. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Dr. Bhatia is supported by the National Institutes of Health, Grant 5T32HL079891, as part of the UCSD Integrated Cardiovascular Epidemiology Fellowship. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Dr. Michos reports Advisory Boards for Amarin, AstraZeneca, Bayer, Boehringer Ingelheim, Esperion, Novartis, Novo Nordisk and Pfizer.
Dr. Tsimikas is a co-inventor of and receives royalties from patents owned by University of California, San Diego, on oxidation-specific antibodies and of biomarkers related to oxidized lipoproteins; and is a co-founder and has an equity interest in Oxitope, Inc and its affiliates, Kleanthi Diagnostics, LLC, and Covicept Therapeutics, Inc.
Dr. Shapiro has participated in scientific advisory boards with the following entities: Amgen; Novartis; Novo Nordisk; and has served as a consultant for Regeneron.
All other authors have nothing to disclose.
Non-standard Abbreviations and Acronyms
- ASCVD
Atherosclerotic cardiovascular disease
- SBP
Systolic blood pressure
- DBP
Diastolic blood pressure
- Lp(a)
Lipoprotein(a)
- ApoB
Apolipoprotein B
- LDL-C
Low-density lipoprotein-cholesterol
- MESA
Multi-Ethnic Study of Atherosclerosis
- HDL-C
High-density lipoprotein-cholesterol
- ACC
American College of Cardiology
- AHA
American Heart Association
References
- 1.Fuchs FD, Whelton PK. High Blood Pressure and Cardiovascular Disease. Hypertension. 2020;75:285–292. doi: 10.1161/HYPERTENSIONAHA.119.14240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Whelton PK, Carey RM, Aronow WS, Casey DE Jr., Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71:e127–e248. doi: 10.1016/j.jacc.2017.11.006 [DOI] [PubMed] [Google Scholar]
- 3.Cheng S, Claggett B, Correia AW, Shah AM, Gupta DK, Skali H, Ni H, Rosamond WD, Heiss G, Folsom AR, et al. Temporal trends in the population attributable risk for cardiovascular disease: the Atherosclerosis Risk in Communities Study. Circulation. 2014;130:820–828. doi: 10.1161/CIRCULATIONAHA.113.008506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grinshtein YU, Ruf R, Shabalin V. Age and gender disparities in the prevalence of lipitension. European Heart Journal. 2021;42. doi: 10.1093/eurheartj/ehab724.2318 [DOI] [Google Scholar]
- 5.Ward NC, Nolde JM, Chan J, Carnagarin R, Watts GF, Schlaich MP. Lipoprotein (a) and Hypertension. Curr Hypertens Rep. 2021;23:44. doi: 10.1007/s11906-021-01161-6 [DOI] [PubMed] [Google Scholar]
- 6.Tsimikas S A Test in Context: Lipoprotein(a): Diagnosis, Prognosis, Controversies, and Emerging Therapies. J Am Coll Cardiol. 2017;69:692–711. doi: 10.1016/j.jacc.2016.11.042 [DOI] [PubMed] [Google Scholar]
- 7.Guan W, Cao J, Steffen BT, Post WS, Stein JH, Tattersall MC, Kaufman JD, McConnell JP, Hoefner DM, Warnick R, et al. Race is a key variable in assigning lipoprotein(a) cutoff values for coronary heart disease risk assessment: the Multi-Ethnic Study of Atherosclerosis. Arterioscler Thromb Vasc Biol. 2015;35:996–1001. doi: 10.1161/ATVBAHA.114.304785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu T, Yoon WS, Lee SR. Recent Updates of Lipoprotein(a) and Cardiovascular Disease. Chonnam Med J. 2021;57:36–43. doi: 10.4068/cmj.2021.57.1.36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Enas EA, Varkey B, Dharmarajan TS, Pare G, Bahl VK. Lipoprotein(a): An independent, genetic, and causal factor for cardiovascular disease and acute myocardial infarction. Indian Heart J. 2019;71:99–112. doi: 10.1016/j.ihj.2019.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu HH, Cao YX, Jin JL, Hua Q, Li YF, Guo YL, Zhu CG, Wu NQ, Dong Q, Li JJ. Lipoprotein (a), hypertension, and cardiovascular outcomes: a prospective study of patients with stable coronary artery disease. Hypertens Res. 2021;44:1158–1167. doi: 10.1038/s41440-021-00668-4 [DOI] [PubMed] [Google Scholar]
- 11.Sechi LA, Kronenberg F, De Carli S, Falleti E, Zingaro L, Catena C, Utermann G, Bartoli E. Association of serum lipoprotein(a) levels and apolipoprotein(a) size polymorphism with target-organ damage in arterial hypertension. JAMA. 1997;277:1689–1695. [PubMed] [Google Scholar]
- 12.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR Jr., Kronmal R, Liu K, et al. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. doi: 10.1093/aje/kwf113 [DOI] [PubMed] [Google Scholar]
- 13.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 14.Grundy SM, Stone NJ, Guideline Writing Committee for the Cholesterol G. 2018 Cholesterol Clinical Practice Guidelines: Synopsis of the 2018 American Heart Association/American College of Cardiology/Multisociety Cholesterol Guideline. Ann Intern Med. 2019;170:779–783. doi: 10.7326/M19-0365 [DOI] [PubMed] [Google Scholar]
- 15.Galle J, Bengen J, Schollmeyer P, Wanner C. Impairment of endothelium-dependent dilation in rabbit renal arteries by oxidized lipoprotein(a). Role of oxygen-derived radicals. Circulation. 1995;92:1582–1589. doi: 10.1161/01.cir.92.6.1582 [DOI] [PubMed] [Google Scholar]
- 16.Boonmark NW, Lou XJ, Yang ZJ, Schwartz K, Zhang JL, Rubin EM, Lawn RM. Modification of apolipoprotein(a) lysine binding site reduces atherosclerosis in transgenic mice. J Clin Invest. 1997;100:558–564. doi: 10.1172/JCI119565 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data used in this study were obtained from the Multi-Ethnic Study of Atherosclerosis (MESA) (https://www.mesa-nhlbi.org) in accordance with their published data access procedures. Dr. Rikhi had full access to all the data in the study and takes responsibility for its integrity and data analysis.


