Abstract
During cotranslational translocation, the signal peptide of a nascent chain binds Sec61 translocon to initiate protein transport through the endoplasmic reticulum (ER) membrane. Our cryo–electron microscopy structure of ribosome-Sec61 shows binding of an ordered heterotetrameric translocon-associated protein (TRAP) complex, in which TRAP-γ is anchored at two adjacent positions of 28S ribosomal RNA and interacts with ribosomal protein L38 and Sec61α/γ. Four transmembrane helices (TMHs) of TRAP-γ cluster with one C-terminal helix of each α, β, and δ subunits. The seven TMH bundle helps position a crescent-shaped trimeric TRAP-α/β/δ core in the ER lumen, facing the Sec61 channel. Further, our in vitro assay establishes the cyclotriazadisulfonamide derivative CK147 as a translocon inhibitor. A structure of ribosome-Sec61-CK147 reveals CK147 binding the channel and interacting with the plug helix from the lumenal side. The CK147 resistance mutations surround the inhibitor. These structures help in understanding the TRAP functions and provide a new Sec61 site for designing translocon inhibitors.
Cryo-EM structures reveal TRAP binding to ribosome-Sec61, and CK147 inhibits Sec61 by arresting the plug helix inside the channel.
INTRODUCTION
About one-third of all eukaryotic proteins are associated with the secretory system that involves the endoplasmic reticulum (ER), Golgi apparatus, trans-Golgi network, and lysosomes (1). To reach their respective final destinations, these proteins enter the secretory pathway at the ER, during which proteins are cotranslationally translocated across or inserted into the ER membrane. Protein cotranslational translocation is a multistep process that involves the signal recognition particle (SRP)–dependent targeting of the ribosome nascent chain (RNC) complex from the cytosol to the ER membrane and subsequent transport of a nascent polypeptide chain from the ribosome through the Sec61 translocon channel. The heterotrimeric Sec61 translocon consists of α, β, and γ subunits. The Sec61α subunit is composed of 10 transmembrane helices (TMHs) that span the ER membrane from the cytosol to the ER lumen. These TMHs define the central channel (or pore), and the structural rearrangement of these TMHs regulates the protein translocation steps. The TMH-bundle contains a movable plug domain at the lumenal side of the translocon and a lateral gate that mediate protein transport across and insertion into the ER membrane, respectively (Fig. 1A). When the RNC complex binds Sec61, the signal peptide (SP) sequence at the N terminus of the nascent polypeptide interacts with the lateral gate and triggers conformational changes, including the displacement of the plug domain and opening of the lateral gate toward the lipid bilayer (2). Once protein translocation is complete, the signal peptidase and oligosaccharyl-transferase (OST) enzymes process the maturation of the translocated preprotein in the ER lumen by SP cleavage and N-linked glycosylation, respectively (3–6).
Fig. 1. Cotranslational translocation across the ER membrane.
(A) Molecular events in the Sec61 translocon channel during the translocation process. The SP (yellow) interacts with the lateral gate (black) of the translocon. This induces the rearrangement of the TMHs of the lateral gate and the plug domain to allow for protein translocation. After SP cleavage by the signal peptidase complex (orange), the transmembrane domain (TMD) is inserted into the lipid bilayer via opening of the lateral gate, whereas the extracellular part of the protein is translocated to the ER lumen. (B) Overview of the different structural states of the Sec61 translocon. Left: Native/closed Sec61 [PDB 5A6U (7)]. Middle: Ribosome-primed Sec61 [PDB 3J7Q (8)]. Right: SP-engaged/opened Sec61 [PDB 3JC2 (2)]. The Sec61α subunit is shown in green, with the TMHs that form the lateral gate (TMH2, TMH3, TMH7, and TMH8) in cyan. The plug domain of Sec61α is in red. The SP in the engaged/opened translocon (right) is in yellow. (C) Comparison of the different structural states. Left: Superposition of the ribosome-primed Sec61 translocon (green/cyan) with the native translocon (gray). Right: Superposition of the SP-engaged translocon (green/cyan) with the ribosome-primed translocon (gray).
Structures representing different functional states of the protein translocation process in the Sec61 translocon (i.e., closed, ribosome-primed, and SP-engaged/opened configuration; Fig. 1B) have been determined by single-particle cryo–electron microscopy (cryo-EM) in the recent past (2, 7–10). Comparisons of these structures revealed the dynamics characteristic of the lateral gate that include the repositioning of TMH2 and TMH3 relative to TMH7 and TMH8 during the translocon gating and the displacement of the plug domain for protein translocation (Fig. 1C). Furthermore, high-resolution cryo-EM structures are available for several accessory proteins such as SRP, the signal peptidase complex, and OST (3, 5, 6, 10). Other accessory translocation proteins, such as the heterotetrameric translocon-associated protein (TRAP) complex, have been identified for which cryo-EM structures are available in the context of ribosome-Sec61 assembly (11). TRAP, composed of α, β, γ, and δ (ssr1 to ssr4) subunits, is thought to be facilitating the translocation of substrates with weak (low hydrophobicity) or proline- and glycine-rich SPs by ensuring the opened conformation of Sec61 (12–16). Furthermore, TRAP might interfere with the final topology of the protein nascent chain (14) and subsequently coordinate with OST for the N-linked glycosylation, which suggests a role of TRAP in the biogenesis of glycoproteins (17–19). The up-regulation of TRAP expression during ER stress advocates for the involvement of TRAP in the ER-associated degradation of improperly folded proteins from the ER lumen to restore ER homeostasis (20). In summary, TRAP is involved in various undetermined functions during protein translocation, maturation, and ER stress (21). The TRAP complex is present in our current eEF2-bound nontranslating ribosome-Sec61 translocon complex; the structure, therefore, implies that TRAP is an integral part of the Sec61 translocon network even before protein synthesis (16, 22).
The protein cotranslational translocation through ER membrane is essential for the metabolism and survival of cells. The up- or down-regulation of the process is associated with several diseases including cancer (23–26). The inhibition of Sec61-dependent protein cotranslational translocation is therefore a valuable therapeutic strategy. Some translocation inhibitors have originated from therapeutic drug screening programs, while others are natural products (27–33). Furthermore, Sec61α subunit mutations confer resistance to translocon inhibitors (34–39). In general, the resistance mutations emerge adjacent to the lateral gate and/or the plug domain of Sec61, which indicate overlapping mechanisms of action by the existing translocation inhibitors and direct impact of these mutations in disrupting the inhibitor binding. The first structure of an inhibited translocon (with mycolactone) has been reported in the recent past (40), followed by the release of two other structures of Sec61 with translocon inhibitors (41, 42), but the binding sites and proposed modes of inhibition differ.
Small-molecule inhibitors of the Sec61 translocon include cotransin (43), apratoxin A (44), coibamide A (37), ipomoeassin F (39), mycolactone (45), and cyclotriazadisulfonamide (CADA) (46). CADA was found as an anti-HIV agent (27) that inhibits the cotranslational translocation of human CD4 (huCD4), a type I membrane protein, via specific and selective interactions with the huCD4 SP (46). Furthermore, proteomic studies identified five additional CADA-sensitive proteins, namely, SORT, ERLEC1, PTK7, DNAJC3, and 4-1BB, and established CADA as a substrate-selective Sec61 inhibitor (47–49). Several CADA derivatives have been evaluated by structure-activity relationship (SAR) studies (50, 51) in which the cellular huCD4 receptor expression was assessed. An optimized CADA derivative CK147 demonstrated improved huCD4 down-modulating activity from micromolar to nanomolar range (51).
In this work, we identified that CK147 inhibits Sec61-dependent cotranslational translocation of huCD4 in vitro. Furthermore, CK147 resistance mutations (D60G, R66G, P83H, and Q127K) are located in the lateral gate and the plug domain of Sec61. Our single-particle cryo-EM structure of ribosome-Sec61 in complex with CK147 reveals that CK147 binds to a site near the plug domain and induces a partially opened conformation of Sec61, and comparison of this structure with that of the ribosome-Sec61 apo complex lacking CK147 shows substantial structural rearrangement of Sec61 upon the inhibitor binding. The apo structure of the ribosome-Sec61 translocon complex reveals the binding of the TRAP-γ subunit and defines its interactions with the ribosome, Sec61, and the remaining subunits of TRAP. This structural information would enhance the current understanding of TRAP’s involvement in the translocation process.
RESULTS
Structure of ribosome-Sec61-TRAP complex
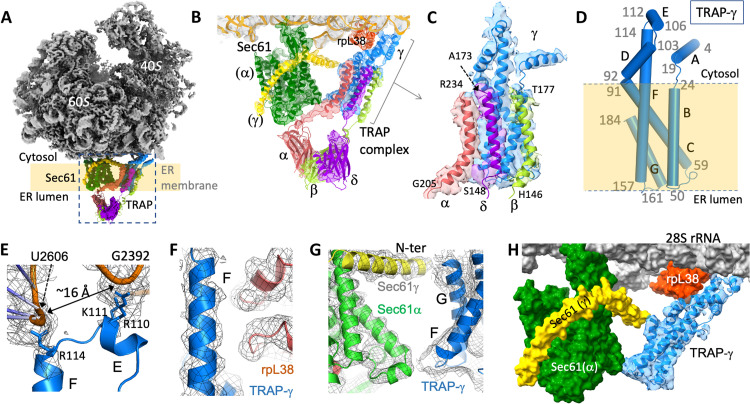
To systematically visualize and characterize the structure of Sec61 in the presence or absence of the translocation inhibitor CK147, we first determined the single-particle cryo-EM structures of an untreated primed ribosome-Sec61 complex in which the Sec61-bound ribosome is in a resting phase, i.e., no peptide being synthesized. The complex was formed by assembling Oryctolagus cuniculus (rabbit) 80S ribosome at the Canis lupus (dog) ER membrane, as described in Materials and Methods. The structure was determined at 2.86-Å resolution (Fig. 2A; fig. S1, A to C; and table S1). The ribosome contains eEF2 that interacts with both the 60S and 40S subunits and stabilizes the complex, as observed earlier (8). Sec61 is well defined by the experimental density, and the local resolution varies from 3.5 to 6 Å (fig. S1, D to G). As expected, the Sec61 translocon adopts the primed conformation in which the plug domain seals the bottom part of the channel near the ER lumen. The density map reveals the binding of a well-ordered TRAP-γ subunit alongside Sec61. Only the 60S ribosome that interacts with both Sec61 and TRAP is modeled into the density; we did not include the atomic coordinates for 40S or eEF2 in the final structure. The models of the ribosome structures of rabbit [Protein Data Bank (PDB): 6MTE] (52) and wild boar (PDB: 3J7Q) (8) were used as templates to fit the 60S part to the experimental density. The available structures (2, 7, 8) and AlphaFold (53) models of the Sec61α and Sec61γ chains were used to fit into the density; the density for Sec61β chain was weak, and therefore, the β chain was not modeled. Two models, one for TRAP-γ and the other for TRAP (α, β, and δ) trimer, were built using AlphaFold and fitted to the cryo-EM density (Fig. 2, B and C, and fig. S1, H to J). The secondary structure elements of the TRAP-γ model could be reliably fitted to the density. The crescent-shaped density accounting for TRAP-α/β/δ trimer in the ER lumen fit the model unambiguously (fig. S1E); however, the map resolution of ~12 Å at the region is not high enough for secondary structure tracing. Therefore, the atomic coordinates for the TRAP-α/β/δ trimer have zero occupancy and zero B factor in the deposited PDB coordinate file, and this part was not included in the final real-space structure refinement cycle.
Fig. 2. Binding of TRAP to ribosome-Sec61 complex.
(A) Cryo-EM density map showing the structure of 80S ribosome (gray)–Sec61 (green)–TRAP (heterotetrameric; α, pink; β, light green; γ, blue; δ, purple) complex. (B) Zoomed view showing the binding of TRAP and Sec61 to the cryo-EM density map. The TRAP-α, TRAP-β, and TRAP-δ subunits are clustered as a trimer in the ER lumen, and the α chain is positioned near the Sec61 channel. The C-terminal helices, one from each TRAP-α, TRAP-β, and TRAP-δ chains, cluster with four TMHs of TRAP-γ. The experimental density map covering Sec61, TRAP-γ, and TRAP-α/β/δ are displayed at the contour levels 2σ, 1.4σ, and 1σ, respectively. (C) Zoomed view showing the stacking of the TRAP C-terminal helices, one from each α, β, and δ chains, with TRAP-γ. (D) Secondary structure elements of TRAP-γ. (E) TRAP-γ interactions with loops of 28S rRNA at two positions that are ~16 Å apart. (F) Relative positioning of the ribosomal protein rpL38 and TRAP-γ. (G) Relative positioning of Sec61α and Sec61γ subunits with respect to TRAP-γ. (H) Molecular assembly of TRAP-γ chain with Sec61 and ribosome, molecular surface for Sec61, and the model for TRAP-γ in the experimental density.
Assembly and interactions of TRAP
The structural assembly of the heterotetrameric TRAP and its interactions in the ribosome-translocon complex can help establish the essential, yet undefined roles that TRAP plays during protein translocation, maturation, and ER stress (11–15, 17, 18, 21). Earlier structural studies, by both single-particle (22) and cryo-tomography (11), have identified the relative positioning of TRAP with respect to the translocon; however, the resolutions of the density maps were not sufficient for tracing the protein chains, which is essential for visualizing the intra- and intermolecular interactions of TRAP. Our structure (Fig. 2, B and C) enables the fitting of the TRAP-γ chain into the experimental density and identifies its molecular interactions with (i) 28S ribosomal RNA (rRNA) and ribosomal protein L38 (rpL38), (ii) α and γ chains of Sec61 (Fig. 2B), and (iii) three C-terminal TMHs, one each from the TRAP α, β, and δ chains (Fig. 2C). The TRAP-γ subunit consists of seven helices of which A, D, and E are cytosolic; B, C, and G are TMHs; and the longest helix F has both cytosolic and transmembrane parts (Fig. 2D). The transmembrane part of TRAP-γ and one C-terminal helix from each TRAP α, β, and δ chains form a seven TMH bundle. The helices B and F of TRAP-γ interface with the TRAP α, β, and δ helices (fig. S1, H to J). The N-terminal parts of the TRAP α, β, and δ chains are clustered as a heterotrimer, which is positioned adjacent to the Sec61 exit channel in the ER lumen (fig. S1K), presumably to interact with the nascent polypeptide chain and/or with other accessory proteins such as OST, as shown earlier (11). The TRAP-α subunit is positioned at the Sec61 channel (Fig. 2B and fig. S1K), and thereby, TRAP-α is expected to have direct contact with nascent proteins protruding from the translocon, which agrees with the finding that TRAP-α is involved in protein biogenesis (54). Two structural studies of a ribosome-Sec61-TRAP complex have been reported after the submission of this article (55, 56). In both structures, determined with translating ribosomes, the C-terminal region of TRAP-α (residues 255 to 266) interacts with ribosomal protein uL29 in contrast to the C-terminal helix interacting with TRAP-γ, which is observed in our structure. This structural difference suggests that TRAP-α is repositioned during protein translocation to interact with the Sec61 TMHs and is directly anchored to the ribosome, presumably for the active involvement of this subunit in translocation initiation.
TRAP-γ is responsible for positioning the TRAP complex at its functional site with respect to the translocon even before translation initiation (Fig. 2, B and C). The positively charged TRAP-γ residues R110 and K111 of helix E and R114 of helix F interact specifically with the sugar-phosphate backbone of the 28S rRNA nucleotides U2606 and C2391-G2392, respectively. The RNA backbones at two interacting sites are ~16 Å apart (Fig. 2E). We hypothesize that the relative positioning of the two positively charged sites of TRAP-γ helps position the TRAP complex in the vicinity of the translocon, i.e., the structure of TRAP-γ at that cytosolic part resembles a “Y-shaped” hook anchoring two RNA “cables” separated by a specific distance of ~16 Å. The F helix acts as the backbone of TRAP-γ extending from its interacting position with 28S rRNA to the lumenal end of the ER membrane, and the F helix is also supported via its interactions with rpL38 in the cytosol (Fig. 2F) and with Sec61α at the lumenal border (Fig. 2G). The C terminus of TRAP-γ is positioned near the N terminus of Sec61γ at the cytosolic side of the ER membrane. The series of observed contacts of TRAP-γ support its precise positioning in both the cytosol and ER membrane regions (Fig. 2H) and help place the trimeric TRAP-α/β/δ at the Sec61 channel exit in the ER lumen (Fig. 2B and fig. S1K).
ER cotranslational translocation is inhibited by CK147
In line with a previous report (51), CK147 (Fig. 3A) exerts a stronger huCD4 down-modulating effect than the lead CADA compound in transiently transfected human embryonic kidney (HEK) 293T cells (Fig. 3B), as evidenced by the maximum reduction in huCD4 expression (95 and 88% for CK147 and CADA, respectively) and the calculated median inhibitory concentration (IC50) values from the concentration-response curves (0.04 and 0.43 μM, respectively). Similar to CADA, CK147 markedly inhibits the cotranslational translocation of huCD4 in a cell-free in vitro translation assay (Fig. 3C, reduced levels of SP-cleaved huCD4 species, open arrowhead, and fig. S2) but has little impact on the ER import of preprolactin (pPL; Fig. 3C). Phenotypic analysis revealed significantly higher cytotoxicity of CK147, with a cytotoxic concentration (CC50) of 0.36 μM in HEK293T cells, whereas CADA hardly affects cell proliferation even at 50 μM concentration (Fig. 3D). Furthermore, CK147 affected the cellular expression of CADA clients more profoundly and reduced the expression of some CADA-resistant proteins in transfected HEK293T cells (fig. S3). However, CK147 maintained some degree of client specificity, as evidenced by the unaffected expression of surface receptors (such as CD58 and CD86) on T-lymphoid MT-4 cells (fig. S4). As expected, the altered cellular levels of the tested receptors correlate with the inhibition of the cotranslational translocation of the pre-proteins, as verified by an in vitro translation assay (fig. S5).
Fig. 3. Effect of CK147 on huCD4 expression and cell proliferation in WT and CK147-resistant cells.
(A) Structures of CADA and CK147. (B) Concentration-response curves of CADA and CK147 for huCD4 down-modulation in HEK293T cells. Cells were transiently transfected with huCD4-tGFP-P2A-RFP and incubated with different compound concentrations. At 24 hours after transfection, tGFP levels were quantified by flow cytometry. Values are means ± SD; n = 3. *P ≤ 0.05; unpaired t tests. (C) Radioblot of the in vitro translation (IVT) of truncated huCD4 and full-length preprolactin (pPL). The constructs were translated in the presence of RRL, sheep rough microsomes, and different concentrations of CK147 or DMSO (1%). Open arrowhead represents the translocated mature protein fraction. The solid arrowhead represents the uncleaved preprotein. The translocation efficiency (%) for each sample is given below the radioblot. (D) Proliferation of HEK293T cells in the presence of CADA and CK147. Cells were treated for 72 hours with increasing compound concentrations. Values are means ± SD; n = 3. *P ≤ 0.05, unpaired t tests. (E) Same as in (D) but for the proliferation of WT HCT116 cells and three CK147-resistant HCT116 clones. Values are means ± SD; n ≥ 3. (F) Cell surface huCD4 expression in WT HCT116 and three CK147-resistant HCT116 clones. Cells were transfected with full-length WT huCD4 and incubated with CADA, and surface huCD4 expression was determined by flow cytometry at 24 hours after transfection. Values are means ± SD; n ≥ 2. (G) Cell surface huCD4 expression in HEK293T cells. Cells were transfected with full-length WT huCD4 together with a WT or mutant Sec61α plasmid and incubated with 0.4 μM CADA or DMSO. After 42 hours of compound treatment, cell surface levels of huCD4 were measured with flow cytometry. Values are means ± SD; n = 3. *P ≤ 0.05, unpaired t tests, compared to WT Sec61α.
Having established CK147 as a more potent and cytotoxic CADA analog, we next generated CK147-resistant cells to identify potential cellular targets of CADA compounds. Briefly, HCT116 cells were grown in the presence of a toxic concentration of CK147 (25 μM), which exerted maximum antiproliferative effects in HEK293T cells (Fig. 3D). We selected three CK147-resistant clones (i.e., clones 10, 13, and 16) for further in-depth phenotypic and genotypic analysis. An untreated control, which went through the same passaging, was also included in our analysis and is referred to as wild-type (WT) HCT116. Similar to the HEK293T cells, the proliferation of WT HCT116 cells is CK147 sensitive, with a CC50 of 0.48 μM (Fig. 3E). In contrast, the selected HCT116 clones showed complete resistance to CK147, as evidenced by the nearly constant proliferation rate at high CK147 concentrations (CC50 values > 50 μM; Fig. 3E). In addition, we evaluated the sensitivity of the CK147-resistant cells to the huCD4 down-modulating effect of CADA. The cell surface huCD4 expression is affected by CADA in a concentration-dependent manner in WT HCT116 cells (IC50 of 0.78 μM; Fig. 3F), which is in line with the effect seen in HEK293T cells (Fig. 3B) and MT-4 cells (IC50 of 0.45 μM; fig. S4) and with previously reported results in other cells (27). In contrast, the CK147-resistant clones no longer (clones 13 and 16) or hardly (clone 10) responded to CADA’s down-modulatory effect on huCD4 expression [IC50 > 50 μM (Fig. 3F)].
CK147 resistance mutations in Sec61α lateral gate and plug domain
The sequence of the Sec61α subunit was analyzed in the resistant clones and aligned to that of WT HCT116 cells. We identified five different heterozygous mutations across the resistant clones, i.e., D60G, R66G, P83H, V102I, and Q127K (Fig. 3G). The amino acid substitutions were all located in one side of the lateral gate, primarily at the interface between TMH2 and TMH3, and in the plug helix. More specifically, in clone 10, the sole amino acid substitution Q127K is located in TMH3. Notably, the same amino acid substitution has been observed in mycolactone resistance cells (40). Clone 13 carried a resistance conferring mutation D60G located in the loop connecting the first two TMHs. This mutation has been identified in previous forward genetic screens as a resistance mutation for other translocation inhibitors, such as decatransin (35) and mycolactone (57). In contrast to the single mutation in most CK147-resistant clones, clone 16 has three Sec61α mutations: R66G, P83H, and V102I (fig. S6). The R66G substitution on the plug helix highlights the importance of the positively charged residue for CK147 binding and plug opening activities. The mutation of R66 has been reported to confer resistance to almost all translocation inhibitors (34–36, 38, 39, 57). The P83H mutation occurs in TMH2, whereas the V102I mutation is in the connecting loop of lateral gate helices TMH2 and TMH3. The V102I mutation site is at the cytosolic side of Sec61α, and it is the only mutation that is located away from the cluster of the remaining mutations in the channel. To provide a full analysis of the translocon, the Sec61β and Sec61γ subunits of the translocon were also sequenced but did not reveal detectable mutations in any of the three CK147-resistant clones.
To validate the detected Sec61 mutations of the CK147-resistant cells, we generated mutants from a plasmid encoding for Sec61α and introduced each mutation individually to Sec61α by site-directed mutagenesis. By transfecting HEK293T cells with the different Sec61α plasmids, we overexpressed the mutant Sec61α in the cells and (transiently) exchanged the endogenous WT protein by each mutant Sec61α counterpart. Resistance was then assessed through huCD4 down-modulation (by coexpressing huCD4 with the mutant Sec61α). As summarized in Fig. 3G and fig. S7, all single mutants, except for V102I, exerted significantly decreased sensitivity to the inhibitor. The lack of resistance for the V102I mutant correlates with its location (i.e., the cytosolic side of Sec61α), distant from the other identified CK147 mutations, and suggests that V102I is a potential compensatory mutation.
Structure of Sec61 before inhibitor binding
As described above, we first obtained a structure of Sec61 in the absence of CK147. The experimental density map for the apo ribosome-Sec61 complex reveals that the Sec61α subunit is in the ribosome-primed conformation with the plug domain sealing the translocon channel (Fig. 4, A and B). Earlier reported structures (PDB IDs: 6MTE and 3J7Q) and an AlphaFold-generated model helped to reliably model Sec61 into the density and added additional secondary structure features toward the lumenal side of the channel. The density toward the lumenal end of Sec61 was lower, yet could trace the protein chain. The AlphaFold model helped in fitting the protein sequence to the density. Here, we have numbered the previously unidentified helices as prime (′ or ″) to the preceding TMH number (Fig. 4A). The structure reveals that the plug helix in the channel is supported from the lumenal end by a newly defined double-stranded β sheet (β3-β4; residues 322 to 340), which is located in the middle of the helix H7′. Similarly, we identified two loop structures (residues 196 to 210 and 235 to 241, respectively) that bifurcate TMH5 and TMH6, respectively, near the lumenal end of the translocon. The THM5 and THM6 region interacts with TRAP-α (fig. S8) and appears to guide the channel opening at the lumenal side of Sec61. The newly identified structural elements near the lumenal end that were disordered in previous Sec61 structures appear to be important for translocon activities. The better resolved density and a reliable AlphaFold model, which could be fitted to the density with high confidence, enabled the identification of these structural elements.
Fig. 4. Conformational change of Sec61 upon CK147 binding.
(A) Sec61α structure in the ribosome-Sec61-TRAP complex without a bound inhibitor; the plug helix is in red. The TMHs are numbered. The new secondary structural elements modeled into the density are annotated as prime (′ or ″) of the preceding TMHs. (B) ER lumenal view of Sec61 showing the density for different structural elements of Sec61. (C) Density for CK147 adjacent to the plug helix (pink) in the ribosome-Sec61-CK147 structure; movie S1 shows a 360° view of CK147 fitting to the experimental density. The docked conformation of CK147 into the site is fitted to the density. (D) Superposition of Sec61 structure with CK147 (colored) and without CK147 (gray) reveals the conformational change upon CK147 (orange) binding. (E) ER lumenal view of the superimposed structures. (F) Repositioning of the plug helix in the pore upon CK147 binding based on the structure superposition in (D).
Structure of CK147-bound Sec61 translocon
Our second structure is of the ribosome-Sec61-CK147 complex. The inhibitor CK147 was added to the Sec61 translocon before the assembly of ribosome-Sec61 complexes, and the sample was spiked with CK147 (10 μM) before the preparation of the cryo-EM grids to ensure high inhibitor occupancy in the complex. A parallel experiment of adding CK147 to a preassembled ribosome-Sec61 complex did not result in binding of the inhibitor as the Sec61α subunit remained in the primed conformation (fig. S9). The cryo-EM data for the CK147-bound complex were also collected on the 200-kV Glacios TEM setup, and the final density map was calculated at 2.79-Å resolution (table S1). The TRAP complex is noticeably less ordered in the CK147-bound structure (fig. S10A) compared to that in the apo structure (fig. S1), suggesting that the inhibitor binding to Sec61α affects the positioning of TRAP. The experimental density for the Sec61 part confirmed the binding of CK147 adjacent to the plug helix inside the Sec61α translocon channel (Fig. 4C and movie S1); the analogous region of the translocon in the apo structure has no such density (Fig. 4B). Superposition of the Sec61 structures with and without CK147 shows substantial structural rearrangement of Sec61 upon CK147 binding. Primarily, the lateral gate helices TMH2 and TMH3 are reoriented, and TMH4 is shifted as the consequence of CK147 binding (Fig. 4, D and E); the CK147-binding site acts as the pivot point for the observed structural aberrations. The C-terminal end of the plug helix (Y63 to L69) is shifted by ~5 Å upon CK147 binding (Fig. 4F).
The CK147-binding site is confined to a region between the plug domain and the lumenal end of the Sec61 channel. The local resolution of the density map in that region of the translocon is ~5 Å (fig. S10, B and C). CK147 can only fit to the density with its sulfonamide groups pointing away from the plug helix. Further, to ascertain the binding mode and conformation of CK147, we applied a molecular docking approach, as discussed in Materials and Methods. The final model of the 60S subunit of the ribosome-Sec61-CK147 structure was fitted against the experimental density map by real-space refinement (table S1). The map-to-model correlation for CK147, the adjacent plug helix, and Sec61 are 0.68, 0.66, and 0.67, respectively, confirming that the inhibitor and surrounding residues have comparable occupancy.
CK147-binding site is adjacent to plug helix
The central 12-membered macrolide ring of CK147 is positioned near the plug helix, and the compound also interacts with the loops connecting either ends of the plug helix (Fig. 5, A and B). These connecting loops are flexible, and in the translocation-competent Sec61 state, the loops assist the plug domain to move out of the channel toward the lumenal end to permit a nascent peptide chain passage through the Sec61 translocon. In the CK147-bound structure, the plug helix and the connecting loops are repositioned to trap a CK147 molecule at the lumenal side of the translocon. The helices TMH2, TMH3, and TMH4 are rearranged to enclose the inhibitor and participate in CK147 binding. The interactions of CK147 with the residues of Sec61 are shown in a two-dimensional (2D) projection Ligplot in fig. S10D. In particular, the plug helix backbone and the side chains of residues M65 and R66 interact extensively with the 12-membered core ring of CK147 (fig. S10, D and E), whereas the nearby residues A59, D60, P61, L69, S71, and R73 interact from either side (Fig. 5A). Apart from the plug domain, the residues Y131 from TMH3 and M136 from the loop connecting TMH3 and TMH4 also interact with CK147.
Fig. 5. Interactions of CK147 and proximity of resistance mutation sites.
(A) Mode of binding of CK147 (orange). The interacting Sec61α side chains are displayed; the plug helix and TMH are in magenta and cyan, respectively. (B) Sec61α molecular surface defines the CK147-binding pocket. (C) Proximity and interactions of the plug helix (magenta) with CK147; the interacting surface of plug with CK147 is in gray. (D) Four of five CK147 resistance mutations D60G, R66G, P83H, and Q127K surround the inhibitor, whereas the V102I mutation is at the cytosolic side of Sec61.
As described earlier, the Sec61α mutations D60G, R66G, P83H, V102I, and Q127K confer CK147 resistance. Except for V102, the remaining mutation sites are in the vicinity of the bound CK147 (Fig. 5D); our mutant V102I Sec61 in HEK293T cells showed no significant impact on the expression of huCD4 (Fig. 3G). The residue D60 and the adjacent A59 and P61 are in the loop preceding the plug helix. All three residues interact with the inhibitor; however, P61 interacts more extensively. Apart from the loss of interactions by the D60G mutation, the main-chain flexibility introduced in the region by D60G presumably disfavors the CK147 binding. The residue R66 interacts extensively with CK147 (fig. S10), and the substantial loss of the inhibitor-protein interactions by the R66G mutation is likely to cause the observed CK147 resistance, as also supported by the huCD4 data of the single R66G mutant (Fig. 3G). The R66G mutation alone has been shown to noticeably resist mycolactone and restore the translocon activity (38); R66 has the most extensive atom-to-atom contacts with CK147 compared to other residues in our structure (fig. S10D). The residue P83 is at the base of TMH2 facing TMH3, and the P83H mutation confers CK147 resistance presumably by rearranging TMH2 and TMH3 that would not be favorable for CK147 binding. The TMH3 residue Q127 is located near the 4-methyl-benzene-sulfonamide moiety of CK147.
DISCUSSION
The plug helix is blocked inside the channel by CK147
Superposition of the CK147- and SP-bound (PDB ID: 3JC2) structures shows that the TMHs are rearranged differently in the two complexes (Fig. 6A). A major difference between both structures is that in the SP-bound Sec61 translocon, the plug domain moves out of the channel (Fig. 1), clearing the path for translocation of the protein. In the inhibitor-bound complex, however, the plug helix remains inside the channel, despite the lateral gate TMHs being rearranged. The Sec61 channel in the ribosome-Sec61 complex must switch from the primed to the SP-bound conformation as the SP from the RNC interacts with Sec61. As discussed earlier, our structural study attempt showed that Sec61 does not adapt the inhibitor-bound conformation if exposed to the inhibitor after the ribosome-Sec61 complex is fully formed; i.e., CK147 should bind Sec61 before the docking of ribosomes to Sec61. However, a previous cell-free in vitro translation/translocation study showed that the lead CADA compound is effective at a post-targeting step of huCD4 RNC to Sec61 (46). Apparently, a paused ribosome presenting the huCD4 SP to Sec61 still permits CADA to inhibit the protein translocation. No ribosome-Sec61 structure is available for such an early targeting state with the SP approaching Sec61. Presumably, the conformational changes triggered by an SP permit CADA binding, i.e., the CK147-bound conformation of Sec61 might be a transient state created for SP binding. Thus, the order of inhibitor addition and/or the features of an RNC play roles in the binding of translocon inhibitors.
Fig. 6. The potential mechanism of translocon inhibition by CK147.
(A) Superposition of SP-bound (blue; PDB ID: 3JC2) and CK147-bound (green/cyan) Sec61α reveals that the lateral gate moves differently in two structures; the SP (yellow) binding moves the plug out of the channel, whereas the plug stays in the pore for CK147 binding. (B and C) Electrostatic potential surface of SP-bound Sec61α (B) and CK147-bound Sec61α (C). The binding of CK147 would lock the Sec61 conformation that is unfavorable for SP binding and protect the channel. (D) Schematic representation of the potential mechanism of inhibition by CK147. The approaching SP (yellow) is unable to displace the plug (magenta) out the channel when CK147 (orange) is bound. The elongated nascent chain cannot translocate to the ER lumen, protrudes from the channel at the ribosome-Sec61 interaction site, and is diverted to the cytosol for degradation by the proteasome.
It is generally accepted that an SP recognizes the plug region of the translocon near the lateral gate. The hydrophobic core of an SP is a crucial determinant to push the plug aside and to permit the nascent peptide passing through the open channel. The SP-bound conformation of Sec61 (Fig. 6B) secures the channel from all sides and permits the nascent polypeptide passage through the ER membrane. The C-terminal end of SP in the looped nascent chain reaches well below in the channel that requires the displacement of the plug. In the CK147-bound Sec61 conformation, the translocon with a rearranged lateral gate is still accessible for a nascent chain from the cytosolic side; however, the inhibitor locks the plug in the channel, which would prevent the peptide entering the ER lumen and would disfavor a correct positioning of the SP helix (Fig. 6, C and D). Thereby, an RNC can potentially destabilize the binding of a weak translocon inhibitor. For example, the characteristic R66G mutation would reduce the inhibitor binding affinity, and the inhibitor can be pushed out to permit SP binding. In addition, our lead CADA compound that has different side groups compared to CK147 (Fig. 3A) is a weak inhibitor, and its binding is reversible (42, 46). This interplay between the inhibitor and SP binding could define the client specificity and selectivity for translocon inhibitors, which may explain the number of substrates that are differentially affected by CADA and CK147 (figs. S3 and S4). Consequently, as CK147 inhibits the ER import and cellular expression of more clients, this will affect the metabolism and survival of cells, resulting in higher cellular toxicity (explaining the lower CC50 values for CK147 as compared to CADA). An example of client specificity and selectivity among the cyclotriazadsulfonamide compounds is CD4 (figs. S3 and S4). Although human and murine CD4 are structurally related type I membrane-anchored receptors with four extracellular immunoglobulin-like domains, they share about 55% amino acid sequence identity. They carry different SPs (in terms of length and hydrophobicity), which affect the cotranslational translocation efficiency (fig. S5), as also shown in previous reports (46, 49). This explains the differential effect on cell surface expression (IC50 values for CK147 are 0.04 and 1.1 μM for human and murine CD4, respectively). The binding affinity of an inhibitor to Sec61 and the intrinsic binding features of an RNC, such as the sequence specificity of an SP for the Sec61 conformational triggering, should be taken into account for finding a protein/SP-specific translocon inhibitor.
Different inhibitor binding sites in Sec61
The CK147-bound Sec61 structure aligns well with a previously reported structure of the mycolactone-bound Sec61 translocon (40). Mycolactone binds at the cytosolic side of the translocon that is ~20 Å away from the CK147-binding site (Fig. 7A). The local resolution near the mycolactone-binding site in our structure is ~3 Å (fig. S10C), and no density is observed in that site, confirming that CK147 does not bind at or near the proposed mycolactone-binding site. The mycolactone resistance mutations are primarily observed in the lateral gate and plug domain of the translocon; thus, they are away from the mycolactone-binding site, but surrounding the CK147-binding pocket, with R66G and Q127K being resistant to both (Fig. 7B). Furthermore, mycolactone and CK147 share a 12-membered macrolide core ring, although the ring compositions and side groups differ. A common conformational state of CK147- and mycolactone-bound Sec61, the resemblance in their core 12-membered ring structure, and the overlapping resistance mutation sites in Sec61α suggest that mycolactone might also bind to a second site, in which the core ring would occupy the CK147-binding site, and its long “Southern chain” would extend toward the SP-binding cleft along the mycolactone resistance mutation sites S82 and T86 in TMH2.
Fig. 7. Binding of mycolactone versus CK147.
(A) The mycolactone (blue)–bound Sec61α (yellow; PDB ID: 6Z3T) aligns well with the CK147 (orange)–bound Sec61α (green), suggesting a common mode of action for both inhibitors. (B) The key mycolactone resistance mutation sites (residues with blue spherical atoms) are rather confined to the CK147-binding pocket; mycolactone is placed on the basis of Sec61α superposition.
A recent cryo-EM study of the Sec61 translocon exposed to a cotransin analog, KZR-8445 containing a large cyclic heptadepsipeptide ring, reveals binding of the inhibitor to the SP-binding cleft (41). Another recent Sec61 structural study of multiple translocation inhibitors, including mycolactone and CADA, showed an overlapping binding site near the lateral gate of Sec61 for all the inhibitors (42). The presented cryo-EM structures, however, were of a post-translocational human/yeast chimeric complex of Sec61/Sec63/Sec71/Sec72 (42). In their apo structure, the authors show a preexisting hydrophobic cavity even in the absence of a translocon inhibitor, and Sec61 is presumed to be in a nonconventional conformational state, which is different from the native, primed, SP-bound, or CK147/mycolactone-bound state of Sec61 (Figs. 1B and 7A). We did not observe the existence of such a pocket in our apo complex or the presence of an additional density patch to account for CK147 binding to that region in our inhibitor-bound structure. The comparison of structures suggest that inhibitors bind to different sites of Sec61 depending on their shape/size and experimental conditions. Follow-up experiments are needed for physiological validation of different binding sites.
Implications of this study
In this study, the structures of CK147-bound Sec61 and apo Sec61 are obtained under identical experimental conditions. The apo structure of the Sec61 translocon-ribosome shows the binding of a well-resolved TRAP complex that reveals the molecular interactions among the TRAP subunits and TRAP interactions with its partners, i.e., the ribosome and the Sec61 translocon. The structure reveals TRAP as an integral part of the translocation machinery at an early stage before translation initiation. The interactions of TRAP with Sec61α and Sec61γ subunits complement the earlier findings that TRAP supports the translocation of proteins with less hydrophobic SPs, and further propose that the protein translocon machinery for the translocation initiation is a multiprotein complex beyond just Sec61. The intra- and intermolecular interactions of TRAP in the cytosol, ER membrane, and ER lumen provide insights into the organizational and functional roles of TRAP. The C-terminal helix of TRAP-α is positioned at the backside of Sec61α (opposite of the lateral gate) and makes a second contact with the translating ribosome in two recent structures of translating ribosome-Sec61-TRAP complexes (55, 56); thus, it is no longer interacting with TRAP-γ as observed in our structure. The structural difference suggests that TRAP-α has a highly flexible linker that repositions the C-terminal helix for interaction with different proteins, such as Sec61α or the C terminus of TRAP-β as measured in living mammalian cells (58), presumably for the precise positioning of TRAP-α for its active role in translocation initiation. We expect that the newly resolved TRAP structures provide the framework for experiments such as cross-linking and systematic functional mutation studies to further identify and validate the roles of different TRAP subunits as well as their intra- and intermolecular interactions at different stages of protein translocation.
We established CK147, an analog of the earlier described CADA compound (51), as a potent inhibitor of cotranslational translocation. In addition, our resistance study finds the Sec61 translocon as the molecular target of CK147. The cryo-EM structure and mutational studies establish the binding of CK147 deep inside the channel, adjacent to the plug helix, and identified a novel Sec61 target site for translocation inhibitor design. The analysis of SAR results on CADA derivatives and other translocon inhibitors in reference to the CK147-binding would help design new translocon inhibitors.
To conclude, this study of a synthetic small molecule, with a 12-membered macrolide ring structure and short side chains, which binds at the plug domain near the lumenal side of the translocon and potently blocks protein translocation, provides several details for fundamental understanding of cotranslational translocation and for inhibitor design. Compared to the recently reported structural studies of Sec61 inhibition, binding of CK147 below the plug domain of Sec61 presents a unique and unexplored site for translocon inhibitors. The different chemical compositions and potential binding sites/modes of translocation inhibitors could pave the way for the structure-based design and discovery of novel, client-specific inhibitors of the Sec61 translocon for the treatment of human diseases.
MATERIALS AND METHODS
Compounds
CADA hydrochloride and the unsymmetrical analog CK147, bearing a cyclohexylmethyl tail and a dimethylaminobenzenesulfonyl side arm, were purchased from TCG Lifesciences Pvt. Ltd. (Kolkata, India), who synthesized the compounds following an earlier described protocol (50, 51). Compounds were dissolved in dimethyl sulfoxide (DMSO) and stored at a stock concentration of 10 mM at room temperature.
Plasmids and molecular cloning
The pcDNA3 expression vector encoding WT huCD4 was a gift from O. Schwarts (Institut Pasteur, Paris, France). The pEGFP-P2A-RFP (red fluorescent protein) expression vector (pEGFP-N1 Clontech backbone) and the pcDNA3 expression vector encoding FLAG-tagged Sec61α were a gift from R. Hegde (MRC Laboratory of Molecular Biology, Cambridge, UK). The sequence for enhanced green fluorescent protein (eGFP) was replaced by that of turbo GFP (tGFP). The tGFP-P2A-RFP and huCD4 fragments were cloned into pcDNA3.1, as described earlier (59). Briefly, inserts and expression vectors containing overlapping DNA ends were amplified using polymerase chain reaction (PCR) with appropriately designed primers. Sec61α mutant plasmids were generated by means of Q5 site-directed mutagenesis (New England Biolabs, Ipswich, MA, USA). All PCRs were performed with the Q5 Hot Start HF DNA Polymerase (New England Biolabs) according to the manufacturer’s protocol. The PCR products were purified either from the agarose gel or from the PCR sample using the NucleoSpin Gel and PCR Clean-up Kit (Macherey Nagel, Düren, Germany). Site-directed mutagenesis was performed with the Q5 site-directed mutagenesis kit (New England Biolabs) or NEBuilder HiFi DNA assembly kit (New England Biolabs) following the manufacturer’s instructions. Next, NEB5α-competent Escherichia coli cells (New England Biolabs) were transformed with the ligated PCR product according to the manufacturer’s instructions. Plasmid DNA was isolated using the NucleoSpin Plasmid Transfection-grade System (Macherey Nagel) supplemented with an endotoxin removal wash. The DNA concentration of all constructs was determined with a NanoDrop 1000 spectrophotometer (Implen, Munich, Germany), and the DNA sequences were confirmed by automated capillary Sanger sequencing (Macrogen Europe).
Cell-free in vitro translation/translocation assay
The EasyXpress linear template kit (Qiagen, Hilden, Germany) was used to amplify and linearize the DNA of interest from the plasmid using PCR. To simplify the analysis of the translated products, we generated truncated huCD4 as shown in fig. S2 (59). PCR products were purified with the NucleoSpin Gel and PCR Clean-up Kit (Macherey Nagel) and transcribed in vitro using T7 RNA polymerase (RiboMAX system, Promega, Madison, WI, USA). RNA was purified from the sample using the NucleoSpin RNA clean-up kit (Macherey Nagel). mRNA transcripts were then translated in rabbit reticulocyte lysate (RRL) (Promega) in the presence of L-35S-methionine (PerkinElmer, Waltham, MA, USA). Translations were performed for 45 min at 30°C in the presence or absence of ovine pancreatic rough microsomes and CK147, supplemented with RNasin (Promega) as described earlier (48). Samples were washed with low-salt buffer [80 mM KOAc, 2 mM Mg(OAc)2, and 50 mM Hepes (pH 7.6)], and radiolabeled proteins were isolated by centrifugation (10 min at 21,382g, 4°C). The radiolabeled proteins were then separated with SDS–polyacrylamide gel electrophoresis on a 4 to 12% Criterion XT bis-tris gels (Bio-Rad, Hercules, CA, USA) in MES buffer (Bio-Rad), detected by phosphor imaging (Cyclone Plus storage phosphor system; PerkinElmer), and quantified using the ImageQuant software.
Transient cell transfection and flow cytometry
Cells were seeded in a six-well plate and incubated overnight to allow adhesion at 37°C before transfection the next day. Lipofectamine LTX (Thermo Fisher Scientific) was used for the transient transfection of cells with a total of 2.5 μg of plasmid DNA, according to the manufacturer’s instructions. Compounds (CADA, CK147, or DMSO) were added 6 hours after transfection, and cells were further incubated at 37°C. For the cell surface staining of huCD4 in the CK147-resistant HCT116 cell clones, cells were collected 24 hours after transfection (i.e., after an 18-hour compound treatment), whereas in the experiments of cotransfection of Sec61α with huCD4, transiently transfected HEK293T cells were collected 48 hours after transfection (i.e., 42 hours of compound treatment). Cells were washed with ice-cold phosphate-buffered saline (PBS) (Thermo Fisher Scientific) containing 2% serum (Global Life Sciences Solutions, Traun, Austria). The cells were then stained with a phycoerythrin-labeled anti-huCD4 antibody (SK3 clone, catalog no. 344606, BioLegend, San Diego, CA, USA) and incubated in the dark for 45 min at 4°C. Excess antibody was removed by washing the cells with ice-cold PBS containing 2% serum. For the flow cytometric analysis of cellular tGFP and RFP levels, no antibody staining was performed. Instead, cells were collected and washed with ice-cold PBS. All cells were finally fixed in PBS containing 1% paraformaldehyde (VWR Life Science brand, Radnor, PA, USA) before data acquisition on either a BD FACSCelesta or a BD FACS Fortessa flow cytometer (BD Biosciences, San Jose, CA, USA) running BD FACSDiva 8.0.1 software. All data were analyzed using the software FlowJo X v10 (BD Biosciences). The data are normalized to the DMSO control (set at 1.00). To compare the down-modulating activity of CADA and CK147, IC50 values were calculated on GraphPad Prism 8 software (San Diego, CA, USA) with a four-parameter concentration-response curve fitting to data from replicate experiments, performed on different days. The absolute IC50 value represented the compound concentration that resulted in 50% reduction in the protein level.
Cell proliferation assay
To analyze the viability of HCT116 or HEK293T cells in the presence of compound, a proliferation assay was performed as described in (36). Cells were seeded in a black 96-multiwell plate at a density of 25,000 cells/ml. After an overnight incubation at 37°C, compound was added and cells were further incubated for 72 hours. Next, the resazurin-based solution Alamar Blue (Thermo Fisher Scientific) was added and the cells were incubated for 3 hours at 37°C to allow for the metabolic conversion of resazurin to resorufin. Using an excitation wavelength between 530 and 560 nm, the resorufin emission signal was recorded at 590 nm with a Tecan Spark microplate reader (Tecan, Männedorf, Switzerland) and analyzed for calculating the cellular proliferation efficiency upon compound treatment. The data were normalized to the DMSO control (set at 1.00). To compare the antiproliferative activity of CADA and CK147, IC50 values were calculated on GraphPad Prism 8 software (San Diego, CA, USA) with a four-parameter concentration-response curve fitting to data from replicate experiments, performed on different days. The absolute IC50 value represented the compound concentration that resulted in 50% reduction in proliferation.
Statistical analysis
Statistical analyses were performed using GraphPad Prism version 9.3.1. Unpaired t test with Welch correction was used for the analysis of the flow cytometry data for huCD4 down-modulation in HEK293T cells and for the proliferation of HEK293T cells in the presence of CADA and CK147. Observed differences were considered significant if the calculated P value was *P ≤ 0.05.
Selection of CK147-resistant HCT116 clones
HCT116 cells were grown in T25 flasks, treated with 25 μM CK147 for 2 weeks, and incubated at 37°C. Every 2 days, the medium was replaced to keep the compound pressure constant. After 2 weeks, cells that survived compound treatment were collected and diluted over different large petri dishes to thoroughly dilute the cell suspension. The cells were then further incubated (under CK147 pressure) at 37°C and microscopically monitored. Once cell colonies started to grow, the CK147-resistant cells were isolated by clonal ring expansion based on a protocol described elsewhere (36). Last, the resistant cells were expanded and cultured in T75 flasks in compound-free medium.
Library preparation and nanopore sequencing
CK147-resistant HCT116 cells were first incubated with medium containing 25 μM CK147 for 72 hours at 37°C. Next, RNA was isolated from 5 × 106 cells using the RNeasy Mini Kit (Qiagen) according to the manufacturer’s protocol and reverse-transcribed into cDNA using random primers from the High-Capacity cDNA Reverse Transcription Kit (Thermo Fisher Scientific). Using specifically designed primers, the Sec61α, Sec61β, and Sec61γ DNA was next PCR-amplified with the Q5 polymerase (Promega) according to the manufacturer’s protocol. Amplified Sec61α, Sec61β, and Sec61γ fragments were purified using the NucleoSpin Gel and PCR Clean-up Kit (Macherey Nagel). The purified DNA was quantified on the Qubit 4 Fluorometer (Thermo Fisher Scientific), and libraries were prepared using the ligation sequencing kit (SQK-LSK 109) from Oxford Nanopore Technologies (ONT) following the manufacturer’s instructions. The amplicons (200 fmol) were first end-repaired with the NEBNext Ultra II end repair kit (New England Biolabs) and then purified using AMPure XP beads (Beckman Coulter). Immediately afterward, the end-prepped amplicons were barcoded using the native barcoding expansion kits (EXP-NBD104 and EXP-NBD114, ONT), and then the barcoded amplicons were pooled all together and purified using AMPure XP beads (Beckman Coulter). The adapter-ligated DNA library was purified with AMPure XP beads and finally eluted in the elution buffer provided by ONT. DNA library (25 fmol) was loaded on an R9.4.1 flow cell and run for 24 to 48 hours on the GridION sequencing platform. Super-accurate base calling, demultiplexing, and adapter trimming were done using the GridION built-in MinKNOW software (v21.11). To obtain the consensus sequences, we used Minimap2 (60) to align the sequencing reads (single-read quality of 15 or higher) to the reference, SAMtools v1.9 (61) to process the mapping files, and Medaka v1.6.0 (ONT software) to obtain the consensus sequences and variant calling. All sequenced fragments in our study had a coverage of >150,000×; the fragments of Sec61α had a coverage of even >500,000×. Only mutations that occurred in at least 10% of the reads were considered.
Isolation of ovine and canine microsomes
ER-derived rough microsomes from canine and ovine pancreas were prepared according to the method of Walter and Blobel (62), except that Hepes (pH 7.6) instead of tetraethylammonium (pH 7.5) was used as the buffer. In addition, in buffer A, in addition to phenylmethylsulfonyl fluoride, the following protease inhibitors were added: aprotinin (10 μg/ml), leupeptin (10 μg/ml), chymostatin (1 μg/ml), and elastatinal (1 μg/ml).
Purification of ribosome-translocon complexes from RRL for cryo-EM
Ribosomes (from RRL), CK147 (10 μM), and dog rough microsomes (0.05 eq/μl) were incubated (in the absence of mRNA) for 45 min at 30°C. Translocon-bound ribosomes were purified from the sample by means of ultracentrifugation as described (3). Briefly, membranes were isolated by pelleting the sample through a sucrose cushion [0.5 M sucrose, 30 mM Hepes/KOH (pH 7.5), 10 mM Mg(OAc)2, 500 mM KOAc, and 1 mM dithiothreitol (DTT)] for 10 min at 95.029g (MLA130 rotor, 4°C). Membrane pellets were then solubilized [30 mM Hepes/KOH, 10 mM Mg(OAc)2, 100 mM KOAc, 1.5% digitonin (MP Biomedicals, Santa Ana, CA, USA), and 1 mM DTT (Sigma-Aldrich, St. Louis, MO, USA) (pH 7.5)] for 1 hour at 4°C. Next, ribosome-translocon complexes were pelleted through a sucrose cushion [0.5 M sucrose, 30 mM Hepes/KOH, 10 mM Mg(OAc)2, 100 mM KOAc, 0.3% digitonin, and 1 mM DTT (pH 7.5)] for 45 min at 560,747g (MLA130 rotor, 4°C). The isolated ribosome-translocon complexes were then resuspended [30 mM Hepes/KOH, 10 mM Mg(OAc)2, 100 mM KOAc, 0.3% digitonin, and 1 mM DTT (pH 7.5)], and the concentration of the 80S ribosome fraction was determined by measuring the 260-nm absorbance (A260) with a NanoDrop 1000 spectrophotometer. Last, samples were used in the cryo-EM studies.
Grid preparation and data collection
Quantifoil Cu300 R1.2/1.3 + 2nm Carbon (ultra-thin carbon) grids were used to prepare vitreous grids of the ribosome-Sec61 complex using Vitrobot Mark IV. The grids were glow-discharged for 30 s at 15-mA current with the chamber pressure set at 0.30 mBar (PELCO easiGlow, Ted Pella). The glow-discharged grids were mounted in the sample chamber of Vitrobot Mark IV at 4°C and 95% relative humidity, blotted, and plunge-frozen in liquid ethane at temperature −172°C. Optimized grids were obtained by applying 2.75 μl of the sample (A260 of 19 and 25.5 for the apo and CK147 inhibited sample, respectively) and blotted for 2.5 s using Whatman grade 2 filter paper with force set to 0. The prepared grids were then clipped and mounted on a 200-kV Glacios TEM (Thermo Fisher Scientific) equipped with autoloader and Falcon 3 direct electron detector as installed in our laboratory. Cryo-EM datasets were collected from the vitrified hydrated grids in counting mode on the Glacios TEM using EPU software version 2.9.0 (Thermo Fisher Scientific). The movies were collected at a nominal magnification of 120,000×, yielding a pixel size of 1.23 Å. Each movie was collected with 40 frames, where each frame received ∼0.8 e/Å2, for a total dose of 32 e/Å2, and subsequently written as a gain-corrected MRC (Medical Research Council, UK) file. The grids of different ribosome-Sec61 complexes were prepared using the same conditions as described above.
Data processing
For both structures, the individual movie frames were motion-corrected and aligned using MotionCor2 (63) as implemented in the Relion 3.1 package (64), and the contrast transfer function (CTF) parameters were estimated by CTFFIND-4 (65). The particles were automatically picked using the reference-free Laplacian-of-Gaussian routine in Relion 3.1. The picked particles were initially classified into 2D and 3D classes in cycles to select a set of good particles. The final 3D classification generated a distinct single class of particles. No additional 3D class representing a different structural state of ribosome was detected. The final set of particles for each structure was used to calculate gold-standard auto-refined maps, which were further improved by B polishing and CTF refinement (table S1); the data processing steps are outlined in figs. S11 and S12. Our attempts of multi-body refinement did not improve the quality of the map at the regions of our interest, i.e., Sec61 and TRAP. All data processing was carried out using Relion 3.1.
Model fitting
The density map for the ribosome-Sec61-TRAP complex was calculated at 2.86-Å resolution (fig. S1). The local resolution of the map varied from 2.5 Å in the ribosome core to 12 Å in the ER lumen. The map revealed the presence of an eEF2-bound ribosome, Sec61, and TRAP. The structure of the rabbit 80S ribosome (PDB: 6MTE and 7ZJX) was used for fitting the 60S part to the density. We did not build the atomic model for eEF2 and the 40S part of ribosome. AlphaFold models generated for Sec61 α and γ chains and the coordinates from a structure of ribosome-Sec61 complex (PDB: 3J7Q) (8) were used for building model into the Sec61 part of the density (fig. S1F). In addition, two AlphaFold models were generated, one for TRAP-γ and the other for TRAP (α, β, and δ). The TRAP-γ subunit is well defined in the map (fig. S1G) at a lower counter level than for the core structure, and the AlphaFold model was fitted to the density. The density adjacent to Sec61 in the ER lumen could fit the AlphaFold-generated N-terminal trimeric complex of TRAP (α, β, and δ) unambiguously (fig. S1, E and K); however, the lower resolution of the density map for the region did not permit the fitting of the heterotrimeric TRAP (α, β, and δ) structure at the secondary structure level, and this part was omitted from the real-space structure refinement using Phenix version 1.20.1-4487 (66).
The above 60S ribosome structure was fitted to the density map of the ribosome-Sec61-CK147 complex. The density for TRAP was present in the map; however, it was poorly ordered. Therefore, no TRAP complex was fitted to this map. The Sec61 conformation altered upon CK147 binding and the α and γ chains were remodeled into the density in the ribosome-Sec61-CK147 map. The local resolution of the CK147-bound Sec61 varied from 3.5 to 6 Å in resolution, and density accounting for the inhibitor was located adjacent to the plug domain where the map resolution is ~5 Å. This region in the apo structure had no density.
Docking of CK147
While the density in the ribosome-Sec61-CK147 map indicated the position of CK147, the conformation of the inhibitor could not be precisely determined. Therefore, molecular docking was carried out using AutoDock Vina (67) as implemented in Chimera v1.15 (68) to help find the most probable conformation of CK147. The docking settings were maintained as default, and a 25.57 × 28.40 × 18.53 Å3 box was set as a cuboid around the region near the plug helix where the density for the inhibitor was found. From top five results based on the docking score, three had similar conformations, and the top docked inhibitor model that fits the density with improved conformational state particularly of the 12-membered ring was chosen. The conformation of CK147 from the docking experiment agreed well with the density map. Although the side groups of CK147 and the side chains of the surrounding residues are not unambiguous because of the low-resolution (~5 Å) density map for the region, the complete model of the region is included in the structure refinement for better assessment of the inhibitor binding. The structure fitted with docked CK147 was refined using Phenix, as described, and the correlation coefficient for CK147 is 0.68 (table S1). The structure figures for the article were produced using ChimeraX v1.2.5 (69) and PyMOL v2.4.1 (The PyMOL Molecular Graphics System, version 2.4.1, Schrödinger LLC.).
Acknowledgments
We thank S. Allan for help with the isolation of sheep and dog microsomes and T. Wawina and B. Vanmechelen for support to the Nanopore sequencing. We thank L. Smalinskaite and R. Hegde for providing the Sec61α plasmid. E.P. expresses gratitude to R. Beckmann and K. Braunger for the helpful training in Sec61 cryo-EM sample preparation.
Funding: K.D. and K.V. acknowledge the internal funding from the Division of Virology and Chemotherapy, Rega Institute, KU Leuven.
Author contributions: E.P., K.V., and K.D. designed the research; E.P., B.D.W., N.R.S., A.C., B.P., J.S., and K.D. performed the research; K.-U.K. and E.H. contributed new reagents/analytic tools; E.P., N.R.S., P.M., K.V., and K.D. analyzed the data; E.P., K.V., and K.D. wrote the paper.
Competing interests: The authors declare that they have no competing interests.
Data and materials availability: The density maps for the ribosome-Sec61-TRAP and ribosome-Sec61-CK147 structures have been deposited to EMDB under the EMDB IDs EMD-15860 and EMD-15863, respectively, and the coordinates have been deposited to PDB under the accession numbers 8B5L and 8B6C, respectively. All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials.
Supplementary Materials
This PDF file includes:
Supplementary Text
Legend for movie S1
Figs. S1 to S12
Tables S1 and S2
Other Supplementary Material for this manuscript includes the following:
Movie S1
REFERENCES AND NOTES
- 1.M. Uhlén, L. Fagerberg, B. M. Hallström, C. Lindskog, P. Oksvold, A. Mardinoglu, Å. Sivertsson, C. Kampf, E. Sjöstedt, A. Asplund, I. Olsson, K. Edlund, E. Lundberg, S. Navani, C. A.-K. Szigyarto, J. Odeberg, D. Djureinovic, J. O. Takanen, S. Hober, T. Alm, P.-H. Edqvist, H. Berling, H. Tegel, J. Mulder, J. Rockberg, P. Nilsson, J. M. Schwenk, M. Hamsten, K. v. Feilitzen, M. Forsberg, L. Persson, F. Johansson, M. Zwahlen, G. v. Heijne, J. Nielsen, F. Pontén, Tissue-based map of the human proteome. Science 347, 1260419 (2015). [DOI] [PubMed] [Google Scholar]
- 2.R. M. Voorhees, R. S. Hegde, Structure of the Sec61 channel opened by a signal sequence. Science 351, 88–91 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.K. Braunger, S. Pfeffer, S. Shrimal, R. Gilmore, O. Berninghausen, E. C. Mandon, T. Becker, F. Forster, R. Beckmann, Structural basis for coupling protein transport and N-glycosylation at the mammalian endoplasmic reticulum. Science 360, 215–219 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.L. Bai, T. Wang, G. Zhao, A. Kovach, H. Li, The atomic structure of a eukaryotic oligosaccharyltransferase complex. Nature 555, 328–333 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.S. Pfeffer, J. Dudek, M. Gogala, S. Schorr, J. Linxweiler, S. Lang, T. Becker, R. Beckmann, R. Zimmermann, F. Forster, Structure of the mammalian oligosaccharyl-transferase complex in the native ER protein translocon. Nat. Commun. 5, 3072 (2014). [DOI] [PubMed] [Google Scholar]
- 6.A. M. Liaci, B. Steigenberger, P. C. Telles de Souza, S. Tamara, M. Grollers-Mulderij, P. Ogrissek, S. J. Marrink, R. A. Scheltema, F. Forster, Structure of the human signal peptidase complex reveals the determinants for signal peptide cleavage. Mol. Cell 81, 3934–3948.e11 (2021). [DOI] [PubMed] [Google Scholar]
- 7.S. Pfeffer, L. Burbaum, P. Unverdorben, M. Pech, Y. Chen, R. Zimmermann, R. Beckmann, F. Forster, Structure of the native Sec61 protein-conducting channel. Nat. Commun. 6, 8403 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.R. M. Voorhees, I. S. Fernandez, S. H. Scheres, R. S. Hegde, Structure of the mammalian ribosome-Sec61 complex to 3.4 A resolution. Cell 157, 1632–1643 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.R. M. Voorhees, R. S. Hegde, Toward a structural understanding of co-translational protein translocation. Curr. Opin. Cell Biol. 41, 91–99 (2016). [DOI] [PubMed] [Google Scholar]
- 10.R. M. Voorhees, R. S. Hegde, Structures of the scanning and engaged states of the mammalian SRP-ribosome complex. eLife 4, e07975 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.S. Pfeffer, J. Dudek, M. Schaffer, B. G. Ng, S. Albert, J. M. Plitzko, W. Baumeister, R. Zimmermann, H. H. Freeze, B. D. Engel, F. Forster, Dissecting the molecular organization of the translocon-associated protein complex. Nat. Commun. 8, 14516 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.T. Kriegler, G. Kiburg, T. Hessa, Translocon-associated protein complex (TRAP) is crucial for co-translational translocation of pre-proinsulin. J. Mol. Biol. 432, 166694 (2020). [DOI] [PubMed] [Google Scholar]
- 13.D. Nguyen, R. Stutz, S. Schorr, S. Lang, S. Pfeffer, H. H. Freeze, F. Forster, V. Helms, J. Dudek, R. Zimmermann, Proteomics reveals signal peptide features determining the client specificity in human TRAP-dependent ER protein import. Nat. Commun. 9, 3765 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.N. Sommer, T. Junne, K.-U. Kalies, M. Spiess, E. Hartmann, TRAP assists membrane protein topogenesis at the mammalian ER membrane. Biochim. Biophys. Acta 1833, 3104–3111 (2013). [DOI] [PubMed] [Google Scholar]
- 15.R. D. Fons, B. A. Bogert, R. S. Hegde, Substrate-specific function of the translocon-associated protein complex during translocation across the ER membrane. J. Cell Biol. 160, 529–539 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.S. Lang, D. Nguyen, P. Bhadra, M. Jung, V. Helms, R. Zimmermann, Signal peptide features determining the substrate specificities of targeting and translocation components in human ER protein import. Front. Physiol. 13, 833540 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.M. E. Losfeld, B. G. Ng, M. Kircher, K. J. Buckingham, E. H. Turner, A. Eroshkin, J. D. Smith, J. Shendure, D. A. Nickerson, M. J. Bamshad, H. H. Freeze, A new congenital disorder of glycosylation caused by a mutation in SSR4, the signal sequence receptor 4 protein of the TRAP complex. Hum. Mol. Genet. 23, 1602–1605 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.B. G. Ng, K. Raymond, M. Kircher, K. J. Buckingham, T. Wood, J. Shendure, D. A. Nickerson, M. J. Bamshad, J. T. Wong, F. P. Monteiro, B. H. Graham, S. Jackson, R. Sparkes, A. E. Scheuerle, S. Cathey, F. Kok, J. B. Gibson, H. H. Freeze, Expanding the molecular and clinical phenotype of SSR4-CDG. Hum. Mutat. 36, 1048–1051 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.C. Phoomak, W. Cui, T. J. Hayman, S.-H. Yu, P. Zhao, L. Wells, R. Steet, J. N. Contessa, The translocon-associated protein (TRAP) complex regulates quality control of N-linked glycosylation during ER stress. Sci. Adv. 7, eabc6364 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.K. Nagasawa, T. Higashi, N. Hosokawa, R. J. Kaufman, K. Nagata, Simultaneous induction of the four subunits of the TRAP complex by ER stress accelerates ER degradation. EMBO Rep. 8, 483–489 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.A. Russo, Understanding the mammalian TRAP complex function(s). Open Biol. 10, 190244 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.J. F. Menetret, R. S. Hegde, M. Aguiar, S. P. Gygi, E. Park, T. A. Rapoport, C. W. Akey, Single copies of Sec61 and TRAP associate with a nontranslating mammalian ribosome. Structure 16, 1126–1137 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.M. Greiner, B. Kreutzer, V. Jung, R. Grobholz, A. Hasenfus, R. F. Stöhr, L. Tornillo, J. Dudek, M. Stöckle, G. Unteregger, J. Kamradt, B. Wullich, R. Zimmermann, Silencing of the SEC62 gene inhibits migratory and invasive potential of various tumor cells. Int. J. Cancer 128, 2284–2295 (2011). [DOI] [PubMed] [Google Scholar]
- 24.M. Linxweiler, J. Linxweiler, M. Barth, J. Benedix, V. Jung, Y. J. Kim, R. M. Bohle, R. Zimmermann, M. Greiner, Sec62 bridges the gap from 3q amplification to molecular cell biology in non-small cell lung cancer. Am. J. Pathol. 180, 473–483 (2012). [DOI] [PubMed] [Google Scholar]
- 25.M. Linxweiler, B. Schick, R. Zimmermann, Let’s talk about Secs: Sec61, Sec62 and Sec63 in signal transduction, oncology and personalized medicine. Signal Transduct. Target. Ther. 2, 17002 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.F. Bochen, H. Adisurya, S. Wemmert, C. Lerner, M. Greiner, R. Zimmermann, A. Hasenfus, M. Wagner, S. Smola, T. Pfuhl, A. Bozzato, B. Al Kadah, B. Schick, M. Linxweiler, Effect of 3q oncogenes SEC62 and SOX2 on lymphatic metastasis and clinical outcome of head and neck squamous cell carcinomas. Oncotarget 8, 4922–4934 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.K. Vermeire, Y. Zhang, K. Princen, S. Hatse, M. F. Samala, K. Dey, H. J. Choi, Y. Ahn, A. Sodoma, R. Snoeck, G. Andrei, E. De Clercq, T. W. Bell, D. Schols, CADA inhibits human immunodeficiency virus and human herpesvirus 7 replication by down-modulation of the cellular CD4 receptor. Virology 302, 342–353 (2002). [DOI] [PubMed] [Google Scholar]
- 28.U. Hommel, H. P. Weber, L. Oberer, H. U. Naegeli, B. Oberhauser, C. A. Foster, The 3D-structure of a natural inhibitor of cell adhesion molecule expression. FEBS Lett. 379, 69–73 (1996). [DOI] [PubMed] [Google Scholar]
- 29.W. Klein, C. Rutz, J. Eckhard, B. Provinciael, E. Specker, M. Neuenschwander, G. Kleinau, P. Scheerer, J. P. von Kries, M. Nazare, K. Vermeire, R. Schulein, Use of a sequential high throughput screening assay to identify novel inhibitors of the eukaryotic SRP-Sec61 targeting/translocation pathway. PLOS One 13, e0208641 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.H. Luesch, S. K. Chanda, R. M. Raya, P. D. DeJesus, A. P. Orth, J. R. Walker, J. C. Izpisúa Belmonte, P. G. Schultz, A functional genomics approach to the mode of action of apratoxin A. Nat. Chem. Biol. 2, 158–167 (2006). [DOI] [PubMed] [Google Scholar]
- 31.S. Cao, A. Norris, J. H. Wisse, J. S. Miller, R. Evans, D. G. Kingston, Ipomoeassin F, a new cytotoxic macrocyclic glycoresin from the leaves of Ipomoea squamosa from the Suriname rainforest. Nat. Prod. Res. 21, 872–876 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.R. A. Medina, D. E. Goeger, P. Hills, S. L. Mooberry, N. Huang, L. I. Romero, E. Ortega-Barria, W. H. Gerwick, K. L. McPhail, Coibamide A, a potent antiproliferative cyclic depsipeptide from the Panamanian marine cyanobacterium Leptolyngbya sp. J. Am. Chem. Soc. 130, 6324–6325 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.C. Demangel, S. High, Sec61 blockade by mycolactone: A central mechanism in Buruli ulcer disease. Biol. Cell 110, 237–248 (2018). [DOI] [PubMed] [Google Scholar]
- 34.A. L. Mackinnon, V. O. Paavilainen, A. Sharma, R. S. Hegde, J. Taunton, An allosteric Sec61 inhibitor traps nascent transmembrane helices at the lateral gate. eLife 3, e01483 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.T. Junne, J. Wong, C. Studer, T. Aust, B. W. Bauer, M. Beibel, B. Bhullar, R. Bruccoleri, J. Eichenberger, D. Estoppey, N. Hartmann, B. Knapp, P. Krastel, N. Melin, E. J. Oakeley, L. Oberer, R. Riedl, G. Roma, S. Schuierer, F. Petersen, J. A. Tallarico, T. A. Rapoport, M. Spiess, D. Hoepfner, Decatransin, a new natural product inhibiting protein translocation at the Sec61/SecYEG translocon. J. Cell Sci. 128, 1217–1229 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.A. O. Paatero, J. Kellosalo, B. M. Dunyak, J. Almaliti, J. E. Gestwicki, W. H. Gerwick, J. Taunton, V. O. Paavilainen, Apratoxin kills cells by direct blockade of the Sec61 protein translocation channel. Cell Chem. Biol. 23, 561–566 (2016). [DOI] [PubMed] [Google Scholar]
- 37.D. Tranter, A. O. Paatero, S. Kawaguchi, S. Kazemi, J. D. Serrill, J. Kellosalo, W. K. Vogel, U. Richter, D. R. Mattos, X. Wan, C. C. Thornburg, S. Oishi, K. L. McPhail, J. E. Ishmael, V. O. Paavilainen, Coibamide A targets Sec61 to prevent biogenesis of secretory and membrane proteins. ACS Chem. Biol. 15, 2125–2136 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.L. Baron, A. O. Paatero, J. D. Morel, F. Impens, L. Guenin-Mace, S. Saint-Auret, N. Blanchard, R. Dillmann, F. Niang, S. Pellegrini, J. Taunton, V. O. Paavilainen, C. Demangel, Mycolactone subverts immunity by selectively blocking the Sec61 translocon. J. Exp. Med. 213, 2885–2896 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.G. Zong, Z. Hu, S. O’Keefe, D. Tranter, M. J. Iannotti, L. Baron, B. Hall, K. Corfield, A. O. Paatero, M. J. Henderson, P. Roboti, J. Zhou, X. Sun, M. Govindarajan, J. M. Rohde, N. Blanchard, R. Simmonds, J. Inglese, Y. Du, C. Demangel, S. High, V. O. Paavilainen, W. Q. Shi, Ipomoeassin F binds Sec61α to inhibit protein translocation. J. Am. Chem. Soc. 141, 8450–8461 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.S. F. Gerard, B. S. Hall, A. M. Zaki, K. A. Corfield, P. U. Mayerhofer, C. Costa, D. K. Whelligan, P. C. Biggin, R. E. Simmonds, M. K. Higgins, Structure of the inhibited state of the Sec translocon. Mol. Cell 79, 406–415 e407 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.S. Rehan, D. Tranter, P. P. Sharp, E. Lowe, J. L. Anderl, T. Muchamuel, V. Abrishami, S. Kuivanen, N. Wenzell, A. Jennings, C. Kalyanaraman, G. B. Craven, T. Strandin, M. Javanainen, O. Vapalahti, M. Jacobson, D. McMinn, C. J. Kirk, J. T. Huiskonen, J. Taunton, V. O. Paavilainen, Signal peptide mimicry primes Sec61 for client-selective inhibition. bioRxiv 2022.07.03.498529 [Preprint]. 5 July 2022. 10.1101/2022.07.03.498529. [DOI]
- 42.S. Itskanov, L. Wang, T. Junne, R. Sherriff, L. Xiao, N. Blanchard, W. Q. Shi, C. Forsyth, D. Hoepfner, M. Spiess, E. Park, A common mechanism of Sec61 translocon inhibition by small molecules. bioRxiv 2022.08.11.503542 [Preprint]. 11 August 2022. 10.1101/2022.08.11.503542. [DOI] [PMC free article] [PubMed]
- 43.J. L. Garrison, E. J. Kunkel, R. S. Hegde, J. Taunton, A substrate-specific inhibitor of protein translocation into the endoplasmic reticulum. Nature 436, 285–289 (2005). [DOI] [PubMed] [Google Scholar]
- 44.Y. Liu, B. K. Law, H. Luesch, Apratoxin a reversibly inhibits the secretory pathway by preventing cotranslational translocation. Mol. Pharmacol. 76, 91–104 (2009). [DOI] [PubMed] [Google Scholar]
- 45.B. S. Hall, K. Hill, M. McKenna, J. Ogbechi, S. High, A. E. Willis, R. E. Simmonds, The pathogenic mechanism of the Mycobacterium ulcerans virulence factor, mycolactone, depends on blockade of protein translocation into the ER. PLOS Pathog. 10, e1004061 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.K. Vermeire, T. W. Bell, V. Van Puyenbroeck, A. Giraut, S. Noppen, S. Liekens, D. Schols, E. Hartmann, K. U. Kalies, M. Marsh, Signal peptide-binding drug as a selective inhibitor of co-translational protein translocation. PLOS Biol. 12, e1002011 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.V. Van Puyenbroeck, E. Claeys, D. Schols, T. W. Bell, K. Vermeire, A proteomic survey indicates sortilin as a secondary substrate of the ER translocation inhibitor cyclotriazadisulfonamide (CADA). Mol. Cell. Proteomics 16, 157–167 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.E. Pauwels, C. Rutz, B. Provinciael, J. Stroobants, D. Schols, E. Hartmann, E. Krause, H. Stephanowitz, R. Schulein, K. Vermeire, A proteomic study on the membrane protein fraction of T cells confirms high substrate selectivity for the ER translocation inhibitor cyclotriazadisulfonamide. Mol. Cell. Proteomics 20, 100144 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.E. Claeys, E. Pauwels, S. Humblet-Baron, B. Provinciael, D. Schols, M. Waer, B. Sprangers, K. Vermeire, Small molecule cyclotriazadisulfonamide abrogates the upregulation of the human receptors CD4 and 4-1BB and suppresses in vitro activation and proliferation of T lymphocytes. Front. Immunol. 12, 650731 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.T. W. Bell, S. Anugu, P. Bailey, V. J. Catalano, K. Dey, M. G. Drew, N. H. Duffy, Q. Jin, M. F. Samala, A. Sodoma, W. H. Welch, D. Schols, K. Vermeire, Synthesis and structure-activity relationship studies of CD4 down-modulating cyclotriazadisulfonamide (CADA) analogues. J. Med. Chem. 49, 1291–1312 (2006). [DOI] [PubMed] [Google Scholar]
- 51.R. Chawla, V. Van Puyenbroeck, N. C. Pflug, A. Sama, R. Ali, D. Schols, K. Vermeire, T. W. Bell, Tuning side arm electronics in unsymmetrical cyclotriazadisulfonamide (CADA) endoplasmic reticulum (ER) translocation inhibitors to improve their human cluster of differentiation 4 (CD4) receptor down-modulating potencies. J. Med. Chem. 59, 2633–2647 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.A. Brown, M. R. Baird, M. C. J. Yip, J. Murray, S. Shao, Structures of translationally inactive mammalian ribosomes. eLife 7, e40486 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.J. Jumper, R. Evans, A. Pritzel, T. Green, M. Figurnov, O. Ronneberger, K. Tunyasuvunakool, R. Bates, A. Žídek, A. Potapenko, A. Bridgland, C. Meyer, S. A. A. Kohl, A. J. Ballard, A. Cowie, B. Romera-Paredes, S. Nikolov, R. Jain, J. Adler, T. Back, S. Petersen, D. Reiman, E. Clancy, M. Zielinski, M. Steinegger, M. Pacholska, T. Berghammer, S. Bodenstein, D. Silver, O. Vinyals, A. W. Senior, K. Kavukcuoglu, P. Kohli, D. Hassabis, Highly accurate protein structure prediction with AlphaFold. Nature 596, 583–589 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.X. Li, O. A. Itani, L. Haataja, K. J. Dumas, J. Yang, J. Cha, S. Flibotte, H. J. Shih, C. E. Delaney, J. Xu, L. Qi, P. Arvan, M. Liu, P. J. Hu, Requirement for translocon-associated protein (TRAP) α in insulin biogenesis. Sci Adv. 5, eaax0292 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.S. Karki, M. Javanainen, D. Tranter, S. Rehan, J. T. Huiskonen, L. Happonen, V. O. Paavilainen, Molecular view of ER membrane remodeling by the Sec61/TRAP translocon. bioRxiv 2022.09.30.510141 [Preprint]. 1 October 2022. 10.1101/2022.09.30.510141. [DOI]
- 56.M. Jaskolowski, A. Jomaa, M. Gamerdinger, S. Shrestha, M. Leibundgut, E. Deuerling, N. Ban, Molecular basis of the TRAP complex function in ER protein biogenesis. bioRxiv 2022.10.04.510795 [Preprint]. 4 October 2022. 10.1101/2022.10.04.510795. [DOI] [PMC free article] [PubMed]
- 57.J. Ogbechi, B. S. Hall, T. Sbarrato, J. Taunton, A. E. Willis, R. C. Wek, R. E. Simmonds, Inhibition of Sec61-dependent translocation by mycolactone uncouples the integrated stress response from ER stress, driving cytotoxicity via translational activation of ATF4. Cell Death Dis. 9, 397 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.M. Sicking, M. Jung, S. Lang, Lights, camera, interaction: Studying protein-protein interactions of the ER protein translocase in living cells. Int. J. Mol. Sci. 22, 10358 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.V. Van Puyenbroeck, E. Pauwels, B. Provinciael, T. W. Bell, D. Schols, K. U. Kalies, E. Hartmann, K. Vermeire, Preprotein signature for full susceptibility to the co-translational translocation inhibitor cyclotriazadisulfonamide. Traffic 21, 250–264 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.H. Li, Minimap2: Pairwise alignment for nucleotide sequences. Bioinformatics 34, 3094–3100 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.H. Li, B. Handsaker, A. Wysoker, T. Fennell, J. Ruan, N. Homer, G. Marth, G. Abecasis, R. Durbin, G. P. D. P. Subgroup, The sequence alignment/map format and SAMtools. Bioinformatics 25, 2078–2079 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.P. Walter, G. Blobel, Preparation of microsomal membranes for cotranslational protein translocation. Methods Enzymol. 96, 84–93 (1983). [DOI] [PubMed] [Google Scholar]
- 63.S. Q. Zheng, E. Palovcak, J. P. Armache, K. A. Verba, Y. Cheng, D. A. Agard, MotionCor2: Anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods 14, 331–332 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.J. Zivanov, T. Nakane, B. O. Forsberg, D. Kimanius, W. J. H. Hagen, E. Lindahl, S. H. W. Scheres, New tools for automated high-resolution cryo-EM structure determination in RELION-3. eLife 7, e42166 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.A. Rohou, N. Grigorieff, CTFFIND4: Fast and accurate defocus estimation from electron micrographs. J. Struct. Biol. 192, 216–221 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.D. Liebschner, P. V. Afonine, M. L. Baker, G. Bunkóczi, V. B. Chen, T. I. Croll, B. Hintze, L. W. Hung, S. Jain, A. J. McCoy, N. W. Moriarty, R. D. Oeffner, B. K. Poon, M. G. Prisant, R. J. Read, J. S. Richardson, D. C. Richardson, M. D. Sammito, O. V. Sobolev, D. H. Stockwell, T. C. Terwilliger, A. G. Urzhumtsev, L. L. Videau, C. J. Williams, P. D. Adams, Macromolecular structure determination using x-rays, neutrons and electrons: Recent developments in Phenix. Acta Crystallogr. D. Struct. Biol. 75, 861–877 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.O. Trott, A. J. Olson, AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 31, 455–461 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.E. F. Pettersen, T. D. Goddard, C. C. Huang, G. S. Couch, D. M. Greenblatt, E. C. Meng, T. E. Ferrin, UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004). [DOI] [PubMed] [Google Scholar]
- 69.T. D. Goddard, C. C. Huang, E. C. Meng, E. F. Pettersen, G. S. Couch, J. H. Morris, T. E. Ferrin, UCSF ChimeraX: Meeting modern challenges in visualization and analysis. Protein Sci. 27, 14–25 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Text
Legend for movie S1
Figs. S1 to S12
Tables S1 and S2
Movie S1