Abstract
Background and Objectives
To determine the prevalence and relative importance of symptoms experienced by children and adults with Friedreich ataxia (FA) and to identify factors associated with a higher burden of disease.
Methods
We conducted qualitative interviews with individuals with FA and caregivers of pediatric individuals with FA to identify potential symptoms of importance to those living with FA. We subsequently performed a cross-sectional study to assess which symptoms have the highest prevalence and importance in FA and to determine which factors are associated with a higher burden of disease.
Results
Thirty-nine participants provided 2,527 quotes regarding the symptomatic burden of FA. Two hundred two individuals (153 individuals with FA and 49 caregivers) participated in a subsequent cross-sectional study. Individuals with FA and caregivers identified impaired coordination, limitations with mobility and walking, inability to do activities, fatigue, and lower extremity weakness as the most prevalent and life-altering symptomatic themes in FA. Muscle stiffness and functional staging for ataxia were associated with the prevalence of symptomatic themes in FA. In addition, the length of smaller GAA expansion and the mean length of both GAA expansions were strongly associated with the onset of symptoms in FA.
Discussion
There are a wide variety of symptoms that affect the lives of individuals with FA. These symptoms, many underrecognized, have different levels of importance and occur at different rates in the FA population. The most common and life altering of these symptoms represent potential targets for future therapeutic interventions.
Friedreich ataxia (FA) is an autosomal recessive neurodegenerative disease that presents with a variety of clinical symptoms, including a loss of coordination (ataxia) in the arms and legs, fatigue, muscle loss, vision impairment, hearing loss, slurred speech, scoliosis, diabetes mellitus, and cardiac issues related to cardiomyopathies and arrhythmias.1,2 The onset of symptoms in individuals with FA typically occurs between 5 and 15 years of age.2 The genetic basis for FA is a homozygous unstable expansion of GAA repeats in the FXN gene on chromosome 9, which causes low expression of the frataxin protein.3,4 Reduced expression of the frataxin protein leads to oxidative stress, mitochondrial damage, and cellular energy failure related to deficient synthesis of key iron-sulfur molecules.5 Together, this results in dysfunction of the peripheral nerves, spinal cord, cerebellum, and heart cells (cardiac myocytes).6 A larger GAA expansion on the smaller FXN allele is associated with more severe disease.7 As novel therapeutics are developed for FA, there is a need to better understand the symptoms and issues that have the greatest effect on the lives of children and adults with this disease. In this study, patient-reported impact of symptoms in FA, we used data from semistructured qualitative interviews and a large cross-sectional study of individuals with FA and caregivers of pediatric individuals with FA to determine the most prevalent and impactful symptoms of this disease.
Methods
Study Participants
Participants included adults with FA aged 18 years and older, children and adolescents with FA aged 8–17 years, and caregivers aged 18 years and older who cared for an individual(s) with FA 0–18 years of age. Participants were recruited from the Friedreich's Ataxia Research Alliance (FARA) registry, the University of Rochester Medical Center, and the Children's Hospital of Philadelphia (CHOP).
Study Design
Phase 1: Semistructured Qualitative Interviews
We conducted interviews with individuals with FA aged 18 years and older, individuals with FA aged 11–17 years, and adult caregivers of pediatric individuals with FA aged 0–18 years. Individuals with FA and caregivers were identified and recruited by the partnering organizations mentioned earlier. Eligible individuals who were interested in participating were subsequently contacted and consented by our study team through phone call. Participants had no relationship or involvement with the study team interviewers before their enrollment in the phase 1 study. During the consent process, participants were provided detailed information about the research team and the purpose of the research. Participants were informed that the study team would be asking questions to determine the symptoms of FA that have the greatest impact on their daily life or the life of the individual who they care for. Interviews were conducted by 3 female study team clinical research coordinators (C.Z., E.W., and B.C.). The study team clinical research coordinators had extensive experience in collecting, coding, and interpreting qualitative data from interviews with participants who experience neuromuscular disease. Using open-ended questions from a comprehensive interview guide, participants were asked to identify the symptoms of FA that have the greatest effect on their lives. Caregivers were asked about the symptoms experienced by the individual(s) with FA for whom they care. Each participant was interviewed by 1 clinical research coordinator. All interviews were conducted over the phone and were audio recorded using Zoom, a Health Insurance Portability and Accountability Act (HIPAA)–compliant conferencing software approved for all patient use by the University of Rochester's IT security team. All interview recordings were transcribed, coded, and analyzed with a qualitative framework technique, triangulation, and an investigator consensus approach.8 Two authors (C.Z. and C.H.) performed the transcript coding and subsequent content analysis. Recurring similar quotes among the interviewees were used to identify potentially relevant symptoms among the patient and caregiver populations. For each of the study populations, common symptoms were categorized into symptomatic themes (concepts representing a group of like symptoms) of FA health.
Phase 2: Two Cross-sectional Survey Studies
After completing phase 1, we performed 2 online cross-sectional studies, one with individuals with FA and the other with caregivers of pediatric individuals with FA to identify what symptoms and issues are of greatest importance to larger sample populations of individuals with FA and caregivers. Two surveys were distributed; one for individuals with FA aged 8 years and older to complete and the other for adult caregivers to complete on behalf of pediatric individuals with FA aged 0–18 years. Individuals with FA and caregivers were recruited through the FARA patient registry. Caregivers of children with FA were also recruited through CHOP. The surveys, administered electronically through Research Electronic Data Capture, a HIPAA-compliant electronic data capture system, were accessible through a public link. Participants were asked to read a information letter and complete a demographic questionnaire before taking the main survey. The main surveys included the potential symptoms of importance identified during the phase 1 qualitative interviews and common symptoms of importance identified in other neurologic disease populations.9-15 The surveys also included demographic, genetic, and functional state questions. The FA patient survey inquired about 245 individual symptoms representing 20 symptomatic themes, and the caregiver survey inquired about 196 individual symptoms representing 20 symptomatic themes. Question selection for the surveys was conducted using an investigator consensus approach with our research team. The surveys were designed to include all potential symptoms of importance while limiting question redundancy. For each individual symptom question, the survey inquired, “How much does the following impact your life now?” for individuals with FA and “How much does the following impact your child's life now?” for caregivers. Participants were provided a 6-point Likert-type scale ranging from 1 to 6. For individuals with FA completing the survey, the Likert scale options consisted of the following: (1) I do not experience this; (2) I experience this, but it does not affect my life; (3) it affects my life a little; (4) it affects my life moderately; (5) it affects my life very much; and (6) it affects my life severely. For caregivers completing the survey, the Likert scale options consisted of the following: (1) He/she does not experience this; (2) he/she experiences this, but it does not affect their life; (3) it affects his/her life a little; (4) it affects his/her life moderately; (5) it affects his/her life very much; and (6) it affects his/her life severely. All participants were given the option to decline to answer any demographic or symptom question. On completing the survey, participants were asked to list and rate the severity of any symptoms not included in the survey. Surveys were collected from individuals with FA from March 23, 2020, to May 2, 2020. Surveys were collected from caregivers from April 3, 2020, to August 3, 2020. This same methodology has previously been used and described during studies of other neurologic disease populations.9-15
Standard Protocol Approvals, Registrations, and Patient Consents
All aspects of this research were approved by the University of Rochester Research Subjects Review Board.
Statistical Analysis
The prevalence and average impact of each symptom and symptomatic theme was calculated for each of the phase 2 sample populations. The average impact is a metric of the relative importance of a symptom or symptomatic theme to an individual and is measured on a 0- to 4-point scale. Numerical values were assigned to each response as follows: 0 = the individual experiences the symptom, but it does not affect the individual's life; 1 = the symptom affects the individual's life a little; 2 = the symptom affects the individual's life moderately; 3 = the symptom affects the individual's life very much; and 4 = the symptom affects the individual's life severely. Average life impact scores for each symptom and symptomatic theme were generated for all participants who reported that they experience the symptom or symptomatic theme. A higher score represents a symptom or symptomatic theme that has a greater effect on the lives of individuals with FA. In addition, a population impact score for each symptom was generated by multiplying the average prevalence by the average impact of the symptom. The possible range for this score is also 0 to 4, with a value of 4 representing a symptom that affects all individuals with FA at the highest level. We have previously used and described these statistical analyses for other neurologic conditions.9-15
Responses from individuals with FA and caregivers on behalf of children with FA were analyzed on the basis of patient age (above vs below the mean reported age), sex (male vs female), age of symptom onset (above vs below the mean reported age), speech (can talk clearly with no changes to their speech vs experiences changes to their speech), muscle stiffness (experiences muscle stiffness vs does not experience muscle stiffness), and functional staging for ataxia (0–4.0 vs >4.0).16 For individuals with FA, an additional analysis was conducted based on employment (on disability vs not on disability). For caregivers of individuals with FA, an additional analysis was conducted based on heart problems (the individual with FA experiences heart problems vs does not experience heart problems).
We obtained the prevalence and average impact of each symptom and symptomatic theme for the entire sample and for each subgroup in both of our sample populations. We used Fisher exact tests to compare the prevalence of each theme across the different subgroups. Statistical significance was defined as a value of p < 0.05. Spearman rank correlations were used to determine the relationship between 3 GAA repeat length variables: the length of the GAA expansion on the smaller allele, the length of the GAA expansion on the larger allele, and the average length of the GAA expansion on both alleles with age of symptom onset, age when first noticed changes in sensation, and functional staging for ataxia. To correct for multiple comparisons, the Benjamini-Hochberg procedure was used with a false discovery rate of 0.05 and 289 test statistics.17 As outlined by this method, the 289 p values were sorted from smallest to largest and the largest value of i such that p(i) ≤ 0.05 i/289 was determined. The null hypotheses associated with the p values p(1), …, p(i) were rejected, resulting in i discoveries.
Data Availability
Additional anonymized data not included in the article or supplemental figures can be obtained through request to the corresponding author.
Results
Phase 1: Semistructured Qualitative Interviews
We conducted qualitative interviews with 15 adults with FA, 14 minors with FA, and 10 FA caregivers. Demographic characteristics of the phase 1 sample population are provided in Table 1. Each interview was 30–60 minutes in length. Through transcript analysis, we identified 2,527 direct quotes identifying the potential symptoms of importance to individuals with FA. Each quote identified was coded as a unique symptom of FA health. An example of how participant quotes were coded as unique symptoms is as follows: when a participant was asked, “what symptoms have the greatest impact on your quality of life or disease burden?,” an adult participant with FA 8 replied, “the greatest one is loss of balance.” This quote was coded as the symptom, impaired balance. Fifteen adult participants with FA provided 1,152 quotes, identifying 433 individual symptoms that have a significant effect on their daily lives. Fourteen participants who were minors with FA provided 571 quotes, identifying 199 individual symptoms that have a significant effect on their daily lives. Ten caregivers of pediatric individuals with FA provided 804 quotes, identifying 284 individual symptoms that they observe having a significant effect on the lives of their children with FA. On completion of phase 1 data collection, all interviews were analyzed for data saturation. Two hundred forty-five symptoms of importance representing 20 symptomatic themes were selected to be included in a survey for patients with FA in phase 2 of the study. One hundred ninety-six symptoms of importance representing the same 20 symptomatic themes were selected to be included in a survey for caregivers of pediatric patients with FA.
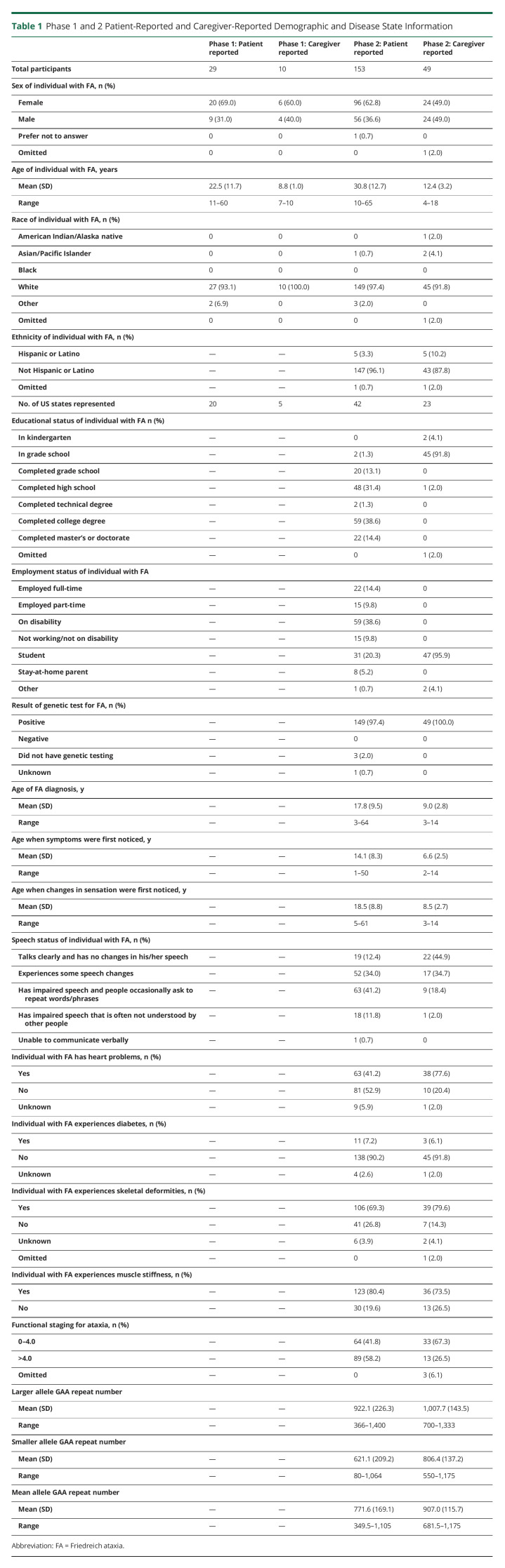
Table 1.
Phase 1 and 2 Patient-Reported and Caregiver-Reported Demographic and Disease State Information
Phase 2: Two Cross-Sectional Studies of Individuals With FA and Caregivers of Pediatric Individuals With FA
Two hundred one individuals with FA and 53 caregivers accessed the survey links to participate in phase 2 of this research. Participant responses were included in the data analysis if the participant completed at least 1 demographic question and 1 survey question. A total of 153 individuals with FA and 49 caregivers satisfied these criteria. Details regarding the demographic information of phase 2 participants are provided in Table 1.
Prevalence of Symptomatic Themes and Symptoms
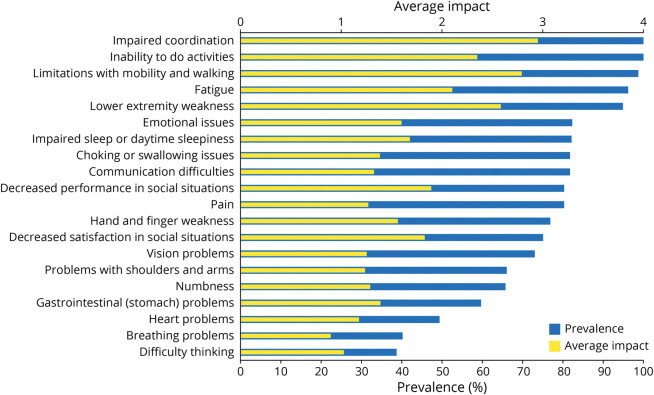
The symptomatic themes that had the greatest prevalence in individuals with FA were impaired coordination (100%), inability to do activities (100%), limitations with mobility and walking (98.7%), fatigue (96.1%), and lower extremity weakness (94.7%). Details regarding the prevalence of all 20 symptomatic themes are provided in Figure 1. Among the 245 symptoms evaluated individually in the patient population with FA, 11 symptoms were experienced by 100% of participants. These included difficulty running, loss of balance, difficulty walking long distances, difficulty playing sports, difficulty standing when your eyes are closed, problems walking, difficulty going downstairs, impaired coordination, difficulty moving quickly, difficulty walking on rough ground, and inability to do activities. Details regarding the prevalence of all 245 symptoms assessed in the patient population with FA are provided in eTable 1 (links.lww.com/WNL/C496).
Figure 1. Prevalence and Average Impact of Symptomatic Themes Reported by Individuals With Friedreich Ataxia.
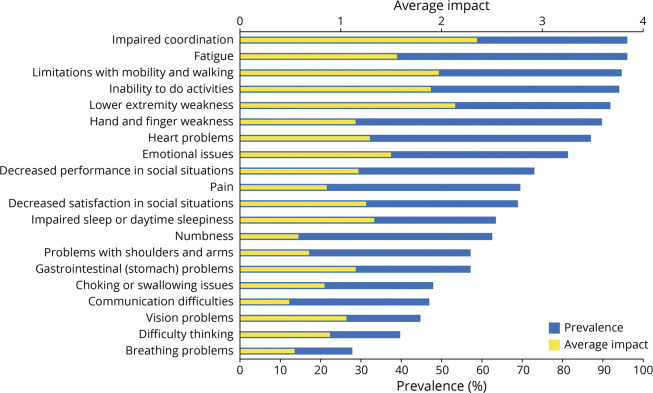
The symptomatic themes that were reported with the greatest prevalence by the FA caregiver population on behalf of patients with FA aged 0–18 years were impaired coordination (95.9%), fatigue (95.9%), limitations with mobility and walking (94.6%), inability to do activities (93.9%), and lower extremity weakness (91.8%). Details regarding the prevalence of all 20 symptomatic themes are provided in Figure 2. Among the 196 symptoms evaluated individually among FA caregivers, 9 symptoms were reported to have a prevalence of >95.5%. These included difficulty playing sports (97.8%), difficulty carrying drinks without spilling (97.7%), frustration (97.7%), difficulty jumping (97.3%), tripping (97.3%), difficulty standing with their eyes closed (97.2%), impaired coordination (95.9%), fatigue (95.9%), and difficulty catching things (95.6%). Details regarding the prevalence of all 196 symptoms assessed in the FA caregiver population are provided in eTable 2 (links.lww.com/WNL/C496).
Figure 2. Prevalence and Average Impact of Symptomatic Themes Reported by Friedreich Ataxia Caregivers.
Average Impact of Symptomatic Themes and Symptoms
Among individuals with FA, the symptomatic themes with the highest average impact scores (0–4) were impaired coordination (2.94), limitations with mobility or walking (2.78), and lower extremity weakness (2.57). Details regarding the average impact scores for all 20 symptomatic themes are provided in Figure 1. Among the 245 individually evaluated symptoms in individuals with FA, the following had the highest average impact scores: impaired balance (3.31), difficulty running (3.25), fatigue with walking distance (3.23), loss of balance (3.22), and difficulty walking long distances (3.12). Details regarding the average impact scores of all 245 symptoms evaluated in the patient population with FA are provided in eTable 1 (links.lww.com/WNL/C496).
Among the FA caregiver population, the symptomatic themes with the highest average impact scores were impaired coordination (2.34), lower extremity weakness (2.13), and limitations with mobility and walking (1.97). Details regarding the average impact scores of all 20 symptomatic themes assessed in the FA caregiver population are provided in Figure 2. Among the 196 individually evaluated symptoms in caregivers on behalf of pediatric patients with FA, the following symptoms had the highest average impact scores: impaired balance (2.90), difficulty standing when their eyes are closed (2.86), difficulty playing sports (2.82), difficulty running (2.77), and difficulty walking long distances (2.71). Details regarding the average impact scores for all 196 individually evaluated symptoms in the FA caregiver population are provided in eTable 2 (links.lww.com/WNL/C496).
Population Impact of Symptomatic Themes and Symptoms
Among individuals with FA, the symptomatic themes with the highest population impact scores (0–4) were impaired coordination (2.94), limitations with mobility and walking (2.74), lower extremity weakness (2.43), and inability to do activities (2.34). Among the individually evaluated symptoms in the patient population with FA, the following had the highest population impact scores: impaired balance (3.25), difficulty running (3.25), loss of balance (3.22), difficulty walking long distances (3.12), and difficulty playing sports (3.05).
Among the FA caregiver population, the symptomatic themes with the highest population impact scores (0–4) were impaired coordination (2.24), lower extremity weakness (1.96), limitations with mobility and walking (1.86), and inability to do activities (1.78). Among the symptoms evaluated individually in the caregiver population, the following had the highest population impact scores: difficulty standing when their eyes are closed (2.78), impaired balance (2.77), difficulty playing sports (2.76), difficulty running (2.62), and difficulty walking long distances (2.57).
Patient-Reported Subgroup Analysis of Symptomatic Theme Prevalence
After correcting for multiple comparisons, there were several symptomatic themes that differed in prevalence based on patient-reported demographic subgroup analysis.
The clinical feature with the greatest association with patient-reported symptomatic burden was muscle stiffness. Twelve of the 20 symptomatic themes assessed were more prevalent in individuals with FA who experienced muscle stiffness vs those who did not. The symptomatic theme with the greatest difference in prevalence was problems with shoulders and arms. Those who experienced muscle stiffness also experienced problems with their shoulders and arms at a rate of 77.4%, while those without muscle stiffness experienced it at 17.9%.
Five of the 20 symptomatic themes assessed were more prevalent in individuals with FA who self-reported being more severe in their functional staging for ataxia (>4.0) vs those who reported as less severe in their functional staging for ataxia (0–4.0).16 The symptomatic theme with the largest difference in prevalence between these subgroups was problems with shoulders and arms. Those with a functional staging for ataxia from 0 to 4.0 experienced problems with shoulders and arms at a rate of 46.9%, while those with a functional staging for ataxia >4.0 experienced this symptomatic theme at a rate of 79.8%.
There were 2 symptomatic themes that differed in prevalence based on patient age, employment status, and speech status. These 2 symptomatic themes were choking and swallowing issues and communication difficulties. Both of these symptomatic themes had a higher prevalence in individuals with FA who were above the mean age (30.8 years), reported their employment status as on disability, and experienced some degree of speech impairment.
There were no differences in patient-reported symptomatic theme prevalence based on sex or age of symptom onset. Of all the symptomatic themes, the presence of choking or swallowing issues was the most likely to differentiate between predefined groups. Full details regarding the symptomatic theme prevalence and p values for patient-reported subgroup analysis are provided in Table 2.
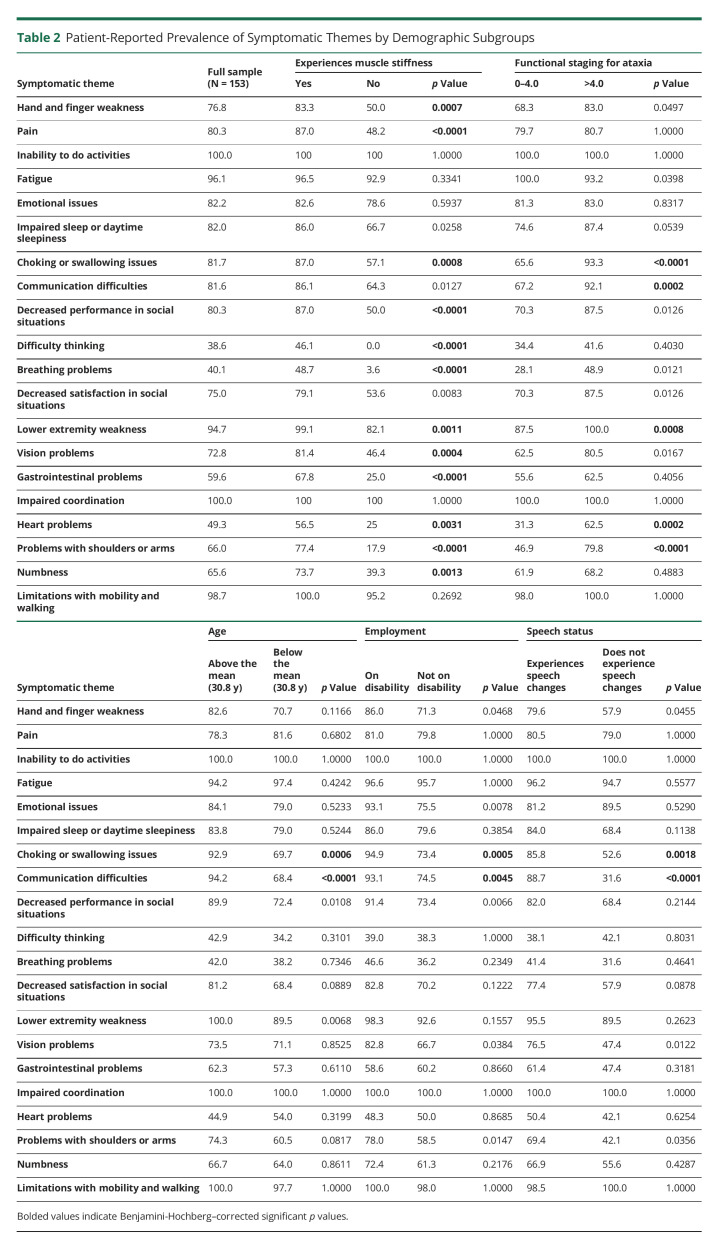
Table 2.
Patient-Reported Prevalence of Symptomatic Themes by Demographic Subgroups
Caregiver-Reported Subgroup Analysis of Symptomatic Theme Prevalence
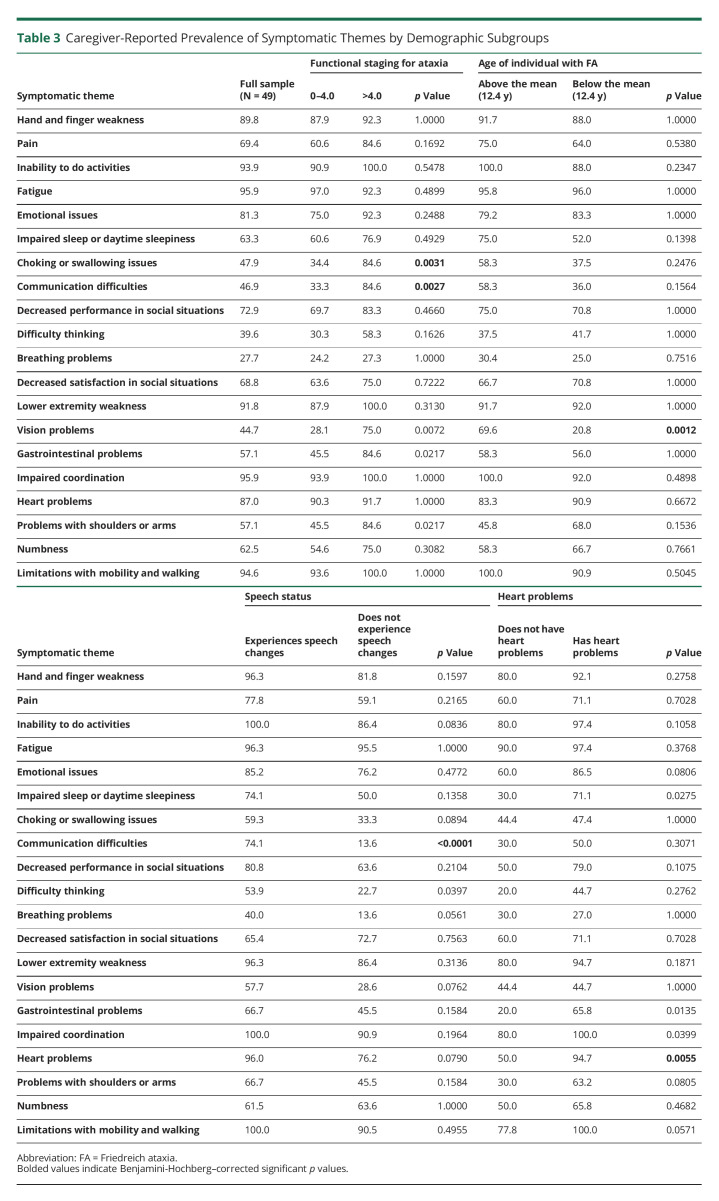
In assessing symptomatic theme prevalence in individuals with FA aged 0–18 years as reported by their caregivers, it was found that several symptomatic themes differed based on demographic subgroup analysis. Two of the 20 symptomatic themes were more prevalent in individuals who were reported as more severe on the functional staging for ataxia scale (>4.0) vs those who were reported as less severe (0–4.0).16 Caregivers who reported that their child had a functional staging for ataxia >4.0 also reported their child experiencing choking or swallowing issues and communication difficulties at a higher rate than those reported at 0–4.0. The symptomatic theme with the greatest difference in prevalence between these subgroups was communication difficulties, which affected children with FA in the >4.0 group at a rate of 84.6%, and children with FA in the 0–4.0 group at a rate of 33.3%.
The only symptomatic theme that differed in prevalence based on the age of pediatric individuals with FA was vision problems. Vision problems affected those above the mean reported age (12.4 years) at a rate of 69.6% and those below the mean reported age at 20.8%.
The only symptomatic theme that differed in prevalence based on speech status was communication difficulties. Pediatric individuals with FA who were reported to have no changes to their speech experienced this symptomatic theme at a rate of 13.6%, while those who were reported to have changes to their speech experienced this symptomatic theme at 74.1%.
There were no differences in caregiver-reported symptomatic theme prevalence based on sex, age of symptom onset, or muscle stiffness. Full details regarding the symptomatic theme prevalence and p values for caregiver-reported subgroup analysis are provided in Table 3.
Table 3.
Caregiver-Reported Prevalence of Symptomatic Themes by Demographic Subgroups
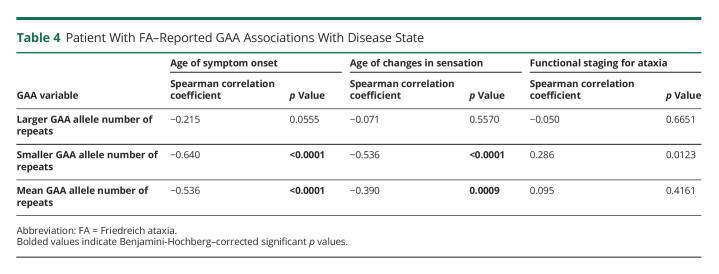
FXN Gene GAA Repeat Length Correlations
Among the FA patient population, there were 4 statistically significant associations with GAA expansion length and age-independent markers of disease burden. The length of the GAA expansion on the smaller allele was associated with patient age of symptom onset and the age at which changes in sensation were first experienced. The mean length of both GAA expansions was also associated with age of symptom onset and age at which changes in sensation were first experienced. Spearman rank correlations and p values for each pair of variables are provided in Table 4.
Table 4.
Patient With FA–Reported GAA Associations With Disease State
Discussion
This research provides one of the largest datasets regarding disease burden in FA and provides baseline data, which can be widely used by researchers, clinicians, and patients who struggle with this disease. In this study, individuals with FA and caregivers of pediatric patients with FA identified the symptoms and symptomatic themes that have the most important and widespread effects on the lives of individuals with FA. In this study, we demonstrate a phenotypic profile in FA that is unique from other neurologic disease populations studied with identical methodology.9-15 These data provide the FA community with valuable information to better understand what symptoms are most important to individuals with FA.
The results of our subgroup analyses provide insight into how certain symptomatic themes differ in prevalence based on FA participant characteristics. We found that the patient-reported prevalence of choking and swallowing issues was associated with muscle stiffness, age, employment status, speech status, and functional staging for ataxia. In addition, we found that the prevalence of communication difficulties was associated with age, employment status, speech status, and functional staging for ataxia. Of note, some symptomatic themes had a very high average prevalence across the entire study population (e.g., impaired coordination, inability to do activities, limitations with mobility and walking, and fatigue), which likely contributed to their inability to differentiate between subgroups.
Individuals with FA and caregivers both identified impaired coordination, limitations with mobility and walking, inability to do activities, fatigue, and lower extremity weakness as the top 5 most prevalent and impactful symptomatic themes. This highlights that in this disease population, the symptomatic themes that are the most common also tend to be the themes that are the most burdensome; a situation that is not overly common in patients with neurologic disease.9-15 In addition, this finding also highlights that the symptoms that caregivers identify in patients at a young age are also those that continue to generate disease burden later in life.
Of interest, the report of muscle stiffness by patients was the clinical feature that had the most widespread association with the prevalence of all symptomatic themes. Additional research will be helpful to further elicit the relationship between this cardinal symptom and the occurrence of other physical, emotional, social, and disease-specific symptoms in FA. In particular, it may be of interest to observe whether the occurrence of these other common life-altering FA symptoms can be changed or lessened by treatments designed to reduce muscle stiffness in this population.
One of the goals of this study was to explore associations between GAA1 and GAA2 repeat lengths in the FXN gene and markers of FA disease severity. Prior reports have hypothesized that the smaller of the 2 GAA expansions has the greatest association with disease progression.7 Using the age of symptom onset, the age at which changes in sensation were first experienced, and functional staging for ataxia as age-independent measures of disease severity, we found that the GAA expansion of the smaller allele and the mean GAA expansion of both alleles had the strongest association with disease severity. Among individuals with FA who self-reported their symptoms, we found that the GAA expansion of the smaller allele was associated with the age of symptom onset and age at which changes to sensation were experienced. As the GAA repeat length of the smaller allele increased, individuals with FA tended to experience symptom onset and changes to sensation at a younger age. We also found that the average length of GAA expansions on both alleles was an indicator of FA phenotype in 2 of the 3 markers of disease burden that were assessed. The average length of GAA expansions on both alleles was inversely correlated with participant age of symptom onset and age at which changes in sensation were experienced. As the average length of GAA expansions on both alleles increased, individuals with FA tended to experience symptom onset and changes to sensation at a younger age. Among individuals with FA who self-reported their symptoms, there were no significant correlations found in the length of the GAA expansion on the larger allele in any markers of disease burden that were analyzed. These findings support prior research that has explored GAA expansion lengths as markers of FA disease phenotype.7
We acknowledge that a limitation of this research is that our sample may not perfectly represent the general population with FA. Those without access to the Internet because of technical or socioeconomic factors were likely underrepresented in our data. Because of the format of the survey and the volume of responses, it is possible that individuals with more advanced FA and caregivers who were too overburdened to complete the survey may be underrepresented in our sample population as well. Despite these limitations, we suspect that our sample population adequately represents individuals with FA and caregivers who would be willing to participate in future research studies. Last, it should be noted that both individuals with FA and caregivers reported their GAA expansion length and functional staging for ataxia. While it is reasonable to believe that they could accurately report both, it is possible that errors in understanding or reporting occurred in the absence of secondary verification.
The results from this research significantly add to existing knowledge of the multifaceted disease burden faced by children and adults with FA. A fundamental understanding of the widespread occurrence and importance of the symptoms that those with FA face in their daily lives is relevant to those who intend to provide clinical care for this population. Knowledge of these symptoms is also relevant for those in the process of developing novel therapeutics for FA and for those who wish to research and study therapies to reduce the symptomatic burden of this disease.
Glossary
- CHOP
Children's Hospital of Philadelphia
- FA
Friedreich ataxia
- FARA
Friedreich's Ataxia Research Alliance
- HIPAA
Health Insurance Portability and Accountability Act
Appendix. Authors
Study Funding
This research was funded by the Friedreich's Ataxia Research Alliance (FARA).
Disclosure
J. Seabury, D. Alexandrou, N. Dilek, B. Greco, and J. Heatwole report no disclosures relevant to the manuscript. J. Larkindale is a paid employee of PepGen Inc. D.R. Lynch receives grant support from the NIH, FDA, Friedreichs Ataxia Research Alliance, Reata Pharmaceuticals, Retrotope, PTC Therapeutics, and Design Pharmaceuticals. C. Park and S. Rosero report no disclosures relevant to the manuscript. S.H. Subramony receives research support from the NIH, FDA, Wyck, Friedreichs Ataxia Research Alliance, Muscular Dystrophy Association, Facioscapulohumeral Muscular Dystrophy Society, National Ataxia Foundation, Reata, PTC, Retrotope, Biohaven, Takeda, Acceleron, Pharnext, Avidity, and Reneo; he has provided consultation or served on advisory boards for Dyne, Avidity, Reata, and Avexis. A. Varma, E. Wagner, S. Walther, J. Weinstein, and M. Wells report no disclosures relevant to the manuscript. C. Zizzi has provided consultation to Recursion Pharmaceuticals. C. Heatwole receives royalties for the use of multiple disease-specific instruments. He has provided consultation to Biogen Idec, Ionis Pharmaceuticals, aTyr Pharma, AMO Pharma, Acceleron Pharma, Cytokinetics, Expansion Therapeutics, Harmony Biosciences, Regeneron Pharmaceuticals, Astellas Pharmaceuticals, AveXis, Recursion Pharmaceuticals, IRIS Medicine, Inc., Takeda Pharmaceutical Company, Scholar Rock, Avidity Biosciences, Novartis Pharmaceuticals Corporation, SwanBio Therapeutics, and the Marigold Foundation; he receives grant support from the Department of Defense, Duchenne UK, Parent Project Muscular Dystrophy, Recursion Pharmaceuticals, Swan Bio Therapeutics, the National Institute of Neurological Disorders and Stroke, the Muscular Dystrophy Association, the Friedreichs Ataxia Research Alliance, Cure Spinal Muscular Atrophy, and the Amyotrophic Lateral Sclerosis Association. Go to Neurology.org/N for full disclosures.
References
- 1.Delatycki MB, Williamson R, Forrest SM. Friedreich ataxia: an overview. J Med Genet. 2000;37(1):1-8. doi: 10.1136/jmg.37.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ghorbani M, Pousset F, Tucker A, et al. Analysis of Friedreich's ataxia patient clinical data reveals importance of accurate GAA repeat determination in disease prognosis and gender differences in cardiac measures. Inform Med Unlocked. 2019;17:100266. doi: 10.1016/j.imu.2019.100266. [DOI] [Google Scholar]
- 3.Schmucker S, Puccio H. Understanding the molecular mechanisms of Friedreich's ataxia to develop therapeutic approaches. Hum Mol Genet. 2010;19(R1):R103-R110. doi: 10.1093/hmg/ddq165. [DOI] [PubMed] [Google Scholar]
- 4.Dürr A, Cossee M, Agid Y, et al. Clinical and genetic abnormalities in patients with Friedreich's ataxia. N Engl J Med. 1996;335(16):1169-1175. doi: 10.1056/NEJM199610173351601. [DOI] [PubMed] [Google Scholar]
- 5.Lynch DR, Farmer JM, Balcer LJ, Wilson RB. Friedreich ataxia: effects of genetic understanding on clinical evaluation and therapy. Arch Neurol. 2002;59(5):743-747. doi: 10.1001/archneur.59.5.743. [DOI] [PubMed] [Google Scholar]
- 6.Koeppen AH. Friedreich's ataxia: pathology, pathogenesis, and molecular genetics. J Neurol Sci. 2011;303(1-2):1-12. doi: 10.1016/j.jns.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Filla A, De Michele G, Cavalcanti F, et al. The relationship between trinucleotide (GAA) repeat length and clinical features in Friedreich ataxia. Am J Hum Genet. 1996;59(3):554-560. [PMC free article] [PubMed] [Google Scholar]
- 8.Walters S. Assessing Quality of Life in Clinical Trials: 2nd edition Analysis and Interpretation. Peter Fayers and Ron Hays (eds). Oxford University Press, 2005, pp. 467, ISBN: 0-19-852769-1. Int J Epidemiol. 2005;34(6):1447-1448. doi: 10.1093/ije/dyi221. [DOI] [Google Scholar]
- 9.Heatwole C, Bode R, Johnson N, et al. Patient-reported impact of symptoms in myotonic dystrophy type 1 (PRISM-1). Neurology. 2012;79(134):348-357. doi: 10.1212/WNL.0b013e318260cbe6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heatwole C, Johnson N, Bode R, et al. Patient-reported impact of symptoms in myotonic dystrophy type 2 (PRISM-2). Neurology. 2015;85(24):2136-2146. doi: 10.1212/WNL.0000000000002225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson NE, Heatwole CR, Dilek N, et al. Quality-of-life in Charcot-Marie-Tooth disease: the patient's perspective. Neuromuscul Disord. 2014;24(11):1018-1023. doi: 10.1016/j.nmd.2014.06.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mongiovi P, Dilek N, Garland C, et al. Patient reported impact of symptoms in spinal muscular atrophy (PRISM-SMA). Neurology. 2018;91(13):e1206-e1214. doi: 10.1212/wnl.0000000000006241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Glidden AM, Luebbe EA, Elson MJ, et al. Patient-reported impact of symptoms in Huntington disease: PRISM-HD. Neurology. 2020;94(19):e2045-e2053. doi: 10.1212/WNL.0000000000008906. [DOI] [PubMed] [Google Scholar]
- 14.Hamel J, Johnson N, Tawil R, et al. Patient-reported symptoms in facioscapulohumeral muscular dystrophy (PRISM-FSHD). Neurology. 2019;93(12):e1180-e1192. doi: 10.1212/WNL.0000000000008123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson NE, Quinn C, Eastwood E, Tawil R, Heatwole CR. Patient-identified disease burden in facioscapulohumeral muscular dystrophy. Muscle Nerve. 2012;46(6):948-950. doi: 10.1002/mus.23529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Subramony SH, May W, Lynch D, et al. Measuring Friedreich ataxia: interrater reliability of a neurologic rating scale. Neurology. 2005;64(7):1261-1262. doi: 10.1212/01.WNL.0000156802.15466.79. [DOI] [PubMed] [Google Scholar]
- 17.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Methodol. 1995;57(1):289-300. doi: 10.1111/j.2517-6161.1995.tb02031.x. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Additional anonymized data not included in the article or supplemental figures can be obtained through request to the corresponding author.