Abstract
Objective(s):
After identifying and recruiting men who have sex with men (MSM) living with HIV and virally unsuppressed, this study attempted to enhance treatment and care via case management to increase the proportion who achieved viral suppression.
Design:
Participants were randomized into one of two study arms: standard of care (SOC) or enhanced case management (CM) intervention. Participants were followed for 12 months with quarterly study assessments, with blood collected for CD4 cell count testing, HIV viral load testing (primary pre-specified outcome), and plasma storage.
Methods:
Participants identified via respondent-driven sampling and direct recruitment and were invited to participate in the randomized controlled trial (RCT). The CM intervention provided a wide range of support services including, health education, clinical care coordination, medication adherence support, and social service assistance. The month-12 assessment included questions about health care utilization, stigma, substance use, and mental health.
Results:
Among the 144 participants virally unsuppressed at baseline, most had had a previous positive HIV test result; were Black, non-Hispanic, gay and bisexual men, aged 22–50. Among the 128 participants at the last study visit, 68 were virally suppressed, with no statistically significant difference between the CM and SOC arms (viral suppression 42% and 53%, respectively; aOR =0.62 [p=0.15; 95% CI: 0.32, 1.2]).
Conclusions:
Reaching targets of at least 90% sustained viral suppression among all PWH will likely require more than an individual-level CM approach that addresses barriers to optimal care and treatment at multiple levels.
Keywords: case management, viral suppression, HIV-positive, MSM, RCT
INTRODUCTION
Since the beginning of the HIV epidemic, gay, bisexual, and other men who have sex with men (MSM) have been the most affected population in the United States (US) [1]. According to a recent Centers for Disease Control and Prevention (CDC) report, 67% of all new infections in the US were diagnosed in MSM [2] even though this group represents, at most, 4–5% of US adult men [1]. Racial and ethnic disparities characterize this epidemic with greatest burdens among Black, Latinx, and multiracial MSM [3]. The HIV Prevention Trials Network (HPTN) 061 study found a 3% incidence rate in Black MSM with a 5.9% incidence rate in those 18 to 29 years old [4]. Additionally, the highest incident rates of HIV infection are among MSM in the Southern US [5]. Racial and geographic disparities among MSM are not limited to HIV prevalence and incidence burdens. Non-white and Southern MSM have lower rates of regular HIV testing, less awareness of HIV status, later presentation for HIV care, poorer adherence to antiretroviral therapy (ART), and lower rates of viral suppression than among other MSM [6,7].
The continuum of care has been used to characterize HIV burden and understand gaps in response to the HIV epidemic [8]. According to CDC estimates, of the 1.2 million estimated persons with HIV (PWH) in the US, 14% do not know they are living with HIV [9], only 50% are in continuous care, and only 56% have sustained viral suppression [9]. Modeling studies point to the need to increase viral suppression among PWH to reduce HIV transmission among MSM in the US [10,11].
While there is some observational evidence that improved care coordination and patient navigation can have a positive impact on HIV care engagement and viral suppression [12] and also evidence for case management reducing sexual risk behaviors and syphilis among MSM with HIV [13], there is a paucity of evidence for enhanced case management having a direct effect on HIV viral suppression in rigorous controlled trials. One study showed that patient navigation, with or without financial incentives, did not have a beneficial effect on HIV viral suppression among hospitalized patients with HIV and substance use [14], with the literature suggesting that multiple factors are associated with lower odds of sustained HIV viral suppression, including demographic factors, drug and alcohol use, mental health symptoms, homelessness, transportation to appointment needs, as well as provider characteristics [15].
To address health disparities and to improve outcomes for all MSM, it is critical to reach higher proportions of MSM with HIV who are not virally suppressed, enhance their linkage to care, and increase their ability to achieve and maintain HIV suppression. These goals are central to the current “Ending the HIV Epidemic: A Plan for The United States” (EHE) [16]. Intervention strategies and packages that can achieve these goals must be acceptable, feasible, and scalable. The HPTN 078 study attempted to address two critical steps in the continuum of care for MSM: (1) enhance outreach and recruitment of MSM with HIV infection, but not virally suppressed, through respondent driven sampling (RDS) and direct recruitment [17], and (2) enhance treatment and care via case management to increase the proportion of MSM who achieved sustained viral suppression. This report is focused on the outcome of the second aim of the HPTN 078 study.
METHODS
Study Design
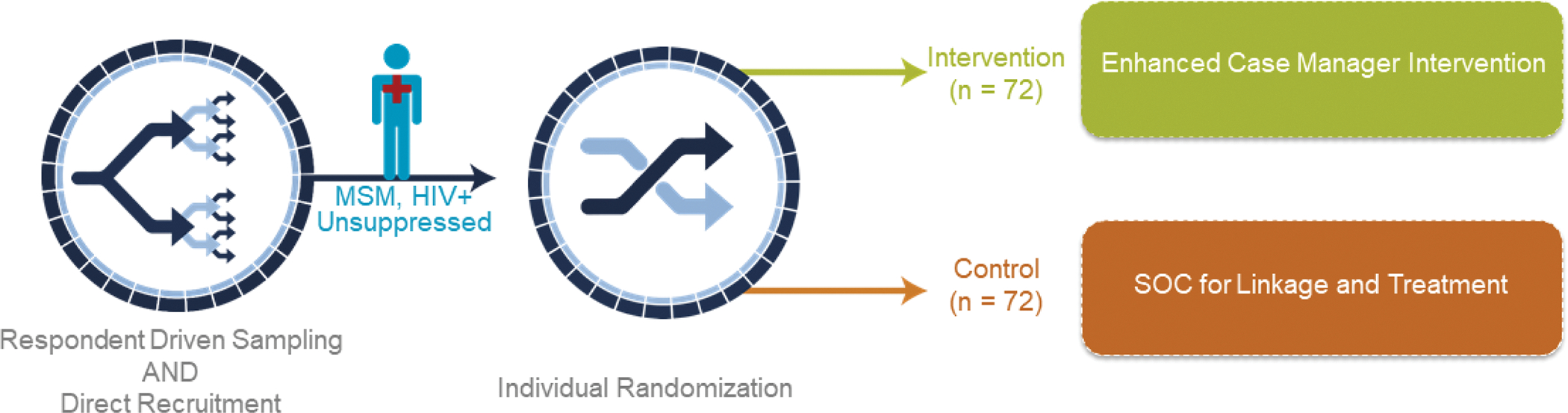
The primary goal of HPTN 078 was to find MSM with HIV who were not virally suppressed and to randomize them into one of two arms receiving either the standard of care (SOC, see below) or an enhanced case management (CM) intervention designed to improve viral suppression rates. RDS and direct recruitment methods were used to identify and recruit participants at clinical research sites in the following cities: Atlanta, GA, Boston, MA, Baltimore, MD, Birmingham, AL [See Beyrer et al., 2021 for a full description of participant outreach, identification, and screening]. The primary endpoint of the study was viral suppression at 12 months. An endpoint of 24 months was originally planned for HPTN 078, but concerns about slow recruitment of viremic men led to the decision to halt recruitment and follow participants for only 12 months.
Study Eligibility
Study enrollment criteria included: age ≥16 years in Birmingham and Boston; and ≥18 years in Atlanta and Baltimore; assigned male sex at birth; have a self-reported history of anal intercourse with another man; be living with HIV and not virally suppressed (defined as having a viral load ≥1000 copies/mL); be able to receive HIV treatment at one of the participating clinics chosen by each site; and have no current plan to relocate in the 24 months following enrollment. Individuals were excluded from the study if they were unable or unwilling to provide consent, were participating in another linkage or ART adherence study, or had any condition that, in the opinion of the site’s Investigator of Record, would make participation in the study unsafe, complicate interpretation of study outcome data, or otherwise interfere with achieving the study objectives. Individuals who self-identified anywhere along the transfeminine spectrum (e.g., transgender women, female, genderqueer, etc.) were included as long as they met study eligibility criteria.
Study Enrollment
At the enrollment visit, participants were consented and randomized (1:1 in blocks of size 2 or 4, randomly chosen, and stratified by study site) to the intervention (CM) or control (SOC) arm of the study (Figure 1). Neither participants nor study personnel were blinded to the intervention assignment. To prevent cross-contamination between the arms, after randomization, all enrollment and follow-up procedures were conducted by the study case manager for those enrolled into the intervention arm and by another staff member for those enrolled into the control arm. Participants in both study arms signed a release of their medical information, provided contact information, were offered the option to have their sexual partners tested for HIV (as a courtesy and potential benefit to study participation), had blood taken for plasma storage, and were provided with referrals for syphilis or hepatitis C (HCV) treatment if they were diagnosed with these conditions during screening. Participants in both study arms also completed a computer-assisted self-interview (CASI) that included questions about substance use and mental health. [Note: All participants completed a CASI at initial screening in which they were asked questions about demographic information, gender and sexual orientation identity, health care coverage, housing stability, and stigma (HIV and sexual orientation) [17]]. For SOC, sites followed their normal procedures to link participants to HIV care. For CM, the intervention began at this visit with the case manager working with the participant to ensure linkage to and engagement in care with their preferred clinic.
Figure 1.
Enhanced case management (CM) intervention improve linkage to care, ART initiation, treatment adherence, and retention in care
Participants attended in-person follow-up study visits at months 3, 6, 9, and 12. At each follow-up visit, contact information was confirmed, social impacts (an undesired change in a person’s relationships, experiences, interactions, rights, and/or community status that occurs as a direct result of participating in a research study) were collected, and blood was collected for CD4 cell count testing, HIV viral load testing, and plasma storage. Participants enrolled in the CM arm met with their case manager and underwent the components of the intervention (see below). The final follow-up visit at month 12 (M12) included the following additional procedures: syphilis testing; completion of a CASI; and optional participation in a semi-structured qualitative interview. All sites provided participants with their syphilis test results and referral for care (if needed) per their normal site procedures. The substance use and mental health data collected (i.e., substance abuse and mental illness symptoms screener [SAMISS]) [18] were recorded separately from other assessments. The protocol developed for the original 24-month data collection cycle had an automatic check procedure in place to ensure complete data collection for the final study visit. When the follow-up duration was changed to 12-months, this automatic check procedure was discontinued. Inadvertently, SAMISS data at M12 were not collected from the Alabama site, which resulted in missing SAMISS data.
Case Management Intervention
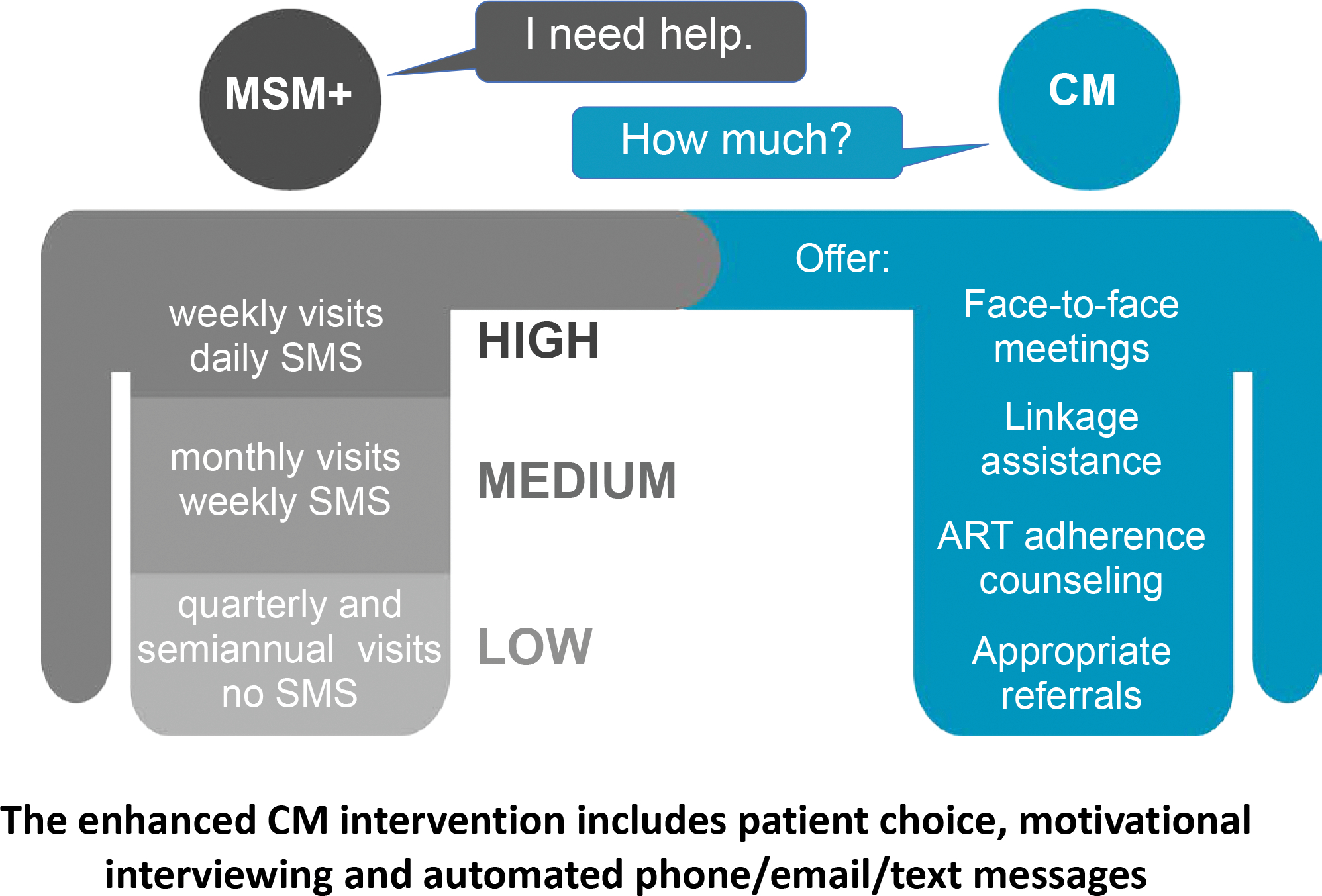
The CM intervention was designed to improve linkage to care, ART initiation, treatment adherence, and retention in care. Each site hired a full-time case manager for the duration of the intervention. To qualify, candidates were required to have at least a bachelor’s degree in a health-related discipline; some counseling experience; at least two years of experience in HIV/AIDS education, clinical care, or case management; and experience working with diverse populations, ideally with the MSM community. As part of the intervention, each case manager was trained to use motivational interviewing (MI), a strength-based, patient-centered counseling approach found to be effective as part of other HIV prevention behavioral interventions [19–23]. Case managers received three face-to-face MI training sessions and were provided with ongoing telephone support from an MI training expert. By using the MI approach, the case managers aimed to strengthen the participants’ motivation for and commitment to change by focusing on participant choice and autonomy for engaging in care and ART adherence. The case managers also provided a wide range of support services including education, clinical care coordination, medication adherence support, and social assistance. When needed, case managers were capable of making appropriate referrals (Figure 2).
Figure 2.
Intervention Assignment Diagram
Additionally, to support participant adherence to HIV treatment and care, the study included a communication platform shown to be effective in other HIV behavioral interventions [24–27], through which participants could choose to receive automated medication adherence and refill reminders, motivational messages, and appointment reminders for both study and non-study visits. The platform was also designed so that participants could ask their case managers for help in response to the automated messages. All messages delivered through this platform could be sent via text, voicemail, or email, and were completely customizable.
A key element of the enhanced CM intervention was that the frequency and type of interactions with the case manager, as well as the tailored messaging, were driven by participants. Their choice ranged from a minimum of contact (only monthly texts and required study visits) to having daily support (Figure 2). Receiving this CM intervention did not preclude receipt of case management provided in the clinical care setting. Thus, participants may have been also receiving SOC case management services, comparable to participants in the SOC arm, in addition to the study interventions.
Standard of Care (SOC)
Individuals in the control arm were provided with SOC linkage to care, ART initiation, ART adherence support, and retention in care at their HIV clinic. It is worth noting that these were highly resourced and good quality care settings affiliated with academic medical centers in each of the four cities, without substantive differences across the sites. They had no interactions with a study case manager, and they did not receive any messages via the communication platform, but all participants in SOC had access to case management in these care sites. Other services were provided in some cases by SOC case managers.
Study Follow-up
For those randomized to the intervention arm, the case manager had contact with each participant at least monthly to confirm their contact information, check-in regarding HIV and ART management, and collect any social impacts due to study participation. Participants could choose if this monthly contact was in person, by text message, email, or phone call. No monthly contact was made by members of the study team with participants randomized to the control arm.
Statistical Analysis
The primary outcome was viral suppression. Individuals who failed to return for follow-up were treated as unsuppressed. Data were summarized as counts and percentages. The analysis of viral suppression by CM intervention arm was intention to treat and based on a logistic regression adjusted for site. Fisher’s exact test was used to evaluate the relationship between viral suppression and other characteristics, and a chi-square test for trend was also conducted for ordinal characteristics. Prespecified secondary predictors of viral suppression are shown in Table 3a, and exploratory predictors are shown in Table 3b. T tests were used to compare the CM intervention intensity (number of encounters, number of activities) between those who were virally suppressed and unsuppressed in the CM intervention arm at 12 months. The study was originally designed to enroll 356 PWH which would have provided 90% power to detect an 18 percentage-point increase in viral suppression at M24 (e.g., 24% to 42%) after accounting for loss to follow-up. Due to slower than anticipated recruitment, enrollment was stopped after 144 individuals were enrolled, follow-up was shortened to 12 months, and the primary outcome was changed to viral suppression at M12.
Table 3a.
Baseline Demographics and Health Characteristics by HIV Viral Load at Month 12
| Overall | Suppressed (<200) | Unsuppressed | Not Collected | p value1 | |
|---|---|---|---|---|---|
|
| |||||
| Participants | 144 | 68 | 60 | 16 | |
|
| |||||
| N (%) | N (%) | n (%) | n (%) | ||
| Age | |||||
| 18–21 | 7 (5) | 3 (4) | 3 (5) | 1 (6) | 0.66 |
| 22–30 | 36 (25) | 16 (24) | 15 (250) | 5 (31) | |
| 31–40 | 34 (24) | 16 (24) | 15 (25) | 3 (19) | |
| 41–50 | 39 (27) | 18 (26) | 17 (28) | 4 (25) | |
| 51–60 | 26 (18) | 15 (22) | 8 (13) | 3 (19) | |
| > 60 | 2 (1) | 0 (0) | 2 (3) | 0 (0) | |
| Mean | 39 | 40 | 39 | 37 | |
|
| |||||
| Race | |||||
|
| |||||
| Native American | 1 (1) | 0 (0) | 0 (0) | 1 (16) | 0.61 |
| Asian | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| Black | 121 (84) | 57 (84) | 53 (88) | 11 (69) | |
| HI/Pacific | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| White | 19 (13) | 9 (13) | 3 (5) | 7 (44) | |
| Other | 8 (6) | 4 (6) | 4 (7) | 0 (0) | |
|
| |||||
| Latino or Hispanic | |||||
|
| |||||
| Yes | 10 (7) | 4 (6) | 6 (10) | 0 (0) | 0.51 |
| No | 134 (93) | 64 (94) | 54 (90) | 16 (100) | |
|
| |||||
| Self-Identified Gender | |||||
|
| |||||
| Male | 139 (97) | 66 (97) | 57 (95) | 16 (100) | 0.59 |
| Female | 1 (1) | 0 (0) | 1 (2) | 0 (0) | |
| Transgender female | 3 (2) | 2 (3) | 1 (2) | 0 (0) | |
| Gender Non-Conforming | 1 (1) | 0 (0) | 1 (2) | 0 (0) | |
|
| |||||
| Sexual Orientation | |||||
|
| |||||
| Gay/Lesbian/Homosexual | 114 (79) | 54 (79) | 47 (78) | 13 (81) | 0.64 |
| Two-spirit | 23 (16) | 9 (13) | 11 (18) | 3 (19) | |
| Straight/Heterosexual | 5 (3) | 4 (6) | 1 (2) | 0 (0) | |
| Other | 2 (1) | 1 (1) | 1 (2) | 0 (0) | |
|
| |||||
| Education | |||||
|
| |||||
| High school diploma or less | 56 (39) | 28 (41) | 20 (33) | 8 (50) | 0.46 |
| Education beyond high school | 88 (61) | 40 (59) | 40 (67) | 8 (50) | |
|
| |||||
| Employment | |||||
|
| |||||
| Full-time employment | 27 (19) | 13 (19) | 11 (18) | 3 (19) | 1.00 |
| Part-time employment | 20 (14) | 10 (15) | 9 (15) | 1 (6) | |
| Not employed | 97 (67) | 45 (66) | 40 (67) | 12 (75) | |
|
| |||||
| Marital Status | |||||
|
| |||||
| Married/civil union/legal partnership | 4 (3) | 0 (0) | 3 (5) | 1 (6) | 0.02 |
| Living with primary or main partner | 16 (11) | 12 (18) | 3 (5) | 1 (6) | |
| Have primary or main partner, not living together | 2 (1) | 1 (1) | 1 (2) | 0 (0) | |
| Single/divorced/widowed | 122 (85) | 55 (81) | 53 (88) | 14 (88) | |
|
| |||||
| Health Care Coverage | |||||
|
| |||||
| Yes | 116 (81) | 54 (79) | 48 (80) | 14 (88) | 1.00 |
| No | 28 (19) | 14 (21) | 12 (20) | 2 (13) | |
|
| |||||
| Stable Housing | |||||
|
| |||||
| Yes | 128 (89) | 63 (93) | 53 (88) | 12 (75) | 0.55 |
| No | 16 (11) | 5 (7) | 7 (12) | 4 (25) | |
|
| |||||
| Substance Use Treatment | |||||
|
| |||||
| Yes | 20 (14) | 9 (13) | 9 (15) | 2 (13) | 0.37 |
| No | 117 (81) | 57 (84) | 46 (77) | 14 (88) | |
| Prefer not to answer | 7 (5) | 2 (3) | 5 (8) | 0 (0) | |
|
| |||||
| Every Taken ART 2 | |||||
|
| |||||
| Yes | 124 (86) | 59 (87) | 52 (87) | 13 (81) | 0.78 |
| No | 18 (13) | 9 (13) | 7 (12) | 2 (13) | |
| Missing | 2 | 0 | 1 | 1 | |
The table shows the baseline demographics and health characteristics of participants based on viral suppression status. Suppressed indicates that the viral load was <200 copies/mL at the month 12 study visit. Numbers in parentheses incidate percentatges, ranges (min/max), or quartiles (Q1, Q3). CM: case manager study arm; SOC: standard of care study arm; N=number; HIV viral load (VL, HIV RNA copies/mL).
Abbreviations: ART: antiretroviral therapy; SAMISS: Substance Abuse and Mental Illness Symptoms Screener; Min: minimum, Max: maximum, 1–5; Q: quartertile.
P-Values are from Fisher exact test. For race, not being mutually exclusive among racial categories, we conducted alternative comparison between Black “Yes/No” and VL suppression status.
Percent of the non-missing number of observations.
Table 3b.
Comparison of Month 12 Virally Suppressed and Non-suppressed on SAMISS Questionnaire – Baseline SAMISS1 and Stigma
| Question | Value2 | Suppressed at Month 12 (VL< 200) | Unsuppressed at Month 12 (VL >= 200) | p value3 | Chi square Test for trend p value4 |
|---|---|---|---|---|---|
|
| |||||
| Q1. How often do you have a drink containing alcohol? | |||||
|
| |||||
| SAMISS not taken | 4/68 (6%) | 1/60 (1%) | 0.49 | 0.53 | |
| Never | 11/68 (16%) | 12/60 (18%) | |||
| Monthly or less | 23/68 (34%) | 18/60 (26%) | |||
| 2–4 times/month | 12/68 (18%) | 18/60 (26%) | |||
| 2–3 times/week | 12/68 (18%) | 7/60 (10%) | |||
| 4 or more times/week | 5/68 (7%) | 3/60 (4%) | |||
| Response not valid | 1/68 (1%) | 1/60 (1%) | |||
|
| |||||
| Q2. How many drinks do you have on a typical day when you are drinking? | |||||
|
| |||||
| SAMISS not taken | 4/68 (6%) | 1/60 (1%) | 0.84 | 0.61 | |
| None | 17/68 (25%) | 17/60 (25%) | |||
| 1 or 2 | 27/68 (40%) | 27/60 (40%) | |||
| 3 or 4 | 15/68 (22%) | 10/60 (15%) | |||
| 5 or 6 | 3/68 (4%) | 4/60 (6%) | |||
| 7–9 | 1/68 (1%) | ||||
| Response not valid | 1/68 (1%) | 1/60 (1%) | |||
|
| |||||
| Q3. How often do you have 4 or more drinks on 1 occasion? | |||||
|
| |||||
| SAMISS not taken | 4/68 (6%) | 1/60 (1%) | 0.20 | 0.59 | |
| Never | 25/68 (37%) | 25/60 (37%) | |||
| Less than monthly | 27/68 (40%) | 17/60 (25%) | |||
| Monthly | 9/68 (13%) | 9/60 (13%) | |||
| Weekly | 1/68 (1%) | 6/60 (9%) | |||
| Daily or almost daily | 2/68 (3%) | 1/60 (1%) | |||
| Response not valid | 1/60 (1%) | ||||
|
| |||||
| Q4. In the past year, how often did you use nonprescription drugs to get high or to change the way you feel? | |||||
|
| |||||
| SAMISS not taken | 4/68 (6%) | 1/60 (1%) | 0.81 | 0.53 | |
| Never | 40/68 (59%) | 31/60 (46%) | |||
| Less than monthly | 9/68 (13%) | 11/60 (16%) | |||
| Monthly | 6/68 (9%) | 8/60 (12%) | |||
| Weekly | 4/68 (6%) | 5/60 (7%) | |||
| Daily or almost daily | 5/68 (7%) | 4/60 (6%) | |||
|
| |||||
| Q5. In the past year, how often did you use drugs prescribed to you or to someone else to get high or change the way you feel? | |||||
|
| |||||
| SAMISS not taken | 4/68 (6%) | 1/60 (1%) | 0.54 | 0.1 | |
| Never | 56/68 (82%) | 46/60 (68%) | |||
| Less than monthly | 6/68 (9%) | 8/60 (12%) | |||
| Monthly | 2/68 (3%) | 3/60 (4%) | |||
| Weekly | 1/60 (1%) | ||||
| Daily or almost daily | 1/60 (1%) | ||||
|
| |||||
| Q6. In the past year, how often did you drink or use drugs more than you meant to? | |||||
|
| |||||
| SAMISS not taken | 4/68 (6%) | 1/60 (1%) | 0.66 | 0.87 | |
| Never | 33/68 (49%) | 32/60 (47%) | |||
| Less than monthly | 19/68 (28%) | 12/60 (18%) | |||
| Monthly | 6/68 (9%) | 9/60 (13%) | |||
| Weekly | 3/68 (4%) | 4/60 (6%) | |||
| Daily or almost daily | 3/68 (4%) | 2/60 (3%) | |||
|
| |||||
| Q7. How often did you feel you wanted or needed to cut down on your drinking or drug use in the past year, and were not able to? | |||||
|
| |||||
| SAMISS not taken | 4/68 (6%) | 1/60 (1%) | 0.91 | 0.89 | |
| Never | 38/68 (56%) | 35/60 (51%) | |||
| Less than monthly | 14/68 (21%) | 11/60 (16%) | |||
| Monthly | 2/68 (3%) | 4/60 (6%) | |||
| Weekly | 5/68 (7%) | 4/60 (6%) | |||
| Daily or almost daily | 5/68 (7%) | 5/60 (7%) | |||
|
| |||||
| Q8. In the past year, when not high or intoxicated, did you ever feel extremely energetic or irritable and more talkative than usual? | |||||
|
| |||||
| SAMISS not taken | 4/68 (6%) | 1/60 (1%) | 0.59 | ||
| Yes | 27/68 (40%) | 22/60 (32%) | |||
| No | 37/68 (54%) | 37/60 (54%) | |||
|
| |||||
| Q9. In the past year, were you ever on medication or antidepressants for depression or nerve problems? | |||||
|
| |||||
| SAMISS not taken | 4/68 (6%) | 1/60 (1%) | 0.18 | ||
| Yes | 17/68 (25%) | 23/60 (34%) | |||
| No | 47/68 (69%) | 36/60 (53%) | |||
|
| |||||
| Q10. In the past year, was there ever a time when you felt sad, blue, or depressed for more than 2 weeks in a row? | |||||
|
| |||||
| SAMISS not taken | 4/68 (6%) | 1/60 (1%) | 0.47 | ||
| Yes | 27/68 (40%) | 29/60 (43%) | |||
| No | 37/68 (54%) | 30/60 (44%) | |||
|
| |||||
| Q11. In the past year, was there ever a time lasting more than 2 weeks when you lost interest in most things or activities that usually give you pleasure? | |||||
|
| |||||
| SAMISS not taken | 4/68 (6%) | 1/60 (1%) | 0.86 | ||
| Yes | 27/68 (40%) | 26/60 (38%) | |||
| No | 37/68 (54%) | 33/60 (49%) | |||
|
| |||||
| Q12. In the past year, did you ever have a period lasting more than 1 month when most of the time you felt worried and anxious? | |||||
|
| |||||
| SAMISS not taken | 4/68 (6%) | 1/60 (1%) | 0.28 | ||
| Yes | 23/68 (34%) | 27/60 (40%) | |||
| No | 41/68 (60%) | 32/60 (47%) | |||
|
| |||||
| Q13. In the past year, did you have a spell or an attack when all of a sudden you felt frightened, anxious, or very uneasy when most people would not be afraid or anxious? | |||||
|
| |||||
| SAMISS not taken | 4/68 (6%) | 1/60 (1%) | 0.72 | ||
| Yes | 26/68 (38%) | 22/60 (32%) | |||
| No | 38/68 (56%) | 37/60 (54%) | |||
|
| |||||
| Q14. In the past year, did you ever have a spell or an attack when for no reason your heart suddenly started to race, you felt faint, or you could not catch your breath? | |||||
|
| |||||
| SAMISS not taken | 4/68 (6%) | 1/60 (1%) | 0.33 | ||
| Yes | 17/68 (25%) | 21/60 (31%) | |||
| No | 47/68 (69%) | 38/60 (56%) | |||
|
| |||||
| Q15. During your lifetime, as a child or adult, have you experienced or witnessed traumatic events that involved harm to yourself or to others? | |||||
|
| |||||
| SAMISS not taken | 4/68 (6%) | 1/60 (1%) | 0.71 | ||
| Yes | 41/68 (60%) | 35/60 (51%) | |||
| No | 23/68 (34%) | 24/60 (35%) | |||
|
| |||||
| Q16. In the past year, have you been troubled by flashbacks, nightmares, or thoughts of the trauma? | |||||
|
| |||||
| SAMISS not taken | 4/68 (6%) | 1/60 (1%) | 0.35 | ||
| Yes | 22/68 (32%) | 23/60 (34%) | |||
| No | 19/68 (28%) | 12/60 (18%) | |||
| Question was skipped | 23/68 (34%) | 24/60 (35%) | |||
|
| |||||
| Q17. In the past 3 months, have you experienced any events or received information that was so upsetting it affected how you cope with everyday life? | |||||
|
| |||||
| SAMISS not taken | 4/68 (6%) | 1/60 (1%) | 0.34 | ||
| Yes | 18/68 (26%) | 22/60 (32%) | |||
| No | 46/68 (68%) | 37/60 (54%) | |||
|
| |||||
| Q18. Have had nightmares about it or thought about it when you did not want to? | |||||
|
| |||||
| SAMISS not taken | 4/68 (6%) | 1/60 (1%) | 0.72 | ||
| Yes | 25/68 (37%) | 25/60 (37%) | |||
| No | 39/68 (57%) | 34/60 (50%) | |||
|
| |||||
| Q19. Tried hard not to think about it or went out of your way to avoid situations that reminded you of it? | |||||
|
| |||||
| SAMISS not taken | 4/68 (6%) | 1/60 (1%) | 0.23 | ||
| Yes | 30/68 (44%) | 34/60 (50%) | |||
| No | 34/68 (50%) | 25/60 (37%) | |||
|
| |||||
| Q20. Were constantly on guard, watchful, or easily startled? | |||||
|
| |||||
| SAMISS not taken | 4/68 (6%) | 1/60 (1%) | 0.72 | ||
| Yes | 25/68 (37%) | 25/60 (37%) | |||
| No | 39/68 (57%) | 34/60 (50%) | |||
|
| |||||
| Q21. Felt numb or detached from others, activities, or your surroundings? | |||||
|
| |||||
| SAMISS not taken | 4/68 (6%) | 1/60 (1%) | 0.72 | ||
| Yes | 28/68 (41%) | 28/60 (41%) | |||
| No | 36/68 (53%) | 31/60 (46%) | |||
|
| |||||
| 1. Internalized Stigma5 | |||||
|
| |||||
| Mean (Min, Max) | 2.1 (1, 5) | 2.0 (1, 5) | 0.40 | ||
| Median (Q1, Q3) | 2 (1, 3) | 2 (1, 3) | |||
|
| |||||
| 2. HIV Stigma6 | |||||
|
| |||||
| Mean (Min, Max) | 2.9 (1, 5) | 2.9 (1, 5) | 0.65 | ||
| Median (Q1, Q3) | 3 (3, 4) | 3 (2, 4) | |||
|
| |||||
| 3. Community Stigma7 | |||||
|
| |||||
| Mean (Min, Max) | 0.3 (0, 1) | 0.3 (0, 1) | 0.33 | ||
| Median (Q1, Q3) | 0 (0, 1) | 0 (0, 1) | |||
The table shows the baseline demographics and health characteristics of participants based on viral suppression status. Suppressed indicates that the viral load was <200 copies/mL at the month 12 study visit. Numbers in parentheses incidate percentatges, ranges (min/max), or quartiles (Q1, Q3). CM: case manager study arm; SOC: standard of care study arm; N=number; HIV viral load (VL, HIV RNA copies/mL).
Abbreviations: ART: antiretroviral therapy; SAMISS: Substance Abuse and Mental Illness Symptoms Screener; Min: minimum, Max: maximum, 1–5; Q: quartertile
SAMISS questions Q22–24 are only available at month 12; CASI questions Q73–79 (engagement in LGBT community) and Q15 (prior ART use) are only available at baseline.
The “missing” category was not included in the statistical test.
If variable/question is categorical then the p value is from Fisher’s exact test for independence; if variable is numeric then the p value is from Wilcoxon rank sum test.
Average exclude variables with value = 99 (prefer not to answer) from the average.
Average exclude variables with value = 99 (prefer not to answer) from the average.
Calculate the percent “Yes” responses among the variables with responses 1 or 2; exclude 77 (don’t know) and 99 (prefer not to answer).
Ethical Review
The local institutional review board for each study site approved the protocol prior to study implementation. Participants provided written informed consent prior to any study procedures. HPTN 078 is registered on ClinicalTrials.gov (NCT02663219).
RESULTS
Sample
We recruited and screened a total of 1305 persons between March 2016 and December 2017; 154 were determined eligible for the study. The primary reasons why people were ineligible were because they were not living with HIV, or were living with HIV, but were virally suppressed (see CONSORT diagram). The study enrolled 144 participants who were living with HIV and virally unsuppressed (defined as having a viral load ≥ 1000 copies/mL); most enrolled participants had a previous positive HIV test result (n=137, 89%). Fifty-seven (40%) participants were recruited via RDS, and 87 (60%) were recruited via direct recruitment. Table 1 shows the baseline demographic and health characteristics of the enrolled participants, by study arm. The average age of participants was 39 years; most were aged 22–50. The study cohort was predominately comprised of Black, non-Hispanic, gay and bisexual men. Most participants were educated, unemployed, single, stably housed, and did not have a history of substance use treatment. There were high rates of HCV and syphilis among the sample [28,29]. The majority had access to healthcare. At baseline, the mean CD4 count was 357 cells/uL, and median HIV-1 viral load was 19,459 copies/mL; 124 (86%) reported previous ART use. Of the 144 enrolled, 143 were alive at M12, and 129 of those (90%) completed the M12 visit. One of the 129 participants did not have viral load data at the final visit (Supplemental Figure 3) and one participant never received any aspect of the CM intervention.
Table 1.
Baseline Demographics and Health Characteristics by Study Arm
| Overall | CM Arm | SOC Arm | |
|---|---|---|---|
|
| |||
| Participants | 144 | 72 | 72 |
|
| |||
| N (%) | n (%) | n (%) | |
|
| |||
| Age | |||
|
| |||
| 18–21 | 7 (5) | 3 (4) | 4 (6) |
| 22–30 | 36 (25) | 21 (29) | 15 (21) |
| 31–40 | 34 (24) | 15 (21) | 19 (26) |
| 41–50 | 39 (27) | 19 (26) | 20 (28) |
| 51–60 | 26 (18) | 13 (18) | 13 (18) |
| > 60 | 2 (1) | 1 (1) | 1 (1) |
| Mean | 39 | 39 | 39 |
|
| |||
| Race 1 | |||
|
| |||
| Native American | 1 (0.7) | 1 (1.4) | 0 (0) |
| Black | 121 (84) | 62 (86.1) | 59 (81.9) |
| White | 19 (13.2) | 9 (12.5) | 10 (13.9) |
| Other | 8 (5.6) | 3 (4.2) | 5 (6.9) |
|
| |||
| Latino/Hispanic | |||
|
| |||
| Yes | 10 (7) | 3 (4) | 7 (10) |
| No | 134 (93) | 69 (96) | 65 (90) |
|
| |||
| Gender | |||
|
| |||
| Male | 139 (97) | 71 (99) | 68 (94) |
| Female | 1 (1) | 0 (0) | 1 (1) |
| Transgender Female | 3 (2) | 1 (1) | 2 (3) |
| Gender Non-Conforming | 1 (1) | 0 (0) | 1 (1) |
|
| |||
| Sexual Orientation | |||
|
| |||
| Gay | 114 (79) | 58 (81) | 56 (78) |
| Bisexual | 23 (16) | 12 (17) | 11 (15) |
| Heterosexual | 5 (3) | 2 (3) | 3 (4) |
| Other | 2 (1) | 0 (0) | 2 (3) |
|
| |||
| Education | |||
|
| |||
| High school diploma or less | 56 (38) | 28 (39) | 28 (39) |
| Education beyond high school | 88 (61) | 44 (62) | 44 (62) |
|
| |||
| Employment | |||
|
| |||
| Full-time | 27 (19) | 13 (18) | 14 (19) |
| Part-time | 20 (14) | 11 (15) | 9 (13) |
| Not employed | 97 (67) | 48 (67) | 49 (68) |
|
| |||
| Marital Status | |||
|
| |||
| Married or living with partner | 20 (14) | 7 (10) | 13 (18) |
| Have primary or main partner, not living together | 2 (1) | 1 (1) | 1 (1) |
| Single/divorced/widowed | 122 (85) | 64 (89) | 58 (81) |
|
| |||
| Health Care Coverage | |||
|
| |||
| Yes | 116 (81) | 59 (82) | 57 (79) |
| No | 28 (19) | 13 (18) | 15 (21) |
|
| |||
| Stable Housing | |||
|
| |||
| Yes | 128 (89) | 65 (90) | 63 (88) |
| No | 16 (11) | 7 (10) | 9 (13) |
|
| |||
| Syphilis Status | |||
|
| |||
| Active | 51 (35) | 27 (38) | 24 (33) |
| Non-active | 13 (9) | 7 (10) | 6 (8) |
| Negative | 80 (56) | 38 (53) | 42 (58) |
|
| |||
| HCV Antibody | |||
|
| |||
| Negative | 122 (85) | 57 (79) | 65 (90) |
| Positive | 22 (15) | 15 (21) | 7 (10) |
|
| |||
| CD4 Count | |||
|
| |||
| < 200 | 42 (29) | 24 (33) | 18 (25) |
| 200 – 350 | 34 (24) | 16 (22) | 18 (25) |
| 351 – 500 | 28 (19) | 16 (22) | 12 (17) |
| > 500 | 40 (28) | 16 (22) | 24 (33) |
| Mean | 357 | 337 | 377 |
|
| |||
| Substance Use | |||
|
| |||
| Yes | 20 (14) | 8 (11) | 12 (17) |
| No | 117 (81) | 60 (83) | 57 (79) |
| Prefer not to answer | 7 (5) | 4 (6) | 3 (4) |
|
| |||
| Ever on ART 2 | |||
| Yes | 124 (87) | 64 (89) | 60 (83) |
| No | 18 (13) | 7 (10) | 11 (15) |
|
| |||
| Missing | 2 | 1 | 1 |
The table shows baseline demographic and health characteristics for study participants in each study arm.
Abbreviations: CM: case manager study arm; SOC: standard of care study arm; N: number; HCV: hepatitis C virus; ART: antiretroviral therapy.
Participants could choose multiple races, if applicable, instead of choosing only one race.
Percent of the non-missing number of observations (Overall, n = 142; CM, n = 71; SOC, n = 71).
Primary Outcome: Viral Load Suppression
Table 2 and Supplemental Figure 4 show data for the primary outcome of viral suppression by study arm, at baseline and across the four quarterly study visits. Of the 144 participants enrolled, viral suppression data were available for 128 participants at the M12 visit. At M12, 47% (n=68) were virally suppressed (defined here as having a viral load <200 copies/mL), with no statistically significant difference between the CM and SOC arms (42% and 53%, respectively; adjusted odds ratio [OR]=0.62 [p=0.15, 95% confidence intervals [CI]=0.32, 1.2]). There were no significant differences in viral suppression between the CM and SOC arms at any of the other quarterly visits. Nearly one-third of the participants achieved viral suppression by the M3 visit. Viral suppression rates increased over time among participants in both arms. In addition, we saw no significant difference in frequency of encounters in those who were unsuppressed compared to those who were suppressed in the CM arm (p=0.43) (Supplementary Table 5). However, there was a significant increase in the number of CM activities in those who were unsuppressed compared to those who were suppressed in the CM arm (p=0.02) (Supplementary Tables 6–7).
Table 2.
Viral Suppression by Visit and Study Arm
| Overall | CM Arm | SOC Arm | |
|---|---|---|---|
|
| |||
| Participants | 144 | 72 | 72 |
|
| |||
| N (%) | n (%) | n (%) | |
|
| |||
| Baseline | 144 | 72 | 72 |
|
| |||
| Suppressed | 0 (0) | 0 (0) | 0 (0) |
| Unsuppressed (VL >= 200) | 144 (100) | 72 (100) | 72 (100) |
| Not done/Not collected | 0 | 0 | 0 |
| Viral load (N = No. unsuppressed) | |||
| Mean VL | 77979 | 60239 | 95718 |
| Median VL | 19459 | 17922 | 22100 |
| 25th | 6368 | 6158 | 7125 |
| 75th | 57573 | 55353 | 63884 |
|
| |||
| 1st Visit, Month 3 1 | 144 | 72 | 72 |
|
| |||
| Suppressed | 41 (28) | 20 (28) | 21 (29) |
| Unsuppressed (VL >= 200) | 60 (42) | 30 (42) | 30 (42) |
| Not done/Not collected | 43 | 22 | 21 |
| Viral load (N = No. unsuppressed) | |||
| Mean VL | 53967 | 54787 | 53148 |
| Median VL | 13047 | 14115 | 11921 |
| 25th | 2526 | 1675 | 2850 |
| 75th | 64250 | 51644 | 73395 |
|
| |||
| 2nd Visit, Month 6 1 | 144 | 72 | 72 |
|
| |||
| Suppressed | 52 (36) | 26 (36) | 26 (36) |
| Unsuppressed (VL >= 200) | 58 (40) | 26 (36) | 32 (44) |
| Not done/Not collected | 34 | 20 | 14 |
| Viral load (N = No. unsuppressed) | |||
| Mean VL | 51608 | 57884 | 46509 |
| Median VL | 20250 | 30700 | 19509 |
| 25th | 5654 | 860 | 3584 |
| 75th | 79721 | 85247 | 72612 |
|
| |||
| 3rd Visit, Month 9 1 | 144 | 72 | 72 |
|
| |||
| Suppressed | 56 (39) | 28 (39) | 28 (39) |
| Unsuppressed (VL >= 200) | 57 (40) | 27 (38) | 30 (42) |
| Not done/Not collected | 31 | 17 | 14 |
| Viral load (N = No. unsuppressed) | |||
| Mean VL | 90685 | 39580 | 136679 |
| Median VL | 21353 | 16572 | 33350 |
| 25th | 4925 | 1461 | 8355 |
| 75th | 86077 | 55214 | 93600 |
|
| |||
| 4th Visit, Month 12 1,2 | 144 | 72 | 72 |
|
| |||
| Suppressed | 68 (47) | 30 (42) | 38 (53) |
| Unsuppressed (VL >= 200) | 60 (42) | 32 (44) | 28 (39) |
| Not done/Not collected | 15 | 10 | 5 |
| Death | 1 | 0 | 1 |
| Viral load (N = No. unsuppressed) | |||
| Mean VL | 60382 | 50123 | 72106 |
| Median VL | 15411 | 21850 | 8305 |
| 25th | 1590 | 3865 | 795 |
| 75th | 62600 | 59950 | 67415 |
The table shows the number of participants based on study visit and study arm. Suppressed indicates that the viral load was <200 copies/mL at the study visit indicated.
Abbreviations: CM: case manager study arm; SOC: standard of care study arm; N=number; HIV viral load (VL, HIV RNA copies/mL).
Adjusted for Site:
Only percents among the non-missing shown.
aOR (95% CI; p): 0.62 (0.15,1.2; p = 0.15)
Predictors of Viral Load Suppression
Given the lack of difference in viral suppression rates between study arms, we next evaluated demographic, health, and psychosocial characteristics that may have been associated with viral suppression. We first evaluated the association of viral suppression with baseline demographic and health characteristics (Supplementary Table 3a) along with self-reported alcohol and substance use, psychiatric symptoms, and stigma (i.e., HIV and sexual orientation) at baseline (Supplementary Table 3b). With the exception of marital status (p=0.02), none of these baseline characteristics were significantly different between men who were vs. were not virally suppressed at M12. We next explored whether self-reported alcohol and substance use, psychiatric symptoms, and stigma were associated with viral suppression at M12 (Table 4). We found that participants who achieved viral suppression reported having four or more drinks on one occasion more frequently than those who were not virally suppressed (p=0.01). Other associations were not statistically significant.
Table 4.
Comparison of Month 12 Virally Suppressed and Non-suppressed on Month 12 SAMISS1 and Stigma
| Question | Value2 | Suppressed at Month12 (vl < 200) | Unsuppressed at Month12 (vl >= 200) | p value3 | Chi square Test for trend p value4 |
|---|---|---|---|---|---|
|
| |||||
| Q1. How often do you have a drink containing alcohol? | n =68 | n = 60 | |||
|
| |||||
| SAMISS not taken | 19 (28%) | 14 (21%) | 0.70 | 0.79 | |
| Never | 10 (15%) | 12 (18%) | |||
| Monthly or less | 21 (31%) | 18 (26%) | |||
| 2–4 times/month | 6 (9%) | 5 (7%) | |||
| 2–3 times/week | 10 (15%) | 6 (9%) | |||
| 4 or more times/week | 1 (1%) | 3 (4%) | |||
| Response not valid/Missing | 1 (1%) | 2 (2%) | |||
|
| |||||
| Q2. How many drinks do you have on a typical day when you are drinking? | |||||
|
| |||||
| SAMISS not taken | 19 (28%) | 14 (21%) | 0.22 | 0.74 | |
| None | 12 (18%) | 17 (25%) | |||
| 1 or 2 | 22 (32%) | 15 (22%) | |||
| 3 or 4 | 11 (16%) | 6 (9%) | |||
| 5 or 6 | 3 (4%) | 6 (9%) | |||
| Response not valid/Missing | 1 (1%) | 2 (2%) | |||
|
| |||||
| Q3. How often do you have 4 or more drinks on 1 occasion? | |||||
|
| |||||
| SAMISS not taken | 19 (28%) | 14 (21%) | 0.01 | 0.83 | |
| Never | 20 (29%) | 26 (38%) | |||
| Less than monthly | 22 (32%) | 6 (9%) | |||
| Monthly | 3 (4%) | 7 (10%) | |||
| Weekly | 3 (4%) | 4 (6%) | |||
| Daily or almost daily | 0 (0%) | 1 (1%) | |||
| Response not valid/Missing | 1 (1%) | 2 (2%) | |||
|
| |||||
| Q4. In the past year, how often did you use nonprescription drugs to get high or to change the way you feel? | |||||
|
| |||||
| SAMISS not taken | 19 (28%) | 14 (21%) | 0.92 | 0.74 | |
| Never | 34 (50%) | 31 (46%) | |||
| Less than monthly | 6 (9%) | 4 (6%) | |||
| Monthly | 3 (4%) | 5 (7%) | |||
| Weekly | 1 (1%) | 1 (1%) | |||
| Daily or almost daily | 4 (6%) | 4 (6%) | |||
| Response missing | 1 (1%) | 1 (1%) | |||
|
| |||||
| Q5. In the past year, how often did you use drugs prescribed to you or to someone else to get high or change the way you feel? | |||||
|
| |||||
| SAMISS not taken | 19 (28%) | 14 (21%) | 0.19 | 0.09 | |
| Never | 41 (60%) | 36 (53%) | |||
| Less than monthly | 6 (9%) | 3 (4%) | |||
| Monthly | 1 (1%) | 2 (3%) | |||
| Weekly | 0 (0%) | 1 (1%) | |||
| Daily or almost daily | 0 (0%) | 3 (4%) | |||
| Response missing | 1 (1%) | 1 (1%) | |||
|
| |||||
| Q6. In the past year, how often did you drink or use drugs more than you meant to? | |||||
|
| |||||
| SAMISS not taken | 19 (28%) | 14 (21%) | 0.09 | 0.29 | |
| Never | 26 (38%) | 27 (40%) | |||
| Less than monthly | 15 (22%) | 6 (9%) | |||
| Monthly | 5 (7%) | 4 (6%) | |||
| Weekly | 2 (3%) | 6 (9%) | |||
| Daily or almost daily | 0 (0%) | 2 (3%) | |||
| Response missing | 1 (1%) | 1 (1%) | |||
|
| |||||
| Q7. How often did you feel you wanted or needed to cut down on your drinking or drug use in the past year, and were not able to? | |||||
|
| |||||
| SAMISS not taken | 19 (28%) | 14 (21%) | 0.75 | 0.93 | |
| Never | 35 (51%) | 31 (46%) | |||
| Less than monthly | 6 (9%) | 6 (9%) | |||
| Monthly | 3 (4%) | 4 (6%) | |||
| Weekly | 1 (1%) | 3 (4%) | |||
| Daily or almost daily | 3 (4%) | 1 (1%) | |||
| Response missing | 1 (1%) | 1 (1%) | |||
|
| |||||
| Q8. In the past year, when not high or intoxicated, did you ever feel extremely energetic or irritable and more talkative than usual? | |||||
|
| |||||
| SAMISS not taken | 19 (28%) | 14 (21%) | 0.83 | ||
| Yes | 17 (25%) | 17 (25%) | |||
| No | 31 (46%) | 28 (41%) | |||
| Response missing | 1 (1%) | 1 (1%) | |||
|
| |||||
| Q9. In the past year, were you ever on medication or antidepressants for depression or nerve problems? | |||||
|
| |||||
| SAMISS not taken | 19 (28%) | 14 (21%) | 0.07 | ||
| Yes | 18 (26%) | 9 (13%) | |||
| No | 30 (44%) | 36 (53%) | |||
| Response missing | 1 (1%) | 1 (1%) | |||
|
| |||||
| Q10. In the past year, was there ever a time when you felt sad, blue, or depressed for more than 2 weeks in a row? | |||||
|
| |||||
| SAMISS not taken | 19 (28%) | 14 (21%) | 0.84 | ||
| Yes | 20 (29%) | 20 (29%) | |||
| No | 28 (41%) | 25 (37%) | |||
| Response missing | 1 (1%) | 1 (1%) | |||
|
| |||||
| Q11. In the past year, was there ever a time lasting more than 2 weeks when you lost interest in most things or activities that usually give you pleasure? | |||||
|
| |||||
| SAMISS not taken | 19 (28%) | 14 (21%) | 0.68 | ||
| Yes | 18 (26%) | 19 (28%) | |||
| No | 30 (44%) | 26 (38%) | |||
| Response missing | 1 (1%) | 1 (1%) | |||
|
| |||||
| Q12. In the past year, did you ever have a period lasting more than 1 month when most of the time you felt worried and anxious? | |||||
|
| |||||
| SAMISS not taken | 19 (28%) | 14 (21%) | 0.83 | ||
| Yes | 21 (31%) | 18 (26%) | |||
| No | 27 (40%) | 27 (40%) | |||
| Response missing | 1 (1%) | 1 (1%) | |||
|
| |||||
| Q13. In the past year, did you have a spell or an attack when all of a sudden you felt frightened, anxious, or very uneasy when most people would not be afraid or anxious? | |||||
|
| |||||
| SAMISS not taken | 19 (28%) | 14 (21%) | 0.83 | ||
| Yes | 18 (26%) | 18 (26%) | |||
| No | 30 (44%) | 27 (40%) | |||
| Response missing | 1 (1%) | 1 (1%) | |||
|
| |||||
| Q14. In the past year, did you ever have a spell or an attack when for no reason your heart suddenly started to race, you felt faint, or you could not catch your breath? | |||||
|
| |||||
| SAMISS not taken | 19 (28%) | 14 (21%) | 0.64 | . | |
| Yes | 11 (16%) | 13 (19%) | |||
| No | 37 (54%) | 32 (47%) | |||
| Response missing | 1 (1%) | 1 (1%) | |||
|
| |||||
| Q15. During your lifetime, as a child or adult, have you experienced or witnessed traumatic events that involved harm to yourself or to others? | |||||
|
| |||||
| SAMISS not taken | 19 (28%) | 14 (21%) | 0.54 | ||
| Yes | 27 (40%) | 22 (32%) | |||
| No | 21 (31%) | 23 (34%) | |||
| Response missing | 1 (1%) | 1 (1%) | |||
|
| |||||
| Q16. In the past year, have you been troubled by flashbacks, nightmares, or thoughts of the trauma? | |||||
|
| |||||
| SAMISS not taken | 19 (28%) | 14 (21%) | 0.78 | ||
| Yes | 13 (19%) | 12 (18%) | |||
| No | 14 (21%) | 10 (15%) | |||
| Question was skipped | 22 (32%) | 24 (35%) | |||
|
| |||||
| Q17. In the past 3 months, have you experienced any events or received information that was so upsetting it affected how you cope with everyday life? | |||||
|
| |||||
| SAMISS not taken | 19 (28%) | 14 (21%) | 0.20 | ||
| Yes | 14 (21%) | 19 (28%) | |||
| No | 34 (50%) | 26 (38%) | |||
| Response missing | 1 ( 1%) | 1 (1%) | |||
|
| |||||
| Q18. Have had nightmares about it or thought about it when you did not want to? | |||||
|
| |||||
| SAMISS not taken | 19 (28%) | 14 (21%) | 0.11 | ||
| Yes | 10 (15%) | 17 (25%) | |||
| No | 38 (56%) | 28 (41%) | |||
| Response missing | 1 (1%) | 1 (1%) | |||
|
| |||||
| Q19. Tried hard not to think about it or went out of your way to avoid situations that reminded you of it? | |||||
|
| |||||
| SAMISS not taken | 19 (28%) | 14 (21%) | 0.41 | ||
| Yes | 22 (32%) | 25 (37%) | |||
| No | 26 (38%) | 20 (29%) | |||
| Response missing | 1 (1%) | 1 (1%) | |||
|
| |||||
| Q20. Were constantly on guard, watchful, or easily startled? | |||||
|
| |||||
| SAMISS not taken | 19 (28%) | 14 (21%) | 1.00 | ||
| Yes | 20 (29%) | 18 (26%) | |||
| No | 28 (41%) | 27 (40%) | |||
| Response missing | 1 (1%) | 1 (1%) | |||
|
| |||||
| Q21. Felt numb or detached from others, activities, or your surroundings? | |||||
|
| |||||
| SAMISS not taken | 19 (28%) | 14 (21%) | 0.68 | ||
| Yes | 20 (29%) | 21 (31%) | |||
| No | 28 (41%) | 24 (35%) | |||
| Response missing | 1 (1%) | 1 (1%) | |||
|
| |||||
| 1. Internalized Stigma5 | |||||
|
| |||||
| Mean (Min, Max) | 2.1 (1, 5) | 2.0 (1, 5) | 0.70 | ||
| Median (Q1, Q3) | 2 (1, 3) | 2 (1, 3) | |||
|
| |||||
| 2. HIV Stigma6 | |||||
|
| |||||
| Mean (Min, Max) | 2.9 (1, 5) | 2.9 (1, 5) | 0.21 | ||
| Median (Q1, Q3) | 3 (3, 4) | 3 (2, 4) | |||
|
| |||||
| 3. Community Stigma7 | |||||
|
| |||||
| Mean (Min, Max) | 0.3 (0, 1) | 0.3 (0, 1) | 0.43 | ||
| Median (Q1, Q3) | 0 (0, 1) | 0 (0, 1) | |||
SAMISS questions Q22–24 are only available at month 12; CASI questions Q73–79 (engagement in LGBT community) and Q15 (prior ART use) are only available at baseline.
The “missing” category was not included in the statistical test.
If variable/question is categorical then the p value is from Fisher’s exact test for independence; if variable is numeric then the p value is from Wilcoxon rank sum test.
A Chi square test for trend is also conducted to take account of the trend of variable values if the categorical variable is ordinal.
Average exclude variables with value = 99 (prefer not to answer) from the average.
Average exclude variables with value = 99 (prefer not to answer) from the average.
Calculate the percent “Yes” responses among the variables with responses 1 or 2; exclude 77 (don’t know) and 99 (prefer not to answer).
The table shows the baseline demographics and health characteristics of participants based on viral suppression status. Suppressed indicates that the viral load was <200 copies/mL at the month 12 study visit. Numbers in parentheses incidate percentatges, ranges (min/max), or quartiles (Q1, Q3). N=number; HIV viral load (VL, HIV RNA copies/mL).
Abbreviations: SAMISS: Substance Abuse and Mental Illness Symptoms Screener; Min: minimum, Max: maximum, 1–5; Q: quartertile.
DISCUSSION
HPTN 078 engaged MSM who were PWH and were not virally suppressed and evaluated the impact of a CM intervention on linkage to care and viral suppression. By the final study visit, nearly half of the men (42% in the CM arm and 54% in the SOC arm) achieved viral suppression, which is a notable achievement for this cohort but falls short of national EHE goals. There was no significant difference in viral suppression between the study arms. Although the trial was underpowered for our original objectives, the 95% CI for the intervention effect does exclude a beneficial effect of CM greater than an odds ratio of 1.20. Furthermore, most participant characteristics (i.e., sociodemographic, health, and psychosocial factors) were not associated with viral suppression after 12 months of follow-up although the power to detect such associations was low.
Most study participants reported a prior HIV diagnosis and were, therefore, aware of their status prior to study participation. Likely reasons for viremia among PWH include not being on ART, poor ART adherence, or treatment failure due to inadequate ART regimen and/or drug resistance. HIV drug resistance was detected in 44 (31%) of 143 individuals at study baseline [30]. Given the recruitment challenges of this often neglected population [17], it is noteworthy that the study team was able to engage these men and retain the majority (90%) of participants in the study for 12 months and that nearly half achieved viral suppression over the course of one year. Also worth noting is that this study was conducted in cities in which EHE efforts were being implemented, perhaps contributing to viral suppression improvements regardless of study arm. It is also possible that study engagement, in and of itself, given the numerous incentivized study visits scheduled, facilitated re-engagement in care and treatment across both study arms, thus, potentially diluting intervention impact and leading to viral suppression for nearly half of these men who were not receiving or adhering to optimal ART when recruited into the study. A study of MSM and transgender women in a sub-Saharan Africa observational cohort (HPTN 075), without an intervention to improve ART use or achieve viral suppression, found that, over 12 months, the proportion of participants using ARV drugs increased from 28.1% to 59.4% and the proportion with VLs <400 copies/mL increased from 21.9% to 57.8%; this demonstrates the feasibility of recruiting a predominantly MSM cohort and achieving increased ART uptake and HIV viral suppression without implementing an intervention [31]. In HPTN 078, we observed that viral suppression incrementally increased over the course of the 12-month observation period. Given the potency of current ART regimens, many people starting ART are able to achieve viral suppression relatively quickly (i.e., within 1–2 months) [32]. However, many of the HPTN 078 study participants took longer to achieve viral suppression with an incremental number achieving viral suppression at 3, 6, and 12 months of observation. Social determinants of health and other factors may have limited access to optimal HIV care and treatment impeding more rapid and sustained viral suppression.
It was surprising that so few of the variables measured in this study were associated with viral suppression. It may be that the variables measured were limited, and/or we did not adequately measure the most relevant and important social determinants of health. A paper was recently published which evaluated qualitative data collected during exit interviews with study participants and study case managers in this study which provides greater insight into our main study findings, an in-depth description of the multiple barriers to adherence faced by this largely “out of care” population, as well as a more nuanced understanding of the benefits and challenges of implementing MI into case management [33–35].
Unfortunately, the CM intervention did not enhance viral suppression in comparison to the SOC arm of the study. We believe that the most likely explanation for the lack of an intervention effect in our study is the fact that the clinical care sites where SOC participants were referred by the study team and received their HIV care were able to provide adequate case management services to most patients, including our study participants. This would mean that the enhancements we provided in the CM intervention (i.e., counselors trained to use MI, use of the communication platform) were not significantly distinct from SOC, nor powerful enough as added components, to make a difference in HIV clinical outcomes among our study participants above and beyond the impact of study participation itself, within a one-year timeframe. It is also possible that individuals with multiple intersecting barriers to viral suppression need more than MI to support their complex care needs. Also, since the frequency and type of interactions with CM, as well as use of the communication platform, was determined by the participants, there was a wide range of intervention exposure. Many participants did not perceive a need for, or want, the enhanced case management that was offered to them. Even when we looked at dose intensity, the number of encounters did not enhance intervention impact on viral suppression. However, participants with a greater number of activities with their case managers were actually less likely to be virally suppressed at the end of the study. One possible explanation for this may be that those who needed a lot of help asked for it and received it from the case managers, but the increased supportive activities remained insufficient for greater achievement of viral suppression. Further, and unfortunately, there was a gap between the availability of participants’ viral load data and the timing of the CM sessions. Thus, the case managers were not able to provide counseling that could have been more closely directed to lack of viral suppression in real time.
The study cohort was comprised predominantly of men who reported that they were aware of their HIV status before being tested in the HPTN 078 study; many of the enrolled participants also reported having had HIV care engagement prior to study participation. The number of younger participants (i.e., aged 21 years and under) was also small; the average age of the participants was 39 years, with most between the ages of 41–50. We do not know whether the CM intervention, with its communication platform, may have improved viral suppression among younger men and among men who were newly diagnosed with HIV. Finally, the intervention was not designed to address broader social determinants of health, including social and structural barriers such as intersectional stigma, racism, and lack of culturally sensitive care from providers in healthcare settings. More comprehensive interventions (e.g., at the community, healthcare facility, interpersonal, and individual levels) may be needed to address the multiple societal and behavioral challenges among disenfranchised MSM in the US.
Conclusion
HPTN 078 was a randomized controlled trial comparing an enhanced CM intervention to SOC in four US cities. The study enrolled participants who were mostly older, non-White, and economically disadvantaged with high unemployment; most reported prior knowledge of their HIV-positive status. All had viral loads >1000 copies/mL at study entry, and nearly half achieved viral suppression (<200 copies/mL) within 12 months with no statistically significant difference between the two study arms. It is possible that engagement in care facilitated through study recruitment and participation alone contributed to viral suppression among many of the participants within the course of one year. Reaching targets of at least 90% sustained viral suppression among PWH will likely require an integrated strategies approach that addresses barriers to optimal care and treatment at multiple levels. Further research is needed to achieve this goal, to help end the HIV epidemic among disenfranchised populations in the US and across the globe.
Supplementary Material
Funding
The HIV Prevention Trials Network is funded by the National Institute of Allergy and Infectious Diseases (UM1AI068619 [HPTN Leadership and Operations Center, PI: Myron Cohen, PhD]), UM1AI068613 [HPTN Statistical and Data Management Center, PI: Deborah Donnell, PhD], UM1AI1068613 [HPTN Laboratory Center, PI: Susan Eshleman, PhD]), with co-funding from the National Institute of Mental Health and the National Institute on Drug Abuse, all components of the U.S. National Institutes of Health. This research was supported by the National Institute of Mental Health to the HIV Center for Clinical and Behavioral Studies at NY State Psychiatric Institute and Columbia University (P30-MH43520; PI: Robert Remien, PhD; T32 MH019139, PI: Theodorus Sandfort, PhD).
Footnotes
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
All procedures performed in studies involving human participants were performed in accordance with the ethical standards of the local institutional review board for each study site that approved the protocol prior to study implementation.
Informed Consent
Informed consent was obtained from all individual participants in the study.
References
- 1.Centers for Disease Control and Prevention. Estimated HIV incidence and prevalence in the United States, 2014–2018; 2020. https://www.cdc.gov/hiv/pdf/library/reports/surveillance/cdc-hiv-surveillance-supplemental-report-vol-25-1.pdf
- 2.Centers for Disease Control and Prevention. HIV among gay and bisexual men; 2017. https://www.cdc.gov/hiv/group/msm/index.html
- 3.HIV.gov. People Living with HIV. 2019.; 2019. https://www.hiv.gov/hiv-basics/overview/data-and-trends/statistics
- 4.Koblin BA, Mayer KH, Eshleman SH, Wang L, Mannheimer S, Del Rio C, et al. Correlates of HIV acquisition in a cohort of Black men who have sex with men in the United States: HIV prevention trials network (HPTN) 061. PloS One 2013; 8:e70413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. HIV in the Southern United States; 2019. https://www.cdc.gov/hiv/pdf/policies/cdc-hiv-in-the-south-issue-brief.pdf
- 6.Millett GA, Peterson JL, Flores SA, Hart TA, Jeffries WL, Wilson PA, et al. Comparisons of disparities and risks of HIV infection in Black and other men who have sex with men in Canada, UK, and USA: A meta-analysis. The Lancet 2012; 380:341–348. [DOI] [PubMed] [Google Scholar]
- 7.Jeffries WL. Trends in Diagnosis of HIV Infection, Linkage to Medical Care, and Viral Suppression Among Men Who Have Sex with Men, by Race/Ethnicity and Age—33 Jurisdictions, United States, 2014–2018. MMWR Morb Mortal Wkly Rep 2020; 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gardner EM, McLees MP, Steiner JF, Del Rio C, Burman WJ. The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clin Infect Dis 2011. sss 52:793–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. HIV in the United States and dependent areas; 2020. https://www.cdc.gov/hiv/pdf/statistics/overview/cdc-hiv-us-ataglance.pdf
- 10.Silhol R, Boily M-C, Dimitrov D, German D, Flynn C, Farley JE, et al. Understanding the HIV Epidemic Among MSM in Baltimore: A Modeling Study Estimating the Impact of Past HIV Interventions and Who Acquired and Contributed to Infections. JAIDS J Acquir Immune Defic Syndr 2020; 84:253–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mitchell KM, Hoots B, Dimitrov D, German D, Flynn C, Farley JE, et al. Improvements in the HIV care continuum needed to meaningfully reduce HIV incidence among men who have sex with men in Baltimore, US: a modelling study for HPTN 078. J Int AIDS Soc 2019; 22:e25246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Irvine MK, Robertson MM, Nash D, Kulkarni SG, Braunstein SL, Levin B. HIV Care Coordination promotes care re-engagement and viral suppression among people who have been out of HIV medical care: an observational effectiveness study using a surveillance-based contemporaneous comparison group. AIDS Res Ther 2021; 18:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xie N, Hu X, Yan H, Ruan L, Liu C, Hu R, et al. Effects of Case Management on Risky Sexual Behaviors and Syphilis Among HIV-Infected Men Who Have Sex With Men in China: A Randomized Controlled Study. Sex Transm Dis 2022; 49:22–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Metsch LR, Feaster DJ, Gooden L, Matheson T, Stitzer M, Das M, et al. Effect of patient navigation with or without financial incentives on viral suppression among hospitalized patients with HIV infection and substance use: a randomized clinical trial. Jama 2016; 316:156–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sheehan DM, Dawit R, Gbadamosi SO, Fennie KP, Li T, Gebrezgi M, et al. Sustained HIV viral suppression among men who have sex with men in the Miami-Dade County Ryan White Program: the effect of demographic, psychosocial, provider and neighborhood factors. BMC Public Health 2020; 20:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fauci AS, Redfield RR, Sigounas G, Weahkee MD, Giroir BP. Ending the HIV epidemic: a plan for the United States. Jama 2019; 321:844–845. [DOI] [PubMed] [Google Scholar]
- 17.Beyrer C, Malone J, Baral S, Wang Z, Rio CD, Mayer KH, et al. Comparing recruitment strategies to engage hard-to-reach men who have sex with men living with HIV with unsuppressed viral loads in four US cities: Results from HPTN 078. J Int AIDS Soc 2021; 24:e25798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abuse S The Substance Abuse and Mental Illness Symptoms Screener (SAMISS)–Key.
- 19.Simoni JM, Pearson CR, Pantalone DW, Marks G, Crepaz N. Efficacy of interventions in improving highly active antiretroviral therapy adherence and HIV-1 RNA viral load: a meta-analytic review of randomized controlled trials. J Acquir Immune Defic Syndr 1999. 2006; 43:S23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DiIorio C, Resnicow K, McDonnell M, Soet J, McCarty F, Yeager K. Using motivational interviewing to promote adherence to antiretroviral medications: a pilot study. J Assoc Nurses AIDS Care 2003; 14:52–62. [DOI] [PubMed] [Google Scholar]
- 21.Safren SA, Otto MW, Worth JL, Salomon E, Johnson W, Mayer K, et al. Two strategies to increase adherence to HIV antiretroviral medication: life-steps and medication monitoring. Behav Res Ther 2001; 39:1151–1162. [DOI] [PubMed] [Google Scholar]
- 22.Samet JH, Horton NJ, Meli S, Dukes K, Tripps T, Sullivan L, et al. A randomized controlled trial to enhance antiretroviral therapy adherence in patients with a history of alcohol problems. Antivir Ther 2005; 10:83–93. [DOI] [PubMed] [Google Scholar]
- 23.Christian P, Laurence B, Bruno S, Catherine T-T, Michel M, Marc S, et al. Efficacy of an educational and counseling intervention on adherence to highly active antiretroviral therapy: French prospective controlled study. HIV Clin Trials 2003; 4:121–131. [DOI] [PubMed] [Google Scholar]
- 24.Huang D, Sangthong R, McNeil E, Chongsuvivatwong V, Zheng W, Yang X. Effects of a phone call intervention to promote adherence to antiretroviral therapy and quality of life of HIV/AIDS patients in Baoshan, China: a randomized controlled trial. AIDS Res Treat 2013; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lester RT, Ritvo P, Mills EJ, Kariri A, Karanja S, Chung MH, et al. Effects of a mobile phone short message service on antiretroviral treatment adherence in Kenya (WelTel Kenya1): a randomised trial. The Lancet 2010; 376:1838–1845. [DOI] [PubMed] [Google Scholar]
- 26.Sidney K, Antony J, Rodrigues R, Arumugam K, Krishnamurthy S, D’souza G, et al. Supporting patient adherence to antiretrovirals using mobile phone reminders: patient responses from South India. AIDS Care 2012; 24:612–617. [DOI] [PubMed] [Google Scholar]
- 27.Lewis MA, Uhrig JD, Bann CM, Harris JL, Furberg RD, Coomes C, et al. Tailored text messaging intervention for HIV adherence: A proof-of-concept study. Health Psychol 2013; 32:248. [DOI] [PubMed] [Google Scholar]
- 28.Irvin R, Gamble T, Malone J, Wang Z, Wilson E, Hughes J, et al. HPTN 078: High prevalence of HCV antibodies among men who have sex with men (MSM). 2019.
- 29.Gamble T, Wang Z, Gaydos C, Batey D, Mayer K, del Rio C, et al. Finding men who have sex with men (MSM) who may be potential amplified HIV transmitters: Results from HPTN 078. 2018.
- 30.Fogel JM, Sivay MV, Cummings V, Wilson EA, Hart S, Gamble T, et al. HIV drug resistance in a cohort of HIV-infected MSM in the United States. Aids 2020; 34:91–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Palumbo PJ, Zhang Y, Clarke W, Breaud A, Sivay M, Cummings V, et al. Uptake of antiretroviral treatment and viral suppression among men who have sex with men and transgender women in sub-Saharan Africa in an observational cohort study: HPTN 075. Int J Infect Dis 1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Colasanti J, Sumitani J, Mehta CC, Zhang Y, Nguyen ML, Del Rio C, et al. Implementation of a rapid entry program decreases time to viral suppression among vulnerable persons living with HIV in the Southern United States. Oxford University Press US; 2018. p. ofy104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brennan-Ing M, Seidel L, Rodgers L, Ernst J, Wirth D, Tietz D, et al. The impact of comprehensive case management on HIV client outcomes. PLoS One 2016; 11:e0148865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kushel M, Colfax G, Ragland K, Heineman A, Palacio H, Bangsberg DR. Case management is associated with improved antiretroviral adherence and CD4+ cell counts in homeless and marginally housed individuals with HIV infection. Clin Infect Dis 2006; 43:234–242. [DOI] [PubMed] [Google Scholar]
- 35.Tolley EE, Hamilton EL, Eley N, Maragh-Bass AC, Okumu E, Balán IC, et al. The role of case management in HIV treatment adherence: HPTN 078. AIDS Behav 2022; :1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


