Abstract
Obesity and diabetes mellitus are considered the most important diseases of the XXI century. Recently, many epidemiological studies have linked exposure to pesticides to the development of obesity and type 2 diabetes mellitus. The role of pesticides and their possible influence on the development of these diseases was investigated by examining the relationship between these compounds and one of the major nuclear receptor families controlling lipid and carbohydrate metabolism: the peroxisome proliferator-activated receptors (PPARs), PPARα, PPARβ/δ, and PPARγ; this was possible through in silico, in vitro, and in vivo assays. The present review aims to show the effect of pesticides on PPARs and their contribution to the changes in energy metabolism that enable the development of obesity and type 2 diabetes mellitus.
1. Introduction
According to the World Health Organization (WHO), obesity affected 13 million people in 2016, and the trend of increasing prevalence is constant, affecting adults and children, regardless of race and social status [1]. Obesity is defined as the loss of balance between the body's energy intake and consumption, leading to the storage of adipose tissue that exceeds its activity and causes hypertrophy and the growth of ectopic adipose tissue [2]. One of the main complications resulting from this metabolic alteration is the development of insulin resistance, leading to type 2 diabetes mellitus. Diabetes mellitus is among the leading causes of death worldwide, ranking ninth in 2019, and was the direct cause of 1.5 million deaths. In large part, these patients were overweight and sedentary. Diabetes mellitus is defined as “a chronic disease that occurs either when the pancreas does not produce enough insulin or when the body cannot effectively use the insulin that is produced” [3]. Both diseases affect a large proportion of the world's population and are interrelated. Obesity is one of the most important risk factors for the development of type 2 diabetes mellitus.
Lately, many epidemiological studies have linked exposure to environmental toxicants such as phthalates, bisphenols, and pesticides to obesity and diabetes [4, 5]. The environmental toxicants capable of promoting lipid accumulation and adipogenesis are known as obesogens; some examples are pesticides such as DDT (dichloro diphenyl trichloroethane), DDE (dichloro diphenyl dichloroethylene), HCH (hexachlorocyclohexane), and chlorpyrifos [6, 7]. On the other hand, most pesticides are endocrine disruptors that alter lipid and carbohydrate metabolism, causing insulin resistance and thus diabetes mellitus [8]. Pesticides such as DDT, DDE, aldicarb, and carbaryl have been linked to the occurrence of diabetes mellitus [9, 10].
Using numerous omics techniques, exposure to pesticides has been linked to the genetic expression of this disease, specifically to one of the key nuclear receptors that control lipid and carbohydrate metabolism: the peroxisome proliferator-activated receptors (PPARs) [6]. PPARs are a family of nuclear receptors of the type II. These receptors bind to a co-repressor protein and when they bind to a ligand, they require a co-activator protein [11], which then forms a complex between the receptor-ligand and retinoid X receptor (RXR), to form a heterodimer, this migrates into the nucleus and binds to the peroxisome proliferator response elements (PPRE), which consist of a sequence of two hexanucleotides (5'-AGGTCA-3') separated by one nucleotide [11, 12]; they enable the transcription of genes that have this sequence in their promoter. Three subtypes have been described so far: PPARα, PPARβ/δ, and PPARγ. PPARα (also known as NRIC1) was originally identified as an orphan receptor activated by peroxisome proliferation [13]. PPARβ or PPAR δ (NR1C2) and PPARγ (NR1C3) have been cloned as activator receptors of many proximal proliferators [14, 15]. PPARγ has two alternative promoters that generate two isoforms expressed in different tissues: PPARγ1 in many different tissues and PPARγ2 specifically in adipose tissue, but this expression can also be induced in other organs by a high-fat diet [15, 16].
The aim of the present review is to describe the differences involved in the activation of PPARs by many pesticides, leading to alterations in fat and carbohydrate metabolism, which could contribute to the development of diseases such as obesity and type 2 diabetes mellitus. To this end, three groups of studies have been made, first in silico studies that help to predict the binding and interaction between the pesticides and the PPARs; second in vitro studies to describe the possible mechanism of action to activate the receptor in specific cell lines; and third in vivo studies to evaluate the global response and the simultaneous use of different pathways in complete organisms.
2. In Silico Predictions: Interaction between Pesticides, Peroxisome Proliferators (PP), and the Peroxisome Proliferator-Activated Receptors (PPARs)
One of the most important points to consider is the description of the docking of the pesticides to the PPAR receptors and their subsequent activation. The interaction of different peroxisomal proliferators (PP) and their PPARs receptors has led to the study of the molecular properties of PP and the active sites of the receptors, this to a better understanding of the binding of both molecules. The in silico experiments have been shown to be sufficient to predict these interactions. They show not only the probability of binding between pesticides and the receptor by software such as ToxCast® [17] or AutoDock Vina® [18], but also the molecular interactions between amino acid residues of PPARs involved in binding and stability of ligand-receptor binding, which promotes better activation. Table 1 summarizes the reports on the prediction of binding and interaction of many pesticides and PPARs, as well as the molecular structures of the pesticides and the software used for prediction.
Table 1.
In silico studies of pesticides and their interaction with PPARs.
| PPAR (subtype) | Pesticide | Chemical clasification | Type of pesticide | Analysis | Structure | Item | Software | Year | References |
|---|---|---|---|---|---|---|---|---|---|
| PPAR γ | Bromuconazole | Triazole | Fungicide | Docking with the raptors |

|
Bind to receptor and act as antagonist | AutoDock Vina | 2021 | Wu et al. [18] |
| Chlorfluazuron | Benzoylurea | Insecticide | Docking with the receptor |

|
Bind to receptor and act as agonist | Discovery Studio 2.5/LigandFit module | 2018 | Ning et al. [20] | |
| Diflubenzuron | Benzoylurea | Insecticide | Docking with the receptor |

|
Bind to receptor and act as agonist | Discovery Studio 2.5/LigandFit module | 2018 | Ning et al. [20] | |
| Flucycloxuron | Benzoylurea | Insecticide | Docking with the receptor |

|
Bind to receptor and act as agonist | Discovery Studio 2.5/LigandFit module | 2018 | Ning et al. [20] | |
| Flufenoxuron | Benzoylurea | Insecticide | Docking with the receptor |

|
Bind to receptor and act as agonist | Discovery Studio 2.5/LigandFit module | 2018 | Ning et al. [20] | |
| PPAR γ | Noviflumuron | Benzoylurea | Insecticide | Docking with the receptor |

|
Bind to receptor and act as agonist | Discovery Studio 2.5/LigandFit module | 2018 | Ning et al. [20] |
| Triphenyltin (TPT) | Organotion | Antifouling | X Rays |

|
Bind ligand-receptor | MOLREP from the CCP4 suite/Coot and REFMAC5 and MolProbity | 2014 | Harada et al. [21] | |
| Tributyltin (TBT) | Organotion | Antifouling | X Rays |

|
Bind ligand-receptor | MOLREP from the CCP4 suite/Coot and REFMAC5 and MolProbity | 2014 | Harada et al. [21] | |
| PPAR γ | Mancozeb | Dithiocarbamate | Fungicide | Docking with the receptor |

|
Bind to receptor and act as agonist | Hex Dock and Patch Dock | 2014 | Bhaskar et al. [78] |
| Imidacloprid | Neonicotinoid | Insecticide | Docking with the receptor |

|
Bind to receptor and act as agonist | Hex Dock and Patch Dock | 2014 | Bhaskar et al. [78] | |
| PPAR α | Fomesafen | Nitrobenzamide | Herbicide | Prediction of bind |

|
Bind to receptor trough QSAR analysis | Sybyl software suite running on an Evans and Sutherland ESV30 | 1997 | Lewis and Lake [19] |
Most of the pesticide structures have a carboxylic group or can form an ion, which let it interact with the residue of aminoacidic of the receptors.
The use of mathematical and computational tools such as quantitative structure-activity relationship (QSAR) models has enabled the prediction of binding between the pesticide and PPARs, for example, fluazinam, a diarylamine used as a fungicide, with the human PPARγ receptor [17]; fomesafen, an herbicide belonging to the nitrobenzamide group, with the PPARα receptor of mice and rats [19]; these interactions considered their molecular chemical characteristics and their physicochemical and biological properties, mainly the size and flexibility of the molecule, electronic distribution, hydrophobicity, hydrogen bonds, and the presence of many pharmacological features related to biological activity.
The application of predictive docking between ligands and receptors has allowed us to assess the molecular level of interactions between the amino acid residues of the active site of the receptor with moieties or functional groups of the structures of pesticides. In the binding of fomesafen and PPARα, the amino acid residues lysine (Lys) is involved in the electrostatic interactions, and methionine (Met), leucine (Leu), and phenylalanine (Phe) favor π–π interactions [19]. Other pesticides evaluated by docking include diflubenzuron, a benzoylurea that inhibits chitin synthesis and is an agonist of the human PPARγ receptor interacting through 18 amino acid residues, highlighting cysteine (Cys 285) [20]. Cys 285 was determined by X-ray crystallography to be essential for the binding between organotin, triphenyltin (TPT), and tributyltin (TBT) with PPARγ, which does not favor a covalent ionic interaction between the tin (Sn) of the pesticides and the sulfur (S) in the ionic state of the amino acid [21]. Besides, the antagonistic interaction between rat PPARγ and bromuconazole, a triazole used as a fungicide, must be due to a close interaction between hydrogen bonds formed between the pesticides and the histidine (His 477) of the receptor, which shares the same amino acid with an anchorage that the pharmacological antagonist GW9662 [18]. So it is being shown that the interaction of some amino acids that are constantly involved in pesticide and receptor binding.
As for the structure of the pesticides described earlier, all of them have the same aromatic ring, except for TBT, which has a lower ability to activate PPARγ. However, its ability to ionize allows stability in the ligand–receptor interaction to produce an ion–π or π–π interaction [18–21], as shown in Table 1. As many authors have suggested the use of this predictive technique makes it possible to define amino acid residues and the moieties and/or functional groups of the pesticides that can facilitate receptor activation, activation levels, possibly biological activity, and identification of their behavior as agonist or antagonist.
3. In Vitro Studies: Binding, Activation, and Mechanism of Action of Pesticides via PPARs Receptors
Cell lines have been the most used to study the binding and biological activation of a ligand to this receptor. They also have the advantage of being accessible in their elaboration and facilitate the understanding of the phenomenon of ligand–receptor integration, so that many pathways can be proposed. The biological effect of the binding of pesticides to the different PPARs, as agonists or antagonists, as well as the biological biomarkers related to the secretion of proteins [22] and/or gene expression [23] transactivated by these nuclear receptors have made it possible to fathom the possible mechanism of action of pesticides with the PPARs [24]. Three main types of cell cultures have been used: those that were transfected, which means that the cell line does not originally express a receptor but is induced [25]; cell lines that express different PPARs, such as the HepG2 line of hepatocytes [26]; and cell lines whose functions depend on activation of the receptor, including the 3T3-L1 cell line of preadipocytes, whose maturation depends on PPARγ [27]. Table 2 shows the reports identified up to this review concerning the different PPARs, the pesticides that activate them, the cell lines used in the studies, and the effect of their activation.
Table 2.
In vitro studies of activation PPARs by pesticides.
| PPAR subtype | Pesticide | Chemical classification | Type of pesticide | Cell culture | Item | Year | References |
|---|---|---|---|---|---|---|---|
| PPARα | Permethrin | Pyrethroid | Insecticide | Primary mouse and human hepatocytes cultures | Activation of PPARα | 2020 | Kondo et al. [125] |
| Methiocarb | Carbamate | Insecticide | Cos-1 cells (transfected) | Activation of PPARα, CYP4A, PXR, CAR | 2016 | Fujino et al. [57] | |
| Carbaryl | Activation of PPARα, PXR, CAR | ||||||
| Deltamethrin | Pyrethroid | Insecticide | Cos-1 cells (transfected) | None activate PPARα | 2019 | Fujino et al. [30] | |
| Cis-Permethrin Cypermethrin | |||||||
| Paraquat | Bipyridine | Herbicide | Primary mouse hepatocytes cultures | Activation of PPARα, regulate lipid homeostasis and dismiss of stress | 2004 | Anderson et al. [66] | |
| PPAR β/δ | 2,4-D | Phenoxy | Herbicide | HepG2 cell line | Increase expres of PPARβ/δ, and CREB (regulator of gluconeogenesis) | 2018 | Sun et al. [26] |
| PPARγ | Endrin | Organochlorine | Insecticide | 3T3-L1 cell line | Up-regulate PPARγ, C/EBPs, FAS, GLUT-4, Adiponectin | 2022 | Seok et al. [24] |
| DDT | Organochlorine | Insecticide | Primary hepatocytes rat | Increase PPARγ | 2021 | Jellali et al. [36] | |
| Permethrin | Pyrethroid | ||||||
| Chlorpyrifos | Organophosphate | Insecticide | 3T3-L1 cell line | Enhance store lipid droplets, up-regulated transcription of PPARγ, C/EBPα and FABP4 | 2020 | Blanco et al. [32] | |
| Permethrin | Pyrethroid | Insecticide | 3T3-L1 cell line | TG accumulation and pre-adipocytes proliferation | 2021 | Kassotis et al. [126] | |
| Cypermethrin | |||||||
| Chlorpyrifos | |||||||
| Organophosphate | |||||||
| Iprodione | Imide | Hepatocyte rat cell line | Up-regulate PPARγ | 2020 | Sohrabi et al. [37] | ||
| Fungicide | |||||||
| Flutolanil | Acid amides | Fungicide | |||||
| Paraquat | Bipyridine | Herbicide | |||||
| DDT | Organochlorine | Insecticide | |||||
| Endosulfan | Insecticide | ||||||
| Methoxychlor | Insecticide | ||||||
| Pentachlorophenol | Insecticide | ||||||
| Quintozene | Fungicide | ||||||
| Toxaphene | Insecticide | ||||||
| Chlorpyrifos | Organophosphate | Insecticide | |||||
| Diazinon | Insecticide | ||||||
| Fibronil | Phenylpyrazole | Fungicide | |||||
| Allethrin | Pyrethroid | Insecticide | |||||
| Bifenthrin | Fungicide | ||||||
| Cyhalothrin | Insecticide | ||||||
| Permethrin | Insecticide | ||||||
| Resmethrin | Insecticide | ||||||
| Atrazine | Triazine | Herbicide | |||||
| Paclobutrazol | Triazole | Herbicide | |||||
| Diuron | Ureas | Herbicide | |||||
| Endrin | Organochlorine | Insecticide | Hepatocyte rat cell line | Down-regulate PPARγ | 2020 | Sohrabi et al. [37] | |
| Propamocarb | Carbamate | Fungicide | |||||
| Rotenone | Heteropentacyclic compound | Insecticide | |||||
| Prallethrin | Pyrethroid | Insecticide | 3T3-L1, OP9, BM-MSC cell lines | Lipid accumulation, activate PPARγ and stimulate the expression of Plin1 | 2020 | Andrews et al. [38] | |
| Allethrin | Pyrethroid | Insecticide | 3T3-L1, OP9, BM-MSC cell lines | Activate transcriptional function of PPARγ | 2020 | Andrews et al. [38] | |
| Fenthion | Organophosphate | Insecticide | |||||
| Fentin | Organotion | Fungicide | |||||
| Quinoxyfen | Quinoline | Fungicide | |||||
| 2-Benzothiazole sulfonic acid | Benzothiazole | Fungicide | Mammalian cells | Bind to PPARγ | 2020 | Neale et. al. [127] | |
| MCPA | Phenoxy | Herbicide | |||||
| TBT | Organotion | Antifouling | THP-1cell line | Activation of PPARγ, increase lipid accumulation and expression of lipid metabolism genes | 2021 | Jie et al. [65] | |
| QpE | Phenoxy | Herbicide | 3T3-L1 cell line | Induce accumulation of lipids via PPARγ | 2019 | Biserni et al. [39] | |
| Glyphosate | Organophosphate | Herbicide | 3T3-L1 cell line | Not induce accumulation of lipids via PPARγ | 2018 | Mesnage et al. [29] | |
| 2,4-D | Phenoxy | Herbicide | |||||
| Dicamba | Chlorophenoxy | Herbicide | |||||
| Mesotrione | Triketone | Herbicide | |||||
| Isoxaflutole | Isoxazole | Herbicide | |||||
| Permethrin | Pyrethroid | Insecticide | 3T3-L1 cell line | Induce adipogenesis via PPARγ | 2019 | Qi et al. [128] | |
| Fibronil | Phenylpyrazole | Fungicide | |||||
| Chlorantraniliprole | Ryanoid | Insecticide | 3T3-L1 cell line | Induce adipogenesis, up-regulate C/EBPα, PPARγ and ACC | 2019 | Yuan et al. [23] | |
| Chlorpyrifos | Organophosphate | Insecticide | HTR8/SVneo cells | Reduce mRNA of PPARγ | 2019 | Ridano et al. [64] | |
| Flubendiamide | Ryanoid | Insecticide | 3T3-L1 cell line | Enhance TG content, increase C/EBPα, PPARγ | 2018 | Sun et al. [129] | |
| Pyraclostrobin | Strobilurin | Fungicide | 3T3-L1 cell line | TG accumulation, without activation of PPARγ, reduce LPL, CEBPα, GLUT4 | 2018 | Luz et al. [40] | |
| Cis-Bifenthrin | Pyrethroid | Insecticide | HepG2 cell line | Lipid accumulation, induce expression of PPARγ, FAS | 2018 | Xiang et al. [58] | |
| QpE | Phenoxy | Herbicide | HepaRG (transfected) | Activate PPARγ | 2018 | Mesnage et al. [37] | |
| Isoxaflutole | Isoxazole | Herbicide | |||||
| Mesotrones | Triketone | Herbicide | |||||
| Glyphosate | Organophosphate | Herbicide | Not activate PPARγ | ||||
| Diazinon | Organophosphate | Insecticide | 3T3-L1 cell line | Increase lipid accumulation, induce transcriptional factors of C/EBPα and PPARγ | 2018 | Smith et al. [33] | |
| Diflubenzuron Chlorfluazuron Flucycloxuron Noviflumuron | Benzoylurea | Insecticide | HepG2 cell line | Exhibited potent PPARγ agonistic activity | 2018 | Ning et al. [20] | |
| Flufenoxuron | |||||||
| TBT | Organotion | Antifouling | Primary adipocytes culture of Onchrorynchus mykiss | Induce lipid accumulation, increase C/EBPα and PPARγ expression | 2017 | Lutfi et al. [43] | |
| TPT | |||||||
| DDT / DDE | Organochlorine | Insecticide | 3T3-L1 cell line | Increase lipid accumulation, PPARγ expression, FAS, C/EBPα, LPL | 2016 | Kim et al. [31] | |
| Fibronil | Phenylpyrazole | Insecticide | 3T3-L1 cell line | Increase lipid accumulation and expression of PPARγ and C/EBPα genes | 2016 | Sun et al. [22] | |
| Glyphosate | Organophosphate | Herbicide | 3T3-L1 cell line | Increase lipid peroxidation, inhibit the induction of PPARγ during differentiation with the commercial presentation, not in pure form | 2016 | Martini et al. [56] | |
| Deltamethrin | Pyrethroid | Insecticide | SH-SY5Y cell line | Decreased PPARγ expression and the receptor protects against pesticide cytotoxicity | 2016 | Ko et al. [68] | |
| TBT | Organotion | Antifouling | MSC cells | Promote adipogenesis via PPARγ receptor but there are others receptors | 2014 | Biemann et al. [60] | |
| Chlorpyrifos | Organophosphate | Insecticide | SH-SY5Y cell line | Activation of PPARγ, dismiss the oxidative stress, inflammation and death cell produced to the pesticide | 2014 | Lee et al. [69] | |
| Rotenone | Heteropentacyclic compound | Insecticide | SH-SY5Y cell line | Activation of PPARγ via rosiglitazone and inhibits the effect of pesticide | 2014 | Corona et al. [70] | |
| TBT | Organotion | Antifouling | MSC cells | Induce PPARγ, FABP4, lipid accumulation, stimulus cellular differentiation | 2011 | Yanik et al. [59] | |
| TPT | |||||||
| Dibutyltin | |||||||
| TBT | Organotion | Antifouling | 3T3-L1 cell line | Increase adipogenic activity but not via PPARγ | 2011 | Penza et al. [52] | |
| Endrin | Organochlorine | Insecticide | 3T3-L1 cell line | Bind to PPARγ, but preferably through to glucocorticoid receptor | 2010 | Sargis et al. [54] | |
| Tolylfluanid | Sulfamide | Fungicide | |||||
| TPT | Organotion | Antifouling | Activate PPARγ | ||||
| TBT | Organotion | Antifouling | 3T3-L1 cell line | Promote adipogenesis and lipid accumulation | 2006 | Grün et al. [130] | |
| TBT | Organotion | Antifouling | 3T3-L1 cell line | Accumulation of lipid but not via PPARγ and increase aP2 | 2005 | Inadera & Shimomura [45] | |
| TBT | Organotion | Antifouling | 3T3-L1 cell line | Activate PPARγ, accumulation of TG and increase adipocyte differentiation | 2005 | Kanayama et al. [44] | |
| TPT | |||||||
| DDT | Organochlorine | Insecticide | 3T3-L1 cell line | Induction of C/EBPα, PPARγ, increase phenotype of adipocytes | 2002 | Moreno-Aliaga & Matsumura [46] | |
| Endrin | Organochlorine | Insecticide | 3T3-L1 cell line | Inhibition of adipocyte differentiation, inhibit C/EBPα but not C/EBPβ and C/EBPδ, reduce PPARγ | 1999 | Moreno-Aliaga & Matsumura [51] | |
| PPARα and PPARγ | DDE | Organochlorine | Insecticide | 3T3-L1 cell line | No affect PPARα neither PPARγ, reduce lipid accumulation inhibit adipocyte differentiation | 2012 | Taxvig et al. [49] |
| Chlorpyrifos | Organophosphate | Insecticide | |||||
| Mancozeb | Dithiocarbamate | Fungicide | |||||
| Prochoraz | Ureas | Fungicide | |||||
| Deltamethrin | Pyrethroid | Insecticide | 3T3-L1 cell line | Activate PPARγ but not PPARα, reduce lipid accumulation | 2012 | Taxvig et al. [49] | |
| Aldrin | Organochlorine | Insecticide | CV-1 cell line transfected with PPARα and PPARγ mouse | ||||
| α-BHC | Insecticide | ||||||
| β-BHC | Insecticide | ||||||
| γ-BHC | Insecticide | ||||||
| δ-BHC | Insecticide | ||||||
| Captan | Fungicide | ||||||
| cis-Chlordane | Insecticide | ||||||
| trans-Chlordane | Insecticide | ||||||
| Chlorobenzilate | Insecticide | ||||||
| Chloropropylate | Insecticide | ||||||
| Chlorothalonil | Fungicide | ||||||
| o,p´-DDT | Insecticide | ||||||
| p,p´-DDT | Insecticide | ||||||
| p,p´-DDE | Insecticide | ||||||
| p,p´-DDD | Insecticide | None have agonistic activity to PPARα and PPARγ | 2006 | Takeuchi et al. [25] | |||
| Dichlobenil | Herbicide | ||||||
| Dicofol | Insecticide | ||||||
| Dieldrin | Insecticide | ||||||
| α-Endosulfan | Insecticide | ||||||
| β-Endosulfan | Insecticide | ||||||
| Endosulfan sulfate | Insecticide | ||||||
| Endrin | Insecticide | ||||||
| Folpet | Fungicide | ||||||
| Fthalide | Fungicide | ||||||
| Heptachlor | Insecticide | ||||||
| Heptachlor epoxide | Insecticide | ||||||
| Methoxychlor | Insecticide | ||||||
| Pentachlorophenol | Insecticide | ||||||
| Quintozene | Fungicide | ||||||
| Acifluorfen | Diphenyl ethers | Herbicide | CV-1 cell line transfected with PPARα and PPARγ mouse | None have agonistic activity to PPARα and PPARγ | 2006 | Takeuchi et al. [25] | |
| Acifluorfen-methyl | Herbicide | ||||||
| Bifenox | Herbicide | ||||||
| Chlomethoxyfen | Herbicide | ||||||
| Chlornitrofen | Herbicide | ||||||
| CNP-amino | Herbicide | ||||||
| Chloroxurone | Herbicide | ||||||
| Diclofop-methyl | Herbicide | CV-1 cell line transfected with PPARα and PPARγ mouse | Induce PPARα and PPARγ | 2006 | Takeuchi et al. [25] | ||
| Fluazifop-butyl | Herbicide | CV-1 cell line transfected with PPARα and PPARγ mouse | None have agonistic activity to PPARα and PPARγ | 2006 | Takeuchi et al. [25] | ||
| Nitrofen | Herbicide | ||||||
| Oxyfluorfen | Herbicide | ||||||
| Acephate | Organophosphate | Insecticide | |||||
| CV-1 cell line transfected with PPARα and PPARγ mouse | None have agonistic activity to PPARα and PPARγ | 2006 | Takeuchi et al. [25] | ||||
| Anilofos | Herbicide | ||||||
| Bromophos-ethyl | Insecticide | ||||||
| Bromophos-methyl | Insecticide | ||||||
| Butamifos | Herbicide | ||||||
| Chlorpyrifos | Insecticide | ||||||
| Chlorpyrifos-methyl | Insecticide | ||||||
| Cyanofenphos | Insecticide | ||||||
| Cyanophos | Insecticide | ||||||
| Diazinon | Insecticide | ||||||
| Dichlofenthion | Insecticide | ||||||
| Dichlorvos | Insecticide | ||||||
| Dimethoate | Insecticide | ||||||
| Dioxabenzofos | Insecticide | ||||||
| Disulfoton | Insecticide | ||||||
| EPN | Insecticide | ||||||
| Edifenphos | Fungicide | ||||||
| Ethion | Insecticide | ||||||
| Ethoprophos | Insecticide | ||||||
| Fenamiphos | Nematicide | ||||||
| Fenchlorphos | Insecticide | ||||||
| Fenitrothion | Insecticide | ||||||
| Fenitrothion oxon | Insecticide | ||||||
| Fensulfothion | Insecticide | ||||||
| Fenthion | Insecticide | ||||||
| Glyphosate | Herbicide | ||||||
| Iprobenfos | Fungicide | ||||||
| Isofenphos | Insecticide | CV-1 cell line transfected with PPARα and PPARγ mouse | None have agonistic activity to PPARα and PPARγ | 2006 | Takeuchi et al. [25] | ||
| Isoxathion | Insecticide | ||||||
| Leptophos | Insecticide | ||||||
| Malathion | Insecticide | ||||||
| Mecarbam | Insecticide | ||||||
| Methamidophos | Insecticide | ||||||
| Methidathion | Insecticide | ||||||
| Methyl-parathion | Insecticide | ||||||
| Monocrotophos | Insecticide | ||||||
| Parathion | Insecticide | ||||||
| Phenthoate | Insecticide | ||||||
| Phorate | Insecticide | ||||||
| Phosalone | Insecticide | ||||||
| Phosmet | Insecticide | ||||||
| Piperophos | Fungicide | ||||||
| Pirimiphos-methyl | Insecticide | ||||||
| Profenofos | Insecticide | ||||||
| Propaphos | Insecticide | ||||||
| Prothiofos | Insecticide | ||||||
| Prothiofos oxon | Insecticide | ||||||
| Pyridaphenthion | Insecticide | ||||||
| Quinalphos | Insecticide | CV-1 cell line transfected with PPARα and PPARγ mouse | None have agonistic activity to PPARα and PPARγ | 2006 | Takeuchi et al. [25] | ||
| Terbufos | Insecticide | ||||||
| Tetrachlorvinphos | Insecticide | ||||||
| Thiometon | Insecticide | ||||||
| Tolclofos-methyl | Fungicide | ||||||
| Tolclofos-methyl oxon | Fungicide | ||||||
| Trichlorfon | Insecticide | ||||||
| Vamidothion | Insecticide | ||||||
| Cyfluthrin | Pyrethroid | Insecticide | CV-1 cell line transfected with PPARα and PPARγ mouse | None have agonistic activity to PPARα and PPARγ | 2006 | Takeuchi et al. [25] | |
| Cyhalothrin | Insecticide | ||||||
| Cypermethrin | Insecticide | ||||||
| Deltamethrin | Insecticide | ||||||
| Etofenprox | Insecticide | ||||||
| Fenvalerate | Insecticide | ||||||
| Flucythrinate | Insecticide | ||||||
| Fluvalinate | Insecticide | ||||||
| Permethrin | Insecticide | ||||||
| Pyrethrin | Insecticide | CV-1 cell line transfected with PPARα and PPARγ mouse | Induce PPARα and PPARγ | 2006 | Takeuchi et al. [25] | ||
| Tefluthrin | Insecticide | CV-1 cell line transfected with PPARα and PPARγ mouse | None have agonistic activity to PPARα and PPARγ | 2006 | Takeuchi et al. [25] | ||
| Tralomethrin | Insecticide | ||||||
| Bendiocarb | Carbamate | Insecticide | CV-1 cell line transfected with PPARα and PPARγ mouse | None have agonistic activity to PPARα and PPARγ | 2006 | Takeuchi et al. [25] | |
| Benomyl | Fungicide | ||||||
| Carbaryl | Insecticide | ||||||
| Carbendazim | Fungicide | ||||||
| Carbofuran | Insecticide | ||||||
| Chlorpropham | Herbicide | ||||||
| Diethofencarb | Fungicide | ||||||
| Dimepiperate | Herbicide | ||||||
| Esprocarb | Herbicide | ||||||
| Ethiofencarb | Insecticide | ||||||
| Fenobucarb | Insecticide | ||||||
| Isoprocarb | Insecticide | ||||||
| Methiocarb | Insecticide | ||||||
| Methomyl | Insecticide | ||||||
| Molinate | Herbicide | ||||||
| Oxamyl | Insecticide | ||||||
| Phenmedipham | Herbicide | ||||||
| Pirimicarb | Insecticide | ||||||
| Pyributicarb | Herbicide | ||||||
| Thiobencarb | Herbicide | ||||||
| Thiobencarb sulfon | Herbicide | ||||||
| Thiram | Fungicide | ||||||
| Alachlor | Acid amides | Herbicide | CV-1 cell line transfected with PPARα and PPARγ mouse | None have agonistic activity to PPARα and PPARγ | 2006 | Takeuchi et al. [25] | |
| Asulam | Herbicide | ||||||
| Cafenstrole | Herbicide | ||||||
| Flutolanil | Fungicide | ||||||
| Mefenacet | Herbicide | ||||||
| Mepronil | Fungicide | ||||||
| Metalaxyl | Fungicide | ||||||
| Metolachlor | Herbicide | ||||||
| Pretilachlor | Herbicide | ||||||
| Propyzamide | Herbicide | ||||||
| Thenylchlor | Herbicide | ||||||
| Anilazine | Triazine | Fungicide | CV-1 cell line transfected with PPARα and PPARγ mouse | None have agonistic activity to PPARα and PPARγ | 2006 | Takeuchi et al. [25] | |
| Atrazine | Herbicide | ||||||
| Metribuzin | Herbicide | ||||||
| Prometon | Herbicide | ||||||
| Prometryn | Herbicide | ||||||
| Simazine | Herbicide | ||||||
| Simetryn | Herbicide | ||||||
| Bensulfuron-methyl | Ureas | Herbicide | |||||
| Daimuron | Herbicide | ||||||
| Diflubenzuron | Insecticide | ||||||
| Diuron | Herbicide | ||||||
| Linuron | Herbicide | ||||||
| Pencycuron | Fungicide | ||||||
| Prochloraz | Fungicide | CV-1 cell line transfected with PPARα and PPARγ mouse | None have agonistic activity to PPARα and PPARγ | 2006 | Takeuchi et al. [25] | ||
| Propanil | Herbicide | ||||||
| Amitraz | Formamidine | Fungicide | CV-1 cell line transfected with PPARα and PPARγ mouse | None have agonistic activity to PPARα and PPARγ | 2006 | Takeuchi et al. [25] | |
| Benfuresate | Benzofuran | Herbicide | |||||
| Bentazone | Benzothiadiazole | Herbicide | |||||
| Benzoximate | Organochlorine | Acaricide | |||||
| Bitertanol | Triazole | Fungicide | |||||
| Bromopropylate | Benzilate | Acaricide | |||||
| Chinomethionat | Quinoxaline | Fungicide | |||||
| Chloridazon | Pyridazinone | Herbicide | |||||
| Dazomet | Thiadiazine | Insecticide | |||||
| Diquat | Bipyridine | Herbicide | |||||
| Fenarimol | Pyrimidine | Fungicide | |||||
| Ferimzone | Pyrimidine | Fungicide | |||||
| Fluazinam | Diarilamine | Fungicide | |||||
| Imazalil | Conazole | Fungicide | CV-1 cell line transfected with PPARα and PPARγ mouse | Induce PPARα and PPARγ | 2006 | Takeuchi et al. [25] | |
| Imidacloprid | Neonicotinoid | Insecticide | |||||
| Iminoctadine | Guanidine | Fungicide | |||||
| Indanofan | Sulfonylurea | Herbicide | |||||
| Ioxynil | Nitrile | Herbicide | CV-1 cell line transfected with PPARα and PPARγ mouse | None have agonistic activity to PPARα and PPARγ | 2006 | Takeuchi et al. [25] | |
| Iprodione | Imide | Fungicide | |||||
| Isoprothiolane | Dithiolane | Fungicide | |||||
| Lenacil | Uracyles | Herbicide | |||||
| MCPA | Phenoxy | Herbicide | |||||
| 2,4-D | Phenoxy | Herbicide | |||||
| Paraquat | Bipyridine | Herbicide | |||||
| Pendimethalin | Dinitroaniline | Herbicide | |||||
| Probenazole | Benoxthiazole | Fungicide | |||||
| Procymidone | Dicarboximide | Fungicide | |||||
| Propiconazole | Triazole | Fungicide | |||||
| Pyrazolynate | Pyrazole | Herbicide | |||||
| Pyrazoxyfen | Pyrazole | Herbicide | |||||
| Pyroquilon | Pyrroloquinoline | Fungicide | CV-1 cell line transfected with PPARα and PPARγ mouse | None have agonistic activity to PPARα and PPARγ | 2006 | Takeuchi et al. [25] | |
| Sethoxydim | Oxime | Herbicide | |||||
| Thiabendazole | Benzimidazole | Fungicide | |||||
| Thiocyclam | Nereistoxin | Insecticide | |||||
| Thiophanate-methyl | Thioureas | Fungicide | |||||
| Triadimefon | Triazole | Fungicide | |||||
| Tricyclazole | Triazole | Fungicide | |||||
| Triflumizole | Imidazole | Fungicide | |||||
| Trifluralin | Dinitroaniline | Herbicide | |||||
| Triforine | Piperazine | Fungicide | |||||
| Vinclozolin | Dicarboximide | Fungicide | |||||
| 2,4-D MCPA | Phenoxy | Herbicide | COS-1 (transfected) | No transactivation receptors | 1999 | Maloney & Waxman [28] | |
| PPARα, PPARβ/δ, PPARγ | Atrazine | Triazine | Herbicide | RK13 (rabbit kidney) transfected | No interaction with the receptors | 2003 | Devos et al. [104] |
Abbreviations: 2,4-D, 2,4-dichlorphenoxyacetic acid; ACC, acetyl Co-A carboxylase; BM-MSC, bone marrow-derived mesenchymal stem cell; C/EBPα, CCAAT enhancer binding protein alpha; C/EBPβ, CCAAT enhancer binding protein beta; CAR, constitutive androstane receptor; CREB, CAMP responsive element binding protein 1; EPN, Ethyl p-nitrophenyl phenylphosphorothioate; FABP4, fatty acid-binding protein 4; FAS, fatty acid synthase; GLUT-4, glucose transporter type 4; LPL, lipoprotein lipase; MCPA, 4-Chloro-o-toloxyacetic acid; DDT, diclorodifeniltricloroetano; DDD, Dichlorodiphenyldichloroethane; DDE, Dichlorodiphenyldichloroethylene; PPARα, peroxisome proliferator-activated receptor alpha; PPARβ/δ, peroxisome proliferator-activated receptor beta or delta; PPARγ, peroxisome proliferator-activated receptor gamma; PXR, pregnane X receptor; QpE, Quizalofop-p-ethyl; TBT, Tributyltin; TG, triglycerides; TPT, Triphenyltin.
3.1. Transactivation of PPARs by Pesticides
To assess the potential biological activity of PPARs through pesticides, two techniques were elaborated; first reporting genes to identify the ability of the complex pesticide-PPAR to bind and translocate to the nucleus cell [25, 28]; and then the recognition and binding to the PPRE via monitoring transcription of regulated genes by the receptors [29]. One of the first analyses of the transactivation of PPARα and PPARγ by the pesticides was carried out in CV-1 cells (kidney cells from monkeys) transfected with the mouse receptors, testing 200 pesticides: 29 organochlorines, 11 diphenyl esters, 59 organophosphates, 12 pyrethroids, 22 carbamates, 11 amides, 7 triazines, 8 ureas, and 44 other groups. The result was that only pyrethrine, imazalil, and diclofob-methyl showed transactivation of PPARα, but none of the 200 pesticides showed transactivation of PPARγ; moreover, these pesticides can activate the RXR, which is also involved in lipid metabolism [25]. These results are consistent with those of 2,4-dichlorophenoxyacetic acid (2,4-D) and 4-chloro-o-toloxyacetic acid (MCPA), previously reported not to transactivate for mouse and human PPARα and PPARγ receptors in transfected COS-1 cells [28]; furthermore, mouse PPARα is more sensitive to the human receptor. Consistent results were observed with pyrethroids: deltamethrin, cis-permethrin, cypermethrin, fenvalerate, allethrin, trans-permethrin, bioresmethrin, and phenothrin, none of which activated the PPARα receptor in transfected COS-1 cells; however, the trans-permethrin metabolites: 3-phenoxybenzoic acid and 3-phenoxybenzaldehyde had agonic activity via PPARα in microarrays [30]. Nevertheless, subsequent studies have shown that many pesticides initially reported as not activators of PPARα and PPARγ can participate in lipid and carbohydrate metabolism, through this receptor as DDT and its metabolite DDE [31], chlorpyrifos [32], diazinon [33], endrin [24], among others.
3.2. Activation of PPARs by Pesticides
Thanks to technological progress, it is now possible to obtain and analyze a large amount of data in a short period of time, making it possible to describe in great detail the changes in the genome, proteome, and metabolome at the cell or tissue level in response to toxic environmental influences. The changes induced by the activation of PPARs due to interaction with pesticides have been linked to the development of obesity and type 2 diabetes mellitus [34, 35]. The analysis is mainly based on the findings of differentially expressed genes (DEGs) [36] and transcription factors (TFs) [37], which are the main group of proteins that have a response to the exposure of a chemical substance and specifically increase biological conditions; the result can be associated through networks that allow to identify central and key genes in many diseases [29].
Alteration of PPARγ expression in the presence of toxaphene, methoxychlor, permethrin, atrazine, DDT, paraquat, and chlorpyrifos was described in microarray (RNA) analysis in a rat hepatocyte model, revealing changes in lipid and carbohydrate metabolism [37]. In another analysis of transcriptomics of HepaRG cells and exposure to quizalofop-p-ethyl, networks of genes associated with metabolic pathways involved in fatty acid degradation were identified. In the presence of isoxaflutole, retinol metabolism and PPARγ signaling were altered, and finally, glyphosate did not alter the expression of PPARγ but decreased large-chain fatty acids (LCFAs) and polyunsaturated fatty acids (PUFAs), suggesting the existence of other receptors involved in lipid metabolism [29]. In a study using the latest technology proposing organ replacement using an organ-on-chip of a rat kidney, the transcriptome and metabolome were analyzed after DDT and permethrin exposure and their mixture. The results show that the conditions assessed produced a hepatic steatosis profile with high expression of PPAR-related genes, fatty acids, lipid metabolism, and steroid biosynthesis; and the mixture had an additive effect on the transport RNA and necrotic/inflammatory profiles [36].
This omics analysis makes it possible to assign all the changes that can be caused by exposure to pesticides without knowing a possible target for the effects on cellular functions. These results have confirmed to a greater extent the changes found in cell cultures are the expression of specific genes controlled by the PPARs. Therefore, the use of this type of technology can make the detection of changes caused by environmental toxicants more efficiently, including those that were not previously foreseeable. However, the cost and specialized equipment make it difficult to use this tool on a larger scale.
3.3. The Biological Effect of PPAR Activation in Lipid Metabolism by the Pesticides
Evaluation of the biological activity of the PPARγ receptor is mainly based on adipocyte differentiation [38], lipid storage in adipose tissue [39], and control of lipid and carbohydrate metabolism [40]. The most commonly used cell line is 3T3-L1 [41]. This strain of preadipocyte is used as a model for initial adipocyte differentiation in assessing activation of the PPARγ receptor [27] and other TFs involved in adipogenesis [42]. However, other cell lines have also been used, such as the OP9 cell line as a model of late adipocyte differentiation [38] and even the use of primary cultures of adipocytes [43]. Less differentiated cells have also been used, such as bone marrow-derived multipotent stromal cells (BM-MSC), which have allowed the evaluation of the role of pesticides on the PPARγ receptor and the other receptors involved in cell differentiation towards the adipocyte lineage [42] and even their possible role in the generation of osteocytes or chondrocytes [38].
To illustrate the biomolecular implications of activation of the PPARγ receptor by pesticides, a brief review of the changes was made and is presented below. The biomarkers used in the preadipocyte cell lines revolve around their differentiation into mature adipocytes. The most important marker is lipid accumulation [27]. Key regulators of adipogenesis that influence and control PPARγ expression include the CCAAT-enhancer-binding protein family (C/EBPs) described as C/EBPα, C/EBPβ, and C/EBPδ, with C/EBPβ being an inducer of C/EBPα, which in turn is an inducer of PPARγ [33]; the use of the aP2 gene (fatty acid binding protein) in mature adipocytes is an indicator of its activation [44, 45]. C/EBPα and PPARγ promote adipogenesis by controlling the expression of ACC (acetyl-CoA carboxylase), FAS/FASN (fatty acid synthase), FAPB4 (fatty acid binding protein 4), LPL (lipoprotein lipase), which are involved in lipogenesis [42].
However, the expression of proteins is not always sufficient to consider them active, as in the case of ACC, which is controlled by a phosphorylation/dephosphorylation process through AMP-activated protein kinase (AMPK) [23, 44]. On the other hand, the evaluation of CYP4A as an early marker of signaling in peroxisome proliferation has been proposed because it has a PPRE sequence in its promoter [47, 48].
Once adipocytes are mature, other biomarkers are used, such as adipokines, hormones that control adipocyte function are involved in the metabolism of lipids and carbohydrates at local and systemic levels. The adipokines most commonly analyzed in the activation of PPARγ by pesticides are: adiponectin, which is only secreted by mature adipocytes, regulates glucose levels, increases insulin sensitivity, and also has anti-inflammatory effects [33]; leptin, which is directly proportional to adipose tissue [49]; resistin, which regulates insulin sensitivity (in humans by macrophages and in mice by adipocytes) [50]; and perilipin, which plays an important role in the mobilization and accumulation of fat in adipose tissue [33]. Other biomarkers associated with the response to PPARγ activation by pesticides include inflammatory biomarkers: IL-6, monocyte chemotherapy protein 1 (MCP1/CCL2), and tumor necrosis factor-alpha (TNF-α), which impair adipocyte differentiation by inhibiting it through the nuclear factor kappa light chain enhancer transcriptional pathway of activating B cells and protein kinase C (NF-/PKC) [51].
Finally, the accumulation of lipids and their subsequent oxidation in mitochondria and peroxisomes lead to high production of reactive oxygen species (ROS), which generate stress in the endoplasmic reticulum (ER) [23] and alter mitochondrial function [40], creating an imbalance in energy homeostasis, factors that have also been studied in adipocyte exposure to pesticides [52].
It is important to consider the presence and activation of another nuclear steroid/thyroid hormone receptors (NR) associated with adipocyte differentiation and/or function, such as the RXR, which functions by forming a heterodimer with PPARs and influences the processes of cell development, differentiation, metabolism, and death [53]; and the glucocorticoid receptor (GR), which induces adipogenesis and induction of insulin resistance in the mature adipocyte [54]. Figure 1 summarizes the relationships between the various biomarkers mentioned in the adipocyte and provides an overview of the relationships and changes reported by pesticide exposure on adipocyte cellular functioning that affect the development of obesity.
Figure 1.
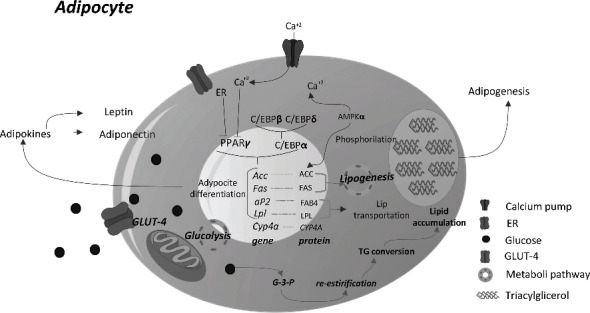
Activation of PPARγ and its interaction with lipid metabolism on the adipocyte. El PPARγ is conditioned to C/EBPα activation, once activated the receptor can recognize and bind to a PEP sequence of the genes of Acc, Fas, Ap2 y Lpl, which are involved with lipogenesis, adipocyte differentiation, lipid accumulation, and adipogenesis; lets the secretion of adipokines, therefore, are used as biomarkers of PPARγ activation. Also, the activation can be blocked by the accumulation of Ca+2 ions and ER activation. Abbreviations: ACC, acetyl Co-A carboxylase; AMPKα, AMP-activated protein kinase; C/EBPα-, CCAAT enhancer binding protein alpha; C/EBP-β, CCAAT enhancer binding protein beta; C/EBP-d, CCAAT enhancer binding protein delta; ER, estrogen receptor; FABP4, fatty acid-binding protein 4; FAS, fatty acid synthase; GLUT-4, glucose transporter type 4; LPL, lipoprotein lipase; PPARγ, peroxisome proliferator-activated receptor gamma; TG, triglycerides.
From the group of pesticides belonging to the organochlorines, the effects of DDT and its main metabolite DDE have been studied. Although its use has been banned in several countries, it is still possible to find it in soil samples and various organisms due to the persistence and the accumulation of its metabolite. For DDT, it has been reported to increase the accumulation of lipids, the expression of PPARγ and C/EBPα protein, and the enzymes FAS and ACC, and leptin [46, 50]; however, in the 3T3-F442A cell line, leptin levels are increased but C/EBPα levels are decreased, possibly leading to late adipocyte differentiation [46]. As for the metabolite DDE, its effect is consistent with that of its parent molecule, as it also increases lipid accumulation, the same enzyme, and adipokines [31] without altering inflammatory markers such as IL-6, MCP-1, and TNF-α [50]. Regarding PPARα receptor activation, no difference in mPPARα expression was detected in the 3T3-L1 lineage [49]. Another organochlorine metabolite studied is oxychloride, a metabolite of chlordane, but it has no effect on adipogenesis or lipolysis in NIH3T3-L1 cells [50]. One more, organochlorine pesticide is dieldrin, whose exposure to NIH3T3-L1 cells increases adiponectin and decreases adipogenesis [50].
Among organophosphate pesticides, diazinon induces the accumulation of lipids and increases the expression of de CEBP, FAS, PPAR, ACC, adiponectin, and perilipin, this last one can be found in mature adipocytes [33]. Fenthion is reported to be a PPARγ agonist in both 3T3-L1 and OP9 cell lines and activates the transcriptional activity of PPARγ [38]. Chlorpyrifos was originally reported as an inhibitor of adipocyte differentiation, decreasing lipid accumulation [55], associated with a decrease in leptin, resistin, and adiponectin secretion [49]; but Blanco et al. in 2020 reported an increase in lipid accumulation and increased expression of C/EBP, PPAR, and FAPB4 in the same cell line, 3T3-L1 [32]. On the other hand, the cyclodiene endrin has been reported to inhibit adipogenesis by inhibiting C/EBP [51] and only modestly stimulating PPARγ activity and to a greater extent GR activity [54], so the effect is not associated with PPARγ. However, Seok et al. in 2022 reported that endrin can activate C/EBPs, PPARγ, glucose transporter type 4 (GLUT-4), adiponectin, and FAS in the late phase of adipogenesis [24]. Glyphosate in its commercial form, but not in its pure form, inhibits PPARγ induction, inhibits proliferation and adipogenesis in 3T3-L1; and in mouse embryo fibroblasts (MEFs), it decreases PPARγ but not C/EBPβ, increases lipid peroxidation and expression of the enzyme superoxide dismutase (SOD) as a process to contain the free radicals and lipids generated during peroxidation [56].
Within the carbamates, methiocarb and carbaryl can activate PPARα [57], while dithiocarbamates such as mancozeb, as antagonists, reduce lipid accumulation and do not affect the expression of either PPARα or PPARγ. As for the imidazoles, prochloraz behaves in the same way as mancozeb as an antagonist [49].
The pyrethroids prallethrin and allethrin have been reported to act as PPARγ agonists to increase the accumulation of lipids in the 3T3-L1 and OP9 lineages, along with suppression of the Osx and Bgalp genes necessary for osteocyte differentiation into MSC lineages. In addition to increase in FAPB4 levels in the 3T3-L1 lineage following prallethrin exposure [38]. In the case of deltamethrin, it was found to be an antagonist of PPARγ by reducing lipid accumulation and adipocyte differentiation of 3T3-L1 [49].
Quinoxyfen, a member of the quinolines, showed agonistic activity for PPARγ in 3T3-L1 cells; however, it suppressed the expression of osteogenic genes in MSC cells, as did the organotoxic agent fentin [38]. Of the phenylpyrazoles, fipronil increases lipid accumulation and expression of C/EBP, PPAR, CCA, FAS, FABP4, and GLUT-4 [22]. Cis-bifenthrin increases the accumulation of lipids in HepG2 cells and the expression of FAS, PPAR, and SCD1 (stearoyl-CoA desaturase-1), which are responsible for the biosynthesis of monounsaturated fatty acids (MUFAs); however, it has also been shown to do so via the pregnane X receptor (PXR) [58].
The most studied group is the organotin compounds, of which the main representatives are TPT and TBT. TPT is reported to activate PPARγ and RXR, increasing lipid accumulation and adipocyte differentiation [44]. Like TBT, it increases lipids accumulation, activates PPARγ, RXR in its homodimeric form [45], LXR, ER [52], and also increases the expression of the gene aP2, as a marker of adipocyte differentiation [44, 45]. In the multipotent bone marrow stromal cells (BMS2), TBT stimulates lipid accumulation and activates the expression of PPARγ, RXR, and LXR receptors, although the PPARγ-RXR heterodimer is required for the adipogenesis process [59]. In the MSC-C3HI0T1/2 cell line, TBT is able to activate PPARγ2, Pref-1, and Sox9, the latter two genes involved in chondrocyte differentiation. However, the presence of dexamethasone decreases the expression of Pref-1 and SOX9, as well as the gene RUNX2, which is involved in osteocyte differentiation [60]. Regarding primary cultures of adipocytes, there is a report of rainbow trout (Oncorhynchus mykiss) adipocytes in which TBT and TPT induce lipid accumulation and increase the expression of PPARγ and C/EBPα, but their activation is not sufficient for complete adipocyte differentiation in this species [43].
The phenoxypropidic acid ester quizalofop-p-ethyl increases PPARγ expression and lipid accumulation and is a potent inducer of adipogenesis in 3T3-L1. However, the mechanism by which this occurs does not entirely dependent on PPARγ [39]. Chlorantraniliprole, a pyrazole, increases triglyceride content and expression of C/EBP, PPAR, and ACC and decreases pAMPK without altering endoplasmic reticulum stress (ERstress) [23]. Within strobilurins, pyraclostrobin accumulates triglycerides without activating PPARγ, LPL, or C/EBPα, so an alternative pathway to that of PPARγ is active, implying a change in mitochondrial function in an attempt by the cell to restore its homeostasis [40].
The study of metabolites derived from pesticides is poorly understood, but for DDE (a metabolite of DDT) in SH-SY5Y cells [31], 3,5,6-trichloropyridinol (TCP) [32] and chlorpyrifos-oxon (CPO) in MCF-7 cells [61], the latter two chlorpyrifos metabolites were reported to have PPARγ-agonizing effects and to promote adipogenesis. The quizalofop-p-ethyl metabolites studied (quizalofopic acid, tetrahydrofurfuryl alcohol, and 2,3-dihydroxyquinoxaline) appear to have no activity on adipose tissue [39]. The plasma hydrolysis metabolite of carbaryl, 1-naphthol, is also able to activate PPARγ. However, the hydrolysis metabolite of methiocarb, metylthio-3,5-xylenol, does not activate PPARγ but decreases the expression of PPARα in the presence of the metabolites: methiocarb sulphoxide and methiocarb sulphone [57].
On the other hand, the effect of mixtures of different pesticides is not as researched rather than that of pesticide metabolites, because of the complexity of selecting truly representative mixtures, doses, and the number of pesticides that can be combined. However, the report on mixtures of quizalofop-p-ethyl with glyphosate, 2,4-D, dicamba, mesotrione, and isoxaflutole does not appear to have any enhancing or inhibitory effect on its adipogenic effect [29].
The effect of the different pesticides on the PPARs receptors present in or possibly derived from cell lines of the adipocyte lineage shows a great diversity of responses, both agonistic and antagonistic, regardless of the structural similarity between the molecules belonging to the same group of pesticides. Furthermore, the direct effect on the genes activated by the PPARs is very obvious, although it is also recognized that they are not the only nuclear receptors involved in the response; and the final consequences of this alteration in lipid metabolism can also be explained by the change in cellular function of organelles such as the mitochondrial and ER. Given that the mechanism of action of pesticides on PPARs affecting lipid metabolism is very complex and diverse, it is difficult to link pesticides directly to the development of obesity, but this link cannot be denied either.
3.4. The Biological Effect of the Activation of PPARs in Carbohydrate Metabolism by Pesticides
The effect of pesticides on the activation of PPARs and carbohydrate metabolism has not been as studied as the liver disturbances in energy metabolism that have been associated to the presence of toxicants. In the literature consulted, only studies concerning the activation of PPARs by pesticides in the HepG2 cell line could be found. Thus, for PPARγ, Ning et al. performed an analysis of 14 pesticides with chitin synthesis inhibitors, 5 of which were found to be potent agonists (diflubenzuron, chlorfluazuron, flucycloxuron, novifluoron, and flufenoxuron). It has been highlighted that diflubenzuron alters energy metabolism by decreasing adenosine triphosphate (ATP) concentrations and increasing those of pyruvate and lactate, two precursor metabolites of the tricarboxylic acid cycle (TAC). The expression of genes encoding enzymes that are part of the TAC such as pyruvate dehydrogenase alpha 1 (PDHA1), oxoglutarate dehydrogenase (OGDH), and citrate synthase (CS) decreases; and with a downward trend in isocitrate dehydrogenase (IDH2) and fumarase (FH), TAC activity decreases. On the other hand, the expression of glycolysis enzymes such as 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 3 (PFKFB3) and lactate dehydrogenase B (LDHB) is increased. It is possible that these changes favor the synthesis of triglycerides, as glycerol precursors are available in large quantities [20].
In the case of the PPARβ/δ receptor and its activation by pesticides, a correlation was found between glucose metabolism in HepG2 cells and the herbicide 2,4-D, which lowers extracellular glucose levels and increases glucose in the hepatocyte, associated with increased expression of FoxO1 (increases expression of gluconeogenic genes), CREB (transcriptional regulator of gluconeogenesis), and PPARs [26].
The effect of pesticides on cells more involved in the systemic regulation of carbohydrate metabolism and serum glucose levels is very low, so it is important to conduct further analyses to understand whether the effect has a direct or indirect impact on the development of type 2 diabetes mellitus by inducing insulin resistance and the subsequent development of the disease, which has been raised by different epidemiological studies [9, 62].
3.5. Alteration of Lipid and Carbohydrate Metabolism by Activation of PPAR by Pesticides in Other Pathological Conditions
Since the expression of PPARs is diverse in the organs that build up organisms, the effect of their activation not only means a change in carbohydrate and fat metabolism in adipose and liver tissue, which is associated with the development of obesity and diabetes but exposure to pesticides and activation of PPARs has also been shown to be involved in other diseases and even to have a possible protective role in other metabolic processes. The following changes: the cell line used in the study and the observed biological effect are also described in Table 2.
The effect of PPARγ activation by pesticides on tumorigenesis and subsequent cancer development was observed by exposing CD1 mouse, rat, and human hepatocytes to permethrin and its metabolites: 3-phenoxybenzoic acid and trans-dichlorochrysanthemic acid. In the presence of permethrin and 3-phenoxybenzoic acid, DNA replication was increased in mouse cells but not in human cells. In addition to increasing the expression of PPARγ in the presence of 3-phenoxybenzoic acid and trans-dichlorochrysanthemic acid in hepatocytes of mice and rats, but not in humans. There is a clear difference in the response to the activation of the receptor in cells of different species [63].
In human reproductive changes, particularly embryo implantation in the uterus, chlorpyrifos has been reported to be able to damage trophoblast function and placental development in the context of decreasing the expression of PPARγ in an extravillous trophoblast cell model (ecCTB) with HTR8/SVneo cells [64].
The alteration of lipid metabolism in macrophages is influenced by the pesticide TBT, which can activate PPARγ, increase lipid accumulation, expression of lipid metabolism genes in human macrophages (THP-1 cells); such as CD36 (a receptor that promotes the entry of fatty acids into the cell), NR1H3/LXRα (regulates the homeostasis of fatty acids and cholesterol), FADS1, FADS2 (catalyze the first step in the synthesis of PUFAs), SREBP-1c (activates hypogenic genes in the liver), ACC (participates in the biosynthesis of fatty acids), FABP4, and FAS [65].
In oxidative stress, PPARα activation may mediate tissue damage due to physical or chemical stress stimuli. Exposure to paraquat increases the presence of CYP4A in primary cultures of mouse hepatocytes of the wild-type genotype and to a greater extent in cells with null PPARα, suggesting regulatory action of PPARα and activation of CYP4A by a different receptor [66].
In the metabolism of lipids in neurons, the metabolite of chlorpyrifos, chlorpyrifos oxon, caused the inhibition of fatty acid amide hydrolase (FAAH), the increase of metabolites of endocannabinoids (eCB), which are agonists of PPARs, as well favored the activation of PPARs in MCF-7 cells and the alteration of lipid metabolism [61].
The activation of PPARs has also been described as a mediator in the damage caused by pesticides that do not activate the receptor or activate it only to a lesser extent, as their effect is abolished by pharmacological agonists of PPARs. PPARγ agonists have been described as dopaminergic neuroprotectors [67], and the most commonly used cell model is SH-SY5Y (human neuroblastoma cells which can differentiate into neurons). Effects of pesticides on this cell line include: deltamethrin decreases the expression of PPARγ and PINK-1 (it is a mitochondrial target involved in protection against ROS) and causes cell death through mitochondria-dependent apoptosis [68]. Chlorpyrifos induces oxidative stress and cell death and also decreases and induces inflammatory genes such as COX-2 and TNF-α [69]. Rotenone increases the proliferation of ROS and decreases the expression of SOD1 [70] and TNF-α by inhibiting mitochondrial complex I [71]. All these effects are reversed with rosiglitazone as a pharmacological agonist of PPARγ.
Assessment of PPARs receptor activation in cell models provides a guide to understand the molecular mechanism by which the interaction and biological responses achieved in the presence of pesticides occur. However, the information that has been reported up to date is insufficient to generate a general mechanism of action that directly correlates pesticide exposure with the activation of PPARs and the development of obesity. Although the general effect on lipid metabolism, and to a lesser extent carbohydrate metabolism, could be a factor in triggering the development of obesity and, as a complication, the development of diabetes. This is because the reported findings are recurrent. However, several of these results may be due to mechanisms unrelated to the activation of PPARs. Besides, the approach of in vitro analyses is limited to a specific cell line, and since the stressful environment might force the cell lines to respond in a way they would not in the presence of other lines, they might mask the responses obtained. Therefore, the use of in vivo models may expand the understanding and framing of a systemic response of PPARs receptor activation by pesticides in the development of obesity and type 2 diabetes mellitus.
4. In Vivo Studies: Activation of PPARs Receptors by Pesticides and Their Subsequent Biological Response
Upstream, animal models have served to evaluate and project the possible effects that might be observed in humans, as what is found in them does not always replicate or approximate the effect observed in humans. In addition, organisms have also been used as sentinel models to assess the degree to which a particular biome is affected by the presence of pesticides or other environmental toxins. Table 3 shows the animal models used to assess exposure to pesticides involved in PPAR receptor activation and their biological effects.
Table 3.
In vivo models of pesticide effect above the PPAR receptors.
| PPAR subtype | Pesticide | Chemical classification | Type of pesticide | Model of study | Item | Year | References |
|---|---|---|---|---|---|---|---|
| PPARα | Methidathion | Organophosphate | Insecticide | Male B6C3F1 mice | These pesticides not active PPARα in a tumorigenesis process | 2022 | Rooney et al. [103] |
| Fenthion | |||||||
| Parathion | |||||||
| Fibronil | Phenylpyrazole | Insecticide | Male albino rats | Up-regulated FABP, ACC1, and PPARα | 2021 | Wasef et al. [80] | |
| Carbendazim | Carbamate | Fungicide | Male zebrafish (Danio rerio) | Level of glucose decreased and PPARα, ACO, CPT1 were not affected | 2020 | Bao et al. [91] | |
| Boscalid | Anilide | Fungicide | Zebrafish (Danio rerio) | Decrease the content of TG and cholesterol by accelerating lipolysis; and inhibiting lipogenesis, via the regulation of PPARα | 2019 | Qian et al. [95] | |
| Permethrin | Pyrethroid | Insecticide | Female C57BL/6N wild-type or PPARα (KO) mice | Increase expression of PPARα in hepatocytes and KO mice the effect decreases | 2019 | Kondo et al. [63] | |
| Propaquizafop | Ariloxiphenoxypropionate | Herbicide | Male SD wild-type or PPARα (KO) rats | PPARα regulates the biochemical and histological changes in the liver in hepatocarcinogenesis | 2018 | Strupp et al. [98] | |
| Propamocarb | Carbamate | Fungicide | Male C57bL/6J mice | Decrease PPARα and increase hepatic bile acids with a change of energy metabolism and the gut microbiota | 2018 | Wu et al.89 | |
| 2,4-D | Organochlorine | Herbicide | Male Sv/129 wild-type or PPARα-null mice | Induce testicular toxicity due to disruption of cholesterol/testosterone homeostasis in Leydig cells via PPARα | 2016 | Harada et al. [109] | |
| Oxadiazon | Oxadiazol | Herbicide | Male C3H/HeNCrl and CAR (KO) mice | PPARα and CAR are involved in the development of liver tumors | 2016 | Kuwata et al. [99] | |
| Toxaphene | Organochlorine | Insecticide | Male B6C3F1 mice | Induce mouse liver tumors, increase CAR, AhR but not PPARα target genes | 2015 | Wan et al. [102] | |
| Myclobutanil | Triazole | Fungicide | Male Wistar Han IGS rats | Perturb fatty acid and steroid metabolism in the liver predominantly through the CAR, PPARα, and PXR signaling pathways. | 2009 | Goetz and Dix [93] | |
| Propiconazole | |||||||
| Triadimefon | |||||||
| Methyl thiophanate | Thioallophanate | Fungicide | Male lizard (Podarcis sicula) | Increase AOX and PPARα | 2006 | Buono et al. [92] | |
| PPARβ/δ | Atrazine | Triazine | Herbicide | Xenopus leavis tadpoles | Increase PPARβ/δ, which is associated with the conversion of lipid and proteins into energy | 2011 | Zaya et al. [94] |
| PPARγ | DDT | Organophosphate | Insecticide | Male SD rats | Decrease PPARγ expression | 2022 | Al-Obaidi [79] |
| DDE | |||||||
| Bromuconazole | Triazole | Fungicide | Male SD rats | Decrease the TG synthesis via inhibiting the PPARγ pathway | 2021 | Wu et al. [18] | |
| TBT | Organotion | Antifouling | Male C57BL/6 mice | Activate PPARγ, increase lipid accumulation and the expression of lipid metabolism | 2021 | Jie et al. [65] | |
| Dieldrin | Organochlorine | Insecticide | Male C57BL/6 mice | No affect the genes regulated by PPARγ in hepatocarcinogenesis | 2020 | Wang et al. [97] | |
| Paraquat | Dipiridile | Herbicide | Male Wistar rats | Activation of PPARγ with pioglitazone, decreases the concentrations of MDA (a lipid peroxidation marker) | 2020 | Amin et al. [107] | |
| Monocrotophos | Organophosphate | Insecticide | Male CFT-Wistar rats | Increase lipid content in the liver, PPARγ, ACC, and FAS | 2020 | Nagaraju et al. [85] | |
| TPT | Organotion | Antifouling | Xenopus tropicalis embryos | TPT exposure reversed some impacts induced by PPARγ overexpression | 2018 | Zhu et al. [110] | |
| TBT | Organotion | Antifouling | Female Wistar rats | Abnormal ovarian adipogenesis with increased cholesterol levels, lipid accumulation, PPARγ, C/EBP-β, and Lipin-1 | 2018 | de Araújo et al. [77] | |
| TBT | Organotion | Antifouling | Male C57bL/6J mice | Increase mRNA expression of the PPARγ target genes Fabp4, Plin1 | 2017 | Baker et al. [76] | |
| Mancozeb | Dithiocarbamate | Fungicide | Swiss albino mice | Affect PPARγ and increased the cholesterol and TG | 2014 | Bhaskar and Mohanty [78] | |
| Imidacloprid | Neonicotinoid | Insecticide | No affinity to PPARγ | ||||
| Paraquat | Dipiridile | Herbicide | Male Wistar rats | Atorvastatin reduces the inflammation produced by pesticide, via PPARγ | 2014 | Malekinejad et al. [131] | |
| Pronamide | Benzamide | Herbicide | Male CD-1 mice | The MoA of hepatocarcinogenesis although to PPARγ and CAR | 2014 | LeBaron et al. [100] | |
| Nitrofen | Diphenyl ether | Herbicide | Pregnant rats and their fetus | Down-regulated PPARγ and altered late gestation possibly due to impair lung development and maturation | 2012 | Gosemann et al. [132] | |
| Paraquat | Dipiridile | Herbicide | PPARγ heterozygous mice (PPARclox/lox/aP2-Cre) | Reduce expression of PPARγ, improve insulin sensitivity, and increased resistance to paraquat-induce oxidative stress | 2008 | Luo et al. [105] | |
| TBT | Organotion | Antifouling | Pregnant C57BL/6J mice and their pups | Increase the number of adipocytes and lipid accumulation through RXR and PPARγ | 2006 | Grün et al. [130] | |
| PPARα PPARγ | Imidacloprid | Neonicotinoids | Insecticide | Zebrafish (Danio rerio) | Inhibit the growth of zebrafish and alters the levels of glycolipid metabolism and oxidative stress; reduce the expression of PPARα and PPARγ | 2021 | Luo et al. [96] |
| Endosulfan sulfate | Organochlorine | Insecticide | Pregnant CD-1 mice and their male pups | In high and low-fat diet, PPARα and its target gene Cpt1a are increased, but not modify PPARγ | 2021 | Yan et al. [87] | |
| Chlorpyrifos | Organophosphate | Insecticide | Male zebrafish (Danio rerio) | Decrease PPARα and PPARγ, due to lipid metabolism disorders that are associated with gut oxidative stress and microbiota dysbiosis | 2019 | Wang et al. [86] | |
| Atrazine | Triazine | Herbicide | Male Kunming mice | Induce nephrotoxicity via modulating CYP450, PPARα, PPARγ, AhR, CAR, and PXR | 2018 | Xia et al. [108] | |
| Lambda cyhalothrin | Pyrethroid | Insecticide | Male albino rats | Up-regulate mRNA expression levels of PPARα, PPARγ, TNF-α FAS, and SREBP-1C | 2016 | Moustafa and Hussein [81] | |
| Triphenyltin | Organotion | Antifouling | Wood frog (Lithobates sylvaticus) | In chronic exposure, increase the expression of PPARα, PPARγ, FAS, and LPL | 2013 | Higley et al. [75] | |
| PPARα PPARγ PPARβ/δ | Glyphosate | Organophosphate | Herbicide | Tilapia (Oreochromis niloticus) | Increase lipid content, alter redox status in liver, the genes involved in ion transport, lipid metabolism, and PPAR signaling pathway | 2022 | Jia et al. [74] |
| Allethrin | Pyrethroids | Insecticide | Male Sprague Dawley rats | No activation of nuclear receptor in liver | 2019 | Fujino et al. [30] | |
| Bioresmethrin | |||||||
| Cis-permetryn | |||||||
| Cypermethrin | |||||||
| Deltamethrin | |||||||
| Fenvalerate | |||||||
| Trans-permetryn | |||||||
| Phenothrin | |||||||
| Difenoconazole | Triazole | Fungicide | Marine medaka (Oryzias melastigma) | Increase the expression of receptor PPARα, PPARβ/δ, PPARγ, and increase lipid levels in muscle but not in liver | 2016 | Dong et al. [73] | |
| Paclobutrazol | Triazole | Fungicide | Male rockfish (Sebasticus marmoratus) | Increase total lipid, TG, TC, free fatty acid and up-regulate PPARα, PPARβ/δ, PPARγ, AR, FAS, FABP4, ACC | 2013 | Sun et al. [72] | |
| Atrazine | Triazine | Herbicide | CFI mice | No interact with the receptors α, β/δ, or γ | 2003 | Devos et al. [104] | |
| Diclofop | Ariloxiphenoxypropionate | Herbicide | Male Wistar rats (Pzh:WIS) | Increase the number of peroxisome and are a rodent PP | 2001 | Palut et al. [101] | |
| Oxadiazon | Oxadiazol | Herbicide | Male SD rats | Peroxisome proliferation only occurred in rats and mice maybe to PPARs activation | 1996 | Richert et al. [83] | |
| Male CD1 mice | |||||||
| Male beagle dogs |
Abbreviations: 2,4-D, 2,4-Dichlorophenoxyacetic acid; ACC, acetyl Co-A carboxylase; ACO, acyl-CoA oxidase; AhR, aryl hydrocarbon receptor; AR, androgen receptor; AOX, alternative oxidase; C/EBP-β, CCAAT enhancer binding protein beta; CAR, constitutive androstane receptor; CPT-1, carnitine palmitoyltransferase I; FABP4, fatty acid-binding protein 4; FAS, fatty acid synthase; KO, knock out; LPL, lipoprotein lipase; MDA, malondialdehyde; PP, peroxisome proliferator; PPARα, peroxisome proliferator-activated receptor alpha; PPARβ/δ, peroxisome proliferator-activated receptor beta or delta; PPARγ, peroxisome proliferator-activated receptor gamma; PXR, pregnane X receptor; SD, Sprague Dawley; SREBP-1C, sterol regulatory element-binding protein 1; TBT, tributyltin; TC, total cholesterol; TG, triglycerides; TNF-α, tumor necrosis factor alpha; TPT, Triphenyltin.
4.1. Changes in Lipid Metabolism in Adipose and Muscle Tissue Involving PPARs due to Pesticides
Lipid metabolism in animal models is assessed by measuring adipose tissue, assessing biomarkers of lipid metabolism in the liver, quantifying triglycerides and cholesterol in serum, and measuring short-chain fatty acids (PFAs) in muscle tissue. Two main animal models were used: aquatic models, which are used to monitor environmental quality, and murine models, which are more focused on clinical implications that can be applied to humans. Then the animal models used to evaluate the activation of PPARs by pesticides involved in lipid metabolism are mentioned.
In the case of triazoles, aquatic animal models are mainly used in the evaluation of their effects, i.e., for paclobutrazol, the rockfish (Sebasticus marmoratus) model was used, in which an increase in the expression of PPARα and PPARβ/δ in the liver and of FAS and ACC1 was observed [72]; for difenoconazole, the marine medaka (Oryzias melastigma) model was used, in which an increase in the expression of PPARα, PPPARγ, and PPARβ/δ was observed in the muscle, but in the liver, only the expression of the first two receptors increased. It is possible that this difference in expression is due to greater oxidation of fatty acids in skeletal muscle tissue and an increase in glucose oxidation in the liver [73].
Glyphosate, in a transcriptomic and proteomic liver analysis of tilapia (Oreochromis niloticus), an increase in lipid content but a decrease in the expression of PPARα was observed, this increase in lipids is probably the result of an imbalance in the redox balance of hepatocytes due to a large amount of intracellular ROS [74].
From the group of organotin compounds, TPT decreases the expression of PPARγ and its correlating genes (Fas, Cyp4b1, Lpl) in the frog embryo model (Lithobates sylvaticus) during the first days of exposure. However, after chronic exposure, this phenomenon reverses and increases the expression of PPARα and PPARγ and related genes; possibly due to an adaptive response to the constant stimulus [75]. Besides, TBT is capable of activating the PPARγ receptor in mouse MSC cells, promoting adipogenesis [76], and increasing adipose tissue mass in adult mice when exposed to the pesticide occurred during mouse fetal development [52]. In the female rat model, it increases adipose tissue weight and increases the accumulation of lipids and cholesterol, as well as the expression of PPARγ and ROS [77].
The use of mouse models to study the activation of PPARs by pesticides can be observed in the analyses performed for mancozeb, a dithiocarbamate, which increased the expression of PPARγ and raised cholesterol and triacylglycerols in the mice serum [78]; however, it was previously reported as an antagonist of PPARγ by not affecting receptor expression and decreasing lipid accumulation in preadipocyte cells [49]. Furthermore, in a mixture of mancozeb and imidacloprid, the increase in cholesterol and triglycerides is enhanced [78]. Including organophosphate, DDT, and DDE could alter adipogenesis processes and reduce PPARγ expression [79]. Another organophosphate, chlorpyrifos promotes obesity but is not related to the expression of PPARγ, it alters mitochondrial function and thermogenesis in mice [7].
Fipronil, an insecticide belonging to the phenylpyrazoles, increased the accumulation of lipids in the liver and altered lipid metabolism by producing an increase in PFAs, which in turn increased the expression of PPARα; and which, when oxidized, increased the concentration of ROS, leading to oxidative stress and activation of inflammatory pathways observed in a rat model [80].
In the case of lambda-cyhalothrin, a pyrethroid capable of activating PPARγ and PPARα receptors in albino rats, it increased the concentration of total lipids, triglycerides, and cholesterol, as well as the inflammatory modulator TNF-α [81]. However, Costa et al. demonstrated the differences between pesticides in species and reported that this inflammatory marker did not change in humans in the presence of α-cypermethrin, another pyrethroid [82].
Also, the different response of different species to PPARγ activation was evident when evaluating the effect of oxadiazon, an oxadiazole herbicide. In mice and rats, it was observed that exposure to pesticide-induced hepatomegaly due to the enlargement of peroxisomes. However, this effect was not observed in dogs, demonstrating a difference in the sensitivity of PP among species [83].
Dicamba, a salt of benzoic acid used as an herbicide, is a structural isomer of 2,4-D known to be a PP that increases the expression of PPARs and beta-oxidation of lipids, in addition to differential expression of CYP4A with respect to rat sex, as an increase was observed only in males [84]. It is clear that the sex of the organisms can also be considered.
The continuous detection of the change in lipid metabolism and the increase in the expression of PPARα and PPARγ in the presence of different pesticides in the models of aquatic organisms has led to their proposal as biomonitors of water quality and to the possibility of monitoring these changes as biomarkers. The differential response between types of pesticide exposure and their effects on lipid metabolism and PPARs expression allows us to propose a broader study of the characteristics of pesticides and/or organisms that make them more susceptible to pesticide exposure response and the activation of PPARs that favor the alteration of lipids involved in the development of obesity.
4.2. Changes in Energy Metabolism in the Liver due to Activation of PPARs by Pesticides
The use of animal models has the advantage that the systemic response to an external stimulus in an organism can be studied. This allows the evaluation of the response of different organs and the compensatory mechanisms of the organism that attempt to minimize and repair the damage caused. Then, various effects of exposure to pesticides on carbohydrate and lipid metabolism as a whole will be described as how both responses relate to the activation of PPARs, as well as their association with the development of obesity and type two diabetes mellitus.
In the organophosphates group, monocrotophos was found to induce glucose intolerance, insulin resistance, and dyslipidemia with hyperinsulinemia in rats, largely due to increased expression of G6FDH (glucose-6-phosphate dehydrogenase) and G3PD (glycerol-3-phosphate), which indirectly promotes the regulation of lipogenesis. The insulin resistance presented is associated with an increase in lipids in the liver, which is favored by increased expression of CCA, FAS, PPARγ (lipogenesis), and a decrease in PPARα (β-oxidation). All the previously described changes together produce the symptoms of hepatic steatosis that occur in patients with obesity [85]. On the other hand, chlorpyrifos alters energy metabolism in the liver by decreasing the expression of pyruvate kinase (PK) and glucokinase (GK) enzymes involved in glycolysis, and by decreasing the expression of PPARα, PPARγ, ACO (acyl-CoA oxidase), FAS, ACC (lipid metabolism); in addition to altering the composition of the gut microbiota, decreasing γ-Proteobacteria, in the zebrafish (Danio rerio) model [86].
Exposure to the organochlorine endosulfan sulfate during pregnancy and early postnatal days in mice resulted in alteration of glucose homeostasis, hepatic lipid metabolism, and gut microbiota; as the expression of PPARα, G6P, GLUT-2 (type 2 glucose transporter) was increased, the opposite effect was observed in the presence of a high-fat diet when biomarkers decreased [87].
Within the group of carbamates, propamocarb has been described to increase GK and decrease PK, PPARα, and genes related to triglyceride and fatty acid synthesis and transport; furthermore, exposure to pesticides has been associated with alteration of the gut microbiota due to alteration of bile acid lipid metabolism, which affects the composition of the microbiota [88, 89]. Another carbamate, carbendazim, which may also be a metabolite of methyl thiophanate and benomyl [90], increases the expression of PPARγ, FAS, hexokinase 1 (HK1) (glycolysis), and PK, in addition to altering the gut microbiota, which decreases the genus Firmicutes and Bacteroidetes, which is associated with obesity [91]. Thiocarbamate, methyl thiophanate, increases PPARα expression, degrades liver glycogen, and increases ACO, an enzyme involved in lipid metabolism and activation of PPARs, in the Gecko model (Podarcis sícula) [92].
In the triazole group, bromuconazole inhibits PPARγ signaling but increases TG, TC, and pyruvate in male rats [18]. Myclobutanil, propiconazole, and triadimefon alter genes regulated by PPARα in male rats, decreasing Cyp4a10, Cyp4a1, and PK, thereby limiting fatty acid biosynthesis and storage [93]. Triazine, a triazide, increases PPARβ/δ expression and decreases lipid storage. This change may be due to the activation of PPARβ/δ redirecting metabolism to energy production as an adaptive response to pesticide exposure in frog tadpoles (Xenopus leavis) [94].
TBT exposure poses a high risk for the development of type 2 diabetes mellitus in mice because it produces insulin resistance, alters hepatic glucose metabolism, increases insulin levels, and decreases serum glucagon levels. Insulin resistance is caused by increased G6P and phosphoenolpyruvate carboxykinase, which are involved in glycogenolysis and gluconeogenesis [34].
Boscalid, an anilide, affects aquatic organisms such as zebrafish (Danio rerio), inhibits their growth and causes liver and kidney damage, increases HK, G6P, and PPARα, promotes β-oxidation and decreases ACC, FAS, TG, TC, and blood glucose [95]. Imidacloprid, a neonicotinoid, inhibits zebrafish growth and alters glycolipid metabolism. It increases the expression levels of PPARγ, PPARα, ACC, FAS, GK, and HK, as well as the inflammatory biomarkers TNF-α, IL-1β, and IL-8, and increases levels in the liver [96].
The above results generally correlate with the increase in lipid storage and lipid oxidation, as well as with the increase in glycogenolysis and gluconeogenesis, and with the development of insulin resistance, which may be the prerequisite for the development of obesity and diabetes. Another factor is the composition of the gut microbiota, which could affect lipid metabolism and promote the development of obesity [91].
4.3. Changes in Energy Pathologies Involving Activation of PPARs by Pesticides
Activation of PPARs by pesticides has also been associated with the development of other metabolic disorders or various pathologies, the most obvious of which is associated with altered energy metabolisms, such as obesity and diabetes. Most notably in their role as PP in the development of tumors and liver cancer, in response to oxidative stress, and in response to renal, reproductive, and developmental toxicity.
Several pesticides have been reported to be PP that increase the concentration of peroxisomes in liver tissue and trigger the development of tumors that eventually lead to carcinogenesis. Nuclear receptor activation is a common mechanism of action in the development of toxicity and carcinogenesis in rodents in non-genotoxic processes [97]. Therefore, activation of PPARs by pesticides has been associated with a possible mechanism of carcinogenesis in rodents but not in humans. Pesticides that activate PPARs and have a carcinogenic effect include propaquizafop, an aryloxyphenoxypropionate that has a hepatocarcinogenic effect via PPARα by increasing the expression of CYP4A and ACO in rats but is not relevant to humans according to the Human Relevance Framework (MOA/HRF) [66, 98]. Oxadiazone, an N-phenyl heterocycle compound, induces tumor development by activating PPARα, inducing CYPa10 and CYP4A [99]. The benzamide pronamide induces liver tumors through the activation of nuclear receptors CAR (constitutive androstane receptor) and PPARα, which induce genes such as Cyp2b10 and Cy4a10, but is not relevant to the mechanism in humans due to quantitative and qualitative differences between mice and humans [100]. Permethrin, a pyrethroid insecticide, produces liver tumors in mice but not in rats by increasing CYP4A expression and activating PPARα, resulting in a mitotic effect [63], although the effect cannot be extrapolated to humans according to the ILSI/PCS (The International Life Sciences Institute/International Program on Chemical Safety) reference frame. And diclofop, a chlorophenoxy herbicide recognized as PP, increases the number of peroxisomes, palmitoyl-CoA oxidase, catalase, and binucleate hepatocytes in rats, effects that have been associated with PPARα activation [101].
On the other hand, there are pesticides capable of producing tumors, but the involvement of PPARs is not involved in the development of the disease. For example, toxaphene, an organochlorine that causes tumors in the liver of mice, increases genes related to oxidative stress and not PPARα [102]. Within the organophosphate group, methidathione produces tumors in the liver of male mice, but the role of PPARα was ruled out by microarray assay [103]. Finally, there are reports of pesticides suppressing tumor development, such as fenthion, which does not increase liver tumor development, and parathion, which suppresses liver cancer development [103]. And atrazine inhibits cell proliferation and cytokine production in the liver and kidney but does not activate PPARs [104]. Overall, the role of PPARα in tumor/cancer development predominates among pesticides that have been reported to be able to do so by enabling the activation of genes that allow peroxisome proliferation and function. However, it is important to recognize that the receptor is not the only one involved in all pesticides.
Regarding oxidative stress and its relation to obesity, it was observed that lower activity of the PPARγ receptor or its deletion tended to reduce the stress and the expression of obesity. For example, the PPARγ2 Pro12Ala polymorphism decreases its activation and leads to a lower body mass index, and increases insulin sensitivity in humans [105]. Therefore, PPARγ-deficient mice are more resistant to oxidative stress and paraquat exposure lethality, likely due to increased expression of antioxidant genes in adipose and skeletal muscle tissues [105]. However, activation of the PPARγ receptor in lung tissue by agonists such as pioglitazone [106] or carvacrol [107] decreases oxidative stress resulting from paraquat inhalation and, consequently, the production of ROS and inflammatory biomarkers such as TNF-α and IL-17, indicating that PPARγ activation elicits a completely different response to the same substance in different organs.
Other effects observed by the activation of PPARS by pesticides include the generation of nephrosis by atrazine and its degradation metabolites, through the activation of PPARα and/or PPARγ receptors, the increase in the expression of CYP4A and its white genes [108]. Testicular toxicity induced by 2,4-D, which is caused by the alteration of PPARα receptor pathways that inhibit cholesterol synthesis in Leydig cells, does not occur in PPARα-null mice [109]. And the induction of malformations, especially in the eye, in frog embryos (Xenopus tropicallis) resulting from exposure to TPT due to overexpression of PPARγ [110].
The effect of activation of PPARs by pesticides in pathologies other than obesity and diabetes allows us to highlight that the alteration of lipid metabolism, its intervention in peroxisome proliferation and its differential response in different organs, its evaluation, and analysis of its role in the metabolism of lipids and carbohydrates are more complex than expected.
5. Discussion
Based on the above information, it can be concluded that the associations between pesticide exposure and the development of obesity and diabetes mellitus in epidemiological studies have promoted the search for the probable molecular mechanism explaining this relationship. To this end, in silico studies were first performed to investigate the interaction between the ligand and the receptor using models such as QSAR or docking. These models revealed that for the PPARγ receptor, the amino acid residue Cys 285 could play a crucial role in the association of pesticides with this receptor, as observed for diflubenzuron [20] or for the organotin compounds TPT and TBT [21]. This interaction was also reported for molecules of a different type such as 1,3-diphenyl-2-propone, volatile organic compounds extracted from a fermented cheonggukjang (Korean food), and the Cys 285 residues of PPARβ/δ and PPARγ [111]. This interaction was confirmed by X-rays to be essential for the interaction between organotin and the PPARY [21]. The chemical structures of the pesticides that form greater stability in the compound and thus greater activity of the receptor are those that contain aromatic rings in their structure and are able to form π–π bonds between the amino acid residues that they contain, conforming receptors and their structures, particularly in an ionized state [19, 21], this has also observed with triclocarban, an antiseptic formerly used in personal care products, by interacting with the receptor mouse and human PPARα through a Cl–π via the amino acid residue phenylalanine (Phe 318) [112]; in the flame retardants bis(2-ethylhexyl)-2,3,4,5-tetrabromophthalate (TBPH) and mono-2(-ethylhexyl)-tetrabromophthalate (MEHP), where the first π-alkyl forms interactions with the amino acid residues histidine (His 199) and proline (Pro 476); and for the second alkyl bonds with the residues methionine (Met 248) and arginine (Arg 260) of the zebrafish PPARγ receptor [113]. This shows that the interaction between the amino acid residues of the receptor and the molecular structures of the pesticides can be given by specific amino acid residues and, moreover, allows specific interactions that enable greater stability.
Since the biological effect of PPAR activation is directly related to the metabolism of lipids and carbohydrates, the main axis of biomarkers studied are the genes containing in their promoter the PPRE and the enzymes/proteins encoded by these genes in the main organs controlling the energetic homeostasis of the organism; the biomarkers used to monitoring PPAR activation through pesticides could be summarized as follows: the biological activation of the PPAR has been observed in adipose tissue by DDT, dieldrin, diazonin, fenthion, and fibronil through the accumulation of lipids as an effect of adipocyte maturation [22, 33, 38, 50]. Also this activation has been observed with organophosphate flame retardants (OPFRs) in inducing adipogenesis as 2-ethylhexyl diphenyl phosphate (EHDPP) [114] and triphenyl phosphate (TPHP) [115]; however, by exploring the mechanism by which pesticides enable this accumulation, it has been shown that the role of PPARγ in this phenomenon is important and consistent, but not unique, as accumulation of lipids is still observed despite blocking the receptor, as in the case of qhizalofop-ethyl [39]; and even the direct role of other receptors in lipid accumulation has been reported, as in the case of dioxin: 2,3,7,8-tetrachlorodibenzo-p-dioxin, which is antagonistic to the aryl hydrocarbon receptor (AhR) and prevents lipid accumulation in association with a decrease in PPARγ [116]. Likewise, the mechanism of lipid accumulation has been shown to be not only due to a process of direct activation of nuclear receptors, as is the case with chlorantraniliprole and pyraclostrobin, which increase oxidative stress in the ER and mitochondria, accordingly, caused by an increase in lipid peroxidation and ROS, and decrease the availability of ATP [23, 40]. In relation to adipogenesis, it has also been observed that the presence of other nuclear receptors is necessary to carry it out, such as RXR, LRX, or ER in organotin: TBT, TPT [45, 52, 59, 60], or the GR for endrin [54], which allows to theorize the existence of a synergistic interaction or enhancer of different receptors by activating a different cascade of regulation and activation of adipogenesis through binding to other receptors also involved in it; for example, TBT forms a covalent bond with the cysteine (Cys) of the active site of the RXR receptor, which activates adipogenesis less efficiently than PPARγ [59]. This could lead to think that the redundant processes present in nature allows to ensure the functioning and maintenance of cells, and even as a regulatory mechanism that overall favors the survival of the organism. In terms of cell differentiation towards the adipocyte lineage, it has been reported that pesticides such as quinoxyfen, fentin, prallethrin, and allethrin show a preference for adipocyte differentiation of MSCs through the PPARγ [38], showing that activation of this receptor is necessary for this outcome by inhibiting genes related to osteocytes or dendrocytes [38].
Antagonists such as deltamethrin, mancozeb, prochloraz, and glyphosate show that complete inhibition of adipogenesis and lipid accumulation corresponds very well with decreased expression of PPARγ and enzymes associated with receptor activation [49, 56]. And that even the effect in the commercial presentation is much greater than in its pure state, as the presence of certain additives can enhance or even increase the activity, as in the case of glyphosate as an antagonist [56] or quizalofop-ethyl as an agonist [39], which has less activity in its pure state. Some additives that have been reported to activate PPARα are toximul [116], a pesticide surfactant, and piperonyl butoxide [118], a pesticide synergist that increases lipid accumulation as well as CYP4A10. Although it is important to highlight that the CYP4A gene contains a PPRE in its promoter, the LXR receptor can also activate this gene [119], revealing an alternative control mechanism and raising the possibility that other genes controlled by PPARs may have a similar mechanism.
In the case of pesticides whose biological activity is controversial because they showed antagonistic activity for PPARγ in initial studies since there is no increase in lipid accumulation, such as chlorpyrifos [49] and endrin [46]; and in later studies, its involvement could be observed by increasing adipogenesis through receptor activation [24, 49, 55]. This discrepancy has been widely discussed and demonstrated in this regard, with results shown to be influenced by the source of strain acquisition, the number of passages in cell culture, and the protocol used during cell differentiation [41]. The protocols used in cell differentiation into adipocytes of the various cell lines use a mixture of a glucocorticoid (dexamethasone), insulin, and 3-isobutyl-1-methylxanthine (MDI), which on the one hand promotes adipogenesis by activating master TFs such as CCAAT-enhancer-binding protein homologous protein (C/EBPs) and PPARγ, and on the other hand, in less differentiated cell lines, inhibits those of other possible lines. In addition to enhancing the stimulus to increase the expression of PPARγ, thereby decanting the differentiation process, as pre-adipocyte cells express low levels of PPARγ [32]. Without the presence of this mixture or any of its components, in the experiments reported, the effect is significantly inhibited or diminished, in addition to obtaining a different pattern of activation and expression of adipogenesis protein. As observed with TBT, the presence of a single element of the MDI mixture favors the expression of adiponectin, perilipin, or C/EBPα in a differential manner [120]. Dexamethasone has been described to decrease the expression of adiponectin (which increases insulin sensitivity) and the transcriptional repression of Pref-1 (a key gene in chondrocyte differentiation); insulin favors the activation of PPARγ; and 3-isobutyl-1-methylxanthine increases the concentration of intracellular cAMP, which is necessary for the activation and increase of C/EBPβ [121]. In addition, the maturity stage of the adipocyte in which the determination is made also affects the observed results. A clear example of this is triterpene, celastrol, which has a stronger effect in the first few days and decreases its effect as it passes through the different stages of maturation [122]. Comparison of results reported to date for pesticides is complicated by the wide variation in methods, which prevents the establishment of a correct mechanism of action for all variants that have an impact. However, it is apparent that they all converge in that activation of PPARγ promotes adipogenesis and lipid accumulation, although the role of the receptor is not yet entirely clear for some pesticides.
The effect of pesticides on PPARs in carbohydrate metabolism has low rates of research, perhaps in large part because it is considered to be the effect of liver injury causing the presence of various toxicants and because the involvement of other receptors in carbohydrate metabolism is more obvious than that of PPARs. However, it has been reiterated that the effect of diflubenzuron on the PPARγ receptor is associated with the decrease of enzymes involved in TCA, reducing their activity and the generation of ATP by this metabolic pathway [20], along with the possibility of increasing the synthesis of triglycerides by increasing the availability of glycerol precursors; and 2,4-D exposure favors the expression of the PPARβ/δ receptor, allowing the reduction of extracellular glucose and increasing glycogen stores [26]. The previous results highlight the need for more evaluation of the effect of pesticides on carbohydrate metabolism via PPARs. Also, exposure to pesticide mixtures remains controversial due to the absorbability and bioaccumulation of individual pesticides [96].
Although the use of different cell lines has allowed to approach the mechanism by which the activation of PPARs by pesticides may favor the development of obesity and diabetes, the use of in vivo models, that is, in animals, has made it possible to analyze the effect of pesticides on several possible target organs simultaneously that were not originally considered and, moreover, to provide a clear approach to the changes that favor the development of disease. The results observed in animal models are very consistent with those observed in vitro experiments. Two main models have been used to evaluate the effects of pesticides on PPARs, aquatic models, and murine models. The first model is used more for environmental monitoring, and the second is in the search for understanding diseases and therapeutic targets.
In aquatic models, it was observed that the greatest effect occurs in muscle tissue by increasing the expression of PPARα, PPRAγ, and PPARβ/δ receptors, increasing lipid storage in muscle and adipose tissue, and increasing lipid metabolism, although this effect is not observed in the liver [73]. In murine models, after exposure to pesticides such as TBT, mancozeb, imidacloprid, lambda-cyhalothrin, fipronil, dicamba, and oxadiazon, an increase in PPARα and/or PPARγ receptor exposure is shown in adipose tissue composition, the presence of dyslipidemia (elevated lipids in the blood) and an increase in lipid metabolism associated with an increase in the concentration of ROS and inflammatory biomarkers in adipose tissue, liver, and muscle [80]. In addition, there is a change in the composition of the gut microbiota, which occurs as a direct change in pesticide exposure, as well as a change in fat metabolism and the formation of ROS [86, 89, 91].
However, not all results are in complete agreement with those observed in vitro experiments. This was the case with mancozeb [78] and lambda-cyhalothrin [81], where no activation of the receptor or generation of inflammatory biomarkers was observed in vitro as shown in the animal model, although it should be noted that the cells and tissues used in the respective experiments are different [82]. Perhaps this is because, in response to toxic exposure, the body attempts to maintain body homeostasis by activating or favoring other receptors that have similar activity to PPARs, such as CAR [72], LXR [72, 73], including estrogen receptors [52], and thyroid receptors [78].
It seems that the alteration of lipid and carbohydrate metabolism because of exposure to pesticides and activation of PPARs is mainly due to an increase in the concentration of lipids, triglycerides, and cholesterol, which are directly related to the increase in the expression of PPARα and PPARγ and the genes activated by these receptors, thus linking them to the development of obesity. On the other hand, the development of insulin resistance is associated with two main processes, the first through the increase in serum lipids and the second through the increase in key enzymes of gluconeogenesis and glycogenolysis (G6P and PKC), leading to an increase in insulin concentration. In a chronic state, insulin resistance develops, leading to the development of type 2 diabetes mellitus.
On the other hand, the recurrently observed increase in oxidative stress and inflammatory biomarkers could be due to the increase of fatty acid in serum, which allows the expression of PPAR receptors, especially in the liver, increasing the accumulation of lipids in this organ, favoring their oxidation, and the consequent production of ROS [77, 80]. The sensitivity of each pesticide to the receptor, the animal species [83], and the genus of the species [84] play an important role in the response obtained during exposure to pesticides.
It is important to highlight that the results presented above link the activation of PPARs receptors in in vitro and in vivo models to the development of obesity and diabetes, due to the interaction, activation, and biological responses (accumulation of lipids, adipogenesis, alteration in carbohydrates metabolism, insulin resistance); mainly with organotin pesticides (TBT and TPT) [21, 34, 43–45, 59, 76], followed by organophosphates (chlorpyrifos and DDT/DDE) [6, 9, 31, 32, 37, 46, 50, 61, 86]; however, the remaining pesticides such as pyrethroids, carbamates, and others show similar trends in terms of effects on PPARs as initially found for organotin and organophosphates. Therefore, further studies are needed to expand and clarify the mechanism by which pesticides might activate PPARs receptors and cause the development of obesity and diabetes mellitus.
Alteration of the gut microbiota has been raised as a possible target in the development of obesity because alteration of the microbiota has been associated with increased oxidation of lipids and consequent activation of PPARs. The oxidation of lipids and the resulting formation of ROS alter the composition of the microbiota. First, direct exposure to pesticides affects the intestinal mucosa and microbiota composition, favoring the increase of strains associated with the presence of obesity [86, 88, 91].
The mechanism of action by which PP pesticides are capable of producing tumors/cancer has been extensively studied, and the role of PPARα activation appears to be important in this process; even though, the results in rodents are not applicable to humans. It is also recognized that this receptor is not the only one involved in tumorigenesis and that the effect of inhibiting the development of tumors and liver cancer may be the effect of exposure to certain pesticides [97, 103]. Finally, the activation of PPARs has also been suggested as a possible therapeutic target in the cognitive symptoms of Gulf War illness [123].
The above data can be summarized in Figure 2, which shows that the effects on energy metabolism in adipose tissue observed in the presence of pesticides occurred in adipose metabolism through activation of nuclear receptors: PPARα, which controls lipid synthesis genes (ACO and CPT1); and the PPARγ receptor, which controls lipid breakdown genes (FAS, ACC, and SREBP1-C). These, when altered, can trigger the accumulation of ROS (due to lipid degradation), which damages DNA, ER, and mitochondrial function, and promotes oxidative stress: the energy metabolism of the cell is altered. These changes promote the secretion of adipokines and inflammatory components such as adiponectin, leptin, TNF-α, and IL-1β, which influence and control the entry and consumption of glucose from cells. Lipid metabolism was also altered by the presence of pesticides, increasing the accumulation and growth of adipose tissue and promoting the formation of new adipocytes (adipogenesis). In addition, macrophages present in the tissue were induced to secrete inflammatory components such as TNF-α, IL-1β, and IL-6. In both cases, a chronic state of inflammation and insulin resistance associated with obesity is promoted. However, since high lipid concentrations prevent the proper functioning of insulin receptors as well as their insufficient synthesis in musculoskeletal tissues, the effect of pesticides at these levels has not yet been studied. On the other hand, changes in energy metabolism were also observed in hepatocytes (Figure 2(b)), in which activation of PPARγ and PPARβ/δ promotes glycolysis but decreases activation of TAC, thereby increasing the presence of precursors in the metabolism of glycerol, favoring the synthesis of triglycerides, promoting the accumulation of lipids and the development of nonalcoholic fatty liver, and altering systemic energy function. In addition, activation of PPARα promotes peroxisome formation, which has been linked to the development of carcinogenic processes in murine models. The development of diabetes under these conditions may be due to the influence of adipokines (leptin and resistin), as they stimulate or reduce the secretion of insulin (a hormone that controls serum glucose levels) [124]. However, the effects of the presence of pesticides have been little studied and their role is unclear. In contrast, in the pancreas, the organ responsible for insulin secretion (Figure 2(c)), the role of activation of PPARs by pesticides is still unknown, as are the effects on signaling and insulin receptor function in muscle tissue (Figure 2(d)). Finally, alteration of the gut microbiota (Figure 2(e)) appears to alter the metabolism of carbohydrates and lipids; they have recently been proposed as another factor in the development of obesity; nevertheless, the exposure to pesticides at these levels is still unclear. The role of PPARs in the development of obesity and diabetes mellitus is more likely to be the alteration of lipid metabolism and, consequently, the development of insulin tolerance, which in turn triggers the development of type 2 diabetes mellitus, although a greater amount of data is still needed to establish this link.
Figure 2.
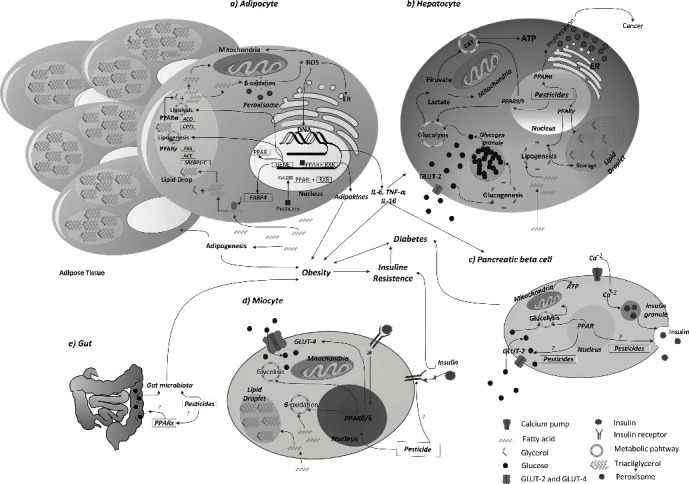
Effects of pesticides on carbohydrate and fat metabolism involving diabetes and obesity through activation of PPARs. Activation of PPARs by pesticides and their effects have been linked to the development of obesity and diabetes in many tissues: adipocytes (a) show mainly PPARα and PPARγ activation to lipid metabolism; in hepatocytes (b) PPARγ is involved in lipid metabolism, PPARα is associated with peroxisome proliferation to cancer and PPAR β/δ in the carbohydrate metabolism; in pancreatic beta cells (c) the role of PPAR affected by pesticides and the function of these cells is not clear; in myocytes (d) PPAR β/δ activation is essential for the regulation of energy metabolism, but the effects of pesticide exposure are unclear; finally, (e) the disruption of the gut microbiota in the development of obesity and diabetes about PPAR activation is unknown. Abbreviations: ACC, acetyl Co-A carboxylase; ACO, acyl-CoA oxidase; AMPKa, AMP-activated protein kinase alpha; ATP, adenosine triphosphate; C/EBP-α, CCAAT enhancer binding protein alpha; C/EBP-δ, CCAAT enhancer binding protein delta; C/EBP-β, CCAAT enhancer binding protein beta; CPT-1, carnitine palmitoyltransferase I; ER, endoplasmic reticulum, FABP4, fatty acid-binding protein 4; FAS, fatty acid synthase; GLUT-2, glucose transporter type 2; GLUT-4, glucose transporter type 4; LPL, lipoprotein lipase; PPARα, peroxisome proliferator-activated receptor alpha; PPARβ/δ, peroxisome proliferator-activated receptor beta/delta; PPARγ, peroxisome proliferator-activated receptor gamma; ROS, reactive oxygen species; RXR, retinoid X receptor; SREBP-1C, sterol regulatory element-binding protein 1, TG, triglycerides.
6. Conclusions
According to the WHO, it is estimated that by 2025, approximately 167 millions of people will deal with obesity and problems linked to overweight such as diabetes, high blood pressure, and dyslipidemia. Environmental toxicants, such as pesticides, have been identified as one of the possible factors favoring the development of these diseases; due to their environmental persistence and their bioaccumulation, the exposure to pesticides can take place everywhere: in schools, offices, at home through different vectors such as the food, drinking and daily use water; also traces of pesticides can be found in air, leaving in this way contact to pesticides in all our surroundings. This is why is so important to research and find more info about the effect of these toxicants in the human body and health. Understanding pesticides and their mechanisms of action could allow finding and proposing therapeutic targets to fight them; given that lipid and carbohydrate metabolisms are involved in obesity and diabetes and being that PPARs are one of the most important nuclear receptors for the control of these metabolic processes, we could prevent or avoid activating these interactions.
The information available up to date does not enable to determine the mechanism of action of a specific chemical group of pesticides because compounds in the same group react differently, e.g., as agonists, antagonists, or with no effect on the receptors. The use of in silico models has allowed the prediction of the interaction between PPARs and pesticides and has been proven to be a very useful tool.
The use of in vitro models with cell lines has been important in elucidating the mechanism of action of pesticides and PPARs, but the discrepancy of the spotted results in different protocols could debate this response. Therefore, the search for alternatives with more precise definitions and lower dispersion of results would allow a better understanding of the effect of pesticides and the mechanism by which they exert this effect.
Finally, in vivo models with animals have made it possible to link the effects of pesticides to lipid metabolism and its effects on the development of obesity through the accumulation of fat that ease insulin resistance and the subsequent development of type 2 diabetes mellitus. Thus, activation of PPARs by pesticides was found to be consistent in most cases; PPARγ receptor activation can lead to adipogenesis and lipogenesis in adipose tissue and liver; lipolysis and proliferation of peroxisomes involved in liver tumor development by the PPARα receptor; and changes in lipid and carbohydrate metabolism to a lesser extent by the PPARβ/δ receptors; however, the changes observed in lipid and even carbohydrate metabolism are not the only ones and are not all exclusively dependent on PPARs, their participation in these metabolic process is essential for the cell function.
The above reviewed statements leave a lot of gaps and opportunities to find more about the alteration of carbohydrate metabolism, and the alteration of pancreatic and muscle-skeleton function associated or not to pesticides, and there are even new implications like the alteration of the gut microbiome associated to the exposure to pesticides and the PPARs leaving many fields in need to be studied with more emphasis.
Acknowledgments
This work was supported by the Consejo Nacional de Ciencia y Tecnología (CONACYT) with a postgraduate grant.
Abbreviations
- 2,4-D:
2,4-Dichlorophenoxyacetic acid
- ACC:
Acetyl-CoA carboxylase
- ACO:
Acyl-CoA oxidase
- AhR:
Aryl hydrocarbon receptor
- AMPKα:
AMP-activated protein kinase alpha
- AOX:
Alternative oxidase
- AR:
Androgen receptor
- Arg:
Arginine
- ATP:
Adenosine triphosphate
- BM-MSC:
Bone marrow-derived multipotent stromal cells
- C/-EBPα:
CCAAT enhancer-binding protein alpha
- C/-EBPβ:
CCAAT enhancer-binding protein beta
- C/-EBPδ:
CCAAT enhancer-binding protein delta
- C/EBPAs:
CCAAT-enhancer-binding proteins
- cAMP:
Cyclic adenosine monophosphate
- CAR:
Constitutive androstane receptor
- TCA:
Tricarboxylic acid cycle
- COS-1 cells:
Fibroblast-like derived from monkey kidney
- CPO:
Chlorpyrifos-oxon
- CPT-1:
Carnitine palmitoyltransferase I
- CREB:
cAMP response element-binding protein
- CS:
Citrate synthase
- CV-1 cells:
Fibroblast cells from the kidney of an African green monkey
- Cys:
Cysteine
- DDD:
Dichloro diphenyldichloroethane
- DDE:
Dichloro diphenyldichloroethylene
- DDT:
Dichloro diphenyltrichloroethane
- DEGs:
Differentially expressed genes
- DHHP:
Diphenyl phosphate
- DNA:
Deoxyribonucleic acid
- eCB:
Metabolites of endocannabinoids
- ecCTB cells:
Extravillous trophoblast cell
- EHDPP:
2-ethylhexyl diphenyl phosphate
- EPN:
Ethyl p-nitrophenyl phenylphosphorothioate
- ER:
Endoplasmic reticulum
- FAAH:
Fatty acid amide hydrolase
- FADS2:
Fatty acid desaturase 2
- FAPB4:
Fatty acid binding protein 4
- FAS/FASN:
Fatty acid synthase
- FH:
Fumarase
- G3PD:
Glycerol-3-phosphate
- G6FDH:
Glucose-6-phosphate dehydrogenase
- GK:
Glucokinase
- GLUT-4:
Glucose transporter type 4
- GLUT-2:
Glucose transporter type 2
- GR:
Glucocorticoid receptor
- HCH:
Hexachlorocyclohexane
- HepaRG cells:
Hepatic cells from human cholangiocarcinoma
- His:
Histidine
- HK1:
Hexokinase 1
- IDH2:
Isocitrate dehydrogenase
- ILSI/PCS:
The International Life Sciences Institute/International Program on Chemical Safety
- KO:
Knock out
- LCFAs:
Large-chain fatty acids
- LDHB:
Lactate dehydrogenase
- Leu:
Leucine
- LpL:
Lipoprotein lipase
- LXR:
Liver X receptor
- Lys:
Lysine
- MCP1/CCL2:
Monocyte chemotherapy protein 1
- MCPA:
4-Chloro-o-toloxyacetic acid
- MDA:
Malondialdehyde
- MEFs:
Mouse embryo fibroblasts
- MEHP:
Mono-2(-ethylhexyl)-tetrabromophthalate
- Met:
Methionine
- MoA:
Mechanism of action
- MSC:
Mesenchymal stem cell
- MUFAs:
Monounsaturated fatty acids
- NR:
Nuclear receptors
- OGDH:
Oxoglutarate dehydrogenase
- OP9 cells:
Cell line derived from mouse bone marrow stromal cells
- OPFRs:
Organophosphate flame retardants
- PDHA1:
Pyruvate dehydrogenase alpha 1
- PFKFB3:
6-Phosphofructo-2-kinase/fructose-2,6-biphosphatase 3
- Phe:
Phenylalanine
- PK:
Pyruvate kinase
- PKC:
Protein kinase C
- PP:
Peroxisome proliferators
- PPARα:
Peroxisome proliferator-activated receptor alpha
- PPARβ/δ:
Peroxisome proliferator-activated receptor beta/delta
- PPARγ:
Peroxisome proliferator-activated receptor gamma
- PPARs:
Peroxisome proliferator-activated receptors
- PPRE:
Peroxisome proliferator response elements
- Pro:
Proline
- PUFAs:
Polyunsaturated fatty acids
- PXR:
Pregnane X receptor
- QpE:
Quizalofop-p-ethyl
- QSAR:
Quantitative structure-activity relationship
- RNA:
Ribonucleic acid
- ROS:
Reactive oxygen species
- RXR:
Retinoid X receptor
- SCD1:
Stearoyl-CoA desaturase-1
- SD:
Sprague Dawley
- SH-SY5Y cells:
Thrice-subcloned cell line derived from the SK-N-SH neuroblastoma cells
- SOD:
Superoxide dismutase
- SREBP-1c:
Sterol regulatory element-binding protein 1
- TAC:
Tricarboxylic acid cycle
- TBPH:
Bis(2-ethylhexyl)-2,3,4,5-tetrabromophthalate
- TBT:
Tributyltin
- TC:
Total cholesterol
- TCP:
3,5,6-Trichloropyridinol
- TFs:
Transcription factors
- TG:
Triglycerides
- TNF-α:
Tumor necrosis factor-alpha
- TPHP:
Triphenyl phosphate
- TPT:
Triphenyltin
- WHO:
World Health Organization
- β-BHC:
Benzene hexachloride beta
- γ-BHC:
Benzene hexachloride gamma
- δ-BHC:
Benzene hexachloride delta.
Data Availability
The review data supporting this Systematic Review are from previously reported studies and datasets, which have been cited. The processed data are available at the U.S. National Institutes of Health's National Library of Medicine (NIH/NLM).
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1. WHO, “Obesity and Overweight,” September 16, 2022, https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight#:~:text=%2Fm2).-,Adults,than%20or%20equal%20to%2030.
- 2.Pellegrinelli V., Carobbio S., Vidal-Puig A. Adipose tissue plasticity: how fat depots respond differently to pathophysiological cues. Diabetologia . 2016;59(6):1075–1088. doi: 10.1007/s00125-016-3933-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. WHO, “Diabetes Overview and Fact Sheet,” September 16, 2022, https://www.who.int/health-topics/diabetes#tab=tab_1.
- 4.Desvergne B., Feige J. N., Casals-Casas C. PPAR-mediated activity of phthalates: a link to the obesity epidemic? Molecular and Cellular Endocrinology . 2009;304(1–2):43–48. doi: 10.1016/j.mce.2009.02.017. [DOI] [PubMed] [Google Scholar]
- 5.Aaseth J., Javorac D., Djordjevic A. B., et al. The role of persistent organic pollutants in obesity: a review of laboratory and epidemiological studies. Toxics . 2022;10(65):3–30. doi: 10.3390/toxics10020065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ren X. M., Kuo Y., Blumberg B. Agrochemicals and obesity. Molecular and Cellular Endocrinology . 2020;515:p. 110926. doi: 10.1016/j.mce.2020.110926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang B., Tsakiridis E. E., Zhang S., et al. The pesticide chlorpyrifos promotes obesity by inhibiting diet-induced thermogenesis in brown adipose tissue. Nature Communications . 2021;12(1):p. 5163. doi: 10.1038/s41467-021-25384-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Le Magueresse-Battistoni B., Labaronne E., Vidal H., Naville D. Endocrine disrupting chemicals in mixture and obesity, diabetes and related metabolic disorders. World Journal of Biological Chemistry . 2017;8(2):108–119. doi: 10.4331/wjbc.v8.i2.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Montgomery M. P., Kamel F., Saldana T. M., Alavanja M. C., Sandler D. P. Incident diabetes and pesticide exposure among licensed pesticide applicators: agricultural health study, 1993-2003. American Journal of Epidemiology . 2008;167(10):1235–1246. doi: 10.1093/aje/kwn028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Everett C. J., Frithsen I. L., Diaz V. A., et al. Association of a polychlorinated dibenzo-p-dioxin, a polychlorinated biphenyl, and DDT with diabetes in the 1999-2002 National Health and Nutrition Examination Survey. Environmental Research . 2007;103(3):413–418. doi: 10.1016/j.envres.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 11.Mangelsdorf D. J., Evans R. M. The RXR heterodimers and orphan receptors. Cell . 1995;83(6):841–850. doi: 10.1016/0092-8674(95)90200-7. [DOI] [PubMed] [Google Scholar]
- 12.Bocher V., Pineda-Torra I., Fruchart J. C., Staels B. PPARs: transcription factors controlling lipid and lipoprotein metabolism. Annals of the New York Academy of Sciences . 2002;967:7–18. doi: 10.1111/j.1749-6632.2002.tb04258.x. [DOI] [PubMed] [Google Scholar]
- 13.Issemann I., Green S. Activation of a member of the steroid hormone receptor superfamily by peroxisome proliferators. Nature . 1990;347(6294):645–650. doi: 10.1038/347645a0. [DOI] [PubMed] [Google Scholar]
- 14.Dreyer C., Krey G., Keller H., et al. Control of the peroxisomal beta-oxidation pathway by a novel family of nuclear hormone receptors. Cell . 1992;68(5):879–887. doi: 10.1016/0092-8674(92)90031-7. [DOI] [PubMed] [Google Scholar]
- 15.Kliewer S. A., Forman B. M., Blumberg B., et al. Differential expression and activation of a family of murine peroxisome proliferator-activated receptors. Proceedings of the National Academy of Sciences of the United States of America . 1994;91(15):7355–7359. doi: 10.1073/pnas.91.15.7355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tontonoz P., Hu E., Graves R. A., Budavari A. I., Spiegelman B. M. mPPAR /2: tissue-specific regulator of an adipocyte enhancer. Genes and Development . 1994;8(10):1224–1234. doi: 10.1101/gad.8.10.1224. [DOI] [PubMed] [Google Scholar]
- 17.Leonard J. A., Nelms M., Craig E., et al. A weight of evidence approach to investigate potential common mechanisms in pesticide groups to support cumulative risk assessment: a case study with dinitroaniline pesticides. Regulatory Toxicology and Pharmacology . 2019;107 doi: 10.1016/j.yrtph.2019.104419. [DOI] [PubMed] [Google Scholar]
- 18.Wu S., Ji X., Wang J., et al. Fungicide bromuconazole has the potential to induce hepatotoxicity at the physiological, metabolomic and transcriptomic levels in rats. Environmental Pollution . 2021;280 doi: 10.1016/j.envpol.2021.116940. [DOI] [PubMed] [Google Scholar]
- 19.Lewis D. F., Lake B. G. Quantitative structure-activity relationship (QSAR) analysis for a series of rodent peroxisome proliferators: interaction with the mouse liver peroxisome proliferator-activated receptor alpha (mPPARalpha) Toxicology In Vitro . 1997;11(1,2):99–105. doi: 10.1016/s0887-2333(96)00067-7. [DOI] [PubMed] [Google Scholar]
- 20.Ning X., Ku T., Gao R., et al. In vitro PPARγ agonistic potential of chitin synthesis inhibitors and their energy metabolism-related hepatotoxicity. The Science of the Total Environment . 2018;615:1126–1132. doi: 10.1016/j.scitotenv.2017.10.016. [DOI] [PubMed] [Google Scholar]
- 21.Harada S., Hiromori Y., Nakamura S., et al. Structural basis for PPARγ transactivation by endocrine-disrupting organotin compounds. Scientific Reports . 2014;5 doi: 10.1038/srep08520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun Q., Qi W., Yang J. J., et al. Fipronil promotes adipogenesis via AMPKα-mediated pathway in 3T3-L1 adipocytes. Food and Chemical Toxicology . 2016;92:217–223. doi: 10.1016/j.fct.2016.04.011. [DOI] [PubMed] [Google Scholar]
- 23.Yuan L., Lin J., Peng Y., Gao R., Sun Q. Chlorantraniliprole induces adipogenesis in 3T3-L1 adipocytes via the AMPKα pathway but not the ER stress pathway. Food Chemistry . 2020;311 doi: 10.1016/j.foodchem.2019.125953. [DOI] [PubMed] [Google Scholar]
- 24.Seok J. W., Park J. Y., Park H. K., Lee H. Endrin potentiates early-stage adipogenesis in 3T3-L1 cells by activating the mammalian target of rapamycin. Life Sciences . 2022;288 doi: 10.1016/j.lfs.2021.120151. [DOI] [PubMed] [Google Scholar]
- 25.Takeuchi S., Matsuda T., Kobayashi S., Takahashi T., Kojima H. In vitro screening of 200 pesticides for agonistic activity via mouse peroxisome proliferator-activated receptor (PPAR)α and PPARγ and quantitative analysis of in vivo induction pathway. Toxicology and Applied Pharmacology . 2006;217(3):235–244. doi: 10.1016/j.taap.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 26.Sun H., Shao W., Liu H., Jiang Z. Exposure to 2,4-dichlorophenoxyacetic acid induced PPARβ-dependent disruption of glucose metabolism in HepG2 cells. Environmental Science and Pollution Research . 2018;25(17):17050–17057. doi: 10.1007/s11356-018-1921-6. [DOI] [PubMed] [Google Scholar]
- 27.Green H., Meuth M. An established pre-adipose cell line and its differentiation in culture. Cell . 1974;3(2):127–133. doi: 10.1016/0092-8674(74)90116-0. [DOI] [PubMed] [Google Scholar]
- 28.Maloney E. K., Waxman D. J. Trans-Activation of PPARalpha and PPARgamma by structurally diverse environmental chemicals. Toxicology and Applied Pharmacology . 1999;161(2):209–218. doi: 10.1006/taap.1999.8809. [DOI] [PubMed] [Google Scholar]
- 29.Mesnage R., Biserni M., Wozniak E., Xenakis T., Mein C. A., Antoniou M. N. Comparison of transcriptome responses to glyphosate, isoxaflutole, quizalofop-p-ethyl and mesotrione in the HepaRG cell line. Toxicology Reports . 2018;5:819–826. doi: 10.1016/j.toxrep.2018.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fujino C., Watanabe Y., Sanoh S., et al. Activation of PXR, CAR and PPARα by pyrethroid pesticides and the effect of metabolism by rat liver microsomes. Heliyon . 2019;5(9, article e02466) doi: 10.1016/j.heliyon.2019.e02466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim J., Sun Q., Yue Y., et al. 4,4′;-dichlorodiphenyltrichloroethane (DDT) and 4,4′;-dichlorodiphenyldichloroethylene (DDE) promote adipogenesis in 3T3-L1 adipocyte cell culture. Pesticide Biochemistry and Physiology . 2016;131:40–45. doi: 10.1016/j.pestbp.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 32.Blanco J., Guardia-Escote L., Mulero M., et al. Obesogenic effects of chlorpyrifos and its metabolites during the differentiation of 3T3-L1 preadipocytes. Food and Chemical Toxicology . 2020;137 doi: 10.1016/j.fct.2020.111171. [DOI] [PubMed] [Google Scholar]
- 33.Smith A., Yu X., Yin L. Diazinon exposure activated transcriptional factors CCAAT-enhancer-binding proteins α (C/EBPα) and peroxisome proliferator-activated receptor γ (PPARγ) and induced adipogenesis in 3T3-L1 preadipocytes. Pesticide Biochemistry and Physiology . 2018;150:48–58. doi: 10.1016/j.pestbp.2018.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu J., Ou K., Chen C., et al. Tributyltin exposure disturbs hepatic glucose metabolism in male mice. Toxicology . 2019;425 doi: 10.1016/j.tox.2019.152242. [DOI] [PubMed] [Google Scholar]
- 35.Celi F. S., Shuldiner A. R. The role of peroxisome proliferator-activated receptor gamma in diabetes and obesity. Current Diabetes Reports . 2002;2(2):179–185. doi: 10.1007/s11892-002-0078-2. [DOI] [PubMed] [Google Scholar]
- 36.Jellali R., Jacques S., Essaouiba A., et al. Investigation of steatosis profiles induced by pesticides using liver organ-on-chip model and omics analysis. Food and Chemical Toxicology . 2021;152 doi: 10.1016/j.fct.2021.112155. [DOI] [PubMed] [Google Scholar]
- 37.Sohrabi S. S., Sohrabi S. M., Rashidipour M., et al. Identification of common key regulators in rat hepatocyte cell lines under exposure of different pesticides. Gene . 2020;739 doi: 10.1016/j.gene.2020.144508. [DOI] [PubMed] [Google Scholar]
- 38.Andrews F. V., Kim S. M., Edwards L., Schlezinger J. J. Identifying adipogenic chemicals: disparate effects in 3T3-L1, OP9 and primary mesenchymal multipotent cell models. Toxicology in Vitro . 2020;67 doi: 10.1016/j.tiv.2020.104904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Biserni M., Mesnage R., Ferro R., et al. Quizalofop-p-ethyl induces adipogenesis in 3T3-L1 adipocytes. Toxicological Sciences . 2019;170(2):452–461. doi: 10.1093/toxsci/kfz097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Luz A. L., Kassotis C. D., Stapleton H. M., Meyer J. N. The high-production volume fungicide pyraclostrobin induces triglyceride accumulation associated with mitochondrial dysfunction, and promotes adipocyte differentiation independent of PPARγ activation, in 3T3-L1 cells. Toxicology . 2018;393:150–159. doi: 10.1016/j.tox.2017.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kassotis C. D., Masse L., Kim S., et al. Characterization of adipogenic chemicals in three different cell culture systems: implications for reproducibility based on cell source and handling. Scientific Reports . 2017;7 doi: 10.1038/srep42104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fu M., Sun T., Bookout A. L., et al. A nuclear receptor atlas: 3T3-L1 adipogenesis. Molecular Endocrinology . 2005;19(10):2437–2450. doi: 10.1210/me.2004-0539. [DOI] [PubMed] [Google Scholar]
- 43.Lutfi E., Riera-Heredia N., Córdoba M., et al. Tributyltin and triphenyltin exposure promotes in vitro adipogenic differentiation but alters the adipocyte phenotype in rainbow trout. Aquatic Toxicology . 2017;188:148–158. doi: 10.1016/j.aquatox.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 44.Kanayama T., Kobayashi N., Mamiya S., Nakanishi T., Nishikawa J. Organotin compounds promote adipocyte differentiation as agonists of the peroxisome proliferator-activated receptor γ/retinoid X receptor pathway. Molecular Pharmacology . 2005;67(3):766–774. doi: 10.1124/mol.104.008409. [DOI] [PubMed] [Google Scholar]
- 45.Inadera H., Shimomura A. Environmental chemical tributyltin augments adipocyte differentiation. Toxicology Letters . 2005;159(3):226–234. doi: 10.1016/j.toxlet.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 46.Moreno-Aliaga M. J., Matsumura F. Effects of 1,1,1-trichloro-2,2-bis(p-chlorophenyl)-ethane (p,p H-DDT) on 3T3-L1 and 3T3-F442A adipocyte differentiation. Biochemical Pharmacology . 2002;63(5):997–1007. doi: 10.1016/s0006-2952(01)00933-9. [DOI] [PubMed] [Google Scholar]
- 47.Sundseth S. S., Waxman D. J. Sex-dependent expression and clofibrate inducibility of cytochrome P4504A fatty acid ω-hydroxylases. Male specificity of liver and kidney CYP4A2 mRNA and tissue-specific regulation by growth hormone and testosterone. Journal of Biological Chemistry . 1992;267(6):3915–3921. [PubMed] [Google Scholar]
- 48.Henderson C. J., Bammler T., Wolf C. R. Deduced amino acid sequence of a murine cytochrome P-450 Cyp4a protein: Developmental and hormonal regulation in liver and kidney. Biochimica et Biophysica Acta . 1994;1200(2):182–190. doi: 10.1016/0304-4165(94)90134-1. [DOI] [PubMed] [Google Scholar]
- 49.Taxvig C., Dreisig K., Boberg J., et al. Differential effects of environmental chemicals and food contaminants on adipogenesis, biomarker release and PPARγ activation. Molecular and Cellular Endocrinology . 2012;361(1–2):106–115. doi: 10.1016/j.mce.2012.03.021. [DOI] [PubMed] [Google Scholar]
- 50.Howell G., Mangum L. Exposure to bioaccumulative organochlorine compounds alter adipogenesis, fatty acid uptake, and adipokine production in NIH3T3-L1 cells. Toxicology in Vitro: An International Journal Published in Association with BIBRA . 2011;25(1):394–402. doi: 10.1016/j.tiv.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Moreno-Aliaga M. J., Matsumura F. Endrin inhibit adipocyte differentiation by selectively altering expression pattern of CCAAT/enhancer binding protein-in 3T3-L1 cells. Molecular Pharmacology . 1999;56(1):91–101. doi: 10.1124/mol.56.1.91. [DOI] [PubMed] [Google Scholar]
- 52.Penza M., Jeremic M., Marrazzo E., et al. The environmental chemical tributyltin chloride (TBT) shows both estrogenic and adipogenic activities in mice which might depend on the exposure dose. Toxicology and Applied Pharmacology . 2011;255(1):65–75. doi: 10.1016/j.taap.2011.05.017. [DOI] [PubMed] [Google Scholar]
- 53.Dawson M. I., Xia Z. The retinoid X receptors and their ligands. Biochimica et Biophysica Acta . 2012;1821(1):21–56. doi: 10.1016/j.bbalip.2011.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sargis R. M., Johnson D. N., Choudhury R. A., Brady M. J. Environmental endocrine disruptors promote adipogenesis in the 3T3-L1 cell line through glucocorticoid receptor activation. Obesity . 2010;18(7):1283–1288. doi: 10.1038/oby.2009.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Megg W. J., Brewer K. L. Weight gain associated with chronic exposure to chlorpyrifos in rats. Journal of Medical Toxicology . 2007;3(3):89–93. doi: 10.1007/BF03160916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Martini C. N., Gabrielli M., Brandani J. N., Vila M. d. C. Glyphosate inhibits PPAR gamma induction and differentiation of preadipocytes and is able to induce oxidative stress. Journal of Biochemical and Molecular Toxicology . 2016;30(8):404–413. doi: 10.1002/jbt.21804. [DOI] [PubMed] [Google Scholar]
- 57.Fujino C., Tamura Y., Tange S., et al. Metabolism of methiocarb and carbaryl by rat and human livers and plasma, and effect on their PXR, CAR and PPARα activities. The Journal of Toxicological Sciences . 2016;41(5):677–691. doi: 10.2131/jts.41.677. [DOI] [PubMed] [Google Scholar]
- 58.Xiang D., Chu T., Li M., Wang Q., Zhu G. Effects of pyrethroid pesticide cis-bifenthrin on lipogenesis in hepatic cell line. Chemosphere . 2018;201:840–849. doi: 10.1016/j.chemosphere.2018.03.009. [DOI] [PubMed] [Google Scholar]
- 59.Yanik S. C., Baker A. H., Mann K. K., Schlezinger J. J. Organotins are potent activators of PPARγ and adipocyte differentiation in bone marrow multipotent mesenchymal stromal cells. Toxicological Sciences . 2011;122(2):476–488. doi: 10.1093/toxsci/kfr140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Biemann R., Fischer B., Blüher M., Navarrete Santos A. Tributyltin affects adipogenic cell fate commitment in mesenchymal stem cells by a PPARγ independent mechanism. Chemico-Biological Interactions . 2014;214(1):1–9. doi: 10.1016/j.cbi.2014.01.021. [DOI] [PubMed] [Google Scholar]
- 61.Herriage S., Chen G., Pope C. Concentration-dependent effects of chlorpyrifos oxon on peroxisome proliferator-activated receptor signaling in MCF-7 cells. Toxicology in Vitro . 2022;78 doi: 10.1016/j.tiv.2021.105268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Everett C. J., Matheson E. M. Biomarkers of pesticide exposure and diabetes in the 1999-2004 national health and nutrition examination survey. Environment International . 2010;36(4):398–401. doi: 10.1016/j.envint.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 63.Kondo M., Miyata K., Nagahori H., et al. Involvement of peroxisome proliferator-activated receptor-alpha in liver tumor production by permethrin in the female mouse. Toxicological Sciences . 2019;168(2):572–596. doi: 10.1093/toxsci/kfz012. [DOI] [PubMed] [Google Scholar]
- 64.Ridano M. E., Racca A. C., Flores-Martín J. B., et al. Effect of chlorpyrifos on human extravillous-like trophoblast cells. Reproductive Toxicology . 2019;90:118–125. doi: 10.1016/j.reprotox.2019.09.001. [DOI] [PubMed] [Google Scholar]
- 65.Jie J., Ling L., Yi Y., et al. Tributyltin triggers lipogenesis in macrophages via modifying PPARγ pathway. Environmental Pollution . 2021;271 doi: 10.1016/j.envpol.2020.116331. [DOI] [PubMed] [Google Scholar]
- 66.Anderson S. P., Howroyd P., Liu J., et al. The transcriptional response to a peroxisome proliferator-activated receptor α agonist includes increased expression of proteome maintenance genes. Journal of Biological Chemistry . 2004;279(50):52390–52398. doi: 10.1074/jbc.M409347200. [DOI] [PubMed] [Google Scholar]
- 67.Xing B., Liu M., Bing G. Neuroprotection with pioglitazone against LPS insult on dopaminergic neurons may be associated with its inhibition of NF-ΚB and JNK activation and suppression of COX-2 activity. Journal of Neuroimmunology . 2007;192(1–2):89–98. doi: 10.1016/j.jneuroim.2007.09.029. [DOI] [PubMed] [Google Scholar]
- 68.Ko J., Park J. H., Park Y. S., Koh H. C. PPAR-γ activation attenuates deltamethrin-induced apoptosis by regulating cytosolic PINK1 and inhibiting mitochondrial dysfunction. Toxicology Letters . 2016;260:8–17. doi: 10.1016/j.toxlet.2016.08.016. [DOI] [PubMed] [Google Scholar]
- 69.Lee J. E., Park J. H., Jang S. J., Koh H. C. Rosiglitazone inhibits chlorpyrifos-induced apoptosis via modulation of the oxidative stress and inflammatory response in SH-SY5Y cells. Toxicology and Applied Pharmacology . 2014;278(2):159–171. doi: 10.1016/j.taap.2014.04.021. [DOI] [PubMed] [Google Scholar]
- 70.Corona J. C., de Souza S. C., Duchen M. R. PPARγ activation rescues mitochondrial function from inhibition of complex I and loss of PINK1. Experimental Neurology . 2014;253:16–27. doi: 10.1016/j.expneurol.2013.12.012. [DOI] [PubMed] [Google Scholar]
- 71.de Nuccio C., Bernardo A., Cruciani C., et al. Peroxisome proliferator activated receptor-γ agonists protect oligodendrocyte progenitors against tumor necrosis factor-alpha-induced damage: effects on mitochondrial functions and differentiation. Experimental Neurology . 2015;271:506–514. doi: 10.1016/j.expneurol.2015.07.014. [DOI] [PubMed] [Google Scholar]
- 72.Sun L., Li J., Zuo Z., Chen M., Wang C. Chronic exposure to paclobutrazol causes hepatic steatosis in male rockfish Sebastiscus marmoratus and the mechanism involved. Aquatic Toxicology . 2013;126:148–153. doi: 10.1016/j.aquatox.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 73.Dong X., Li Y., Zhang L., et al. Influence of difenoconazole on lipid metabolism in marine medaka (Oryzias Melastigma) Ecotoxicology . 2016;25(5):982–990. doi: 10.1007/s10646-016-1655-5. [DOI] [PubMed] [Google Scholar]
- 74.Jia R., Hou Y., Feng W., Li B., Zhu J. Alterations at biochemical, proteomic and transcriptomic levels in liver of tilapia (Oreochromis Niloticus) under chronic exposure to environmentally relevant level of glyphosate. Chemosphere . 2022;294 doi: 10.1016/j.chemosphere.2022.133818. [DOI] [PubMed] [Google Scholar]
- 75.Higley E., Tompsett A. R., Giesy J. P., Hecker M., Wiseman S. Effects of triphenyltin on growth and development of the wood frog (Lithobates Sylvaticus) Aquatic Toxicology . 2013;144-145:155–161. doi: 10.1016/j.aquatox.2013.09.029. [DOI] [PubMed] [Google Scholar]
- 76.Baker A. H., Wu T. H., Bolt A. M., et al. Tributyltin alters the bone marrow microenvironment and suppresses B cell development. Toxicological sciences: An Official Journal of the Society of Toxicology . 2017;158(1):63–75. doi: 10.1093/toxsci/kfx067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.de Araújo J. F. P., Podratz P. L., Sena G. C., et al. The obesogen tributyltin induces abnormal ovarian adipogenesis in adult female rats. Toxicology Letters . 2018;295:99–114. doi: 10.1016/j.toxlet.2018.06.1068. [DOI] [PubMed] [Google Scholar]
- 78.Bhaskar R., Mohanty B. Pesticides in mixture disrupt metabolic regulation: In silico and in vivo analysis of cumulative toxicity of mancozeb and imidacloprid on body weight of mice. General and Comparative Endocrinology . 2014;205:226–234. doi: 10.1016/j.ygcen.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 79.Al-Obaidi Z. A. F., Erdogan C. S., Sümer E., et al. Investigation of obesogenic effects of hexachlorobenzene, DDT and DDE in male rats. General and Comparative Endocrinology . 2022;327 doi: 10.1016/j.ygcen.2022.114098. [DOI] [PubMed] [Google Scholar]
- 80.Wasef L., Nassar A. M. K., El-Sayed Y. S., et al. The potential ameliorative impacts of cerium oxide nanoparticles against fipronil-induced hepatic steatosis. Scientific Reports . 2021;11(1):p. 1310. doi: 10.1038/s41598-020-79479-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Moustafa G. G., Hussein M. M. Lambda cyhalothrin toxicity induces alterations in lipogenic genes and inflammatory factors in rat liver. Japanese Journal of Veterinary Research . 2016;64(1):25–38. [PubMed] [Google Scholar]
- 82.Costa C., Rapisarda V., Catania S., et al. Cytokine patterns in greenhouse workers occupationally exposed to α-cypermethrin: An observational study. Environmental Toxicology and Pharmacology . 2013;36(3):796–800. doi: 10.1016/j.etap.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 83.Richert L., Price S., Chesne C., Maita K., Carmichael N. Comparison of the induction of hepatic peroxisome proliferation by the herbicide oxadiazon in vivo rats, mice, and dogs and in vitro in rat and human hepatocytes. Toxicology and Applied Pharmacology . 1996;141(1):35–43. doi: 10.1006/taap.1996.0257. [DOI] [PubMed] [Google Scholar]
- 84.Espandiari P., Thomas V. A., Glauert H. P., et al. The herbicide dicamba (2-methoxy-3,6-dichlorobenzoic acid) is a peroxisome proliferator in rats. Fundamental and Applied Toxicology . 1995;26(1):85–90. doi: 10.1006/faat.1995.1077. [DOI] [PubMed] [Google Scholar]
- 85.Nagaraju R., Joshi A. K. R., Vamadeva S. G., Rajini P. S. Deregulation of hepatic lipid metabolism associated with insulin resistance in rats subjected to chronic monocrotophos exposure. Journal of Biochemical and Molecular Toxicology . 2020;34(8):p. e22506. doi: 10.1002/jbt.22506. [DOI] [PubMed] [Google Scholar]
- 86.Wang X., Shen M., Zhou J., Jin Y. Chlorpyrifos disturbs hepatic metabolism associated with oxidative stress and gut microbiota dysbiosis in adult zebrafish. Comparative Biochemistry and Physiology. Toxicology and Pharmacology . 2019;216:19–28. doi: 10.1016/j.cbpc.2018.11.010. [DOI] [PubMed] [Google Scholar]
- 87.Yan J., Wang D., Meng Z., et al. Effects of incremental endosulfan sulfate exposure and high fat diet on lipid metabolism, glucose homeostasis and gut microbiota in mice. Environmental Pollution . 2021;268 doi: 10.1016/j.envpol.2020.115697. [DOI] [PubMed] [Google Scholar]
- 88.Wu S., Jin C., Wang Y., Fu Z., Jin Y. Exposure to the fungicide propamocarb causes gut microbiota dysbiosis and metabolic disorder in mice. Environmental Pollution . 2018;237:775–783. doi: 10.1016/j.envpol.2017.10.129. [DOI] [PubMed] [Google Scholar]
- 89.Wu S., Luo T., Wang S., et al. Chronic exposure to fungicide propamocarb induces bile acid metabolic disorder and increases trimethylamine in C57BL/6J mice. The Science of the Total Environment . 2018;642:341–348. doi: 10.1016/j.scitotenv.2018.06.084. [DOI] [PubMed] [Google Scholar]
- 90.Jin Y., Zeng Z., Wu Y., Zhang S., Fu Z. Oral exposure of mice to carbendazim induces hepatic lipid metabolism disorder and gut microbiota dysbiosis. Toxicological Sciences . 2015;147(1):116–126. doi: 10.1093/toxsci/kfv115. [DOI] [PubMed] [Google Scholar]
- 91.Bao Z., Zhao Y., Wu A., et al. Sub-chronic carbendazim exposure induces hepatic glycolipid metabolism disorder accompanied by gut microbiota dysbiosis in adult zebrafish (Daino Rerio) The Science of the Total Environment . 2020;739 doi: 10.1016/j.scitotenv.2020.140081. [DOI] [PubMed] [Google Scholar]
- 92.Buono S., Cristiano L., D’Angelo B., Cimini A., Putti R. PPARα mediates the effects of the pesticide methyl thiophanate on liver of the lizard Podarcis sicula. Comparative Biochemistry and Physiology . 2007;145(3):306–314. doi: 10.1016/j.cbpc.2006.12.016. [DOI] [PubMed] [Google Scholar]
- 93.Goetz A. K., Dix D. J. Mode of action for reproductive and hepatic toxicity inferred from a genomic study of triazole antifungals. Toxicological Sciences . 2009;110(2):449–462. doi: 10.1093/toxsci/kfp098. [DOI] [PubMed] [Google Scholar]
- 94.Zaya R. M., Amini Z., Whitaker A. S., Ide C. F. Exposure to atrazine affects the expression of key genes in metabolic pathways integral to energy homeostasis in Xenopus laevis tadpoles. Aquatic Toxicology . 2011;104(3–4):254–262. doi: 10.1016/j.aquatox.2011.04.022. [DOI] [PubMed] [Google Scholar]
- 95.Qian L., Zhang J., Chen X., et al. Toxic effects of boscalid in adult zebrafish (Danio rerio) on carbohydrate and lipid metabolism. Environmental Pollution . 2019;247:775–782. doi: 10.1016/j.envpol.2019.01.054. [DOI] [PubMed] [Google Scholar]
- 96.Luo T., Weng Y., Huang Z., Zhao Y., Jin Y. Combined hepatotoxicity of imidacloprid and microplastics in adult zebrafish: Endpoints at gene transcription. Comparative Biochemistry and Physiology . 2021;246 doi: 10.1016/j.cbpc.2021.109043. [DOI] [PubMed] [Google Scholar]
- 97.Wang Z., Wu Q., Li X., Klaunig J. E. Constitutive androstane receptor (CAR) mediates dieldrin-induced liver tumorigenesis in mouse. Archives of Toxicology . 2020;94(8):2873–2884. doi: 10.1007/s00204-020-02781-8. [DOI] [PubMed] [Google Scholar]
- 98.Strupp C., Bomann W. H., Spézia F., et al. A human relevance investigation of PPARα-mediated key events in the hepatocarcinogenic mode of action of propaquizafop in rats. Regulatory Toxicology and Pharmacology . 2018;95:348–361. doi: 10.1016/j.yrtph.2018.04.005. [DOI] [PubMed] [Google Scholar]
- 99.Kuwata K., Inoue K., Ichimura R., et al. Constitutive active/androstane receptor, peroxisome proliferator-activated receptor α, and cytotoxicity are involved in oxadiazon-induced liver tumor development in mice. Food and Chemical Toxicology: An International Journal Published for the British Industrial Biological Research Association . 2016;88:75–86. doi: 10.1016/j.fct.2015.12.017. [DOI] [PubMed] [Google Scholar]
- 100.LeBaron M. J., Rasoulpour R. J., Gollapudi B. B., et al. Characterization of nuclear receptor-mediated murine hepatocarcinogenesis of the herbicide pronamide and its human relevance. Toxicological Sciences . 2014;142(1):74–92. doi: 10.1093/toxsci/kfu155. [DOI] [PubMed] [Google Scholar]
- 101.Palut D., Ludwicki J. K., Kostka G., et al. Studies of early hepatocellular proliferation and peroxisomal proliferation in wistar rats treated with herbicide diclofop. Toxicology . 2001;158(3):119–126. doi: 10.1016/s0300-483x(00)00371-1. [DOI] [PubMed] [Google Scholar]
- 102.Wan Z., Neal B. H., Lamb J. C., Klaunig J. E. Mechanistic investigation of toxaphene induced mouse liver tumors. Toxicological Sciences . 2015;147(2):549–561. doi: 10.1093/toxsci/kfv151. [DOI] [PubMed] [Google Scholar]
- 103.Rooney J., Wehmas L. C., Ryan N., et al. Genomic comparisons between hepatocarcinogenic and non-hepatocarcinogenic organophosphate insecticides in the mouse liver. Toxicology . 2022;465 doi: 10.1016/j.tox.2021.153046. [DOI] [PubMed] [Google Scholar]
- 104.Devos S., de Bosscher K., Staels B., et al. Inhibition of cytokine production by the herbicide atrazine. Search for nuclear receptor targets. Biochemical Pharmacology . 2003;65(2):303–308. doi: 10.1016/s0006-2952(02)01507-1. [DOI] [PubMed] [Google Scholar]
- 105.Luo W., Cao J., Li J., He W. Adipose tissue-specific PPARγ deficiency increases resistance to oxidative stress. Experimental Gerontology . 2008;43(3):154–163. doi: 10.1016/j.exger.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 106.Amin F., Menarzia A., Kazerani H. R., Shakeri F., Boskadaby M. H. Systemic inflammation and oxidative stress induced by inhaled paraquat in rat improved by carvacol, possible role of PPARγ receptors. BioFactors . 2021;47(5):778–787. doi: 10.1002/biof.1761. [DOI] [PubMed] [Google Scholar]
- 107.Amin F., Memarzia A., Kazerani H. R., Boskabady M. H. Carvacrol and zataria multiflora influenced the PPARγ agonist effects on systemic inflammation and oxidative stress induced by inhaled paraquat in rat. Iranian Journal of Basic Medical Sciences . 2020;23(7):930–936. doi: 10.22038/ijbms.2020.45962.10648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Xia J., Lin J., Li X. N., et al. Atrazine-induced environmental nephrosis was mitigated by lycopene via modulating nuclear xenobiotic receptors-mediated response. Journal of Nutritional Biochemistry . 2018;51:80–90. doi: 10.1016/j.jnutbio.2017.09.006. [DOI] [PubMed] [Google Scholar]
- 109.Harada Y., Tanaka N., Ichikawa M., et al. PPARα-dependent cholesterol/testosterone disruption in Leydig cells mediates 2,4-dichlorophenoxyacetic acid-induced testicular toxicity in mice. Archives of Toxicology . 2016;90(12):3061–3071. doi: 10.1007/s00204-016-1669-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhu J., Huang X., Jiang H., et al. The role of PPARγ in embryonic development of Xenopus tropicalis under triphenyltin-induced teratogenicity. Science of the Total Environment . 2018;633:1245–1252. doi: 10.1016/j.scitotenv.2018.03.313. [DOI] [PubMed] [Google Scholar]
- 111.Arulkumar R., Jung H. J., Noh S. G., Park D., Chung H. Y. Cheonggukjang-specific component 1,3-diphenyl-2-propanone as a novel PPARα/γ dual agonist: An in vitro and in silico study. International Journal of Molecular Sciences . 2021;22(19) doi: 10.3390/ijms221910884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Xie W., Zhang W., Ren J., et al. Metabonomics indicates inhibition of fatty acid synthesis, β-oxidation, and tricarboxylic acid cycle in triclocarban-induced cardiac metabolic alterations in male mice. Journal of Agricultural and Food Chemistry . 2018;66(6):1533–1542. doi: 10.1021/acs.jafc.7b05220. [DOI] [PubMed] [Google Scholar]
- 113.Guo W., Han J., Wu S., et al. Bis(2-ethylhexyl)-2,3,4,5-tetrabromophthalate affects lipid metabolism in zebrafish larvae via DNA methylation modification. Environmental Science and Technology . 2019;54(1):355–363. doi: 10.1021/acs.est.9b05796. [DOI] [PubMed] [Google Scholar]
- 114.Sun W., Duan X., Chen H., Zhang L., Sun H. Adipogenic activity of 2-ethylhexyl diphenyl phosphate via peroxisome proliferator-activated receptor γ pathway. The Science of the Total Environment . 2020;711 doi: 10.1016/j.scitotenv.2019.134810. [DOI] [PubMed] [Google Scholar]
- 115.Kim S., Rabhi N., Blum B. C., et al. Triphenyl phosphate is a selective PPARg modulator that does not induce brite adipogenesis in vitro and in vivo. Archives of Toxicology . 2020;94(9):3087–3103. doi: 10.1007/s00204-020-02815-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ashida H., Harada K., Mishima S., et al. Luteolin suppresses TCDD-induced wasting syndrome in a cultured adipocyte model. Pesticide Biochemistry and Physiology . 2015;120:14–20. doi: 10.1016/j.pestbp.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 117.Upham J., Acott P. D., O’Regan P., et al. The pesticide adjuvant, ToximulTM, alters hepatic metabolism through effects on downstream targets of PPARα. Biochimica et Biophysica Acta . 2007;1772(9):1057–1064. doi: 10.1016/j.bbadis.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 118.Lake B. G., Price R. J., Scott M. P., et al. Piperonyl butoxide: Mode of action analysis for mouse liver tumor formation and human relevance. Toxicology . 2020;439 doi: 10.1016/j.tox.2020.152465. [DOI] [PubMed] [Google Scholar]
- 119.Stulnig T. M., Steffensen K. R., Gao H., et al. Novel roles of liver X receptors exposed by gene expression profiling in liver and adipose tissue. Molecular Pharmacology . 2002;62(6):1299–1305. doi: 10.1124/mol.62.6.1299. [DOI] [PubMed] [Google Scholar]
- 120.Regnier S. M., El-Hashani E., Kamau W., et al. Tributyltin differentially promotes development of a phenotypically distinct adipocyte. Obesity . 2015;23(9):1864–1871. doi: 10.1002/oby.21174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hua Y., Ke S., Wang Y., et al. Prolonged treatment with 3-Isobutyl-1-methylxanthine improves the efficiency of differentiating 3T3-L1 cells into adipocytes. Analytical Biochemistry . 2016;507:18–20. doi: 10.1016/j.ab.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 122.Choi S. K., Park S., Jang S., et al. Cascade regulation of PPARγ(2) and C/EBPα signaling pathways by celastrol impairs adipocyte differentiation and stimulates lipolysis in 3T3-L1 adipocytes. Metabolism: Clinical and Experimental . 2016;65(5):646–654. doi: 10.1016/j.metabol.2016.01.009. [DOI] [PubMed] [Google Scholar]
- 123.Abdullah L., Evans J. E., Montague H., et al. Chronic elevation of phosphocholine containing lipids in mice exposed to Gulf War agents pyridostigmine bromide and permethrin. Neurotoxicology and Teratology . 2013;40:74–84. doi: 10.1016/j.ntt.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 124.Kim W. K., Bae K.-H., Lee S. C., Oh K.-J. “The latest insights into adipokines in diabetes” Journal of. Clinical Medicine . 2019;8(11):1874–1879. doi: 10.3390/jcm8111874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kondo M., Kikumoto H., Osimitz T. G., et al. An evaluation of the human relevance of the liver tumors observed in female mice treated with permethrin based on mode of action. Toxicological Sciences . 2020;175(1):50–63. doi: 10.1093/toxsci/kfaa017. [DOI] [PubMed] [Google Scholar]
- 126.Kassotis C. D., Hoffman K., Phillips A. L., et al. Characterization of adipogenic, PPARγ, and TRβ activities in house dust extracts and their associations with organic contaminants. The Science of the Total Environment . 2021;758 doi: 10.1016/j.scitotenv.2020.143707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Neale P. A., Braun G., Brack W., et al. Assessing the mixture effects in vitro bioassays of chemicals occurring in small agricultural streams during rain events. Environmental Science and Technology . 2020;54(13):8280–8290. doi: 10.1021/acs.est.0c02235. [DOI] [PubMed] [Google Scholar]
- 128.Qi W., Clark J. M., Suvorov A., Park Y. Ivermectin decreases triglyceride accumulation by inhibiting adipogenesis of 3T3-L1 preadipocytes. Food and Chemical Toxicology . 2019;131 doi: 10.1016/j.fct.2019.110576. [DOI] [PubMed] [Google Scholar]
- 129.Sun Q., Lin J., Peng Y., Gao R., Peng Y. Flubendiamide enhances adipogenesis and inhibits AMPKα in 3T3-L1 adipocytes. Molecules . 2018;23(11) doi: 10.3390/molecules23112950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Grün F., Watanabe H., Zamanian Z., et al. Endocrine-disrupting organotin compounds are potent inducers of adipogenesis in vertebrates. Molecular Endocrinology . 2006;20(9):2141–2155. doi: 10.1210/me.2005-0367. [DOI] [PubMed] [Google Scholar]
- 131.Malekinejad H., Khoramjouy M., Hobbenaghi R., Amniattalab A. Atorvastatin attenuates the paraquat-induced pulmonary inflammation via PPARγ receptors: A new indication for atorvastatin. Pesticide Biochemistry and Physiology . 2014;114(1):79–89. doi: 10.1016/j.pestbp.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 132.Gosemann J. H., Doi T., Kutasy B., et al. Alterations of peroxisome proliferator-activated receptor γ and monocyte chemoattractant protein 1 gene expression in the nitrofen-induced hypoplastic lung. Journal of Pediatric Surgery . 2012;47(5):847–851. doi: 10.1016/j.jpedsurg.2012.01.038. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The review data supporting this Systematic Review are from previously reported studies and datasets, which have been cited. The processed data are available at the U.S. National Institutes of Health's National Library of Medicine (NIH/NLM).


