Abstract
Social evolution is tightly linked to dispersal decisions, but the ecological and social factors selecting for philopatry or dispersal often remain obscure. Elucidating selection mechanisms underlying alternative life histories requires measurement of fitness effects in the wild. We report on a long-term field study of 496 individually marked cooperatively breeding fish, showing that philopatry is beneficial as it increases breeding tenure and lifetime reproductive success in both sexes. Dispersers predominantly join established groups and end up in smaller groups when they ascend to dominance. Life history trajectories are sex specific, with males growing faster, dying earlier, and dispersing more, whereas females more likely inherit a breeding position. Increased male dispersal does not seem to reflect an adaptive preference but rather sex-specific differences in intrasexual competition. Cooperative groups may thus be maintained because of inherent benefits of philopatry, of which females seem to get the greater share in social cichlids.
Life history trajectories may differ between males and females, even if both sexes would benefit most from the same strategy.
INTRODUCTION
The link between dispersal and cooperation is a fundamental component of several major transitions in the evolution of life, including the formation of animal societies and multicellular organisms (1, 2). Delaying or completely foregoing dispersal has been identified as a key characteristic in the transition from mere group living to more complex forms of sociality and cooperation (3–5). However, only a minority of animal species has evolved cooperative societies (among vertebrates, ~9% of birds, ~0.5% of mammals, ~0.1% of fishes) (6), and most animal systems are characterized by early dispersal and independent breeding. Cooperative breeders, in contrast, typically show delayed dispersal combined with alloparental care. The crucial evolutionary question is under which conditions individuals will benefit from extended philopatry and potentially costly cooperation, if dispersing early and maximizing own reproduction appear to be more generally adaptive (3, 7).
Where prolonged philopatry has evolved, it is assumed to be either beneficial by increasing fitness when staying in the natal territory (8) or to result from constraints on dispersal and independent breeding (9), for instance, through habitat saturation (10) or enhanced predation risk (11). Group formation resulting from philopatry is thought to select for cooperation and alloparental care mainly through inclusive fitness benefits (6) achieved by supporting close relatives (12). In addition, direct fitness benefits from philopatry may accrue, for example, through increased foraging efficiency (3), enhanced survival by group membership (13), some reproductive share within the group (14, 15), or from the eventual inheritance of a breeding position (16, 17). If group membership is contingent on cooperation because subordinates must compensate for the costs they incur on dominants to be tolerated (“pay to stay”) (18–20), then direct benefits will result in selection for cooperation in connection with philopatry (21), particularly where within-group relatedness is low or heterogeneous (22, 23). The relative importance of these various explanations for philopatry and cooperation within groups has been extensively investigated (24–28) and debated (3, 5, 6, 29–32), and it is affected by the quality of the environment (7, 33).
Fitness consequences of dispersal and philopatry
Understanding the fitness outcomes of dispersal decisions in cooperative breeders is crucial to resolve this debate (6, 7). If extended philopatry in the natal group enhances the fitness of individuals and is hence adaptive, then there should be competition for being able to remain in the natal territory (34), and high-quality individuals are expected to preferentially stay home (8). In contrast, if dispersal provides higher fitness, for instance, through increased prospects of own reproduction (35) or affiliation with groups providing greater protection (36), then delayed or foregone dispersal likely reflects a best-of-a-bad-job decision caused by environmental or social constraints (37). Here, high-quality individuals are expected to choose the dispersal option (9). It is important to consider, however, that dispersal itself is often costly (38), which may limit the probability that individuals will be able to reproduce independently and successfully (39).
The fitness outcomes of philopatry and dispersal may be sex specific (40). Cooperative species with sex biases in dispersal propensity (41) are therefore particularly suited to study the ecological and social conditions favoring one or the other life history strategy, i.e., dispersal or philopatry (42–44). This is because in these systems, the costs and benefits of either strategy can be scrutinized without confounding factors that have been shown to affect interspecific comparisons, e.g., mating system variation or environmental stochasticity (45). Specifically, in cooperatively breeding species, sex-biased dispersal skews within-group relatedness (46), resulting in sex differences in the potential to reap indirect fitness benefits, which thus affects the payoffs of cooperative behavior (22, 47). Sex-specific direct fitness benefits of philopatry or dispersal may also result from differential opportunities for own reproduction and/or resource inheritance (16, 42), which, in turn, may cause sex-specific cooperation propensity in a pay-to-stay scenario (48, 49). Thus, explorations of the links between sex biases in dispersal and cooperative behavior can elucidate the ecological and social drivers of transitions to complex societies (42–44). However, the fact that a species shows sex-biased dispersal does not necessarily imply the presence of sex-specific payoffs of dispersal or philopatry; dispersal may be sex-biased even if both sexes similarly benefit from the same life history strategy, i.e., from dispersing or remaining philopatric (41). In these cases, it is intriguing to understand the causes that prevent one sex more often than the other from adopting the preferred life history strategy, leading to sex biases despite similar payoffs. This could reveal constraints either on dispersal (9) or on philopatry (34). To gauge the relative importance of benefits and constraints involved in dispersal and philopatry, it is thus beneficial to study their fitness consequences in cooperative systems in which (i) dispersal is sex-biased; (ii) ecology, sociality, and relatedness structures are sufficiently well understood; and (iii) experimental and longitudinal approaches are feasible to enable testing alternative hypotheses of the evolution of delayed/foregone dispersal and cooperation. The cooperatively breeding cichlid fish Neolamprologus pulcher fulfills all these requirements.
Cooperative cichlids
N. pulcher is a substrate-breeding cichlid species endemic to Lake Tanganyika (50). Groups of these fish defend territories year round in which individuals find shelters essential for breeding and protection from predators (15). Territories cluster in colonies from a few to over 100 groups located close to one another (14, 51). Dispersing individuals preferentially move to more central territories (52), likely because a group’s location within a colony has important effects on individual fitness prospects: Proximity to neighboring groups provides antipredator protection (51, 53) and reproductive benefits (36), and it influences reproductive sharing (14) and relatedness patterns among group members (54). Groups are composed of a dominant breeder pair largely monopolizing reproduction and several subordinate helpers of both sexes and of varying age and size (51, 55–58). Subordinates help raising the dominants’ broods to be tolerated in a territory (i.e., they pay to stay) (59–65). Access to shelters and protection by larger group members is crucial for subordinate survival (11, 58). Large males can monopolize several territories, rendering them polygynous (66), and in accordance with this, the operational sex ratio in our study population was approximately 1:2 in favor of females (67). Because of frequent dispersal and breeder turnover, relatedness between dominants and subordinates declines with the subordinates’ age, and sexually mature helpers are largely unrelated to their respective dominant territory holders (22). Consequently, indirect fitness gains during the subordinate stage are negligible compared to the direct fitness fish reap once they have obtained a dominant position (36). Nevertheless, subordinates may participate in reproduction, thus gaining some direct fitness before ascending to dominance [e.g., (14); reviewed in (15)]. Furthermore, subordinates either queue for dominance in their natal territory or disperse to increase their chances of acquiring dominance (16, 22, 57). Because groups are organized in a size-based hierarchy, larger subordinates are more likely to gain dominance (16, 68). Dispersal in N. pulcher is sex- and size-biased; males disperse more often than females, and large fish disperse more often than small group members (69). Dispersal typically covers only short distances in this species [less than 10 m; (16); see below for additional information regarding sex, size, and distance effects in dispersal].
Experiments in the laboratory (58) and field (11) revealed predation as an ecological constraint on dispersal, which likely affects the sexes differentially as predation risk declines with increasing body size (11, 58). Males grow larger in this species (15) and should thus suffer less mortality costs of dispersal. Hence, males are suggested to more easily reap the potential benefits of dispersal in the form of increasing their relative rank within the group (70) or potentially ascending directly to dominance (16). Dispersers might also improve the prospects of future reproductive success by joining groups having more helpers or being located in more favorable areas of a colony, i.e., with closer neighbors (36, 53). Benefits of philopatry have been suggested by experimental manipulation of predation risk and a sequential settlement experiment (57, 58). These benefits might be higher for females, because more frequent turnover among male breeders and a higher male dispersal propensity skew within-group relatedness in favor of females (16, 22), and female breeders suffer less from parentage losses to subordinates (14, 15, 71, 72). Reduced philopatry in males is likely linked to enhanced intrasexual competition among males compared to females (73) despite an unbiased sex ratios at birth (15): Only half the breeding positions exist for males due to the polygynous mating pattern (67), males lose more parentage to competitors (14), and male reproduction is most strongly limited by their access to fertilizable eggs [(74); because of competition with other males (67) and stolen fertilizations (14, 15)]. It is currently unclear how philopatry and dispersal affect fitness in both sexes under natural conditions. This gap is due to a lack of individual-level, long-term data on fitness correlates in the natural habitat of these cooperatively breeding fish (15).
Aims
Three hypotheses feature prominently for the explanation of sex-specific dispersal: resource competition, local mate competition, and inbreeding avoidance (40). Whereas the latter does not make clear predictions about which sex should disperse (40), the other two allow specific predictions based on the ecological, social, or reproductive benefits (8) or constraints (9) the sexes face. To clarify which of these benefits or constraints favor male- or female-biased dispersal, which, in turn, may affect cooperation (75), it is necessary to assess the fitness effects of the varying life history trajectories in both sexes. To this end, we report on longitudinal life history data of individually marked N. pulcher, observed in their natural habitat in southern Lake Tanganyika, revealing their dispersal decisions in dependence of size, age, sex, and social status, with special emphasis on the entailing consequences for important correlates of fitness (e.g., estimates of dominance tenure and lifetime reproductive success; note that even if our study only provides estimates of fitness correlates, we refer to these as “fitness” for simplicity’s sake throughout). This aims at revealing major ecological and social factors selecting for philopatry or dispersal in males and females of this cooperatively breeding fish species, elucidating potential factors that promote their advanced sociality.
RESULTS
To study long-term survival, dispersal decisions, and respective fitness consequences, we surveyed 496 individually marked N. pulcher for a large proportion of their total lifetime (for each analysis we report the exact criteria for data inclusion and details of model selection and fit in the respective supplement).
Life history trajectories of males and females
Growth
Recaptures in two consecutive years of 195 individual fish allowed for estimates of annual growth (fig. S1A). Males grew faster and to a larger maximum size than females [linear mixed-effects model (LME): likelihood ratio test (LRT) = 188.8 and P < 0.001; Fig. 1 and fig. S2A], and annual growth generally declined with increasing body size (LME: LRT = 424.72 and P < 0.001; Fig. 1 and fig. S2A).
Fig. 1. Annual growth of 195 individually marked N. pulcher caught in the wild.
Increases in standard length [SL (in centimeters)] between two consecutive years (y axis) plotted as a function of SL in the first of these 2 years (x axis). Annual growth declined with increasing size at a similar rate in both sexes. Males (blue) had a higher size-specific growth rate and grew to a larger size than females (yellow). In both sexes, dispersing individuals (dashed lines; females, upward-pointing triangles; males, diamonds) grew at a faster size-specific rate than individuals that stayed in their territory throughout the observation period (solid lines; females, downward-pointing triangles; males, squares). Points give individual measurements and are slightly offset if overlapping to improve visibility. Statistical details are given in the main text and in sections S1 and S2.
Survival
Older (and thus larger) fish of both sexes had higher survival probabilities [binomial generalized linear model (bGLM): LRT = 8.36 and P = 0.004; n = 496 fish; fig. S1A). Females had higher annual survival chances than males (bGLM: LRT = 5.14 and P = 0.023). On average females lived longer than males [Poisson generalized linear model (pGLM): LRT = 18.06 and P < 0.001; Fig. 2 and sections S3 and S4]. The mean age at death (±standard deviation) was 2.6 (±1.4) years for males and 3.3 (±1.5) years for females; the oldest age estimate at death was 8 years for a female (Fig. 2).
Fig. 2. The estimated age at death in full years of all 496 individual N. pulcher considered in this study.
Columns give the absolute counts (left y axis), while density curves show the respective proportions of all fish in the current study [e.g., 60 males estimated to have died at age 1 (blue bar; left y axis) from a total of 232 males, i.e., ~26% (blue line; right y axis)]. Females (yellow) had on average longer lives than males (blue). Statistical details are given in the main text and in sections S3 and S4.
Attainment of breeder status
Males were larger when ascending to dominance [binomial generalized linear mixed-effects model (bGLMM): LRT = 168.9 and P < 0.001; Fig. 3; n = 761 observations of 496 individuals; section S5]. This is reflected by the difference between the sexes in the size at which they had a 50% chance of having obtained dominance (as predicted by the respective bGLMM), which was 4.77 cm for females and 5.23 cm for males. Males were younger than females when reaching a dominant breeder position [pGLM: scaled deviance (sd) = 4.54 and P = 0.033; n = 80 fish, which ascended to dominance during the observation period; section S11]. Overall, females were more likely to be observed as dominants than males [174 of 264 females (66%) were observed to hold a dominant breeder position, compared to 114 of 232 males (49%); χ2 test: χ2 = 13.58 and P < 0.001].
Fig. 3. A fish’s probability of being the dominant breeder in its group.
Dominance status (y axis; 0, subordinate; 1, dominant) is plotted as a function of body size [lower x axis; SL (in centimeters)] for 761 measurements of size and social status of females (yellow) and males (blue). Solid lines represent values predicted by the respective generalized bGLMM. The upper x axis indicates the corresponding estimated ages for both sexes. The horizontal gray line identifies the 0.5 probability of being dominant and the dashed lines denote the respective intersection with the predicted curves. In both sexes, the probability of being dominant increased with increasing size, but males ascended to dominance at a larger size than females, whereas the corresponding age estimates were similar between the sexes. Points give individual measurements and are slightly offset to improve visibility. Statistical details are given in the main text and in section S5.
Dispersal
Males were more likely to disperse than females (bGLMM: LRT = 6.67 and P = 0.01; n = 1029 observations of 490 individual fish; see section S6 for dispersal definition and detection), but dispersal distances did not significantly differ between the sexes (LME: LRT = 2.91 and P = 0.09; n = 103 dispersals; see Materials and Methods and section S7 for details and a discussion of dispersal tracing). When dispersing, males were larger than females [LME: LRT = 10.33 and P = 0.001; n = 103 dispersals; section S8; median (range) standard length (SL): males, 5.4 cm (3.3 to 6.5); females, 4.8 cm (3.8 to 5.7)]. In contrast, the age at dispersal did not differ between the sexes [pGLMM: LRT = 1.32 and P = 0.25; median (range) in years: males, 2 (1 to 6); females, 2 (1 to 5)]. The overwhelming majority of dispersals resulted in fish joining already established groups (97 cases), whereas only seven instances of dispersal targeted a recently founded group (6.7% of all dispersals; section S6). Twelve of 66 dispersing subordinates kept their subordinate status after dispersal (18%), and males more often than females retained their subordinate status when dispersing as subordinates [1 of 27 dispersing females remained subordinate (4%), whereas 11 of 39 dispersing males did (28%); section S6].
Territory inheritance
Females were approximately twice as likely as males to inherit the dominant position in a territory in which they had initially been recorded as subordinates: 18 of 43 females (42%) compared to 8 of 37 males (22%; Fisher’s exact test, P = 0.06).
Fitness consequences of dispersal for males and females
Effects on growth
In both sexes, dispersers grew faster than fish that did not disperse (LME: LRT = 14.25 and P < 0.001; n = 268 annual growth measurements of 195 individual fish; Fig. 1 and sections S1-S2).
Effects on survival
Among recaptured fish, females lived longer than males (pGLM: LRT = 4.97 and P = 0.026; n = 197 individual fish; section S9), but dispersal did not influence estimated age at death (LRT = 0.14 and P = 0.14).
Effects on breeder status
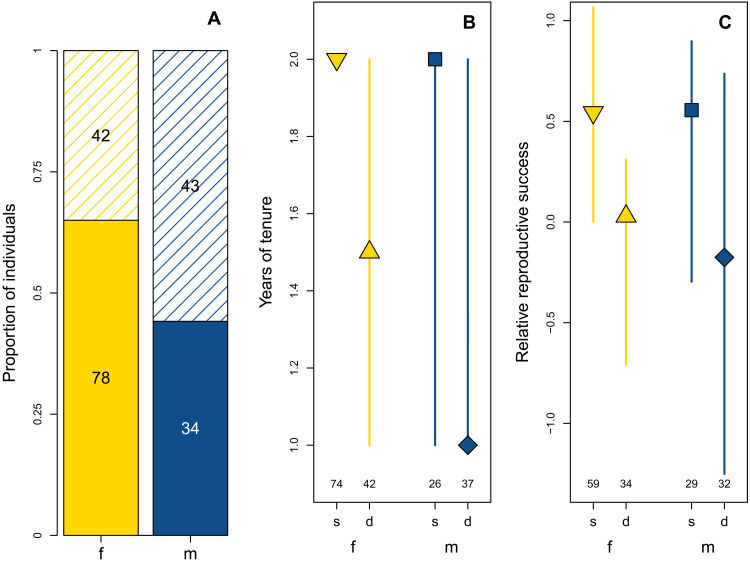
For initially subordinate fish, dispersal increased their chances of ascending to dominance, irrespective of sex (bGLM: LRT = 8.46 and P = 0.004; n = 99 individual fish; section S10), but dispersers were of a similar age as philopatric individuals when acquiring dominance (pGLM: sd = 1.34 and P = 0.25; n = 80 individual fish; section S11). Dominance tenure of dispersers was shortened compared to philopatric fish, for males and females alike (pGLM: sd = 6.2 and P = 0.013; n = 179 individual fish; Fig. 4 and section S12). Most philopatric individuals held tenure for 2 years (65 of 100 philopatric fish; fig. S12), while most dispersing fish held tenure for only 1 year (41 of 79 dispersers; fig. S12). Nevertheless, among fish with long tenure (3 to 4 years), dispersers were as numerous as nondispersers (18 individuals each). The difference in tenure times between dispersers and nondispersers was thus most prominent in the first year of attaining dominance, resulting in an overall different distribution of tenure duration between fish that followed different life history strategies (χ2 test: χ2 = 31.96 and P < 0.001).
Fig. 4. The propensity for dispersal and its fitness-related consequences for female and male N. pulcher.
In all three plots, females (f) are represented in yellow and males (m) in blue. Nondispersing individuals (s) are represented by solid-fill areas of bars in (A), and by downward-pointing triangles (females) and squares (males) in (B) and (C). Dispersing individuals (d) are represented by dashed areas of bars in (A), and by upward-pointing triangles (females) and diamonds (males) in (B) and (C). Numbers in all plots show the sample sizes representing individual fish. Males had a higher propensity for dispersal than females, as shown in (A) for the 197 recaptured individuals, but both sexes suffered similar costs of dispersal as shown in (B) and (C). (B) shows the recorded tenure times of 179 individuals that were observed as dominant and recaptured at least once. Dispersers had shorter dominance tenure than nondispersers. (C) denotes the relative reproductive success, i.e., sex- and status-specific Z scores of estimated reproductive success, for 154 individuals of which their dispersal decisions and at least one estimate of their relative reproductive success were known. Dispersers had significantly lower relative reproductive success than nondispersers. Statistical details are given in the main text and in sections S6, S12, and S14.
Effects on group size
The effect of dispersal on changes in group size was influenced by dispersal coinciding with changes in social status of the focal fish (LME: LRT = 10.83 and P = 0.004; n = 75 individual fish; Fig. 5 and section S13). Fish that gained dominance via dispersal (dispersal type “SD,” subordinate to dominant) joined groups smaller than their group of origin (Wilcoxon matched-pairs signed-ranks test: V = 629 and P = 0.003), while dispersal without status change (dispersal types “SS,” subordinate to subordinate, and “DD,” dominant to dominant) did not lead to a significant change in group size (V = 145.5 and P = 0.12).
Fig. 5. The change in group size experienced by dispersing fish.
Plotted is group size after dispersal minus group size prior to dispersal (y axis). Positive values thus indicate that fish dispersed into groups that were larger than their group of origin, while negative values indicate a reduction in group size due to dispersal. Females are represented in yellow (f; triangles) and males in blue (m; diamonds). Data were sorted according to the three observed types of dispersal (SS, subordinate to subordinate; SD, subordinate to dominant; DD, dominant to dominant). The type of dispersal affected the ensuing changes in group size, with fish ascending to dominance from subordinate status via dispersal (SD) experiencing a significant reduction in group size. Symbols indicate median values and vertical lines show interquartile ranges. Numbers below symbols give the respective sample sizes (numbers of individual fish). Statistical details are given in the main text and in section S13.
Effects on reproductive success
Lifetime reproductive success of male and female dispersers was reduced compared to fish of the same sex that did not disperse (LM: t = −2.15 and P = 0.03; n = 154 individual fish; Fig. 4 and section S14). These estimates were based on individual survival and per-group offspring counts, modified by estimates of average parentage of group members and within-group reproductive competition (the data were Z-transformed to allow for comparisons between fish of different sexes and status that were observed at different times; see Materials and Methods). The differences in reproductive success between dispersers and nondispersers of both sexes were mostly accrued during an individual’s dominance tenure (LM: t = −2.46 and P = 0.013; n = 130 individual dominant fish; section S15). Estimates of reproductive success during the subordinate life stage did not differ between dispersing and philopatric fish (LM: t = −0.2 and P = 0.84; n = 75 individual subordinates; section S16).
Social organization
Group size
Large group size did not significantly affect growth rates (LME: LRT = 3.42 and P = 0.065; n = 195 individual fish; fig. S2A) or dominance tenure (pGLM: sd = 2.96 and P = 0.085; n = 179 individual fish; fig. S12). Large group size enhanced the reproductive success of dominants (LM: t = 2.8 and P = 0.005; n = 130 individual dominant fish; section S15) but not that of subordinates (LM: t = 1.8 and P = 0.07; n = 75 individual fish; section S16). Overall lifetime reproductive success was increased in larger groups (LM: t = 3.23 and P = 0.001; n = 154 individual fish; see section S14 for details). Group size neither affected the likelihood to disperse (bGLMM: LRT = 0.44 and P = 0.51; n = 1029 observations of 490 individual fish; section S6) nor to acquire dominance (bGLM: LRT = 0.24 and P = 0.62; n = 99 individual fish; section S10).
Spatial structure
The probability of ascending to dominance was higher for subordinate members of more isolated groups (bGLM: LRT = 4.17 and P = 0.041; n = 99 individual subordinate fish; section S10). None of the other aspects of dispersal decisions and life history trajectories we investigated was influenced by local population density, i.e., a group’s nearest-neighbor distance. Dispersal typically covered short distances (fig. S7A); hence, it rarely involved areas with different territory densities and did not alter the effects of between-group sociality (see Introduction) (14, 36, 52–54).
DISCUSSION
Our data reveal clear negative fitness effects of dispersal in N. pulcher: In both sexes, fish that dispersed had shorter dominance tenure and lower reproductive success in comparison to nondispersers (Fig. 4). Nevertheless, dispersers grew faster than philopatric individuals (Fig. 1), which is in accordance with previous studies (58); subordinates reduce cooperation shortly before dispersing, which enhances growth (61, 76). Furthermore, large male helpers have been shown to retard growth to receive less aggression and remain tolerated (73). The growth differences between philopatric helpers and dispersers we found are hence likely reflecting strategic decisions associated with dispersal. While dispersal increased the probability of attaining dominance, there was no difference among dispersing and nondispersing fish regarding the age at which they ascended to dominance, but dispersers had shorter dominance tenure (Fig. 4). None of these effects were observed to be sex-specific. Together, these findings imply that philopatry is beneficial to both sexes in N. pulcher. However, dispersal was biased toward males (Fig. 4), the larger and faster growing sex (Fig. 1), thereby contradicting the prediction that more competitive individuals should succeed in choosing the more beneficial option.
Fitness benefits of philopatry
Our results suggest that delayed or foregone dispersal in N. pulcher is beneficial because of greater fitness rewards of philopatry (8), which, in this system, typically accrue from direct fitness gains such as increased survival (11, 36, 58), participation in reproduction (14, 77–79), and territory inheritance (16, 22, 68, 69). As large N. pulcher helpers are rarely related to the offspring the breeders produce due to group membership dynamics (22), sexually mature subordinates will typically obtain little indirect fitness benefits from continued philopatry and alloparental care (36, 80). However, cooperation is a prerequisite of prolonged philopatry because dominants require “payment” from subordinate group members [(20, 23, 59, 60, 63–65, 81); reviewed in (15)]. Hence, philopatric individuals must cooperate at sufficiently high levels to avoid eviction from their group (18, 19, 21). In these pay-to-stay scenarios, selection for philopatry coincides with selection for cooperation, because under the threat of eviction, helping dominants is an essential means for subordinate group members to gain access to the benefits of staying in their territory (82).
The fundamental selective force in the ecology of these fish is predation risk, which favors group living and constrains dispersal and independent breeding (11, 15, 51, 58, 83). This is corroborated by our findings that fish tended to disperse late in their lives after attaining a large size and that less than 10% of successful dispersal events coincided with the formation of a group. As group size is a crucial determinant of success by enhancing survival and productivity in these fish (36, 83, 84), dispersers aiming to breed should attempt to join an existing group rather than trying to found one, which was the case in 92% of dispersals where the disperser took over the dominant breeding position.
Despite the differential dispersal propensity of males and females (16, 22, 59, 69), (Fig. 4A), dispersal did not show sex-specific fitness effects (Fig. 4, B and C). This indicates that the observed sex bias in dispersal is not the result of adaptive dispersal decisions in either sex but rather stems from differential constraints on the ability to remain philopatric (34). Given that dispersal is male-biased in this species, we expected that males would suffer less or gain more from dispersal. Our data showing a lack of sex-specific effects of dispersal on fitness correlates, including reproductive success, growth, age at ascension to dominance, dominance tenure, and longevity, suggest that individuals benefit from philopatry irrespective of their sex, which corroborates the “benefits of philopatry” hypothesis (8). The fact that males are less philopatric than females may reflect the sex-specific costs subordinates impose on breeders (72, 73), rather than intersexual competition for philopatry among subordinates. Male subordinates experience enhanced eviction risk due to the higher costs they impose on male breeders compared to the costs of female subordinates incurred on female dominants (14, 15, 59, 71, 85). This is corroborated by the retarded growth of large male helpers, leaving a size margin to the dominant male breeder (73). Therefore, male helpers are constrained in size by the reproductive competition with male breeders, which is implemented by the latter’s aggression. This does not apply in females (79). In contrast, there is no evidence in N. pulcher that certain positive helper effects are sex-specific, e.g., with female subordinates being more beneficial than males, a situation found in Seychelles warblers (86).
Why disperse?
Given the high costs of dispersal, an obvious question is why individuals disperse in the first place, assuming that they could potentially facilitate extended philopatry in their natal territory through continually maintaining or enhancing cooperation (21). The most likely explanation is that individuals may strategically join another group to increase their reproductive potential (44, 70). In N. pulcher, the ability of subordinates to successfully reproduce in their natal territory is rather limited, with only about 5 to 15% of offspring produced by group members other than the breeding pair [(14); reviewed in (15)], and dominants’ reproduction is strongly influenced by group traits (36). Hence, dispersal may either increase the chances to reproduce at all or the amount of reproduction an individual can expect in the future. Dispersers may thus switch groups without changes to their role or dominance status. This may yield benefits from higher group quality (e.g., larger group size) and, in the case of subordinate dispersal (dispersal type SS in our sample), advancement in the queue toward dominance. Our data suggest that if individuals disperse without ensuing status change, then the dispersing individuals tend to join groups that are larger than their groups of origin (Fig. 5), which corroborates results from laboratory experiments (87). Even if we did not determine a significant change in the short-term reproductive output of dispersing breeders (fig. S17), group size affected productivity (table S13.2), which confirmed results from previous experimental field work showing that large group size enhances offspring survival (84). In addition, large group size raises survival prospects (11, 83), which is consistent with the enhanced alloparental care of helpers in small groups as this may augment group size (88). All this might suggest that dispersal without status change serves to improve both short- and long-term fitness perspectives (36). Alternatively, individuals may disperse to ascend from subordinate to breeder status (dispersal type SD), which, in N. pulcher, typically resulted in joining a smaller group than the group of origin (Fig. 5). Even if small group size might enhance growth, this effect was weak (see fig. S2A), and smaller group size entails reduced productivity and survival prospects. Therefore, together with the reduced dominance tenure and overall diminished reproductive success across all dispersers compared to philopatric individuals (Fig. 4, B and C), advancing to breeder status via dispersal reflects a strategy that assures some immediate reproduction at the cost of reduced lifetime fitness prospects due to lower fecundity and shortened reproductive life span. Hence, dispersing to gain breeder status is advantageous where chances of successful philopatry and ascension to dominance in the natal territory are low, e.g., because dominance queues are long (16, 70).
Sex-specific life history trajectories
Our results suggest that male and female N. pulcher follow different life history trajectories. Males had lower recapture rates indicating lower survival, and they had shorter expected life spans compared to females, despite males growing faster than females and attaining a larger body size (Fig. 1), which reduces predation risk (11). Males reached dominance at a similar age (albeit larger size) as females, and the age at dispersal did not differ between the sexes. However, males were less likely to ascend to dominance via territory inheritance, and they were larger than females at the point of dispersal. All this coincides with the facultatively polygynous mating pattern of this species. The operational sex ratio of about 1:2 in favor of females in our study population reflects the sex-specific mortality differences, which, in turn, may enhance the polygyny potential at the colony level (67).
These divergent life histories affect within-group relatedness of N. pulcher, which is lower among males (22). This may suggest sex-specific feedbacks between dispersal behavior, cooperation, and relatedness: The less dispersive sex enjoys increased within-group relatedness and consequently has greater incentives to cooperate (5, 75, 89, 90). This, in turn, reduces the benefits of dispersal [e.g., (24, 42, 43, 91); but see (92)]. Our study shows that these plausible causal relationships may not always apply: Despite similar costs of dispersal to both sexes in N. pulcher (Fig. 4), males and females clearly differ in their dispersal propensity, and they follow divergent life history trajectories. These fish either grow slowly, remain small, queue for inheritance, and live long (females: “live slow, die old”), or they grow fast and to a large size, disperse for independent breeding opportunities, and die at a relatively young age (males: “live fast, die young”). Nevertheless, both sexes invest similarly in cooperative behavior (15, 58). That being said, the lower relatedness among breeders and their male subordinates (22) may increase the potential for male-male conflict within groups (59, 71, 72, 85). The higher dispersal rates of male subordinates may hence reflect either increased eviction rates or the voluntary decision of male subordinates to leave the territory (20, 21), which may be the outcome of a negotiation process between breeders and helpers where the outside options prevail for male subordinates (32, 93). In addition, dispersal may serve to spread genes (69) or to reduce kin competition, particularly as close kin tend to cooperate less in N. pulcher (23), but evaluating these aspects is outside the scope of the current manuscript.
In conclusion, this long-term field study of individually marked cooperatively breeding fish indicates that transitions to complex societies in cichlids may involve both ecological constraints (9) and benefits of philopatry (8). Group living in N. pulcher is obligate because of exceptionally high predation risk, which constrains the possibility to breed independently (15). Moreover, philopatry is the preferable alternative to dispersal, yielding enhanced fitness. Dispersal may, however, serve to ensure at least some immediate reproduction at the cost of long-term success. The access to the benefits of philopatry is contingent on cooperative investment in alloparental care, territory maintenance, and defence, as subordinates must pay to stay. The fitness benefits of philopatry apply to both sexes, but dispersal is sex-biased nonetheless. Apparently, male subordinates are more constrained to remain philopatric than females due to the higher intrasexual competition among males (see Introduction for additional details) (67). Our work thus highlights potential pitfalls when assuming a straightforward functional relationship between dispersal patterns and cooperation in complex societies (1, 5, 31, 75), underlining the importance of incorporating a species’ wider ecology in analyses of the drivers of advanced sociality (7, 32, 33).
MATERIALS AND METHODS
Field methods
Focal fish were caught and surveyed off the Zambian coast of Lake Tanganyika at depths between 8 and 12 m near Kasakalawe Point, west of Mpulungu (8°46.849′S, 31°04.882′E), from 2009 to 2015. The fish originated from four distinct colonies in which all N. pulcher territories were permanently marked (36). Before capture, each fish’s current home territory and social status was assessed during 5-min observations using scuba diving. The status of each individual was defined as either dominant or subordinate in its home territory; in N. pulcher, dominance hierarchies are linear and stable (94), allowing for reliable assessments of within-group relationships on the basis of relative body size and antagonistic interactions (14). An individual’s dominance tenure was then calculated as the sum of years during which it was observed as dominant. Fish were caught with hand nets and plexiglass tubes, and their size (SL to the nearest millimeters) and sex were visually determined upon capture (95). Captured fish received subcutaneous visible implant elastomer marks (Northwest Marine Technologies) (95), and we took a small tissue sample from their dorsal fin (see below). Subsequently, the fish were released back into their home territory and observed for another 5 min. Approximately 24 hours later, we checked each individual again. No fish were lost during this time period, and most fish resumed normal behavior (i.e., swimming freely, feeding, and/or interacting with other fish) already within the 5-min survey upon release. We caught and marked fish during four field seasons, each extending from September to December, in 2009, 2011, 2012, and 2013. In following field seasons (2010, 2012, 2013, 2014, and 2015), we checked all territories in the colonies where we had marked fish. We recaptured all fish bearing elastomer marks, recorded their size, status, and current home territory, and we took a small tissue sample to verify identity via DNA fingerprinting by analyzing 13 microsatellites (70, 96). We furthermore collected tissue samples for genetic identification also from a large number of fish that were not observed to bear marks but were large enough to potentially have been marked in the previous year. Identity was determined unequivocally via genetic identification.
During each field season, we assessed the composition of each group in which a marked individual was found (36), and its distance to the nearest-neighboring N. pulcher group [distances were measured in meters (to the nearest 0.1 m) from territory center to territory center] (for polygynous males, we used the shortest distance to any foreign territory rather than distances between territories that were defended by the same male). In addition, in 2011, 2012, and 2013, we counted the number of juveniles (i.e., fish of 0.5- to 1.5-cm SL; determined on the basis of size estimates and morphological characteristics; see fig. S14A for details and explanation) in each territory as a proxy of recent reproductive output. These measures allowed us to calculate dispersal distances and reproductive success (see below). We assumed marked fish that could not be recaptured in a given year to be dead, because (i) long-distance dispersal is very rare in this species (fig. S7A) (69); (ii) in each field season, we surveyed a large proportion of fish in the local population, including colonies in which we had not marked fish for this study; (iii) we have experienced fish retaining their marks for 5+ years in both the laboratory and field; and (iv) in each field season, we collected tissue samples for genetic identification also from a large number of fish that were not observed to bear marks. Thus, we consider it unlikely that we missed fish that had lost their marks, remained undetected, or dispersed outside of our working range.
Calculation of reproductive success
The most precise measure of the reproductive success of individuals involves the genotyping of offspring and potential parents. In our study, this was not possible because the capture of small group members requires destruction of the shelters, which dissolves groups (14, 22, 97) and hence would have obstructed the collection of long-term life history data. Fortunately, several field studies of fish from the same population provided good estimates of the reproductive share within N. pulcher groups (14, 96, 97), revealing that reproductive success is greatly biased toward the dominants (15, 36). Because of differences in the reproductive potential of the two sexes [the polygynous mating pattern renders a much higher maximum reproductive success for males than for females (67)] and annual variation in colony-wide reproductive success, we standardized the reproductive success estimates of all marked individuals by transforming our counts of juveniles below helper size in each group into sex- and year-specific Z scores. Note that this estimates only the direct reproductive success of individuals, omitting any potential indirect fitness gains by helper effects on relatives. First, we calculated each individual’s expected share in its group’s reproduction based on the most recent parentage study in the same population (14): We assumed that dominant males sired 76% of offspring and that dominant females were the mothers of 82% of offspring. We further assumed that large (i.e., mature; >3.5-cm SL) subordinates in a group equally shared either 11% (males) or 5% (females) of their group’s reproductive success (assigning the remaining reproductive success to “unknown” individuals) (14). While it is possible that dispersal affects reproductive sharing in groups (54), these effects are unlikely to explain the patterns of reproductive success and dispersal behavior we report here (fig. S14B). Hence, we had information about an individual’s sex, social status, and reproductive success in a given year in 355 cases. On the basis of these, we calculated sex- and status-specific average annual reproductive success, i.e., average reproductive success in a given year of dominant females, dominant males, subordinate females, and subordinate males, respectively. For each individual, we then calculated its relative reproductive success throughout the observation period: sex- and status-specific Z scores were calculated for each individual and each year in which it was observed and then summed over the number of observation years [i.e., , where xijk is the reproductive success of individual i of sex j and status k, is the average annual reproductive success of individuals of sex j and status k, and Sjk is the standard deviation of annual reproductive success of individuals of sex j and status k (98)]. We calculated Z scores for each individual throughout the observation period as a measure of its relative reproductive success compared to other individuals of the same sex and status (dominant or subordinate) observed at the same time.
Sample size and recaptures
We marked a total of 496 fish for this study, 264 females and 232 males. We recaptured 197 of these fish at least once (i.e., at least approximately 1 year after they had initially been marked; 120 females and 77 males). Recapture numbers for consecutive years declined (fig. S1A; see the “Field methods” section above and section S6 for why this likely represents an effect of mortality): 135 fish were recaptured once (80 females and 55 males), 50 fish were recaptured in 2 years (36 females and 14 males), and 12 fish were recaptured in 3 years (4 females and 8 males). Thus, we recorded 271 recapture events involving 197 different individuals, 268 of which took place in consecutive years (i.e., three fish were recaptured after a “gap year”), for 266 of which we had full information about the respective group sizes during both measurements. In total, we had information about a fish’s social status and size in a given year in 761 cases. We recorded each individual’s status (alive or missing, dominant or subordinate, and dispersed or philopatric) up to two times per field season, once during its initial capture or recapture at the beginning of the field season (September to October) and once close to the end of the field season (November to December). For 1250 of these observations, which included 490 individual fish, we had information about an individual’s sex, size, social status, its group’s size, and its group’s nearest-neighbor distance. This allowed us to detect survival and dispersal within and between field seasons (see section S6 for further details). We recorded a total of 104 dispersals, for 103 of which we had information about the respective individual’s groups before and after dispersing. Of these, 46 dispersal events were realized by 42 individual females, and 58 dispersal events were realized by 45 individual males. The majority of these events was detected between field seasons (98 dispersal events detected as a change in territory from 1 year to the next), with only 6 dispersals being observed during a given field season (i.e., a change in territory in the same year; 4 dispersals occurred in the field season during which a given fish was initially captured and marked, and 2 dispersals occurred in the field season during which a given fish was recaptured for the first time).
Potential limitations
Some degree of uncertainty about individual life histories is inherent in long-term field studies like this. Estimates of dispersal and mortality in a natural population are prone to error because nearby dispersals are easier to determine than long-range dispersals, and the latter may lead to dispersers being missed and erroneously scored as dead. This would bias estimates of dispersal distances (section S6) and mortality. However, in our case, dispersal distances have been shown to be generally small by previous studies involving large-scale genetic screening (69, 99), and there were no local populations within the known dispersal range of these fish that we did not thoroughly check routinely for marked individuals. Another limitation to datasets collected in nature is that individual life histories are unknown before first capture, thereby somewhat truncating the information (e.g., about whether previous dispersal had occurred or for how long the current social status had already been kept). Hence, it is possible that numbers of dispersal events are underestimated, either because dispersal had occurred before an individual’s inclusion in the study or because it remained undetected. To reduce the potential bias introduced by these truncations and limitations, we only included those “philopatric” or “dispersing” individuals in our analyses of the consequences of dispersal that were recaptured at least once. This ensures that we observed them for a similar stretch of time, crucially for the period during which most dispersals occurred, i.e., across field seasons (section S6). This resulted in quite balanced sample sizes for dispersers and philopatric group members (sections S10 to S16). Estimates of reproductive success and relatedness effects rely on some extrapolation of factors that cannot be measured for each individual under field conditions. Fortunately, in our case, estimates about the magnitude of sex-specific reproductive shares of subordinates were available from the same population [Kasakalawe (14)] in addition to the results from several experimental studies [summarized in (15)], and the relatedness between large, sexually mature subordinates and the dominant breeders in their territories is very low (22, 80). Because this previous information could be considered in our assessment process, the chances that our reproductive success estimates are biased in a particular direction are small. Last, complex datasets from a natural setting are notoriously difficult to analyze because of missing values, zero inflation, and unbalanced sample sizes, to name but a few issues that arise. We think to have chosen the most appropriate and meaningful analysis procedures, and we also provide additional information about each analysis in the Supplementary Materials.
Statistical analyses
All analyses were performed using R version 3.5.2 (100). Normally distributed data were analyzed using LM or LME, and nonnormally distributed data were analyzed using GLM or GLMM. GLMs and GLMMs were fitted either with logarithmic link function (log link; approximately Poisson-distributed data) or with logistic link function (logit link; binomially distributed data). Mixed-effects models were fitted using the R package lme4 (101), and model fit was checked with DHARMa (102). See the Supplementary Materials for details of the models, model selection, and model fit.
Ethical statement
All applicable international, national, and institutional guidelines for the care and use of animals were followed. The field work reported here complied with Zambian laws and was carried out in agreement with local authorities under the Memorandum of Understanding issued by the Department of Fisheries: Ministry of Agriculture and Cooperatives, Zambia, dated 20 March 2009.
Acknowledgments
We dedicate this work to the late A. Mwewa, whose help and advice were invaluable to us. The contributions of P. Brena, G. Cassola, L. Chapuis, J. Flury, M. Freiburghaus, F. Heussler, I. Keller, S. Meindl, J. Riederer, and J. Walker were crucial for our data collection. We thank D. Sinyinza, T. Banda, and the staff at the Department of Fisheries in Mpulungu, Zambia, for logistic support and the Zambian Ministry of Agriculture and Cooperatives for the study permission. We are grateful to C. Mwewa and the staff at the Tanganyika Science Lodge for their hospitality. J. Allen, J. Savage, and three anonymous referees provided important insights.
Funding: Funding was provided by SNF grants 31003A_122511, 310030B_138660, and 31003A_156152 to M.T. and 31003A_166470 to J.G.F.
Author contributions: A.J., M.Z., and M.T. conceived the study. J.G.F. and M.T. secured the funding. A.J., M.Z., and D.J. coordinated the fieldwork. A.J., M.Z., D.J., J.G.F., and M.T. collected the data. D.B. and D.J. conducted genetical analyses. A.J. analyzed the data and drafted the first version of the manuscript, which was revised by M.T. All authors contributed to revisions and approved the final version of the manuscript.
Competing interests: The authors declare that they have no competing interests.
Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and the Supplementary Materials.
Supplementary Materials
This PDF file includes:
Legend for data S1
Figs. S1 to S18
Tables S1 to S18
Other Supplementary Material for this manuscript includes the following:
Data file S1
REFERENCES AND NOTES
- 1.M. E. Hochberg, D. J. Rankin, M. Taborsky, The coevolution of cooperation and dispersal in social groups and its implications for the emergence of multicellularity. BMC Evol. Biol. 8, 238 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.S. A. West, R. M. Fisher, A. Gardner, E. T. Kiers, Major evolutionary transitions in individuality. Proc. Natl. Acad. Sci. U.S.A. 112, 10112–10119 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.W. D. Koenig, F. A. Pitelka, W. J. Carmen, R. L. Mumme, M. T. Stanback, The evolution of delayed dispersal in cooperative breeders. Q. Rev. Biol. 67, 111–150 (1992). [DOI] [PubMed] [Google Scholar]
- 4.M. Griesser, S. M. Drobniak, S. Nakagawa, C. A. Botero, Family living sets the stage for cooperative breeding and ecological resilience in birds. PLOS Biol. 15, e20000483 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.C. Mullon, L. Keller, L. Lehmann, Social polymorphism is favoured by the co-evolution of dispersal with social behaviour. Nat. Ecol. Evol. 2, 132–140 (2018). [DOI] [PubMed] [Google Scholar]
- 6.W. D. Koenig, J. L. Dickinson, Eds., Cooperative Breeding in Vertebrates: Studies of Ecology, Evolution, and Behavior (Cambridge University Press, 2015). [Google Scholar]
- 7.I. García-Ruiz, A. Quiñones, M. Taborsky, The evolution of cooperative breeding by direct and indirect fitness effects. Sci. Adv. 8, eabl7853 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.P. B. Stacey, J. D. Ligon, The Benefits-of-Philopatry Hypothesis for the evolution of cooperative breeding: Variation in territory quality and group size effects. Am. Nat. 137, 831–846 (1991). [Google Scholar]
- 9.S. T. Emlen, The evolution of helping. I. An ecological constraints model. Am. Nat. 119, 29–39 (1982). [Google Scholar]
- 10.P. B. Stacey, Habitat saturation and communal breeding in the acorn woodpecker. Anim. Behav. 27, 1153–1166 (1979). [Google Scholar]
- 11.D. Heg, Z. Bachar, L. Brouwer, M. Taborsky, Predation risk is an ecological constraint for helper dispersal in a cooperatively breeding cichlid. Proc. R. Soc. B Biol. Sci. 271, 2367–2374 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.W. D. Hamilton, The genetical evolution of social behaviour. I. J. Theor. Biol. 7, 1–16 (1964). [DOI] [PubMed] [Google Scholar]
- 13.S. A. Kingma, Direct benefits explain interspecific variation in helping behaviour among cooperatively breeding birds. Nat. Commun. 8, 1094 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.J. K. Hellmann, I. Y. Ligocki, C. M. O’Connor, A. R. Reddon, K. A. Garvy, S. E. Marsh-Rollo, H. L. Gibbs, S. Balshine, I. M. Hamilton, Reproductive sharing in relation to group and colony-level attributes in a cooperative breeding fish. Proc. R. Soc. B Biol. Sci. 282, 20150954 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.M. Taborsky, in Cooperative Breeding in Vertebrates: Studies of Ecology, Evolution and Behavior, W. D. Koenig, J. L. Dickinson, Eds. (Cambridge University Press, 2016), pp. 272–293. [Google Scholar]
- 16.K. A. Stiver, J. Fitzpatrick, J. K. Desjardins, S. Balshine, Sex differences in rates of territory joining and inheritance in a cooperatively breeding cichlid fish. Anim. Behav. 71, 449–456 (2006). [Google Scholar]
- 17.E. Leadbeater, J. M. Carruthers, J. P. Green, N. S. Rosser, J. Field, Nest inheritance is the missing source of direct fitness in a primitively eusocial insect. Science 333, 874–876 (2011). [DOI] [PubMed] [Google Scholar]
- 18.H. Kokko, R. A. Johnstone, J. Wright, The evolution of parental and alloparental effort in cooperatively breeding groups: When should helpers pay to stay? Behav. Ecol. 13, 291–300 (2002). [Google Scholar]
- 19.I. M. Hamilton, M. Taborsky, Unrelated helpers will not fully compensate for costs imposed on breeders when they pay to stay. Proc. R. Soc. B Biol. Sci. 272, 445–454 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.A. E. Quiñones, G. S. van Doorn, I. Pen, F. J. Weissing, M. Taborsky, Negotiation and appeasement can be more effective drivers of sociality than kin selection. Philos. Trans. R. Soc. B Biol. Sci. 371, 20150089 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.R. A. Johnstone, M. A. Cant, Reproductive skew and the threat of eviction: A new perspective. Proc. R. Soc. B Biol. Sci. 266, 275–279 (1999). [Google Scholar]
- 22.P. Dierkes, D. Heg, M. Taborsky, E. Skubic, R. Achmann, Genetic relatedness in groups is sex-specific and declines with age of helpers in a cooperatively breeding cichlid. Ecol. Lett. 8, 968–975 (2005). [DOI] [PubMed] [Google Scholar]
- 23.M. Zöttl, D. Heg, N. Chervet, M. Taborsky, Kinship reduces alloparental care in cooperative cichlids where helpers pay-to-stay. Nat. Commun. 4, 1341 (2013). [DOI] [PubMed] [Google Scholar]
- 24.S. G. Pruett-Jones, M. J. Lewis, Sex ratio and habitat limitation promote delayed dispersal in superb fairy-wrens. Nature 348, 541–542 (1990). [Google Scholar]
- 25.J. Komdeur, Importance of habitat saturation and territory quality for evolution of cooperative breeding in the Seychelles warbler. Nature 358, 493–495 (1992). [Google Scholar]
- 26.M. A. Du Plessis, Obligate cavity-roosting as a constraint on dispersal of green (red-billed) woodhoopoes: Consequences for philopatry and the likelihood of inbreeding. Oecologia 90, 205–211 (1992). [DOI] [PubMed] [Google Scholar]
- 27.J. R. Walters, P. D. Doerr, J. H. Carter III, Delayed dispersal and reproduction as a life-history tactic in cooperative breeders: Fitness calculations from red-cockaded woodpeckers. Am. Nat. 139, 623–643 (1992). [Google Scholar]
- 28.V. Baglione, D. Canestrari, J. M. Marcos, J. Ekman, Experimentally increased food resources in the natal territory promote offspring philopatry and helping in cooperatively breeding carrion crows. Proc. R. Soc. B Biol. Sci. 273, 1529–1535 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.N. Perrin, L. Lehmann, Is sociality driven by the costs of dispersal or the benefits of philopatry? A role for kin-discrimination mechanisms. Am. Nat. 158, 471–483 (2001). [DOI] [PubMed] [Google Scholar]
- 30.R. Covas, M. Griesser, Life history and the evolution of family living in birds. Proc. R. Soc. B Biol. Sci. 274, 1349–1357 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.A. Gardner, K. R. Foster, in Ecology of Social Evolution, J. Korb, J. Heinze, Eds. (Springer, 2008), pp. 1–36. [Google Scholar]
- 32.M. Taborsky, M. A. Cant, J. Komdeur, The Evolution of Social Behaviour (Cambridge University Press, ed. 1, 2021). [Google Scholar]
- 33.S.-F. Shen, S. T. Emlen, W. D. Koenig, D. R. Rubenstein, The ecology of cooperative breeding behaviour. Ecol. Lett. 20, 708–720 (2017). [DOI] [PubMed] [Google Scholar]
- 34.J. Ekman, S. Eggers, M. Griesser, Fighting to stay: The role of sibling rivalry for delayed dispersal. Anim. Behav. 64, 453–459 (2002). [Google Scholar]
- 35.J. Komdeur, A. Huffstadt, W. Prast, G. Castle, R. Mileto, J. Wattel, Transfer experiments of Seychelles warblers to new islands: Changes in dispersal and helping behaviour. Anim. Behav. 49, 695–708 (1995). [Google Scholar]
- 36.A. Jungwirth, M. Taborsky, First- and second-order sociality determine survival and reproduction in cooperative cichlids. Proc. R. Soc. B Biol. Sci. 282, 20151971 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.A. D. C. MacColl, B. J. Hatchwell, Determinants of lifetime fitness in a cooperative breeder, the long-tailed tit Aegithalos caudatus. J. Anim. Ecol. 73, 1137–1148 (2004). [Google Scholar]
- 38.D. Bonte, H. Van Dyck, J. M. Bullock, A. Coulon, M. Delgado, M. Gibbs, V. Lehouck, E. Matthysen, K. Mustin, M. Saastamoinen, N. Schtickzelle, V. M. Stevens, S. Vandewoestijne, M. Baguette, K. Barton, T. G. Benton, A. Chaput-Bardy, J. Clobert, C. Dytham, T. Hovestadt, C. M. Meier, S. C. F. Palmer, C. Turlure, J. M. J. Travis, Costs of dispersal. Biol. Rev. Camb. Philos. Soc. 87, 290–312 (2012). [DOI] [PubMed] [Google Scholar]
- 39.M. Nevoux, D. Arlt, M. Nicoll, C. Jones, K. Norris, The short- and long-term fitness consequences of natal dispersal in a wild bird population. Ecol. Lett. 16, 438–445 (2013). [DOI] [PubMed] [Google Scholar]
- 40.X.-Y. Li, H. Kokko, Sex-biased dispersal: A review of the theory. Biol. Rev. Camb. Philos. Soc. 94, 721–736 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.A. Trochet, E. A. Courtois, V. M. Stevens, M. Baguette, A. Chaine, D. S. Schmeller, J. Clobert, Evolution of sex-biased dispersal. Q. Rev. Biol. 91, 297–320 (2016). [DOI] [PubMed] [Google Scholar]
- 42.T. H. Clutton-Brock, A. F. Russell, L. L. Sharpe, A. J. Young, Z. Balmforth, G. M. McIlrath, Evolution and development of sex differences in cooperative behavior in meerkats. Science 297, 253–256 (2002). [DOI] [PubMed] [Google Scholar]
- 43.D. S. Richardson, T. Burke, J. Komdeur, Direct benefits and the evolution of female-biased cooperative breeding in Seychelles warblers. Evolution 56, 2313–2321 (2002). [DOI] [PubMed] [Google Scholar]
- 44.D. A. Williams, K. N. Rabenold, Male-biased dispersal, female philopatry, and routes to fitness in a social corvid. J. Anim. Ecol. 74, 150–159 (2005). [Google Scholar]
- 45.D. R. Rubenstein, P. Abbot, Eds., Comparative Social Evolution (Cambridge University Press, ed. 1, 2017). [Google Scholar]
- 46.A. P. H. Bose, L. Koch, J. Dabernig-Heinz, J. Grimm, K. M. Sefc, A. Jordan, Patterns of sex-biased dispersal are consistent with social and ecological constraints in a group-living cichlid fish. BMC Ecol. Evol. 22, 21 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.E. C. Berg, J. M. Madie, T. A. Langen, A. F. Russell, Reverse sex-biased philopatry in a cooperative bird: Genetic consequences and a social cause. Mol. Ecol. 18, 3486–3499 (2009). [DOI] [PubMed] [Google Scholar]
- 48.R. Schürch, D. Heg, Life history and behavioral type in the highly social cichlid Neolamprologus pulcher. Behav. Ecol. 21, 588–598 (2010). [Google Scholar]
- 49.D. Josi, D. Heg, T. Takeyama, D. Bonfils, D. A. Konovalov, J. G. Frommen, M. Kohda, M. Taborsky, Age- and sex-dependent variation in relatedness corresponds to reproductive skew, territory inheritance, and workload in cooperatively breeding cichlids. Evolution 75, 2881–2897 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.N. Duftner, K. M. Sefc, S. Koblmüller, W. Salzburger, M. Taborsky, C. Sturmbauer, Parallel evolution of facial stripe patterns in the Neolamprologus brichardi/pulcher species complex endemic to Lake Tanganyika. Mol. Phylogenet. Evol. 45, 706–715 (2007). [DOI] [PubMed] [Google Scholar]
- 51.F. Groenewoud, J. G. Frommen, D. Josi, H. Tanaka, A. Jungwirth, M. Taborsky, Predation risk drives social complexity in cooperative breeders. Proc. Natl. Acad. Sci. U.S.A. 113, 4104–4109 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.D. Heg, Z. Heg-Bachar, L. Brouwer, M. Taborsky, Experimentally induced helper dispersal in colonially breeding cooperative cichlids. Environ. Biol. Fishes 83, 191–206 (2008). [Google Scholar]
- 53.A. Jungwirth, D. Josi, J. Walker, M. Taborsky, Benefits of coloniality: Communal defence saves anti-predator effort in cooperative breeders. Funct. Ecol. 29, 1218–1224 (2015). [Google Scholar]
- 54.J. K. Hellmann, M. G. Sovic, H. L. Gibbs, A. R. Reddon, C. M. O’Connor, I. Y. Ligocki, S. Marsh-Rollo, S. Balshine, I. M. Hamilton, Within-group relatedness is correlated with colony-level social structure and reproductive sharing in a social fish. Mol. Ecol. 25, 4001–4013 (2016). [DOI] [PubMed] [Google Scholar]
- 55.M. Taborsky, D. Limberger, Helpers in fish. Behav. Ecol. Sociobiol. 8, 143–145 (1981). [Google Scholar]
- 56.S. Balshine, B. Leach, F. Neat, H. Reid, M. Taborsky, N. Werner, Correlates of group size in a cooperatively breeding cichlid fish (Neolamprologus pulcher). Behav. Ecol. Sociobiol. 50, 134–140 (2001). [Google Scholar]
- 57.D. Heg, S. Rothenberger, R. Schürch, Habitat saturation, benefits of philopatry, relatedness, and the extent of co-operative breeding in a cichlid. Behav. Ecol. 22, 82–92 (2011). [Google Scholar]
- 58.M. Taborsky, Broodcare helpers in the cichlid fish Lamprologus brichardi: Their costs and benefits. Anim. Behav. 32, 1236–1252 (1984). [Google Scholar]
- 59.M. Taborsky, Breeder-helper conflict in a cichlid fish with broodcare helpers: An experimental analysis. Behaviour 95, 45–75 (1985). [Google Scholar]
- 60.R. Bergmüller, M. Taborsky, Experimental manipulation of helping in a cooperative breeder: Helpers ‘pay to stay’ by pre-emptive appeasement. Anim. Behav. 69, 19–28 (2005). [Google Scholar]
- 61.R. Bergmüller, D. Heg, M. Taborsky, Helpers in a cooperatively breeding cichlid stay and pay or disperse and breed, depending on ecological constraints. Proc. R. Soc. B Biol. Sci. 272, 325–331 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.M. Zöttl, J. G. Frommen, M. Taborsky, Group size adjustment to ecological demand in a cooperative breeder. Proc. R. Soc. B Biol. Sci. 280, 20122772 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.S. Fischer, M. Zöttl, F. Groenewoud, B. Taborsky, Group-size-dependent punishment of idle subordinates in a cooperative breeder where helpers pay to stay. Proc. R. Soc. B Biol. Sci. 281, 20140184 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.J. Naef, M. Taborsky, Commodity-specific punishment for experimentally induced defection in cooperatively breeding fish. R. Soc. Open Sci. 7, 191808 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.J. Naef, M. Taborsky, Punishment controls helper defence against egg predators but not fish predators in cooperatively breeding cichlids. Anim. Behav. 168, 137–147 (2020). [Google Scholar]
- 66.D. Limberger, Pairs and harems in a cichlid fish, Lamprologus brichardi. Z. Tierpsychol. 62, 115–144 (1983). [Google Scholar]
- 67.A. Jungwirth, P. F. Brena, I. Keller, M. Taborsky, Polygyny affects paternal care, but not survival, pair stability, and group tenure in a cooperative cichlid. Behav. Ecol. 27, 592–600 (2016). [Google Scholar]
- 68.S. Balshine-Earn, F. C. Neat, H. Reid, M. Taborsky, Paying to stay or paying to breed? Field evidence for direct benefits of helping behavior in a cooperatively breeding fish. Behav. Ecol. 9, 432–438 (1998). [Google Scholar]
- 69.K. A. Stiver, J. K. Desjardins, J. Fitzpatrick, B. D. Neff, J. S. Quinn, S. Balshine, Evidence for size and sex-specific dispersal in a cooperatively breeding cichlid fish. Mol. Ecol. 16, 2974–2984 (2007). [DOI] [PubMed] [Google Scholar]
- 70.A. Jungwirth, J. Walker, M. Taborsky, Prospecting precedes dispersal and increases survival chances in cooperatively breeding cichlids. Anim. Behav. 106, 107–114 (2015). [Google Scholar]
- 71.D. Heg, E. Jutzeler, D. Bonfils, J. S. Mitchell, Group composition affects male reproductive partitioning in a cooperatively breeding cichlid. Mol. Ecol. 17, 4359–4370 (2008). [DOI] [PubMed] [Google Scholar]
- 72.J. S. Mitchell, E. Jutzeler, D. Heg, M. Taborsky, Gender differences in the costs that subordinate group members impose on dominant males in a cooperative breeder. Ethology 115, 1162–1174 (2009). [Google Scholar]
- 73.D. Heg, N. Bender, I. M. Hamilton, Strategic growth decisions in helper cichlids. Proc. R. Soc. B Biol. Sci. 271(Suppl 6), S505–S508 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.J. K. Desjardins, J. Fitzpatrick, K. A. Stiver, G. Vanderkraak, S. Balshine, Costs and benefits of polygyny in the cichlid Neolamprologus pulcher. Anim. Behav. 75, 1771–1779 (2008). [Google Scholar]
- 75.R. A. Johnstone, M. A. Cant, Sex differences in dispersal and the evolution of helping and harming. Am. Nat. 172, 318–330 (2008). [DOI] [PubMed] [Google Scholar]
- 76.M. Zöttl, L. Chapuis, M. Freiburghaus, M. Taborsky, Strategic reduction of help before dispersal in a cooperative breeder. Biol. Lett. 9, 20120878 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.P. Dierkes, M. Taborsky, U. Kohler, Reproductive parasitism of broodcare helpers in a cooperatively breeding fish. Behav. Ecol. 10, 510–515 (1999). [Google Scholar]
- 78.D. Heg, R. Bergmüller, D. Bonfils, O. Otti, Z. Bachar, R. Burri, G. Heckel, M. Taborsky, Cichlids do not adjust reproductive skew to the availability of independent breeding options. Behav. Ecol. 17, 419–429 (2006). [Google Scholar]
- 79.D. Heg, I. M. Hamilton, Tug-of-war over reproduction in a cooperatively breeding cichlid. Behav. Ecol. Sociobiol. 62, 1249–1257 (2008). [Google Scholar]
- 80.K. A. Stiver, P. Dierkes, M. Taborsky, H. L. Gibbs, S. Balshine, Relatedness and helping in fish: Examining the theoretical predictions. Proc. R. Soc. B Biol. Sci. 272, 1593–1599 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.D. Heg, M. Taborsky, Helper response to experimentally manipulated predation risk in the cooperatively breeding cichlid Neolamprologus pulcher. PLOS ONE 5, e10784 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.A. J. Gaston, The evolution of group territorial behavior and cooperative breeding. Am. Nat. 112, 1091–1100 (1978). [Google Scholar]
- 83.D. Heg, L. Brouwer, Z. Bachar, M. Taborsky, Large group size yields group stability in the cooperatively breeding cichlid Neolamprologus pulcher. Behaviour 142, 1615–1641 (2005). [Google Scholar]
- 84.L. Brouwer, D. Heg, M. Taborsky, Experimental evidence for helper effects in a cooperatively breeding cichlid. Behav. Ecol. 16, 667–673 (2005). [Google Scholar]
- 85.J. S. Mitchell, E. Jutzeler, D. Heg, M. Taborsky, Dominant members of cooperatively-breeding groups adjust their behaviour in response to the sexes of their subordinates. Behaviour 146, 1665–1686 (2009). [Google Scholar]
- 86.M. Hammers, S. A. Kingma, L. G. Spurgin, K. Bebbington, H. L. Dugdale, T. Burke, J. Komdeur, D. S. Richardson, Breeders that receive help age more slowly in a cooperatively breeding bird. Nat. Commun. 10, 1301 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.A. R. Reddon, D. Balk, S. Balshine, Sex differences in group-joining decisions in social fish. Anim. Behav. 82, 229–234 (2011). [Google Scholar]
- 88.I. García-Ruiz, M. Taborsky, Group augmentation on trial: Helpers in small groups enhance antipredator defence of eggs. Biol. Lett. 18, 20220170 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.A. Gardner, Sex-biased dispersal of adults mediates the evolution of altruism among juveniles. J. Theor. Biol. 262, 339–345 (2010). [DOI] [PubMed] [Google Scholar]
- 90.R. A. Johnstone, M. A. Cant, J. Field, Sex-biased dispersal, haplodiploidy and the evolution of helping in social insects. Proc. R. Soc. B Biol. Sci. 279, 787–793 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.A. F. Russell, Dispersal costs set the scene for helping in an atypical avian cooperative breeder. Proc. R. Soc. B Biol. Sci. 268, 95–99 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.C. J. Blackmore, R. Peakall, R. Heinsohn, The absence of sex-biased dispersal in the cooperatively breeding grey-crowned babbler. J. Anim. Ecol. 80, 69–78 (2011). [DOI] [PubMed] [Google Scholar]
- 93.M. Taborsky, J. G. Frommen, C. Riehl, Correlated pay-offs are key to cooperation. Philos. Trans. R. Soc. B Biol. Sci. 371, 20150084 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.C. J. Dey, A. R. Reddon, C. M. O’Connor, S. Balshine, Network structure is related to social conflict in a cooperatively breeding fish. Anim. Behav. 85, 395–402 (2013). [Google Scholar]
- 95.A. Jungwirth, V. Balzarini, M. Zöttl, A. Salzmann, M. Taborsky, J. G. Frommen, Long-term individual marking of small freshwater fish: The utility of Visual Implant Elastomer tags. Behav. Ecol. Sociobiol. 73, 49 (2019). [Google Scholar]
- 96.R. Bruintjes, D. Bonfils, D. Heg, M. Taborsky, Paternity of subordinates raises cooperative effort in cichlids. PLOS ONE 6, e25673 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.P. Dierkes, M. Taborsky, R. Achmann, Multiple paternity in the cooperatively breeding fish Neolamprologus pulcher. Behav. Ecol. Sociobiol. 62, 1581–1589 (2008). [Google Scholar]
- 98.H. Chubb, J. M. Simpson, The use of Z-scores in paediatric cardiology. Ann. Pediatr. Cardiol. 5, 179–184 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.K. A. Stiver, P. Dierkes, M. Taborsky, S. Balshine, Dispersal patterns and status change in a co-operatively breeding cichlid Neolamprologus pulcher: Evidence from microsatellite analyses and behavioural observations. J. Fish Biol. 65, 91–105 (2004). [Google Scholar]
- 100.R Development Core Team, R: A language and environment for statistical computing. R Foundation for Statistical Computing (2013); www.r-project.org/.
- 101.D. Bates, M. Maechler, B. M. Bolker, S. Walker, lme4: Linear mixed-effects models using Eigen and S4. R package version 1.1-7 (2013); http://cran.r-project.org/package=lme4).
- 102.F. Hartig, DHARMa: Residual diagnostics for hierarchical (multi-level/mixed) regression models. R Package (2020).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Legend for data S1
Figs. S1 to S18
Tables S1 to S18
Data file S1