Summary
Background
Brexanolone has rapid, long-lasting, and remarkable efficacy in the treatment of post-partum depression (PPD). We test the hypothesis that brexanolone inhibits proinflammatory modulators and macrophage activation in PPD patients, which may promote clinical recovery.
Methods
PPD patients (N = 18) provided blood samples before and after brexanolone infusion according to the FDA-approved protocol. Patients were unresponsive to prior treatment before brexanolone therapy. Serum was collected to determine neurosteroid levels and whole blood cell lysates were examined for inflammatory markers and in vitro responses to the inflammatory activators lipopolysaccharide (LPS) and imiquimod (IMQ).
Findings
Brexanolone infusion altered multiple neuroactive steroid levels (N = 15–18), reduced levels of inflammatory mediators (N = 11) and inhibited their response to inflammatory immune activators (N = 9–11). Specifically, brexanolone infusion reduced whole blood cell tumor necrosis factor-α (TNF-α, p = 0.003), and interleukin-6 (IL-6, p = 0.04) and these effects were correlated with HAM-D score improvement (TNF-α, p = 0.049; IL-6, p = 0.02). Furthermore, brexanolone infusion prevented LPS and IMQ-induced elevation of TNF-α (LPS: p = 0.02; IMQ: p = 0.01), IL-1β (LPS: p = 0.006; IMQ: p = 0.02) and IL-6 (LPS: p = 0.009; IMQ: p = 0.01), indicating inhibition of toll-like receptor (TLR)4 and TLR7 responses. Finally, inhibition of TNF-α, IL-1β and IL-6 responses to both LPS and IMQ were correlated with HAM-D score improvements (p < 0.05).
Interpretation
Brexanolone actions involve inhibition of inflammatory mediator production and inhibition of inflammatory responses to TLR4 and TLR7 activators. The data suggest that inflammation plays a role in post-partum depression and that inhibition of inflammatory pathways contributes to the therapeutic efficacy of brexanolone.
Funding
The Foundation of Hope, Raleigh, NC and UNC School of Medicine, Chapel Hill.
Keywords: [3α,5α]-3-hydroxy-pregnan-20-one (3α,5α-THP); Cytokines; Toll-like receptors; Hamilton rating scale for depression (HAM-D)
Research in context.
Evidence before this study
The mechanisms of the long-lasting and strong therapeutic efficacy of brexanolone in postpartum depression are unknown. Brexanolone is an intravenous preparation of the endogenous neuroactive steroid allopregnanolone, which has potent activity as a positive modulator of GABAA receptors, is an inhibitor of the hypothalamic-pituitary stress response and inhibits inflammatory pathways in macrophages and brain mediated by toll-like receptors (TLR4 and TLR7). Brexanolone infusion for 60 h produces therapeutic actions within 8–10 h that last up to 90 days. It is unknown which actions account for its therapeutic effects and these investigations have relevance to the core pathology of post-partum depression, as well other forms of depression.
Added value of this study
The data show that brexanolone infusion alters multiple neuroactive steroid levels, inhibits production of inflammatory mediators TNF-α and IL-6 and inhibits inflammatory responses to lipopolysaccharide (LPS) and imiquimod (IMQ) in immune cells in the blood in association with clinical improvement. This data directly links a mechanism of brexanolone action to its therapeutic efficacy.
Implications of all the available evidence
Together with previous studies showing that depression can be associated with elevations of pro-inflammatory modulators in blood, these results implicate the TLR4 and TLR7 inflammatory pathways along with TNF-α, IL-1β and IL-6 in the etiology of post-partum depression and suggest that other inhibitors of these pathways may have therapeutic efficacy in both post-partum and major depression.
Introduction
Postpartum depression (PPD), defined here as a major depressive episode starting in the third trimester of pregnancy or within 4 weeks after delivery, is the one of the most common complications of childbirth, with prevalence estimates around 15%.1, 2, 3 In 2019, brexanolone became the first Food and Drug Administration (FDA) approved treatment for PPD.4 Brexanolone is an intravenous formulation of allopregnanolone, a derivative of progesterone, and a highly active neurosteroid. The drug is given via intravenous infusion over 60 h, and patients’ mood dramatically improves compared to placebo with a low non-response rate and duration of action up to 9 months.2,5,6 In light of these rapid and sustained clinical improvements, we posited that investigation of its mechanisms of action would also advance our understanding of the etiology of postpartum depression.
Like many psychiatric disorders, PPD likely has biological and psychosocial components. Indeed, PPD has unique features involving dysregulation of the hypothalamic-pituitary-adrenal (HPA) stress axis, proinflammatory signaling, and GABA circuitry.7 Previous work has shown that allopregnanolone (specifically–[3α,5α]-3-hydroxy-pregnan-20-one; 3α,5α-THP) has anti-inflammatory effects in macrophages and the brain via inhibition of toll-like receptor-4 (TLR4) and TLR7 pathway activation.8,9 Indeed, elevations in proinflammatory cytokines IL-6 (>27%; median levels: 0.70 pg/ml/healthy subjects and 0.89 pg/ml/PPD patients), TNF-α (12.5%; median levels: 2.4 pg/ml/healthy subjects and 2.7 pg/ml/PPD patients) and other modulators in plasma of PPD patients at 6–12 weeks post-partum compared to healthy subjects have been reported.10 Elevation in IL-1β (∼122%) was also reported in urine of women with PPD symptoms (16.2 pg/ml) on day 28 post-partum compared to levels in women without depressive symptoms (7.3 pg/ml).11 Elevations in proinflammatory modulators have been also reported in other forms of depression.12, 13, 14, 15 However, it is unknown if the inhibition of inflammation through these pathways contributes to the therapeutic efficacy of brexanolone in PPD. We hypothesized that brexanolone inhibits proinflammatory neuroimmune signaling and/or responses to inflammatory activators in PPD patients to promote clinical recovery. Better understanding the therapeutic mechanisms of this drug may lead to faster development of additional effective treatments that optimize patient care while minimizing the need for hospital admission and treatment cost.
Methods
Participants, brexanolone administration procedure, and HAM-D score evaluation
Participants
This study received institutional review board approval for recruited patients in the University of North Carolina (UNC), Chapel Hill. Informed consent from all participants was obtained. Subjects in this study met criteria for infusion as outlined in UNC's Clinical Brexanolone Treatment Program,6 which offers access to this treatment for women with moderate to severe PPD with HAM-D scores greater or equal to 18. Eighteen post-partum depression participants aged between 24 and 41 years of age volunteered for the study. By definition, the PPD would have started during the third trimester or within 4 weeks of delivery, and yet on average these women received the brexanolone infusion at 3.4 months postpartum. Most of these women were in outpatient treatment for their depression, with 17 out of the 18 participants being on some sort of psychotropic regimen to address their PPD. Despite this treatment in the community, these cases still met criteria for moderate to severe PPD and thus brexanolone treatment was warranted. See Table 1 for patient demographics including the concurrent psychotropic medications used by number and class. No patients in the study were cigarette smokers.
Table 1.
Demographic and clinical data of study patients with post-partum depression (PPD).
| Mean age (years) | 31 (24–41) N = 18 (%) |
||
| Race/ethnicity | |||
| Non-Hispanic white | 16 (88) | ||
| Hispanic white | 1 (6) | ||
| Other, Non-Hispanic | 1 (6) | ||
| Marital status | |||
| Married | 16 (88) | ||
| Domestic partner | 1 (6) | ||
| Legally separated | 1 (6) | ||
| Patient characteristics | Statistic N = 18 (%) |
HAM-D score baseline (SD) | HAM-D score post infusion (SD) |
| Presence of psychiatric comorbidity/historya | |||
| No | 0 | – | – |
| Yes | 18 (100) | 23 (5) | 11 (7) |
| Concurrent psychiatric treatment | |||
| None | 1 (6) | 23 (0) | 12 (0) |
| Single psychotropic | 4 (22) | 25 (3) | 8 (3) |
| Two psychotropics | 4 (22) | 22 (3) | 12 (7) |
| Three psychotropics | 5 (28) | 22 (7) | 10 (8) |
| Four psychotropics | 4 (22) | 25 (4) | 15 (7) |
| Psychotropic class typeb | |||
| SSRI | 12 (67) | ||
| SGA | 9 (50) | ||
| Mood stabilizer | 5 (28) | ||
| Benzodiazepine | 3 (17) | ||
| Anxiolytic | 3 (17) | ||
| SNRI | 2 (11) | ||
| SARI | 2 (11) | ||
| NDRI | 2 (11) | ||
| Antihistamine | 2 (11) | ||
| Beta blocker | 1 (6) | ||
| Alpha blocker | 1 (6) | ||
| Anti-epileptic | 1 (6) |
SD: standard deviation.
Psychiatric Comorbidity/History includes: anxiety (67%), previous PPD episodes (39%), past major depressive episodes (33%), and PTSD (22%).
SSRI (selective serotonin reuptake inhibitor): sertraline, escitalopram, fluoxetine. SGA (second generation antipsychotic): quetiapine, aripiprazole, lurasidone, olanzapine. Mood stabilizer: lithium. Benzodiazepines: clonazepam, ativan. Anxiolytic: buspar. SNRI (serotonin norepinephrine reuptake inhibitor): desvenlafaxine, duloxetine. SARI (serotonin 2 antagonist/reuptake inhibitor): trazodone. NDRI (norepinephrine dopamine reuptake inhibitor): Wellbutrin. Antihistamine: vistaril, Benadryl. Beta blocker: propranolol. Alpha blocker: prazosin. Anti-epileptic: pregabalin.
Brexanolone administration procedure
Brexanolone injection is a sterile solution of 5 mg/ml allopregnanolone in 250 mg/ml sulfobutylether-β-cyclo-dextrin, buffered with citrate and diluted with sterile water. Each patient received a continuous infusion according to the FDA-approved protocol: 30 μg/kg per h (0–4 h); 60 μg/kg per h (4–24 h); 90 μg/kg per h (24–52 h); 60 μg/kg per h (52–56 h); 30 μg/kg per h (56–60 h). Patients were treated in a medically-supervised setting for 72 h, consisting of 60 h of study drug infusion and an additional 12 h for completion of assessments. Blood was drawn approximately 1 h before (9-10 am) and 6 h after (10-11 am) brexanolone infusion. No fasting was required. Meals were provided at the usual intervals throughout hospitalization, but not prior to the pre-brexanolone or post-brexanolone blood draws on admission day or discharge day.
The Hamilton rating scale for depression (HAM-D) score evaluation
The HAM-D, a 17-item diagnostic questionnaire, was used to measure depression severity ∼2 h before and 6 h after brexanolone infusion. HAM-D consists of individual ratings related to depressed mood, feelings of guilt, suicide, insomnia, agitation, anxiety, somatic and genital symptoms, hypochondriasis, loss of weight, and insight. Higher HAM-D scores indicate more severe depression.16 We report the % HAM-D score improvement as the % decrease in HAM-D score 6 h following brexanolone infusion compared to HAM-D scores measured 1 h prior to brexanolone infusion.
Serum preparation and neuroactive steroid measurements
Blood was collected in Vacutainer® Glass Serum Tubes (BD, 366430), allowed to clot for 30–60 min at room temperature, then centrifuged at 1300×g at 20°C for 10 min. Serum was collected in 2 ml polypropylene tubes, then stored at −80°C until assay. Serum was assayed by gas chromatography-mass spectrometry (GCMS) as previously described,17 with extraction modifications.18 Briefly, 300 μl of serum was applied to a preconditioned Strata™-X 33 μm column (Phenomenex, 8B-S100-UBL), washed with water, 30% methanol in water, dried on a vacuum manifold, and washed again with n-hexane. Steroids were eluted with acetonitrile into glass vials treated with Sigmacote® (Sigma-Aldrich, SL2) and dried down. Samples were derivatized with heptafluorobutyric acid in ethyl acetate, dried down, and transferred to deactivated glass inserts. The samples were diluted in 10 μl of heptane, and 2 μl was injected into the GCMS in duplicate. GCMS settings and analysis were performed as previously described17 which allowed simultaneous measurement of allopregnanolone, pregnanolone (3α,5β-THP), allotetrahydrodeoxycorticosterone (3α,5α-THDOC), pregnenolone, 3α,5α-androsterone and 3α,5α-androstan-diol in PPD patient serum. Other pregnane steroids such as the 3β-reduced derivatives of allopregnanolone and THDOC were not measured. Results are expressed as picograms/milliliter (pg/ml).
Whole blood collection for in vitro whole-blood immune challenge
Whole blood (∼8 ml/subject) was drawn in sodium heparin coated Vacutainer® Plastic Tubes (BD, 367878). Immediately after blood drawing, the tubes were gently inverted 8–10 times to prevent breakage of red blood cells, but to ensure proper mixture with sodium heparin to prevent clotting and put on ice to minimize blood cell degeneration. Blood was kept on ice <1 h for transfer from the hospital to the laboratory before experimentation.
Baseline measurements of TNF-α, IL-6, IL-1β, and IP-10
Whole blood cell lysates were prepared by adding 500 μl of radioimmunoprecipitation (RIPA) buffer (Sigma, Cat. #R0278) supplemented with protease inhibitor cocktails (ratio 1:100) (Sigma, Cat. #P8340) to 500 μl of whole blood (ratio 1:1) and the mixtures were immediately centrifuged (9500×g; 4°C) for 5 min. Pellets were isolated by gently removing the supernatant and the supernatant residuals. RIPA buffer supplemented with protease inhibitor cocktails (100 μl) was added to the cell pellets. The mixtures were vortexed, kept on ice for 15 min, sonicated twice for 30 s at 25% output power with a Sonicator ultrasonic processor (Misonix, Inc., Farmingdale, NY) and centrifuged (14,000×g; 4°C) for 30 min. Cell lysates were kept at −80°C until the levels of TNF-α, IL-6, IL-1β, and IP-10 were assessed by enzyme-linked immunosorbent assays (ELISAs).
In vitro stimulation of whole blood by lipopolysaccharide and imiquimod and cell lysate preparation
To avoid blood contamination, under sterile conditions, 1000 μl of blood was added into each well of low adhesion 24-well plates (Corning, #3473) on ice. To prevent protein degradation, 5 μl of cell culture specific protease inhibitor (Sigma, #P1860) (ratio 1:200) was added into each well. The selective agonists for TLR4 [lipopolysaccharide (LPS); 10 μg/ml] (Cat. #L9641, Lot # 071M4120V, Sigma-Aldrich, Saint Louis, MO, USA), and TLR7 [imiquimod (IMQ); 30 μg/ml] (Cat. #tlrl-imqs, InvivoGen, San Diego, CA, USA) were added and the plates were gently transferred to cell culture incubator (37°C, 5% CO2) and incubated for 4 h. RIPA buffer (1000 μl) supplemented with protease inhibitor cocktails (ratio 1:100) (Sigma, Cat. #P8340) was added into each well (ratio 1:1) on ice and the mixtures were immediately centrifuged (9500×g; 4°C) for 5 min. Cell pellets were collected by gently removing supernatants and the supernatant residuals. RIPA buffer supplemented with protease inhibitor cocktails (100 μl) was added to the cell pellets. The mixtures were vortexed, kept on ice for 15 min, sonicated twice for 30 s at 25% output power with a Sonicator ultrasonic processor, and centrifuged (14,000×g; 4°C) for 30 min. Cell lysates were kept at −80°C until the levels of TNF-α, IL-6, and IL-1β were assessed by ELISAs.
Enzyme-linked immunosorbent assay (ELISA)
Total protein levels were determined by the bicinchoninic acid assay (BCA, Thermo Fisher Scientific, Waltham, MA, USA, Cat.# 23228 and Cat.# 1859078). Protein extracts were assayed with ELISA kits (Raybiotech, Norcross, GA, USA) for TNF-α (Cat. # ELH-TNFa-CL-1), IL-6 (Cat. # ELH-IL6-CL-1), IL-1β (Cat. # ELH-IL1b-1), and IP-10 (Cat.# ELH-IP10-1) as per the manufacturer's instructions. Results are expressed as picograms/milligram total protein (pg/mg).
Statistics
Heparinized whole blood for chemokine/cytokine level evaluation in cell lysates and serum for neurosteroid level evaluation were collected from 18 PPD patients (Table 1). GCMS steroid measures were excluded if a pre-treatment value was not detected. The evaluated inflammatory chemokine/cytokines were not detectible at baseline before brexanolone administration in cell lysates obtained from 4 patients and the samples from the patients were excluded from the analysis. If in vitro LPS and IMQ treatments (n = 3, 5 respectively) did not increase the levels of evaluated cytokines compared with in culture baseline levels in cell lysates, these samples were excluded from the analysis, since the immune cells were biologically inactive.
Each steroid or chemokine/cytokine data set were first tested for normal (Gaussian) distribution using the Kolmogorov–Smirnov normality test and if any of the groups failed the normality test, the Multiple Wilcoxon matched-pairs signed rank tests with Bonferroni-Dunn corrections (MLT Wilcoxon BFD) for multiple comparisons were applied. The mean values (pg/ml for steroids, and pg/mg total protein for chemokine/cytokines), standard error of mean (SEM), 95% confidence intervals (CI) of mean, median values of differences and % CI of differences, sum of signed ranks (W), p and adjusted p values were examined. The levels of evaluated cytokines in culture at baseline and following in vitro treatments with LPS or IMQ were examined in parallel. Then, the values of the baseline adjusted LPS- or IMQ-induced levels of the cytokines in the pre- or post-brexanolone condition, were subjected to statistical analysis.
Linear regression analysis was used to examine whether there is a significant correlation between the % change of each steroid or chemokine/cytokine and the % decrease (improvement) in HAM-D score where the predictor was the change in the steroid or chemokine/cytokine (an independent variable) and the outcome was the change in HAM-D score (a dependent variable). The coefficient of determination (R2 value), F statistic, degrees of freedom, and p value were examined. GraphPad Prism 9.4.1 was used for the statistical analysis. p < 0.05 was considered as statistically significant.
Ethics statement
This study received institutional review board approval (#20-1944) for the study at the University of North Carolina School of Medicine, School of Medicine, Chapel Hill.
Role of funders
The Foundation of Hope had no role in study design, data collection, analysis or reporting.
Results
Participant characteristics
Eighteen PPD patients aged between 24 and 41 years of age participated in the study. All patients were between 5 weeks and 6 months postpartum, with an average of 3.4 months, at the time of receiving the 60 h inpatient brexanolone dosing protocol. Brexanolone was generally well tolerated. All patients except one patient were under concurrent psychiatric treatments with at least one psychotropic drug but were unresponsive to concurrent medications prior to brexanolone therapy. The patient characteristics are presented in Table 1. The mean HAM-D score value at baseline was 23 (standard deviation (SD) 5) and was 11 (SD 7) after brexanolone infusion.
Brexanolone infusion alters multiple neuroactive steroid levels in PPD patient serum
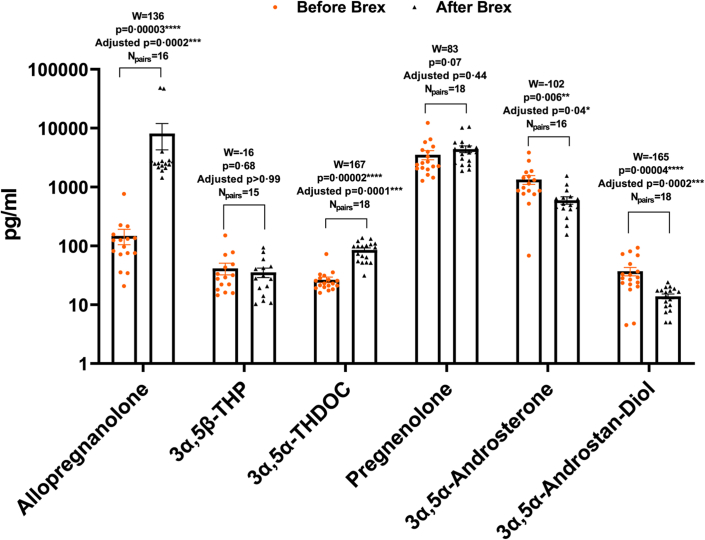
Perinatal depression has been associated with decreased allopregnanolone levels during pregnancy.19 The levels of allopregnanolone, 3α,5β-THP, 3α,5α-THDOC, pregnenolone, 3α,5α-androsterone and 3α,5α-androstan-diol in serum before and following brexanolone infusion were simultaneously examined. The data values of the steroid groups were tested for normal (Gaussian) distribution using the Kolmogorov–Smirnov normality test. Since the values did not represent normal distribution, the MLT Wilcoxon BFD for the multiple comparisons were applied to determine the effects of brexanolone infusion on the levels of the steroids. The mean values (pg/ml), SEM, 95% CI of mean for each steroid before and following brexanolone infusion were evaluated. The levels of allopregnanolone before brexanolone infusion were 147.6 ± 43.47 pg/ml (95% CI 54.98–240.3; N = 16) and following infusion were 8125 ± 3852 pg/ml (95% CI −85.55 to 16336; N = 16). The levels of 3α,5β-THP before brexanolone infusion were 41.30 ± 9.23 pg/ml (95% CI 21.51–61.09; N = 15) and following infusion were 35.35 ± 6.49 pg/ml (95% CI 21.43–49.28; N = 15). The levels of 3α,5α-THDOC before brexanolone infusion were 26.51 ± 2.99 pg/ml (95% CI 20.20–32.81; N = 18) and following infusion were 85.19 ± 7.22 pg/ml (95% CI 69.97–100.40; N = 18). The levels of pregnenolone before brexanolone infusion were 3528 ± 624.4 pg/ml (95% CI 2210–4845; N = 18) and following infusion were 4415 ± 584.6 pg/ml (95% CI 3181–5648; N = 18). The levels of 3α,5α-androsterone before brexanolone infusion were 1335 ± 230.1 pg/ml (95% CI 844.1–1825; N = 16) and following infusion were 596.7 ± 86.29 pg/ml (95% CI 412.8–780.7; N = 16). The levels of 3α,5α-androstan-diol before brexanolone infusion were 36.99 ± 6.00 pg/ml (95% CI 24.34–49.65; N = 18) and following infusion were 13.85 ± 1.32 pg/ml (95% CI 11.07–16.63; N = 18). To determine if there are significant differences in the steroid levels in serum before vs. following brexanolone infusion, the median values of differences and % CI of differences, W, p and adjusted p values were examined. As expected, brexanolone infusion increased the levels of allopregnanolone (+2342 pg/ml; 97.87% CI 2057–2725; W = 136, p = 0.00003, adjusted p = 0.0002 [MLT Wilcoxon BFD], Npairs = 16). Surprisingly, brexanolone infusion also increased 3α,5α-THDOC (+72.60 pg/ml; 96.91% CI 36.10–76.30; W = 167, p = 0.00002, adjusted p = 0.0001 [MLT Wilcoxon BFD], Npairs = 18), while decreasing 3α,5α-androsterone (−662.1 pg/ml; 97.87% CI −1155 to −173.3; W = −102, p = 0.006, adjusted p = 0.04 [MLT Wilcoxon BFD], Npairs = 16) and 3α,5α-androstan-diol (−14.95 pg/ml; 96.91% CI −31.90 to −11.60; W = −165, p = 0.00004, adjusted p = 0.0002 [MLT Wilcoxon BFD], Npairs = 18). Brexanolone infusion did not change the levels of pregnenolone (+862.7 pg/ml; 96.91% CI −120.9 to 1788; W = 83, p = 0.07, adjusted p = 0.44 [MLT Wilcoxon BFD], Npairs = 18) and 3α,5β-THP (−5.60 pg/ml; 96.48% CI −11.30 to 9.90; W = −16, p = 0.68, adjusted p > 0.99 [MLT Wilcoxon BFD], Npairs = 15) (Fig. 1). To determine if the % change in each steroid after brexanolone infusion correlated with the % decrease (improvement) in HAM-D score, linear regression analysis was applied and the coefficient of determination (R2 value), F statistic, degrees of freedom, and p value were examined. No effects were found (Fig. S1).
Fig. 1.
Brexanolone infusion alters multiple steroid levels in PPD patient serum. Serum was collected ∼1 h pre- and 6 h post-brexanolone (Brex) infusion (60 h) and simultaneously analyzed for the levels of allopregnanolone, pregnanolone (3α,5β-THP), allotetrahydrodeoxycorticosterone (3α,5α-THDOC), pregnenolone, 3α,5α-androsterone and 3α,5α-androstan-diol by gas chromatography-mass spectrometry (GCMS). Since the data values did not represent normal (Gaussian) distribution using the Kolmogorov–Smirnov normality test, Multiple Wilcoxon matched-pairs signed rank tests with Bonferroni-Dunn correction (MLT Wilcoxon BFD) for the multiple comparisons were applied to determine the effects of brexanolone infusion on the levels of the neurosteroids. The median values of differences in picograms/milliliter (pg/ml), % confidence intervals (CI) of differences, sum of signed ranks (W), p and adjusted p values were examined. Brexanolone infusion increased the levels of allopregnanolone (Npairs = 16) and 3α, 5α-THDOC (Npairs = 18), while decreasing 3α,5α-androsterone (Npairs = 16) and 3α,5α-androstan-diol (Npairs = 18). The changes in the levels of 3α,5β-THP (Npairs = 15) and pregnenolone (Npairs = 18) after brexanolone infusion were not statistically significant. The W values, p and adjusted p values are presented in the figure. ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001; ∗∗∗∗p < 0.0001 [MLT Wilcoxon BFD].
Brexanolone infusion inhibits IL-6 and TNF-α, but not interferon γ-induced protein 10 (IP-10) in blood cells of PPD patients
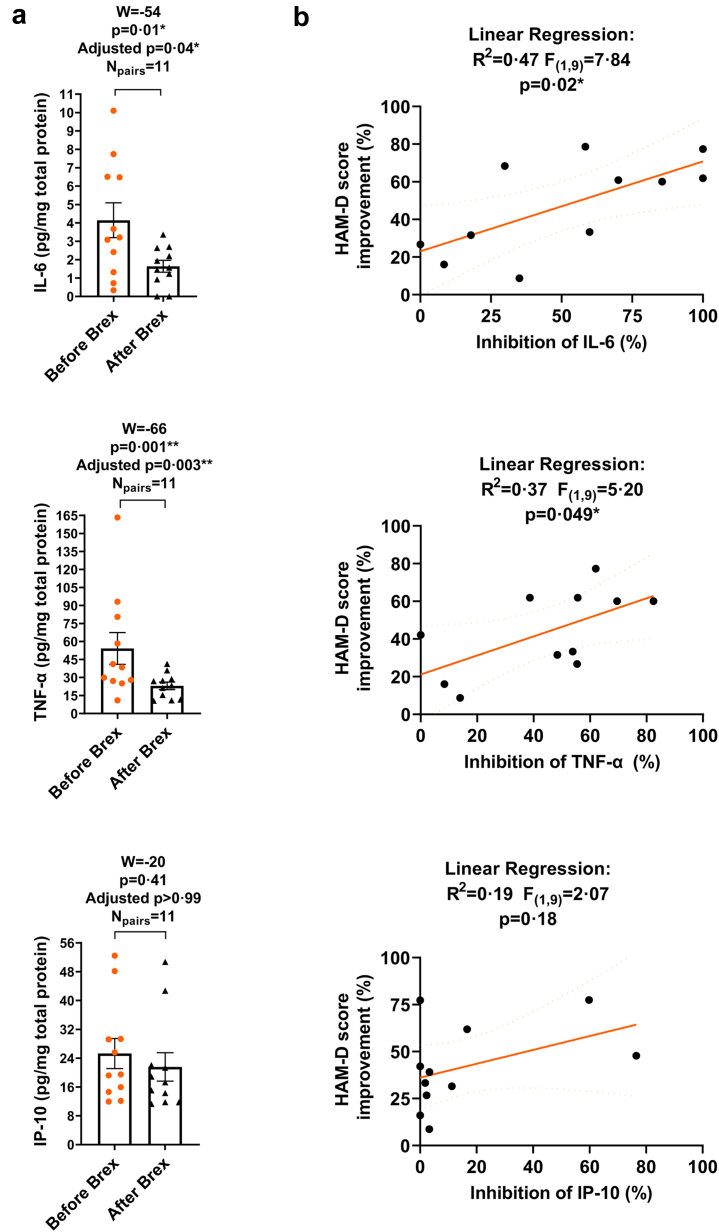
Postpartum depression as well as other forms of depression have been associated with increases in the levels of inflammatory cytokines TNF-α, IL-6, and IL-1β10,12, 13, 14, 15 that are indicative of TLR signal activation.20 Cell lysates were isolated from whole blood of PPD patients ∼1 h before and 6 h after 60 h duration of brexanolone infusion and the levels of the cytokines IL-6, TNF-α, IL-1β and the chemokine IP-10 were examined by ELISAs. Since the TNF-α and IP-10 values did not pass the Kolmogorov–Smirnov normality test, the MLT Wilcoxon BFD for the multiple comparisons were applied to determine the effects of brexanolone infusion on the levels of the chemokine/cytokines in blood cells. The mean values (pg/mg total protein), SEM, 95% CI of mean for each chemokine/cytokine before and following brexanolone infusion were first evaluated. The levels of IL-6 before brexanolone infusion were 4.14 ± 0.95 pg/mg (95% CI 2.03–6.26; N = 11) and following infusion were 1.64 ± 0.32 pg/mg (95% CI 0.91–2.36; N = 11). The levels of TNF-α before brexanolone infusion were 54.17 ± 13.23 pg/mg (95% CI 24.68–83.66; N = 11) and following infusion were 23.03 ± 3.10 pg/mg (95% CI 16.14–29.93; N = 11). The levels of IP-10 before brexanolone infusion were 25.29 ± 4.17 pg/mg (95% CI 16.00–34.59; N = 11) and following infusion were 21.57 ± 3.95 pg/mg (95% CI 12.77–30.37; N = 11). IL-1β was not detectible at baseline in most samples (data not shown). To determine if there are significant differences in the chemokine/cytokine levels in blood cells post- vs. pre-brexanolone infusion, the median values of differences and % CI of differences, W, p and adjusted p values were examined. We found that brexanolone infusion inhibited IL-6 (−0.85 pg/mg; 98.83% CI −7.75 to −0.31; W = −54, p = 0.01, adjusted p = 0.04 [MLT Wilcoxon BFD], Npairs = 11) and TNF-α (−19.95 pg/mg; 98.83% CI −51.81 to −2.51; W = −66, p = 0.001, adjusted p = 0.003 [MLT Wilcoxon BFD], Npairs = 11), but not IP-10 (−0.39 pg/mg; 98.83% CI −17.53 to 6.11; W = −20, p = 0.41, adjusted p > 0.99 [MLT Wilcoxon BFD], Npairs = 11) (Fig. 2a). Linear regression analysis was applied to determine if the % change in each chemokine/cytokine after brexanolone infusion correlated with the % decrease (improvement) in HAM-D score. The inhibition (%) of IL-6 (R2 = 0.47, F(1,9) = 7.84, p = 0.02 [Linear regression]) was positively correlated with the % decrease (improvement) in HAM-D score. The inhibition (%) of TNF-α (R2 = 0.37, F(1,9) = 5.20, p = 0.049 [Linear regression]) was also positively correlated with the % decrease (improvement) in HAM-D score. The % change in IP-10 (R2 = 0.19, F(1,9) = 2.07, p = 0.18 [Linear regression]) did not correlate with the % decrease (improvement) in HAM-D score (Fig. 2b).
Fig. 2.
Brexanolone infusion inhibits IL-6 and TNF-α, but not IP-10 in blood cells of post-partum depression (PPD) patients. The inhibition of IL-6 and TNF-α correlates with HAM-D score improvement after brexanolone infusion. Cell lysates were isolated from whole blood of PPD patients ∼1 h before and 6 h after 60 h duration of brexanolone (Brex) infusion and the levels of IL-6, TNF-α, and IP-10 were examined by ELISAs. a: Since the groups with TNF-α and IP-10 values did not pass the Kolmogorov–Smirnov normality test, the Multiple Wilcoxon matched-pairs signed rank tests with Bonferroni-Dunn correction for the multiple comparisons were applied to determine the effects of brexanolone infusion on the levels of IL-6, TNF-α, and IP-10. The median values of differences in picograms/milligram total protein (pg/mg), % confidence intervals (CI) of differences, sum of signed ranks (W), p and adjusted p values were examined. Brexanolone infusion decreased the levels of IL-6 (Npairs = 11) and TNF-α (Npairs = 11), but not IP-10 (Npairs = 11). The W values, p and adjusted p values are presented in the figure. ∗p < 0.05; ∗∗p < 0.01, [MLT Wilcoxon BFD] b: Linear regression analysis was applied to examine whether the % inhibition in IL-6, TNF-α, or IP-10 after brexanolone infusion correlated with the % decrease (improvement) in HAM-D score. The coefficient of determination (R2 value), F statistic, degrees of freedom, and p value were examined. The inhibition (%) of both IL-6 and TNF-α positively correlated with the % decrease (improvement) in HAM-D score after brexanolone infusion. The % change in IP-10 did not correlate with the % decrease (improvement) in HAM-D score. ∗p < 0.05, [Linear regression analysis].
Brexanolone infusion inhibits activation of TLR4 and TLR7 inflammatory signals in whole blood cells in vitro
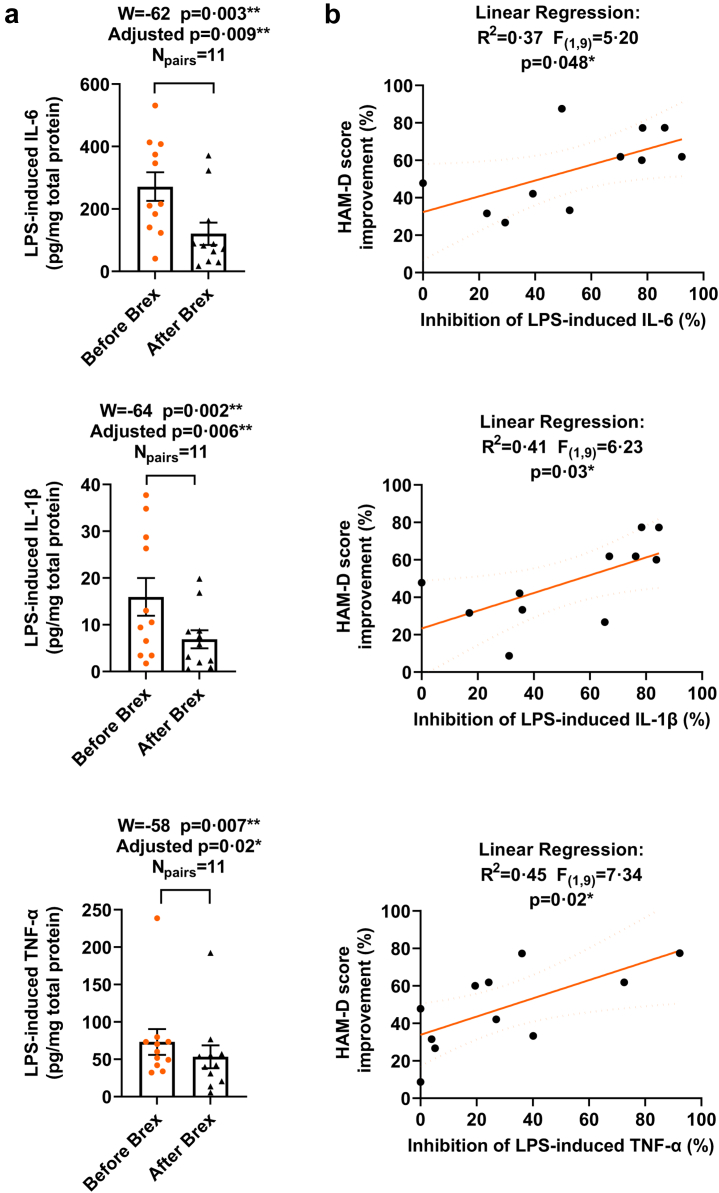
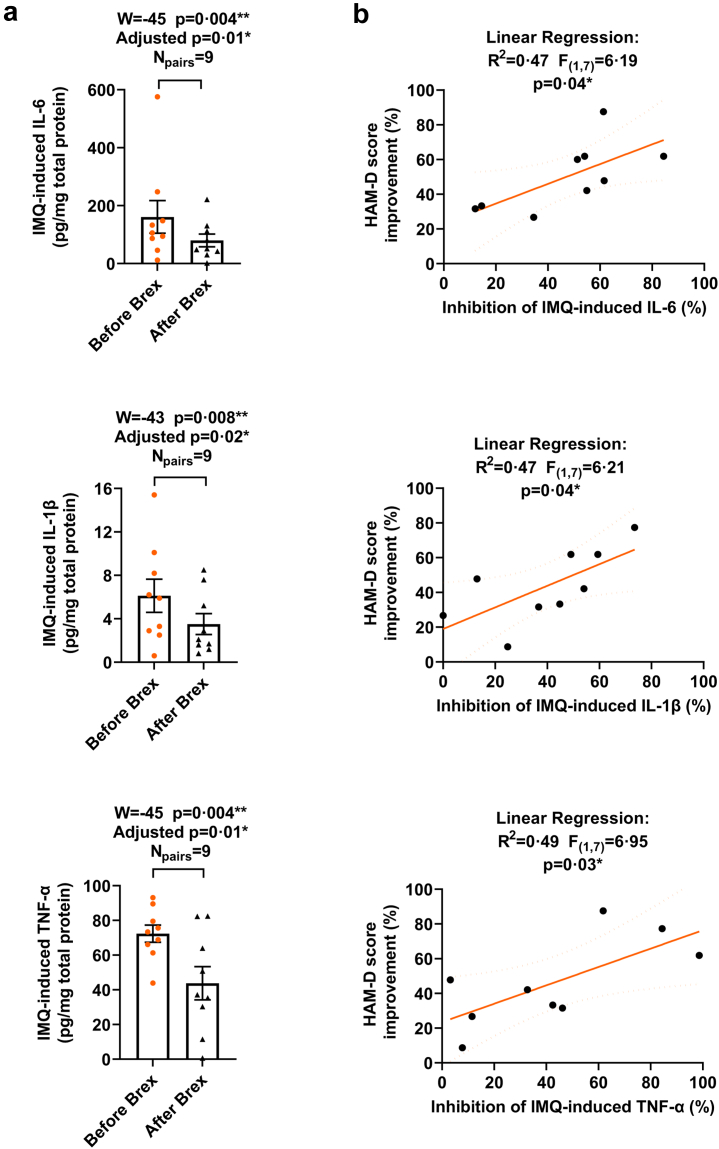
To estimate the effect of brexanolone on blood cell response to inflammatory agonists that specifically activate TLR4 or TLR7 inflammatory pathways, whole blood collected before and after brexanolone administration was exposed to LPS (TLR4 signal activator; 10 μg/ml; 37°C; 4 h) or IMQ (TLR7 signal activator; 30 μg/ml; 37°C; 4 h) in cell culture in vitro. The levels of IL-6, IL-1β, and TNF-α in culture at baseline, and following the in vitro exposure to LPS or IMQ were examined in isolated cell lysates by ELISAs. Since the values of the baseline adjusted LPS or IMQ-induced levels of the cytokines in the pre- or post-brexanolone condition, did not represent normal (Gaussian) distribution (the Kolmogorov–Smirnov normality test), the MLT Wilcoxon BFD for the multiple comparisons were applied. The mean values (pg/mg total protein), SEM, 95% CI of mean for each LPS- or IMQ-induced cytokine before and following brexanolone infusion were evaluated. The levels of IL-6 before brexanolone infusion were 271.4 ± 45.63 pg/mg (95% CI 169.8–373.1; N = 11) and 161.0 ± 56.41 pg/mg (95% CI 30.88–291.1; N = 9) after the exposure to LPS and IMQ, respectively and following brexanolone infusion were 120.6 ± 35.89 pg/mg (95% CI 40.64–200.6; N = 11) and 79.73 ± 22.13 pg/mg (95% CI 28.69–130.80; N = 9) after the exposure to LPS and IMQ, respectively. The levels of IL-1β before brexanolone infusion were 15.95 ± 4.03 pg/mg (95% CI 6.97–24.93; N = 11) and 6.12 ± 1.53 pg/mg (95% CI 2.60–9.65; N = 9) after the exposure to LPS and IMQ, respectively and following brexanolone infusion were 6.88 ± 1.93 pg/mg (95% CI 2.58–11.19; N = 11) and 3.51 ± 0.96 pg/mg (95% CI 1.30–5.73; N = 9) after the exposure to LPS and IMQ, respectively. The levels of TNF-α before brexanolone infusion were 73.13 ± 17.28 pg/mg (95% CI 34.63–111.6; N = 11) and 72.37 ± 4.96 pg/mg (95% CI 60.93–83.80; N = 9) after the exposure to LPS and IMQ, respectively and following brexanolone infusion were 53.37 ± 15.26 pg/mg (95% CI 19.37–87.38; N = 11) and 43.81 ± 9.61 pg/mg (95% CI 21.65–65.97; N = 9) after the exposure to LPS and IMQ, respectively. To determine if there are significant differences in the LPS- or IMQ-induced cytokine levels in blood cells post- vs. pre-brexanolone infusion, the median values of differences and % CI of differences, W, p and adjusted p values were examined. Brexanolone infusion partially inhibited the LPS-induced elevation of IL-6 (−106.3 pg/mg; 98.83% CI −319.0 to −11.90; W = −62, p = 0.003, adjusted p = 0.009 [MLT Wilcoxon BFD], Npairs = 11), IL-1β (−4.50 pg/mg; 98.83% CI −27.30 to −1.80; W = −64, p = 0.002, adjusted p = 0.006 [MLT Wilcoxon BFD], Npairs = 11), and TNF-α (−17.76 pg/mg; 98.83% CI −46.46 to −0.01; W = −58, p = 0.007, adjusted p = 0.02 [MLT Wilcoxon BFD], Npairs = 11) (Fig. 3a). Brexanolone infusion also partially inhibited the IMQ-induced elevation of IL-6 (−44.50 pg/mg; 96.09% CI −136.1 to −13.70; W = −45, p = 0.004, adjusted p = 0.01 [MLT Wilcoxon BFD], Npairs = 9), IL-1β (−2.50 pg/mg; 96.09% CI −4.30 to −0.40; W = −43, p = 0.008, p = 0.02 [MLT Wilcoxon BFD], Npairs = 9), and TNF-α (−26.05 pg/mg; 96.09% CI −49.17 to −7.04; W = −45, p = 0.004, adjusted p = 0.01 [MLT Wilcoxon BFD], Npairs = 9) (Fig. 4a). These results suggest inhibition of the inflammatory response to TLR4 and TLR7 activators following brexanolone infusion. Linear regression analysis was applied to examine whether the % inhibition in the LPS- or IMQ-induced IL-6, IL-1β, and TNF-α after brexanolone infusion correlated with the % decrease (improvement) in HAM-D score. The inhibition (%) of LPS-induced IL-6 (R2 = 0.37, F(1,9) = 5.20, p = 0.048 [Linear regression]), IL-1β (R2 = 0.41, F(1,9) = 6.23, p = 0.03 [Linear regression]), and TNF-α (R2 = 0.45, F(1,9) = 7.34, p = 0.02 [Linear regression]) were each positively correlated with the % decrease (improvement) in HAM-D score after brexanolone infusion (Fig. 3b). The inhibition (%) of the IMQ-induced IL-6 (R2 = 0.47, F(1,7) = 6.19, p = 0.04 [Linear regression]), IL-1β (R2 = 0.47, F(1,7) = 6.21, p = 0.04 [Linear regression]), and TNF-α (R2 = 0.49, F(1,7) = 6.95, p = 0.03 [Linear regression]) was positively correlated with the % decrease (improvement) in HAM-D score after brexanolone infusion (Fig. 4b).
Fig. 3.
Brexanolone infusion alters in vitro blood cell responses to the TLR4 inflammatory agonist lipopolysaccharide (LPS) as defined by the levels of cytokines IL-6, IL-1β, and TNF-α. The inhibition of the LPS-induced IL-6, IL-1β, and TNF-α correlates with HAM-D score improvement after brexanolone infusion. Whole blood drawn from post-partum depression (PPD) patients ∼1 h before and 6 h after 60 h duration of brexanolone (Brex) infusion was exposed to LPS (10 μg/ml; 37 °C; 4 h) in cell culture in vitro. The levels of IL-6, IL-1β, and TNF-α in culture at baseline, and following the in vitro exposure to LPS were examined in isolated cell lysates by ELISAs. a: Since the values of the baseline adjusted LPS-induced levels of the cytokines in the pre- or post-brexanolone condition, did not represent normal (Gaussian) distribution (the Kolmogorov–Smirnov normality test), the Multiple Wilcoxon matched-pairs signed rank tests with Bonferroni-Dunn correction for the multiple comparisons were applied. The median values of differences in picograms/milligram total protein (pg/mg) and % confidence intervals (CI) of differences were examined. The levels of IL-6 (Npairs = 11), IL-1β (Npairs = 11), and TNF-α (Npairs = 11) were significantly reduced in the post-brexanolone (After Brex) condition when compared with the pre-brexanolone (Before Brex) condition. The W values, p and adjusted p values are presented in the figure. ∗p < 0.05; ∗∗p < 0.01, [MLT Wilcoxon BFD] b: The linear regression analysis was applied to examine whether the % inhibition in LPS-induced IL-6, IL-1β, or TNF-α after brexanolone infusion were correlated with the % decrease (improvement) in HAM-D score. The coefficient of determination (R2 value), F statistic, degrees of freedom, and p value were examined. The inhibition (%) of LPS-induced IL-6, IL-1β, and TNF-α were each positively correlated with the % decrease (improvement) in HAM-D score after brexanolone infusion. ∗p < 0.05, [Linear regression analysis].
Fig. 4.
Brexanolone infusion alters in vitro blood cell responses to the TLR7 inflammatory agonist imiquimod (IMQ) as defined by the levels of cytokines IL-6, IL-1β, and TNF-α. The inhibition of the IMQ-induced IL-6, IL-1β, and TNF-α correlates with HAM-D score improvement after brexanolone infusion. Whole blood drawn from post-partum depression (PPD) patients ∼1 h before and 6 h after 60 h duration of brexanolone (Brex) infusion was exposed to IMQ (30 μg/ml; 37 °C; 4 h) in cell culture in vitro. The levels of IL-6, IL-1β, and TNF-α in culture at baseline, and following the in vitro exposure to IMQ were examined in isolated cell lysates by ELISAs. a: Since the values of the baseline adjusted IMQ-induced levels of the cytokines in the pre- or post-brexanolone condition, did not represent normal (Gaussian) distribution (the Kolmogorov–Smirnov normality test), the Multiple Wilcoxon matched-pairs signed rank tests with Bonferroni-Dunn correction for the multiple comparisons were applied. The median values of differences in picograms/milligram total protein (pg/mg) and % confidence intervals (CI) of differences were examined. The levels of IL-6 (Npairs = 9), IL-1β (Npairs = 9), and TNF-α (Npairs = 9) were significantly reduced in the post-brexanolone (After Brex) condition when compared with the pre-brexanolone (Before Brex) condition. The W values, p and adjusted p values are presented in the figure. ∗p < 0.05, ∗∗p < 0.01, [MLT Wilcoxon BFD]. b: Linear regression analysis was applied to examine whether the % inhibition in the IMQ-induced IL-6, IL-1β, or TNF-α after brexanolone infusion correlated with the % decrease (improvement) in HAM-D score. The coefficient of determination (R2 value), F statistic, degrees of freedom, and p value were examined. The inhibition (%) of the IMQ-induced IL-6, IL-1β, and TNF-α were positively correlated with the % decrease (improvement) in HAM-D score after brexanolone infusion. ∗p < 0.05, [Linear regression analysis].
Discussion
The data indicate that brexanolone infusion inhibits inflammatory signaling in circulation of PPD patients 6 h following infusion, and these effects are correlated with its therapeutic efficacy measured by the reduction in HAM-D scores. Furthermore, immune cells isolated from whole blood samples of PPD patients also exhibited inhibition of responses to the inflammatory activators LPS and IMQ following brexanolone infusion, compared to their activation prior to treatment. The changes in response to LPS and IMQ were also correlated with HAM-D score improvement in the patients.
These data implicate alterations in inflammatory signaling in the therapeutic efficacy of brexanolone as well increase our understanding of the potential etiologies of post-partum depression. This study demonstrates inhibition of proinflammatory cytokines and inhibition of responses to inflammatory activators in PPD patients and may help to explain the antidepressant actions of brexanolone following treatment.
The study was limited by several experimental constraints. The lack of a placebo control group restricted our analysis to the effects of brexanolone in PPD and did not allow us to specifically assess the etiology of PPD. The lack of analysis at various intervals following treatment prevented us from determining if the immune response was inhibited or desensitized by brexanolone therapy. Future studies will address these limitations. Due to the relatively small sample sizes in the study, the results should be replicated in further studies.
The present study focused on brexanolone inhibition of IL-6, TNF-α and IL-1β since several (although not all) previous studies have shown these markers of inflammation are elevated in PPD10,11,21,22 as well as other forms of depression.12, 13, 14, 15 The fact that the same markers found increased in PPD (TNF-α, IL-1β and IL-6)10,11 were inhibited following brexanolone therapy, and this inhibition was correlated with therapeutic response, suggests that inflammation plays a role in the etiology of PPD. However, there is also evidence that not all depression symptoms are linked to inflammation.23 Moreover, four subjects (from eighteen subjects) were left out of the analyses in this study because they did not exhibit elevation of the evaluated inflammatory markers in blood cells, raising further questions regarding the role of these particular inflammatory markers and/or blood cell inflammation in PPD. Therefore, further studies are clearly warranted.
Allopregnanolone has previously been shown to have pleotropic actions in the CNS,24,25 which include positive allosteric modulation of GABAA receptors,26 inhibition of the hypothalamic-pituitary-adrenal axis27 as well as inhibition of pro-inflammatory and neuroimmune activation in macrophages and the brain,8,9,28 all of which likely contribute to alterations in brain network function.25 Importantly, this study directly links a specific mechanism of action to therapeutic response in PPD patients.
As expected, brexanolone infusion increased serum levels of allopregnanolone. However, changes in allopregnanolone levels following brexanolone infusion were not predictive of HAM-D score improvement. This finding is consistent with the observation that the therapeutic effects of brexanolone infusion are long-lasting (up to 90 days post-infusion), despite the fact that allopregnanolone levels return to baseline within days of infusion.2,5,6 Furthermore, brexanolone infusion induced unexpected changes in the serum levels of several other neuroactive steroids, including 3α,5α-THDOC, 3α,5α-androsterone, and 3α,5α-androstan-diol. The observation that brexanolone alters the levels of multiple neurosteroids is consistent with evidence that allopregnanolone may act at nuclear pregnane-X receptors to change the expression of steroidogenic genes.29 However, the mechanism of such divergent effects on various steroids will require further study.
Brexanolone infusion is remarkable for its fast, long-acting, and highly efficacious therapeutic actions.2,5,6 The efficacy of brexanolone in moderate and severe PPD has been transformative in setting the bar for the ideal standard of care for this devastating condition and highlights the need for rapid and long-acting therapies.6 Understanding the mechanisms of its actions is important for further development of more cost-effective medication that does not require IV infusion and medical supervision. Furthermore, depression is a common ailment in other patient populations, including major depression outside of the perinatal period and co-morbid with many other neuropsychiatric conditions, that involve elevations in pro-inflammatory signaling. Therefore, the ability of brexanolone to inhibit inflammatory signaling through both TLR4 and TLR7 receptors and to inhibit immune cell responses to inflammatory activators may represent an important breakthrough in the treatment of depression associated with numerous conditions.28,30
Contributors
Study design: IB, RP, SMB and ALM; Clinical approvals and data collection: RP and HK; Data collection: IB, GB and TKO; Data verification: IB, RP, TKO, ALM. Drafted manuscript: IB, RP, TKO, ALM; All authors edited and approved the manuscript.
Data sharing statement
Deidentified participant data, study protocol, and informed consent forms are available upon reasonable request.
Declaration of interests
ALM and IB declare a U.S. provisional patent on the anti-inflammatory effects of allopregnanolone and related steroids. ALM and SMB have previously received research funding from Sage Therapeutics for other projects. SMB has received consulting fees from Ancora Bio, Modern Health and Web MD. The authors declare no other potential conflicts of interest.
Acknowledgements
This work was funded by a grant from the Foundation of Hope, Raleigh, NC and support from the UNC School of Medicine, Chapel Hill, NC to ALM. We thank Professor Kai Xia at the Mental Health Informatics and Analytics Core in the Department of Psychiatry, UNC School of Medicine for statistical guidance on the data in the report.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.ebiom.2023.104473.
Appendix A. Supplementary data
References
- 1.Gavin N.I., Gaynes B.N., Lohr K.N., Meltzer-Brody S., Gartlehner G., Swinson T. Perinatal depression: a systematic review of prevalence and incidence. Obstet Gynecol. 2005;106(5 Pt 1):1071–1083. doi: 10.1097/01.AOG.0000183597.31630.db. [DOI] [PubMed] [Google Scholar]
- 2.Meltzer-Brody S., Colquhoun H., Riesenberg R., et al. Brexanolone injection in post-partum depression: two multicentre, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet. 2018;392(10152):1058–1070. doi: 10.1016/S0140-6736(18)31551-4. [DOI] [PubMed] [Google Scholar]
- 3.Arifin S.R.M., Cheyne H., Maxwell M. Review of the prevalence of postnatal depression across cultures. AIMS Public Health. 2018;5(3):260–295. doi: 10.3934/publichealth.2018.3.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Canady V.A. FDA approves first drug for postpartum depression treatment. Ment Health Wkly. 2019;29(12):6. [Google Scholar]
- 5.Kanes S., Colquhoun H., Gunduz-Bruce H., et al. Brexanolone (SAGE-547 injection) in post-partum depression: a randomised controlled trial. Lancet. 2017;390(10093):480–489. doi: 10.1016/S0140-6736(17)31264-3. [DOI] [PubMed] [Google Scholar]
- 6.Patterson R., Krohn H., Richardson E., Kimmel M., Meltzer-Brody S. A brexanolone treatment Program at an academic medical center: patient selection, 90-day posttreatment outcomes, and lessons learned. J Acad Consult Liaison Psychiatry. 2022;63(1):14–22. doi: 10.1016/j.jaclp.2021.08.001. [DOI] [PubMed] [Google Scholar]
- 7.Meltzer-Brody S., Rubinow D. In: Women's mood disorders. Cox E., editor. Springer; Cham: 2021. An overview of perinatal mood and anxiety disorders: epidemiology and etiology. [Google Scholar]
- 8.Balan I., Aurelian L., Schleicher R., Boero G., O'Buckley T., Morrow A.L. Neurosteroid allopregnanolone (3 alpha,5 alpha-THP) inhibits inflammatory signals induced by activated MyD88-dependent toll-like receptors. Transl Psychiatry. 2021;11(1):145. doi: 10.1038/s41398-021-01266-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Balan I., Beattie M.C., O'Buckley T.K., Aurelian L., Morrow A.L. Endogenous neurosteroid (3⍺,5⍺)3-hydroxypregnan-20-one inhibits toll-like-4 receptor activation and pro-inflammatory signaling in macrophages and brain. Sci Rep. 2019;9(1):1220. doi: 10.1038/s41598-018-37409-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Achtyes E., Keaton S.A., Smart L., et al. Inflammation and kynurenine pathway dysregulation in post-partum women with severe and suicidal depression. Brain Behav Immun. 2020;83:239–247. doi: 10.1016/j.bbi.2019.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corwin E.J., Johnston N., Pugh L. Symptoms of postpartum depression associated with elevated levels of interleukin-1 beta during the first month postpartum. Biol Res Nurs. 2008;10(2):128–133. doi: 10.1177/1099800408323220. [DOI] [PubMed] [Google Scholar]
- 12.Bhattacharya A., Derecki N.C., Lovenberg T.W., Drevets W.C. Role of neuro-immunological factors in the pathophysiology of mood disorders. Psychopharmacology (Berl) 2016;233(9):1623–1636. doi: 10.1007/s00213-016-4214-0. [DOI] [PubMed] [Google Scholar]
- 13.Dantzer R., O'Connor J.C., Freund G.G., Johnson R.W., Kelley K.W. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9(1):46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Howren M.B., Lamkin D.M., Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med. 2009;71(2):171–186. doi: 10.1097/PSY.0b013e3181907c1b. [DOI] [PubMed] [Google Scholar]
- 15.Rizavi H.S., Ren X., Zhang H., Bhaumik R., Pandey G.N. Abnormal gene expression of proinflammatory cytokines and their membrane-bound receptors in the lymphocytes of depressed patients. Psychiatry Res. 2016;240:314–320. doi: 10.1016/j.psychres.2016.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hamilton M. Springer; Berlin Heidelberg: 1986. The Hamilton rating scale for depression. [Google Scholar]
- 17.Porcu P., O'Buckley T.K., Alward S.E., et al. Simultaneous quantification of GABAergic 3 alpha,5 alpha/3alpha,5 beta neuroactive steroids in human and rat serum. Steroids. 2009;74:463–473. doi: 10.1016/j.steroids.2008.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Snelling C., Tanchuck-Nipper M.A., Ford M.M., et al. Quantification of ten neuroactive steroids in plasma in Withdrawal Seizure-Prone and -Resistant mice during chronic ethanol withdrawal. Psychopharmacology (Berl) 2014;231(17):3401–3414. doi: 10.1007/s00213-014-3618-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Osborne L.M., Gispen F., Sanyal A., Yenokyan G., Meilman S., Payne J.L. Lower allopregnanolone during pregnancy predicts postpartum depression: an exploratory study. Psychoneuroendocrinology. 2017;79:116–121. doi: 10.1016/j.psyneuen.2017.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pandey G.N., Rizavi H.S., Ren X., Bhaumik R., Dwivedi Y. Toll-like receptors in the depressed and suicide brain. J Psychiatr Res. 2014;53:62–68. doi: 10.1016/j.jpsychires.2014.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kendall-Tackett K. A new paradigm for depression in new mothers: the central role of inflammation and how breastfeeding and anti-inflammatory treatments protect maternal mental health. Int Breastfeed J. 2007;2:6. doi: 10.1186/1746-4358-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yim I.S., Tanner Stapleton L.R., Guardino C.M., Hahn-Holbrook J., Dunkel Schetter C. Biological and psychosocial predictors of postpartum depression: systematic review and call for integration. Annu Rev Clin Psychol. 2015;11:99–137. doi: 10.1146/annurev-clinpsy-101414-020426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frank P., Jokela M., Batty G.D., Cadar D., Steptoe A., Kivimaki M. Association between systemic inflammation and individual symptoms of depression: a pooled analysis of 15 population-based cohort studies. Am J Psychiatry. 2021;178(12):1107–1118. doi: 10.1176/appi.ajp.2021.20121776. [DOI] [PubMed] [Google Scholar]
- 24.Boero G., Porcu P., Morrow A.L. Pleiotropic actions of allopregnanolone underlie therapeutic benefits in stress-related disease. Neurobiol Stress. 2020;12 doi: 10.1016/j.ynstr.2019.100203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morrow A.L., Balan I., Boero G. Mechanisms underlying recovery from postpartum depression following brexanolone therapy. Biol Psychiatry. 2022;91(3):252–253. doi: 10.1016/j.biopsych.2021.11.006. [DOI] [PubMed] [Google Scholar]
- 26.Antonoudiou P., Colmers P.L.W., Walton N.L., et al. Allopregnanolone mediates affective switching through modulation of oscillatory states in the basolateral amygdala. Biol Psychiatry. 2022;91(3):283–293. doi: 10.1016/j.biopsych.2021.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boero G., Tyler R.E., Todd C.A., et al. (3 alpha, 5 alpha)3-hydroxypregnan-20-one (3alpha, 5alpha-THP) regulation of hypothalamic and extrahypothalamic corticotropin releasing factor (CRF): sexual dimorphism and brain region specificity in Sprague Dawley rats. Neuropharmacology. 2021;186 doi: 10.1016/j.neuropharm.2021.108463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Balan I., Aurelian L., Williams K.S., Campbell B., Meeker R.B., Morrow A.L. Inhibition of human macrophage activation via pregnane neurosteroid interactions with toll-like receptors: sex differences and structural requirements. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.940095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frye C.A., Koonce C.J., Walf A.A. Role of pregnane xenobiotic receptor in the midbrain ventral tegmental area for estradiol- and 3 alpha,5 alpha-THP-facilitated lordosis of female rats. Psychopharmacology (Berl) 2014;231(17):3365–3374. doi: 10.1007/s00213-013-3406-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pinna G. Allopregnanolone (1938–2019): a trajectory of 80 years of outstanding scientific achievements. Neurobiol Stress. 2020;13 doi: 10.1016/j.ynstr.2020.100246. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.