Objective:
To define benchmark cutoffs for redo liver transplantation (redo-LT).
Background:
In the era of organ shortage, redo-LT is frequently discussed in terms of expected poor outcome and wasteful resources. However, there is a lack of benchmark data to reliably evaluate outcomes after redo-LT.
Methods:
We collected data on redo-LT between January 2010 and December 2018 from 22 high-volume transplant centers. Benchmark cases were defined as recipients with model of end stage liver disease (MELD) score ≤25, absence of portal vein thrombosis, no mechanical ventilation at the time of surgery, receiving a graft from a donor after brain death. Also, high-urgent priority and early redo-LT including those for primary nonfunction (PNF) or hepatic artery thrombosis were excluded. Benchmark cutoffs were derived from the 75th percentile of the medians of all benchmark centers.
Results:
Of 1110 redo-LT, 373 (34%) cases qualified as benchmark cases. Among these cases, the rate of postoperative complications until discharge was 76%, and increased up to 87% at 1-year, respectively. One-year overall survival rate was excellent with 90%. Benchmark cutoffs included Comprehensive Complication Index CCI® at 1-year of ≤72, and in-hospital and 1-year mortality rates of ≤13% and ≤15%, respectively. In contrast, patients who received a redo-LT for PNF showed worse outcomes with some values dramatically outside the redo-LT benchmarks.
Conclusion:
This study shows that redo-LT achieves good outcome when looking at benchmark scenarios. However, this figure changes in high-risk redo-LT, as for example in PNF. This analysis objectifies for the first-time results and efforts for redo-LT and can serve as a basis for discussion about the use of scarce resources.
Keywords: benchmark, retransplantation, redo liver transplantation
The availability of liver transplantation (LT) has revolutionized the treatment of many patients with advanced liver diseases and liver cancer.1–3 This success has generated a dramatic shortage of available organs with the consequence to consider marginal (also called extended criteria) grafts. The use of these livers, however, carries an increased risk for graft failure, with the potential need also for secondary transplants.4,5 Such redo liver transplants (redo-LT) after initial failure are generally perceived to be associated with outrageous cost, and several transplant physicians may consider redo procedures as futile or unethical, in view of scarce resources.6,7 Importantly, however, redo-LT may vary highly in terms of indications, for example, for recurrence of the underlying disease versus acute graft failure.
It therefore seems crucial to have objective benchmark values for these challenging procedures, serving as references to compare with primary LT, or higher-risk population requiring a redo-LT.
Accordingly, in this study, we aim to establish clinically relevant thresholds gathered in high-volume centers on 3 continents. For this purpose, an ideal cohort serving as benchmark redo-LT cohort was defined using a well-established methodology previously used to assess primary LT,8,9 and other major procedures.10–17 The benchmark values were subsequently used to assess outcome of redo-LT in recipients with severe liver disease stages, and in patients requiring an emergency high-risk redo-LT, such as those with primary nonfunction (PNF).
METHODS
Study Design
Benchmarks in redo-LT were established according to a standardized methodology as previously reported for other complex surgical procedures,8–17 and critically refined by a panel of experts through a Delphi consensus finding process.18
International high-volume LT reference centers were selected based on a caseload of ≥50 LT per year, having published in the field of LT, and holding a comprehensive prospective patient database covering a minimum follow-up of 2 years. The final collaborative group included 22 centers: 13 from Europe, 8 from North America, and 1 from South America. No Asian center could be included due to the small number of available cadaveric grafts.
Study Population and Case Selection
The centers provided details of all adults (18 years and above) redo-LT they performed between January 2010 and December 2018. Third or more LT and redo-LT with combined other organ transplantations, living donors, split grafts or domino livers were excluded.
Following our previous benchmark analysis for primary LT8 we defined ideal redo-LT by excluding all cases, which were listed with high urgent priority and/or underwent redo-LT within the first 30 days after primary LT, thus including all cases with PNF or acute hepatic artery thrombosis (HAT). Furthermore, we considered in the benchmark cohort only redo-LT with liver grafts from brain death donors, and on recipients with a relatively low laboratory model of end stage liver disease (labMELD) score ≤25, with no life support, according to previous studies.19–23 Finally, we also excluded technical difficult scenarios such as recipient portal vein thrombosis (Supplementary Digital Content Table 1, Supplemental Digital Content 1, http://links.lww.com/SLA/E109).24
Comparison Cohorts
To test the derived thresholds from the benchmark cohort, we created several comparator groups with different risk profiles and compared their outcomes with those of the benchmark group.
Finally, we compared each benchmark value with previous studies in primary LT.8,9
Data Collection, Follow-up, and Outcome
Investigators of participating centers entered deidentified recipient, graft, and outcome-specific data into a predesigned spreadsheet and forwarded them via a secure file transfer (https://transfer.usz.ch/) to the local investigator at the University Hospital Zurich, Switzerland, who checked the data for completeness.
Postoperative complications were collected at 5 postoperative time points (discharge, 3, 6, 12, and 24 mo) and graded by severity according to the Clavien-Dindo system.25,26 Cumulative morbidity was summarized by the Comprehensive Complication Index CCI®.27 According to the inaugural study on primary LT,8 which showed that grade 1 complications have only minimal impact on the patient care and do not influence the CCI®, we did not record grade 1 complications. Thus, the complication rates we report hereafter correspond to complications grade ≥2.
The study protocol was approved by the Cantonal Ethics Committee of Zurich, Switzerland, and by the institutional review boards of participating centers.
Benchmark Values and Cutoffs
We selected 20 benchmark values, most of which were similar to the previous reported primary LT benchmark study.8 They included duration of recipient-hepatectomy and whole transplantation surgery, number of blood transfusions until 24 hours postoperative, length of intensive care unit and hospital stay, newly need for renal replacement therapy after redo-LT until discharge, PNF (defined as graft failure resulting in death or third transplantation within 7 days of redo-LT excluding other causes of graft failure such as vascular thrombosis, rejection, or recurrent disease) and intra-abdominal bleeding. Any complications and severe (Clavien-Dindo grade ≥3) complications, the CCI®, biliary complications, HAT, second redo-LT and graft and patient survival were presented with benchmark cutoffs at discharge, 3 months, 6 months, and 1 year.
To determine benchmark cutoffs, median values of the continuous outcome variables and proportions of the categorical outcome variables were calculated separately for each participating center. Based on these center-specific median and proportion values, the 75th percentile of each outcome indicator was considered the benchmark cutoff, and thus the “best achievable” result.10,18
Statistical Analysis
Discrete variables were described using counts (percent), and continuous variables were described using medians [with interquartile range (IQR)]. The Pearson product-moment correlation coefficient was used to explore surgical volume-outcome correlations. Statistical analysis were performed using the R software 4.1.1 (R Foundation, Vienna, Austria).28
RESULTS
Benchmark Cohort and Cutoffs
We identified 373 (34%) benchmark cases from 17 centers of 1110 redo-LT, performed by 22 centers over the 9-year study period (Supplementary Digital Content Figure 1, Supplemental Digital Content 1, http://links.lww.com/SLA/E109). The proportion of benchmark cases varied widely among centers (range: 0%–60%) (Fig. 1). Baseline characteristics of benchmark and nonbenchmark patients are presented in Supplementary Digital Content Table 2 (Supplemental Digital Content 1, http://links.lww.com/SLA/E109).
FIGURE 1.
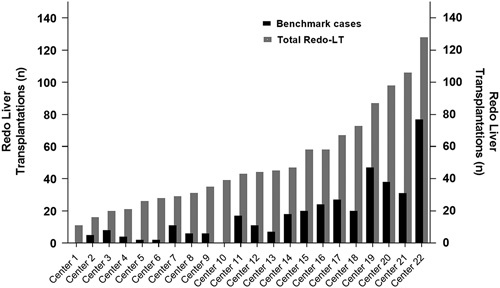
Distribution of redo liver transplantation among transplant centers. There is substantial variation in the proportion of benchmark cases among the 22 expert centers.
Benchmark recipients consisted of predominantly male patients (222 of 373; 60%), displayed a median age of 50 years (IQR: 39–59), and a median labMELD score of 17 points (IQR: 11–22). The median donor age in the benchmark cohort was 49 years (IQR: 37–61), the median cold ischemia time was 7.5 hours (IQR: 6.1–9.2 hours). The main indications for redo-LT included biliary complications (42%), recurrence of the underlying liver disease (32%), late arterial complications (24%), and rejection (18%). One- and 2-year overall survival rates were excellent with 90% and 88%, respectively. The rate of postoperative complications until discharge was high with 76%, and increased up to 87% at 1 year, respectively. Of note, PNF occurred only in 9 (2.4%) of these benchmark redo-LT. Looking at the cumulative burden of morbidity, median CCI® at discharge was 29.6 (IQR: 20.9–51.7) and increased to 44.9 (IQR: 20.9–73.6) at 1 year. The resulting benchmark cutoffs are listed in Table 1.
TABLE 1.
Benchmark Cutoffs in Redo Liver Transplantation
| Perioperative Course | ||||
| Recipient hepatectomy duration | ≤4.0 h | |||
| Operation duration | ≤8.1 h | |||
| Blood transfusions within 24 h after surgery | ≤8 units RBC | |||
| Newly need for dialysis | ≤20% | |||
| Intensive care unit stay | ≤6 d | |||
| Hospital stay | ≤21 d | |||
| Postoperative morbidity and mortality | Discharge | 3 mo | 6 mo | 1 y |
| Any complication | ≤94% | ≤100% | ≤100% | ≤100% |
| ≥Grade 3a complication | ≤60% | ≤65% | ≤71% | ≤72% |
| CCI® | ≤40 | ≤48 | ≤52 | ≤72 |
| Primary nonfunction | ≤2.7% | ≤2.7% | ≤2.7% | ≤2.7% |
| Intra-abdominal bleeding | ≤23% | ≤23% | ≤23% | ≤23% |
| Any biliary complication | ≤15% | ≤20% | ≤24% | ≤30% |
| Anastomotic stricture | ≤4% | ≤14% | ≤17% | ≤25% |
| Nonanastomotic stricture | 0% | ≤5% | ≤5% | ≤5% |
| Biliary leakage | ≤9% | ≤9% | ≤9% | ≤9% |
| Any arterial complication | ≤6% | ≤15% | ≤15% | ≤15% |
| Hepatic artery thrombosis* | ≤3.2% | ≤6.5% | ≤6.5% | ≤6.5% |
| Graft-loss | ≤16% | ≤19% | ≤20% | ≤20% |
| Redo redo liver transplantation | ≤8% | ≤9% | ≤11% | ≤11% |
| Mortality | ≤13% | ≤13% | ≤14% | ≤15% |
Values are the 75th percentile of centers median.
Hepatic artery thrombosis (HAT) are usually divided into early (within the first month postoperatively) and late (after 1 mo postoperatively) HAT depending on the timing of their occurrence. Taking this into account, the benchmark values are 5% and 0%, respectively.
RBC indicates red blood cells.
Influence of Center Volume on Outcome Performance
A significant correlation was observed between center volume and center-specific outcome parameters, with decreasing postoperative morbidity CCI® at 1 year in correlation with increasing caseload (Pearson R=−0.55, P=0.0082; Fig. 2).
FIGURE 2.
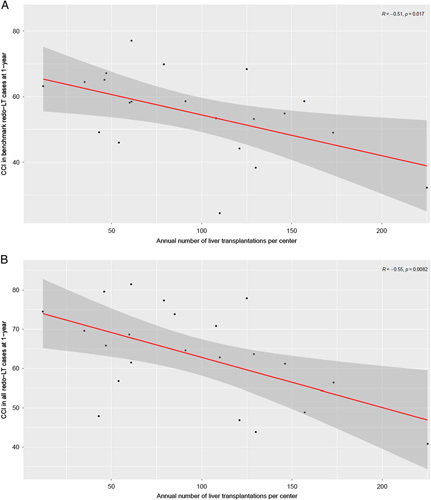
Pearson correlation between transplant center volume and center-specific surgical outcome. There is a highly significant correlation between the annual liver transplant caseload per center and the center-specific CCI® at 1 year in (A) benchmark redo-LT cases and (B) all redo-LT, respectively.
Validation of the Benchmark Criteria
To verify the relevance of the selected benchmark criteria, we compared postoperative outcomes between the benchmark and nonbenchmark cohort. The complication rate at 1 year was 87% in the group of benchmark cases and reached 96% in nonbenchmark cases. The nonbenchmark patient profile represented an odds ratio of 3.3 (95% confidence interval: 2.1–5.2, P<0.001) for the development of any complications during the first postoperative year (Supplementary Digital Content Figure 2, Supplemental Digital Content 1, http://links.lww.com/SLA/E109).
Higher-risk Cohorts
The newly determined benchmark cutoffs were subsequentially compared with the outcomes of redo-LT in recipients with different risk profiles. First, we looked at sicker recipients represented by a labMELD score ≥30 (Table 2). In this cohort of 112 patients with a median labMELD score of 34 (IQR: 32–37), 92 (82%) showed at least one severe complication at 1 year (benchmark 72%), resulting in a median CCI® at 1 year of 60 (IQR: 40–96) (benchmark 72). In-hospital mortality was 16% (18 patients) (benchmark 13%) and increased to 21% (23 patients) at 1-year follow up (benchmark 15%).
TABLE 2.
Outcomes after Redo Liver Transplantation in 2 Higher-Risk Groups Compared With Benchmark Cutoffs
| MELD ≥30 (n=112) | Redo-LT for PNF (n=143) | Redo-LT Benchmark Cutoff | |
|---|---|---|---|
| Perioperative course | |||
| Recipient hepatectomy duration, h | 2.8 (2.0–4.2) | 1.3 (0.6–2.2) | ≤4.0 |
| Operation duration, h | 7.8 (6.6–9.0) | 5.0 (3.9–6.1) | ≤8.1 |
| Blood transfusion, units of RBC | 7 (4–17) | 3 (0–6) | ≤8 |
| Newly need for dialysis | 35 (31) | 31 (22) | ≤20% |
| Intensive care unit stay, d | 6 (3–10) | 14 (6–26) | ≤6 |
| Hospital stay, d | 20 (12–38) | 27 (16–46) | ≤21 |
| Postoperative morbidity and mortality at 1 y | |||
| Any complication | 103 (92) | 141 (99) | ≤100% |
| ≥Grade 3a complication | 92 (82) | 130 (91) | ≤72% |
| CCI® | 60 (40–96) | 86 (58–100) | ≤72 |
| Primary nonfunction | 4 (3.6) | 18 (12.6) | ≤2.7% |
| Intra-abdominal Bleeding | 31 (28) | 26 (18) | ≤23% |
| Any biliary complication | 22 (20) | 21 (15) | ≤30% |
| Anastomotic stricture | 15 (13) | 12 (8.4) | ≤25% |
| Nonanastomotic stricture | 3 (2.7) | 0 (0) | ≤5% |
| Biliary leakage | 9 (8.0) | 9 (6.3) | ≤9% |
| Hepatic artery thrombosis | 5 (4.5) | 5 (3.5) | ≤6.5% |
| Graft-loss | 29 (26) | 58 (41) | ≤20% |
| Redo redo liver transplantation | 8 (7) | 3 (2) | ≤11% |
| Mortality | 23 (21) | 58 (41) | ≤15% |
Data shown as median and IQR or number and proportion (%).
RBC indicates red blood cells.
In a second step, we compared the benchmark values with the outcomes of an emergency retransplant group, which consisted of 143 recipients who underwent urgent redo-LT because of PNF, and found dramatically worse outcomes (Table 2). For example, in-hospital mortality rate was almost 3 times the benchmark value (36 vs. 13%), and median CCI® at discharge was 70 (IQR: 44–100) (benchmark 40). Of note, 14 patients (10%) received a liver graft from circulatory death donor.
To address technically challenging situations, we analyzed additional 54 cases with recipient PVT (Supplementary Digital Content Table 3, Supplemental Digital Content 1, http://links.lww.com/SLA/E109). Again, 1-year morbidity (CCI® 65, IQR: 44–99) and mortality (24%) were well above the benchmark cutoffs of 45 and 10%, respectively.
Comparison With Primary LT Benchmark Cutoffs
Finally, we compared the benchmark cutoffs of elective redo-LT with the previously reported benchmarks for primary donation after brain death (DBD) and donation after circulatory death (DCD) liver transplants, respectively (Table 3).8,9 For all outcome parameters, the cutoff values of redo-LT were higher than those of primary LT. For example, the CCI® benchmark cutoff at 1 year for redo-LT was 30 points higher than for DBD LT, and 33 points higher than for DCD LT. The difference was also striking for the mortality benchmark cutoff, which was ≤15% for redo-LT, compared with ≤9% for DBD LT and ≤9.6% for DCD LT. In contrast, the difference of benchmark values in transplant-specific complications such as biliary complications or HAT were less impressive.
TABLE 3.
Benchmark Cutoffs for Redo Liver Transplantation Compared With Primary Liver Transplantation
| Redo-LT | Primary DBD LT | Primary DCD LT | |
|---|---|---|---|
| Perioperative course | |||
| Operation duration | ≤8.1 h | ≤6 h | ≤6.8 h |
| Blood transfusions | ≤8 units of RBC | ≤3 units of RBC | ≤3 units of RBC |
| Newly need for dialysis | ≤20% | ≤8% | ≤9.6% |
| Intensive care unit stay | ≤6 d | ≤4 d | ≤3 d |
| Hospital stay | ≤21 d | ≤18 d | ≤16 d |
| Postoperative morbidity and mortality at 1 y | |||
| Any complication | ≤100% | ≤94% | ≤95% |
| ≥Grade 3a complication | ≤72% | ≤59% | ≤66% |
| CCI® | ≤72 | ≤42 | ≤39 |
| Primary nonfunction | ≤2.7% | NA | ≤2.5% |
| Intra-abdominal bleeding | ≤23% | NA | ≤10% |
| Any biliary complication | ≤30% | ≤28% | NA |
| Hepatic artery thrombosis | ≤6.5% | ≤4.4% | ≤4.5% |
| Graft-loss | ≤20% | ≤11% | ≤14.4% |
| Mortality | ≤15% | ≤9% | ≤9.6% |
Values are the 75th percentile of centers median.
NA indicates not available; RBC, red blood cells.
DISCUSSION
This international, multicenter study defines new benchmark values after redo-LT by using a well-established benchmark methodology.10,18,29 While the results corroborate the poorer outcome when compared with primary LT, this risk is considerably less in elective redo-LT compared with emergencies or complex scenarios. This novel information may help in the critical controversial discussion whether to offer a second chance for receiving a liver in sick patients.
A key element of benchmarking is the definition of an appropriate benchmark cohort. Ideally, the cohort should consist of low-risk cases, although the term low-risk must be defined for each index operation. In the previous benchmark studies for primary LT,8,9 recipient-specific and donor-specific as well as technical criteria have proven useful, and we adopted most of them in the current study. A recipient labMELD score cutoff of a maximum of 20 points was, however, not reasonable in our study because recipients requiring redo-LT present with more advanced disease stages, recognizable by the higher median labMELD score of 24 points (IOR: 16–32) in our retransplant cohort; for example, compared with 14 points (IQR: 10–19) in the cohort of the DCD benchmark study from the United Kingdom.9 Accordingly, the median CCI® at 1 year increased from 45 points in recipients with a labMELD score ≤25 to 60 in those with a labMELD score >25 (P<0.001), and 1-year mortality rate from 9.9% to 20% (P=0.001), supporting the decision to use an optimal cutoff of labMELD 25 in this study.
A particular feature of transplantation is the dependence of outcomes on organ quality, especially in high-risk patients. For this reason, we excluded all partial livers from the outset and included only DBD transplants in our benchmark cohort. However, it is striking, that although our data came from well-established center databases, donor-specific and graft-specific data were often unknown. For example, information on donor steatosis was available in only half of the cases (n=505) making this parameter unsuitable for distinguishing between benchmark and nonbenchmark cases. Data for cold ischemia time and donor age were also missing in about 10% of cases each. Furthermore, cases with available donor-data showed a relatively homogeneous distribution [median cold ischemia time 7.2 hour (IQR: 5.8–8.7 hours) and median donor age of 48 years (IQR: 33–60]). In view of these circumstances, we decided to limit the benchmark criteria for redo-LT predominantly on recipient parameters.
Benchmark values are designed to support practice. It is therefore clear that we cannot consider all confounders from the benchmark cohort without being too restrictive compromising clinical relevance. This is for example illustrated by the comparison of hospitalized and nonhospitalized patients within the benchmark group. Applying hospitalization as an exclusion criterion would shrink the benchmark cohort by additional 88 patients to only 285 patients, representing only one quarter of the total redo-LT cohort. Nevertheless, it is important to mention that the benchmark patients, who were at home before redo-LT had superior outcomes close to those with primary LT. With an in-hospital and 1-year mortality of 4.6% and 7.7%, respectively, they were well within the benchmark values of primary LT. This is a good example about how new insights can be provided through benchmark studies.
Another key element of benchmarking is center selection. Centers participating in the establishment of benchmark values should be reference centers.18 Criteria such as center volume can be seen as surrogate markers for center expertise. The 17 centers represented in our benchmark cohort performed a median of 108 LT/year (IQR: 60–130 LT/year), fulfilling the recommended minimum caseload of 50 LT/year.18 Recently, surgeon volume was added as a new surrogate marker of quality.18,30 In our study the median number of LT per surgeon was 19 cases/year (IQR: 14–22 LT/year). However, the significance of this number in a study for redo-LT is questionable, as it is common practice in most transplantation centers that such difficult surgery is performed by 2 staff surgeons, typically involving the most experienced members of the team.
It is further noteworthy to mention, that a follow-up of at least 12 months after primary LT is necessary to adequately assess the morbidity of surgery.8 Consistently, benchmark cutoffs for CCI® and biliary complications increase significantly after 6 months up to 1 year postoperatively (eg, CCI® from 52 to 72 points and biliary complications from 24 to 30, respectively) underlining the need for a minimum follow-up of 1 year also for redo-LT.8
Accordingly, with a very high 1-year benchmark morbidity of 100%, and a benchmark mortality of 15%, the best achievable results in redo-LT are expectedly inferior compared with primary LT8,9 and also compared with other major liver10,13 and abdominal surgeries.11,12,14–16 Only a benchmark study looking at surgery for perihilar cholangiocarcinoma, as presented last year in the ESA meeting, had comparable high morbidity and mortality rates.17
Benchmark redo-LT disclosed however a lower risk compared to emergency redo-LT, as for example in PNF cases, where surgeons are confronted with severe time issues due to the lack of available methods to bridge liver failure. This crisis scenario compromises the acceptance of marginal livers for such sickest recipients. Even, in this cohort, livers from donors after circulatory death were accepted in 10% of patients of the PNF cases. Mortality rates exploded consecutively to 36% at discharge and 40% after 1 year, respectively. The situation is different for redo-LT due to early HAT. Here, most outcomes were only slightly outside or even within the benchmark cutoffs and should therefore not be equated with the results of other emergency redo-LT.
Another question relates to the correlation of center volume to surgical outcomes. We found a strong correlation between the annual liver transplant caseload and the outcome in redo-LT. To a lesser extent, this correlation also exists between redo-LT caseload and surgical outcome. This second correlation may, however, relate to the total center volume since a higher redo-LT caseload occurred mostly in higher volume centers in this benchmark cohort (Supplementary Digital Content Figure 3, Supplemental Digital Content 1, http://links.lww.com/SLA/E109).
This study has inherent limitations. Due to the retrospective character, complications may have been recorded differently with potential underestimation of complications. This is, however, minimized by omitting recording grade 1 according to the conclusions from the previous benchmark study in LT, which show no influence of grade 1 complication on the calculation of CCI® or other endpoints. We also had little information regarding graft quality, such as steatosis, therefore such information remains poorly defined in the benchmark analysis. We present, however, the largest cohort of redo-LT cases worldwide, enabling the establishment of credible reference thresholds for many postoperative endpoints, importantly including morbidity.
In conclusion, this multicentric study provides novel benchmark values for redo-LT, which may serve as reference for evaluating other groups of redo-LT, and particularly higher risk scenarios like PNF. The study, however, suggests that outcomes are highly acceptable for ideal (benchmark) retransplant candidates, justifying redo-LT, even at a time of severe organ shortage.
Supplementary Material
ACKNOWLEDGMENTS
The authors convey their appreciation for the help in preparing the manuscript to Dr Milo Puhan, University of Zurich and Dr Ashish Saharia, Houston Methodist Hospital, Houston and they thank Susanne Gaal from the University of Zurich, for contributing to the coordination of the study.
DISCUSSANTS
Johann Pratschke (Berlin, Germany)
At first, I very much appreciate the privilege to be the first discussant of this study on benchmarking in redo liver transplantation (redo-LT). I would like to congratulate the authors for preparing this international analysis. As we heard during Ms. Abbassi’s talk, the authors have collected data from 1110 redo-LT. Out of this cohort, 34% qualified as benchmark cases. They could show that outcomes are excellent when patient selection is “ideal.” While reading this manuscript, my first impression was that, after all, we know from our everyday work with transplant patients that recipients in good general condition, who are nonhospitalized, low-MELD, and transplanted using high quality DBD allografts from relatively young donors with short cold ischemia, will normally have excellent outcomes. Obviously, this should not differ much from those with primary LTs. Nowadays, emergency redo-LTs and complex cases represent borderline indications, sometimes, with devastating outcomes. Therefore, the message of this study is rather predictable. However, the strong side of the paper is the large sample size of 22 international centers.
I have the following questions:
First, especially in the current MELD era, and due to the increasing pressures of organ shortages and financial constraints, futility is increasingly in the limelight in clinical research. Should we really focus on defining benchmarks in potentially high-risk scenarios, such as redo-LT, or would it be more clinically relevant or appropriate to define futility cut-off values instead?
Second, do the authors think that the defined benchmarks could be extended by the modulation of various modifiable recipient or donor risk factors without negatively impacting the outcomes and “downstaging” higher risk patients to benchmark outcome levels?
Third, although this is a large multi-center study, some countries and regions with major transplant programs were underrepresented, e.g. only 1 South American center and 0 centers from Spain, Brazil, Germany, Australia were included. This center selection carries some potential bias. Could you please comment on this?
Response from Pierre-Alain Clavien (Zurich, Switzerland)
Many thanks, Professor Pratschke, for your insights and questions. Regarding your first point on the predictable results and somewhat lack of novelty of these findings, I must emphasize that the topic of redo-LT remains highly controversial, and many cases are still turned down in many centers, simply because the risk is considered to be too high. We believe, therefore, that the well-established methodology of benchmarking offers objective and clinically relevant data on outcome, particularly enabling comparisons among different categories of redo-LT. Our main objective here was to present solid data on redo-LTs. Your second point suggests that we should focus on futility criteria. Many attempts were made at identifying futility criteria, but no consensus was ever reached. While the multicentric study on benchmark cases demonstrates that most patients survive with a good functioning liver at a decent follow-up, the comparison with redo-LT due to primary nonfunction (PNF) discloses a much poorer outcome, in contrast to hepatic artery thrombosis (HAT). However, defining futility cut-offs for such complex and dramatic situations would be highly problematic in ethical terms and hardly applicable in today's world.
With regard to your second question on modulating or “downstaging” risk factors, the reality is that we can only intervene to a limited extent. For example, we are unable to simply extubate patients, make them younger, or influence the MELD score. The same is, unfortunately, true for the optimization of donor risk factors. We can reduce ischemia times, but we cannot make grafts younger, or reduce steatosis. We still must accept what we get, particularly for the emergency scenario of PNF.
Finally, regarding the distribution of centers worldwide, many centers could not be included since they failed to meet the required caseload. Most centers in some parts of the world, such as Asia, focused on living donation, which was excluded from our analysis, and lastly, some qualifying centers failed to supply the data. We would, however, like to state that we included 22 large centers, providing 1110 cases of redo-LT, including 373 benchmark cases, which we believe offer robust information.
Tomoaki Kato (New York, USA)
Congratulations on the effort and your excellent paper. However, I have a hard time accepting primary nonfunction as a high risk in redo-LTs. As transplant surgeons, we made a rule that, if we selected an organ and made a bad choice, causing the patient to suffer the consequences of it, we should then prioritize them for a re-transplant. On the other hand, we know that there are some patients with very bad intraoperative courses, such as massive bleeding. Even if a good organ goes in, it can still become a primary nonfunction. In such cases, re-transplant is probably high risk; however, in cases clearly caused by organ selection, they may not necessarily be high risk for a re-transplant. So, do you differentiate between these two in your analysis?
Response from Pierre-Alain Clavien (Zurich, Switzerland)
Thank you very much, Professor Kato, for your important remarks. Regarding your first question on the somewhat liability of the transplant surgeon for redo-LT in case of PNF, we cannot ignore the almost 50% mortality rate from this benchmark study. While we are not presenting this as a futility criterion, centers must decide whether to proceed or not, also thinking about organ utility. Of course, any experienced team knows that the quality of the organ may influence outcome. If you add a severe steatotic graft to the balance of risk, you may only expect a dismal outcome. So, we are confronted with this dilemma, and redo-LT in a PNF scenario remains a decision for each individual center to make. Now, hopefully, the new data available in this paper can help facilitate the decision-making process.
Christiane Bruns (Cologne, Germany)
Thank you very much for the presentation. I do have a quite similar question. You used the values of the 75th percentile of recipients as a benchmark for redo-LT, and then, compared this collective to regular transplant recipients. Did you also determine the values of the 75th percentile as a benchmark for the respective transplanted organs?
Response from Pierre-Alain Clavien (Zurich, Switzerland)
Thank you, Professor Bruns, for this question, and this very nicely touches on the rationale of the novel benchmark study and its use. Benchmark values gathered in “ideal scenarios” offer a basis for various outcome parameters. However, at this point, we could not establish benchmark values for offered organs, as the registered data is incomplete, e.g., we lack data on donor liver histology. Our study design was, therefore, restrictive, excluding any donor livers with additional donor warm ischemia, or livers with additional technical difficulties, eg, partial grafts.
Footnotes
The authors report no conflicts of interest.
This study is supported by the LGID (Liver and Gastrointestinal Disease) Foundation.
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.annalsofsurgery.com.
Contributor Information
Daniel Gero, Email: Daniel.Gero@usz.ch.
Xavier Muller, Email: xmuller.ucl@gmail.com.
Alba Bueno, Email: alba.buenojimenez@nhs.net.
Wojciech Figiel, Email: w.figiel@yahoo.es.
Fabien Robin, Email: fabien.robin@chu-rennes.fr.
Sophie Laroche, Email: laroche.sp@gmail.com.
Benjamin Picard, Email: benjamin.picard@aphp.fr.
Sadhana Shankar, Email: sadhana.shankar@nhs.net.
Tommy Ivanics, Email: tommy.Ivanics@uhn.ca.
Marjolein van Reeven, Email: m.vanreeven@erasmusmc.nl.
Otto B. van Leeuwen, Email: o.b.van.leuuwen@umcg.nl;Susanne.Gaal@usz.ch.
Hillary J. Braun, Email: hillary.braun@ucsf.edu;Susanne.Gaal@usz.ch.
Diethard Monbaliu, Email: diethard.monbaliu@uzleuven.be.
Antoine Breton, Email: antoinejoe.breton@gmail.com.
Neeta Vachharajani, Email: navachharajani@wustl.edu.
Eliano Bonaccorsi Riani, Email: eliano.bonaccorsi@saintluc.uclouvain.be.
Greg Nowak, Email: greg.nowak@regionstockholm.se.
Robert R. McMillan, Email: rrmcmillan@houstonmethodist.org;Susanne.Gaal@usz.ch.
Samir Abu-Gazala, Email: Samir.Abu-Gazala@Pennmedicine.upenn.edu.
Amit Nair, Email: Amit_Nair@urmc.rochester.edu.
Rocio Bruballa, Email: rocio.bruballa@hiba.org.ar.
Flavio Paterno, Email: fp227@njms.rutgers.edu.
Deborah Weppler Sears, Email: SEARSD@ccf.org.
Antonio D. Pinna, Email: pinnaa@ccf.org.
James V. Guarrera, Email: james.guarrera@njms.rutgers.edu.
Eduardo de Santibañes, Email: eduardo.desantibanes@hospitalitaliano.org.ar;Susanne.Gaal@usz.ch.
Martin de Santibañes, Email: martin.desantibanes@hospitalitaliano.org.ar.
Roberto Hernandez-Alejandro, Email: Roberto_Hernandez@URMC.Rochester.edu.
Kim Olthoff, Email: kim.olthoff@uphs.upenn.edu.
R Mark Ghobrial, Email: rmghobrial@houstonmethodist.org;Susanne.Gaal@usz.ch.
Bo-Göran Ericzon, Email: bo-goran.ericzon@ki.se.
Olga Ciccarelli, Email: olga.ciccarelli@saintluc.uclouvain.be.
William C. Chapman, Email: chapmanw@wustl.edu.
Jean-Yves Mabrut, Email: jean-yves.mabrut@chu-lyon.fr;Susanne.Gaal@usz.ch.
Jacques Pirenne, Email: Jacques.Pirenne@uzleuven.be.
Nancy L. Ascher, Email: nancy.ascher@ucsf.edu.
Robert J. Porte, Email: r.j.porte@umcg.nl;Susanne.Gaal@usz.ch.
Vincent E. de Meijer, Email: v.e.de.meijer@umcg.nl.
Wojciech G. Polak, Email: w.polak@erasmusmc.nl.
Gonzalo Sapisochin, Email: gonzalo.sapisochin@uhn.ca.
Magdy Attia, Email: magdy.attia@nhs.net.
Emmanuel Weiss, Email: emmanuel.weiss@aphp.fr.
René A. Adam, Email: rene.adam@aphp.fr;Susanne.Gaal@usz.ch.
Daniel Cherqui, Email: daniel.cherqui@aphp.fr;Susanne.Gaal@usz.ch.
Karim Boudjema, Email: karim.boudjema@chu-rennes.fr.
Krzysztof Zieniewicz, Email: krzysztof.zieniewicz@wum.edu.pl.
Wayel Jassem, Email: wayel.jassem@kcl.ac.uk.
Philipp Dutkowski, Email: philipp.dutkowski@usz.ch;Susanne.Gaal@usz.ch.
Pierre-Alain Clavien, Email: clavien@access.uzh.ch;Susanne.Gaal@usz.ch.
REFERENCES
- 1. Santopaolo F, Lenci I, Milana M, et al. Liver transplantation for hepatocellular carcinoma: where do we stand? World J Gastroenterol. 2019;25:2591–2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ostojic A, Mrzljak A, Mikulic D. Liver transplantation for benign liver tumors. World J Hepatol. 2021;13:1098–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Spolverato G, Bagante F, Tsilimigras DI, et al. Liver transplantation in patients with liver metastases from neuroendocrine tumors. Minerva Chir. 2019;74:399–406. [DOI] [PubMed] [Google Scholar]
- 4. Merion RM, Goodrich NP, Feng S. How can we define expanded criteria for liver donors? J Hepatol. 2006;45:484–488. [DOI] [PubMed] [Google Scholar]
- 5. Bodzin AS, Baker TB. Liver transplantation today: where we are now and where we are going. Liver Transpl. 2018;24:1470–1475. [DOI] [PubMed] [Google Scholar]
- 6. Berumen J, Hemming A. Liver retransplantation: how much is too much? Clin Liver Dis. 2017;21:435–447. [DOI] [PubMed] [Google Scholar]
- 7. Llado L, Lopez-Dominguez J, Ramos E, et al. Is liver retransplantation justified in the current era? Cir Esp (Engl Ed). 2021;99:339–345. [DOI] [PubMed] [Google Scholar]
- 8. Muller X, Marcon F, Sapisochin G, et al. Defining benchmarks in liver transplantation: a multicenter outcome analysis determining best achievable results. Ann Surg. 2018;267:419–425. [DOI] [PubMed] [Google Scholar]
- 9. Schlegel A, van Reeven M, Croome K, et al. A multicentre outcome analysis to define global benchmarks for donation after circulatory death liver transplantation. J Hepatol. 2022;76:371–382. [DOI] [PubMed] [Google Scholar]
- 10. Rossler F, Sapisochin G, Song G, et al. Defining benchmarks for major liver surgery: a multicenter analysis of 5202 living liver donors. Ann Surg. 2016;264:492–500. [DOI] [PubMed] [Google Scholar]
- 11. Schmidt HM, Gisbertz SS, Moons J, et al. Defining benchmarks for transthoracic esophagectomy: a multicenter analysis of total minimally invasive esophagectomy in low risk patients. Ann Surg. 2017;266:814–821. [DOI] [PubMed] [Google Scholar]
- 12. Sanchez-Velazquez P, Muller X, Malleo G, et al. Benchmarks in pancreatic surgery: a novel tool for unbiased outcome comparisons. Ann Surg. 2019;270:211–218. [DOI] [PubMed] [Google Scholar]
- 13. Raptis DA, Linecker M, Kambakamba P, et al. Defining benchmark outcomes for ALPPS. Ann Surg. 2019;270:835–841. [DOI] [PubMed] [Google Scholar]
- 14. Raptis DA, Sanchez-Velazquez P, Machairas N, et al. Defining benchmark outcomes for pancreatoduodenectomy with portomesenteric venous resection. Ann Surg. 2020;272:731–737. [DOI] [PubMed] [Google Scholar]
- 15. Gero D, Raptis DA, Vleeschouwers W, et al. Defining global benchmarks in bariatric surgery: a retrospective multicenter analysis of minimally invasive Roux-en-Y gastric bypass and sleeve gastrectomy. Ann Surg. 2019;270:859–867. [DOI] [PubMed] [Google Scholar]
- 16. Gero D, Vannijvel M, Okkema S, et al. Defining global benchmarks in elective secondary bariatric surgery comprising conversional, revisional, and reversal procedures. Ann Surg. 2021;274:821–828. [DOI] [PubMed] [Google Scholar]
- 17. Mueller M, Breuer E, Mizuno T, et al. Perihilar cholangiocarcinoma—novel benchmark values for surgical and oncological outcomes from 24 expert centers. Ann Surg. 2021;274:780–788. [DOI] [PubMed] [Google Scholar]
- 18. Gero D, Muller X, Staiger RD, et al. How to establish benchmarks for surgical outcomes? A checklist based on an international expert Delphi consensus. Ann Surg. 2022;275:115–120. [DOI] [PubMed] [Google Scholar]
- 19. Croome KP, Lee DD, Perry DK, et al. Comparison of longterm outcomes and quality of life in recipients of donation after cardiac death liver grafts with a propensity-matched cohort. Liver Transpl. 2017;23:342–351. [DOI] [PubMed] [Google Scholar]
- 20. Dubbeld J, Hoekstra H, Farid W, et al. Similar liver transplantation survival with selected cardiac death donors and brain death donors. Br J Surg. 2010;97:744–753. [DOI] [PubMed] [Google Scholar]
- 21. Goldberg DS, Karp SJ, McCauley ME, et al. Interpreting outcomes in DCDD liver transplantation: first report of the multicenter IDOL consortium. Transplantation. 2017;101:1067–1073. [DOI] [PubMed] [Google Scholar]
- 22. Laing RW, Scalera I, Isaac J, et al. Liver transplantation using grafts from donors after circulatory death: a propensity score-matched study from a single center. Am J Transplant. 2016;16:1795–1804. [DOI] [PubMed] [Google Scholar]
- 23. Dutkowski P, Oberkofler CE, Slankamenac K, et al. Are there better guidelines for allocation in liver transplantation? A novel score targeting justice and utility in the model for end-stage liver disease era. Ann Surg. 2011;254:745–753; discussion 753. [DOI] [PubMed] [Google Scholar]
- 24. Ghabril M, Agarwal S, Lacerda M, et al. Portal vein thrombosis is a risk factor for poor early outcomes after liver transplantation: analysis of risk factors and outcomes for portal vein thrombosis in waitlisted patients. Transplantation. 2016;100:126–133. [DOI] [PubMed] [Google Scholar]
- 25. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Clavien PA, Barkun J, de Oliveira ML, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250:187–196. [DOI] [PubMed] [Google Scholar]
- 27. Slankamenac K, Graf R, Barkun J, et al. The comprehensive complication index: a novel continuous scale to measure surgical morbidity. Ann Surg. 2013;258:1–7. [DOI] [PubMed] [Google Scholar]
- 28. Team RC. A Language And Environment For Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2014. [Google Scholar]
- 29. Staiger RD, Schwandt H, Puhan MA, et al. Improving surgical outcomes through benchmarking. Br J Surg. 2019;106:59–64. [DOI] [PubMed] [Google Scholar]
- 30. Terho P, Sallinen V, Leppaniemi A, et al. Does the surgeon’s caseload affect the outcome in laparoscopic cholecystectomy for acute cholecystitis? Surg Laparosc Endosc Percutan Tech. 2020;30:522–528. [DOI] [PubMed] [Google Scholar]


