Abstract
This multicenter observational study included 171 COVID-19 adult patients hospitalized in the ICUs of nine hospitals in Lombardy (Northern Italy) from December, 1st 2021, to February, 9th 2022. During the study period, the Delta/Omicron variant ratio of cases decreased with a delay of two weeks in ICU patients compared to that in the community; a higher proportion of COVID-19 unvaccinated patients was infected by Delta than by Omicron whereas a higher rate of COVID-19 boosted patients was Omicron-infected. A higher number of comorbidities and a higher comorbidity score in ICU critically COVID-19 inpatients was positively associated with the Omicron infection as well in vaccinated individuals. Although people infected by Omicron have a lower risk of severe disease than those infected by Delta variant, the outcome, including the risk of ICU admission and the need for mechanical ventilation due to infection by Omicron versus Delta, remains uncertain. The continuous monitoring of the circulating SARS-CoV-2 variants remains a milestone to counteract this pandemic.
Keywords: SARS-CoV-2, Intensive care unit (ICU), Severe infections, COVID-19, Omicron variant, Delta variant, Variant of Concern (VOC), Italy
Introduction
Assessing the clinical severity of infection with new SARS-CoV-2 variants is crucial to ensure the public health response in terms of control measures and mitigation strategies and to support clinicians in patients’ management - particularly in case of severe disease. Since its emergence in November 2021, the SARS-CoV-2 Omicron variant of concern (VOC) (lineage B.1.1.529) has rapidly spread, replacing SARS-CoV-2 Delta (lineage B.1.617.2) and its AY.xx sub-lineages in most European countries [1]. Preliminary findings have indicated that the effectiveness of COVID-19 vaccines is significantly lower against Omicron compared to the Delta variant [2,3]. On the contrary, recent studies have suggested that Omicron infection is associated with less severe disease as it has a lower replication efficiency in lungs than other SARS-CoV-2 variants [4,5]. A reduction of 36–73% in the risk of hospitalization for Omicron compared with Delta has been reported [5,6].
This study aimed at comparing the epidemiological features of Omicron and Delta variants in patients tested positive for SARS-CoV-2 RNA who required intensive care unit (ICU) for COVID-19 in Lombardy (Northern Italy, nearly 10 million inhabitants) during the period of transition from Delta (December 2021) to Omicron dominance (January 2022).
Material and methods
Study population
This multicentre study included adult patients (> 18 years old) hospitalized in the ICUs of nine hospitals in Lombardy from 1st December 2021 to 9th February 2022. Respiratory specimens were collected for SARS-CoV-2 diagnosis and processed at the Microbiology and Virology laboratory of each hospital using commercial multiplex real-time RT-PCR assays. Genotyping of SARS-CoV-2 VOCs was carried out by means of real-time RT-PCR screening tests targeting specific single nucleotide polymorphisms in the S gene, or by whole genome sequencing or partial sequencing of the S gene.
For each patient, the following information were collected: (1) age and gender; (2) date of hospitalization; (3) COVID-19 vaccination status (i.e. no vaccinated, completed vaccination with two doses ≥120 days before, completed vaccination with two doses <120 days before, completed vaccination with booster doses ≥7 days before); (5) presence or absence of underlying comorbidities defined to increase the risk of severe COVID-19 (6). These underlying comorbidities were divided into: (i) medium risk (individual with diseases or other conditions that cause a moderate risk of severe COVID-19) and including diabetes mellitus, obesity (BMI ≥ 30 kg/m2), autoimmune diseases, chronic cardiac diseases and hypertension, chronic lung disease; (ii) high risk (individuals with diseases or conditions that carry a high risk of severe COVID-19, also in younger people) and including chronic renal diseases, solid organ transplant, active malignancy, and immunocompromised patients.
Statistical analyses
Categorical variables were summarized by use of frequency distributions and compared by use of Pearson's χ² test and Fisher Exact tests and t-test and one-way ANOVA were used for comparing continuous independent variables. The frequency was expressed as crude proportion with corresponding 95% confidence interval (95% CI) calculated by Mid-P exact test assuming a normal distribution. The risk of infection was expressed as the number of individuals with Omicron or Delta laboratory-confirmed infection out of the total number of individuals. The conditional maximum-likelihood estimate (CMLE) of odds ratios (OR) with corresponding 95% CI were calculated.
The study population was categorized according to the age in the following age groups (years): age groups (years): 18–29, 30–44, 45–54, 55–64, 65–74, 75–84. An overall comorbidity score weighting the impact of comorbidities was assigned and summed one point for each comorbidity of medium risk and two points for each comorbidity of high risk [6].
The proportion of males in the study population was compared with those of the Italian population by using exact probability binomial level [7]); Differences were considered significant at a p-value <0.05. Statistical analysis was performed using GraphPad Prism version 8.0 for Windows (GraphPad Software, San Diego, CA, USA). This study did not require ethical approval as it is based on routine surveillance data on COVID-19.
Results
A total of 205 adult patients were included in the study and in 171/205 (83.4%) of these patients, the identification of SARS-CoV-2 VOC was successfully performed whereas in 34/205 (16.6%) this information was not available due to low viral load or unavailability of the respiratory samples, thus excluded from further analyses. Table 1 shows the characteristics of the study patients. Overall, 119 (78.9%) Delta/Delta-like and 36 (21.1%) Omicron cases were observed. The median age of patients was 62 years (range 21–84 years: Inter Quartile Range [IQR]: 16 years), and no difference (p = 0.39) in age was observed between patients infected by Delta (median 62 years, range 38–84 years; IQR: 17 years) and those infected by Omicron (median 61.5 years, range 21–79 years; IQR: 16.7 years). The 65–74 years age group was the most represented for both Delta (n = 46; 34.1%) and Omicron (n = 9; 30%) (Table 1). Overall, the proportion of males (120/171; 70.2%) was significantly higher than that of the Italian population (48.7%; p < 0.001) a similar proportion of males was observed in ICU patient groups infected by Delta (67.4%) and Omicron (80.6%; p = 0.13) (Table 1).
Table 1.
Characteristics of patients hospitalized in ICU with detected SARS-CoV-2 variants in Lombardy (Italy), 1st December 2021 - 9th February 2022.
| Categories | All 171; 100% | Delta 135; 78.9% | Omicron 36; 21.1% | |
|---|---|---|---|---|
| Median age (range; IQR*) | 62 (21–84;16) | 62 (38–84;17) | 61.5 (21–79;16.7) | |
| Age group (years) | 18–29 | 1 (0.6%) | 0 (0%) | 1 (2.8%) |
| 30–44 | 5 (2.9%) | 4 (3%) | 1 (2.8%) | |
| 45–54 | 41 (24%) | 31 (23%) | 7 (23.3) | |
| 55–64 | 48 (28.1%) | 39 (28.9%) | 8 (26.7) | |
| 65–74 | 58 (33.9%) | 46 (34.1%) | 9 (30.0) | |
| 75–84 | 18 (10.5%) | 15 (11.1%) | 3 (10.0) | |
| Gender | Female | 51 (29.8%) | 44 (32.6%) | 7 (19.4%) |
| Male | 120 (70.2%) | 91 (67.4%) | 29 (80.6%) | |
| Week of surveillance | 48–2021 | 7 (4.1%) | 7 (5.2%) | 0 (0) |
| 49–2021 | 18 (10.5%) | 18 (13.3%) | 0 (0) | |
| 50–2021 | 18 (10.5%) | 18 (13.3%) | 0 (0) | |
| 51–2021 | 29 (17%) | 28 (20.7%) | 1 (3.3%) | |
| 52–2021 | 30 (17.5%) | 29 (21.5%) | 1 (2.8%) | |
| 1–2022 | 17 (9.9%) | 11 (8.1%) | 6 (16.7%) | |
| 2–2022 | 23 (13.5%) | 15 (11.1%) | 8 (22.2%) | |
| 3–2022 | 18 (10.5%) | 6 (4.4%) | 12 (33.3%) | |
| 4–2022 | 7 (4.1%) | 2 (1.5%) | 5 (8.3%) | |
| 5–2022 | 3 (1.8%) | 0 (0%) | 3 (3.3%) | |
| 7–2022 | 1 (0.6%) | 1 (0.7%) | 1 (2.8%) | |
| Comorbidities | No | 52 (30.4%) | 44 (32.6%) | 8 (22.2%) |
| Yes | 119 (69.6%) | 91 (67.4%) | 28 (77.8%) | |
| 1 | 45 (26.3%) | 38 (28.1%) | 7 (19.4%) | |
| 2–3 | 65 (34.5%) | 44 (32.6%) | 15 (41.7%) | |
| >3 | 14 (8.8%) | 9 (6.7%) | 5 (16.7%) | |
| COVID-19 vaccination | No | 98 (57.3%) | 83 (61.5%) | 15 (41.7%) |
| Yes | 69 (40.4%) | 48 (35.6%) | 21 (58.3%) | |
| Unknown | 4 (2.3%) | 4 (3.0%) | 0 (0) | |
| 1 dose | 4 (5.8%) | 3 (6.3%) | 1 (4.8%) | |
| 2 doses <120 days | 6 (8.7%) | 4 (8.3%) | 2 (9.5%) | |
| 2 doses >120 days | 41 (59.4%) | 34 (70.8%) | 7 (33.3%) | |
| 3 doses | 18 (26.1%) | 7 (14.6%) | 11 (35.6%) | |
| Intubated | No | 27 (15.8%) | 21 (15.6%) | 6 (16.7%) |
| Yes | 144 (84.2%) | 114 (84.4%) | 30 (83.3%) |
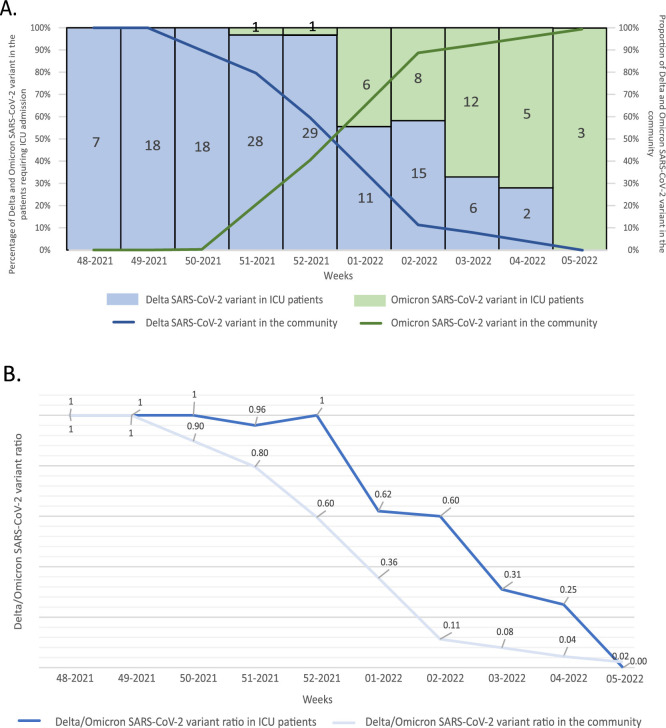
In order to compare the prevalence of VOCs infecting COVID-19 patients requiring ICU with variants circulating in the general population during the same period, VOCs point-prevalence data from national/regional surveys were considered [8]. According to temporal distribution Delta cases were overcame by Omicron cases since week 1 of 2022 (Fig. 1 A). Delta cases peaked at week 51 of 2021 (29 cases; 21.5% of all Delta cases) while the peak of Omicron cases was at week 3–2022 (12 cases, 33.3% of all Omicron cases) (Table 1). No Omicron cases were observed in ICU inpatients hospitalized from week 48–2021 to week 50–2021. The Delta/Omicron ratio of cases decreased more rapidly in the general population than in our series of ICU patients. In detail, the Delta/Omicron ratio dropped below the value of 0.5 between week 52 of 2021 and 01 of 2022 in the general population, while in our ICU patients between week 2 and 3–2022 with a delay of at least two weeks (Fig. 1B).
Fig. 1.
(A) Percentage of ICU cases by infecting SARS-CoV-2 VOC (left) and proportion of Delta and Omicron cases by week of sampling (right, data obtained from national/regional point-prevalence flash survey (8)) Lombardy (Italy), 1st December 2021 – 9th February 2022. Number of ICU cases is reported within each bar. (B) Delta/Omicron ratio in our series of COVID-19 ICU patients as compared to the general population (data from national/regional point-prevalence surveys (8)).
Overall, 52 out of 171 (30.4%) patients had no comorbidities, whereas of the remaining 119 (66.9%) patients with comorbidities, 26.3% (n = 45) had one comorbidity, 34.5% (n = 65) two/three comorbidities, and 8.8% (n = 14) showed more than three comorbidities (Table 1).
The percentage of ICU inpatients with more than three comorbidities was statistical higher in the Omicron-infected group than that observed in the Delta-infected group (16.7% vs 6.7%; p < 0.001). By estimating the comorbidity score the mean score of comorbidities in ICU patients infected by Omicron was statistically higher than that observed in patients infected by Delta (1.8 vs 0.8; p = 0.004). The mean score of comorbidities in ICU patients with vaccination (at least one dose) was significantly higher than that identified in ICU patients with no vaccination (1.4 vs 0.8, p = 0.005). No difference in the mean score of comorbidities was observed in patients aged less than 64 years and those older than 65 years (0.9 vs 1.2; p = 0.16). No difference in the risk of infection by Delta (OR: 1.5; 95%CI: 0.9–2.4) neither Omicron (OR: 0.5; 95% CI: 0.2–1.3) was observed between individuals 64 years old or younger and those over 65 years.
Overall, only 69/171 (40.4%) ICU patients were COVID-19 vaccinated (Table 1). This proportion is significantly lower (p < 0.001) than the percentage of the Italian adult population receiving at least two doses of vaccine (88.4%) [9]. Among ICU Delta-positive inpatients, 61.5% (83/135) was COVID-19 unvaccinated whereas among ICU Omicron-positive inpatients, 41.7% (15/36) was COVID-19 unvaccinated (p = 0.2) (Table 1). The percentage of ICU COVID-19 boosted individuals was statistically higher in the group of Omicron-infected inpatients than that observed in the Delta-infected group (35.6% vs 14.6%; p < 0.001).
Overall, 144 out of 171 (84.2%) patients had been intubated requiring invasive mechanical ventilation with no correlation related to the Delta (114/135; 84.4%) and Omicron (30/36; 83.3%) infection.
Discussion
In most European countries, the epidemiological scenario of SARS-CoV-2 VOCs has rapidly changed since the beginning of December 2021 [1], when the Delta variant - which was largely predominant in the last six-eight months – was entirely replaced by the Omicron [5,6,10]. The mutational pattern of Omicron exhibited a greater genetic diversity compared to other VOCs that had been circulating previously [11], thus raising major concerns on its transmissibility, severity, and immune response escape ability. This swift and complete replacement of circulating VOCs led to an unpredicted rising in the number of cases primarily due to the higher transmissibility of Omicron, which is estimated to be 2.7–3.7 times more infectious than Delta variant in vaccinated and boosted people [12]. As expected, in the present study the consequence of the Omicron variant in COVID-19 patients requiring ICU were delayed by about two weeks compared to its circulation in the community, reflecting the timing for SARS-CoV-2 worsening symptoms and ICU admission (10–14 days).
A greater proportion of unvaccinated patients was infected by Delta than by Omicron; interestingly, in our ICU inpatients, the rate of ICU COVID-19 boosted individuals was statistically higher in the group of Omicron-infected inpatients than that observed in the Delta-infected group, in line with data uncovered the Omicron immune-evasive properties [2,3,12].
The percentage of ICU inpatients with more than three comorbidities was statistical higher in the Omicron-infected group than that observed in the Delta-infected group; moreover, the mean score of comorbidities was higher in patients infected by Omicron compared to Delta patients as well as in vaccinated compared to unvaccinated patients; similarly, a recent study has underlined that total number of comorbidities in critically COVID-19 hospitalized patients was positively associated with the Omicron group [14].
It has been demonstrated that people infected by Omicron have a lower risk of severe disease than those infected by Delta variant: in fact, both the incidence of ICU admission [15] and the mortality [16] decrease with the Omicron variant in comparison to Delta. Nevertheless, the outcome, including the risk of ICU admission and the need for mechanical ventilation due to infection by Omicron versus Delta remains uncertain. The outcome from SARS-CoV-2 infection however could be related to individual characteristics such as age, comorbidities, and prior immunity from vaccination or could be driven by viral markers of pathogenicity or individual biomarkers [13]. Among the limitations of the present study is that no follow-up data were available for the patients analyzed. Additionally, no comparisons with data obtained from patients admitted to general hospital wards (different from ICU) were performed.
Conclusions
Despite the not increased pathogenicity of breakthrough SARS-CoV-2 Omicron VOC infections from vaccinated individuals, the exceptionally high transmission levels of this variant have resulted in a significant increase in hospitalization, continuing to pose overwhelming demands on health care systems in most countries, and possibly leading to significant morbidity, particularly in vulnerable populations. The circulation of this more transmissible but less severe variant in countries with high vaccination coverage has endorsed the best clinical scenario in the worst epidemiological situation (nearly 90 million cases in less than one month worldwide). The continuous monitoring of the circulating SARS-CoV-2 variants remains a milestone to counteract this pandemic.
Author's contributions
AP, EP and FB designed the paper. LP, FC, AV, MRG, VM, CF, MA, DF, AB, FM, FN, APC, GR, AC, SMIM carried out the analysis. FM, GG, AC, FLL, ML, LC, RK, RR acquired and manage clinical data. AP, LP and EP conducted statistical analyses and created graphics. AP, LP, and EP wrote the manuscript, which was then reviewed and approved by the other authors. The members of the COVID-19 laboratory group collected the samples and performed SARS-CoV-2 typing while the ICU clinicians’ group collected and managed clinical data. AP and EP had full access to all data in the study, and the corresponding author had final responsibility for the decision to submit for publication.
Declaration of Competing Interest
None declared.
Acknowledgments
Acknowledgments
We thank Mrs Daniela Sartori for manuscript editing and to all members of the COVID-19 laboratories and ICU clinicians groups.
Funding
No external funding was received.
References
- 1.European Centre for Disease Prevention and Control. Weekly epidemiological update: omicron variant of concern (VOC) – week 2 (data as of 20 January 2022) EU/EEA https://www.ecdc.europa.eu/en/news-events/weekly-epidemiological-update-omicron-variant-concern-voc-week-2-data-20-january-2022.
- 2.Andrews N., Stowe J., Kirsebom F., Toffa S., Rickeard T., Gallagher E., Gower C., Kall M., Groves N., O'Connell A.M., Simons D., Blomquist P.B., Zaidi A., Nash S., Iwani Binti Abdul Aziz N., Thelwall S., Dabrera G., Myers R., Amirthalingam G., Gharbia S., Barrett J.C., Elson R., Ladhani S.N., Ferguson N., Zambon M., Campbell C.N.J., Brown K., Hopkins S., Chand M., Ramsay M., Lopez Bernal J. Covid-19 vaccine effectiveness against the Omicron (B.1.1.529) variant. N Engl J Med. 2022;386(16):1532–1546. doi: 10.1056/NEJMoa2119451. Apr 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dejnirattisai W., Huo J., Zhou D., Zahradník J., Supasa P., Liu C., et al. SARS-CoV-2 Omicron-B.1.1.529 leads to widespread escape from neutralizing antibody responses. Cell. 2022;185(3):467–484. doi: 10.1016/j.cell.2021.12.046. Feb 3e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iuliano A.D., Brunkard J.M., Boehmer T.K., Peterson E., Adjei S., Binder A.M., et al. Trends in disease severity and health care utilization during the early Omicron variant period compared with previous SARS-CoV-2 high transmission periods - United States, December 2020-January 2022. MMWR Morb Mortal Wkly Rep. 2022;71(4):146–152. doi: 10.15585/mmwr.mm7104e4. Jan 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lewnard J.A., Hong V.X., Patel M.M., Kahn R., Lipsitch M., Tartof S.Y. Clinical outcomes associated with SARS-CoV-2 Omicron (B.1.1.529) variant and BA.1/BA.1.1 or BA.2 subvariant infection in Southern California. Nat Med. 2022;28(9):1933–1943. doi: 10.1038/s41591-022-01887-z. Sep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Veneti L., Bøås H., Bråthen Kristoffersen A., Stålcrantz J., Bragstad K., Hungnes O., et al. Reduced risk of hospitalisation among reported COVID-19 cases infected with the SARS-CoV-2 Omicron BA.1 variant compared with the Delta variant, Norway, December 2021 to January 2022. Eurosurveillance. 2022;27(4) doi: 10.2807/1560-7917.ES.2022.27.4.2200077. 01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Demo-Geodemo. Available at: https://www.tuttitalia.it/statistiche/popolazione-eta-sesso-stato-civile-2021. (Last accessed 09-02-2022).
- 8.Istituto Superiore di Sanità (ISS). Monitoraggio delle varianti del virus SARS-CoV-2 di interesse in sanità pubblica in Italia. Available at: https://www.epicentro.iss.it/coronavirus/sars-cov-2-monitoraggio-varianti-indagini-rapide. Last accessed 09-02-2022.
- 9.Ministero della Salute. Report vaccni anti-COVID-19 https://www.governo.it/it/cscovid19/report-vaccini/. (Last accessed 09-02-2022).
- 10.Ulloa A.C., Buchan S.A., Daneman N., Brown K.A. Estimates of SARS-CoV-2 Omicron variant severity in Ontario, Canada. JAMA. 2022;327(13):1286–1288. doi: 10.1001/jama.2022.2274. Apr 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kumar S., Thambiraja T.S., Karuppanan K., Subramaniam G. Omicron and Delta variant of SARS-CoV-2: acomparative computational study of spike protein. J Med Virol. 2021:1–9. doi: 10.1002/jmv.27526. [DOI] [PubMed] [Google Scholar]
- 12.Lyngse F.P., Kirkeby C.T., Denwood M., Christiansen L.E., Mølbak K., Møller C.H., Skov R.L., Krause T.G., Rasmussen M., Sieber R.N., Johannesen T.B., Lillebaek T., Fonager J., Fomsgaard A., Møller F.T., Stegger M., Overvad M., Spiess K., Mortensen L.H. Household transmission of SARS-CoV-2 Omicron variant of concern subvariants BA.1 and BA.2 in Denmark. Nat Commun. 2022;13(1):5760. doi: 10.1038/s41467-022-33498-0. Sep 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fagyas M., Fejes Z., Sütő R., Nagy Z., Székely B., Pócsi M., et al. Circulating ACE2 activity predicts mortality and disease severity in hospitalized COVID-19 patients. Int J Infect Dis. 2022;115:8–16. doi: 10.1016/j.ijid.2021.11.028. Feb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Corriero A., Ribezzi M., Mele F., Angrisani C., Romaniello F., Daleno A., Loconsole D., Centrone F., Chironna M., Brienza N. COVID-19 variants in critically ill patients: a comparison of the Delta and Omicron variant profiles. Infect Dis Rep. 2022;14(3):492–500. doi: 10.3390/idr14030052. Jun 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vieillard-Baron A., Flicoteaux R., Salmona M., Chariot A., De Maupeou D'Ableiges B., Darmon M., Batteux F., APHP Reality Research Group Omicron Variant in the critical care units of paris metropolitan area the reality research group. Am J Respir Crit Care Med. 2022 doi: 10.1164/rccm.202202-0411LE. ù. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sigal A., Milo R., Jassat W. Estimating disease severity of Omicron and Delta SARS-CoV-2 infections. Nat Rev Immunol. 2022;22:267–269. doi: 10.1038/s41577-022-00720-5. [DOI] [PMC free article] [PubMed] [Google Scholar]