Abstract
COVID-19 convalescent plasma (CCP) could improve the clinical outcome of COVID-19 patients when high-titer CCP is administered in early stages of disease. However, CCP donors have a risk profile like first-time donors, pathogen reduction treatment (PRT) may mitigate such risk but should not impact CCP quality. The current study aims to assess the impact of PRT-technologies available in Saudi Arabia on the neutralizing activity of CCP.
Study design
and Methods: CCP was collected from eligible donors by plasmapheresis. The neutralization titer was determined with an in-house microneutralization assay (MNA) using a local SARS-CoV-2 clinical isolate. Selected units were split and subject to PRT with amotosalen/UVA (AS) or Riboflavin/UVB (RB) (pairwise side-by-side comparison) followed by a second MNA analysis. 51 CCP units were collected, 27 were included in the analysis reaching the minimum MNA titer of 1:40 (4 reached high titer (≥1:250)). 27 CCP units were treated with AS and 14 with RB, the median MNA pre-treatment titer was 1:80 (1:40–640). The impact of AS and RB PRT on CCP neutralizing activity was not significantly different, nor in the total analysis neither in the pairwise comparison (94.6 vs 96.4 % retention, p > 0.05). No correlation of titer and blood group was observed, but a trend for increasing MNA titer with donor age, choosing donors with an age > 45 years would increase the number of high-titer CCP donors. The difference in impact of AS and RB on CCP MNA titer was below the limit of detection of the assay (0.5-fold).
Keywords: COVID-19, Convalescent plasma, Pathogen reduction, Neutralization assay
1. Introduction
Convalescent plasma (CP) is often the only potential treatment option for newly emerging diseases [1]. After more than 2 years of the COVID-19 pandemic, multiple clinical trials with CCP have been conducted, mostly treating critically ill COVID-19 patients with a very heterogenous quality of CCP, not showing a significant benefit for the patient in summary [2], [3], [4], [5]. However, recent studies report a significant impact of CCP-treatment on mortality and length of stay when well-characterized high-titer CCP is administered in early stages of infection before ventilation or oxygen support [6], [7], [8]. It was also reported that the administration of high titer CCP in early stages of disease to outpatients significantly reduced disease progression and hospitalization rate [9]. Despite the development of therapeutic antibodies, CCP may be in case of newly emerging SARS-CoV-2 variants also an important future treatment option [10], [11]. Since CCP-donors are like first-time donors with an elevated risk for window-period transmission of blood borne viruses, pathogen reduction treatment (PRT) may be a way mitigating such risk. In Saudi Arabia, the NAT/serology positivity rate for transfusion-transmissible infections was 8.7 % in 2020, with HBV as most prevalent marker, followed by HCV and Treponema [12] Transmission despite NAT/serology testing is occasionally reported from multiple countries, for example two recent cases of HCV transmission in Germany [13], nine cases of HBV transmission in Slovenia [14] and transfusion of an HIV contaminated unit in France [15]. Furthermore there are concerns regarding a blood-transmissible potential future variant, even there is currently no evidence for SARS-CoV-2 blood transmissibility [16] (efficient inactivation of SARS-CoV-2 in plasma has been shown with amtosalen/UVA (AS) [17] and riboflavin/UVB (RB) [18] technologies). Studies conducted to date assessing the impact of PRT on CCP have several weaknesses, in particular small sample numbers and a non-standardized methodology, making it difficult to assess differences between technologies [19]. The aim of our study was the assessment of the impact of locally available PRT-methods for plasma (AS and RB) on the neutralizing activity of CPP, analyzed with a neutralization assay using a local SARS-CoV-2 clinical isolate.
2. Methods
2.1. CCP collection and storage
CCP donors were qualified by the following criteria based on the European Commission Guidance on collection, testing processing, storage and distribution and monitored use of CCP: 18–65 years of age male and nulliparous female donors, prior laboratory confirmed SARS-CoV-2 infection, ≥14 days without symptoms after diagnosis and negative NAT, standard donor criteria for plasmapheresis plasma donation. 630 mL (600 mL + 5 % safety margin) of CCP (incl. anticoagulant) was collected from eligible donors with a Trima Accel plasmapheresis device (Terumo BCT, Lakewood, U.S.A.). The plasma was stored under room temperature until PRT for max. 8 h. Directly after PRT-treatment, the plasma was transferred to a −30 °C freezer and stored at −30 °C until use.
2.2. Pathogen reduction treatment
The following common guard bands for PRT were applied: RBC count <4 × 109/L, WBC count ≤ 1 × 109/L, platelet count ≤2.1 × 1012/L. CCP was treated with amotosalen/UVA technology using the INTERCEPT Blood System Processing Set for Plasma and the INTERCEPT Illuminator (Cerus Corporation, Concord, U.S.A.) or Riboflavin/UVB technology using the Mirasol Plasma Disposable Kit and the Mirasol Illuminator (Terumo BCT) according to the manufacturers instructions.
2.3. Determination of CCP neutralizing activity
Our in-house microneutralization assay (MNA) was used as described previously [20]. Briefly, the CCP samples were serially diluted and mixed with an equal volume of Dulbeccos Modified Eagle Medium (DMEM) containing SARS-CoV-2 (isolate SARS-CoV-2/human/SAU/85791C/2020) with a viral load of 100 TCID50 to inoculate confluent Vero E6 cells (ATCC# CRL-1586). MN titers were determined by the highest dilution preventing a cytopathic effect (CPE). Since the dilution steps were 1:2, the limit of detection of the assay were differences between study arms of < 0.5-fold.
2.4. Side-by-side comparison of PRT methods
For side-by-side comparison experiments, after taking a pre-treatment sample, each CCP unit was distributed between the two systems (400 mL for AS and 200 mL for RB) according to the manufacturers guard bands. PRT was conducted simultaneously. After PRT plasma treatment and before freezing, a 12 mL sample was taken sterile using a vacutainer with polypropylene tubes (Becton-Dickinson, U.S.A.) from each plasma unit. All plasma units and samples were frozen in the same freezer. The CCP units were later used in clinical practice on demand.
2.5. Statistical analysis
Statistical analysis was conducted applying the two-sample (unpaired) t-test analyzing whether samples from two independent populations have different means, p-values <0.05 were considered significant.
2.6. Ethical Board Review
The study was approved by the unit of biomedical ethics of the King Abdulaziz University (registration number HA-02-J-008).
3. Results
In total we collected 51 CCP units (05/2020–12/2020) from different donors. 27 units (53 %) having an MNA titer of ≥ 1:40, were included in the analysis (which we consider the minimum titer allowing the detection of potential loss of neutralizing activity post PRT taking the limit of the assay (1:20) into account). The median age of the total donor population was 32 years (14−58).
3.1. The impact of PRT on CPP neutralizing activity
In total (including side-by-side comparisons) 27 PRT experiments with AS and 14 with RB were conducted, the units had a median MNA pre-treatment titer of 1:80 (1:40–640). 4 CCP units fulfilled the US FDA definition of high titer CCP (a neutralization titer of >1:250). The preservation of neutralizing activity (including samples of all available pathogen-reduced units, also the ones treated with only one PRT) assessed with a microneutralization assay pre- and post PRT was not significantly different between the AS and RB arm ( Table 1). In a side-by-side comparison approach, 14 CCP units were divided between AS and RB, treated simultaneously and preservation of neutralizing activity assessed by MSA was compared. In 13 of 14 analyses, the full neutralizing activity was preserved with both PRT technologies. There was also no significant difference in the preservation of neutralizing activity between AS and RB PRT of CCP in the pairwise analysis ( Table 2).
Table 1.
Preservation of CCP neutralizing activity post PRT (including all units, also of collections only treated with one PRT).
| Preservation nAB post AS-treatment (%) | Preservation nAB post RB-treatment (%) | |
|---|---|---|
| n | 27 | 14 |
| mean | 94.4 | 96.7 |
| SD | 20.0 | 12.9 |
| p-value | 0.659 | |
nAB (neutralizing antibodies), SD (standard deviation)
Table 2.
Paired side-by-side comparison of the impact of PRT on CCP neutralizing activity (including only paired units, pathogen reduced by both technologies).
| CCP Unit # | Pre-treatment NT50 | Post-AS-treatment (%) | Post-RB-treatment (%) |
|---|---|---|---|
| 1 | 1:40 | 100 | 100 |
| 2 | 1:40 | 100 | 100 |
| 3 | 1:40 | 100 | 100 |
| 4 | 1:160 | 100 | 100 |
| 5 | 1:160 | 100 | 100 |
| 6 | 1:320 | 100 | 100 |
| 7 | 1:160 | 25 | 50 |
| 8 | 1:80 | 100 | 100 |
| 9 | 1:40 | 100 | 100 |
| 10 | 1:40 | 100 | 100 |
| 11 | 1:40 | 100 | 100 |
| 12 | 1:160 | 100 | 100 |
| 13 | 1:40 | 100 | 100 |
| 14 | 1:40 | 100 | 100 |
| mean | 94.6 | 96.4 | |
| SD | 20.0 | 13.4 | |
| p-value | 0.807 | ||
3.2. Donor characteristics correlating with CCP titer
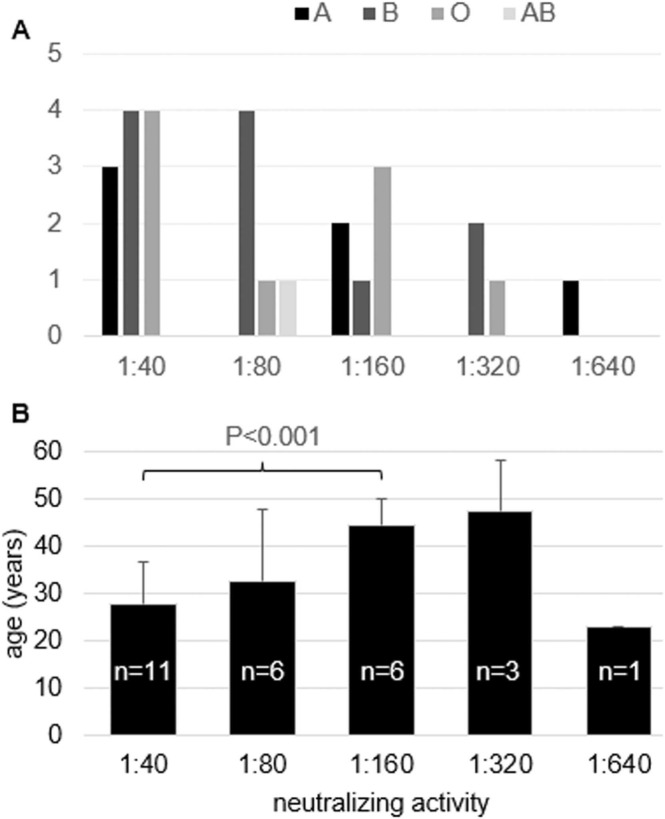
Since only 7.8 % of our donations fulfilled the definition of high-titer CCP (≥1:250)[21] ( Fig. 1), we assessed donor characteristics potentially correlating with higher titer CCP to improve our donor specifications. In contrast to previously published data, we did not observe a correlation between ABO blood group and CCP MNA titer, the ABO blood groups were distributed broadly (Fig. 1 A). However, that may also be due to the small sample number. But we observed in line with previously published data a correlation between donor age and MNA titer. The antibody titer shows a tendency to increase with age (Fig. 1 B), but this trend is not statistically significant (likely due to small sample numbers), with the exception of the difference between the age of 1:40 titer CCP donors (44.5 ± 5.6 years) and 1:160 titer CCP donors (27.9 ± 8.6 years) (p < 0.001).
Fig. 1.
Correlation of CCP neutralizing activity and donor characteristics.
4. Discussion
We did not find a statistically significant difference between the two PRT technologies, nor in the comparison analysis of all collected units, neither in the paired side-by-side comparison, regarding the impact on neutralizing activity. Since the limit of detection of the MNA is < 0.5-fold, potential differences below 0.5-fold cannot be excluded. A previous side-by-side comparison study reported a significantly higher decrease of neutralizing antibodies quality and quantity post RB-treatment compared to AS and Methylene Blue (MB) treatment [22]. The difference to our study may be explained by the usage of different neutralization assays. The higher loss of antibody quantity post RB-treatment compared to other PRT methods in that study is in line with former reports of 13 %−32 % IgG loss post RB-treatment [19]. AS and RB technology use different mode of actions, while AS crosslinks nucleic acids with a photochemical reaction using amotosalen and UVA light, RB damages nucleic acids with a photodynamic reaction involving UVB/UVC light and reactive oxygen species [23]. The different modes of action impact labile plasma proteins differently, RB treatment leads to a significantly increased prolonged coagulation time compared to other technologies [24]. Short-wave UVB light in the absorption spectrum of proteins is also considered damaging proteins additionally [25], [26]. However, with respect to coagulation factor recovery, a recent review considers pathogen reduced plasma with all commercially available technologies acceptable in quality [27]. Comparing both technologies we also noticed differences in processing and handling. The AS processing set allows the treatment of an apheresis collection with a single set (max. volume 650 mL) saving time and consumables, while for RB treatment two processing sets are needed (max. volume 360 mL). However, the AS technology has an additional removal step of photochemicals (Compound Adsorption Device, CAD) taking approx. 10 min. The time to treatment post collection is longer with AS PRT compared to RB PRT (20 h vs. 8 h), allowing more process flexibility, since the preservation of labile plasma proteins is not playing a role during the production of CCP.
We noticed that only 4 of 51 donations (7.8 %) fulfilled the criterion of high-titer CCP (≥1:250) preferred for effective treatment [21]. We found no correlation of donor ABO blood group with CCP titer but observed a non-significant trend of increased donor age and increased CCP titer (with the exception that the age of donors with a 1:160 titer was significantly higher compared to donors with a 1:40 titer). Previous studies reported a lower likelihood of obtaining high-titer CCP from donors with blood group O [28], [29], however that may not be visible in our analysis due to low sample numbers. A study from Brazil reported no correlation of high-titer CCP donors with ABO blood group, but with the body mass index (BMI), observing increased titers with increased obesity [30]. Correlation of high-titer CCP with increasing age was reported in multiple studies [28], a recent study from the UK reported the highest likelihood for obtaining hight-titer CCP from hospitalized older male donors [31]. These studies point towards linkage of COVID-19 disease severity (hospitalization) with a higher likelihood of high-titer neutralizing antibodies, and increasing age, obesity and non-O blood group are risk factors for disease severity which may used as surrogate markers to choose CCP donors.
In conclusion we did not observe a significant difference of the impact of between PRT technologies on the neutralizing activity of CCP until the limit of detection of our assay (<0.5-fold), but differences in processing and handling which should be considered when choosing such technology.
Funding Sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CRediT authorship contribution statement
Salwa Hindawi: Performed the research study. Salwa hindawi and Esam Azhar: Designed the research study. Esam Azhar and Sherif El-Kafrawy: Contributed to essential reagents and testing. Myasim Tilmisani and Omar Assiri: Collected the plasma and contributed to its preservation and transportation. Hani Samadani and Mohammad Raml: Contributed to plasma sampling and processing. Salwa Hindawi, Maha Badawi and Tarek Elgemmezi: Analyzed the data. Maha Badawi and Tarek Elgemmezi: Wrote the manuscript. All authors reviewed and approved the final draft.
Acknowledgements
We thank Abdulrehman Algosaibi Co. for technical support as well as Marcus Picard-Maureau for critical reading of the manuscript and helpful comments.
Fig. 1: Correlation of CCP neutralizing activity and donor characteristics. The number of donors with a specific ABO-blood group was linked to the MNA titer of CCP respectively (A). The average age of each donor population with a specific MNA titer of CCP was calculated with standard deviation (error bars). The only significant difference between two groups is indicated.
References
- 1.Casadevall A., Pirofski L.A. The convalescent sera option for containing COVID-19. J Clin Invest. 2020;130:1545–1548. doi: 10.1172/JCI138003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jorda A., Kussmann M., Kolenchery N., Siller-Matula J.M., Zeitlinger M., Jilma B., et al. Convalescent plasma treatment in patients with Covid-19: a systematic review and meta-analysis. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.817829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Axfors C., Janiaud P., Schmitt A.M., Van't Hooft J., Smith E.R., Haber N.A., et al. Association between convalescent plasma treatment and mortality in COVID-19: a collaborative systematic review and meta-analysis of randomized clinical trials. BMC Infect Dis. 2021;21:1170. doi: 10.1186/s12879-021-06829-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gupta T., Kannan S., Kalra B., Thakkar P. Systematic review and meta-analysis of randomised controlled trials testing the safety and efficacy of convalescent plasma in the treatment of coronavirus disease 2019 (COVID-19): evidence-base for practise and implications for research. Transfus Med. 2021;31 doi: 10.1111/tme.12803. 409-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bargay-Lleonart J., Sarubbo F., Arrizabalaga M., Guerra J.M., Borras J., El Haji K., et al. Reinforcement of the standard therapy with two infusions of convalescent plasma for patients with COVID-19: a randomized clinical trial. J Clin Med. 2022:11. doi: 10.3390/jcm11113039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Joyner M.J., Carter R.E., Senefeld J.W., Klassen S.A., Mills J.R., Johnson P.W., et al. Convalescent plasma antibody levels and the risk of death from Covid-19. N Engl J Med. 2021;384 doi: 10.1056/NEJMoa2031893. 1015-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sanz C., Nomdedeu M., Pereira A., Sauleda S., Alonso R., Bes M., et al. Efficacy of early transfusion of convalescent plasma with high-titer SARS-CoV-2 neutralizing antibodies in hospitalized patients with COVID-19. Transfusion. 2022 doi: 10.1111/trf.16863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Avendano-Sola C., Ramos-Martinez A., Munez-Rubio E., Ruiz-Antoran B., Malo de Molina R., Torres F., et al. A multicenter randomized open-label clinical trial for convalescent plasma in patients hospitalized with COVID-19 pneumonia. J Clin Invest. 2021:131. doi: 10.1172/JCI152740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sullivan D.J., Gebo K.A., Shoham S., Bloch E.M., Lau B., Shenoy A.G., et al. Early outpatient treatment for Covid-19 with convalescent plasma. N Engl J Med. 2022 doi: 10.1056/NEJMoa2119657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bellusci L., Golding H., Khurana S. Therapeutic potential of convalescent plasma and SARS-CoV-2 hyperimmune immunoglobulins against BQ.1, BQ.1.1 and XBB variants. J Clin Invest. 2023 doi: 10.1172/JCI168583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schrezenmeier H., Hoffmann S., Hofmann H., Appl T., Jahrsdorfer B., Seifried E., et al. Immune plasma for the treatment of COVID-19: lessons learned so far. Hamostaseologie. 2023;43:67–74. doi: 10.1055/a-1987-3682. [DOI] [PubMed] [Google Scholar]
- 12.Alsughayyir J., Almalki Y., Alburayk I., Alalshaik M., Aljoni I., Kandel M., et al. Prevalence of transfusion-transmitted infections in Saudi Arabia blood donors: a nationwide, cross-sectional study. Saudi Med J. 2022;43 doi: 10.15537/smj.2022.43.12.20220634. 1363-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Himmelsbach K., Mueller S., Kress J., Fiedler S.A., Miskey C., Ivics Z., et al. Second hepatitis C virus transmission by blood components since introduction of mandatory NAT screening in Germany. Transfusion. 2023;63 doi: 10.1111/trf.17224. 339-47. [DOI] [PubMed] [Google Scholar]
- 14.Candotti D., Assennato S.M., Laperche S., Allain J.P., Levicnik-Stezinar S. Multiple HBV transfusion transmissions from undetected occult infections: revising the minimal infectious dose. Gut. 2019;68 doi: 10.1136/gutjnl-2018-316490. 313-21. [DOI] [PubMed] [Google Scholar]
- 15.Cappy P., Barlet V., Lucas Q., Tinard X., Pillonel J., Gross S., et al. Transfusion of HIV-infected blood products despite highly sensitive nucleic acid testing. Transfusion. 2019;59 doi: 10.1111/trf.15203. 2046-53. [DOI] [PubMed] [Google Scholar]
- 16.Mawalla W.F., Njiro B.J., Bwire G.M., Nasser A., Sunguya B. No evidence of SARS-CoV-2 transmission through transfusion of human blood products: A systematic review. EJHaem. 2021. [DOI] [PMC free article] [PubMed]
- 17.Azhar E.I., Hindawi S.I., El-Kafrawy S.A., Hassan A.M., Tolah A.M., Alandijany T.A., et al. Amotosalen and ultraviolet A light treatment efficiently inactivates severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in human plasma. Vox Sang. 2020 doi: 10.1111/vox.13043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ragan I., Hartson L., Pidcoke H., Bowen R., Goodrich R. Pathogen reduction of SARS-CoV-2 virus in plasma and whole blood using riboflavin and UV light. PLoS One. 2020;15 doi: 10.1371/journal.pone.0233947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Focosi D., Franchini M. Impact of pathogen-reduction technologies on COVID-19 convalescent plasma potency. Transfus Clin Et Biol: J De la Soc Fr De Transfus Sang. 2021;28:132–134. doi: 10.1016/j.tracli.2021.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alandijany T.A., El-Kafrawy S.A., Tolah A.M., Sohrab S.S., Faizo A.A., Hassan A.M., et al. Development and optimization of in-house ELISA for detection of human IgG Antibody to SARS-CoV-2 full length spike protein. Pathogens. 2020:9. doi: 10.3390/pathogens9100803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Farnsworth C.W., Case J.B., Hock K., Chen R.E., O'Halloran J.A., Presti R., et al. Assessment of serological assays for identifying high titer convalescent plasma. Transfusion. 2021;61 doi: 10.1111/trf.16580. 2658-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kostin A.I., Lundgren M.N., Bulanov A.Y., Ladygina E.A., Chirkova K.S., Gintsburg A.L., et al. Impact of pathogen reduction methods on immunological properties of the COVID-19 convalescent plasma. Vox Sang. 2021;116 doi: 10.1111/vox.13056. 665-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prowse C.V. Component pathogen inactivation: a critical review. Vox Sang. 2013;104:183–199. doi: 10.1111/j.1423-0410.2012.01662.x. [DOI] [PubMed] [Google Scholar]
- 24.Coene J., Devreese K., Sabot B., Feys H.B., Vandekerckhove P., Compernolle V. Paired analysis of plasma proteins and coagulant capacity after treatment with three methods of pathogen reduction. Transfusion. 2014;54:1321–1331. doi: 10.1111/trf.12460. [DOI] [PubMed] [Google Scholar]
- 25.Sonego G., Abonnenc M., Crettaz D., Lion N., Tissot J.D., Prudent M. Irreversible oxidations of platelet proteins after riboflavin-UVB pathogen inactivation. Transfus Clin Et Biol: J De la Soc Fr De Transfus Sang. 2020;27:36–42. doi: 10.1016/j.tracli.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 26.Kaiser-Guignard J., Canellini G., Lion N., Abonnenc M., Osselaer J.C., Tissot J.D. The clinical and biological impact of new pathogen inactivation technologies on platelet concentrates. Blood Rev. 2014;28:235–241. doi: 10.1016/j.blre.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 27.Wasiluk T., Rogowska A., Boczkowska-Radziwon B., Zebrowska A., Bolkun L., Piszcz J., et al. Maintaining plasma quality and safety in the state of ongoing epidemic - the role of pathogen reduction. Transfus Apher Sci: J World Apher Assoc: J Eur Soc Haemapheresis. 2021;60 doi: 10.1016/j.transci.2020.102953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Focosi D., Franchini M. Clinical predictors of SARS-CoV-2 neutralizing antibody titers in COVID-19 convalescents: implications for convalescent plasma donor recruitment. Eur J Haematol. 2021;107:24–28. doi: 10.1111/ejh.13630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hayes C., Rubenstein W., Gibb D., Klapper E., Tanaka J., Pepkowitz S. Blood group O convalescent plasma donations have significantly lower levels of SARS-CoV-2 IgG antibodies compared to blood group A donations. Transfusion. 2021;61:2245–2249. doi: 10.1111/trf.16524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wendel S., Fontao-Wendel R., Fachini R., Candelaria G., Scuracchio P., Achkar R., et al. A longitudinal study of convalescent plasma (CCP) donors and correlation of ABO group, initial neutralizing antibodies (nAb), and body mass index (BMI) with nAb and anti-nucleocapsid (NP) SARS-CoV-2 antibody kinetics: proposals for better quality of CCP collections. Transfusion. 2021;61 doi: 10.1111/trf.16323. 1447-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mehew J., Johnson R., Roberts D., Griffiths A., Harvala H. Convalescent plasma for COVID-19: donor demographic factors associated high neutralising antibody titres. Transfus Med. 2022 doi: 10.1111/tme.12868. [DOI] [PMC free article] [PubMed] [Google Scholar]