Abstract
Secretory pathway Ca2+/Mn2+ ATPase 1 (SPCA1) actively transports cytosolic Ca2+ and Mn2+ into the Golgi lumen, playing a crucial role in cellular calcium and manganese homeostasis. Detrimental mutations of the ATP2C1 gene encoding SPCA1 cause Hailey-Hailey disease. Here, using nanobody/megabody technologies, we determined cryo–electron microscopy structures of human SPCA1a in the ATP and Ca2+/Mn2+-bound (E1-ATP) state and the metal-free phosphorylated (E2P) state at 3.1- to 3.3-Å resolutions. The structures revealed that Ca2+ and Mn2+ share the same metal ion–binding pocket with similar but notably different coordination geometries in the transmembrane domain, corresponding to the second Ca2+-binding site in sarco/endoplasmic reticulum Ca2+-ATPase (SERCA). In the E1-ATP to E2P transition, SPCA1a undergoes similar domain rearrangements to those of SERCA. Meanwhile, SPCA1a shows larger conformational and positional flexibility of the second and sixth transmembrane helices, possibly explaining its wider metal ion specificity. These structural findings illuminate the unique mechanisms of SPCA1a-mediated Ca2+/Mn2+ transport.
Cryo-EM structures of SPCA1a present the mechanisms of its wide metal ion recognition and Ca2+/Mn2+ transport.
INTRODUCTION
Newly synthesized secretory proteins are translocated and folded in the endoplasmic reticulum (ER) with the assistance of various types of ER-resident molecular chaperones and modification enzymes. Correctly folded proteins are transported to the Golgi to undergo further posttranslational modification and quality control and then sorted at the trans-Golgi network (TGN) before transportation to their destination (1). In the early secretory pathway comprising the ER and Golgi, maintaining homeostasis of luminal and cytosolic Ca2+ lays the foundation for proper protein synthesis (2). Sarco(endo)plasmic Ca2+–adenosine triphosphatase (ATPase) (SERCA) and secretory pathway Ca2+/Mn2+-ATPase (SPCA) play essential roles in sustaining higher concentrations of luminal Ca2+ in the ER and Golgi apparatus (2, 3). High-resolution structures of SERCA have been determined for a number of intermediate states, leading to a deep understanding of the detailed mechanisms of its Ca2+ transport cycle (4, 5). However, the mechanism of Ca2+ homeostasis in the Golgi apparatus (3), which also likely acts as an intracellular calcium storage compartment, is less well understood than that in the ER. In this context, whereas several regulins with a single-spanning transmembrane (TM) helix, such as phospholamban and sarcolipin, are known to regulate the Ca2+-transporting activity of SERCA (6), these regulatory TM peptides have not been identified for SPCA1a.
In contrast to SERCA localized in the ER and cis-Golgi, SPCA is present in the trans-Golgi and TGN for Ca2+ uptake, generating a large Ca2+ gradient concentration between the ER (~500 μM) and Golgi (~100 μM) (3, 7). Loss of SPCA1 is known to cause Golgi stress and apoptosis (8). In addition, loss-of-function mutations in the ATP2C1 (i.e., SPCA1) gene can cause Hailey-Hailey disease (HHD), a skin disorder characterized by persistent blisters and erosion that is inherited in an autosomal dominant manner (9). Homozygous knockout of the ATP2C1 gene in mice proved to be embryonic lethal due to failed neural tube closure (10). However, the mechanism by which decreased SPCA1 protein abundance affects keratocytes and neuroepithelial cells remains elusive.
Previous biochemical studies demonstrated that SPCA1 is unique in that it transports both Mn2+ and Ca2+, with a higher affinity for the former than the latter (11). Mn2+ is a crucial cofactor for many enzymes, including glutamine synthetase (12) and pyruvate carboxylase (13). O-linked glycosylation, an essential event for most secretory proteins, also requires Mn2+ in the Golgi (14). However, excess intracellular Mn2+ is toxic (15). Hitherto, SPCA1a is the most clearly identified Golgi-resident Mn2+ pump (11, 16–19). Previous work demonstrated that excessive Mn2+ uptake prevents neuronal and glial survival by inhibiting the Ca2+ transport activity of SPCA1 and inducing Golgi fragmentation via Golgi stress (20). Mn2+ accumulation to toxic levels in the mouse brain likely correlates with high expression of SPCA1 (21), suggesting that SPCA1 serves as a vital Mn2+ transporter in the Golgi membrane that engages in Mn2+ detoxification by actively pumping cytosolic Mn2+ into the secretory pathway (17, 22).
Although the functions of SPCA1 and the effects of its deficiency have been studied (10, 11, 23–25), the overall mechanism of operation remains elusive due to a lack of structural information. It is widely accepted that P-type ATPases such as SERCA (4, 26, 27) follow a post-Albers cycle (28, 29) during which two distinct states (E1 and E2) are generated. Numerous structural, biochemical, biophysical, and computational studies established that SERCA functions according to the scheme E1 → E1-ATP → E1P-ADP → E2P → E2-Pi → E2 (30), although the exact steps of entry/binding of adenosine triphosphate (ATP) and release of adenosine diphosphate (ADP) and Ca2+ remain controversial (26, 31–34). The similar functions and high amino acid sequence identity between SERCA and SPCA (fig. S1) suggest similar mechanisms of operation in metal ion transport. To gain detailed insight into the ion transport mechanism of SPCA, we here determined cryo–electron microscopy (cryo-EM) structures of human SPCA1a in two intermediate states at 3.1- to 3.3-Å resolution: the E1-ATP state using β,γ-methyleneadenosine 5′-triphosphate (AMPPCP), a nonhydrolyzable analog of ATP, in the presence of Ca2+ or Mn2+, and the E2P state using BeF3−, a phosphate group mimic, in the absence of these metal ions.
As predicted, the present cryo-EM analyses revealed that the overall arrangement of the cytosolic and TM domains in SPCA1a resembles that in SERCA and that the location and amino acid residue geometry of the Ca2+/Mn2+-binding site in SPCA1a are nearly superimposable with those of the second Ca2+-binding site (site II) in SERCA2b. Despite these structural similarities, SPCA1a engages in fewer interactions between cytosolic domains, especially in the E1-Ca2+/Mn2+-ATP state, and undergoes similar rearrangements of both cytosolic and TM domains during the transition from E1-ATP to E2P states than occurs in SERCA. Moreover, the larger conformational and positional flexibility of TM2 and TM6 in SPCA1a compared with SERCA may help to explain the wider metal selectivity of SPCA1a. The present cryo-EM analyses provide important insights into the mechanisms of SPCA1a-mediated Ca2+/Mn2+ transport to the Golgi apparatus.
RESULTS
Cryo-EM structure analysis of human SPCA1a
Purified SPCA1a retained Ca2+- and Mn2+-dependent ATPase activity, exhibiting the release of the phosphate group as monitored by EnzCheck phosphate assay (Fig. 1A). SPCA1a showed much-enhanced ATPase activity with Co2+ and Ni2+, as well as with Ca2+ and Mn2+, compared to that without those divalent metal ions, suggesting the capacity of the Ca2+/Mn2+ pump to recognize other divalent metal ions as substrates. Although the physiological concentrations of intracellular labile Mn2+, Co2+, and Ni2+ remain elusive, these biochemical results suggest the wide metal specificity of SPCA1a.
Fig. 1. Cryo-EM maps and modeled structures of human SPCA1a in the E1-Ca2+/Mn2+-ATP and E2P states.
(A) ATPase activities of purified human SPCA1a in the presence of 10 μM Ca2+ (Ca), 10 μM Ca2+ + 446 nM megabody 14 (Ca + Mb14), 10 μM Mn2+ (Mn), 10 μM Co2+ (Co), or 10 μM Ni2+ (Ni) and in the absence of them (CTRL). All measurements were made in triplicate. (B) Domain organization of SPCA1a. SPCA1a contains three cytosolic domains, A-domain (orange), P-domain (blue), and N-domain (hot pink), and TM1–2, TM3–4, and TM5–10 helix clusters (green, violet, and wheat, respectively). (C to E) Cryo-EM density maps of SPCA1a in the E1-Ca2+-AMPPCP (C), E1-Mn2+-AMPPCP (D), and E2-BeF3− (E) states. (F to H) Ribbon diagrams of cryo-EM structures of SPCA1a in the E1-Ca2+-AMPPCP (F), E1-Mn2+-AMPPCP (G), and E2-BeF3− (H) states. The cytoplasmic domains (A-, P-, and N-domains), TM subdomains (TM1–2, TM3–4, and TM5–10), and the head domain of the megabody are colored as in (C) to (E). Ligands (AMPPCP, BeF3−, Ca2+, and Mn2+) bound to SPCA1a are highlighted in circles.
According to the PhosphoPlus database, SPCA1a is ubiquitylated at Lys8 (A-domain) and Lys496 (N-domain). Consistently, we verified the marked ubiquitylation of endogenous SPCA1a by Western blotting using an anti-ubiquitin antibody (fig. S2A). By contrast, overexpressed dodecapeptide from human podoplanin (PA)-tagged SPCA1a was ubiquitylated only partially, indicating a much lower ubiquitylation efficiency compared to the endogenous protein. As expected, ubiquitylated species were completely removed after treatment with Usp2cc enzyme (35) (fig. S2, C to E). The resulting homogeneously deubiquitinated SPCA1a sample was used for cryo-EM single-particle analysis (fig. S2, F and G).
We performed cryo-EM analyses of SPCA1a in the E1-Ca2+/Mn2+-AMPPCP and E2-BeF3− states as mimics of the E1-Ca2+/Mn2+-ATP and E2P states, respectively. During data analysis, we found that whereas the A-domain of SPCA1a was visible in some two-dimensional (2D)–averaged images of the E2P state (figs. S3 and S4A), it was barely visible in any 2D-averaged images of the E1-AMPPCP state (fig. S4B). Presumably due to the lack of density of the A-domain in the 2D-averaged maps, 3D reconstruction of the E1-Ca2+/Mn2+-AMPPCP state was unsuccessful. We thus predicted that an additional protein moiety might compensate for the apparent loss of the A-domain, facilitating 3D reconstruction. We first tested human cofilin-1 (hCFL-1) as a binder protein since it is known to bind the P-domain of SPCA1a (25). However, no interaction was observed between purified hCFL-1 and SPCA1a in our in vitro assay using size exclusion chromatography (SEC; fig. S5, A and B).
We then decided to screen a nanobody that binds specifically and tightly to SPCA1a using a yeast surface-displayed nanobody library (36). The ability of nanobodies to bind SPCA1a was first evaluated by flow cytometry (fig. S6, A and B). Nanobodies with a high fluorescein isothiocyanate (FITC)–positive percentage (≥10%) were chosen for sequencing and were found to share the same sequence. Clone No.14, named Nb14, was eventually selected and used in subsequent steps. The open reading frame of Nb14 was subcloned into the pET151b vector with an N-terminal pelB signal peptide to produce the nanobody in the periplasm of Escherichia coli (fig. S7, A to C). The binding performance of the recombinant nanobody was further assessed by SEC in the presence of Ca2+ or Mn2+ (fig. S8, A and B). The complex of Nb14 and purified human SPCA1a was subjected to cryo-EM single-particle analysis.
While the E1-AMPPCP state of SPCA1a in complex with Nb14 generated a cryo-EM map at an overall resolution of 3.4 Å, particles showing clear density for the A-domain only accounted for 8.45% of total particles (fig. S9, A and D). Therefore, we performed extra rounds of classification to exclude particles without the visible A-domain and reattempted 3D reconstruction (fig. S10A). Consequently, Nb14 was found to bind the N-domain of SPCA1a (fig. S10D). We surmised that the increased A-domain visibility in the E1-AMPPCP state is likely due to the bound Nb14 with a molecular mass of ~17 kDa; hence, we predicted that increasing the nanobody size would further facilitate 2D and 3D classification, eventually generating an even higher resolution map of SPCA1a. To this end, we converted Nb14 to a megabody (37) by fusing a scaffold protein to Nb14 while retaining the binding affinity for SPCA1a. c7HopQ was used as a scaffold protein to generate megabody 14 (Mb14) with a molecular weight of ~56 kDa (fig. S7, D to F). The SEC analysis confirmed its capability to bind SPCA1a (fig. S8, C to F). The biochemical analysis demonstrated that Mb14 binding abolished the Ca2+-dependent ATPase activity of SPCAC1a (Fig. 1A), suggesting that bound Mb14 may have blocked the conformation and/or dynamics of the A-domain during the catalytic cycle. Consistently, the usage of Mb14 greatly increased the proportion of SPCA1a particles with a clearly visible A-domain (from 8.45 to 48.95% or 53.87%; fig. S9, B to D) and improved the map resolution (from 3.4 to 3.1 Å; figs. S11 and S12). Therefore, cryo-EM maps of the SPCA1a-Mb14 complex were used for subsequent structure modeling and refinement. By conducting further 3D variability analysis using cryoSPARC (38), we identified domain rotations for each of the Ca2+- and Mn2+-bound E1-AMPPCP states, leading to the reconstruction of at least three subclass structures (figs. S11 and S12). In this work, subclass 2 of the Ca2+-bound form and subclass 1 of the Mn2+-bound form were chosen for structure description and comparison since they showed the clearest density maps in almost the entire region among all subclasses (figs. S11D and S12D).
Overall structure of SPCA1a
The present cryo-EM analysis revealed that, like SERCA, SPCA1a shares the typical architecture of P-type ATPases, with 10 TM helices (TM1 to TM10) and three cytosolic domains, including the actuator (A), nucleotide-binding (N), and phosphorylation (P) domains (Fig. 1, B to H). Mb14 was found to bind the N-domain in the E1-Ca2+/Mn2+-AMPPCP state (Fig. 1, C and D, light green). The TM domain of SPCA1a consists of three TM helix clusters (TM1–2, TM3–4, and TM5–10), similar to SERCA (Fig. 1F and fig. S13A).
Despite the similar arrangements of the cytosolic domains and TM helices, superimposition of the cryo-EM structures of SPCA1a and SERCA2b revealed different structural features, especially in the loop regions on both the cytosolic and luminal sides, and interactions between cytosolic domains. As predicted from amino acid sequence alignment (fig. S1), the nine cytosolic loops and four luminal loops of SPCA1a are shorter than those of SERCA2b due to the deletion of SERCA2b-specific residues (Fig. 2, A and D). In this connection, whereas most of the loops are exposed to the solvent without strong interactions with other domains in SPCA1a, loop 9 in the N-domain of SERCA2b is much longer than its counterpart in SPCA1a, and Leu577 in this loop appears to make hydrophobic contacts with Leu485 within the same domain of the E1-Ca2+-AMPPCP state of SERCA2b (27). In addition, this longer loop forms hydrogen bonds (H-bonds) with parts of the A-domain in the Ca2+-unbound E2 state of SERCA (fig. S14). We also found that loop 3 in the A-domain of SERCA2b contains an insertion of Thr171, and this residue forms H-bonds with Glu486 of the N-domain in the E1-Ca2+-AMPPCP state (Fig. 2A, inset). Thus, while the longer loops in the A- and N-domains of SERCA2b appear to contribute to stabilizing interdomain interactions within the cytosolic head piece, the interdomain interactions seem much weaker in SPCA1a due to the lack of the loops.
Fig. 2. Superimposition of cryo-EM structures of human SPCA1a and SERCA2b.
(A) Cryo-EM structures of SPCA1a (blue) and SERCA2b (salmon, PDB ID: 6LLE) in the E1-Ca2+-AMPPCP state were aligned such that the RMSD values of their P-domains are minimized. The longer loops of SERCA2b compared with those in SPCA1a are colored fluorescent green and numbered sequentially according to amino acid sequence. The circled inset shows interactions between Glu486 (N-domain) and Thr171 (A-domain). (B) H-bond network formed between cytosolic domains in the E1-2Ca2+-AMPPCP state of SERCA2b. (C) H-bond network formed between cytosolic domains in the E1-Ca2+-AMPPCP state of SPCA1a. (D) Cryo-EM structures of SPCA1a and SERCA2b (PDB ID: 6LLY) in the E2P state were aligned such that the RMSD values of their P-domains are minimized. (E) H-bond network formed between cytosolic domains in the E2-BeF3− state of SERCA2b. (F) H-bond network formed between cytosolic domains in the E2-BeF3− state of SPCA1a.
In line with this, while eight interdomain H-bonds are formed at the interfaces of the A-, N-, and P-domains in the E1-Ca2+-AMPPCP state of SERCA2b, only three are present in the corresponding state of SPCA1a (Fig. 2, B and C). The reduced interdomain interactions in SPCA1a appear consistent with the unstable nature of its A-domain in the E1-AMPPCP state. By contrast, the A-domain of SPCA1a was clearly identified in the E2-BeF3− state even without the nanobody/megabody (fig. S4A). Consistently, the number of interdomain H-bonds in SPCA1a is comparable to that in SERCA2b (Fig. 2, E and F). Thus, while the cytosolic domain arrangement is similar between SPCA1a and SERCA, the mode of interactions at the cytosolic domain interfaces is different between these two Ca2+ pumps, possibly explaining the unstable nature of the A-domain in the E1-AMPPCP state of SPCA1a.
Structural basis of ATP binding and phosphorylation in SPCA1a
The cryo-EM structures revealed that AMPPCP is located at the N-domain and P-domain boundary in SPCA1a (Figs. 1, F and G, and 3, A and B), like in SERCA1a (26) and SERCA2b (27). In the E1-Ca2+-AMPPCP state, the ATP-binding pocket consists of Thr352, Glu427, Ser456, Lys480, Gly571, Asp572, and Arg619 (Fig. 3A). An AMPPCP molecule in the Mn2+-bound state is located at almost the same position as in the Ca2+-bound state (Fig. 3B). To compare the location of AMPPCP in SPCA1a and SERCA, we focused on the geometry of residues surrounding AMPPCP bound to SPCA1a and SERCA2b (fig. S15). By measuring the distances between the center of the AMPPCP molecule and the Cα atoms of the surrounding residues (fig. S15, A and C), we found that the mode of ATP binding in SPCA1a is highly similar to that in SERCA2b (fig. S15, B and D).
Fig. 3. ATP- and metal ion–binding sites of human SPCA1a.
(A and B) ATP-binding site of SPCA1a in the E1-Ca2+-AMPPCP (A) and E1-Mn2+-AMPPCP (B) states. ATP-binding amino acid residues are shown in stick representation. Residues belonging to the A-, N-, and P-domains are colored orange, hot pink, and light blue, respectively. Electron density for the bound AMPPCP molecule is represented as mesh. (C) Phosphorylation site of SPCA1a in the E2-BeF3− state. Amino acid residues involved in interactions with the phosphoryl group are represented by sticks. Residues belonging to the A-, N-, and P-domains are colored orange, hot pink, and light blue, respectively. Cryo-EM density for AMPPCP and BeF3− is represented as mesh. (D and E) Metal ion–binding sites of SPCA1a in the E1-Ca2+-AMPPCP (D) and E1-Mn2+-AMPPCP (E) states. Amino acid residues located at the Ca2+- or Mn2+-binding pocket are shown in stick representation. (F) Comparison of the distances between the Oδ atom of Asp742 and Ca2+ (or Mn2+) in the E1-Ca2+-AMPPCP (light green) and E1-Mn2+-AMPPCP (pink) states. Structures are aligned for residues Val303-Ile306. Note that the Cα and Oδ atoms of Asp742 get closer to Mn2+ by 0.25 and 0.63 Å, respectively, compared to those in the Ca2+-bound state. (G to I) Top (i.e., cytosolic) view of the TM domain of SPCA1a in the E1-Ca2+-AMPPCP state (G), the E2P state (H), and SERCA2b in the E1-Ca2+-AMPPCP state (I). Amino acid residues involved in Ca2+ binding are represented by sticks. Ca2+ ions are shown as green spheres. Structures were aligned for the TM7–TM10 cluster in a conventional manner.
In the E2-BeF3− state (Fig. 1, E and H), no metal ion is bound to the TM domain. A BeF3− molecule is covalently bridged to Asp350 with the assistance of a nearby Mg2+ ion coordinated by Asp350 and Asp644 (Fig. 3C). Notably, the Thr189-Gly190-Glu191 (TGE) loop is located adjacent to Asp350 (Fig. 3C), as seen in other P-type ATPases in the E2P state (26, 39, 40) (also see below for a description of the movement of the TGE loop).
Structural basis of Ca2+/Mn2+ binding in SPCA1a
Well-resolved density was observed for the metal-binding pocket in the TM domain of SPCA1a in the E1-Ca2+/Mn2+-AMPPCP state, demonstrating that the Ca2+/Mn2+-binding site is primarily constituted by TM4 and TM6 (Fig. 3, F and G). In the Ca2+-bound state, the main-chain carbonyl groups of Val303, Ala304, and Ile306 in TM4 and the Oδ atoms of Asn738 and Asp742 in TM6 coordinate to the Ca2+ ion with distances of 2.36 to 2.44 Å, adopting a square pyramid geometry. In addition, the Oɛ atom of Glu308 in TM4 appears to weakly bind to the metal ion as a sixth ligand with a distance of 3.12 Å, forming an octahedral-like Ca2+ complex (Fig. 3D), as observed in one of the Ca2+-binding sites (site II) of SERCA1a (26) and SERCA2b (27) (Fig. 3I).
Notably, Mn2+ occupies the same binding site in SPCA1a as Ca2+ (Fig. 3, D and E), forming a similar coordination structure to the Ca2+-bound state. The Mn2+-bound state also has a square pyramid geometry, in which the main-chain carbonyl groups of Val303, Ala304, and Ile306 in TM4 and the side-chain Oδ atoms of Asn738 and Asp742 coordinate to Mn2+ with distances of 2.15 to 2.55 Å. However, the side chain of Glu308 is located more apart from Mn2+ compared to the Ca2+-bound state, not allowing an octahedral-like complex. Since the ion radius of Mn2+ (~80 pm) is smaller than that of Ca2+ (~100 pm), capturing Mn2+ requires TM4 and TM6 to move closer to each other. In support of this, the main-chain Cα and side-chain Oδ atoms of Asp742 in TM6 get closer to Mn2+ by 0.25 and 0.63 Å, respectively, than to Ca2+ (Fig. 3F), forming a more compact metal-binding complex. This closer geometry between Mn2+ and its surrounding residues likely explains the higher affinity for Mn2+ than for Ca2+, as revealed by a previous biochemical study using a liposomal SPCA1a (11). Unlike SERCA, SPCA1 lacks another metal-binding pocket (site I), likely due to amino acid substitutions in TM6 (e.g., T798M, E907D, E770A, and N767S). The lack of site I may provide TM6 with positional flexibility due to the increased vacant space and thereby allow it to more closely approach TM4 to bind Mn2+ at site II (Fig. 3, E to H).
In the metal unbound state (i.e. E2-BeF3− state), the metal-binding cavity gets wider as a result of the rotation and rearrangement of TM4 and TM6 (movie S1), which drives Met741 and Asp742 in TM6 away from the ion-binding site (Fig. 3, G and H, and fig. S13, B and C). This structural insight seems to nicely explain how Ca2+/Mn2+ are released from SPCA1a.
Conformational transition from E1-Ca2+ (Mn2+)-ATP to E2P states in SPCA1a
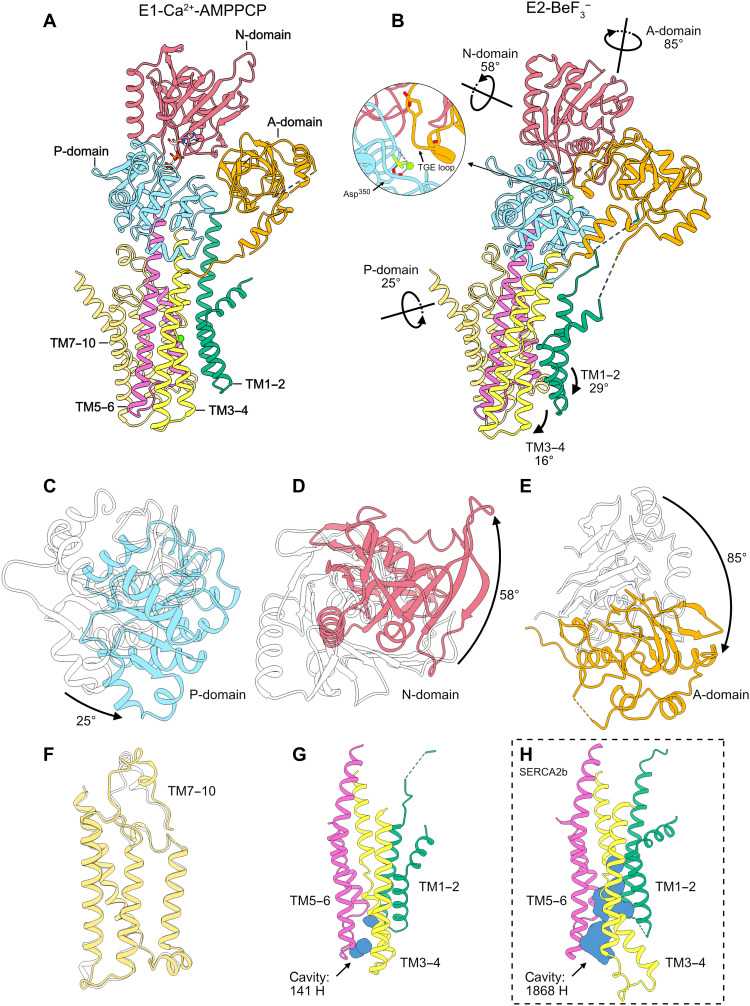
To gain further insight into the mechanisms of Ca2+/Mn2+ transport conducted by SPCA1a, we compared cryo-EM structures of its E1-AMPPCP and E2P states (Fig. 4, A to G). Given that, except for the metal-binding site, overall structures of SPCA1a in E1-Ca2+-AMPPCP and E1-Mn2+-AMPPCP states are almost identical to each other, with a root mean square deviation (RMSD) value of 0.983 Å, we used the structure of the E1-Ca2+-AMPPCP state exclusively for comparison with the E2P state. We found that during the transition from E1-AMPPCP to E2P states, the N-domain rotates away from the P-domain by 58° around the indicated axis (Fig. 4, B and D). In addition to N-domain rotation, the A-domain rotates by 85° around the indicated axis (Fig. 4, B and E), which drives the 189TGE loop closer to Asp350 to prevent the rebinding of ADP (Fig. 4B, inset), as seen in other P-type ATPases. Although SPCA1a undergoes a similar cytosolic domain rearrangement to SERCA, the luminal Ca2+ release gate constituted by TM3–4 and TM5–6 is much narrower in the E2P state of SPCA1a than in the corresponding state of SERCA2b (Fig. 4, G and H).
Fig. 4. Domain rearrangements of human SPCA1a during the transition from E1-ATP to E2P states.
(A and B) Relocation of cytosolic domains and TM helices during the transition from E1-Ca2+-AMPPCP (A) to E2P (B) states. Rotation angles and axes calculated in ChimeraX are represented as black arrows and lines, respectively. A-, N-, and P-domains and TM1–2, TM3–4, TM5–6, and TM7–10 clusters are colored orange, hot pink, light blue, green, yellow, violet, and dark yellow, respectively. (C to F) Close-up views of cytosolic domain rearrangement during the transition from E1-Ca2+-AMPPCP to E2P states, in which P-, N-, and A-domains and the TM7–10 cluster are highlighted in (C), (D), (E), and (F), respectively. Structures were aligned for the TM7–10 cluster in a conventional manner. Structures of SPCA1a in E1-Ca2+-AMPPCP and E2-BeF3− states are represented as transparent and colored models, respectively. (G and H) Cavities formed by TM1–2, TM3–4, and TM5–6 of SPCA1a (G) and SERCA2b (H) in the E2-BeF3− state. Cavities shown as blue surfaces were calculated with pyKVFinder (https://github.com/LBC-LNBio/pyKVFinder), and their sizes are represented by the number of hydrogen atoms that can be accommodated in the cavity.
To quantitatively compare the domain movements in SPCA1a and SERCA during the transition from the E1-AMPPCP to E2P states, we quantified the rotation angles of the main-chain Cα atoms in the cytosolic and TM domains and performed a statistical analysis of SERCA2b, SERCA1a, and SPCA1a (fig. S16). Since the rotational direction of each domain varies, the rotation axes were determined separately for A-, N-, and P-domains and for TM1–2, TM3–4, TM5–6, and TM7–10. Figure S17 shows a schematic representation of our rotation angle calculation. It is apparent that during the transition from the E1-AMPPCP to E2P states, the A- and N-domains undergo similar rotation to those in SERCAs, while the P-domain and TM5–6 of SPCA1a rotate by larger angles than in SERCAs. In contrast, the TM1–2 of SPCA1a rotate by a smaller angle than in SERCAs (fig. S16). These observations indicate that, overall, SPCA1a undergoes a similar domain rearrangement to SERCAs during the E1-ATP to E2P transition, while the TM1–2, TM5–6, and P-domain display different relocation behaviors between these two pumps.
Another striking difference between SPCA1a and SERCA2b was seen in TM2. TM2 of the E2P state of SPCA1a could only be modeled for residues Phe100 to Glu125, while the corresponding part of SERCA2b (Phe88 to Lys120) could be modeled entirely and was found to be longer than that in SPCA1a (fig. S18). In the E2P state, Tyr122-Glu125 of SPCA1a is twisted concomitant with the A-domain rotation (fig. S18A), while the corresponding helix of SERCA2b remains straight (fig. S18B). It is, however, notable that the TM2 twisting is seen in the E2·Pi state of SERCA2b, i.e., immediately after the dephosphorylation (fig. S19C) (41). Given that TM2 is directly linked to the A-domain, the observed twist around the cytosolic end of TM2 in SPCA1a suggests that TM2 is one of the key elements that modulate the metal ion transport by this Ca2+/Mn2+ ATPase (see also Discussion).
HHD mutations
Our present study also provides structural information for understanding mutations causing HHD. We mapped known missense mutations associated with HHD (23) on the cryo-EM structure of human SPCA1a in the E1-Ca2+-AMPPCP state (Fig. 5). Notably, the mutations are located throughout the entire SPCA1a protein chain. Most mutations occur around the metal-binding pocket (16 mutations) and in the P-domain (15 mutations), suggesting that these mutations affect the metal transport activity, efficiency of ATP hydrolysis, and/or phosphorylation of Asp350 of SPCA1a. In support of this, a previous study (42) reported that D742Y mutation (on TM6) at the metal-binding pocket eliminated the ability to bind both Ca2+ and Mn2+, and G309C mutation (on TM4) adjacent to the metal-binding pocket abolished Mn2+ binding, but the affinity for Ca2+ was retained, suggesting that Gly309 plays a role in Mn2+ selectivity. As an additional note, it is known that the symptoms of the HHD can develop at certain ages, mostly 19 to 65 years old (43), suggesting that patients carrying the mutations do not show obvious symptoms until the ages. In other words, the mutations are not always the sole reasons for the HHD, and other acquired environmental, accidental, or stochastic factors may be involved in this pathology. Nevertheless, the present cryo-EM structures provide molecular insight into the functional defects that can be caused by HHD mutations.
Fig. 5. HHD-associated mutations in SPCA1.
HHD-associated missense mutations (red spheres) are mapped onto the cryo-EM structure of SPCA1a in the E1-Ca2+-AMPPCP state.
DISCUSSION
In the present study, cryo-EM structures were determined at near-atomic resolutions for human SPCA1a, a Golgi-resident Ca2+/Mn2+ pump, in the E1-Ca2+/Mn2+-ATP and E2P states. The structures provided important insights into how Ca2+ and Mn2+ ions are specifically bound to the TM domain and transported to the Golgi lumen by SPCA1a in conjunction with marked rearrangements of the cytosolic and TM domains.
Unlike other Ca2+-transporting ATPases, such as SERCA and plasma membrane Ca2+ ATPase (PMCA), SPCA1a is ubiquitylated at Lys8 in the A-domain and Lys496 in the N-domain. However, the physiological significance of SPCA1a ubiquitylation is unknown. It should be noted that when SPCA1a was overexpressed, the proportion of ubiquitylated SPCA1a was much smaller than that of the nonubiquitylated form (fig. S2A). Monoubiquitylation plays a unique role in 26S proteasome degradation (44) and locking the conformation of the FANCD2-FANCI heterodimer (45). Therefore, monoubiquitylation is considered a versatile mechanism in protein quality control and conformational regulation. Together with the largely different ubiquitylation patterns observed for overexpressed and endogenous SPCA1a proteins (fig. S2A), and the mislocalization of overexpressed SPCA1a (fig. S2B), these results imply that native ubiquitylation of SPCA1a may play a role in specifying the cellular localization, binding partners, and hence physiological function of SPCA1. In this context, we failed to detect an interaction between SPCA1a and hCFL-1 in our in vitro experiments using purified proteins (fig. S5), although previous studies reported their functional interaction (25).
The Mn2+ transport activity of SPCA1a was first confirmed by observing the inhibitory effect of MnCl2 on Ca2+ transport activity (16, 42). The present cryo-EM structure of SPCA1a in the E1-Mn2+-AMPPCP state revealed the mechanism of Mn2+ binding. Previous studies on plasma membrane ATPase-related 1 (PMR1), a yeast homolog of SPCA1a, suggested the presence of a possible selective gate controlling Mn2+ entry, formed by Gln783 and Val335 (46, 47). Although these two residues are conserved in SPCA1a (Gln747 and Val314 in SPCA1a, respectively; fig. S1), Val314 has been found to be located far from Gln747 (fig. S20A). Instead, Gln747 appears to form a hydrophobic gate with Val313 and Val316 in the E2P state (fig. S20B), which may serve as a Mn2+ selection filter. To compare TM helix interactions between SERCA1a, SERCA2b, and SPCA1a, a quantitative analysis was performed by counting the number of H-bonds formed between different TM helices (Fig. 6A and fig. S20C). Whereas TM6 forms 10 and 11 H-bonds with neighboring TMs in SERCA1a and SERCA2b, respectively, only five interdomain H-bonds were found for TM6 of SPCA1a in the E1-Mn2+-AMPPCP state (Fig. 6, A to D), and similar observations were made for the E2P state (Fig. 6, A and E to G). Interdomain interactions involving TM6 are depicted in Fig. 6 (B to G). It is thus obvious that smaller numbers of H-bonds are formed between TM6 and its neighboring TM helices in SPCA1a, compared to those in SERCA1a and SERCA2b. These structural features suggest that TM6 of SPCA1a may be more mobile than in SERCAs, possibly explaining the capability to bind Mn2+ and Ca2+ (Fig. 3, D to G) and show the wide metal specificity (Fig. 1A). The Q747A gain-of-function mutation enhanced the SPCA1-mediated uptake of Mn2+ (17), while the corresponding Q783A mutation in yeast PMR1 blocked Mn2+ transport (46, 47), suggesting that the Mn2+ transport activity of SPCA1a is highly sensitive to the residues or local structure surrounding the metal-binding site.
Fig. 6. H-bond analysis based on cryo-EM structures of SPCA1a.
(A) Number of H-bonds formed between each TM helix and its neighboring regions in the E1-Ca2+/Mn2+-AMPPCP (blue) and E2-BeF3− (salmon) states of SERCA1a (s1a), SERCA2b (s2b), and SPCA1a (spca). (B to G) Close-up views of interdomain H-bonds involving TM6 in the E1-Mn2+-AMPPCP state of SPCA1a (B), the E1-2Ca2+-AMPPCP state of SERCA1a (C; PDB ID: 3AR2), the E1-2Ca2+-AMPPCP state of SERCA2 (D; PDB ID: 6LLE), the E2P state of SPCA1a (E), the E2P state of SERCA1a (F; PDB ID: 3B9B), and the E2P state of SERCA2b (G; PDB ID: 6LLY). Ca2+ and Mn2+ ions are represented as green and purple spheres, respectively. Residues of TM5, TM6, and TM9 are colored yellow, green, and magenta, respectively. TM helix numbers are indicated in parentheses: 5–6 in Asn730 (5–6) in (B) refers to the loop between TM5 and TM6. 9−10 in Phe957 (9−10) in (C) and Phe956 (9−10) in (D) refer to the loop between TM9 and TM10.
We compared the subclass structures of the E1-Ca2+-AMPPCP state determined by 3D variability analysis using cryoSPARC (fig. S21). Of note, three cytosolic domains move jointly in the same directions in the E1-Ca2+-AMPPCP state (fig. S21, A to D), mimicking the onset of the transition from E1-ATP to E2P. Similar behavior was also observed for SERCA2b T1032stop (27), a SERCA2b mutant lacking the luminal C-terminal tail (C-tail) and displaying the higher ATPase activity than the wild type (48). Thus, the removal of the C-tail resulted in heterogeneous conformational subclasses in both E1-2Ca2+-ATP and E2P states of SERCA2b. SPCA1a with 10 TM helices and no subsequent extension segments is unlikely to undergo conformational regulation observed with SERCA2b. In this context, the density of the A-domain was less clear in the Mn2+-bound state than in the Ca2+-bound one (figs. S11 and S12), suggesting the greater dynamics of the A-domain in the former state. Given that the location and position of the cytosolic domains are closely coupled to those of the TM helices in SPCA1a, as seen in other ATPases (4), the different dynamic properties of the A-domain in the Ca2+- and Mn2+-bound states may explain the different turnover rates in the Ca2+- and Mn2+-dependent ATPase cycles of SPCA1a (Fig. 1A).
In the transition from E1-ATP to E2P states, we found that TM2 of SPCA1a is partially twisted on the cytosolic side, while TM2 of SERCA2b remains straight (fig. S18). Detailed comparison of TM helix movements induced by the E1-ATP to E2P transition revealed that whereas TM3 of SERCA2b rotates with its cytosolic end anchored (fig. S22, A to C), the whole part of TM3 rotates away from the original position in SPCA1a (fig. S22, D to F). In addition, a loop of the P-domain approaches the twisted site of TM2 in the E2P state of SPCA1a, forming a close contact between Lys333 (P-domain) and Ser124 (twisted site of TM2; fig. S22E, inset). Although SERCA2b also contains a similar contact between Val333 (P-domain) and Gln108 (TM2; fig. S22B, inset), its TM2 remains straight in the E2P state.
This variable behavior of TM2 may suggest its potential regulatory role in the Ca2+/Mn2+ transport of SPCA1. TM2 twisting also appears to happen in SERCA1 in E2-derivative states (E2, E2·Pi, E2-ATP, E2ADP, etc.; fig. S22, G to P). A salt bridge is formed between Glu113 and Arg334 (fig. S22, G to P, inset), possibly compensating for the structural stability impaired by TM2 twisting in the E2-derivative states. According to the structure-based sequence alignment, Glu113 and Arg334 in SERCA1 correspond to Glu125 and Arg333 of SPCA1a, respectively, indicating that these two charged residues are conserved in SPCA1a (fig. S1).
We note that TM2 twisting happens in Na+/K+ ATPase and H+/K+ ATPase as in SPCA1a and SERCA1 (fig. S23, A and B), and that, in almost all cases, the twist is seen in the E2-derivative states. By contrast, TM2 twisting was not observed in ATPases with other functions than metal ion transport, such as ATP13A with polyamine transport activity and P4-ATPase with lipid translocation activity (fig. S23, C and D) (39, 49). Thus, the TM2 twisting seems likely to be an essential event of metal transporting P-type ATPases.
Previous molecular dynamics simulation works demonstrated that in SERCA, the kinked part of TM1 and the middle region of TM4 constitute a primary Ca2+ access point, where Asp59 (TM1) and Glu309 (TM4) likely serve as critical residues for Ca2+ attraction (50–52). The superimpositions of TM1, TM2, and TM4 between SERCA1a, SERCA2b, and SPCA1a in the E1-Ca2+-AMPPCP state demonstrate that the relative positions of TM1, TM2, and TM4 are almost identical among these three pumps and that Asp59 and Glu309 in SERCA correspond to Asn81 and Glu308 in SPCA1a (fig. S19A). In the E2P state, however, TM1 moves closer to TM4 in SPCA1a than in SERCA1a and SERCA2b (fig. S19B). In the meantime, the cytosolic side of TM2 gets twisted in SPCA1a, whereas the corresponding part of SERCA1a and SERCA2b remain intact (fig. S19B). Notably, the TM domain arrangement of SPCA1a in the E2P state is highly similar to that in the E2·Pi state of SERCA. Thus, the TM2 twisting seems to be closely coupled to the rearrangement of its neighboring TM1 and TM4, and thereby regulates the entry and release of Ca2+ in both SPCA1a and SERCA. In this context, the closure of the luminal Ca2+ release gate is seen in the E2P state of SPCA1a (Fig. 4G), whereas SERCA2b undergoes this gate closure in the subsequent E2·Pi state (53). These structural findings may suggest that SPCA1a releases the metal ions at earlier steps than SERCA. Future structural studies of SPCA1a in other intermediate states, in combination with computational and mutational analyses based on structural and phenotypic information, will thoroughly reveal the mechanisms of metal ion recognition and release by SPCA1a.
MATERIALS AND METHODS
Expression of SPCA1a
A PiggyBac Cumate Switch Inducible Vector harboring the coding region of human SPCA1 isoform 1a, with a PA-tag (GVAMPGAEDDVV) and a tobacco etch virus (TEV) cleavage site at the N terminus, was introduced into human embryonic kidney (HEK) 293T cells along with the Super PiggyBac Transposase Expression Vector (System Bioscience, LLC, CA, USA). The resultant cell line inducibly expressing SPCA1a was cultured in Dulbecco’s modified Eagle’s medium supplemented with 4% inactivated fetal calf serum and incubated in a humidified incubator with 5% CO2 at 37°C. After 2 days of incubation, expression of SPCA1a was induced with cumate (150 μg/ml) and 50 nM phorbol 12-myristate 13-acetate (PMA). Cells were incubated at 37°C for another 30 to 50 hours, harvested by centrifugation at 1000 to 2000g for 15 min, frozen in liquid N2, and stored at −80°C before purification.
Immunofluorescence
To analyze the localization of overexpressed SPCA1a in cells, the inducible HEK293T cells were plated onto poly-l-lysine–coated coverslips. Expression of SPCA1a was induced with cumate (150 μg/ml) and 50 nM PMA. Cells were fixed with phosphate-buffered saline (PBS) containing 4% paraformaldehyde, permeabilized with PBS containing 0.1% Triton X-100, and blocked with PBS containing 2% fetal calf serum in PBS for 45 min at room temperature. Cells were then incubated with antibodies against PA-tag [Fujifilm-Wako, Sendai, Miyagi, Japan; 1:5000 for immunofluorescence (IF)] and GM130 (PM061; MBL, Tokyo, Japan; 1:2000 for IF) overnight at 4°C. CF488-conjugated anti-rabbit immunoglobulin G (IgG) and CF568-conjugated anti-rat IgG antibodies (Biotium, San Francisco Bay Area, CA, USA; 1:2000 for IF) were used as secondary antibodies. Nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI) (Nacalai, Kyoto, Japan; 1:10,000 for IF). Fluorescent images were obtained using an FV1000 confocal microscope (Olympus, Tokyo, Japan) equipped with a UPLSAPO 60× silicon oil-immersion objective lens (numerical aperture, 1.30).
Immunoprecipitation
HEK293T cells from a 6-cm dish were harvested, washed twice with PBS, and resuspended in 1 ml of lysis buffer comprising 50 mM Hepes-NaOH (pH 7.0), 100 mM NaCl, 20% glycerol, 1 mM CaCl2, 1 mM MgCl2, 1% n-dodecyl-β-d-maltoside (DDM), and 1/100 Protease Inhibitor Cocktail (Nacalai). The solution was gently mixed by rotation at 4°C for 1 hour and then centrifuged at 17,000g for 10 min. Supernatant (800 μl) was collected and gently mixed with 1 μg of anti-ATP2C1 antibody (WH0027032M1; Sigma-Aldrich, Burlington, MA, USA) by rotation at 4°C for 1 hour. Forty-microliter slurry of protein G Sepharose, prewashed with Wash buffer comprising 50 mM Hepes-NaOH (pH 7.0), 100 mM NaCl, 20% glycerol, 1 mM CaCl2, 1 mM MgCl2, 0.02% glyco-diosgenin (GDN), and 1/100 Protease Inhibitor Cocktail (Nacalai), was added into the supernatant and gently mixed by rotation at 4°C for 1 hour. The protein G Sepharose was then washed five times with 500 μl of Wash buffer and terminated by adding 40 μl of SDS buffer for the subsequent SDS–polyacrylamide gel electrophoresis.
Purification of Usp2cc
The Usp2cc deubiquitination enzyme was purified according to a previous report (35). In brief, referring to the amino acid sequence of GenBank accession AAV27298.1, the DNA sequence of Usp2cc was optimized for expression in E. coli, ordered from Eurofins Genomics (Tokyo, Japan), and inserted into the pET15b vector by Gibson Assembly. After purification using an Ni-NTA affinity column (35), SEC was carried out on a Superdex 75 10/300 GL column (GE Healthcare, Chicago, IL, USA) at 4°C with buffer containing 50 mM MES (pH 7.4), 300 mM NaCl, 20% glycerol, and 1 mM dithiothreitol (DTT). Peak fractions were pooled, concentrated, and stored at −80°C before use.
Purification of SPCA1a
All purification steps were conducted at 4°C. To prepare Ca2+-bound SPCA1a, harvested cells were resuspended in Buffer A comprising 50 mM Hepes-NaOH (pH 7.0), 100 mM NaCl, 20% glycerol, 1 mM CaCl2, 1 mM MgCl2, 1 mM DTT, 1 mM phenylmethylsulfonyl fluoride (PMSF), and 1/100 Protease Inhibitor Cocktail (Nacalai) and were homogenized using a Dounce homogenizer. After homogenization, 1% (w/v) DDM was added to solubilize membranes. The solution was gently mixed by rotation at 4°C for 1.5 hours and then centrifuged at 22,300g for 1 hour to remove insoluble material. The supernatant was centrifuged again for another 15 min in a new tube to remove remaining impurities, and the supernatant was incubated for 3 hours with 5 ml of anti–PA-tag Antibody Beads (Fujifilm-Wako), preequilibrated with Buffer B [50 mM Hepes-NaOH (pH 7.0), 100 mM NaCl, 20% glycerol, 1 mM CaCl2, 1 mM MgCl2, and 1 mM DTT]. Beads were washed with 50 ml of Buffer B + 0.02% GDN (Anatrace, Maumee, OH, USA), followed by elution with 10 ml of Buffer B + 0.02% GDN and PA peptide (0.2 mg/ml), followed by 40 ml of Buffer B + 0.02% GDN. The elution fraction was concentrated using an Amicon Ultracel -50K centrifugal filter (Merck, Darmstadt, Germany). The concentrated SPCA1a was treated with 200 to 300 pmol of Usp2cc at 4°C for 1 hour to cleave off ubiquitin, and the ubiquitin-free SPCA1a was purified by SEC on a Superose 6 Increase 10/300 GL column (GE Healthcare) at 4°C preequilibrated with buffer containing 50 mM MES (pH 6.0), 100 mM NaCl, 1 mM CaCl2, 1 mM MgCl2, 1 mM DTT, and 0.02% GDN. The nanobody (or megabody) complex with SPCA1a was prepared by mixing SPCA1a with nanobody (or megabody) at a quantity ratio of 1:1.5 to 1:2 (a 1:1 molar ratio should also work), along with 200 to 300 pmol of Usp2cc at 4°C for 1 hour, and this was subjected to SEC purification. Peak fractions were pooled, concentrated to 2 to 6 mg/ml using an Amicon Ultracel -100K centrifugal filter (Merck), and kept on ice before use.
To prepare Mn2+-bound SPCA1a for cryo-EM analysis, the purification procedures were slightly modified as follows. Buffer A was replaced by Buffer A_Mn [50 mM Hepes-NaOH (pH 7.0), 100 mM NaCl, 20% glycerol, 2 mM EGTA, 1 mM MgCl2, 1 mM DTT, 1 mM PMSF, and 1/100 Protease Inhibitor Cocktail]. After solubilization with Buffer A_Mn + 1% DDM, 1 mM MnCl2 was added to the sample before centrifugation. During the purification with anti–PA-tag Antibody Beads (Fujifilm-Wako), Buffer B was replaced by Buffer B_Mn [Hepes-NaOH (pH 7.0), 100 mM NaCl, 20% glycerol, 1 mM MnCl2, 1 mM MgCl2, and 1 mM DTT]. At the last purification step by SEC, the running SEC buffer was replaced by the SEC_Mn buffer [50 mM MES (pH 6.0), 100 mM NaCl, 1 mM MnCl2, 1 mM MgCl2, 1 mM DTT, and 0.02% GDN].
Nanobody selection from the library
Nanobody selection was performed following the recommended protocol (36) with some modifications. In brief, 5 × 109, 3 × 107, 2 × 107, and 2 × 107 induced yeast cells were used for the first, second, third, and fourth rounds, respectively. The working volume was 5 ml for the first round and 1 ml for subsequent rounds. Before each round of magnetic-activated cell sorting (MACS) selection, a preclearing step was performed, in which induced yeast cells were washed and resuspended in buffer [50 mM MES (pH 6.0), 100 mM NaCl, 1 mM CaCl2, 1 mM MgCl2, 1 mM DTT, 0.02% GDN, and 20 μM AMPPCP], incubated with 200 nM anti–PA-FITC [Fujifilm-Wako, in-house FITC-labeled with the Pierce FITC Antibody Labeling Kit (Thermo Fisher Scientific, Waltham, MA, USA)] antibody and anti-FITC microbeads (Miltenyi, Bergisch Gladbach, North Rhine-Westphalia, Germany) at 4°C for 40 min, and then passed through an LD column to remove clones nonspecifically bound to beads and anti–PA-FITC antibody. The precleared yeast cells were then incubated with a preincubated complex of PA-SPCA1a and anti–PA-FITC at 4°C for 1 hour and passed through an LS column (Miltenyi) to collect binders. Successively lower concentrations of PA-SPCA1a from the second round (500, 500, 100, and 20 nM) were used for selection to enrich nanobodies with higher binding affinity. The collected yeast cells were then recovered and induced for subsequent selections. After four rounds of MACS selection, yeast cells were stained with anti–PA-FITC and sorted into 96-well plates by fluorescence-activated cell sorting (FACS) using a BD FACSMelody cell sorter (BD Biosciences, Franklin Lakes, NJ, USA). Clones were stained with anti–PA-FITC and analyzed by flow cytometry with an Accuri C6 flow cytometer (BD Biosciences) to screen for binders.
Expression and purification of nanobodies
Nanobody genes were isolated from yeast cells using the GenTLE (from Yeast) High Recovery Kit (Takara, Kyoto, Japan), sequenced, and cloned into the expression vector pET151 with a pelB signal peptide inserted at the N terminus, resulting in the pET151_pelB_Nb14 construct. The construct was transformed into E. coli BL21 (DE3), and cells were grown in Terrific Broth and induced with 1 mM isopropyl β-d-thiogalactopyranoside (IPTG) at 25°C for 15 hours when the absorbance at 660 nm (OD660) reached 0.6 to 0.8. After culturing, cells were washed with 1× PBS, pelleted, and resuspended in 100 ml of sucrose buffer [500 mM sucrose, 200 mM tris (pH 8), and 0.5 mM EDTA]. After stirring on ice for 15 min, 200 ml of chilled deionized water was added to release periplasmic nanobody by stirring for a further 45 min. The lysate was then mixed with 150 mM NaCl, 2 mM MgCl2, and 20 mM imidazole and centrifuged at 22,300g at 4°C for 20 min. The supernatant was mixed with 5 ml of Ni-NTA resin (Qiagen, Hilden, Düsseldorf, Germany), preequilibrated with normal buffer (150 mM NaCl and 2 mM MgCl2), and stirred on ice for 1 hour. The mixture was then applied to a column and washed twice with a high-salt buffer [20 mM Hepes (pH 7.5), 500 mM NaCl, and 20 mM imidazole] and then twice with NH7.5 buffer [20 mM Hepes (pH 7.5) and 100 mM NaCl] + 20 mM imidazole. Nanobodies were eluted with NH7.5 buffer + 400 mM imidazole and purified by SEC on a Superdex200 10/300 GL increase column (GE Healthcare).
Expression and purification of megabodies
The scaffold protein c7HopQ was used to generate a megabody, as previously reported (37). Briefly, the DNA sequence of c7HopQ was inserted into the pET151_pelB_Nb14 vector as described in the Supplementary Materials. After transforming the construct into E. coli BL21 (DE3), cells were grown in Terrific Broth and induced with 1 mM IPTG at 25°C for 20 hours after reaching OD660 ~ 1. Induced cells were washed with 1× PBS, pelleted, and resuspended in 100 ml of sucrose buffer [500 mM sucrose, 200 mM tris (pH 8), and 0.5 mM EDTA]. After stirring on ice for 20 min, 200 ml of chilled deionized water was added to release periplasmic megabody by stirring for a further 45 min. The lysate was mixed with 150 mM NaCl, 2 mM MgCl2, and 20 mM imidazole and centrifuged at 22,300g at 4°C for 20 min. The supernatant was mixed with 5 ml of Ni-NTA resin (Qiagen) preequilibrated with normal buffer (150 mM NaCl and 2 mM MgCl2) and stirred on ice for 1 hour. The mixture was applied to an open column and washed twice with a high-salt buffer [20 mM Hepes (pH 7.5), 500 mM NaCl, and 20 mM imidazole] and then twice with NH7.5 buffer [20 mM Hepes (pH 7.5) and 100 mM NaCl] + 20 mM imidazole. Megabodies were eluted with NH7.5 buffer + 400 mM imidazole and purified by SEC on a Superdex200 10/300 GL increase column (GE Healthcare).
ATPase activity assay
The SPCA1a protein used for the ATPase activity assay was purified essentially as described above. However, we used the buffer deprived of CaCl2 throughout the purification processes. The reaction mixture composed of 100 mM Hepes (pH 7.0), 100 mM NaCl, 0.02% GDN, 1 mM EGTA, 5 mM ATP, and appropriate combinations of MgCl2 and CaCl2 (0.000944 M CaCl2 and 0.011916 M MgCl2), MnCl2 (0.001037 M MnCl2 and 0.011907 M MgCl2), CoCl2 (0.001029 M CoCl2 and 0.0119148 M MgCl2), or NiSO4 (0.0010375 M NiSO4 and 0.0119073 M MgCl2) to fix the final Ca2+, Mn2+, Co2+, or Ni2+ concentration to 10 μM. After preincubation at 37°C for 10 min, the ATP hydrolysis reaction was initiated by adding 200 nM SPCA1a proteins. To generate free metal ions at 10 μM, a publicly available program WEBMAX EXTENDED (https://somapp.ucdmc.ucdavis.edu/pharmacology/bers/maxchelator/webmaxc/webmaxcE.htm) was used for calculation. The amount of inorganic phosphate released from the reactions was quantified using the EnzCheck Phosphate Assay Kit (Thermo Fisher Scientific). The absorbance at 360 nm (A360) was measured with a U-3900 spectrophotometer (Hitachi, Chiyoda, Tokyo, Japan). For the ATPase activity with 10 μM MnCl2, CoCl2, and NiCl2, 100 nM SPCA1a protein was used due to the highest limit of A360 of the spectrophotometer used.
Grid preparation for cryo-EM
Purified SPCA1a proteins were mixed with inhibitor solutions at final concentrations of 1 mM AMPPCP for the E1-Ca2+/Mn2+-AMPPCP form and 1 mM BeSO4, 5 mM NaF, and 5 mM EGTA for the E2-BeF3− form. After incubation for 1 hour on ice, protein solutions (3 μl) were applied to a freshly glow-discharged Quantifoil honey carbon grid (R 1.2/1.3, Cu, 300 mesh) using a Vitrobot Mark IV instrument (Thermo Fisher Scientific) at 4°C with a blotting time of 4 s under 100% humidity conditions. Grids were plunge-frozen in liquid ethane.
Cryo-EM data collection
For the E1-Ca2+-AMPPCP state of SPCA1a complexed with Nb14, data were collected using a Krios G4 microscope (Thermo Fisher Scientific) operated at 300 kV; 3789 movies were acquired using a Gatan K3 Summit direct electron detector with a Gatan Quantum-LS Energy Filter (Gatan, Pleasanton, California, USA) in electron counting mode. Movies were collected at a magnification of ×105,000 with a calibrated pixel size of 0.83 Å per pixel (University of Tokyo, Japan). The electron flux was set to an accumulated exposure of 49 e−/Å2 at the specimen. Data were automatically acquired by the image shift method using SerialEM software (54) with a defocus range from −1.8 to −0.84 μm.
For the E1-Ca2+-AMPPCP state of SPCA1a complexed with Mb14, data were collected using a CRYOARM 300 II microscope (JEOL, Akishima, Tokyo, Japan) operated at 300 kV; 5600 movies were acquired using a Gatan K3 Summit direct electron detector with an in-column Omega energy filter in electron counting mode. Movies were collected at a magnification of ×60,000 with a calibrated pixel size of 0.788 Å per pixel (Tohoku Medical Megabank Organization, Tohoku University, Japan). The electron flux was set to an accumulated exposure of 50 e−/Å2 at the specimen. Data were automatically acquired by the image shift method using SerialEM software, with a defocus range from −1.6 to −0.8 μm.
For the E1-Mn2+-AMPPCP state of SPCA1a complexed with Mb14, data were collected using a CRYOARM 300 II microscope (JEOL) operated at 300 kV; 6075 movies were acquired using a Gatan K3 Summit direct electron detector with an in-column Omega energy filter in electron counting mode. Movies were collected at a magnification of ×60,000 with a calibrated pixel size of 0.788 Å per pixel (Mega bank). The electron flux was set to an accumulated exposure of 50 e−/Å2 at the specimen. Data were automatically acquired by the image shift method using SerialEM software, with a defocus range from −1.6 to −0.8 μm.
For the E2-BeF3− form of SPCA1a, data were collected using a Titan Krios G3i microscope (Thermo Fisher Scientific) operated at 300 kV; 3501 movies were acquired using a Gatan K3 Summit direct electron detector with a Gatan Quantum-LS Energy Filter (GIF) in electron counting mode. Movies were collected at a magnification of ×105,000 with a calibrated pixel size of 0.83 Å per pixel (University of Tokyo, Japan). The electron flux was set to an accumulated exposure of 49 e−/Å2 at the specimen. Data were automatically acquired by the image shift method using SerialEM software, with a defocus range from −1.8 to −0.8 μm.
Cryo-EM data processing
Motion correction and Bayesian polishing were performed in RELION-3 (55), and other steps were performed in cryoSPARC (56). Dose-fractionated movies were subjected to beam-induced motion correction (implemented in RELION-3) and then imported into cryoSPARC.
For the E1-Ca2+-AMPPCP state of SPCA1a complexed with Nb14, contrast transfer function (CTF) estimation was performed through patch CTF estimation implemented in cryoSPARC. Particles were picked using a cryoSPARC Blob picker and extracted with downsampling to a pixel size of 3.32 Å per pixel. These particles were subjected to several rounds of 2D classification. The best particles were selected and subjected to cryoSPARC template picker, and again extracted for several rounds of 2D classification. Best particles were then subjected to ab initio reconstruction and heterogeneous refinement. Particles belonging to the best class were reextracted with a pixel size of 1.0375 Å per pixel and subjected to another three rounds of ab initio reconstruction and heterogeneous refinement, resulting in 142,884 particles and a map at a global resolution of 3.4 Å. These particles were exported to RELION-3 for Bayesian polishing and imported back into cryoSPARC for nonuniform refinement along with local defocus refinement and global CTF refinement, resulting in an improved resolution of 3.3 Å. The improved map was then subjected to local resolution estimation and filtered using a local filtering function.
For the E1-Ca2+-AMPPCP state of SPCA1a complexed with Mb14, CTF estimation was performed through patch CTF estimation implemented in cryoSPARC. Particles were picked using a cryoSPARC Blob picker and extracted with downsampling to a pixel size of 3.32 Å per pixel. These particles were subjected to several rounds of 2D classification. The best particles were selected, subjected to cryoSPARC template picker, and again extracted for several rounds of 2D classification. The best particles were then subjected to ab initio reconstruction and heterogeneous refinement. Particles belonging to the best class were reextracted with a pixel size of 0.985 Å per pixel and subjected to nonuniform refinement, resulting in 385,430 particles and a map at a global resolution of 3.1 Å. These particles were exported to RELION-3 for Bayesian polishing and imported back into cryoSPARC for nonuniform refinement and 3D variability analysis, divided into seven frames using the intermediate mode in 3D variability analysis. The first, fourth, and seventh frames resulting from 3D variability analysis were chosen as subclass 1, subclass 2, and subclass 3, respectively. The chosen particles were then subjected to nonuniform refinement along with local defocus refinement and global CTF refinement, resulting in resolutions of 3.1, 3.12, and 3.14, respectively. The improved maps were subjected to local resolution estimation and filtered using a local filtering function.
For the E1-Mn2+-AMPPCP state of SPCA1a complexed with Mb14, CTF estimation was performed through patch CTF estimation implemented in cryoSPARC. Particles were picked using a cryoSPARC Blob picker and extracted with downsampling to a pixel size of 3.32 Å per pixel. These particles were subjected to several rounds of 2D classification. The best particles were selected and subjected to cryoSPARC template picker and again extracted for several rounds of 2D classification. The best particles were then subjected to ab initio reconstruction and heterogeneous refinement. Particles belonging to the best class were reextracted with a pixel size of 0.985 Å per pixel and subjected to nonuniform refinement, resulting in 318,234 particles and a map at a global resolution of 3.20 Å. These particles were exported to RELION-3 for Bayesian polishing and imported back into cryoSPARC for nonuniform refinement and 3D variability analysis, divided into five frames by the intermediate mode in 3D variability analysis. The first, third, and fifth frames resulting from 3D variability analysis were chosen as subclass 1, subclass 2, and subclass 3, respectively. The chosen particles were then subjected to nonuniform refinement along with global CTF refinement, resulting in resolutions of 3.19, 3.11, and 3.25 Å, respectively. The improved maps were subjected to local resolution estimation and filtered using a local filtering function.
For the E2-BeF3− form, CTF estimation was performed with CTFFIND4 (57). Particles were picked using the Blob picker in cryoSPARC and extracted with downsampling to a pixel size of 4.4 Å per pixel. These particles were subjected to five rounds of 2D classification and one round of ab initio reconstruction. The best particles were selected, subjected to the template picker in cryoSPARC, and again extracted for five rounds of 2D classification and one round of ab initio reconstruction. The best particles were reextracted with a pixel size of 1.17 Å per pixel and subjected to local CTF refinement and nonuniform refinement (58), resulting in a map at a global resolution of 3.4 Å. These particles were exported to RELION-3 for Bayesian polishing using pyem scripts (59) and imported back into cryoSPARC for nonuniform refinement, resulting in an improved resolution of 3.3 Å.
Model building and validation
The SPCA1a structure predicted by AlphaFold (60) (UniProt: P98194) for the E2-BeF3− state, and a Swiss homology model for the E1-Ca2+/Mn2+-AMPPCP state were used as the initial atomic models. The models were fitted into the refined density maps using UCSF Chimera (61) and built using Coot (62). Because of the poor density, the following sequence regions were not modeled: N–19, 67–69, 205–210, and 905–919 of SPCA1a and 135–139 of the nanobody head piece in the E1-Ca2+-AMPPCP state subclass 2; N–22, 67–68, 205–210, and 905–919 of SPCA1a and 135–139 of the nanobody head piece in the E1-Mn2+-AMPPCP state subclass 1; N–16, 65–70, 128–134, 205–208, and 904–919 in the E2-BeF3− state. Model refinement was performed using phenix.real_space_refine (63) and validated with phenix.molprobity (64) in Phenix, as summarized in Table 1. Structure figures were prepared using UCSF ChimeraX (65).
Table 1. Cryo-EM data statistics.
| Nano-Ca2+-AMPPCP | Mega-Ca2+-AMPPCP | Mega-Mn2+-AMPPCP | BeF3− | |||||
|---|---|---|---|---|---|---|---|---|
| Subclass 1 | Subclass 2 | Subclass 3 | Subclass 1 | Subclass 2 | Subclass 3 | |||
| Data collection and processing | ||||||||
| Magnification | 105,000 | 60,000 | 105,000 | |||||
| Voltage (kV) | 300 | |||||||
| Microscope | Titan Krios G4 | CRYOARM 300 II | Titan Krios G3i | |||||
| Detector | K3 summit | |||||||
| Electron exposure (e−/Å) | 49 | 50 | 49 | |||||
| Defocus range (μm) | −1.8 to −0.84 | −1.6 to −0.8 | −1.8 to −0.8 | |||||
| Pixel size (Å) | 0.83 | 0.788 | 0.83 | |||||
| Symmetry imposed | C1 | |||||||
| Number of movies | 3789 | 5600 | 6075 | 3501 | ||||
| Initial particle images (no.) | 2,892,219 | 4,104,763 | 5,030,147 | 1,945,070 | ||||
| Final particle images (no.) | 142,884 | 91,849 | 305,692 | 102,741 | 151,371 | 298,318 | 161,692 | 260,172 |
| Map resolution (Å) | 3.36 | 3.1 | 3.12 | 3.14 | 3.19 | 3.11 | 3.25 | 3.3 |
| FSC threshold | 0.143 | |||||||
| Map resolution range (Å) | 2.84–30 | 2.71–30 | 2.13–30 | 2.71–30 | 2.72–30 | 2.66–30 | 2.7–30 | 2.80–30 |
| Map sharpening B factor (Å2) | −104.88 | −81.86 | −111.48 | −85.61 | −100 | −163.59 | −132.53 | −115.5 |
| Model building and refinement | ||||||||
| Model resolution (Å) | BLANK | 3.3 | 3.3 | 3.3 | 3.5 | BLANK | 3.3 | |
| FSC threshold | 0.5 | |||||||
| Model composition | ||||||||
| Atoms | BLANK | 15,470 (H: 7804) | 7666 | 7678 | 7649 | BLANK | 6672 | |
| Amino acid residues | 999 | 999 | 1001 | 997 | 871 | |||
| Ligands | CA:1 ACP:1 |
MN:1 ACP:1 |
MG:1 BEF:1 |
|||||
| Root mean square deviations | ||||||||
| Bond lengths (Å) | BLANK | 0.005 | 0.006 | 0.005 | 0.005 | BLANK | 0.003 | |
| Bond angles (°) | 1.115 | 1.075 | 1.029 | 0.912 | 0.529 | |||
| Validation | ||||||||
| Molprobity score | BLANK | 1.28 | 1.6 | 1.42 | 1.77 | BLANK | 1.33 | |
| Clashscore | 4.52 | 8.86 | 7.62 | 9.01 | 6.05 | |||
| Rotamer outliers (%) | 0.12 | 0.35 | 0.35 | 1.66 | 0.13 | |||
| EMRinger | 1.88 | 1.63 | 1.64 | 0.39 | 2.11 | |||
| Ramachandran plot | ||||||||
| Favored (%) | BLANK | 97.78 | 97.37 | 98.18 | 97.37 | BLANK | 98.38 | |
| Allowed (%) | 2.22 | 2.63 | 1.82 | 2.63 | 1.62 | |||
| Outliers (%) | 0 | 0 | 0 | 0 | 0 | |||
| CaBLAM outliers (%) | 1.23 | 1.33 | 1.43 | 1.02 | 1.29 | |||
Main-chain rotation analysis
SPCA1a (Mb14, Ca2+, subclass 2), SERCA1a [Protein Data Bank (PDB) ID: 3AR2], and SERCA2b (PDB ID: 6LLE) were used as structures in the E1-Ca2+-AMPPCP state. SPCA1a (E2-BeF3−), SERCA1a (PDB ID: 3B9B), and SERCA2b (PDB ID: 6LLY) were used as structures in the E2-BeF3− state. Domains were defined as A-, N-, and P-domains and TM1–2, TM3–4, TM5–6, and TM7–10. Structures were aligned over TM7–10 for calculating the main-chain rotation. The rotation axes and the center points of the axes were calculated in ChimeraX. 3D coordinates of the E1-Ca2+-AMPPCP models and E2P state models, and those of the center point of rotation axes, were merged and cleaned using in-house scripts. Angles were calculated between two vectors from the center point of the rotation axes to Cα atoms in E1-Ca2+-AMPPCP and E2-BeF3− states.
H-bond analysis
H-bonds were detected in UCSF ChimeraX (version 1.4). H-bonds formed between side-chain atoms and other atoms in different domains were counted, while H-bonds formed between side-chain (or main-chain) atoms and other atoms in the same domains were excluded using in-house scripts.
Other software and packages used in this study
Statistical analysis and figure preparation were performed in RStudio version 2021.9.0.351 (RStudio Inc.) with R version 4.1.0 installed. Various R packages were used, including tidyverse (1.3.1), ggplot2 (3.3.5), ggpubr (0.4.0), rstatix (0.7.0), and CytoExploreR (1.1.0). pyKVFinder (66) was used for cavity calculation. center_of_geometry() in the MDAnalysis package (www.mdanalysis.org/) was used for determining the center of AMPPCP (67, 68).
Acknowledgments
We thank Y. Sakamaki from the Kikkawa laboratory at the University of Tokyo and K. Kobayashi from the Nureki laboratory at the University of Tokyo for assistance in cryo-EM data collection. We are also grateful to T. Osaki of the Ikeuchi laboratory at the Institute of Industrial Science, University of Tokyo, for assistance with FACS. We thank T. Yokoyama, K. Nanatani, J. Inoue, S. Koshiba, and M. Yamamoto for management of the cryo-EM facility at Tohoku University medical megabank organization.
Funding: This work was supported by funding from AMED-CREST (21gm1410006h0001) to K.I., JSPS KAKENHI to K.I. (18H03978, 21H04758, and 21H05247), the Basis for Supporting Innovative Drug Discovery and Life Science Research (BINDS) from the Japan Agency for Medical Research and Development (AMED) under grant number JP19am0101115 (support number 1025), and the Pioneering Research Support Project for PhD Students from Tohoku University (grant number J210002135) to Z.C.
Author contributions: Z.C. designed and performed almost all experiments, image processing, structure modeling, and data analysis. H.H. and M.I. established the expression and purification system for recombinant human SPCA1a. Z.C. optimized the protocol. Y.D. assisted in yeast handling and flow cytometry. S.W. and M.K. assisted in cryo-EM data collection at the University of Tokyo and Tohoku University, as well as image processing, structure modeling, and structure refinement. K.I. supervised the project. Z.C. and K.I. prepared the manuscript. Z.C., S.W., and K.I. discussed the results and critically reviewed the manuscript.
Competing interests: The authors declare that they have no competing interests.
Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. The cryo-EM maps of the complex of SPCA1a and Mb14 in the E1-Ca2+/Mn2+-AMPPCP state and SPCA1a alone in the E2-BeF3− state have been deposited in the Electron Microscopy Data Bank under accession code EMD-33711 (Ca, subclass 1), EMD-33712 (Ca, subclass 2), EMD-33713 (Ca, subclass 3), EMD-33714 (Mn, subclass 1), and EMD-33717 (E2P). Atomic coordinates of the complex of SPCA1a and Mb14 in the E1-Ca2+/Mn2+-AMPPCP state and SPCA1a alone in the E2-BeF3− state have been deposited in the Protein Data Bank under accession codes 7YAG (Ca, subclass 1), 7YAH (Ca, subclass 2), 7YAI (Ca, subclass 3), 7YAJ (Mn, subclass 1), and 7YAM (E2P). Scripts used in data analysis have been deposited at Zenodo (https://doi.org/10.5281/zenodo.7475696) and Github (https://github.com/ZhenghaoChenAtGit/spca1a_2022).
Supplementary Materials
This PDF file includes:
Supplementary Text
Figs. S1 to S23
Other Supplementary Material for this : manuscript includes the following:
Movie S1
REFERENCES AND NOTES
- 1.G. Griffiths, K. Simons, The trans Golgi Network: Sorting at the exit site of the Golgi complex. Science 234, 438–443 (1986). [DOI] [PubMed] [Google Scholar]
- 2.J. Krebs, L. B. Agellon, M. Michalak, Ca2+ homeostasis and endoplasmic reticulum (ER) stress: An integrated view of calcium signaling. Biochem. Biophys. Res. Commun. 460, 114–121 (2015). [DOI] [PubMed] [Google Scholar]
- 3.P. Pizzo, V. Lissandron, P. Capitanio, T. Pozzan, Ca2+ signalling in the Golgi apparatus. Cell Calcium 50, 184–192 (2011). [DOI] [PubMed] [Google Scholar]
- 4.M. Dyla, M. Kjærgaard, H. Poulsen, P. Nissen, Structure and mechanism of P-type ATPase ion pumps. Annu. Rev. Biochem. 89, 583–603 (2020). [DOI] [PubMed] [Google Scholar]
- 5.R. Aguayo-Ortiz, L. M. Espinoza-Fonseca, Linking biochemical and structural states of SERCA: Achievements, challenges, and new Opportunities. Int. J. Mol. Sci. 21, 4146 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.N. Rathod, J. J. Bak, J. O. Primeau, M. E. Fisher, L. M. Espinoza-Fonseca, M. J. Lemieux, H. S. Young, Nothing regular about the regulins: Distinct functional properties of SERCA transmembrane peptide regulatory subunits. Int. J. Mol. Sci. 22, 8891 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.J. Suzuki, K. Kanemaru, M. Iino, Genetically encoded fluorescent indicators for organellar calcium imaging. Biophys. J. 111, 1119–1131 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.G. W. Okunade, M. L. Miller, M. Azhar, A. Andringa, L. P. Sanford, T. Doetschman, V. Prasad, G. E. Shull, Loss of the Atp2c1 secretory pathway Ca2+-ATPase (SPCA1) in mice causes Golgi stress, apoptosis, and midgestational death in homozygous embryos and squamous cell tumors in adult heterozygotes*. J. Biol. Chem. 282, 26517–26527 (2007). [DOI] [PubMed] [Google Scholar]
- 9.Z. Hu, J. M. Bonifas, J. Beech, G. Bench, T. Shigihara, H. Ogawa, S. Ikeda, T. Mauro, E. H. Epstein, Mutations in ATP2C1, encoding a calcium pump, cause Hailey-Hailey disease. Nat. Genet. 24, 61–65 (2000). [DOI] [PubMed] [Google Scholar]
- 10.J. M. Brown, M. J. García-García, The secretory pathway calcium ATPase 1 (SPCA1) controls neural tube closure by regulating cytoskeletal dynamics. Development 145, dev170019 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.J. Chen, S. Smaardijk, C.-A. Mattelaer, F. Pamula, I. Vandecaetsbeek, J. Vanoevelen, F. Wuytack, E. Lescrinier, J. Eggermont, P. Vangheluwe, An N-terminal Ca2+−binding motif regulates the secretory pathway Ca2+/Mn2+−transport ATPase SPCA1. J. Biol. Chem. 294, 7878–7891 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.F. C. Wedler, R. B. Denman, Glutamine synthetase: The major Mn(II) enzyme in mammalian brain. Curr. Top Cell Regul. 24, 153–169 (1984). [DOI] [PubMed] [Google Scholar]
- 13.S. Xiang, L. Tong, Crystal structures of human and Staphylococcus aureus pyruvate carboxylase and molecular insights into the carboxyltransfer reaction. Nat. Struct. Mol. Biol. 15, 295–302 (2008). [DOI] [PubMed] [Google Scholar]
- 14.R. J. Kaufman, M. Swaroop, P. Murtha-Riel, Depletion of manganese within the secretory pathway inhibits O-linked glycosylation in mammalian cells. Biochemistry 33, 9813–9819 (1994). [DOI] [PubMed] [Google Scholar]
- 15.C. W. Olanow, Manganese-induced Parkinsonism and Parkinson’s disease. Ann. N. Y. Acad. Sci. 1012, 209–223 (2004). [DOI] [PubMed] [Google Scholar]
- 16.V. K. Ton, D. Mandal, C. Vahadji, R. Rao, Functional expression in yeast of the human secretory pathway Ca2+, Mn2+-ATPase defective in Hailey-Hailey disease. J. Biol. Chem. 277, 6422–6427 (2002). [DOI] [PubMed] [Google Scholar]
- 17.S. Mukhopadhyay, A. D. Linstedt, Identification of a gain-of-function mutation in a Golgi P-type ATPase that enhances Mn2+ efflux and protects against toxicity. Proc. Natl. Acad. Sci. U.S.A. 108, 858–863 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.J. Stribny, L. Thines, A. Deschamps, P. Goffin, P. Morsomme, The human Golgi protein TMEM165 transports calcium and manganese in yeast and bacterial cells. J. Biol. Chem. 295, 3865–3874 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.A.-S. Roy, S. Miskinyte, A. Garat, A. Hovnanian, M.-A. Krzewinski-recchi, F. Foulquier, SPCA1 governs the stability of TMEM165 in Hailey-Hailey disease. Biochimie 174, 159–170 (2020). [DOI] [PubMed] [Google Scholar]
- 20.M. R. Sepúlveda, F. Wuytack, A. M. Mata, High levels of Mn2+ inhibit secretory pathway Ca2+/Mn2+-ATPase (SPCA) activity and cause Golgi fragmentation in neurons and glia. J. Neurochem. 123, 824–836 (2012). [DOI] [PubMed] [Google Scholar]
- 21.M. R. Sepúlveda, T. Dresselaers, P. Vangheluwe, W. Everaerts, U. Himmelreich, A. M. Mata, F. Wuytack, Evaluation of manganese uptake and toxicity in mouse brain during continuous MnCl2 administration using osmotic pumps. Contrast Media Mol. Imaging 7, 426–434 (2012). [DOI] [PubMed] [Google Scholar]
- 22.S. Leitch, M. Feng, S. Muend, L. T. Braiterman, A. L. Hubbard, R. Rao, Vesicular distribution of secretory pathway Ca2+-ATPase isoform 1 and a role in manganese detoxification in liver-derived polarized cells. Biometals 24, 159–170 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.M. Micaroni, G. Giacchetti, R. Plebani, G. G. Xiao, L. Federici, ATP2C1 gene mutations in Hailey–Hailey disease and possible roles of SPCA1 isoforms in membrane trafficking. Cell Death Dis. 7, –e2259 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.H.-H. H. Hoffmann, W. M. Schneider, V. A. Blomen, M. A. Scull, A. Hovnanian, T. R. Brummelkamp, C. M. Rice, Diverse viruses require the calcium transporter SPCA1 for maturation and spread. Cell Host Microbe 22, 460–470.e5 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.C. Kienzle, N. Basnet, A. H. Crevenna, G. Beck, B. Habermann, N. Mizuno, J. von Blume, Cofilin recruits F-actin to SPCA1 and promotes Ca2+−mediated secretory cargo sorting. J. Cell Biol. 206, 635–654 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.C. Toyoshima, M. Nakasako, H. Nomura, H. Ogawa, Crystal structure of the calcium pump of sarcoplasmic reticulum at 2.6 Å resolution. Nature 405, 647–655 (2000). [DOI] [PubMed] [Google Scholar]
- 27.Y. Zhang, M. Inoue, A. Tsutsumi, S. Watanabe, T. Nishizawa, K. Nagata, M. Kikkawa, K. Inaba, Cryo-EM structures of SERCA2b reveal the mechanism of regulation by the luminal extension tail. Sci. Adv. 6, eabb0147 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.R. L. Post, C. Hegyvary, S. Kume, Activation by adenosine triphosphate in the phosphorylation kinetics of sodium and potassium ion transport adenosine triphosphatase. J. Biol. Chem. 247, 6530–6540 (1972). [PubMed] [Google Scholar]
- 29.R. W. Albers, Biochemical aspects of active transport. Annu. Rev. Biochem. 36, 727–756 (1967). [DOI] [PubMed] [Google Scholar]
- 30.M. G. Palmgren, P. Nissen, P-type ATPases. Annu. Rev. Biophys. 40, 243–266 (2011). [DOI] [PubMed] [Google Scholar]
- 31.Y. Kabashima, H. Ogawa, R. Nakajima, C. Toyoshima, What ATP binding does to the Ca2+ pump and how nonproductive phosphoryl transfer is prevented in the absence of Ca2. Proc. Natl. Acad. Sci. U.S.A. 117, 18448–18458 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Y. Zhang, S. Watanabe, A. Tsutsumi, H. Kadokura, M. Kikkawa, K. Inaba, Cryo-EM analysis provides new mechanistic insight into ATP binding to Ca2+-ATPase SERCA2b. EMBO J. 40, e108482 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.M. Dyla, D. S. Terry, M. Kjaergaard, T. L.-M. Sørensen, J. Lauwring Andersen, J. P. Andersen, C. Rohde Knudsen, R. B. Altman, P. Nissen, S. C. Blanchard, Dynamics of P-type ATPase transport revealed by single-molecule FRET. Nature 551, 346–351 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Y. Zhang, K. Inaba, Structural basis of the conformational and functional regulation of human SERCA2b, the ubiquitous endoplasmic reticulum calcium pump. Bioessays 44, e2200052 (2022). [DOI] [PubMed] [Google Scholar]
- 35.A.-M. Catanzariti, T. A. Soboleva, D. A. Jans, P. G. Board, R. T. Baker, An efficient system for high-level expression and easy purification of authentic recombinant proteins. Protein Sci. 13, 1331–1339 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.C. McMahon, A. S. Baier, R. Pascolutti, M. Wegrecki, S. Zheng, J. X. Ong, S. C. Erlandson, D. Hilger, S. G. F. Rasmussen, A. M. Ring, A. Manglik, A. C. Kruse, Yeast surface display platform for rapid discovery of conformationally selective nanobodies. Nat. Struct. Mol. Biol. 25, 289–296 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.T. Uchański, S. Masiulis, B. Fischer, V. Kalichuk, U. López-Sánchez, E. Zarkadas, M. Weckener, A. Sente, P. Ward, A. Wohlkönig, T. Zögg, H. Remaut, J. H. Naismith, H. Nury, W. Vranken, A. R. Aricescu, E. Pardon, J. Steyaert, Megabodies expand the nanobody toolkit for protein structure determination by single-particle cryo-EM. Nat. Methods 18, 60–68 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.A. Punjani, D. J. Fleet, 3D variability analysis: Resolving continuous flexibility and discrete heterogeneity from single particle cryo-EM. J. Struct. Biol. 213, 107702 (2021). [DOI] [PubMed] [Google Scholar]
- 39.S. I. Sim, S. von Bülow, G. Hummer, E. Park, Structural basis of polyamine transport by human ATP13A2 (PARK9). Mol. Cell 81, 4635–4649.e8 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.R. M. Bitter, S. Oh, Z. Deng, S. Rahman, R. K. Hite, P. Yuan, Structure of the Wilson disease copper transporter ATP7B. Sci. Adv. 8, eabl5508 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Y. Zhang, C. Kobayashi, X. Cai, S. Watanabe, A. Tsutsumi, M. Kikkawa, Y. Sugita, K. Inaba, Multiple sub-state structures of SERCA2b reveal conformational overlap at transition steps during the catalytic cycle. Cell Rep. 41, 111760 (2022). [DOI] [PubMed] [Google Scholar]
- 42.R. J. Fairclough, L. Dode, J. Vanoevelen, J. P. Andersen, L. Missiaen, L. Raeymaekers, F. Wuytack, A. Hovnanian, Effect of Hailey-Hailey disease mutations on the function of a new variant of human secretory pathway Ca2+/Mn2+-ATPase (hSPCA1). J. Biol. Chem. 278, 24721–24730 (2003). [DOI] [PubMed] [Google Scholar]
- 43.Genetic and Rare Diseases Information Center, Hailey-Hailey disease (2021);https://rarediseases.info.nih.gov/diseases/6559/hailey-hailey-disease.
- 44.O. Braten, I. Livneh, T. Ziv, A. Admon, I. Kehat, L. H. Caspi, H. Gonen, B. Bercovich, A. Godzik, S. Jahandideh, L. Jaroszewski, T. Sommer, Y. T. Kwon, M. Guharoy, P. Tompa, A. Ciechanover, Numerous proteins with unique characteristics are degraded by the 26S proteasome following monoubiquitination. Proc. Natl. Acad. Sci. U.S.A. 113, E4639–E4647 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.P. Alcón, S. Shakeel, Z. A. Chen, J. Rappsilber, K. J. Patel, L. A. Passmore, FANCD2–FANCI is a clamp stabilized on DNA by monoubiquitination of FANCD2 during DNA repair. Nat. Struct. Mol. Biol. 27, 240–248 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.D. Mandal, T. B. Woolf, R. Rao, Manganese selectivity of pmr1, the yeast secretory pathway ion pump, is defined by residue gln783 in transmembrane segment 6: Residue Asp778 is essential for cation transport. J. Biol. Chem. 275, 23933–23938 (2000). [DOI] [PubMed] [Google Scholar]
- 47.D. Mandal, S. J. Rulli, R. Rao, Packing interactions between transmembrane helices alter ion selectivity of the yeast Golgi Ca2+/Mn2+-ATPase PMR1. J. Biol. Chem. 278, 35292–35298 (2003). [DOI] [PubMed] [Google Scholar]
- 48.M. Inoue, N. Sakuta, S. Watanabe, Y. Zhang, K. Yoshikaie, Y. Tanaka, R. Ushioda, Y. Kato, J. Takagi, T. Tsukazaki, K. Nagata, K. Inaba, Structural basis of sarco/endoplasmic reticulum Ca2+-ATPase 2b regulation via transmembrane helix interplay. Cell Rep. 27, 1221–1230.e3 (2019). [DOI] [PubMed] [Google Scholar]
- 49.J. Tillinghast, S. Drury, D. Bowser, A. Benn, K. P. K. Lee, Structural mechanisms for gating and ion selectivity of the human polyamine transporter ATP13A2. Mol. Cell 81, 4650–4662.e4 (2021). [DOI] [PubMed] [Google Scholar]
- 50.A. Das, H. Rui, R. Nakamoto, B. Roux, Conformational transitions and alternating-access mechanism in the sarcoplasmic reticulum calcium pump. J. Mol. Biol. 429, 647–666 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.C. Toyoshima, Structural aspects of ion pumping by Ca2+-ATPase of sarcoplasmic reticulum. Arch. Biochem. Biophys. 476, 3–11 (2008). [DOI] [PubMed] [Google Scholar]
- 52.M. Musgaard, L. Thøgersen, B. Schiøtt, E. Tajkhorshid, Tracing cytoplasmic Ca2+ ion and water access points in the Ca2+-ATPase. Biophys. J. 102, 268–277 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.A. Zhang, H. Yu, C. Liu, C. Song, The Ca2+ permeation mechanism of the ryanodine receptor revealed by a multi-site ion model. Nat. Commun. 11, 922 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.D. N. Mastronarde, SerialEM: A program for automated tilt series acquisition on Tecnai microscopes using prediction of specimen position. Microsc. Microanal. 9, 1182–1183 (2003). [Google Scholar]
- 55.J. Zivanov, T. Nakane, B. O. Forsberg, D. Kimanius, W. J. Hagen, E. Lindahl, S. H. Scheres, New tools for automated high-resolution cryo-EM structure determination in RELION-3. eLife 7, e42166 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.A. Punjani, J. L. Rubinstein, D. J. Fleet, M. A. Brubaker, cryoSPARC: Algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods 14, 290–296 (2017). [DOI] [PubMed] [Google Scholar]
- 57.A. Rohou, N. Grigorieff, CTFFIND4: Fast and accurate defocus estimation from electron micrographs. J. Struct. Biol. 192, 216–221 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.A. Punjani, H. Zhang, D. J. Fleet, Non-uniform refinement: Adaptive regularization improves single-particle cryo-EM reconstruction. Nat. Methods 17, 1214–1221 (2020). [DOI] [PubMed] [Google Scholar]
- 59.D. Asarnow, E. Palovcak, Y. Cheng, UCSF pyem v0.5. (2019); doi: 10.5281/zenodo.3576630. [DOI]
- 60.J. Jumper, R. Evans, A. Pritzel, T. Green, M. Figurnov, O. Ronneberger, K. Tunyasuvunakool, R. Bates, A. Žídek, A. Potapenko, A. Bridgland, C. Meyer, S. A. A. Kohl, A. J. Ballard, A. Cowie, B. Romera-Paredes, S. Nikolov, R. Jain, J. Adler, T. Back, S. Petersen, D. Reiman, E. Clancy, M. Zielinski, M. Steinegger, M. Pacholska, T. Berghammer, S. Bodenstein, D. Silver, O. Vinyals, A. W. Senior, K. Kavukcuoglu, P. Kohli, D. Hassabis, Highly accurate protein structure prediction with AlphaFold. Nature 596, 583–589 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.E. F. Pettersen, T. D. Goddard, C. C. Huang, G. S. Couch, D. M. Greenblatt, E. C. Meng, T. E. Ferrin, UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004). [DOI] [PubMed] [Google Scholar]
- 62.P. Emsley, B. Lohkamp, W. G. Scott, K. Cowtan, Features and development of Coot. Acta Crystallogr. D. Biol. Crystallogr. 66, 486–501 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.P. V. Afonine, B. K. Poon, R. J. Read, O. V. Sobolev, T. C. Terwilliger, A. Urzhumtsev, P. D. Adams, Real-space refinement in PHENIX for cryo-EM and crystallography. Acta Crystallogr. D. Struct. Biol. 74, 531–544 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.C. J. Williams, J. J. Headd, N. W. Moriarty, M. G. Prisant, L. L. Videau, L. N. Deis, V. Verma, D. A. Keedy, B. J. Hintze, V. B. Chen, S. Jain, S. M. Lewis, W. B. Arendall III, J. Snoeyink, P. D. Adams, S. C. Lovell, J. S. Richardson, D. C. Richardson, MolProbity: More and better reference data for improved all-atom structure validation. Protein Sci. 27, 293–315 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.T. D. Goddard, C. C. Huang, E. C. Meng, E. F. Pettersen, G. S. Couch, J. H. Morris, T. E. Ferrin, U. C. S. F. ChimeraX, UCSF ChimeraX: Meeting modern challenges in visualization and analysis. Protein Sci. 27, 14–25 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.J. V. . d. S. Guerra, H. V. Ribeiro-Filho, G. E. Jara, L. O. Bortot, J. G. d. C. Pereira, P. S. Lopes-de-Oliveira, pyKVFinder: An efficient and integrable Python package for biomolecular cavity detection and characterization in data science. BMC Bioinformatics 22, 607 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.N. Michaud-Agrawal, E. J. Denning, T. B. Woolf, O. Beckstein, MDAnalysis: A toolkit for the analysis of molecular dynamics simulations. J. Comput. Chem. 32, 2319–2327 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.R. J. Gowers, M. Linke, J. Barnoud, T. J. E. Reddy, M. N. Melo, S. L. Seyler, J. Domański, D. L. Dotson, S. Buchoux, I. M. Kenney, O. Beckstein, MDAnalysis: A Python package for the rapid analysis of molecular dynamics simulations. Proc. 15th Python Sci. Conf., 98–105 (2016). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Text
Figs. S1 to S23
Movie S1