Abstract
Background
Cardiovascular disease (CVD) is the leading course of disease-related death in both developed and developing countries. Atherosclerosis is main pathology of CVD, and its severity is thought to be related to trimethylamine N-oxide (TMAO) level in plasma. Therefore, it is necessary to deeply understand the synergistic patterns between TMAO and other contribution variables to atherosclerosis, allowing for effective and timely monitoring or intervention.
Methods
A total of 359 participants were recruited in our study, including 190 atherosclerosis patients, 82 MI or stroke patients, 68 non-atherosclerosis controls and 19 healthy controls. Information on their risk associated with atherosclerosis and plasma TMAO concentration were collected. LASSO regression, multivariate analysis and univariate analysis were then performed to confirm the correlation between TMAO level and risk factors of atherosclerosis.
Results
Compared to patients and non-atherosclerosis controls, healthy participants had a normal BMI range (lower than 24), lower triglyceride concentration, and healthy lifestyle habits (no smoking and low salt diet). However, under backgrounds of statins treatment and balanced dietary preferences, TMAO levels were not significantly different among patients, non-atherosclerosis controls and healthy controls. Using LASSO regression model, four indicators was identified to have contribution to TMAO levels, including diabetes, atherosclerosis, low-density lipoprotein and total cholesterol. Subsequent univariate analysis further confirmed that the presence or absence of diabetes had a decisive effect on patients' plasma TMAO levels, even though they had been taking statin lipid-lowering drugs for a long time.
Conclusion
Diabetics have abnormally high plasma TMAO levels even under continuous statins treatment, which may contribute to the development and progression of atherosclerosis. Therefore, it is necessary to focus on monitoring TMAO levels in diabetic patients to reduce adverse cardiovascular events in diabetic patients.
Keywords: Trimethylamine N-Oxide, Atherosclerosis, LASSO regression, Diabetes, Risk factors
1. Introduction
Atherosclerosis is the main pathological feature of cardiovascular diseases included but not limited to myocardial infarctions (MI) and stroke, therefore responsibly for 31% annual death cases in worldwide [1,2]. With the improved prosperity of dietary habits and continuing tobacco usage, atherosclerotic disease maintain “morbidity extension” worldwide, especially in developing countries [3]. In addition to cardiovascular diseases, the global spread of atherosclerotic disease contributes to the burden in middle-aged on arthritis, depression and other chronic disease, which will further rob laborers of their mobility, cognition or communicative competence [3]. There is therefore an urgent need to develop sensitive atherosclerosis biomarkers for disease monitoring and progress evaluation.
The increase of triglyceride-enriched lipoprotein and the decrease of high-density lipoprotein (HDL) are the main patterns of dyslipidemia in patients with atherosclerotic cardiovascular disease [4]. Although low-density lipoprotein (LDL) levels are considered to be major risk factor for cardiovascular disease [5,6], efficient and inexpensive therapies, such as statins, have contributed to reduce LDL levels across the board [3,4]. Indeed, a large proportion of patients with atherosclerosis have normal plasma LDL levels [7]. Nevertheless, other risk factors, such as obesity, diabetes and dietary preferences of high carbohydrate, still contribute to high morbidity to atherosclerosis [3,4,8,9]. In spite of advances in controlling these risk factors in the United States [10], the prevalence of above patients groups still increased by 35% from the 1990s to the 2010s [11]. To better monitor the progression of atherosclerotic disease, the Pooled Cohort Risk Equations (PCE) for atherosclerotic cardiovascular disease was established in the Guideline on the Assessment of Cardiovascular Risk (2013), containing parameters of total cholesterol, LDL-cholesterol and diabetes, etc. [12,13] However, accumulating evidences indicated overestimation of risk predictions in both Asian and European population [14,15], and reasons for these overestimation are unclear. Further introduction of atherosclerosis pathogenic parameters to supplement and/or modify the equation should be a valuable strategy.
In the last decade, trimethylamine N-oxide (TMAO), an intestinal microbiota-associated metabolite, shown to be major adverse factors for atherosclerotic cardiovascular disease [[16], [17], [18]]. The main mechanism of TMAO to atherosclerosis involve the increase of platelet hyperreactivity through altered Ca2+ signaling [18], and the promoting of insulin resistance through binding and activating PERK protein [19]. A prospective study involving 4007 patients shown that increased plasma TMAO concentration was associated with an increased risk of adverse cardiovascular events [20]. Model animal experiments have further elucidated that targeted inhibition of TMAO formation can inhibit the progression of atherosclerosis [21]. Recent evidence, however, suggests that the pathogenicity of TMAO for atherosclerosis appears to be significantly overestimated [22]. One possible explanation for these cases is that patients with high TMAO levels also have diabetes and/or hypertension [23]. These two diseases are major risk factors for atherosclerosis [3]. Therefore, further exploration of the correlation between TMAO levels and atherosclerosis risk factors could help improve its effectiveness in disease surveillance and progression assessment of atherosclerosis.
In this study, we focused on the correlation between plasma TMAO levels and risk factors of atherosclerosis in atherosclerosis-related diseases. Therefore, 359 participants were prospectively recruited, containing 19 healthy controls, 68 non-atherosclerosis controls, 190 atherosclerosis patients, and 82 MI or stroke patients. Their atherosclerosis risk information was collected and their plasma TMAO concentration were detected. LASSO regression, multivariate analysis and univariate analysis were performed to demonstrate the correlation between TMAO level and risk factors of atherosclerosis.
2. Methods and materials
2.1. Study population
From May 2019 to May 2020, 359 subjects from the Affiliated Hospital of Chengde Medical University were retrospectively enrolled for TMAO level analysis. Among them, 19 are healthy controls, 68 are non-atherosclerosis controls, 190 are atherosclerosis patients, 82 are MI or stroke patients. All subjects have face-to-face interviewed by well-trained cardiologist using a written questionnaire (Supplementary information 3), and focus on their medical history, medication history, diet preference, living habits and physical indexes. For those diabetics, their latest blood glucose indicators, including fasting blood glucose, glycosylated hemoglobin (HbA1c) and 2 hPG, was additionally collected from the hospital case system. Among them, subjects with fasting plasma glucose ≥7.0 mM can be confirmed as diabetes. For suspected diabetic patients, 2 hPG and/or HbA1c were further detected, and patients with 2 hPG >11.1 mM or HbA1c >6.5% were confirmed as diabetes. Subsequently, according to clinical manifestations, all diabetic patients were further confirmed as type two diabetes mellitus (T2DM). Healthy subjects without history of thrombosis, atherosclerosis, stroke, diabetes, chronic kidney disease and cancer are defined as healthy controls. All suspected patients, except healthy controls, underwent arteriography, and were divided into atherosclerosis and non-atherosclerosis groups according to the results. Peripheral blood samples and baseline information of the participants were collected and used in this study under the supervision of the Ethics Committee of the Affiliated Hospital of Chengde Medical University. One week before blood collection, all subjects suspended the use of antibiotics. Drawing on previous studies, all subjects stopped eating fish and seafood 48 h before sampling [24], and did not use loop diuretics during treatment (our cohort have excluded potential loop diuretics users, such as heart failure patients) [25].
2.2. Sample collection and detection
Peripheral blood samples were collected using EDTA-K2 anticoagulant tubes from overnight fasting participants. Then, anticoagulant tubes were reversed several times and centrifuged at 300 g to separate the plasma. Each plasma sample was divided into 3 parts. One part was sent to the Department of Clinical Laboratory for blood lipid test. Another part of plasma was used for TMAO detection. The rest part of the plasma samples was stored at −80 °C for outlier verification. Permission for the use of these samples was obtained from Ethics Committee of the Affiliated Hospital of Chengde Medical University.
2.3. TMAO detection
LC-MS/MS based stable isotope internal standard strategy was used for quantifying TMAO concentration in plasma samples. Specifically, 200 μL plasma sample was extracted using 650 μL of acetonitrile solution containing 80 ng/mL TMAO-d9. After centrifugation (1000 g, 3 min), the supernatant was collected and evaporated under N2 atmosphere. Then, the residue was resuspended with 200 μL DI water, and 160 μL of it was taken for subsequent detection. LC-MS/MS analysis was performed using ACQUITY UPLC I-Class/Xevo TQD system equipped with ACQUITY UPLC HSS T3 chromatographic column (2.1 × 100 mm; Waters, USA). The mobile phases consisted of (A) 0.1% formic acid aqueous solution and (B) pure methyl alcohol. The flow rate of mobile phases was 0.3 mL/min. The elution of TMAO was conducted according to the following gradient: 0 min 2% B; 0–1.0 min 2% B; 1.0–2.5 min 50% B; 2.5–5.0 min 2% B. 10 μL sample volume was used for the injection. Electrospray ionization in positive ion mode was operated for TMAO detection using the following parameters: capillary voltages 3.5 kV; cone voltage 30 V; collision energy 18 V; desolvation temperature 350 °C; desolvation gas flow 650 L/h. Characteristic ion pairs for TMAO and TMAO-d9 detection were m/z 76 → 58 and m/z 85 → 66, respectively.
2.4. LASSO regression analysis
LASSO regression analysis was performed to select variables that potentially contributed to TMAO levels. To determine the penalty factor, a 10-fold cross validation test was carried out, resulting in an optimal lambda for variables filtering based on the principles reported by Li et al. [26].
2.5. Statistical analysis
Statistical analysis for this study was carried out using SPSS 19.0 software. Continuous variables were presented as median (interquartile range). Discrete variables were regard as categorical variables and presented as a percentage of eligible subjects. Continuous variables and discrete variables were compared using Student t-test or nonparametric test when appropriate [20,23,27,28]. ANOVA design with a post hoc test or Kruskal-Wallis H test were selected for evaluating statistic difference among 3 or more groups according to whether or not the distribution was normal [23,27]. Only p value in these test methods lower than 0.05 was considered statistically significance. Graphical plots were generated using Origin 2018 software or Python project.
3. Results
3.1. Patient characteristics
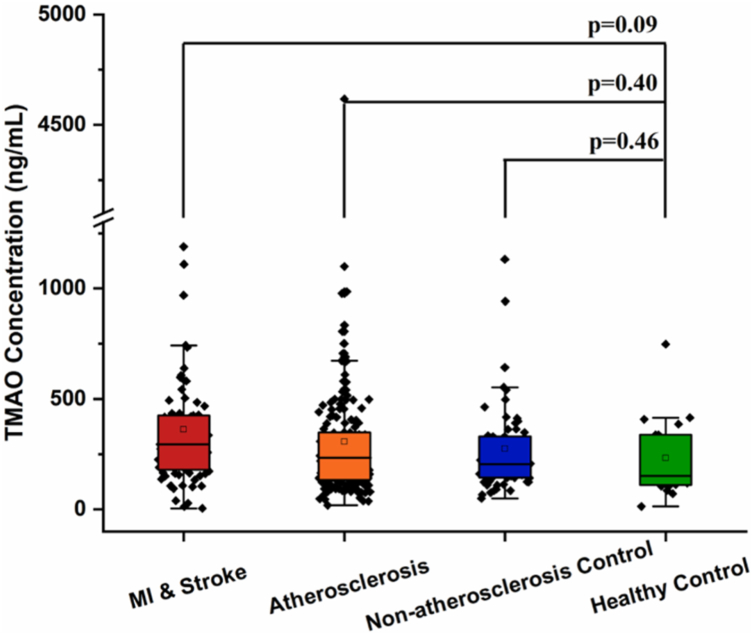
From May 2019 to May 2020, we recruited 359 participants from the Affiliated Hospital of Chengde Medical University in China. Among them, 19 are healthy controls, 68 are non-atherosclerosis controls, 190 are atherosclerosis patients, 82 are MI or stroke patients. The main baseline characteristics of these four groups are shown in Table 1. Ten indicators are found differently enriched among these four groups, including BMI, dietary preference of salty food, smoking, hypertension, diabetes, hyperlipidemia, atherosclerosis, MI, stroke and triglyceride (Table 1). Specifically, compared to non-atherosclerosis controls and patient groups, the healthy controls show a normal BMI range (lower than 24), lower triglyceride concentration, and healthy lifestyle habits (no smoking and low salt diet). However, there were no significant differences between the four groups in terms of other life-style habits, physical indexes and lipid indicators. Even the indicator of TMAO concentration, which we focused on in this study, was no significant difference among the four groups (Table 1 and Fig. 1).
Table 1.
Clinical and blood lipids characteristics of the cohort.
| Variables | Total (N = 359) | MI & Stroke Patients (N = 82) | Atherosclerosis Patients (N = 190) | Non-atherosclerosis Controls (N = 68) | Healthy Controls (N = 19) | p Value |
|---|---|---|---|---|---|---|
| Age, yrs | 59 (53–64) | 61 (56–66) | 59 (53–64) | 59 (53–64) | 55 (49–61) | 0.08 |
| Male, % | 61.8 | 67.1 | 64.2 | 51.5 | 52.6 | 0.16 |
| BMI | 25.9 (23.8–28.2) | 25.0 (23.2–28.1) | 26.2 (24.2–28.1) | 26.8 (24.1–28.7) | 22.9 (21.6–24.2) | <0.01 |
| Red meat, % | 34.5 | 34.1 | 38.4 | 27.9 | 21.1 | 0.28 |
| White meat, % | 8.1 | 4.9 | 11.6 | 2.9 | 5.3 | 0.12 |
| Sweet food, % | 22.3 | 23.2 | 19.5 | 23.5 | 42.1 | 0.13 |
| Salty food, % | 48.5 | 47.6 | 54.7 | 35.3 | 36.8 | 0.04 |
| Spicy food, % | 24.8 | 19.5 | 25.8 | 26.5 | 31.6 | 0.65 |
| Greasy food, % | 35.7 | 30.5 | 38.9 | 35.3 | 26.3 | 0.44 |
| Smoker, % | 44.0 | 54.9 | 45.3 | 29.4 | 36.8 | 0.02 |
| Drinker, % | 33.4 | 41.5 | 32.6 | 25.0 | 36.8 | 0.19 |
| Hypertension, % | 56.5 | 59.8 | 57.4 | 66.2 | 0.0 | <0.01 |
| Diabetes, % | 21.2 | 29.3 | 19.5 | 22.1 | 0.0 | <0.01 |
| Hyperlipidemia, % | 45.4 | 42.7 | 44.7 | 63.2 | 0.0 | <0.01 |
| Atherosclerosis, % | 64.3 | 50.0 | 100.0 | 0.0 | 0.0 | <0.01 |
| MI, % | 8.6 | 37.8 | 0.0 | 0.0 | 0.0 | <0.01 |
| Stroke, % | 15.6 | 68.3 | 0.0 | 0.0 | 0.0 | <0.01 |
| TCHO, mM | 4.00 (3.36–4.78) | 3.91 (3.37–4.51) | 4.07 (3.41–5.04) | 4.12 (3.35–4.71) | 4.01 (3.17–4.49) | 0.55 |
| HDL, mM | 1.02 (0.79–1.3) | 0.90 (0.73–1.29) | 1.05 (0.87–1.36) | 0.96 (0.67–1.13) | 1.09 (0.83–1.22) | 0.13 |
| LDL, mM | 2.17 (1.67–2.7) | 2.08 (1.58–2.44) | 2.21 (1.75–2.83) | 2.22 (1.65–2.75) | 2.21 (1.72–2.82) | 0.33 |
| Triglyceride, mM | 1.54 (1.05–2.47) | 1.62 (1.15–2.38) | 1.46 (1.04–2.34) | 2.00 (1.22–3.10) | 0.98 (0.83–1.31) | 0.01 |
| TMAO, ng/mL | 233 (142–368) | 294 (181–424) | 234 (132–349) | 204 (143–329) | 151 (110–338) | 0.28 |
Values are median (interquartile range) or percentage. The p value for the comparison among four groups was obtained by ANOVA design (with a post hoc test) or Kruskal-Wallis H test depending on whether the variables obey normal distribution.
BMI = body mass index, MI = myocardial infarction, TCHO = total cholesterol, HDL = high-density lipoprotein, LDL = low-density lipoprotein, TMAO = trimethylamine N-oxide.
Fig. 1.
Comparison of plasma TMAO level among healthy controls, non-atherosclerosis controls, atherosclerosis patients and MI/stroke patients. The p value for the comparison between every two groups was obtained by Student t-test. Error bars, transverse-line and quadrate in box plots indicate 1.5 IQR, median and mean, respectively.
3.2. LASSO regression of TMAO with baseline data
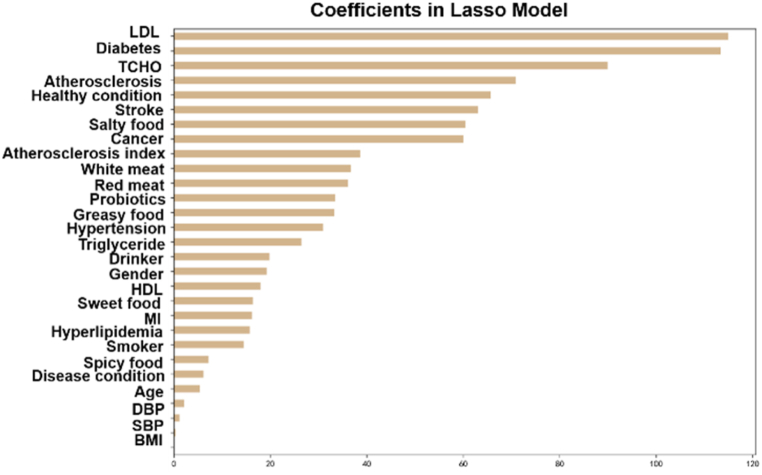
Because TMAO has a clear pathogenesis to atherosclerosis and has been confirmed by a large number of studies [16,19,21], we considered that some baseline characteristics may affect the accuracy of TMAO in indicating atherosclerosis development. Therefore, we next conducted a regression analysis using the least absolute shrinkage and selection operator (LASSO) to discover the potential contributing factors to TMAO concentration. Due to the severe imbalance in our baseline data, containing 9 continuous variables and 20 discrete variables (Supplementary information 1, Table S1), we temporarily eliminate all the patient data containing missing values to improve the accuracy of the LASSO regression. As shown in Fig. 2, diabetes and atherosclerosis state of patients are the main contributory discrete variables, and levels of LDL and TCHO are the main continuous variables. Health status of patients is the fifth significant variable, but it has been verified no significant contribution to TMAO level (Fig. 1).
Fig. 2.
Lasso coefficients of all predictors.
3.3. Model validation of LASSO regression
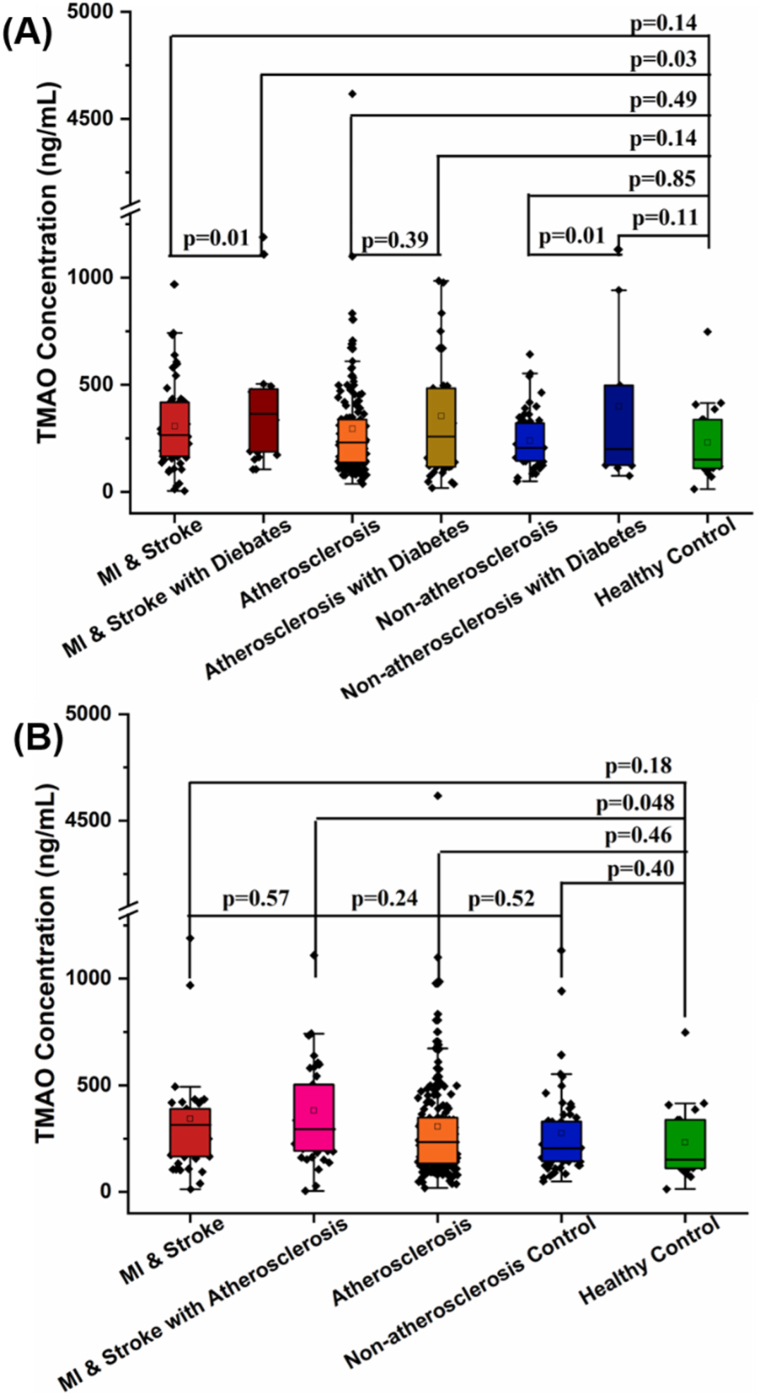
Based on the patients' status of diabetes, all participants in groups of MI & stroke, atherosclerosis and non-atherosclerosis control were further divided into diabetic group and non-diabetic group (Fig. 3A). With this phenotyping, patients in MI & stroke group can be clearly distinguished with the healthy controls, with p value of 0.01. More importantly, among MI & stroke patients and non-atherosclerosis controls, patients with diabetes also have higher levels of TMAO concentration relative to their non-diabetes counterparts, with p value of 0.01. On the other hand, by phenotyping based on patients' status of atherosclerosis, only patients with atherosclerosis in MI & stroke group have statistical difference compared to the healthy controls (Fig. 3B). Meanwhile, no significant differences in TMAO levels were found when comparing these MI or stroke combined atherosclerosis patients with pure MI, stroke or atherosclerosis patients (Fig. 3B). Moreover, comparing pure atherosclerosis patients with non-atherosclerosis control groups, also no significant TMAO level difference presents between them (Fig. 3B).
Fig. 3.
Comparison of plasma TMAO level among patients and controls with (A) diabetes and (B) atherosclerosis phenotyping. The p value for the comparison between every two groups was obtained by Student t-test. Error bars, transverse-line and quadrate in box plots indicate 1.5 IQR, median and mean, respectively.
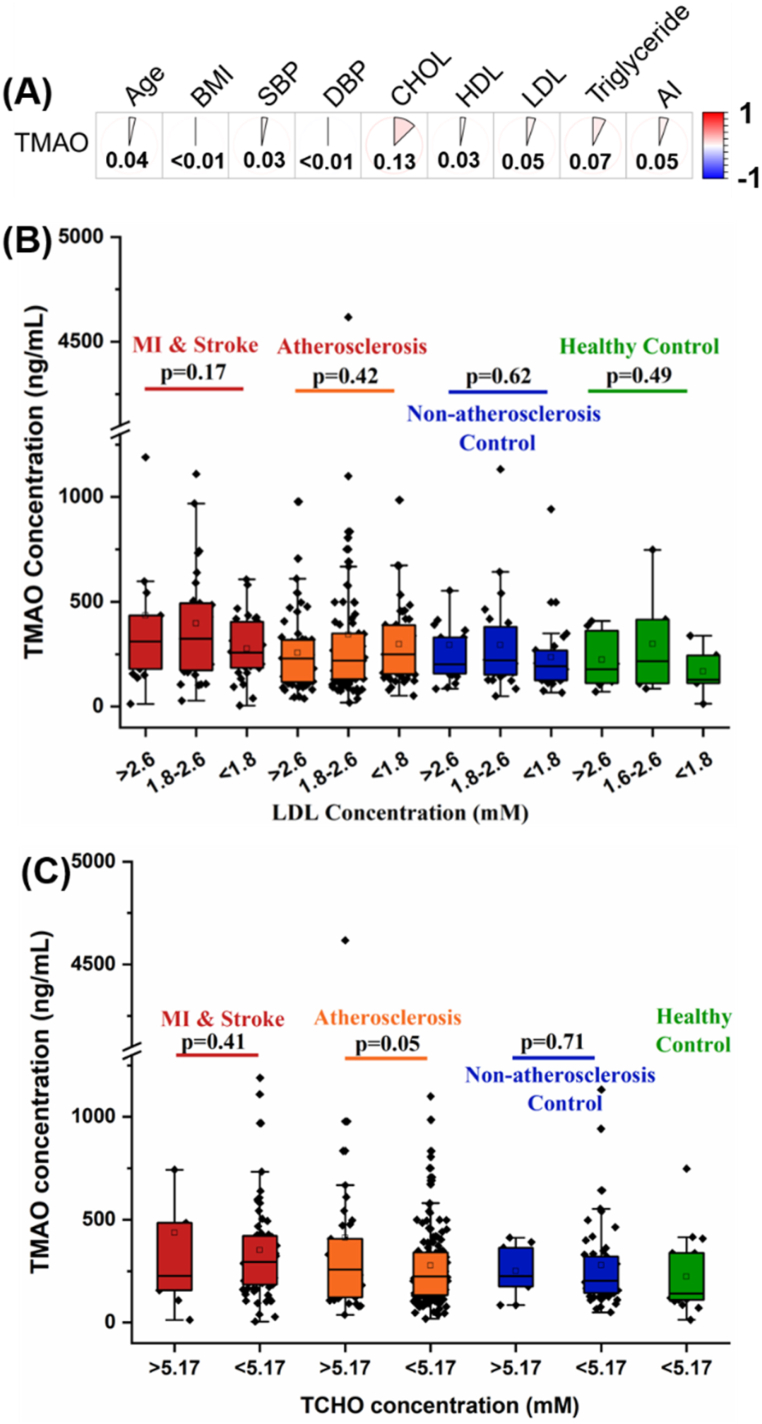
To verify the correlation of TMAO level with 9 continuous variables (age, BMI, AI, SBP, DBP, and level of CHOL, HDL, LDL and triglyceride), a Pearson correlation analysis was performed. As shown in Fig. 4A, the Pearson correlation coefficients (r) of all 9 continuous variables to TMAO level are lower than 0.2, indicating negligible correlation. Indeed, for the main two contributory variables, LDL and TCHO, there are no significant difference in TMAO concentration observed within the four groups after phenotyping based on LDL or TCHO concentration (Fig. 4B–C).
Fig. 4.
(A) Pearson correlation analysis with TMAO concentration and 9 continuous variables. Comparison of plasma TMAO level among patients and controls based on the level of (B) LDL and (C) TCHO. The p value for the comparison between every two groups or every three groups was obtained by Student t-test or ANOVA design (with a post hoc test), respectively. Error bars, transverse-line and quadrate in box plots indicate 1.5 IQR, median and mean, respectively.
4. Discussion
The spread of atherosclerosis is a worldwide healthy problem. Unfortunately, existing models for monitoring atherosclerosis lack sufficient sensitivity and it is necessary to supplement or modify contribution variables of atherosclerosis on the basis of the existing models. TMAO, as an emerging biomarker, has made remarkable achievements in monitoring long-term adverse events in atherosclerosis. However, emerging evidences also suggests that TMAO may act on atherosclerosis as a secondary consequence of risk factors for atherosclerosis, such as hypertension and hyperlipidemia. Therefore, it is necessary to further clarify the complex relationship between TMAO and atherosclerosis, atherosclerosis-related adverse events (mainly MI and stroke) and atherosclerosis risk factors, which can improve the effectiveness and sensitivity of TMAO as a monitoring indicator for the development of atherosclerosis.
In this study, 359 participants were recruited from the Affiliated Hospital of Chengde Medical University (China), including 19 healthy controls, 68 non-atherosclerosis controls, 190 atherosclerosis patients, 82 MI or stroke patients. First, we tried to demonstrate the correlation between TMAO levels and the development of atherosclerosis. However, there were no statistically significant differences in TMAO levels in atherosclerotic patients, non-atherosclerotic controls, MI or stroke patients compared with healthy controls (Fig. 1). Based on previous findings, only when TMAO concentration above 6.18 μM (appr. 460 ng/mL), there is significant increase on cardiovascular adverse events [20]. However, in our cohort, the median TMAO levels in the four groups are 142–368 ng/mL (appr. 1.91–4.94 μM), and upper quartiles of TMAO levels in these four groups are all lower than 460 ng/mL, indicating a negligible pathogenic contribution of TMAO to atherosclerosis development.
Much previously reported TMAO levels in atherosclerosis associated cardiovascular diseases were significantly higher than our results [23,[28], [29], [30]]. For example, Senthong et al. reported a study on TMAO level in American MI population (recruitment time was 2012–2014, recruited subjects were elderly aged about 60 with ≥50% vessel stenosis), in which the median TMAO level was 5.5 μM (appr. 409 ng/mL) [23]. In addition, Suzuki et al. reported a TMAO pathogenicity research in European MI population (recruitment time was 2004–2007, recruited subjects were elderly aged 53–81 on the acute attacking of MI), in which the TMAO level was 4.6–6.4 μM (appr. 342–476 ng/mL) [29]. On the other hand, Xu et al. reported TMAO level research in Han Chinese population suffered acute cerebral ischemia stroke (recruitment time was 2018–2019, recruited subjects were elderly aged 51–75 on the attacking of stroke), in which the TMAO level was 1682–3636 ng/mL (appr. 22.6–48.8 μM) [30]. However, in our study, we focused on the atherosclerotic receiving long-term drug treatment. The thrombosis in myocardial infarction (TIMI) flow grade in 82.1% of patients (279/340) was grade 3 (Supplementary information 1, Table S1), indicating that the distal coronary artery of patients was completely filled and rapidly eliminated. According to Spearman correlation analysis, TIMI flow grade did not have correlation with subjects' TMAO level (Supplementary information 2, Fig. S1). Moreover, in addition to healthy controls, 93.8% of participants (319/340) were treated with one or more lipid lowering or blood pressure lowering drugs, including statins, aspirin, clopidogrel and metformin (Supplementary information 2, Table S1). Statins, in particular, were used for lipid-lowering treatment in 92.4% of patients (314/340) (Supplementary information 2, Fig. S3). According to the latest studies, statins can alter users' intestinal microbiota and thence decrease their plasma TMAO level [31,32]. Although metformin has similar efficacy on TMAO lowering [33], only 5% of subjects in our cohort received this drug, which not enough to explain the overall level decrease of TMAO in our cohort. Similar drug regimens were also reported in the previous atherosclerosis associated TMAO studies in Han Chinese population [34,35]. Waleed et al. recruited 73 newly diagnosed 18–75 aged non-ST-segment elevation MI patients during 2019–2020, giving aspirin and clopidogrel antiplatelet therapy to them, and their plasma TMAO levels were detected as 0.42–0.78 μM (appr. 31–58 ng/mL) [34]. Sheng et al. recruited 335 newly diagnosed ≥18 aged ST-segment elevation MI patients during 2017–2018, giving aspirin and ticagrelor or clopidogrel therapy to them, and their plasma TMAO levels were detected as 1.34–3.90 μM (appr. 99.7–290.3 ng/mL) [35]. These two TMAO related studies under statin treating background all shown characteristics of low plasma TMAO levels similar to our cohort.
For atherosclerosis patients in Han Chinese population without drug treatment, it also been reported to have a low TMAO levels (median concentration of 2.7 μM, n = 322) [36], which may be related to the unique dietary habits of the Chinese people. TMAO levels in human body are mainly determined by dietary intake of cholinergic foods [28], inclusive of red meat [27,37], fish in white meat [38], dairy products [39], and most recently reported whole-grains cereals [40]. In our cohort, we focused on the impact of red meat, white meat and dairy products (mainly probiotics) on TMAO levels. However, there was no statistical difference in these TMAO-associated dietary preference among subjects in the four groups (Table 1 and Supplementary information 1, Table S1). Based on previous finding, human plasma TMAO level was sensitive to red meat intake, which can return to a lower level within 4 weeks once red meat intaking was stopped [27]. Therefore, such undifferentiated TMAO-derived diet preferences in our cohort may be conducive to proving a balance dietary background and thence to reflecting the direct relationship between TMAO and cardiovascular disease. Of course, in subsequent studies, specific TMAO-derived diet intake of subjects should be recorded more accurately, and the average TMAO level of each subject should be obtained through multiple samples, so as to draw more accurate conclusions about the impact of dietary preferences on TMAO level.
Previous studies have confirmed that TMAO levels have independent association with atherosclerotic burden [23,28]. The main mechanism of TMAO to atherosclerosis involve the increase of platelet hyperreactivity through altered Ca2+ signaling [18], and the promotion of insulin resistance by binding and activating PERK protein [19]. Wang et al. reported that plasma TMAO levels were positive correlation with atherosclerotic burden [28]. However, patients with high TMAO levels always have diabetes and/or hypertension [23]. That is, TMAO may coupling with other risk factors of atherosclerosis to facilitate the development of atherosclerosis. Indeed, some atherosclerosis-related diseases do presented high TMAO levels in patients, such as diabetes [41] and chronic kidney disease [42]. To thoroughly identify the contributing indicators of TMAO levels, LASSO regression analysis was performed using TMAO as dependent variable and baseline characteristics of cohort as independent variables. As shown in Fig. 2, diabetes and atherosclerosis state of patients were the main contributory discrete variables, and levels of LDL and TCHO were the main continuous variables.
Based on the patients' status of diabetes, all participants in groups of MI & stroke, atherosclerosis and non-atherosclerosis control are further divided into diabetes group and non-diabetes group (Fig. 3A). With this phenotyping, diabetes patients in MI & stroke group can be clearly distinguished from healthy controls, with a p-value of 0.01. More importantly, among MI & stroke group and non-atherosclerosis group, patients with diabetes have higher level of TMAO concentrations, with a p-value of 0.01. Indeed, the association between TMAO levels and the incident of diabetes was previously widely reported [43,44], and the prevalence of diabetes increased by 54% per 372 ng/mL (abbr. 5 μM) increment of plasma TMAO levels [44].
At present, the exact mechanism of TMAO-induced diabetes pathogenesis is still unclear. Partial evidence suggests that dietary TMAO can induce adipose tissue inflammation and block hepatic insulin signaling, an important contributor to insulin resistance and type 2 diabetes [45]. Furthermore, high levels of plasma TMAO can reduce the expression of proteins involved in bile acid synthesis and transport, thereby reducing the size of total bile acid pool [46]. Importantly, bile acid, as the ligands for FXR and TGR5, can intervene in glucoses metabolism through multiple pathways [47], such as regulating diabetes-associated NLRP3 inflammasome activation through the TGR5-cAMP-PKA axis [48]. More importantly, when the vessel wall is stimulated by risk factors for atherosclerosis, the NLRP3 inflammasome will be activated to initiate the release of the downstream inflammatory factor IL-1β, and at the same time promote the expression of IL-6 and IL-8 in an autocrine manner, which intensifies the development of atherosclerosis lesions [49]. Therefore, in the process of atherosclerosis progression, TMAO is more likely to cooperate with diabetes risk factors to promote atherosclerotic disease progression and atherosclerotic adverse events.
In addition to diabetes factors, atherosclerosis was also used as subtyping evidence to elucidate the actual contribution of TMAO in patients with MI or stroke. Only patients with atherosclerosis in MI & stroke group were significantly different compared to healthy controls (Fig. 3B). Meanwhile, compared these atherosclerosis patients with pure MI & stroke patients or pure atherosclerosis patients, no significant difference in TMAO level can be found. Moreover, compared atherosclerosis group with non-atherosclerosis controls, there was also no significant difference in TMAO levels between them. Similar results were also reported at previous atherosclerosis associated studies, which was inclusive of Han Chinese population [36], American population [22], and European population [50]. More importantly, TMAO levels of atherosclerotic in these studies generally lower than 6.18 μM (appr. 460 ng/mL). In contrast, the positive association between TMAO and atherosclerosis progression usually occurs at higher TMAO levels (close to 6.18 μM or 460 ng/mL) [20,23,28,29]. Considering the low level of TMAO in the Chinese population, the prognostic value of TMAO in patients with pure atherosclerosis may not be fully reflected, but it has great application potential in patients with atherosclerosis and diabetes.
Although TMAO has been identified as a causative factor for atherosclerosis and diabetes, the prognostic application of TMAO in atherosclerosis patients with diabetes has not been focused until recently [51,52]. Part of evidences show that, TMAO level is an independent risk factor associated with adverse event in type 1 diabetes and type 2 diabetes, which can lead to CVD events and even death [41,52]. In our cohort, all of diabetics are type 2 diabetes (Supplementary information 2, Table S2), and 27.6% of them (21/76) with TMAO levels >460 ng/mL (Supplementary information 1, Table S1), indicating a potential high CVD risk of them. However, based on Cardona et al.’ study, type 2 diabetes patients with poor glycemic control will blunt the prognostic value of TMAO [51]. Interestingly, in our cohort of diabetes subjects, blood glucose indexes (fasting blood glucose, HbA1c and 2 hPG) were not present perceptible correlation with TMAO levels (Supplementary information 2, Fig. S2). Consequently, when evaluating the prognosis of atherosclerosis patients with diabetes, TMAO level and insulin resistance should be considered comprehensively for more accurate results.
LDL and TCHO levels were also main contributors to TMAO levels. Unlike diabetes and atherosclerosis risk factors, LDL and TCHO levels are continuous variables. As most of variables in the LASSO model are discrete variables, this may distort the regression results on continuous variables. Therefore, for further validating the correlation between TMAO levels and these two lipid indictors, a Pearson correlation analysis was performed. As shown in Fig. 4A, the Pearson correlation coefficient (r) of LDL, TCHO and other risk factors of atherosclerosis were all lower than 0.2, indicating negligible correlation. Moreover, no significant difference in TMAO concentration can be observed for LDL or TCHO subtyping in the four groups (Fig. 4B–C). Consequently, in our cohort, subjects' LDL and TCHO levels may have negligible effect on their plasma TMAO levels.
This study has several limitations. First, this is a single-center cross-sectional study. Multi-center parallel validation and follow-up on a large time scale will further enhance the evidence level for the conclusion of this study. Second, the proportion of diabetes subjects in non-atherosclerosis group, atherosclerosis group and MI & stroke group is small relative to their intra-group non-diabetes counterparts. Further expansion of the number of subjects is needed in order to better balance the difference in the number of subjects intra- and inter-groups. Three, fine dietary management (including the precise intake of fish, choline and carnitine within 48 h) and comprehensive intestinal microbiota information need to be involved in order to minimize the “background value” introduced by non-disease factors [24,46]. Finally, detailed imaging data (e.g., intravascular photoacoustic tomography, high-resolution vessel wall magnetic resonance imaging, and magnetic resonance spectroscopy) should also be included in the analysis, showing the vulnerability, compositional characteristics and metabolic profile of atherosclerotic plaques to better explain differences in stratification results.
5. Conclusions
Cardiovascular disease (CVD) is the leading course of disease-related death in both developed and developing countries with atherosclerosis as its main pathology. Although TMAO had been shown to contribute to the development and progression of atherosclerosis, we found no significant difference of TMAO concentration among atherosclerosis patients, MI or stroke patients, non-atherosclerosis controls and healthy controls. However, using LASSO regression model to elucidate the correlation between TMAO and atherosclerosis risk factors, four contributor candidates were successfully identified to have correlation to TMAO levels, including disease status of diabetes or atherosclerosis, and blood lipid indicators level of low-density lipoprotein cholesterol or total cholesterol. Subsequent univariate analysis further confirmed that the presence or absence of diabetes had a decisive effect on plasma TMAO levels. Considering the definite pathogenesis of TMAO to atherosclerosis, this high levels of TMAO in diabetes patients may contribute to the development and progression of atherosclerosis. Therefore, it is necessary to focus on monitoring TMAO levels in diabetic patients in order to reduce adverse cardiovascular events.
Ethics approval and consent to participate
The study was approved by the Ethics Committee of the Affiliated Hospital of Chengde Medical University (IRB approval number was CYFYLL2021099). All patients provided written informed consent for this study. All methods in this study carried out in accordance with the Declaration of Helsinki. Permission to use the patient's samples was obtained from the Ethics Committee of the Affiliated Hospital of Chengde Medical University.
Author contribution statement
Hao Liang: Performed the experiments; Contributed reagents, materials, analysis tools or data.
Anqi Yu: Analyzed and interpreted the data.
Zheng Wang: Performed the experiments.
Na Zhang; Qingsong Wang; Haichao Gao: Contributed reagents, materials, analysis tools or data.
Junhui Gao: Conceived and designed the experiments.
Xinjun Wang: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Hong Wang: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
Dr. Xinjun Wang was supported by China postdoctoral science foundation [2022M722412] and the scientific instrument application methods project of Shang-hai Science and technology innovation action plan [19142200800].
Data availability statement
The detail baseline information of our cohort is available in the Supplementary information 1. Detail drug regimen of non-atherosclerosis controls, atherosclerosis patients and MI & stroke patients is shown in the Supplementary information 2. Detail case report form is shown in the Supplementary infor-mation 3.
Consent for publication
Not applicable.
Declaration of interest's statement
The authors declare no competing interests.
Acknowledgements
Xinjun Wang especially wishes to thank Lei Liu and Huidi Zhang from Biotecan Medical Diagnostics Co., Ltd for their assistance in subject design and TMAO detection, respectively.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e13657.
Contributor Information
Junhui Gao, Email: jhgao68@163.com.
Xinjun Wang, Email: xjwang16@fudan.edu.cn.
Hong Wang, Email: wanghong659@163.com.
Abbreviations
- CVD
cardiovascular disease
- MI
myocardial infarctions
- HDL
high-density lipoprotein
- LDL
low-density lipoprotein
- TCHO
total cholesterol
- TMAO
trimethylamine N-oxide
- PCE
the Pooled Cohort Risk Equations
- AI
atherosclerosis index
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Benjamin E.J., et al. Heart disease and stroke statistics-2019 update: a report from the American heart association. Circulation. 2019;139:e56–e528. doi: 10.1161/cir.0000000000000659. [DOI] [PubMed] [Google Scholar]
- 2.Williams J.W., et al. Single cell RNA sequencing in atherosclerosis research. Circ. Res. 2020;126:1112–1126. doi: 10.1161/circresaha.119.315940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Libby P. The changing landscape of atherosclerosis. Nature. 2021;592:524–533. doi: 10.1038/s41586-021-03392-8. [DOI] [PubMed] [Google Scholar]
- 4.Nordestgaard B.G., Varbo A. Triglycerides and cardiovascular disease. Lancet. 2014;384:626–635. doi: 10.1016/s0140-6736(14)61177-6. [DOI] [PubMed] [Google Scholar]
- 5.Pirillo A., et al. Global epidemiology of dyslipidaemias. Nat. Rev. Cardiol. 2021 doi: 10.1038/s41569-021-00541-4. [DOI] [PubMed] [Google Scholar]
- 6.Mayyas F., Bani Omar E. Plasma lipoprotein (a) and tissue plasminogen activator are associated with increased risk of atherosclerotic cardiovascular disease. Heliyon. 2022;8 doi: 10.1016/j.heliyon.2022.e09836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu D., et al. Low-density lipoprotein cholesterol 4: the notable risk factor of coronary artery disease development. Front. Cardiovasc. Med. 2021;8 doi: 10.3389/fcvm.2021.619386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miyawaki D., et al. Maternal high-fat diet promotes calcified atherosclerotic plaque formation in adult offspring by enhancing transformation of VSMCs to osteochondrocytic-like phenotype. Heliyon. 2022;8 doi: 10.1016/j.heliyon.2022.e10644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lokpo S.Y., et al. The pattern of dyslipidaemia and factors associated with elevated levels of non-HDL-cholesterol among patients with type 2 diabetes mellitus in the Ho municipality: a cross sectional study. Heliyon. 2022;8 doi: 10.1016/j.heliyon.2022.e10279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mensah G.A., et al. The global burden of cardiovascular diseases and risk factors: 2020 and beyond. J. Am. Coll. Cardiol. 2019;74:2529–2532. doi: 10.1016/j.jacc.2019.10.009. [DOI] [PubMed] [Google Scholar]
- 11.Moore J.X., et al. Metabolic syndrome prevalence by race/ethnicity and sex in the United States, national health and nutrition examination survey, 1988-2012. Prev. Chronic Dis. 2017;14:E24. doi: 10.5888/pcd14.160287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muntner P., et al. Validation of the atherosclerotic cardiovascular disease Pooled Cohort risk equations. JAMA. 2014;311:1406–1415. doi: 10.1001/jama.2014.2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goff D.C., Jr., et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American college of cardiology/American heart association task force on practice guidelines. J. Am. Coll. Cardiol. 2014;63:2935–2959. doi: 10.1016/j.jacc.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rodriguez F., et al. Atherosclerotic cardiovascular disease risk prediction in disaggregated asian and hispanic subgroups using electronic health records. J. Am. Heart Assoc. 2019;8 doi: 10.1161/jaha.118.011874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mora S., et al. Evaluation of the pooled cohort risk equations for cardiovascular risk prediction in a multiethnic cohort from the Women's health initiative. JAMA Intern. Med. 2018;178:1231–1240. doi: 10.1001/jamainternmed.2018.2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berger M., et al. Trimethylamine N-oxide and adenosine diphosphate-induced platelet reactivity are independent risk factors for cardiovascular and all-cause mortality. Circ. Res. 2020;126:660–662. doi: 10.1161/circresaha.119.316214. [DOI] [PubMed] [Google Scholar]
- 17.Schuett K., et al. Trimethylamine-N-oxide and heart failure with reduced versus preserved ejection fraction. J. Am. Coll. Cardiol. 2017;70:3202–3204. doi: 10.1016/j.jacc.2017.10.064. [DOI] [PubMed] [Google Scholar]
- 18.Zhu W., et al. Gut microbial metabolite TMAO enhances platelet hyperreactivity and thrombosis risk. Cell. 2016;165:111–124. doi: 10.1016/j.cell.2016.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen S., et al. Trimethylamine N-oxide binds and activates PERK to promote metabolic dysfunction. Cell Metabol. 2019;30:1141–1151.e1145. doi: 10.1016/j.cmet.2019.08.021. [DOI] [PubMed] [Google Scholar]
- 20.Tang W.H., et al. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N. Engl. J. Med. 2013;368:1575–1584. doi: 10.1056/NEJMoa1109400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Z., et al. Non-lethal inhibition of gut microbial trimethylamine production for the treatment of atherosclerosis. Cell. 2015;163:1585–1595. doi: 10.1016/j.cell.2015.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meyer K.A., et al. Microbiota-dependent metabolite trimethylamine N-oxide and coronary artery calcium in the coronary artery risk development in young adults study (CARDIA) J. Am. Heart Assoc. 2016;5 doi: 10.1161/jaha.116.003970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Senthong V., et al. Plasma trimethylamine N-oxide, a gut microbe-generated phosphatidylcholine metabolite, is associated with atherosclerotic burden. J. Am. Coll. Cardiol. 2016;67:2620–2628. doi: 10.1016/j.jacc.2016.03.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dambrova M., et al. Meldonium decreases the diet-increased plasma levels of trimethylamine N-oxide, a metabolite associated with atherosclerosis. J. Clin. Pharmacol. 2013;53:1095–1098. doi: 10.1002/jcph.135. [DOI] [PubMed] [Google Scholar]
- 25.Li D.Y., et al. Loop diuretics inhibit renal excretion of trimethylamine N-oxide. JACC Basic Transl. Sci. 2021;6:103–115. doi: 10.1016/j.jacbts.2020.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li Y.M., et al. A LASSO-derived risk model for long-term mortality in Chinese patients with acute coronary syndrome. J. Transl. Med. 2020;18:157. doi: 10.1186/s12967-020-02319-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Z., et al. Impact of chronic dietary red meat, white meat, or non-meat protein on trimethylamine N-oxide metabolism and renal excretion in healthy men and women. Eur. Heart J. 2019;40:583–594. doi: 10.1093/eurheartj/ehy799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Z., et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472:57–63. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Suzuki T., et al. Trimethylamine N-oxide and risk stratification after acute myocardial infarction. Clin. Chem. 2017;63:420–428. doi: 10.1373/clinchem.2016.264853. [DOI] [PubMed] [Google Scholar]
- 30.Xu D., et al. The relationship of large-artery atherothrombotic stroke with plasma trimethylamine N-oxide level and blood lipid-related indices: a cross-sectional comparative study. BioMed Res. Int. 2021;2021 doi: 10.1155/2021/5549796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kummen M., et al. Rosuvastatin alters the genetic composition of the human gut microbiome. Sci. Rep. 2020;10:5397. doi: 10.1038/s41598-020-62261-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vourakis M., et al. The role of gut microbiota on cholesterol metabolism in atherosclerosis. Int. J. Mol. Sci. 2021;22 doi: 10.3390/ijms22158074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuka J., et al. Metformin decreases bacterial trimethylamine production and trimethylamine N-oxide levels in db/db mice. Sci. Rep. 2020;10 doi: 10.1038/s41598-020-71470-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Waleed K.B., et al. Trimethylamine N-oxide is associated with coronary atherosclerotic burden in non-ST-segment myocardial infarction patients: SZ-NSTEMI prospective cohort study. Rev. Cardiovasc. Med. 2021;22:231–238. doi: 10.31083/j.rcm.2021.01.299. [DOI] [PubMed] [Google Scholar]
- 35.Sheng Z., et al. Relation of circulating trimethylamine N-oxide with coronary atherosclerotic burden in patients with ST-segment elevation myocardial infarction. Am. J. Cardiol. 2019;123:894–898. doi: 10.1016/j.amjcard.2018.12.018. [DOI] [PubMed] [Google Scholar]
- 36.Yin J., et al. Dysbiosis of gut microbiota with reduced trimethylamine-N-oxide level in patients with large-artery atherosclerotic stroke or transient ischemic attack. J. Am. Heart Assoc. 2015;4 doi: 10.1161/jaha.115.002699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krüger R., et al. Associations of current diet with plasma and urine TMAO in the KarMeN study: direct and indirect contributions. Mol. Nutr. Food Res. 2017;61 doi: 10.1002/mnfr.201700363. [DOI] [PubMed] [Google Scholar]
- 38.Yin X., et al. The relationship between fish intake and urinary trimethylamine-N-oxide. Mol. Nutr. Food Res. 2020;64 doi: 10.1002/mnfr.201900799. [DOI] [PubMed] [Google Scholar]
- 39.Rohrmann S., et al. Plasma concentrations of trimethylamine-N-oxide are directly associated with dairy food consumption and low-grade inflammation in a German adult population. J. Nutr. 2016;146:283–289. doi: 10.3945/jn.115.220103. [DOI] [PubMed] [Google Scholar]
- 40.Costabile G., et al. Plasma TMAO increase after healthy diets: results from 2 randomized controlled trials with dietary fish, polyphenols, and whole-grain cereals. Am. J. Clin. Nutr. 2021 doi: 10.1093/ajcn/nqab188. [DOI] [PubMed] [Google Scholar]
- 41.Winther S.A., et al. Utility of plasma concentration of trimethylamine N-oxide in predicting cardiovascular and renal complications in individuals with type 1 diabetes. Diabetes Care. 2019;42:1512–1520. doi: 10.2337/dc19-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ravid J.D., et al. Uraemic solutes as therapeutic targets in CKD-associated cardiovascular disease. Nat. Rev. Nephrol. 2021;17:402–416. doi: 10.1038/s41581-021-00408-4. [DOI] [PubMed] [Google Scholar]
- 43.Roy S., et al. Plasma trimethylamine-N-oxide and impaired glucose regulation: results from the oral infections, glucose intolerance and insulin resistance study (ORIGINS) PLoS One. 2020;15 doi: 10.1371/journal.pone.0227482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhuang R., et al. Gut microbe-generated metabolite trimethylamine N-oxide and the risk of diabetes: a systematic review and dose-response meta-analysis. Obes. Rev. 2019;20:883–894. doi: 10.1111/obr.12843. [DOI] [PubMed] [Google Scholar]
- 45.Gao X., et al. Dietary trimethylamine N-oxide exacerbates impaired glucose tolerance in mice fed a high fat diet. J. Biosci. Bioeng. 2014;118:476–481. doi: 10.1016/j.jbiosc.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 46.Koeth R.A., et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 2013;19:576–585. doi: 10.1038/nm.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lefebvre P., et al. Role of bile acids and bile acid receptors in metabolic regulation. Physiol. Rev. 2009;89:147–191. doi: 10.1152/physrev.00010.2008. [DOI] [PubMed] [Google Scholar]
- 48.Guo C., et al. Bile acids control inflammation and metabolic disorder through inhibition of NLRP3 inflammasome. Immunity. 2016;45:802–816. doi: 10.1016/j.immuni.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 49.Kinnunen K., et al. Lysosomal destabilization activates the NLRP3 inflammasome in human umbilical vein endothelial cells (HUVECs) J. Cell Commun. Signal. 2017;11:275–279. doi: 10.1007/s12079-017-0396-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bordoni L., et al. Trimethylamine N-oxide and the reverse cholesterol transport in cardiovascular disease: a cross-sectional study. Sci. Rep. 2020;10 doi: 10.1038/s41598-020-75633-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cardona A., et al. Trimethylamine N-oxide and incident atherosclerotic events in high-risk individuals with diabetes: an ACCORD trial post hoc analysis. BMJ Open Diabetes Res. Care. 2019;7 doi: 10.1136/bmjdrc-2019-000718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Croyal M., et al. Plasma trimethylamine N-oxide and risk of cardiovascular events in patients with type 2 diabetes. J. Clin. Endocrinol. Metabol. 2020;105 doi: 10.1210/clinem/dgaa188. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The detail baseline information of our cohort is available in the Supplementary information 1. Detail drug regimen of non-atherosclerosis controls, atherosclerosis patients and MI & stroke patients is shown in the Supplementary information 2. Detail case report form is shown in the Supplementary infor-mation 3.