Objective:
To define benchmark values for liver transplantation (LT) in patients with perihilar cholangiocarcinoma (PHC) enabling unbiased comparisons.
Background:
Transplantation for PHC is used with reluctance in many centers and even contraindicated in several countries. Although benchmark values for LT are available, there is a lack of specific data on LT performed for PHC.
Methods:
PHC patients considered for LT after Mayo-like protocol were analyzed in 17 reference centers in 2 continents over the recent 5-year period (2014–2018). The minimum follow-up was 1 year. Benchmark patients were defined as operated at high-volume centers (≥50 overall LT/year) after neoadjuvant chemoradiotherapy, with a tumor diameter <3 cm, negative lymph nodes, and with the absence of relevant comorbidities. Benchmark cutoff values were derived from the 75th to 25th percentiles of the median values of all benchmark centers.
Results:
One hundred thirty-four consecutive patients underwent LT after completion of the neoadjuvant treatment. Of those, 89.6% qualified as benchmark cases. Benchmark cutoffs were 90-day mortality ≤5.2%; comprehensive complication index at 1 year of ≤33.7; grade ≥3 complication rates ≤66.7%. These values were better than benchmark values for other indications of LT. Five-year disease-free survival was largely superior compared with a matched group of nodal negative patients undergoing curative liver resection (n=106) (62% vs 32%, P<0.001).
Conclusion:
This multicenter benchmark study demonstrates that LT offers excellent outcomes with superior oncological results in early stage PHC patients, even in candidates for surgery. This provocative observation should lead to a change in available therapeutic algorithms for PHC.
Keywords: benchmarks, CCI® , complications, liver transplantation, Mayo-protocol, outcomes, perihilar cholangiocarcinoma
Perihilar cholangiocarcinoma (PHC) is one of the most dismal diseases with a near zero 3-year survival when patients are not candidate for surgery,1,2 and only 10% to 40% 5-year survival in large series of resected PHC.3–5 Attempts to use liver transplantation (LT) in patients with unresectable PHC started in the early development of the procedure in the 1980s with catastrophic results including early recurrence of the cancer with unacceptable short-term survival.6–8 This led to consider PHC as a futile indication for LT and a waste of the scarce resource of available organs.8 However, based on the observation that some patients treated with radiation achieved a long-term survival benefit, the Mayo group (Rochester, MN) introduced the concept of neoadjuvant therapy combining chemo- and radiotherapy followed by LT in a selective group of patients with PHC deemed not candidate for a curative resection.9 They subsequently reported staggering 5-year survival rates over 75% in patients with underlying primary sclerosing cholangitis (PSC) and over 50% in those with de novo PHC.10 Despite these excellent results, only a few centers around the world adopted the Mayo or a Mayo-like protocol with similar encouraging results,11 whereas many other centers or even countries like the UK, Germany and Japan eliminated LT as a therapeutic option. Those hesitant care-givers based their arguments on the too high risk for recurrent disease in the context of mandatory immunosuppression, organ shortage, and the burden of a neoadjuvant protocol. The topic therefore remains highly controversial with an urgent need for unbiased and convincing data.
Pushing the controversies further, one may wonder whether LT could even be superior in the favorable scenario of resectable PHC. Considering some available evidence, this approach may not be foolish.12,13 Curative surgery for PHC remains one of the most challenging procedures requiring major hepatectomies associated with a high postoperative morbidity and a reported benchmark cutoff mortality of 13% at 3 months as best achievable outcome reference in a study presented at the ESA meeting 2021 including only low-risk (benchmark) cases.14 Further limitations included the high incidence of positive margins after so-called “curative resection” with a benchmark cutoff at 43% followed by recurrent disease in nearly two-thirds of the patients at 1 year.14
Therefore, two controversial issues arise: First, is neoadjuvant chemoradiotherapy followed by LT justified at all in selected patients with PHC not candidate for resection? And second, might LT be superior to resection in selected patients both in terms of postoperative and oncological courses? Those debates remain unsolved in the literature due to the heterogeneity of the disease, patient selection leading to misleading comparisons of nonuniform cohorts, often small sample size and high dropout rates, and eventually the absence of reference values for many outcome parameters.
We therefore intended to evaluate, whether LT is justified in patients with unresectable PHC. Second, we aimed to analyze outcome performance in early stage PHC, comparing neoadjuvant chemoradiotherapy followed by LT with liver resection. For both questions, we used a novel approach of benchmarking looking at optimal cases operated at expert centers around the globe, which allows to provide clinically relevant benchmark values and enables unbiased comparisons after complex procedures.12–15
METHODS
Study Design
To establish benchmarks for outcome indicators in LT for PHC in patients subjected to a neoadjuvant protocol, we followed a standardized methodology,16 previously applied and reported for curative surgery in PHC14 and other complex procedures.12–15,16–19 International high-volume centers were selected based on (a) case load ≥50 LT per year or ≥250 over the defined 5-year study period, (b) previously published in the area of LT, and (c) maintained a comprehensive prospective patient database covering at least a 1-year follow-up.
Separate cohorts of patients were analyzed to test the respective benchmark values: 1 cohort targeted benchmark-PHC cases fulfilling the Mayo criteria, which underwent curative resection.14 Additional cohorts included high-risk PHC patients with major comorbidities who underwent LT, benchmark patients who underwent LT for other indications12 and patients transplanted for PHC outside of a Mayo/modified Mayo neoadjuvant protocol.
Study Population
Patients who underwent LT for PHC over the 5-year study period (January 2014–December 2018) and who presented with a localized tumor (<3 cm) without distant or lymph node metastases and underwent neoadjuvant chemoradiotherapy according to a Mayo or modified Mayo protocol were included. Benchmark patients were those without major comorbidities excluding American Society of Anesthesiologists (ASA) class ≥3; obesity (body mass index ≥35 kg/m2); chronic cardiovascular, pulmonary or renal diseases and the use of certain medications for diabetes and coagulation (Table 1). Patient and outcome data were collected and stored in an encrypted and anonymized online data registry provided by the University Hospital of Zurich (www.phc4transplant.org). The data were checked for completeness by 2 main investigators (E.B. and M.M.). Ethical approval from the Cantonal ethical commission of Zurich (KEK 2020-03052, 2017-00309) and from each respective center were obtained.
TABLE 1.
Inclusion and Exclusion Criteria for The Low-risk Cohort Treated in High-volume Centers
| Inclusion criteria |
| Age ≥18 yr |
| Scheduled for liver transplantation for unresectable/PSC-related PHC according to Mayo criteria |
| Benchmark criteria |
| Liver transplantation with neoadjuvant chemoradiotherapy according to (modified) Mayo protocol |
| No distant or lymph node metastases (based on final pathology) |
| No major comorbidities defined as follows: |
| Medical exclusion criteria |
| ASA classification ≥3 |
| Body mass index ≥35 kg/m2 |
| Cardiac disease defined as: |
| CHF onset or exacerbation in 30 d before surgery |
| History of angina pectoris within 1 mo of surgery |
| Myocardial infarction within 6 mo before surgery |
| History of percutaneous coronary intervention or cardiac surgery |
| Atrial fibrillation |
| Chronic renal failure MDRD ≥Stage 3: GRF<60 mL/min/1.73 m2 or serum creatinine >1.8 mg/dL or 160 mmol/L |
| Chronic obstructive pulmonary disease with FEV1<80% |
| Use of anticoagulants: |
| NOACs |
| Vitamin K antagonist |
| Clopidogrel |
| Diabetes mellitus ≥2 oral antidiabetic drugs or insulin |
CHF, congestive heart failure; FEV, forced expiratory volume in 1 second; GFR, glomerular filtration rate; MDRD, modification of diet in renal disease; NOAC, non-vitamin K antagonist oral anticoagulant.
Indicators for Outcome
Eighteen clinically relevant outcome variables for surgical and oncological qualities were analyzed at discharge, 3, 6, and 12 months after LT,20,21 namely hospital and intensive care unit (ICU) stay as well as LT-specific complications such as biliary complications or graft-loss. Oncological treatment quality is represented by response to chemoradiation and R0-rates. Each postoperative complication was collected and ranked using the Clavien-Dindo grading system,22,23 grade 1 complications were included to ensure comparability with the resection cohort. The comprehensive complication index (CCI®)24,25 was used to express the cumulative burden of morbidity indicated by a continuous numeric scale ranging from 0 (no complication) to 100 (death). For each outcome variable, a benchmark value was calculated and defined as the best achievable result for LT for PHC.
Statistical Analysis
Because of the high variability in the number of benchmark cases among centers, we modified the methodology considering Benchmark cutoffs from the 75th percentile (for values indicating worse outcome) up to the 25th percentile (for indicators of good outcome) of the median value of each participating center. Details about the statistical analyses are listed on Supplementary Table 1, Supplemental Digital Content 1, http://links.lww.com/SLA/E110. All tests were 2-sided, and P<0.05 was considered statistically significant. Statistical analysis was performed using R Statistical Software (Version 4.0.2; R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
The collaborative group consisted of 17 high-volume centers from 2 continents (8 from North America and 9 from Europe). A total of 134 consecutive patients with PHC underwent a LT over the 5-year study period, of which 120 (89.6%) qualified as low-risk (benchmark) cases, constituting the study population (Supplementary Figure 1, Supplemental Digital Content 1, http://links.lww.com/SLA/E110). This cohort displayed a median recipient age of 51 (R: 18–72) years with a predominance of male patients (70%) and a median Lab-MELD score of 10. The dropout of patients before constituting the study population (ie, underwent LT) was available in only 4 of the 17 centers. The dropout in these centers was 28% (52 patients out of 187 patients) mostly relating to tumor progression and positive lymph nodes during the staging laparotomy. The median follow-up of the benchmark cohort was 26 months (interquartile range (IQR): 15.75-42) with a minimum of one year follow-up available in each patient except for 4 patients, who were lost to follow-up.
Of 67 patients (55.8%) presenting with PSC as underlying disease, 2 had additional hepatolithiasis and 1 patient had a secondary sclerosing cholangitis due to a longstanding biliary obstruction. The standard Mayo protocol (external beam radiotherapy to 45 Gy and continuous 5-FU, followed by intraluminal biliary brachytherapy and protracted capecitabine) was applied in 88.1% of cases, whereas the remaining patients received a slightly modified chemoradiation protocol. Half of all patients displayed a complete radiologic response to the neoadjuvant treatment, 8% a marked and 15% a moderate response. The majority (61%) of patients received a liver from a brain-dead donor, 8% from an extended criteria donor and 30% underwent a living-donor liver transplantation. The median duration of surgery was 6.5 hours (IQR: 5.2–8.2) and the median intraoperative blood loss was 700 mL (IQR: 425–1025) with a median transfusion requirement of 2 units of red blood cells. Median length of ICU and hospital stay were short with 1.4 (IQR: 1–4) and 7 (IQR: 6–13) days, respectively. Most patients (78%) developed at least 1 complication, 54% suffered more than 1 complication and more than half (63%) experienced severe complications requiring intervention (grade ≥3). Correspondingly, the CCI® at 1 year was 33.5 (IQR: 20.8–49.9).
Curative resection (R0) was achieved in 94% of cases. The majority of tumors were classified as Bismuth-Corlette type IV26 or B4,T2,M0,N0 according to a new staging system.27 Overall, 1- and 5-year patient survival were 92% (actual survival) and 55% (actuarial survival), respectively. Other relevant patient characteristics are listed on Supplementary Table 2, Supplemental Digital Content 1, http://links.lww.com/SLA/E110.
Benchmark Values
Benchmark cutoffs, covering the 75th to the 25th percentile of the medians of each center, were calculated for 18 LT-specific peri- and postoperative parameters indicating the best achievable results for each variable (Table 2). For example, the benchmark for R0-resection margin and 90-day mortality rates were ≥80.0% and ≤5.2%, respectively. The cutoff for severe (grade ≥3) complications is ≤66.7%, whereas the benchmark value for cumulative morbidity, expressed by the CCI® was ≤33.7. Of note, the overall cumulative patient morbidity did not increase after 6-month follow-up. The cutoffs for long-term (5 year) disease-free survival (DFS) and overall survival (OS) were ≥43.8% and ≥60.0%, respectively.
TABLE 2.
Benchmark Values and Medians of All Centers After LT for PHC, Compared With Benchmark Values After Resection for PHC and All Other Indications for LT
| Benchmark Cutoffs | PHC LT Benchmark | PHC Resection Benchmark14 | LT (All Indications) Benchmark12 |
|---|---|---|---|
| Dropout rate | ≤31% | — | — |
| Operation time (h) | ≤9 | ≤8 | ≤6 |
| ICU stay (d) | ≤4 | ≤2 | ≤4 |
| Hospital stay (d) | ≤18 | ≤19 | ≤18 |
| Postoperative morbidity at 12 mo | |||
| Any complication | ≤79.8% | ≤87% | ≤94% |
| Clavien-Dindo grade ≥3 | ≤66.7% | ≤70% | ≤59% |
| CCI® | ≤33.7 | ≤30.5 | ≤42.1 |
| 1-year hospital readmission rate | ≤67% | ≤31% | — |
| Graft-loss | ≤0% | — | ≤11% |
| EAD | ≤7.2% | — | — |
| HAT | ≤13.2% | — | ≤4.4% |
| Relaparotomy rate (30 d) | ≤39.3% | ≤19% | — |
| Postoperative mortality | |||
| 3-month mortality | ≤5.2% | ≤13% | ≤4% |
| Oncological outcomes | |||
| R0-resection margin rate | ≥80.0% | ≥56.7% | — |
| Overall survival rate: | |||
| 1 yr | ≥92.4% | ≥77.5% | ≥91% |
| 2 yr | ≥66.7% | ≥61.5% | — |
| 5 yr | ≥60.0% | ≥39.7% | — |
| Disease-free survival rate: | |||
| 1 yr | ≥68.8% | ≥33.3% | — |
| 2 yr | ≥54.2% | ≥7.9% | — |
| 5 yr | ≥43.8% | ≥0% | — |
EAD indicates early allograft dysfunction; ICU, Intensive care unit; HAT, hepatic artery thrombosis
The benchmark values from transplant patients with PHC were well within the benchmark cutoffs of LT for other indications (eg, cirrhosis, HCC, and hepatitis),12 as illustrated by comparable 1-year survival (≥92.4%), median CCI® (≤33.7), or hospital stay (≤18 d) (Table 2).
Comparing LT to Curative Resection
When comparing the transplanted unresectable low-risk cohort to a matched low-risk cohort of upfront curatively resected Bismuth-Corlette IV PHC patients (ie, node-negative), from the same 5-year study period (n=106), a significantly better 5-year DFS of 50.2% versus 17.4%, P<0.001 was observed in the LT group (Fig. 1). Consistently, a better OS (56.3% vs 39.9%, P=0.07), although not statistically significant, was identified. After exclusion of all PSC patients the differences in DFS remained highly significant (49.9% vs 18.1%, P=0.005), and the difference in OS remained, although not significant (56.4% vs 39.7%, P=0.4). There was also no difference in the rates of major complications (grade ≥3: 72.7% vs 74.6%, P=0.8), but a higher 3-month mortality rate in the resection group (3% vs 7%, P=0.17), however, not significant.
FIGURE 1.
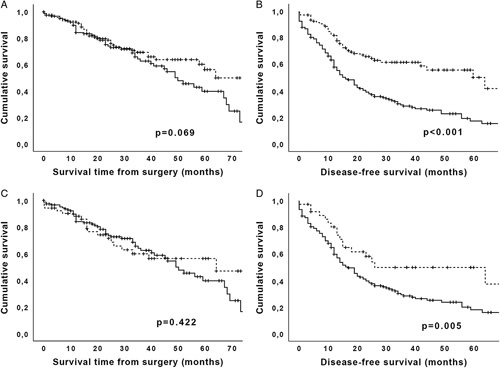
Comparison of survival data from matched resected and transplanted cohorts. A, OS of resected Bismuth Type IV benchmark cases (solid line) versus transplanted (dashed line) unresectable PHC cases: 5-year survival is 56.3% versus 39.9%, P=0.07. B, Five-year DFS of 50.2% versus 17.4%, P<0.001. C, OS after exclusion of all cases with underlying PSC. D, DFS after exclusion of all cases with underlying PSC.
Outcome Performance of a “Higher-risk” Cohort
To test the relevance of the identified benchmark values, 14 high-risk patients not fulfilling the benchmark criteria, but treated in the same benchmark centers of this study were analyzed. Those patients presented with obesity class II to III, severe metabolic and/or cardiovascular disease, rendering them a particularly delicate patient population. Nonetheless, most outcome parameters of this subcohort remained within the benchmark values, in particular with a 64.3% rate of severe complications (grade ≥3) (benchmark value ≤66.7%) and R0 rates of 81.8% (benchmark ≥80%). The relaparotomy rate was, however, higher at 45.5%, that is, outside of the benchmark of ≤39.3% and 76.4% of this population was still disease-free after 1-year follow-up (benchmark value ≥68.8%). Of note, OS was poorer with a median of 21 months (95% confidence interval 9–33) and a 5-year survival of 34.6% (benchmark value ≥60.0%).
LT without Neoadjuvant Chemoradiation
While some centers perform a slightly modified Mayo protocol, several centers completely omit neoadjuvant chemoradiation and opted for upfront LT in the cases of unresectable PHC (n=27 patients). Although these cases were excluded from the benchmark study, outcome measures were analyzed separately. Most perioperative parameters were inferior compared with the LT cohort with chemoradiation but still within the benchmark cutoffs, as R0 rates (81% vs 94%; benchmark ≥80.0%), ICU-stay (4 vs 1.4 days, benchmark ≤4 days), or relaparotomy rates (27% vs 24%, benchmark ≤39.3%) (Supplementary Table 2, Supplemental Digital Content 1, http://links.lww.com/SLA/E110). Only the 5-year DFS of 42% was below the expected benchmark cutoff of ≥44%. Of note, those centers also list significant rates of dropout patients up to 50%, that showed tumor progression while on the waiting list.
DISCUSSION
This is the first study defining benchmark values for PHC subjected to a standardized neoadjuvant chemoradiation protocol followed by LT. We made 2 main observations. First, all benchmark parameters for LT for unresectable PHC lie within the previously reported cutoff values for LT, performed for other indications. Second, LT for PHC offers benchmark values far superior compared with those identified in similarly selected patients treated by upfront liver resection. This includes lower complication rates and strikingly superior oncological outcomes in the neoadjuvant treated LT group.
Currently, the gold standard curative therapy for PHC is liver resection, which disclosed, however, disturbingly poor benchmark values as reported at last year’s (2021) ESA meeting,14 including 13% 3-month mortality, and 78% tumor recurrence rates at 1 year in the ideal benchmark scenario. In addition, the reported incidence of R0-resection in resected patients was only 45%. A need for improvements in patient selection as well as oncological and surgical approaches seems therefore obvious.
In contrast to liver resection, total hepatectomy, that is, LT, is logically an attractive alternative to overcome most of the issues related to a complex surgery in the setting of a locally invasive tumor with a high potential to leave tumor behind, and remains feasible even in unresectable disease with vascular encasement. LT is, however, not performed in most countries including the UK, Germany, or Japan, with the argument of high recurrence rates and unacceptable survival under immunosuppression. Such policy is in marked contrast to the inaugural publication from the Mayo group in 2000.28 Gradually, this protocol has gained acceptance in the United States (US) for patients with unresectable early stage PHC, which has been approved as a standard diagnosis eligible for MELD exception points in 2012. Adoption outside of the US has remained fretting. Accordingly, only few centers in Europe have adopted a Mayo-like protocol combining neoadjuvant chemoradiation and LT as a putative curative option in selected cases of unresectable PHC.29–31 Of note, a retrospective multicenter study from the US32 suggested a survival benefit with LT over resection, but with only limited caseload and a lack of uniform treatment protocols among centers. Another meta-analysis with pooled data sets remained unconvincing due to the heterogenous patient cohorts collected over a >20-year observation period and including distinct entities like intrahepatic cholangiocarcinoma, metastatic diseases, as well as mixing resectable and unresectable PHC.11
Our data instead demonstrates that LT for PHC offers similar or even better outcomes than LT performed for any other indication, including HCC or various causes of cirrhosis.12 In fact, all relevant outcome measures, for example, the rate of postoperative complications, graft-loss, or hepatic artery thrombosis (HAT), were below the previously reported benchmark cutoffs for LT.12 Only the median duration of surgery (7 hours) was slightly above the benchmark value of ≤6 hours, perhaps due to additional technical challenges related to the neoadjuvant radiotherapy and considering that a third of cases received a partial graft from a living donation requiring complex biliary reconstruction. Of note, almost all complications in LT for PHC occurred within the first 6 postoperative months, unlike LT for other indications, where many complications still occurred after 6 months up to 1 year.12 It is unclear whether this better outcome relates to a fitter patient cohort or stricter patient or graft selections. Although the follow-up must cover 1 year for LT for other etiologies, a 6-month follow-up seems sufficient to assess LT in patients with PHC.
A limitation of this study is the lack of consistent reporting of dropout cases in both LT and resection groups. Therefore, a formal intention-to-treat analysis could not be performed. Such information is also notoriously poorly reported in the literature. Available data on LT for PHC suggest a dropout ranging between 20% and 50%.11,33–35 In our benchmark study this information was properly reported only in 4 centers indicating a dropout of about a third of the patients.
Similar information is also absent in most series of resection for PHC with many patients never reaching resection due to tumor progression; for example, during the waiting time after portal vein embolization or the discovery at surgery of previously undetected liver metastasis or peritoneal carcinomatosis. In addition, available data indicate failed curative resection in more than a third of the cases and documented local recurrence at 1 year in more than half of the “so called” curative resections.14,36–37 Such scenarios are even more harmful to patients than dropout before LT. The only definite results can come from a randomized trial, which unfortunately seems not feasible. Accordingly, a recent multicentric randomized controlled trial (RCT) from France comparing LT versus liver resection in resectable PHC patients (NCT02232932) was prematurely terminated, due to a failure of recruitment (personal communication: E. Vibert, Paris, France).
Another limitation of LT for cancer patients is the lack of available organs.38 Such patients experience waiting times up to 15 months depending on allocation policies and donation rates39 leading to unacceptable dropout rates due to tumor progression.11,32,33 This logically leads to an increased use of marginal grafts, for example, livers donated after circulatory death or fatty livers, where machine liver perfusion may help to secure viability or repair of such risky grafts.40 The best alternative may reside in living donation, which was used in only a third of the benchmark cohort. The Mayo group showed excellent long-term survival rates using living-donor grafts for PHC arising in PSC,41 despite a slightly increased risk for vascular and biliary complications. LT in patients with a resectable tumor should be performed only in countries with short waiting time (eg,<2 months in Norway) or with the availability of living donation. For example, at the Swiss HPB and Transplant center in Zurich, Switzerland, we consider LT in this population only with the confirmed availability of a living donor.
One relevant topic remains the role and ideal components of the neoadjuvant therapy for LT in PHC patients. Many centers have modified the Mayo protocol, for example in omitting the brachytherapy to minimize the risk of hepatic artery thrombosis. The ideal protocol remains to be defined. Some centers, such as in the Netherlands,42 have questioned the role of any neoadjuvant therapy in patients with PHC. The data are too scarce to definitively address this issue, but our current study suggests that omitting any neoadjuvant protocol increases the risk of positive margin with poorer DFS. This may additionally lead to fast track-related issues such as losing the benefit for the assessment of the natural history of the disease, thereby offering LT to patients with a too aggressive tumor biology.
In summary, this study demonstrates that neoadjuvant chemoradiation associated to LT must be considered in selected patients with unresectable PHC, and countries that currently still prohibit this approach should reconsider their guidelines. LT should also belong to the standard of care for selective node-negative patients potentially candidate for a resection. These patients should, at the very least, be informed about the potential superiority of LT, particularly with the use of living-donor transplantation. National and center algorithms including policies for organ allocation should be reconsidered to enable patients with PHC to undergo LT.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Neeta Vachharajani, Washington University School of Medicine, St. Louis MO and Marieke de Boer, University Medical Center Groningen, Groningen the Netherlands for their help in preparing the manuscript, and Mrs. Susanne Gaal from the University Hospital Zurich, Zurich Switzerland, for the coordination of the study. Finally, the authors convey their appreciation to Milo A. Puhan, MD, from the Epidemiology, Biostatistics and Prevention Institute, University of Zurich, Zurich, Switzerland for his valuable advice on the methodology and Beat Müllhaupt, from the Department of Gastroenterology and Hepatology, University Hospital Zurich, Zurich Switzerland for careful revision of the manuscript.
DISCUSSANTS
Irinel Popescu (Bucharest, Romania)
This is an important study on a hot topic in liver transplantation: the treatment of perihilar cholangiocarcinoma (PHC). The authors report optimal oncological results after liver transplantation (LT) using a neoadjuvant approach as a new standard of care in selected patients with negative node involvement, even in candidates for liver resection.
However, a high dropout rate was recorded (31%). Most patients dropped out because of tumor progression (40%) or positive staging laparotomy (38%). Median overall survival in this cohort was 10 months under palliative chemotherapy; the cause of death was rapid progression of disease in most cases. Perhaps, more refined selection criteria, based on molecular profile and liquid biopsy, could reduce this dropout rate. For the time being, the dropout rate can be considered more as an empiric marker of tumor aggressiveness, and, even in the few potentially resectable cases, it was not necessarily a drawback.
The study group includes a significant number of patients with Klatskin on primary sclerosing cholangitis (PSC) (55.8%). It looks already well established that liver transplantation should always be the first option in patients with Klatskin on PSC, treating both the tumor and the underlying disease, as we do in hepatocellular carcinoma on cirrhosis. This should also be the case for Klatskin with vascular encasement; of those, 51 (73%) were staged node-negative on preoperative imaging, and may, therefore, have qualified and benefitted from transplantation. When the subgroup of transplanted patients without PSC was compared against resection, no significant difference in survival between the 2 surgical treatments was found (P=0.422), even though disease-free survival was in favor of transplantation (P=0.005). Therefore, in this subgroup, further studies are required.
Most probably, the overall indications for transplantation in PHC will expand, provided an adequate source of donor could be found. An ethical strategy would be the use in an initial phase of living and marginal donors; this will allow for a larger RCT series, which will improve the statistical significance of the results.
I conclude by congratulating the authors for the standardization of an essential treatment strategy in Klatskin tumors, aimed at significantly improving oncological results.
Response From Pierre-Alain Clavien (Zurich, Switzerland)
Thank you very much, Prof. Popescu, for raising these interesting points. I would like to comment on some of your remarks. First, drop-outs before LT are a recurring, well-documented issue for PHC using Mayo or Mayo-like protocols. As seen in our study, around 20% to 30% of patients are removed from the transplant list due to tumor progression during the neoadjuvant therapy.
However, dropout rates in patients scheduled for a curative resection are rarely reported, although those rates are comparable or even higher than those for LT, especially when including aborted surgery due to too extensive diseases, and peritoneal carcinomatosis or resection leaving behind a macroscopic tumor (R2 resection). Thus, an important focus for the future is early patient selection, and the availability of novel tumor markers in serum or biopsy might help to select the optimal patient for the respective therapies.
In PHC patients with underlying PSC or vascular encasement, we can all agree that LT is the only curative option. This novel benchmark study also nicely shows that patients with de novo resectable PHC can substantially profit from LT, demonstrated by lower tumor recurrence rates and lower peri-/postoperative morbidity and mortality, compared to a similar group of patients undergoing resection.
In a time of severe organ shortage, we strongly support the use of living donation to permit a timely transplantation and, indeed, this is the only option in areas where the waiting time for an organ is indecently long, as is the case in Zurich, Switzerland. I doubt, however, that a conclusive RCT will ever be performed due to slow recruitment, causing a long study period due to the rarity of the disease as well as varying local treatment approaches, and patient choice will remain major issues. For the time being, we must rely on robust and multicentric data, such as those in this benchmark study.
Hugo Pinto Marques (Lisbon, Portugal)
Thank you very much, Prof. Clavien, for this provocative study. So, considering your results, what is your future attitude towards resectable patients with PHC. Are you proposing upfront liver transplantation for now?
Response From Pierre-Alain Clavien (Zurich, Switzerland)
If we have a patient with PHC, the first question we ask ourselves is whether he/she is a candidate for liver transplantation following the Mayo criteria including a strict lymph node negative status. If both resection and liver transplantation remain an option, we must inform the patient about the results of this study by emphasizing that their chance of survival is twice as high with a LT than with a resection. If the patient opts for LT, we must inform them of the need for a living donor.
Pål-Dag Line (Oslo, Norway)
Thank you for a very nice study. I have questions regarding two details. The first one regards the fairly high incidence of arterial thrombosis. The Mayo Clinic usually prescribed the use of vascular conduits for vascular reconstruction due to the radiation. How was this in this study sample? Second, were all centers able to use endoluminal brachytherapy, or was this delivered by stereotactic radiation? We have found it extremely difficult to do intraluminal, having switched to stereotactic radiation.
Response From Pierre-Alain Clavien (Zurich, Switzerland)
Thank you, Prof. Line, for these relevant questions. In the early phase of their experience with liver transplantation, the Mayo group experienced a high incidence of late vascular complications (3–11 months postoperatively), such as hepatic artery thrombosis, portal vein thrombosis or stenosis (Mantel et al, Liver Transpl, 2007). By using donor iliac artery grafts, they could avoid these complications in whole grafts; however, they saw earlier arterial complications, including hepatic artery thrombosis, in living-donor recipients. Similarly, we recognized this risk and omitted brachytherapy in our modified Mayo protocol to reduce the cumulative dose of irradiation on the vessels. In the current study, about one-third of centers used only external beam, displaying slightly lower rates of hepatic artery thrombosis (11.8% vs 15.6%, ns).
Antonio Pinna (Weston, FL, USA)
This is a great paper, though very controversial. Starting from today, it tells me that I should no longer resect a PHC. Instead, I should only opt for a liver transplant. I have a few questions to challenge this. How do you explain the difference in outcomes between East and West using the same resectability criteria? You demonstrate very good results with liver transplantation; however, in Japan, liver resection demonstrates the same outcome with the same tumor criteria. Could it be that early morbidity and mortality after liver resection in the Western and Eastern world are making the real difference?
Second, I suppose that there is an issue in the methodology when comparing resectable patients that are going for transplantation. When we decide on a resection, we see the patient, complete the staging, and then, we resect. Based on the same tumor criteria, if a patient is selected for transplant, they face a longer waiting time, potentially, with an in vivo selection of less aggressive tumors. I think that this potential bias needs to be addressed. Having said that, I think that this is an important paper that can change the Western approach to the treatment of selected PHC.
Response From Pierre-Alain Clavien (Zurich, Switzerland)
Thank you, Prof. Pinna, for these key comments. Indeed, Prof. Nagino and his colleagues from Nagoya, Japan, reported an amazing low postoperative mortality of 2% at 3 months, compared to a 13% benchmark mortality, as reported last year at ESA, and published in the last November issue (Mueller et al, Ann Surg. 2021). One question was whether we should have separate benchmark values for Japan versus the rest of the world. We concluded that a benchmark study must offer global values. In fact, the data from Nagoya was a major part of the reported benchmark study. Now, to answer your question, indeed, if a patient with a resectable PHC is treated in Nagoya, Japan, then he/she should undergo a resection, not a transplantation. In other locations, a PHC patient candidate for a transplantation should be offered this option.
Second, regarding a potential selection bias explaining the better outcome in the transplantation group, due to a longer observation time and natural selection, is a well taken point. I do not believe, however, that this explains the superiority of transplantation. For example, the procedure-related outcome, in terms of postoperative morbidity and mortality, strongly favors liver transplantation, though a closer look at the natural history may impact on long-term oncological results.
Johann Pratschke (Berlin, Germany)
This was a great presentation on a very important issue. We joined forces with Amsterdam to evaluate our resections for Klatskin tumors together. These papers were often rejected because the rate of complications was not comparable to the Japanese groups, which is difficult to argue from the perspective of a Western center. As you already know, since 2018, the pro-duct002 study, which evaluates the potential value of liver transplantation for borderline/nonresectable perihilar cholangiocarcinoma, has been running in Germany. The inclusion criteria are based on Mayo. With 14 transplant centers participating, we have an average recruitment of 1 patient per year. So, this is just to set the scene for how many patients we are talking about here. Please comment on this.
Response From Pierre-Alain Clavien (Zurich, Switzerland)
Thank you, Prof. Pratschke, for these comments. You are talking about the classical indications for LT, that is, the nonresectable lymph node negative cases. Indeed, this is a rare condition, but it is not as sporadic as you suggest. According to an incidence of 1 to -2/100,000, and a rate of 2% potentially eligible for LT, according to stringent Mayo criteria (as reported by De Vreede et al, 2000, Liver Transpl), we expect 15 to 30 cases annually in Germany alone. In Zurich, for example, we transplant 1 to 2 cases of nonresectable PHC per year. If we were to add the number of resectable, potential transplant candidates to this, the figure would be higher. Nevertheless, the number of patients per center remains low, requiring multicentric collaborations and tight centralization policies to optimize outcome. This was the rationale behind using such multicentric benchmark methodology, in order to enable conclusive observations leading to the recommendation to consider LT in selective resection scenarios.
Eric Vibert (Villejuif France)
I would like to comment on the feasibility of a RCT comparing resection versus transplantation in patients with PHC. The only RCT ever attempted to give definite results by directly comparing LT versus liver resection in resectable PHC patients (NCT02232932) was recently conducted in 18 French centers, and unfortunately, it was terminated, as it failed to recruit the required number of patients. We also observed an unexpected drop-out rate of 55% in the first 20 patients enrolled in the LT arm.
Response From Pierre-Alain Clavien (Zurich, Switzerland)
Thank you, Dr. Vibert, for this precious information, and congratulations on your initiative to attempt such a challenging RCT. We concluded that we may not be able to obtain a higher level of evidence than the one provided in this benchmark study. Only a large registry, including all resectable and unresectable PHC patients with sufficient follow-up, will provide more accurate data on survival benefits and true drop-out rates for resection and LT.
Footnotes
E.B. and M.M. contributed equally and shared first authorship.
The authors report no conflicts of interest.
This study is supported by the LGID (Liver and Gastrointestinal Disease) Foundation and the Yvonne Jacob Foundation.
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.annalsofsurgery.com.
Contributor Information
Eva Breuer, Email: Eva.Breuer@usz.ch.
Matteo Mueller, Email: Matteo.Mueller@usz.ch.
Majella B. Doyle, Email: doylem@wustl.edu.
Liu Yang, Email: yang.Liu@mayo.edu.
Sarwa Darwish Murad, Email: s.darwishmurad@erasmusmc.nl.
Imran J. Anwar, Email: imran.anwar@duke.edu.
Shaheed Merani, Email: shaheed.merani@unmc.edu.
Ashley Limkemann, Email: Ashley.Limkemann@osumc.edu.
Heithem Jeddou, Email: Heithem.JEDDOU@chu-rennes.fr.
Steven C. Kim, Email: kims@surgery.wisc.edu.
Victor López-López, Email: victorrelopez@gmail.com.
Ahmed Nassar, Email: ahmed.nassar@emory.edu.
Frederik J.H. Hoogwater, Email: f.j.h.hoogwater@umcg.nl.
Eric Vibert, Email: eric.vibert.pbr@gmail.com.
Michelle L. De Oliveira, Email: michelleldeol@yahoo.com.
Daniel Cherqui, Email: daniel.cherqui@pbr.aphp.fr.
Robert J. Porte, Email: r.j.porte@umcg.nl;Susanne.Gaal@usz.ch.
Joseph F. Magliocca, Email: jmaglio@emory.edu.
Lutz Fischer, Email: l.fischer@uke.de.
Constantino Fondevila, Email: constantino.fondevila@gmail.com.
Krzysztof Zieniewicz, Email: kzienie@amwaw.edu.pl.
Pablo Ramírez, Email: pablo.ramirez@carm.es.
David P. Foley, Email: foley@surgery.wisc.edu.
Karim Boudjema, Email: karim.boudjema@chu-rennes.fr.
Austin D. Schenk, Email: austin.schenk@osumc.edu.
Alan N. Langnas, Email: alangnas@unmc.edu.
Stuart Knechtle, Email: stuart.knechtle@duke.edu.
Wojciech G. Polak, Email: w.polak@erasmusmc.nl.
C. Burcin Taner, Email: taner.burcin@mayo.edu.
William C. Chapman, Email: chapmanw@wustl.edu.
Charles B. Rosen, Email: rosen.charles@mayo.edu.
Gregory J. Gores, Email: gores.gregory@mayo.edu.
REFERENCES
- 1. Kuvshinoff BW, Armstrong JG, Fong Y, et al. Palliation of irresectable hilar cholangiocarcinoma with biliary drainage and radiotherapy. Br J Surg. 1995;82:1522–1525. [DOI] [PubMed] [Google Scholar]
- 2. Foo ML, Gunderson LL, Bender CE, et al. External radiation therapy and transcatheter iridium in the treatment of extrahepatic bile duct carcinoma. Int J Radiat Oncol Biol Phys. 1997;39:929–935. [DOI] [PubMed] [Google Scholar]
- 3. Matsuo K, Rocha FG, Ito K, et al. The Blumgart preoperative staging system for hilar cholangiocarcinoma: analysis of resectability and outcomes in 380 patients. J Am Coll Surg. 2012;215:343–355. [DOI] [PubMed] [Google Scholar]
- 4. Nagino M, Ebata T, Yokoyama Y, et al. Evolution of surgical treatment for perihilar cholangiocarcinoma: a single-center 34-year review of 574 consecutive resections. Ann Surg. 2013;258:129–140. [DOI] [PubMed] [Google Scholar]
- 5. Nuzzo G, Giuliante F, Ardito F, et al. Improvement in perioperative and long-term outcome after surgical treatment of hilar cholangiocarcinoma: results of an Italian multicenter analysis of 440 patients. Arch Surg. 2012;147:26–34. [DOI] [PubMed] [Google Scholar]
- 6. Meyer CG, Penn I, James L. Liver transplantation for cholangiocarcinoma: results in 207 patients. Transplantation. 2000;69:1633–1637. [DOI] [PubMed] [Google Scholar]
- 7. Robles R, Figueras J, Turrion VS, et al. Spanish experience in liver transplantation for hilar and peripheral cholangiocarcinoma. Ann Surg. 2004;239:265–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Goldstein RM, Stone M, Tillery GW, et al. Is liver transplantation indicated for cholangiocarcinoma? Am J Surg. 1993;166:768–771; discussion 771-2. [DOI] [PubMed] [Google Scholar]
- 9. Darwish Murad S, Kim WR, Harnois DM, et al. Efficacy of neoadjuvant chemoradiation, followed by liver transplantation, for perihilar cholangiocarcinoma at 12 US centers. Gastroenterology. 2012;143:88–98 e3; quiz e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rosen CB. Transplantation versus resection for hilar cholangiocarcinoma: an argument for shifting paradigms for resectable disease in annals of surgery 2018. Ann Surg. 2018;267:808–809. [DOI] [PubMed] [Google Scholar]
- 11. Cambridge WA, Fairfield C, Powell JJ, et al. Meta-analysis and meta-regression of survival after liver transplantation for unresectable perihilar cholangiocarcinoma. Ann Surg. 2021;273:240–250. [DOI] [PubMed] [Google Scholar]
- 12. Muller X, Marcon F, Sapisochin G, et al. Defining benchmarks in liver transplantation: a multicenter outcome analysis determining best achievable results. Ann Surg. 2018;267:419–425. [DOI] [PubMed] [Google Scholar]
- 13. Rossler F, Sapisochin G, Song G, et al. Defining benchmarks for major liver surgery: a multicenter analysis of 5202 living liver donors. Ann Surg. 2016;264:492–500. [DOI] [PubMed] [Google Scholar]
- 14. Mueller M, Breuer E, Mizuno T, et al. Perihilar cholangiocarcinoma—novel benchmark values for surgical and oncological outcomes from 24 expert centers. Ann Surg. 2021;274:780–788. [DOI] [PubMed] [Google Scholar]
- 15. Raptis DA, Linecker M, Kambakamba P, et al. Defining benchmark outcomes for ALPPS. Ann Surg. 2019;270:835–841. [DOI] [PubMed] [Google Scholar]
- 16. Gero D, Muller X, Staiger RD, et al. How to establish benchmarks for surgical outcomes? A checklist based on an International Expert Delphi Consensus. Ann Surg. 2022;275:115–120. [DOI] [PubMed] [Google Scholar]
- 17. Sanchez-Velazquez P, Muller X, Malleo G, et al. Benchmarks in pancreatic surgery: a novel tool for unbiased outcome comparisons. Ann Surg. 2019;270:211–218. [DOI] [PubMed] [Google Scholar]
- 18. Schmidt HM, Gisbertz SS, Moons J, et al. Defining benchmarks for transthoracic esophagectomy: a multicenter analysis of total minimally invasive esophagectomy in low risk patients. Ann Surg. 2017;266:814–821. [DOI] [PubMed] [Google Scholar]
- 19. Gero D, Raptis DA, Vleeschouwers W, et al. Defining global benchmarks in bariatric surgery: a retrospective multicenter analysis of minimally invasive Roux-en-Y gastric bypass and sleeve gastrectomy. Ann Surg. 2019;270:859–867. [DOI] [PubMed] [Google Scholar]
- 20. Vonlanthen R, Clavien PA. What factors affect mortality after surgery? Lancet. 2012;380:1034–1036. [DOI] [PubMed] [Google Scholar]
- 21. Dimick JB, Osborne NH, Hall BL, et al. Risk adjustment for comparing hospital quality with surgery: how many variables are needed? J Am Coll Surg. 2010;210:503–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Clavien PA, Barkun J, de Oliveira ML, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250:187–196. [DOI] [PubMed] [Google Scholar]
- 24. Slankamenac K, Graf R, Barkun J, et al. The comprehensive complication index: a novel continuous scale to measure surgical morbidity. Ann Surg. 2013;258:1–7. [DOI] [PubMed] [Google Scholar]
- 25. Clavien PA, Vetter D, Staiger RD, et al. The Comprehensive Complication Index (CCI): added value and clinical perspectives 3 years “Down the Line”. Ann Surg. 2017;265:1045–1050. [DOI] [PubMed] [Google Scholar]
- 26. Bismuth H, Corlette MB. Intrahepatic cholangioenteric anastomosis in carcinoma of the hilus of the liver. Surg Gynecol Obstet. 1975;140:170–178. [PubMed] [Google Scholar]
- 27. Deoliveira ML, Schulick RD, Nimura Y, et al. New staging system and a registry for perihilar cholangiocarcinoma. Hepatology. 2011;53:1363–1371. [DOI] [PubMed] [Google Scholar]
- 28. De Vreede I, Steers JL, Burch PA, et al. Prolonged disease-free survival after orthotopic liver transplantation plus adjuvant chemoirradiation for cholangiocarcinoma. Liver Transpl. 2000;6:309–316. [DOI] [PubMed] [Google Scholar]
- 29. Mansour JC, Aloia TA, Crane CH, et al. Hilar cholangiocarcinoma: expert consensus statement. HPB (Oxford). 2015;17:691–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Heimbach JK, Gores GJ, Nagorney DM, et al. Liver transplantation for perihilar cholangiocarcinoma after aggressive neoadjuvant therapy: a new paradigm for liver and biliary malignancies? Surgery. 2006;140:331–334. [DOI] [PubMed] [Google Scholar]
- 31. Heimbach JK, Gores GJ, Haddock MG, et al. Predictors of disease recurrence following neoadjuvant chemoradiotherapy and liver transplantation for unresectable perihilar cholangiocarcinoma. Transplantation. 2006;82:1703–1707. [DOI] [PubMed] [Google Scholar]
- 32. Ethun CG, Lopez-Aguiar AG, Anderson DJ, et al. Transplantation versus resection for hilar cholangiocarcinoma: an argument for shifting treatment paradigms for resectable disease. Ann Surg. 2018;267:797–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Loveday BPT, Knox JJ, Dawson LA, et al. Neoadjuvant hyperfractionated chemoradiation and liver transplantation for unresectable perihilar cholangiocarcinoma in Canada. J Surg Oncol. 2018;117:213–219. [DOI] [PubMed] [Google Scholar]
- 34. Marchan EM, Landry JC. Neoadjuvant chemoradiation followed by orthotopic liver transplantation in cholangiocarcinomas: the emory experience. J Gastrointest Oncol. 2016;7:248–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Darwish Murad S, Kim WR, Therneau T, et al. Predictors of pretransplant dropout and posttransplant recurrence in patients with perihilar cholangiocarcinoma. Hepatology. 2012;56:972–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Buettner S, van Vugt JLA, Gaspersz MP, et al. Survival after resection of perihilar cholangiocarcinoma in patients with lymph node metastases. HPB (Oxford). 2017;19:735–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Watson MD, Baimas-George MR, Passeri MJ, et al. Effect of margin status on survival after resection of hilar cholangiocarcinoma in the modern era of adjuvant therapies. Am Surg. 2021;87:1496–1503. [DOI] [PubMed] [Google Scholar]
- 38. Petrowsky H, Fritsch R, Guckenberger M, et al. Modern therapeutic approaches for the treatment of malignant liver tumours. Nat Rev Gastroenterol Hepatol. 2020;17:755–772. [DOI] [PubMed] [Google Scholar]
- 39. Burra P, Samuel D, Sundaram V, et al. Limitations of current liver donor allocation systems and the impact of newer indications for liver transplantation. J Hepatol. 2021;75(suppl 1)):S178–S190. [DOI] [PubMed] [Google Scholar]
- 40. Sousa Da Silva RX, Weber A, Dutkowski P, et al. Machine perfusion in liver transplantation. Hepatology. 2022;1–19. Available at: https://doi.org/10.1002/hep.32546. [DOI] [PubMed] [Google Scholar]
- 41. Tan EK, Rosen CB, Heimbach JK, et al. Living donor liver transplantation for perihilar cholangiocarcinoma: outcomes and complications. J Am Coll Surg. 2020;231:98–110. [DOI] [PubMed] [Google Scholar]
- 42. Vugts JJA, Gaspersz MP, Roos E, et al. Eligibility for liver transplantation in patients with perihilar cholangiocarcinoma. Ann Surg Oncol. 2021;28:1483–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]


