Abstract
The tumor microenvironment consists of diverse, complex etiological factors. The matrix component of pancreatic ductal adenocarcinoma (PDAC) plays an important role not only in physical properties such as tissue rigidity but also in cancer progression and therapeutic responsiveness. Although significant efforts have been made to model desmoplastic PDAC, existing models could not fully recapitulate the etiology to mimic and understand the progression of PDAC. Here, two major components in desmoplastic pancreatic matrices, hyaluronic acid- and gelatin-based hydrogels, are engineered to provide matrices for tumor spheroids composed of PDAC and cancer-associated fibroblasts (CAF). Shape analysis profiles reveals that incorporating CAF contributes to a more compact tissue formation. Higher expression levels of markers associated with proliferation, epithelial to mesenchymal transition, mechanotransduction, and progression are observed for cancer-CAF spheroids cultured in hyper desmoplastic matrix-mimicking hydrogels, while the trend can be observed when those are cultured in desmoplastic matrix-mimicking hydrogels with the presence of transforming growth factor-β1 (TGF-β1). The proposed multicellular pancreatic tumor model, in combination with proper mechanical properties and TGF-β1 supplement, makes strides in developing advanced pancreatic models for resembling and monitoring the progression of pancreatic tumors, which could be potentially applicable for realizing personalized medicine and drug testing applications.
Keywords: Pancreatic cancer, Desmoplasia, Fibrosis, Extracellular matrix, Tumor microenvironment
Graphical abstract
In this study, two major components in desmoplastic pancreatic matrices, hyaluronic acid- and gelatin-based hydrogels, are engineered to provide matrices for tumor spheroids composed of pancreatic cancer and cancer-associated fibroblasts (CAF). By using this multicellular microtumor model, mechanical factors (rigidity) and biological factors (hybrid matrices, CAF, TGF-beta stimulation) affecting the tumor microenvironment are evaluated to analyze the tumoral changes.
Highlights
-
•
The matrix of pancreatic ductal carcinoma plays important roles in cancer progression and therapy response.
-
•
Cancer associated fibroblasts, mechanical properties, and TGF-β signaling are required for building pancreatic tumor models.
-
•
Cancer spheroids cultured in desmoplasia mimicking HAMA/GelMA gels become more progressive phenotypes with TGF-β signaling.
1. Introduction
Pancreatic ductal adenocarcinoma (PDAC) is a highly aggressive, lethal tumor with a 5-year survival rate of less than 10% [1]. Although various multimodal treatments (surgery, adjuvant therapy, radiotherapy, and immunotherapy) have been proposed to treat pancreatic cancer, the mortality rate is still high. In terms of responsiveness to anticancer therapy, PDAC has unique biological properties such as the development of anticancer drug resistance, decreased accessibility/efficacy of therapeutic agents due to dense extracellular matrix (ECM) deposition, and high metastasis rate [2,3]. For decades, various studies have been conducted using in vitro models to explore the mechanism of tumor development and to evaluate the efficacy of newly developed therapeutic strategies [4]. However, since PDAC progression includes complex spatiotemporal interactions such as cell-microenvironment and cell-cell interactions, there are many limitations to analyzing these interactions using simple two-dimensional (2D) in vitro models [5]. The introduction of 3D in vitro tumor models (3D cell spheroids, cell encapsulation) has made it possible to replicate the biophysics of tumors and their surrounding microenvironment more accurately [6]. However, as advances in tumor biology deepen our understanding of tumor complexity, several considerations remain for developing personalized and precise biomimetic PDAC modeling [7].
Among the factors that can influence tumor progression, the abundant “desmoplastic fibrous stroma” in PDAC is recognized as the main contributor to the inefficacy of anticancer therapies [8]. Desmoplasia comprises various ECM components such as hyaluronic acid (HA), collagen, laminin, and fibronectin, as well as cellular components (stroma) such as cancer-associated fibroblasts (CAF) and immune cells (tumor-associated macrophages, neutrophils, regulatory T cells). Remarkably, 80% of the total volume of PDAC comes from the stromal ECM, which is a mixture of various ECM components rather than a single component, which affects tumor cell survival, epithelial-mesenchymal transition (EMT), and malignancy [9,10]. EMT is a process by which cancerous cells suppress their epithelial features and transition to mesenchymal ones, which helps them to acquire mobility and metastasize. Collagens are the most abundant component of the ECM in PDAC, making up over 90% of ECM protein signals [11]. Specifically, collagen I is responsible for a majority of the desmoplastic reaction that can lead to pro-tumorigenic effects associated with lower rates of patient survival [12]. Although gelatin cannot be regarded as a faithful representative of collagen biochemistry, it has been used in literature as a tunable hydrogel to model the ECM and encapsulate a range of cells. Due to the fact gelatin can be modified and methacrylated, gelatin methacryloyl (GelMA) was employed over collagen in this study in order to generate photo-crosslinkable HA methacryloyly (HAMA)-GelMA gels as well as tune their mechanical properties to see the effects on stiffness on cellular processes while still acting as an ECM mimic material as reported. Gelatin and GelMA retain the arginine-glycine-aspartic acid (RGD) and MMP peptide sequences that favors cell behaviors while possessing good biocompatibility. Therefore, it is used in this study as a representative and as a tuneable matrix material. 1% HAMA was chosen based on the fact that HAMA has a rapid crosslinking time, by increasing the HAMA concentration the hydrogels crosslink too fast and give mechanical properties that do not match the desired stiffnesses. The values chosen in the study were based on literature results of the in vivo values as well as the reported phenomena that occur in vivo in regard to biological processes. In addition to collagen I, HA is another large component of the desmoplastic ECM of PDAC that leads to the activation of various intracellular signaling pathways, thus promoting both cell survival and invasion [13,14]. A tumor model fabricated with hybrid ECM involving HA was shown to exhibit low chemotherapeutic agent sensitivity similar to in vivo and had an effect on the promotion of tumor cell migration [15,16]. These results indicate the need for a 3D cell encapsulation technique based on tumor tissue-specific hybrid ECM rather than using a single ECM to model biomimetic tumor development in vitro.
In addition to the ECM constituting desmoplasia of PDAC, stromal cells, such as pancreatic astrocytes (PSCs) and CAF, play an important role in maintaining the desmoplastic tumor microenvironment (TME). CAFs and activated PSCs found near ductal tumor masses are responsible for the excessive accumulation of ECM that can embed PDAC malignant cells within a densely packed fibrotic barrier [[8], [9], [10]]. In malignancy, CAF subtypes contribute to cancer metastasis by promoting tumor cell proliferation and/or activating ECM remodeling causing a stiffer ECM that induces tumor cell migration [17,18]. Specifically, CAFs can be activated by PDAC cells to express type I collagen, and subsequently activate focal adhesion kinase (FAK) in cancer stem cells to induce cancer progression [19]. Therefore, to investigate the factors regulating PDAC progression, it is essential to consider both 1) the effect of stromal composition and 2) the interactions between cancer cells and adjacent stromal cells, including CAFs.
In PDAC tumor models, biological/mechanical modulation and analysis at the “tissue level” rather than the simple cell level will be meaningful in predicting and analyzing tumor behavior. Analysis of tumor phenotypes following changes in the mechanical properties of micro-tumor models or stimulation of specific signaling pathways will help infer mechanisms of cancer proliferation and progression. Previous studies have shown that culturing pancreatic cancer cell lines on substrates with different stiffness can induce changes in tumor EMT levels, including changes in vimentin (VIM) and E-CAD expression, as well as induce chemical resistance [20]. Moreover, the stromal stiffness of pancreatic tissue differs in health, tumor metastasis, and desmoplastic stages, indicating a progressively increasing stromal mechanical strength as the tumor develops [[20], [21], [22], [23]]. The finding that the stiffness of these tissues differs with the progression of PDAC may have the potential to correlate with a gradual decline in the therapeutic efficacy of PDAC. Although there have been analyses at the single cell level, and according to the stiffness of the matrix in vivo, changes at the multicellular microtissue level based on the PDAC-specific ECM composition have not yet been fully elucidated. Taken together, multicellular spheroids within hybrid ECM hydrogels with varying mechanical properties that promote cell interactions may be essential for analyzing the ultimate PDAC phenotype through different mechanical environments (healthy for degenerating tumors) and biological stimulation (TGF-β1).
In this study, we hypothesized that establishing a 3D in vitro cancer model containing multicellular spheroids within a hybrid ECM hydrogel could be important for analyzing PDAC phenotypic changes during tumor progression. The ECM hydrogels we propose here reflects the different conditions of the pancreas (healthy/precancerous, cancerous/desmoplastic and hyperdesmoplastic) from a stiffness perspective. We hypothesize that changing the ECM stiffness effect the PDAC cancer progression. To this end, we first prepared multicellular spheroids composed of cancer cells and CAFs, and then encapsulated them with hybrid ECM containing HAMA/GelMA with tunable mechanical properties. Using the combination of the crosslinkable ECM hydrogels GelMA and HAMA, we were able to represent the major ECM composition of PDAC, as well as reproduce the increase in PDAC stiffness by tuning the degree of crosslinking. Using this 3D organotypic tumor model platform, we were able to explore how the mechanical properties, cell-cell interaction, and TGF-β1 induction synergistically regulate pancreatic cancer cell states and matrix fibrosis. Immunofluorescence staining and quantitative real-time polymerase chain reaction (qRT-PCR) were used to evaluate the expression of key proteins and genes associated with pancreatic cancer EMT, metastasis, and matrix fibrosis in the presence of TGF-β1 signaling activation. We expect that our understanding of the mechanical and biological stimuli for modulating matrix stiffness of elaborately fabricated 3D multicellular PDACs will shed light on biomedical applications for tumor modeling and therapeutic developments.
2. Experimental section
2.1. Methacryloyl modification of HA and gelatin
Methacrylolyl modification of HA was performed according to the literature [24,25]. Sodium hyaluronate (750 kDa - 1 MDa, LifeCore) was purchased in dry powder form and dissolved in distilled water to obtain 1% (w/v) HA solution. Dimethylformamide (DMF) was added (DMF: water 1:1) to the HA solution. This was performed to decrease the viscosity and increase the miscibility of methacrylic anhydride (MAA). The solution was stirred and cooled to +4 °C in an ice bath. MAA was added dropwise while stirring the HA solution in the ice bath. MAA was added 3x molar excess to HA to obtain methacrylolyl modification of the hydroxyl residues of the N-Acetylglucosamine (GlcNAc) repeating unit of the polymer backbone. The pH of the reaction medium was maintained at pH = 9 by constant monitoring and the addition of 1 M NaOH. The reaction was continued for 6 h to stabilize the pH. The reaction solution was kept on ice overnight, and the ingredients were transferred to dialysis tubing the following day. Samples were dialyzed against distilled water for a week. After dialysis samples were centrifuged, the supernatant was freeze-dried to obtain HAMA. HAMA was kept at −20 °C until intended use.
Methacrylolyl modification of the gelatin was performed according to our group's previous protocol [26]. Type A porcine gelatin (300 bloom, Sigma G1890) was used for the experiments. Gelatin was dissolved at 50 °C in Dulbecco's phosphate-buffered saline (DPBS; GIBCO) to obtain 10% (w/v) gelatin in DPBS. MAA was added to the gelatin solution in a controlled manner using a syringe pump with a dispense rate of 0.5 mL/min to obtain 2x molar excess. After adding the MAA, the reaction was continued at 50 °C under constant stirring for 1 h and then stopped by adding the same volume of DPBS and making pH = 7.4. After finalizing the methacrylation reaction, samples were centrifuged, and the supernatants were dialyzed against distilled water. After dialysis, the aqueous reaction solution was freeze-dried to obtain GelMA. Samples were kept at 4 °C until intended use.
2.2. Characterization of HAMA using proton NMR
The NMR characterization was performed by 16 scans at 400 MHz. The incorporation of methacrylate groups into HA and the degree of methacrylation were analyzed from the obtained NMR spectra. Deuterium oxide (D2O, 20 mg/mL) was used to dissolve the HA and HAMA samples. The relative integration of the methacrylate protons (5.6 and 6.0 ppm) to the methyl protons in HA (1.8 ppm) was used to compute the methacrylation degree of the polymer (DoM) [27].
2.3. Preparation of bicomponent HAMA/GelMA network hydrogels
A solution was prepared to contain 1% (w/v) photoinitiator (Irgacure, 2-Hydroxy-4′-(2-hydroxyethoxy)-2-methylpropiophenone), 1% HAMA, and 10% GelMA in DPBS or cell growth media. Initially, the photoinitiator was dissolved at 50 °C under constant agitation. Afterward, HAMA was added to the solution and was dissolved at 4 °C overnight. Finally, GelMA was added to the solution and was dissolved at 50 °C under constant agitation to obtain the prepolymer solution for the HAMA/GelMA bicomponent network hydrogel. HAMA/GelMA solution was UV crosslinked using a 365 nm point light source (Omnicure 2000) with 1 W/cm2 power in PDMS molds. Gels with a spectrum of Young's moduli were obtained by irradiating the samples 1–20 s. Samples were further characterized for mechanical and rheological properties.
Biocompatibility of the HAMA/GelMA was tested using human dermal fibroblasts, as a CAF model, using PrestoBlue assay described in the cell culture section.
2.4. Mechanical characterization of HAMA/GelMA, HAMA, and GelMA hydrogels
For the assessment of bulk compression modulus of the HAMA/GelMA/HAMA/GelMA hydrogels, disk-shaped samples (10 mm diameter x 1.5 mm thickness) crosslinked at 1–20 s were compressed at a rate of 1 mm min−1 using a mechanical tester (Instron 5943, Instron Int. Ltd., MA, USA) using the Bluehill version 3 software at 25 °C. The linear (elastic deformation) region of the stress-strain curves were employed to calculate compressive modulus of the hydrogels.
2.5. Rheological characterization of HAMA/GelMA, HAMA, and GelMA hydrogels
The rheological properties of HAMA, GelMA, and HAMA/GelMA hydrogels were assessed by a Rheometer (Anton Paar, MCR 302). Shear stress, temperature sweep, viscosity, and storage moduli were measured with a 25 mm sandblasted parallel-plate geometry. Frequency sweeps were conducted at 0.1–10 Hz under a fixed oscillatory strain of 1% at 25 °C. Temperature sweeps were conducted at 1 Hz and a fixed strain of 1% from 10 to 40 °C. Viscosity experiments were conducted at 25 °C with a shear rate of 0.001–10 1/s. Oscillatory step strain experiments were conducted at 1 Hz at 25 °C with cycles 1, 3, and 5 at a strain of 1% and cycles 2 and 4 at 200% strain.
For the in situ UV crosslinking kinetic assessment, the UV light source Omnicure 1000 (Lumen Dynamics LDGI) was routed through a 5 mm optical fiber to irradiate the bottom of the samples across a glass substrate. The UV intensity was explored at 0.5, 1, and 2 W/cm2, and 10 s after the data collection started, the UV light source was initiated. The storage and loss modulus were recorded by Anton Paar Rheocompass software.
2.6. Characterization of the hydrogels using scanning electron microscope
Samples were freeze dried and then attached to the scanning electron microscope (SEM) stubs and coated with gold for 30 s under vacuum. Samples were imaged using Zeiss Supra V 40VP SEM under high vacuum. Images were analyzed using Ima geJ for pore size measurements.
2.7. Cell culture
Pancreatic cancer cell line (ASPC-1) and CAF cells (ATCC #CRL-1682, ATCC #PCS-201-012) were obtained from the American Type Culture Collection (ATCC). ASPC-1 cells were chosen based on their metastatic traits and to model PDAC allowing us to observe the effects on the EMT processes [28]. Cells were cultured in RPMI, and DMEM High glucose media, respectively. All cell culture media were prepared by fetal bovine serum (FBS) with a final concentration of 10% (v/v) and Penicillin-Streptomycin (50 mg/mL streptomycin in 0.9% NaCl and 50,000 U/mL penicillin in 0.9% NaCl) with a final concentration of 1% (v/v). Cells were thawed in a 37 °C water bath and added to a tube with 9 ml of warm media. The suspension was centrifuged at 1000 rpm for 5 min, and the pellet was resuspended in fresh media and transferred to a clean cell culture flask. Cells were cultured until reaching 80% confluency and removed from the flask with the help of Trypsin (Trypsin-EDTA (0.05%), phenol red), and the trypsin activity was stopped by adding fresh culture media containing FBS. The cells were centrifuged, resuspended, and then counted using Countess II Automated Cell Counter for downstream experiments.
2.8. Preparation of cancer spheroids
Aggrewell400 plates were used to obtain cancer spheroids. ASPC-1 was used for the experiments, and CAFs were also incorporated in the spheroid structure to mimic the interaction between cancer and CAFs. Spheroids were prepared according to the manufacturer's protocol. Briefly, the Aggrewell400 plate was incubated with Anti-Adherence Solution (STEMCELL Technologies) to obtain an ultra-low-adhesion (ULA) surface. After incubation, the wells were washed with distilled water twice and air-dried in a laminar flow hood. According to the protocol, to obtain 200 cells/microwell, 2.4 × 105 cells consisting of cancer cells and CAFs in a 10:1 ratio were added to each well of the 24 well plates (1200 microwells/well). The cells were cultured for 3 days to form spheroids, and the generated spheroids were harvested afterward for multicellular tumor spheroids.
2.9. Characterization of cancer spheroids
Cancer spheroid viability was characterized using Live-Dead assay and PrestoBlue assay during the 10-day culture on the Aggrewell400 platform. Live dead staining was performed according to the manufacturer's protocol (Invitrogen, L3224). Briefly, calcein AM dye was added to the culture media for a final concentration of 2 μM and Ethidium Homodimer for 4 μM. Samples were incubated for 15 min and imaged using a Zeiss Axiovert microscope equipped for fluorescence imaging. PrestoBlue assay was performed according to the manufacturer's protocol (Invitrogen A13262). Briefly, PrestoBlue reagent was added to the growth media at 10% (v/v) concentration and was incubated with the spheroids for 2 h at 37 °C. At the end of the incubation, 200 μl of the reacted PrestoBlue solution was transferred to a black bottom 96 well plate for fluorescence analysis. The samples were excited at 540 nm, and the emission was read at 610 nm. Samples were prepared at quadruplicates, and the average fluorescence intensity (FI) was calculated for each sample. The blank with no spheroids was subtracted from each sample to obtain the final FI values. Results were plotted using GraphPad Prism 9.2.0.
The fluorescence images of the spheroids were used to characterize size and shape parameters. Calcein AM channel was used for the analysis. Images were converted to grayscale, applied threshold, and binarized using ImageJ Fiji. Each spheroids outline was captured using a magic wand tool and was measured using the Measure tab. Area, perimeter, circularity, and solidity parameters were used to compare spheroids formed at different conditions.
2.10. Preparation of multicellular tumor spheroids
The spheroids were collected from the Aggrewell400 plates, transferred to a 15 ml conical tube, and centrifuged. 100 μl of HAMA/GelMA was crosslinked at the bottom of 24 well cell inserts with 3 μm pore size. The spheroids were pipetted in 15 μl volume onto the hydrogel as a base on top of the transwell mesh, and another layer of 100 μL of HAMA/GelMA was added, mixed with the spheroids, and crosslinked. Multicellular tumor spheroids on the transwell inserts were placed in the well plate, and 2 ml of media was added. The samples were cultured for up to 14 days, and media was changed every 3 days.
2.11. Characterization of multicellular tumor spheroids
Multicellular tumor spheroids were characterized using PrestoBlue, Calcein staining, and cell tracker. PrestoBlue and Calcein staining were performed according to the protocol described above. For cell tracker experiments, two trackers were chosen: tracker green CMFDA and tracker orange CMRA (Invitrogen) for cancer and CAFs, respectively. Cells were harvested from well plates and then counted. Dyes were prepared in 10 μM concentration in growth media without the supplement of serum, and cells were incubated for 1 h. Cells were then transferred to fresh media and incubated further for 30 min before transferring into Aggrewell400 plates. Spheroids were formed after cell tracker staining. Cells labeled with cell trackers formed spheroids, and multicellular tumor spheroids were imaged at days 1, 3, and 7 for cancer and CAFs interaction using Zeiss LSM 770 inverted confocal microscope.
2.12. Immunohistochemistry
Multicellular tumor spheroids were fixed at days 3 and 7 using 4% paraformaldehyde (PFA) (v/v) in PBS. Samples were permeabilized using 1% Triton X-100 solution in PBS (v/v). Samples were then blocked using 1% bovine serum albumin (BSA) solution in PBS (w/v). E-cadherin (ab1416, Abcam), Vimentin (ab45936, Abcam), α-smooth muscle actin (α-SMA) (ab5694, Abcam), and YAP (SC-271134, Santa Cruz) was used as primary antibodies. Samples were incubated with primary antibodies overnight at 4 °C. Alexa Fluor goat anti-mouse 488 and goat anti-rabbit 554 secondary antibodies were added to the samples and incubated for 3 h at 37 °C. Samples were stained for DAPI and imaged using Zeiss LSM 770 inverted confocal microscope.
Alternatively, samples were stained with Alexa Fluor 488 Phalloidin and DAPI to show the F-actin cytoskeleton.
2.13. TGF-β treatment
TGF-β treatment was performed for fibroblasts in 2D and 3D constructs. In the literature 2.5–10 ng TGF-β was used for stimulating the fibroblasts to induce fibrosis and CAF phenotype [29]. We have chosen 10 ng/ml TGF-β according to Bordignon et al. [29]. Initially, 10,000 cells were seeded onto 24 well plates and cultured for 24 h for cell adhesion. Afterward, 10 ng/ml TGF-β was added to the cells (one group was not treated for negative control). The samples were stained with calcein and ethidium homodimer for viability analysis (n = 3). Samples were also fixed and stained for α-SMA for fibrotic phenotype (n = 3). α-SMA intensity was characterized for 3 days (Image J Fiji). Secondly, fibroblast spheroids were formed in Aggrewell400 plates and treated with 10 ng/ml TGF-β for 3 days. Spheroids were imaged for viability and α-SMA staining. Finally, cancer + CAF spheroids were prepared and treated with 10 ng/ml TGF-β with and without hydrogels. Spheroids without hydrogels were characterized for viability and E-CAD, VIM, and α-SMA staining. Spheroids with hydrogels were stained additionally for YAP for focal adhesion and mechanotransduction response andqRT-PCR were performed to the samples.
2.14. qRT-PCR analysis
In vitro culture of multicellular tumor spheroids in HAMA/GelMA hydrogels were continued for days 3 and 14. The samples were then removed from cell culture and washed with PBS before RNA extraction. A RNeasy plus mini kit was used to extract total RNA (Qiagen, CA, USA). Total RNA was reverse-transcribed into cDNA using the QuantiTect Reverse Transcription Kit. qRT-PCR was employed to measure the expression of the target genes. QuantStudio 3 software (Thermo Fisher Scientific) was used to calculate the Ct values from the melt curves. The reaction conditions were 5 min initial denaturation at 95 °C; 5 s recurring denaturation at 95 °C; and 10s amplification at 60 °C up to 45 cycles. The 2−ΔΔCt method was used. Shortly, the difference between the Ct values of the target gene and the house keeping gene (HKG) were calculated (ΔCt), followed by the calculation of ΔΔCt values by subtraction the ΔCt of control from the sample to normalize the relative expression levels of all mRNAs. Two HKG were selected: with glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and β-actin. Table S1 shows the primer sequences for the targeted genes.
2.15. Statistical analysis
Statistical analysis of the results were performed using GraphPad Prism 9 software. Image analysis was performed by a normality test followed by a parametric or non-parametric analysis of variance (ANOVA) test. Post-hoc analyses were performed using Tukey's test. For multiparametric analysis multiple parameter ANOVA analysis was performed.
3. Results and discussion
3.1. Pancreatic cancer and CAF spheroids to mimic 3D tumors in vitro
In this study, we constructed a "3D PDAC model" and analyzed the biological response of multicellular (PDAC/CAF) components depending on the stiffness conditions within the hybrid ECM (Gelatin/HA) composition in the development and progression of PDAC, as shown in Fig. 1A. As the first step of this study, spheroids composed of cancer cells and CAFs were prepared, and the incubation time was optimized through size and shape analysis. As shown in Fig. 1B(ii), multicellular spheroids were produced on a non-adhesive microwell platform (Aggrewell400). Through F-actin and nuclear staining, it was confirmed that multicellular spheroids were successfully fabricated (Fig. 1B(iii)). To analyze the cell distribution within spheroids, cancer cells (green) and CAFs (orange) were labeled with cell trackers (Fig. 1B(i)). In 2D culture, the fluorescence signals of cancer cells and CAFs overlap in a discrete pattern, but in 3D spheroids, the CAFs are located around the spheroids. Next, the expression level of E-CAD (epithelial cell marker)/VIM (mesenchymal cell marker) was analyzed to determine the optimal ratio of cancer cells to CAF (Fig. S1). As a result, cell proliferation and marker expressions were low at a high ratio of more than 50% of CAF. Interestingly, the cancer cell:CAF ratio of 9:1 showed high expression of both E-CAD and VIM. We set this ratio as a condition to maintain both the initial phenotype of cancer cells and CAF (which may be affected by TME stress).
Fig. 1.
Schematic of pancreatic cancer development and cancer spheroid production. A- PDAC development with cancer microenvironment alterations. B- Cancer only and Cancer + CAFs were formed into spheroids in Aggrewell400 microplates (cancer: green, CAF: orange; F-actin: Alexa Fluor 488-Phalloidin, Nucleus: DAPI). C- Spheroids were stained for live/dead dye and imaged for days 1, 3, and 7 (Green: Calcein-live, Red: Ethidium homodimer-dead, scale bar: 500 μm, insert scale bar: 100 μm). D- Size analysis of the spheroids from micrographs for days 1, 2, 3, 4, and 7 (Two-Way ANOVA, *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, and ****p ≤ 0.0001). E− Shape analysis of the spheroids from micrographs for days 1, 2, 3, 4, and 7 (*p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, and ****p ≤ 0.0001).
We acknowledge that the 9:1 ratio condition, which is a cancer cell: CAF ratio based on the preliminary data from which we analyzed cell-to-cell interactions in this study, cannot fully reflect the cell-to-cell ratio mainly observed in PDAC patient tissues. However, the ratio of the cancer cell:CAF observed in actual patients may vary depending on the individual, and accordingly, drug responsiveness may be observed differently. In other words, the phenotype analysis of tumors in the 3D hybrid ECD/multicellular model system, which is the goal of our present study, is not to find one optimal representative cell ratio, but to determine whether different cell ratios can affect progression. Based on the findings and model systems established in the study, it will be possible to further demonstrate whether different ratios between cancer cells and CAFs can influence progression.
Hoffmann et al. (2015) discussed the spheroids compactness in their study and concluded that the compactness varies from cell source to cell source and the addition of the fibroblasts enhances the compactness [30]. They used an equal ratio of fibroblasts to cancer cells. In another study, cancer cell to stromal cell ratios of 10:1 to 1:10 was tested and the higher CAF ratios showed higher Young's moduli [31]. However, when the compactness was compared of two cancer cell lines they have used, one responded to increased stromal cell ratio with increased compactness, on the other hand another cell line showed no change in spheroids compactness. These two studies show that the spheroid compactness is not directly correlated with the ratio of the stromal cells but also the cell type itself. Our results show that the addition of CAFs with a cancer:CAFs ratio of 10:1 still contributed to the spheroid compactness and this ratio is acceptable. Similar to our results, Monteiro et al. prepared an in vitro PDAC multicellular model with a ratio of mouse PDAC cells to human CAF ratio of 8:2 [32]. Another study reported that an increase in the CAF ratio could ultimately affect the susceptibility of tumor models to anticancer agents [33]. Since we aimed to see tissue level changes by environmental stress and fibrosis signaling stimulation, we determined the cell ratio that can maximize the phenotype of each cell component as the spheroid formation ratio. Combining these results, we selected a cancer cell-to-CAF ratio of 9:1 to explore the role of mechanical properties and TGF-β signaling in regulating pancreatic cancer progression associated with stem, EMT, and mechanotransduction.
To analyze the viability over time, multicellular spheroid samples were stained with live/dead staining and observed for 7 days (Fig. 1C; Green – Live cell, Red – Dead cell). Both cancer and Cancer + CAF spheroids maintained high viability throughout the experimental period (7 days). In addition, PrestoBlue viability assay was performed to quantitatively analyze the change in viability over time (Fig. S2). A significant difference in cell viability between the Cancer group and Cancer + CAF was observed from the incubation on Day 3. A higher fluorescence signal (proliferation) was observed in Cancer + CAF than in cancer cells alone, and the difference in viability peaked at Day 7. Moreover, during the incubation of multicellular spheroids for up to Day 7, the size and shape of the spheroids changed over time. To analyze this in more detail, we analyzed the size and shape of the spheroid over time (Fig. 1D and E). We found that tumor spheroids with CAFs have a smaller area and higher circularity compared to those without CAFs for 7 days in culture, indicating that the inclusion of CAFs in tumor spheroids might affect cell-cell interactions. In addition, spheroid perimeter and solidity analysis showed that the Cancer cell + CAF group had a lower perimeter and higher solidity than the cancer cell only group (Fig. S3).
From our results, it is noted that the Cancer + CAF spheroids have higher spheroid compactness (lower spheroid region) and higher circularity compared to the Cancer-only spheroids. Hwang et al., reported that when generating tumor spheroids with different pancreatic cancer cell lines, the circularity may differ between cell lines [34]. The circularity of PDAC cell lines was found to be lowest for MiaPaca-2, intermediate between ASPC-1 and PANC-1, and highest for Capan-1 and BXPC-3. In particular, the mesenchymal phenotype of the cell line was associated with the circularity of the spheroids (high circularity and mesenchymal phenotype). In another study, Monteiro et al. designed single and cocultures of PANC-1 and CAF spheroids [32]. They found that the PANC-1 spheroids exhibited high circularity from the outset, but low compactness and no significant differences over time. On the other hand, PANC-1/CAF-spheroids had a low initial circularity but increased the circularity and compactness of the spheroids over time. Similar to these studies, our results showed that cancer spheroids composed of ASPC-1 exhibited intermediate circularity (range: 0.2–0.6) and the differences in circularity and compactness between groups were significant from Day 3. These results indicate that Cancer + CAF spheroids have more mesenchymal phenotypes with the addition of CAF, and that a certain amount of time is required for each cell to interact. Based on these results, Day 3, the time point when differences in compactness and circularity were observed in Cancer + CAF spheroids, was set as the pre-incubation time before integration into hybrid ECM.
3.2. Preparation and characterization of HAMA/GelMA matrices
The medical and scientific community recognizes that PDAC is characterized by a unique desmoplastic reaction in which an increased deposition of a dense and crosslinked ECM occurs as the disease progresses [35]. This stromal component of PDAC accounts for up to 80% of the tumor mass and is responsible for making PDAC one of the stiffest malignancies, and several magnitudes stiffer than healthy pancreatic tissue [36]. The stiffness of pancreatic tissue may vary depending on the extent of the desmoplastic tumor progression [[20], [21], [22], [23]]. For example, the stroma stiffness of pancreatic tissue can reach about 0.5–1 kPa in healthy cases and about 4–12 kPa in the in desmoplastic PDAC stroma [37,38]. This high stiffness of PDAC matrices is reported to increase the formation of invadopodia in the invading cancer cells and could be, therefore, an important aspect when designing tumor models with better recapitulation ability [39,40]. Considering these points, it is critical to include spheroids within a matrix where one can control the mechanical stability and tunability and where the ECM production can be closely replicated.
To achieve desmoplastic ECM mimicry, we employed two hydrogel components representing the actual tumor matrix; gelatin substituting collagen type I and HA as itself. Both biopolymers were methacrylated to achieve a tunable crosslinking scheme (Fig. 2A). HAMA and GelMA were dissolved in a growth medium to obtain 1% HAMA and 10% GelMA solutions, and then the solutions were irradiated with UV to obtain a HAMA-GelMA bicomponent network hydrogel. HAMA has a fast crosslinking and therefore anything over 1% would become over-crosslinked. The 1%–10% ratio of HAMA to GelMA was chosen based on the abilitity to achieve certain stiffness values. The effect of UV crosslinking intensity on the storage modulus of the HAMA/GelMA hydrogel exhibited that the storage modulus increased with increasing UV intensity (Fig. 2B). Moreover, as the light intensity increased, the less time it took for the storage modulus to plateau; therefore, we chose 1 W/cm2 to crosslink our HAMA/GelMA hydrogels for the rest of the experiments.
Fig. 2.
HAMA and GelMA hydrogel synthesis and characterization. A- Schematic of HAMA, GelMA, and HAMA/GelMA. B- Analysis of HAMA/GelMA storage modulus using different UV power outputs during real-time crosslinking (n = 3). C- Compressive modulus of HAMA/GelMA hydrogels (1% HAMA, 10% GelMA composition) irradiated with 1 W/cm2s for 2, 5 and 10 s (n = 3, One-Way ANOVA, *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, and ****p ≤ 0.0001). D- Rheological analysis (change of storage modulus during frequency sweeps) of uncrosslinked (0 s), 2, 5, and 10 s crosslinked HAMA/GelMA hydrogels (1% HAMA, 10% GelMA composition) irradiated with 1 W/cm2s of UV (n = 3). E− Rheological analysis (temperature sweeps) of uncrosslinked (0 s), 2, 5, and 10 s crosslinked HAMA/GelMA hydrogels (1% HAMA, 10% GelMA composition) irradiated with 1 W/cm2s of UV (n = 3). F- SEM images of HAMA/GelMA crosslinked for 2, 5, and 10s (Scale bar: 100 μm, blue arrows: macropores, orange arrows: micropores). G- Pore size distribution of 2, 5, and 10s HAMA/GelMA hydrogels measured from SEM images after freeze-drying (n ≥ 5, One-Way ANOVA, *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, and ****p ≤ 0.0001).
Based on previously reported values of the mechanical properties of healthy (∼0.5–1 kPa), and stroma of desmoplastic PDAC (greater than 4–12 kPa), we set a crosslinking time for hybrid ECM hydrogels that can provide appropriate modulus values [20,21,[36], [37], [38],41]. Compressive modulus measurements results have shown that crosslinking times of 2 s (1–3 kPa; mean 3 kPa), 5 s (10–15 kPa; mean 15 kPa) and 10 s (30–40 kPa; mean 36 kPa) (Fig. 2C). These results showed that the mechanical strength of "healthy tissues to precancerous transition phase" can be modeled when the crosslinking time is 2 s, and "precancerous transition phase to around stiffness values of desmoplastic PDAC stroma" can be modeled in the case of 5 s. In addition, in order to analyze the biological changes of the multicellular organoid PDAC model under more severe mechanical strength conditions, a more rigid hyperdesmoplastic condition than that of desmoplastic was implemented through a crosslinking time condition of 10 s. In addition, the rheological characterization including frequency sweep (Fig. 2D and S4) and temperature sweep showing the crossover points between the storage and loss modulus (Fig. 2E and S4) was performed on HAMA only, GelMA only, and HAMA/GelMA (1%HAMA/10%GelMA) with different crosslinking time (0, 2, 5, 10s).
As with the compressive modulus analysis, the increase in crosslinking time resulted in an increase of the storage modulus of the 1% HAMA/10% GelMA group (Fig. 2D; 10s > 5s > 2s > 0s). Interestingly, the increase of the hybrid hydrogel storage modulus in the frequency sweep was similar to that of 1% HAMA rather than 10% GelMA at 2s and 5s crosslinking times (Fig. S4). On the other hand, in the case of temperature sweep, the 1% HAMA/10% GelMA group showed a significant decrease in the storage modulus of the uncrosslinked condition and 2s as the temperature increased, whereas the 5s and 10s showed relatively small fluctuations in the temperature increase (Fig. 2E). In particular, when comparing this trend with the individual hydrogel component, the degree of temperature reactivity in the hybrid ECM hydrogel seems to be due to 10% GelMA rather than 1% HAMA (Fig. S4).
In our results, HAMA shows a combination of viscous and elastic behavior in the rheological analysis with an average storage modulus 10 Pa before crosslinking, and 500–1000 Pa after crosslinking for 2–10 s. It also has a very low loss modulus when not crosslinked (<0.01 Pa). Similar to our results, Poldervaart et al. reported that HAMA showed a viscous behavior before photocrosslinking, but more elastic after photocrosslinking [42]. The HAMA solution also shows crossing over of the storage and loss modulus in the amplitude sweeps, demonstrating the shear thinning behavior and the transition from elastic to viscous phase. Also, similar to our results, HAMA did not show significant temperature responsiveness. On the other hand, in our results, uncrosslinked GelMA was temperature responsiveness and showed gel-sol transition around 32 °C. The thermal reactivity of GelMA was not only observed in the hybrid ECM hydrogel, but also preserved to some extent after crosslinking justifying our ratio of HAMA to GelMA. GelMA's rheological properties were well studied in the literature [43,44]. Han et al. demonstrated a similar trend where uncrosslinked GelMA showed gel-sol transition around 32 °C but the crosslinked GelMA showed better temperature stability maintaining their storage modulus around body temperature (37 °C) [44]. In our previous study, we showed that mechanical properties of spheroids-incorporating GelMA-based hydrogels were not significantly different from those without spheroids [45]. For this reason, we believe the presence of spheroids inside hydrogels may not lead to a notable difference of mechanical properties compared to hydrogels without spheroids.
In addition, to analyze the effect of crosslinking time on dried porosity, HAMA/GelMA hydrogels were freeze dried and then imaged through SEM (Fig. 2F). Macropores are defined as pore sizes greater than 50 μm while micropores are less than 2 μm. In all crosslinking time conditions, the freeze-dried hybrid ECM hydrogel had a large number of pores inside. In particular, as a result of pore size analysis, it shows ∼2 times higher macropore size for the hydrogel crosslinked for 10 s compared to the gel crosslinked for 2 and 5 s (Fig. 2G). However, we observed that micropore sizes decreased with increasing the crosslinking time, which is in line with the higher mechanical properties with longer UV crosslinking.
3.3. Characterization of the cancer microtumor models composed of cancer and CAF spheroids embedded into HAMA/GelMA hydrogels with different stiffnesses
In tumor progression, TMEs provide a unique physical and biochemical niche for tumor growth [46]. To implement a TME composed of complex and diverse cellular/ECM components, in this study, we tuned the cellular, ECM and tissue stiffness aspects of the tumor niche and analyzed the concomitant biological response of the microtumor model. To this end, tumor/CAF spheroids were embedded into a tunable HAMA/GelMA hybrid hydrogel, and the degree of crosslinking was adjusted to simulate normal, desmoplastic, and hyperdesmoplastic matrices. Live/dead viability assays and metabolic activity assays were performed to assess viability within three different stiffnesses of HAMA/GelMA hydrogels (3, 15, 36 kPa) over time (Fig. 3A). In both live/dead assay and PrestoBlue metabolic activity assay, it was confirmed that HAMA/GelMA hydrogel under experimental conditions did not adversely affect cell viability. In all conditions, viability was observed above 95% at day 1 and cell proliferation increased over time. Interestingly, the spheroids composed of mixture of cancer cells and CAFs showed no significant difference in viability on day 7, whereas the spheroids composed only of cancer cells showed differences in viability according to the stiffness of the HAMA/GelMA hybrid hydrogel. In particular, the viability increased as the stiffness of the HAMA/GelMA hybrid hydrogel decreased (Fig. 3A, Fig. S6). These results suggest that both the presence of CAF and the stiffness of the embedding gel can influence the proliferation of cellular components within the microtumor model. Bauer et al. demonstrated that CAFs are mechanosensitive, as the substrate stiffness increases up to a critical value (20–40 kPa). In this study, CAFs responded to substrate stiffness by applying more force to the hydrogels as well as secreting cytokines that activate the TGF-β pathway [47]. Calvo et al. also tested CAFs with different substrate stiffness that as the substrate stiffness increased, the number of YAP (a mechanosensitive transcription factor that translocated to the nucleus and activated genes regulating matrix regulation)-positive nuclei increased, demonstrating the mechanosensitive nature of CAF in cancer [48]. This study supports our finding that the group with CAF can adapt to the environment, maintaining relatively constant viability, even with increased stiffness of the TME, such as desmoplastic and hyperdesmoplastic conditions, compared to the group without CAF.
Fig. 3.
Analysis of the cancer microtissue models prepared using Cancer + CAFs spheroids embedded into HAMA/GelMA hydrogels with tuned stiffness. A- Calcein staining of the spheroids in HAMA/GelMA hydrogels and PrestoBlue proliferation analysis of the samples (n ≥ 2, Two-Way ANOVA, *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, and ****p ≤ 0.0001). B– F-actin staining of the micro-cancer tissue models (Red: Alexa fluor 488 Phalloidin, scale bar: 100 μm). C- Spheroid size and shape analysis in HAMA/GelMA 2, 5, 10s for days 3, 5 and 7 of micro-cancer tissue preparation (One-Way ANOVA, *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, and ****p ≤ 0.0001). D- Cell tracker staining of the micro-cancer tissue models (Cancer: green, CAF: red). E− CAF to cancer ratio per spheroid within different hydrogel stiffnesses for a 7-day period. (One-Way ANOVA, *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, and ****p ≤ 0.0001).
Next, we performed shape analysis of spheroids within HAMA/GelMA hydrogels of different stiffnesses to monitor the effect of CAF presence on the area and circularity of tumor spheroids (Fig. 3B and C). The spheroids were stained for F-actin to observe their morphology. In our results, the mean diameter was 311 μm (±43) for cancer only spheroids and 250 μm (±23) for cancer cells + CAF spheroids. Past studies have reported that spheroids that are too small (<200 μm) are inadequate to create physiological conditions and spheroids that are too large (>500 μm) contain hypoxic or necrotic cores due to difficulties in nutrient supply [[49], [50], [51], [52]]. Based on these studies, our spheroid size in the range of 220–350 μm is suitable for microtumor model studies.
In the quantitative shape analysis, spheroids embedded in HAMA/GelMA hybrid hydrogels with or without CAF (Fig. 3C, Fig. S5) showed a similar trend to those not encapsulated in hydrogels (Fig. 1D and E). Spheroids composed of a mixture of cancer and CAF were more circular with smaller areas compared to those without CAF. Interestingly, the morphological changes (both size and circularity) of spheroids with stiffness were more pronounced in the cancer cell only spheroid groups than in the cancer cell + CAF spheroid groups. On Day 3 and Day 5, cancer cell-specific spheroids had a larger spheroid area as the compressive modulus of the HAMA/GelMA hybrid hydrogel decreased. However, during the same experimental period, cancer cell + CAF spheroids did not show a significant difference in spheroid area. In addition, the cancer cell + CAF spheroids in the HAMA/GelMA 15 kPa hybrid hydrogel showed the high variation with time (the highest circularity on Day 3, the lowest circularity on Day 5). On the other hand, on Day 7, there was no significant difference between groups in terms of circularity, but the size of the spheroid was the largest in the 15 kPa group. These results suggest that fluctuations in cell proliferation and cellular behavior may occur when multicellular spheroids are cultured at a stiffness close to the desmoplastic ECM of PDAC (15 kPa). On the other hand, high stiffness (∼36 kPa), such as in the hyperdesmoplastic stiffness environment, maintained a small spheroid area during the whole period of the experiment, with and without CAF, and did not show large variability in circularity. We acknowledge that analysis of spheroid area and circularity was performed using 2D images from fluorescence and confocal microscopes, which indicates the complex 3D structures of spheroids may not be captured. Nevertheless, we believe that the averaged shape information from a number of spheroids could represent the complex 3D structures of spheroids. These results suggest that both the degree of mechanical stiffness and the presence of CAF can influence tumor behavior when modeling desmoplastic ECM.
These trends for cancer cells + CAF spheroids differed from the trends for cancer only spheroids, suggesting that the incorporation of CAFs interacting with cancer cells causing a remodeling in their ECMs in cancer spheroids could be required for recapitulating the TME for building better 3D PDAC models. Similar to the results, when cancer spheroids are cultured on hydrogels with stiffness in the range of 90–1050 Pa, the spheroid size can be reduced in harder hydrogels [53,54].
Furthermore, we performed area analysis studies between cancer cells and CAFs to analyze the distribution of each cellular component in spheroids embedded in HAMA/GelMA hybrid hydrogels with different stiffness. To this end, the cancer cells and CAFs were labeled with cell trackers of different colors (Fig. 3D). The CAFs were distributed in the microtumor model periphery, especially in the stiffer gels on Day 1, closely nesting the cancer core, similar to what occurs in vivo. In the quantitative analysis result of the live cell trackers, the CAF signal showed a general increase over time from Day 1 to Day 7. In particular, the most significant changes of CAF coverage of the spheroids were observed in the stiffest hydrogels between days 1, 3, and 7. The highest CAF area ratio was also observed in the stiffest gel at day 7 (Fig. 3E). This result may also correlate with the observation of CAFs at the periphery of the stiff hydrogels. Yakavets et al. showed that after 24 h of spheroid formation, the live cell tracker signal coming from the fibroblasts migrated from the center of the spheroids to the shell [55]. Apte et al. also showed the dense desmoplastic stroma rich in CAFs surrounding the PDAC tumor nodules proving a spatial arrangement of cancer cells and CAFs in tumors [56]. Taken together, our results showed that the Cancer + CAF spheroids remained highly compact and mechanically robust, indicating that CAFs establish close cell-cell contacts among one another with the tumor core.
3.4. Role of mechanical properties in regulating cancer cell phenotypes
CAFs are dynamic and differentiate into heterogeneous cell groups in tissue fibroblasts, the incorporation of CAFs into tumor models may lead to changes in the phenotype of the microtumor model [57]. In particular, we observed that the inclusion of CAFs in HAMA/GelMA hybrid hydrogels with different stiffnesses promoted different levels of cell-cell interactions, resulting in different shape profiles (area and circularity) and area ratios of the cells. Changes in the mechanical environment of TME can trigger activation of ion channels or protein kinases in the tumor niche at the short-term level, and affect gene transcription and phenotype in the long-term [58]. Based on these results, we hypothesized that various mechanical properties (healthy to precancerous; 3 kPa, precancerous to desmoplastic; 15 kPa and hyperdesmoplastic pancreatic tumor matrix; 36 kPa) could affect cancer cell behavior through mechanotransduction and activation of EMT-related signaling pathways.
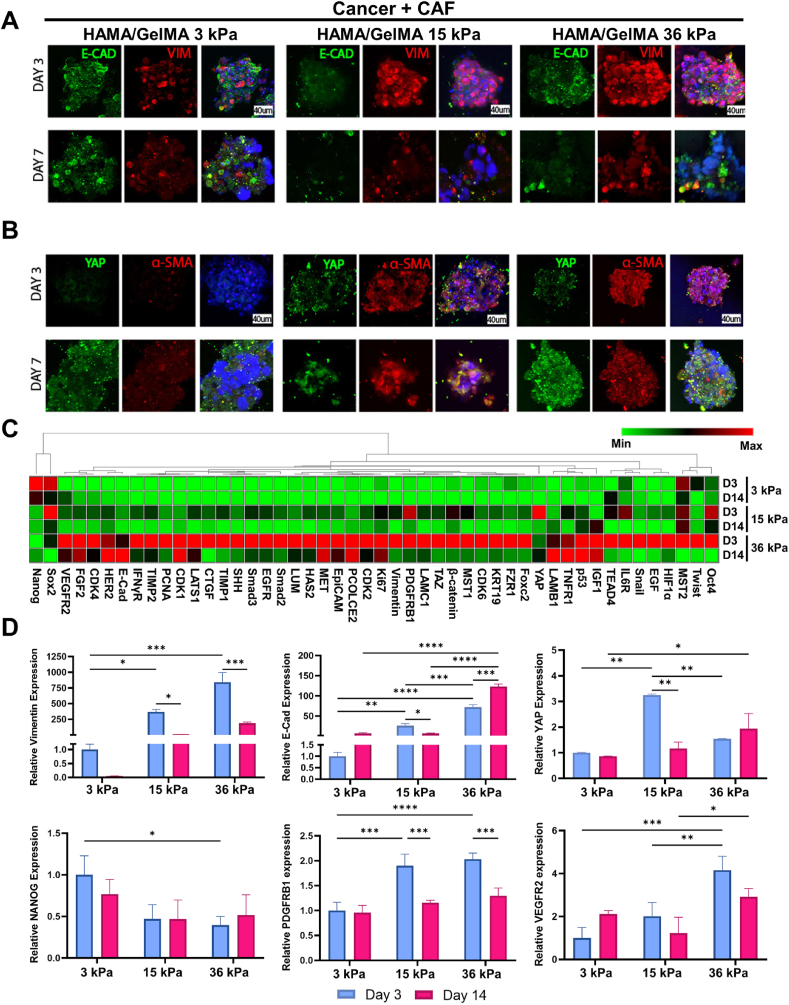
To address this hypothesis, confocal microscopy imaging was first performed for evaluating expression levels of markers associated with mechanotransduction (YAP) and EMT (E-CAD, VIM, and α-SMA) for Cancer + CAF spheroids encapsulated in HAMA + GelMA hydrogels with different compressive modulus (3, 15, and 36 kPa) on Day 3 and 7 (Fig. 4A and B). E-CAD is a cell adhesion protein that can be observed in cell-cell adhesion. Cancer + CAF spheroids cultured in HAMA/GelMA hydrogels with 15 and 36 kPa showed lower levels of E-CAD for 7 days compared to those cultured in gels with 3 kPa compressive modulus. Expression of VIM (intermediate filament & mesenchymal marker) and YAP (core effector of the Hippo pathway) is mechanosensitive and increases pancreatic cancer with increased matrix stiffness [23,59]. The spheroids express higher levels of YAP on Day 7 compared to those cultured for 3 days, especially for the HAMA/GelMA with 36 kPa condition. VIM expression levels for Cancer + CAF spheroids (15 and 36 kPa) appeared to peak on Day 3, and then decreased on Day 7. α-SMA is a marker of fibrosis in tissue fibroblasts and also known for one of EMT markers for cancer cells [60,61]. We observed that expression levels of α-SMA for Cancer + CAF spheroids cultured in HAMA/GelMA hydrogels with compressive modulus of 15 and 36 kPa seemed to peak on Day 3 and then maintained until Day 7 when compared to those cultured in hybrid hydrogels with 3 kPa. Together, Cancer + CAF spheroids cultured in higher mechanical properties (more rigid hydrogels, e.g., 15 and 36 kPa) exhibited less expression levels of E-CAD (undergoing EMT for 15 & 36 kPa) but higher expression levels of YAP (peak on day 7 for 36 kPa), VIM (peak on day 3 for 15 and 36 kPa), and α-SMA (15 and 36 kPa stiffness conditions for 7 days) compared to those cultured in hydrogels with 3 kPa compressive modulus (healthy pancreatic tissues). These results indicate that mechanical properties may play a critical role in regulating cellular mechanotransduction and EMT of pancreatic cancer cells to be more progressive phenotypes.
Fig. 4.
Immunostaining and RT-qPCR analysis of the micro-cancer tissue models. A- E-CAD and VIM staining of Cancer + CAFs micro-cancer tissue models (red: VIM, green: E-CAD, blue: DAPI, scale bar: 40 μm). B- α-SMA and YAP staining Cancer + CAFs micro-cancer tissue models (red: α-SMA, green: YAP, blue: DAPI, scale bar: 40 μm). C- qRT PCR analysis and heatmap of the genes for micro-cancer tissue models prepared by 3, 15, 36 kPa compressive modulus of HAMA/GelMA on days 3 and 14 (red: maximal change, green: minimal change). D- Fold changes of the selected genes (VIM, E-Cad, YAP, Nanog, PDGFRB1, and VEGFR2) (Two-Way ANOVA, *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, and ****p ≤ 0.0001).
To collect quantitative data, we performed gene expression analysis for Cancer + CAF spheroids cultured in HAMA/GelMA hydrogels with different stiffness (3, 15, 36 kPa) on day 3 and 14 using qRT-PCR. A panel of markers associated with stemness and CSCs (Nanog-Oct4), and progression/EMT/ mechanotransduction (Twust-COL2) were evaluated (Fig. 4C and D). When comparing the shape characterization and gene analysis on day 14 with earlier time points, the shape parameters were more sensitive to early changes while gene expression results were more significant at later days of the culture. It is observed that Cancer + CAF spheroids cultured in soft HAMA/GelMA hybrid hydrogels (3 and 15 kPa compressive modulus) on Day 3 showed higher expression levels of stemness markers compared to those cultured in stiff hydrogels (36 kPa), except for Oct4 expression. Moreover, it is noted that the condition representing the hyperdesmoplastic matrices for pancreatic tumors (36 kPa) promoted higher levels of progression/EMT/mechanotransduction markers (VIM, E-Cad, PDGFRB1, VEGFR2) on Day 3. Interestingly, Cancer + CAF spheroids cultured in hydrogels crosslinked for 15 kPa displayed higher expression levels of mechanotransduction gene (YAP) compared to those in the 36 kPa condition on Day 3 (Fig. 4D). These results clearly correspond to the expression results of protein markers (Fig. 4A and B) and indicate that increased mechanical properties of the matrices give rise to decreased stemness together with increased marker expression associated with tumor progressions in early time points.
3.5. Combinatorial effect of mechanical properties and TGF-β signaling on controlling cancer cell states
While the matrix components play a large role in the TME characteristics, TGF-β signaling in pancreatic cancer cells was also found to play a crucial role in regulating pancreatic tumor states and their behaviors [62]. TGF-β is a known central mediator of fibrogenesis and a key molecule in the activation of the fibrotic program [63]. TGF-β-associated signaling pathways could regulate tumor malignancy by modulating EMT for various cancer types, which is one of the major features of metastasis for tumor progression [64]. For example, TGF-β is known to induce the transcription of E-CAD repressors such as Snail and SIP-1 thereby resulting in E-CAD loss and the epithelial to mesenchymal transition [65]. During this transition, cells undergoing EMT can obtain invasive and migratory properties to become cancer stem cell-like phenotypes that can self-renew and initiate tumor formation [67].
In addition, TGF-β can binds to serine/threonine kinase receptors and causes phosphorylation to form a heteromeric complex, which regulates the transcription of fibrosis/mesenchymal-related genes such as VIM when complexed with the nucleus [66]. Moreover, TGF-β-associated signaling pathways could play an important role in CAF activation affecting their tissue synthesis and proliferation in TME. While some models in the literature study the effects of TGF-β in spheroid models, it is important to investigate the interplay between mechanical properties of TME and TGF-β signaling to precisely understand the combinatorial roles of those factors and potentially design better models for PDAC.
Therefore, our next question investigated how the induction of TGF-β signaling pathway could regulate shape profiles. Cancer-CAF occupancy ratios, and even mechanical properties-mediated marker expression associated with tumor progressions. To answer the question, we first assessed shape profiles and area ratios of cancer-CAF spheroids cultured in HAMA/GelMA hybrid hydrogels with different mechanical properties with the induction of TGF-β (Fig. 5A–C). Cancer and CAFs labeled with green and red cell trackers, respectively, showed both cells were mixed well to form tumor spheroids (Fig. 5A). In addition, the shape analysis displayed that the area of the spheroids did not change with increased gel stiffness while the circularity of the spheroids decreased as the gel stiffness was increased at all time points, which differs from the result without the presence of TGF-β as shown in Fig. 4C (Fig. 5B). Furthermore, the cancer-CAF area ratio measurements showed that the only significant CAF response was observed in medium stiffness gels under TGF-β treatment (Fig. 5C). These results are different compared to no TGF-β treatment where gel stiffness showed more significant changes on CAFs (Fig. 3E). With cancer phenotype changes and ECM remodeling, the variation of mechanical properties-mediated marker expression levels was observed in Fig. 4C and D. We found that the expression trend (E-CAD, VIM, YAP, and α-SMA) observed for cancer-CAF spheroids in stiffer hydrogels (15 and 36 kPa of compressive modulus) without the TGF-β addition (Fig. 4A and B) was similar to the trend with the TGF-β induction (Fig. 5D and E). Notable changes were observed for TGF-β signaling-induced Cancer + CAF spheroids in soft hydrogels (3 kPa), showing higher expression levels of YAP and α-SMA on day 3 comparable to stiff conditions (15 & 36 kPa), but their expression levels of YAP and α-SMA were decreased on Day 7. This indicates that the extra induction of TGF-β signaling may play a bigger role in Cancer + CAF spheroids cultured in healthy pancreatic matrices compared to stiff ones.
Fig. 5.
Analysis of the TGF-β treated micro-cancer tissue models for cell tracker, shape, area ratio, immunohistochemistry, and qRT-PCR. A- Cell tracker staining of the TGF-β treated micro-cancer tissue models (Cancer: green, CAF: red). B- Spheroid size and shape analysis in TGF-β treated HAMA/GelMA 3, 15, 36 kPa of compressive modulus for days 3, 5 and 7 of micro-cancer tissue model preparation (One-Way ANOVA, *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, and ****p ≤ 0.0001). C- CAF to cancer ratio per spheroid treated with TGF-β within different hydrogel stiffnesses for a 7-day period. (One-Way ANOVA, *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, and ****p ≤ 0.0001). D- E-CAD and VIM staining of Cancer + CAF micro-cancer tissue model treated with the TGF-β (red: VIM, green: E-CAD, blue: DAPI, scale bar: 40 μm). E− α-SMA and YAP staining Cancer + CAF micro-cancer tissue model (red: α-SMA, green: YAP, blue: DAPI, scale bar: 40 μm). F- qRT PCR analysis and heatmap of the genes for the TGF-β treated micro-cancer tissue model prepared by 3, 15, 36 kPa compressive modulus of HAMA/GelMA at days 3 and 14 (red: maximal change, green: minimal change). G- Fold changes of the selected genes (VIM, E-CAD, and YAP) for the TGF-β treated micro-cancer tissue model (Two-Way ANOVA, *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, and ****p ≤ 0.0001).
To investigate the combinatorial effect of mechanical properties and TGF-β signaling induction on pancreatic tumor progression-related behaviors, we assessed the transcriptomic profiles of Cancer + CAF spheroids cultured in HAMA/GelMA hybrid hydrogels (healthy, desmoplastic, and hyper desmoplastic pancreatic tumor matrices) with TGF-β addition. We employed the same panel of markers associated with stemness and CSCs (Nanog-Oct4), progression/EMT/mechanotransduction (Twist- COL2) as shown in Fig. 4C (Fig. 5F and G). Higher expression levels of stemness markers were observed for Cancer + CAF spheroids cultured in soft hydrogels, which is in line with the result without TGF-β induction as displayed in Fig. 4C. Moreover, it was noted that when inducing TGF-β signaling, Cancer + CAF spheroids cultured in hydrogels with 36 kPa mimicking the desmoplastic pancreatic matrices promote higher levels of markers associated with progression/EMT/mechanotransduction, which does not correspond to the outcomes without the TGF-β signaling addition as shown in Fig. 4C. It was observed that Cancer + CAF spheroids cultured in stiffer HAMA/GelMA hydrogels exhibited elevated expression of progression/EMT/mechanotransduction markers without TGF-β induction (hyper desmoplastic > desmoplastic > healthy), while those spheroids that cultured in the combination of hydrogels mimicking desmoplastic pancreatic matrices and TGF-β signaling induction tended to possess more mechanosensitive, mesenchymal, and progressive characteristics. TGF-β is activated in CAFs through mechanotransduction pathways but it also activated fibroblasts to transform into CAFs [60]. From our findings we observed that the chemical TGF-β stimulation precludes the mechanosensing pathways in a way that, when we added the biochemical stimulation, the cells no longer respond to TME physical factors but overwhelmed by the activation of the TGF-β directly. It is of key importance that we were able to show that, in the absence of the TGF-β stimulation, TME mechanical factors determine cell phenotype. On the other hand, a strong stimulus like TGF-β has an impactful effect in comparison to the mechanical cues and controls cell phenotype directly.
4. Conclusions
Using HAMA/GelMA hydrogels resembling pancreatic tissues, this study demonstrates why the various factors within the TME, such as i) the presence of CAFs, ii) mechanical properties (healthy/precancerous, precancerous/desmoplastic, hyperdesmoplastic), and iii) induction of TGF-β signaling is required for building pancreatic tumor models. Cancer + CAF spheroids cultured in stiffer hydrogels mimicking hyper desmoplastic matrices show decreased levels of stemness markers but increased levels of progression/EMT/mechanotransduction markers without the supplement of TGF-β. However, we reveal that pancreatic spheroids cultured in hydrogels mimicking desmoplastic pancreatic matrices become more progressive phenotypes with the presence of TGF-β signaling. Our findings here may help guide further advanced designs of pancreatic tumor models required to precisely understand the role of microenvironments in promoting progression of tumors, and potentially employed for developing personalized pancreatic tumor models. We proposed that the combination of mechanical properties and growth factor presence could promote pancreatic tumor progression when mimicking the mechanical properties of the native PDAC microenvironment. However, we acknowledge that it is possible that the molecular species including proteins and small molecules play an important role in these different matrices in vivo. Nevertheless, based on the findings from the study, we revealed that the proposed PDAC model system mimicking mechanical properties of the native PDAC microenvironment could induce pancreatic cancer progression. We also acknowledge that multiple cell sources have not been considered since the present study is designed to establish proof of concept for modeling of PDAC microenvironments. However, we believe that using different pancreatic cancer cell lines as well as primary cancer cells will be required for our further in-depth studies to closely and realistically mimic the parameters chosen for the design of PDAC models. In particular, by using this hybrid hydrogel model to grow patient-derived pancreatic cancer cells, it will be possible to evaluate drug response and identify potential gene mutations to predict effective treatment options. Future studies also include testing pancreatic cancer drug treatments on our model and comparing the treatment outcomes to in vivo studies.
Author contributions
M.E. and N.F. contributed equally as first authors. M.E. performed the in vitro and material synthesis and immunofluorescence staining. N.F. performed material synthesis, characterization, microtissue construction, and immunofluorescence staining. Both first authors equally wrote the manuscript. N.R.B, R.H., A.C. helped with data collection, material characterization, mechanical testing, and drawing schematics. M.M., M.M, Y. L, J.S., H-J.C, Y.Z., H.K, M.R.D helped with writing manuscript, revision, and reference. A.K., J.L., H-J.K conceptualized the idea, supervised the research, and contributed to manuscript preparation and revision. All the authors discussed the results and commented on the manuscript.
Declaration of competing interest
The authors declare no conflict of interest.
Acknowledgements
Dr. M. Ermis and Dr. N. Falcone contributed equally to this work. The authors gratefully acknowledge the funding by the National Institutes of Health (HL140951, HL137193, CA257558, DK130566). Dr. M. Ermis acknowledges The Scientific and Technological Research Council of Turkiye for 2219-International Postdoctoral Research Fellowship Program.
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bioactmat.2023.02.005.
Contributor Information
Ali Khademhosseini, Email: khademh@terasaki.org.
Junmin Lee, Email: junmin@postech.ac.kr.
Han-Jun Kim, Email: hanjun@korea.ac.kr.
Appendix A. Supplementary data
The following are the Supplementary data to this article.
References
- 1.Bengtsson A., Andersson R., Ansari D. The actual 5-year survivors of pancreatic ductal adenocarcinoma based on real-world data. Sci. Rep. 2020;10(1) doi: 10.1038/s41598-020-73525-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hosein A.N., Brekken R.A., Maitra A. Pancreatic cancer stroma: an update on therapeutic targeting strategies. Nat. Rev. Gastroenterol. Hepatol. 2020;17(8):487–505. doi: 10.1038/s41575-020-0300-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carvalho T.M., et al. Tumor microenvironment features and chemoresistance in pancreatic ductal adenocarcinoma: insights into targeting physicochemical barriers and metabolism as therapeutic approaches. Cancers. 2021;13(23):6135. doi: 10.3390/cancers13236135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Katt M.E., et al. In vitro tumor models: advantages, disadvantages, variables, and selecting the right platform. Front. Bioeng. Biotechnol. 2016;4(12) doi: 10.3389/fbioe.2016.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salvatore V., et al. The tumor microenvironment promotes cancer progression and cell migration. Oncotarget. 2017;8(6):9608–9616. doi: 10.18632/oncotarget.14155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ware M.J., et al. Generation of an in vitro 3D PDAC stroma rich spheroid model. Biomaterials. 2016;108:129–142. doi: 10.1016/j.biomaterials.2016.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heinrich M.A., et al. Translating complexity and heterogeneity of pancreatic tumor: 3D in vitro to in vivo models. Adv. Drug Deliv. Rev. 2021;174:265–293. doi: 10.1016/j.addr.2021.04.018. [DOI] [PubMed] [Google Scholar]
- 8.Pothula S.P., et al. Pancreatic stellate cells: aiding and abetting pancreatic cancer progression. Pancreatology. 2020;20(3):409–418. doi: 10.1016/j.pan.2020.01.003. [DOI] [PubMed] [Google Scholar]
- 9.Schnittert J., Bansal R., Prakash J. Targeting pancreatic stellate cells in cancer. Trends in Cancer. 2019;5(2):128–142. doi: 10.1016/j.trecan.2019.01.001. [DOI] [PubMed] [Google Scholar]
- 10.Suklabaidya S., et al. Experimental models of pancreatic cancer desmoplasia. Lab. Invest. 2018;98(1):27–40. doi: 10.1038/labinvest.2017.127. [DOI] [PubMed] [Google Scholar]
- 11.Tian C., et al. Proteomic analyses of ECM during pancreatic ductal adenocarcinoma progression reveal different contributions by tumor and stromal cells. Proc. Natl. Acad. Sci. USA. 2019;116(39):19609–19618. doi: 10.1073/pnas.1908626116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weniger M., Honselmann K.C., Liss A.S. The extracellular matrix and pancreatic cancer: a complex relationship. Cancers. 2018;10(9):316. doi: 10.3390/cancers10090316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferrara B., et al. The extracellular matrix in pancreatic cancer: description of a complex network and promising therapeutic options. Cancers. 2021;13(17):4442. doi: 10.3390/cancers13174442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Masugi Y. The desmoplastic stroma of pancreatic cancer: multilayered levels of heterogeneity, clinical significance, and therapeutic opportunities. Cancers. 2022;14(13):3293. doi: 10.3390/cancers14133293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mazzocchi A., et al. Pleural effusion aspirate for use in 3D lung cancer modeling and chemotherapy screening. ACS Biomater. Sci. Eng. 2019;5(4):1937–1943. doi: 10.1021/acsbiomaterials.8b01356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu H.-Y., Korc M., Lin C.-C. Biomimetic and enzyme-responsive dynamic hydrogels for studying cell-matrix interactions in pancreatic ductal adenocarcinoma. Biomaterials. 2018;160:24–36. doi: 10.1016/j.biomaterials.2018.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pereira B.A., et al. CAF subpopulations: a new reservoir of stromal targets in pancreatic cancer. Trends in Cancer. 2019;5(11):724–741. doi: 10.1016/j.trecan.2019.09.010. [DOI] [PubMed] [Google Scholar]
- 18.Belhabib I., et al. Extracellular matrices and cancer-associated fibroblasts: targets for cancer diagnosis and therapy? Cancers. 2021;13(14):3466. doi: 10.3390/cancers13143466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Begum A., et al. Direct interactions with cancer-associated fibroblasts lead to enhanced pancreatic cancer stem cell function. Pancreas. 2019;48(3):329. doi: 10.1097/MPA.0000000000001249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rice A.J., et al. Matrix stiffness induces epithelial–mesenchymal transition and promotes chemoresistance in pancreatic cancer cells. Oncogenesis. 2017;6(7) doi: 10.1038/oncsis.2017.54. e352-e352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nabavizadeh A., et al. Noninvasive Young's modulus visualization of fibrosis progression and delineation of pancreatic ductal adenocarcinoma (PDAC) tumors using Harmonic Motion Elastography (HME) in vivo. Theranostics. 2020;10(10):4614–4626. doi: 10.7150/thno.37965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lachowski D., et al. Substrate rigidity controls activation and durotaxis in pancreatic stellate cells. Sci. Rep. 2017;7(1):2506. doi: 10.1038/s41598-017-02689-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liot S., et al. Stroma involvement in pancreatic ductal adenocarcinoma: an overview focusing on extracellular matrix proteins. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.612271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Busatto C.A., et al. Oil-in-microgel strategy for enzymatic-triggered release of hydrophobic drugs. J. Colloid Interface Sci. 2017;493:356–364. doi: 10.1016/j.jcis.2017.01.029. [DOI] [PubMed] [Google Scholar]
- 25.Chandrasekharan A., et al. In situ photocrosslinkable hyaluronic acid‐based surgical glue with tunable mechanical properties and high adhesive strength. J. Polym. Sci. Polym. Chem. 2019;57(4):522–530. [Google Scholar]
- 26.Nichol J.W., et al. Cell-laden microengineered gelatin methacrylate hydrogels. Biomaterials. 2010;31(21):5536–5544. doi: 10.1016/j.biomaterials.2010.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oudshoorn M.H.M., et al. Synthesis of methacrylated hyaluronic acid with tailored degree of substitution. Polymer. 2007;48(7):1915–1920. [Google Scholar]
- 28.Deer E.L., et al. Phenotype and genotype of pancreatic cancer cell lines. Pancreas. 2010;39(4):425–435. doi: 10.1097/MPA.0b013e3181c15963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bordignon P., et al. Dualism of FGF and TGF-β signaling in heterogeneous cancer-associated fibroblast activation with ETV1 as a critical determinant. Cell Rep. 2019;28(9):2358–2372. e6. doi: 10.1016/j.celrep.2019.07.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoffmann O.I., et al. Impact of the spheroid model complexity on drug response. J. Biotechnol. 2015;205:14–23. doi: 10.1016/j.jbiotec.2015.02.029. [DOI] [PubMed] [Google Scholar]
- 31.Zarubova J., et al. Cell‐taxi: mesenchymal cells carry and transport clusters of cancer cells. Small. 2022;18(50) doi: 10.1002/smll.202203515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Monteiro M.V., et al. Stratified 3D microtumors as organotypic testing platforms for screening pancreatic cancer therapies. Small Methods. 2021;5(5) doi: 10.1002/smtd.202001207. [DOI] [PubMed] [Google Scholar]
- 33.Brumskill S., et al. Inclusion of cancer-associated fibroblasts in drug screening assays to evaluate pancreatic cancer resistance to therapeutic drugs. J. Physiol. Biochem. 2023;79:223–234. doi: 10.1007/s13105-021-00857-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hwang H.J., et al. Multiplex quantitative analysis of stroma-mediated cancer cell invasion, matrix remodeling, and drug response in a 3D co-culture model of pancreatic tumor spheroids and stellate cells. J. Exp. Clin. Cancer Res. 2019;38(1):258. doi: 10.1186/s13046-019-1225-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pandol S., et al. Desmoplasia of pancreatic ductal adenocarcinoma. Clin. Gastroenterol. Hepatol. 2009;7(11):S44–S47. doi: 10.1016/j.cgh.2009.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.MacCurtain B.M., et al. Pancreatic ductal adenocarcinoma: relating biomechanics and prognosis. J. Clin. Med. 2021;10(12):2711. doi: 10.3390/jcm10122711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rice A., et al. Matrix stiffness induces epithelial–mesenchymal transition and promotes chemoresistance in pancreatic cancer cells. Oncogenesis. 2017;6(7) doi: 10.1038/oncsis.2017.54. e352-e352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tomás-Bort E., et al. 3D approaches to model the tumor microenvironment of pancreatic cancer. Theranostics. 2020;10(11):5074. doi: 10.7150/thno.42441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rubiano A., et al. Viscoelastic properties of human pancreatic tumors and in vitro constructs to mimic mechanical properties. Acta Biomater. 2018;67:331–340. doi: 10.1016/j.actbio.2017.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nabavizadeh A., et al. Noninvasive Young's modulus visualization of fibrosis progression and delineation of pancreatic ductal adenocarcinoma (PDAC) tumors using Harmonic Motion Elastography (HME) in vivo. Theranostics. 2020;10(10):4614. doi: 10.7150/thno.37965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nguyen A.V., et al. Stiffness of pancreatic cancer cells is associated with increased invasive potential. Integrative biology. 2016;8(12):1232–1245. doi: 10.1039/c6ib00135a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Prata J.E., et al. Complex fluids based on methacrylated hyaluronic acid. Biomacromolecules. 2010;11(3):769–775. doi: 10.1021/bm901373x. [DOI] [PubMed] [Google Scholar]
- 43.Young A.T., White O.C., Daniele M.A. Rheological properties of coordinated physical gelation and chemical crosslinking in gelatin methacryloyl (GelMA) hydrogels. Macromol. Biosci. 2020;20(12) doi: 10.1002/mabi.202000183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Han L., et al. Mussel-inspired cryogels for promoting wound regeneration through photobiostimulation, modulating inflammatory responses and suppressing bacterial invasion. Nanoscale. 2019;11(34):15846–15861. doi: 10.1039/c9nr03095f. [DOI] [PubMed] [Google Scholar]
- 45.Lee J., et al. A heart‐breast cancer‐on‐a‐chip platform for disease modeling and monitoring of cardiotoxicity induced by cancer chemotherapy. Small. 2021;17(15) doi: 10.1002/smll.202004258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Murgai M., Giles A., Kaplan R. Physiological, tumor, and metastatic niches: opportunities and challenges for targeting the tumor microenvironment. Crit. Rev. Oncog. 2015;20(3–4) doi: 10.1615/critrevoncog.2015013668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bauer J., et al. Increased stiffness of the tumor microenvironment in colon cancer stimulates cancer associated fibroblast-mediated prometastatic activin A signaling. Sci. Rep. 2020;10(1):1–11. doi: 10.1038/s41598-019-55687-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Calvo F., et al. Mechanotransduction and YAP-dependent matrix remodelling is required for the generation and maintenance of cancer-associated fibroblasts. Nat. Cell Biol. 2013;15(6):637–646. doi: 10.1038/ncb2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pinto B., et al. Three-dimensional spheroids as in vitro preclinical models for cancer research. Pharmaceutics. 2020;12(12):1186. doi: 10.3390/pharmaceutics12121186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cavo M., et al. A synergic approach to enhance long-term culture and manipulation of MiaPaCa-2 pancreatic cancer spheroids. Sci. Rep. 2020;10(1):1–11. doi: 10.1038/s41598-020-66908-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Han S.J., Kwon S., Kim K.S. Challenges of applying multicellular tumor spheroids in preclinical phase. Cancer Cell Int. 2021;21(1):1–19. doi: 10.1186/s12935-021-01853-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gunay G., et al. The effects of size and shape of the ovarian cancer spheroids on the drug resistance and migration. Gynecol. Oncol. 2020;159(2):563–572. doi: 10.1016/j.ygyno.2020.09.002. [DOI] [PubMed] [Google Scholar]
- 53.Li Y., Kumacheva E. Hydrogel microenvironments for cancer spheroid growth and drug screening. Sci. Adv. 2018;4(4):eaas8998. doi: 10.1126/sciadv.aas8998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu J., et al. Soft fibrin gels promote selection and growth of tumorigenic cells. Nat. Mater. 2012;11(8):734–741. doi: 10.1038/nmat3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yakavets I., et al. Advanced co-culture 3D breast cancer model for investigation of fibrosis induced by external stimuli: Optimization study. Sci. Rep. 2020;10(1):1–11. doi: 10.1038/s41598-020-78087-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Apte M., et al. Desmoplastic reaction in pancreatic cancer: role of pancreatic stellate cells. Pancreas. 2004;29(3):179–187. doi: 10.1097/00006676-200410000-00002. [DOI] [PubMed] [Google Scholar]
- 57.Louault K., Li R.-R., DeClerck Y.A. Cancer-associated fibroblasts: understanding their heterogeneity. Cancers. 2020;12(11):3108. doi: 10.3390/cancers12113108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chin L., et al. Mechanotransduction in cancer. Current opinion in chemical engineering. 2016;11:77–84. doi: 10.1016/j.coche.2016.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang W., et al. Matrix stiffness and its influence on pancreatic diseases. Biochim. Biophys. Acta, Rev. Cancer. 2021;1876(1) doi: 10.1016/j.bbcan.2021.188583. [DOI] [PubMed] [Google Scholar]
- 60.Shi X., et al. Transforming growth factor-β signaling in fibrotic diseases and cancer-associated fibroblasts. Biomolecules. 2020;10(12):1666. doi: 10.3390/biom10121666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pan J.-J., Yang M.-H. The role of epithelial-mesenchymal transition in pancreatic cancer. J. Gastrointest. Oncol. 2011;2(3):151. doi: 10.3978/j.issn.2078-6891.2011.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Melzer C., et al. The role of TGF-β and its crosstalk with RAC1/RAC1b signaling in breast and pancreas carcinoma. Cell Commun. Signal. 2017;15(1):19. doi: 10.1186/s12964-017-0175-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Biernacka A., Dobaczewski M., Frangogiannis N.G. TGF-β signaling in fibrosis. Growth Factors. 2011;29(5):196–202. doi: 10.3109/08977194.2011.595714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rajagopal M.U., et al. TGFβ drives metabolic Perturbations during epithelial mesenchymal Transition in pancreatic cancer: TGFβ induced EMT in PDAC. Cancers. 2021;13(24):6204. doi: 10.3390/cancers13246204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Andl C.D., et al. Coordinated functions of E-cadherin and transforming growth factor β receptor II in vitro and in vivo. Cancer Res. 2006;66(20):9878–9885. doi: 10.1158/0008-5472.CAN-05-4157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wu Y., et al. TGFβ1 regulation of vimentin gene expression during differentiation of the C2C12 skeletal myogenic cell line requires Smads, AP-1 and Sp1 family members. Biochim. Biophys. Acta Mol. Cell Res. 2007;1773(3):427–439. doi: 10.1016/j.bbamcr.2006.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Safa A.R. Epithelial-mesenchymal transition: a hallmark in pancreatic cancer stem cell migration, metastasis formation, and drug resistance. Journal of Cancer Metastasis and Treatment. 2020;6:36. doi: 10.20517/2394-4722.2020.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.