Abstract
Objectives
Zinc is considered an essential multipurpose trace element because of its ability to act as a cofactor and signaling molecule. As reported in earlier studies of pediatric respiratory infection management, zinc exhibits potent immunoregulatory and antiviral properties, but its effects on pediatric patients with COVID-19 remain unknown. The aim of this study was to determine the extent to which zinc supplementation improves COVID-19 symptoms, length of hospitalization and, to determine how zinc supplementation impacts ICU admission, in-hospital mortality, need for ventilation, duration of ventilation, need for vasopressors, development of liver injury, or respiratory failure.
Methods
Pediatric patients younger than 18 years with confirmed COVID-19 infection during the study period (March 1, 2020, to December 31, 2021) were recruited for this retrospective cohort study. The study population was divided into two arms (zinc/no zinc supplementation as an adjunct to standard therapy).
Results
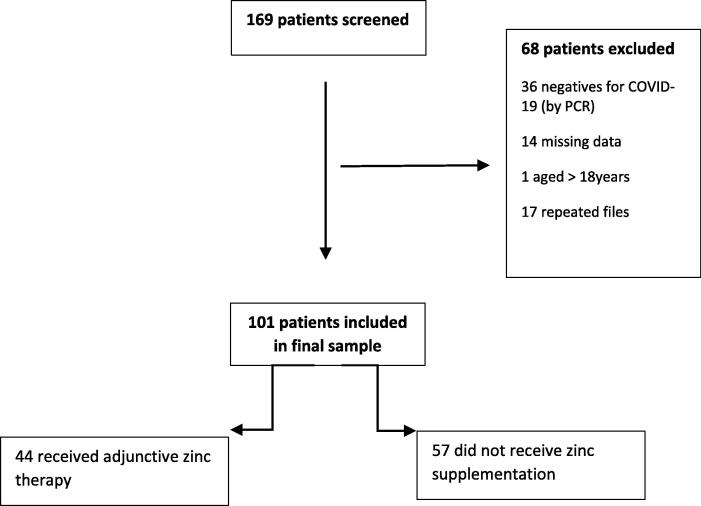
Of 169 hospitalized patients who were screened, 101 met the inclusion criteria. No statistically significant association was found between the administration of zinc as adjunctive therapy and symptom reduction, intensive care unit (ICU) admission, or mortality (p = 0.105; p = 0.941, and p = 0.073, respectively). However, zinc supplementation was associated with a statistically significant reduction in respiratory failure and length of hospitalization (p = 0.004 and p = 0.017, respectively), also, zinc administration was associated with elevated serum creatinine (p = 0.01*)
Conclusions
Among pediatric patients with COVID-19, zinc supplementation was associated with shorter hospital stay. However, there was no significant difference between the two groups in terms of symptom improvement, in-hospital mortality, or ICU admission. In addition, the study raises question about the possibility of kidney injury as indicated by high levels of serum creatinine.
Keywords: Zinc, Dietary Supplements, COVID-19, Child, Creatinine
1. Introduction
The outbreak of the new coronavirus was first reported in December 2019. (Carlos et al., 2020, Tang et al., 2020) The World Health Organization (WHO) declared the outbreak of Coronavirus Disease 2019 as a global pandemic on March 11, 2020. (Huang et al, 2020) COVID-19 is a highly contagious infectious disease transmitted by indirect contact with an infected surface or directly by close contact (<1.5 m) with an infected person. COVID-19 causes a severe acute respiratory syndrome that ranges from asymptomatic to severe and life-threatening (Coronavirus disease (covid-19), 2020, Huang et al., 2020, Shereen et al., 2020).
Severe symptoms are associated with high mortality, acute respiratory distress syndrome (ARDS), and acute cardiac, liver, and kidney injuries. (Shereen et al., 2020, Wu and McGoogan, 2020) Most reported cases of COVID-19 are in adults rather than pediatric patients. (Ding et al., 2020, Tagarro et al., 2021) In a multicenter, cross-sectional retrospective study conducted in six centers in Saudi Arabia found that only 4.76% of the patients had moderate-to-severe disease with the rest either asymptomatic or had mild disease. (AlGhamdi et al, 2022) According to the currently available evidence, pediatric COVID-19 accounts for 0.8%–2% of global cases. (Qiu et al., 2020, Lu et al., 2020) In children, COVID-19 triggers an inflammatory immune response that can lead to life-threatening complications such as multisystem inflammatory syndrome (MIS-C). (Riphagen et al., 2020, Verdoni et al., 2020) This inflammatory immune response is associated with a proinflammatory cytokine storm and immune dysregulation, producing acute respiratory distress syndrome (ARDS) and leading eventually to multiorgan failure. (Azkur et al., 2020, Hamer et al., 2020, Jordan et al., 2020, Su et al., 2016, Weiss and Leibowitz, 2011, Wu et al., 2020) However, most pediatric patients exhibit mild to moderate symptoms and are hospitalized less often than adults. (Wang E, Brar K,2020).
Zinc is an essential trace element for maintenance of immunological response, (Lee SR, 2018) with recognized benefits when used directly or indirectly against respiratory viruses. It has also been observed to improve the treatment of pneumonia in children, (Acevedo-Murillo et al., 2019, Barnett et al., 2010, Skalny et al., 2020) and elevated intracellular zinc levels directly inhibit viral RNA-dependent polymerase in cases of SARS-CoV. (Barnard et al., 2007, Read et al., 2019, Pormohammad et al., 2020, Te Velthuis et al., 2010) Zinc can also have a synergistic effect when combined with anti-viral drugs to combat SARS-CoV. (Kumar et al,2020).
Another study investigated the effect of zinc sulfate in adult patients with COVID-19 when added to hydroxychloroquine and azithromycin. They reported an increased frequency of patients discharged and decreased need for ventilation or admission to ICU when compared to patients who received the standard therapy alone. (Carlucci et al,2020) In another recent study examining the effect of zinc sulfate as an adjunctive therapy in adult patients with COVID-19, zinc supplementation was found to have a potential survival benefit. However, the results were not statistically significant, and the use of zinc was linked to an increased risk of acute kidney injury (AKI) during ICU stay.(Al Sulaiman K et al, 2021) In a study of more than three thousand hospitalized adult COVID-19 patients who received zinc supplements, Jennifer et al. reported an increased rate of discharge to home and a 24% reduction in risk of in-hospital mortality but no significant reduction in all-cause mortality.(Jothimani D et al, 2020) There are some reports of an association between low serum zinc levels and severe ARDS among patients with COVID-19. (Gonçalves et al, 2020) Other evidence suggests that low baseline zinc levels in COVID −19 patients are associated with prolonged hospitalization and increased mortality when compared to COVID-19 patients with normal zinc levels, but there are currently no data on the improving effect of zinc supplements following admission. (Frontera et al, 2020) Another meta-analyses were conducted to investigate the effect of micronutrients such as zinc, vitamin C, and vitamin D in term of mortality in adult patients infected with COVID-19, they found zinc supplementation was not linked with reduction in mortality. (Beran et al, 2022) To date, too little attention has been paid to the role of zinc supplementation as an adjunctive therapy for pediatric COVID-19 patients. As the first attempt to investigate this issue, the aim of the present study was to evaluate the efficacy of zinc supplements as an adjunctive therapy for hospitalized pediatric patients with COVID-19 in terms of symptom reduction from day 1 to discharge or death, ICU admission, respiratory failure, and liver injury.
2. Method
2.1. Study design
A retrospective cohort study of hospitalized pediatric patients with COVID-19 was conducted between March 1, 2020, and December 31, 2021. Patients were included in the study if they were hospitalized younger than 18 years of age, with a confirmed positive PCR test for COVID-19. Patients were excluded if there was missing data (such as lost medical records, or no details written regarding the patient COVID-19 symptoms).
2.2. Setting
The study was conducted at the Maternity and Children’s Hospital, Makkah, Saudi Arabia. This tertiary government hospital in the western region of Saudi Arabia has a bed capacity of more than four hundred. As the largest pediatric hospital in Makkah region, it provides health services for neonates, pediatric patients, and pregnant women. The study subjects were recruited from pediatric medical wards, the pediatric intensive care unit, the neonate intensive care unit, and the pediatric emergency room during the period March 2020–December 2021.
2.3. Study population
The study population was divided into two groups: those who received zinc supplement from the day of hospital admission at dose of 1 mg per kg daily as an adjunct to standard therapy and those who received standard therapy alone according to Saudi ministry of health Protocol for Patients Confirmed with COVID-19. (Saudi MoH Protocol, 2023).
2.4. Primary objective
To determine the extent to which zinc supplementation improves COVID-19 symptoms (nasal discharge, sneezing, cough, dyspnea, diarrhea, vomiting or abdominal pain, anosmia, headache, conjunctivitis, cutaneous rash, neurological seizure), and length of hospitalization.
2.5. Secondary objective
To determine how zinc supplementation impacts ICU admission, in-hospital mortality, need for ventilation, duration of ventilation, need for vasopressors, development of liver injury, and development of respiratory failure.
2.6. Ethic al approval
Was obtained from the Ministry of Health Institutional Review Board, Makkah, Saudi Arabia (Number: H-02-k-076–0222-667). Informed consent was not required, as this was a retrospective non-intervention study. All research procedures followed the relevant guidelines and regulations.
2.7. Data collection
Data inserted in an Excel sheet included patient demographics, medication, laboratory tests, comorbidities, signs, symptoms, length of hospitalization, ICU admission, mortality, and need for ventilation. The demographic data included gender, age, nationality, and weight. Medications refers to zinc, oxygen, antivirals, antibiotics, and steroids. Laboratory tests included serum creatinine (Scr), erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), alanine aminotransferase (ALT), and international normalized ratio (INR as indication for liver failure or impairment). Comorbidities included diabetes, hypertension, cardiovascular disease, chronic kidney disease, and liver dysfunction. Symptoms included nasal discharge, sneezing, cough, dyspnea, diarrhea, vomiting or abdominal pain, anosmia, headache, conjunctivitis, cutaneous rash, neurological (seizure), and hypoxemia.
2.8. Data management and statistical analysis
The data were analyzed using Statistical Package for the Social Sciences (SPSS) version 23. Categorical variables are presented as frequencies and percentages. The Shapiro-Wilk test was used to assess for normality; all numerical variables were found to be non-normally distributed, including age, length of hospital stays, inflammatory parameters, and laboratory findings. A chi-square test was used to detect any association between the categorical variables. The Mann-Whitney U test was used to determine the difference in numerical factors between the two groups. Univariate logistic regression was used to determine the predictability of laboratory results regarding the incidence of respiratory failure or lung abnormality, need for mechanical ventilation, and need for ICU admission. The level of significance was set at 0.05.
3. Results
3.1. Sociodemographic profiles
In total, 101 patients were included in the study (See Fig. 1).Table 1 shows the sociodemographic profiles of the included patients. Most of the patients were males (57.4%) and (42.6%) were females. of which 69.3% were Saudis. The median age was 32 months; and the interquartile range was 66. Regarding comorbidities, six patients (5.9%) had cardiovascular disease, and four (4%) had liver disease. Table 2 shows the patients’ clinical manifestations on day one of hospital admission and on day of discharge. On day one, the most frequently observed clinical manifestations were fever (98 patients or 97%); diarrhea, vomiting or abdominal pain (53 patients or 52.5%); and sneezing (40 patients or 39.6%). Seven patients (6.9%) were asymptomatic on day one. On day of discharge, 93 patients (95.88%) were asymptomatic, and none had fever, sneezing, hypoxemia, nasal discharge, cutaneous rash, or headache. However, four patients (4.12%) had diarrhea, vomiting, or abdominal pain; two (2.06%) had dyspnea; two (2.06%) had a cough; one (1.03%) had seizures; one (1.06%) had anosmia; and one (1.03%) had conjunctivitis.
Fig. 1.
Study overview.
Table 1.
Participant sociodemographic profile (N = 101).
| Demographical Characteristics | N | % | ||||
|---|---|---|---|---|---|---|
| Gender | n | % | ||||
| Male | 58 | 57.40 | ||||
| Female | 43 | 42.60 | ||||
| Nationality | ||||||
| Saudi | 70 | 69.30 | ||||
| Non-Saudi | 31 | 30.70 | ||||
| Comorbidities | ||||||
| Cardiovascular disease Liver disease Diabetes Chronic kidney disease Hypertension |
6 | 5.9 | ||||
| 4 | 4 | |||||
| 3 | 3 | |||||
| 3 | 3 | |||||
| 1 | 1 | |||||
| Age (months) | ||||||
| Median | 32 | |||||
| Interquartile range | 66 | |||||
Table 2.
Clinical manifestations.
| Clinical manifestation | At day 1 (N = 101) |
At day of discharge (n = 97) |
||
|---|---|---|---|---|
| N | % | n | % | |
| Fever | 98 | 97 | 0 | 0.00 |
| Diarrhea, vomiting or abdominal pain | 53 | 52.5 | 4 | 4.12 |
| Sneezing | 40 | 39.6 | 0 | 0.00 |
| Dyspnea | 36 | 35.6 | 2 | 2.06 |
| Cough | 28 | 27.7 | 2 | 2.06 |
| Neurological (seizure) | 21 | 20.8 | 1 | 1.03 |
| Hypoxemia | 18 | 17.8 | 0 | 0.00 |
| Anosmia | 17 | 16.8 | 1 | 1.03 |
| Nasal discharge | 9 | 8.9 | 0 | 0.00 |
| Cutaneous rash | 9 | 8.9 | 0 | 0.00 |
| Asymptomatic | 7 | 6.9 | 93 | 95.88 |
| Headache | 7 | 6.9 | 0 | 0.00 |
| Conjunctivitis | 2 | 2 | 1 | 1.03 |
3.2. Laboratory parameter profile
Table 3 summarizes patient inflammatory parameters and laboratory profiles. The median ESR (erythrocyte sedimentation rate) was 27.3 mm/h, and the interquartile range was 31.9. The median CRP (C-reactive protein) was 1.6 mg/dl, and the interquartile range was 3.72. The median WBC (white blood cell count) was 10.52 103/IU, and the interquartile range was 7.24. Among those tested for D-dimer (mg/L), 59 (92.2%) returned a positive result, and 5 (7.8%) returned a negative result. The median ALT (alanine transaminase) was 18.25 IU/L, and the interquartile range was 16.67. The median INR (international normalized ratio) was 1.07, and the interquartile range was 0.2. The median Scr (Serum Creatinine) was 36.65 ummol/L, and the interquartile range was 13.14. In terms of treatment, 95 patients (94.1%) received antibiotics; 44 (43.6%) received zinc; 39 (38.6%) received steroids; 34 (33.7%) received anti-viral medications; 27 (26.7%) received oxygen; and 9 (8.9%) received vasopressors.
Table 3.
Inflammatory parameters and participant laboratory profiles (N = 101).
| Inflammatory parameters | Normal level | |
|---|---|---|
| ESR (erythrocyte sedimentation rate) mm/h (n = 85) | < 10 mm/hr | |
| Median | 27.3 | |
| Interquartile range | 31.9 | |
| CRP (C-reactive protein) mg/dl (n = 90) | < 1.6 mg/dl | |
| Median | 1.6 | |
| Interquartile range | 3.72 | |
| WBC (white blood cell count) 103/IU (n = 100) | 0–3 days: 7 –25.7 4–60 days: 3.6 –15.4 61–365 days: 2.5 – 17.8 366–730 days: 3.6 – 16.4 2–6 years 5.0 – 15.5 7–18 years 4.5 – 13.5 |
|
| Median | 10.52 | |
| Interquartile range | 7.24 | |
| D-Dimer mg/L (n = 64) | ||
| Positive | 59 (92.2%) | |
| Negative | 5 (7.8%) | |
| Laboratory profiles | Normal level | |
| ALT (alanine transaminase) IU/L (n = 97) | ≤ 25 U/L for male ≤ 22 U/L for female |
|
| Median | 18.25 | |
| Interquartile range | 16.67 | |
| INR (international normalized ratio) (n = 68) | 1–1.08 | |
| Median | 1.07 | |
| Interquartile range | 0.2 | |
| Scr (serum creatinine) mmol/L (n = 98) | Infant 15–55 Child 25–60 |
|
| Median | 36.65 | |
| Interquartile range | 13.14 | |
3.3. COVID-19 complication profile
Twenty-three (22.8%) of the participating patients had respiratory failure or lung abnormality; 21 (20.8%) were admitted to the ICU; 11 (10.9%) needed mechanical ventilation; and 4 (4%) had liver injury. It is worth mentioning that the median length of hospital stay was 6 days; the interquartile range was 6, and the mean was 9.36 ± 11.07.
3.4. Factors associated with zinc supplementation
Table 4 presents factors associated with the use of zinc as an adjunct treatment for COVID-19. Those who received zinc as an adjunct treatment were significantly older than those who did not (median age = 51 (vs 21); interquartile range = 94 (vs 62)) (p < 0.001). Incidence of fever on the first day of infection was significantly lower among patients who used the zinc as an adjunct treatment (93.2% vs 100%) (p = 0.045) as compared to those who did not. It was also observed that administering zinc notably lowered the incidence of insomnia as compared to those who did not receive zinc (4.5% vs 26.3%) (p = 0.004). One the day of discharge serum creatinine values were significantly higher among those who received zinc as an adjunct treatment as compared to those who did not (median serum creatinine = 38.24 (vs 34.4); interquartile range = 13.33 (vs 12.1) (p = 0.01). Incidence of respiratory failure or lung abnormality was significantly higher among those who did not receive zinc as an adjunct treatment when compared to those who received zinc (33.3% vs 9.1%) (p = 0.004). Length of hospital stay was also significantly lower among those who received zinc as an adjunct treatment (p = 0.017) (median hospital length of stay = 5 days (vs 7 days); interquartile range = 4.75 (vs 8)). Factors not significantly associated with the use of zinc as an adjunct treatment for COVID-19 included gender, nationality, clinical manifestations (other than fever or anosmia), asymptomatic on day of discharge, inflammatory parameters, and laboratory results (other than serum creatinine), ICU admission, liver injury, need for mechanical ventilation, and outcome. Supplementary Tables 1A–C show the results of univariate logistic regression (lab results as predictors of need for ICU admission, need for mechanical ventilation, and respiratory failure or lung abnormality, respectively, as secondary to COVID-19 infection in pediatric patients). None of the lab results in any of the logistic regression models could significantly predict need for ICU admission, need for mechanical ventilation, respiratory failure, or lung abnormality.
Table 4.
Factors associated with zinc supplements and outcomes.
| Factor | Zinc supplement treatment |
P-value | |
|---|---|---|---|
| Received | Not received | ||
| Sociodemographic profile n (%) | |||
| Gender | 0.130 | ||
| Male | 29 (65.9) | 29 (50.9) | |
| Female | 15 (34.1) | 28 (49.1) | |
| Nationality n (%) | 0.276 | ||
| Saudi | 33 (75) | 37 (64.9) | |
| Non-Saudi | 11 (25) | 20 (35.1) | |
| Age in months: median (interquartile range) | 51 (94) | 21 (62) | < 0.001* |
| Clinical manifestations at day 1 (of hospital admission) n (%) | |||
| Fever | 0.045* | ||
| Yes | 41 (93.2) | 57 (1 0 0) | |
| No | 3 (6.8) | 0 (0) | |
| Asymptomatic | 0.407 | ||
| Yes | 2 (4.5) | 5 (8.8) | |
| No | 42 (95.5) | 52 (91.2) | |
| Nasal discharge | 0.517 | ||
| Yes | 3 (6.8) | 6 (10.5) | |
| No | 41 (93.2) | 51 (89.5) | |
| Sneezing | 0.518 | ||
| Yes | 19 (43.2) | 21 (36.8) | |
| No | 25 (58.8) | 36 (63.2) | |
| Cough | 0.719 | ||
| Yes | 13 (29.5) | 15 (26.3) | |
| No | 31 (70.5) | 42 (73.7) | |
| Dyspnea | 0.894 | ||
| Yes | 16 (36.4) | 20 (35.1) | |
| No | 28 (63.6) | 37 (64.9) | |
| Diarrhea, vomiting or abdominal pain | 0.714 | ||
| Yes | 24 (54.5) | 29 (50.9) | |
| No | 20 (45.5) | 28 (49.1) | |
| Anosmia | 0.004* | ||
| Yes | 2 (4.5) | 15 (26.3) | |
| No | 42 (95.5) | 42 (73.7) | |
| Headache | 0.123 | ||
| Yes | 5 (11.4) | 2 (3.5) | |
| No | 39 (88.6) | 55 (96.5) | |
| Conjunctivitis | 0.104 | ||
| Yes | 2 (4.5) | 0 (0) | |
| No | 42 (95.5) | 57 (1 0 0) | |
| Cutaneous rash | 0.517 | ||
| Yes | 3 (6.8) | 6 (10.5) | |
| No | 41 (93.2) | 51 (89.5) | |
| Neurological (seizure) | 0.159 | ||
| Yes | 12 (27.3) | 9 (15.8) | |
| No | 32 (72.7) | 48 (84.2) | |
| Hypoxemia | 0.334 | ||
| Yes | 6 (13.6) | 12 (21.1) | |
| No | 38 (86.4) | 45 (78.9) | |
| Clinical manifestations at day of discharge: median (interquartile range) | |||
| Asymptomatic | 0.105 | ||
| Yes | 43 (97.7) | 51 (89.5) | |
| No | 1 (2.3) | 6 (10.5) | |
| Inflammatory parameters and participant laboratory profiles: median (interquartile range) upon discharge | |||
| ESR (erythrocyte sedimentation rate) | 22.5 (33.77) | 34.5 (37.5) | 0.393 |
| CRP (C-reactive protein) | 1.61 (3.74) | 0.9 (3.9) | 0.981 |
| WBC (white blood cell count) | 10.19 (7.3) | 10.8 (7.21) | 0.783 |
| ALT (alanine transaminase) | 19 (15.97) | 17.5 (17) | 0.113 |
| INR (international normalized ratio) | 1.06 (0.2) | 1.1 (0.27) | 0.315 |
| Scr (serum creatinine) | 38.24 (13.33) | 34.4 (12.1) | 0.01* |
| D-Dimer | 0.141 | ||
| Yes | 27 (87.1%) | 32 (97%) | |
| No | 4 (12.9%) | 1 (3%) | |
| Complications: n (%) | |||
| ICU admission | 0.941 | ||
| Yes | 9 (20.5) | 12 (21.1) | |
| No | 35 (79.5) | 45 (78.9) | |
| Liver injury | 0.791 | ||
| Yes | 2 (50) | 2 (50) | |
| No | 42 (43.3) | 55 (56.7) | |
| Need for mechanical ventilation | 0.610 | ||
| Yes | 4 (9.1) | 7 (12.3) | |
| No | 40 (90.9) | 50 (87.7) | |
| Respiratory Failure or Lung Abnormality | 0.004* | ||
| Yes | 4 (9.1) | 19 (33.3) | |
| No | 40 (90.9) | 38 (66.7) | |
| Duration of hospitalization (days) from ER admission until discharge or death (median, interquartile range) | 5 (4.75) | 7 (8) | 0.017* |
| Outcomes | |||
| Death | 0 (0) | 4 (7) | 0.073 |
| Resolution of infection | 44 (1 0 0) | 53 (93) | |
*Significance level 0.05.
4. Discussion
The present study is the first to investigate the efficacy of zinc supplementation in pediatric patients with COVID-19. The findings indicate a positive association between patients there a symptomatic on day of discharge and zinc supplementation as adjunct therapy. While all the patients in the zinc supplementation group witnessed resolution of their infection, this was not statistically significant. These findings align with previous study of adult patients, which found no association between the use of zinc as adjunctive therapy and being asymptomatic on day of discharge. (Abd-Elsalam et al, 2020) However, the present findings failed to confirm the statistically significant improvement in symptoms reported in Elalfy et al.’s adult study, which compared routine supportive treatment to the effect of a combination of ivermectin, nitazoxanide, and ribavirin plus zinc supplementation. They reported significantly higher viral clearance from the nasopharynx and asymptomatic in the combined treatment group (antiviral plus zinc supplementation). (Elalfy et al, 2021) A retrospective observational study that investigated the effect on length of hospital stay of a combination of hydroxychloroquine, azithromycin, and zinc sulfate as compared to hydroxychloroquine and azithromycin showed that the addition of zinc sulfate had no effect on length of stay. (Carlucci et al, 2020) In our study, however, the length of hospital stay was significantly shorter in the zinc group. Another retrospective observational study by Carlucci et al. showed no difference in overall mortality rates for hydroxychloroquine and azithromycin plus zinc sulfate versus hydroxychloroquine and azithromycin alone. (Wessels et al, 2020) Moreover, our results align with Al Sulaiman et al.’s finding that 30-day mortality was lower among adult patients who received zinc supplementation, although this was not statistically significant. (Carlucci et al,2020) We also found that mortality rates were lower among patients who received zinc supplement than among those who did not receive zinc; again, however, the difference was not statistically significant. The present findings also align with previous evidence that ICU admission and need for mechanical ventilation are not significantly associated with the use of zinc as an adjunct treatment for COVID-19. (Abd-Elsalam et al., 2020, Al Sulaiman et al., 2021) However, we found that the incidence of respiratory failure or lung abnormality was significantly lower when zinc was used as an adjunct treatment. This aligns with earlier evidence that zinc supplementation helps to improve mucociliary clearance, strengthens epithelium integrity, and reduces viral replication, so reducing lung damage. (Wessels I et al, 2020) Contrary to Carlucci et al.’s finding that hydroxychloroquine and azithromycin plus zinc sulfate was not associated with a significantly different rise in serum creatinine as compared to other COVID-19 patients who received hydroxychloroquine and azithromycin alone,. (Carlucci et al,2020) we observed that serum creatinine increased significantly when zinc was used as an adjunct treatment.
5. Conclusions
In this retrospective cohort study, pediatric patients with COVID-19 were treated with an adjunctive therapy of zinc sulfate, and the results were compared to standard therapy. The zinc intervention significantly reduced respiratory failure, lung abnormality, and length of hospitalization. However, as compared to the standard therapy, the intervention failed to significantly improve symptoms, in-hospital mortality, or ICU admission. The results also raise a question about the relationship between zinc administration and elevation of serum creatinine.
6. Study limitations and recommendations
As this was an observational retrospective study, the analysis may have been impacted by confounding variables. In addition, the relatively small sample size, no measurement of serum concentration for zinc, and the fact that this was a single-center study limits generalizability, a multicenter and larger sample size study is strongly recommended to validate the present findings.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Peer review under responsibility of King Saud University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jsps.2023.02.011.
Contributor Information
Ghufran Ibrahim Alhajjaji, Email: Ghufran.alhajjaji@gmail.com, Galhajjaji@moh.gov.sa.
Nouf Alotaibi, Email: Nealotaibi@uqu.edu.sa.
Nada Abutaleb, Email: Ph.nada.abutaleb@gmail.com, NASafhi@moh.gov.sa.
Mishal M. Alotaibi, Email: mmiteb@psmmc.med.sa.
Abdulrahman Alhajjaji, Email: Ph.alhajjaji@gmail.com, S437002470@st.uqu.edu.sa.
Abdulmalik S. Alotaibi, Email: assalotaibi@uqu.edu.sa.
Bashayer Alshehail, Email: bmalshehail@iau.edu.sa.
Moawad E. Alotaibi, Email: me.alotaibi@psmmc.med.sa.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- Abd-Elsalam S, Soliman S, Esmail ES, et al. Do Zinc Supplements Enhance the Clinical Efficacy of Hydroxychloroquine? A Randomized, Multicenter Trial. 2020;199(10):3642-3646 [DOI] [PMC free article] [PubMed] [Retracted]
- Acevedo-Murillo J.A., García León M.L., Firo-Reyes V., et al. Zinc supplementation promotes a th1 response and improves clinical symptoms in fewer hours in children with pneumonia younger than 5 years old. a randomized controlled clinical trial. Front. Pediatr. 2019;7:431. doi: 10.3389/fped.2019.00431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Sulaiman K., Aljuhani O., Al Shaya A., et al. Evaluation of zinc sulfate as an adjunctive therapy in COVID-19 critically ill patients: a two-center propensity-score matched study. Crit. Care. 2021;25(1) doi: 10.1186/s13054-021-03785-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AlGhamdi A., Al Talhi Y., Al Najjar A., Sobhi A., Al Juaid A., Ibrahim A., Alshengeti A., Al-Hebshi A., Farahat F., Al Qurainees G., Al Saif M., Hamdan N., Al Jehani S., Al Mansouri W., AlDabbagh M. Epidemiology, clinical characteristics and risk factors of COVID-19 among children in Saudi Arabia: a multicenter chart review study. BMC Pediatr. 2022 Feb 12;22(1):86. doi: 10.1186/s12887-021-02959-8. PMID: 35151286; PMCID: PMC8840071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azkur A.K., Akdis M., Azkur D., et al. Immune response to SARS-COV-2 and mechanisms of immunopathological changes in Covid-19. Allergy. 2020;75(7):1564–1581. doi: 10.1111/all.14364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnard D.L., Wong M.-H., Bailey K., et al. Effect of oral Gavage treatment with znal42 and other metallo-ion formulations on influenza A H5N1 and H1N1 virus infections in mice. Antiviral Chem. Chemother. 2007;18(3):125–132. doi: 10.1177/095632020701800302. [DOI] [PubMed] [Google Scholar]
- Barnett J.B., Hamer D.H., Meydani S.N. Low zinc status: a new risk factor for pneumonia in the elderly? Nutr. Rev. 2010;68(1):30–37. doi: 10.1111/j.1753-4887.2009.00253.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beran A, Mhanna M, Srour O, Ayesh H, Stewart JM, Hjouj M, Khokher W, Mhanna AS, Ghazaleh D, Khader Y, Sayeh W, Assaly R. Clinical significance of micronutrient supplements in patients with coronavirus disease 2019: A comprehensive systematic review and meta-analysis. Clin Nutr ESPEN. 2022 Apr;48:167-177. doi: 10.1016/j.clnesp.2021.12.033. Epub 2022 Jan 13. PMID: 35331487; PMCID: PMC8755558. [DOI] [PMC free article] [PubMed]
- Carlos W.G., Dela Cruz C.S., Cao B., et al. Novel wuhan (2019-ncov) coronavirus. Am. J. Respir. Crit. Care Med. 2020;201(4):7–8. doi: 10.1164/rccm.2014P7. [DOI] [PubMed] [Google Scholar]
- Carlucci P.M., Ahuja T., Petrilli C., et al. Zinc sulfate in combination with a zinc ionophore may improve outcomes in hospitalized COVID-19 patients. J. Med. Microbiol. 2020;69(10):1228–1234. doi: 10.1099/jmm.0.001250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coronavirus disease (covid-19), 2020. World Health Organization. https://www.who.int/news-room/q-a-detail/q-a-coronaviruses. [accessed 15th October 2021].
- Ding Q., Lu P., Fan Y., et al. The clinical characteristics of pneumonia patients coinfected with 2019 novel coronavirus and influenza virus in Wuhan, China. J. Med. Virol. 2020;92(9):1549–1555. doi: 10.1002/jmv.25781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elalfy H, Besheer T, El-Mesery A, et al. Effect of a combination of nitazoxanide, ribavirin, and ivermectin plus zinc supplement (MANS.NRIZ study) on the clearance of mild COVID-19. 2021;93(5):3176-3183 [DOI] [PMC free article] [PubMed]
- Frontera JA, Rahimian JO, Yaghi S, et al. Treatment with zinc is associated with reduced in-hospital mortality among COVID-19 patients: A multi-center cohort study. 2020.
- Gonçalves T.J., Gonçalves S.E., Guarnieri A., et al. Association between low zinc levels and severity of acute respiratory distress syndrome by new coronavirus SARS-COV-2. Nutr. Clin. Pract. 2020;36(1):186–191. doi: 10.1002/ncp.10612. [DOI] [PubMed] [Google Scholar]
- Hamer M., Kivimäki M., Gale C.R., et al. Lifestyle risk factors, inflammatory mechanisms, and covid-19 hospitalization: a community-based cohort study of 387,109 adults in UK. Brain Behav. Immun. 2020;87:184–187. doi: 10.1016/j.bbi.2020.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan R.E., Adab P., Cheng K.K. Covid-19: Risk factors for severe disease and death. BMJ. 2020;1198 doi: 10.1136/bmj.m1198. [DOI] [PubMed] [Google Scholar]
- Jothimani D., Kailasam E., Danielraj S., et al. Covid-19: poor outcomes in patients with zinc deficiency. Int. J. Infect. Dis. 2020;100:343–349. doi: 10.1016/j.ijid.2020.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A., Kubota Y., Chernov M., et al. Potential role of zinc supplementation in prophylaxis and treatment of COVID-19. Med. Hypotheses. 2020;144 doi: 10.1016/j.mehy.2020.109848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.R. Critical role of zinc as either an antioxidant or a prooxidant in cellular systems. Oxid. Med. Cell. Longev. 2018;2018:1–11. doi: 10.1155/2018/9156285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X, Zhang L, Du H, Zhang J, Li YY, Qu J, et al.; Chinese Pediatric Novel Coronavirus Study Team. SARS-COV-2 infection in children. New England Journal Med 2020; 382: 1663-1665. [DOI] [PMC free article] [PubMed]
- Pormohammad A., Monych N., Turner R. Zinc and SARS-COV-2: a molecular modeling study of Zn interactions with RNA-dependent RNA-polymerase and 3c-like proteinase enzymes. Int. J. Mol. Med. 2020;47(1):326–340. doi: 10.3892/ijmm.2020.4790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu H., Wu J., Hong L., et al. Clinical and epidemiological features of 35 children with corona virus disease 2019 (COVID-19) in Zhejiang, China. an observational cohort studies. Lancet Infect. Dis. 2020;20:689–696. doi: 10.1016/S1473-3099(20)30198-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read S.A., Obeid S., Ahlenstiel C., et al. The role of zinc in antiviral immunity. Adv. Nutr. 2019;10(4):696–710. doi: 10.1093/advances/nmz013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riphagen S., Gomez X., Gonzalez-Martinez C., et al. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet. 2020;395(10237):1607–1608. doi: 10.1016/S0140-6736(20)31094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saudi MoH Protocol for Patients Suspected of/Confirmed with COVID-19, 2022. Ministry of health (MOH). https://www.moh.gov.sa/en/Ministry/MediaCenter/Publications/Pages/covid19.aspx[accessed 18th January 2023].
- Shereen M.A., Khan S., Kazmi A., et al. Covid-19 infection: emergence, transmission, and characteristics of human coronaviruses. J. Adv. Res. 2020;24:91–98. doi: 10.1016/j.jare.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skalny A., Rink L., Ajsuvakova O., et al. Zinc and respiratory tract infections: perspectives for covid-19 (review) Int. J. Mol. Med. 2020;46:17–26. doi: 10.3892/ijmm.2020.4575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su S., Wong G., Shi W., et al. Epidemiology, genetic recombination, and pathogenesis of Coronaviruses. Trends Microbiol. 2016;24(6):490–502. doi: 10.1016/j.tim.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagarro A., Epalza C., Santos M., et al. Screening and severity of coronavirus disease 2019 (covid- 19) in children in Madrid, Spain. JAMA Pediatr. 2021;175(3):316–317. doi: 10.1001/jamapediatrics.2020.1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang D., Tou J., Wang J., et al. Prevention and control strategies for emergency, limited-term, and elective operations in pediatric surgery during the epidemic period of covid-19. World J. Pediatr. Surg. 2020;3(1) doi: 10.1136/wjps-2020-000122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Te Velthuis A.J., van den Worm S.H., Sims A.C., et al. Zn2+ inhibits coronavirus and arterivirus RNA polymerase activity in vitro and zinc ionophores block the replication of these viruses in cell culture. PLoS Pathog. 2010;6(11) doi: 10.1371/journal.ppat.1001176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdoni L., Mazza A., Gervasoni A., et al. An outbreak of severe kawasaki-like disease at the Italian epicentre of the SARS-COV-2 epidemic: an observational cohort study. Lancet. 2020;395(10239):1771–1778. doi: 10.1016/S0140-6736(20)31103-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang E., Brar K. Covid-19 in children: an epidemiology study from China. J. Aller. Clin. Immunol.: Pract. 2020;8(6):2118–2120. doi: 10.1016/j.jaip.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S.R., Leibowitz J.L. Coronavirus pathogenesis. Adv. Virus Res. 2011:85–164. doi: 10.1016/B978-0-12-385885-6.00009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessels I., Rolles B., Rink L. The potential impact of zinc supplementation on COVID-19 pathogenesis. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.01712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (covid-19) outbreak in China. JAMA. 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- Wu D., Wu T., Liu Q., et al. The SARS-COV-2 outbreak: what we know. Int. J. Infect. Dis. 2020;94:44–48. doi: 10.1016/j.ijid.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.