Abstract
Background and Objectives
Although alpha-synuclein–related pathology is the hallmark of dementia with Lewy bodies (DLB), cerebrovascular and Alzheimer disease pathologies are common in patients with DLB. Little is known about the contribution of these pathologies to neurodegeneration in DLB. We investigated associations of cerebrovascular, β-amyloid, and tau biomarkers with gray matter (GM) volume in patients with probable DLB.
Methods
We assessed patients with probable DLB and cognitively unimpaired (CU) controls with 11C-Pittsburgh compound B (PiB) and 18F-flortaucipir PET as markers of β-amyloid and tau, respectively. MRI was used to assess white matter hyperintensity (WMH) volume (a marker of cerebrovascular lesion load) and regional GM volume (a marker of neurodegeneration). We used correlations and analysis of covariance (ANCOVA) in the entire cohort and structural equation models (SEMs) in patients with DLB to investigate associations of WMH volume and regional β-amyloid and tau PET standardized uptake value ratios (SUVrs) with regional GM volume.
Results
We included 30 patients with DLB (69.3 ± 10.2 years, 87% men) and 100 CU controls balanced on age and sex. Compared with CU controls, patients with DLB showed a lower GM volume across all cortical and subcortical regions except for the cuneus, putamen, and pallidum. A larger WMH volume was associated with a lower volume in the medial and orbital frontal cortices, insula, fusiform cortex, and thalamus in patients with DLB. A higher PiB SUVr was associated with a lower volume in the inferior temporal cortex, while flortaucipir SUVr did not correlate with GM volume. SEMs showed that a higher age and absence of the APOE ε4 allele were significant predictors of higher WMH volume, and WMH volume in turn was a significant predictor of GM volume in medial and orbital frontal cortices, insula, and inferior temporal cortex. By contrast, we observed 2 distinct paths for the fusiform cortex, with age having an effect through PiB and flortaucipir SUVr on one path and through WMH volume on the other path.
Discussion
Patients with probable DLB have widespread cortical atrophy, most of which is likely influenced by alpha-synuclein–related pathology. Although cerebrovascular, β-amyloid, and tau pathologies often coexist in probable DLB, their contributions to neurodegeneration seem to be region specific.
Dementia with Lewy bodies (DLB) is the second most common neurodegenerative dementia, after Alzheimer disease (AD). While alpha-synuclein–related pathology is the main hallmark of DLB, cerebrovascular and AD pathologies are also common in patients with DLB.1-3 It is likely that these coexisting pathologies contribute to the neurodegeneration in DLB. However, their contribution to regional atrophy and whether their effects are isolated or joint are largely unknown. One reason for this knowledge gap is the lack of studies combining cerebrovascular, AD, and neurodegeneration biomarkers in the same cohort. Another reason is that these pathologies can also be present in cognitively unimpaired (CU) individuals. Hence, whether findings are specific to DLB or ubiquitous to the aging population is unclear.
Some studies have shown individual associations of cerebrovascular and AD biomarkers with neurodegeneration in DLB.4-9 White matter hyperintensities (WMH) are widely used MRI biomarkers of cerebrovascular disease,10 and gray matter (GM) volume on MRI is a common marker of neurodegeneration. In imaging studies, AD pathology is commonly assessed through different tracers for β-amyloid and tau neurofibrillary tangles (NFTs) on PET. Only 3 studies have investigated the association of WMH with neurodegeneration in DLB.4,6,9 In one of these, we recently showed that a higher WMH volume was associated with thinner orbitofrontal, retrosplenial, and posterior cingulate cortices and a smaller volume with the thalamus and pallidum.4 Two earlier studies showed that higher visual rating scores of WMH were associated with reduced global brain volume and higher visual rating scores of medial temporal atrophy.6,9 For AD biomarkers, some studies did not find associations between amyloid PET and GM volumes,7 while other studies reported significant associations.5,8 The only longitudinal tau PET study showed that an increase in tau in lateral occipital and temporoparietal cortices was associated with increased rates of neurodegeneration in DLB over time.11 However, none of these studies, including our own, combined all biomarkers in the same cohort, so that it is still unclear how cerebrovascular and AD biomarkers together associate with neurodegeneration and whether these associations are specific to DLB or are a feature of aging. Answering these questions would identify DLB-specific targets for future trials and improve the currently low clinical detection rate of DLB.12
Our overall goal was to investigate the associations of cerebrovascular and AD biomarkers with neurodegeneration in patients with DLB. Our first objective was to assess univariate associations of WMH, β-amyloid, and tau biomarkers with regional GM volume in patients with DLB. We also investigated these associations in an age-balanced and sex-balanced CU group for comparison. Our second objective was to assess multivariable associations of WMH, β-amyloid, and tau biomarkers with regional GM volume in patients with DLB using structural equation models (SEMs). We addressed the question of isolated vs joint associations of WMH and AD biomarkers with GM volume, in the context of important modifiers such as age and APOE genotype. We hypothesized that WMH and AD biomarkers would have an association with GM volume in DLB, but the spatial patterns would be different.
Methods
Participants
We included consecutive patients with probable DLB12,13 who were at mild to moderate clinical stages. Patients were recruited at the Mayo Clinic Alzheimer Disease Research Center (ADRC)14 between November 2017 and October 2020. Clinical evaluations included a neurologic examination and standardized instruments for the assessment of cognitive performance and activities of daily living. We selected the Mini-Mental State Examination (MMSE) to characterize the cohort for global cognitive performance. We also assessed clinical features characteristic of DLB, which are as follows: (1) Unified Parkinson Disease Rating Scale, part III (UPDRS-III) for parkinsonism; (2) visual hallucinations characterized by being fully formed, not restricted to a single episode and not related to another medical issue; (3) cognitive fluctuations defined as a score of 3 or 4 on the Mayo Fluctuations Questionnaire15; and (4) probable REM sleep behavior disorder (RBD) based on the International Classification of Sleep Disorders-II diagnostic criteria.16 Clinical diagnosis was established by a consensus committee including behavioral neurologists, neuropsychologists, and study coordinators.
In addition, we included a CU group from the population-based MCSA, which was balanced on age and sex with the DLB group.
All patients with probable DLB and CU individuals were required to have 3T MRI, β-amyloid, and tau PET scans as per inclusion criteria. Exclusion criteria for the DLB and CU groups were a history of traumatic brain injury, hydrocephalus, or intracranial mass and a history of chemotherapy, head radiation therapy, or substance abuse. DLB participants with neurologic or psychiatric disorders other than DLB were excluded. CU participants with any neurologic or psychiatric disease were excluded.
PET and MRI Biomarkers: Data Acquisition and Image Processing
PET imaging was performed on Siemens and General Electric PET/CT scanners. The tracers 11C-Pittsburgh compound B (PiB) and flortaucipir (18F-AV-1451) were used to assess β-amyloid and tau pathologies, respectively, following the procedures described in Ferreira et al.2 High-resolution 3D T1-weighted magnetization prepared rapid gradient echo and axial T2-weighted fluid-attenuated inversion recovery (FLAIR) MRI sequences were acquired on 3T scanners (Siemens) for the assessment of GM volume and WMH, respectively.
All PET and MRI scans were visually inspected for technical quality. The study setup from PET and MRI scans to statistical analysis is represented in Figure 1 and described in detail in eMethods in the Supplement (links.lww.com/WNL/C497). In brief, SPM12 was used to segment gray and white matter from T1-weighted MRIs and to rigidly align PET images with them.17 Using Mayo Clinic Adult Lifespan Template (MCALT) atlases, we determined β-amyloid and tau positivity by obtaining the respective cortical PiB and flortaucipir retention standardized uptake value ratio (SUVr) of β-amyloid and tau meta-regions of interest (ROIs) (see eMethods). Meta-ROI SUVrs were classified as normal (−) or abnormal (+) using a cut point of ≥1.48 for PiB and a cut point of ≥1.25 for flortaucipir.18 In total, MCALT atlases provided estimations of PiB and flortaucipir SUVr across 23 ROIs (Figure 1 and eTable 1 in the Supplement).
Figure 1. Study Setup: Biomarkers, MCALT Atlas, and Statistical Analyses.
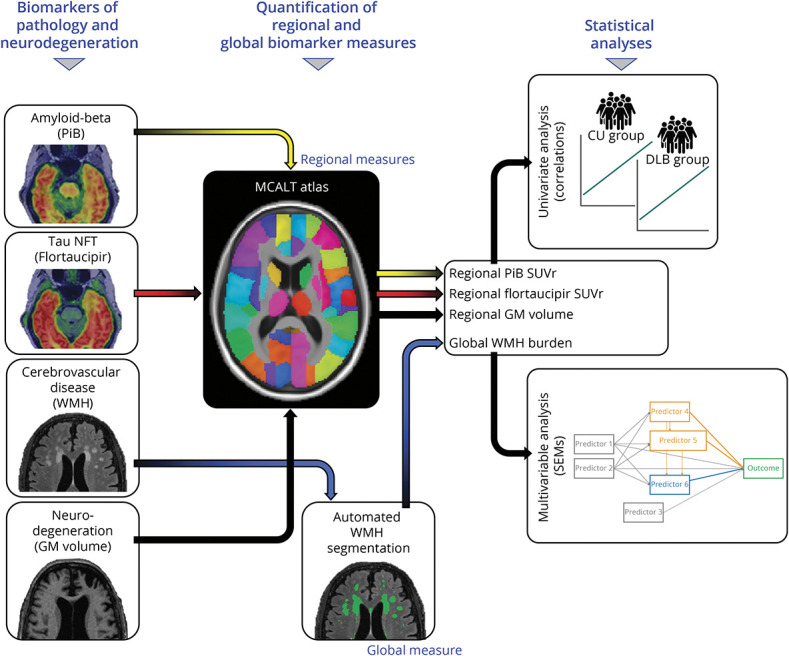
All PET and MRI scans were visually inspected for technical quality. PiB and flortaucipir PET scans were rigidly aligned to individuals' T1-weighted MRIs. Using MCALT atlases, we determined β-amyloid and tau positivity. WMH were assessed on FLAIR MRI using a fully automated segmentation method. For regional GM volume, we propagated MCALT atlases to individuals' native T1-weighted MRIs. Hence, the MCALT output for PiB, flortaucipir, and GM volume provided regional measures base on the atlases, while the WMH output represented a global estimation of WMH volume. PiB, flortaucipir, WMH, and GM volume were analyzed using univariate and multivariable methods. Analyses were primarily conducted in the DLB group, and univariate analyses were replicated in the CU group only for those regions that resulted significant in the DLB group, for comparison. Univariate analyses (correlations) were set up in a region-to-region fashion for PiB and flortaucipir and in a global WMH volume to regional GM volume fashion for WMH. In multivariable analyses (SEMs), PiB, flortaucipir, and WMH were included together as predictors, and GM volume was the outcome variable. Abbreviations: CU = cognitively unimpaired; DLB = dementia with Lewy bodies; GM = gray matter; MCALT = Mayo Clinic Adult Lifespan Template; NFT = neurofibrillary tangles; PiB = Pittsburgh compound B; WMH = white matter hyperintensities; SEMs = structural equation models; SUVr = standardized uptake value ratios.
The WMH volume was quantified from 3D FLAIR images using a fully automated updated version of a method previously described19 and explained in detail in eMethods in the Supplement (links.lww.com/WNL/C497).
For regional GM volume, we propagated MCALT atlases to individuals' native space, and regional volumetric estimations across the cortical mantle and subcortical GM structures were calculated. Left and right ROIs were combined, giving a total of 21 cortical ROIs and 7 subcortical ROIs. The total intracranial volume (TIV) was estimated from the tissue probabilities and included in statistical models involving GM volume, to account for between-subject variability in head size.
Statistical Analysis
Demographic and clinical characteristics were reported using mean values and standard deviations for continuous variables, medians and interquartile range for the strongly skewed WMH volume, and counts and percentages for categorical variables. Group differences were tested with t tests for continuous variables and chi-square tests for categorical variables. PiB SUVr, flortaucipir SUVr, and WMH volume were analyzed with a log transformation because of skewness.
We used ANCOVA (age and TIV as covariates) to test for differences in regional GM volume between the DLB and CU groups. p values were adjusted with the false discovery rate method for multiple testing.
Univariate associations of PiB and flortaucipir SUVr and WMH volume with regional GM volume were assessed through partial Spearman correlations, adjusting for age and TIV. These correlations were set up in a region-to-region fashion for PiB and flortaucipir (e.g., PiB and flortaucipir SUVr in the middle frontal cortical region were correlated with GM volume in the middle frontal cortical region). For WMH, we used a global estimation of WMH volume, so that correlations were set up in a global WMH volume to regional GM volume fashion. This was performed to replicate previous studies where GM volume has often been correlated with biomarkers separately, in a univariate manner.4-9 Furthermore, by comparing univariate and multivariable results, we could approximate pathologic underpinnings of neurodegeneration (i.e., observe competitive effects in multivariable analysis in comparison with findings from univariate analysis—how introducing biomarkers in a multivariable model modifies the univariate association of a given biomarker with GM volume). Correlations were explorative to identify relevant regions for SEMs. Differences in slopes were tested through linear regression with an interaction term for group by biomarker of pathology toward the prediction of regional GM volume (TIV was added as an adjustment variable).
Complex multivariable associations of PiB and flortaucipir SUVr and WMH volume with regional GM volume were assessed with SEMs. Once again, PiB, flortaucipir, and GM volume were regional estimations, while WMH represented a global estimation of WMH volume. We included age and APOE genotype as important exogenous predictors of AD and CVD biomarkers and GM volume in DLB. The TIV was also added to the SEMs as a confounding factor of GM volume. Figure 2A shows the general form of our SEMs, and details of these models are further described in eMethods in the Supplement (links.lww.com/WNL/C497). MPlus version 8 was used to fit the SEMs.20
Figure 2. Structural Equation Models (SEMs).
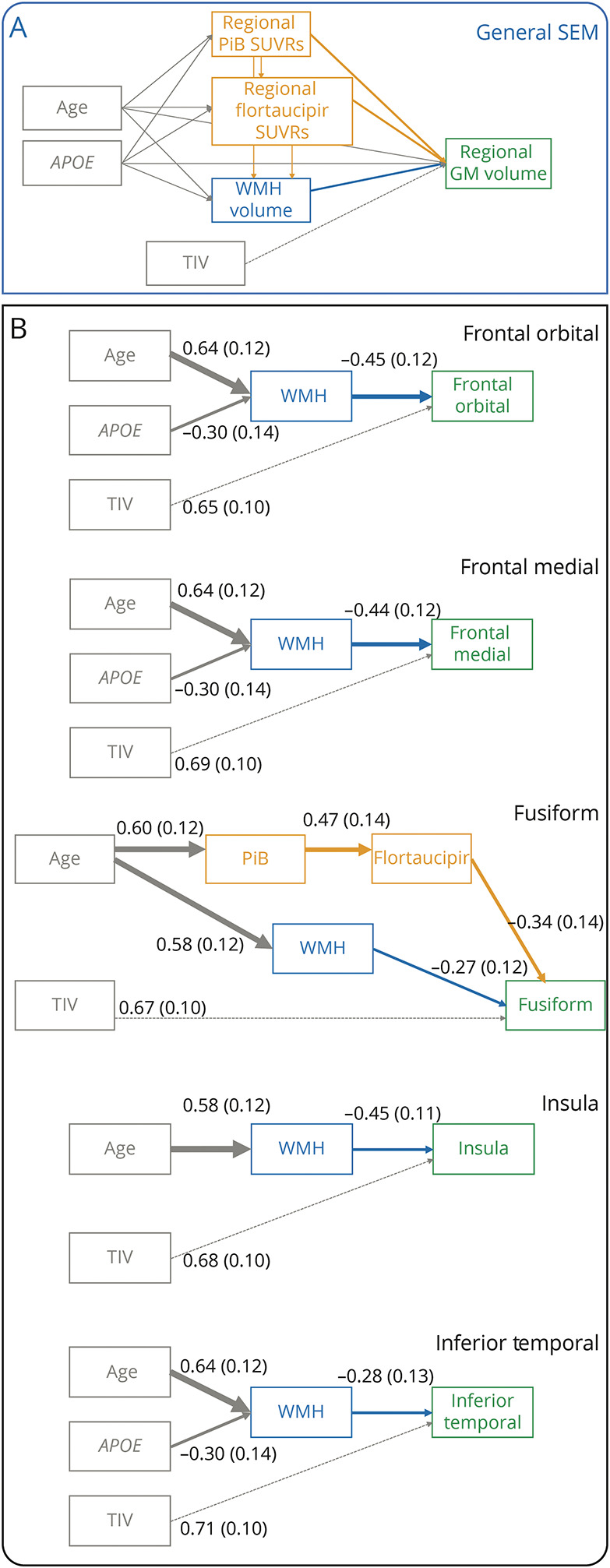
In all SEMs, PiB, flortaucipir, and GM volume were regional estimations and WMH represented a global estimation of WMH volume. We included age and APOE genotype as important modifiers of PiB, flortaucipir, WMH, and GM volume in DLB. The TIV was also added to the SEMs as a confounding factor of GM volume. (A) General form of our SEMs. (B) SEMs for regions of interest. Arrows that join nodes are direct effects, and arrows that join nodes but pass through intervening variables are indirect effects. The intervening variables are mediators. Effects come from linear regression models and represent regression coefficients (and standard errors between brackets). Coefficients were standardized per standard deviation change in each variable to facilitate interpretation across predictors. The unit for age is decades. TIV was scaled to TIV/1,000. Thickness in all arrows reflects the strength of regression coefficients, except for that of the TIV. The TIV is represented as an unweighted dashed line because this variable was not interpreted; it was included only in the SEMs to calculate the effects of PiB, flortaucipir, and WMH volume independent of TIV. Abbreviations: APOE = Apolipoprotein E; DLB = dementia with Lewy bodies; GM = gray matter; PiB = Pittsburgh compound B; SUVr = standardized uptake value ratios; WMH = white matter hyperintensities; TIV = total intracranial volume; SEM = structural equation model.
Thalamus, caudate, putamen, pallidum, and substantia innominata were excluded from all analyses involving PiB and flortaucipir because of off-target binding in the thalamus and basal ganglia and the small size of the substantia innominata for PET resolution. A p value ≤ 0.05 (2-tailed) was deemed significant in all the analyses.
Standard Protocol Approvals, Registrations, and Patient Consents
The Mayo Clinic Institutional Review Board approved the study (17-011339). Informed consent on participation was obtained from all patients or a surrogate according to the Declaration of Helsinki.
Data Availability
Anonymized data will be shared by request from a qualified investigator in accordance with the Mayo ADRC data sharing protocol.
Results
Cohort Characteristics
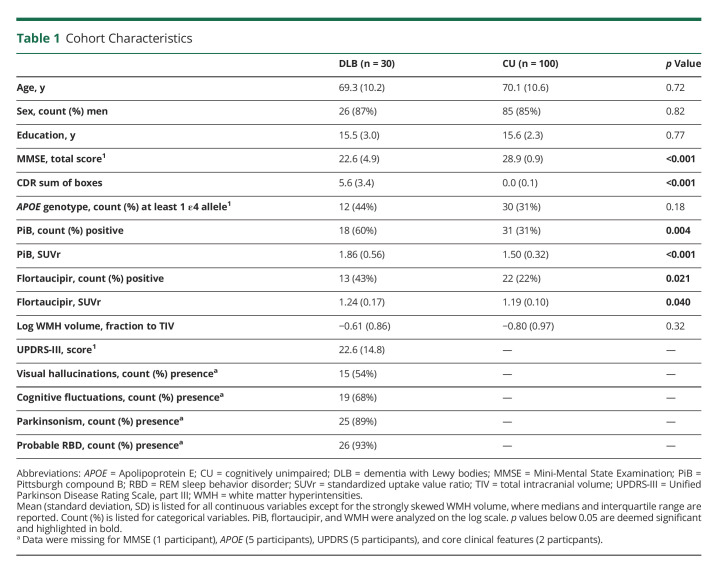
A total of 130 individuals fulfilled our selection criteria (30 individuals with probable DLB and 100 CU individuals). Table 1 summarizes key characteristics of the groups. By design, the DLB and CU groups were comparable in age and sex distribution. Both groups were also comparable in years of education. MMSE scores were significantly lower and CDR sum of boxes significantly higher in the DLB group than in the CU group. Regarding clinical DLB features, 54% of patients with DLB had visual hallucinations, 68% had cognitive fluctuations, 89% had parkinsonism, and 93% had probable RBD. The average (SD) UPDRS-III score in the DLB group was 22.6 (14.8).
Table 1.
Cohort Characteristics
The frequency of patients with DLB with positive PiB (60%) and flortaucipir (43%) scans was significantly higher than that of CU individuals (PiB: 31%, flortaucipir: 22%). The log-transformed WMH volume in the DLB group was qualitatively higher than that in the CU group, but it did not reach statistical significance (p = 0.32).
Regional GM Volume Differences Between DLB and CU Groups
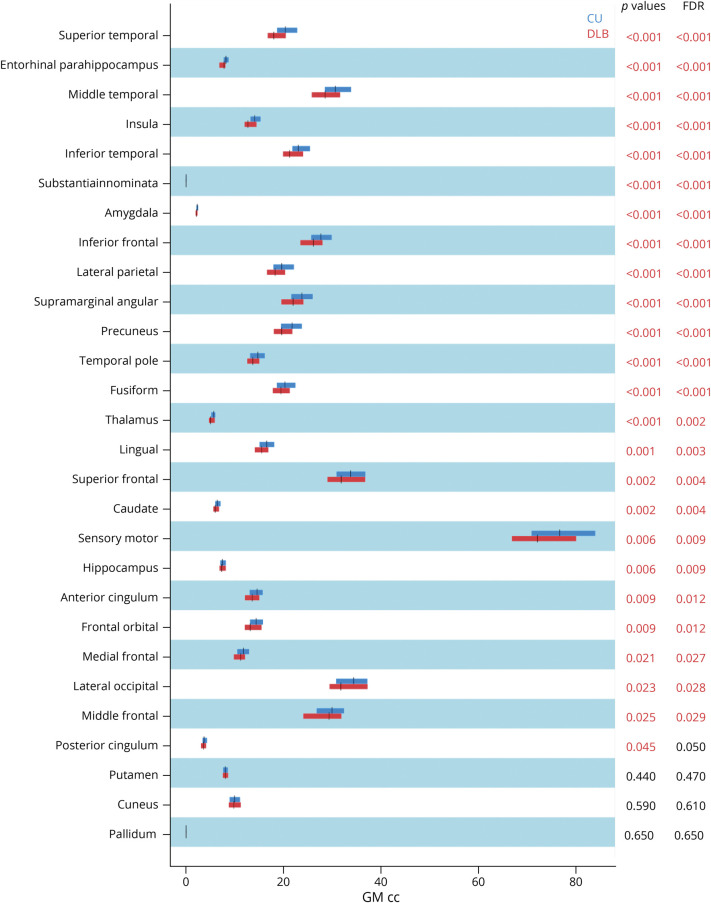
Patients with DLB showed lower GM volume than CU individuals across all cortical and subcortical regions except for the cuneus, putamen, and pallidum (Figure 3).
Figure 3. Differences in Regional GM Volume Between DLB and CU Groups.
p values are based on ANCOVA adjusting for age and TIV. Figure shows original and FDR-adjusted p values. Significant p values in red. Abbreviations: cc = cubic centimeters; CU = cognitively unimpaired group; DLB = dementia with Lewy bodies group; GM = gray matter; FDR = false discovery rate.
Univariate Associations of PiB, Flortaucipir, and WMH With Regional GM Volume
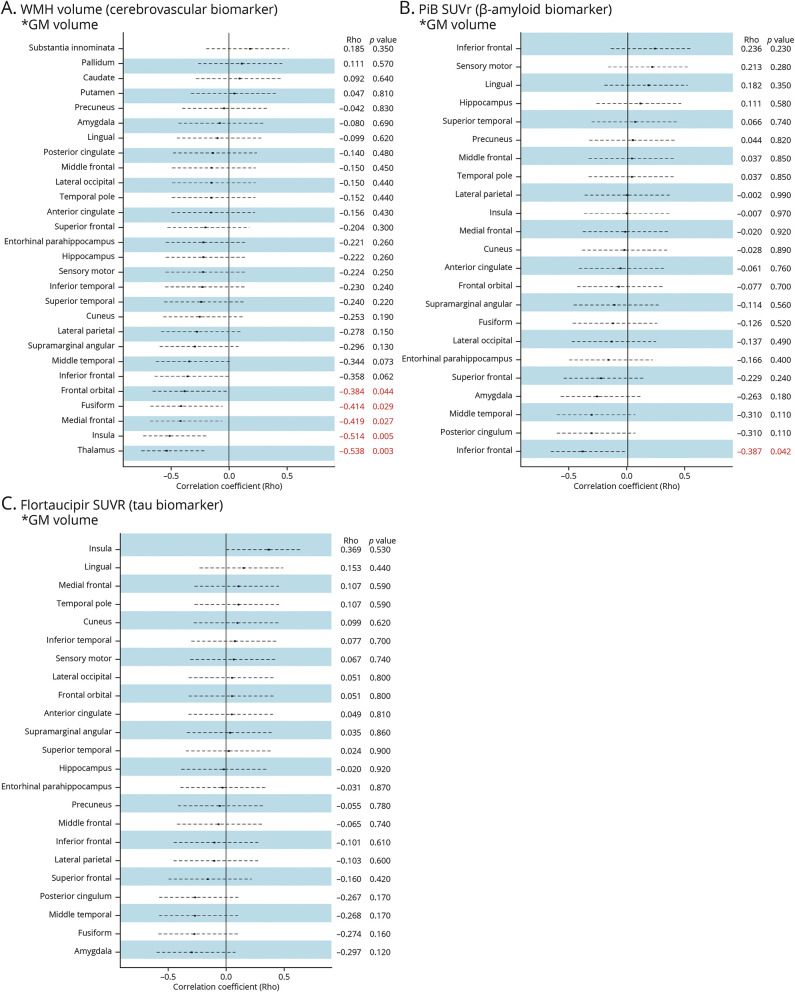
Figure 4 shows forest plots of partial Spearman correlations in the DLB group, adjusted for age and TIV. For significant associated regions, we also reported the results from the CU group for comparison, including brain maps and scatter plots for visualization (Figure 5).
Figure 4. Univariate Associations (Partial Spearman Correlations) of WMH, PiB, and Flortaucipir With Regional GM Volume in DLB.
The forest plots show the correlation coefficient from partial Spearman correlations adjusting for age and total intracranial volume. Dashed lines show 95% confidence intervals. Significant results (p < 0.05) are marked in red. (A) For WMH, correlations are calculated for the association of global WMH volume with GM volume in a given region (e.g., for hippocampus, global WMH volume with GM volume in the hippocampus). For PiB (B) and flortaucipir (C), correlations are calculated for the association of PiB or flortaucipir SUVr in a given region with GM volume in the same region (e.g., for hippocampus, PiB SUVr in the hippocampus with GM volume in the hippocampus). Abbreviations: GM = gray matter; PiB = Pittsburgh compound B; SUVr = Standardized uptake value ratios, WMHs = white matter hyperintensities.
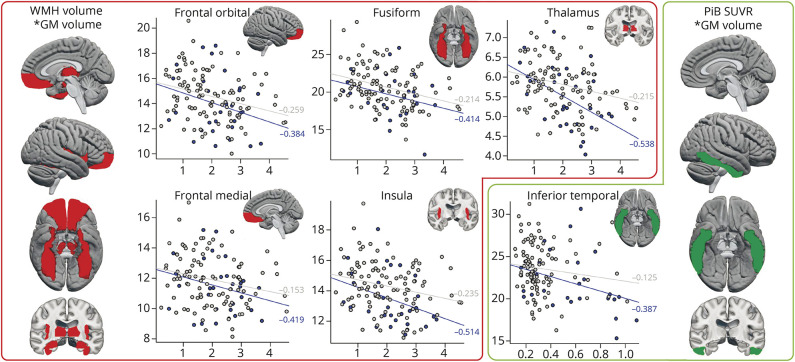
Figure 5. Brain Maps and Scatter Plots of Associations of WMH and PiB With Regional GM Volume in DLB.
Side brain maps give an overall overview of the regions that were statistically significant in univariate analysis (partial Spearman correlations for PiB SUVr and WMH volume with regional GM volume). The scatter plots show the associations between log transformed PiB SUVr or log transformed WMH volume with regional GM volume, separately for DLB patients (blue dots and blue regression lines) and CU individuals (gray dots and gray regression lines). Spearman correlation coefficients (partial) are displayed by the regression lines. Abbreviations: CU = cognitively unimpaired; DLB = dementia with Lewy bodies; GM = gray matter; PiB = Pittsburgh compound B; SUVr = Standardized uptake value ratios, WMH = white matter hyperintensities.
Regarding WMH, a larger WMH volume was significantly associated with lower volume in medial and orbital frontal cortices, insula, fusiform cortex, and thalamus in patients with DLB, after adjusting for age and TIV. All these associations were also statistically significant in the CU group except for the association between WMH volume and medial frontal cortex (γ = −0.153, p = 0.13). However, correlation coefficients tended to be stronger in the DLB group (correlation coefficients between −0.384 to −0.538) than in the CU group (correlation coefficients between −0.125 to −0.259) (Figure 5). This observation was supported by a statistically significant interaction for the thalamus volume (p = 0.043), indicating that the correlation between WMH and thalamus volumes was stronger in the DLB group.
Regarding PiB, higher PiB SUVrs were significantly associated with lower volume in the inferior temporal cortex in patients with DLB, after adjusting for age and TIV (γ = −0.387, p = 0.042), but this association was not significant in the CU group (γ = −0.125, p = 0.220) (Figure 5). We did not find significant associations between flortaucipir SUVr and GM volume in the DLB group after adjusting for age and TIV (Figure 4).
Structural Equation Models for Associations of PiB, Flortaucipir, and WMH With Regional GM Volume in Patients With DLB
SEM results for the regions that were significant in the correlation analyses have been listed in Table 2 and presented in Figure 2B.
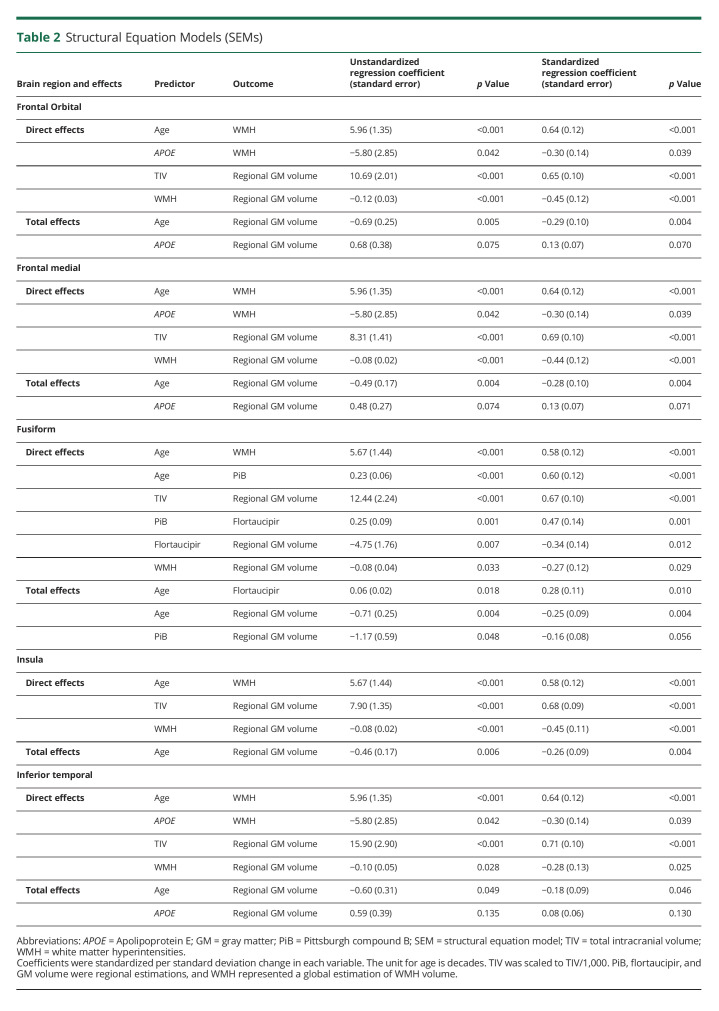
Table 2.
Structural Equation Models (SEMs)
WMH volume predicted GM volume in medial and orbital frontal cortices, insula, and inferior temporal cortex. In medial frontal, orbital frontal, and inferior temporal cortices, the effect of WMH volume was a mediation of the effects of age and APOE genotype over GM volume. An older age and absence of the APOE ε4 allele were significant predictors of a higher WMH volume, and a higher WMH volume in turn was a significant predictor of lower GM volume. In the insula, older age was a significant predictor of higher WMH volume, and a higher WMH volume in turn was a significant predictor of lower GM volume.
The SEM result for fusiform cortex was completely different. We observed 2 distinct paths: one path with the effect of age on fusiform volume through PiB and flortaucipir and another path with the effect of age on fusiform volume through WMH volume. The standardized direct effect of age on both PiB and WMH volume was of similar magnitude (0.58 vs 0.60) (Table 2).
Discussion
The paucity of studies combining biomarkers of cerebrovascular disease, β-amyloid, tau, and neurodegeneration in DLB has hindered our understanding of how copathologies influence neurodegeneration in this common dementia syndrome. Previous single biomarker studies have provided relevant data, but whether these pathologies act independently or in combination and their effects are specific to DLB or shared with the aging population is largely unknown. In this study, we demonstrated that although β-amyloid and tau biomarker positivity and WMH were frequent in our DLB group, their contributions to GM volume were focal and region specific. WMH were associated with GM volume in frontal areas, fusiform cortex, and thalamus, whereas the tau biomarker was associated with GM volume in the fusiform cortex, as a mediator for the effect of the β-amyloid biomarker.
We first demonstrated that, when compared with CU participants, patients with DLB had reduced volume across all cortical and subcortical GM regions except for the cuneus, putamen, and pallidum. This was important for the interpretation of our subsequent analyses as potential pathologic underpinnings of brain atrophy in DLB. We found a pattern of GM atrophy that replicates the findings from a large multicenter study, which showed that widespread cortical atrophy characterizes the majority of patients with DLB.21 Although smaller single-center DLB studies may not have been adequately powered to capture widespread atrophy, the overview of findings recapitulates the pattern of atrophy observed in our study.8,22-31
Mounting evidence shows that atrophy is more prominent and widespread in patients with DLB with concomitant AD pathology.22,24-26,32,33 Similarly, atrophy in DLB seems greater in patients with higher WMH burden,4,6,9 but the WMH literature is still scant. The important question of whether WMH and AD biomarkers have isolated or synergistic effects on GM volume in DLB is still unanswered. The important question of whether WMH and AD biomarkers have isolated or synergistic effects on GM volume in DLB is still unanswered, since no previous studies have investigated these associations.
Among the investigated biomarkers, univariate analyses showed that WMH volume was associated with GM volume in more ROIs than β-amyloid and tau biomarkers. This observation was clearly supported by the multivariable analysis (SEMs). These findings suggest that WMH contribute to atrophy as much as AD biomarkers in our cohort of patients with mild to moderate DLB. Whether this observation is specific to DLB or shared with the aging population is an important question. Our univariate analysis showed that a larger WMH volume was associated with lower volume in medial and orbital frontal cortices, insula, fusiform cortex, and thalamus in patients with DLB. All these associations, except for that of the medial frontal cortex, were also significant in the CU group. However, the strength of the associations was higher in the DLB group than in the CU group, supported by a statistically significant interaction between diagnostic group and WMH volume for the thalamus. In addition, patients with DLB had significantly lower GM volumes than the CU group in all these regions. Based on these results, we consider the possibility that WMH have an influence on these regions in the aging brain, but the association and its detrimental effect over brain integrity is higher in magnitude in DLB. An interesting finding from the SEMs is that the relatively narrow age range in this cohort did not have a direct effect on regional GM volume in any of the tested models. Instead, the effect of age was through WMH volume in all the GM regions except for the fusiform cortex, where a second path through β-amyloid and tau biomarkers was also observed in DLB. Although we cannot exclude that a wider age range or higher statistical power might reveal direct effects of age on GM volume, our current finding suggests that a higher age alone likely does not cause the neurodegeneration observed in DLB. Instead, age-related increases in WMH and AD copathology seem to influence part of the neurodegeneration in DLB.
The effects of WMH and AD biomarkers were region specific. Our univariate and multivariable analyses suggest a predilection of cerebrovascular disease (i.e., WMH) for fronto-insular-thalamic regions, while β-amyloid and tau pathologies seemed to target temporal areas extending to occipital cortex (fusiform and inferior temporal cortices). Previous DLB studies reported associations of β-amyloid with neurodegeneration in temporal and occipital cortices, including fusiform and inferior temporal areas.5,8,32 We recently showed that a longitudinal increase in tau-PET SUVr was associated with longitudinal atrophy rates in cortical areas overlapping with fusiform and inferior temporal cortices.11 Studies investigating both β-amyloid and tau biomarkers are scant in DLB. Van der Zande and colleagues25 combined β-amyloid and tau CSF biomarkers and showed an association with greater medial temporal atrophy in DLB. We also reported that neuropathologic evidence of AD pathology in DLB was associated with greater hippocampal atrophy at baseline34 and with hippocampal and temporoparietal atrophy over 2 years of follow-up.24 However, none of these previous studies investigated AD biomarkers in combination with WMH, in association with regional GM volumes. When all these biomarkers were modeled together in SEMs in this study, the univariate association of β-amyloid with inferior temporal cortex was taken over by WMH volume in mediating age-related and APOE-related effects. β-Amyloid positivity and high WMH volume have been associated with increased neurodegeneration in temporal lobe in patients with AD.35 Furthermore, β-amyloid and WMH volume increase with age in DLB.2,4 Hence, it is possible that our SEM for the inferior temporal cortex captured the most prominent effect of WMH volume over the weaker effect of β-amyloid and tau in our cohort of patients with mild to moderate DLB. By contrast, neuropathologic studies have shown the effect of NFT density over GM volume. The less prominent effect observed in this study may be due to a higher sensitivity of neuropathologic measures to NFT or that neuropathologic studies include older patients with DLB at more advanced stages of the disease and with higher levels of tau pathology and GM degeneration. In all other SEMs, WMH volume had a more central role than β-amyloid in the prediction of GM volume, often in paths involving indirect effects of age and APOE genotype. In addition, we observed that the absence of the APOE ε4 allele was a significant predictor of higher WMH volume. A previous study showed that regional brain atrophy and clinical disease severity is greater in patients with DLB who are carriers of the APOE ε4 allele.36 Because a higher WMH volume is also associated with greater brain atrophy and clinical disease severity,4,6,9 we believe that patients with a high WMH volume who are APOE ε4 carriers may tend to be too impaired to be eligible for this study on patients with mild to moderate DLB. However, further research explaining this finding is needed.
The SEM for the fusiform cortex showed combined effects of tau (through β-amyloid) and WMH volume. Again, these effects were mediations of the indirect effect of age over GM volume. Based on separate biomarker studies,4,37,38 we recently suggested a spatial coexistence of tau with cerebrovascular disease in temporoposterior brain regions in DLB.4 This study helps clarify that both tau and cerebrovascular biomarkers independently contribute to the neurodegeneration in the fusiform cortex in DLB. Indeed, longitudinal biomarker studies in DLB point to greater rates of atrophy in the fusiform cortex in patients with DLB with higher PiB SUVrs at baseline,5 concomitant AD pathology,24 and higher rates of increase in tau-PET SUVr.11 In light of the available evidence, it is possible that WMH volume is associated with fusiform cortex, but accumulation of amyloid-related tau pathology could be the main driver of the neurodegeneration in fusiform cortex over time in DLB.11
By contrast, previous studies showed that β-amyloid and tau do not seem to drive neurodegeneration in frontal cortex in DLB,5,26,32 although we cannot exclude that patients with DLB with concomitant AD pathology and neurodegeneration in frontal cortex would be too impaired to participate in such studies. Our current findings show that when WMH and β-amyloid and tau biomarkers are modeled together in a cohort of patients with mild to moderate DLB, WMH emerged as the main driver of GM volume in frontal cortex. One of our recent studies showed the vulnerability of frontal lobes to cerebrovascular disease (i.e., WMH) in DLB.4 In this study, the frontal cortical regions that were uniquely associated with WMH volume were located in medial and orbital frontal cortices and in the insula, which has a large medial frontal portion. The thalamus, which is strongly connected to the frontal cortex, was also uniquely associated with WMH volume. Therefore, our current analyses replicate the findings from our earlier study4 and extend the knowledge in confirming that these associations with WMH volume are independent of AD biomarkers in patients with mild to moderate DLB. Insula and medial frontal regions are innervated by cholinergic pathways, which are heavily targeted by cerebrovascular disease.39 The thalamus also receives cholinergic input.40 We previously suggested vascular-related cholinergic neurodegeneration as a potential mechanism driving neurodegeneration in frontal areas and thalamus in DLB.4 This hypothesis could be extended to the insula, as shown in the current study. The finding of neurodegeneration in the insula associated with cerebrovascular disease but not with β-amyloid or tau is of interest, helping to better understand pathologic correlates of a GM region that has recently been included as a potential biomarker in the diagnostic criteria of prodromal DLB.41 Prodromal DLB includes early atrophy in the insula, anterior cingulum, and medial-orbital frontal areas,27,42,43 and a recent longitudinal study showed faster degeneration of GM regions that receive dense cholinergic innervation, in prodromal DLB.44 Hence, the actual contribution of cerebrovascular disease to all these findings in patients with prodromal DLB and DLB deserve more attention.
Altogether, this study suggests unique and joint contributions of WMH and AD biomarkers toward focal GM atrophy in DLB. Still, when compared with the CU group, we observed GM differences in many areas other than those associated with WMH and AD biomarkers. Hence, the primary influence of alpha-synuclein–related pathology on GM volume revealed by neuropathologic studies remains to be investigated in vivo once a reliable topographical biomarker of alpha-synuclein is developed. In the meantime, our current findings add to the emerging notion that therapies targeting AD and cerebrovascular pathologies may be considered in patients with DLB for preventing neurodegenerative changes.
This study has some limitations. First, the lack of reliable topographical biomarkers of alpha-synuclein–related pathology hinders in vivo assessment of the hallmark pathology in DLB. Because we found that our patients with DLB had lower GM volume than CU individuals across many regions in which we did not see any contribution of WMH or β-amyloid and tau biomarkers, we assumed that alpha-synuclein–related pathology likely was the main contributor to neurodegeneration. However, this remains to be demonstrated. The lack of an alpha-synuclein biomarker also limits the ability to support the clinical diagnosis of DLB. Reassuringly, autopsy confirmation rates at our center are 89%.3 In this study, autopsy examinations were available for 5 cases, with a DLB diagnosis being confirmed in 4 of them. Second, GM volume is a gross structural measure of neurodegeneration, and this study could be expanded to investigate other topographical markers that inform about the metabolic and microstructural components of neurodegeneration such as 18F-fluorodeoxyglucose-PET, diffusion tensor imaging, or functional MRI, ideally using longitudinal designs. Third, statistical power was lower for patients with DLB than for the CU group. We thus anticipated that it would take stronger associations to become statistically significant in the DLB group. To circumvent this problem, we favored uncorrected p values in our univariate analysis and reported coefficients instead of conducting severe corrections for multiple testing. We thus consider our univariate correlation analysis as explorative to serve the purpose of identifying relevant regions for the SEMs. Fourth, the CU group was balanced on age and sex with the DLB group, and it is thus not representative of the general population. Fifth, regional neurodegeneration of GM correlates with remote cortical β-amyloid.8 We did not explore such associations because region-to-region effects are more common and possibly more relevant for tau-PET SUVr.45 Finally, we included WMH volume as a surrogate marker of cerebrovascular disease.10 Although vascular pathology contributes to WMH in our cohort,46 some WMH may lack the vascular specificity. In addition, there are other aspects of cerebrovascular disease that may influence neurodegeneration independent of WMH volume. For example, infarcts may influence neurodegeneration.47 Although infarcts are observed in patients with DLB, the frequency is not higher than in CU individuals.3 Given the size of our study cohort, we had limited power to investigate the association of infarcts with GM volume. In addition, neuropathologic studies have also emphasized the role of cerebral amyloid angiopathy in DLB.48 We focused this study on biomarkers, and an interesting prospect for the future is investigating associations with clinical endpoints. Our previous single biomarker studies suggest that WMH and AD biomarkers could jointly influence clinical phenotype and cognitive performance in DLB.2,3,5,11,24
We conclude that patients with probable DLB had widespread cortical atrophy, most of which likely is influenced by alpha-synuclein–related pathology. In addition, atrophy in specific regions was associated with concomitant cerebrovascular and AD biomarkers. The association of cerebrovascular disease with neurodegeneration was more extensive than that of β-amyloid and tau biomarkers in our cohort of patients with mild to moderate probable DLB and included GM areas that receive substantial cholinergic innervation. In turn, amyloid-related tau pathology was associated with neurodegeneration in the fusiform cortex. All these effects were mediations of indirect effects of age and APOE genotype. Modeling different biomarkers of pathology advances our understanding about underpinnings of neurodegeneration in DLB. As these findings get replicated and established, such understanding may become central in guiding new individualized therapeutic strategies, paving the way for precision medicine in DLB.
Acknowledgment
The authors thank AVID Radiopharmaceuticals, Inc, for provision of the AV-1451 precursor, chemistry production, advice and oversight, and Food and Drug Administration regulatory cross-filing permission and documentation needed for this work. They particularly thank the patients and their family members for participating in this research.
Glossary
- AD
Alzheimer disease
- ADRC
Alzheimer Disease Research Center
- CU
cognitively unimpaired
- DLB
dementia with Lewy bodies
- FLAIR
fluid-attenuated inversion recovery
- GM
gray matter
- MCALT
Mayo Clinic Adult Lifespan Template
- MMSE
Mini-Mental State Examination
- NFT
neurofibrillary tangle
- PiB
Pittsburgh compound B
- RBD
REM sleep behavior disorder
- SEM
structural equation model
- TIV
total intracranial volume
- UPDRS
Unified Parkinson Disease Rating Scale
- WMH
white matter hyperintensity
Appendix. Authors
Study Funding
This study was supported by the NIH (U01- NS100620, P50-AG016574, P30-AG62677, U01-AG006786, R37-AG011378, R01-AG041851, R01-AG040042, C06-RR018898, and R01-NS080820), Foundation Dr. Corinne Schuler, the Mangurian Foundation for Lewy Body Research, the Elsie and Marvin Dekelboum Family Foundation, the Little Family Foundation, the Ted Turner and Family Foundation LBD Functional Genomics Program, the Robert H. and Clarice Smith and Abigail Van Buren Alzheimer's Disease Research Program, Center for Innovative Medicine (CIMED), the Swedish Brain Foundation (Hjärnfonden), the regional agreement on medical training and clinical research (ALF) between Stockholm County Council and Karolinska Institutet, the Swedish Alzheimer Foundation (Alzheimerfonden), Neurofonden, the Swedish Dementia Funding (Demensfonden), Karolinska Institutet travel grants, Funding for Research from Karolinska Institutet, the Gun och Bertil Stohnes Foundation, the Gamla Tjänarinnor Foundation, and the Foundation for Geriatric Diseases at Karolinska Institutet. The sponsors played no role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
Disclosure
D. Ferreira, S.A. Przybelski, and T.G. Lesnick report no disclosures relevant to the manuscript. C.G. Schwarz receives research support from the NIH. P. Diaz-Galvan reports no disclosures relevant to the manuscript. J. Graff-Radford serves on the editorial board for Neurology and receives research support from NIH. M.L. Senjem owns or has owned stock in medical-related companies, unrelated to the current work, within the past 36 months: Align Technology, Inc., Inovio Pharmaceuticals, Inc., Mesa Laboratories, Inc., Johnson and Johnson, LHC Group, Inc., Natus Medical Inc., and Varex Imaging Corporation. J.A. Fields reports no disclosures relevant to the manuscript. D.S. Knopman serves on a Data Safety Monitoring Board for the DIAN study, has served on a Data Safety monitoring Board for a tau therapeutic for Biogen but received no personal compensation, is a site investigator in Biogen aducanumab trials, is an investigator in clinical trials sponsored by Lilly Pharmaceuticals and the University of Southern California, serves as a consultant for Samus Therapeutics, Roche, Magellan Health and Alzeca Biosciences but receives no personal compensation, and receives research support from the NIH. D.T. Jones reports no disclosures relevant to the manuscript. R. Savica reports no disclosures relevant to the manuscript. T. Ferman receives funding from the Mangurian Foundation for Lewy body research and NIH. NR Graff-Radford reports no disclosures relevant to the manuscript. V.J. Lowe serves as a consultant for Bayer Schering Pharma, Piramal Life Sciences, Life Molecular Imaging, Eisai Inc., AVID Radiopharmaceuticals, and Merck Research and receives research support from GE Healthcare, Siemens Molecular Imaging, AVID Radiopharmaceuticals, and the NIH (NIA, NCI). C.R. Jack has consulted for Lily and serves on an independent data monitoring board for Roche and as a speaker for Eisai, but he receives no personal compensation from any commercial entity. He receives research support from NIH and the Alexander Family Alzheimer's Disease Research Professorship of the Mayo Clinic. R.C. Petersen serves as a consultant for Roche, Inc., Merck, Inc., Biogen, Inc., Eisai, Inc., Genentech, Inc., and Nestle, Inc., served on a DSMB for Genentech, receives royalties from Oxford University Press and UpToDate, and receives NIH funding. E. Westman reports no disclosures relevant to the manuscript. B.F. Boeve has served as an investigator for clinical trials sponsored by Biogen and Alector. He receives royalties from the publication of a book entitled Behavioral Neurology of Dementia (Cambridge Medicine, 2017). He serves on the Scientific Advisory Board of the Tau Consortium. He receives research support from NIH, the Mayo Clinic Dorothy and Harry T. Mangurian Jr. Lewy Body Dementia Program, the Little Family Foundation, and the Ted Turner and Family Foundation LBD Functional Genomics Program. K. Kantarci consults for Biogen, receives research support from Avid Radiopharmaceuticals and Eli Lilly, and receives funding from NIH and Alzheimer's Drug Discovery Foundation. Go to Neurology.org/N for full disclosures.
References
- 1.Irwin DJ, Grossman M, Weintraub D, et al. Neuropathological and genetic correlates of survival and dementia onset in synucleinopathies: a retrospective analysis. Lancet Neurol. 2017;16(1):55-65. doi: 10.1016/s1474-4422(16)30291-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferreira D, Przybelski SA, Lesnick TG, et al. β-Amyloid and tau biomarkers and clinical phenotype in dementia with Lewy bodies. Neurology. 2020;95(24):e3257–e3268. doi: 10.1212/wnl.0000000000010943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sarro L, Tosakulwong N, Schwarz CG, et al. An investigation of cerebrovascular lesions in dementia with Lewy bodies compared to Alzheimer's disease. Alzheimer's Demen. 2017;13(3):257-266. doi: 10.1016/j.jalz.2016.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferreira D, Nedelska Z, Graff-Radford J, et al. Cerebrovascular disease, neurodegeneration, and clinical phenotype in dementia with Lewy bodies. Neurobiol Aging. 2021;105:252-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sarro L, Senjem ML, Lundt ES, et al. Amyloid-β deposition and regional grey matter atrophy rates in dementia with Lewy bodies. Brain. 2016;139(10):2740-2750. doi: 10.1093/brain/aww193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barber R, Gholkar A, Scheltens P, Ballard C, McKeith IG, O'Brien JT. MRI volumetric correlates of white matter lesions in dementia with Lewy bodies and Alzheimer's disease. Int J Geriatr Psychiatry. 2000;15(10):911-916. doi: [DOI] [PubMed] [Google Scholar]
- 7.Donaghy PC, Firbank MJ, Thomas AJ, et al. Clinical and imaging correlates of amyloid deposition in dementia with Lewy bodies. Mov Disord. 2018;33(7):1130-1138. doi: 10.1002/mds.27403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mak E, Donaghy PC, Mckiernan E, et al. Beta amyloid deposition maps onto hippocampal and subiculum atrophy in dementia with Lewy bodies. Neurobiol Aging. 2019;73:74-81. doi: 10.1016/j.neurobiolaging.2018.09.004 [DOI] [PubMed] [Google Scholar]
- 9.Joki H, Higashiyama Y, Nakae Y, et al. White matter hyperintensities on MRI in dementia with Lewy bodies, Parkinson's disease with dementia, and Alzheimer's disease. J Neurol Sci. 2018;385:99-104. doi: 10.1016/j.jns.2017.12.018 [DOI] [PubMed] [Google Scholar]
- 10.Wardlaw JM, Smith EE, Biessels GJ, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013;12(8):822-838. doi: 10.1016/s1474-4422(13)70124-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen Q, Przybelski SA, Senjem ML, et al. Longitudinal tau positron emission tomography in dementia with lewy bodies. Movement Disord. 2022;37(6):1256-1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McKeith I, Boeve BF, Dickson DW, et al. Diagnosis and management of dementia with Lewy bodies: fourth report of the DLB Consortium. Neurology. 2017;65:1863-1872. [DOI] [PubMed] [Google Scholar]
- 13.McKeith IG, Dickson DW, Lowe J, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65(12):1863-1872. doi: 10.1212/01.wnl.0000187889.17253.b1 [DOI] [PubMed] [Google Scholar]
- 14.Roberts RO, Geda YE, Knopman DS, et al. The Mayo Clinic Study of Aging: design and sampling, participation, baseline measures and sample characteristics. Neuroepidemiology. 2008;30(1):58-69. doi: 10.1159/000115751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferman TJ, Smith GE, Boeve BF, et al. DLB fluctuations: specific features that reliably differentiate DLB from AD and normal aging. Neurology. 2004;62(2):181-187. doi: 10.1212/wnl.62.2.181 [DOI] [PubMed] [Google Scholar]
- 16.Boeve BF, Molano JR, Ferman TJ, et al. Validation of the Mayo Sleep Questionnaire to screen for REM sleep behavior disorder in an aging and dementia cohort. Sleep Med. 2011;12(5):445-453. doi: 10.1016/j.sleep.2010.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jack CRJ, Lowe VJ, Senjem ML, et al. 11C-PiB and structural MRI provide complementary information in imaging of Alzheimer's disease and amnestic mild cognitive impairment. Brain. 2008;131(3):665-680. doi: 10.1093/brain/awm336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jack CR, Wiste HJ, Botha H, et al. The bivariate distribution of amyloid-β and tau: relationship with established neurocognitive clinical syndromes. Brain. 2019;142(10):3230-3242. doi: 10.1093/brain/awz268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raz L, Jayachandran M, Tosakulwong N, et al. Thrombogenic microvesicles and white matter hyperintensities in postmenopausal women. Neurology. 2013;80(10):911-918. doi: 10.1212/wnl.0b013e3182840c9f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muthén LK, Muthén BO. Mplus User's Guide, Eighth Edition: Muthén & Muthén; 1998-2017. [Google Scholar]
- 21.Oppedal K, Ferreira D, Cavallin L, et al. A signature pattern of cortical atrophy in dementia with Lewy bodies: a study on 333 patients from the European DLB consortium. Alzheimer's Demen. 2019;15(3):400-409. doi: 10.1016/j.jalz.2018.09.011 [DOI] [PubMed] [Google Scholar]
- 22.Whitwell JL, Weigand SD, Shiung MM, et al. Focal atrophy in dementia with Lewy bodies on MRI: a distinct pattern from Alzheimer's disease. Brain. 2007;130(3):708-719. doi: 10.1093/brain/awl388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nedelska Z, Ferman TJ, Boeve BF, et al. Pattern of brain atrophy rates in autopsy-confirmed dementia with Lewy bodies. Neurobiol Aging. 2015;36(1):452-461. doi: 10.1016/j.neurobiolaging.2014.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van der Zande JJ, Steenwijk MD, ten Kate M, Wattjes MP, Scheltens P, Lemstra AW. Gray matter atrophy in dementia with Lewy bodies with and without concomitant Alzheimer's disease pathology. Neurobiol Aging. 2018;71:171-178. doi: 10.1016/j.neurobiolaging.2018.07.005 [DOI] [PubMed] [Google Scholar]
- 25.Ye R, Touroutoglou A, Brickhouse M, et al. Topography of cortical thinning in the Lewy body diseases. NeuroImage Clin. 2020;26:102196. doi: 10.1016/j.nicl.2020.102196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blanc F, Colloby SJ, Philippi N, et al. Cortical thickness in dementia with lewy bodies and Alzheimer's disease: a comparison of prodromal and dementia stages. PLoS One. 2015;10(6):e0127396. doi: 10.1371/journal.pone.0127396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Watson R, Colloby SJ, Blamire AM, O'Brien JT. Subcortical volume changes in dementia with Lewy bodies and Alzheimer's disease. A comparison with healthy aging. Int Psychogeriatrics. 2016;28(4):529-536. doi: 10.1017/s1041610215001805 [DOI] [PubMed] [Google Scholar]
- 28.Burton EJ, Karas G, Paling SM, et al. Patterns of cerebral atrophy in dementia with lewy bodies using voxel-based morphometry. Neuroimage. 2002;17(2):618-630. doi: 10.1006/nimg.2002.1197 [DOI] [PubMed] [Google Scholar]
- 29.Takahashi R, Ishii K, Miyamoto N, et al. Measurement of gray and white matter atrophy in dementia with Lewy bodies using diffeomorphic anatomic registration through exponentiated lie algebra: a comparison with conventional voxel-based morphometry. Am J Neuroradiology. 2010;31(10):1873-1878. doi: 10.3174/ajnr.a2200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Watson R, Blamire AM, Colloby SJ, O'Brien JT. Assessment of regional gray matter loss in dementia with Lewy bodies : a surface-based MRI analysis. Am J Geriatr Psychiatry. 2015;23(1):38-46. doi: 10.1016/j.jagp.2014.07.005 [DOI] [PubMed] [Google Scholar]
- 31.Watson R, O'Brien JT, Barber R, Blamire AM. Patterns of gray matter atrophy in dementia with Lewy bodies: a voxel-based morphometry study. Int Psychogeriatrics. 2012;24(4):532-540. doi: 10.1017/s1041610211002171 [DOI] [PubMed] [Google Scholar]
- 32.Abdelnour C, Ferreira D, Oppedal K, et al. The combined effect of amyloid-β and tau biomarkers on brain atrophy in dementia with Lewy bodies. NeuroImage Clin. 2020;27:102333. doi: 10.1016/j.nicl.2020.102333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abdelnour C, Ferreira D, van de Beek M, et al. Parsing heterogeneity within dementia with Lewy bodies using clustering of biological, clinical, and demographic data. Alzheimer's Res Ther. 2022;14(1):14. doi: 10.1186/s13195-021-00946-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kantarci K, Ferman TJ, Boeve BF, et al. Focal atrophy on MRI and neuropathologic classification of dementia with Lewy bodies. Neurology. 2012;79(6):553-560. doi: 10.1212/wnl.0b013e31826357a5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ferreira D, Shams S, Cavallin L, et al. The contribution of small vessel disease to subtypes of Alzheimer's disease: a study on cerebrospinal fluid and imaging biomarkers. Neurobiol Aging. 2018;70:18-29. doi: 10.1016/j.neurobiolaging.2018.05.028 [DOI] [PubMed] [Google Scholar]
- 36.Saeed U, Mirza SS, MacIntosh BJ, et al. APOE-ε4 associates with hippocampal volume, learning, and memory across the spectrum of Alzheimer's disease and dementia with Lewy bodies. Alzheimer's Demen. 2018;14(9):1137-1147. doi: 10.1016/j.jalz.2018.04.005 [DOI] [PubMed] [Google Scholar]
- 37.Kantarci K, Lowe VJ, Boeve BF, et al. AV-1451 tau and β-amyloid positron emission tomography imaging in dementia with Lewy bodies. Ann Neurol. 2017;81(1):58-67. doi: 10.1002/ana.24825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nedelska Z, Schwarz CG, Boeve BF, et al. White matter integrity in dementia with Lewy bodies: a voxel-based analysis of diffusion tensor imaging. Neurobiol Aging. 2015;36(6):2010-2017. doi: 10.1016/j.neurobiolaging.2015.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nemy M, Cedres N, Grothe MJ, et al. Cholinergic white matter pathways make a stronger contribution to attention and memory in normal aging than cerebrovascular health and nucleus basalis of Meynert. Neuroimage. 2020;211:116607. doi: 10.1016/j.neuroimage.2020.116607 [DOI] [PubMed] [Google Scholar]
- 40.Mesulam MM. Cholinergic circuitry of the human nucleus basalis and its fate in Alzheimer's disease. J Comp Neurol. 2013;521(18):4124-4144. doi: 10.1002/cne.23415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McKeith IG, Ferman TJ, Thomas AJ, et al. Research criteria for the diagnosis of prodromal dementia with Lewy bodies. Neurology. 2020;94(17):743-755. doi: 10.1212/wnl.0000000000009323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Blanc F, Colloby SJ, Cretin B, et al. Grey matter atrophy in prodromal stage of dementia with Lewy bodies and Alzheimer's disease. Alzheimer's Res Ther. 2016;8(1):31. doi: 10.1186/s13195-016-0198-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roquet D, Noblet V, Anthony P, et al. Insular atrophy at the prodromal stage of dementia with Lewy bodies: a VBM DARTEL study. Sci Rep. 2017;7(1):9437. doi: 10.1038/s41598-017-08667-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kantarci K, Nedelska Z, Chen Q, et al. Longitudinal atrophy in prodromal dementia with Lewy bodies points to cholinergic degeneration. Brain Commun. 2022;4(2):fcac013. doi: 10.1093/braincomms/fcac013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.LaPoint MR, Chhatwal JP, Sepulcre J, Johnson KA, Sperling RA, Schultz AP. The association between tau PET and retrospective cortical thinning in clinically normal elderly. Neuroimage. 2017;157:612-622. doi: 10.1016/j.neuroimage.2017.05.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Murray ME, Vemuri P, Preboske GM, et al. A quantitative postmortem MRI design sensitive to white matter hyperintensity differences and their relationship with underlying pathology. J Neuropathol Exp Neurol. 2012;71(12):1113-1122. doi: 10.1097/nen.0b013e318277387e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Raman MR, Preboske GM, Przybelski SA, et al. Antemortem MRI findings associated with microinfarcts at autopsy. Neurology 2014;82(22):1951-1958. doi: 10.1212/wnl.0000000000000471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jellinger KA. Significance of cerebral amyloid angiopathy and other co-morbidities in Lewy body diseases. J Neural Transm. 2021;128(5):687-699. doi: 10.1007/s00702-021-02345-9 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data will be shared by request from a qualified investigator in accordance with the Mayo ADRC data sharing protocol.