Abstract
Appropriate expression of fear in the face of threats in the environment is essential for survival. The sustained expression of fear in the absence of threat signals is a central pathological feature of trauma- and anxiety-related disorders. Our understanding of the neural circuitry that controls fear inhibition coalesces around the amygdala, hippocampus, and prefrontal cortex. By discussing thalamic and sub-thalamic influences on fear-related learning and expression in this review, we suggest a more inclusive neurobiological framework that expands our canonical view of fear. First, we visit how fear-related learning and expression is influenced by the aforementioned canonical brain regions. Next, we review emerging data that shed light on new roles for thalamic and subthalamic nuclei in fear-related learning and expression. Then, we highlight how these neuroanatomical hubs can modulate fear via integration of sensory and salient stimuli, gating information flow and calibrating behavioral responses, as well as maintaining and updating memory representations. Finally, we propose that the presence of this thalamic and sub-thalamic neuroanatomy in parallel with the tripartite prefrontal cortex-amygdala-hippocampus circuit allows for dynamic modulation of information based on interoceptive and exteroceptive signals.
1. Introduction
Fear is an emotional state that is induced when imminent danger or threat is perceived by an organism. Observed across many species, fear-related behaviors allow an organism to be vigilant, evaluate threat and respond appropriately. In contrast to these adaptive properties of fear, fear responses can become maladaptive when they cannot be controlled in the absence of threat or danger. Fear expressed toward stimuli that do not themselves signal threat (fear generalization) and fear expressed toward stimuli after they cease to be threats (deficits in fear extinction) are two debilitating and highly prevalent dimensions of trauma- and anxiety-related disorders such as post-traumatic stress disorder (PTSD) and generalized anxiety disorder (GAD) (1–5). Suppressing fear generalization and rescuing deficits in extinction learning requires an appreciation for the neurobiological mechanisms that govern the expression and inhibition of fear in normative and disrupted states.
The expression and inhibition of fear are accomplished by a network of brain regions that integrate sensory information and threat assessment with appropriate behavioral output. A wealth of research has provided strong evidence that cortico-limbic networks make important contributions to fear inhibition. More specifically, canonical fear-related neural circuitry comprising of the amygdala, hippocampus, and prefrontal cortex have received the most attention for their roles in regulating fear-related behaviors (6–11). However, recently, new technologies like activity-based circuit mapping, optogenetics, chemogenetics and in vivo recordings of neural activity are making a case for more nuanced control of fear by brain regions outside of this canon. Most notably, rapidly accumulating data are highlighting that thalamic and sub-thalamic brain regions that were traditionally viewed as being mere relays of information flow in the brain, do in fact, make important contributions to various dimensions of fear-related behavior. In this review, we discuss the newly appreciated influences of these brain regions on fear generalization and extinction. We conclude by integrating them into the established neuroanatomical canon to expand our understanding of the neurobiological underpinnings of trauma- and anxiety-related disorders.
Fear generalization and fear extinction are studied via the use of Pavlovian classical conditioning. To study both constructs, a neutral stimulus is paired with an aversive unconditional stimulus (US) that by itself results in an unconditional response. For example, presentation of a specific auditory or visual cue is paired with a mild aversive experience such as an electric shock or air puff. Exposure to the neutral stimulus after an association has been formed between its presentation and the US will elicit a robust conditional fear response; resulting in the neutral stimulus being called a conditional stimulus (CS). In humans, this fear response is measured in the form of an enhanced startle reflex or skin conductance. In rodents, complete cessation of movement or freezing is used as a proxy for fear. To study fear generalization, animals are trained to distinguish between a neutral stimulus that has come to be associated with an aversive outcome (CS+) and another neutral stimulus that is not associated with threat (CS-). Fear generalization manifests as fear responses to the CS+ and the CS- and is a debilitating dimension of PTSD (1, 2, 5, 12). To study the extinction of fear responses, after conditioning, multiple presentations of the CS+ but now without the aversive US and the learning of this new association, CS+ but no aversive outcome, is assayed, by measuring fear toward future presentations of the CS+. Appropriate extinction of fear is expressed as a steady decline in fear towards the CS+ presentations in the absence of the US. In rodents, prior exposure to stress impairs extinction learning (13–17) and consequently the ability to inhibit fear to the now non-threatening CS+. Individuals living with trauma- and anxiety-related disorders like PTSD and GAD show deficits in extinction learning and a consequent inability to inhibit fear although the CS+ is no longer associated with the aversive US (1, 2, 5, 12, 18–20).
Another key dimension of fear observed in trauma-related disorders like PTSD is the expression of fear towards salient cues that are not predictive of trauma. For example, a tone present during the traumatic event that does not predict the occurrence of the threat can elicit fear (21–23) and give rise to the pathological and intrusive nature of traumatic memories. The neurobiology underlying this clinically relevant construct is beginning to be explored in animal models (24–26). As such, the potential contributions of specific thalamic and subthalamic circuits to this important endophenotype of PTSD are yet to be determined and beyond the scope of this review that focuses on fear generalization and extinction.
2. The canonical tripartite circuit and fear.
Our appreciation for neurobiological mechanisms that underlie fear generalization and fear extinction is centered around the functioning of the canonical tripartite circuit consisting of the amygdala, prefrontal cortex, and hippocampus (Fig. 1). In this section, we provide a brief overview of the literature highlighting the contributions of the canonical circuit to expression of appropriate fear responses. We refer the reader to (1, 2, 4, 8, 9, 11, 27–31) for more comprehensive analyses of the contributions of these brain regions to fear.
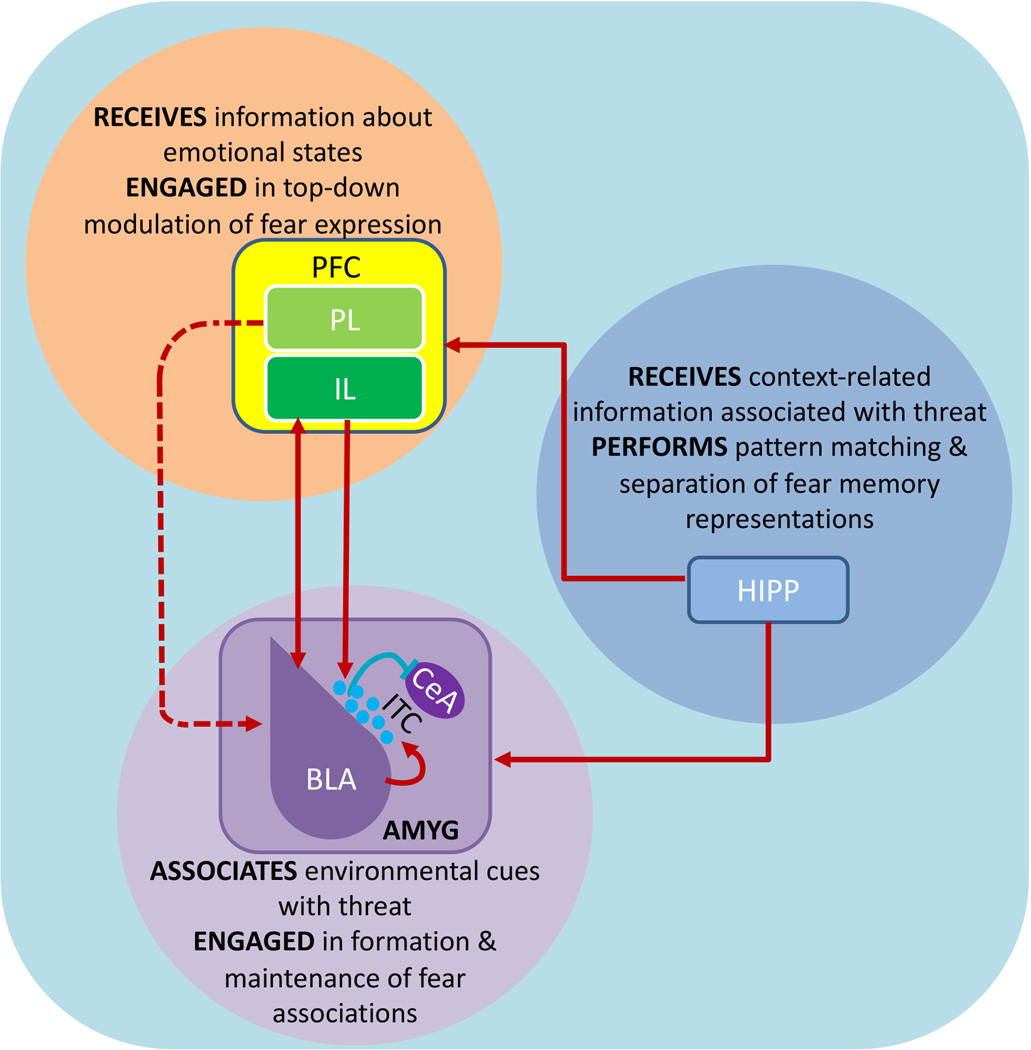
Figure 1: Canonical circuitry mediating fear inhibition.
The amygdala, prefrontal cortex and hippocampus make up the canonical tripartite circuit that influences fear-related behavior. As one of the prominent sites of CS-US convergence, the BLA contains dedicated neuronal ensembles that track the emotional valence of CS. In the presence of a neutral or extinguished CS, the BLA indirectly acts on the CeA through the ITC GABAergic cluster to inhibit fear. Additionally, the reciprocal communication between IL and BLA can enhance inhibition of fear responses. Although the IL inputs to ITC are sparse, these inputs are known to inhibit CeA output. However, the role of PL→BLA pathway in fear inhibition remains unclear. Bidirectional communication between the hippocampus and amygdala is essential for contextual regulation of fear. The hippocampus initiates pattern separation processes that facilitate distinction between safety and danger. Through inputs to the mPFC (both PL and IL), the hippocampus can then modulate fear expression. Coordinated activity in the tripartite circuit is essential for expression of appropriate fear responses. Synchronization of neuronal oscillations between BLA, hippocampus and PFC is presumed to support fear inhibition. Solid red arrows in the figure indicate activation of an excitatory pathway and dashed arrows indicate suppression of the pathway. Solid blue lines indicate inhibitory connections. PFC: prefrontal cortex; PL: prelimbic prefrontal cortex; IL: infralimbic prefrontal cortex; HIPP: hippocampus; AMYG: amygdala; BLA: basolateral amygdala; ITC: intercalated GABAergic interneurons; CeA: central amygdala
2.1. Fear generalization
Sensory information related to the CS and US converges in the basolateral amygdala (BLA) and synaptic plasticity at glutamatergic synapses in the region is essential to achieve clear delineation between fear and safety. Discrete neuronal subpopulations in the BLA dubbed ‘cue-specific neurons’ are dedicated to encoding precision of fear memories by selectively increasing activity in response to reinforced cue (CS+) compared to a non-reinforced cue (CS-) (32, 33). Information regarding the CS-US pairing is then sent from the BLA directly to the central amygdala (CeA) or indirectly through the GABAergic intercalated interneurons (ITCs) of the amygdala. The CeA has genetically distinct, and functionally defined, neuronal subpopulations gating the specificity of learned fear memories. Here, a CRF+ cell population in the CeA mediates fear discrimination by selectively responding to CS+ only associated with weak US intensities (34). A separate PKCδ+ GABAergic cell population can modulate fear generalization by increasing fear towards CS- (35, 36). The CeA then projects to downstream hypothalamic and brainstem regions that generate the autonomic, behavioral, and endocrine responses associated with fear.
Fear responses are closely regulated by the medial prefrontal cortex (mPFC) that communicates bidirectionally with the BLA and CeA. Activity-dependent plasticity within the mPFC is required for successful fear discriminative learning, suggesting a top-down control of fear generalization (33, 37–39). Particularly, in rodents, there is division-specific functional delineation within the mPFC, where the prelimbic (PL) and infralimbic (IL) cortices are separately implicated in fear expression and fear inhibition respectively. During discriminative fear learning, distinct experience-specific prefrontal neuronal ensembles are recruited to distinguish between safe and dangerous stimuli. While activation of the IL supports fear discrimination, activation of PL promotes fear generalization (40–42). Consistent with the idea of dissociable roles of PL and IL in fear discrimination, Corches et al., reported that PL neuronal activity tracks both conditional and generalized fear responses, and IL neurons ramp up activity specifically during successful fear discrimination (43).
The ability to discriminate and not generalize also relies on recognition of learned fear associations in specific contexts and limiting fear responses to similar environments. The hippocampus encodes the contextual information regarding where the fearful experience occurred and has bidirectional connectivity with the amygdala and the prefrontal cortex. The stress-sensitive hippocampal subfields CA1, CA2, CA3, and DG (dentate gyrus) are crucial for pattern separation and completion processes (44–47) that allow representations of threat and safety to be stored in a distinct, non-overlapping manner. The CA1 is required for adaptive generalization of fear memories from partial cues through the pattern completion process (48). The DG-CA3 circuit performs pattern separation for accurate encoding of similar experiences and resolving uncertain threats. Deletion of the GluN1 subunit of NMDA receptors in DG cells causes deficits in discrimination learning (49). Similarly, inhibition of neural activity in the DG during retrieval of fear memories leads to overgeneralization of fear to safe contexts (50), supporting the idea that failure in pattern separation processes leads to inaccurate activation of threat representations (2, 47).
Clinically, the distinction between safety and danger is blurred in individuals suffering from stress- and anxiety-related disorders such as post-traumatic stress disorder (PTSD) and generalized anxiety disorder (GAD). The neurobiological mechanisms underlying the observed overgeneralization of trauma-related memories involve hyperactivity in the amygdala, impaired prefrontal top-down control and reduced hippocampal activation. Heightened activity in the amygdala occurs in concert with reduced top-down inhibitory control of amygdala function by the ventromedial prefrontal cortex (vmPFC). Studies in human participants diagnosed with PTSD report reduced activation of the vmPFC and decreased functional connectivity between the vmPFC and amygdala (51–56), thereby leading to exaggerated responses to negative emotional stimuli (57–59). Activation of the vmPFC is specifically required for inhibiting fear responses to non-threatening stimuli that least resemble the CS+. This is particularly deficient in the case of Generalized Anxiety Disorder, where neural reactivity of vmPFC remains largely unchanged in response to the CS+ as well as other perceptually dissimilar stimuli, leading to the loss of distinction between threat and safety (60–62). This hypoactivity in the vmPFC is also inversely correlated with PTSD symptom severity. The substantial reduction in hippocampal volume (63–66) seen after exposure to severe stress and trauma is thought to contribute to fear generalization. High-resolution fMRI imaging analysis reveal association between damage to hippocampal subfields CA1, CA2, CA3/DG and PTSD symptoms (67–69). Therefore, clinical studies provide robust evidence that functioning of the tripartite circuit is heavily disrupted in stress- and anxiety-related disorders.
2.2. Fear extinction
A cardinal feature of stress- and anxiety-related disorders is the deficit in extinction learning, where repeated exposure to the CS+ even in the absence of US continues to elicit fear responses. The neural ensembles embedded within the trisynaptic circuit are re-engaged to extinguish fear memories. Electrophysiological studies have shown that distinct neuronal subsets within the BLA become active during high fear states compared to low fear states. While one set of neurons called ‘fear neurons’ increase their activity in response to the CS+ with fear learning, another set of BLA neurons called ‘extinction neurons’ show increased response to the CS+ after extinction training (35, 36, 70). These ‘extinction neurons’ send long-range projections to the mPFC, where the PL-projecting BLA neurons signal fear expression and the IL-projecting BLA neurons signal fear extinction (71). Accumulating evidence suggests that acquisition of fear extinction also involves remodeling of inhibitory interneurons in the BLA, shifting the balance from ensembles encoding threat signaling to extinction (72–74). Moreover, inhibition of fear responses following extinction learning requires top-down cortical modulation of the amygdala. Strong amygdala-prefrontal synchrony at the end of fear learning has been implicated in resistance to extinction of fear memories (75). The IL cortex directly activates the GABAergic ITCs which drive feed forward inhibition of CeA output neurons to suppress fear expression (76–80). Notably, recruitment of ITCs can also be enhanced alternatively through potentiation of excitatory inputs from the BLA (81).
Expression of fear extinction does not merely rely on the cortico-amygdalar communication, but also on contextual information from the hippocampus. Gating of fear responses after extinction relies on hippocampal inputs to PL (82, 83). A recent study by Lacagnina and colleagues (44) explored the possibility of dedicated cell populations within the hippocampal DG subfield that control fear and extinction memories. Using activity-dependent neural tagging and targeted optogenetic manipulations, the authors found that extinction training involves active suppression of DG neurons encoding fear acquisition and establishment of another distinct set of DG neurons encoding the extinction memory. This raises the possibility that the interaction between fear and extinction representations in the DG could potentially determine resistance to extinction. Coordinated activity between the BLA, hippocampus and PFC is presumed to support inhibition of fear responses.
Neuroimaging data in humans have consistently shown that functional deficits at any of these three nodes of the tripartite circuit can lead to extinction deficits. Impaired extinction in individuals suffering from PTSD is associated with increased amygdala activation during learning as well as retrieval of extinction memories (52, 53, 84, 85). In line with the hypothesis that hyperactivity in the amygdala is caused by defective inhibition from the vmPFC, individuals diagnosed with PTSD and GAD show reduced activation of the PFC and consequently, impaired recall of extinction memories (85, 86). Furthermore, a failure to engage the hippocampus can also potentially lead to deficits in appropriate inhibition of fear responses in a context-dependent manner (85, 87, 88).
Among current therapeutic interventions, exposure-based therapies are considered to be the most effective in reducing symptom severity of trauma- and anxiety-related disorders (89–91). Exposure-based therapy utilizes fear extinction mechanisms, where patients are repeatedly and systematically exposed to trauma-associated stimuli in a non-threatening environment to reduce traumatic fear responses. The gradual reduction in fear achieved through exposure-based therapies is considered to involve reshaping cellular and molecular processes as well as functional connectivity within the tripartite circuit (92, 93). However, such exposure-based therapies are quite aversive to patients, with dropout rates estimated to be around 50% or more (94–96). Understanding the neural mechanisms that operate outside the tripartite circuit to modulate emotional responses might help identify effective therapeutic interventions that are less likely to trigger aversiveness.
3. Thalamic and sub-thalamic influences on fear.
Emerging literature on thalamic and sub-thalamic brain regions suggest that interoceptive and exteroceptive information must be integrated across multiple sites within the nervous system for fear to be appropriately expressed or inhibited in dynamic environments. Thalamic and subthalamic regions have the potential to synchronize neural activity across multiple nodes of cortical and subcortical networks according to attentional demands in situations of safety versus danger. The normative entrenched view of these regions as mere relay centers that transfer sensorimotor information to the cortex, is changing. Instead, evidence points to the more refined view of them functioning as ‘switch boards’ where multiple streams of sensory and salient information are integrated and targeted to specific, segregated subsets of cortical and subcortical structures for computation of appropriate behavioral outcomes.
As a hub that coordinates activity across cortical and subcortical networks, the thalamus is sensitive to perturbations in physiological and psychological states. This functional complexity of thalamic nuclei is apparent in individuals with damage to thalamic neuroanatomy, who express profound impairments in inhibitory control accompanied by deficits spanning emotional and cognitive domains (97–102). The thalamus becomes activated across varied emotional states such as happiness, sadness, and disgust, (103) suggesting that the thalamus participates in processing salient emotions irrespective of their valence. Aberrations in thalamic activity has been reported in patients suffering from stress- and anxiety-related disorders (54, 88, 104–107). For instance, patients with PTSD exhibit thalamic hypoactivity. A meta-analysis of traumatic processing in PTSD patients found critical thalamic mediation of cortical crosstalk during the recall of traumatic stimuli (108). The severity and progression of PTSD is characterized by disrupted thalamic connectivity (109, 110), suggesting that thalamic and subthalamic regions play a fundamental role in controlling activity across multiple regions in states of high fear.
In the following section, we provide an overview of the critical contributions of thalamic nuclei in processing fear starting with their ability to integrate multimodal sensory information, modulate flow of information based on ongoing situational demands, and maintenance of adaptive fear associations for future encounters. We focus on these three key mechanistic features by which the thalamic and subthalamic circuits influence the canonical tripartite circuit for fine-tuning threat responses (Fig. 2). First, we consider the role of auditory thalamus and paraventricular thalamus in integration of multimodal sensory information and emotional states. Next, we discuss gating of threat-related information in the thalamic reticular nucleus and zona incerta. Finally, we examine how thalamic regions support plasticity in canonical fear circuits using nucleus reuniens and mediodorsal thalamus as examples.
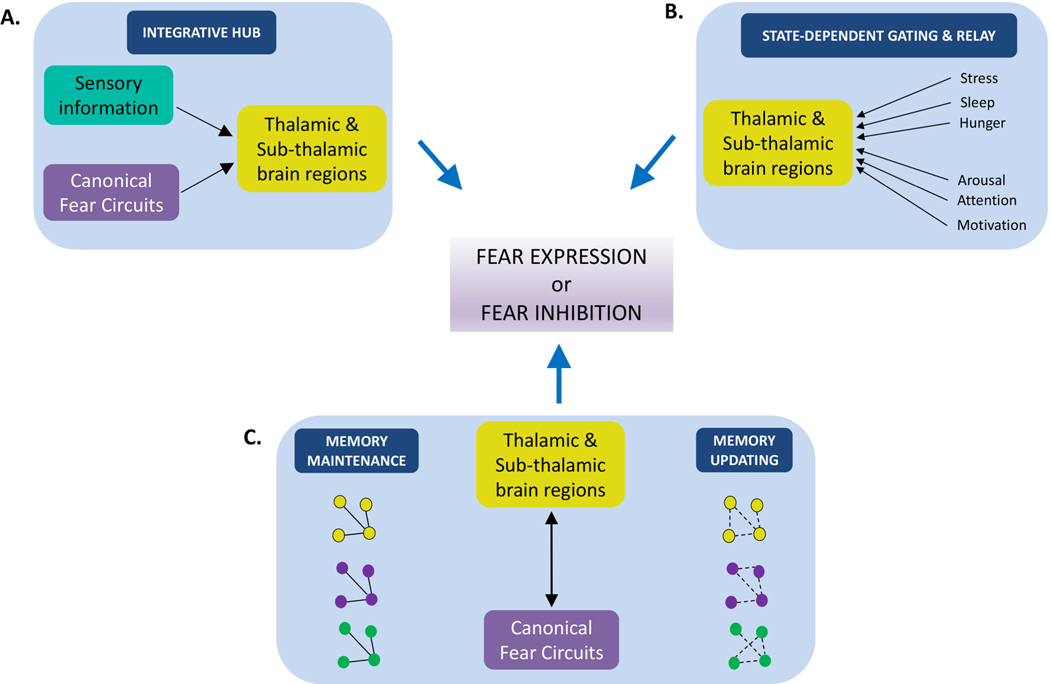
Figure 2: Thalamic and sub-thalamic mechanisms modulating fear.
A) The thalamus and subthalamus function as a central integrative unit that synthesizes multimodal sensory information from the cortical networks and fear-related information from canonical fear circuitry (Fig. 1) to modulate behavioral outcomes. Convergence of sensory (auditory, visual, somatosensory and nociceptive) inputs in the thalamus and sub-thalamus allows for fine-tuning of sensory information based on emotional relevance. In section 4, we discuss the role of auditory thalamus and paraventricular nucleus in this context.
B) More than a mere relay, the thalamus and sub-thalamus functions as a ‘modulator’ that can alter information flow in a state-dependent manner. Activity of thalamic and subthalamic neurons is known to change dynamically during interoceptive states linked to stress, sleep, hunger, arousal, motivation, and attentional states. Further, these neurons can control forebrain activation and deactivation through inhibition of cortical information flow, as well as employing coincident activity of multiple converging inputs to overcome the inhibitory gate when necessary. Thus, associative and state-dependent information from the thalamic and sub-thalamic nuclei can influence fear output. In section 5, we examine the gating mechanisms employed by thalamic reticular nucleus and subthalamic zona incerta.
C) Thalamic and sub-thalamic circuits contribute to the maintenance (left) and updating (right) of fear memory representations by sustaining neuronal activity through reciprocal connectivity with the canonical fear circuits. Activity of cell assemblies (green, blue and orange circles connected with solid lines) in the canonical fear circuits recruited to the memory trace, is sustained through thalamic activity. Functional dissociation experiments suggest a strong role for thalamus in maintenance of memories via activity in thalamocortical loops. In addition to active maintenance of learned information (as evident in the thalamic reuniens discussed in section 6.1), updating of stored memory representations (green, blue and orange circles connected with dashed lines) in the canonical fear circuits also requires dynamic engagement of the thalamus (e.g., the mediodorsal thalamus discussed in section 6.2). This helps in incorporating new information rapidly based on appropriate environmental as well as internal contexts.
4. Integrative hub for linking sensory and emotional information (Fig. 2A and Fig. 3A)
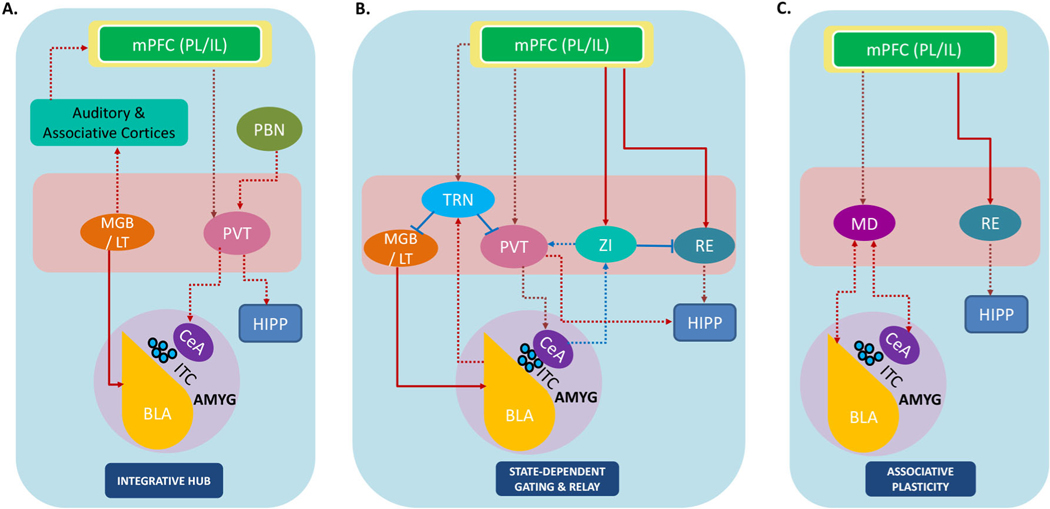
Figure 3: Thalamic and subthalamic modulation of tripartite circuitry.
Schematic representation of direct connections between thalamic/subthalamic nuclei (in pink rectangle) and the canonical tripartite circuit including the medial prefrontal cortex (mPFC), hippocampus (HIPP) and amygdala (AMYG). The thalamic and subthalamic regions are engaged in processing fear starting from the early stages of sensory processing to choosing appropriate behavioral strategies. Through their extensive connectivity, these highly distributed, parallel thalamic pathways play a critical role in reinforcing and fine-tuning activity in the tripartite fear circuit.
A. The auditory thalamus and paraventricular thalamus are integrative hubs that synthesize multimodal sensory information and emotional states. The auditory thalamus (MGB/LT) is engaged in multisensory integration through its communication with the primary auditory cortex (AC) and associative cortices, as well as the BLA. The PVT integrates cognitive information from the mPFC, and nociceptive and stress-related information from the PBN and is well-positioned to influence threat-related behavioral responses through its projections to the BLA and HIPP.
B. The thalamic reticular nucleus (TRN) and zona incerta (ZI) facilitate gating of sensory signals and calibration of fear responses according to ongoing situational demands. The TRN directly influences thalamic signaling through its inhibitory connections to the auditory thalamus and PVT, whereas the ZI controls activity in the RE and PVT. This inhibition between thalamic nuclei may allow for selective state-dependent engagement of the tripartite circuit. The functional role of connections between these inhibitory nuclei and tripartite circuit remains to be fully understood.
C. The thalamic reuniens (RE) and mediodorsal (MD) nuclei are critical hubs that coordinate functional interactions across the tripartite circuit. The RE contributes towards the precise establishment and maintenance of associative memories, through its connections to the mPFC and HIPP. The MD, with its extensive connections to the amygdala and mPFC, might be involved in tracking the behavioral relevance of threat-related signals and selecting appropriate behavioral strategies.
Red lines indicate excitatory connections and blue lines indicate inhibitory connections. Dotted lines indicate anatomical pathways that are yet to be examined in the context of fear inhibition. MGB: medial geniculate body; LT: lateral thalamus; TRN: thalamic reticular nucleus; PVT: paraventricular nucleus of the thalamus; RE: thalamic nucleus reuniens; ZI: zona incerta; MD: mediodorsal thalamus; PL: prelimbic prefrontal cortex; IL: infralimbic prefrontal cortex; BLA: basolateral amygdala; ITC: intercalated GABAergic interneurons; CeA: central amygdala
The thalamus receives a rich network of sensory inputs. The convergence of cortical, subcortical and brainstem inputs onto the same target thalamic nucleus (111–113) enables the thalamus to exert strong influence on ascending sensorimotor information. The ability of thalamus to then multiplex different streams of incoming sensory information allows for fine calibration of sensory processing. Thalamic integration of CS and US related information allows for increased cue sensitivity required for proper fear inhibition (114, 115). The cue-specific information from the thalamus is then utilized by the tripartite circuit to form fear associations and suppress non-specific information (114, 116–120).
4.1. Sensory integration in the auditory thalamus
The thalamus controls the transformation of complex sensory signals into behaviorally relevant information, made available to amygdala. Here, we discuss the auditory thalamus as an example of how thalamic processing refines detection of fearful stimuli. Such thalamic routes for dynamic processing of sensory stimuli extends to the visual and somatosensory systems (121). The auditory thalamus is not just a primary relay station for auditory signals en route to the cortex, it acts as an integrative site for processing multimodal information. CS-related auditory information reaches the amygdala via thalamo-amygdala as well as thalamo-cortical-amygdala projections. These amygdala-projecting higher order thalamic neurons lie within the lateral thalamus (LT) including the medial geniculate body (MGB), posterior intralaminar nucleus (PIN) and suprageniculate nucleus (SG). These two complementary thalamic projections converge in the BLA, providing complex auditory representations necessary to maintain specificity of fear responses. Thalamic neurons communicating with the amygdala act as drivers of fear memory formation. Recent studies have shown that (114, 117) plasticity in these thalamic neurons can directly evoke stimulus-specific responses in the amygdala. Silencing of thalamic inputs to the amygdala results in diminished fear discrimination capabilities. Lesioning of the MGB causes fear generalization as well as impaired extinction, with sustained responding to the CS- even after going through fear extinction training (122–124). As expected, lesioning of the region can disrupt learning-associated synaptic plasticity in the BLA.
Neurons in the MGB develop CS-specific tuning plasticity where the largest response is allocated to the tone associated with the US (114, 125, 126). This shift in tuning of the receptive field occurs only when the CS is paired with the US, suggesting that the MGB performs complex multimodal sensory processing. Indeed, neurons in the MGB respond to both the auditory information (CS) and the aversive somatosensory stimulation (US) (127, 128). Although stable CS-specific plasticity occurs at the level of individual neurons, the distinguishing feature of MGB compared to amygdala is that the population level auditory representation of the CS tones is transient, highlighting its complex role in driving downstream neuronal plasticity and dynamic functional influence of amygdala to generate appropriate behavioral outcomes. Thalamic neurons compute experience-dependent information to facilitate fine tuning of cortical representations and enhance contrast between safety and danger (CS+ vs CS-) leading to appropriate balance between fear expression and inhibition.
4.2. Integration of emotional states and threat information in the paraventricular nucleus of thalamus
The PVT is a dorsal midline thalamic nucleus that is potently activated in response to stress and emotional arousal. The PVT receives inputs from mPFC, insular cortex, ventral hippocampus, and parabrachial nucleus, and sends dense projections to the nucleus accumbens (NAc), bed nucleus of stria terminalis and central amygdala (CeA) (129, 130). This makes the PVT an ideal candidate to integrate interoceptive, homeostatic and contextual signals and guide the appropriate adaptive responses to threat. The PVT plays a crucial role in expression of fear memories (131–135). Neurons in the PVT become tone-responsive 24 hours after fear conditioning and show persistent increase in their spontaneous firing rate (131). Retrieval of fear memories 1 week after learning requires recruitment of PVT to the fear network. The PVT closely interacts with the tripartite circuit to calibrate future behavioral responses. Silencing of prelimbic inputs to the PVT and PVT projections to the CeA causes impairments in retrieval of week-old fear memories. Indeed, inhibition of PVT interferes with learning-induced synaptic plasticity that occurs in the amygdala (132). Similarly, in the context of extinction, silencing of infralimbic inputs to the PVT and PVT projections to the CeA causes impairments in retrieval of week-old extinction memories (135). The PVT is, thus, capable of modulating fear based on prior experience and salience of the presented cues (136). It is plausible that the PVT engages distinct circuits for expression of fear compared to extinction of fear. However, this hypothesis remains to be tested.
A recent study by Ma et al., 2021 adds strength to the notion of that the PVT can differentially engage downstream circuits, switching between active avoidance and passive freezing behaviors (137). Neurons in the posterior PVT (pPVT) show increase in activity during threat avoidance but remains suppressed during expression of freezing behavior. Notably, the pPVT neurons projecting to the NAc and the CeA are anatomically and functionally segregated such that the pPVT→NAc pathway facilitates active coping strategies while the pPVT→CeA pathway facilitates passive strategies. Therefore, when faced with threats, the PVT enables the selection of adaptive behaviors in a state- and experience- dependent manner.
One of the potential pathways through which aversive signals reach the PVT is the parabrachial nucleus (PBN). Activation of the PBN-PVT pathway causes animals to exhibit fear and anxiety-like behaviors even in the absence of threats (138). This is consistent with the notion that the PVT is broadly responsive to aversive states and regulates fear-related behaviors (136–141). Lesion studies indicate that the PVT is required to mediate neuroendocrine responses to chronic stress (142, 143). The PVT, therefore, integrates threat-related signals with information regarding the emotional state of the animal to decide on the appropriate behavioral strategy.
5. Gating of sensory signals to modulate fear (Fig. 2B and Fig. 3B)
Gating mechanisms modulate information flow between distributed circuits, creating a blueprint for neuronal activity patterns required to produce desirable behavior output. Thalamic gating mechanisms allow for fine-tuning of cue- and context- related sensory information (144–147). In the amygdala, thalamic afferents contacting the inhibitory interneurons mediate sensory gating during learning CS-US fear associations (80, 148, 149). Convergent CS and US information in the amygdala is strengthened by thalamic afferents, thereby, potentially conferring cue-specificity to distinct cell populations within the amygdala (32). Additionally, thalamic control over the amygdala interneurons could modulate rhythmic gamma-frequency oscillations in the amygdala that are essential for fear discrimination and extinction (150, 151).
A single thalamic nucleus is functionally connected to multiple cortical and subcortical regions and can thereby, drive efficient input-specific changes in dendritic excitability through inhibition or disinhibition mechanisms. In this section, we examine the thalamic gating functions by focusing on two main inhibitory systems, the thalamic reticular nucleus (TRN) and the sub-thalamic zona incerta (ZI).
5.1. Gatekeeping of threat-related sensory signals by the thalamic reticular nucleus
The TRN, a layer of GABAergic neurons encapsulating the anterolateral division of the thalamus, provides a major source of inhibition to thalamocortical neurons. As a dynamic inhibitory hub, the TRN gates information flow from different thalamic nuclei to the cortex in a modality-specific manner and is well-positioned to modulate fear expression. Thus, recruitment of TRN to the fear network allows for calibration of fear responses through its connections with the cortex and amygdala. The TRN shapes cortical activity patterns through its inhibition of the MGB. Desynchronization of MGB neurons by the TRN reduces the likelihood of eliciting population-level responses in the auditory cortex (152). Additionally, based on inputs received from the BLA, the TRN can suppress spontaneous neuronal activity in the MGB (116). This subsequently causes amplification of tone-evoked responses in the auditory cortex and can potentially facilitate appropriate fear associations. Indeed, TRN neurons specifically increase their spiking responses to the CS during extinction learning (153). Optogenetic activation of the TRN during extinction learning facilitates the extinction process, while inhibition disrupts it. TRN’s control of extinction-related fear inhibition is achieved through suppression of neuronal activity in midline thalamic nuclei (including the paraventricular and mediodorsal thalamus) that synapse with the CeA. Thus, the TRN through its connections to the cortical and subcortical networks enables selection of relevant threat-related sensory information and shapes appropriate behavioral expression of fear.
5.2. Calibration of fear responses by the zona incerta
The zona incerta (ZI), present directly beneath the thalamus, is a highly interconnected structure with complex chemical heterogeneity and widespread connections throughout the brain. Consistent with its extensive connectivity, the ZI has been implicated in modulating a wide variety of functions including sensorimotor integration, risk assessment and behavioral flexibility under fearful or stressful states. Blocking synaptic transmission in the ZI disrupts fear learning and subsequent recall of fear memories (154, 155). Under stressful conditions such as fear conditioning at high shock intensities, chemogenetic activation of ZI can abolish fear generalization. The observed reduction in fear response is due to dampening of fear response to the neutral stimulus while leaving the response to the aversive stimulus unaltered, suggesting a definitive role for ZI in mediating graded fear responses.
This remarkable level of association between ZI activation and specificity of fear responses could indicate that the ZI is a site for integration of sensory and salient information. Indeed, neurons in the ZI are responsive to a range of sensory stimuli (115, 156–161), allowing them to integrate incoming information from the environment and choose the appropriate behavioral strategy. Multi-channel recordings and c-fos studies from the ZI have revealed that the neurons respond to auditory, visual, and somatosensory cues. Early lesioning studies demonstrated the crucial role of ZI in sensory discrimination (115, 162–164). Interestingly, deep brain stimulation of the ZI can ameliorate symptoms of anxiety and depression, and enhance discrimination between fearful and non-fearful faces in a subset of Parkinsonian patients (162). The ability of ZI to discern sensory signals is made possible, in part, through incoming cortical information. During extinction of conditional fear responses, neuronal activity in the rostral ZI ramps up to generate a reduction in freezing behavior. This increase in activity is abolished with silencing of the mPFC, suggesting that ZI gates behavioral responses based on threat-related information from the cortex (154).
The incoming stimulus- and threat-related cortical information, paired with the ability to modulate thalamic nuclei, makes ZI an ideal substrate to synchronize and gates thalamic activity to alter fear behaviors. The ZI exerts inhibitory control over “higher order” thalamic nuclei such as posterior complex of the thalamus, and nucleus reuniens. For instance, based on cortical somatosensory information, PV+ ZI neurons communicate with the medial posterior complex of the thalamus to enhance defensive flight behaviors elicited by tactile stimulation. On the other hand, the GABA+ ZI neurons collates multisensory signals, enhances motivation, and drives predatory hunting behavior. In the context of learned fear, stimulation of GABA+ cells in the ZI effectively suppresses fear responses and enhances fear discrimination by acting mainly through its efferents in the thalamic reuniens (115, 165). Together, these studies suggest that the ZI can regulate fear behaviors by engaging distinct circuits based on environmental threats and the internal state of the animal. The ZI can modulate sensory thresholds depending on arousal states (166–168). The “state-dependent gating” hypothesis suggests that the ZI receives information regarding sleep and wakefulness from the brain stem cholinergic system and modulates activity in higher-order thalamic nuclei. Mounting evidence suggests that the ZI is not only sensitive to arousal states, but also to other interoceptive (physiological?) cues such as stress and hunger (157, 169–171), potentially through its connections to hypothalamus and insular cortex. Therefore, it is plausible that the ZI facilitates selective engagement of the tripartite circuit based on ongoing state-dependent demands like sleep, hunger and stress. However, this idea is yet to be tested empirically.
6. Associative plasticity (Fig. 2C and Fig. 3C)
Processing threat-related signals not only requires engagement of sensorimotor integration and gating of relevant sensory information, but also establishing long-term maintenance of learned associations between multimodal sensory signals and the aversive outcomes. Learning-dependent plasticity in thalamic circuits is essential to establish, maintain and update fear memories. This section aims to analyze the role of specialized thalamic nuclei such as the reuniens (RE), and mediodorsal thalamus in associative learning.
6.1. Bridging interactions across the tripartite circuit through thalamic reuniens
RE is a ventral midline thalamic nucleus reciprocally connected to the mPFC and hippocampus, and controls specificity and persistence of fear memories (172–174). Muscimol-induced inactivation of RE following a weak fear conditioning procedure enhances fear memory consolidation and fear generalization (173). Notably, the firing pattern in the RE seems to differentially contribute to fear generalization. Phasic stimulation of the RE during fear acquisition leads to increased fear generalization while tonic stimulation reduces generalization of fear memories (174). This suggests that activity patterns in the RE are directly related to suppression of fear responses. Importantly, activity-dependent brain mapping studies have revealed increased cfos expression in the RE following extinction learning and recall (175, 176). Subsequently, inactivation of the RE prior to extinction training prevents extinction learning while RE inactivation prior to retrieval impairs retrieval of the extinction memories (177). Together, these studies suggest that RE should be online to achieve suppression of fear memories.
Within the tripartite circuit, suppression of fear responses is known to require activation of the mPFC that in turn then drives inhibition of the hippocampus. The connection through the RE has been suggested to mediate this cortical control of hippocampal processing. The RE sends axon collaterals to mPFC and the hippocampus (178–180), forming a disynaptic link that can potentially coordinate neural activity between the two structures. Consistent with this idea, inactivation of mPFC inputs to the RE has effects similar to that of RE inactivation, i.e., leads to an increase in fear generalization (174). The RE is then well-positioned to exert its effects on the hippocampal-dependent memories by altering the excitability of the hippocampus. Through its excitatory inputs to the CA1, combined with the ability to activate local inhibitory neurons (181, 182), RE can elicit both feedforward excitation as well as inhibition of hippocampal activity. Effectively, this allows the RE to toggle between hippocampal activity states to potentially facilitate consolidation or updating of fear memories.
Additionally, the RE is able to bring about suppression of fear responses through its excitatory connections to the amygdala. A recent study has uncovered a role for the poorly understood IL→RE→BLA pathway in extinction of remote memories (183). Using a closed-loop optogenetic approach, the authors show that increased activity in the RE facilitates suppression of fear during remote fear extinction. Pathway specific inhibition of RE-BLA projections impairs remote fear extinction and reduces extinction-associated plasticity at the synapses. Therefore, it is evident that the RE encodes and transmits safety-related information to the tripartite circuit. Further research is required to delineate the mechanisms through which the RE coordinates functional interactions within the tripartite circuit and the extent to which these interactions contribute to fear inhibition.
6.2. Maintenance and updating of fear memories in the mediodorsal thalamus
The mediodorsal thalamus (MD) plays a multifaceted role in cognitive processing and is specifically involved in maintenance of fear memories through persistent thalamocortical activity. Well-placed to influence the tripartite circuit, the MD communicates extensively with the mPFC and amygdala (184–187). Early studies found that lesioning of MD interfered with Pavlovian conditioning using eyeblink and heart rate conditioning paradigms in rabbits (188–190). The lesions only mildly affect the animals’ ability to learn the distinction between CS+ and CS-. However, when the animals are subjected to reversal learning where the CS- is newly reinforced taking the form of CS+ and the CS+ becomes CS-, MD lesions cause dramatic impairments in acquisition of the reversal task. This suggests that the communication between MD and its cortical targets might be important for learning complex associations. Yet it remains to be determined how MD activity might contribute to fear discrimination.
Due to the dense excitatory reciprocal connectivity with mPFC, MD has been linked to behavioral flexibility and thought to play a role in extinguishing learned fear memories. MD shows increase in tonic firing during extinction learning that is directly related to the animal’s performance during extinction recall (191). When low frequency stimulation is applied to MD prior to extinction learning, it prevents prefrontal synaptic excitability to return to baseline levels and suppresses fear extinction (192, 193). On the other hand, high frequency stimulation of MD causes LTP-like changes in prefrontal excitability resulting in enhanced extinction recall. These electrophysiological findings suggest that plasticity in the MD-mPFC pathway is essential for maintenance of extinction memories. Interestingly, these thalamocortical neurons can modulate their firing pattern between tonic and bursting modes to alter fear extinction (191). While increase in burst firing of MD neurons suppresses fear extinction, tonic firing facilitates it. In tonic firing mode these neurons act as faithful relays where groups of neurons fire synchronously to facilitate effective transfer of information to cortical neurons. Moreover, a recent study indicated that MD neurons can drive feedforward inhibition in the BLA to suppress fear responses (187). Thus, the MD might be particularly important for facilitating synchronizing activity across the tripartite circuit to maintain multimodal fear memory representations and their behavioral relevance.
7. Future directions & Conclusion
In discussing emerging findings on thalamic and subthalamic function, we collate evidence of distributed thalamic and subthalamic nodes that regulate several important and distinct aspects of fear inhibition. Unlike labeled lines in sensory processing, circuits involved in fear inhibition are distributed, thereby increasing the probability of integration of information from multiple sources. Fear-related information is indeed processed simultaneously along parallel channels and fear associations are encoded in distributed areas across the brain, challenging the canonical role of tripartite circuitry in fear processing. Thalamic and subthalamic regions multiplex multi-modal sensory information, integrate this information with input from the canonical brain regions that signal salience, and drive distinct motor and autonomic responses via divergent downstream pathways to alter fear expression and inhibition. Endowed with extensive connectivity across the brain, these regions modify sensory information based on exteroceptive and interoceptive demands. Thalamic and sub-thalamic gating can effectively drive differential engagement of distributed, downstream fear ensembles, and engender behavioral flexibility in dynamic environments. During states of increased emotional intensity such as high fear/stress situations, these regions bias the cognitive strategies based on prior experience and current status to choose appropriate behavioral outcomes. The active involvement of thalamus in maintaining fear associations through thalamocortical loops highlights parallel streams of information processing outside the tripartite fear circuit. The thalamic and subthalamic networks engaged in fear processing are not merely alternative and redundant pathways, as previously thought. Accumulating evidence suggest that these networks play a dynamic role in switching fear behaviors such that certain circuits become dominant based on ongoing demands. Gaining deeper insights into how brain-wide networks contribute to behavioral flexibility and appropriate fear expression can inform us about the neurobiological and physiological basis of stress, fear and anxiety.
Highlights.
Fear generalization and deficits in extinction learning are debilitating dimensions of trauma- and anxiety-related disorders.
Our neurobiological understanding of these dimensions comes from a focus on the amygdala, prefrontal cortex and hippocampus.
Traditionally viewed as relay stations, thalamic and sub-thalamic brain regions are becoming increasingly appreciated for playing nuanced roles in fear generalization and extinction learning.
Including thalamic and sub-thalamic regions in the conversation with the amygdala, prefrontal cortex and hippocampus expands the neurobiological canon of fear generalization and extinction learning.
From a more inclusive neuroanatomical framework comes better opportunities to identify therapeutic strategies to suppress fear generalization and rescue deficits in fear extinction.
Funding and Disclosures.
BGD is supported by R01MH120133 which included support of AV and R56MH128427 from the US National Institute of Health. Support to BGD is also provided by the Department of Pediatrics at USC Keck School of Medicine, the Division of Endocrinology at Children’s Hospital Los Angeles, the Developmental Neuroscience and Neurogenetics Program at The Saban Research Institute, and the Canadian Institute for Advanced Research.
Footnotes
The authors have no competing financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Dunsmoor JE, Paz R, Fear Generalization and Anxiety: Behavioral and Neural Mechanisms. Biol Psychiatry 78, 336–343 (2015). [DOI] [PubMed] [Google Scholar]
- 2.Dymond S, Dunsmoor JE, Vervliet B, Roche B, Hermans D, Fear Generalization in Humans: Systematic Review and Implications for Anxiety Disorder Research. Behav Ther 46, 561–582 (2015). [DOI] [PubMed] [Google Scholar]
- 3.Jovanovic T, Kazama A, Bachevalier J, Davis M, Impaired safety signal learning may be a biomarker of PTSD. Neuropharmacology 62, 695–704 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jovanovic T, Ressler KJ, How the neurocircuitry and genetics of fear inhibition may inform our understanding of PTSD. Am J Psychiatry 167, 648–662 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaczkurkin AN et al. , Neural Substrates of Overgeneralized Conditioned Fear in PTSD. Am J Psychiatry 174, 125–134 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ehrlich I. et al. , Amygdala inhibitory circuits and the control of fear memory. Neuron 62, 757–771 (2009). [DOI] [PubMed] [Google Scholar]
- 7.Gross CT, Canteras NS, The many paths to fear. Nat Rev Neurosci 13, 651–658 (2012). [DOI] [PubMed] [Google Scholar]
- 8.Herry C. et al. , Neuronal circuits of fear extinction. Eur J Neurosci 31, 599–612 (2010). [DOI] [PubMed] [Google Scholar]
- 9.Maren S, Quirk GJ, Neuronal signalling of fear memory. Nat Rev Neurosci 5, 844–852 (2004). [DOI] [PubMed] [Google Scholar]
- 10.Orsini CA, Maren S, Neural and cellular mechanisms of fear and extinction memory formation. Neurosci Biobehav Rev 36, 1773–1802 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tovote P, Fadok JP, Luthi A, Neuronal circuits for fear and anxiety. Nat Rev Neurosci 16, 317–331 (2015). [DOI] [PubMed] [Google Scholar]
- 12.Jasnow AM, Lynch JF 3rd, Gilman TL, Riccio DC, Perspectives on fear generalization and its implications for emotional disorders. J Neurosci Res 95, 821–835 (2017). [DOI] [PubMed] [Google Scholar]
- 13.Maren S, Holmes A, Stress and Fear Extinction. Neuropsychopharmacology 41, 58–79 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maroun M. et al. , Fear extinction deficits following acute stress associate with increased spine density and dendritic retraction in basolateral amygdala neurons. Eur J Neurosci 38, 2611–2620 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miracle AD, Brace MF, Huyck KD, Singler SA, Wellman CL, Chronic stress impairs recall of extinction of conditioned fear. Neurobiol Learn Mem 85, 213–218 (2006). [DOI] [PubMed] [Google Scholar]
- 16.Raio CM, Brignoni-Perez E, Goldman R, Phelps EA, Acute stress impairs the retrieval of extinction memory in humans. Neurobiol Learn Mem 112, 212–221 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raio CM, Phelps EA, The influence of acute stress on the regulation of conditioned fear. Neurobiol Stress 1, 134–146 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blechert J, Michael T, Vriends N, Margraf J, Wilhelm FH, Fear conditioning in posttraumatic stress disorder: evidence for delayed extinction of autonomic, experiential, and behavioural responses. Behav Res Ther 45, 2019–2033 (2007). [DOI] [PubMed] [Google Scholar]
- 19.VanElzakker MB, Dahlgren MK, Davis FC, Dubois S, Shin LM, From Pavlov to PTSD: the extinction of conditioned fear in rodents, humans, and anxiety disorders. Neurobiol Learn Mem 113, 3–18 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wessa M, Flor H, Failure of extinction of fear responses in posttraumatic stress disorder: evidence from second-order conditioning. Am J Psychiatry 164, 1684–1692 (2007). [DOI] [PubMed] [Google Scholar]
- 21.Brewin CR, Holmes EA, Psychological theories of posttraumatic stress disorder. Clin Psychol Rev 23, 339–376 (2003). [DOI] [PubMed] [Google Scholar]
- 22.Brewin CR, Dalgleish T, Joseph S, A dual representation theory of posttraumatic stress disorder. Psychol Rev 103, 670–686 (1996). [DOI] [PubMed] [Google Scholar]
- 23.Desmedt A, Marighetto A, Piazza PV, Abnormal Fear Memory as a Model for Posttraumatic Stress Disorder. Biol Psychiatry 78, 290–297 (2015). [DOI] [PubMed] [Google Scholar]
- 24.Al Abed AS et al. , Preventing and treating PTSD-like memory by trauma contextualization. Nat Commun 11, 4220 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Desmedt A, (Re)contextualizing the Trauma to Prevent or Treat PTSD-Related Hypermnesia. Chronic Stress (Thousand Oaks) 5, 24705470211021073 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaouane N. et al. , Glucocorticoids can induce PTSD-like memory impairments in mice. Science 335, 1510–1513 (2012). [DOI] [PubMed] [Google Scholar]
- 27.Asok A, Kandel ER, Rayman JB, The Neurobiology of Fear Generalization. Front Behav Neurosci 12, 329 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Herry C, Johansen JP, Encoding of fear learning and memory in distributed neuronal circuits. Nat Neurosci 17, 1644–1654 (2014). [DOI] [PubMed] [Google Scholar]
- 29.Krabbe S, Grundemann J, Luthi A, Amygdala Inhibitory Circuits Regulate Associative Fear Conditioning. Biol Psychiatry 83, 800–809 (2018). [DOI] [PubMed] [Google Scholar]
- 30.Lissek S. et al. , Neural substrates of classically conditioned fear-generalization in humans: a parametric fMRI study. Soc Cogn Affect Neurosci 9, 1134–1142 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Likhtik E, Johansen JP, Neuromodulation in circuits of aversive emotional learning. Nat Neurosci 22, 1586–1597 (2019). [DOI] [PubMed] [Google Scholar]
- 32.Ghosh S, Chattarji S, Neuronal encoding of the switch from specific to generalized fear. Nat Neurosci 18, 112–120 (2015). [DOI] [PubMed] [Google Scholar]
- 33.Grosso A, Santoni G, Manassero E, Renna A, Sacchetti B, A neuronal basis for fear discrimination in the lateral amygdala. Nat Commun 9, 1214 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sanford CA et al. , A Central Amygdala CRF Circuit Facilitates Learning about Weak Threats. Neuron 93, 164–178 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Botta P. et al. , Regulating anxiety with extrasynaptic inhibition. Nat Neurosci 18, 14931500 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haubensak W. et al. , Genetic dissection of an amygdala microcircuit that gates conditioned fear. Nature 468, 270–276 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pollack GA et al. , Cued fear memory generalization increases over time. Learn Mem 25, 298–308 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vieira PA et al. , Prefrontal NMDA receptors expressed in excitatory neurons control fear discrimination and fear extinction. Neurobiol Learn Mem 119, 52–62 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vieira PA et al. , Prefrontal consolidation supports the attainment of fear memory accuracy. Learn Mem 21, 394–405 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bayer H, Bertoglio LJ, Infralimbic cortex controls fear memory generalization and susceptibility to extinction during consolidation. Sci Rep 10, 15827 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scarlata MJ et al. , Chemogenetic stimulation of the infralimbic cortex reverses alcoholinduced fear memory overgeneralization. Sci Rep 9, 6730 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vanvossen AC et al. , Newly acquired and reactivated contextual fear memories are more intense and prone to generalize after activation of prelimbic cortex NMDA receptors. Neurobiol Learn Mem 137, 154–162 (2017). [DOI] [PubMed] [Google Scholar]
- 43.Corches A. et al. , Differential fear conditioning generates prefrontal neural ensembles of safety signals. Behav Brain Res 360, 169–184 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lacagnina AF et al. , Distinct hippocampal engrams control extinction and relapse of fear memory. Nat Neurosci 22, 753–761 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Leutgeb JK, Leutgeb S, Moser MB, Moser EI, Pattern separation in the dentate gyrus and CA3 of the hippocampus. Science 315, 961–966 (2007). [DOI] [PubMed] [Google Scholar]
- 46.Yassa MA, Stark CE, Pattern separation in the hippocampus. Trends Neurosci 34, 515–525 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kheirbek MA, Klemenhagen KC, Sahay A, Hen R, Neurogenesis and generalization: a new approach to stratify and treat anxiety disorders. Nat Neurosci 15, 1613–1620 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhou H. et al. , The interhemispheric CA1 circuit governs rapid generalisation but not fear memory. Nat Commun 8, 2190 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McHugh TJ et al. , Dentate gyrus NMDA receptors mediate rapid pattern separation in the hippocampal network. Science 317, 94–99 (2007). [DOI] [PubMed] [Google Scholar]
- 50.Bernier BE et al. , Dentate Gyrus Contributes to Retrieval as well as Encoding: Evidence from Context Fear Conditioning, Recall, and Extinction. J Neurosci 37, 63596371 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bremner JD et al. , Neural correlates of memories of childhood sexual abuse in women with and without posttraumatic stress disorder. Am J Psychiatry 156, 1787–1795 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rauch SL, Shin LM, Phelps EA, Neurocircuitry models of posttraumatic stress disorder and extinction: human neuroimaging research--past, present, and future. Biol Psychiatry 60, 376–382 (2006). [DOI] [PubMed] [Google Scholar]
- 53.Bremner JD et al. , Positron emission tomographic imaging of neural correlates of a fear acquisition and extinction paradigm in women with childhood sexual-abuse-related post-traumatic stress disorder. Psychol Med 35, 791–806 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Etkin A, Wager TD, Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry 164, 1476–1488 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shin LM et al. , Regional cerebral blood flow in the amygdala and medial prefrontal cortex during traumatic imagery in male and female Vietnam veterans with PTSD. Arch Gen Psychiatry 61, 168–176 (2004). [DOI] [PubMed] [Google Scholar]
- 56.Shin LM, Rauch SL, Pitman RK, Amygdala, medial prefrontal cortex, and hippocampal function in PTSD. Ann N Y Acad Sci 1071, 67–79 (2006). [DOI] [PubMed] [Google Scholar]
- 57.Babaev O, Piletti Chatain C, Krueger-Burg D, Inhibition in the amygdala anxiety circuitry. Exp Mol Med 50, 18 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McLaughlin KA et al. , Amygdala response to negative stimuli predicts PTSD symptom onset following a terrorist attack. Depress Anxiety 31, 834–842 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stevens JS et al. , Amygdala Reactivity and Anterior Cingulate Habituation Predict Posttraumatic Stress Disorder Symptom Maintenance After Acute Civilian Trauma. Biol Psychiatry 81, 1023–1029 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cha J. et al. , Circuit-wide structural and functional measures predict ventromedial prefrontal cortex fear generalization: implications for generalized anxiety disorder. J Neurosci 34, 4043–4053 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Greenberg T, Carlson JM, Cha J, Hajcak G, Mujica-Parodi LR, Neural reactivity tracks fear generalization gradients. Biol Psychol 92, 2–8 (2013). [DOI] [PubMed] [Google Scholar]
- 62.Greenberg T, Carlson JM, Cha J, Hajcak G, Mujica-Parodi LR, Ventromedial prefrontal cortex reactivity is altered in generalized anxiety disorder during fear generalization. Depress Anxiety 30, 242–250 (2013). [DOI] [PubMed] [Google Scholar]
- 63.Kitayama N, Vaccarino V, Kutner M, Weiss P, Bremner JD, Magnetic resonance imaging (MRI) measurement of hippocampal volume in posttraumatic stress disorder: a meta-analysis. J Affect Disord 88, 79–86 (2005). [DOI] [PubMed] [Google Scholar]
- 64.Levy-Gigi E, Szabo C, Richter-Levin G, Keri S, Reduced hippocampal volume is associated with overgeneralization of negative context in individuals with PTSD. Neuropsychology 29, 151–161 (2015). [DOI] [PubMed] [Google Scholar]
- 65.Logue MW et al. , Smaller Hippocampal Volume in Posttraumatic Stress Disorder: A Multisite ENIGMA-PGC Study: Subcortical Volumetry Results From Posttraumatic Stress Disorder Consortia. Biol Psychiatry 83, 244–253 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stein MB, Koverola C, Hanna C, Torchia MG, McClarty B, Hippocampal volume in women victimized by childhood sexual abuse. Psychol Med 27, 951–959 (1997). [DOI] [PubMed] [Google Scholar]
- 67.Chen LW et al. , Smaller hippocampal CA1 subfield volume in posttraumatic stress disorder. Depress Anxiety 35, 1018–1029 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Postel C. et al. , Variations in response to trauma and hippocampal subfield changes. Neurobiol Stress 15, 100346 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang Z. et al. , Magnetic resonance imaging of hippocampal subfields in posttraumatic stress disorder. Arch Gen Psychiatry 67, 296–303 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Herry C. et al. , Switching on and off fear by distinct neuronal circuits. Nature 454, 600606 (2008). [DOI] [PubMed] [Google Scholar]
- 71.Senn V. et al. , Long-range connectivity defines behavioral specificity of amygdala neurons. Neuron 81, 428–437 (2014). [DOI] [PubMed] [Google Scholar]
- 72.Davis P, Zaki Y, Maguire J, Reijmers LG, Cellular and oscillatory substrates of fear extinction learning. Nat Neurosci 20, 1624–1633 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kasugai Y. et al. , Structural and Functional Remodeling of Amygdala GABAergic Synapses in Associative Fear Learning. Neuron 104, 781–794 e784 (2019). [DOI] [PubMed] [Google Scholar]
- 74.Trouche S, Sasaki JM, Tu T, Reijmers LG, Fear extinction causes target-specific remodeling of perisomatic inhibitory synapses. Neuron 80, 1054–1065 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Livneh U, Paz R, Amygdala-prefrontal synchronization underlies resistance to extinction of aversive memories. Neuron 75, 133–142 (2012). [DOI] [PubMed] [Google Scholar]
- 76.Berretta S, Pantazopoulos H, Caldera M, Pantazopoulos P, Pare D, Infralimbic cortex activation increases c-Fos expression in intercalated neurons of the amygdala. Neuroscience 132, 943–953 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cho JH, Deisseroth K, Bolshakov VY, Synaptic encoding of fear extinction in mPFCamygdala circuits. Neuron 80, 1491–1507 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Likhtik E, Popa D, Apergis-Schoute J, Fidacaro GA, Pare D, Amygdala intercalated neurons are required for expression of fear extinction. Nature 454, 642–645 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Marek R, Strobel C, Bredy TW, Sah P, The amygdala and medial prefrontal cortex: partners in the fear circuit. J Physiol 591, 2381–2391 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Asede D, Bosch D, Luthi A, Ferraguti F, Ehrlich I, Sensory inputs to intercalated cells provide fear-learning modulated inhibition to the basolateral amygdala. Neuron 86, 541554 (2015). [DOI] [PubMed] [Google Scholar]
- 81.Amano T, Unal CT, Pare D, Synaptic correlates of fear extinction in the amygdala. Nat Neurosci 13, 489–494 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bouton ME, Context, ambiguity, and unlearning: sources of relapse after behavioral extinction. Biol Psychiatry 52, 976–986 (2002). [DOI] [PubMed] [Google Scholar]
- 83.Sotres-Bayon F, Sierra-Mercado D, Pardilla-Delgado E, Quirk GJ, Gating of fear in prelimbic cortex by hippocampal and amygdala inputs. Neuron 76, 804–812 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Linnman C, Zeffiro TA, Pitman RK, Milad MR, An fMRI study of unconditioned responses in post-traumatic stress disorder. Biol Mood Anxiety Disord 1, 8 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Milad MR et al. , Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biol Psychiatry 66, 1075–1082 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Garfinkel SN et al. , Impaired contextual modulation of memories in PTSD: an fMRI and psychophysiological study of extinction retention and fear renewal. J Neurosci 34, 13435–13443 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rougemont-Bucking A. et al. , Altered processing of contextual information during fear extinction in PTSD: an fMRI study. CNS Neurosci Ther 17, 227–236 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Suarez-Jimenez B. et al. , Neural signatures of conditioning, extinction learning, and extinction recall in posttraumatic stress disorder: a meta-analysis of functional magnetic resonance imaging studies. Psychol Med 50, 1442–1451 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.van Rooij SJH, Jovanovic T, Impaired inhibition as an intermediate phenotype for PTSD risk and treatment response. Prog Neuropsychopharmacol Biol Psychiatry 89, 435–445 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Craske MG et al. , Optimizing inhibitory learning during exposure therapy. Behav Res Ther 46, 5–27 (2008). [DOI] [PubMed] [Google Scholar]
- 91.Morrison FG, Ressler KJ, From the neurobiology of extinction to improved clinical treatments. Depress Anxiety 31, 279–290 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhu X. et al. , Exposure-based therapy changes amygdala and hippocampus restingstate functional connectivity in patients with posttraumatic stress disorder. Depress Anxiety 35, 974–984 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hauner KK, Mineka S, Voss JL, Paller KA, Exposure therapy triggers lasting reorganization of neural fear processing. Proc Natl Acad Sci U S A 109, 9203–9208 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Loerinc AG et al. , Response rates for CBT for anxiety disorders: Need for standardized criteria. Clin Psychol Rev 42, 72–82 (2015). [DOI] [PubMed] [Google Scholar]
- 95.Zayfert C. et al. , Exposure utilization and completion of cognitive behavioral therapy for PTSD in a “real world” clinical practice. J Trauma Stress 18, 637–645 (2005). [DOI] [PubMed] [Google Scholar]
- 96.Storm MP, Christensen KS, Comparing treatments for post-traumatic stress disorder - a systematic review. Dan Med J 68, (2021). [PubMed] [Google Scholar]
- 97.Bogousslavsky J, Regli F, Uske A, Thalamic infarcts: clinical syndromes, etiology, and prognosis. Neurology 38, 837–848 (1988). [DOI] [PubMed] [Google Scholar]
- 98.Carrera E, Bogousslavsky J, The thalamus and behavior: effects of anatomically distinct strokes. Neurology 66, 1817–1823 (2006). [DOI] [PubMed] [Google Scholar]
- 99.Cheung CC, Lee TM, Yip JT, King KE, Li LS, The differential effects of thalamus and basal ganglia on facial emotion recognition. Brain Cogn 61, 262–268 (2006). [DOI] [PubMed] [Google Scholar]
- 100.Van der Werf YD et al. , Deficits of memory, executive functioning and attention following infarction in the thalamus; a study of 22 cases with localised lesions. Neuropsychologia 41, 1330–1344 (2003). [DOI] [PubMed] [Google Scholar]
- 101.Wilkos E, Brown TJ, Slawinska K, Kucharska KA, Social cognitive and neurocognitive deficits in inpatients with unilateral thalamic lesions - pilot study. Neuropsychiatr Dis Treat 11, 1031–1038 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Van Der Werf YD et al. , Neuropsychological correlates of a right unilateral lacunar thalamic infarction. J Neurol Neurosurg Psychiatry 66, 36–42 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lane RD, Reiman EM, Ahern GL, Schwartz GE, Davidson RJ, Neuroanatomical correlates of happiness, sadness, and disgust. Am J Psychiatry 154, 926–933 (1997). [DOI] [PubMed] [Google Scholar]
- 104.Lanius RA et al. , Neural correlates of traumatic memories in posttraumatic stress disorder: a functional MRI investigation. Am J Psychiatry 158, 1920–1922 (2001). [DOI] [PubMed] [Google Scholar]
- 105.Yan X. et al. , Spontaneous brain activity in combat related PTSD. Neurosci Lett 547, 1–5 (2013). [DOI] [PubMed] [Google Scholar]
- 106.Lanius RA et al. , Recall of emotional states in posttraumatic stress disorder: an fMRI investigation. Biol Psychiatry 53, 204–210 (2003). [DOI] [PubMed] [Google Scholar]
- 107.Lanius RA et al. , Functional connectivity of dissociative responses in posttraumatic stress disorder: a functional magnetic resonance imaging investigation. Biol Psychiatry 57, 873–884 (2005). [DOI] [PubMed] [Google Scholar]
- 108.Ramage AE et al. , A coordinate-based meta-analytic model of trauma processing in posttraumatic stress disorder. Hum Brain Mapp 34, 3392–3399 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ben-Zion Z. et al. , Multi-domain potential biomarkers for post-traumatic stress disorder (PTSD) severity in recent trauma survivors. Transl Psychiatry 10, 208 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Steuwe C. et al. , Effect of direct eye contact in women with PTSD related to interpersonal trauma: Psychophysiological interaction analysis of connectivity of an innate alarm system. Psychiatry Res 232, 162–167 (2015). [DOI] [PubMed] [Google Scholar]
- 111.Groh A. et al. , Convergence of cortical and sensory driver inputs on single thalamocortical cells. Cereb Cortex 24, 3167–3179 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rouiller EM, Welker E, A comparative analysis of the morphology of corticothalamic projections in mammals. Brain Res Bull 53, 727–741 (2000). [DOI] [PubMed] [Google Scholar]
- 113.Rovo Z, Ulbert I, Acsady L, Drivers of the primate thalamus. J Neurosci 32, 1789417908 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Barsy B. et al. , Associative and plastic thalamic signaling to the lateral amygdala controls fear behavior. Nat Neurosci 23, 625–637 (2020). [DOI] [PubMed] [Google Scholar]
- 115.Venkataraman A. et al. , Modulation of fear generalization by the zona incerta. Proc Natl Acad Sci U S A 116, 9072–9077 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Aizenberg M. et al. , Projection from the Amygdala to the Thalamic Reticular Nucleus Amplifies Cortical Sound Responses. Cell Rep 28, 605–615 e604 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Taylor JA et al. , Single cell plasticity and population coding stability in auditory thalamus upon associative learning. Nat Commun 12, 2438 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tye KM, Stuber GD, de Ridder B, Bonci A, Janak PH, Rapid strengthening of thalamo-amygdala synapses mediates cue-reward learning. Nature 453, 1253–1257 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Weinberger NM, The medial geniculate, not the amygdala, as the root of auditory fear conditioning. Hear Res 274, 61–74 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yang Y. et al. , Selective synaptic remodeling of amygdalocortical connections associated with fear memory. Nat Neurosci 19, 1348–1355 (2016). [DOI] [PubMed] [Google Scholar]
- 121.Briggs F, Usrey WM, Emerging views of corticothalamic function. Curr Opin Neurobiol 18, 403–407 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Jarrell TW, Gentile CG, Romanski LM, McCabe PM, Schneiderman N, Involvement of cortical and thalamic auditory regions in retention of differential bradycardiac conditioning to acoustic conditioned stimuli in rabbits. Brain Res 412, 285294 (1987). [DOI] [PubMed] [Google Scholar]
- 123.Poremba A, Gabriel M, Medial geniculate lesions block amygdalar and cingulothalamic learning-related neuronal activity. J Neurosci 17, 8645–8655 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Antunes R, Moita MA, Discriminative auditory fear learning requires both tuned and nontuned auditory pathways to the amygdala. J Neurosci 30, 9782–9787 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Edeline JM, Weinberger NM, Associative retuning in the thalamic source of input to the amygdala and auditory cortex: receptive field plasticity in the medial division of the medial geniculate body. Behav Neurosci 106, 81–105 (1992). [DOI] [PubMed] [Google Scholar]
- 126.Bakin JS, Weinberger NM, Classical conditioning induces CS-specific receptive field plasticity in the auditory cortex of the guinea pig. Brain Res 536, 271–286 (1990). [DOI] [PubMed] [Google Scholar]
- 127.Bordi F, LeDoux JE, Response properties of single units in areas of rat auditory thalamus that project to the amygdala. II. Cells receiving convergent auditory and somatosensory inputs and cells antidromically activated by amygdala stimulation. Exp Brain Res 98, 275–286 (1994). [DOI] [PubMed] [Google Scholar]
- 128.Bordi F, LeDoux JE, Response properties of single units in areas of rat auditory thalamus that project to the amygdala. I. Acoustic discharge patterns and frequency receptive fields. Exp Brain Res 98, 261–274 (1994). [DOI] [PubMed] [Google Scholar]
- 129.Kirouac GJ, The Paraventricular Nucleus of the Thalamus as an Integrating and Relay Node in the Brain Anxiety Network. Front Behav Neurosci 15, 627633 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Penzo MA, Gao C, The paraventricular nucleus of the thalamus: an integrative node underlying homeostatic behavior. Trends Neurosci 44, 538–549 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Do-Monte FH, Quinones-Laracuente K, Quirk GJ, A temporal shift in the circuits mediating retrieval of fear memory. Nature 519, 460–463 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Penzo MA et al. , The paraventricular thalamus controls a central amygdala fear circuit. Nature 519, 455–459 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Padilla-Coreano N, Do-Monte FH, Quirk GJ, A time-dependent role of midline thalamic nuclei in the retrieval of fear memory. Neuropharmacology 62, 457–463 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Chen M, Bi LL, Optogenetic Long-Term Depression Induction in the PVT-CeL Circuitry Mediates Decreased Fear Memory. Mol Neurobiol 56, 4855–4865 (2019). [DOI] [PubMed] [Google Scholar]
- 135.Tao Y. et al. , Projections from Infralimbic Cortex to Paraventricular Thalamus Mediate Fear Extinction Retrieval. Neurosci Bull 37, 229–241 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zhu Y. et al. , Dynamic salience processing in paraventricular thalamus gates associative learning. Science 362, 423–429 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ma J. et al. , Divergent projections of the paraventricular nucleus of the thalamus mediate the selection of passive and active defensive behaviors. Nat Neurosci 24, 1429–1440 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zhu YB et al. , PBN-PVT projections modulate negative affective states in mice. Elife 11, (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zhu Y, Wienecke CF, Nachtrab G, Chen X, A thalamic input to the nucleus accumbens mediates opiate dependence. Nature 530, 219–222 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Beas BS et al. , The locus coeruleus drives disinhibition in the midline thalamus via a dopaminergic mechanism. Nat Neurosci 21, 963–973 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Gao C. et al. , Two genetically, anatomically and functionally distinct cell types segregate across anteroposterior axis of paraventricular thalamus. Nat Neurosci 23, 217–228 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Bhatnagar S. et al., A cholecystokinin-mediated pathway to the paraventricular thalamus is recruited in chronically stressed rats and regulates hypothalamic-pituitary-adrenal function. J Neurosci 20, 5564–5573 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Bhatnagar S, Huber R, Nowak N, Trotter P, Lesions of the posterior paraventricular thalamus block habituation of hypothalamic-pituitary-adrenal responses to repeated restraint. J Neuroendocrinol 14, 403–410 (2002). [DOI] [PubMed] [Google Scholar]
- 144.Wolff M, Morceau S, Folkard R, Martin-Cortecero J, Groh A, A thalamic bridge from sensory perception to cognition. Neurosci Biobehav Rev 120, 222–235 (2021). [DOI] [PubMed] [Google Scholar]
- 145.Halassa MM, Acsady L, Thalamic Inhibition: Diverse Sources, Diverse Scales. Trends Neurosci 39, 680–693 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Grundemann J, Distributed coding in auditory thalamus and basolateral amygdala upon associative fear learning. Curr Opin Neurobiol 67, 183–189 (2021). [DOI] [PubMed] [Google Scholar]
- 147.Yoshii T, The Role of the Thalamus in Post-Traumatic Stress Disorder. Int J Mol Sci 22, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wolff SB et al. , Amygdala interneuron subtypes control fear learning through disinhibition. Nature 509, 453–458 (2014). [DOI] [PubMed] [Google Scholar]
- 149.Krabbe S. et al. , Adaptive disinhibitory gating by VIP interneurons permits associative learning. Nat Neurosci 22, 1834–1843 (2019). [DOI] [PubMed] [Google Scholar]
- 150.Stujenske JM, Likhtik E, Topiwala MA, Gordon JA, Fear and safety engage competing patterns of theta-gamma coupling in the basolateral amygdala. Neuron 83, 919–933 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Courtin J, Karalis N, Gonzalez-Campo C, Wurtz H, Herry C, Persistence of amygdala gamma oscillations during extinction learning predicts spontaneous fear recovery. Neurobiol Learn Mem 113, 82–89 (2014). [DOI] [PubMed] [Google Scholar]
- 152.Ibrahim BA et al. , Corticothalamic gating of population auditory thalamocortical transmission in mouse. Elife 10, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Lee JH et al. , The rostroventral part of the thalamic reticular nucleus modulates fear extinction. Nat Commun 10, 4637 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Chou XL et al. , Inhibitory gain modulation of defense behaviors by zona incerta. Nat Commun 9, 1151 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zhou M. et al. , A central amygdala to zona incerta projection is required for acquisition and remote recall of conditioned fear memory. Nat Neurosci 21, 1515–1519 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Dopfel D. et al. , Individual variability in behavior and functional networks predicts vulnerability using an animal model of PTSD. Nat Commun 10, 2372 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lkhagvasuren B. et al. , Distribution of Fos-immunoreactive cells in rat forebrain and midbrain following social defeat stress and diazepam treatment. Neuroscience 272, 3457 (2014). [DOI] [PubMed] [Google Scholar]
- 158.Otake K, Kin K, Nakamura Y, Fos expression in afferents to the rat midline thalamus following immobilization stress. Neurosci Res 43, 269–282 (2002). [DOI] [PubMed] [Google Scholar]
- 159.Porro CA et al. , Independent time courses of supraspinal nociceptive activity and spinally mediated behavior during tonic pain. Pain 104, 291–301 (2003). [DOI] [PubMed] [Google Scholar]
- 160.Ueyama T. et al. , Estrogen alters c-Fos response to immobilization stress in the brain of ovariectomized rats. Brain Res 1084, 67–79 (2006). [DOI] [PubMed] [Google Scholar]
- 161.Zhao ZD et al., Zona incerta GABAergic neurons integrate prey-related sensory signals and induce an appetitive drive to promote hunting. Nat Neurosci 22, 921–932 (2019). [DOI] [PubMed] [Google Scholar]
- 162.Burrows AM et al. , Limbic and motor function comparison of deep brain stimulation of the zona incerta and subthalamic nucleus. Neurosurgery 70, 125–130; discussion 130121 (2012). [DOI] [PubMed] [Google Scholar]
- 163.Legg CR, Visual discrimination impairments after lesions in zona incerta or lateral terminal nucleus of accessory optic tract. Brain Res 177, 461–478 (1979). [DOI] [PubMed] [Google Scholar]
- 164.Thompson R, Bachman MK, Zona Incerta - Link between the Visual Cortical Sensory System and the Brain-Stem Motor System. Physiol Psychol 7, 251–253 (1979). [Google Scholar]
- 165.Venkataraman A. et al. , Incerto-thalamic modulation of fear via GABA and dopamine. Neuropsychopharmacology 46, 1658–1668 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Bartho P, Freund TF, Acsady L, Selective GABAergic innervation of thalamic nuclei from zona incerta. Eur J Neurosci 16, 999–1014 (2002). [DOI] [PubMed] [Google Scholar]
- 167.Trageser JC et al. , State-dependent gating of sensory inputs by zona incerta. J Neurophysiol 96, 1456–1463 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Trageser JC, Keller A, Reducing the uncertainty: gating of peripheral inputs by zona incerta. J Neurosci 24, 8911–8915 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Liu K. et al. , Lhx6-positive GABA-releasing neurons of the zona incerta promote sleep. Nature 548, 582–587 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Zhang X, van den Pol AN, Rapid binge-like eating and body weight gain driven by zona incerta GABA neuron activation. Science 356, 853–859 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Zhou H, Xiang W, Huang M, Inactivation of Zona Incerta Blocks Social Conditioned Place Aversion and Modulates Post-traumatic Stress Disorder-Like Behaviors in Mice. Front Behav Neurosci 15, 743484 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ramanathan KR, Ressler RL, Jin J, Maren S, Nucleus Reuniens Is Required for Encoding and Retrieving Precise, Hippocampal-Dependent Contextual Fear Memories in Rats. J Neurosci 38, 9925–9933 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Troyner F, Bicca MA, Bertoglio LJ, Nucleus reuniens of the thalamus controls fear memory intensity, specificity and long-term maintenance during consolidation. Hippocampus 28, 602–616 (2018). [DOI] [PubMed] [Google Scholar]
- 174.Xu W, Sudhof TC, A neural circuit for memory specificity and generalization. Science 339, 1290–1295 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Ramanathan KR, Jin J, Giustino TF, Payne MR, Maren S, Prefrontal projections to the thalamic nucleus reuniens mediate fear extinction. Nat Commun 9, 4527 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Silva BA, Burns AM, Graff J, A cFos activation map of remote fear memory attenuation. Psychopharmacology (Berl) 236, 369–381 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Ramanathan KR, Maren S, Nucleus reuniens mediates the extinction of contextual fear conditioning. Behav Brain Res 374, 112114 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Vertes RP, Hoover WB, Szigeti-Buck K, Leranth C, Nucleus reuniens of the midline thalamus: link between the medial prefrontal cortex and the hippocampus. Brain Res Bull 71, 601–609 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Hoover WB, Vertes RP, Collateral projections from nucleus reuniens of thalamus to hippocampus and medial prefrontal cortex in the rat: a single and double retrograde fluorescent labeling study. Brain Struct Funct 217, 191–209 (2012). [DOI] [PubMed] [Google Scholar]
- 180.Varela C, Kumar S, Yang JY, Wilson MA, Anatomical substrates for direct interactions between hippocampus, medial prefrontal cortex, and the thalamic nucleus reuniens. Brain Struct Funct 219, 911–929 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Dolleman-Van der Weel MJ, Witter MP, Nucleus reuniens thalami innervates gamma aminobutyric acid positive cells in hippocampal field CA1 of the rat. Neurosci Lett 278, 145–148 (2000). [DOI] [PubMed] [Google Scholar]
- 182.Goswamee P, Leggett E, McQuiston AR, Nucleus Reuniens Afferents in Hippocampus Modulate CA1 Network Function via Monosynaptic Excitation and Polysynaptic Inhibition. Front Cell Neurosci 15, 660897 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Silva BA et al. , A thalamo-amygdalar circuit underlying the extinction of remote fear memories. Nat Neurosci 24, 964–974 (2021). [DOI] [PubMed] [Google Scholar]
- 184.Vertes RP, Analysis of projections from the medial prefrontal cortex to the thalamus in the rat, with emphasis on nucleus reuniens. J Comp Neurol 442, 163–187 (2002). [DOI] [PubMed] [Google Scholar]
- 185.Aggleton JP, Mishkin M, Projections of the amygdala to the thalamus in the cynomolgus monkey. J Comp Neurol 222, 56–68 (1984). [DOI] [PubMed] [Google Scholar]
- 186.Timbie C, Garcia-Cabezas MA, Zikopoulos B, Barbas H, Organization of primate amygdalar-thalamic pathways for emotions. PLoS Biol 18, e3000639 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Baek J. et al. , Neural circuits underlying a psychotherapeutic regimen for fear disorders. Nature 566, 339–343 (2019). [DOI] [PubMed] [Google Scholar]
- 188.Buchanan SL, Mediodorsal thalamic lesions impair differential Pavlovian heart rate conditioning. Exp Brain Res 73, 320–328 (1988). [DOI] [PubMed] [Google Scholar]
- 189.Powell DA, Churchwell J, Mediodorsal thalamic lesions impair trace eyeblink conditioning in the rabbit. Learn Mem 9, 10–17 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Buchanan SL, Thompson RH, Mediodorsal thalamic lesions and Pavlovian conditioning of heart rate and eyeblink responses in the rabbit. Behav Neurosci 104, 912–918 (1990). [DOI] [PubMed] [Google Scholar]
- 191.Lee S. et al. , Bidirectional modulation of fear extinction by mediodorsal thalamic firing in mice. Nat Neurosci 15, 308–314 (2011). [DOI] [PubMed] [Google Scholar]
- 192.Herry C, Vouimba RM, Garcia R, Plasticity in the mediodorsal thalamo-prefrontal cortical transmission in behaving mice. J Neurophysiol 82, 2827–2832 (1999). [DOI] [PubMed] [Google Scholar]
- 193.Herry C, Garcia R, Prefrontal cortex long-term potentiation, but not long-term depression, is associated with the maintenance of extinction of learned fear in mice. J Neurosci 22, 577–583 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]