Abstract
Purpose
Primary pulmonary lympho-epithelioma-like carcinoma (PPLELC) is a rare subtype of primary non-small cell lung cancer (NSCLC). Currently, there is still lack of research data on anti-angiogenic therapy of advanced PPLELC. The purpose of this study was to investigate the efficacy and safety of anti-angiogenic therapy combined with chemotherapy compared with traditional chemotherapy for these patients.
Methods
Advanced PPLELC patients admitted to six grade A hospitals from January 2013 to January 2021 were selected. The patients received anti-angiogenic therapy combined with chemotherapy (AT group) or chemotherapy (CT group) alone.
Results
A total of 65 patients were included in this study, including 31 patients in the AT group treated with anti-angiogenic therapy combined with chemotherapy and 34 patients in the CT group treated with chemotherapy alone. As of October 1, 2021, the median progression-free survival (PFS) in the AT group was 11.2 months [95% confidence interval (CI), 5.9–16.5]. The median PFS in the CT group was 7.0 months [95%CI, 5.1–8.9] [Hazard Ratio (HR), 0.49; 95%CI, 0.29–0.83; P = 0.008]. The 1-year PFS rates were 41.9% and 17.6%, respectively. The overall response rates (ORR) of two groups were 45.2% (95% CI, 0.27–0.64), 38.2% (95% CI, 0.21–0.56), (P = 0.571). The disease control rates (DCR) of two groups were 93.5% (95% CI, 0.84–1.03), 88.2% (95% CI, 0.77–1.00), (P = 0.756).
Conclusion
Among patients with advanced PPLELC, the PFS of patients with anti-angiogenic therapy combined with chemotherapy is better than that of patients with chemotherapy alone. Anti-angiogenic therapy combined with chemotherapy is an optional treatment scheme.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00432-022-03935-0.
Keywords: PPLELC, Anti-angiogenic therapy, Efficacy and safety, Retrospective study
Introduction
Lympho-epitheliomatoid carcinoma is a rare epithelial neoplasm originating mostly in the nasopharynx and also in the foregut (Ambrosio et al. 2011; Anand et al. 2018). Primary pulmonary lympho-epithelioma-like carcinoma is a rare subtype of primary non-small cell lung cancer that histologically resembles undifferentiated nasopharyngeal carcinoma (NPC) (Hayashi et al. 2012), the incidence in all cases of non-small cell lung cancer is about 0.7% (Anand et al. 2018; Xie et al. 2020). It was previously classified as a variant of large cell carcinoma (Travis et al. 2006), then reclassified as other and unclassified cancers in the 2015 World Health Organization (WHO) Classification of Tumors of the Lung (Travis et al. 2015).
First described by Bégin et al. (1987), PPLELC has been considered to be closely associated with Epstein–Barr virus (EBV) infection (Han et al. 2000). About 1600 cases have been reported worldwide in the past 33 years since the discovery (Kim et al. 2016; Darrason et al. 2019; Hong et al. 2019; Wu et al. 2020; Chen et al. 2021), mainly focused on the past five years, reporting mainly in Asia, especially Hong Kong, Taiwan, Guangdong and other regions. PPLELC usually affects never-smokers, is gender-neutral, and is younger than non-small cell lung cancer (Lin et al. 2016). Similar to other types of lung cancer, the prognosis is good for most patients diagnosed early with lung lympho-epithelioid, with a median overall survival of approximately 107 months and a 5-year survival of approximately 60%, compared with a mean survival of 13 months for patients with non-lung lympho-epithelioid (He et al. 2015; Liang et al. 2012).
The main treatment strategy for early PPLELC is surgery (Lin et al. 2016; Ho et al. 2006). For patients with distant metastasis, surgical treatment is not possible, and a variety of treatment modes are usually accepted, including chemotherapy, radiotherapy and immunotherapy (Kumar et al. 2017; Narayanan et al. 2019; Zhou et al. 2019; Tang et al. 2020; Qiu et al. 2019). Several treatments have been reported in the literature, but the treatment of PPLELC is empirical due to its rarity. However, there is still a lack of research data on anti-angiogenic therapy in advanced PPLELC. Therefore, this study aims to investigate the effect of anti-angiogenic therapy on advanced PPLELC patients.
Materials and methods
Patients
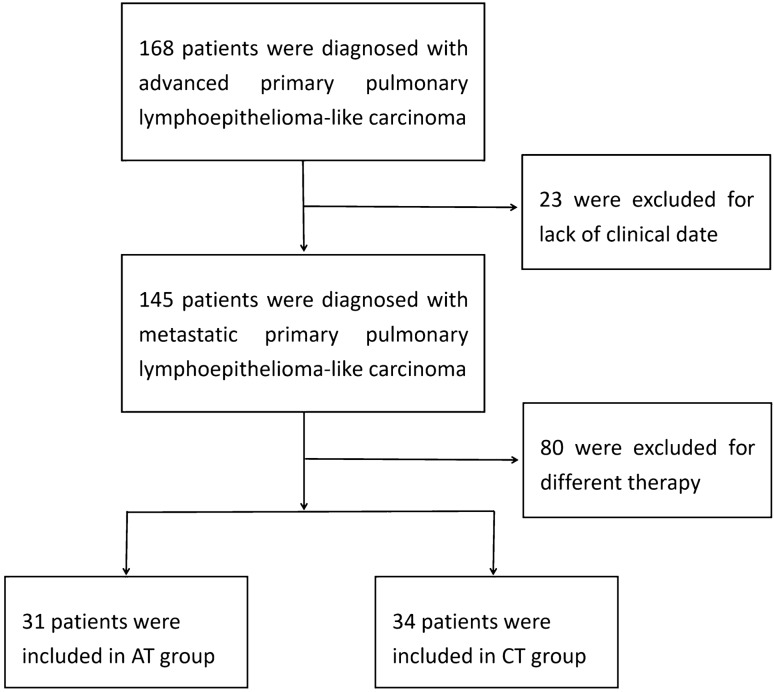
Patients aged 18 years or older, stage IIIB-IV, diagnosed as lung lympho-epithelioid carcinoma according to the 2015 WHO histological classification criteria for lung tumors, and without allergenic EGFR or ALK mutations; According to the Solid Tumor Response Assessment Criteria (RECIST), version 1.1 has at least one measurable lesion. This retrospective study collected data of PPLELC patients from Nanfang Hospital affiliated to Southern Medical University, Zhujiang Hospital affiliated to Southern Medical University, The First Affiliated Hospital of Guangzhou University of Traditional Chinese Medicine, Cancer Hospital of Sun Yat-sen University, Cancer Hospital affiliated to Guangzhou Medical University, Three Gorges Hospital affiliated to Chongqing University from January 2013 to January 2021. 65 patients with advanced PPLELC were eventually included in the study (Fig. 1).
Fig. 1.
Study enrollment. A total of 168 patients diagnosed with advanced PPLELC in six grade A hospitals from January 2013 to January 2021 were selected, and 65 patients were eventually included. Due to the lack of clinical data, 23 patients were excluded. 80 patients were excluded because the treatment regimen did not meet the requirements of the study. Finally, of the 65 included patients, 31 patients were included in the AT group combined with first-line anti-angiogenesis therapy, and 34 patients were included in the CT group with first-line chemotherapy
Experimental design and treatment
In this real-world retrospective study, patients were divided into AT group treated with anti-angiogenic therapy combined with chemotherapy and CT group treated with chemotherapy. AT group received anti-angiogenic therapy, including bevacizumab (n = 22), endu (n = 6) or anlotinib (n = 3), the chemotherapy regimen included paclitaxel (n = 14), pemetrexed (n = 10) or gemcitabine (n = 7) combined with platinum or mono-therapy. The chemotherapy regimen in CT group included paclitaxel (n = 19), pemetrexed (n = 10) or gemcitabine (n = 5) combined with platinum or mono-therapy.
Assessment
The tumor response assessment program is conducted every two to four cycles. Tumor response was assessed as complete response (CR), partial response (PR), disease stability (SD), or disease progression (PD) according to RECIST version 1.1. Adverse events and laboratory abnormalities were graded according to the National Cancer Institute Standard for Common Terminology for Adverse Events, Version 4.0.
Statistical analysis
Efficacy was assessed in the enrolled population and safety was assessed in the treated population. Kaplan–Meier methods were used to estimate progression-free survival. T test was used for measurement data (age) and Chi-square test (Cartesian test) was used for counting data. Univariate Cox regression was used to screen individual risk factors. The independent prognostic covariate of PPLELC patients was determined by multivariate Cox regression model and forward procedure. Cox proportional risk regression was used for univariate and multivariate survival analyses. All statistical analyses were performed in IBM SPSS Statistics (Version 19.0, Armonk, NY, USA). When P value was less than 0.05, the difference was considered statistically significant.
Results
Patient characteristics
The median age of patients was 54 years (interquartile range, 48–62 years). There were 38 female patients (58.5%) and 27 male patients (41.5%). ECOG score was 0 in 24 patients (36.9%) and 1 in 41 patients (63.1%). 18 patients (27.7%) were smokers, and 10 patients (15.4%) had a family history. There were 10 patients (15.4%) in stage IIIB and 55 patients (84.6%) in stage IV. Patients with single organ metastasis accounted for 19 (29.2%), and patients with multiple organ metastasis accounted for 46 (70.8%). The most common tumor sites were middle lobe of right lung and lower lobe of right lung, with 18 (27.7%) and 17 (26.1%) patients, respectively. There were 61 patients (95.3%) who were positive for Epstein–Barr virus, 15 patients (23.1%) had received radiotherapy, and 4 patients (6.2%) with gene mutations, including 3 gene mutations in the AT group, which were NRAS Q61L (14.5%) missense mutation, SMAD4 K110Ter (19.5%) mutation and FGFR3–TACC3 (F17:T10) fusion. MYC amplification was found in 1 case in CT group. There was no significant difference between the two groups at baseline (P > 0.05). (Table1).
Table 1.
Baseline characteristics
| All patients (n = 65) | AT group (n = 31) | CT group (n = 34) | P | |
|---|---|---|---|---|
| Age, years Median(IQR) | 54 (48–62) | 55 (48.5–61.5) | 54 (48–61.25) | 0.892 |
| Sex | 0.285 | |||
| Female | 27 (41.5%) | 15 (48.4%) | 12 (35.3%) | |
| Male | 38 (58.5%) | 16 (51.6%) | 22 (64.7%) | |
| ECOG performance status | 0.592 | |||
| 0 | 24 (36.9%) | 12 (38.7%) | 11 (32.3%) | |
| 1 | 41 (63.1%) | 19 (61.3%) | 23 (67.6%) | |
| Smokers | 18 (27.7%) | 10 (32.3%) | 8 (23.5%) | 0.432 |
| Family history | 10 (15.4%) | 3 (9.7%) | 7 (20.6%) | 0.309 |
| TNM | 0.382 | |||
| III B | 10 (15.4%) | 3 (9.7%) | 7 (20.6%) | |
| IV | 55 (84.6%) | 28 (90.3%) | 27 (79.4%) | |
| Metastases | 0.260 | |||
| Single organ | 19 (29.2%) | 7 (22.6%) | 12 (35.3%) | |
| Multiple organ | 46 (70.8%) | 24 (77.4%) | 22 (64.7%) | |
| Tumor location | 0.903 | |||
| Left upper lobe of lung | 10 (15.4%) | 5 (16.1%) | 5 (14.7%) | |
| Left lower lobe of lung | 15 (23.1%) | 8 (25.8%) | 7 (20.6%) | |
| Right upper lobe of lung | 5 (7.7%) | 2 (6.5%) | 3 (8.8%) | |
| Right middle lobe of lung | 18 (27.7%) | 7 (22.6%) | 11 (32.4%) | |
| Right lower lobe of lung | 17 (26.1%) | 9 (29.0%) | 8 (23.5%) | |
| EB( +) | 61 (95.3%) | 29 (93.5%) | 32 (94.1%) | 1.0 |
| Radiotherapy | 15 (23.1%) | 6 (19.3%) | 9 (26.5%) | 0.496 |
| Gene mutation | 4 (6.2%) | 3 (9.7%) | 1 (2.9%) | 0.540 |
Data are median number (IQR) or n (%). IQR interquartile range. Data from all patients who were enrolled in this study
Efficacy
The minimum follow-up time was about 1.4 months. Median follow-up time was 18 months in the AT group (95% CI, 13.5–26) and 19 months in the CT group (95% CI, 13–28). AT the end of the follow-up period, 11 patients (35.5%) in the AT group and 12 patients (35.3%) in the CT group continued treatment. Anti-angiogenic therapy was administered either as up-front or salvage therapy (first-line n = 18, ≥ second-line n = 13, cross-line n = 7), while chemotherapy was used in the first-line therapy.
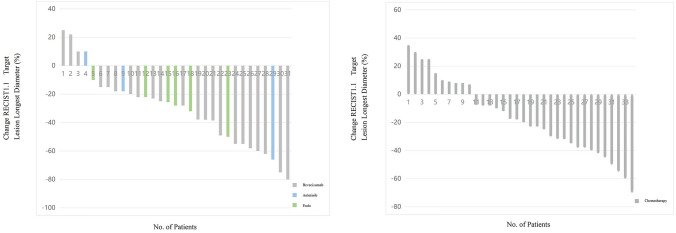
There were no CR cases in both AT group and CT group. In AT group, 14 patients (45.2%) reached PR, 15 patients (48.3%) reached SD, and 2 patients (6.5%) did not respond to treatment. In CT group, 13 patients (38.2%) reached PR, 17 patients (50.0%) reached SD, and 4 patients (11.8%) did not respond to treatment. The ORR of AT group and CT group were 14 (45.2%, 0.27–0.64) and 13 (38.2%, 0.21–0.56), P = 0.571; DCR were 29 (93.5%, 0.84–1.03) and 30 (88.2%, 0.77–1.00), P = 0.756 (Table 2, Fig. 2).
Table 2.
Efficacy of treatments
| AT group (n = 31) | CT group (n = 34) | P | |
|---|---|---|---|
| Best overall response | 0.763 | ||
| Complete response | 0 | 0 | |
| Partial response | 14 (45.2%) | 13 (38.2%) | |
| Stable disease | 15 (48.3%) | 17 (50.0%) | |
| Progressive disease | 2 (6.5%) | 4 (11.8%) | |
| Objective response | 14 (45.2%, 0.27–0.64) | 13 (38.2%, 0.21–0.56) | 0.571 |
| Disease control | 29 (93.5%, 0.84–1.03) | 30 (88.2%, 0.77–1.00) | 0.756 |
Data are n (%). Confirmed complete and partial responses were assessed by the investigator according to the Response Evaluation Criteria in Solid Tumors, version 1.1
Fig. 2.
Waterfall diagram of optimal efficacy evaluation for patients in the AT group and CT group. A In the AT group, 15 patients achieved SD status, 14 patients achieved PR status, and 2 patients did not respond to treatment. B In the CT group, 17 patients achieved SD status, 13 patients achieved PR status, and 4 patients did not respond to treatment
The median PFS was 11.2 months in the AT group [95% CI, 5.9–16.5] and 7.0 months in the CT Group [95% CI, 5.1–8.9] [HR, 0.49; 95% CI, 0.29–0.83; P = 0.008]. The 1-year PFS rates were 41.9% and 17.6%, respectively (Fig. 3, Supplement Table 1).
Fig. 3.
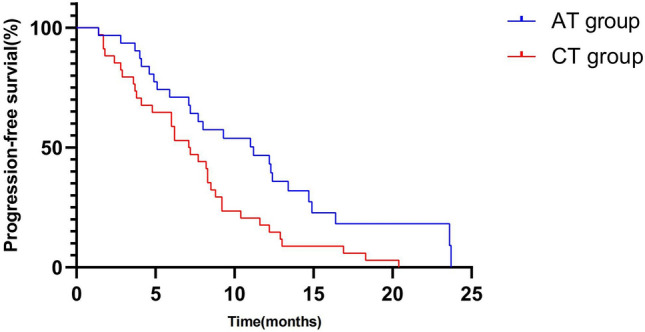
PFS in AT group and CT group. Median PFS was 11.2 months in the AT group [95% CI, 5.9–16.5] and 7.0 months in the CT group [95% CI, 5.1–8.9] [HR, 0.49; 95% CI, 0.29–0.83; P = 0.008]
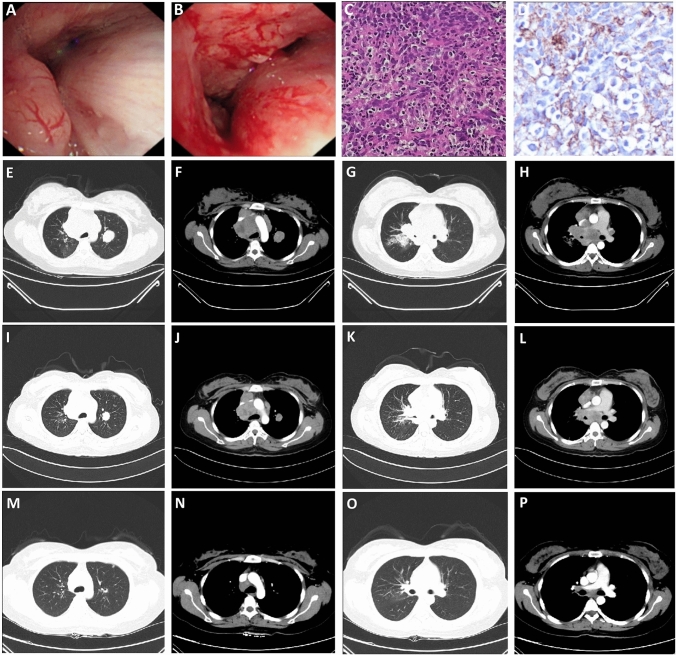
In this study, we reported a 59-year-old female patient with FGFR3–TACC3 (F17:T10) fusion, no smoking history, tumor located in the right lung with lymphatic, liver, and bone metastases, clinical stage T4N3M1, pathologically confirmed as PPLELC, Genetic analysis revealed FGFR3–TACC3 (F17:T10) fusion without EGFR, ALK and other conventional mutations. The patient was treated with anlotinib in combination with albumin–paclitaxel and carboplatin at the physician's recommendation, achieving disease control and PFS for 8 months (Fig. 4).
Fig. 4.
Radiographic images of the patient with FGFR3–TACC3 (F17:T10) fusion. A, B Fiber-bronchoscopy results in October 2018 indicated the growth of new organisms in the right main bronchus; C HE × 400 times, the results showed that some tumor cells were arranged in nests, with abundant cytoplasm, red staining, obvious nuclear atypia, interstitial fibrous hyperplasia accompanied by a large number of lymphocytes. D Immuno-histochemical × 400 times: PD-L1(22C3) CPS = 80; E–H Enhanced chest computed tomography images in October 2018, mass soft tissue density shadow near hilum in the upper lobe of right lung, with obstructive pneumonia, multiple lymph node metastases in mediastinum and bilateral hilum, and double lung metastases (E and G are pulmonary window, F and H are mediastinal window); I–L Enhanced chest computed tomography images in February 2019 showed that the mass soft tissue density shadow near hilum in the upper lobe of right lung was smaller than before, obstructive pneumonia was better than before, multiple lymph node metastases in mediastinum and bilateral hilum were smaller than before, and double lung metastases were smaller than before (I and K are lung window, J and L are mediastinal window); M–P Enhanced chest computed tomography images in June 2019 showed that the mass soft tissue density shadow near hilum in the upper lobe of the right lung was smaller than before, obstructive pneumonia was better than before, multiple lymph node metastases in mediastinum and bilateral hilum were smaller than before, and double lung metastases were smaller and less than before (M and O are lung window, N and P are mediastinal window)
Safety
Treatment-related adverse events (both hematological and non-hematological poisoning events) occurred more frequently in the AT group than in the CT group. In the AT group, there were 17 cases (54.8%) of grade 3 and 8 cases (25.8%) of grade 4 treatment-related adverse events. There were 13 cases (38.2%) of grade 3 and 12 cases (35.3%) of grade 4 treatment-related adverse reactions in the CT group. The most common adverse reactions in AT group were decreased appetite 7 (22.6%), anemia 8 (25.8%), leukopenia 14 (45.2%), neutropenia 16 (51.6%), and decreased platelet 8 (25.8%). Anemia 10 (29.4%), leukopenia 14 (41.2%), neutropenia 15 (44.1%), and thrombocytopenia 10 (29.4%) were most common in the CT group (Table 3).
Table 3.
Treatment-related adverse events
| AT group (n = 31) | CT group (n = 34) | |||||||
|---|---|---|---|---|---|---|---|---|
| Grade 1–2 | Grade 3 | Grade 4 | ALL | Grade1–2 | Grade3 | Grade4 | ALL | |
| Fatigue | 4 (12.9%) | 0 | 0 | 4 (12.9%) | 2 (5.9%) | 0 | 0 | 2 (5.9%) |
| Nausea | 6 (19.3%) | 0 | 0 | 6 (19.3%) | 5 (14.7%) | 0 | 0 | 5 (14.7%) |
| Vomiting | 4 (12.9%) | 0 | 0 | 4 (12.9%) | 4 (11.8%) | 0 | 0 | 4 (11.8%) |
| Anorexia | 7 (22.6%) | 0 | 0 | 7 (22.6%) | 6 (17.6%) | 0 | 0 | 6 (17.6%) |
| Peripheral sensory neuropathy | 2 (6.5%) | 0 | 0 | 2 (6.5%) | 2 (5.9%) | 0 | 0 | 2 (5.9%) |
| Hypertension | 2 (6.5%) | 2 (6.5%) | 0 | 4 (12.9%) | 0 | 0 | 0 | 0 |
| Proteinuria | 2 (6.5%) | 0 | 0 | 2 (6.5%) | 0 | 0 | 0 | 0 |
| Anemia | 8 (25.8%) | 0 | 0 | 8 (25.8%) | 10 (29.4%) | 0 | 0 | 10 (29.4%) |
| White blood cell decreased | 10 (32.3%) | 4 (12.9%) | 0 | 14 (45.2%) | 9 (26.4%) | 2 (5.9%) | 3 (8.8%) | 14 (41.2%) |
| Neutrophil count decreased | 2 (6.5%) | 6 (19.3%) | 8 (25.8%) | 16 (51.6%) | 3 (8.8%) | 3 (8.8%) | 9 (26.4%) | 15 (44.1%) |
| Lymphocyte count decreased | 4 (12.9%) | 2 (6.5%) | 6 (19.3%) | 3 (8.8%) | 3 (8.8%) | 0 | 6 (17.6%) | |
| platelet count decreased | 6 (19.3%) | 2 (6.5%) | 0 | 8 (25.8%) | 6 (17.6%) | 4 (11.8%) | 0 | 10 (29.4%) |
| ALT increased | 2 (6.5%) | 0 | 0 | 2 (6.5%) | 0 | 1 (2.9%) | 0 | 1 (2.9%) |
| AST increased | 2 (6.5%) | 0 | 0 | 2 (6.5%) | 2 (5.9%) | 0 | 0 | 2 (5.9%) |
| Blood bilirubin increased | 2 (6.5%) | 0 | 0 | 2 (6.5%) | 2 (5.9%) | 0 | 0 | 2 (5.9%) |
| Rash maculo-papular | 5 (16.1%) | 0 | 0 | 5 (16.1%) | 2 (5.9%) | 0 | 0 | 2 (5.9%) |
| Tracheal fistula | 0 | 1 (3.2%) | 0 | 1 (3.2%) | 0 | 0 | 0 | 0 |
| Overall | 68 | 17 | 8 | 93 | 56 | 13 | 12 | 81 |
Discussion
PPLELC is a rare subtype of NSCLC. In this era of targeted therapy and checkpoint inhibitors for non-small cell lung cancer, anti-angiogenic drugs still play an important role (Shukla et al. 2020). However, the prognosis of advanced PPLELC is poor, due to the lack of drug treatment data, the role of anti-angiogenic therapy in PPLELC is unknown. It is worth exploring the effect of anti-angiogenic therapy in PPLELC.
Induction of angiogenesis is one of the 10 characteristics of malignant tumors. Angiogenesis provides essential nutrients for tumor growth and is an important prerequisite for tumor hematogenous dissemination. The ECOG-4599 study established the status of bevacizumab combined with first-line chemotherapy (Sandler et al. 2007). Small molecule tyrosine kinase inhibitor (TKI) drugs targeting angiogenesis have become one of the research hotspots in recent years. The biggest feature of small molecule drugs is that their target coverage is more comprehensive. In addition to VEGF/R and other pathways, they can also cover PDGF/R, FGF/FGFR and other pathways.
In this study, a patient with FGFR3–TACC3 fusion was reported. In general, operable FGFR3 gene fusion is found relatively commonly in glioma and bladder cancer (Wu et al. 2013), FGFR3–TACC3 fusion has been reported in 2.5% of NPC (Yuan et al. 2014). However, FGFR3 alteration was rarely observed in NSCLC, and was detected in 0.1% of adenocarcinomas and 0.6% of squamous cell carcinomas, respectively (AACR Project GENIE Consortium 2017; Qin et al. 2019). The prevalence of FGFR3 in LELC was 4% (Chau et al. 2065). FGFR3–TACC3 is reported to be a relapsing drug resistance mechanism that can bypass EGFR blockade by all generations of EGFR TKI in NSCLC (Ou et al. 2017). In this study, FGFR3–TACC3 fusion patients were treated with small molecule multi-target TKI, which inhibited VEGFR, PDGFR, FGFR and c-Kit targets, showing anti-tumor angiogenesis and tumor growth inhibition. PFS benefits were obtained in combination with chemotherapy, suggesting that FGFR3 aberrations may represent an opportunity to target therapy in PPLELC.
However, anti-angiogenic therapy alone does not significantly improve patient outcomes, as the removal of blood vessels transforms tumor cells into a hypoxic-tolerant phenotype. Combination of anti-angiogenic therapy with other therapies, including chemotherapy, radiotherapy, immunotherapy and anti-EGFR therapy, has good efficacy due to the vaso-normalization effect induced by anti-angiogenic agents (Tian et al. 2020). Radiation alone as the sole means of treating cancer often triggers the angiogenesis pathway, leading to upregulation of multiple angiogenic growth factors, and anti-angiogenic inhibitors can help overcome this fact (Rani and Prabhu 2021). Randomized Phase III studies have also shown that treatment with antiangiogenic agents in combination with programmed cell death ligand 1 (PD-L1) antibodies significantly improves survival compared with standard therapy in renal cell carcinoma (RCC), NSCLC and hepatocellular carcinoma (HCC) (Hack et al. 2020). However, there are still many problems to be solved, such as how to choose a more reasonable combination partner for anti-angiogenic therapy, the timing, recommended dosage and the proportion of anti-angiogenic inhibitor combination therapy (Tian et al. 2020). In this study, anti-angiogenic therapy combined with chemotherapy in patients with advanced PPLELC was included. The results showed that compared with chemotherapy alone, it can improve the short-term efficacy of patients. It is well known that immunotherapy can improve the long-term prognosis of patients. If anti-angiogenic combined with immunotherapy and chemotherapy is a better choice, it is worth further exploration.
Our study found that anti-angiogenic therapy combined with chemotherapy was more beneficial than chemotherapy alone in patients with advanced PPLELC, but there was no way to screen the dominant population, and some patients did not respond to anti-angiogenic therapy combined with chemotherapy. Both targeted therapy and immunotherapy have corresponding markers to accurately identify the population, but there is no recognized marker in the field of anti-angiogenic therapy. Bevacizumab is a monoclonal antibody with a clear target of VEGF, so it is logical that VEGF expression might predict benefit. However, clinical studies have found that VEGF expression does not predict the benefit of bevacizumab addition (Otrock et al. 2011). The tumor vasculature is a target for antiangiogenic drugs, but quantitative or qualitative measures of the vasculature cannot be used as biomarkers of efficacy because there is no standardized method for measuring micro-vessel density and there is considerable potential for selection and observer bias. The detection of vascular phenotype and tumor cell genotype is still in the stage of preclinical research (Jubb et al. 2006). Compared to using the tissue biomarkers to select effective anti-angiogenic drugs, the studies of discover and validation in the non-invasive, dynamic biomarker were at a more advanced stage, including measuring the circulating protein related to angiogenesis, circulating microRNAs, secrete body outside, circulating endothelial cells and or estimates of progenitor cells and vascular imaging (Paolo et al. 2020).
Our study has some limitations. This study was from a retrospective cohort and the number of included patients was limited. This is due to the rarity of PPLELC and the small number of patients, even fewer patients in the advanced stage of metastasis. Moreover, the emergence and application of different anti-angiogenic drugs in clinical practice will affect the results. Differences in patients' chemotherapy regimens also affected the results. However, this study first reported the value of anti-angiogenic therapy in advanced PPLELC. Data were collected from multiple centers in this study, and patients came from areas with high PPLELC incidence, which has certain advantages.
In conclusion, compared with the current treatment of patients with advanced PPLELC, anti-angiogenic therapy is clinically beneficial and sufficiently safe, and the number of patients should be further expanded or the follow-up period extended to further observe the survival benefit.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors are grateful to all staff at the study centre who contributed to this study.
Author contributions
HJB: Conceptualization; Data curation; Resources; Writing—original draft; Writing—review & editing. LZM, XXL, MGY: Methodology; Visualization; Writing—review & editing. BSZ, JZ, GBP, XTL: Resources; Validation; Visualization. YHF, HHB: Formal analysis; Methodology; Software; Writing—original draft. SDM: Funding acquisition; Project administration; Supervision; Visualization. All authors read and approved the final manuscript.
Funding
This work was supported by the Antitumor Angiogenesis Targeted Therapy Research Fund of the Chinese Society of Clinical Oncology (Y-S2016-004), the Nanfang Hospital Dean's Fund (2018B013), the Wu Jieping Medical Foundation (320.6750.19061) and the General project of Chongqing Natural Science Foundation (cstc2021jcyj-msxmX0950).
Data availability
Datasets generated and analyzed during the study are available from HB on reasonable request.
Code availability
Not applicable.
Declarations
Conflict of interest
All authors declare no conflicts of interest associated with this manuscript.
Ethics approval
Because of the nature of retrospective design and patient anonymization, the ethical board of the Institutional Ethics Committee of the Southern medical university Nanfang Hospital approved the retrospective study.
Consent to participate
The need for written informed consent was waived due to the retrospective nature of the study.
Consent for publication
All authors have consented to publication of the results presented in this manuscript.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Hejing Bao, Ling Zhen Ma, Xiaoli Lin, and Mengge Yu contributed equally.
References
- AACR Project GENIE Consortium AACR Project GENIE: powering precision medicine through an international consortium. Cancer Discov. 2017;7(8):818–831. doi: 10.1158/2159-8290.CD-17-0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrosio MR, Rocca BJ, Onorati M, et al. Lymphoepithelioma-like carcinoma of the ovary. Int J Surg Pathol. 2011;19(4):514–517. doi: 10.1177/1066896909354336. [DOI] [PubMed] [Google Scholar]
- Anand A, Zayac A, Curtiss C, Graziano S. Pulmonary lymphoepithelioma-like carcinoma disguised as squamous cell carcinoma. J Thorac Oncol. 2018;13(5):e75–e76. doi: 10.1016/j.jtho.2017.11.133. [DOI] [PubMed] [Google Scholar]
- Bégin LR, Eskandari J, Joncas J, Panasci L. Epstein-Barr virus related lymphoepithelioma-like carcinoma of lung. J Surg Oncol. 1987;36(4):280–283. doi: 10.1002/jso.2930360413. [DOI] [PubMed] [Google Scholar]
- Chau SL, Tong JH, Chow C, et al. Distinct molecular landscape of Epstein-Barr virus associated pulmonary lymphoepithelioma-like carcinoma revealed by genomic sequencing. Cancers (basel) 2020;12(8):2065. doi: 10.3390/cancers12082065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Gu C, Chen X, et al. Clinicopathological and prognostic analyses of 86 resected pulmonary lymphoepithelioma-like carcinomas. J Surg Oncol. 2021;123(2):544–552. doi: 10.1002/jso.26276. [DOI] [PubMed] [Google Scholar]
- Darrason M, Martin A, Soussan M, et al. Immunotherapy for LELC: case report and a focused review. Clin Lung Cancer. 2019;20(3):e393–e401. doi: 10.1016/j.cllc.2018.12.008. [DOI] [PubMed] [Google Scholar]
- Di Paolo V, Colletti M, Ferruzzi V, et al. Circulating biomarkers for tumor angiogenesis: where are we? Curr Med Chem. 2020;27(14):2361–2380. doi: 10.2174/0929867325666180821151409. [DOI] [PubMed] [Google Scholar]
- Hack SP, Zhu AX, Wang Y. Augmenting anticancer immunity through combined targeting of angiogenic and PD-1/PD-L1 pathways: challenges and opportunities. Front Immunol. 2020;11:598877. doi: 10.3389/fimmu.2020.598877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han AJ, Xiong M, Zong YS. Association of Epstein-Barr virus with lymphoepithelioma-like carcinoma of the lung in southern China. Am J Clin Pathol. 2000;114(2):220–226. doi: 10.1309/148K-ND54-6NJX-NA61. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Haba R, Tanizawa J, et al. Cytopathologic features and differential diagnostic considerations of primary lymphoepithelioma-like carcinoma of the lung. Diagn Cytopathol. 2012;40(9):820–825. doi: 10.1002/dc.21670. [DOI] [PubMed] [Google Scholar]
- He J, Shen J, Pan H, Huang J, Liang W, He J. Pulmonary lymphoepithelioma-like carcinoma: a surveillance, epidemiology, and end results database analysis. J Thorac Dis. 2015;7(12):2330–2338. doi: 10.3978/j.issn.2072-1439.2015.12.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho JC, Wong MP, Lam WK. Lymphoepithelioma-like carcinoma of the lung. Respirology. 2006;11(5):539–545. doi: 10.1111/j.1440-1843.2006.00910.x. [DOI] [PubMed] [Google Scholar]
- Hong S, Liu D, Luo S, et al. The genomic landscape of Epstein-Barr virus-associated pulmonary lymphoepithelioma-like carcinoma. Nat Commun. 2019;10(1):3108. doi: 10.1038/s41467-019-10902-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jubb AM, Oates AJ, Holden S, Koeppen H. Predicting benefit from anti-angiogenic agents in malignancy. Nat Rev Cancer. 2006;6(8):626–635. doi: 10.1038/nrc1946. [DOI] [PubMed] [Google Scholar]
- Kim C, Rajan A, DeBrito PA, Giaccone G. Metastatic lymphoepithelioma-like carcinoma of the lung treated with nivolumab: a case report and focused review of literature. Transl Lung Cancer Res. 2016;5(6):720–726. doi: 10.21037/tlcr.2016.11.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V, Dave V, Harris J, Huang Y. Response of advanced stage recurrent lymphoepithelioma-like carcinoma to nivolumab. Immunotherapy. 2017;9(12):955–961. doi: 10.2217/imt-2017-0067. [DOI] [PubMed] [Google Scholar]
- Liang Y, Wang L, Zhu Y, et al. Primary pulmonary lymphoepithelioma-like carcinoma: fifty-two patients with long-term follow-up. Cancer. 2012;118(19):4748–4758. doi: 10.1002/cncr.27452. [DOI] [PubMed] [Google Scholar]
- Lin Z, Situ D, Chang X, et al. Surgical treatment for primary pulmonary lymphoepithelioma-like carcinoma. Interact Cardiovasc Thorac Surg. 2016;23(1):41–46. doi: 10.1093/icvts/ivw064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan A, Knollmann FD, Walby JAS, Lim S, Gandara DR, Riess JW. EBV-positive primary pulmonary lymphoepithelioma-like carcinoma response to PD-L1 blockade. Clin Lung Cancer. 2019;20(3):e238–e241. doi: 10.1016/j.cllc.2018.12.015. [DOI] [PubMed] [Google Scholar]
- Otrock ZK, Hatoum HA, Musallam KM, Awada AH, Shamseddine AI. Is VEGF a predictive biomarker to anti-angiogenic therapy? Crit Rev Oncol Hematol. 2011;79(2):103–111. doi: 10.1016/j.critrevonc.2010.07.008. [DOI] [PubMed] [Google Scholar]
- Ou SI, Horn L, Cruz M, et al. Emergence of FGFR3-TACC3 fusions as a potential by-pass resistance mechanism to EGFR tyrosine kinase inhibitors in EGFR mutated NSCLC patients. Lung Cancer. 2017;111:61–64. doi: 10.1016/j.lungcan.2017.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin A, Johnson A, Ross JS, et al. Detection of known and novel FGFR fusions in non-small cell lung cancer by comprehensive genomic profiling. J Thorac Oncol. 2019;14(1):54–62. doi: 10.1016/j.jtho.2018.09.014. [DOI] [PubMed] [Google Scholar]
- Qiu ZX, Zhou P, Wang K. Primary pulmonary lymphoepithelioma-like carcinoma response favorably to nivolumab: a case report. Onco Targets Ther. 2019;12:8595–8600. doi: 10.2147/OTT.S219512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rani V, Prabhu A. Combining angiogenesis inhibitors with radiation: advances and challenges in cancer treatment. Curr Pharm Des. 2021;27(7):919–931. doi: 10.2174/1381612826666201002145454. [DOI] [PubMed] [Google Scholar]
- Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer [published correction appears in N Engl J Med. 2007 Jan 18;356(3):318] N Engl J Med. 2006;355(24):2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- Shukla NA, Yan MN, Hanna N. The story of angiogenesis inhibitors in non-small-cell lung cancer: the past, present, and future. Clin Lung Cancer. 2020;21(4):308–313. doi: 10.1016/j.cllc.2020.02.024. [DOI] [PubMed] [Google Scholar]
- Tang Z, Fang R, Tong G, Liu P, Ou Z, Tang Y. Overcoming resistance to anti-PD-1 immunotherapy in lymphoepithelioma-like carcinoma: a case report and review of the literature. Lung Cancer. 2020;146:335–340. doi: 10.1016/j.lungcan.2020.06.027. [DOI] [PubMed] [Google Scholar]
- Tian W, Cao C, Shu L, Wu F. Anti-angiogenic therapy in the treatment of non-small cell lung cancer. Onco Targets Ther. 2020;13:12113–12129. doi: 10.2147/OTT.S276150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis WD, Garg K, Franklin WA, et al. Bronchioloalveolar carcinoma and lung adenocarcinoma: the clinical importance and research relevance of the 2004 World Health Organization pathologic criteria. J Thorac Oncol. 2006;1(9 Suppl):S13–S19. doi: 10.1097/01243894-200611001-00004. [DOI] [PubMed] [Google Scholar]
- Travis WD, Brambilla E, Burke AP, Marx A, Nicholson AG. Introduction to the 2015 world health organization classification of tumors of the lung, pleura, thymus, and heart. J Thorac Oncol. 2015;10(9):1240–1242. doi: 10.1097/JTO.0000000000000663. [DOI] [PubMed] [Google Scholar]
- Wu YM, Su F, Kalyana-Sundaram S, et al. Identification of targetable FGFR gene fusions in diverse cancers. Cancer Discov. 2013;3(6):636–647. doi: 10.1158/2159-8290.CD-13-0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Wang W, Zhou P, et al. Primary pulmonary lymphoepithelioma-like carcinoma is characterized by high PD-L1 expression, but low tumor mutation burden. Pathol Res Pract. 2020;216(8):153043. doi: 10.1016/j.prp.2020.153043. [DOI] [PubMed] [Google Scholar]
- Xie Z, Liu L, Lin X, et al. A multicenter analysis of genomic profiles and PD-L1 expression of primary lymphoepithelioma-like carcinoma of the lung. Mod Pathol. 2020;33(4):626–638. doi: 10.1038/s41379-019-0391-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan L, Liu ZH, Lin ZR, Xu LH, Zhong Q, Zeng MS. Recurrent FGFR3-TACC3 fusion gene in nasopharyngeal carcinoma. Cancer Biol Ther. 2014;15(12):1613–1621. doi: 10.4161/15384047.2014.961874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou N, Lin Y, Peng X, Wang Y, Wang Y. Thorough survey and analysis of pulmonary lymphoepithelioma-like carcinoma in Macau and multimodality treatment for advanced disease. Lung Cancer. 2019;138:116–123. doi: 10.1016/j.lungcan.2019.10.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Datasets generated and analyzed during the study are available from HB on reasonable request.
Not applicable.