Abstract
Prostatic zinc content is a known biomarker for discriminating normal healthy tissue from benign prostatic hyperplasia (BPH) and prostate cancer (PCa). Given that zinc content is not readily measured without a tissue biopsy, we have been exploring noninvasive imaging methods to detect these diagnostic differences using a zinc-responsive MRI contrast agent. During imaging studies in mice, we observed that a bolus of glucose stimulates secretion of zinc from the prostate of fasted mice. This discovery allowed the use of a Gd-based zinc sensor to detect differential zinc secretion in regions of healthy versus malignant prostate tissue in a transgenic adenocarcinoma mouse model of PCa. Here, we used a zinc-responsive MRI agent to detect zinc release across the prostate during development of malignancy and confirm the loss of total tissue zinc by synchrotron radiation X-ray fluorescence (μSR-XRF). Quantitative μSR-XRF results show that the lateral lobe of the mouse prostate uniquely accumulates high concentrations of zinc, 1.06 ± 0.08 mM, and that the known loss of zinc content in the prostate is only observed in the lateral lobe during development of PCa. Additionally, we confirm that lesions identified by a loss of zinc secretion indeed represent malignant neoplasia and that the relative zinc concentration in the lesion is reduced to 0.370 ± 0.001 mM. The μSR-XRF data also provided insights into the mechanism of zinc secretion by showing that glucose promotes movement of zinc pools (~1 mM) from the glandular lumen of the lateral lobe of the mouse prostate into the stromal/smooth muscle surrounding the glands. Co-localization of zinc and gadolinium in the stromal/smooth muscle areas as detected by μSR-XRF confirm that glucose initiates secretion of zinc from intracellular compartments into the extracellular spaces of the gland where it binds to the Gd-based agent and albumin promoting MR image enhancement.
Graphical Abstract

INTRODUCTION
Metals play a crucial role in many cellular processes and are heterogeneously distributed within the cell and organs.1 In mitochondria, for example, iron–sulfur clusters act as redox catalysts in the electron transport chain and as catalytic sites in TCA cycle enzymes.2 Cellular respiration, free radical detoxification, and cross-linking of collagen and elastin are regulated by copper-containing enzymes, and both iron and copper play critical roles in homeostasis of reactive oxygen species (ROS).3 In addition to its well-known structural role in zinc finger biochemistry, divalent zinc also serves as a regulatory messenger ion within cells and between cells4 and is stored and released along with hormones, enzymes, or metabolites released by secretory cells.5 Tissues such as the endocrine and exocrine pancreas, mammary glands, brain, and prostate are known to contain high levels of zinc, and zinc dysregulation is tightly linked to altered pathological states.6–13 Many different types of zinc-targeted MRI and optical sensors have been developed14–19 in an effort to monitor zinc dysregulation, aid in disease diagnosis, or identify therapeutic targets;20 however, few have been proven to work in vivo.
One of the most widely studied zinc-containing tissues is the pancreas. In the endocrine pancreas, zinc is packaged with insulin in β-cell granules, which then exocytose into the extracellular space surrounding β-cells in response to an increase in plasma glucose.5 In an effort to image insulin secretion from the intact pancreas in vivo, we designed a Gd-based zinc-responsive agent that binds with serum albumin only in the presence of excess zinc ions and used it to image insulin secretion (via zinc release) from the rodent pancreas.21–23 Given that the pancreas releases insulin and zinc only after stimulation by an increase in blood glucose levels, we refer to this stimulatory process as glucose-stimulated zinc secretion (GSZS). This technology has been used to image an increased functional release of insulin and expansion of the mouse pancreas after mice were placed on a high-fat diet for a period of 12 weeks.21 During those studies, we observed by MRI that the prostate also responds to a sudden increase in blood glucose by releasing zinc ions into the surrounding stromal tissue. Given that the prostate contains the highest levels of zinc in the body, and that zinc levels are markedly decreased in prostate cancer (PCa),24 we tested the possibility of using MRI to monitor the zinc status of prostate tissues in anticipation of detecting differential zinc release between normal versus malignant prostate tissues.25 This was indeed evident in TRAMP mice, a widely studied model of PCa,26 as hypointense regions of the prostate were later shown by histology to correspond to malignant tissue. Although this is consistent with the known decrease in total zinc content in PCa, the question remained: Does the MRI observation simply reflect the total zinc content of prostate tissue or does it reflect the loss of ability of PCa cells to secrete zinc ions in response to a glucose bolus? Therefore, in an effort to further understand these MRI observations, we turned to high-resolution synchrotron radiation X-ray fluorescence (μSR-XRF) studies of prostate tissue samples from the same animals studied by MRI to obtain elemental maps of zinc and gadolinium before and after exposure to high glucose. X-ray fluorescence (XRF) methods have been used to quantify the distribution of metals in tissues and in various other samples ranging from geology, to electronics, to materials science. This work highlights the advantage of using both MRI and μSR-XRF to characterize the distribution and trafficking of zinc in healthy and malignant prostate tissue.
RESULTS AND DISCUSSION
In this study, we used a combination of MRI to monitor glucose-stimulated zinc secretion (GSZS) in the mouse prostate followed by resection of the prostate, fixation, tissue sectioning, and analysis by μSR-XRF to obtain elemental maps of the tissue distribution of zinc and gadolinium in healthy versus cancerous mouse prostate tissues. For MRI sensing, we used a Gd-DO3A derivative containing a single zinc binding moiety (Figure 1A). This zinc sensor, referred to as GdL2 in a previous publication,22 has a rather modest zinc binding affinity of KD(Zn) = 2.35 μM, as measured by a competitive binding assay in the absence of albumin. Once a single zinc ion binds to GdL2, the resulting complex forms a ternary complex with serum albumin, which results in slowing of molecular rotation and an amplification of the longitudinal T1 relaxivity (r1) of water protons at 1.5 T from 4.7 ± 0.1 mM−1 s−1 to 11.1 ± 0.4 mM−1 s−1.
Figure 1.

(A) Schematic to illustrate the mechanism of MRI zinc detection. (B) Current study design and sample preparation. After resection of the organ, 50 and 10 μm sections were cut and mounted for μSR-XRF and hematoxylin and eosin (H&E) staining, respectively.
Zinc secretion from the prostate is initiated by a bolus injection of glucose in fasted mice followed by intravenous injection of GdL2. Given that imaging agents like GdL2 are thought to remain extracellular after injection, the MR image enhancement detected in the prostate has largely been shown to reflect zinc ions secreted from the prostate initiated by the sudden increase in extracellular glucose.25 Since total tissue zinc content may not necessarily parallel release of zinc as initiated by glucose (GSZS), the present study was designed to measure GSZS by MRI in healthy and TRAMP mouse prostates followed by resection of the prostate and analysis of metal content and distribution by μSR-XRF. Healthy C57BL6 mice and 20–23 week-old TRAMP mice were fasted overnight (at least 12 h) before receiving a bolus of either 2.2 mmol/kg glucose or saline intraperitoneally and 0.07 mmol/kg GdL2 intravenously. As summarized in Figure 1B, T1-weighted MRI scans of the prostate were collected sequentially over ~7 min followed by removal of the animal from the magnet, prostate resection and freezing, and preparation of mouse prostate whole mounts on XRF-compatible Ultralene film for μSR-XRF.
Injection of glucose and GdL2 resulted in dramatic image enhancement of the prostate in fasted healthy mice (Figure 2A). Image enhancement is not detected without the addition of glucose.25 Although GdL2 has a much lower affinity for zinc than the agent used in our previous publication, which contains two zinc binding arms,25 this result shows that a substantial amount of zinc is secreted from the prostate in response to a sudden increase in glucose, enough to occupy the single binding site on GdL2 and promote detectable complexation with albumin. In 20–23-week-old TRAMP mice, somewhat lower image enhancement is seen from some regions of prostate tissue, plus there are clear hypo-intense regions indicative of reduced zinc flux in those areas. We previously reported that these hypo-intense regions correspond to malignant tissue confirmed by immunohistochemistry.25 A quantitative analysis of these regions, summarized in the box-whisker plot of Figure 2B, shows that the contrast-to-noise ratio (prostate versus muscle tissues) in the healthy animals at 7 min after GSZS was 15 ± 4.5 versus 8.5 ± 3.7 (p < 0.05) in TRAMP mice. This substantial decrease in CNR is consistent with reduced zinc secretion from the hypointense regions of the TRAMP prostate. Given the known relationship between zinc content in healthy (high zinc) versus cancerous prostate tissue (low zinc),24,27,28 this imaging result could reflect either a loss of ability to secrete zinc ions in cancerous tissues or simply that less zinc is available in the tissue to be secreted. In an effort to differentiate between these possibilities, these tissue samples were frozen by exposure to liquid nitrogen-chilled isopentane and prepared for quantitative elemental analyses by μSR-XRF. μSR-XRF images of the prepared prostate tissue slices were collected at the Diamond Light Source, Harwell, UK, by rastering the 50-μm-thick sample with 11 and 8.2 keV beams through the prostate section to obtain elemental maps of zinc, gadolinium, and phosphorus (Figure 2C). The H&E stain shows a poorly differentiated tumor in the lateral lobe of the prostate. The phosphorus map (blue) outlines the phospholipid membranes. The gadolinium map (red) shows that the contrast agent has limited access to the center of the dense tumor core, while the zinc map (green) shows that the zinc content in the tumor core is lower compared to the surrounding tissue. The average zinc content in tumor tissue was 0.370 ± 0.003 mM, while the average zinc content across all other regions was quite variable at 0.40 ± 0.02 mM.
Figure 2.

In vivo GSZS MRI identification of malignant prostate lesions and μSR-XRF validation of zinc and gadolinium content. (A) T1-weighted gradient echo images of healthy C57BL6 and 21-week-old TRAMP mice prior to and 7 min after receiving a bolus of 0.07 mmol/kg GdL2 plus 2.2 mmol/kg glucose. The inset in the TRAMP mouse image shows a hypo-intense region in the lateral/ventral lobe, consistent with a nascent tumor. (B) Image analysis of ROIs drawn along the prostates of healthy and TRAMP animals. The contrast-to-noise ratio of prostate versus muscle tissue indicates a loss of GSZS in the TRAMP animal (N = 4 healthy, N = 6 TRAMP, *p < 0.05). (C) Representative H&E stains and μSR-XRF images of prostate tissue samples show a poorly differentiated tumor in the lateral lobe and the distribution of zinc, gadolinium, and phosphorus in those same slices. Concentration bars for zinc and gadolinium correspond to absolute quantified values in millimolar; absolute concentrations for P were not obtained due to low energy of the fluorescent signal.
Quantitative XRF maps were also measured on prostate tissues from wild-type (WT) mice that had not received glucose or GdL2. The μSR-XRF map in Figure 3A shows that the lateral lobe has much more zinc than the other prostate lobes. A magnified image of the lateral lobe shows that zinc accumulates in the glandular wall (which includes secretory epithelial cells) and the supporting stromal tissue. In animals that received GdL2 but no additional glucose, the XRF images (Figure 3B) show that GdL2 distributes in the surrounding stroma and interstitial spaces but not in the inner luminal, zinc-rich spaces. The phosphorus maps clearly define the glandular wall that separates zinc from GdL2. Figure 3C shows images from another mouse that received both glucose and GdL2. Here, the glandular lumen is relatively free of zinc, while the stromal and interstitial spaces contain both zinc and gadolinium. These μSR-XRF data confirm the MRI results by directly showing that a bolus of glucose administered to fasted mice stimulates movement of zinc from the glandular lumen to the stromal and interstitial spaces where it comes into contact with GdL2. Similarly, μSR-XRF prostate images from TRAMP mice given both glucose and GdL2 showed a similar zinc and gadolinium colocalization in the stromal smooth muscle surrounding the glands, but little zinc in the interstitial spaces (Figure 3D). To quantify the elemental distributions in the respective prostatic lobes, ROIs guided by the adjacent H&E sections were drawn to identify the ventral, lateral, and dorsal lobes (Figure 4A) following previously published guidelines.29 Here, we analyzed zinc, gadolinium, iron, and copper in an effort to determine if other elements play significant roles in the development of PCa in conjunction with zinc. Analyses of these data (Figure 4B) indicate that the lateral lobe of the WT mouse prostate contains the most zinc, 1.06 ± 0.08 mM, compared to the ventral and dorsal lobes, 0.3 ± 0.1 mM and 0.509 ± 0.003, respectively (p = 0.002). For the animals that received GdL2, there was no significant difference in the distribution of the agent across lobes as seen in the quantified gadolinium bar graphs. Furthermore, the known loss of zinc in PCa was found to be localized only in the lateral lobe of the TRAMP mice, decreasing to 0.38 ± 0.02 mM (p-value <0.05). Conversely, iron, copper, and gadolinium concentrations did not differ across all lobes, treatment paradigms, or between healthy versus TRAMP animals. While malignant prostate cells are known to accumulate less zinc due to downregulated import transporters,30 the μSR-XRF data show that the greatest reduction in zinc is seen largely in the lateral lobe. In mice, this lobe is embryologically homologous to the peripheral zone of the human prostate where PCa is most often seen.31
Figure 3.
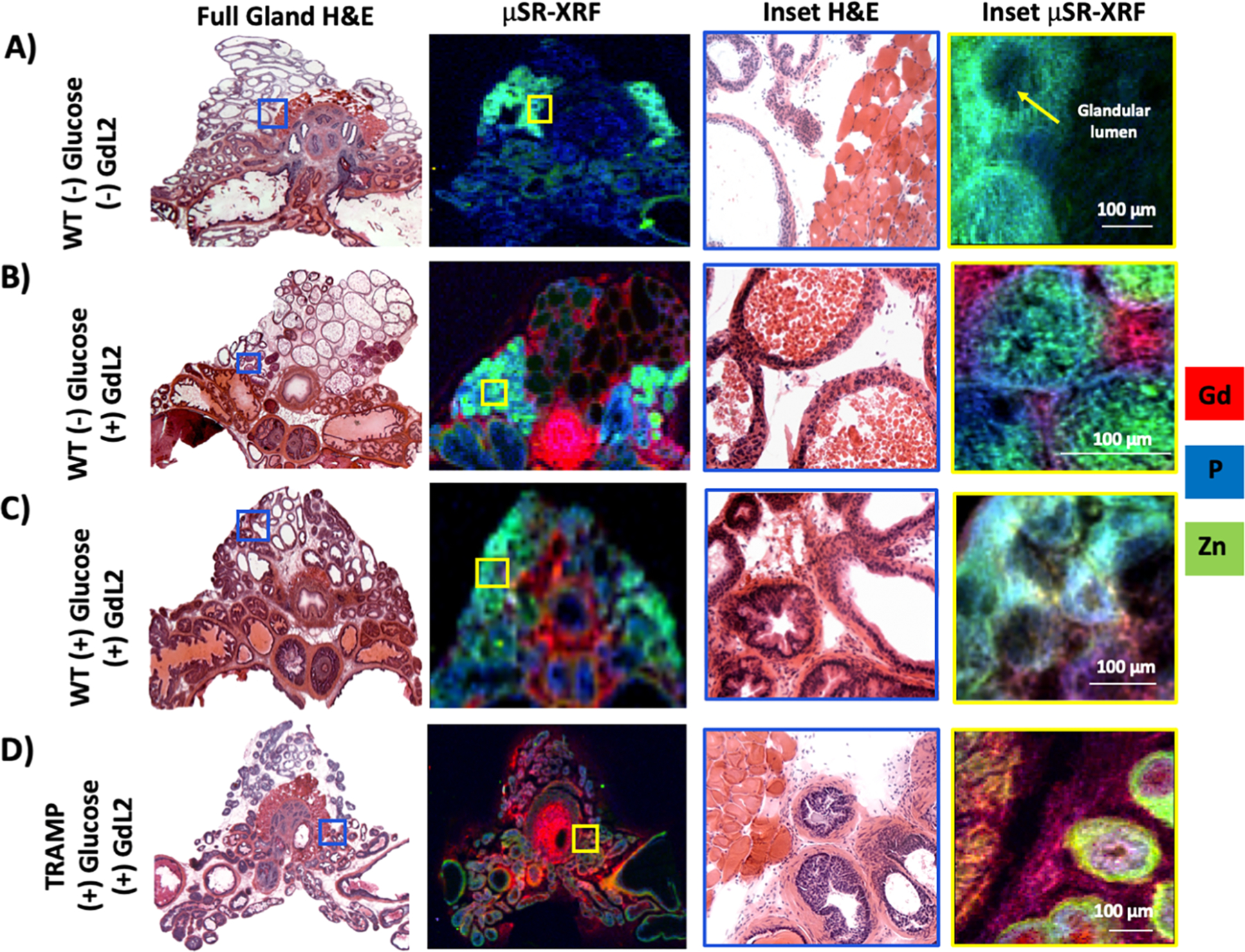
Glucose-stimulated movement of zinc pools in the prostate. (A) μSR-XRF of WT C57Bl6 animals without any glucose or GdL2 illustrates the endogenous distribution of zinc. (B) μSR-XRF of WT animals only receiving 0.07 mmol/kg GdL2 shows the distribution of zinc and gadolinium in the prostate. High resolution μSR-XRF shows glands found in the lateral lobe full of zinc-rich secretions. Phosphorus membrane seen in blue delineates the separation between intraglandular zinc and stromal gadolinium. (C) μSR-XRF of WT animals receiving both glucose and GdL2 shows that the distribution of zinc continues to concentrate in the lateral lobe, but at the glandular level, zinc is now secreted from the glands into the stromal space where it comes in contact with gadolinium. (D) μSR-XRF of 20–23-week-old TRAMP receiving glucose and GdL2 illustrates the relative reduction of zinc in the entire gland; at the glandular level, zinc is found predominantly in the smooth muscle surrounding the gland, and not distributed in the stroma as seen in C.
Figure 4.

Quantification of element concentration in different prostate lobes. (A) Mouse prostate section illustrating the ventral (yellow), lateral (gray), dorsal (blue), and anterior (green) lobes. Close inspection of histological structures and the decision chart from ref 29 allowed consistent identification of lobular structures. (B) Quantified concentrations of zinc, gadolinium, copper, and iron, categorized by prostate lobe for healthy naïve WT animals (N = 3), WT mice receiving GdL2 and no glucose (N = 3), WT mice receiving GdL2 and glucose (N = 3), and 20–23-week-old TRAMP receiving GdL2 and glucose (N = 10).
By outlining the glandular compartments separate from the stromal/smooth muscle compartments in the lateral prostate, we were able to quantify the local metal concentrations in the prostate of animals with or without prior injection of glucose (Figure 5A). The quantitative measures of zinc and gadolinium (Figure 5B) show that glucose effectively stimulates movement of ~1 mM zinc from the glandular lumen to the stromal compartment (1.5 ± 0.8 mM in the glandular lumen prior to glucose stimulation and 0.5 ± 0.1 mM in the lumen after glucose stimulation, p < 0.05). Furthermore, a significant loss of zinc in the glandular lumen was observed in TRAMP mice after glucose stimulation (0.51 ± 0.09 mM, p < 0.01). This also suggests that the mechanism(s) of intraglandular zinc transport initiated by glucose may not be completely disrupted in malignant tissues. However, the trafficking of zinc from the intraglandular space into the surrounding smooth muscle and surrounding stroma remains unknown. Figure 5B also shows that the amount of Gd in the glandular and stromal compartments is not altered upon glucose stimulation. This confirms that the contrast enhancement detected in the prostate by MRI during GSZS is solely driven by the movement of zinc ions. Some movement of copper was also detected in the healthy prostate of animals after receiving glucose. Figure S1 shows the glandular and stromal distribution of copper and copper and zinc as an overlay. The quantified copper concentration in the stromal compartment was slightly impacted by glucose stimulation. Although not statistically significant (p value = 0.06), it is possible that in a study powered to detect more fine copper concentration differences, this movement of copper pools may also prove meaningful.
Figure 5.

Glandular and stromal distribution of elements. (A) H&E stained section of prostate glands illustrating the glandular lumen (yellow line) and smooth muscle surrounding the gland as stroma (blue dotted line). (B) The distribution of zinc and gadolinium in WT mice receiving GdL2 and either saline or glucose and in TRAMP mice receiving GdL2 and glucose. *p < 0.05, **p < 0.01.
Given the confirmed secretion of such large concentrations of zinc, we postulated that we could perhaps improve detection by modifying the affinity of the probe for zinc. Both zinc and citrate are known to be elevated in the healthy prostate,9 so it would not be unreasonable to assume that zinc may be weakly associated with citrate (KD(Zn) = 10 μM)28,32 and that both are co-secreted during GSZS. Should this be true, one would expect to find differences between a low and a high affinity zinc probe due to competition between the probe and citrate. This hypothesis was tested by performing the same experiments shown here with a high affinity zinc probe, GdL1, KD(Zn) = 118 nM (Figure S2A). In this case, one detects selective MR contrast enhancement of the lateral lobe in healthy TRAMP mice (Figure S2B), consistent with μSR-XRF zinc maps (Figure 3B). TRAMP mice were monitored over time and as predicted by μSR-XRF, in fully developed PCa, the only significant loss of enhancement was observed in the lateral lobe (Figure 2SB). Although citrate is likely to be the best candidate to accompany secretion of zinc from the prostate, we cannot discard the possibility of other zinc chaperones. Figure S2C shows that the glandular lumen of healthy prostate is not only zinc rich, but also rich in sulfur. These data suggest that upon glucose stimulation, these glands secrete their luminal zinc along with an unknown sulfur-rich chaperone. Cysteine-rich secretory proteins (CRISPs), and particularly CRISP-3, have been shown to be localized in the prostate as a secretory protein and upregulated in prostate cancer.33,34 Cysteine residues can display high affinity for zinc and its complexation may contribute to the correct overall protein structure and function.35 These observations evidently highlight the importance of zinc in the regulation and function of complex secretory systems such as the prostate.
CONCLUSIONS
In this work, we demonstrate that glucose-stimulated movement of zinc ions from intracellular compartments into extracellular spaces in the mouse prostate tissues can be mapped by use of synchrotron radiation X-ray fluorescence. Glucose promotes trafficking of large concentrations of zinc (~1 mM) from the glandular lumen to the smooth muscle/stromal compartments of the healthy mouse prostate and confirms the loss of zinc in the malignant prostate. In the mouse prostate, the lateral lobe appears to be the only lobe that undergoes zinc depletion during malignant transformation. The μSR-XRF results confirm the earlier MRI observation of GSZS and provide a quantitative measure on these zinc movements. Together, these findings highlight the potential for metal content and trafficking as biomarkers of prostate disease.
Supplementary Material
ACKNOWLEDGMENTS
The authors would like to acknowledge the Diamond Light Source for time on Beamline I18 under Proposal [SP14747].
Funding
Financial support from the National Institutes of Health (DK-095416), the Cancer Prevention and Research Institute of Texas (RP180178), and the Robert A. Welch Foundation (AT-584) are gratefully acknowledged.
Footnotes
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.inorgchem.9b01132.
Experimental details for the synthesis of the compounds, in vivo MRI, μSR-XRF ex vivo imaging, elemental concentration mapping, statistical analyses, and additional μSR-XRF images and analyses of prostate samples (PDF)
The authors declare no competing financial interest.
REFERENCES
- (1).Hare DJ; New EJ; de Jonge MD; McColl G Imaging metals in biology: balancing sensitivity, selectivity and spatial resolution. Chem. Soc. Rev 2015, 44 (17), 5941–58. [DOI] [PubMed] [Google Scholar]
- (2).Stehling O; Lill R The role of mitochondria in cellular iron-sulfur protein biogenesis: mechanisms, connected processes, and diseases. Cold Spring Harbor Perspect. Biol 2013, 5 (8), a011312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Polishchuk R; Lutsenko S Golgi in copper homeostasis: a view from the membrane trafficking field. Histochem. Cell Biol 2013, 140 (3), 285–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Maret W Zinc in Cellular Regulation: The Nature and Significance of ″Zinc Signals”. Int. J. Mol. Sci 2017, 18 (11), 2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Kelleher SL; McCormick NH; Velasquez V; Lopez V Zinc in specialized secretory tissues: roles in the pancreas, prostate, and mammary gland. Adv. Nutr 2011, 2 (2), 101–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Lyubartseva G; Smith JL; Markesbery WR; Lovell MA Alterations of zinc transporter proteins ZnT-1, ZnT-4 and ZnT-6 in preclinical Alzheimer’s disease brain. Brain Pathol 2010, 20 (2), 343–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Farquharson MJ; Al-Ebraheem A; Falkenberg G; Leek R; Harris AL; Bradley DA The distribution of trace elements Ca, Fe, Cu and Zn and the determination of copper oxidation state in breast tumour tissue using muSRXRF and muXANES. Phys. Med. Biol 2008, 53 (11), 3023–37. [DOI] [PubMed] [Google Scholar]
- (8).Beker Aydemir T; Chang S-M; Guthrie GJ; Maki AB; Ryu M-S; Karabiyik A; Cousins RJ Zinc transporter ZIP14 functions in hepatic zinc, iron and glucose homeostasis during the innate immune response (endotoxemia). PLoS One 2012, 7 (10), No. e48679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Costello LC; Franklin RB Novel role of zinc in the regulation of prostate citrate metabolism and its implications in prostate cancer. Prostate 1998, 35 (4), 285–96. [DOI] [PubMed] [Google Scholar]
- (10).Costello LC; Franklin RB A Review of the Current Status and Concept of the Emerging Implications of Zinc and Zinc Transporters in the Development of Pancreatic Cancer. Pancreat. Disord. Ther 2013, S4, DOI: 10.4172/2165-7092.S4-002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Yang J; Zhang Y; Cui X; Yao W; Yu X; Cen P; Hodges SE; Fisher WE; Brunicardi FC; Chen C; Yao Q; Li M Gene profile identifies zinc transporters differentially expressed in normal human organs and human pancreatic cancer. Curr. Mol. Med 2013, 13 (3), 401–409. [PMC free article] [PubMed] [Google Scholar]
- (12).Kelleher SL; Seo YA; Lopez V Mammary gland zinc metabolism: regulation and dysregulation. Genes Nutr 2009, 4 (2), 83–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Wang G; Biswas AK; Ma W; Kandpal M; Coker C; Grandgenett PM; Hollingsworth MA; Jain R; Tanji K; Lοpez-Pintado S; Borczuk A; Hebert D; Jenkitkasemwong S; Hojyo S; Davuluri RV; Knutson MD; Fukada T; Acharyya S Metastatic cancers promote cachexia through ZIP14 upregulation in skeletal muscle. Nat. Med 2018, 24 (6), 770–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).De Leon-Rodriguez LM; Lubag AJ; Lopez JA; Andreude-Riquer G; Alvarado-Monzon JC; Sherry AD A second generation MRI contrast agent for imaging zinc ions in vivo. MedChemComm 2012, 3 (4), 480–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Li D; Chen S; Bellomo EA; Tarasov AI; Kaut C; Rutter GA; Li WH Imaging dynamic insulin release using a fluorescent zinc indicator for monitoring induced exocytotic release (ZIMIR). Proc. Natl. Acad. Sci. U. S. A 2011, 108 (52), 21063–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Wahsner J; Gale EM; Rodriguez-Rodriguez A; Caravan P Chemistry of MRI Contrast Agents: Current Challenges and New Frontiers. Chem. Rev 2019, 119, 957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Ghosh SK; Kim P; Zhang XA; Yun SH; Moore A; Lippard SJ; Medarova Z A novel imaging approach for early detection of prostate cancer based on endogenous zinc sensing. Cancer Res 2010, 70 (15), 6119–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Isaac M; Pallier A; Szeremeta F; Bayle PA; Barantin L; Bonnet CS; Seneque O MRI and luminescence detection of Zn(2+) with a lanthanide complex-zinc finger peptide conjugate. Chem. Commun. (Cambridge, U. K.) 2018, 54 (53), 7350–7353. [DOI] [PubMed] [Google Scholar]
- (19).Zhang XA; Lovejoy KS; Jasanoff A; Lippard SJ Water-soluble porphyrins as a dual-function molecular imaging platform for MRI and fluorescence zinc sensing. Proc. Natl. Acad. Sci. U. S. A 2007, 104 (26), 10780–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Franklin RB; Zou J; Zheng Y; Naslund MJ; Costello LC Zinc Ionophore (Clioquinol) Inhibition of Human ZIP1-Deficient Prostate Tumor Growth in the Mouse Ectopic Xenograft Model: A Zinc Approach for the Efficacious Treatment of Prostate Cancer. Int. J. Cancer Clin Res 2016, 3 (1), DOI: 10.23937/2378-3419/3/1/1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Lubag AJ; De Leon-Rodriguez LM; Burgess SC; Sherry AD Noninvasive MRI of beta-cell function using a Zn2+-responsive contrast agent. Proc. Natl. Acad. Sci. U. S. A 2011, 108 (45), 18400–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Martins AF; Clavijo Jordan V; Bochner F; Chirayil S; Paranawithana N; Zhang S; Lo ST; Wen X; Zhao P; Neeman M; Sherry AD Imaging insulin secretion from the mouse pancreas by MRI is improved by use of a zinc-responsive MRI sensor with lower affinity for Zn2+ ions. J. Am. Chem. Soc 2018, 140, 17456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Yu J; Martins AF; Preihs C; Clavijo Jordan V; Chirayil S; Zhao P; Wu Y; Nasr K; Kiefer GE; Sherry AD Amplifying the sensitivity of zinc(II) responsive MRI contrast agents by altering water exchange rates. J. Am. Chem. Soc 2015, 137 (44), 14173–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Zaichick V; Sviridova TV; Zaichick SV Zinc in the human prostate gland: normal, hyperplastic and cancerous. Int. Urol. Nephrol 1997, 29 (5), 565–74. [DOI] [PubMed] [Google Scholar]
- (25).Clavijo Jordan MV; Lo ST; Chen S; Preihs C; Chirayil S; Zhang S; Kapur P; Li WH; De Leon-Rodriguez LM; Lubag AJ; Rofsky NM; Sherry AD Zinc-sensitive MRI contrast agent detects differential release of Zn(II) ions from the healthy vs. malignant mouse prostate. Proc. Natl. Acad. Sci. U. S. A 2016, 113 (37), E5464–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Hurwitz AA; Foster BA; Allison JP; Greenberg NM; Kwon ED The TRAMP mouse as a model for prostate cancer. Current Protocols in Immunology; John Wiley & Sons, Inc.: Hoboken, NJ, 2001; Chapter 20, Unit 20, p 5. [DOI] [PubMed] [Google Scholar]
- (27).Cortesi M; Fridman E; Volkov A; Shilstein S; Chechik R; Breskin A; Vartsky D; Kleinman N; Kogan G; Moriel E; Gladysh V; Huszar M; Ramon J; Raviv G Clinical assessment of the cancer diagnostic value of prostatic zinc: a comprehensive needle-biopsy study. Prostate 2008, 68 (9), 994–1006. [DOI] [PubMed] [Google Scholar]
- (28).Costello LC; Franklin RB Zinc is decreased in prostate cancer: an established relationship of prostate cancer! JBIC, J. Biol. Inorg. Chem 2011, 16 (1), 3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Oliveira DS; Dzinic S; Bonfil AI; Saliganan AD; Sheng S; Bonfil RD The mouse prostate: a basic anatomical and histological guideline. Bosn J. Basic Med. Sci 2015, 16 (1), 8–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Desouki MM; Geradts J; Milon B; Franklin RB; Costello LC hZip2 and hZip3 zinc transporters are down regulated in human prostate adenocarcinomatous glands. Mol. Cancer 2007, 6, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Fong LY; Jing R; Smalley KJ; Wang ZX; Taccioli C; Fan S; Chen H; Alder H; Huebner K; Farber JL; Fiehn O; Croce CM Human-like hyperplastic prostate with low ZIP1 induced solely by Zn deficiency in rats. Proc. Natl. Acad. Sci. U. S. A 2018, 115 (47), E11091–E11100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Dubi N; Gheber L; Fishman D; Sekler I; Hershfinkel M Extracellular zinc and zinc-citrate, acting through a putative zinc-sensing receptor, regulate growth and survival of prostate cancer cells. Carcinogenesis 2008, 29 (9), 1692–700. [DOI] [PubMed] [Google Scholar]
- (33).Kosari F; Asmann YW; Cheville JC; Vasmatzis G Cysteine-rich secretory protein-3: a potential biomarker for prostate cancer. Cancer Epidemiol. Biomarkers Prev 2002, 11 (11), 1419–26. [PubMed] [Google Scholar]
- (34).Pathak BR; Breed AA; Deshmukh P; Mahale SD Androgen receptor mediated epigenetic regulation of CRISP3 promoter in prostate cancer cells. J. Steroid Biochem. Mol. Biol 2018, 181, 20–27. [DOI] [PubMed] [Google Scholar]
- (35).Pace NJ; Weerapana E Zinc-binding cysteines: diverse functions and structural motifs. Biomolecules 2014, 4 (2), 419–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


