Abstract
PURPOSE:
Although multigene panel testing (MGPT) is increasingly utilized in clinical practice, there remain limited data on patient-reported outcomes.
METHODS:
BRCA 1/2 negative patients were contacted and offered MGPT. Patients completed pre- and post-test counseling, and surveys assessing cognitive, affective and behavioral outcomes at baseline, post-disclosure and 6 and 12 months.
RESULTS:
Of 317 eligible BRCA1/2 negative patients who discussed the study with research staff, 249 (79%) enrolled. Decliners were more likely to be older, non-white, and recruited by mail or email. Ninety-five percent of enrolled patients proceeded with MGPT. There were no significant changes in anxiety, depression, cancer specific distress or uncertainty post-disclosure. There were significant but small increases in knowledge, cancer-specific distress and depression at 6–12 months. Uncertainty declined over time. Those with a VUS had significant decreases in uncertainty but also small increases in cancer specific distress at 6 and 12 months. Among those with a positive result, medical management recommendations changed in 26% of cases and 2.6% of all tested.
CONCLUSION:
Most BRCA1/2 negative patients have favorable psychosocial outcomes after receipt of MGPT results, although small increases in depression and cancer-specific worry may exist and may vary by result. Medical management changed in few patients.
Keywords: multigene panel testing, genetic testing outcomes, cancer genetic testing, cancer prevention and screening, delivery of genetic services
INTRODUCTION
Multigene panels, including high and moderate penetrance cancer susceptibility genes are increasingly being used in clinical practice.1–3 Multigene panels can identify additional pathogenic variants1; 4–6, but are also associated with a high rate of Variants of Uncertain Significance (VUS). Risk estimates for many low- and moderate-penetrant genes and optimal medical management are still evolving.3; 5; 7 Thus, there remain questions about the clinical and patient related risks of incorporating multigene panel testing (MGPT) into clinical practice and a need for evidence to guide best practice.8; 9 How best to counsel patients for multiple and variable genes and support informed decision making remains unclear given the potential for misunderstanding results, inappropriate screening or prophylactic surgery and increased psychosocial distress related to uncertainty or informational overload10–14.
There are relatively few studies reporting patient reported cognitive, affective and behavioral outcomes of clinical incorporation of MGPT.2; 3; 15–17 In addition, it is not well described how many individuals with prior negative testing for BRCA1/2 are interested in further testing. In the METEOR (Multiplex tEsTing for Evaluation Of breast cancer Risk) study, we sought to evaluate patients’ interest in MGPT among BRCA1/2 negative patients. Second, we sought to evaluate patient reported outcomes and frequency of changes to medical management to better understand the potential risks and benefits of incorporating MGPT into clinical practice.
MATERIALS and METHODS
Participants
Institutional Review Board approval was obtained. Eligible participants were >17 years old, English speaking and had negative BRCA1/2 clinical testing. Study staff identified BRCA1/2 negative individuals from the clinical registry and clinic schedules from January 2014 through January 2015. Patients who were eligible were approached in clinic, and/or sent a letter or email. Participants were informed that they could have testing for additional cancer susceptibility genes, including APC, ATM, BARD1, BMPR1A, BRIP1, CDH1, CDK4, CDKN2A, CHEK2, EPCAM [large rearrangements only], MLH1, MSH2, MSH6, MUTYH, NBN, PALB2, PMS2, PTEN, RAD51C, RAD51D, SMAD4, STK11 and TP53. The cost of testing was covered by Myriad Genetics and the cost of counseling by institutional research funds. Testing could also be ordered clinically but at the time coverage for panel testing in this patient population was variable.
Study Procedures
Participants completed informed consent and were scheduled for in-person pre-test counseling with a genetic counselor (n=5 genetic counselors). A tiered-binned genetic counseling approach was used18. In this model, tier 1 “indispensable” information is presented to all patients, and additional information (tier 2) is provided to support variable informational needs among diverse patient populations. “Binning” clinically relevant information into groups is used to minimize information overload, support informed decision-making and facilitate adaptive responses to testing.18 Sessions were audiotaped and genetic counselors completed a checklist for each session.
After pre-test counseling, patients could proceed with the 25-gene panel or elect to undergo testing of select genes based on their personal and family history. Participants were scheduled for an in-person disclosure visit where results were shared by the genetic counselor and participants met with a physician with expertise in cancer genetics (n=2 physicians).
Measures
Surveys completed at baseline (T0), within 7 days prior to pre-test counseling), within 7 days after pre-test counseling (T1), within 7 days after result disclosure (T2) and at 6 (T3) and 12 (T4) months after disclosure evaluated constructs informed by a conceptual model19 grounded in the Self-Regulation Theory of Health Behavior.20 Our model proposes that uptake of genetic testing and response to (e.g. psychosocial adjustment) and use of (performance of risk reduction behaviors) genetic information are products of an individual’s understanding and perception of the disease threat.19; 21
Knowledge of genetic disease
Knowledge of genetic disease was evaluated (T0-T4) using an adapted version of the Cancer Genetics Knowledge scale and ClinSeq knowledge scale,22–24 evaluating knowledge of inheritance and test interpretation (8 items); and benefits (4 items) and limitations (7 items) of MGPT (Cronbach’s α=0.78–0.82 across T0-T4).2; 15
Psychosocial adjustment
Psychosocial adjustment was evaluated with 3 measures. 1) State anxiety (T0-T2) was measured with the 20-item State Inventory of the State-Trait Anxiety Inventory25,26 (Cronbach’s α=0.95–0.96). 2) General anxiety and depression (T0-T4) were assessed with the 7-item Hospital Anxiety and Depression Scale (HADS)27 (Cronbach’s α=0.85–0.89 and 0.83–0.88). 3) Cancer specific distress (T0-T4) was evaluated with 14 items of Impact of Events Scale28 (Cronbach’s α=0.89).
Satisfaction with genetic services
Satisfaction with genetic services was measured (T1-T2) with a 9-item scale evaluating participants’ perceptions of their genetic counseling and testing experience29,30 (Cronbach’s α=0.84–0.96).
Uncertainty
Uncertainty was assessed (T0-T4) using a 3-item scale adapted from the Multidimensional Impact of Cancer Risk Assessment Question (MICRA)31 (Cronbach’s α=0.77–0.83).
Perceived Utility
Perceived Utility was assessed (T1-T4) with a novel scale developed to evaluate patient perceptions of the utility of genetic testing, comprised of 12 items evaluating medical and personal utility for self and relatives, on a 5-point Likert scale (Cronbach’s α=0.94–0.96).
Behavioral intention
Behavioral intention (T0, T1) was included as an early surrogate of performance of screening and risk reduction behaviors and included intent to perform mammography, breast MRI, and prophylactic mastectomy. Patients could mark “not applicable” if the screening behavior had not been recommended for them. Additionally, those who had prior mastectomy for treatment or prevention were not asked questions about breast cancer screening.
Statistical Analysis
We chose the sample size to allow us to estimate uptake of MGPT among enrollees with sufficient precision, where precision is defined as one-half the width of a 95% confidence interval. For an anticipated 245 enrollees, we expected that we could estimate uptake with a precision of 6.5% or smaller. To examine longitudinal change, we used linear regressions estimated by Generalized Estimating Equations assuming exchangeable working correlation matrices to account for repeated temporal measures within participants. We included categorical time in the models via indicator yes/no variables, and also entered time and covariate interactions when examining temporal trends. We used Wald tests of model coefficients (coef) to test for differences between time points. We used logistic regressions to assess covariates that were associated with declining participation and failure to complete follow-up surveys. We used Raghunathan and colleagues’ multiple imputation methods to impute both missing baseline data and longitudinal response data and figures to visually represent changes over time.32 The multiple imputation method flexibly allows for categorical, continuous, and count variables. We used Rubin’s combining rules as implemented in STATA (Statacorp, College Station, Texas) with 25 datasets in the imputation process.33 P-values between 0.01 and 0.05 based on two-sided hypothesis tests were considered statistically significant. P-values less than 0.01 were considered more strongly significant after partially accounting for multiple hypothesis testing.
RESULTS
Participants and uptake of multigene panel testing
Six hundred fifteen eligible individuals with prior negative BRCA1/2 testing (and no panel testing) were identified from our clinic registries. The mean time from initial BRCA 1/2 testing was 4.57 years (SD 3.81). Potential participants were approached in clinic by their physician or genetic counselor and referred to the study team to discuss the study in more detail (n=168), were contacted by phone by a research staff member in advance of an upcoming visit (n=12) or were sent a letter by mail or email with study information (n=435). All potential participants received multiple phone calls to attempt to discuss the study with research staff. Research staff were able to speak with 317 potential participants about the study and option for additional MGPT. This included 77% (129/168) of those approached in clinic by their provider, 100% (12/12) of those called in advance of their clinic visit and 40% (176/435) who were mailed a letter. Among those who had a discussion about the study with research staff, thirty-three declined participation (10%), and 15 (5%) expressed initial interest and verbal consent but never proceeded (i.e. passive decliners). Twenty potential participants were ineligible after contact. Two hundred forty-nine individuals (79% of eligible participants who discussed the study with research staff, 41% of those contacted) enrolled (Figure 1). Reasons for declining among those who actively declined included not being interested in additional testing (n=13), being concerned about uncertainty or unclear utility or risk of distress (n=10), concerned about research burden (n=5), poor timing (n=3) and cost of the medical follow-up visit (n=2).
Figure 1: Study Consort.
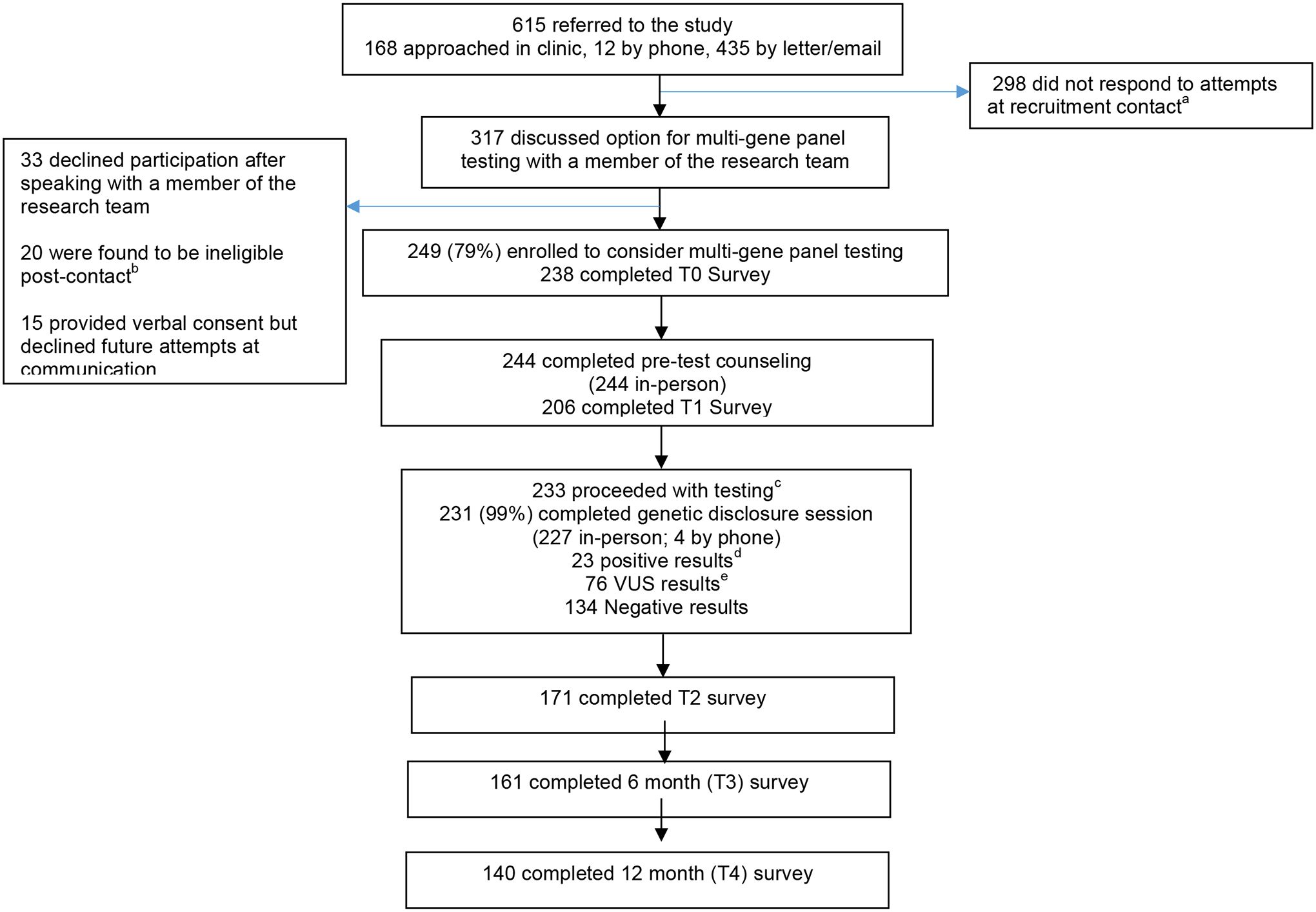
a Includes individuals who provided permission to be contacted during a clinic visit but never responded to recruitment attempts
b Ineligibility due to: offered a competing study (7), already had panel testing (6), previously tested positive for a genetic mutation (3), did not have prior BRCA1/2 testing (2), deceased (1), clinician request (1)
c three participants proceeded with a targeted custom panels.
d APC (9), ATM (4), BRCA1 (2), BARD1, CDH1, CHEK2 , APC/CHEK2, MUTYH (het), NBN, PALB2, TP53
e ATM (18), APC (7), CHEK2 (6), APC/CHEK2 (3), BRIP1 (3), RAD51D (3), SMAD4 (3), ATM/NBN (2), BMPR1A (2), BRCA1 (2), CHD1 (2), MSH6 (2), NBN (2), PMS2 (2), RAD51C (2), APC/CDH1, ATM/PMS2, ATM/BMPR1A, ATM/TP53, BARD1, BARD1/MSH2, BRCA1/MUTYH, BRCA2, BRIP1/CDKN2A, BRIP1/CHEK2, CDK4/CHEK2, CDKN2A, CDKN2A/TP53, CHEK2/STK11, MLH1, MUTYH/RAD51D, STK11.
Participants who enrolled had a mean age of 55.3 years old, 90% were white, 98% female, 73% had a college education or higher and 81% had a personal history of cancer (Table 1). In multivariable analysis, older age (OR = .98/year, CI 0.97–1.00/year, p=0.019), non-white (OR = 3.11, CI 1.72–5.62, p=<0.001) and recruited by mail or email (OR 3.14, CI 2.11–4.66, p<0.001) were associated with declining the option to enroll and consider additional MGPT. Those who actively declined were more likely to have a history of cancer (OR = 10.69, CI 1.41–80.91, p=0.02), as compared to those who passively declined. Ninety-five percent of participants who met with a genetic counselor proceeded with testing. Almost all participants elected for the 26-gene panel, while 3 elected for a custom panel to minimize uncertainty. All but two participants elected to receive their genetic panel results (Figure 1). Results were available on average in 12 days (range 7–20). Those who did not complete T1 surveys were more likely to be other race (OR 5.9, p=0.04 for non-completion compared to whites) and unaffected (i.e. personal history of cancer, OR 8.4, p=0.05). At T2 and T3, the only factor associated with not completing surveys was being affected (OR 10.5, p=0.002, OR 4.6, p=0.003 respectively). Therefore, we use multiple imputation to account for differences in attrition.
Table 1.
Characteristics of those contacted for multi-gene panel testing by decision to enroll or decline
| Consented (N=249) | |
|---|---|
| Range | 27–87 |
| Race/Ethnicity | |
| White | 225 (90) |
| Black/African American | 10 (4) |
| Hispanic/Latino | 6 (2) |
| Other | 7 (3) |
| Unknown | 1 (1) |
| Gender | |
| Female | 243 (98) |
| Male | 6 (2) |
| Education | |
| Graduate school | 104 (42) |
| College graduate | 78 (31) |
| Some college | 24 (10) |
| High School or Less | 16 (6) |
| Missing/Declined | 27 (11) |
| Marital Status | |
| Married or living as married | 178 (71) |
| Years From Initial BRCA1/2 Testing | 4.57 (3.82) (0.01–18.34) |
| Personal History of Cancer | |
| No | 47 (19) |
| Yes | 202 (81) |
| Breast | 158 (78) |
| Ovarian | 7 (3) |
| Other | 9 (4) |
| Multiple | 28 (14) |
| Missing | 0 |
| Number of FDR/SDR with Cancer | |
| Number of FDR or SDR with breast cancer | 1.41 (1.21) |
| Number of FDR or SDR with ovarian cancer | 0.23 (0.55) |
| Number of FDR or SDR with an “other” cancer | 2.42 (1.76) |
Patient reported short-term and longitudinal cognitive and affective outcomes with multigene panel testing
Knowledge increased significantly, although the changes were small and may not be clinically significant. Perceived utility decreased significantly after pre-test counseling (Figures 2A, 2F, Supplemental Table 1). There were no significant changes in state or general anxiety, depression, cancer specific distress, or uncertainty after receipt of results, as compared to baseline (Figures. 2B–E).
Figure 2: Longitudinal Change in Patient Reported Cognitive and Affective Outcomes in ALL PARTICIPANTS and BY RESULT.
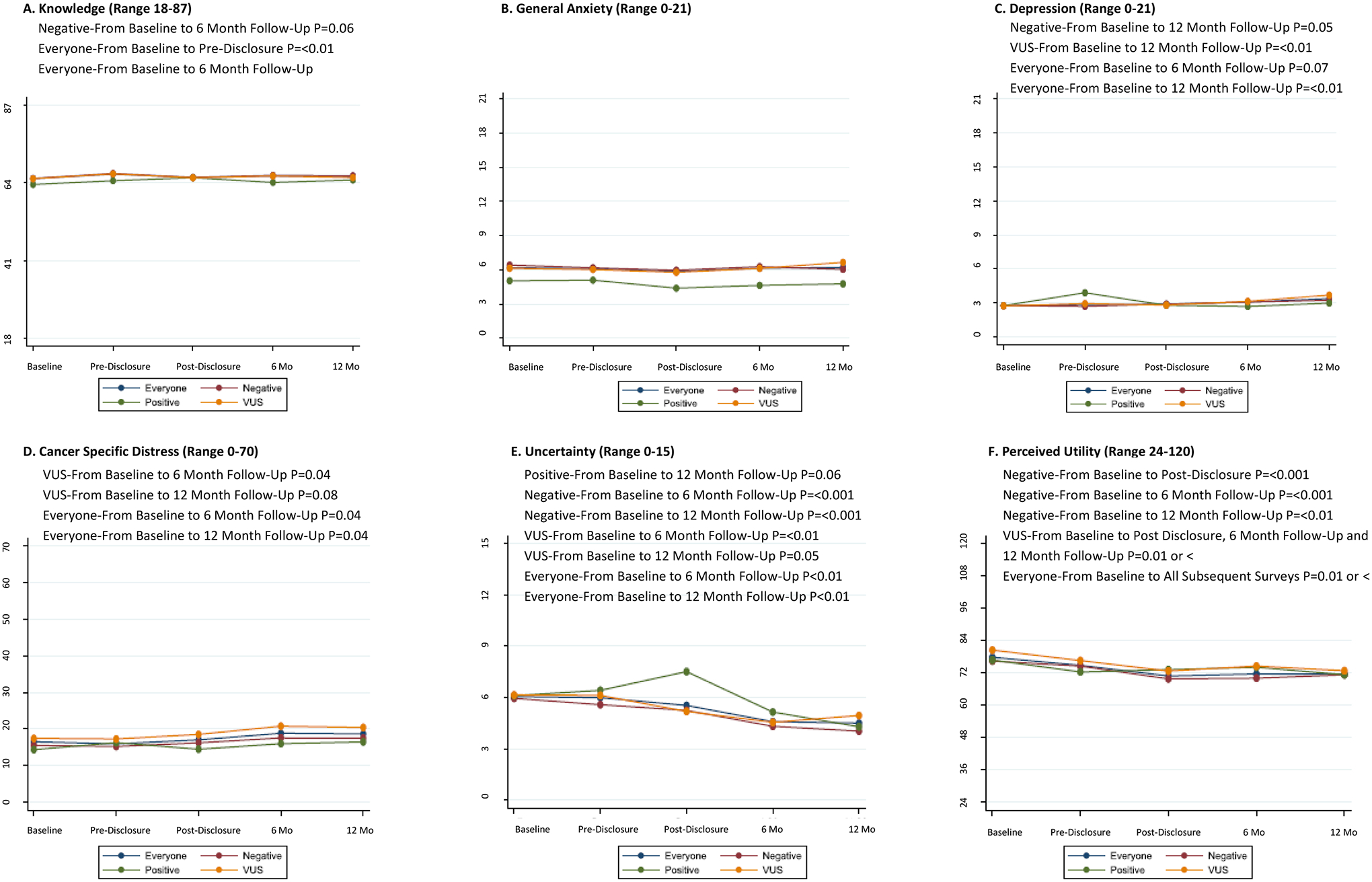
Everyone = All Participants
Negative = Uninformative Negative Result
Positive = Positive Result
VUS = Variant of Uncertain Significance Result
Baseline=0–7 Days after consent, Pre-Disclosure=0–7 Days after pre-test counseling, Post-Disclosure=0–7 Days after result disclosure counseling, 6 Mo=6 Months after disclosure counseling, 12 Mo=12 Months after disclosure counseling.
Did not examine Baseline to Pre-Disclosure changes since the participants did not know results at the time of Pre-Disclosure.
All findings not statistically significant unless indicated.
Knowledge increased minimally, but significantly from baseline to 6 months post-disclosure (Figure 2A). State anxiety, (not shown) and general anxiety did not change significantly over time (Figure 2B). In contrast, cancer-specific distress increased significantly from baseline to 6 and 12 months, although the changes were small and may not be clinically significant (Figure 2D). Similarly, depression increased significantly from baseline to 12 months34, although the mean score was below the clinical cut-off of 8 for clinically significant depression (Figure 2C). Both uncertainty and perceived utility declined significantly from baseline to 6 and 12 months (Figures 2E, 2F). Outcomes did not differ in analyses evaluating for different genetic counselors.
Patient reported short-term and longitudinal outcomes with multigene panel testing by test result and participant characteristics
In secondary analyses evaluating outcomes by test result, there were no significant changes in knowledge, state or general anxiety, depression, cancer-specific distress or uncertainty among any of the test result subgroups after receipt of results, as compared to baseline (Figures 2A–2E). Perceived utility did not change significantly after receipt of results for those with a positive result, but declined significantly after receipt of result among those with an uninformative and VUS result (Figure 2F).
There were no longitudinal changes in knowledge or general anxiety among any of the result subgroups (Figure 2A, 2B). Those with a positive result (n=23) did not have any significant increases in other outcomes including cancer-specific distress, depression or perceived utility. Uncertainty declined over time but changes were not statistically significant. Those with an uninformative result (n=134) had no significant change in cancer-specific distress, but a small but significant increase in depression from baseline to 12 months, which again was well below clinically significant cut-offs. They had significant declines in uncertainty and perceived utility. Patients with a VUS (n=76) had significant increases in cancer-specific distress from baseline to 6 and 12 months, significant declines in depression from baseline to 12 months, and significant declines in uncertainty and perceived utility from baseline to 6 and 12 months (Figures 2C–2F).
In full regression models, older age (coef −0.09 per one-year increase, p=0.001) and non-white race (coef=−3.6, p=0.005) were generally associated with lower knowledge, but did not change significantly over time when examining univariable covariate by time interaction models. Cancer-specific distress was generally higher in men (full model coef=15.1, p=0.002), and those with a personal history of cancer (coef 8.4, p<0.001), but lower with increasing age (coef= −0.2, p=0.008). Cancer-specific distress did not change significantly over time for those with a personal history of cancer or by age, but increased for men at 12 months (univariable covariate by time interaction coef 26.2 at T4, versus T0, p<0.001).
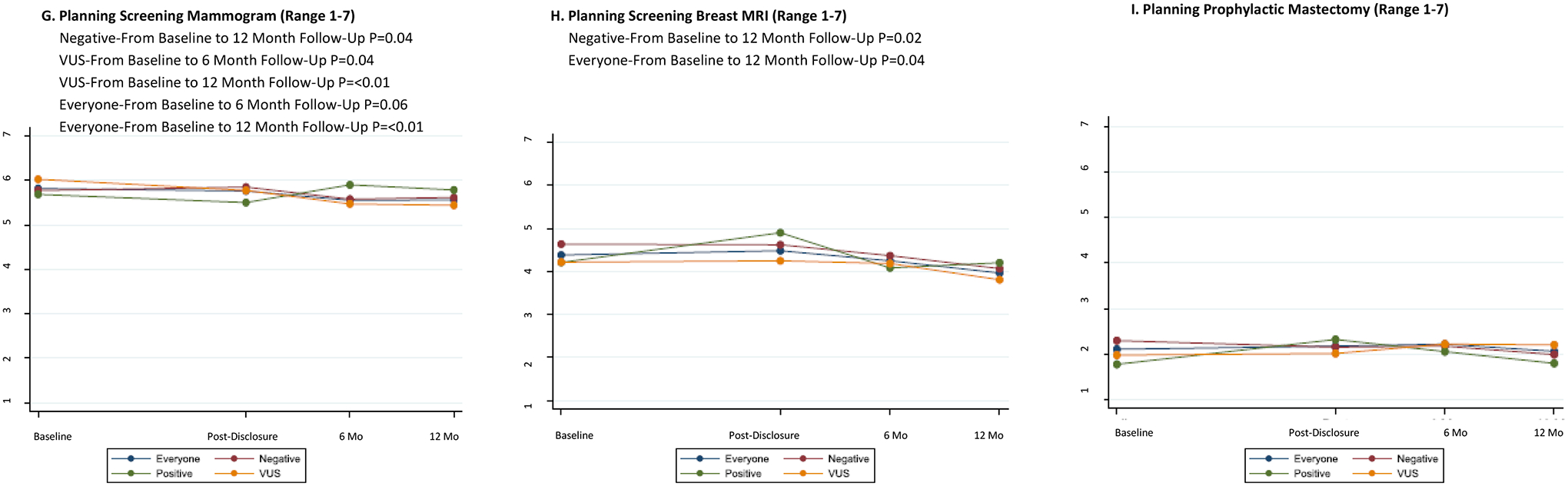
Behavioral intention and medical management recommendations after multigene panel testing
Intention to perform mammogram, breast MRI or prophylactic mastectomy did not change significantly from baseline to after result disclosure (Figure 2G–I). Overtime, intent to perform a mammogram declined significantly from baseline to 6 and 12 months, and intent to perform MRI declined significantly from baseline to 12 months (Figures 2G, 2H). There was no significant change in intent to perform prophylactic mastectomy (Figure 2I).
In exploratory analyses by test result, intent to perform mammography declined significantly among those with a negative or VUS result, but did not change significantly for those with a positive result (Figure 2G). Intent to perform screening breast MRI declined significantly at 6 and 12 months only among those with a negative result (Figure 2H). There were no significant changes in intent to perform prophylactic mastectomy in any of the result subgroups (Figure 2I).
Among the 23 patients with a positive result, medical management recommendations changed in 6 (26%) cases and 2.6% of all tested. (Supplementary Table 2). Of note, for two patients, their insurers did not cover large genomic rearrangement testing at the time of initial BRCA1/2 testing and they were found to have large genomic rearrangements. Apart from these two BRCA findings, medical management change in 4 (15%) of cases. In 7 (30%) cases, the positive result had the potential to change management but did not, either because of uncertainty regarding risks and appropriate medical management in moderate penetrance genes (n=4) or the patient had already had risk reducing mastectomy (n=3). See Supplementary Table 1 for additional details. In 10 cases (43%), we identified low penetrant variants in APC or monoallelic mutations in MYH, where increasing colonoscopy frequency to every five years has been recommended, but is based on limited data35.
DISCUSSION
Despite increasing use of MGPT in clinical practice, there are relatively limited studies evaluating comprehensive patient reported outcomes (PROs). To our knowledge this is the largest longitudinal study of PROs in clinical patients undergoing MGPT after negative BRCA1/2 testing. These data in BRCA1/2 negative patients, reveal no significant short-term increases in distress and increased knowledge after pre-test counseling. Additionally, they demonstrate that there is a small, but real, chance that there will be a change in medical management for the patients. Given a large population of patients with prior negative testing for BRCA 1/2 (without panel testing), these results can help inform decision making regarding the value of further panel testing.
These results are consistent with the COGENT study, a multi-center randomized trial of phone compared to in-person disclosure of germline genetic test results, which included patients undergoing MGPT, the RESPECT study, a study evaluating the uptake and outcomes of returning MGPT research results to research participants,15; 36 and a recently published study in breast cancer patients focused on cancer worry exclusively.17 While we found greater increase in uncertainty among patients undergoing MGPT as compared to targeted testing in the COGENT study,2 longitudinal data from the COGENT study and these data reveal declines in uncertainty over time.37 This suggests that while more complex MGPT may be associated with increased uncertainty for some patients after receipt of results, uncertainty is likely to diminish over time. Of note, we did find small increases in cancer-specific distress and depression at 6 and 12 months in this study, which is in contrast to longitudinal data from the COGENT study where there were no significant increases in these outcomes among the subgroup who had MGPT.37 This may be related to the fact that patients in METEOR have already lived with the uncertainty of negative results and are now embarking on a second round of testing, as opposed to COGENT where MGPT was done as their first testing experience. Although the increases in this study were small, and may not be sufficiently large enough changes to be clinically significant, they do suggest value in continued evaluation of longitudinal affective outcomes among a wide range of patients, and in different testing scenarios, as MGPT is increasingly utilized. Additionally, secondary analyses revealed greater cancer specific distress in patients with a personal history of cancer and significant increases in longitudinal cancer-specific distress in men, although these may not be large enough to be clinically significant, and they should be confirmed in larger samples. Secondary analyses also revealed lower knowledge that persisted among older and non-white patients. Attention to these subgroups may be important as we continue to use MGPT in clinical care.
Equally important, and consistent with the COGENT study,15; 37 we did not identify increased distress in the short- term or long-term among the subset with a positive result. Another subgroup of interest are patients who receive a VUS result, given that approximately 30% of patients who undergo MGPT receive a VUS result, which can be difficult for providers and patients to understand.3 In this study, patients with a VUS result had increases in cancer-specific distress and depression at 6 and 12 months, despite corresponding declines in uncertainty. This is in contrast to the COGENT study where there were no significant differences in this outcome among those with a VUS,37 and Katz et al. who found no impact of test result on cancer worry after MGPT in breast cancer patients.17 Given conflicting data and different measures and populations across the studies to date, continued evaluation of outcomes by test result, including both those with a positive and VUS result, and in representative clinical populations remains important.
Longitudinal data on performance of health behaviors after testing is needed to fully understand the clinical utility of incorporating MGPT into clinical practice. While many studies have suggested that MGPT identifies more genetic mutations than targeted testing and therefore has clinical utility in a large proportion of patients,1; 4–6 we have found that MGPT currently changes medical management recommendations in relatively few patients compared to management based on personal and family history.36; 38 It would also be useful for future studies to compare medical management changes among those who get MGPT compared to a population that has not had testing. Equally important, there have been concerns that MGPT could be associated with greater misunderstanding, false reassurance or inappropriate use of screening or prophylactic surgery, particularly among those with a VUS result.10; 39 In this study, with post-test counseling by both a GC and a physician, we did not find an increase in intention to have prophylactic mastectomy after MGPT, consistent with a recent study reporting no increase in prophylactic surgery after MGPT in breast cancer patients.3 We did find that intent to perform mammography and screening breast MRI declined over time, specifically among those with a VUS or uninformative negative result. Further study of behavioral outcomes among all subgroups and based on what is indicated according to their personal and family history is needed to confirm that MGPT results are being used appropriately. Equally important, whole exome testing is starting to enter the clinical arena, potentially introducing greater uncertainty and risks for distress or misunderstanding. Thus, it will be important to also study patient reported outcomes with even more expanded testing.
While these data suggest favorable cognitive and affective outcomes among interested patients, who undergo pre- and post-test counseling utilizing the tiered-binned model, it is important to acknowledge that there are some patients who decline testing or receipt of results. Some patients expressed concerns about risk of distress and uncertainty, supporting the value of pre-test counseling to ascertain patient preferences, particularly as the clinical utility of large panels remains unclear. In contrast to a recent study reporting high hypothetical interest in MGPT among BRCA1/2 negative participants,40 many patients in this study did not respond to the invitation to receive multigene panel testing. It is possible that uptake of testing after already completing prior BRCA1/2 testing is lower than when multigene panel testing is offered at initial testing, due to testing fatigue or other testing experiences. Equally important, we found higher uptake when patients had a discussion with a research staff member or their physician, suggesting that re-contacting people in writing may not be the most effective strategy for offering updated MGPT. Additionally, we found that non-white patients were more likely to not respond to the invitation for MGPT, raising the possibility for disparities in uptake of testing, consistent with previously published studies.41; 42. Additionally, a study that surveyed patients retrospectively after MGPT did find higher distress and lower satisfaction among non-white patients, providing strong rationale for additional studies to determine if outcomes differ for minority populations.39
We acknowledge several limitations of this study. This is a single-site study and results may not be representative of more diverse clinical settings and populations. We don’t know if non-responders received the invitation and understood what was being offered, and our findings may represent a more select group of patients and may not be representative of all clinical patients eligible for MGPT. Those who participated were predominantly white, women with a college education and further data in diverse clinical populations are needed. Additionally, most of the patients had a personal history of cancer and the counseling and testing were covered by the study, which may limit generalizability to some clinical populations. Subgroup analyses by test result include relatively small sample sizes and would benefit from confirmation. There was some loss to attrition which could impact outcomes. This study included multiple outcomes to explore which may be most salient to inform primary and secondary outcomes for future research. Thus we did not correct for multiple hypotheses. In many cases the overall change was relatively small and well below clinical cut-offs for clinically significant findings (where available). Thus, these significant changes may not be clinically significant for most patients. Many of our positive results were low penetrant variants, which could explain our favorable affective outcomes. Our findings were in the setting of in-person pre-test and post-test counseling, using the tiered binned counseling model, and findings may not be similar with different counseling or delivery approaches. We have not included the potential impact of findings on family members in this discussion. This issue is complex as the finding of a CHEK2, for example, may not change recommendations for family members in the presence of a strong family history. Additional research on the downstream impact of testing on change of medical management in family members is needed.
These prospective data suggest favorable short-term and longitudinal patient reported cognitive and affective outcomes after multigene panel testing, among interested patients and with pre and post-test genetic counseling. Additional studies evaluating longitudinal behavioral and screening outcomes, and psychosocial and behavioral outcomes by test subgroup are needed. Additionally, attention to disparities in uptake and outcomes among non-white patients may be important as multigene panel testing is more widely adopted in clinical care.
Supplementary Material
FUNDING/ACKNOWLEDGEMENTS:
Supported by the Rooney Family Fund. The cost of genetic testing was covered by Myriad Genetics. Myriad Genetics did not otherwise provide financial support for the study.
Footnotes
CONFLICT OF INTEREST NOTIFICATION PAGE:
Dr. Domchek has received honoraria from AstraZeneca, Clovis and Bristol Myers Squibb. Dr. Bradbury has received honoraria from AstraZeneca and Merck.
DATA AVAILABILITY STATEMENT:
The datasets used and/or analyzed during the current study are not publicly available and are securely in the possession of the corresponding author, who has full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
REFERENCES
- 1.Yorczyk A, Robinson LS, and Ross TS (2015). Use of panel tests in place of single gene tests in the cancer genetics clinic. Clinical genetics 88, 278–282. [DOI] [PubMed] [Google Scholar]
- 2.Hall MJ, Patrick-Miller LJ, Egleston BL, Domchek SM, Daly MB, Ganschow P, Grana G, Olopade OI, Fetzer D, Brandt A, et al. (2018). Use and Patient-Reported Outcomes of Clinical Multigene Panel Testing for Cancer Susceptibility in the Multicenter Communication of Genetic Test Results by Telephone Study. JCO Precision Oncology, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kurian AW, Ward KC, Hamilton AS, Deapen DM, Abrahamse P, Bondarenko I, Li Y, Hawley ST, Morrow M, Jagsi R, et al. (2018). Uptake, Results, and Outcomes of Germline Multiple-Gene Sequencing After Diagnosis of Breast Cancer. JAMA Oncol 4, 1066–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Desmond A, Kurian AW, Gabree M, Mills MA, Anderson MJ, Kobayashi Y, Horick N, Yang S, Shannon KM, Tung N, et al. (2015). Clinical Actionability of Multigene Panel Testing for Hereditary Breast and Ovarian Cancer Risk Assessment. JAMA oncology 1, 943–951. [DOI] [PubMed] [Google Scholar]
- 5.LaDuca H, Stuenkel AJ, Dolinsky JS, Keiles S, Tandy S, Pesaran T, Chen E, Gau CL, Palmaer E, Shoaepour K, et al. (2014). Utilization of multigene panels in hereditary cancer predisposition testing: analysis of more than 2,000 patients. Genetics in medicine : official journal of the American College of Medical Genetics 16, 830–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pearlman R, Frankel WL, Swanson B, Zhao W, Yilmaz A, Miller K, Bacher J, Bigley C, Nelsen L, Goodfellow PJ, et al. (2017). Prevalence and Spectrum of Germline Cancer Susceptibility Gene Mutations Among Patients With Early-Onset Colorectal Cancer. JAMA oncology 3, 464–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pedersen IS, Schmidt AY, Bertelsen B, Ernst A, Andersen CLT, Kruse T, Rossing M, and Thomassen M (2018). A Danish national effort of BRCA1/2 variant classification. Acta oncologica 57, 159–162. [DOI] [PubMed] [Google Scholar]
- 8.Katki HA, Greene MH, and Isabel Achatz M (2018). Testing Positive on a Multigene Panel Does Not Suffice to Determine Disease Risks. Journal of the National Cancer Institute. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kurian AW, and Ford JM (2015). Multigene Panel Testing in Oncology Practice: How Should We Respond? JAMA Oncol 1, 277–278. [DOI] [PubMed] [Google Scholar]
- 10.Kurian AW, Li Y, Hamilton AS, Ward KC, Hawley ST, Morrow M, McLeod MC, Jagsi R, and Katz SJ (2017). Gaps in Incorporating Germline Genetic Testing Into Treatment Decision-Making for Early-Stage Breast Cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 35, 2232–2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bradbury AR, Patrick-Miller L, and Domchek S (2015). Multiplex genetic testing: reconsidering utility and informed consent in the era of next-generation sequencing. Genet Med 17, 97–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lorans M, Dow E, Macrae FA, Winship IM, and Buchanan DD (2018). Update on Hereditary Colorectal Cancer: Improving the Clinical Utility of Multigene Panel Testing. Clinical colorectal cancer. [DOI] [PubMed] [Google Scholar]
- 13.Robson ME, Bradbury AR, Arun B, Domchek SM, Ford JM, Hampel HL, Lipkin SM, Syngal S, Wollins DS, and Lindor NM (2015). American Society of Clinical Oncology Policy Statement Update: Genetic and Genomic Testing for Cancer Susceptibility. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 33, 3660–3667. [DOI] [PubMed] [Google Scholar]
- 14.Boyar SR, and Shapiro CL (2016). Ready, Fire, Aim: Addressing Issues Associated With Multigene Panel Testing for Cancer Susceptibility. Oncology 30, 800, 807. [PubMed] [Google Scholar]
- 15.Bradbury AR, Patrick-Miller LJ, Egleston BL, Hall MJ, Domchek SM, Daly MB, Ganschow P, Grana G, Olopade OI, Fetzer D, et al. (2018). Randomized Noninferiority Trial of Telephone vs In-Person Disclosure of Germline Cancer Genetic Test Results. Journal of the National Cancer Institute 110, 985–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bradbury AR, Patrick-Miller LJ, Egleston BL, DiGiovanni L, Brower J, Harris D, Stevens EM, Maxwell KN, Kulkarni A, Chavez T, et al. (2015). Patient feedback and early outcome data with a novel tiered-binned model for multiplex breast cancer susceptibility testing. Genet Med. [DOI] [PubMed] [Google Scholar]
- 17.Katz SJ, Ward KC, Hamilton AS, Abrahamse P, Hawley ST, and Kurian AW (2018). Association of Germline Genetic Test Type and Results With Patient Cancer Worry After Diagnosis of Breast Cancer. JCO Precision Oncology, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bradbury AR, Patrick-Miller L, Long J, Powers J, Stopfer J, Forman A, Rybak C, Mattie K, Brandt A, Chambers R, et al. (2015). Development of a tiered and binned genetic counseling model for informed consent in the era of multiplex testing for cancer susceptibility. Genetics in medicine : official journal of the American College of Medical Genetics 17, 485–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patrick-Miller LJ, Egleston BL, Fetzer D, Forman A, Bealin L, Rybak C, Peterson C, Corbman M, Albarracin J, Stevens E, et al. (2014). Development of a communication protocol for telephone disclosure of genetic test results for cancer predisposition. JMIR research protocols 3, e49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leventhal H, Benyamini Y, Brownlee S, Diefenbach M, Leventhal EA, and Patrick-Miller L (1997). Perceptions of Health and Illness: Current Research and Applications. In Illness representations: theoretical foundations, Petrie KJ and Weinman JA, eds. (Amsterdam, Harwood; ), pp 19–46. [Google Scholar]
- 21.Shiloh S (2006). Illness representations, self-regulation, and genetic counseling: a theoretical review. Journal of genetic counseling 15, 325–337. [DOI] [PubMed] [Google Scholar]
- 22.Kaphingst KA, McBride CM, Wade C, Alford SH, Reid R, Larson E, Baxevanis AD, and Brody LC (2012). Patients’ understanding of and responses to multiplex genetic susceptibility test results. Genetics in medicine : official journal of the American College of Medical Genetics. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lerman C, Narod S, Schulman K, Hughes C, Gomez-Caminero A, Bonney G, Gold K, Trock B, Main D, Lynch J, et al. (1996). BRCA1 testing in families with hereditary breast-ovarian cancer. A prospective study of patient decision making and outcomes. Jama 275, 1885–1892. [PubMed] [Google Scholar]
- 24.Kelly K, Leventhal H, Marvin M, Toppmeyer D, Baran J, and Schwalb M (2004). Cancer genetics knowledge and beliefs and receipt of results in Ashkenazi Jewish individuals receiving counseling for BRCA1/2 mutations. Cancer Control 11, 236–244. [DOI] [PubMed] [Google Scholar]
- 25.Speilberger CD, Gorsuch RL, Lushene R, Vagg PR, and Jacobs GA (1983). Manual for the State-Trait Anxiety Inventory.(Palo Alto, CA: Consulting Psychologists Press; ). [Google Scholar]
- 26.Hamilton JG, Lobel M, and Moyer A (2009). Emotional distress following genetic testing for hereditary breast and ovarian cancer: a meta-analytic review. Health psychology : official journal of the Division of Health Psychology, American Psychological Association 28, 510–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zigmond AS, and Snaith RP (1983). The hospital anxiety and depression scale. Acta Psychiatr Scand 67, 361–370. [DOI] [PubMed] [Google Scholar]
- 28.Horowitz M, Wilner N, and Alvarez W (1979). Impact of Event Scale: a measure of subjective stress. Psychosomatic medicine 41, 209–218. [DOI] [PubMed] [Google Scholar]
- 29.Pieterse AH, van Dulmen AM, Beemer FA, Bensing JM, and A. MGEM (2007). Cancer genetic counseling: Communication and counselees’ post-visit satisfaction, cognitions, anxiety, and needs fulfillment. Journal of genetic counseling 16, 85–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patrick-Miller L, Egleston BL, Daly M, Stevens E, Fetzer D, Forman A, Bealin L, Rybak C, Peterson C, Corbman M, et al. (2013). Implementation and outcomes of telephone disclosure of clinical BRCA1/2 test results. Patient education and counseling 93, 413–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cella D, Hughes C, Peterman A, Chang CH, Peshkin BN, Schwartz MD, Wenzel L, Lemke A, Marcus AC, and Lerman C (2002). A brief assessment of concerns associated with genetic testing for cancer: the Multidimensional Impact of Cancer Risk Assessment (MICRA) questionnaire. Health psychology : official journal of the Division of Health Psychology, American Psychological Association 21, 564–572. [PubMed] [Google Scholar]
- 32.Raghunathan TE, Lepkowski JM, Van Hoewyk J, and Solenberger P (2001). A multivariate technique for multiplying imputing missing values using a sequence of regression models. Survey Methodology 27, 85–95. [Google Scholar]
- 33.S. JL (1997). Analysis of Incomplete Multivariate Data.(Boca Raton, FL: Chapman and Hall; ). [Google Scholar]
- 34.Bjelland I, Dahl AA, Haug TT, and Neckelmann D (2002). The validity of the Hospital Anxiety and Depression Scale. An updated literature review. Journal of psychosomatic research 52, 69–77. [DOI] [PubMed] [Google Scholar]
- 35.Katona BW, Yurgelun MB, Garber JE, Offit K, Domchek SM, Robson ME, and Stadler ZK (2018). A counseling framework for moderate-penetrance colorectal cancer susceptibility genes. Genet Med. [DOI] [PubMed] [Google Scholar]
- 36.Bradbury AR, Patrick-Miller L, Egleston BL, Maxwell KN, DiGiovanni L, Brower J, Fetzer D, Gaieski JB, Brandt A, McKenna D, et al. (2018). Returning Individual Genetic Research Results to Research Participants: Uptake and Outcomes Among Patients With Breast Cancer. JCO Precision Oncology, 1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kilbride MK, Egleston BL, Hall MJ, Patrick-Miller LJ, Domchek SM, Daly MB, Ganschow P, Grana G, Olopade OI, Fetzer D, et al. (2018. (submitted).). Longitudinal cognitive, affective and behavioral outcomes in a randomized non-inferiority trial of in-person versus telephone disclosure of germline cancer genetic test results.
- 38.Bradbury AR, Patrick-Miller LJ, Egleston BL, DiGiovanni L, Brower J, Harris D, Stevens EM, Maxwell KN, Kulkarni A, Chavez T, et al. (2016). Patient feedback and early outcome data with a novel tiered-binned model for multiplex breast cancer susceptibility testing. Genetics in medicine : official journal of the American College of Medical Genetics 18, 25–33. [DOI] [PubMed] [Google Scholar]
- 39.Lumish HS, Steinfeld H, Koval C, Russo D, Levinson E, Wynn J, Duong J, and Chung WK (2017). Impact of Panel Gene Testing for Hereditary Breast and Ovarian Cancer on Patients. J Genet Couns 26, 1116–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vicuna B, Delaney HD, Flores KG, Ballinger L, Royce M, Dayao Z, Pal T, and Kinney AY (2018). Preferences for multigene panel testing for hereditary breast cancer risk among ethnically diverse BRCA-uninformative families. Journal of community genetics 9, 81–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Allford A, Qureshi N, Barwell J, Lewis C, and Kai J (2014). What hinders minority ethnic access to cancer genetics services and what may help? European journal of human genetics : EJHG 22, 866–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hann KEJ, Freeman M, Fraser L, Waller J, Sanderson SC, Rahman B, Side L, Gessler S, Lanceley A, and team P.s. (2017). Awareness, knowledge, perceptions, and attitudes towards genetic testing for cancer risk among ethnic minority groups: a systematic review. BMC public health 17, 503. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are not publicly available and are securely in the possession of the corresponding author, who has full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.


