Abstract
Purpose:
Historically, open techniques have been favored over minimally invasive approaches for complex surgeries. We aimed to identify differences in perioperative outcomes, surgical footprints, and complication rates in patients undergoing either open or robotic reoperative partial nephrectomy.
Materials and Methods:
A retrospective review of patients undergoing reoperative partial nephrectomy was performed. Patients were assigned to cohorts based on current and prior surgical approaches: open after open, open after minimally invasive surgery, robotic after open, and robotic after minimally invasive surgery cohorts. Perioperative outcomes were compared among cohorts. Factors contributing to complications were assessed.
Results:
A total of 192 patients underwent reoperative partial nephrectomy, including 103 in the open after open, 10 in the open after minimally invasive surgery, 47 in the robotic after open, and 32 in the robotic after minimally invasive surgery cohorts. The overall and major complication (grade ≥3) rates were 65% and 19%, respectively. The number of blood transfusions, overall complications, and major complications were significantly lower in robotic compared to open surgical cohorts. On multivariate analysis, the robotic approach was protective against major complications (OR 0.3, p = 0.02) and estimated blood loss was predictive (OR 1.03, p = 0.004). Prior surgical approach was not predictive for major complications.
Conclusions:
Reoperative partial nephrectomy is feasible using both open and robotic approaches. While the robotic approach was independently associated with fewer major complications, prior approach was not, implying that prior surgical approaches are less important to perioperative outcomes and in contributing to the overall surgical footprint.
Keywords: robotics, minimally invasive surgical procedures, carcinoma, renal cell, von Hippel-Lindau disease
Partial nephrectomy (PN) remains the preferred treatment for patients with small renal masses and for patients at risk for recurrent renal cell carcinoma (RCC) due to hereditary forms of kidney cancer.1 Minimally invasive surgery (MIS) has shown improved length of stay and estimated blood loss (EBL) compared to open surgery while maintaining equivalent oncologic efficacy.2 However, for patients who have undergone prior partial nephrectomies, the obliteration of surgical planes and subsequent adhesions can create a hostile surgical field. Traditionally, surgeons have opted for an open surgical approach when facing more complex operations; however, there are few data to support the use of open or MIS techniques over the other in this scenario.
High quality evidence of outcomes in reoperative renal surgery is lacking, with most data being limited to small case series. Furthermore, the use of MIS rather than open surgery in these complex reoperations has an even greater dearth of information. Reoperative partial nephrectomy (RePN) via open approach was shown to be feasible; however, initial reports demonstrated a high rate of complication of 20% to 50% in all patients and in those with solitary kidneys, respectively.3,4 However, as experience with this operation grew, surgeons were able to obtain similar perioperative outcomes and complication rates when matching reoperative surgical outcomes to index surgical outcomes.5 Outcomes of laparoscopic PN in reoperative surgery, however, have been mixed with some series showing conversion rates to open approach as high as 40% while others demonstrated similar outcomes to procedures performed in nonoperated fields.6,7 Fortunately with increasing experience and the incorporation of the robotic surgical platform, outcomes comparable to PN in nonoperated fields have been reported, though most of these series are limited by decidedly small sample sizes of fewer than 30 patients.8,9
It is also unclear if the operative approach, open or MIS, utilized in the index surgery alters subsequent operations on that renal unit. This concept of a “surgical footprint,” in which a surgical intervention in a particular operative field can affect future surgeries, may play a role in reoperative outcomes. Evidence from other surgical specialties has shown that a larger surgical footprint (ie more complete dissection, presence of an anastomosis) has led to increased postoperative complications.10 Currently it is unclear if the surgical footprint differs between open and MIS surgery as the data are inconsistent across studies.10–12 However, the preponderance of evidence suggests that a higher number of reoperations on a kidney leads to increased complications. Consequently, to minimize the likelihood of complications, a better understanding of the impact of the surgical footprint is needed.13
The primary goal of this study is to identify any differences in perioperative outcomes between the robotic and open approach to RePN. The next goal is to assess if the prior surgical approach caused a distinct surgical footprint, causing differences in outcomes. The final aim of this study is to identify which factors lead to increased numbers of significant complications. We hypothesize that MIS outcomes would be noninferior to open outcomes in patients undergoing RePN and that a prior MIS approach would contribute to a smaller surgical footprint compared to prior open surgery.
MATERIALS AND METHODS
An Institutional Review Board-approved prospectively maintained registry was queried for all patients from January 2010 to July 2019 who underwent extirpative renal surgery after having prior surgery on the ipsilateral kidney (IRB No. NCI-97-C-0147). Prior surgeries were defined as minimally invasive if they were laparoscopic or robotic PN. Patients’ prior surgical histories were reviewed, and patients were subsequently stratified into 4 cohorts: open after open surgery, open after MIS only, robotic after open surgery, robotic after MIS only (fig. 1). The cohorts are defined by the surgical approach utilized in prior renal surgery and the surgical approach used for the surgery currently being evaluated. Any history of prior open PN would automatically place the patient in a prior open surgery cohort.
Figure 1.
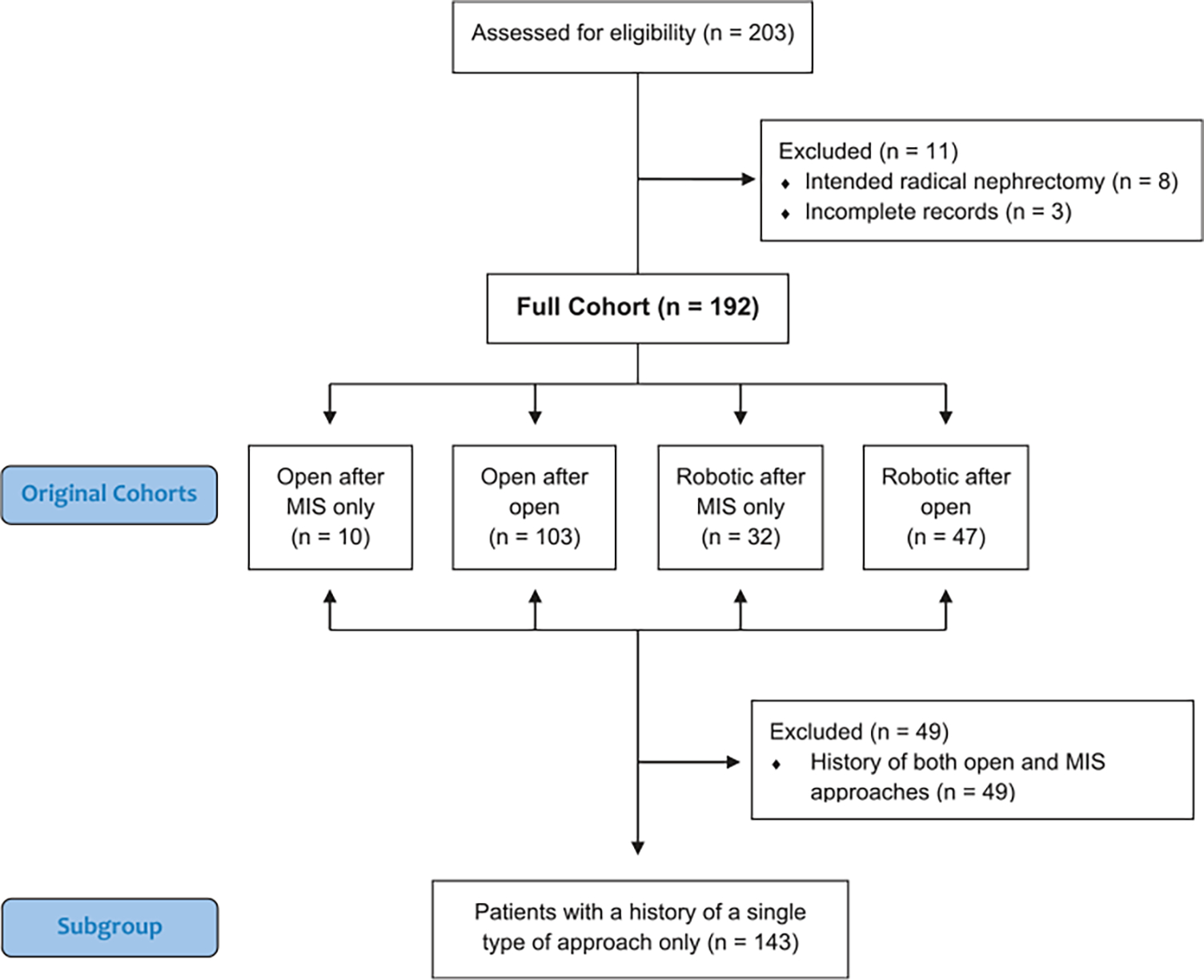
Patient cohorts. All patients were originally assigned to cohorts based on prior surgical history and current surgical approach. Any history of open surgery would place patient in prior open cohort. To remove confounding factors when assessing role of prior surgery’s effect on surgical footprints, new separate groups were created. Given that some patients had both prior open and MIS surgery, subgroup analysis was performed in patients who had history of only open partial nephrectomies or history of only prior minimally invasive partial nephrectomies.
Patient information was extracted including demographic, perioperative, and pathological outcomes. Complications were defined utilizing the Clavien-Dindo classification schema.14 A single aspect of the classification system was modified due to the fact that all patients undergoing renal surgery at our institution are routinely monitored in the intensive care unit (ICU) until postoperative day 1. This ICU stay was not considered a complication unless patients required ICU level care due to life-threatening complication as addressed by the Clavien-Dindo schema. Glomerular filtration rate (GFR) was estimated using the Modification of Diet in Renal Disease equation. Associations between cohorts, demographic data, perioperative data, and outcomes were evaluated with the use of univariate and multivariate linear and logistic regression, 1-way analysis of variance, and Kruskal-Wallis H test for the 4-cohort analysis. Similar analysis was performed utilizing Pearson’s chisquared test for categorical variables or Student’s t-test and Wilcoxon-Mann-Whitney test for continuous variables for the remaining analysis. Statistical analysis was performed with STATA 16 (StataCorp, College Station, Texas). A p value of <0.05 was considered significant.
RESULTS
From January 2010 to July 2019, a total of 672 patients underwent PN at our institution. Of these, 192 patients who had prior partial nephrectomies were identified and included in the final analysis (fig. 1). Demographic data were similar among all cohorts with only tumor laterality differing among the groups (table 1). The cohorts were divided as follows: 103 patients were in the open after open cohort, 10 were in the open after MIS only cohort, 47 were in the robotic after open cohort, and 32 were in the robotic after MIS only cohort. The proportion of robotic procedures increased during the study period. While only 24% (24 of 98) of procedures in the first half of the study period were performed via robotic approach, 59% (55 of 94) were performed via a robotic approach in the second half. A significant notable difference was the open after open cohort having an increased number of prior mean procedures at 2.0 (p <0.001). The majority of patients had a known hereditary cancer predisposition syndrome. A total of 124 patients (65%) experienced a complication while 36 patients (19%) experienced a grade ≥3 complication.
Table 1.
Patient demographics
| All Pts | Open after MIS Only | Open after Open | Robotic after MIS Only | Robotic after Open | p Value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| No. pts | 192 | 10 | 103 | 32 | 47 | ||||||
| Median yrs age (range) | 48 | (27–79) | 52 | (27–79) | 50 | (27–77) | 47 | (27–69) | 46 | (28–68) | 0.5 |
| No. sex (%): | 0.9 | ||||||||||
| Male | 123 | (64) | 6 | (60) | 68 | (66) | 21 | (66) | 28 | (60) | |
| Female No. race (%): | 69 | (36) | 4 | (40) | 35 | (34) | 11 | (34) | 19 | (40) | |
| No. race (%): | 0.6 | ||||||||||
| White, nonHispanic | 152 | (79) | 7 | (70) | 78 | (76) | 28 | (88) | 39 | (83) | |
| Black/African American | 116 | 0 | (0) | 77 | 13 | 36 | |||||
| Asian | 95 | 0 | (0) | 77 | 0 | (0) | 24 | ||||
| Other | 2,010 | 3 | (30) | 1,111 | 39 | 36 | |||||
| No. hereditary predisposition syndrome (%): | 0.2 | ||||||||||
| von Hippel Lindau | 156 | (81) | 7 | (70) | 88 | (85) | 22 | (69) | 39 | (83) | |
| Birt-Hogg-Dubé | 6 | (3) | 0 | (0) | 3 | (3) | 2 | (6) | 1 | (2) | |
| Hereditary leiomyomatosis + RCC | 4 | (2) | 1 | (10) | 2 | (2) | 0 | (0) | 1 | (2) | |
| Other | 23 | (12) | 2 | (20) | 9 | (9) | 6 | (19) | 6 | (13) | |
| Sporadic | 2 | (1) | 0 | (0) | 1 | (1) | 2 | (6) | 0 | (0) | |
| No. predominant tumor histology (%): | 0.3 | ||||||||||
| Clear cell | 168 | (88) | 7 | (70) | 94 | (91) | 28 | (88) | 40 | (85) | |
| Papillary type 1 | 7 | (4) | 1 | (10) | 2 | (2) | 2 | (6) | 2 | (4) | |
| Hybrid oncocytic/chromophobe tumor | 6 | (3) | 0 | (0) | 3 | (3) | 2 | (6) | 1 | (2) | |
| Hereditary leiomyomatosis + RCC-associated RCC | 4 | (2) | 1 | (10) | 2 | (2) | 0 | (0) | 1 | (2) | |
| Other | 6 | (3) | 1 | (10) | 2 | (2) | 0 | (0) | 3 | (6) | |
| No. American Society of Anesthesiologists® score (%): | 0.994 | ||||||||||
| 1 | 0 | (0) | 0 | (0) | 0 | (0) | 0 | (0) | 0 | (0) | |
| 2 | 1 | (0.5) | 0 | (0) | 0 | (0) | 1 | (3) | 0 | (0) | |
| 3 | 189 | (98) | 10 | (100) | 103 | (100) | 29 | (91) | 47 | (100) | |
| 4 | 2 | (1) | 0 | (0) | 0 | (0) | 2 | (6) | 0 | (0) | |
| No. laterality (%): | 0.03 | ||||||||||
| Lt | 94 | (49) | 5 | (50) | 48 | (47) | 23 | (72) | 18 | (38) | |
| Rt | 98 | (51) | 5 | (50) | 55 | (53) | 9 | (28) | 29 | (62) | |
| Mean±SD No. prior ipsilat procedures | 1.7±0.9 | 1.3±0.5 | 2±0.9 | 1.3±0.8 | 1.5±0.7 | 0.0001 | |||||
| Median No. prior ipsilat procedures (IQR) | 1 | (1–2) | 1 | (1–2) | 2 | (1–2) | 1 | (1–1) | 1 | (1–2) | 0.0001 |
| No. history of ablation (%): | 0.6 | ||||||||||
| No | 157 | (82) | 8 | (80) | 81 | (79) | 28 | (88) | 40 | (85) | |
| Yes | 35 | (18) | 2 | (20) | 22 | (21) | 4 | (13) | 7 | (15) | |
Intraoperative outcomes demonstrated notable differences among the cohorts (table 2). Units of packed red blood cells (pRBCs) transfused intraoperatively (p = 0.03) were significantly lower in both robotic surgery arms. The median duration of operation was 388 minutes with no considerable difference noted among the cohorts. There was, however, a significant difference in the number of tumors resected at time of operation. The overall median number of tumors resected per procedure was 5, with both open surgical cohorts having significantly more tumors resected (p = 0.01). One patient (0.5%) in the robotic after open cohort required a conversion to radical nephrectomy while 6 patients (7.6%) in the robotic cohorts required conversion to open surgery.
Table 2.
Perioperative factors and outcomes by cohort
| All Pts | Open after MIS Only | Open after Open | Robotic after MIS Only | Robotic after Open | p Value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| No. pts | 192 | 10 | 103 | 32 | 47 | ||||||
| Intraop characteristics and outcomes: | |||||||||||
| No. radical nephrectomy conversions (%) | 1 | (0.5) | 0 | (0) | 0 | (0) | 0 | (0) | 1 | (2) | 0.5 |
| No. robotic to open conversions (%) | 6 | (3.1) | Not applicable | Not applicable | 2 | (6.3) | 4 | (8.5) | 0.5 | ||
| Median mins duration of surgery (IQR) | 388 | (339–460) | 414 | (256–475) | 380 | (333–432) | 421 | (356–472) | 384 | (338–489) | 0.5 |
| Median ml EBL (IQR) | 2,000 | (1,100–3,500) | 2,100 | (850–3,500) | 2,100 | (1,200–4,000) | 1,750 | (750–3,000) | 1,900 | (800–2,750) | 0.1 |
| Median units intraop pRBCs transfused (IQR) | 3 | (0–6) | 2 | (1–3) | 41 | (1–8) | 2 | (0–4) | 2 | (0–4) | 0.03 |
| No. hilar clamping utilized (%) | 42 | (22) | 5 | (50) | 21 | (20) | 5 | (16) | 11 | (23) | 0.2 |
| Median No. tumors resected (IQR) | 5 | (3–10) | 6 | (1–15) | 7 | (4–12) | 4 | (2–6) | 4 | (2–8) | 0.01 |
| Postop outcomes: | |||||||||||
| Median units postop pRBCs transfused (IQR) | 0 | (0–2) | 1 | (1–2) | 2 | (0–2) | 0 | (0–1) | 0 | (0–0) | <0.001 |
| No. pts experiencing any grade Clavien complication (%) | 124 | (65) | 8 | (80) | 83 | (81) | 17 | (53) | 16 | (34) | <0.001 |
| No. pts experiencing grade ≥3 Clavien complication (%) | 36 | (19) | 2 | (20) | 26 | (25) | 4 | (13) | 4 | (9) | 0.07 |
| Mean±SD No. grade ≥3 Clavien complications | 0.3±0.8 | 0.2±0.4 | 0.5±1.0 | 0.1±0.3 | 0.08±0.3 | 0.046 | |||||
| Median % 3-mos change in glomerular filtration rate (IQR) | −7.2 (−17.1–3.5) | −2.8 (−18.1–11.1) | −6.7 (−19.1–4.1) | −6.1 (−13.1–4.4) | −8.5 (−17.1–2.1) | 0.7 | |||||
Postoperatively, the number of pRBC transfusions (p <0.001), mean number of grade ≥3 complications (p = 0.046), and number of patients experiencing any complication (p <0.001) were significantly lower in both robotic surgery cohorts compared to the open surgical cohorts. The number of patients who experienced a grade ≥3 complication was significantly lower in only the robotic after open cohort (OR 0.3, p = 0.02, table 3). There were no differences in change in 3-month median change in estimated GFR (p = 0.4).
Table 3.
Univariate analysis of perioperative factors and outcomes by presence of grade ≥3 complication
| No Grade ≥3 Complications | Grade ≥3 Complication | Odds Ratio (95% CI) | p Value | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| No. pts | 156 | 36 | |||||
| Demographic: | |||||||
| Median yrs age (range) | 48 | (27–79) | 51 | (28–69) | 1.01 | (1.0–1.04) | 0.5 |
| No. von Hippel Lindau diagnosis (%) | 126 | (81) | 30 | (83) | 0.8 | (0.3–2.2) | 0.7 |
| Median No. prior ipsilat procedures (IQR) | 1 | (1–2) | 2 | (1–2) | 1.3 | (0.9–1.9) | 0.2 |
| No. cohort (%): | |||||||
| Open after MIS only | 8 | (5) | 2 | (6) | 0.7 | (0.2–3.7) | 0.7 |
| Open after open | 77 | (49) | 26 | (72) | Reference | Reference | |
| Robotic after MIS only | 28 | (18) | 4 | (11) | 0.4 | (0.1–1.3) | 0.1 |
| Robotic after open | 43 | (28) | 4 | (11) | 0.3 | (0.1–0.8) | 0.02 |
| Intraop characteristics and outcomes: | |||||||
| Median mins duration surgery (IQR) | 379 | (325–441) | 445 | (373–493) | 1.005 | (1.001–1.008) | 0.01 |
| Median ml EBL (IQR) | 1,850 | (1,000–3,000) | 3,500 | (1,600–5,000) | 1.0004 | (1.0002–1.0005) | <0.001 |
| No. hilar clamping utilized (%) | 37 | (24) | 5 | (14) | 0.5 | (0.2–1.4) | 0.2 |
| Median No. tumors resected (IQR) | 5 | (2–9) | 8 | (5–13) | 1.04 | (1.0002–1.09) | 0.07 |
When assessing for factors associated with a patient developing a grade ≥3 complication, several demographic and intraoperative variables were found to be significant. Surgery duration (OR 1.005, 95% CI 1.001–1.008, p = 0.01), surgical approach utilized (OR 0.3, 95% CI 0.1–0.8, p = 0.02), and number of prior open procedures (OR 1.6, 95% CI 1.1–2.4, p = 0.02) were significant risk factors (table 3 and fig. 2). For perioperative factors, EBL (OR 1.0004, 95% CI 1.0002–1.0005, p <0.001), pRBCs transfused intra and postoperatively (OR 1.2, 95% CI 1.1–1.3, p <0.001 and OR 1.5, 95% CI 1.2e–1.8, p <0.001, respectively), and the percent change in 3-month GFR were also associated with the presence of grade ≥3 complication (OR 0.97, 95% CI 0.95e0.99, p = 0.02).
Figure 2.
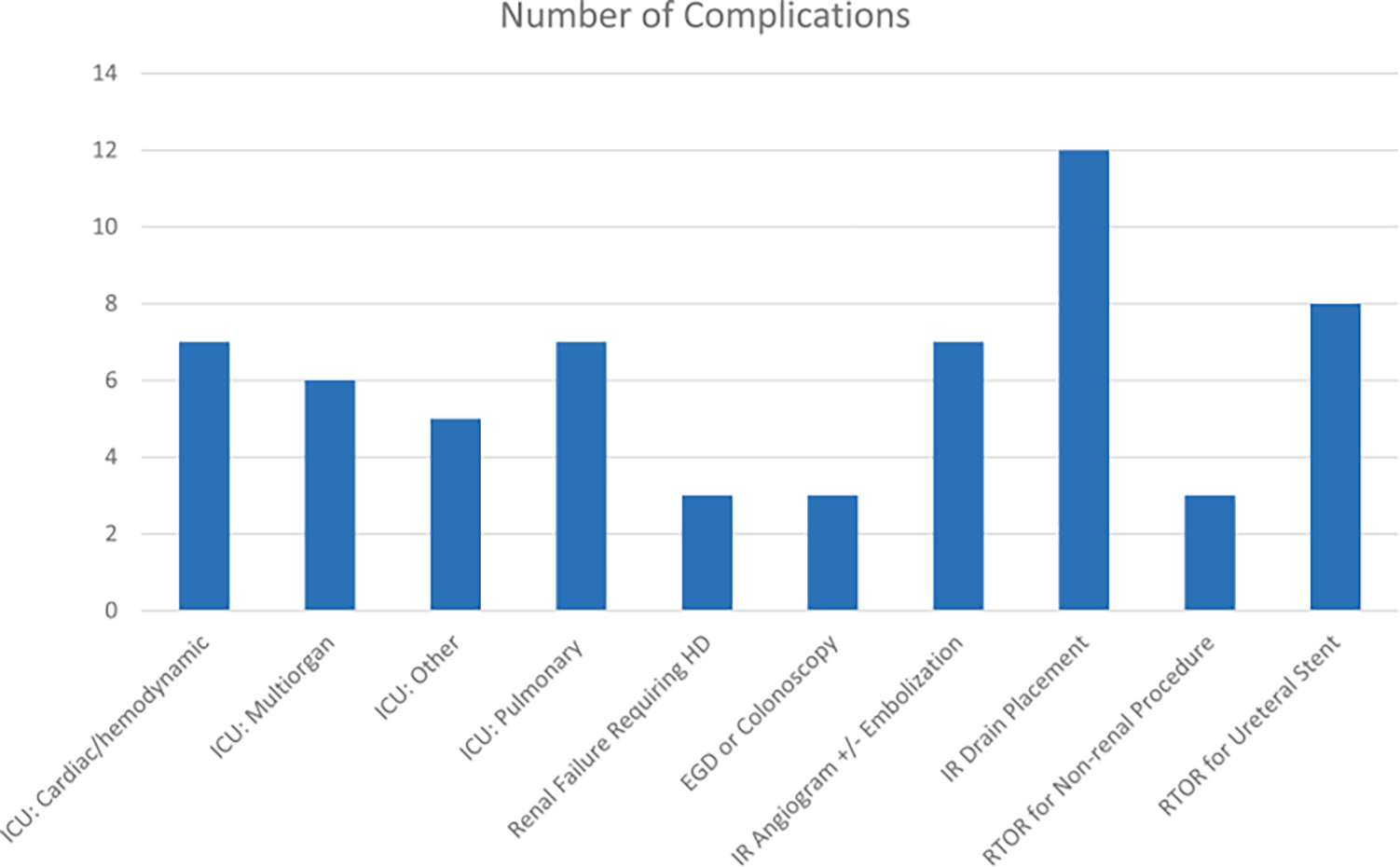
Grade ≥3 complications by cause. HD, hemodialysis. EGD, esophagogastroduodenoscopy. IR, interventional radiology. RTOR, return to operating room.
Multivariate models demonstrated that EBL (OR per 100 ml: 1.03, 95% CI 1.009–1.05; p = 0.005) was predictive of which patients experienced a grade ≥3 complication while being part of the robotic after open cohort was protective (OR 0.2, 95% CI 0.07–0.9, p = 0.03) (supplementary table 1, https://www.jurology.com). The model was subsequently adjusted, and the 4-cohort design was replaced with the current surgical approach utilized and a history of open surgery as separate variables. Similarly, EBL (OR per 100 ml: 1.03, 95% CI 1.009–1.05; p = 0.004) was predictive of grade ≥3 complications while currently undergoing robotic surgery was protective (OR 0.3, 95% CI 0.1–0.8, p = 0. 02; table 4). Notably a history of open procedures (OR 0.8, p = 0.7) and the number of tumors resected (OR 1.0, p = 0.4) were not associated.
Table 4.
Multivariate analysis of risk factors for grade ≥3 complication
| Risk Factor | Logistic Coefficient | Odds Ratio | 95% CI | p Value |
|---|---|---|---|---|
|
| ||||
| Surgery duration | 0.003 | 1.003 | 0.998–1.008 | 0.2 |
| Current surgical approach: robotic | −1.3 | 0.3 | 0.1–0.8 | 0.02 |
| History of prior open surgery | −0.2 | 0.8 | 0.3–2.4 | 0.7 |
| No. prior ipsilat procedures | −0.04 | 1.0 | 0.6–1.5 | 0.9 |
| No. tumors resected | −0.03 | 1.0 | 0.9–1.04 | 0.4 |
| EBL (per 100 ml) | 0.03 | 1.03 | 1.009–1.05 | 0.004 |
| Constant | −2.8 | 0.07 | ||
Given that some patients had both prior open and MIS surgery, a subgroup analysis was performed in patients who had a history of only open PN or a history of only prior MIS PN. On multivariate analysis, only EBL was found to be predictive of a grade ≥3 complication (OR per 100 ml: 1.03, 95% CI 1.008–1.06, p = 0.007, supplementary table 2, https://www.jurology.com).
DISCUSSION
RePN is a challenging surgery. Normal surgical planes can become altered or altogether obliterated and peri-hilar fibrosis can make vascular dissection a hazardous ordeal (fig. 3). This study is the largest to comprehensively describe the outcomes of patients undergoing complex RePN. Most notably, these results demonstrate that in experienced hands, RePNs are both feasible and safe. Of the 192 RePNs performed, only 1 patient (0.5%) required a radical nephrectomy, which was necessitated due to a vascular injury. Of the 79 attempted robotic RePN, 6 (7.6%) required conversion to open approach. These results help clarify that repeat surgery, independent of the approach, is feasible without compromising the final outcome of a successful operation. Current literature suggests that robotic RePN is likely feasible; however, with fewer than 40 patients reported in the literature, power to make meaningful conclusions is lacking. The favorable results of the 79 patients in our robotic cohorts clearly demonstrates the feasibility of robotic RePN.
Figure3.
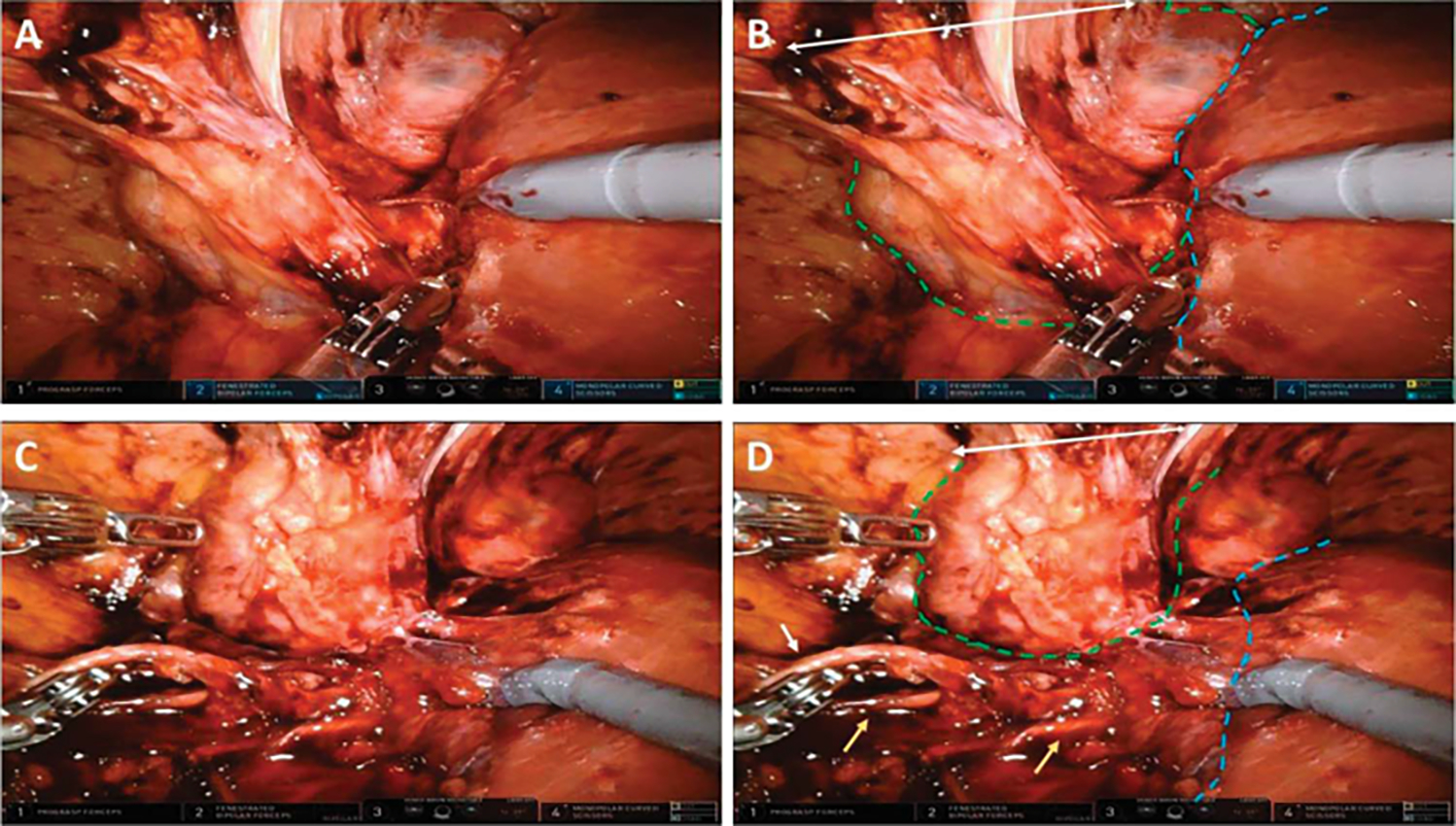
Intraoperative view of robotic after open PN demonstrating characteristic peri and paranephric adhesions to anterior abdominal wall and surrounding organs and tissue. A and B, right kidney (outlined in green dashes) adherent to liver (outlined in blue dashes) and adherent to prior incision site on anterior abdominal wall (white double arrow). C and D, same patient after further dissection. White arrow indicates ureter, yellow arrow indicates right gonadal veins.
The results among the cohorts are split by current surgical approach, with both robotic cohorts experiencing decreased need for intra and postoperative transfusions, fewer patients experiencing a complication, and lower number of grade ≥3 complications experienced per patient and only the robotic after open cohort experiencing fewer patients with a grade ≥3 complication. On both multivariate models, 2 major risk factors were identified as significant contributors to experiencing a grade ≥3 complication: EBL and current robotic surgery. The subgroup analysis demonstrated that a history of open only or MIS only surgery was not a factor in predicting the presence of a grade ≥3 complication. These results suggest that the prior surgical approaches are less important in determining perioperative outcomes, and therefore less important in the overall surgical footprint. Likely it is the extent of tissue plane manipulation or dissection, whether open or robotic, that plays a greater role in the surgical footprint as is echoed in prior literature.10,15
EBL as a factor contributing to increased high grade complications is logical, as many times these patients require considerable intraoperative blood products and fluid resuscitation leading to ICU level care due to longer times weaning ventilation and other secondary issues such as pre-renal acute kidney injury. However, it is unclear if EBL is merely a predictor of the complexity of the operation and may reflect unaccounted for variables such as tumor complexity or perirenal fibrosis. Unfortunately, no validated renal mass complexity scoring system exists for patients with multifocal tumors and therefore tumor complexity could not be quantified.16
It is important to note that while the size of the surgical footprint vis-à-vis open versus minimally invasive procedures did not seem to impact outcomes, the surgical technique utilized by surgeons at our institution incorporates several factors in minimizing the footprint of surgery regardless of approach. For instance, Gerota’s fascia is preserved and reapproximated at the end of each case when technically feasible. Additionally, hilar dissection is minimized, and the renal vein and artery are often not skeletonized in order to potentially limit fibrosis during subsequent procedures. Similarly, the hilum is not clamped in the majority of cases and renovascular occlusion is reserved for deeper tumors or those located near the hilum.
This study has its limitations. The study is retrospective and comprised mostly of a relatively uncommon group of patients with known or suspected hereditary renal cell carcinoma; however, the challenges and complications arising from prior surgery are not different from those with sporadic kidney cancer. There is also likely a considerable selection bias in choosing operative approach, and indeed, the open after open RePN cohort had the most prior procedures. The vast majority of patients underwent tumor enucleations in order to spare as much parenchyma as this benefits a population which is afflicted with multiple, recurrent masses. Also, the patients’ prior surgeries consisted of a heterogeneous mix of approaches with many having had both MIS or open surgery in the past. Furthermore, some patients may have had a history of focally ablative procedures. In order to standardize this group, we hypothesized that open surgery would have the most manipulation of surgical planes and therefore our definition of prior open surgery was a patient who ever had a prior open PN in the ipsilateral renal unit. The purpose of the subgroup analysis was to address this limitation by allowing for a purer assessment of the effects of prior surgical approaches, but it came at the cost of statistical power as the patient cohort size was considerably reduced. Therefore, the favorable surgical outcomes in robotic surgery do not necessarily tout robotic assisted surgery as a safer approach, but rather demonstrate its safety and feasibility in the reoperative setting. However, even with its drawbacks, this study remains the largest study analyzing patients undergoing RePN and their outcomes.
CONCLUSIONS
In patients with a history of prior ipsilateral PN, the use of robotic approach to PN is safe, feasible, and may have improved perioperative outcomes over open surgery. The surgical footprint of prior surgeries is likely less due to the surgical approach utilized, but rather the amount of tissue plane manipulation and dissection that was performed at that time.
Supplementary Material
Acknowledgments
This research was supported by the Intramural Research Program of the National Cancer Institute, National Institutes of Health.
Abbreviations and Acronyms
- EBL
estimated blood loss
- GFR
glomerular filtration rate
- ICU
intensive care unit
- MIS
minimally invasive surgery
- PN
partial nephrectomy
- pRBC
packed red blood cell
- RCC
renal cell carcinoma
- RePN
reoperative partial nephrectomy
Contributor Information
Sandeep Gurram, Urologic Oncology Branch, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
Nicholas A. Friedberg, Department of Urology, George Washington University Medical School, Washington, District of Columbia Urologic Oncology Branch, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
Chirag Gordhan, Department of Urology, George Washington University Medical School, Washington, District of Columbia; Urologic Oncology Branch, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
Winston Li, Urologic Oncology Branch, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
Michael A. Ahdoot, Urologic Oncology Branch, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, Maryland
Jillian Egan, Department of Urology, MedStar Georgetown University Hospital, Washington, District of Columbia; Urologic Oncology Branch, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
Nitin K. Yerram, Urologic Oncology Branch, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, Maryland
Gennady Bratslavsky, Department of Urology, SUNY Upstate Medical Center, Syracuse, New York; Urologic Oncology Branch, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
Adam R. Metwalli, Division of Urology, Department of Surgery, Howard University College of Medicine, Washington, District of Columbia Urologic Oncology Branch, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
W. Marston Linehan, Urologic Oncology Branch, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
Mark W. Ball, Urologic Oncology Branch, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
REFERENCES
- 1.Campbell S, Uzzo RG, Allaf ME et al. : Renal mass and localized renal cancer: AUA guideline. J Urol 2017; 198: 520. [DOI] [PubMed] [Google Scholar]
- 2.Gill IS, Kavoussi LR, Lane BR et al. : Comparison of 1,800 laparoscopic and open partial nephrectomies for single renal tumors. J Urol 2007; 178: 41. [DOI] [PubMed] [Google Scholar]
- 3.Johnson A, Sudarshan S, Liu J et al. : Feasibility and outcomes of repeat partial nephrectomy. J Urol 2008; 180: 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu NW, Khurana K, Sudarshan S et al. : Repeat partial nephrectomy on the solitary kidney: surgical, functional and oncological outcomes. J Urol 2010; 183: 1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Magera JS Jr, Frank I, Lohse CM et al. : Analysis of repeat nephron sparing surgery as a treatment option in patients with a solid mass in a renal remnant. J Urol 2008; 179: 853. [DOI] [PubMed] [Google Scholar]
- 6.Boris RS, Gupta GN, Benson JS et al. : Feasibility and outcomes of laparoscopic renal intervention after prior open ipsilateral retroperitoneal surgery. J Endourol 2013; 27: 196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Turna B, Aron M, Frota R et al. : Feasibility of laparoscopic partial nephrectomy after previous ipsilateral renal procedures. Urology 2008; 72: 584. [DOI] [PubMed] [Google Scholar]
- 8.Autorino R, Khalifeh A, Laydner H et al. : Repeat robot-assisted partial nephrectomy (RAPN): feasibility and early outcomes. BJU Int 2013; 111: 767. [DOI] [PubMed] [Google Scholar]
- 9.Watson MJ, Sidana A, Diaz AW et al. : Repeat robotic partial nephrectomy: characteristics, complications, and renal functional outcomes. J Endourol 2016; 30: 1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fantola G, Brunaud L, Nguyen-Thi PL et al. : Risk factors for postoperative complications in robotic general surgery. Updates Surg 2017; 69: 45. [DOI] [PubMed] [Google Scholar]
- 11.Guan B, Li J, Yang W et al. : Reoperative thyroid surgery: can endoscopic areola approach be used? Surg Endosc 2017; 31: 1296. [DOI] [PubMed] [Google Scholar]
- 12.Tolboom RC, Draaisma WA and Broeders IA: Evaluation of conventional laparoscopic versus robot-assisted laparoscopic redo hiatal hernia and antireflux surgery: a cohort study. J Robot Surg 2016; 10: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bratslavsky G, Liu JJ, Johnson AD et al. : Salvage partial nephrectomy for hereditary renal cancer: feasibility and outcomes. J Urol 2008; 179: 67. [DOI] [PubMed] [Google Scholar]
- 14.Dindo D, Demartines N and Clavien PA: Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004; 240: 205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buchs NC, Addeo P, Bianco FM et al. : Perioperative risk assessment in robotic general surgery: lessons learned from 884 cases at a single institution. Arch Surg 2012; 147: 701. [DOI] [PubMed] [Google Scholar]
- 16.Chalfin H, Yerram NK, Gurram S et al. : A novel multiplex score to predict outcomes of partial nephrectomy for multiple tumors. J Clin Oncol, suppl., 2020; 38: 656. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


