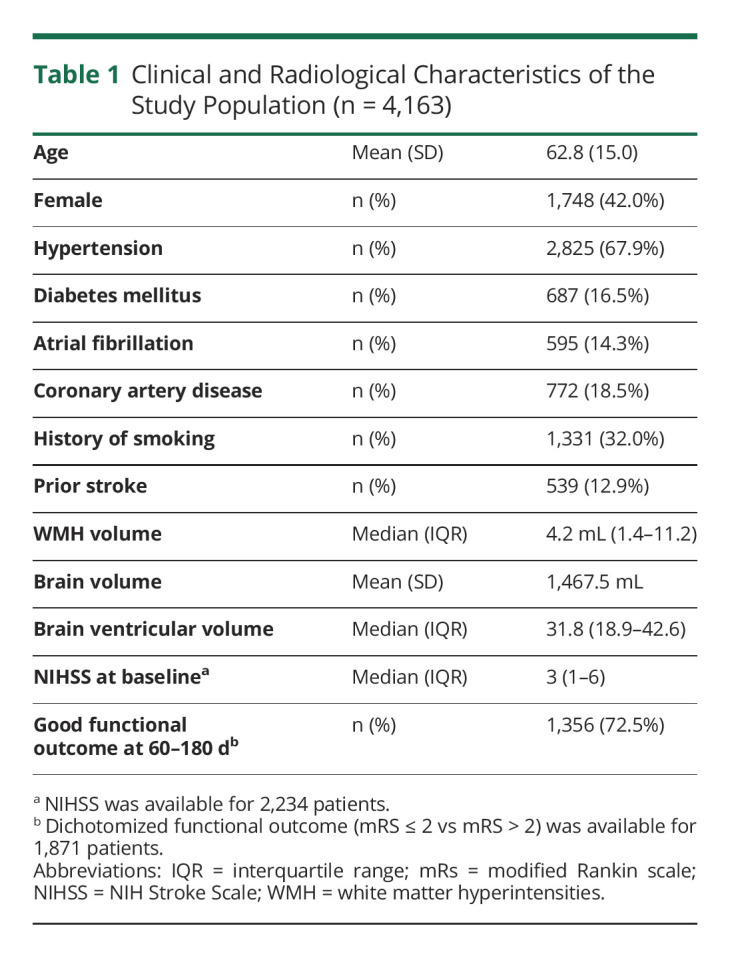
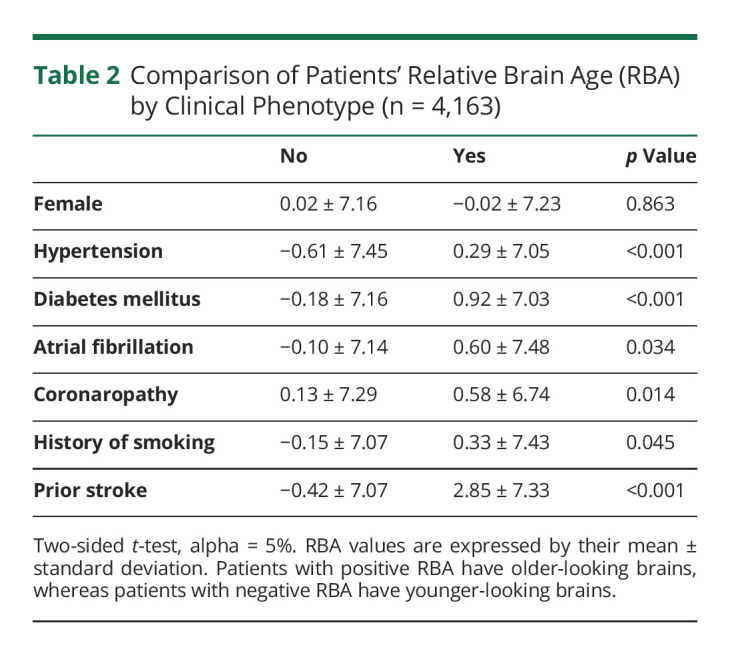
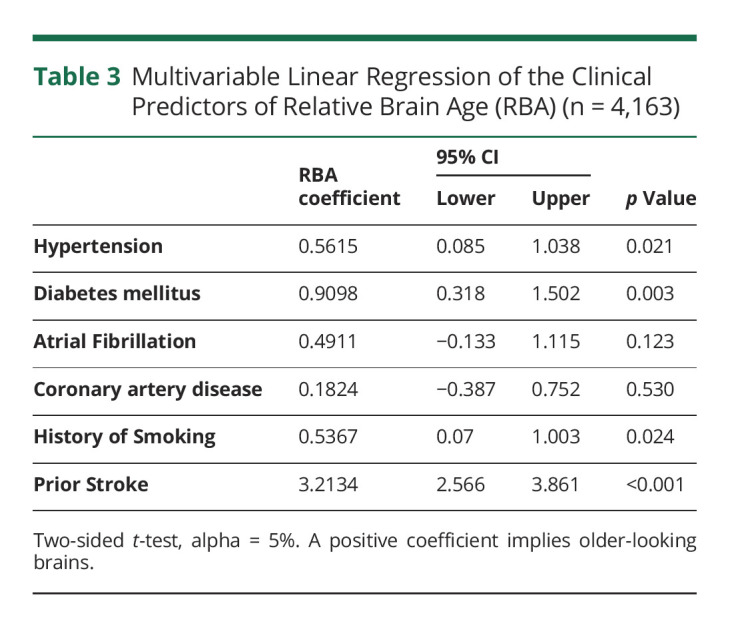
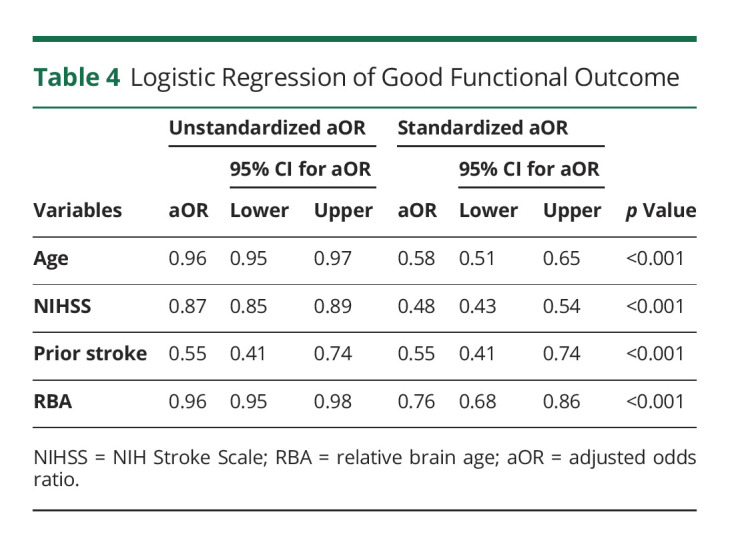
Martin Bretzner
Martin Bretzner, MD
1From the J. Philip Kistler Stroke Research Center (M.B., A.K.B., M.D.S., S.H., A. Dalca, K.D., A.-K.G., M.R.E., P.M.R., M.N., R.W.R., C.W., N.S.R.), A.A. Martinos Center for Biomedical Imaging (A. Dalca, O.W.), and Henry and Allison McCance Center for Brain Health (J. Rosand), Massachusetts General Hospital, Harvard Medical School, Boston; Lille Neuroscience & Cognition (M.B., X.L., R. Lopes, G.K.), Inserm, CHU Lille, U1172 and Institut Pasteur de Lille (M.G.), CNRS, Inserm, CHU Lille, US 41 - UMS 2014 - PLBS, Lille University, France; Computer Science and Artificial Intelligence Lab (A. Dalca, C.W., P.G.), Massachusetts Institute of Technology, Cambridge; Division of Preventive Medicine (P.M.R.), Department of Medicine, Brigham and Women's Hospital, Boston, MA; Department of Medicine (O.R.B.), Division of Neurology, University of British Columbia, Vancouver, Canada; Department of Neurology (J.W.C., S.J.K.), University of Maryland School of Medicine and Veterans Affairs Maryland Health Care System, Baltimore, MD; School of Medical Sciences (A. Donatti, A. Sousa), University of Campinas (UNICAMP) and the Brazilian Institute of Neuroscience and Neurotechnology (BRAINN), Campinas, São Paulo; Departments of Neurosurgery (C.G.) and Neurology (R.Z.), Geisinger, Danville, PA; Department of Neurosurgery (C.G.), Christian Doppler Klinik, Paracelsus Medical University, Salzburg, Austria; Division of Emergency Medicine (Laura Heitsch), Washington University School of Medicine, St. Louis; Department of Neurology (Laura Heitsch, C.-L.P.), Washington University School of Medicine & Barnes-Jewish Hospital, St. Louis, MO; Department of Clinical Neuroscience (L. Holmegaard, K.J., T.M.S., T.T.), Institute of Neuroscience and Physiology, Sahlgrenska Academy at University of Gothenburg, Sweden; Department of Neurology, Sahlgrenska University Hospital, Gothenburg, Sweden; Department of Neurology (J.J.-C.), Neurovascular Research Group (NEUVAS), IMIM-Hospital del Mar (Institut Hospital del Mar d'Investigacions M`ediques), Universitat Autonoma de Barcelona, Spain; Department of Neurosciences (R. Lemmens), Experimental Neurology and Leuven Research Institute for Neuroscience and Disease (LIND), KU Leuven - University of Leuven, Belgium; Department of Neurology (R. Lemmens), Laboratory of Neurobiology, VIB Vesalius Research Center, University Hospitals Leuven, Belgium; School of Medicine and Public Health (C.R.L.), University of Newcastle, New South Wales; Department of Neurology, John Hunter Hospital, Newcastle, New South Wales, Australia; Division of Endocrinology (P.F.M.), Diabetes and Nutrition, Department of Medicine, University of Maryland School of Medicine, Baltimore; Department of Pharmacotherapy and Translational Research and Center for Pharmacogenomics (C.W.M.), University of Florida, Gainesville; Department of Neurology (J.F.M.), Mayo Clinic, Jacksonville, FL; Klinik und Poliklinik für Neurologie (A.R.), Universitätsmedizin Rostock, Germany; Department of Neurology (S.R., R.S.), Clinical Division of Neurogeriatrics, Medical University Graz, Austria; Center for Genomic Medicine (J. Rosand), Massachusetts General Hospital, Boston; Broad Institute (J. Rosand), Cambridge, MA; Department of Neurology and Evelyn F. McKnight Brain Institute (J. Roquer, T.R., R.L.S./M.S.), Miller School of Medicine, University of Miami, FL; Institute of Cardiovascular Research (P.S.), Royal Holloway University of London (ICR2UL), UK St Peter's and Ashford Hospitals, Egham, United Kingdom; Department of Neurology (A. Slowik), Jagiellonian University Medical College, Krakow, Poland; Division of Neurocritical Care & Emergency Neurology (D.S.), Department of Neurology, Helsinki University Central Hospital, Finland; Stroke Division (V.T.), Florey Institute of Neuroscience and Mental Health, Heidelberg; Department of Neurology (V.T.), Austin Health, Heidelberg, Australia; Departments of Radiology (A.V.) and Neurology and Rehabilitation Medicine (D.W.), University of Cincinnati College of Medicine, OH; Department of Clinical Sciences Lund, Radiology (J.W.) and Neurology (A.G.L.), Lund University, Sweden; Department of Radiology, Neuroradiology, Skåne University Hospital, Malmö, Sweden; Departments of Neurology and Public Health Sciences (B.B.W.), University of Virginia, Charlottesville, VA; University of Technology Sydney (J.M.), Australia; Section of Neurology (A.G.L.), Skåne University Hospital, Lund, Sweden; Department of Laboratory Medicine (C.J.), Institute of Biomedicine, the Sahlgrenska Academy, University of Gothenburg, Sweden; and Department of Clinical Genetics and Genomics (C.J.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden.
1,✉,
Anna K Bonkhoff
Anna K Bonkhoff, MD
1From the J. Philip Kistler Stroke Research Center (M.B., A.K.B., M.D.S., S.H., A. Dalca, K.D., A.-K.G., M.R.E., P.M.R., M.N., R.W.R., C.W., N.S.R.), A.A. Martinos Center for Biomedical Imaging (A. Dalca, O.W.), and Henry and Allison McCance Center for Brain Health (J. Rosand), Massachusetts General Hospital, Harvard Medical School, Boston; Lille Neuroscience & Cognition (M.B., X.L., R. Lopes, G.K.), Inserm, CHU Lille, U1172 and Institut Pasteur de Lille (M.G.), CNRS, Inserm, CHU Lille, US 41 - UMS 2014 - PLBS, Lille University, France; Computer Science and Artificial Intelligence Lab (A. Dalca, C.W., P.G.), Massachusetts Institute of Technology, Cambridge; Division of Preventive Medicine (P.M.R.), Department of Medicine, Brigham and Women's Hospital, Boston, MA; Department of Medicine (O.R.B.), Division of Neurology, University of British Columbia, Vancouver, Canada; Department of Neurology (J.W.C., S.J.K.), University of Maryland School of Medicine and Veterans Affairs Maryland Health Care System, Baltimore, MD; School of Medical Sciences (A. Donatti, A. Sousa), University of Campinas (UNICAMP) and the Brazilian Institute of Neuroscience and Neurotechnology (BRAINN), Campinas, São Paulo; Departments of Neurosurgery (C.G.) and Neurology (R.Z.), Geisinger, Danville, PA; Department of Neurosurgery (C.G.), Christian Doppler Klinik, Paracelsus Medical University, Salzburg, Austria; Division of Emergency Medicine (Laura Heitsch), Washington University School of Medicine, St. Louis; Department of Neurology (Laura Heitsch, C.-L.P.), Washington University School of Medicine & Barnes-Jewish Hospital, St. Louis, MO; Department of Clinical Neuroscience (L. Holmegaard, K.J., T.M.S., T.T.), Institute of Neuroscience and Physiology, Sahlgrenska Academy at University of Gothenburg, Sweden; Department of Neurology, Sahlgrenska University Hospital, Gothenburg, Sweden; Department of Neurology (J.J.-C.), Neurovascular Research Group (NEUVAS), IMIM-Hospital del Mar (Institut Hospital del Mar d'Investigacions M`ediques), Universitat Autonoma de Barcelona, Spain; Department of Neurosciences (R. Lemmens), Experimental Neurology and Leuven Research Institute for Neuroscience and Disease (LIND), KU Leuven - University of Leuven, Belgium; Department of Neurology (R. Lemmens), Laboratory of Neurobiology, VIB Vesalius Research Center, University Hospitals Leuven, Belgium; School of Medicine and Public Health (C.R.L.), University of Newcastle, New South Wales; Department of Neurology, John Hunter Hospital, Newcastle, New South Wales, Australia; Division of Endocrinology (P.F.M.), Diabetes and Nutrition, Department of Medicine, University of Maryland School of Medicine, Baltimore; Department of Pharmacotherapy and Translational Research and Center for Pharmacogenomics (C.W.M.), University of Florida, Gainesville; Department of Neurology (J.F.M.), Mayo Clinic, Jacksonville, FL; Klinik und Poliklinik für Neurologie (A.R.), Universitätsmedizin Rostock, Germany; Department of Neurology (S.R., R.S.), Clinical Division of Neurogeriatrics, Medical University Graz, Austria; Center for Genomic Medicine (J. Rosand), Massachusetts General Hospital, Boston; Broad Institute (J. Rosand), Cambridge, MA; Department of Neurology and Evelyn F. McKnight Brain Institute (J. Roquer, T.R., R.L.S./M.S.), Miller School of Medicine, University of Miami, FL; Institute of Cardiovascular Research (P.S.), Royal Holloway University of London (ICR2UL), UK St Peter's and Ashford Hospitals, Egham, United Kingdom; Department of Neurology (A. Slowik), Jagiellonian University Medical College, Krakow, Poland; Division of Neurocritical Care & Emergency Neurology (D.S.), Department of Neurology, Helsinki University Central Hospital, Finland; Stroke Division (V.T.), Florey Institute of Neuroscience and Mental Health, Heidelberg; Department of Neurology (V.T.), Austin Health, Heidelberg, Australia; Departments of Radiology (A.V.) and Neurology and Rehabilitation Medicine (D.W.), University of Cincinnati College of Medicine, OH; Department of Clinical Sciences Lund, Radiology (J.W.) and Neurology (A.G.L.), Lund University, Sweden; Department of Radiology, Neuroradiology, Skåne University Hospital, Malmö, Sweden; Departments of Neurology and Public Health Sciences (B.B.W.), University of Virginia, Charlottesville, VA; University of Technology Sydney (J.M.), Australia; Section of Neurology (A.G.L.), Skåne University Hospital, Lund, Sweden; Department of Laboratory Medicine (C.J.), Institute of Biomedicine, the Sahlgrenska Academy, University of Gothenburg, Sweden; and Department of Clinical Genetics and Genomics (C.J.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden.
1,
Markus D Schirmer
Markus D Schirmer, PhD
1From the J. Philip Kistler Stroke Research Center (M.B., A.K.B., M.D.S., S.H., A. Dalca, K.D., A.-K.G., M.R.E., P.M.R., M.N., R.W.R., C.W., N.S.R.), A.A. Martinos Center for Biomedical Imaging (A. Dalca, O.W.), and Henry and Allison McCance Center for Brain Health (J. Rosand), Massachusetts General Hospital, Harvard Medical School, Boston; Lille Neuroscience & Cognition (M.B., X.L., R. Lopes, G.K.), Inserm, CHU Lille, U1172 and Institut Pasteur de Lille (M.G.), CNRS, Inserm, CHU Lille, US 41 - UMS 2014 - PLBS, Lille University, France; Computer Science and Artificial Intelligence Lab (A. Dalca, C.W., P.G.), Massachusetts Institute of Technology, Cambridge; Division of Preventive Medicine (P.M.R.), Department of Medicine, Brigham and Women's Hospital, Boston, MA; Department of Medicine (O.R.B.), Division of Neurology, University of British Columbia, Vancouver, Canada; Department of Neurology (J.W.C., S.J.K.), University of Maryland School of Medicine and Veterans Affairs Maryland Health Care System, Baltimore, MD; School of Medical Sciences (A. Donatti, A. Sousa), University of Campinas (UNICAMP) and the Brazilian Institute of Neuroscience and Neurotechnology (BRAINN), Campinas, São Paulo; Departments of Neurosurgery (C.G.) and Neurology (R.Z.), Geisinger, Danville, PA; Department of Neurosurgery (C.G.), Christian Doppler Klinik, Paracelsus Medical University, Salzburg, Austria; Division of Emergency Medicine (Laura Heitsch), Washington University School of Medicine, St. Louis; Department of Neurology (Laura Heitsch, C.-L.P.), Washington University School of Medicine & Barnes-Jewish Hospital, St. Louis, MO; Department of Clinical Neuroscience (L. Holmegaard, K.J., T.M.S., T.T.), Institute of Neuroscience and Physiology, Sahlgrenska Academy at University of Gothenburg, Sweden; Department of Neurology, Sahlgrenska University Hospital, Gothenburg, Sweden; Department of Neurology (J.J.-C.), Neurovascular Research Group (NEUVAS), IMIM-Hospital del Mar (Institut Hospital del Mar d'Investigacions M`ediques), Universitat Autonoma de Barcelona, Spain; Department of Neurosciences (R. Lemmens), Experimental Neurology and Leuven Research Institute for Neuroscience and Disease (LIND), KU Leuven - University of Leuven, Belgium; Department of Neurology (R. Lemmens), Laboratory of Neurobiology, VIB Vesalius Research Center, University Hospitals Leuven, Belgium; School of Medicine and Public Health (C.R.L.), University of Newcastle, New South Wales; Department of Neurology, John Hunter Hospital, Newcastle, New South Wales, Australia; Division of Endocrinology (P.F.M.), Diabetes and Nutrition, Department of Medicine, University of Maryland School of Medicine, Baltimore; Department of Pharmacotherapy and Translational Research and Center for Pharmacogenomics (C.W.M.), University of Florida, Gainesville; Department of Neurology (J.F.M.), Mayo Clinic, Jacksonville, FL; Klinik und Poliklinik für Neurologie (A.R.), Universitätsmedizin Rostock, Germany; Department of Neurology (S.R., R.S.), Clinical Division of Neurogeriatrics, Medical University Graz, Austria; Center for Genomic Medicine (J. Rosand), Massachusetts General Hospital, Boston; Broad Institute (J. Rosand), Cambridge, MA; Department of Neurology and Evelyn F. McKnight Brain Institute (J. Roquer, T.R., R.L.S./M.S.), Miller School of Medicine, University of Miami, FL; Institute of Cardiovascular Research (P.S.), Royal Holloway University of London (ICR2UL), UK St Peter's and Ashford Hospitals, Egham, United Kingdom; Department of Neurology (A. Slowik), Jagiellonian University Medical College, Krakow, Poland; Division of Neurocritical Care & Emergency Neurology (D.S.), Department of Neurology, Helsinki University Central Hospital, Finland; Stroke Division (V.T.), Florey Institute of Neuroscience and Mental Health, Heidelberg; Department of Neurology (V.T.), Austin Health, Heidelberg, Australia; Departments of Radiology (A.V.) and Neurology and Rehabilitation Medicine (D.W.), University of Cincinnati College of Medicine, OH; Department of Clinical Sciences Lund, Radiology (J.W.) and Neurology (A.G.L.), Lund University, Sweden; Department of Radiology, Neuroradiology, Skåne University Hospital, Malmö, Sweden; Departments of Neurology and Public Health Sciences (B.B.W.), University of Virginia, Charlottesville, VA; University of Technology Sydney (J.M.), Australia; Section of Neurology (A.G.L.), Skåne University Hospital, Lund, Sweden; Department of Laboratory Medicine (C.J.), Institute of Biomedicine, the Sahlgrenska Academy, University of Gothenburg, Sweden; and Department of Clinical Genetics and Genomics (C.J.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden.
1,
Sungmin Hong
Sungmin Hong, PhD
1From the J. Philip Kistler Stroke Research Center (M.B., A.K.B., M.D.S., S.H., A. Dalca, K.D., A.-K.G., M.R.E., P.M.R., M.N., R.W.R., C.W., N.S.R.), A.A. Martinos Center for Biomedical Imaging (A. Dalca, O.W.), and Henry and Allison McCance Center for Brain Health (J. Rosand), Massachusetts General Hospital, Harvard Medical School, Boston; Lille Neuroscience & Cognition (M.B., X.L., R. Lopes, G.K.), Inserm, CHU Lille, U1172 and Institut Pasteur de Lille (M.G.), CNRS, Inserm, CHU Lille, US 41 - UMS 2014 - PLBS, Lille University, France; Computer Science and Artificial Intelligence Lab (A. Dalca, C.W., P.G.), Massachusetts Institute of Technology, Cambridge; Division of Preventive Medicine (P.M.R.), Department of Medicine, Brigham and Women's Hospital, Boston, MA; Department of Medicine (O.R.B.), Division of Neurology, University of British Columbia, Vancouver, Canada; Department of Neurology (J.W.C., S.J.K.), University of Maryland School of Medicine and Veterans Affairs Maryland Health Care System, Baltimore, MD; School of Medical Sciences (A. Donatti, A. Sousa), University of Campinas (UNICAMP) and the Brazilian Institute of Neuroscience and Neurotechnology (BRAINN), Campinas, São Paulo; Departments of Neurosurgery (C.G.) and Neurology (R.Z.), Geisinger, Danville, PA; Department of Neurosurgery (C.G.), Christian Doppler Klinik, Paracelsus Medical University, Salzburg, Austria; Division of Emergency Medicine (Laura Heitsch), Washington University School of Medicine, St. Louis; Department of Neurology (Laura Heitsch, C.-L.P.), Washington University School of Medicine & Barnes-Jewish Hospital, St. Louis, MO; Department of Clinical Neuroscience (L. Holmegaard, K.J., T.M.S., T.T.), Institute of Neuroscience and Physiology, Sahlgrenska Academy at University of Gothenburg, Sweden; Department of Neurology, Sahlgrenska University Hospital, Gothenburg, Sweden; Department of Neurology (J.J.-C.), Neurovascular Research Group (NEUVAS), IMIM-Hospital del Mar (Institut Hospital del Mar d'Investigacions M`ediques), Universitat Autonoma de Barcelona, Spain; Department of Neurosciences (R. Lemmens), Experimental Neurology and Leuven Research Institute for Neuroscience and Disease (LIND), KU Leuven - University of Leuven, Belgium; Department of Neurology (R. Lemmens), Laboratory of Neurobiology, VIB Vesalius Research Center, University Hospitals Leuven, Belgium; School of Medicine and Public Health (C.R.L.), University of Newcastle, New South Wales; Department of Neurology, John Hunter Hospital, Newcastle, New South Wales, Australia; Division of Endocrinology (P.F.M.), Diabetes and Nutrition, Department of Medicine, University of Maryland School of Medicine, Baltimore; Department of Pharmacotherapy and Translational Research and Center for Pharmacogenomics (C.W.M.), University of Florida, Gainesville; Department of Neurology (J.F.M.), Mayo Clinic, Jacksonville, FL; Klinik und Poliklinik für Neurologie (A.R.), Universitätsmedizin Rostock, Germany; Department of Neurology (S.R., R.S.), Clinical Division of Neurogeriatrics, Medical University Graz, Austria; Center for Genomic Medicine (J. Rosand), Massachusetts General Hospital, Boston; Broad Institute (J. Rosand), Cambridge, MA; Department of Neurology and Evelyn F. McKnight Brain Institute (J. Roquer, T.R., R.L.S./M.S.), Miller School of Medicine, University of Miami, FL; Institute of Cardiovascular Research (P.S.), Royal Holloway University of London (ICR2UL), UK St Peter's and Ashford Hospitals, Egham, United Kingdom; Department of Neurology (A. Slowik), Jagiellonian University Medical College, Krakow, Poland; Division of Neurocritical Care & Emergency Neurology (D.S.), Department of Neurology, Helsinki University Central Hospital, Finland; Stroke Division (V.T.), Florey Institute of Neuroscience and Mental Health, Heidelberg; Department of Neurology (V.T.), Austin Health, Heidelberg, Australia; Departments of Radiology (A.V.) and Neurology and Rehabilitation Medicine (D.W.), University of Cincinnati College of Medicine, OH; Department of Clinical Sciences Lund, Radiology (J.W.) and Neurology (A.G.L.), Lund University, Sweden; Department of Radiology, Neuroradiology, Skåne University Hospital, Malmö, Sweden; Departments of Neurology and Public Health Sciences (B.B.W.), University of Virginia, Charlottesville, VA; University of Technology Sydney (J.M.), Australia; Section of Neurology (A.G.L.), Skåne University Hospital, Lund, Sweden; Department of Laboratory Medicine (C.J.), Institute of Biomedicine, the Sahlgrenska Academy, University of Gothenburg, Sweden; and Department of Clinical Genetics and Genomics (C.J.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden.
1,
Adrian Dalca
Adrian Dalca, PhD
1From the J. Philip Kistler Stroke Research Center (M.B., A.K.B., M.D.S., S.H., A. Dalca, K.D., A.-K.G., M.R.E., P.M.R., M.N., R.W.R., C.W., N.S.R.), A.A. Martinos Center for Biomedical Imaging (A. Dalca, O.W.), and Henry and Allison McCance Center for Brain Health (J. Rosand), Massachusetts General Hospital, Harvard Medical School, Boston; Lille Neuroscience & Cognition (M.B., X.L., R. Lopes, G.K.), Inserm, CHU Lille, U1172 and Institut Pasteur de Lille (M.G.), CNRS, Inserm, CHU Lille, US 41 - UMS 2014 - PLBS, Lille University, France; Computer Science and Artificial Intelligence Lab (A. Dalca, C.W., P.G.), Massachusetts Institute of Technology, Cambridge; Division of Preventive Medicine (P.M.R.), Department of Medicine, Brigham and Women's Hospital, Boston, MA; Department of Medicine (O.R.B.), Division of Neurology, University of British Columbia, Vancouver, Canada; Department of Neurology (J.W.C., S.J.K.), University of Maryland School of Medicine and Veterans Affairs Maryland Health Care System, Baltimore, MD; School of Medical Sciences (A. Donatti, A. Sousa), University of Campinas (UNICAMP) and the Brazilian Institute of Neuroscience and Neurotechnology (BRAINN), Campinas, São Paulo; Departments of Neurosurgery (C.G.) and Neurology (R.Z.), Geisinger, Danville, PA; Department of Neurosurgery (C.G.), Christian Doppler Klinik, Paracelsus Medical University, Salzburg, Austria; Division of Emergency Medicine (Laura Heitsch), Washington University School of Medicine, St. Louis; Department of Neurology (Laura Heitsch, C.-L.P.), Washington University School of Medicine & Barnes-Jewish Hospital, St. Louis, MO; Department of Clinical Neuroscience (L. Holmegaard, K.J., T.M.S., T.T.), Institute of Neuroscience and Physiology, Sahlgrenska Academy at University of Gothenburg, Sweden; Department of Neurology, Sahlgrenska University Hospital, Gothenburg, Sweden; Department of Neurology (J.J.-C.), Neurovascular Research Group (NEUVAS), IMIM-Hospital del Mar (Institut Hospital del Mar d'Investigacions M`ediques), Universitat Autonoma de Barcelona, Spain; Department of Neurosciences (R. Lemmens), Experimental Neurology and Leuven Research Institute for Neuroscience and Disease (LIND), KU Leuven - University of Leuven, Belgium; Department of Neurology (R. Lemmens), Laboratory of Neurobiology, VIB Vesalius Research Center, University Hospitals Leuven, Belgium; School of Medicine and Public Health (C.R.L.), University of Newcastle, New South Wales; Department of Neurology, John Hunter Hospital, Newcastle, New South Wales, Australia; Division of Endocrinology (P.F.M.), Diabetes and Nutrition, Department of Medicine, University of Maryland School of Medicine, Baltimore; Department of Pharmacotherapy and Translational Research and Center for Pharmacogenomics (C.W.M.), University of Florida, Gainesville; Department of Neurology (J.F.M.), Mayo Clinic, Jacksonville, FL; Klinik und Poliklinik für Neurologie (A.R.), Universitätsmedizin Rostock, Germany; Department of Neurology (S.R., R.S.), Clinical Division of Neurogeriatrics, Medical University Graz, Austria; Center for Genomic Medicine (J. Rosand), Massachusetts General Hospital, Boston; Broad Institute (J. Rosand), Cambridge, MA; Department of Neurology and Evelyn F. McKnight Brain Institute (J. Roquer, T.R., R.L.S./M.S.), Miller School of Medicine, University of Miami, FL; Institute of Cardiovascular Research (P.S.), Royal Holloway University of London (ICR2UL), UK St Peter's and Ashford Hospitals, Egham, United Kingdom; Department of Neurology (A. Slowik), Jagiellonian University Medical College, Krakow, Poland; Division of Neurocritical Care & Emergency Neurology (D.S.), Department of Neurology, Helsinki University Central Hospital, Finland; Stroke Division (V.T.), Florey Institute of Neuroscience and Mental Health, Heidelberg; Department of Neurology (V.T.), Austin Health, Heidelberg, Australia; Departments of Radiology (A.V.) and Neurology and Rehabilitation Medicine (D.W.), University of Cincinnati College of Medicine, OH; Department of Clinical Sciences Lund, Radiology (J.W.) and Neurology (A.G.L.), Lund University, Sweden; Department of Radiology, Neuroradiology, Skåne University Hospital, Malmö, Sweden; Departments of Neurology and Public Health Sciences (B.B.W.), University of Virginia, Charlottesville, VA; University of Technology Sydney (J.M.), Australia; Section of Neurology (A.G.L.), Skåne University Hospital, Lund, Sweden; Department of Laboratory Medicine (C.J.), Institute of Biomedicine, the Sahlgrenska Academy, University of Gothenburg, Sweden; and Department of Clinical Genetics and Genomics (C.J.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden.
1,
Kathleen Donahue
Kathleen Donahue, BS
1From the J. Philip Kistler Stroke Research Center (M.B., A.K.B., M.D.S., S.H., A. Dalca, K.D., A.-K.G., M.R.E., P.M.R., M.N., R.W.R., C.W., N.S.R.), A.A. Martinos Center for Biomedical Imaging (A. Dalca, O.W.), and Henry and Allison McCance Center for Brain Health (J. Rosand), Massachusetts General Hospital, Harvard Medical School, Boston; Lille Neuroscience & Cognition (M.B., X.L., R. Lopes, G.K.), Inserm, CHU Lille, U1172 and Institut Pasteur de Lille (M.G.), CNRS, Inserm, CHU Lille, US 41 - UMS 2014 - PLBS, Lille University, France; Computer Science and Artificial Intelligence Lab (A. Dalca, C.W., P.G.), Massachusetts Institute of Technology, Cambridge; Division of Preventive Medicine (P.M.R.), Department of Medicine, Brigham and Women's Hospital, Boston, MA; Department of Medicine (O.R.B.), Division of Neurology, University of British Columbia, Vancouver, Canada; Department of Neurology (J.W.C., S.J.K.), University of Maryland School of Medicine and Veterans Affairs Maryland Health Care System, Baltimore, MD; School of Medical Sciences (A. Donatti, A. Sousa), University of Campinas (UNICAMP) and the Brazilian Institute of Neuroscience and Neurotechnology (BRAINN), Campinas, São Paulo; Departments of Neurosurgery (C.G.) and Neurology (R.Z.), Geisinger, Danville, PA; Department of Neurosurgery (C.G.), Christian Doppler Klinik, Paracelsus Medical University, Salzburg, Austria; Division of Emergency Medicine (Laura Heitsch), Washington University School of Medicine, St. Louis; Department of Neurology (Laura Heitsch, C.-L.P.), Washington University School of Medicine & Barnes-Jewish Hospital, St. Louis, MO; Department of Clinical Neuroscience (L. Holmegaard, K.J., T.M.S., T.T.), Institute of Neuroscience and Physiology, Sahlgrenska Academy at University of Gothenburg, Sweden; Department of Neurology, Sahlgrenska University Hospital, Gothenburg, Sweden; Department of Neurology (J.J.-C.), Neurovascular Research Group (NEUVAS), IMIM-Hospital del Mar (Institut Hospital del Mar d'Investigacions M`ediques), Universitat Autonoma de Barcelona, Spain; Department of Neurosciences (R. Lemmens), Experimental Neurology and Leuven Research Institute for Neuroscience and Disease (LIND), KU Leuven - University of Leuven, Belgium; Department of Neurology (R. Lemmens), Laboratory of Neurobiology, VIB Vesalius Research Center, University Hospitals Leuven, Belgium; School of Medicine and Public Health (C.R.L.), University of Newcastle, New South Wales; Department of Neurology, John Hunter Hospital, Newcastle, New South Wales, Australia; Division of Endocrinology (P.F.M.), Diabetes and Nutrition, Department of Medicine, University of Maryland School of Medicine, Baltimore; Department of Pharmacotherapy and Translational Research and Center for Pharmacogenomics (C.W.M.), University of Florida, Gainesville; Department of Neurology (J.F.M.), Mayo Clinic, Jacksonville, FL; Klinik und Poliklinik für Neurologie (A.R.), Universitätsmedizin Rostock, Germany; Department of Neurology (S.R., R.S.), Clinical Division of Neurogeriatrics, Medical University Graz, Austria; Center for Genomic Medicine (J. Rosand), Massachusetts General Hospital, Boston; Broad Institute (J. Rosand), Cambridge, MA; Department of Neurology and Evelyn F. McKnight Brain Institute (J. Roquer, T.R., R.L.S./M.S.), Miller School of Medicine, University of Miami, FL; Institute of Cardiovascular Research (P.S.), Royal Holloway University of London (ICR2UL), UK St Peter's and Ashford Hospitals, Egham, United Kingdom; Department of Neurology (A. Slowik), Jagiellonian University Medical College, Krakow, Poland; Division of Neurocritical Care & Emergency Neurology (D.S.), Department of Neurology, Helsinki University Central Hospital, Finland; Stroke Division (V.T.), Florey Institute of Neuroscience and Mental Health, Heidelberg; Department of Neurology (V.T.), Austin Health, Heidelberg, Australia; Departments of Radiology (A.V.) and Neurology and Rehabilitation Medicine (D.W.), University of Cincinnati College of Medicine, OH; Department of Clinical Sciences Lund, Radiology (J.W.) and Neurology (A.G.L.), Lund University, Sweden; Department of Radiology, Neuroradiology, Skåne University Hospital, Malmö, Sweden; Departments of Neurology and Public Health Sciences (B.B.W.), University of Virginia, Charlottesville, VA; University of Technology Sydney (J.M.), Australia; Section of Neurology (A.G.L.), Skåne University Hospital, Lund, Sweden; Department of Laboratory Medicine (C.J.), Institute of Biomedicine, the Sahlgrenska Academy, University of Gothenburg, Sweden; and Department of Clinical Genetics and Genomics (C.J.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden.
1,
Anne-Katrin Giese
Anne-Katrin Giese, MD
1From the J. Philip Kistler Stroke Research Center (M.B., A.K.B., M.D.S., S.H., A. Dalca, K.D., A.-K.G., M.R.E., P.M.R., M.N., R.W.R., C.W., N.S.R.), A.A. Martinos Center for Biomedical Imaging (A. Dalca, O.W.), and Henry and Allison McCance Center for Brain Health (J. Rosand), Massachusetts General Hospital, Harvard Medical School, Boston; Lille Neuroscience & Cognition (M.B., X.L., R. Lopes, G.K.), Inserm, CHU Lille, U1172 and Institut Pasteur de Lille (M.G.), CNRS, Inserm, CHU Lille, US 41 - UMS 2014 - PLBS, Lille University, France; Computer Science and Artificial Intelligence Lab (A. Dalca, C.W., P.G.), Massachusetts Institute of Technology, Cambridge; Division of Preventive Medicine (P.M.R.), Department of Medicine, Brigham and Women's Hospital, Boston, MA; Department of Medicine (O.R.B.), Division of Neurology, University of British Columbia, Vancouver, Canada; Department of Neurology (J.W.C., S.J.K.), University of Maryland School of Medicine and Veterans Affairs Maryland Health Care System, Baltimore, MD; School of Medical Sciences (A. Donatti, A. Sousa), University of Campinas (UNICAMP) and the Brazilian Institute of Neuroscience and Neurotechnology (BRAINN), Campinas, São Paulo; Departments of Neurosurgery (C.G.) and Neurology (R.Z.), Geisinger, Danville, PA; Department of Neurosurgery (C.G.), Christian Doppler Klinik, Paracelsus Medical University, Salzburg, Austria; Division of Emergency Medicine (Laura Heitsch), Washington University School of Medicine, St. Louis; Department of Neurology (Laura Heitsch, C.-L.P.), Washington University School of Medicine & Barnes-Jewish Hospital, St. Louis, MO; Department of Clinical Neuroscience (L. Holmegaard, K.J., T.M.S., T.T.), Institute of Neuroscience and Physiology, Sahlgrenska Academy at University of Gothenburg, Sweden; Department of Neurology, Sahlgrenska University Hospital, Gothenburg, Sweden; Department of Neurology (J.J.-C.), Neurovascular Research Group (NEUVAS), IMIM-Hospital del Mar (Institut Hospital del Mar d'Investigacions M`ediques), Universitat Autonoma de Barcelona, Spain; Department of Neurosciences (R. Lemmens), Experimental Neurology and Leuven Research Institute for Neuroscience and Disease (LIND), KU Leuven - University of Leuven, Belgium; Department of Neurology (R. Lemmens), Laboratory of Neurobiology, VIB Vesalius Research Center, University Hospitals Leuven, Belgium; School of Medicine and Public Health (C.R.L.), University of Newcastle, New South Wales; Department of Neurology, John Hunter Hospital, Newcastle, New South Wales, Australia; Division of Endocrinology (P.F.M.), Diabetes and Nutrition, Department of Medicine, University of Maryland School of Medicine, Baltimore; Department of Pharmacotherapy and Translational Research and Center for Pharmacogenomics (C.W.M.), University of Florida, Gainesville; Department of Neurology (J.F.M.), Mayo Clinic, Jacksonville, FL; Klinik und Poliklinik für Neurologie (A.R.), Universitätsmedizin Rostock, Germany; Department of Neurology (S.R., R.S.), Clinical Division of Neurogeriatrics, Medical University Graz, Austria; Center for Genomic Medicine (J. Rosand), Massachusetts General Hospital, Boston; Broad Institute (J. Rosand), Cambridge, MA; Department of Neurology and Evelyn F. McKnight Brain Institute (J. Roquer, T.R., R.L.S./M.S.), Miller School of Medicine, University of Miami, FL; Institute of Cardiovascular Research (P.S.), Royal Holloway University of London (ICR2UL), UK St Peter's and Ashford Hospitals, Egham, United Kingdom; Department of Neurology (A. Slowik), Jagiellonian University Medical College, Krakow, Poland; Division of Neurocritical Care & Emergency Neurology (D.S.), Department of Neurology, Helsinki University Central Hospital, Finland; Stroke Division (V.T.), Florey Institute of Neuroscience and Mental Health, Heidelberg; Department of Neurology (V.T.), Austin Health, Heidelberg, Australia; Departments of Radiology (A.V.) and Neurology and Rehabilitation Medicine (D.W.), University of Cincinnati College of Medicine, OH; Department of Clinical Sciences Lund, Radiology (J.W.) and Neurology (A.G.L.), Lund University, Sweden; Department of Radiology, Neuroradiology, Skåne University Hospital, Malmö, Sweden; Departments of Neurology and Public Health Sciences (B.B.W.), University of Virginia, Charlottesville, VA; University of Technology Sydney (J.M.), Australia; Section of Neurology (A.G.L.), Skåne University Hospital, Lund, Sweden; Department of Laboratory Medicine (C.J.), Institute of Biomedicine, the Sahlgrenska Academy, University of Gothenburg, Sweden; and Department of Clinical Genetics and Genomics (C.J.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden.
1,
Mark R Etherton
Mark R Etherton, MD, PhD
1From the J. Philip Kistler Stroke Research Center (M.B., A.K.B., M.D.S., S.H., A. Dalca, K.D., A.-K.G., M.R.E., P.M.R., M.N., R.W.R., C.W., N.S.R.), A.A. Martinos Center for Biomedical Imaging (A. Dalca, O.W.), and Henry and Allison McCance Center for Brain Health (J. Rosand), Massachusetts General Hospital, Harvard Medical School, Boston; Lille Neuroscience & Cognition (M.B., X.L., R. Lopes, G.K.), Inserm, CHU Lille, U1172 and Institut Pasteur de Lille (M.G.), CNRS, Inserm, CHU Lille, US 41 - UMS 2014 - PLBS, Lille University, France; Computer Science and Artificial Intelligence Lab (A. Dalca, C.W., P.G.), Massachusetts Institute of Technology, Cambridge; Division of Preventive Medicine (P.M.R.), Department of Medicine, Brigham and Women's Hospital, Boston, MA; Department of Medicine (O.R.B.), Division of Neurology, University of British Columbia, Vancouver, Canada; Department of Neurology (J.W.C., S.J.K.), University of Maryland School of Medicine and Veterans Affairs Maryland Health Care System, Baltimore, MD; School of Medical Sciences (A. Donatti, A. Sousa), University of Campinas (UNICAMP) and the Brazilian Institute of Neuroscience and Neurotechnology (BRAINN), Campinas, São Paulo; Departments of Neurosurgery (C.G.) and Neurology (R.Z.), Geisinger, Danville, PA; Department of Neurosurgery (C.G.), Christian Doppler Klinik, Paracelsus Medical University, Salzburg, Austria; Division of Emergency Medicine (Laura Heitsch), Washington University School of Medicine, St. Louis; Department of Neurology (Laura Heitsch, C.-L.P.), Washington University School of Medicine & Barnes-Jewish Hospital, St. Louis, MO; Department of Clinical Neuroscience (L. Holmegaard, K.J., T.M.S., T.T.), Institute of Neuroscience and Physiology, Sahlgrenska Academy at University of Gothenburg, Sweden; Department of Neurology, Sahlgrenska University Hospital, Gothenburg, Sweden; Department of Neurology (J.J.-C.), Neurovascular Research Group (NEUVAS), IMIM-Hospital del Mar (Institut Hospital del Mar d'Investigacions M`ediques), Universitat Autonoma de Barcelona, Spain; Department of Neurosciences (R. Lemmens), Experimental Neurology and Leuven Research Institute for Neuroscience and Disease (LIND), KU Leuven - University of Leuven, Belgium; Department of Neurology (R. Lemmens), Laboratory of Neurobiology, VIB Vesalius Research Center, University Hospitals Leuven, Belgium; School of Medicine and Public Health (C.R.L.), University of Newcastle, New South Wales; Department of Neurology, John Hunter Hospital, Newcastle, New South Wales, Australia; Division of Endocrinology (P.F.M.), Diabetes and Nutrition, Department of Medicine, University of Maryland School of Medicine, Baltimore; Department of Pharmacotherapy and Translational Research and Center for Pharmacogenomics (C.W.M.), University of Florida, Gainesville; Department of Neurology (J.F.M.), Mayo Clinic, Jacksonville, FL; Klinik und Poliklinik für Neurologie (A.R.), Universitätsmedizin Rostock, Germany; Department of Neurology (S.R., R.S.), Clinical Division of Neurogeriatrics, Medical University Graz, Austria; Center for Genomic Medicine (J. Rosand), Massachusetts General Hospital, Boston; Broad Institute (J. Rosand), Cambridge, MA; Department of Neurology and Evelyn F. McKnight Brain Institute (J. Roquer, T.R., R.L.S./M.S.), Miller School of Medicine, University of Miami, FL; Institute of Cardiovascular Research (P.S.), Royal Holloway University of London (ICR2UL), UK St Peter's and Ashford Hospitals, Egham, United Kingdom; Department of Neurology (A. Slowik), Jagiellonian University Medical College, Krakow, Poland; Division of Neurocritical Care & Emergency Neurology (D.S.), Department of Neurology, Helsinki University Central Hospital, Finland; Stroke Division (V.T.), Florey Institute of Neuroscience and Mental Health, Heidelberg; Department of Neurology (V.T.), Austin Health, Heidelberg, Australia; Departments of Radiology (A.V.) and Neurology and Rehabilitation Medicine (D.W.), University of Cincinnati College of Medicine, OH; Department of Clinical Sciences Lund, Radiology (J.W.) and Neurology (A.G.L.), Lund University, Sweden; Department of Radiology, Neuroradiology, Skåne University Hospital, Malmö, Sweden; Departments of Neurology and Public Health Sciences (B.B.W.), University of Virginia, Charlottesville, VA; University of Technology Sydney (J.M.), Australia; Section of Neurology (A.G.L.), Skåne University Hospital, Lund, Sweden; Department of Laboratory Medicine (C.J.), Institute of Biomedicine, the Sahlgrenska Academy, University of Gothenburg, Sweden; and Department of Clinical Genetics and Genomics (C.J.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden.
1,
Pamela M Rist
Pamela M Rist, ScD
1From the J. Philip Kistler Stroke Research Center (M.B., A.K.B., M.D.S., S.H., A. Dalca, K.D., A.-K.G., M.R.E., P.M.R., M.N., R.W.R., C.W., N.S.R.), A.A. Martinos Center for Biomedical Imaging (A. Dalca, O.W.), and Henry and Allison McCance Center for Brain Health (J. Rosand), Massachusetts General Hospital, Harvard Medical School, Boston; Lille Neuroscience & Cognition (M.B., X.L., R. Lopes, G.K.), Inserm, CHU Lille, U1172 and Institut Pasteur de Lille (M.G.), CNRS, Inserm, CHU Lille, US 41 - UMS 2014 - PLBS, Lille University, France; Computer Science and Artificial Intelligence Lab (A. Dalca, C.W., P.G.), Massachusetts Institute of Technology, Cambridge; Division of Preventive Medicine (P.M.R.), Department of Medicine, Brigham and Women's Hospital, Boston, MA; Department of Medicine (O.R.B.), Division of Neurology, University of British Columbia, Vancouver, Canada; Department of Neurology (J.W.C., S.J.K.), University of Maryland School of Medicine and Veterans Affairs Maryland Health Care System, Baltimore, MD; School of Medical Sciences (A. Donatti, A. Sousa), University of Campinas (UNICAMP) and the Brazilian Institute of Neuroscience and Neurotechnology (BRAINN), Campinas, São Paulo; Departments of Neurosurgery (C.G.) and Neurology (R.Z.), Geisinger, Danville, PA; Department of Neurosurgery (C.G.), Christian Doppler Klinik, Paracelsus Medical University, Salzburg, Austria; Division of Emergency Medicine (Laura Heitsch), Washington University School of Medicine, St. Louis; Department of Neurology (Laura Heitsch, C.-L.P.), Washington University School of Medicine & Barnes-Jewish Hospital, St. Louis, MO; Department of Clinical Neuroscience (L. Holmegaard, K.J., T.M.S., T.T.), Institute of Neuroscience and Physiology, Sahlgrenska Academy at University of Gothenburg, Sweden; Department of Neurology, Sahlgrenska University Hospital, Gothenburg, Sweden; Department of Neurology (J.J.-C.), Neurovascular Research Group (NEUVAS), IMIM-Hospital del Mar (Institut Hospital del Mar d'Investigacions M`ediques), Universitat Autonoma de Barcelona, Spain; Department of Neurosciences (R. Lemmens), Experimental Neurology and Leuven Research Institute for Neuroscience and Disease (LIND), KU Leuven - University of Leuven, Belgium; Department of Neurology (R. Lemmens), Laboratory of Neurobiology, VIB Vesalius Research Center, University Hospitals Leuven, Belgium; School of Medicine and Public Health (C.R.L.), University of Newcastle, New South Wales; Department of Neurology, John Hunter Hospital, Newcastle, New South Wales, Australia; Division of Endocrinology (P.F.M.), Diabetes and Nutrition, Department of Medicine, University of Maryland School of Medicine, Baltimore; Department of Pharmacotherapy and Translational Research and Center for Pharmacogenomics (C.W.M.), University of Florida, Gainesville; Department of Neurology (J.F.M.), Mayo Clinic, Jacksonville, FL; Klinik und Poliklinik für Neurologie (A.R.), Universitätsmedizin Rostock, Germany; Department of Neurology (S.R., R.S.), Clinical Division of Neurogeriatrics, Medical University Graz, Austria; Center for Genomic Medicine (J. Rosand), Massachusetts General Hospital, Boston; Broad Institute (J. Rosand), Cambridge, MA; Department of Neurology and Evelyn F. McKnight Brain Institute (J. Roquer, T.R., R.L.S./M.S.), Miller School of Medicine, University of Miami, FL; Institute of Cardiovascular Research (P.S.), Royal Holloway University of London (ICR2UL), UK St Peter's and Ashford Hospitals, Egham, United Kingdom; Department of Neurology (A. Slowik), Jagiellonian University Medical College, Krakow, Poland; Division of Neurocritical Care & Emergency Neurology (D.S.), Department of Neurology, Helsinki University Central Hospital, Finland; Stroke Division (V.T.), Florey Institute of Neuroscience and Mental Health, Heidelberg; Department of Neurology (V.T.), Austin Health, Heidelberg, Australia; Departments of Radiology (A.V.) and Neurology and Rehabilitation Medicine (D.W.), University of Cincinnati College of Medicine, OH; Department of Clinical Sciences Lund, Radiology (J.W.) and Neurology (A.G.L.), Lund University, Sweden; Department of Radiology, Neuroradiology, Skåne University Hospital, Malmö, Sweden; Departments of Neurology and Public Health Sciences (B.B.W.), University of Virginia, Charlottesville, VA; University of Technology Sydney (J.M.), Australia; Section of Neurology (A.G.L.), Skåne University Hospital, Lund, Sweden; Department of Laboratory Medicine (C.J.), Institute of Biomedicine, the Sahlgrenska Academy, University of Gothenburg, Sweden; and Department of Clinical Genetics and Genomics (C.J.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden.
1,
Marco Nardin
Marco Nardin, BS
1From the J. Philip Kistler Stroke Research Center (M.B., A.K.B., M.D.S., S.H., A. Dalca, K.D., A.-K.G., M.R.E., P.M.R., M.N., R.W.R., C.W., N.S.R.), A.A. Martinos Center for Biomedical Imaging (A. Dalca, O.W.), and Henry and Allison McCance Center for Brain Health (J. Rosand), Massachusetts General Hospital, Harvard Medical School, Boston; Lille Neuroscience & Cognition (M.B., X.L., R. Lopes, G.K.), Inserm, CHU Lille, U1172 and Institut Pasteur de Lille (M.G.), CNRS, Inserm, CHU Lille, US 41 - UMS 2014 - PLBS, Lille University, France; Computer Science and Artificial Intelligence Lab (A. Dalca, C.W., P.G.), Massachusetts Institute of Technology, Cambridge; Division of Preventive Medicine (P.M.R.), Department of Medicine, Brigham and Women's Hospital, Boston, MA; Department of Medicine (O.R.B.), Division of Neurology, University of British Columbia, Vancouver, Canada; Department of Neurology (J.W.C., S.J.K.), University of Maryland School of Medicine and Veterans Affairs Maryland Health Care System, Baltimore, MD; School of Medical Sciences (A. Donatti, A. Sousa), University of Campinas (UNICAMP) and the Brazilian Institute of Neuroscience and Neurotechnology (BRAINN), Campinas, São Paulo; Departments of Neurosurgery (C.G.) and Neurology (R.Z.), Geisinger, Danville, PA; Department of Neurosurgery (C.G.), Christian Doppler Klinik, Paracelsus Medical University, Salzburg, Austria; Division of Emergency Medicine (Laura Heitsch), Washington University School of Medicine, St. Louis; Department of Neurology (Laura Heitsch, C.-L.P.), Washington University School of Medicine & Barnes-Jewish Hospital, St. Louis, MO; Department of Clinical Neuroscience (L. Holmegaard, K.J., T.M.S., T.T.), Institute of Neuroscience and Physiology, Sahlgrenska Academy at University of Gothenburg, Sweden; Department of Neurology, Sahlgrenska University Hospital, Gothenburg, Sweden; Department of Neurology (J.J.-C.), Neurovascular Research Group (NEUVAS), IMIM-Hospital del Mar (Institut Hospital del Mar d'Investigacions M`ediques), Universitat Autonoma de Barcelona, Spain; Department of Neurosciences (R. Lemmens), Experimental Neurology and Leuven Research Institute for Neuroscience and Disease (LIND), KU Leuven - University of Leuven, Belgium; Department of Neurology (R. Lemmens), Laboratory of Neurobiology, VIB Vesalius Research Center, University Hospitals Leuven, Belgium; School of Medicine and Public Health (C.R.L.), University of Newcastle, New South Wales; Department of Neurology, John Hunter Hospital, Newcastle, New South Wales, Australia; Division of Endocrinology (P.F.M.), Diabetes and Nutrition, Department of Medicine, University of Maryland School of Medicine, Baltimore; Department of Pharmacotherapy and Translational Research and Center for Pharmacogenomics (C.W.M.), University of Florida, Gainesville; Department of Neurology (J.F.M.), Mayo Clinic, Jacksonville, FL; Klinik und Poliklinik für Neurologie (A.R.), Universitätsmedizin Rostock, Germany; Department of Neurology (S.R., R.S.), Clinical Division of Neurogeriatrics, Medical University Graz, Austria; Center for Genomic Medicine (J. Rosand), Massachusetts General Hospital, Boston; Broad Institute (J. Rosand), Cambridge, MA; Department of Neurology and Evelyn F. McKnight Brain Institute (J. Roquer, T.R., R.L.S./M.S.), Miller School of Medicine, University of Miami, FL; Institute of Cardiovascular Research (P.S.), Royal Holloway University of London (ICR2UL), UK St Peter's and Ashford Hospitals, Egham, United Kingdom; Department of Neurology (A. Slowik), Jagiellonian University Medical College, Krakow, Poland; Division of Neurocritical Care & Emergency Neurology (D.S.), Department of Neurology, Helsinki University Central Hospital, Finland; Stroke Division (V.T.), Florey Institute of Neuroscience and Mental Health, Heidelberg; Department of Neurology (V.T.), Austin Health, Heidelberg, Australia; Departments of Radiology (A.V.) and Neurology and Rehabilitation Medicine (D.W.), University of Cincinnati College of Medicine, OH; Department of Clinical Sciences Lund, Radiology (J.W.) and Neurology (A.G.L.), Lund University, Sweden; Department of Radiology, Neuroradiology, Skåne University Hospital, Malmö, Sweden; Departments of Neurology and Public Health Sciences (B.B.W.), University of Virginia, Charlottesville, VA; University of Technology Sydney (J.M.), Australia; Section of Neurology (A.G.L.), Skåne University Hospital, Lund, Sweden; Department of Laboratory Medicine (C.J.), Institute of Biomedicine, the Sahlgrenska Academy, University of Gothenburg, Sweden; and Department of Clinical Genetics and Genomics (C.J.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden.
1,
Robert W Regenhardt
Robert W Regenhardt, MD, PhD
1From the J. Philip Kistler Stroke Research Center (M.B., A.K.B., M.D.S., S.H., A. Dalca, K.D., A.-K.G., M.R.E., P.M.R., M.N., R.W.R., C.W., N.S.R.), A.A. Martinos Center for Biomedical Imaging (A. Dalca, O.W.), and Henry and Allison McCance Center for Brain Health (J. Rosand), Massachusetts General Hospital, Harvard Medical School, Boston; Lille Neuroscience & Cognition (M.B., X.L., R. Lopes, G.K.), Inserm, CHU Lille, U1172 and Institut Pasteur de Lille (M.G.), CNRS, Inserm, CHU Lille, US 41 - UMS 2014 - PLBS, Lille University, France; Computer Science and Artificial Intelligence Lab (A. Dalca, C.W., P.G.), Massachusetts Institute of Technology, Cambridge; Division of Preventive Medicine (P.M.R.), Department of Medicine, Brigham and Women's Hospital, Boston, MA; Department of Medicine (O.R.B.), Division of Neurology, University of British Columbia, Vancouver, Canada; Department of Neurology (J.W.C., S.J.K.), University of Maryland School of Medicine and Veterans Affairs Maryland Health Care System, Baltimore, MD; School of Medical Sciences (A. Donatti, A. Sousa), University of Campinas (UNICAMP) and the Brazilian Institute of Neuroscience and Neurotechnology (BRAINN), Campinas, São Paulo; Departments of Neurosurgery (C.G.) and Neurology (R.Z.), Geisinger, Danville, PA; Department of Neurosurgery (C.G.), Christian Doppler Klinik, Paracelsus Medical University, Salzburg, Austria; Division of Emergency Medicine (Laura Heitsch), Washington University School of Medicine, St. Louis; Department of Neurology (Laura Heitsch, C.-L.P.), Washington University School of Medicine & Barnes-Jewish Hospital, St. Louis, MO; Department of Clinical Neuroscience (L. Holmegaard, K.J., T.M.S., T.T.), Institute of Neuroscience and Physiology, Sahlgrenska Academy at University of Gothenburg, Sweden; Department of Neurology, Sahlgrenska University Hospital, Gothenburg, Sweden; Department of Neurology (J.J.-C.), Neurovascular Research Group (NEUVAS), IMIM-Hospital del Mar (Institut Hospital del Mar d'Investigacions M`ediques), Universitat Autonoma de Barcelona, Spain; Department of Neurosciences (R. Lemmens), Experimental Neurology and Leuven Research Institute for Neuroscience and Disease (LIND), KU Leuven - University of Leuven, Belgium; Department of Neurology (R. Lemmens), Laboratory of Neurobiology, VIB Vesalius Research Center, University Hospitals Leuven, Belgium; School of Medicine and Public Health (C.R.L.), University of Newcastle, New South Wales; Department of Neurology, John Hunter Hospital, Newcastle, New South Wales, Australia; Division of Endocrinology (P.F.M.), Diabetes and Nutrition, Department of Medicine, University of Maryland School of Medicine, Baltimore; Department of Pharmacotherapy and Translational Research and Center for Pharmacogenomics (C.W.M.), University of Florida, Gainesville; Department of Neurology (J.F.M.), Mayo Clinic, Jacksonville, FL; Klinik und Poliklinik für Neurologie (A.R.), Universitätsmedizin Rostock, Germany; Department of Neurology (S.R., R.S.), Clinical Division of Neurogeriatrics, Medical University Graz, Austria; Center for Genomic Medicine (J. Rosand), Massachusetts General Hospital, Boston; Broad Institute (J. Rosand), Cambridge, MA; Department of Neurology and Evelyn F. McKnight Brain Institute (J. Roquer, T.R., R.L.S./M.S.), Miller School of Medicine, University of Miami, FL; Institute of Cardiovascular Research (P.S.), Royal Holloway University of London (ICR2UL), UK St Peter's and Ashford Hospitals, Egham, United Kingdom; Department of Neurology (A. Slowik), Jagiellonian University Medical College, Krakow, Poland; Division of Neurocritical Care & Emergency Neurology (D.S.), Department of Neurology, Helsinki University Central Hospital, Finland; Stroke Division (V.T.), Florey Institute of Neuroscience and Mental Health, Heidelberg; Department of Neurology (V.T.), Austin Health, Heidelberg, Australia; Departments of Radiology (A.V.) and Neurology and Rehabilitation Medicine (D.W.), University of Cincinnati College of Medicine, OH; Department of Clinical Sciences Lund, Radiology (J.W.) and Neurology (A.G.L.), Lund University, Sweden; Department of Radiology, Neuroradiology, Skåne University Hospital, Malmö, Sweden; Departments of Neurology and Public Health Sciences (B.B.W.), University of Virginia, Charlottesville, VA; University of Technology Sydney (J.M.), Australia; Section of Neurology (A.G.L.), Skåne University Hospital, Lund, Sweden; Department of Laboratory Medicine (C.J.), Institute of Biomedicine, the Sahlgrenska Academy, University of Gothenburg, Sweden; and Department of Clinical Genetics and Genomics (C.J.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden.
1,
Xavier Leclerc
Xavier Leclerc, MD, PhD
1From the J. Philip Kistler Stroke Research Center (M.B., A.K.B., M.D.S., S.H., A. Dalca, K.D., A.-K.G., M.R.E., P.M.R., M.N., R.W.R., C.W., N.S.R.), A.A. Martinos Center for Biomedical Imaging (A. Dalca, O.W.), and Henry and Allison McCance Center for Brain Health (J. Rosand), Massachusetts General Hospital, Harvard Medical School, Boston; Lille Neuroscience & Cognition (M.B., X.L., R. Lopes, G.K.), Inserm, CHU Lille, U1172 and Institut Pasteur de Lille (M.G.), CNRS, Inserm, CHU Lille, US 41 - UMS 2014 - PLBS, Lille University, France; Computer Science and Artificial Intelligence Lab (A. Dalca, C.W., P.G.), Massachusetts Institute of Technology, Cambridge; Division of Preventive Medicine (P.M.R.), Department of Medicine, Brigham and Women's Hospital, Boston, MA; Department of Medicine (O.R.B.), Division of Neurology, University of British Columbia, Vancouver, Canada; Department of Neurology (J.W.C., S.J.K.), University of Maryland School of Medicine and Veterans Affairs Maryland Health Care System, Baltimore, MD; School of Medical Sciences (A. Donatti, A. Sousa), University of Campinas (UNICAMP) and the Brazilian Institute of Neuroscience and Neurotechnology (BRAINN), Campinas, São Paulo; Departments of Neurosurgery (C.G.) and Neurology (R.Z.), Geisinger, Danville, PA; Department of Neurosurgery (C.G.), Christian Doppler Klinik, Paracelsus Medical University, Salzburg, Austria; Division of Emergency Medicine (Laura Heitsch), Washington University School of Medicine, St. Louis; Department of Neurology (Laura Heitsch, C.-L.P.), Washington University School of Medicine & Barnes-Jewish Hospital, St. Louis, MO; Department of Clinical Neuroscience (L. Holmegaard, K.J., T.M.S., T.T.), Institute of Neuroscience and Physiology, Sahlgrenska Academy at University of Gothenburg, Sweden; Department of Neurology, Sahlgrenska University Hospital, Gothenburg, Sweden; Department of Neurology (J.J.-C.), Neurovascular Research Group (NEUVAS), IMIM-Hospital del Mar (Institut Hospital del Mar d'Investigacions M`ediques), Universitat Autonoma de Barcelona, Spain; Department of Neurosciences (R. Lemmens), Experimental Neurology and Leuven Research Institute for Neuroscience and Disease (LIND), KU Leuven - University of Leuven, Belgium; Department of Neurology (R. Lemmens), Laboratory of Neurobiology, VIB Vesalius Research Center, University Hospitals Leuven, Belgium; School of Medicine and Public Health (C.R.L.), University of Newcastle, New South Wales; Department of Neurology, John Hunter Hospital, Newcastle, New South Wales, Australia; Division of Endocrinology (P.F.M.), Diabetes and Nutrition, Department of Medicine, University of Maryland School of Medicine, Baltimore; Department of Pharmacotherapy and Translational Research and Center for Pharmacogenomics (C.W.M.), University of Florida, Gainesville; Department of Neurology (J.F.M.), Mayo Clinic, Jacksonville, FL; Klinik und Poliklinik für Neurologie (A.R.), Universitätsmedizin Rostock, Germany; Department of Neurology (S.R., R.S.), Clinical Division of Neurogeriatrics, Medical University Graz, Austria; Center for Genomic Medicine (J. Rosand), Massachusetts General Hospital, Boston; Broad Institute (J. Rosand), Cambridge, MA; Department of Neurology and Evelyn F. McKnight Brain Institute (J. Roquer, T.R., R.L.S./M.S.), Miller School of Medicine, University of Miami, FL; Institute of Cardiovascular Research (P.S.), Royal Holloway University of London (ICR2UL), UK St Peter's and Ashford Hospitals, Egham, United Kingdom; Department of Neurology (A. Slowik), Jagiellonian University Medical College, Krakow, Poland; Division of Neurocritical Care & Emergency Neurology (D.S.), Department of Neurology, Helsinki University Central Hospital, Finland; Stroke Division (V.T.), Florey Institute of Neuroscience and Mental Health, Heidelberg; Department of Neurology (V.T.), Austin Health, Heidelberg, Australia; Departments of Radiology (A.V.) and Neurology and Rehabilitation Medicine (D.W.), University of Cincinnati College of Medicine, OH; Department of Clinical Sciences Lund, Radiology (J.W.) and Neurology (A.G.L.), Lund University, Sweden; Department of Radiology, Neuroradiology, Skåne University Hospital, Malmö, Sweden; Departments of Neurology and Public Health Sciences (B.B.W.), University of Virginia, Charlottesville, VA; University of Technology Sydney (J.M.), Australia; Section of Neurology (A.G.L.), Skåne University Hospital, Lund, Sweden; Department of Laboratory Medicine (C.J.), Institute of Biomedicine, the Sahlgrenska Academy, University of Gothenburg, Sweden; and Department of Clinical Genetics and Genomics (C.J.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden.
1,
Renaud Lopes
Renaud Lopes, PhD
1From the J. Philip Kistler Stroke Research Center (M.B., A.K.B., M.D.S., S.H., A. Dalca, K.D., A.-K.G., M.R.E., P.M.R., M.N., R.W.R., C.W., N.S.R.), A.A. Martinos Center for Biomedical Imaging (A. Dalca, O.W.), and Henry and Allison McCance Center for Brain Health (J. Rosand), Massachusetts General Hospital, Harvard Medical School, Boston; Lille Neuroscience & Cognition (M.B., X.L., R. Lopes, G.K.), Inserm, CHU Lille, U1172 and Institut Pasteur de Lille (M.G.), CNRS, Inserm, CHU Lille, US 41 - UMS 2014 - PLBS, Lille University, France; Computer Science and Artificial Intelligence Lab (A. Dalca, C.W., P.G.), Massachusetts Institute of Technology, Cambridge; Division of Preventive Medicine (P.M.R.), Department of Medicine, Brigham and Women's Hospital, Boston, MA; Department of Medicine (O.R.B.), Division of Neurology, University of British Columbia, Vancouver, Canada; Department of Neurology (J.W.C., S.J.K.), University of Maryland School of Medicine and Veterans Affairs Maryland Health Care System, Baltimore, MD; School of Medical Sciences (A. Donatti, A. Sousa), University of Campinas (UNICAMP) and the Brazilian Institute of Neuroscience and Neurotechnology (BRAINN), Campinas, São Paulo; Departments of Neurosurgery (C.G.) and Neurology (R.Z.), Geisinger, Danville, PA; Department of Neurosurgery (C.G.), Christian Doppler Klinik, Paracelsus Medical University, Salzburg, Austria; Division of Emergency Medicine (Laura Heitsch), Washington University School of Medicine, St. Louis; Department of Neurology (Laura Heitsch, C.-L.P.), Washington University School of Medicine & Barnes-Jewish Hospital, St. Louis, MO; Department of Clinical Neuroscience (L. Holmegaard, K.J., T.M.S., T.T.), Institute of Neuroscience and Physiology, Sahlgrenska Academy at University of Gothenburg, Sweden; Department of Neurology, Sahlgrenska University Hospital, Gothenburg, Sweden; Department of Neurology (J.J.-C.), Neurovascular Research Group (NEUVAS), IMIM-Hospital del Mar (Institut Hospital del Mar d'Investigacions M`ediques), Universitat Autonoma de Barcelona, Spain; Department of Neurosciences (R. Lemmens), Experimental Neurology and Leuven Research Institute for Neuroscience and Disease (LIND), KU Leuven - University of Leuven, Belgium; Department of Neurology (R. Lemmens), Laboratory of Neurobiology, VIB Vesalius Research Center, University Hospitals Leuven, Belgium; School of Medicine and Public Health (C.R.L.), University of Newcastle, New South Wales; Department of Neurology, John Hunter Hospital, Newcastle, New South Wales, Australia; Division of Endocrinology (P.F.M.), Diabetes and Nutrition, Department of Medicine, University of Maryland School of Medicine, Baltimore; Department of Pharmacotherapy and Translational Research and Center for Pharmacogenomics (C.W.M.), University of Florida, Gainesville; Department of Neurology (J.F.M.), Mayo Clinic, Jacksonville, FL; Klinik und Poliklinik für Neurologie (A.R.), Universitätsmedizin Rostock, Germany; Department of Neurology (S.R., R.S.), Clinical Division of Neurogeriatrics, Medical University Graz, Austria; Center for Genomic Medicine (J. Rosand), Massachusetts General Hospital, Boston; Broad Institute (J. Rosand), Cambridge, MA; Department of Neurology and Evelyn F. McKnight Brain Institute (J. Roquer, T.R., R.L.S./M.S.), Miller School of Medicine, University of Miami, FL; Institute of Cardiovascular Research (P.S.), Royal Holloway University of London (ICR2UL), UK St Peter's and Ashford Hospitals, Egham, United Kingdom; Department of Neurology (A. Slowik), Jagiellonian University Medical College, Krakow, Poland; Division of Neurocritical Care & Emergency Neurology (D.S.), Department of Neurology, Helsinki University Central Hospital, Finland; Stroke Division (V.T.), Florey Institute of Neuroscience and Mental Health, Heidelberg; Department of Neurology (V.T.), Austin Health, Heidelberg, Australia; Departments of Radiology (A.V.) and Neurology and Rehabilitation Medicine (D.W.), University of Cincinnati College of Medicine, OH; Department of Clinical Sciences Lund, Radiology (J.W.) and Neurology (A.G.L.), Lund University, Sweden; Department of Radiology, Neuroradiology, Skåne University Hospital, Malmö, Sweden; Departments of Neurology and Public Health Sciences (B.B.W.), University of Virginia, Charlottesville, VA; University of Technology Sydney (J.M.), Australia; Section of Neurology (A.G.L.), Skåne University Hospital, Lund, Sweden; Department of Laboratory Medicine (C.J.), Institute of Biomedicine, the Sahlgrenska Academy, University of Gothenburg, Sweden; and Department of Clinical Genetics and Genomics (C.J.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden.
1,
Morgan Gautherot
Morgan Gautherot, PhD
1From the J. Philip Kistler Stroke Research Center (M.B., A.K.B., M.D.S., S.H., A. Dalca, K.D., A.-K.G., M.R.E., P.M.R., M.N., R.W.R., C.W., N.S.R.), A.A. Martinos Center for Biomedical Imaging (A. Dalca, O.W.), and Henry and Allison McCance Center for Brain Health (J. Rosand), Massachusetts General Hospital, Harvard Medical School, Boston; Lille Neuroscience & Cognition (M.B., X.L., R. Lopes, G.K.), Inserm, CHU Lille, U1172 and Institut Pasteur de Lille (M.G.), CNRS, Inserm, CHU Lille, US 41 - UMS 2014 - PLBS, Lille University, France; Computer Science and Artificial Intelligence Lab (A. Dalca, C.W., P.G.), Massachusetts Institute of Technology, Cambridge; Division of Preventive Medicine (P.M.R.), Department of Medicine, Brigham and Women's Hospital, Boston, MA; Department of Medicine (O.R.B.), Division of Neurology, University of British Columbia, Vancouver, Canada; Department of Neurology (J.W.C., S.J.K.), University of Maryland School of Medicine and Veterans Affairs Maryland Health Care System, Baltimore, MD; School of Medical Sciences (A. Donatti, A. Sousa), University of Campinas (UNICAMP) and the Brazilian Institute of Neuroscience and Neurotechnology (BRAINN), Campinas, São Paulo; Departments of Neurosurgery (C.G.) and Neurology (R.Z.), Geisinger, Danville, PA; Department of Neurosurgery (C.G.), Christian Doppler Klinik, Paracelsus Medical University, Salzburg, Austria; Division of Emergency Medicine (Laura Heitsch), Washington University School of Medicine, St. Louis; Department of Neurology (Laura Heitsch, C.-L.P.), Washington University School of Medicine & Barnes-Jewish Hospital, St. Louis, MO; Department of Clinical Neuroscience (L. Holmegaard, K.J., T.M.S., T.T.), Institute of Neuroscience and Physiology, Sahlgrenska Academy at University of Gothenburg, Sweden; Department of Neurology, Sahlgrenska University Hospital, Gothenburg, Sweden; Department of Neurology (J.J.-C.), Neurovascular Research Group (NEUVAS), IMIM-Hospital del Mar (Institut Hospital del Mar d'Investigacions M`ediques), Universitat Autonoma de Barcelona, Spain; Department of Neurosciences (R. Lemmens), Experimental Neurology and Leuven Research Institute for Neuroscience and Disease (LIND), KU Leuven - University of Leuven, Belgium; Department of Neurology (R. Lemmens), Laboratory of Neurobiology, VIB Vesalius Research Center, University Hospitals Leuven, Belgium; School of Medicine and Public Health (C.R.L.), University of Newcastle, New South Wales; Department of Neurology, John Hunter Hospital, Newcastle, New South Wales, Australia; Division of Endocrinology (P.F.M.), Diabetes and Nutrition, Department of Medicine, University of Maryland School of Medicine, Baltimore; Department of Pharmacotherapy and Translational Research and Center for Pharmacogenomics (C.W.M.), University of Florida, Gainesville; Department of Neurology (J.F.M.), Mayo Clinic, Jacksonville, FL; Klinik und Poliklinik für Neurologie (A.R.), Universitätsmedizin Rostock, Germany; Department of Neurology (S.R., R.S.), Clinical Division of Neurogeriatrics, Medical University Graz, Austria; Center for Genomic Medicine (J. Rosand), Massachusetts General Hospital, Boston; Broad Institute (J. Rosand), Cambridge, MA; Department of Neurology and Evelyn F. McKnight Brain Institute (J. Roquer, T.R., R.L.S./M.S.), Miller School of Medicine, University of Miami, FL; Institute of Cardiovascular Research (P.S.), Royal Holloway University of London (ICR2UL), UK St Peter's and Ashford Hospitals, Egham, United Kingdom; Department of Neurology (A. Slowik), Jagiellonian University Medical College, Krakow, Poland; Division of Neurocritical Care & Emergency Neurology (D.S.), Department of Neurology, Helsinki University Central Hospital, Finland; Stroke Division (V.T.), Florey Institute of Neuroscience and Mental Health, Heidelberg; Department of Neurology (V.T.), Austin Health, Heidelberg, Australia; Departments of Radiology (A.V.) and Neurology and Rehabilitation Medicine (D.W.), University of Cincinnati College of Medicine, OH; Department of Clinical Sciences Lund, Radiology (J.W.) and Neurology (A.G.L.), Lund University, Sweden; Department of Radiology, Neuroradiology, Skåne University Hospital, Malmö, Sweden; Departments of Neurology and Public Health Sciences (B.B.W.), University of Virginia, Charlottesville, VA; University of Technology Sydney (J.M.), Australia; Section of Neurology (A.G.L.), Skåne University Hospital, Lund, Sweden; Department of Laboratory Medicine (C.J.), Institute of Biomedicine, the Sahlgrenska Academy, University of Gothenburg, Sweden; and Department of Clinical Genetics and Genomics (C.J.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden.
1,
Clinton Wang
Clinton Wang, PhD
1From the J. Philip Kistler Stroke Research Center (M.B., A.K.B., M.D.S., S.H., A. Dalca, K.D., A.-K.G., M.R.E., P.M.R., M.N., R.W.R., C.W., N.S.R.), A.A. Martinos Center for Biomedical Imaging (A. Dalca, O.W.), and Henry and Allison McCance Center for Brain Health (J. Rosand), Massachusetts General Hospital, Harvard Medical School, Boston; Lille Neuroscience & Cognition (M.B., X.L., R. Lopes, G.K.), Inserm, CHU Lille, U1172 and Institut Pasteur de Lille (M.G.), CNRS, Inserm, CHU Lille, US 41 - UMS 2014 - PLBS, Lille University, France; Computer Science and Artificial Intelligence Lab (A. Dalca, C.W., P.G.), Massachusetts Institute of Technology, Cambridge; Division of Preventive Medicine (P.M.R.), Department of Medicine, Brigham and Women's Hospital, Boston, MA; Department of Medicine (O.R.B.), Division of Neurology, University of British Columbia, Vancouver, Canada; Department of Neurology (J.W.C., S.J.K.), University of Maryland School of Medicine and Veterans Affairs Maryland Health Care System, Baltimore, MD; School of Medical Sciences (A. Donatti, A. Sousa), University of Campinas (UNICAMP) and the Brazilian Institute of Neuroscience and Neurotechnology (BRAINN), Campinas, São Paulo; Departments of Neurosurgery (C.G.) and Neurology (R.Z.), Geisinger, Danville, PA; Department of Neurosurgery (C.G.), Christian Doppler Klinik, Paracelsus Medical University, Salzburg, Austria; Division of Emergency Medicine (Laura Heitsch), Washington University School of Medicine, St. Louis; Department of Neurology (Laura Heitsch, C.-L.P.), Washington University School of Medicine & Barnes-Jewish Hospital, St. Louis, MO; Department of Clinical Neuroscience (L. Holmegaard, K.J., T.M.S., T.T.), Institute of Neuroscience and Physiology, Sahlgrenska Academy at University of Gothenburg, Sweden; Department of Neurology, Sahlgrenska University Hospital, Gothenburg, Sweden; Department of Neurology (J.J.-C.), Neurovascular Research Group (NEUVAS), IMIM-Hospital del Mar (Institut Hospital del Mar d'Investigacions M`ediques), Universitat Autonoma de Barcelona, Spain; Department of Neurosciences (R. Lemmens), Experimental Neurology and Leuven Research Institute for Neuroscience and Disease (LIND), KU Leuven - University of Leuven, Belgium; Department of Neurology (R. Lemmens), Laboratory of Neurobiology, VIB Vesalius Research Center, University Hospitals Leuven, Belgium; School of Medicine and Public Health (C.R.L.), University of Newcastle, New South Wales; Department of Neurology, John Hunter Hospital, Newcastle, New South Wales, Australia; Division of Endocrinology (P.F.M.), Diabetes and Nutrition, Department of Medicine, University of Maryland School of Medicine, Baltimore; Department of Pharmacotherapy and Translational Research and Center for Pharmacogenomics (C.W.M.), University of Florida, Gainesville; Department of Neurology (J.F.M.), Mayo Clinic, Jacksonville, FL; Klinik und Poliklinik für Neurologie (A.R.), Universitätsmedizin Rostock, Germany; Department of Neurology (S.R., R.S.), Clinical Division of Neurogeriatrics, Medical University Graz, Austria; Center for Genomic Medicine (J. Rosand), Massachusetts General Hospital, Boston; Broad Institute (J. Rosand), Cambridge, MA; Department of Neurology and Evelyn F. McKnight Brain Institute (J. Roquer, T.R., R.L.S./M.S.), Miller School of Medicine, University of Miami, FL; Institute of Cardiovascular Research (P.S.), Royal Holloway University of London (ICR2UL), UK St Peter's and Ashford Hospitals, Egham, United Kingdom; Department of Neurology (A. Slowik), Jagiellonian University Medical College, Krakow, Poland; Division of Neurocritical Care & Emergency Neurology (D.S.), Department of Neurology, Helsinki University Central Hospital, Finland; Stroke Division (V.T.), Florey Institute of Neuroscience and Mental Health, Heidelberg; Department of Neurology (V.T.), Austin Health, Heidelberg, Australia; Departments of Radiology (A.V.) and Neurology and Rehabilitation Medicine (D.W.), University of Cincinnati College of Medicine, OH; Department of Clinical Sciences Lund, Radiology (J.W.) and Neurology (A.G.L.), Lund University, Sweden; Department of Radiology, Neuroradiology, Skåne University Hospital, Malmö, Sweden; Departments of Neurology and Public Health Sciences (B.B.W.), University of Virginia, Charlottesville, VA; University of Technology Sydney (J.M.), Australia; Section of Neurology (A.G.L.), Skåne University Hospital, Lund, Sweden; Department of Laboratory Medicine (C.J.), Institute of Biomedicine, the Sahlgrenska Academy, University of Gothenburg, Sweden; and Department of Clinical Genetics and Genomics (C.J.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden.
1,
Oscar R Benavente
Oscar R Benavente, MD
1From the J. Philip Kistler Stroke Research Center (M.B., A.K.B., M.D.S., S.H., A. Dalca, K.D., A.-K.G., M.R.E., P.M.R., M.N., R.W.R., C.W., N.S.R.), A.A. Martinos Center for Biomedical Imaging (A. Dalca, O.W.), and Henry and Allison McCance Center for Brain Health (J. Rosand), Massachusetts General Hospital, Harvard Medical School, Boston; Lille Neuroscience & Cognition (M.B., X.L., R. Lopes, G.K.), Inserm, CHU Lille, U1172 and Institut Pasteur de Lille (M.G.), CNRS, Inserm, CHU Lille, US 41 - UMS 2014 - PLBS, Lille University, France; Computer Science and Artificial Intelligence Lab (A. Dalca, C.W., P.G.), Massachusetts Institute of Technology, Cambridge; Division of Preventive Medicine (P.M.R.), Department of Medicine, Brigham and Women's Hospital, Boston, MA; Department of Medicine (O.R.B.), Division of Neurology, University of British Columbia, Vancouver, Canada; Department of Neurology (J.W.C., S.J.K.), University of Maryland School of Medicine and Veterans Affairs Maryland Health Care System, Baltimore, MD; School of Medical Sciences (A. Donatti, A. Sousa), University of Campinas (UNICAMP) and the Brazilian Institute of Neuroscience and Neurotechnology (BRAINN), Campinas, São Paulo; Departments of Neurosurgery (C.G.) and Neurology (R.Z.), Geisinger, Danville, PA; Department of Neurosurgery (C.G.), Christian Doppler Klinik, Paracelsus Medical University, Salzburg, Austria; Division of Emergency Medicine (Laura Heitsch), Washington University School of Medicine, St. Louis; Department of Neurology (Laura Heitsch, C.-L.P.), Washington University School of Medicine & Barnes-Jewish Hospital, St. Louis, MO; Department of Clinical Neuroscience (L. Holmegaard, K.J., T.M.S., T.T.), Institute of Neuroscience and Physiology, Sahlgrenska Academy at University of Gothenburg, Sweden; Department of Neurology, Sahlgrenska University Hospital, Gothenburg, Sweden; Department of Neurology (J.J.-C.), Neurovascular Research Group (NEUVAS), IMIM-Hospital del Mar (Institut Hospital del Mar d'Investigacions M`ediques), Universitat Autonoma de Barcelona, Spain; Department of Neurosciences (R. Lemmens), Experimental Neurology and Leuven Research Institute for Neuroscience and Disease (LIND), KU Leuven - University of Leuven, Belgium; Department of Neurology (R. Lemmens), Laboratory of Neurobiology, VIB Vesalius Research Center, University Hospitals Leuven, Belgium; School of Medicine and Public Health (C.R.L.), University of Newcastle, New South Wales; Department of Neurology, John Hunter Hospital, Newcastle, New South Wales, Australia; Division of Endocrinology (P.F.M.), Diabetes and Nutrition, Department of Medicine, University of Maryland School of Medicine, Baltimore; Department of Pharmacotherapy and Translational Research and Center for Pharmacogenomics (C.W.M.), University of Florida, Gainesville; Department of Neurology (J.F.M.), Mayo Clinic, Jacksonville, FL; Klinik und Poliklinik für Neurologie (A.R.), Universitätsmedizin Rostock, Germany; Department of Neurology (S.R., R.S.), Clinical Division of Neurogeriatrics, Medical University Graz, Austria; Center for Genomic Medicine (J. Rosand), Massachusetts General Hospital, Boston; Broad Institute (J. Rosand), Cambridge, MA; Department of Neurology and Evelyn F. McKnight Brain Institute (J. Roquer, T.R., R.L.S./M.S.), Miller School of Medicine, University of Miami, FL; Institute of Cardiovascular Research (P.S.), Royal Holloway University of London (ICR2UL), UK St Peter's and Ashford Hospitals, Egham, United Kingdom; Department of Neurology (A. Slowik), Jagiellonian University Medical College, Krakow, Poland; Division of Neurocritical Care & Emergency Neurology (D.S.), Department of Neurology, Helsinki University Central Hospital, Finland; Stroke Division (V.T.), Florey Institute of Neuroscience and Mental Health, Heidelberg; Department of Neurology (V.T.), Austin Health, Heidelberg, Australia; Departments of Radiology (A.V.) and Neurology and Rehabilitation Medicine (D.W.), University of Cincinnati College of Medicine, OH; Department of Clinical Sciences Lund, Radiology (J.W.) and Neurology (A.G.L.), Lund University, Sweden; Department of Radiology, Neuroradiology, Skåne University Hospital, Malmö, Sweden; Departments of Neurology and Public Health Sciences (B.B.W.), University of Virginia, Charlottesville, VA; University of Technology Sydney (J.M.), Australia; Section of Neurology (A.G.L.), Skåne University Hospital, Lund, Sweden; Department of Laboratory Medicine (C.J.), Institute of Biomedicine, the Sahlgrenska Academy, University of Gothenburg, Sweden; and Department of Clinical Genetics and Genomics (C.J.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden.
1,
John W Cole
John W Cole, MD, MS
1From the J. Philip Kistler Stroke Research Center (M.B., A.K.B., M.D.S., S.H., A. Dalca, K.D., A.-K.G., M.R.E., P.M.R., M.N., R.W.R., C.W., N.S.R.), A.A. Martinos Center for Biomedical Imaging (A. Dalca, O.W.), and Henry and Allison McCance Center for Brain Health (J. Rosand), Massachusetts General Hospital, Harvard Medical School, Boston; Lille Neuroscience & Cognition (M.B., X.L., R. Lopes, G.K.), Inserm, CHU Lille, U1172 and Institut Pasteur de Lille (M.G.), CNRS, Inserm, CHU Lille, US 41 - UMS 2014 - PLBS, Lille University, France; Computer Science and Artificial Intelligence Lab (A. Dalca, C.W., P.G.), Massachusetts Institute of Technology, Cambridge; Division of Preventive Medicine (P.M.R.), Department of Medicine, Brigham and Women's Hospital, Boston, MA; Department of Medicine (O.R.B.), Division of Neurology, University of British Columbia, Vancouver, Canada; Department of Neurology (J.W.C., S.J.K.), University of Maryland School of Medicine and Veterans Affairs Maryland Health Care System, Baltimore, MD; School of Medical Sciences (A. Donatti, A. Sousa), University of Campinas (UNICAMP) and the Brazilian Institute of Neuroscience and Neurotechnology (BRAINN), Campinas, São Paulo; Departments of Neurosurgery (C.G.) and Neurology (R.Z.), Geisinger, Danville, PA; Department of Neurosurgery (C.G.), Christian Doppler Klinik, Paracelsus Medical University, Salzburg, Austria; Division of Emergency Medicine (Laura Heitsch), Washington University School of Medicine, St. Louis; Department of Neurology (Laura Heitsch, C.-L.P.), Washington University School of Medicine & Barnes-Jewish Hospital, St. Louis, MO; Department of Clinical Neuroscience (L. Holmegaard, K.J., T.M.S., T.T.), Institute of Neuroscience and Physiology, Sahlgrenska Academy at University of Gothenburg, Sweden; Department of Neurology, Sahlgrenska University Hospital, Gothenburg, Sweden; Department of Neurology (J.J.-C.), Neurovascular Research Group (NEUVAS), IMIM-Hospital del Mar (Institut Hospital del Mar d'Investigacions M`ediques), Universitat Autonoma de Barcelona, Spain; Department of Neurosciences (R. Lemmens), Experimental Neurology and Leuven Research Institute for Neuroscience and Disease (LIND), KU Leuven - University of Leuven, Belgium; Department of Neurology (R. Lemmens), Laboratory of Neurobiology, VIB Vesalius Research Center, University Hospitals Leuven, Belgium; School of Medicine and Public Health (C.R.L.), University of Newcastle, New South Wales; Department of Neurology, John Hunter Hospital, Newcastle, New South Wales, Australia; Division of Endocrinology (P.F.M.), Diabetes and Nutrition, Department of Medicine, University of Maryland School of Medicine, Baltimore; Department of Pharmacotherapy and Translational Research and Center for Pharmacogenomics (C.W.M.), University of Florida, Gainesville; Department of Neurology (J.F.M.), Mayo Clinic, Jacksonville, FL; Klinik und Poliklinik für Neurologie (A.R.), Universitätsmedizin Rostock, Germany; Department of Neurology (S.R., R.S.), Clinical Division of Neurogeriatrics, Medical University Graz, Austria; Center for Genomic Medicine (J. Rosand), Massachusetts General Hospital, Boston; Broad Institute (J. Rosand), Cambridge, MA; Department of Neurology and Evelyn F. McKnight Brain Institute (J. Roquer, T.R., R.L.S./M.S.), Miller School of Medicine, University of Miami, FL; Institute of Cardiovascular Research (P.S.), Royal Holloway University of London (ICR2UL), UK St Peter's and Ashford Hospitals, Egham, United Kingdom; Department of Neurology (A. Slowik), Jagiellonian University Medical College, Krakow, Poland; Division of Neurocritical Care & Emergency Neurology (D.S.), Department of Neurology, Helsinki University Central Hospital, Finland; Stroke Division (V.T.), Florey Institute of Neuroscience and Mental Health, Heidelberg; Department of Neurology (V.T.), Austin Health, Heidelberg, Australia; Departments of Radiology (A.V.) and Neurology and Rehabilitation Medicine (D.W.), University of Cincinnati College of Medicine, OH; Department of Clinical Sciences Lund, Radiology (J.W.) and Neurology (A.G.L.), Lund University, Sweden; Department of Radiology, Neuroradiology, Skåne University Hospital, Malmö, Sweden; Departments of Neurology and Public Health Sciences (B.B.W.), University of Virginia, Charlottesville, VA; University of Technology Sydney (J.M.), Australia; Section of Neurology (A.G.L.), Skåne University Hospital, Lund, Sweden; Department of Laboratory Medicine (C.J.), Institute of Biomedicine, the Sahlgrenska Academy, University of Gothenburg, Sweden; and Department of Clinical Genetics and Genomics (C.J.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden.
1,
Amanda Donatti
Amanda Donatti, PhD
1From the J. Philip Kistler Stroke Research Center (M.B., A.K.B., M.D.S., S.H., A. Dalca, K.D., A.-K.G., M.R.E., P.M.R., M.N., R.W.R., C.W., N.S.R.), A.A. Martinos Center for Biomedical Imaging (A. Dalca, O.W.), and Henry and Allison McCance Center for Brain Health (J. Rosand), Massachusetts General Hospital, Harvard Medical School, Boston; Lille Neuroscience & Cognition (M.B., X.L., R. Lopes, G.K.), Inserm, CHU Lille, U1172 and Institut Pasteur de Lille (M.G.), CNRS, Inserm, CHU Lille, US 41 - UMS 2014 - PLBS, Lille University, France; Computer Science and Artificial Intelligence Lab (A. Dalca, C.W., P.G.), Massachusetts Institute of Technology, Cambridge; Division of Preventive Medicine (P.M.R.), Department of Medicine, Brigham and Women's Hospital, Boston, MA; Department of Medicine (O.R.B.), Division of Neurology, University of British Columbia, Vancouver, Canada; Department of Neurology (J.W.C., S.J.K.), University of Maryland School of Medicine and Veterans Affairs Maryland Health Care System, Baltimore, MD; School of Medical Sciences (A. Donatti, A. Sousa), University of Campinas (UNICAMP) and the Brazilian Institute of Neuroscience and Neurotechnology (BRAINN), Campinas, São Paulo; Departments of Neurosurgery (C.G.) and Neurology (R.Z.), Geisinger, Danville, PA; Department of Neurosurgery (C.G.), Christian Doppler Klinik, Paracelsus Medical University, Salzburg, Austria; Division of Emergency Medicine (Laura Heitsch), Washington University School of Medicine, St. Louis; Department of Neurology (Laura Heitsch, C.-L.P.), Washington University School of Medicine & Barnes-Jewish Hospital, St. Louis, MO; Department of Clinical Neuroscience (L. Holmegaard, K.J., T.M.S., T.T.), Institute of Neuroscience and Physiology, Sahlgrenska Academy at University of Gothenburg, Sweden; Department of Neurology, Sahlgrenska University Hospital, Gothenburg, Sweden; Department of Neurology (J.J.-C.), Neurovascular Research Group (NEUVAS), IMIM-Hospital del Mar (Institut Hospital del Mar d'Investigacions M`ediques), Universitat Autonoma de Barcelona, Spain; Department of Neurosciences (R. Lemmens), Experimental Neurology and Leuven Research Institute for Neuroscience and Disease (LIND), KU Leuven - University of Leuven, Belgium; Department of Neurology (R. Lemmens), Laboratory of Neurobiology, VIB Vesalius Research Center, University Hospitals Leuven, Belgium; School of Medicine and Public Health (C.R.L.), University of Newcastle, New South Wales; Department of Neurology, John Hunter Hospital, Newcastle, New South Wales, Australia; Division of Endocrinology (P.F.M.), Diabetes and Nutrition, Department of Medicine, University of Maryland School of Medicine, Baltimore; Department of Pharmacotherapy and Translational Research and Center for Pharmacogenomics (C.W.M.), University of Florida, Gainesville; Department of Neurology (J.F.M.), Mayo Clinic, Jacksonville, FL; Klinik und Poliklinik für Neurologie (A.R.), Universitätsmedizin Rostock, Germany; Department of Neurology (S.R., R.S.), Clinical Division of Neurogeriatrics, Medical University Graz, Austria; Center for Genomic Medicine (J. Rosand), Massachusetts General Hospital, Boston; Broad Institute (J. Rosand), Cambridge, MA; Department of Neurology and Evelyn F. McKnight Brain Institute (J. Roquer, T.R., R.L.S./M.S.), Miller School of Medicine, University of Miami, FL; Institute of Cardiovascular Research (P.S.), Royal Holloway University of London (ICR2UL), UK St Peter's and Ashford Hospitals, Egham, United Kingdom; Department of Neurology (A. Slowik), Jagiellonian University Medical College, Krakow, Poland; Division of Neurocritical Care & Emergency Neurology (D.S.), Department of Neurology, Helsinki University Central Hospital, Finland; Stroke Division (V.T.), Florey Institute of Neuroscience and Mental Health, Heidelberg; Department of Neurology (V.T.), Austin Health, Heidelberg, Australia; Departments of Radiology (A.V.) and Neurology and Rehabilitation Medicine (D.W.), University of Cincinnati College of Medicine, OH; Department of Clinical Sciences Lund, Radiology (J.W.) and Neurology (A.G.L.), Lund University, Sweden; Department of Radiology, Neuroradiology, Skåne University Hospital, Malmö, Sweden; Departments of Neurology and Public Health Sciences (B.B.W.), University of Virginia, Charlottesville, VA; University of Technology Sydney (J.M.), Australia; Section of Neurology (A.G.L.), Skåne University Hospital, Lund, Sweden; Department of Laboratory Medicine (C.J.), Institute of Biomedicine, the Sahlgrenska Academy, University of Gothenburg, Sweden; and Department of Clinical Genetics and Genomics (C.J.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden.
1,
Christoph Griessenauer
Christoph Griessenauer, MD
1From the J. Philip Kistler Stroke Research Center (M.B., A.K.B., M.D.S., S.H., A. Dalca, K.D., A.-K.G., M.R.E., P.M.R., M.N., R.W.R., C.W., N.S.R.), A.A. Martinos Center for Biomedical Imaging (A. Dalca, O.W.), and Henry and Allison McCance Center for Brain Health (J. Rosand), Massachusetts General Hospital, Harvard Medical School, Boston; Lille Neuroscience & Cognition (M.B., X.L., R. Lopes, G.K.), Inserm, CHU Lille, U1172 and Institut Pasteur de Lille (M.G.), CNRS, Inserm, CHU Lille, US 41 - UMS 2014 - PLBS, Lille University, France; Computer Science and Artificial Intelligence Lab (A. Dalca, C.W., P.G.), Massachusetts Institute of Technology, Cambridge; Division of Preventive Medicine (P.M.R.), Department of Medicine, Brigham and Women's Hospital, Boston, MA; Department of Medicine (O.R.B.), Division of Neurology, University of British Columbia, Vancouver, Canada; Department of Neurology (J.W.C., S.J.K.), University of Maryland School of Medicine and Veterans Affairs Maryland Health Care System, Baltimore, MD; School of Medical Sciences (A. Donatti, A. Sousa), University of Campinas (UNICAMP) and the Brazilian Institute of Neuroscience and Neurotechnology (BRAINN), Campinas, São Paulo; Departments of Neurosurgery (C.G.) and Neurology (R.Z.), Geisinger, Danville, PA; Department of Neurosurgery (C.G.), Christian Doppler Klinik, Paracelsus Medical University, Salzburg, Austria; Division of Emergency Medicine (Laura Heitsch), Washington University School of Medicine, St. Louis; Department of Neurology (Laura Heitsch, C.-L.P.), Washington University School of Medicine & Barnes-Jewish Hospital, St. Louis, MO; Department of Clinical Neuroscience (L. Holmegaard, K.J., T.M.S., T.T.), Institute of Neuroscience and Physiology, Sahlgrenska Academy at University of Gothenburg, Sweden; Department of Neurology, Sahlgrenska University Hospital, Gothenburg, Sweden; Department of Neurology (J.J.-C.), Neurovascular Research Group (NEUVAS), IMIM-Hospital del Mar (Institut Hospital del Mar d'Investigacions M`ediques), Universitat Autonoma de Barcelona, Spain; Department of Neurosciences (R. Lemmens), Experimental Neurology and Leuven Research Institute for Neuroscience and Disease (LIND), KU Leuven - University of Leuven, Belgium; Department of Neurology (R. Lemmens), Laboratory of Neurobiology, VIB Vesalius Research Center, University Hospitals Leuven, Belgium; School of Medicine and Public Health (C.R.L.), University of Newcastle, New South Wales; Department of Neurology, John Hunter Hospital, Newcastle, New South Wales, Australia; Division of Endocrinology (P.F.M.), Diabetes and Nutrition, Department of Medicine, University of Maryland School of Medicine, Baltimore; Department of Pharmacotherapy and Translational Research and Center for Pharmacogenomics (C.W.M.), University of Florida, Gainesville; Department of Neurology (J.F.M.), Mayo Clinic, Jacksonville, FL; Klinik und Poliklinik für Neurologie (A.R.), Universitätsmedizin Rostock, Germany; Department of Neurology (S.R., R.S.), Clinical Division of Neurogeriatrics, Medical University Graz, Austria; Center for Genomic Medicine (J. Rosand), Massachusetts General Hospital, Boston; Broad Institute (J. Rosand), Cambridge, MA; Department of Neurology and Evelyn F. McKnight Brain Institute (J. Roquer, T.R., R.L.S./M.S.), Miller School of Medicine, University of Miami, FL; Institute of Cardiovascular Research (P.S.), Royal Holloway University of London (ICR2UL), UK St Peter's and Ashford Hospitals, Egham, United Kingdom; Department of Neurology (A. Slowik), Jagiellonian University Medical College, Krakow, Poland; Division of Neurocritical Care & Emergency Neurology (D.S.), Department of Neurology, Helsinki University Central Hospital, Finland; Stroke Division (V.T.), Florey Institute of Neuroscience and Mental Health, Heidelberg; Department of Neurology (V.T.), Austin Health, Heidelberg, Australia; Departments of Radiology (A.V.) and Neurology and Rehabilitation Medicine (D.W.), University of Cincinnati College of Medicine, OH; Department of Clinical Sciences Lund, Radiology (J.W.) and Neurology (A.G.L.), Lund University, Sweden; Department of Radiology, Neuroradiology, Skåne University Hospital, Malmö, Sweden; Departments of Neurology and Public Health Sciences (B.B.W.), University of Virginia, Charlottesville, VA; University of Technology Sydney (J.M.), Australia; Section of Neurology (A.G.L.), Skåne University Hospital, Lund, Sweden; Department of Laboratory Medicine (C.J.), Institute of Biomedicine, the Sahlgrenska Academy, University of Gothenburg, Sweden; and Department of Clinical Genetics and Genomics (C.J.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden.
1,
Laura Heitsch
Laura Heitsch, MD
1From the J. Philip Kistler Stroke Research Center (M.B., A.K.B., M.D.S., S.H., A. Dalca, K.D., A.-K.G., M.R.E., P.M.R., M.N., R.W.R., C.W., N.S.R.), A.A. Martinos Center for Biomedical Imaging (A. Dalca, O.W.), and Henry and Allison McCance Center for Brain Health (J. Rosand), Massachusetts General Hospital, Harvard Medical School, Boston; Lille Neuroscience & Cognition (M.B., X.L., R. Lopes, G.K.), Inserm, CHU Lille, U1172 and Institut Pasteur de Lille (M.G.), CNRS, Inserm, CHU Lille, US 41 - UMS 2014 - PLBS, Lille University, France; Computer Science and Artificial Intelligence Lab (A. Dalca, C.W., P.G.), Massachusetts Institute of Technology, Cambridge; Division of Preventive Medicine (P.M.R.), Department of Medicine, Brigham and Women's Hospital, Boston, MA; Department of Medicine (O.R.B.), Division of Neurology, University of British Columbia, Vancouver, Canada; Department of Neurology (J.W.C., S.J.K.), University of Maryland School of Medicine and Veterans Affairs Maryland Health Care System, Baltimore, MD; School of Medical Sciences (A. Donatti, A. Sousa), University of Campinas (UNICAMP) and the Brazilian Institute of Neuroscience and Neurotechnology (BRAINN), Campinas, São Paulo; Departments of Neurosurgery (C.G.) and Neurology (R.Z.), Geisinger, Danville, PA; Department of Neurosurgery (C.G.), Christian Doppler Klinik, Paracelsus Medical University, Salzburg, Austria; Division of Emergency Medicine (Laura Heitsch), Washington University School of Medicine, St. Louis; Department of Neurology (Laura Heitsch, C.-L.P.), Washington University School of Medicine & Barnes-Jewish Hospital, St. Louis, MO; Department of Clinical Neuroscience (L. Holmegaard, K.J., T.M.S., T.T.), Institute of Neuroscience and Physiology, Sahlgrenska Academy at University of Gothenburg, Sweden; Department of Neurology, Sahlgrenska University Hospital, Gothenburg, Sweden; Department of Neurology (J.J.-C.), Neurovascular Research Group (NEUVAS), IMIM-Hospital del Mar (Institut Hospital del Mar d'Investigacions M`ediques), Universitat Autonoma de Barcelona, Spain; Department of Neurosciences (R. Lemmens), Experimental Neurology and Leuven Research Institute for Neuroscience and Disease (LIND), KU Leuven - University of Leuven, Belgium; Department of Neurology (R. Lemmens), Laboratory of Neurobiology, VIB Vesalius Research Center, University Hospitals Leuven, Belgium; School of Medicine and Public Health (C.R.L.), University of Newcastle, New South Wales; Department of Neurology, John Hunter Hospital, Newcastle, New South Wales, Australia; Division of Endocrinology (P.F.M.), Diabetes and Nutrition, Department of Medicine, University of Maryland School of Medicine, Baltimore; Department of Pharmacotherapy and Translational Research and Center for Pharmacogenomics (C.W.M.), University of Florida, Gainesville; Department of Neurology (J.F.M.), Mayo Clinic, Jacksonville, FL; Klinik und Poliklinik für Neurologie (A.R.), Universitätsmedizin Rostock, Germany; Department of Neurology (S.R., R.S.), Clinical Division of Neurogeriatrics, Medical University Graz, Austria; Center for Genomic Medicine (J. Rosand), Massachusetts General Hospital, Boston; Broad Institute (J. Rosand), Cambridge, MA; Department of Neurology and Evelyn F. McKnight Brain Institute (J. Roquer, T.R., R.L.S./M.S.), Miller School of Medicine, University of Miami, FL; Institute of Cardiovascular Research (P.S.), Royal Holloway University of London (ICR2UL), UK St Peter's and Ashford Hospitals, Egham, United Kingdom; Department of Neurology (A. Slowik), Jagiellonian University Medical College, Krakow, Poland; Division of Neurocritical Care & Emergency Neurology (D.S.), Department of Neurology, Helsinki University Central Hospital, Finland; Stroke Division (V.T.), Florey Institute of Neuroscience and Mental Health, Heidelberg; Department of Neurology (V.T.), Austin Health, Heidelberg, Australia; Departments of Radiology (A.V.) and Neurology and Rehabilitation Medicine (D.W.), University of Cincinnati College of Medicine, OH; Department of Clinical Sciences Lund, Radiology (J.W.) and Neurology (A.G.L.), Lund University, Sweden; Department of Radiology, Neuroradiology, Skåne University Hospital, Malmö, Sweden; Departments of Neurology and Public Health Sciences (B.B.W.), University of Virginia, Charlottesville, VA; University of Technology Sydney (J.M.), Australia; Section of Neurology (A.G.L.), Skåne University Hospital, Lund, Sweden; Department of Laboratory Medicine (C.J.), Institute of Biomedicine, the Sahlgrenska Academy, University of Gothenburg, Sweden; and Department of Clinical Genetics and Genomics (C.J.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden.
1,
Lukas Holmegaard
Lukas Holmegaard, MD
1From the J. Philip Kistler Stroke Research Center (M.B., A.K.B., M.D.S., S.H., A. Dalca, K.D., A.-K.G., M.R.E., P.M.R., M.N., R.W.R., C.W., N.S.R.), A.A. Martinos Center for Biomedical Imaging (A. Dalca, O.W.), and Henry and Allison McCance Center for Brain Health (J. Rosand), Massachusetts General Hospital, Harvard Medical School, Boston; Lille Neuroscience & Cognition (M.B., X.L., R. Lopes, G.K.), Inserm, CHU Lille, U1172 and Institut Pasteur de Lille (M.G.), CNRS, Inserm, CHU Lille, US 41 - UMS 2014 - PLBS, Lille University, France; Computer Science and Artificial Intelligence Lab (A. Dalca, C.W., P.G.), Massachusetts Institute of Technology, Cambridge; Division of Preventive Medicine (P.M.R.), Department of Medicine, Brigham and Women's Hospital, Boston, MA; Department of Medicine (O.R.B.), Division of Neurology, University of British Columbia, Vancouver, Canada; Department of Neurology (J.W.C., S.J.K.), University of Maryland School of Medicine and Veterans Affairs Maryland Health Care System, Baltimore, MD; School of Medical Sciences (A. Donatti, A. Sousa), University of Campinas (UNICAMP) and the Brazilian Institute of Neuroscience and Neurotechnology (BRAINN), Campinas, São Paulo; Departments of Neurosurgery (C.G.) and Neurology (R.Z.), Geisinger, Danville, PA; Department of Neurosurgery (C.G.), Christian Doppler Klinik, Paracelsus Medical University, Salzburg, Austria; Division of Emergency Medicine (Laura Heitsch), Washington University School of Medicine, St. Louis; Department of Neurology (Laura Heitsch, C.-L.P.), Washington University School of Medicine & Barnes-Jewish Hospital, St. Louis, MO; Department of Clinical Neuroscience (L. Holmegaard, K.J., T.M.S., T.T.), Institute of Neuroscience and Physiology, Sahlgrenska Academy at University of Gothenburg, Sweden; Department of Neurology, Sahlgrenska University Hospital, Gothenburg, Sweden; Department of Neurology (J.J.-C.), Neurovascular Research Group (NEUVAS), IMIM-Hospital del Mar (Institut Hospital del Mar d'Investigacions M`ediques), Universitat Autonoma de Barcelona, Spain; Department of Neurosciences (R. Lemmens), Experimental Neurology and Leuven Research Institute for Neuroscience and Disease (LIND), KU Leuven - University of Leuven, Belgium; Department of Neurology (R. Lemmens), Laboratory of Neurobiology, VIB Vesalius Research Center, University Hospitals Leuven, Belgium; School of Medicine and Public Health (C.R.L.), University of Newcastle, New South Wales; Department of Neurology, John Hunter Hospital, Newcastle, New South Wales, Australia; Division of Endocrinology (P.F.M.), Diabetes and Nutrition, Department of Medicine, University of Maryland School of Medicine, Baltimore; Department of Pharmacotherapy and Translational Research and Center for Pharmacogenomics (C.W.M.), University of Florida, Gainesville; Department of Neurology (J.F.M.), Mayo Clinic, Jacksonville, FL; Klinik und Poliklinik für Neurologie (A.R.), Universitätsmedizin Rostock, Germany; Department of Neurology (S.R., R.S.), Clinical Division of Neurogeriatrics, Medical University Graz, Austria; Center for Genomic Medicine (J. Rosand), Massachusetts General Hospital, Boston; Broad Institute (J. Rosand), Cambridge, MA; Department of Neurology and Evelyn F. McKnight Brain Institute (J. Roquer, T.R., R.L.S./M.S.), Miller School of Medicine, University of Miami, FL; Institute of Cardiovascular Research (P.S.), Royal Holloway University of London (ICR2UL), UK St Peter's and Ashford Hospitals, Egham, United Kingdom; Department of Neurology (A. Slowik), Jagiellonian University Medical College, Krakow, Poland; Division of Neurocritical Care & Emergency Neurology (D.S.), Department of Neurology, Helsinki University Central Hospital, Finland; Stroke Division (V.T.), Florey Institute of Neuroscience and Mental Health, Heidelberg; Department of Neurology (V.T.), Austin Health, Heidelberg, Australia; Departments of Radiology (A.V.) and Neurology and Rehabilitation Medicine (D.W.), University of Cincinnati College of Medicine, OH; Department of Clinical Sciences Lund, Radiology (J.W.) and Neurology (A.G.L.), Lund University, Sweden; Department of Radiology, Neuroradiology, Skåne University Hospital, Malmö, Sweden; Departments of Neurology and Public Health Sciences (B.B.W.), University of Virginia, Charlottesville, VA; University of Technology Sydney (J.M.), Australia; Section of Neurology (A.G.L.), Skåne University Hospital, Lund, Sweden; Department of Laboratory Medicine (C.J.), Institute of Biomedicine, the Sahlgrenska Academy, University of Gothenburg, Sweden; and Department of Clinical Genetics and Genomics (C.J.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden.
1,
Katarina Jood
Katarina Jood, MD
1From the J. Philip Kistler Stroke Research Center (M.B., A.K.B., M.D.S., S.H., A. Dalca, K.D., A.-K.G., M.R.E., P.M.R., M.N., R.W.R., C.W., N.S.R.), A.A. Martinos Center for Biomedical Imaging (A. Dalca, O.W.), and Henry and Allison McCance Center for Brain Health (J. Rosand), Massachusetts General Hospital, Harvard Medical School, Boston; Lille Neuroscience & Cognition (M.B., X.L., R. Lopes, G.K.), Inserm, CHU Lille, U1172 and Institut Pasteur de Lille (M.G.), CNRS, Inserm, CHU Lille, US 41 - UMS 2014 - PLBS, Lille University, France; Computer Science and Artificial Intelligence Lab (A. Dalca, C.W., P.G.), Massachusetts Institute of Technology, Cambridge; Division of Preventive Medicine (P.M.R.), Department of Medicine, Brigham and Women's Hospital, Boston, MA; Department of Medicine (O.R.B.), Division of Neurology, University of British Columbia, Vancouver, Canada; Department of Neurology (J.W.C., S.J.K.), University of Maryland School of Medicine and Veterans Affairs Maryland Health Care System, Baltimore, MD; School of Medical Sciences (A. Donatti, A. Sousa), University of Campinas (UNICAMP) and the Brazilian Institute of Neuroscience and Neurotechnology (BRAINN), Campinas, São Paulo; Departments of Neurosurgery (C.G.) and Neurology (R.Z.), Geisinger, Danville, PA; Department of Neurosurgery (C.G.), Christian Doppler Klinik, Paracelsus Medical University, Salzburg, Austria; Division of Emergency Medicine (Laura Heitsch), Washington University School of Medicine, St. Louis; Department of Neurology (Laura Heitsch, C.-L.P.), Washington University School of Medicine & Barnes-Jewish Hospital, St. Louis, MO; Department of Clinical Neuroscience (L. Holmegaard, K.J., T.M.S., T.T.), Institute of Neuroscience and Physiology, Sahlgrenska Academy at University of Gothenburg, Sweden; Department of Neurology, Sahlgrenska University Hospital, Gothenburg, Sweden; Department of Neurology (J.J.-C.), Neurovascular Research Group (NEUVAS), IMIM-Hospital del Mar (Institut Hospital del Mar d'Investigacions M`ediques), Universitat Autonoma de Barcelona, Spain; Department of Neurosciences (R. Lemmens), Experimental Neurology and Leuven Research Institute for Neuroscience and Disease (LIND), KU Leuven - University of Leuven, Belgium; Department of Neurology (R. Lemmens), Laboratory of Neurobiology, VIB Vesalius Research Center, University Hospitals Leuven, Belgium; School of Medicine and Public Health (C.R.L.), University of Newcastle, New South Wales; Department of Neurology, John Hunter Hospital, Newcastle, New South Wales, Australia; Division of Endocrinology (P.F.M.), Diabetes and Nutrition, Department of Medicine, University of Maryland School of Medicine, Baltimore; Department of Pharmacotherapy and Translational Research and Center for Pharmacogenomics (C.W.M.), University of Florida, Gainesville; Department of Neurology (J.F.M.), Mayo Clinic, Jacksonville, FL; Klinik und Poliklinik für Neurologie (A.R.), Universitätsmedizin Rostock, Germany; Department of Neurology (S.R., R.S.), Clinical Division of Neurogeriatrics, Medical University Graz, Austria; Center for Genomic Medicine (J. Rosand), Massachusetts General Hospital, Boston; Broad Institute (J. Rosand), Cambridge, MA; Department of Neurology and Evelyn F. McKnight Brain Institute (J. Roquer, T.R., R.L.S./M.S.), Miller School of Medicine, University of Miami, FL; Institute of Cardiovascular Research (P.S.), Royal Holloway University of London (ICR2UL), UK St Peter's and Ashford Hospitals, Egham, United Kingdom; Department of Neurology (A. Slowik), Jagiellonian University Medical College, Krakow, Poland; Division of Neurocritical Care & Emergency Neurology (D.S.), Department of Neurology, Helsinki University Central Hospital, Finland; Stroke Division (V.T.), Florey Institute of Neuroscience and Mental Health, Heidelberg; Department of Neurology (V.T.), Austin Health, Heidelberg, Australia; Departments of Radiology (A.V.) and Neurology and Rehabilitation Medicine (D.W.), University of Cincinnati College of Medicine, OH; Department of Clinical Sciences Lund, Radiology (J.W.) and Neurology (A.G.L.), Lund University, Sweden; Department of Radiology, Neuroradiology, Skåne University Hospital, Malmö, Sweden; Departments of Neurology and Public Health Sciences (B.B.W.), University of Virginia, Charlottesville, VA; University of Technology Sydney (J.M.), Australia; Section of Neurology (A.G.L.), Skåne University Hospital, Lund, Sweden; Department of Laboratory Medicine (C.J.), Institute of Biomedicine, the Sahlgrenska Academy, University of Gothenburg, Sweden; and Department of Clinical Genetics and Genomics (C.J.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden.
1,
Jordi Jimenez-Conde
Jordi Jimenez-Conde, MD
1From the J. Philip Kistler Stroke Research Center (M.B., A.K.B., M.D.S., S.H., A. Dalca, K.D., A.-K.G., M.R.E., P.M.R., M.N., R.W.R., C.W., N.S.R.), A.A. Martinos Center for Biomedical Imaging (A. Dalca, O.W.), and Henry and Allison McCance Center for Brain Health (J. Rosand), Massachusetts General Hospital, Harvard Medical School, Boston; Lille Neuroscience & Cognition (M.B., X.L., R. Lopes, G.K.), Inserm, CHU Lille, U1172 and Institut Pasteur de Lille (M.G.), CNRS, Inserm, CHU Lille, US 41 - UMS 2014 - PLBS, Lille University, France; Computer Science and Artificial Intelligence Lab (A. Dalca, C.W., P.G.), Massachusetts Institute of Technology, Cambridge; Division of Preventive Medicine (P.M.R.), Department of Medicine, Brigham and Women's Hospital, Boston, MA; Department of Medicine (O.R.B.), Division of Neurology, University of British Columbia, Vancouver, Canada; Department of Neurology (J.W.C., S.J.K.), University of Maryland School of Medicine and Veterans Affairs Maryland Health Care System, Baltimore, MD; School of Medical Sciences (A. Donatti, A. Sousa), University of Campinas (UNICAMP) and the Brazilian Institute of Neuroscience and Neurotechnology (BRAINN), Campinas, São Paulo; Departments of Neurosurgery (C.G.) and Neurology (R.Z.), Geisinger, Danville, PA; Department of Neurosurgery (C.G.), Christian Doppler Klinik, Paracelsus Medical University, Salzburg, Austria; Division of Emergency Medicine (Laura Heitsch), Washington University School of Medicine, St. Louis; Department of Neurology (Laura Heitsch, C.-L.P.), Washington University School of Medicine & Barnes-Jewish Hospital, St. Louis, MO; Department of Clinical Neuroscience (L. Holmegaard, K.J., T.M.S., T.T.), Institute of Neuroscience and Physiology, Sahlgrenska Academy at University of Gothenburg, Sweden; Department of Neurology, Sahlgrenska University Hospital, Gothenburg, Sweden; Department of Neurology (J.J.-C.), Neurovascular Research Group (NEUVAS), IMIM-Hospital del Mar (Institut Hospital del Mar d'Investigacions M`ediques), Universitat Autonoma de Barcelona, Spain; Department of Neurosciences (R. Lemmens), Experimental Neurology and Leuven Research Institute for Neuroscience and Disease (LIND), KU Leuven - University of Leuven, Belgium; Department of Neurology (R. Lemmens), Laboratory of Neurobiology, VIB Vesalius Research Center, University Hospitals Leuven, Belgium; School of Medicine and Public Health (C.R.L.), University of Newcastle, New South Wales; Department of Neurology, John Hunter Hospital, Newcastle, New South Wales, Australia; Division of Endocrinology (P.F.M.), Diabetes and Nutrition, Department of Medicine, University of Maryland School of Medicine, Baltimore; Department of Pharmacotherapy and Translational Research and Center for Pharmacogenomics (C.W.M.), University of Florida, Gainesville; Department of Neurology (J.F.M.), Mayo Clinic, Jacksonville, FL; Klinik und Poliklinik für Neurologie (A.R.), Universitätsmedizin Rostock, Germany; Department of Neurology (S.R., R.S.), Clinical Division of Neurogeriatrics, Medical University Graz, Austria; Center for Genomic Medicine (J. Rosand), Massachusetts General Hospital, Boston; Broad Institute (J. Rosand), Cambridge, MA; Department of Neurology and Evelyn F. McKnight Brain Institute (J. Roquer, T.R., R.L.S./M.S.), Miller School of Medicine, University of Miami, FL; Institute of Cardiovascular Research (P.S.), Royal Holloway University of London (ICR2UL), UK St Peter's and Ashford Hospitals, Egham, United Kingdom; Department of Neurology (A. Slowik), Jagiellonian University Medical College, Krakow, Poland; Division of Neurocritical Care & Emergency Neurology (D.S.), Department of Neurology, Helsinki University Central Hospital, Finland; Stroke Division (V.T.), Florey Institute of Neuroscience and Mental Health, Heidelberg; Department of Neurology (V.T.), Austin Health, Heidelberg, Australia; Departments of Radiology (A.V.) and Neurology and Rehabilitation Medicine (D.W.), University of Cincinnati College of Medicine, OH; Department of Clinical Sciences Lund, Radiology (J.W.) and Neurology (A.G.L.), Lund University, Sweden; Department of Radiology, Neuroradiology, Skåne University Hospital, Malmö, Sweden; Departments of Neurology and Public Health Sciences (B.B.W.), University of Virginia, Charlottesville, VA; University of Technology Sydney (J.M.), Australia; Section of Neurology (A.G.L.), Skåne University Hospital, Lund, Sweden; Department of Laboratory Medicine (C.J.), Institute of Biomedicine, the Sahlgrenska Academy, University of Gothenburg, Sweden; and Department of Clinical Genetics and Genomics (C.J.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden.
1,
Steven J Kittner
Steven J Kittner, MD
1From the J. Philip Kistler Stroke Research Center (M.B., A.K.B., M.D.S., S.H., A. Dalca, K.D., A.-K.G., M.R.E., P.M.R., M.N., R.W.R., C.W., N.S.R.), A.A. Martinos Center for Biomedical Imaging (A. Dalca, O.W.), and Henry and Allison McCance Center for Brain Health (J. Rosand), Massachusetts General Hospital, Harvard Medical School, Boston; Lille Neuroscience & Cognition (M.B., X.L., R. Lopes, G.K.), Inserm, CHU Lille, U1172 and Institut Pasteur de Lille (M.G.), CNRS, Inserm, CHU Lille, US 41 - UMS 2014 - PLBS, Lille University, France; Computer Science and Artificial Intelligence Lab (A. Dalca, C.W., P.G.), Massachusetts Institute of Technology, Cambridge; Division of Preventive Medicine (P.M.R.), Department of Medicine, Brigham and Women's Hospital, Boston, MA; Department of Medicine (O.R.B.), Division of Neurology, University of British Columbia, Vancouver, Canada; Department of Neurology (J.W.C., S.J.K.), University of Maryland School of Medicine and Veterans Affairs Maryland Health Care System, Baltimore, MD; School of Medical Sciences (A. Donatti, A. Sousa), University of Campinas (UNICAMP) and the Brazilian Institute of Neuroscience and Neurotechnology (BRAINN), Campinas, São Paulo; Departments of Neurosurgery (C.G.) and Neurology (R.Z.), Geisinger, Danville, PA; Department of Neurosurgery (C.G.), Christian Doppler Klinik, Paracelsus Medical University, Salzburg, Austria; Division of Emergency Medicine (Laura Heitsch), Washington University School of Medicine, St. Louis; Department of Neurology (Laura Heitsch, C.-L.P.), Washington University School of Medicine & Barnes-Jewish Hospital, St. Louis, MO; Department of Clinical Neuroscience (L. Holmegaard, K.J., T.M.S., T.T.), Institute of Neuroscience and Physiology, Sahlgrenska Academy at University of Gothenburg, Sweden; Department of Neurology, Sahlgrenska University Hospital, Gothenburg, Sweden; Department of Neurology (J.J.-C.), Neurovascular Research Group (NEUVAS), IMIM-Hospital del Mar (Institut Hospital del Mar d'Investigacions M`ediques), Universitat Autonoma de Barcelona, Spain; Department of Neurosciences (R. Lemmens), Experimental Neurology and Leuven Research Institute for Neuroscience and Disease (LIND), KU Leuven - University of Leuven, Belgium; Department of Neurology (R. Lemmens), Laboratory of Neurobiology, VIB Vesalius Research Center, University Hospitals Leuven, Belgium; School of Medicine and Public Health (C.R.L.), University of Newcastle, New South Wales; Department of Neurology, John Hunter Hospital, Newcastle, New South Wales, Australia; Division of Endocrinology (P.F.M.), Diabetes and Nutrition, Department of Medicine, University of Maryland School of Medicine, Baltimore; Department of Pharmacotherapy and Translational Research and Center for Pharmacogenomics (C.W.M.), University of Florida, Gainesville; Department of Neurology (J.F.M.), Mayo Clinic, Jacksonville, FL; Klinik und Poliklinik für Neurologie (A.R.), Universitätsmedizin Rostock, Germany; Department of Neurology (S.R., R.S.), Clinical Division of Neurogeriatrics, Medical University Graz, Austria; Center for Genomic Medicine (J. Rosand), Massachusetts General Hospital, Boston; Broad Institute (J. Rosand), Cambridge, MA; Department of Neurology and Evelyn F. McKnight Brain Institute (J. Roquer, T.R., R.L.S./M.S.), Miller School of Medicine, University of Miami, FL; Institute of Cardiovascular Research (P.S.), Royal Holloway University of London (ICR2UL), UK St Peter's and Ashford Hospitals, Egham, United Kingdom; Department of Neurology (A. Slowik), Jagiellonian University Medical College, Krakow, Poland; Division of Neurocritical Care & Emergency Neurology (D.S.), Department of Neurology, Helsinki University Central Hospital, Finland; Stroke Division (V.T.), Florey Institute of Neuroscience and Mental Health, Heidelberg; Department of Neurology (V.T.), Austin Health, Heidelberg, Australia; Departments of Radiology (A.V.) and Neurology and Rehabilitation Medicine (D.W.), University of Cincinnati College of Medicine, OH; Department of Clinical Sciences Lund, Radiology (J.W.) and Neurology (A.G.L.), Lund University, Sweden; Department of Radiology, Neuroradiology, Skåne University Hospital, Malmö, Sweden; Departments of Neurology and Public Health Sciences (B.B.W.), University of Virginia, Charlottesville, VA; University of Technology Sydney (J.M.), Australia; Section of Neurology (A.G.L.), Skåne University Hospital, Lund, Sweden; Department of Laboratory Medicine (C.J.), Institute of Biomedicine, the Sahlgrenska Academy, University of Gothenburg, Sweden; and Department of Clinical Genetics and Genomics (C.J.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden.
1,
Robin Lemmens
Robin Lemmens, MD
1From the J. Philip Kistler Stroke Research Center (M.B., A.K.B., M.D.S., S.H., A. Dalca, K.D., A.-K.G., M.R.E., P.M.R., M.N., R.W.R., C.W., N.S.R.), A.A. Martinos Center for Biomedical Imaging (A. Dalca, O.W.), and Henry and Allison McCance Center for Brain Health (J. Rosand), Massachusetts General Hospital, Harvard Medical School, Boston; Lille Neuroscience & Cognition (M.B., X.L., R. Lopes, G.K.), Inserm, CHU Lille, U1172 and Institut Pasteur de Lille (M.G.), CNRS, Inserm, CHU Lille, US 41 - UMS 2014 - PLBS, Lille University, France; Computer Science and Artificial Intelligence Lab (A. Dalca, C.W., P.G.), Massachusetts Institute of Technology, Cambridge; Division of Preventive Medicine (P.M.R.), Department of Medicine, Brigham and Women's Hospital, Boston, MA; Department of Medicine (O.R.B.), Division of Neurology, University of British Columbia, Vancouver, Canada; Department of Neurology (J.W.C., S.J.K.), University of Maryland School of Medicine and Veterans Affairs Maryland Health Care System, Baltimore, MD; School of Medical Sciences (A. Donatti, A. Sousa), University of Campinas (UNICAMP) and the Brazilian Institute of Neuroscience and Neurotechnology (BRAINN), Campinas, São Paulo; Departments of Neurosurgery (C.G.) and Neurology (R.Z.), Geisinger, Danville, PA; Department of Neurosurgery (C.G.), Christian Doppler Klinik, Paracelsus Medical University, Salzburg, Austria; Division of Emergency Medicine (Laura Heitsch), Washington University School of Medicine, St. Louis; Department of Neurology (Laura Heitsch, C.-L.P.), Washington University School of Medicine & Barnes-Jewish Hospital, St. Louis, MO; Department of Clinical Neuroscience (L. Holmegaard, K.J., T.M.S., T.T.), Institute of Neuroscience and Physiology, Sahlgrenska Academy at University of Gothenburg, Sweden; Department of Neurology, Sahlgrenska University Hospital, Gothenburg, Sweden; Department of Neurology (J.J.-C.), Neurovascular Research Group (NEUVAS), IMIM-Hospital del Mar (Institut Hospital del Mar d'Investigacions M`ediques), Universitat Autonoma de Barcelona, Spain; Department of Neurosciences (R. Lemmens), Experimental Neurology and Leuven Research Institute for Neuroscience and Disease (LIND), KU Leuven - University of Leuven, Belgium; Department of Neurology (R. Lemmens), Laboratory of Neurobiology, VIB Vesalius Research Center, University Hospitals Leuven, Belgium; School of Medicine and Public Health (C.R.L.), University of Newcastle, New South Wales; Department of Neurology, John Hunter Hospital, Newcastle, New South Wales, Australia; Division of Endocrinology (P.F.M.), Diabetes and Nutrition, Department of Medicine, University of Maryland School of Medicine, Baltimore; Department of Pharmacotherapy and Translational Research and Center for Pharmacogenomics (C.W.M.), University of Florida, Gainesville; Department of Neurology (J.F.M.), Mayo Clinic, Jacksonville, FL; Klinik und Poliklinik für Neurologie (A.R.), Universitätsmedizin Rostock, Germany; Department of Neurology (S.R., R.S.), Clinical Division of Neurogeriatrics, Medical University Graz, Austria; Center for Genomic Medicine (J. Rosand), Massachusetts General Hospital, Boston; Broad Institute (J. Rosand), Cambridge, MA; Department of Neurology and Evelyn F. McKnight Brain Institute (J. Roquer, T.R., R.L.S./M.S.), Miller School of Medicine, University of Miami, FL; Institute of Cardiovascular Research (P.S.), Royal Holloway University of London (ICR2UL), UK St Peter's and Ashford Hospitals, Egham, United Kingdom; Department of Neurology (A. Slowik), Jagiellonian University Medical College, Krakow, Poland; Division of Neurocritical Care & Emergency Neurology (D.S.), Department of Neurology, Helsinki University Central Hospital, Finland; Stroke Division (V.T.), Florey Institute of Neuroscience and Mental Health, Heidelberg; Department of Neurology (V.T.), Austin Health, Heidelberg, Australia; Departments of Radiology (A.V.) and Neurology and Rehabilitation Medicine (D.W.), University of Cincinnati College of Medicine, OH; Department of Clinical Sciences Lund, Radiology (J.W.) and Neurology (A.G.L.), Lund University, Sweden; Department of Radiology, Neuroradiology, Skåne University Hospital, Malmö, Sweden; Departments of Neurology and Public Health Sciences (B.B.W.), University of Virginia, Charlottesville, VA; University of Technology Sydney (J.M.), Australia; Section of Neurology (A.G.L.), Skåne University Hospital, Lund, Sweden; Department of Laboratory Medicine (C.J.), Institute of Biomedicine, the Sahlgrenska Academy, University of Gothenburg, Sweden; and Department of Clinical Genetics and Genomics (C.J.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden.
1,
Christopher R Levi
Christopher R Levi, PhD
1From the J. Philip Kistler Stroke Research Center (M.B., A.K.B., M.D.S., S.H., A. Dalca, K.D., A.-K.G., M.R.E., P.M.R., M.N., R.W.R., C.W., N.S.R.), A.A. Martinos Center for Biomedical Imaging (A. Dalca, O.W.), and Henry and Allison McCance Center for Brain Health (J. Rosand), Massachusetts General Hospital, Harvard Medical School, Boston; Lille Neuroscience & Cognition (M.B., X.L., R. Lopes, G.K.), Inserm, CHU Lille, U1172 and Institut Pasteur de Lille (M.G.), CNRS, Inserm, CHU Lille, US 41 - UMS 2014 - PLBS, Lille University, France; Computer Science and Artificial Intelligence Lab (A. Dalca, C.W., P.G.), Massachusetts Institute of Technology, Cambridge; Division of Preventive Medicine (P.M.R.), Department of Medicine, Brigham and Women's Hospital, Boston, MA; Department of Medicine (O.R.B.), Division of Neurology, University of British Columbia, Vancouver, Canada; Department of Neurology (J.W.C., S.J.K.), University of Maryland School of Medicine and Veterans Affairs Maryland Health Care System, Baltimore, MD; School of Medical Sciences (A. Donatti, A. Sousa), University of Campinas (UNICAMP) and the Brazilian Institute of Neuroscience and Neurotechnology (BRAINN), Campinas, São Paulo; Departments of Neurosurgery (C.G.) and Neurology (R.Z.), Geisinger, Danville, PA; Department of Neurosurgery (C.G.), Christian Doppler Klinik, Paracelsus Medical University, Salzburg, Austria; Division of Emergency Medicine (Laura Heitsch), Washington University School of Medicine, St. Louis; Department of Neurology (Laura Heitsch, C.-L.P.), Washington University School of Medicine & Barnes-Jewish Hospital, St. Louis, MO; Department of Clinical Neuroscience (L. Holmegaard, K.J., T.M.S., T.T.), Institute of Neuroscience and Physiology, Sahlgrenska Academy at University of Gothenburg, Sweden; Department of Neurology, Sahlgrenska University Hospital, Gothenburg, Sweden; Department of Neurology (J.J.-C.), Neurovascular Research Group (NEUVAS), IMIM-Hospital del Mar (Institut Hospital del Mar d'Investigacions M`ediques), Universitat Autonoma de Barcelona, Spain; Department of Neurosciences (R. Lemmens), Experimental Neurology and Leuven Research Institute for Neuroscience and Disease (LIND), KU Leuven - University of Leuven, Belgium; Department of Neurology (R. Lemmens), Laboratory of Neurobiology, VIB Vesalius Research Center, University Hospitals Leuven, Belgium; School of Medicine and Public Health (C.R.L.), University of Newcastle, New South Wales; Department of Neurology, John Hunter Hospital, Newcastle, New South Wales, Australia; Division of Endocrinology (P.F.M.), Diabetes and Nutrition, Department of Medicine, University of Maryland School of Medicine, Baltimore; Department of Pharmacotherapy and Translational Research and Center for Pharmacogenomics (C.W.M.), University of Florida, Gainesville; Department of Neurology (J.F.M.), Mayo Clinic, Jacksonville, FL; Klinik und Poliklinik für Neurologie (A.R.), Universitätsmedizin Rostock, Germany; Department of Neurology (S.R., R.S.), Clinical Division of Neurogeriatrics, Medical University Graz, Austria; Center for Genomic Medicine (J. Rosand), Massachusetts General Hospital, Boston; Broad Institute (J. Rosand), Cambridge, MA; Department of Neurology and Evelyn F. McKnight Brain Institute (J. Roquer, T.R., R.L.S./M.S.), Miller School of Medicine, University of Miami, FL; Institute of Cardiovascular Research (P.S.), Royal Holloway University of London (ICR2UL), UK St Peter's and Ashford Hospitals, Egham, United Kingdom; Department of Neurology (A. Slowik), Jagiellonian University Medical College, Krakow, Poland; Division of Neurocritical Care & Emergency Neurology (D.S.), Department of Neurology, Helsinki University Central Hospital, Finland; Stroke Division (V.T.), Florey Institute of Neuroscience and Mental Health, Heidelberg; Department of Neurology (V.T.), Austin Health, Heidelberg, Australia; Departments of Radiology (A.V.) and Neurology and Rehabilitation Medicine (D.W.), University of Cincinnati College of Medicine, OH; Department of Clinical Sciences Lund, Radiology (J.W.) and Neurology (A.G.L.), Lund University, Sweden; Department of Radiology, Neuroradiology, Skåne University Hospital, Malmö, Sweden; Departments of Neurology and Public Health Sciences (B.B.W.), University of Virginia, Charlottesville, VA; University of Technology Sydney (J.M.), Australia; Section of Neurology (A.G.L.), Skåne University Hospital, Lund, Sweden; Department of Laboratory Medicine (C.J.), Institute of Biomedicine, the Sahlgrenska Academy, University of Gothenburg, Sweden; and Department of Clinical Genetics and Genomics (C.J.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden.
1,
Patrick F McArdle
Patrick F McArdle, PhD
1From the J. Philip Kistler Stroke Research Center (M.B., A.K.B., M.D.S., S.H., A. Dalca, K.D., A.-K.G., M.R.E., P.M.R., M.N., R.W.R., C.W., N.S.R.), A.A. Martinos Center for Biomedical Imaging (A. Dalca, O.W.), and Henry and Allison McCance Center for Brain Health (J. Rosand), Massachusetts General Hospital, Harvard Medical School, Boston; Lille Neuroscience & Cognition (M.B., X.L., R. Lopes, G.K.), Inserm, CHU Lille, U1172 and Institut Pasteur de Lille (M.G.), CNRS, Inserm, CHU Lille, US 41 - UMS 2014 - PLBS, Lille University, France; Computer Science and Artificial Intelligence Lab (A. Dalca, C.W., P.G.), Massachusetts Institute of Technology, Cambridge; Division of Preventive Medicine (P.M.R.), Department of Medicine, Brigham and Women's Hospital, Boston, MA; Department of Medicine (O.R.B.), Division of Neurology, University of British Columbia, Vancouver, Canada; Department of Neurology (J.W.C., S.J.K.), University of Maryland School of Medicine and Veterans Affairs Maryland Health Care System, Baltimore, MD; School of Medical Sciences (A. Donatti, A. Sousa), University of Campinas (UNICAMP) and the Brazilian Institute of Neuroscience and Neurotechnology (BRAINN), Campinas, São Paulo; Departments of Neurosurgery (C.G.) and Neurology (R.Z.), Geisinger, Danville, PA; Department of Neurosurgery (C.G.), Christian Doppler Klinik, Paracelsus Medical University, Salzburg, Austria; Division of Emergency Medicine (Laura Heitsch), Washington University School of Medicine, St. Louis; Department of Neurology (Laura Heitsch, C.-L.P.), Washington University School of Medicine & Barnes-Jewish Hospital, St. Louis, MO; Department of Clinical Neuroscience (L. Holmegaard, K.J., T.M.S., T.T.), Institute of Neuroscience and Physiology, Sahlgrenska Academy at University of Gothenburg, Sweden; Department of Neurology, Sahlgrenska University Hospital, Gothenburg, Sweden; Department of Neurology (J.J.-C.), Neurovascular Research Group (NEUVAS), IMIM-Hospital del Mar (Institut Hospital del Mar d'Investigacions M`ediques), Universitat Autonoma de Barcelona, Spain; Department of Neurosciences (R. Lemmens), Experimental Neurology and Leuven Research Institute for Neuroscience and Disease (LIND), KU Leuven - University of Leuven, Belgium; Department of Neurology (R. Lemmens), Laboratory of Neurobiology, VIB Vesalius Research Center, University Hospitals Leuven, Belgium; School of Medicine and Public Health (C.R.L.), University of Newcastle, New South Wales; Department of Neurology, John Hunter Hospital, Newcastle, New South Wales, Australia; Division of Endocrinology (P.F.M.), Diabetes and Nutrition, Department of Medicine, University of Maryland School of Medicine, Baltimore; Department of Pharmacotherapy and Translational Research and Center for Pharmacogenomics (C.W.M.), University of Florida, Gainesville; Department of Neurology (J.F.M.), Mayo Clinic, Jacksonville, FL; Klinik und Poliklinik für Neurologie (A.R.), Universitätsmedizin Rostock, Germany; Department of Neurology (S.R., R.S.), Clinical Division of Neurogeriatrics, Medical University Graz, Austria; Center for Genomic Medicine (J. Rosand), Massachusetts General Hospital, Boston; Broad Institute (J. Rosand), Cambridge, MA; Department of Neurology and Evelyn F. McKnight Brain Institute (J. Roquer, T.R., R.L.S./M.S.), Miller School of Medicine, University of Miami, FL; Institute of Cardiovascular Research (P.S.), Royal Holloway University of London (ICR2UL), UK St Peter's and Ashford Hospitals, Egham, United Kingdom; Department of Neurology (A. Slowik), Jagiellonian University Medical College, Krakow, Poland; Division of Neurocritical Care & Emergency Neurology (D.S.), Department of Neurology, Helsinki University Central Hospital, Finland; Stroke Division (V.T.), Florey Institute of Neuroscience and Mental Health, Heidelberg; Department of Neurology (V.T.), Austin Health, Heidelberg, Australia; Departments of Radiology (A.V.) and Neurology and Rehabilitation Medicine (D.W.), University of Cincinnati College of Medicine, OH; Department of Clinical Sciences Lund, Radiology (J.W.) and Neurology (A.G.L.), Lund University, Sweden; Department of Radiology, Neuroradiology, Skåne University Hospital, Malmö, Sweden; Departments of Neurology and Public Health Sciences (B.B.W.), University of Virginia, Charlottesville, VA; University of Technology Sydney (J.M.), Australia; Section of Neurology (A.G.L.), Skåne University Hospital, Lund, Sweden; Department of Laboratory Medicine (C.J.), Institute of Biomedicine, the Sahlgrenska Academy, University of Gothenburg, Sweden; and Department of Clinical Genetics and Genomics (C.J.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden.
1,
Caitrin W McDonough
Caitrin W McDonough, PhD
1From the J. Philip Kistler Stroke Research Center (M.B., A.K.B., M.D.S., S.H., A. Dalca, K.D., A.-K.G., M.R.E., P.M.R., M.N., R.W.R., C.W., N.S.R.), A.A. Martinos Center for Biomedical Imaging (A. Dalca, O.W.), and Henry and Allison McCance Center for Brain Health (J. Rosand), Massachusetts General Hospital, Harvard Medical School, Boston; Lille Neuroscience & Cognition (M.B., X.L., R. Lopes, G.K.), Inserm, CHU Lille, U1172 and Institut Pasteur de Lille (M.G.), CNRS, Inserm, CHU Lille, US 41 - UMS 2014 - PLBS, Lille University, France; Computer Science and Artificial Intelligence Lab (A. Dalca, C.W., P.G.), Massachusetts Institute of Technology, Cambridge; Division of Preventive Medicine (P.M.R.), Department of Medicine, Brigham and Women's Hospital, Boston, MA; Department of Medicine (O.R.B.), Division of Neurology, University of British Columbia, Vancouver, Canada; Department of Neurology (J.W.C., S.J.K.), University of Maryland School of Medicine and Veterans Affairs Maryland Health Care System, Baltimore, MD; School of Medical Sciences (A. Donatti, A. Sousa), University of Campinas (UNICAMP) and the Brazilian Institute of Neuroscience and Neurotechnology (BRAINN), Campinas, São Paulo; Departments of Neurosurgery (C.G.) and Neurology (R.Z.), Geisinger, Danville, PA; Department of Neurosurgery (C.G.), Christian Doppler Klinik, Paracelsus Medical University, Salzburg, Austria; Division of Emergency Medicine (Laura Heitsch), Washington University School of Medicine, St. Louis; Department of Neurology (Laura Heitsch, C.-L.P.), Washington University School of Medicine & Barnes-Jewish Hospital, St. Louis, MO; Department of Clinical Neuroscience (L. Holmegaard, K.J., T.M.S., T.T.), Institute of Neuroscience and Physiology, Sahlgrenska Academy at University of Gothenburg, Sweden; Department of Neurology, Sahlgrenska University Hospital, Gothenburg, Sweden; Department of Neurology (J.J.-C.), Neurovascular Research Group (NEUVAS), IMIM-Hospital del Mar (Institut Hospital del Mar d'Investigacions M`ediques), Universitat Autonoma de Barcelona, Spain; Department of Neurosciences (R. Lemmens), Experimental Neurology and Leuven Research Institute for Neuroscience and Disease (LIND), KU Leuven - University of Leuven, Belgium; Department of Neurology (R. Lemmens), Laboratory of Neurobiology, VIB Vesalius Research Center, University Hospitals Leuven, Belgium; School of Medicine and Public Health (C.R.L.), University of Newcastle, New South Wales; Department of Neurology, John Hunter Hospital, Newcastle, New South Wales, Australia; Division of Endocrinology (P.F.M.), Diabetes and Nutrition, Department of Medicine, University of Maryland School of Medicine, Baltimore; Department of Pharmacotherapy and Translational Research and Center for Pharmacogenomics (C.W.M.), University of Florida, Gainesville; Department of Neurology (J.F.M.), Mayo Clinic, Jacksonville, FL; Klinik und Poliklinik für Neurologie (A.R.), Universitätsmedizin Rostock, Germany; Department of Neurology (S.R., R.S.), Clinical Division of Neurogeriatrics, Medical University Graz, Austria; Center for Genomic Medicine (J. Rosand), Massachusetts General Hospital, Boston; Broad Institute (J. Rosand), Cambridge, MA; Department of Neurology and Evelyn F. McKnight Brain Institute (J. Roquer, T.R., R.L.S./M.S.), Miller School of Medicine, University of Miami, FL; Institute of Cardiovascular Research (P.S.), Royal Holloway University of London (ICR2UL), UK St Peter's and Ashford Hospitals, Egham, United Kingdom; Department of Neurology (A. Slowik), Jagiellonian University Medical College, Krakow, Poland; Division of Neurocritical Care & Emergency Neurology (D.S.), Department of Neurology, Helsinki University Central Hospital, Finland; Stroke Division (V.T.), Florey Institute of Neuroscience and Mental Health, Heidelberg; Department of Neurology (V.T.), Austin Health, Heidelberg, Australia; Departments of Radiology (A.V.) and Neurology and Rehabilitation Medicine (D.W.), University of Cincinnati College of Medicine, OH; Department of Clinical Sciences Lund, Radiology (J.W.) and Neurology (A.G.L.), Lund University, Sweden; Department of Radiology, Neuroradiology, Skåne University Hospital, Malmö, Sweden; Departments of Neurology and Public Health Sciences (B.B.W.), University of Virginia, Charlottesville, VA; University of Technology Sydney (J.M.), Australia; Section of Neurology (A.G.L.), Skåne University Hospital, Lund, Sweden; Department of Laboratory Medicine (C.J.), Institute of Biomedicine, the Sahlgrenska Academy, University of Gothenburg, Sweden; and Department of Clinical Genetics and Genomics (C.J.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden.
1,
James F Meschia
James F Meschia, MD
1From the J. Philip Kistler Stroke Research Center (M.B., A.K.B., M.D.S., S.H., A. Dalca, K.D., A.-K.G., M.R.E., P.M.R., M.N., R.W.R., C.W., N.S.R.), A.A. Martinos Center for Biomedical Imaging (A. Dalca, O.W.), and Henry and Allison McCance Center for Brain Health (J. Rosand), Massachusetts General Hospital, Harvard Medical School, Boston; Lille Neuroscience & Cognition (M.B., X.L., R. Lopes, G.K.), Inserm, CHU Lille, U1172 and Institut Pasteur de Lille (M.G.), CNRS, Inserm, CHU Lille, US 41 - UMS 2014 - PLBS, Lille University, France; Computer Science and Artificial Intelligence Lab (A. Dalca, C.W., P.G.), Massachusetts Institute of Technology, Cambridge; Division of Preventive Medicine (P.M.R.), Department of Medicine, Brigham and Women's Hospital, Boston, MA; Department of Medicine (O.R.B.), Division of Neurology, University of British Columbia, Vancouver, Canada; Department of Neurology (J.W.C., S.J.K.), University of Maryland School of Medicine and Veterans Affairs Maryland Health Care System, Baltimore, MD; School of Medical Sciences (A. Donatti, A. Sousa), University of Campinas (UNICAMP) and the Brazilian Institute of Neuroscience and Neurotechnology (BRAINN), Campinas, São Paulo; Departments of Neurosurgery (C.G.) and Neurology (R.Z.), Geisinger, Danville, PA; Department of Neurosurgery (C.G.), Christian Doppler Klinik, Paracelsus Medical University, Salzburg, Austria; Division of Emergency Medicine (Laura Heitsch), Washington University School of Medicine, St. Louis; Department of Neurology (Laura Heitsch, C.-L.P.), Washington University School of Medicine & Barnes-Jewish Hospital, St. Louis, MO; Department of Clinical Neuroscience (L. Holmegaard, K.J., T.M.S., T.T.), Institute of Neuroscience and Physiology, Sahlgrenska Academy at University of Gothenburg, Sweden; Department of Neurology, Sahlgrenska University Hospital, Gothenburg, Sweden; Department of Neurology (J.J.-C.), Neurovascular Research Group (NEUVAS), IMIM-Hospital del Mar (Institut Hospital del Mar d'Investigacions M`ediques), Universitat Autonoma de Barcelona, Spain; Department of Neurosciences (R. Lemmens), Experimental Neurology and Leuven Research Institute for Neuroscience and Disease (LIND), KU Leuven - University of Leuven, Belgium; Department of Neurology (R. Lemmens), Laboratory of Neurobiology, VIB Vesalius Research Center, University Hospitals Leuven, Belgium; School of Medicine and Public Health (C.R.L.), University of Newcastle, New South Wales; Department of Neurology, John Hunter Hospital, Newcastle, New South Wales, Australia; Division of Endocrinology (P.F.M.), Diabetes and Nutrition, Department of Medicine, University of Maryland School of Medicine, Baltimore; Department of Pharmacotherapy and Translational Research and Center for Pharmacogenomics (C.W.M.), University of Florida, Gainesville; Department of Neurology (J.F.M.), Mayo Clinic, Jacksonville, FL; Klinik und Poliklinik für Neurologie (A.R.), Universitätsmedizin Rostock, Germany; Department of Neurology (S.R., R.S.), Clinical Division of Neurogeriatrics, Medical University Graz, Austria; Center for Genomic Medicine (J. Rosand), Massachusetts General Hospital, Boston; Broad Institute (J. Rosand), Cambridge, MA; Department of Neurology and Evelyn F. McKnight Brain Institute (J. Roquer, T.R., R.L.S./M.S.), Miller School of Medicine, University of Miami, FL; Institute of Cardiovascular Research (P.S.), Royal Holloway University of London (ICR2UL), UK St Peter's and Ashford Hospitals, Egham, United Kingdom; Department of Neurology (A. Slowik), Jagiellonian University Medical College, Krakow, Poland; Division of Neurocritical Care & Emergency Neurology (D.S.), Department of Neurology, Helsinki University Central Hospital, Finland; Stroke Division (V.T.), Florey Institute of Neuroscience and Mental Health, Heidelberg; Department of Neurology (V.T.), Austin Health, Heidelberg, Australia; Departments of Radiology (A.V.) and Neurology and Rehabilitation Medicine (D.W.), University of Cincinnati College of Medicine, OH; Department of Clinical Sciences Lund, Radiology (J.W.) and Neurology (A.G.L.), Lund University, Sweden; Department of Radiology, Neuroradiology, Skåne University Hospital, Malmö, Sweden; Departments of Neurology and Public Health Sciences (B.B.W.), University of Virginia, Charlottesville, VA; University of Technology Sydney (J.M.), Australia; Section of Neurology (A.G.L.), Skåne University Hospital, Lund, Sweden; Department of Laboratory Medicine (C.J.), Institute of Biomedicine, the Sahlgrenska Academy, University of Gothenburg, Sweden; and Department of Clinical Genetics and Genomics (C.J.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden.
1,
Chia-Ling Phuah
Chia-Ling Phuah, MD
1From the J. Philip Kistler Stroke Research Center (M.B., A.K.B., M.D.S., S.H., A. Dalca, K.D., A.-K.G., M.R.E., P.M.R., M.N., R.W.R., C.W., N.S.R.), A.A. Martinos Center for Biomedical Imaging (A. Dalca, O.W.), and Henry and Allison McCance Center for Brain Health (J. Rosand), Massachusetts General Hospital, Harvard Medical School, Boston; Lille Neuroscience & Cognition (M.B., X.L., R. Lopes, G.K.), Inserm, CHU Lille, U1172 and Institut Pasteur de Lille (M.G.), CNRS, Inserm, CHU Lille, US 41 - UMS 2014 - PLBS, Lille University, France; Computer Science and Artificial Intelligence Lab (A. Dalca, C.W., P.G.), Massachusetts Institute of Technology, Cambridge; Division of Preventive Medicine (P.M.R.), Department of Medicine, Brigham and Women's Hospital, Boston, MA; Department of Medicine (O.R.B.), Division of Neurology, University of British Columbia, Vancouver, Canada; Department of Neurology (J.W.C., S.J.K.), University of Maryland School of Medicine and Veterans Affairs Maryland Health Care System, Baltimore, MD; School of Medical Sciences (A. Donatti, A. Sousa), University of Campinas (UNICAMP) and the Brazilian Institute of Neuroscience and Neurotechnology (BRAINN), Campinas, São Paulo; Departments of Neurosurgery (C.G.) and Neurology (R.Z.), Geisinger, Danville, PA; Department of Neurosurgery (C.G.), Christian Doppler Klinik, Paracelsus Medical University, Salzburg, Austria; Division of Emergency Medicine (Laura Heitsch), Washington University School of Medicine, St. Louis; Department of Neurology (Laura Heitsch, C.-L.P.), Washington University School of Medicine & Barnes-Jewish Hospital, St. Louis, MO; Department of Clinical Neuroscience (L. Holmegaard, K.J., T.M.S., T.T.), Institute of Neuroscience and Physiology, Sahlgrenska Academy at University of Gothenburg, Sweden; Department of Neurology, Sahlgrenska University Hospital, Gothenburg, Sweden; Department of Neurology (J.J.-C.), Neurovascular Research Group (NEUVAS), IMIM-Hospital del Mar (Institut Hospital del Mar d'Investigacions M`ediques), Universitat Autonoma de Barcelona, Spain; Department of Neurosciences (R. Lemmens), Experimental Neurology and Leuven Research Institute for Neuroscience and Disease (LIND), KU Leuven - University of Leuven, Belgium; Department of Neurology (R. Lemmens), Laboratory of Neurobiology, VIB Vesalius Research Center, University Hospitals Leuven, Belgium; School of Medicine and Public Health (C.R.L.), University of Newcastle, New South Wales; Department of Neurology, John Hunter Hospital, Newcastle, New South Wales, Australia; Division of Endocrinology (P.F.M.), Diabetes and Nutrition, Department of Medicine, University of Maryland School of Medicine, Baltimore; Department of Pharmacotherapy and Translational Research and Center for Pharmacogenomics (C.W.M.), University of Florida, Gainesville; Department of Neurology (J.F.M.), Mayo Clinic, Jacksonville, FL; Klinik und Poliklinik für Neurologie (A.R.), Universitätsmedizin Rostock, Germany; Department of Neurology (S.R., R.S.), Clinical Division of Neurogeriatrics, Medical University Graz, Austria; Center for Genomic Medicine (J. Rosand), Massachusetts General Hospital, Boston; Broad Institute (J. Rosand), Cambridge, MA; Department of Neurology and Evelyn F. McKnight Brain Institute (J. Roquer, T.R., R.L.S./M.S.), Miller School of Medicine, University of Miami, FL; Institute of Cardiovascular Research (P.S.), Royal Holloway University of London (ICR2UL), UK St Peter's and Ashford Hospitals, Egham, United Kingdom; Department of Neurology (A. Slowik), Jagiellonian University Medical College, Krakow, Poland; Division of Neurocritical Care & Emergency Neurology (D.S.), Department of Neurology, Helsinki University Central Hospital, Finland; Stroke Division (V.T.), Florey Institute of Neuroscience and Mental Health, Heidelberg; Department of Neurology (V.T.), Austin Health, Heidelberg, Australia; Departments of Radiology (A.V.) and Neurology and Rehabilitation Medicine (D.W.), University of Cincinnati College of Medicine, OH; Department of Clinical Sciences Lund, Radiology (J.W.) and Neurology (A.G.L.), Lund University, Sweden; Department of Radiology, Neuroradiology, Skåne University Hospital, Malmö, Sweden; Departments of Neurology and Public Health Sciences (B.B.W.), University of Virginia, Charlottesville, VA; University of Technology Sydney (J.M.), Australia; Section of Neurology (A.G.L.), Skåne University Hospital, Lund, Sweden; Department of Laboratory Medicine (C.J.), Institute of Biomedicine, the Sahlgrenska Academy, University of Gothenburg, Sweden; and Department of Clinical Genetics and Genomics (C.J.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden.
1,
Arndt Rolfs
Arndt Rolfs, MD
1From the J. Philip Kistler Stroke Research Center (M.B., A.K.B., M.D.S., S.H., A. Dalca, K.D., A.-K.G., M.R.E., P.M.R., M.N., R.W.R., C.W., N.S.R.), A.A. Martinos Center for Biomedical Imaging (A. Dalca, O.W.), and Henry and Allison McCance Center for Brain Health (J. Rosand), Massachusetts General Hospital, Harvard Medical School, Boston; Lille Neuroscience & Cognition (M.B., X.L., R. Lopes, G.K.), Inserm, CHU Lille, U1172 and Institut Pasteur de Lille (M.G.), CNRS, Inserm, CHU Lille, US 41 - UMS 2014 - PLBS, Lille University, France; Computer Science and Artificial Intelligence Lab (A. Dalca, C.W., P.G.), Massachusetts Institute of Technology, Cambridge; Division of Preventive Medicine (P.M.R.), Department of Medicine, Brigham and Women's Hospital, Boston, MA; Department of Medicine (O.R.B.), Division of Neurology, University of British Columbia, Vancouver, Canada; Department of Neurology (J.W.C., S.J.K.), University of Maryland School of Medicine and Veterans Affairs Maryland Health Care System, Baltimore, MD; School of Medical Sciences (A. Donatti, A. Sousa), University of Campinas (UNICAMP) and the Brazilian Institute of Neuroscience and Neurotechnology (BRAINN), Campinas, São Paulo; Departments of Neurosurgery (C.G.) and Neurology (R.Z.), Geisinger, Danville, PA; Department of Neurosurgery (C.G.), Christian Doppler Klinik, Paracelsus Medical University, Salzburg, Austria; Division of Emergency Medicine (Laura Heitsch), Washington University School of Medicine, St. Louis; Department of Neurology (Laura Heitsch, C.-L.P.), Washington University School of Medicine & Barnes-Jewish Hospital, St. Louis, MO; Department of Clinical Neuroscience (L. Holmegaard, K.J., T.M.S., T.T.), Institute of Neuroscience and Physiology, Sahlgrenska Academy at University of Gothenburg, Sweden; Department of Neurology, Sahlgrenska University Hospital, Gothenburg, Sweden; Department of Neurology (J.J.-C.), Neurovascular Research Group (NEUVAS), IMIM-Hospital del Mar (Institut Hospital del Mar d'Investigacions M`ediques), Universitat Autonoma de Barcelona, Spain; Department of Neurosciences (R. Lemmens), Experimental Neurology and Leuven Research Institute for Neuroscience and Disease (LIND), KU Leuven - University of Leuven, Belgium; Department of Neurology (R. Lemmens), Laboratory of Neurobiology, VIB Vesalius Research Center, University Hospitals Leuven, Belgium; School of Medicine and Public Health (C.R.L.), University of Newcastle, New South Wales; Department of Neurology, John Hunter Hospital, Newcastle, New South Wales, Australia; Division of Endocrinology (P.F.M.), Diabetes and Nutrition, Department of Medicine, University of Maryland School of Medicine, Baltimore; Department of Pharmacotherapy and Translational Research and Center for Pharmacogenomics (C.W.M.), University of Florida, Gainesville; Department of Neurology (J.F.M.), Mayo Clinic, Jacksonville, FL; Klinik und Poliklinik für Neurologie (A.R.), Universitätsmedizin Rostock, Germany; Department of Neurology (S.R., R.S.), Clinical Division of Neurogeriatrics, Medical University Graz, Austria; Center for Genomic Medicine (J. Rosand), Massachusetts General Hospital, Boston; Broad Institute (J. Rosand), Cambridge, MA; Department of Neurology and Evelyn F. McKnight Brain Institute (J. Roquer, T.R., R.L.S./M.S.), Miller School of Medicine, University of Miami, FL; Institute of Cardiovascular Research (P.S.), Royal Holloway University of London (ICR2UL), UK St Peter's and Ashford Hospitals, Egham, United Kingdom; Department of Neurology (A. Slowik), Jagiellonian University Medical College, Krakow, Poland; Division of Neurocritical Care & Emergency Neurology (D.S.), Department of Neurology, Helsinki University Central Hospital, Finland; Stroke Division (V.T.), Florey Institute of Neuroscience and Mental Health, Heidelberg; Department of Neurology (V.T.), Austin Health, Heidelberg, Australia; Departments of Radiology (A.V.) and Neurology and Rehabilitation Medicine (D.W.), University of Cincinnati College of Medicine, OH; Department of Clinical Sciences Lund, Radiology (J.W.) and Neurology (A.G.L.), Lund University, Sweden; Department of Radiology, Neuroradiology, Skåne University Hospital, Malmö, Sweden; Departments of Neurology and Public Health Sciences (B.B.W.), University of Virginia, Charlottesville, VA; University of Technology Sydney (J.M.), Australia; Section of Neurology (A.G.L.), Skåne University Hospital, Lund, Sweden; Department of Laboratory Medicine (C.J.), Institute of Biomedicine, the Sahlgrenska Academy, University of Gothenburg, Sweden; and Department of Clinical Genetics and Genomics (C.J.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden.
1,
Stefan Ropele
Stefan Ropele, MD
1From the J. Philip Kistler Stroke Research Center (M.B., A.K.B., M.D.S., S.H., A. Dalca, K.D., A.-K.G., M.R.E., P.M.R., M.N., R.W.R., C.W., N.S.R.), A.A. Martinos Center for Biomedical Imaging (A. Dalca, O.W.), and Henry and Allison McCance Center for Brain Health (J. Rosand), Massachusetts General Hospital, Harvard Medical School, Boston; Lille Neuroscience & Cognition (M.B., X.L., R. Lopes, G.K.), Inserm, CHU Lille, U1172 and Institut Pasteur de Lille (M.G.), CNRS, Inserm, CHU Lille, US 41 - UMS 2014 - PLBS, Lille University, France; Computer Science and Artificial Intelligence Lab (A. Dalca, C.W., P.G.), Massachusetts Institute of Technology, Cambridge; Division of Preventive Medicine (P.M.R.), Department of Medicine, Brigham and Women's Hospital, Boston, MA; Department of Medicine (O.R.B.), Division of Neurology, University of British Columbia, Vancouver, Canada; Department of Neurology (J.W.C., S.J.K.), University of Maryland School of Medicine and Veterans Affairs Maryland Health Care System, Baltimore, MD; School of Medical Sciences (A. Donatti, A. Sousa), University of Campinas (UNICAMP) and the Brazilian Institute of Neuroscience and Neurotechnology (BRAINN), Campinas, São Paulo; Departments of Neurosurgery (C.G.) and Neurology (R.Z.), Geisinger, Danville, PA; Department of Neurosurgery (C.G.), Christian Doppler Klinik, Paracelsus Medical University, Salzburg, Austria; Division of Emergency Medicine (Laura Heitsch), Washington University School of Medicine, St. Louis; Department of Neurology (Laura Heitsch, C.-L.P.), Washington University School of Medicine & Barnes-Jewish Hospital, St. Louis, MO; Department of Clinical Neuroscience (L. Holmegaard, K.J., T.M.S., T.T.), Institute of Neuroscience and Physiology, Sahlgrenska Academy at University of Gothenburg, Sweden; Department of Neurology, Sahlgrenska University Hospital, Gothenburg, Sweden; Department of Neurology (J.J.-C.), Neurovascular Research Group (NEUVAS), IMIM-Hospital del Mar (Institut Hospital del Mar d'Investigacions M`ediques), Universitat Autonoma de Barcelona, Spain; Department of Neurosciences (R. Lemmens), Experimental Neurology and Leuven Research Institute for Neuroscience and Disease (LIND), KU Leuven - University of Leuven, Belgium; Department of Neurology (R. Lemmens), Laboratory of Neurobiology, VIB Vesalius Research Center, University Hospitals Leuven, Belgium; School of Medicine and Public Health (C.R.L.), University of Newcastle, New South Wales; Department of Neurology, John Hunter Hospital, Newcastle, New South Wales, Australia; Division of Endocrinology (P.F.M.), Diabetes and Nutrition, Department of Medicine, University of Maryland School of Medicine, Baltimore; Department of Pharmacotherapy and Translational Research and Center for Pharmacogenomics (C.W.M.), University of Florida, Gainesville; Department of Neurology (J.F.M.), Mayo Clinic, Jacksonville, FL; Klinik und Poliklinik für Neurologie (A.R.), Universitätsmedizin Rostock, Germany; Department of Neurology (S.R., R.S.), Clinical Division of Neurogeriatrics, Medical University Graz, Austria; Center for Genomic Medicine (J. Rosand), Massachusetts General Hospital, Boston; Broad Institute (J. Rosand), Cambridge, MA; Department of Neurology and Evelyn F. McKnight Brain Institute (J. Roquer, T.R., R.L.S./M.S.), Miller School of Medicine, University of Miami, FL; Institute of Cardiovascular Research (P.S.), Royal Holloway University of London (ICR2UL), UK St Peter's and Ashford Hospitals, Egham, United Kingdom; Department of Neurology (A. Slowik), Jagiellonian University Medical College, Krakow, Poland; Division of Neurocritical Care & Emergency Neurology (D.S.), Department of Neurology, Helsinki University Central Hospital, Finland; Stroke Division (V.T.), Florey Institute of Neuroscience and Mental Health, Heidelberg; Department of Neurology (V.T.), Austin Health, Heidelberg, Australia; Departments of Radiology (A.V.) and Neurology and Rehabilitation Medicine (D.W.), University of Cincinnati College of Medicine, OH; Department of Clinical Sciences Lund, Radiology (J.W.) and Neurology (A.G.L.), Lund University, Sweden; Department of Radiology, Neuroradiology, Skåne University Hospital, Malmö, Sweden; Departments of Neurology and Public Health Sciences (B.B.W.), University of Virginia, Charlottesville, VA; University of Technology Sydney (J.M.), Australia; Section of Neurology (A.G.L.), Skåne University Hospital, Lund, Sweden; Department of Laboratory Medicine (C.J.), Institute of Biomedicine, the Sahlgrenska Academy, University of Gothenburg, Sweden; and Department of Clinical Genetics and Genomics (C.J.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden.
1,
Jonathan Rosand
Jonathan Rosand, MD, MSc
1From the J. Philip Kistler Stroke Research Center (M.B., A.K.B., M.D.S., S.H., A. Dalca, K.D., A.-K.G., M.R.E., P.M.R., M.N., R.W.R., C.W., N.S.R.), A.A. Martinos Center for Biomedical Imaging (A. Dalca, O.W.), and Henry and Allison McCance Center for Brain Health (J. Rosand), Massachusetts General Hospital, Harvard Medical School, Boston; Lille Neuroscience & Cognition (M.B., X.L., R. Lopes, G.K.), Inserm, CHU Lille, U1172 and Institut Pasteur de Lille (M.G.), CNRS, Inserm, CHU Lille, US 41 - UMS 2014 - PLBS, Lille University, France; Computer Science and Artificial Intelligence Lab (A. Dalca, C.W., P.G.), Massachusetts Institute of Technology, Cambridge; Division of Preventive Medicine (P.M.R.), Department of Medicine, Brigham and Women's Hospital, Boston, MA; Department of Medicine (O.R.B.), Division of Neurology, University of British Columbia, Vancouver, Canada; Department of Neurology (J.W.C., S.J.K.), University of Maryland School of Medicine and Veterans Affairs Maryland Health Care System, Baltimore, MD; School of Medical Sciences (A. Donatti, A. Sousa), University of Campinas (UNICAMP) and the Brazilian Institute of Neuroscience and Neurotechnology (BRAINN), Campinas, São Paulo; Departments of Neurosurgery (C.G.) and Neurology (R.Z.), Geisinger, Danville, PA; Department of Neurosurgery (C.G.), Christian Doppler Klinik, Paracelsus Medical University, Salzburg, Austria; Division of Emergency Medicine (Laura Heitsch), Washington University School of Medicine, St. Louis; Department of Neurology (Laura Heitsch, C.-L.P.), Washington University School of Medicine & Barnes-Jewish Hospital, St. Louis, MO; Department of Clinical Neuroscience (L. Holmegaard, K.J., T.M.S., T.T.), Institute of Neuroscience and Physiology, Sahlgrenska Academy at University of Gothenburg, Sweden; Department of Neurology, Sahlgrenska University Hospital, Gothenburg, Sweden; Department of Neurology (J.J.-C.), Neurovascular Research Group (NEUVAS), IMIM-Hospital del Mar (Institut Hospital del Mar d'Investigacions M`ediques), Universitat Autonoma de Barcelona, Spain; Department of Neurosciences (R. Lemmens), Experimental Neurology and Leuven Research Institute for Neuroscience and Disease (LIND), KU Leuven - University of Leuven, Belgium; Department of Neurology (R. Lemmens), Laboratory of Neurobiology, VIB Vesalius Research Center, University Hospitals Leuven, Belgium; School of Medicine and Public Health (C.R.L.), University of Newcastle, New South Wales; Department of Neurology, John Hunter Hospital, Newcastle, New South Wales, Australia; Division of Endocrinology (P.F.M.), Diabetes and Nutrition, Department of Medicine, University of Maryland School of Medicine, Baltimore; Department of Pharmacotherapy and Translational Research and Center for Pharmacogenomics (C.W.M.), University of Florida, Gainesville; Department of Neurology (J.F.M.), Mayo Clinic, Jacksonville, FL; Klinik und Poliklinik für Neurologie (A.R.), Universitätsmedizin Rostock, Germany; Department of Neurology (S.R., R.S.), Clinical Division of Neurogeriatrics, Medical University Graz, Austria; Center for Genomic Medicine (J. Rosand), Massachusetts General Hospital, Boston; Broad Institute (J. Rosand), Cambridge, MA; Department of Neurology and Evelyn F. McKnight Brain Institute (J. Roquer, T.R., R.L.S./M.S.), Miller School of Medicine, University of Miami, FL; Institute of Cardiovascular Research (P.S.), Royal Holloway University of London (ICR2UL), UK St Peter's and Ashford Hospitals, Egham, United Kingdom; Department of Neurology (A. Slowik), Jagiellonian University Medical College, Krakow, Poland; Division of Neurocritical Care & Emergency Neurology (D.S.), Department of Neurology, Helsinki University Central Hospital, Finland; Stroke Division (V.T.), Florey Institute of Neuroscience and Mental Health, Heidelberg; Department of Neurology (V.T.), Austin Health, Heidelberg, Australia; Departments of Radiology (A.V.) and Neurology and Rehabilitation Medicine (D.W.), University of Cincinnati College of Medicine, OH; Department of Clinical Sciences Lund, Radiology (J.W.) and Neurology (A.G.L.), Lund University, Sweden; Department of Radiology, Neuroradiology, Skåne University Hospital, Malmö, Sweden; Departments of Neurology and Public Health Sciences (B.B.W.), University of Virginia, Charlottesville, VA; University of Technology Sydney (J.M.), Australia; Section of Neurology (A.G.L.), Skåne University Hospital, Lund, Sweden; Department of Laboratory Medicine (C.J.), Institute of Biomedicine, the Sahlgrenska Academy, University of Gothenburg, Sweden; and Department of Clinical Genetics and Genomics (C.J.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden.
1,
Jaume Roquer
Jaume Roquer, MD
1From the J. Philip Kistler Stroke Research Center (M.B., A.K.B., M.D.S., S.H., A. Dalca, K.D., A.-K.G., M.R.E., P.M.R., M.N., R.W.R., C.W., N.S.R.), A.A. Martinos Center for Biomedical Imaging (A. Dalca, O.W.), and Henry and Allison McCance Center for Brain Health (J. Rosand), Massachusetts General Hospital, Harvard Medical School, Boston; Lille Neuroscience & Cognition (M.B., X.L., R. Lopes, G.K.), Inserm, CHU Lille, U1172 and Institut Pasteur de Lille (M.G.), CNRS, Inserm, CHU Lille, US 41 - UMS 2014 - PLBS, Lille University, France; Computer Science and Artificial Intelligence Lab (A. Dalca, C.W., P.G.), Massachusetts Institute of Technology, Cambridge; Division of Preventive Medicine (P.M.R.), Department of Medicine, Brigham and Women's Hospital, Boston, MA; Department of Medicine (O.R.B.), Division of Neurology, University of British Columbia, Vancouver, Canada; Department of Neurology (J.W.C., S.J.K.), University of Maryland School of Medicine and Veterans Affairs Maryland Health Care System, Baltimore, MD; School of Medical Sciences (A. Donatti, A. Sousa), University of Campinas (UNICAMP) and the Brazilian Institute of Neuroscience and Neurotechnology (BRAINN), Campinas, São Paulo; Departments of Neurosurgery (C.G.) and Neurology (R.Z.), Geisinger, Danville, PA; Department of Neurosurgery (C.G.), Christian Doppler Klinik, Paracelsus Medical University, Salzburg, Austria; Division of Emergency Medicine (Laura Heitsch), Washington University School of Medicine, St. Louis; Department of Neurology (Laura Heitsch, C.-L.P.), Washington University School of Medicine & Barnes-Jewish Hospital, St. Louis, MO; Department of Clinical Neuroscience (L. Holmegaard, K.J., T.M.S., T.T.), Institute of Neuroscience and Physiology, Sahlgrenska Academy at University of Gothenburg, Sweden; Department of Neurology, Sahlgrenska University Hospital, Gothenburg, Sweden; Department of Neurology (J.J.-C.), Neurovascular Research Group (NEUVAS), IMIM-Hospital del Mar (Institut Hospital del Mar d'Investigacions M`ediques), Universitat Autonoma de Barcelona, Spain; Department of Neurosciences (R. Lemmens), Experimental Neurology and Leuven Research Institute for Neuroscience and Disease (LIND), KU Leuven - University of Leuven, Belgium; Department of Neurology (R. Lemmens), Laboratory of Neurobiology, VIB Vesalius Research Center, University Hospitals Leuven, Belgium; School of Medicine and Public Health (C.R.L.), University of Newcastle, New South Wales; Department of Neurology, John Hunter Hospital, Newcastle, New South Wales, Australia; Division of Endocrinology (P.F.M.), Diabetes and Nutrition, Department of Medicine, University of Maryland School of Medicine, Baltimore; Department of Pharmacotherapy and Translational Research and Center for Pharmacogenomics (C.W.M.), University of Florida, Gainesville; Department of Neurology (J.F.M.), Mayo Clinic, Jacksonville, FL; Klinik und Poliklinik für Neurologie (A.R.), Universitätsmedizin Rostock, Germany; Department of Neurology (S.R., R.S.), Clinical Division of Neurogeriatrics, Medical University Graz, Austria; Center for Genomic Medicine (J. Rosand), Massachusetts General Hospital, Boston; Broad Institute (J. Rosand), Cambridge, MA; Department of Neurology and Evelyn F. McKnight Brain Institute (J. Roquer, T.R., R.L.S./M.S.), Miller School of Medicine, University of Miami, FL; Institute of Cardiovascular Research (P.S.), Royal Holloway University of London (ICR2UL), UK St Peter's and Ashford Hospitals, Egham, United Kingdom; Department of Neurology (A. Slowik), Jagiellonian University Medical College, Krakow, Poland; Division of Neurocritical Care & Emergency Neurology (D.S.), Department of Neurology, Helsinki University Central Hospital, Finland; Stroke Division (V.T.), Florey Institute of Neuroscience and Mental Health, Heidelberg; Department of Neurology (V.T.), Austin Health, Heidelberg, Australia; Departments of Radiology (A.V.) and Neurology and Rehabilitation Medicine (D.W.), University of Cincinnati College of Medicine, OH; Department of Clinical Sciences Lund, Radiology (J.W.) and Neurology (A.G.L.), Lund University, Sweden; Department of Radiology, Neuroradiology, Skåne University Hospital, Malmö, Sweden; Departments of Neurology and Public Health Sciences (B.B.W.), University of Virginia, Charlottesville, VA; University of Technology Sydney (J.M.), Australia; Section of Neurology (A.G.L.), Skåne University Hospital, Lund, Sweden; Department of Laboratory Medicine (C.J.), Institute of Biomedicine, the Sahlgrenska Academy, University of Gothenburg, Sweden; and Department of Clinical Genetics and Genomics (C.J.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden.
1,
Tatjana Rundek
Tatjana Rundek, MD, PhD
1From the J. Philip Kistler Stroke Research Center (M.B., A.K.B., M.D.S., S.H., A. Dalca, K.D., A.-K.G., M.R.E., P.M.R., M.N., R.W.R., C.W., N.S.R.), A.A. Martinos Center for Biomedical Imaging (A. Dalca, O.W.), and Henry and Allison McCance Center for Brain Health (J. Rosand), Massachusetts General Hospital, Harvard Medical School, Boston; Lille Neuroscience & Cognition (M.B., X.L., R. Lopes, G.K.), Inserm, CHU Lille, U1172 and Institut Pasteur de Lille (M.G.), CNRS, Inserm, CHU Lille, US 41 - UMS 2014 - PLBS, Lille University, France; Computer Science and Artificial Intelligence Lab (A. Dalca, C.W., P.G.), Massachusetts Institute of Technology, Cambridge; Division of Preventive Medicine (P.M.R.), Department of Medicine, Brigham and Women's Hospital, Boston, MA; Department of Medicine (O.R.B.), Division of Neurology, University of British Columbia, Vancouver, Canada; Department of Neurology (J.W.C., S.J.K.), University of Maryland School of Medicine and Veterans Affairs Maryland Health Care System, Baltimore, MD; School of Medical Sciences (A. Donatti, A. Sousa), University of Campinas (UNICAMP) and the Brazilian Institute of Neuroscience and Neurotechnology (BRAINN), Campinas, São Paulo; Departments of Neurosurgery (C.G.) and Neurology (R.Z.), Geisinger, Danville, PA; Department of Neurosurgery (C.G.), Christian Doppler Klinik, Paracelsus Medical University, Salzburg, Austria; Division of Emergency Medicine (Laura Heitsch), Washington University School of Medicine, St. Louis; Department of Neurology (Laura Heitsch, C.-L.P.), Washington University School of Medicine & Barnes-Jewish Hospital, St. Louis, MO; Department of Clinical Neuroscience (L. Holmegaard, K.J., T.M.S., T.T.), Institute of Neuroscience and Physiology, Sahlgrenska Academy at University of Gothenburg, Sweden; Department of Neurology, Sahlgrenska University Hospital, Gothenburg, Sweden; Department of Neurology (J.J.-C.), Neurovascular Research Group (NEUVAS), IMIM-Hospital del Mar (Institut Hospital del Mar d'Investigacions M`ediques), Universitat Autonoma de Barcelona, Spain; Department of Neurosciences (R. Lemmens), Experimental Neurology and Leuven Research Institute for Neuroscience and Disease (LIND), KU Leuven - University of Leuven, Belgium; Department of Neurology (R. Lemmens), Laboratory of Neurobiology, VIB Vesalius Research Center, University Hospitals Leuven, Belgium; School of Medicine and Public Health (C.R.L.), University of Newcastle, New South Wales; Department of Neurology, John Hunter Hospital, Newcastle, New South Wales, Australia; Division of Endocrinology (P.F.M.), Diabetes and Nutrition, Department of Medicine, University of Maryland School of Medicine, Baltimore; Department of Pharmacotherapy and Translational Research and Center for Pharmacogenomics (C.W.M.), University of Florida, Gainesville; Department of Neurology (J.F.M.), Mayo Clinic, Jacksonville, FL; Klinik und Poliklinik für Neurologie (A.R.), Universitätsmedizin Rostock, Germany; Department of Neurology (S.R., R.S.), Clinical Division of Neurogeriatrics, Medical University Graz, Austria; Center for Genomic Medicine (J. Rosand), Massachusetts General Hospital, Boston; Broad Institute (J. Rosand), Cambridge, MA; Department of Neurology and Evelyn F. McKnight Brain Institute (J. Roquer, T.R., R.L.S./M.S.), Miller School of Medicine, University of Miami, FL; Institute of Cardiovascular Research (P.S.), Royal Holloway University of London (ICR2UL), UK St Peter's and Ashford Hospitals, Egham, United Kingdom; Department of Neurology (A. Slowik), Jagiellonian University Medical College, Krakow, Poland; Division of Neurocritical Care & Emergency Neurology (D.S.), Department of Neurology, Helsinki University Central Hospital, Finland; Stroke Division (V.T.), Florey Institute of Neuroscience and Mental Health, Heidelberg; Department of Neurology (V.T.), Austin Health, Heidelberg, Australia; Departments of Radiology (A.V.) and Neurology and Rehabilitation Medicine (D.W.), University of Cincinnati College of Medicine, OH; Department of Clinical Sciences Lund, Radiology (J.W.) and Neurology (A.G.L.), Lund University, Sweden; Department of Radiology, Neuroradiology, Skåne University Hospital, Malmö, Sweden; Departments of Neurology and Public Health Sciences (B.B.W.), University of Virginia, Charlottesville, VA; University of Technology Sydney (J.M.), Australia; Section of Neurology (A.G.L.), Skåne University Hospital, Lund, Sweden; Department of Laboratory Medicine (C.J.), Institute of Biomedicine, the Sahlgrenska Academy, University of Gothenburg, Sweden; and Department of Clinical Genetics and Genomics (C.J.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden.
1,
Ralph L Sacco
Ralph L Sacco, MD, MS
1From the J. Philip Kistler Stroke Research Center (M.B., A.K.B., M.D.S., S.H., A. Dalca, K.D., A.-K.G., M.R.E., P.M.R., M.N., R.W.R., C.W., N.S.R.), A.A. Martinos Center for Biomedical Imaging (A. Dalca, O.W.), and Henry and Allison McCance Center for Brain Health (J. Rosand), Massachusetts General Hospital, Harvard Medical School, Boston; Lille Neuroscience & Cognition (M.B., X.L., R. Lopes, G.K.), Inserm, CHU Lille, U1172 and Institut Pasteur de Lille (M.G.), CNRS, Inserm, CHU Lille, US 41 - UMS 2014 - PLBS, Lille University, France; Computer Science and Artificial Intelligence Lab (A. Dalca, C.W., P.G.), Massachusetts Institute of Technology, Cambridge; Division of Preventive Medicine (P.M.R.), Department of Medicine, Brigham and Women's Hospital, Boston, MA; Department of Medicine (O.R.B.), Division of Neurology, University of British Columbia, Vancouver, Canada; Department of Neurology (J.W.C., S.J.K.), University of Maryland School of Medicine and Veterans Affairs Maryland Health Care System, Baltimore, MD; School of Medical Sciences (A. Donatti, A. Sousa), University of Campinas (UNICAMP) and the Brazilian Institute of Neuroscience and Neurotechnology (BRAINN), Campinas, São Paulo; Departments of Neurosurgery (C.G.) and Neurology (R.Z.), Geisinger, Danville, PA; Department of Neurosurgery (C.G.), Christian Doppler Klinik, Paracelsus Medical University, Salzburg, Austria; Division of Emergency Medicine (Laura Heitsch), Washington University School of Medicine, St. Louis; Department of Neurology (Laura Heitsch, C.-L.P.), Washington University School of Medicine & Barnes-Jewish Hospital, St. Louis, MO; Department of Clinical Neuroscience (L. Holmegaard, K.J., T.M.S., T.T.), Institute of Neuroscience and Physiology, Sahlgrenska Academy at University of Gothenburg, Sweden; Department of Neurology, Sahlgrenska University Hospital, Gothenburg, Sweden; Department of Neurology (J.J.-C.), Neurovascular Research Group (NEUVAS), IMIM-Hospital del Mar (Institut Hospital del Mar d'Investigacions M`ediques), Universitat Autonoma de Barcelona, Spain; Department of Neurosciences (R. Lemmens), Experimental Neurology and Leuven Research Institute for Neuroscience and Disease (LIND), KU Leuven - University of Leuven, Belgium; Department of Neurology (R. Lemmens), Laboratory of Neurobiology, VIB Vesalius Research Center, University Hospitals Leuven, Belgium; School of Medicine and Public Health (C.R.L.), University of Newcastle, New South Wales; Department of Neurology, John Hunter Hospital, Newcastle, New South Wales, Australia; Division of Endocrinology (P.F.M.), Diabetes and Nutrition, Department of Medicine, University of Maryland School of Medicine, Baltimore; Department of Pharmacotherapy and Translational Research and Center for Pharmacogenomics (C.W.M.), University of Florida, Gainesville; Department of Neurology (J.F.M.), Mayo Clinic, Jacksonville, FL; Klinik und Poliklinik für Neurologie (A.R.), Universitätsmedizin Rostock, Germany; Department of Neurology (S.R., R.S.), Clinical Division of Neurogeriatrics, Medical University Graz, Austria; Center for Genomic Medicine (J. Rosand), Massachusetts General Hospital, Boston; Broad Institute (J. Rosand), Cambridge, MA; Department of Neurology and Evelyn F. McKnight Brain Institute (J. Roquer, T.R., R.L.S./M.S.), Miller School of Medicine, University of Miami, FL; Institute of Cardiovascular Research (P.S.), Royal Holloway University of London (ICR2UL), UK St Peter's and Ashford Hospitals, Egham, United Kingdom; Department of Neurology (A. Slowik), Jagiellonian University Medical College, Krakow, Poland; Division of Neurocritical Care & Emergency Neurology (D.S.), Department of Neurology, Helsinki University Central Hospital, Finland; Stroke Division (V.T.), Florey Institute of Neuroscience and Mental Health, Heidelberg; Department of Neurology (V.T.), Austin Health, Heidelberg, Australia; Departments of Radiology (A.V.) and Neurology and Rehabilitation Medicine (D.W.), University of Cincinnati College of Medicine, OH; Department of Clinical Sciences Lund, Radiology (J.W.) and Neurology (A.G.L.), Lund University, Sweden; Department of Radiology, Neuroradiology, Skåne University Hospital, Malmö, Sweden; Departments of Neurology and Public Health Sciences (B.B.W.), University of Virginia, Charlottesville, VA; University of Technology Sydney (J.M.), Australia; Section of Neurology (A.G.L.), Skåne University Hospital, Lund, Sweden; Department of Laboratory Medicine (C.J.), Institute of Biomedicine, the Sahlgrenska Academy, University of Gothenburg, Sweden; and Department of Clinical Genetics and Genomics (C.J.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden.
1,
Reinhold Schmidt
Reinhold Schmidt, MD
1From the J. Philip Kistler Stroke Research Center (M.B., A.K.B., M.D.S., S.H., A. Dalca, K.D., A.-K.G., M.R.E., P.M.R., M.N., R.W.R., C.W., N.S.R.), A.A. Martinos Center for Biomedical Imaging (A. Dalca, O.W.), and Henry and Allison McCance Center for Brain Health (J. Rosand), Massachusetts General Hospital, Harvard Medical School, Boston; Lille Neuroscience & Cognition (M.B., X.L., R. Lopes, G.K.), Inserm, CHU Lille, U1172 and Institut Pasteur de Lille (M.G.), CNRS, Inserm, CHU Lille, US 41 - UMS 2014 - PLBS, Lille University, France; Computer Science and Artificial Intelligence Lab (A. Dalca, C.W., P.G.), Massachusetts Institute of Technology, Cambridge; Division of Preventive Medicine (P.M.R.), Department of Medicine, Brigham and Women's Hospital, Boston, MA; Department of Medicine (O.R.B.), Division of Neurology, University of British Columbia, Vancouver, Canada; Department of Neurology (J.W.C., S.J.K.), University of Maryland School of Medicine and Veterans Affairs Maryland Health Care System, Baltimore, MD; School of Medical Sciences (A. Donatti, A. Sousa), University of Campinas (UNICAMP) and the Brazilian Institute of Neuroscience and Neurotechnology (BRAINN), Campinas, São Paulo; Departments of Neurosurgery (C.G.) and Neurology (R.Z.), Geisinger, Danville, PA; Department of Neurosurgery (C.G.), Christian Doppler Klinik, Paracelsus Medical University, Salzburg, Austria; Division of Emergency Medicine (Laura Heitsch), Washington University School of Medicine, St. Louis; Department of Neurology (Laura Heitsch, C.-L.P.), Washington University School of Medicine & Barnes-Jewish Hospital, St. Louis, MO; Department of Clinical Neuroscience (L. Holmegaard, K.J., T.M.S., T.T.), Institute of Neuroscience and Physiology, Sahlgrenska Academy at University of Gothenburg, Sweden; Department of Neurology, Sahlgrenska University Hospital, Gothenburg, Sweden; Department of Neurology (J.J.-C.), Neurovascular Research Group (NEUVAS), IMIM-Hospital del Mar (Institut Hospital del Mar d'Investigacions M`ediques), Universitat Autonoma de Barcelona, Spain; Department of Neurosciences (R. Lemmens), Experimental Neurology and Leuven Research Institute for Neuroscience and Disease (LIND), KU Leuven - University of Leuven, Belgium; Department of Neurology (R. Lemmens), Laboratory of Neurobiology, VIB Vesalius Research Center, University Hospitals Leuven, Belgium; School of Medicine and Public Health (C.R.L.), University of Newcastle, New South Wales; Department of Neurology, John Hunter Hospital, Newcastle, New South Wales, Australia; Division of Endocrinology (P.F.M.), Diabetes and Nutrition, Department of Medicine, University of Maryland School of Medicine, Baltimore; Department of Pharmacotherapy and Translational Research and Center for Pharmacogenomics (C.W.M.), University of Florida, Gainesville; Department of Neurology (J.F.M.), Mayo Clinic, Jacksonville, FL; Klinik und Poliklinik für Neurologie (A.R.), Universitätsmedizin Rostock, Germany; Department of Neurology (S.R., R.S.), Clinical Division of Neurogeriatrics, Medical University Graz, Austria; Center for Genomic Medicine (J. Rosand), Massachusetts General Hospital, Boston; Broad Institute (J. Rosand), Cambridge, MA; Department of Neurology and Evelyn F. McKnight Brain Institute (J. Roquer, T.R., R.L.S./M.S.), Miller School of Medicine, University of Miami, FL; Institute of Cardiovascular Research (P.S.), Royal Holloway University of London (ICR2UL), UK St Peter's and Ashford Hospitals, Egham, United Kingdom; Department of Neurology (A. Slowik), Jagiellonian University Medical College, Krakow, Poland; Division of Neurocritical Care & Emergency Neurology (D.S.), Department of Neurology, Helsinki University Central Hospital, Finland; Stroke Division (V.T.), Florey Institute of Neuroscience and Mental Health, Heidelberg; Department of Neurology (V.T.), Austin Health, Heidelberg, Australia; Departments of Radiology (A.V.) and Neurology and Rehabilitation Medicine (D.W.), University of Cincinnati College of Medicine, OH; Department of Clinical Sciences Lund, Radiology (J.W.) and Neurology (A.G.L.), Lund University, Sweden; Department of Radiology, Neuroradiology, Skåne University Hospital, Malmö, Sweden; Departments of Neurology and Public Health Sciences (B.B.W.), University of Virginia, Charlottesville, VA; University of Technology Sydney (J.M.), Australia; Section of Neurology (A.G.L.), Skåne University Hospital, Lund, Sweden; Department of Laboratory Medicine (C.J.), Institute of Biomedicine, the Sahlgrenska Academy, University of Gothenburg, Sweden; and Department of Clinical Genetics and Genomics (C.J.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden.
1,
Pankaj Sharma
Pankaj Sharma, MD, PhD, FRCP
1From the J. Philip Kistler Stroke Research Center (M.B., A.K.B., M.D.S., S.H., A. Dalca, K.D., A.-K.G., M.R.E., P.M.R., M.N., R.W.R., C.W., N.S.R.), A.A. Martinos Center for Biomedical Imaging (A. Dalca, O.W.), and Henry and Allison McCance Center for Brain Health (J. Rosand), Massachusetts General Hospital, Harvard Medical School, Boston; Lille Neuroscience & Cognition (M.B., X.L., R. Lopes, G.K.), Inserm, CHU Lille, U1172 and Institut Pasteur de Lille (M.G.), CNRS, Inserm, CHU Lille, US 41 - UMS 2014 - PLBS, Lille University, France; Computer Science and Artificial Intelligence Lab (A. Dalca, C.W., P.G.), Massachusetts Institute of Technology, Cambridge; Division of Preventive Medicine (P.M.R.), Department of Medicine, Brigham and Women's Hospital, Boston, MA; Department of Medicine (O.R.B.), Division of Neurology, University of British Columbia, Vancouver, Canada; Department of Neurology (J.W.C., S.J.K.), University of Maryland School of Medicine and Veterans Affairs Maryland Health Care System, Baltimore, MD; School of Medical Sciences (A. Donatti, A. Sousa), University of Campinas (UNICAMP) and the Brazilian Institute of Neuroscience and Neurotechnology (BRAINN), Campinas, São Paulo; Departments of Neurosurgery (C.G.) and Neurology (R.Z.), Geisinger, Danville, PA; Department of Neurosurgery (C.G.), Christian Doppler Klinik, Paracelsus Medical University, Salzburg, Austria; Division of Emergency Medicine (Laura Heitsch), Washington University School of Medicine, St. Louis; Department of Neurology (Laura Heitsch, C.-L.P.), Washington University School of Medicine & Barnes-Jewish Hospital, St. Louis, MO; Department of Clinical Neuroscience (L. Holmegaard, K.J., T.M.S., T.T.), Institute of Neuroscience and Physiology, Sahlgrenska Academy at University of Gothenburg, Sweden; Department of Neurology, Sahlgrenska University Hospital, Gothenburg, Sweden; Department of Neurology (J.J.-C.), Neurovascular Research Group (NEUVAS), IMIM-Hospital del Mar (Institut Hospital del Mar d'Investigacions M`ediques), Universitat Autonoma de Barcelona, Spain; Department of Neurosciences (R. Lemmens), Experimental Neurology and Leuven Research Institute for Neuroscience and Disease (LIND), KU Leuven - University of Leuven, Belgium; Department of Neurology (R. Lemmens), Laboratory of Neurobiology, VIB Vesalius Research Center, University Hospitals Leuven, Belgium; School of Medicine and Public Health (C.R.L.), University of Newcastle, New South Wales; Department of Neurology, John Hunter Hospital, Newcastle, New South Wales, Australia; Division of Endocrinology (P.F.M.), Diabetes and Nutrition, Department of Medicine, University of Maryland School of Medicine, Baltimore; Department of Pharmacotherapy and Translational Research and Center for Pharmacogenomics (C.W.M.), University of Florida, Gainesville; Department of Neurology (J.F.M.), Mayo Clinic, Jacksonville, FL; Klinik und Poliklinik für Neurologie (A.R.), Universitätsmedizin Rostock, Germany; Department of Neurology (S.R., R.S.), Clinical Division of Neurogeriatrics, Medical University Graz, Austria; Center for Genomic Medicine (J. Rosand), Massachusetts General Hospital, Boston; Broad Institute (J. Rosand), Cambridge, MA; Department of Neurology and Evelyn F. McKnight Brain Institute (J. Roquer, T.R., R.L.S./M.S.), Miller School of Medicine, University of Miami, FL; Institute of Cardiovascular Research (P.S.), Royal Holloway University of London (ICR2UL), UK St Peter's and Ashford Hospitals, Egham, United Kingdom; Department of Neurology (A. Slowik), Jagiellonian University Medical College, Krakow, Poland; Division of Neurocritical Care & Emergency Neurology (D.S.), Department of Neurology, Helsinki University Central Hospital, Finland; Stroke Division (V.T.), Florey Institute of Neuroscience and Mental Health, Heidelberg; Department of Neurology (V.T.), Austin Health, Heidelberg, Australia; Departments of Radiology (A.V.) and Neurology and Rehabilitation Medicine (D.W.), University of Cincinnati College of Medicine, OH; Department of Clinical Sciences Lund, Radiology (J.W.) and Neurology (A.G.L.), Lund University, Sweden; Department of Radiology, Neuroradiology, Skåne University Hospital, Malmö, Sweden; Departments of Neurology and Public Health Sciences (B.B.W.), University of Virginia, Charlottesville, VA; University of Technology Sydney (J.M.), Australia; Section of Neurology (A.G.L.), Skåne University Hospital, Lund, Sweden; Department of Laboratory Medicine (C.J.), Institute of Biomedicine, the Sahlgrenska Academy, University of Gothenburg, Sweden; and Department of Clinical Genetics and Genomics (C.J.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden.
1,
Agnieszka Slowik
Agnieszka Slowik, MD, PhD
1From the J. Philip Kistler Stroke Research Center (M.B., A.K.B., M.D.S., S.H., A. Dalca, K.D., A.-K.G., M.R.E., P.M.R., M.N., R.W.R., C.W., N.S.R.), A.A. Martinos Center for Biomedical Imaging (A. Dalca, O.W.), and Henry and Allison McCance Center for Brain Health (J. Rosand), Massachusetts General Hospital, Harvard Medical School, Boston; Lille Neuroscience & Cognition (M.B., X.L., R. Lopes, G.K.), Inserm, CHU Lille, U1172 and Institut Pasteur de Lille (M.G.), CNRS, Inserm, CHU Lille, US 41 - UMS 2014 - PLBS, Lille University, France; Computer Science and Artificial Intelligence Lab (A. Dalca, C.W., P.G.), Massachusetts Institute of Technology, Cambridge; Division of Preventive Medicine (P.M.R.), Department of Medicine, Brigham and Women's Hospital, Boston, MA; Department of Medicine (O.R.B.), Division of Neurology, University of British Columbia, Vancouver, Canada; Department of Neurology (J.W.C., S.J.K.), University of Maryland School of Medicine and Veterans Affairs Maryland Health Care System, Baltimore, MD; School of Medical Sciences (A. Donatti, A. Sousa), University of Campinas (UNICAMP) and the Brazilian Institute of Neuroscience and Neurotechnology (BRAINN), Campinas, São Paulo; Departments of Neurosurgery (C.G.) and Neurology (R.Z.), Geisinger, Danville, PA; Department of Neurosurgery (C.G.), Christian Doppler Klinik, Paracelsus Medical University, Salzburg, Austria; Division of Emergency Medicine (Laura Heitsch), Washington University School of Medicine, St. Louis; Department of Neurology (Laura Heitsch, C.-L.P.), Washington University School of Medicine & Barnes-Jewish Hospital, St. Louis, MO; Department of Clinical Neuroscience (L. Holmegaard, K.J., T.M.S., T.T.), Institute of Neuroscience and Physiology, Sahlgrenska Academy at University of Gothenburg, Sweden; Department of Neurology, Sahlgrenska University Hospital, Gothenburg, Sweden; Department of Neurology (J.J.-C.), Neurovascular Research Group (NEUVAS), IMIM-Hospital del Mar (Institut Hospital del Mar d'Investigacions M`ediques), Universitat Autonoma de Barcelona, Spain; Department of Neurosciences (R. Lemmens), Experimental Neurology and Leuven Research Institute for Neuroscience and Disease (LIND), KU Leuven - University of Leuven, Belgium; Department of Neurology (R. Lemmens), Laboratory of Neurobiology, VIB Vesalius Research Center, University Hospitals Leuven, Belgium; School of Medicine and Public Health (C.R.L.), University of Newcastle, New South Wales; Department of Neurology, John Hunter Hospital, Newcastle, New South Wales, Australia; Division of Endocrinology (P.F.M.), Diabetes and Nutrition, Department of Medicine, University of Maryland School of Medicine, Baltimore; Department of Pharmacotherapy and Translational Research and Center for Pharmacogenomics (C.W.M.), University of Florida, Gainesville; Department of Neurology (J.F.M.), Mayo Clinic, Jacksonville, FL; Klinik und Poliklinik für Neurologie (A.R.), Universitätsmedizin Rostock, Germany; Department of Neurology (S.R., R.S.), Clinical Division of Neurogeriatrics, Medical University Graz, Austria; Center for Genomic Medicine (J. Rosand), Massachusetts General Hospital, Boston; Broad Institute (J. Rosand), Cambridge, MA; Department of Neurology and Evelyn F. McKnight Brain Institute (J. Roquer, T.R., R.L.S./M.S.), Miller School of Medicine, University of Miami, FL; Institute of Cardiovascular Research (P.S.), Royal Holloway University of London (ICR2UL), UK St Peter's and Ashford Hospitals, Egham, United Kingdom; Department of Neurology (A. Slowik), Jagiellonian University Medical College, Krakow, Poland; Division of Neurocritical Care & Emergency Neurology (D.S.), Department of Neurology, Helsinki University Central Hospital, Finland; Stroke Division (V.T.), Florey Institute of Neuroscience and Mental Health, Heidelberg; Department of Neurology (V.T.), Austin Health, Heidelberg, Australia; Departments of Radiology (A.V.) and Neurology and Rehabilitation Medicine (D.W.), University of Cincinnati College of Medicine, OH; Department of Clinical Sciences Lund, Radiology (J.W.) and Neurology (A.G.L.), Lund University, Sweden; Department of Radiology, Neuroradiology, Skåne University Hospital, Malmö, Sweden; Departments of Neurology and Public Health Sciences (B.B.W.), University of Virginia, Charlottesville, VA; University of Technology Sydney (J.M.), Australia; Section of Neurology (A.G.L.), Skåne University Hospital, Lund, Sweden; Department of Laboratory Medicine (C.J.), Institute of Biomedicine, the Sahlgrenska Academy, University of Gothenburg, Sweden; and Department of Clinical Genetics and Genomics (C.J.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden.
1,
Alessandro Sousa
Alessandro Sousa, MD
1From the J. Philip Kistler Stroke Research Center (M.B., A.K.B., M.D.S., S.H., A. Dalca, K.D., A.-K.G., M.R.E., P.M.R., M.N., R.W.R., C.W., N.S.R.), A.A. Martinos Center for Biomedical Imaging (A. Dalca, O.W.), and Henry and Allison McCance Center for Brain Health (J. Rosand), Massachusetts General Hospital, Harvard Medical School, Boston; Lille Neuroscience & Cognition (M.B., X.L., R. Lopes, G.K.), Inserm, CHU Lille, U1172 and Institut Pasteur de Lille (M.G.), CNRS, Inserm, CHU Lille, US 41 - UMS 2014 - PLBS, Lille University, France; Computer Science and Artificial Intelligence Lab (A. Dalca, C.W., P.G.), Massachusetts Institute of Technology, Cambridge; Division of Preventive Medicine (P.M.R.), Department of Medicine, Brigham and Women's Hospital, Boston, MA; Department of Medicine (O.R.B.), Division of Neurology, University of British Columbia, Vancouver, Canada; Department of Neurology (J.W.C., S.J.K.), University of Maryland School of Medicine and Veterans Affairs Maryland Health Care System, Baltimore, MD; School of Medical Sciences (A. Donatti, A. Sousa), University of Campinas (UNICAMP) and the Brazilian Institute of Neuroscience and Neurotechnology (BRAINN), Campinas, São Paulo; Departments of Neurosurgery (C.G.) and Neurology (R.Z.), Geisinger, Danville, PA; Department of Neurosurgery (C.G.), Christian Doppler Klinik, Paracelsus Medical University, Salzburg, Austria; Division of Emergency Medicine (Laura Heitsch), Washington University School of Medicine, St. Louis; Department of Neurology (Laura Heitsch, C.-L.P.), Washington University School of Medicine & Barnes-Jewish Hospital, St. Louis, MO; Department of Clinical Neuroscience (L. Holmegaard, K.J., T.M.S., T.T.), Institute of Neuroscience and Physiology, Sahlgrenska Academy at University of Gothenburg, Sweden; Department of Neurology, Sahlgrenska University Hospital, Gothenburg, Sweden; Department of Neurology (J.J.-C.), Neurovascular Research Group (NEUVAS), IMIM-Hospital del Mar (Institut Hospital del Mar d'Investigacions M`ediques), Universitat Autonoma de Barcelona, Spain; Department of Neurosciences (R. Lemmens), Experimental Neurology and Leuven Research Institute for Neuroscience and Disease (LIND), KU Leuven - University of Leuven, Belgium; Department of Neurology (R. Lemmens), Laboratory of Neurobiology, VIB Vesalius Research Center, University Hospitals Leuven, Belgium; School of Medicine and Public Health (C.R.L.), University of Newcastle, New South Wales; Department of Neurology, John Hunter Hospital, Newcastle, New South Wales, Australia; Division of Endocrinology (P.F.M.), Diabetes and Nutrition, Department of Medicine, University of Maryland School of Medicine, Baltimore; Department of Pharmacotherapy and Translational Research and Center for Pharmacogenomics (C.W.M.), University of Florida, Gainesville; Department of Neurology (J.F.M.), Mayo Clinic, Jacksonville, FL; Klinik und Poliklinik für Neurologie (A.R.), Universitätsmedizin Rostock, Germany; Department of Neurology (S.R., R.S.), Clinical Division of Neurogeriatrics, Medical University Graz, Austria; Center for Genomic Medicine (J. Rosand), Massachusetts General Hospital, Boston; Broad Institute (J. Rosand), Cambridge, MA; Department of Neurology and Evelyn F. McKnight Brain Institute (J. Roquer, T.R., R.L.S./M.S.), Miller School of Medicine, University of Miami, FL; Institute of Cardiovascular Research (P.S.), Royal Holloway University of London (ICR2UL), UK St Peter's and Ashford Hospitals, Egham, United Kingdom; Department of Neurology (A. Slowik), Jagiellonian University Medical College, Krakow, Poland; Division of Neurocritical Care & Emergency Neurology (D.S.), Department of Neurology, Helsinki University Central Hospital, Finland; Stroke Division (V.T.), Florey Institute of Neuroscience and Mental Health, Heidelberg; Department of Neurology (V.T.), Austin Health, Heidelberg, Australia; Departments of Radiology (A.V.) and Neurology and Rehabilitation Medicine (D.W.), University of Cincinnati College of Medicine, OH; Department of Clinical Sciences Lund, Radiology (J.W.) and Neurology (A.G.L.), Lund University, Sweden; Department of Radiology, Neuroradiology, Skåne University Hospital, Malmö, Sweden; Departments of Neurology and Public Health Sciences (B.B.W.), University of Virginia, Charlottesville, VA; University of Technology Sydney (J.M.), Australia; Section of Neurology (A.G.L.), Skåne University Hospital, Lund, Sweden; Department of Laboratory Medicine (C.J.), Institute of Biomedicine, the Sahlgrenska Academy, University of Gothenburg, Sweden; and Department of Clinical Genetics and Genomics (C.J.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden.
1,
Tara M Stanne
Tara M Stanne, MD
1From the J. Philip Kistler Stroke Research Center (M.B., A.K.B., M.D.S., S.H., A. Dalca, K.D., A.-K.G., M.R.E., P.M.R., M.N., R.W.R., C.W., N.S.R.), A.A. Martinos Center for Biomedical Imaging (A. Dalca, O.W.), and Henry and Allison McCance Center for Brain Health (J. Rosand), Massachusetts General Hospital, Harvard Medical School, Boston; Lille Neuroscience & Cognition (M.B., X.L., R. Lopes, G.K.), Inserm, CHU Lille, U1172 and Institut Pasteur de Lille (M.G.), CNRS, Inserm, CHU Lille, US 41 - UMS 2014 - PLBS, Lille University, France; Computer Science and Artificial Intelligence Lab (A. Dalca, C.W., P.G.), Massachusetts Institute of Technology, Cambridge; Division of Preventive Medicine (P.M.R.), Department of Medicine, Brigham and Women's Hospital, Boston, MA; Department of Medicine (O.R.B.), Division of Neurology, University of British Columbia, Vancouver, Canada; Department of Neurology (J.W.C., S.J.K.), University of Maryland School of Medicine and Veterans Affairs Maryland Health Care System, Baltimore, MD; School of Medical Sciences (A. Donatti, A. Sousa), University of Campinas (UNICAMP) and the Brazilian Institute of Neuroscience and Neurotechnology (BRAINN), Campinas, São Paulo; Departments of Neurosurgery (C.G.) and Neurology (R.Z.), Geisinger, Danville, PA; Department of Neurosurgery (C.G.), Christian Doppler Klinik, Paracelsus Medical University, Salzburg, Austria; Division of Emergency Medicine (Laura Heitsch), Washington University School of Medicine, St. Louis; Department of Neurology (Laura Heitsch, C.-L.P.), Washington University School of Medicine & Barnes-Jewish Hospital, St. Louis, MO; Department of Clinical Neuroscience (L. Holmegaard, K.J., T.M.S., T.T.), Institute of Neuroscience and Physiology, Sahlgrenska Academy at University of Gothenburg, Sweden; Department of Neurology, Sahlgrenska University Hospital, Gothenburg, Sweden; Department of Neurology (J.J.-C.), Neurovascular Research Group (NEUVAS), IMIM-Hospital del Mar (Institut Hospital del Mar d'Investigacions M`ediques), Universitat Autonoma de Barcelona, Spain; Department of Neurosciences (R. Lemmens), Experimental Neurology and Leuven Research Institute for Neuroscience and Disease (LIND), KU Leuven - University of Leuven, Belgium; Department of Neurology (R. Lemmens), Laboratory of Neurobiology, VIB Vesalius Research Center, University Hospitals Leuven, Belgium; School of Medicine and Public Health (C.R.L.), University of Newcastle, New South Wales; Department of Neurology, John Hunter Hospital, Newcastle, New South Wales, Australia; Division of Endocrinology (P.F.M.), Diabetes and Nutrition, Department of Medicine, University of Maryland School of Medicine, Baltimore; Department of Pharmacotherapy and Translational Research and Center for Pharmacogenomics (C.W.M.), University of Florida, Gainesville; Department of Neurology (J.F.M.), Mayo Clinic, Jacksonville, FL; Klinik und Poliklinik für Neurologie (A.R.), Universitätsmedizin Rostock, Germany; Department of Neurology (S.R., R.S.), Clinical Division of Neurogeriatrics, Medical University Graz, Austria; Center for Genomic Medicine (J. Rosand), Massachusetts General Hospital, Boston; Broad Institute (J. Rosand), Cambridge, MA; Department of Neurology and Evelyn F. McKnight Brain Institute (J. Roquer, T.R., R.L.S./M.S.), Miller School of Medicine, University of Miami, FL; Institute of Cardiovascular Research (P.S.), Royal Holloway University of London (ICR2UL), UK St Peter's and Ashford Hospitals, Egham, United Kingdom; Department of Neurology (A. Slowik), Jagiellonian University Medical College, Krakow, Poland; Division of Neurocritical Care & Emergency Neurology (D.S.), Department of Neurology, Helsinki University Central Hospital, Finland; Stroke Division (V.T.), Florey Institute of Neuroscience and Mental Health, Heidelberg; Department of Neurology (V.T.), Austin Health, Heidelberg, Australia; Departments of Radiology (A.V.) and Neurology and Rehabilitation Medicine (D.W.), University of Cincinnati College of Medicine, OH; Department of Clinical Sciences Lund, Radiology (J.W.) and Neurology (A.G.L.), Lund University, Sweden; Department of Radiology, Neuroradiology, Skåne University Hospital, Malmö, Sweden; Departments of Neurology and Public Health Sciences (B.B.W.), University of Virginia, Charlottesville, VA; University of Technology Sydney (J.M.), Australia; Section of Neurology (A.G.L.), Skåne University Hospital, Lund, Sweden; Department of Laboratory Medicine (C.J.), Institute of Biomedicine, the Sahlgrenska Academy, University of Gothenburg, Sweden; and Department of Clinical Genetics and Genomics (C.J.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden.
1,
Daniel Strbian
Daniel Strbian, MD
1From the J. Philip Kistler Stroke Research Center (M.B., A.K.B., M.D.S., S.H., A. Dalca, K.D., A.-K.G., M.R.E., P.M.R., M.N., R.W.R., C.W., N.S.R.), A.A. Martinos Center for Biomedical Imaging (A. Dalca, O.W.), and Henry and Allison McCance Center for Brain Health (J. Rosand), Massachusetts General Hospital, Harvard Medical School, Boston; Lille Neuroscience & Cognition (M.B., X.L., R. Lopes, G.K.), Inserm, CHU Lille, U1172 and Institut Pasteur de Lille (M.G.), CNRS, Inserm, CHU Lille, US 41 - UMS 2014 - PLBS, Lille University, France; Computer Science and Artificial Intelligence Lab (A. Dalca, C.W., P.G.), Massachusetts Institute of Technology, Cambridge; Division of Preventive Medicine (P.M.R.), Department of Medicine, Brigham and Women's Hospital, Boston, MA; Department of Medicine (O.R.B.), Division of Neurology, University of British Columbia, Vancouver, Canada; Department of Neurology (J.W.C., S.J.K.), University of Maryland School of Medicine and Veterans Affairs Maryland Health Care System, Baltimore, MD; School of Medical Sciences (A. Donatti, A. Sousa), University of Campinas (UNICAMP) and the Brazilian Institute of Neuroscience and Neurotechnology (BRAINN), Campinas, São Paulo; Departments of Neurosurgery (C.G.) and Neurology (R.Z.), Geisinger, Danville, PA; Department of Neurosurgery (C.G.), Christian Doppler Klinik, Paracelsus Medical University, Salzburg, Austria; Division of Emergency Medicine (Laura Heitsch), Washington University School of Medicine, St. Louis; Department of Neurology (Laura Heitsch, C.-L.P.), Washington University School of Medicine & Barnes-Jewish Hospital, St. Louis, MO; Department of Clinical Neuroscience (L. Holmegaard, K.J., T.M.S., T.T.), Institute of Neuroscience and Physiology, Sahlgrenska Academy at University of Gothenburg, Sweden; Department of Neurology, Sahlgrenska University Hospital, Gothenburg, Sweden; Department of Neurology (J.J.-C.), Neurovascular Research Group (NEUVAS), IMIM-Hospital del Mar (Institut Hospital del Mar d'Investigacions M`ediques), Universitat Autonoma de Barcelona, Spain; Department of Neurosciences (R. Lemmens), Experimental Neurology and Leuven Research Institute for Neuroscience and Disease (LIND), KU Leuven - University of Leuven, Belgium; Department of Neurology (R. Lemmens), Laboratory of Neurobiology, VIB Vesalius Research Center, University Hospitals Leuven, Belgium; School of Medicine and Public Health (C.R.L.), University of Newcastle, New South Wales; Department of Neurology, John Hunter Hospital, Newcastle, New South Wales, Australia; Division of Endocrinology (P.F.M.), Diabetes and Nutrition, Department of Medicine, University of Maryland School of Medicine, Baltimore; Department of Pharmacotherapy and Translational Research and Center for Pharmacogenomics (C.W.M.), University of Florida, Gainesville; Department of Neurology (J.F.M.), Mayo Clinic, Jacksonville, FL; Klinik und Poliklinik für Neurologie (A.R.), Universitätsmedizin Rostock, Germany; Department of Neurology (S.R., R.S.), Clinical Division of Neurogeriatrics, Medical University Graz, Austria; Center for Genomic Medicine (J. Rosand), Massachusetts General Hospital, Boston; Broad Institute (J. Rosand), Cambridge, MA; Department of Neurology and Evelyn F. McKnight Brain Institute (J. Roquer, T.R., R.L.S./M.S.), Miller School of Medicine, University of Miami, FL; Institute of Cardiovascular Research (P.S.), Royal Holloway University of London (ICR2UL), UK St Peter's and Ashford Hospitals, Egham, United Kingdom; Department of Neurology (A. Slowik), Jagiellonian University Medical College, Krakow, Poland; Division of Neurocritical Care & Emergency Neurology (D.S.), Department of Neurology, Helsinki University Central Hospital, Finland; Stroke Division (V.T.), Florey Institute of Neuroscience and Mental Health, Heidelberg; Department of Neurology (V.T.), Austin Health, Heidelberg, Australia; Departments of Radiology (A.V.) and Neurology and Rehabilitation Medicine (D.W.), University of Cincinnati College of Medicine, OH; Department of Clinical Sciences Lund, Radiology (J.W.) and Neurology (A.G.L.), Lund University, Sweden; Department of Radiology, Neuroradiology, Skåne University Hospital, Malmö, Sweden; Departments of Neurology and Public Health Sciences (B.B.W.), University of Virginia, Charlottesville, VA; University of Technology Sydney (J.M.), Australia; Section of Neurology (A.G.L.), Skåne University Hospital, Lund, Sweden; Department of Laboratory Medicine (C.J.), Institute of Biomedicine, the Sahlgrenska Academy, University of Gothenburg, Sweden; and Department of Clinical Genetics and Genomics (C.J.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden.
1,
Turgut Tatlisumak
Turgut Tatlisumak, MD
1From the J. Philip Kistler Stroke Research Center (M.B., A.K.B., M.D.S., S.H., A. Dalca, K.D., A.-K.G., M.R.E., P.M.R., M.N., R.W.R., C.W., N.S.R.), A.A. Martinos Center for Biomedical Imaging (A. Dalca, O.W.), and Henry and Allison McCance Center for Brain Health (J. Rosand), Massachusetts General Hospital, Harvard Medical School, Boston; Lille Neuroscience & Cognition (M.B., X.L., R. Lopes, G.K.), Inserm, CHU Lille, U1172 and Institut Pasteur de Lille (M.G.), CNRS, Inserm, CHU Lille, US 41 - UMS 2014 - PLBS, Lille University, France; Computer Science and Artificial Intelligence Lab (A. Dalca, C.W., P.G.), Massachusetts Institute of Technology, Cambridge; Division of Preventive Medicine (P.M.R.), Department of Medicine, Brigham and Women's Hospital, Boston, MA; Department of Medicine (O.R.B.), Division of Neurology, University of British Columbia, Vancouver, Canada; Department of Neurology (J.W.C., S.J.K.), University of Maryland School of Medicine and Veterans Affairs Maryland Health Care System, Baltimore, MD; School of Medical Sciences (A. Donatti, A. Sousa), University of Campinas (UNICAMP) and the Brazilian Institute of Neuroscience and Neurotechnology (BRAINN), Campinas, São Paulo; Departments of Neurosurgery (C.G.) and Neurology (R.Z.), Geisinger, Danville, PA; Department of Neurosurgery (C.G.), Christian Doppler Klinik, Paracelsus Medical University, Salzburg, Austria; Division of Emergency Medicine (Laura Heitsch), Washington University School of Medicine, St. Louis; Department of Neurology (Laura Heitsch, C.-L.P.), Washington University School of Medicine & Barnes-Jewish Hospital, St. Louis, MO; Department of Clinical Neuroscience (L. Holmegaard, K.J., T.M.S., T.T.), Institute of Neuroscience and Physiology, Sahlgrenska Academy at University of Gothenburg, Sweden; Department of Neurology, Sahlgrenska University Hospital, Gothenburg, Sweden; Department of Neurology (J.J.-C.), Neurovascular Research Group (NEUVAS), IMIM-Hospital del Mar (Institut Hospital del Mar d'Investigacions M`ediques), Universitat Autonoma de Barcelona, Spain; Department of Neurosciences (R. Lemmens), Experimental Neurology and Leuven Research Institute for Neuroscience and Disease (LIND), KU Leuven - University of Leuven, Belgium; Department of Neurology (R. Lemmens), Laboratory of Neurobiology, VIB Vesalius Research Center, University Hospitals Leuven, Belgium; School of Medicine and Public Health (C.R.L.), University of Newcastle, New South Wales; Department of Neurology, John Hunter Hospital, Newcastle, New South Wales, Australia; Division of Endocrinology (P.F.M.), Diabetes and Nutrition, Department of Medicine, University of Maryland School of Medicine, Baltimore; Department of Pharmacotherapy and Translational Research and Center for Pharmacogenomics (C.W.M.), University of Florida, Gainesville; Department of Neurology (J.F.M.), Mayo Clinic, Jacksonville, FL; Klinik und Poliklinik für Neurologie (A.R.), Universitätsmedizin Rostock, Germany; Department of Neurology (S.R., R.S.), Clinical Division of Neurogeriatrics, Medical University Graz, Austria; Center for Genomic Medicine (J. Rosand), Massachusetts General Hospital, Boston; Broad Institute (J. Rosand), Cambridge, MA; Department of Neurology and Evelyn F. McKnight Brain Institute (J. Roquer, T.R., R.L.S./M.S.), Miller School of Medicine, University of Miami, FL; Institute of Cardiovascular Research (P.S.), Royal Holloway University of London (ICR2UL), UK St Peter's and Ashford Hospitals, Egham, United Kingdom; Department of Neurology (A. Slowik), Jagiellonian University Medical College, Krakow, Poland; Division of Neurocritical Care & Emergency Neurology (D.S.), Department of Neurology, Helsinki University Central Hospital, Finland; Stroke Division (V.T.), Florey Institute of Neuroscience and Mental Health, Heidelberg; Department of Neurology (V.T.), Austin Health, Heidelberg, Australia; Departments of Radiology (A.V.) and Neurology and Rehabilitation Medicine (D.W.), University of Cincinnati College of Medicine, OH; Department of Clinical Sciences Lund, Radiology (J.W.) and Neurology (A.G.L.), Lund University, Sweden; Department of Radiology, Neuroradiology, Skåne University Hospital, Malmö, Sweden; Departments of Neurology and Public Health Sciences (B.B.W.), University of Virginia, Charlottesville, VA; University of Technology Sydney (J.M.), Australia; Section of Neurology (A.G.L.), Skåne University Hospital, Lund, Sweden; Department of Laboratory Medicine (C.J.), Institute of Biomedicine, the Sahlgrenska Academy, University of Gothenburg, Sweden; and Department of Clinical Genetics and Genomics (C.J.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden.
1,
Vincent Thijs
Vincent Thijs, PhD
1From the J. Philip Kistler Stroke Research Center (M.B., A.K.B., M.D.S., S.H., A. Dalca, K.D., A.-K.G., M.R.E., P.M.R., M.N., R.W.R., C.W., N.S.R.), A.A. Martinos Center for Biomedical Imaging (A. Dalca, O.W.), and Henry and Allison McCance Center for Brain Health (J. Rosand), Massachusetts General Hospital, Harvard Medical School, Boston; Lille Neuroscience & Cognition (M.B., X.L., R. Lopes, G.K.), Inserm, CHU Lille, U1172 and Institut Pasteur de Lille (M.G.), CNRS, Inserm, CHU Lille, US 41 - UMS 2014 - PLBS, Lille University, France; Computer Science and Artificial Intelligence Lab (A. Dalca, C.W., P.G.), Massachusetts Institute of Technology, Cambridge; Division of Preventive Medicine (P.M.R.), Department of Medicine, Brigham and Women's Hospital, Boston, MA; Department of Medicine (O.R.B.), Division of Neurology, University of British Columbia, Vancouver, Canada; Department of Neurology (J.W.C., S.J.K.), University of Maryland School of Medicine and Veterans Affairs Maryland Health Care System, Baltimore, MD; School of Medical Sciences (A. Donatti, A. Sousa), University of Campinas (UNICAMP) and the Brazilian Institute of Neuroscience and Neurotechnology (BRAINN), Campinas, São Paulo; Departments of Neurosurgery (C.G.) and Neurology (R.Z.), Geisinger, Danville, PA; Department of Neurosurgery (C.G.), Christian Doppler Klinik, Paracelsus Medical University, Salzburg, Austria; Division of Emergency Medicine (Laura Heitsch), Washington University School of Medicine, St. Louis; Department of Neurology (Laura Heitsch, C.-L.P.), Washington University School of Medicine & Barnes-Jewish Hospital, St. Louis, MO; Department of Clinical Neuroscience (L. Holmegaard, K.J., T.M.S., T.T.), Institute of Neuroscience and Physiology, Sahlgrenska Academy at University of Gothenburg, Sweden; Department of Neurology, Sahlgrenska University Hospital, Gothenburg, Sweden; Department of Neurology (J.J.-C.), Neurovascular Research Group (NEUVAS), IMIM-Hospital del Mar (Institut Hospital del Mar d'Investigacions M`ediques), Universitat Autonoma de Barcelona, Spain; Department of Neurosciences (R. Lemmens), Experimental Neurology and Leuven Research Institute for Neuroscience and Disease (LIND), KU Leuven - University of Leuven, Belgium; Department of Neurology (R. Lemmens), Laboratory of Neurobiology, VIB Vesalius Research Center, University Hospitals Leuven, Belgium; School of Medicine and Public Health (C.R.L.), University of Newcastle, New South Wales; Department of Neurology, John Hunter Hospital, Newcastle, New South Wales, Australia; Division of Endocrinology (P.F.M.), Diabetes and Nutrition, Department of Medicine, University of Maryland School of Medicine, Baltimore; Department of Pharmacotherapy and Translational Research and Center for Pharmacogenomics (C.W.M.), University of Florida, Gainesville; Department of Neurology (J.F.M.), Mayo Clinic, Jacksonville, FL; Klinik und Poliklinik für Neurologie (A.R.), Universitätsmedizin Rostock, Germany; Department of Neurology (S.R., R.S.), Clinical Division of Neurogeriatrics, Medical University Graz, Austria; Center for Genomic Medicine (J. Rosand), Massachusetts General Hospital, Boston; Broad Institute (J. Rosand), Cambridge, MA; Department of Neurology and Evelyn F. McKnight Brain Institute (J. Roquer, T.R., R.L.S./M.S.), Miller School of Medicine, University of Miami, FL; Institute of Cardiovascular Research (P.S.), Royal Holloway University of London (ICR2UL), UK St Peter's and Ashford Hospitals, Egham, United Kingdom; Department of Neurology (A. Slowik), Jagiellonian University Medical College, Krakow, Poland; Division of Neurocritical Care & Emergency Neurology (D.S.), Department of Neurology, Helsinki University Central Hospital, Finland; Stroke Division (V.T.), Florey Institute of Neuroscience and Mental Health, Heidelberg; Department of Neurology (V.T.), Austin Health, Heidelberg, Australia; Departments of Radiology (A.V.) and Neurology and Rehabilitation Medicine (D.W.), University of Cincinnati College of Medicine, OH; Department of Clinical Sciences Lund, Radiology (J.W.) and Neurology (A.G.L.), Lund University, Sweden; Department of Radiology, Neuroradiology, Skåne University Hospital, Malmö, Sweden; Departments of Neurology and Public Health Sciences (B.B.W.), University of Virginia, Charlottesville, VA; University of Technology Sydney (J.M.), Australia; Section of Neurology (A.G.L.), Skåne University Hospital, Lund, Sweden; Department of Laboratory Medicine (C.J.), Institute of Biomedicine, the Sahlgrenska Academy, University of Gothenburg, Sweden; and Department of Clinical Genetics and Genomics (C.J.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden.
1,
Achala Vagal
Achala Vagal, MD
1From the J. Philip Kistler Stroke Research Center (M.B., A.K.B., M.D.S., S.H., A. Dalca, K.D., A.-K.G., M.R.E., P.M.R., M.N., R.W.R., C.W., N.S.R.), A.A. Martinos Center for Biomedical Imaging (A. Dalca, O.W.), and Henry and Allison McCance Center for Brain Health (J. Rosand), Massachusetts General Hospital, Harvard Medical School, Boston; Lille Neuroscience & Cognition (M.B., X.L., R. Lopes, G.K.), Inserm, CHU Lille, U1172 and Institut Pasteur de Lille (M.G.), CNRS, Inserm, CHU Lille, US 41 - UMS 2014 - PLBS, Lille University, France; Computer Science and Artificial Intelligence Lab (A. Dalca, C.W., P.G.), Massachusetts Institute of Technology, Cambridge; Division of Preventive Medicine (P.M.R.), Department of Medicine, Brigham and Women's Hospital, Boston, MA; Department of Medicine (O.R.B.), Division of Neurology, University of British Columbia, Vancouver, Canada; Department of Neurology (J.W.C., S.J.K.), University of Maryland School of Medicine and Veterans Affairs Maryland Health Care System, Baltimore, MD; School of Medical Sciences (A. Donatti, A. Sousa), University of Campinas (UNICAMP) and the Brazilian Institute of Neuroscience and Neurotechnology (BRAINN), Campinas, São Paulo; Departments of Neurosurgery (C.G.) and Neurology (R.Z.), Geisinger, Danville, PA; Department of Neurosurgery (C.G.), Christian Doppler Klinik, Paracelsus Medical University, Salzburg, Austria; Division of Emergency Medicine (Laura Heitsch), Washington University School of Medicine, St. Louis; Department of Neurology (Laura Heitsch, C.-L.P.), Washington University School of Medicine & Barnes-Jewish Hospital, St. Louis, MO; Department of Clinical Neuroscience (L. Holmegaard, K.J., T.M.S., T.T.), Institute of Neuroscience and Physiology, Sahlgrenska Academy at University of Gothenburg, Sweden; Department of Neurology, Sahlgrenska University Hospital, Gothenburg, Sweden; Department of Neurology (J.J.-C.), Neurovascular Research Group (NEUVAS), IMIM-Hospital del Mar (Institut Hospital del Mar d'Investigacions M`ediques), Universitat Autonoma de Barcelona, Spain; Department of Neurosciences (R. Lemmens), Experimental Neurology and Leuven Research Institute for Neuroscience and Disease (LIND), KU Leuven - University of Leuven, Belgium; Department of Neurology (R. Lemmens), Laboratory of Neurobiology, VIB Vesalius Research Center, University Hospitals Leuven, Belgium; School of Medicine and Public Health (C.R.L.), University of Newcastle, New South Wales; Department of Neurology, John Hunter Hospital, Newcastle, New South Wales, Australia; Division of Endocrinology (P.F.M.), Diabetes and Nutrition, Department of Medicine, University of Maryland School of Medicine, Baltimore; Department of Pharmacotherapy and Translational Research and Center for Pharmacogenomics (C.W.M.), University of Florida, Gainesville; Department of Neurology (J.F.M.), Mayo Clinic, Jacksonville, FL; Klinik und Poliklinik für Neurologie (A.R.), Universitätsmedizin Rostock, Germany; Department of Neurology (S.R., R.S.), Clinical Division of Neurogeriatrics, Medical University Graz, Austria; Center for Genomic Medicine (J. Rosand), Massachusetts General Hospital, Boston; Broad Institute (J. Rosand), Cambridge, MA; Department of Neurology and Evelyn F. McKnight Brain Institute (J. Roquer, T.R., R.L.S./M.S.), Miller School of Medicine, University of Miami, FL; Institute of Cardiovascular Research (P.S.), Royal Holloway University of London (ICR2UL), UK St Peter's and Ashford Hospitals, Egham, United Kingdom; Department of Neurology (A. Slowik), Jagiellonian University Medical College, Krakow, Poland; Division of Neurocritical Care & Emergency Neurology (D.S.), Department of Neurology, Helsinki University Central Hospital, Finland; Stroke Division (V.T.), Florey Institute of Neuroscience and Mental Health, Heidelberg; Department of Neurology (V.T.), Austin Health, Heidelberg, Australia; Departments of Radiology (A.V.) and Neurology and Rehabilitation Medicine (D.W.), University of Cincinnati College of Medicine, OH; Department of Clinical Sciences Lund, Radiology (J.W.) and Neurology (A.G.L.), Lund University, Sweden; Department of Radiology, Neuroradiology, Skåne University Hospital, Malmö, Sweden; Departments of Neurology and Public Health Sciences (B.B.W.), University of Virginia, Charlottesville, VA; University of Technology Sydney (J.M.), Australia; Section of Neurology (A.G.L.), Skåne University Hospital, Lund, Sweden; Department of Laboratory Medicine (C.J.), Institute of Biomedicine, the Sahlgrenska Academy, University of Gothenburg, Sweden; and Department of Clinical Genetics and Genomics (C.J.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden.
1,
Johan Wasselius
Johan Wasselius, MD, PhD
1From the J. Philip Kistler Stroke Research Center (M.B., A.K.B., M.D.S., S.H., A. Dalca, K.D., A.-K.G., M.R.E., P.M.R., M.N., R.W.R., C.W., N.S.R.), A.A. Martinos Center for Biomedical Imaging (A. Dalca, O.W.), and Henry and Allison McCance Center for Brain Health (J. Rosand), Massachusetts General Hospital, Harvard Medical School, Boston; Lille Neuroscience & Cognition (M.B., X.L., R. Lopes, G.K.), Inserm, CHU Lille, U1172 and Institut Pasteur de Lille (M.G.), CNRS, Inserm, CHU Lille, US 41 - UMS 2014 - PLBS, Lille University, France; Computer Science and Artificial Intelligence Lab (A. Dalca, C.W., P.G.), Massachusetts Institute of Technology, Cambridge; Division of Preventive Medicine (P.M.R.), Department of Medicine, Brigham and Women's Hospital, Boston, MA; Department of Medicine (O.R.B.), Division of Neurology, University of British Columbia, Vancouver, Canada; Department of Neurology (J.W.C., S.J.K.), University of Maryland School of Medicine and Veterans Affairs Maryland Health Care System, Baltimore, MD; School of Medical Sciences (A. Donatti, A. Sousa), University of Campinas (UNICAMP) and the Brazilian Institute of Neuroscience and Neurotechnology (BRAINN), Campinas, São Paulo; Departments of Neurosurgery (C.G.) and Neurology (R.Z.), Geisinger, Danville, PA; Department of Neurosurgery (C.G.), Christian Doppler Klinik, Paracelsus Medical University, Salzburg, Austria; Division of Emergency Medicine (Laura Heitsch), Washington University School of Medicine, St. Louis; Department of Neurology (Laura Heitsch, C.-L.P.), Washington University School of Medicine & Barnes-Jewish Hospital, St. Louis, MO; Department of Clinical Neuroscience (L. Holmegaard, K.J., T.M.S., T.T.), Institute of Neuroscience and Physiology, Sahlgrenska Academy at University of Gothenburg, Sweden; Department of Neurology, Sahlgrenska University Hospital, Gothenburg, Sweden; Department of Neurology (J.J.-C.), Neurovascular Research Group (NEUVAS), IMIM-Hospital del Mar (Institut Hospital del Mar d'Investigacions M`ediques), Universitat Autonoma de Barcelona, Spain; Department of Neurosciences (R. Lemmens), Experimental Neurology and Leuven Research Institute for Neuroscience and Disease (LIND), KU Leuven - University of Leuven, Belgium; Department of Neurology (R. Lemmens), Laboratory of Neurobiology, VIB Vesalius Research Center, University Hospitals Leuven, Belgium; School of Medicine and Public Health (C.R.L.), University of Newcastle, New South Wales; Department of Neurology, John Hunter Hospital, Newcastle, New South Wales, Australia; Division of Endocrinology (P.F.M.), Diabetes and Nutrition, Department of Medicine, University of Maryland School of Medicine, Baltimore; Department of Pharmacotherapy and Translational Research and Center for Pharmacogenomics (C.W.M.), University of Florida, Gainesville; Department of Neurology (J.F.M.), Mayo Clinic, Jacksonville, FL; Klinik und Poliklinik für Neurologie (A.R.), Universitätsmedizin Rostock, Germany; Department of Neurology (S.R., R.S.), Clinical Division of Neurogeriatrics, Medical University Graz, Austria; Center for Genomic Medicine (J. Rosand), Massachusetts General Hospital, Boston; Broad Institute (J. Rosand), Cambridge, MA; Department of Neurology and Evelyn F. McKnight Brain Institute (J. Roquer, T.R., R.L.S./M.S.), Miller School of Medicine, University of Miami, FL; Institute of Cardiovascular Research (P.S.), Royal Holloway University of London (ICR2UL), UK St Peter's and Ashford Hospitals, Egham, United Kingdom; Department of Neurology (A. Slowik), Jagiellonian University Medical College, Krakow, Poland; Division of Neurocritical Care & Emergency Neurology (D.S.), Department of Neurology, Helsinki University Central Hospital, Finland; Stroke Division (V.T.), Florey Institute of Neuroscience and Mental Health, Heidelberg; Department of Neurology (V.T.), Austin Health, Heidelberg, Australia; Departments of Radiology (A.V.) and Neurology and Rehabilitation Medicine (D.W.), University of Cincinnati College of Medicine, OH; Department of Clinical Sciences Lund, Radiology (J.W.) and Neurology (A.G.L.), Lund University, Sweden; Department of Radiology, Neuroradiology, Skåne University Hospital, Malmö, Sweden; Departments of Neurology and Public Health Sciences (B.B.W.), University of Virginia, Charlottesville, VA; University of Technology Sydney (J.M.), Australia; Section of Neurology (A.G.L.), Skåne University Hospital, Lund, Sweden; Department of Laboratory Medicine (C.J.), Institute of Biomedicine, the Sahlgrenska Academy, University of Gothenburg, Sweden; and Department of Clinical Genetics and Genomics (C.J.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden.
1,
Daniel Woo
Daniel Woo, MD
1From the J. Philip Kistler Stroke Research Center (M.B., A.K.B., M.D.S., S.H., A. Dalca, K.D., A.-K.G., M.R.E., P.M.R., M.N., R.W.R., C.W., N.S.R.), A.A. Martinos Center for Biomedical Imaging (A. Dalca, O.W.), and Henry and Allison McCance Center for Brain Health (J. Rosand), Massachusetts General Hospital, Harvard Medical School, Boston; Lille Neuroscience & Cognition (M.B., X.L., R. Lopes, G.K.), Inserm, CHU Lille, U1172 and Institut Pasteur de Lille (M.G.), CNRS, Inserm, CHU Lille, US 41 - UMS 2014 - PLBS, Lille University, France; Computer Science and Artificial Intelligence Lab (A. Dalca, C.W., P.G.), Massachusetts Institute of Technology, Cambridge; Division of Preventive Medicine (P.M.R.), Department of Medicine, Brigham and Women's Hospital, Boston, MA; Department of Medicine (O.R.B.), Division of Neurology, University of British Columbia, Vancouver, Canada; Department of Neurology (J.W.C., S.J.K.), University of Maryland School of Medicine and Veterans Affairs Maryland Health Care System, Baltimore, MD; School of Medical Sciences (A. Donatti, A. Sousa), University of Campinas (UNICAMP) and the Brazilian Institute of Neuroscience and Neurotechnology (BRAINN), Campinas, São Paulo; Departments of Neurosurgery (C.G.) and Neurology (R.Z.), Geisinger, Danville, PA; Department of Neurosurgery (C.G.), Christian Doppler Klinik, Paracelsus Medical University, Salzburg, Austria; Division of Emergency Medicine (Laura Heitsch), Washington University School of Medicine, St. Louis; Department of Neurology (Laura Heitsch, C.-L.P.), Washington University School of Medicine & Barnes-Jewish Hospital, St. Louis, MO; Department of Clinical Neuroscience (L. Holmegaard, K.J., T.M.S., T.T.), Institute of Neuroscience and Physiology, Sahlgrenska Academy at University of Gothenburg, Sweden; Department of Neurology, Sahlgrenska University Hospital, Gothenburg, Sweden; Department of Neurology (J.J.-C.), Neurovascular Research Group (NEUVAS), IMIM-Hospital del Mar (Institut Hospital del Mar d'Investigacions M`ediques), Universitat Autonoma de Barcelona, Spain; Department of Neurosciences (R. Lemmens), Experimental Neurology and Leuven Research Institute for Neuroscience and Disease (LIND), KU Leuven - University of Leuven, Belgium; Department of Neurology (R. Lemmens), Laboratory of Neurobiology, VIB Vesalius Research Center, University Hospitals Leuven, Belgium; School of Medicine and Public Health (C.R.L.), University of Newcastle, New South Wales; Department of Neurology, John Hunter Hospital, Newcastle, New South Wales, Australia; Division of Endocrinology (P.F.M.), Diabetes and Nutrition, Department of Medicine, University of Maryland School of Medicine, Baltimore; Department of Pharmacotherapy and Translational Research and Center for Pharmacogenomics (C.W.M.), University of Florida, Gainesville; Department of Neurology (J.F.M.), Mayo Clinic, Jacksonville, FL; Klinik und Poliklinik für Neurologie (A.R.), Universitätsmedizin Rostock, Germany; Department of Neurology (S.R., R.S.), Clinical Division of Neurogeriatrics, Medical University Graz, Austria; Center for Genomic Medicine (J. Rosand), Massachusetts General Hospital, Boston; Broad Institute (J. Rosand), Cambridge, MA; Department of Neurology and Evelyn F. McKnight Brain Institute (J. Roquer, T.R., R.L.S./M.S.), Miller School of Medicine, University of Miami, FL; Institute of Cardiovascular Research (P.S.), Royal Holloway University of London (ICR2UL), UK St Peter's and Ashford Hospitals, Egham, United Kingdom; Department of Neurology (A. Slowik), Jagiellonian University Medical College, Krakow, Poland; Division of Neurocritical Care & Emergency Neurology (D.S.), Department of Neurology, Helsinki University Central Hospital, Finland; Stroke Division (V.T.), Florey Institute of Neuroscience and Mental Health, Heidelberg; Department of Neurology (V.T.), Austin Health, Heidelberg, Australia; Departments of Radiology (A.V.) and Neurology and Rehabilitation Medicine (D.W.), University of Cincinnati College of Medicine, OH; Department of Clinical Sciences Lund, Radiology (J.W.) and Neurology (A.G.L.), Lund University, Sweden; Department of Radiology, Neuroradiology, Skåne University Hospital, Malmö, Sweden; Departments of Neurology and Public Health Sciences (B.B.W.), University of Virginia, Charlottesville, VA; University of Technology Sydney (J.M.), Australia; Section of Neurology (A.G.L.), Skåne University Hospital, Lund, Sweden; Department of Laboratory Medicine (C.J.), Institute of Biomedicine, the Sahlgrenska Academy, University of Gothenburg, Sweden; and Department of Clinical Genetics and Genomics (C.J.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden.
1,
Ona Wu
Ona Wu, PhD
1From the J. Philip Kistler Stroke Research Center (M.B., A.K.B., M.D.S., S.H., A. Dalca, K.D., A.-K.G., M.R.E., P.M.R., M.N., R.W.R., C.W., N.S.R.), A.A. Martinos Center for Biomedical Imaging (A. Dalca, O.W.), and Henry and Allison McCance Center for Brain Health (J. Rosand), Massachusetts General Hospital, Harvard Medical School, Boston; Lille Neuroscience & Cognition (M.B., X.L., R. Lopes, G.K.), Inserm, CHU Lille, U1172 and Institut Pasteur de Lille (M.G.), CNRS, Inserm, CHU Lille, US 41 - UMS 2014 - PLBS, Lille University, France; Computer Science and Artificial Intelligence Lab (A. Dalca, C.W., P.G.), Massachusetts Institute of Technology, Cambridge; Division of Preventive Medicine (P.M.R.), Department of Medicine, Brigham and Women's Hospital, Boston, MA; Department of Medicine (O.R.B.), Division of Neurology, University of British Columbia, Vancouver, Canada; Department of Neurology (J.W.C., S.J.K.), University of Maryland School of Medicine and Veterans Affairs Maryland Health Care System, Baltimore, MD; School of Medical Sciences (A. Donatti, A. Sousa), University of Campinas (UNICAMP) and the Brazilian Institute of Neuroscience and Neurotechnology (BRAINN), Campinas, São Paulo; Departments of Neurosurgery (C.G.) and Neurology (R.Z.), Geisinger, Danville, PA; Department of Neurosurgery (C.G.), Christian Doppler Klinik, Paracelsus Medical University, Salzburg, Austria; Division of Emergency Medicine (Laura Heitsch), Washington University School of Medicine, St. Louis; Department of Neurology (Laura Heitsch, C.-L.P.), Washington University School of Medicine & Barnes-Jewish Hospital, St. Louis, MO; Department of Clinical Neuroscience (L. Holmegaard, K.J., T.M.S., T.T.), Institute of Neuroscience and Physiology, Sahlgrenska Academy at University of Gothenburg, Sweden; Department of Neurology, Sahlgrenska University Hospital, Gothenburg, Sweden; Department of Neurology (J.J.-C.), Neurovascular Research Group (NEUVAS), IMIM-Hospital del Mar (Institut Hospital del Mar d'Investigacions M`ediques), Universitat Autonoma de Barcelona, Spain; Department of Neurosciences (R. Lemmens), Experimental Neurology and Leuven Research Institute for Neuroscience and Disease (LIND), KU Leuven - University of Leuven, Belgium; Department of Neurology (R. Lemmens), Laboratory of Neurobiology, VIB Vesalius Research Center, University Hospitals Leuven, Belgium; School of Medicine and Public Health (C.R.L.), University of Newcastle, New South Wales; Department of Neurology, John Hunter Hospital, Newcastle, New South Wales, Australia; Division of Endocrinology (P.F.M.), Diabetes and Nutrition, Department of Medicine, University of Maryland School of Medicine, Baltimore; Department of Pharmacotherapy and Translational Research and Center for Pharmacogenomics (C.W.M.), University of Florida, Gainesville; Department of Neurology (J.F.M.), Mayo Clinic, Jacksonville, FL; Klinik und Poliklinik für Neurologie (A.R.), Universitätsmedizin Rostock, Germany; Department of Neurology (S.R., R.S.), Clinical Division of Neurogeriatrics, Medical University Graz, Austria; Center for Genomic Medicine (J. Rosand), Massachusetts General Hospital, Boston; Broad Institute (J. Rosand), Cambridge, MA; Department of Neurology and Evelyn F. McKnight Brain Institute (J. Roquer, T.R., R.L.S./M.S.), Miller School of Medicine, University of Miami, FL; Institute of Cardiovascular Research (P.S.), Royal Holloway University of London (ICR2UL), UK St Peter's and Ashford Hospitals, Egham, United Kingdom; Department of Neurology (A. Slowik), Jagiellonian University Medical College, Krakow, Poland; Division of Neurocritical Care & Emergency Neurology (D.S.), Department of Neurology, Helsinki University Central Hospital, Finland; Stroke Division (V.T.), Florey Institute of Neuroscience and Mental Health, Heidelberg; Department of Neurology (V.T.), Austin Health, Heidelberg, Australia; Departments of Radiology (A.V.) and Neurology and Rehabilitation Medicine (D.W.), University of Cincinnati College of Medicine, OH; Department of Clinical Sciences Lund, Radiology (J.W.) and Neurology (A.G.L.), Lund University, Sweden; Department of Radiology, Neuroradiology, Skåne University Hospital, Malmö, Sweden; Departments of Neurology and Public Health Sciences (B.B.W.), University of Virginia, Charlottesville, VA; University of Technology Sydney (J.M.), Australia; Section of Neurology (A.G.L.), Skåne University Hospital, Lund, Sweden; Department of Laboratory Medicine (C.J.), Institute of Biomedicine, the Sahlgrenska Academy, University of Gothenburg, Sweden; and Department of Clinical Genetics and Genomics (C.J.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden.
1,
Ramin Zand
Ramin Zand, MD
1From the J. Philip Kistler Stroke Research Center (M.B., A.K.B., M.D.S., S.H., A. Dalca, K.D., A.-K.G., M.R.E., P.M.R., M.N., R.W.R., C.W., N.S.R.), A.A. Martinos Center for Biomedical Imaging (A. Dalca, O.W.), and Henry and Allison McCance Center for Brain Health (J. Rosand), Massachusetts General Hospital, Harvard Medical School, Boston; Lille Neuroscience & Cognition (M.B., X.L., R. Lopes, G.K.), Inserm, CHU Lille, U1172 and Institut Pasteur de Lille (M.G.), CNRS, Inserm, CHU Lille, US 41 - UMS 2014 - PLBS, Lille University, France; Computer Science and Artificial Intelligence Lab (A. Dalca, C.W., P.G.), Massachusetts Institute of Technology, Cambridge; Division of Preventive Medicine (P.M.R.), Department of Medicine, Brigham and Women's Hospital, Boston, MA; Department of Medicine (O.R.B.), Division of Neurology, University of British Columbia, Vancouver, Canada; Department of Neurology (J.W.C., S.J.K.), University of Maryland School of Medicine and Veterans Affairs Maryland Health Care System, Baltimore, MD; School of Medical Sciences (A. Donatti, A. Sousa), University of Campinas (UNICAMP) and the Brazilian Institute of Neuroscience and Neurotechnology (BRAINN), Campinas, São Paulo; Departments of Neurosurgery (C.G.) and Neurology (R.Z.), Geisinger, Danville, PA; Department of Neurosurgery (C.G.), Christian Doppler Klinik, Paracelsus Medical University, Salzburg, Austria; Division of Emergency Medicine (Laura Heitsch), Washington University School of Medicine, St. Louis; Department of Neurology (Laura Heitsch, C.-L.P.), Washington University School of Medicine & Barnes-Jewish Hospital, St. Louis, MO; Department of Clinical Neuroscience (L. Holmegaard, K.J., T.M.S., T.T.), Institute of Neuroscience and Physiology, Sahlgrenska Academy at University of Gothenburg, Sweden; Department of Neurology, Sahlgrenska University Hospital, Gothenburg, Sweden; Department of Neurology (J.J.-C.), Neurovascular Research Group (NEUVAS), IMIM-Hospital del Mar (Institut Hospital del Mar d'Investigacions M`ediques), Universitat Autonoma de Barcelona, Spain; Department of Neurosciences (R. Lemmens), Experimental Neurology and Leuven Research Institute for Neuroscience and Disease (LIND), KU Leuven - University of Leuven, Belgium; Department of Neurology (R. Lemmens), Laboratory of Neurobiology, VIB Vesalius Research Center, University Hospitals Leuven, Belgium; School of Medicine and Public Health (C.R.L.), University of Newcastle, New South Wales; Department of Neurology, John Hunter Hospital, Newcastle, New South Wales, Australia; Division of Endocrinology (P.F.M.), Diabetes and Nutrition, Department of Medicine, University of Maryland School of Medicine, Baltimore; Department of Pharmacotherapy and Translational Research and Center for Pharmacogenomics (C.W.M.), University of Florida, Gainesville; Department of Neurology (J.F.M.), Mayo Clinic, Jacksonville, FL; Klinik und Poliklinik für Neurologie (A.R.), Universitätsmedizin Rostock, Germany; Department of Neurology (S.R., R.S.), Clinical Division of Neurogeriatrics, Medical University Graz, Austria; Center for Genomic Medicine (J. Rosand), Massachusetts General Hospital, Boston; Broad Institute (J. Rosand), Cambridge, MA; Department of Neurology and Evelyn F. McKnight Brain Institute (J. Roquer, T.R., R.L.S./M.S.), Miller School of Medicine, University of Miami, FL; Institute of Cardiovascular Research (P.S.), Royal Holloway University of London (ICR2UL), UK St Peter's and Ashford Hospitals, Egham, United Kingdom; Department of Neurology (A. Slowik), Jagiellonian University Medical College, Krakow, Poland; Division of Neurocritical Care & Emergency Neurology (D.S.), Department of Neurology, Helsinki University Central Hospital, Finland; Stroke Division (V.T.), Florey Institute of Neuroscience and Mental Health, Heidelberg; Department of Neurology (V.T.), Austin Health, Heidelberg, Australia; Departments of Radiology (A.V.) and Neurology and Rehabilitation Medicine (D.W.), University of Cincinnati College of Medicine, OH; Department of Clinical Sciences Lund, Radiology (J.W.) and Neurology (A.G.L.), Lund University, Sweden; Department of Radiology, Neuroradiology, Skåne University Hospital, Malmö, Sweden; Departments of Neurology and Public Health Sciences (B.B.W.), University of Virginia, Charlottesville, VA; University of Technology Sydney (J.M.), Australia; Section of Neurology (A.G.L.), Skåne University Hospital, Lund, Sweden; Department of Laboratory Medicine (C.J.), Institute of Biomedicine, the Sahlgrenska Academy, University of Gothenburg, Sweden; and Department of Clinical Genetics and Genomics (C.J.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden.
1,
Bradford B Worrall
Bradford B Worrall, MD, MSc
1From the J. Philip Kistler Stroke Research Center (M.B., A.K.B., M.D.S., S.H., A. Dalca, K.D., A.-K.G., M.R.E., P.M.R., M.N., R.W.R., C.W., N.S.R.), A.A. Martinos Center for Biomedical Imaging (A. Dalca, O.W.), and Henry and Allison McCance Center for Brain Health (J. Rosand), Massachusetts General Hospital, Harvard Medical School, Boston; Lille Neuroscience & Cognition (M.B., X.L., R. Lopes, G.K.), Inserm, CHU Lille, U1172 and Institut Pasteur de Lille (M.G.), CNRS, Inserm, CHU Lille, US 41 - UMS 2014 - PLBS, Lille University, France; Computer Science and Artificial Intelligence Lab (A. Dalca, C.W., P.G.), Massachusetts Institute of Technology, Cambridge; Division of Preventive Medicine (P.M.R.), Department of Medicine, Brigham and Women's Hospital, Boston, MA; Department of Medicine (O.R.B.), Division of Neurology, University of British Columbia, Vancouver, Canada; Department of Neurology (J.W.C., S.J.K.), University of Maryland School of Medicine and Veterans Affairs Maryland Health Care System, Baltimore, MD; School of Medical Sciences (A. Donatti, A. Sousa), University of Campinas (UNICAMP) and the Brazilian Institute of Neuroscience and Neurotechnology (BRAINN), Campinas, São Paulo; Departments of Neurosurgery (C.G.) and Neurology (R.Z.), Geisinger, Danville, PA; Department of Neurosurgery (C.G.), Christian Doppler Klinik, Paracelsus Medical University, Salzburg, Austria; Division of Emergency Medicine (Laura Heitsch), Washington University School of Medicine, St. Louis; Department of Neurology (Laura Heitsch, C.-L.P.), Washington University School of Medicine & Barnes-Jewish Hospital, St. Louis, MO; Department of Clinical Neuroscience (L. Holmegaard, K.J., T.M.S., T.T.), Institute of Neuroscience and Physiology, Sahlgrenska Academy at University of Gothenburg, Sweden; Department of Neurology, Sahlgrenska University Hospital, Gothenburg, Sweden; Department of Neurology (J.J.-C.), Neurovascular Research Group (NEUVAS), IMIM-Hospital del Mar (Institut Hospital del Mar d'Investigacions M`ediques), Universitat Autonoma de Barcelona, Spain; Department of Neurosciences (R. Lemmens), Experimental Neurology and Leuven Research Institute for Neuroscience and Disease (LIND), KU Leuven - University of Leuven, Belgium; Department of Neurology (R. Lemmens), Laboratory of Neurobiology, VIB Vesalius Research Center, University Hospitals Leuven, Belgium; School of Medicine and Public Health (C.R.L.), University of Newcastle, New South Wales; Department of Neurology, John Hunter Hospital, Newcastle, New South Wales, Australia; Division of Endocrinology (P.F.M.), Diabetes and Nutrition, Department of Medicine, University of Maryland School of Medicine, Baltimore; Department of Pharmacotherapy and Translational Research and Center for Pharmacogenomics (C.W.M.), University of Florida, Gainesville; Department of Neurology (J.F.M.), Mayo Clinic, Jacksonville, FL; Klinik und Poliklinik für Neurologie (A.R.), Universitätsmedizin Rostock, Germany; Department of Neurology (S.R., R.S.), Clinical Division of Neurogeriatrics, Medical University Graz, Austria; Center for Genomic Medicine (J. Rosand), Massachusetts General Hospital, Boston; Broad Institute (J. Rosand), Cambridge, MA; Department of Neurology and Evelyn F. McKnight Brain Institute (J. Roquer, T.R., R.L.S./M.S.), Miller School of Medicine, University of Miami, FL; Institute of Cardiovascular Research (P.S.), Royal Holloway University of London (ICR2UL), UK St Peter's and Ashford Hospitals, Egham, United Kingdom; Department of Neurology (A. Slowik), Jagiellonian University Medical College, Krakow, Poland; Division of Neurocritical Care & Emergency Neurology (D.S.), Department of Neurology, Helsinki University Central Hospital, Finland; Stroke Division (V.T.), Florey Institute of Neuroscience and Mental Health, Heidelberg; Department of Neurology (V.T.), Austin Health, Heidelberg, Australia; Departments of Radiology (A.V.) and Neurology and Rehabilitation Medicine (D.W.), University of Cincinnati College of Medicine, OH; Department of Clinical Sciences Lund, Radiology (J.W.) and Neurology (A.G.L.), Lund University, Sweden; Department of Radiology, Neuroradiology, Skåne University Hospital, Malmö, Sweden; Departments of Neurology and Public Health Sciences (B.B.W.), University of Virginia, Charlottesville, VA; University of Technology Sydney (J.M.), Australia; Section of Neurology (A.G.L.), Skåne University Hospital, Lund, Sweden; Department of Laboratory Medicine (C.J.), Institute of Biomedicine, the Sahlgrenska Academy, University of Gothenburg, Sweden; and Department of Clinical Genetics and Genomics (C.J.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden.
1,
Jane Maguire
Jane Maguire, PhD
1From the J. Philip Kistler Stroke Research Center (M.B., A.K.B., M.D.S., S.H., A. Dalca, K.D., A.-K.G., M.R.E., P.M.R., M.N., R.W.R., C.W., N.S.R.), A.A. Martinos Center for Biomedical Imaging (A. Dalca, O.W.), and Henry and Allison McCance Center for Brain Health (J. Rosand), Massachusetts General Hospital, Harvard Medical School, Boston; Lille Neuroscience & Cognition (M.B., X.L., R. Lopes, G.K.), Inserm, CHU Lille, U1172 and Institut Pasteur de Lille (M.G.), CNRS, Inserm, CHU Lille, US 41 - UMS 2014 - PLBS, Lille University, France; Computer Science and Artificial Intelligence Lab (A. Dalca, C.W., P.G.), Massachusetts Institute of Technology, Cambridge; Division of Preventive Medicine (P.M.R.), Department of Medicine, Brigham and Women's Hospital, Boston, MA; Department of Medicine (O.R.B.), Division of Neurology, University of British Columbia, Vancouver, Canada; Department of Neurology (J.W.C., S.J.K.), University of Maryland School of Medicine and Veterans Affairs Maryland Health Care System, Baltimore, MD; School of Medical Sciences (A. Donatti, A. Sousa), University of Campinas (UNICAMP) and the Brazilian Institute of Neuroscience and Neurotechnology (BRAINN), Campinas, São Paulo; Departments of Neurosurgery (C.G.) and Neurology (R.Z.), Geisinger, Danville, PA; Department of Neurosurgery (C.G.), Christian Doppler Klinik, Paracelsus Medical University, Salzburg, Austria; Division of Emergency Medicine (Laura Heitsch), Washington University School of Medicine, St. Louis; Department of Neurology (Laura Heitsch, C.-L.P.), Washington University School of Medicine & Barnes-Jewish Hospital, St. Louis, MO; Department of Clinical Neuroscience (L. Holmegaard, K.J., T.M.S., T.T.), Institute of Neuroscience and Physiology, Sahlgrenska Academy at University of Gothenburg, Sweden; Department of Neurology, Sahlgrenska University Hospital, Gothenburg, Sweden; Department of Neurology (J.J.-C.), Neurovascular Research Group (NEUVAS), IMIM-Hospital del Mar (Institut Hospital del Mar d'Investigacions M`ediques), Universitat Autonoma de Barcelona, Spain; Department of Neurosciences (R. Lemmens), Experimental Neurology and Leuven Research Institute for Neuroscience and Disease (LIND), KU Leuven - University of Leuven, Belgium; Department of Neurology (R. Lemmens), Laboratory of Neurobiology, VIB Vesalius Research Center, University Hospitals Leuven, Belgium; School of Medicine and Public Health (C.R.L.), University of Newcastle, New South Wales; Department of Neurology, John Hunter Hospital, Newcastle, New South Wales, Australia; Division of Endocrinology (P.F.M.), Diabetes and Nutrition, Department of Medicine, University of Maryland School of Medicine, Baltimore; Department of Pharmacotherapy and Translational Research and Center for Pharmacogenomics (C.W.M.), University of Florida, Gainesville; Department of Neurology (J.F.M.), Mayo Clinic, Jacksonville, FL; Klinik und Poliklinik für Neurologie (A.R.), Universitätsmedizin Rostock, Germany; Department of Neurology (S.R., R.S.), Clinical Division of Neurogeriatrics, Medical University Graz, Austria; Center for Genomic Medicine (J. Rosand), Massachusetts General Hospital, Boston; Broad Institute (J. Rosand), Cambridge, MA; Department of Neurology and Evelyn F. McKnight Brain Institute (J. Roquer, T.R., R.L.S./M.S.), Miller School of Medicine, University of Miami, FL; Institute of Cardiovascular Research (P.S.), Royal Holloway University of London (ICR2UL), UK St Peter's and Ashford Hospitals, Egham, United Kingdom; Department of Neurology (A. Slowik), Jagiellonian University Medical College, Krakow, Poland; Division of Neurocritical Care & Emergency Neurology (D.S.), Department of Neurology, Helsinki University Central Hospital, Finland; Stroke Division (V.T.), Florey Institute of Neuroscience and Mental Health, Heidelberg; Department of Neurology (V.T.), Austin Health, Heidelberg, Australia; Departments of Radiology (A.V.) and Neurology and Rehabilitation Medicine (D.W.), University of Cincinnati College of Medicine, OH; Department of Clinical Sciences Lund, Radiology (J.W.) and Neurology (A.G.L.), Lund University, Sweden; Department of Radiology, Neuroradiology, Skåne University Hospital, Malmö, Sweden; Departments of Neurology and Public Health Sciences (B.B.W.), University of Virginia, Charlottesville, VA; University of Technology Sydney (J.M.), Australia; Section of Neurology (A.G.L.), Skåne University Hospital, Lund, Sweden; Department of Laboratory Medicine (C.J.), Institute of Biomedicine, the Sahlgrenska Academy, University of Gothenburg, Sweden; and Department of Clinical Genetics and Genomics (C.J.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden.
1,
Arne G Lindgren
Arne G Lindgren, MD, PhD
1From the J. Philip Kistler Stroke Research Center (M.B., A.K.B., M.D.S., S.H., A. Dalca, K.D., A.-K.G., M.R.E., P.M.R., M.N., R.W.R., C.W., N.S.R.), A.A. Martinos Center for Biomedical Imaging (A. Dalca, O.W.), and Henry and Allison McCance Center for Brain Health (J. Rosand), Massachusetts General Hospital, Harvard Medical School, Boston; Lille Neuroscience & Cognition (M.B., X.L., R. Lopes, G.K.), Inserm, CHU Lille, U1172 and Institut Pasteur de Lille (M.G.), CNRS, Inserm, CHU Lille, US 41 - UMS 2014 - PLBS, Lille University, France; Computer Science and Artificial Intelligence Lab (A. Dalca, C.W., P.G.), Massachusetts Institute of Technology, Cambridge; Division of Preventive Medicine (P.M.R.), Department of Medicine, Brigham and Women's Hospital, Boston, MA; Department of Medicine (O.R.B.), Division of Neurology, University of British Columbia, Vancouver, Canada; Department of Neurology (J.W.C., S.J.K.), University of Maryland School of Medicine and Veterans Affairs Maryland Health Care System, Baltimore, MD; School of Medical Sciences (A. Donatti, A. Sousa), University of Campinas (UNICAMP) and the Brazilian Institute of Neuroscience and Neurotechnology (BRAINN), Campinas, São Paulo; Departments of Neurosurgery (C.G.) and Neurology (R.Z.), Geisinger, Danville, PA; Department of Neurosurgery (C.G.), Christian Doppler Klinik, Paracelsus Medical University, Salzburg, Austria; Division of Emergency Medicine (Laura Heitsch), Washington University School of Medicine, St. Louis; Department of Neurology (Laura Heitsch, C.-L.P.), Washington University School of Medicine & Barnes-Jewish Hospital, St. Louis, MO; Department of Clinical Neuroscience (L. Holmegaard, K.J., T.M.S., T.T.), Institute of Neuroscience and Physiology, Sahlgrenska Academy at University of Gothenburg, Sweden; Department of Neurology, Sahlgrenska University Hospital, Gothenburg, Sweden; Department of Neurology (J.J.-C.), Neurovascular Research Group (NEUVAS), IMIM-Hospital del Mar (Institut Hospital del Mar d'Investigacions M`ediques), Universitat Autonoma de Barcelona, Spain; Department of Neurosciences (R. Lemmens), Experimental Neurology and Leuven Research Institute for Neuroscience and Disease (LIND), KU Leuven - University of Leuven, Belgium; Department of Neurology (R. Lemmens), Laboratory of Neurobiology, VIB Vesalius Research Center, University Hospitals Leuven, Belgium; School of Medicine and Public Health (C.R.L.), University of Newcastle, New South Wales; Department of Neurology, John Hunter Hospital, Newcastle, New South Wales, Australia; Division of Endocrinology (P.F.M.), Diabetes and Nutrition, Department of Medicine, University of Maryland School of Medicine, Baltimore; Department of Pharmacotherapy and Translational Research and Center for Pharmacogenomics (C.W.M.), University of Florida, Gainesville; Department of Neurology (J.F.M.), Mayo Clinic, Jacksonville, FL; Klinik und Poliklinik für Neurologie (A.R.), Universitätsmedizin Rostock, Germany; Department of Neurology (S.R., R.S.), Clinical Division of Neurogeriatrics, Medical University Graz, Austria; Center for Genomic Medicine (J. Rosand), Massachusetts General Hospital, Boston; Broad Institute (J. Rosand), Cambridge, MA; Department of Neurology and Evelyn F. McKnight Brain Institute (J. Roquer, T.R., R.L.S./M.S.), Miller School of Medicine, University of Miami, FL; Institute of Cardiovascular Research (P.S.), Royal Holloway University of London (ICR2UL), UK St Peter's and Ashford Hospitals, Egham, United Kingdom; Department of Neurology (A. Slowik), Jagiellonian University Medical College, Krakow, Poland; Division of Neurocritical Care & Emergency Neurology (D.S.), Department of Neurology, Helsinki University Central Hospital, Finland; Stroke Division (V.T.), Florey Institute of Neuroscience and Mental Health, Heidelberg; Department of Neurology (V.T.), Austin Health, Heidelberg, Australia; Departments of Radiology (A.V.) and Neurology and Rehabilitation Medicine (D.W.), University of Cincinnati College of Medicine, OH; Department of Clinical Sciences Lund, Radiology (J.W.) and Neurology (A.G.L.), Lund University, Sweden; Department of Radiology, Neuroradiology, Skåne University Hospital, Malmö, Sweden; Departments of Neurology and Public Health Sciences (B.B.W.), University of Virginia, Charlottesville, VA; University of Technology Sydney (J.M.), Australia; Section of Neurology (A.G.L.), Skåne University Hospital, Lund, Sweden; Department of Laboratory Medicine (C.J.), Institute of Biomedicine, the Sahlgrenska Academy, University of Gothenburg, Sweden; and Department of Clinical Genetics and Genomics (C.J.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden.
1,
Christina Jern
Christina Jern, MD
1From the J. Philip Kistler Stroke Research Center (M.B., A.K.B., M.D.S., S.H., A. Dalca, K.D., A.-K.G., M.R.E., P.M.R., M.N., R.W.R., C.W., N.S.R.), A.A. Martinos Center for Biomedical Imaging (A. Dalca, O.W.), and Henry and Allison McCance Center for Brain Health (J. Rosand), Massachusetts General Hospital, Harvard Medical School, Boston; Lille Neuroscience & Cognition (M.B., X.L., R. Lopes, G.K.), Inserm, CHU Lille, U1172 and Institut Pasteur de Lille (M.G.), CNRS, Inserm, CHU Lille, US 41 - UMS 2014 - PLBS, Lille University, France; Computer Science and Artificial Intelligence Lab (A. Dalca, C.W., P.G.), Massachusetts Institute of Technology, Cambridge; Division of Preventive Medicine (P.M.R.), Department of Medicine, Brigham and Women's Hospital, Boston, MA; Department of Medicine (O.R.B.), Division of Neurology, University of British Columbia, Vancouver, Canada; Department of Neurology (J.W.C., S.J.K.), University of Maryland School of Medicine and Veterans Affairs Maryland Health Care System, Baltimore, MD; School of Medical Sciences (A. Donatti, A. Sousa), University of Campinas (UNICAMP) and the Brazilian Institute of Neuroscience and Neurotechnology (BRAINN), Campinas, São Paulo; Departments of Neurosurgery (C.G.) and Neurology (R.Z.), Geisinger, Danville, PA; Department of Neurosurgery (C.G.), Christian Doppler Klinik, Paracelsus Medical University, Salzburg, Austria; Division of Emergency Medicine (Laura Heitsch), Washington University School of Medicine, St. Louis; Department of Neurology (Laura Heitsch, C.-L.P.), Washington University School of Medicine & Barnes-Jewish Hospital, St. Louis, MO; Department of Clinical Neuroscience (L. Holmegaard, K.J., T.M.S., T.T.), Institute of Neuroscience and Physiology, Sahlgrenska Academy at University of Gothenburg, Sweden; Department of Neurology, Sahlgrenska University Hospital, Gothenburg, Sweden; Department of Neurology (J.J.-C.), Neurovascular Research Group (NEUVAS), IMIM-Hospital del Mar (Institut Hospital del Mar d'Investigacions M`ediques), Universitat Autonoma de Barcelona, Spain; Department of Neurosciences (R. Lemmens), Experimental Neurology and Leuven Research Institute for Neuroscience and Disease (LIND), KU Leuven - University of Leuven, Belgium; Department of Neurology (R. Lemmens), Laboratory of Neurobiology, VIB Vesalius Research Center, University Hospitals Leuven, Belgium; School of Medicine and Public Health (C.R.L.), University of Newcastle, New South Wales; Department of Neurology, John Hunter Hospital, Newcastle, New South Wales, Australia; Division of Endocrinology (P.F.M.), Diabetes and Nutrition, Department of Medicine, University of Maryland School of Medicine, Baltimore; Department of Pharmacotherapy and Translational Research and Center for Pharmacogenomics (C.W.M.), University of Florida, Gainesville; Department of Neurology (J.F.M.), Mayo Clinic, Jacksonville, FL; Klinik und Poliklinik für Neurologie (A.R.), Universitätsmedizin Rostock, Germany; Department of Neurology (S.R., R.S.), Clinical Division of Neurogeriatrics, Medical University Graz, Austria; Center for Genomic Medicine (J. Rosand), Massachusetts General Hospital, Boston; Broad Institute (J. Rosand), Cambridge, MA; Department of Neurology and Evelyn F. McKnight Brain Institute (J. Roquer, T.R., R.L.S./M.S.), Miller School of Medicine, University of Miami, FL; Institute of Cardiovascular Research (P.S.), Royal Holloway University of London (ICR2UL), UK St Peter's and Ashford Hospitals, Egham, United Kingdom; Department of Neurology (A. Slowik), Jagiellonian University Medical College, Krakow, Poland; Division of Neurocritical Care & Emergency Neurology (D.S.), Department of Neurology, Helsinki University Central Hospital, Finland; Stroke Division (V.T.), Florey Institute of Neuroscience and Mental Health, Heidelberg; Department of Neurology (V.T.), Austin Health, Heidelberg, Australia; Departments of Radiology (A.V.) and Neurology and Rehabilitation Medicine (D.W.), University of Cincinnati College of Medicine, OH; Department of Clinical Sciences Lund, Radiology (J.W.) and Neurology (A.G.L.), Lund University, Sweden; Department of Radiology, Neuroradiology, Skåne University Hospital, Malmö, Sweden; Departments of Neurology and Public Health Sciences (B.B.W.), University of Virginia, Charlottesville, VA; University of Technology Sydney (J.M.), Australia; Section of Neurology (A.G.L.), Skåne University Hospital, Lund, Sweden; Department of Laboratory Medicine (C.J.), Institute of Biomedicine, the Sahlgrenska Academy, University of Gothenburg, Sweden; and Department of Clinical Genetics and Genomics (C.J.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden.
1,
Polina Golland
Polina Golland, PhD
1From the J. Philip Kistler Stroke Research Center (M.B., A.K.B., M.D.S., S.H., A. Dalca, K.D., A.-K.G., M.R.E., P.M.R., M.N., R.W.R., C.W., N.S.R.), A.A. Martinos Center for Biomedical Imaging (A. Dalca, O.W.), and Henry and Allison McCance Center for Brain Health (J. Rosand), Massachusetts General Hospital, Harvard Medical School, Boston; Lille Neuroscience & Cognition (M.B., X.L., R. Lopes, G.K.), Inserm, CHU Lille, U1172 and Institut Pasteur de Lille (M.G.), CNRS, Inserm, CHU Lille, US 41 - UMS 2014 - PLBS, Lille University, France; Computer Science and Artificial Intelligence Lab (A. Dalca, C.W., P.G.), Massachusetts Institute of Technology, Cambridge; Division of Preventive Medicine (P.M.R.), Department of Medicine, Brigham and Women's Hospital, Boston, MA; Department of Medicine (O.R.B.), Division of Neurology, University of British Columbia, Vancouver, Canada; Department of Neurology (J.W.C., S.J.K.), University of Maryland School of Medicine and Veterans Affairs Maryland Health Care System, Baltimore, MD; School of Medical Sciences (A. Donatti, A. Sousa), University of Campinas (UNICAMP) and the Brazilian Institute of Neuroscience and Neurotechnology (BRAINN), Campinas, São Paulo; Departments of Neurosurgery (C.G.) and Neurology (R.Z.), Geisinger, Danville, PA; Department of Neurosurgery (C.G.), Christian Doppler Klinik, Paracelsus Medical University, Salzburg, Austria; Division of Emergency Medicine (Laura Heitsch), Washington University School of Medicine, St. Louis; Department of Neurology (Laura Heitsch, C.-L.P.), Washington University School of Medicine & Barnes-Jewish Hospital, St. Louis, MO; Department of Clinical Neuroscience (L. Holmegaard, K.J., T.M.S., T.T.), Institute of Neuroscience and Physiology, Sahlgrenska Academy at University of Gothenburg, Sweden; Department of Neurology, Sahlgrenska University Hospital, Gothenburg, Sweden; Department of Neurology (J.J.-C.), Neurovascular Research Group (NEUVAS), IMIM-Hospital del Mar (Institut Hospital del Mar d'Investigacions M`ediques), Universitat Autonoma de Barcelona, Spain; Department of Neurosciences (R. Lemmens), Experimental Neurology and Leuven Research Institute for Neuroscience and Disease (LIND), KU Leuven - University of Leuven, Belgium; Department of Neurology (R. Lemmens), Laboratory of Neurobiology, VIB Vesalius Research Center, University Hospitals Leuven, Belgium; School of Medicine and Public Health (C.R.L.), University of Newcastle, New South Wales; Department of Neurology, John Hunter Hospital, Newcastle, New South Wales, Australia; Division of Endocrinology (P.F.M.), Diabetes and Nutrition, Department of Medicine, University of Maryland School of Medicine, Baltimore; Department of Pharmacotherapy and Translational Research and Center for Pharmacogenomics (C.W.M.), University of Florida, Gainesville; Department of Neurology (J.F.M.), Mayo Clinic, Jacksonville, FL; Klinik und Poliklinik für Neurologie (A.R.), Universitätsmedizin Rostock, Germany; Department of Neurology (S.R., R.S.), Clinical Division of Neurogeriatrics, Medical University Graz, Austria; Center for Genomic Medicine (J. Rosand), Massachusetts General Hospital, Boston; Broad Institute (J. Rosand), Cambridge, MA; Department of Neurology and Evelyn F. McKnight Brain Institute (J. Roquer, T.R., R.L.S./M.S.), Miller School of Medicine, University of Miami, FL; Institute of Cardiovascular Research (P.S.), Royal Holloway University of London (ICR2UL), UK St Peter's and Ashford Hospitals, Egham, United Kingdom; Department of Neurology (A. Slowik), Jagiellonian University Medical College, Krakow, Poland; Division of Neurocritical Care & Emergency Neurology (D.S.), Department of Neurology, Helsinki University Central Hospital, Finland; Stroke Division (V.T.), Florey Institute of Neuroscience and Mental Health, Heidelberg; Department of Neurology (V.T.), Austin Health, Heidelberg, Australia; Departments of Radiology (A.V.) and Neurology and Rehabilitation Medicine (D.W.), University of Cincinnati College of Medicine, OH; Department of Clinical Sciences Lund, Radiology (J.W.) and Neurology (A.G.L.), Lund University, Sweden; Department of Radiology, Neuroradiology, Skåne University Hospital, Malmö, Sweden; Departments of Neurology and Public Health Sciences (B.B.W.), University of Virginia, Charlottesville, VA; University of Technology Sydney (J.M.), Australia; Section of Neurology (A.G.L.), Skåne University Hospital, Lund, Sweden; Department of Laboratory Medicine (C.J.), Institute of Biomedicine, the Sahlgrenska Academy, University of Gothenburg, Sweden; and Department of Clinical Genetics and Genomics (C.J.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden.
1,
Grégory Kuchcinski
Grégory Kuchcinski, MD, PhD
1From the J. Philip Kistler Stroke Research Center (M.B., A.K.B., M.D.S., S.H., A. Dalca, K.D., A.-K.G., M.R.E., P.M.R., M.N., R.W.R., C.W., N.S.R.), A.A. Martinos Center for Biomedical Imaging (A. Dalca, O.W.), and Henry and Allison McCance Center for Brain Health (J. Rosand), Massachusetts General Hospital, Harvard Medical School, Boston; Lille Neuroscience & Cognition (M.B., X.L., R. Lopes, G.K.), Inserm, CHU Lille, U1172 and Institut Pasteur de Lille (M.G.), CNRS, Inserm, CHU Lille, US 41 - UMS 2014 - PLBS, Lille University, France; Computer Science and Artificial Intelligence Lab (A. Dalca, C.W., P.G.), Massachusetts Institute of Technology, Cambridge; Division of Preventive Medicine (P.M.R.), Department of Medicine, Brigham and Women's Hospital, Boston, MA; Department of Medicine (O.R.B.), Division of Neurology, University of British Columbia, Vancouver, Canada; Department of Neurology (J.W.C., S.J.K.), University of Maryland School of Medicine and Veterans Affairs Maryland Health Care System, Baltimore, MD; School of Medical Sciences (A. Donatti, A. Sousa), University of Campinas (UNICAMP) and the Brazilian Institute of Neuroscience and Neurotechnology (BRAINN), Campinas, São Paulo; Departments of Neurosurgery (C.G.) and Neurology (R.Z.), Geisinger, Danville, PA; Department of Neurosurgery (C.G.), Christian Doppler Klinik, Paracelsus Medical University, Salzburg, Austria; Division of Emergency Medicine (Laura Heitsch), Washington University School of Medicine, St. Louis; Department of Neurology (Laura Heitsch, C.-L.P.), Washington University School of Medicine & Barnes-Jewish Hospital, St. Louis, MO; Department of Clinical Neuroscience (L. Holmegaard, K.J., T.M.S., T.T.), Institute of Neuroscience and Physiology, Sahlgrenska Academy at University of Gothenburg, Sweden; Department of Neurology, Sahlgrenska University Hospital, Gothenburg, Sweden; Department of Neurology (J.J.-C.), Neurovascular Research Group (NEUVAS), IMIM-Hospital del Mar (Institut Hospital del Mar d'Investigacions M`ediques), Universitat Autonoma de Barcelona, Spain; Department of Neurosciences (R. Lemmens), Experimental Neurology and Leuven Research Institute for Neuroscience and Disease (LIND), KU Leuven - University of Leuven, Belgium; Department of Neurology (R. Lemmens), Laboratory of Neurobiology, VIB Vesalius Research Center, University Hospitals Leuven, Belgium; School of Medicine and Public Health (C.R.L.), University of Newcastle, New South Wales; Department of Neurology, John Hunter Hospital, Newcastle, New South Wales, Australia; Division of Endocrinology (P.F.M.), Diabetes and Nutrition, Department of Medicine, University of Maryland School of Medicine, Baltimore; Department of Pharmacotherapy and Translational Research and Center for Pharmacogenomics (C.W.M.), University of Florida, Gainesville; Department of Neurology (J.F.M.), Mayo Clinic, Jacksonville, FL; Klinik und Poliklinik für Neurologie (A.R.), Universitätsmedizin Rostock, Germany; Department of Neurology (S.R., R.S.), Clinical Division of Neurogeriatrics, Medical University Graz, Austria; Center for Genomic Medicine (J. Rosand), Massachusetts General Hospital, Boston; Broad Institute (J. Rosand), Cambridge, MA; Department of Neurology and Evelyn F. McKnight Brain Institute (J. Roquer, T.R., R.L.S./M.S.), Miller School of Medicine, University of Miami, FL; Institute of Cardiovascular Research (P.S.), Royal Holloway University of London (ICR2UL), UK St Peter's and Ashford Hospitals, Egham, United Kingdom; Department of Neurology (A. Slowik), Jagiellonian University Medical College, Krakow, Poland; Division of Neurocritical Care & Emergency Neurology (D.S.), Department of Neurology, Helsinki University Central Hospital, Finland; Stroke Division (V.T.), Florey Institute of Neuroscience and Mental Health, Heidelberg; Department of Neurology (V.T.), Austin Health, Heidelberg, Australia; Departments of Radiology (A.V.) and Neurology and Rehabilitation Medicine (D.W.), University of Cincinnati College of Medicine, OH; Department of Clinical Sciences Lund, Radiology (J.W.) and Neurology (A.G.L.), Lund University, Sweden; Department of Radiology, Neuroradiology, Skåne University Hospital, Malmö, Sweden; Departments of Neurology and Public Health Sciences (B.B.W.), University of Virginia, Charlottesville, VA; University of Technology Sydney (J.M.), Australia; Section of Neurology (A.G.L.), Skåne University Hospital, Lund, Sweden; Department of Laboratory Medicine (C.J.), Institute of Biomedicine, the Sahlgrenska Academy, University of Gothenburg, Sweden; and Department of Clinical Genetics and Genomics (C.J.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden.
1,
Natalia S Rost
Natalia S Rost, MD, MPH
1From the J. Philip Kistler Stroke Research Center (M.B., A.K.B., M.D.S., S.H., A. Dalca, K.D., A.-K.G., M.R.E., P.M.R., M.N., R.W.R., C.W., N.S.R.), A.A. Martinos Center for Biomedical Imaging (A. Dalca, O.W.), and Henry and Allison McCance Center for Brain Health (J. Rosand), Massachusetts General Hospital, Harvard Medical School, Boston; Lille Neuroscience & Cognition (M.B., X.L., R. Lopes, G.K.), Inserm, CHU Lille, U1172 and Institut Pasteur de Lille (M.G.), CNRS, Inserm, CHU Lille, US 41 - UMS 2014 - PLBS, Lille University, France; Computer Science and Artificial Intelligence Lab (A. Dalca, C.W., P.G.), Massachusetts Institute of Technology, Cambridge; Division of Preventive Medicine (P.M.R.), Department of Medicine, Brigham and Women's Hospital, Boston, MA; Department of Medicine (O.R.B.), Division of Neurology, University of British Columbia, Vancouver, Canada; Department of Neurology (J.W.C., S.J.K.), University of Maryland School of Medicine and Veterans Affairs Maryland Health Care System, Baltimore, MD; School of Medical Sciences (A. Donatti, A. Sousa), University of Campinas (UNICAMP) and the Brazilian Institute of Neuroscience and Neurotechnology (BRAINN), Campinas, São Paulo; Departments of Neurosurgery (C.G.) and Neurology (R.Z.), Geisinger, Danville, PA; Department of Neurosurgery (C.G.), Christian Doppler Klinik, Paracelsus Medical University, Salzburg, Austria; Division of Emergency Medicine (Laura Heitsch), Washington University School of Medicine, St. Louis; Department of Neurology (Laura Heitsch, C.-L.P.), Washington University School of Medicine & Barnes-Jewish Hospital, St. Louis, MO; Department of Clinical Neuroscience (L. Holmegaard, K.J., T.M.S., T.T.), Institute of Neuroscience and Physiology, Sahlgrenska Academy at University of Gothenburg, Sweden; Department of Neurology, Sahlgrenska University Hospital, Gothenburg, Sweden; Department of Neurology (J.J.-C.), Neurovascular Research Group (NEUVAS), IMIM-Hospital del Mar (Institut Hospital del Mar d'Investigacions M`ediques), Universitat Autonoma de Barcelona, Spain; Department of Neurosciences (R. Lemmens), Experimental Neurology and Leuven Research Institute for Neuroscience and Disease (LIND), KU Leuven - University of Leuven, Belgium; Department of Neurology (R. Lemmens), Laboratory of Neurobiology, VIB Vesalius Research Center, University Hospitals Leuven, Belgium; School of Medicine and Public Health (C.R.L.), University of Newcastle, New South Wales; Department of Neurology, John Hunter Hospital, Newcastle, New South Wales, Australia; Division of Endocrinology (P.F.M.), Diabetes and Nutrition, Department of Medicine, University of Maryland School of Medicine, Baltimore; Department of Pharmacotherapy and Translational Research and Center for Pharmacogenomics (C.W.M.), University of Florida, Gainesville; Department of Neurology (J.F.M.), Mayo Clinic, Jacksonville, FL; Klinik und Poliklinik für Neurologie (A.R.), Universitätsmedizin Rostock, Germany; Department of Neurology (S.R., R.S.), Clinical Division of Neurogeriatrics, Medical University Graz, Austria; Center for Genomic Medicine (J. Rosand), Massachusetts General Hospital, Boston; Broad Institute (J. Rosand), Cambridge, MA; Department of Neurology and Evelyn F. McKnight Brain Institute (J. Roquer, T.R., R.L.S./M.S.), Miller School of Medicine, University of Miami, FL; Institute of Cardiovascular Research (P.S.), Royal Holloway University of London (ICR2UL), UK St Peter's and Ashford Hospitals, Egham, United Kingdom; Department of Neurology (A. Slowik), Jagiellonian University Medical College, Krakow, Poland; Division of Neurocritical Care & Emergency Neurology (D.S.), Department of Neurology, Helsinki University Central Hospital, Finland; Stroke Division (V.T.), Florey Institute of Neuroscience and Mental Health, Heidelberg; Department of Neurology (V.T.), Austin Health, Heidelberg, Australia; Departments of Radiology (A.V.) and Neurology and Rehabilitation Medicine (D.W.), University of Cincinnati College of Medicine, OH; Department of Clinical Sciences Lund, Radiology (J.W.) and Neurology (A.G.L.), Lund University, Sweden; Department of Radiology, Neuroradiology, Skåne University Hospital, Malmö, Sweden; Departments of Neurology and Public Health Sciences (B.B.W.), University of Virginia, Charlottesville, VA; University of Technology Sydney (J.M.), Australia; Section of Neurology (A.G.L.), Skåne University Hospital, Lund, Sweden; Department of Laboratory Medicine (C.J.), Institute of Biomedicine, the Sahlgrenska Academy, University of Gothenburg, Sweden; and Department of Clinical Genetics and Genomics (C.J.), Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden.
1;
and the MRI-GENIE and GISCOME Investigators and the International Stroke Genetics Consortium1