Summary
Hepatocellular carcinoma (HCC) is a fatal malignant tumor, but effective clinical interventions are limited. PLGA/PEI-mediated DNA vaccine encoding the dual targets of high-mobility group box 1 (HMGB1) or GPC3 was developed for HCC treatment. Compared with PLGA/PEI-GPC3 immunization, PLGA/PEI-HMGB1/GPC3 co-immunization significantly inhibited the subcutaneous tumor growth, while increasing the infiltration of CD8+T cells and DCs. Furthermore, the PLGA/PEI-HMGB1/GPC3 vaccine induced a strong CTL effect and promoted functional CD8+T cell proliferation. Intriguingly, the depletion assay proved that the therapeutic effect PLGA/PEI-HMGB1/GPC3 vaccine was dependent on antigen-specific CD8+T cell immune responses. In the rechallenge experiment, PLGA/PEI-HMGB1/GPC3 vaccine provided a long-lasting resistance to the growth of the contralateral tumor by inducing the memory CD8+T cell responses. Collectively, PLGA/PEI-HMGB1/GPC3 vaccine could induce a strong and long-lasting CTL effect and inhibit the tumor progression or re-attack. Therefore, the combined co-immunization of PLGA/PEI-HMGB1/GPC3 might be served as an effective anti-tumor strategy against HCC.
Subject areas: immunology, cancer
Graphical abstract

Highlights
-
•
PLGA/PEI-HMGB1/GPC3 inhibited tumor growth and promoted the immune cell infiltration
-
•
CD8+T cells were required for the therapeutic effect of PLGA/PEI-HMGB1/GPC3
-
•
PLGA/PEI-HMGB1/GPC3 induced the long-lasting anti-tumor effect by memory CD8+T cells
Immunology; Cancer.
Introduction
Hepatocellular carcinoma (HCC) ranks as the sixth most commonly diagnosed cancer worldwide, with a poor five-year survival rate.1,2 The diagnosis and interventional therapy of patients with HCC are usually influenced by many complicated factors, including the volume of solid tumor, the various pathological features, and the incidence of vascular invasion.3 Unfortunately, more than half of patients with HCC experience recurrences and are diagnosed with extensive metastases in multiple organs.4 Currently, the mainstream treatment strategy for HCC focuses on surgery, radiotherapy, or systemic therapy including chemotherapy, immune-enhancing cytokines, and monoclonal antibodies.5,6 Although these strategies prolong the survival of patients to some extent, the high recurrence rate and potential clinical adverse events make it necessary to design novel targeted approaches to control this dreaded disease.
Tumor vaccines, especially therapeutic ones, bring great promise for cancer immunotherapy and promote the induction of cytotoxic T lymphocyte (CTL) responses.7,8 Moreover, different immunization strategies, such as DNA,9 RNA,10 protein,11 or peptide,12 have been tested on a large scale in clinical or preclinical research. DNA vaccination that generates the cytotoxic effect by mediating the expression of antigenic proteins has gained significant attention in cancer therapy.13 Glypican-3 (GPC3) is a secreted form of heparan sulfate proteoglycan or cell membrane protein anchored by glycosylphosphatidylinositol, which is considered as a diagnostic marker for HCC, because it is found to be highly expressed in malignant liver tissues and detected in serum.14,15 In addition, GPC3 is undetectable in normal tissues thus demonstrating that GPC3 can serve as an important therapeutic target for HCC immunotherapy.16 Until recently, a series of DC or peptide-based vaccines targeting GPC3 have achieved promising effects in treating HCC.17,18 Although significant progress has been made in developing novel vaccine therapy for HCC, the concerns related to safety and effectiveness are still challenging.19 Therefore, designing novel and powerful strategies for HCC interventions is urgent.
High-mobility group protein 1 (HMGB1) plays a vital role in inflammation-associated diseases including malignant tumors by promoting the expression of pro-inflammatory cytokines.20 High HMGB1 levels are frequently identified in various solid tumor types including renal,21 liver, lung,22 colorectal23 and ductal breast cancer,24 thus suggesting that the aberrant expression of HMGB1 is closely associated with the genesis and progression of the malignant disease. Furthermore, it has been found that blocking extracellular HMGB1 inhibits tumor growth by regulating immune cell infiltration in microenvironment and promotes checkpoint inhibitor-based immunotherapy.25 Considering the complex function of HMGB1 in cell migration, invasion, and metastasis, HMGB1 as a novel therapeutic target is being developed in cancer treatment.26 In addition, HMGB1 acts as a DNA binding protein that exert its downstream activity by regulating the TLR9 signaling pathway.27 Moreover, HMGB1 released from damaged cells or activated macrophages can manipulate the magnitude of the adaptive immune response through the successful recruitment of various pro-inflammatory cells.28 Intriguingly, HMGB1 could specifically promote the maturity and migration of dendritic cells (DCs), thereby promoting the differentiation of Th1 response and proliferation of T cells.29 Besides, the endogenous-derived HMGB1 can also function as an immune adjuvant via improving the immunogenicity of ovalbumin protein,30 thus indicating that HMGB1 can also potentially serve as an adjuvant for vaccine strategy. Therefore, we inferred that the incorporation of HMGB1 into the DNA vaccine could be used as a tumor antigen or immune adjuvant to enlarge the antigen-specific immune responses.
DNA vaccine should be able to transfect host cells, so as to promote the expression of administered antigens by an effective delivery system. Our previous study has demonstrated that PLGA/PEI-mediated DNA delivery system is able to promote DNA vaccine to penetrate the biological barriers and transfer the plasmid into the nucleus of the target cell to express it stably.31 In this study, our result also demonstrated that PLGA/PEI-HMGB1/GPC3 vaccine could be efficiently expressed in vitro and in vivo. The immunogenicity and therapy effect of the PLGA/PEI-HMGB1/GPC3 vaccine was evaluated in the HCC model. PLGA/PEI-HMGB1/GPC3 vaccines could induce affective and long-lasting DC-mediated antigen-specific CD8+T cell immune responses, which can effectively resist tumor progression and re-attack. Therefore, PLGA/PEI-HMGB1/GPC3 might be an effective anti-tumor strategy against HCC.
Results
PLGA/PEI-HMGB1/GPC3 vaccine could be efficiently expressed both in vitro and in vivo
The PLGA/PEI-HMGB1/GPC3 nanoparticle-based vaccine was constructed in accordance with the procedures described in the STAR Methods section. To confirm the delivery efficacy of PLGA/PEI nanoparticles for mediating the expression of encoded genes under in vitro and in vivo settings. Firstly, the flow cytometry technique was employed to assess the expression after the transient transfection assay in vitro. It was observed that either PLGA/PEI-HMGB1 or PLGA/PEI-GPC3 displayed the high-level expression of HMGB1 or GPC3 in 293T cells respectively (Figures 1A–1D) compared with the PLGA/PEI-Vector. Secondly, to validate the expression of the gene delivered by PLGA/PEI in vivo, PLGA/PEI-HMGB1 or PLGA/PEI-GPC3 vaccine was injected intramuscularly, and PLGA/PEI-vector was used as the control group. Afterward, the injected muscles were excised and subjected to western blot assay. The western blot result showed the abundant existence of HMGB1 and GPC3 at the corresponding molecular weight (Figures 1E and 1F). Collectively, these results indicated that PLGA/PEI-HMGB1/GPC3 nanoparticle-based vaccine could be efficiently expressed in vitro and in vivo.
Figure 1.
The expression of PLGA/PEI-GPC3 and PLGA/PEI-HMGB1 vaccine
(A and B) The protein expression of HMGB1 or GPC3 was evaluated by flow cytometry in pHMGB1-transfected or pGPC3-transfected 293T cells.
(C and D) Statistical analysis of the proportions of the positive cells in A and B.
(E and F) The mice were intramuscularly administered PLGA/PEI-GPC3 and PLGA/PEI-HMGB1 vaccines. The injected muscular tissues were collected at 72 h after immunization. In vivo protein expression of HMGB1 and GPC3 was determined by western blot assay in muscular tissues. Data are from one representative experiment of three performed and presented as the mean ± SD, ∗∗∗∗p < 0.001.
Co-immunization of PLGA/PEI-HMGB1/GPC3 inhibits the growth of the subcutaneous tumor and promotes the infiltration of immune cells
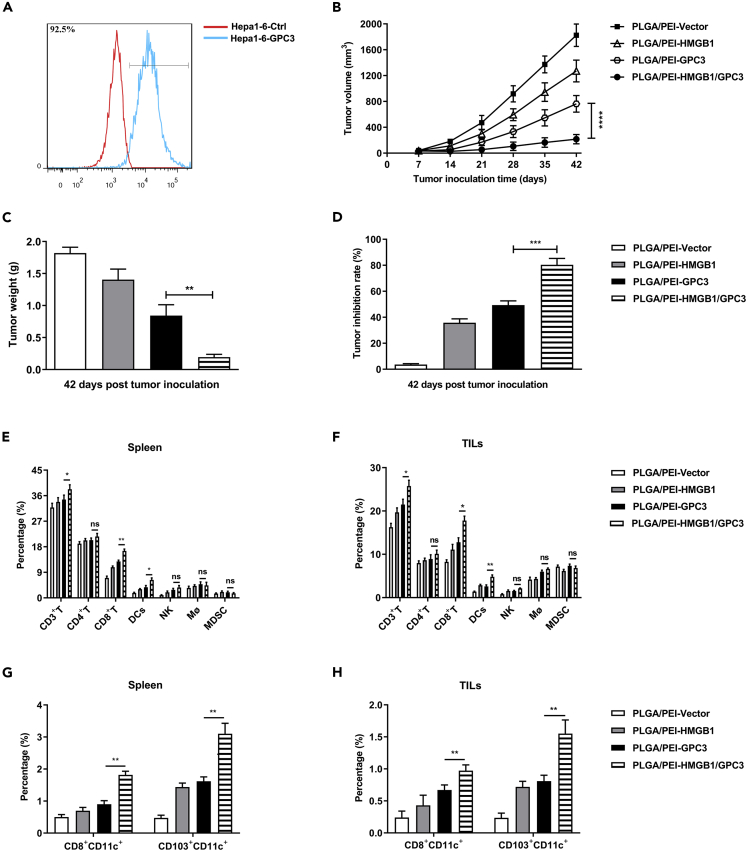
The stable cell line Hepa1-6-hGPC3 was established and the expression of hGPC3 was detected by flow cytometry. The result revealed that Hepa1-6-hGPC3 cells showed a significant increase of hGPC3 expression compared with control cells (Figure 2A). In order to determine the therapeutic effect of the PLGA/PEI-HMGB1/GPC3 vaccine, the primary murine models were established by subcutaneous incubation of Hepa1-6-hGPC3 cells. Next, mice with tumors were randomly divided into four groups, and then the nanoparticle vaccines were used for intramuscular vaccination. The tumor volume was monitored every week until 42 days after the tumor inoculation (Figure 2B). In parallel, the mice immunized with PLGA/PEI-HMGB1/GPC3 showed significantly lower tumor weight and higher tumor inhibition rate over their counterparts in PLGA/PEI-GPC3 group (Figures 2C and 2D). The tumor microenvironment is composed of heterogeneous subsets of immune cells, including T cells, macrophage (Mφ), DCs, and myeloid-derived suppressor cells (MDSCs), and their frequencies could be served as indicators of therapeutic effects. Therefore, to identify the effector cells that can suppress tumorigenesis, we detected the immune cell subsets in spleens and tumors using flow cytometry (Figures 2E and 2F). In comparison to the PLGA/PEI-GPC3, PLGA/PEI-HMGB1/GPC3 elicited a higher percentage of CD3+T, CD8+T cells, and DCs, which might induce a cytotoxic effect against the tumor. DCs have a unique capacity to perform antigen presentation processes, including endocytosis, processing, and presentation. Both CD8+DCs and CD103+DCs play a vital role in activating CD8+T cell anti-tumor immune responses in mice.32 Remarkably, compared with the single vaccine group, mice immunized with PLGA/PEI-HMGB1/GPC3 vaccine had an increased frequency of CD8+CD11C+DCs and CD103+CD11C+DCs (Figures 2G and 2H). These results indicated that PLGA/PEI-HMGB1/GPC3 vaccine could effectively inhibit tumor growth and increase the infiltration of immune cells.
Figure 2.
Anti-tumor effect of PLGA/PEI-HMGB1/GPC3 vaccine in the subcutaneous Hepa1-6-hGPC3 tumor model
(A) The frequency of hGPC3-positive cells was assessed by flow cytometry in Hepa1-6 cells infected with hGPC3-lentivirus. C57BL/6 mice were s.c. implanted with 5×106 Hepa1-6-hGPC3 cells on the right flank. One week following inoculation, the mice were randomly sub-grouped and respectively vaccinated with PLGA/PEI-Vector, PLGA/PEI-HMGB1, PLGA/PEI-GPC3, or PLGA/PEI-HMGB1/GPC3 vaccines.
(B) Tumor growth was recorded once a week from day 7 to day 42.
(C) Tumor weights.
(D) Tumor inhibition rate.
(E and F) Immune cell subsets, including CD3+T, CD8+T, CD4+T, DCs, NK, Mφ, and MDSCs, were quantified by flow cytometry in splenocytes and TILs.
(G and H) The frequency of CD8+CD11C+ or CD103+CD11C+ in the splenocytes and TILs was detected by flow cytometry. Data are from one representative experiment of three performed and presented as the mean ± SD, ∗∗p < 0.01; ∗∗∗p < 0.001.
The PLGA/PEI-HMGB1/GPC3 vaccine induces potent CTL response
The potential mechanism of therapeutic tumor vaccines may be mainly due to the generation of cytotoxic responses against the genes of interest. In order to verify the existence and intensity of the anticipated CTL response, we assessed the antigen-specific immune response through sequential proliferation, ELISPOT, and cytokine release experiments. The splenocytes stimulated with GPC3 protein showed superior proliferative ability, which was confirmed by the increased ratios of EdU+CD8+T cells in the PLGA/PEI-HMGB1/GPC3 group compared with the PLGA/PEI-GPC3 group (Figures 3A and 3B). Moreover, with respect to the secretion of Th1 cytokines, the frequencies of IL-2+CD8+T, IFN-γ+CD8+T, TNF-α+CD8+T cells were observed to be statistically higher in PLGA/PEI-HMGB1/GPC3 group than that in single vaccination group (Figures 3C and 3D), thus suggesting that dual target vaccine could significantly elevate the cytotoxicity via CTL effect. Moreover, an increased level of IFN-γ-producing CD8+T cells was observed by the ELISPOT experiment in PLGA/PEI-HMGB1/GPC3 group (Figure 3E). In addition, the effector cells obtained from the dual target vaccine group almost completely cleared the target cells, thus displaying the potent cytotoxicity of antigen-specific CD8+T cells (Figure 3F). These observations demonstrated that PLGA/PEI-HMGB1/GPC3 vaccine could trigger a potent CTL response against the malignant tumor cells.
Figure 3.
CTL effect induced by PLGA/PEI-HMGB1/GPC3 co-immunization
The lymphocytes obtained from the spleens were restimulated with GPC3 protein (10 μg/mL) ex vivo.
(A and B) The proliferation ability of CD8+T was assessed by EdU assay.
(C and D) The frequencies of IFN-γ+CD8+T, IL-2+CD8+T, or TNF-α+CD8+T cells were analyzed by flow cytometry in GPC3 protein stimulated lymphocytes following incubation with 500 ng/mL Ionomycin, 50 ng/mL PMA, and 1× Brefeldin A for 5h.
(E) The number of IFN-γ secreting CD8+T cells was assessed by ELISPOT assay.
(F) A co-culture experiment evaluated the antigen clearance effect. Data are from one representative experiment of three performed and presented as the mean ± SD, ∗∗p < 0.01.
The anti-tumor effect induced by PLGA/PEI-HMGB1/GPC3 vaccine is CD8+T cell dependent
In order to determine the role of CD8+T cells in the anti-tumor responses induced by the PLGA/PEI-HMGB1/GPC3 vaccine, the depletion assay of CD8+T cells was conducted in vivo. First of all, we verified the CD8 depletion effect through flow cytometry and confirmed that the CD8α mAb could significantly reduce the proportion of CD8+T cells and CD8+DCs cells in spleens and tumors (Figures 4A and 4B). The extensive CD8+T cell infiltration induced by PLGA/PEI-HMGB1/GPC3 vaccine was also eliminated (Figure 4C). Accordingly, the depletion of CD8+T cells almost eliminated the anti-tumor effect of this vaccine, as evidenced by the comparison of tumor volume (Figure 4D), tumor weight (Figure 4E), and tumor inhibition rate (Figure 4F) between groups. The result of the CD8 depletion assay suggested the indispensable role of CD8+ T cells in the therapeutic efficacy against tumors.
Figure 4.
The anti-tumor effect induced by PLGA/PEI-HMGB1/GPC3 vaccine was CD8+T cell dependent
For the CD8+T cell depletion assay, 0.5 mg CD8α antibody was administered two days before the tumor establishment and repeated on day 5 and day 12 after the first dose of vaccine.
(A and B) The ratio of CD8+T or CD8+CD11C+ subsets from the splenocytes or TILs was assessed by flow cytometry.
(C) CD8+T infiltration was detected by IHC assay (×200 magnification). 5×106 Hepa1-6-GPC3 cells were s.c. implanted in naive mice or PLGA/PEI-HMGB1-GPC3 vaccine recipients.
(D) Tumor growth curves in vivo. The mice were euthanized on day 42, and tumor weight.
(E) as well as tumor inhibition rate.
(F) were measured. Data are from one representative experiment of three performed and presented as the mean ± SD, ∗∗p < 0.01; ∗∗∗p < 0.001.
PLGA/PEI-HMGB1/GPC3 vaccine induces a long-lasting protective immunity against HCC
Overall, the above-mentioned results indicated that the mice immunized with PLGA/PEI-HMGB1/GPC3 vaccine could elicit potent Th1 cytokine secreted by CTLs. We hypothesized that the controlled-release pattern of PLGA/PEI vehicles might induce memory T cell response, which was correlated with the long-lasting protective immunity against tumors. Hence, mice immunized with PLGA/PEI-HMGB1/GPC3 vaccines were rechallenged contra-laterally with Hepa1-6-GPC3 cells. The tumor progression was monitored within 70 days and the tumor growth in the PLGA/PEI-HMGB1/GPC3 group was substantially suppressed compared with the naive mice (Figure 5A). Furthermore, the PLGA/PEI-HMGB1/GPC3 vaccine recipients displayed a 100% survival rate thus showing strong resistance to tumor progression (Figure 5B). The challenge results were highly indicative of a memory T cell response elicited by PLGA/PEI-HMGB1/GPC3 vaccine against the GPC3 antigen. In this regard, 42 days after initial tumor inoculation, the subsets of memory CD8+T cells were defined with CD44 and CD62L by flow cytometry analysis (Figure 5C), in which memory T cells were identified by naive (CD44lowCD62Lhigh), effector memory (CD44highCD62Llow), and central memory (CD44highCD62Lhigh). Interestingly, compared with mice immunized with PLGA/PEI-GPC3, the percentage of naive CD8+T cells in the PLGA/PEI-HMGB1/GPC3 group was efficiently reduced, while the effector or central memory CD8+T cells were found to have a statistical increase in PLGA/PEI-HMGB1/GPC3 group (Figures 5D–5F). Consequently, the long-acting protective effect against the tumor could be predominantly attributed to the potent antigen clearance effect by memory T cell immune response.
Figure 5.
The induction of long-lasting anti-tumor effects by PLGA/PEI-HMGB1-GPC3 vaccine
Mice that received PLGA/PEI-HMGB1-GPC3 vaccine were rechallenged with Hepa1-6-hGPC3 cells on the left flank accompanied by naive mice as the control group.
(A) Growth curve of individual mice over time in 70 days.
(B) The percentage survival of the control group and PLGA/PEI-HMGB1-GPC3 immunized group.
(C) Representative images of flow cytometry and statistical analysis of CD8+T cell cells labeled by CD44 and CD62L in the splenocytes from various vaccine immunization groups.
(D–F) The frequencies of Naive: CD44-/CD62Lhigh, Central memory: CD44+/CD62Lhigh, Effector memory: CD44+/CD62Llow CD8+CD11C+ or CD103+CD11C+ in and TILs were analyzed statistically. Data are from one representative experiment of three performed and presented as the mean ± SD, ∗∗p < 0.01.
Discussion
Implementing immunization with DNA vaccine represents an important strategy for eliciting cellular and humoral immune responses against foreign antigens.33 DNA vaccines could effectively generate an antigen-specific response in the host owing to the cellular uptake and expression of the delivered genes in vivo.34 However, the therapeutic efficacy of the DNA vaccine was far from satisfactory due to the limited delivery efficacy and degradation of administered antigenic materials.35 Consequently, studies conducted in recent times have primarily focused on the approaches attempting to enhance the immunogenicity of DNA vaccines, especially the nanotechnology-based delivery vectors for improving the immunogenicity of antigens.36
The potential application of biodegradable polyester particles as delivery systems has opened new avenues in DNA vaccination. Biodegradable particle materials represented by PLGA occupy a dominant position in this field, because they have the advantage of a controlled release profile and shielding effect, which can ensure the intactness of administered antigens and avoid needing more time.37 Contributed to the high transfection efficiency and low cost, the cationic polymers PEI has become one of the most widely used synthetic gene delivery tools in vivo.38 However, the apparent toxicity of PEI can hinder its possible application in mucosal vaccines, especially in the condition of pulmonary or nasal delivery.39 In this case, the complex of PLGA conjugated PEI eliminates the toxicity via the substantial positive charge that has aggregated on the cell membrane. Previous study has confirmed that PLGA/PEI-based encapsulation of antigen sequence displayed no toxic effect and was highly proficient in DNA delivery.31 Our results showed that the PLGA-PEI-based nanoparticles can mediate the expression of both adjuvant molecules and antigens in vivo and thus can contribute to the induction of remarkable therapeutic effects. In the course of these experiments, no abnormal inflammatory changes were observed in mice immunized with vaccines, which confirmed the biosafety of the PLGA/PEI-HMGB1/GPC3 nanoparticle vaccine.
DCs are unique professional antigen-presenting cells specialized for capturing and subsequent processing of various antigens for priming the differentiation and proliferation of T cells.40 The induction and maturity of CD11+DCs are critical for the initiation of strong antigen-specific immune responses and persistent maintenance of protective immunity.41 PLGA/PEI-mediated DNA vaccine could also shield the delivered antigens from degradation to further improve their uptake into DCs and induce the maturity of DCs.31 The results of the present study showed that PLGA/PEI-HMGB1/GPC3 vaccine can significantly promote the ratio of CD8+DCs and CD103+DCs, thus indicating that the nanoparticle can sufficiently bolster the magnitude of the CTL effect via DCs-mediated anti-tumor response. In addition, the design of a vaccine strategy targeting DCs might offer great promise for enhancing the immune response against the tumor. Consistent with our study, the nanoparticle-vectored vaccine has displayed a sustained antigen release pattern accompanied by effective induction of DCs subsets.
The cytotoxic CD8+T cells act as the major executor for implementing anti-tumor response among the distinct immune subpopulations.42 Unfortunately, the infiltrating CD8+T cells can also unexpectedly develop into an immune energy state contributed by the immunosuppressive tumor microenvironment, which can greatly compromise the proliferation and cytokine release ability of CD8+T cells.43 However, in this study we observed that the PLGA/PEI-HMGB1/GPC3 vaccine strengthened the CTL effect via the augmentation of proliferative and Th1 cytokine secretion including that of IFN-γ, TNF-α, and IL-2, thus suggesting that the vaccine was capable to recover the activity of intra-tumor CD8+T cells. Overall, these results indicated that the CD8+T cells are indispensable for the therapeutic efficacy of the PLGA/PEI-HMGB1/GPC3 vaccine. Although there was no significant difference in the induction of CD4 T cells between the PLGA/PEI-GPC3 group and PLGA/PEI-HMGB1/GPC3, CD4+T cells play an indispensable role in the recruitment of cytotoxic T cells and the generation of immune memory. Concurrently, the triple time immunization also elicited a long-lasting protective effect from the memory T cell accompanied by lower naive T cells. An elevated number of antigen-primed memory T cells was found to be potent enough to resist tumor rechallenge, as reflected by the prolonged survival of mice. The difference in therapeutic efficacy to the tumor challenge in settings of three times vaccination could be attributed to the ratio of memory T cells as well as the ceaseless exposure to the tumor antigens arising from the slow-release effect of PLGA/PEI materials.
To summarize, PLGA/PEI delivery system-based HMGB1 and GPC3 vaccine were developed for treating HCC. The results indicated that PLGA/PEI-HMGB1/GPC3 vaccine efficiently promoted the expression of administered antigens and enhanced the induction of CD8+DCs or CD103+DCs in vivo. Intramuscularly immunization with PLGA/PEI-HMGB1/GPC3 significantly suppressed tumor progression in subcutaneously implanted tumor model by triggering a potent antigen-specific CTL response. Taken together, delivering dual target antigens by PLGA/PEI nanoparticle could serve as a promising platform with huge potential for clinical translation in treating HCC and other solid tumors.
Limitations of the study
The mechanism of HMGB1 in vaccine effect is still unclear. Therefore, single-cell sequencing and other techniques are needed to further explore its infiltration and regulation of immune cell subsets. In addition, the therapeutic effect of the PLGA/PEI-HMGB1/GPC3 vaccine needs to be further verified and studied in other models, such as the metastasis model and liver orthotopic model.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Rabbit anti-mouse/human HMGB1 | Beyotime | Cat#AG2167 |
| Rabbit anti-human GPC3 | Proteintech | Cat#25175-1-AP; RRID:AB_2879942 |
| Rabbit anti-mouse/human GAPDH | Cell Signaling Technology | Cat#5174; RRID:AB_10622025 |
| Rat anti-mouse CD8α | eBioscience | Cat# 14-0081-82; RRID:AB_467087 |
| HRP-conjugated goat anti-rabbit IgG H&L secondary antibody | VICMED | Cat# VSA27 |
| PerCP/Cyanine5.5-labeled anti-mouse CD8α | Biolegend | Cat# 100734; RRID:AB_2075238 |
| PE-labeled anti-mouse CD103 | Biolegend | Cat# 121406; RRID:AB_1133989 |
| APC-labeled anti-mouse CD11c | Biolegend | Cat# 117310; RRID:AB_313779 |
| APC-labeled anti-mouse IFN-γ | Biolegend | Cat# 505810; RRID:AB_315404 |
| PE-labeled anti-mouse IL-2 | Biolegend | Cat# 503808; RRID:AB_315302 |
| FITC-labeled anti-mouse TNF-α | Biolegend | Cat# 506304; RRID:AB_315425 |
| APC-labeled anti-human GPC3 | SinoBiological | Cat# 100393-R024-A; RRID:AB_2860068 |
| Alexa Fluor 647-conjugated goat anti-rabbit IgG(H + L) secondary antibody | VICMED | Cat# VA1024 |
| Bacterial and virus strains | ||
| pCDH-CMV-MCS-EF1-Puro-hGPC3 | Stored in the lab | N/A |
| Biological samples | ||
| Mouse tumor tissues | Stored in the lab | N/A |
| Chemicals and recombinant proteins | ||
| PLGA | Sigma-Aldrich | Cat# P2066 |
| PEI | Sigma-Aldrich | Cat# 408727 |
| Human GPC3 | Stored in the lab | N/A |
| Critical commercial assays | ||
| BeyoClick EdU Cell Proliferation Kit | Beyotime | Cat# C0081L |
| Mouse IFN-γ precoated ELISPOT kit | Dakewe | Cat# 2210002 |
| Experimental models: Cell lines | ||
| HEK293T | ATCC | N/A |
| Hepa1-6 | Beyotime | Cat# C7355 |
| Experimental models: Organisms/strains | ||
| C57BL/6 mice; female | Animal Center of Xuzhou Medical University | N/A |
| Recombinant DNA | ||
| pcDNA3.1-HMGB1 | This paper | N/A |
| pcDNA3.1-hGPC3 | Miaoling Bio | N/A |
| Software and algorithms | ||
| GraphPad Prism version 8.0 | GraphPad | N/A |
| FlowJo software version 7.6 | FlowJo | N/A |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Dafei Chai (chaidafei@xzhmu.edu.cn).
Materials availability
This study did not generate new unique reagent.
Experimental model and subject details
Cell lines.
Plasmids.
Mouse strains.
Methods details
Plasmid construct
The mouse HMGB1 cDNA sequence was obtained from the open reading frame (ORF) cDNA (Public Plasmid Library, PPL; Biogot biotechnology) by PCR assay. Thereafter, the amplified DNA product was subjected to agarose gel electrophoresis and purified by DNA Gel Extraction Kit (Axygen, AP-GX-250) in accordance with the manufacturer’s instructions. The extracted DNA fragments were then sub-cloned into the pcDNA3.1 vector using the restriction enzymes Nhe I and EcoR I to obtain the plasmid pcDNA3.1-HMGB1 (pHMGB1). The plasmid pcDNA3.1-hGPC3 (pGPC3) was constructed from Miaoling Bio (P5074). The reliability of the insert was confirmed by DNA sequencing. Subsequently, the recombinant plasmids were transformed into Escherichia coli (DH5α) competent cells, and proliferated in LB medium containing 100 μg/mL ampicillin overnight. The plasmids were isolated and purified from the bacteria by EndoFree Plasmid kit (Qiagen, 12362) and were identified by gel electrophoresis again.
Vaccine preparation
As described previously, PLGA/PEI nanoparticle-based vaccines are prepared by the W/O/W double emulsion solvent evaporation technique.44 In brief, 200 mg PLGA (Sigma-Aldrich, P2066) was dissolved in 2 mL dichloromethane by ultrasonic wave. Thereafter, 3 mL polyvinyl alcohol (PVA) solution was poured into a W/O single emulsion, and ultrasonic treatment was carried out for 10 minutes. Then, using a homogenizer, the resulting solution was added dropwise to 50 mL of 1% PVA solution containing 2% isopropyl alcohol within 60 minutes. The preparation was centrifuged for 40 minutes at 8000 g to obtain the purified PLGA nanoparticles. The particles were washed twice to remove foreign protein. Add PEI (Sigma-Aldrich, 408727) solution (2 mg/mL) to the PLGA solution (1.5 mg/mL) with gentle stirring for 15s. After that, incubate the solution at room temperature for 1h to ensure uniform encapsulation efficiency. The solution was then incubated at room temperature for 1h to ensure uniform encapsulation rate. Finally, the antigen plasmid was mixed with polycationic nanoparticles, and the PLGA/PEI-DNA complex was obtained by gentle oscillation. Before inoculation, the poly complex was incubated for 30 minutes at room temperature.
Animals and cell lines
Six-week-old female C57BL/6 mice were obtained from the Animal Center of Xuzhou Medical University and housed in a specific-pathogen-free (SPF) environment. All the protocols during the whole process of the experiment in vivo were approved by the Animal Care and Use Committee of Xuzhou Medical University. Mouse HCC cell line Hepa1-6 was purchased from Beyotime, and HEK293T cells were purchased from American Type Culture Collection (ATCC). The Hepa1-6-hGPC3 cell line was established by using the recombinant pCDH-CMV-MCS-EF1-Puro-hGPC3 lentivirus. Hepa1-6 (Beyotime, C7355), Hepa1-6-hGPC3, and HEK293T cell lines were cultured in DMEM medium containing 10% FBS (FBS, ExCell Bio, FSP500) and 1×penicillin-streptomycin (Sangon Biotech, E607011-0100) at 37°C with 5% CO2 atmosphere.
Western blot analysis
The same amount of the protein was separated by 10% SDS-PAGE, electrically transferred to polyvinylidene fluoride (PVDF) membranes, and then blocked in 5% non-fat dry milk for 2 hours. The membranes were immunoblotted with primary antibodies against HMGB1 (Beyotime, AG2167), GPC3 (Proteintech, 25175-1-AP), and GAPDH (Cell Signaling Technology, #5174), and incubated overnight at 4°C. The immunoblotted membranes were then washed twice with TBST and incubated with HRP-conjugated secondary antibodies (VICMED, VSA27) at room temperature for 1h. Thereafter, the membranes were again washed with PBST for another 30 minutes and then were visualized by Super-Signal Enhancer Chemiluminescent (ECL plus) Substrate (Nature Biosciences, TE0015). The images were acquired by an ECL detection machine (Bio-Rad).
Tumor challenge and vaccine immunization
Mice with 5×106 Hepa1-6-hGPC3 cells were subcutaneously injected into the right flank of mice, and then mice were immunized with 50 μg PLGA/PEI-Vector, PLGA/PEI-HMGB1, PLGA/PEI-GPC3 vaccine respectively, and PLGA/PEI-HMGB1/GPC3 at a dose of 100 μg. For PLGA/PEI-Vector, PLGA/PEI-HMGB1, PLGA/PEI-GPC3 vaccine immunized group, mice received additional 50 μg PLGA/PEI-Vector to ensure that the total DNA amount was 100 μg. Then, the booster administration was repeated twice on the 10th and 20th days after the initial vaccine immunization, respectively. The tumor sizes were monitored with a caliper and calculated once a week by using the formula V= (width2×length)/2. If the implants reach 2000 mm3 in volume, mice will be euthanized with CO2. The tumor mass was photographed, and the corresponding weight was recorded. For a rechallenge experiment, the mice that had cleared the tumor as described above were rechallenged subcutaneously with 5×106 Hepa1-6-GPC3 cells on the contralateral side. The age-controlled naive mice were used as a control.
Preparation of splenocytes and TILs
The spleens were aseptically removed, homogenized, and lysed with ACK (Ammonium-Chloride-Potassium) lysis buffer. The splenocytes were cultured in RPMI containing 200 U/mL IL-2. In parallel, the dissected tumors were cut into pieces and digested with collagenase to generate single-cell suspensions. TILs (Tumor-infiltrating leukocytes) were isolated from the single-cell suspensions with 33% Percoll (VicMed, VIC1555) by density gradient centrifugation.
Flow cytometry
To detect the expression of pHMGB1 or pGPC3 in vitro, 293T cells were transfected with pHMGB1, pGPC3 or vector with lipofectamine 2000 (Invitrogen, Cat#11668019) for 48 h according to the manufacturer’s protocol. The pHMGB1-transfected cells were performed intracellular staining using anti-mouse HMGB1 and Alexa Fluor 647-conjugated goat anti-rabbit IgG(H + L) secondary antibody (VICMED, VA1024); pGPC3-transfected cells was subjected to surface staining using anti-human GPC3 (APC labeled, SinoBiological, 100393-R024-A).
The lymphocytes were harvested from the spleen of the vaccinated mice at the endpoint. The cells were then stained with murine monoclonal antibodies (mAbs) against CD8α (PerCP/Cyanine5.5-labeled, Biolegend, Cat#100734), CD103 (PE-labeled, Biolegend, Cat#121406), and CD11c (APC-labeled, Biolegend, Cat#117310). For the cytokine release assay, the lymphocytes were cultured in the presence of GPC3 protein. They were further stimulated with PMA (50 ng/mL, Sigma-Aldrich, P1585), Ionomycin (500 ng/mL, Sigma-Aldrich, I9657-1MG), and Brefeldin A (1×BFA, eBioscience, 00-4506-51) respectively for the last 5h. Then the cells were intracellularly stained with IFN-γ (APC-labeled, Biolegend, Cat#505810), IL-2 (PE-labeled, Biolegend, Cat#503808), and TNF-α (FITC-labeled, Biolegend, Cat#506304). FlowJo software (Version 7.6) was used to analyze the data from the FACSCanto II Flow Cytometer (BD Biosciences, USA).
CD8+T cells proliferation assay
The cell proliferation rate was examined by a BeyoClick EdU Cell Proliferation Kit (Beyotime, C0081L). The splenocytes were inoculated into 48-well plates and cultured in RPMI medium containing 50 U/mL IL-2 and GPC3 protein (10 μg/mL). After incubation at 37°C for 5 days, the cells were labeled with 2 × EdU for 2 hours and then stained with CD8 antibody (PerCP labeled, Biolegend). Following fixation, the cells were permeabilized and rinsed with 1×Perm/wash buffer (BD Biosciences, 554723). Thereafter, the cells were co-cultured with 70 μL click reaction solution at room temperature for 1h. Lastly, the cells were washed with 1×permeabilization buffer and suspended in PBS. The proportion of EdU+CD8+T cells was analyzed by flow cytometry and was presented as the proliferation rate.
Immunohistochemistry (IHC)
The tumor tissues were fixed, embedded, and cut into 5 μm sections in turn. Then, paraffin removal and epitope recovery were carried out by a heat-induced scheme on sections. Sections were blocked with 10% BSA at room temperature for 1 hour, and incubated with rat anti-mouse CD 8α antibody (eBioscience, 14-0081-82) at 4°C overnight. Next, the horseradish peroxidase-conjugated goat anti-rat secondary antibody (Zhongshan Biotech, PV9004) was incubated with the section at room temperature for 1 hour. Finally, it was developed with DAB detection kit (Zhongshan Biotech, ZLI-9018), and the nucleus was stained with hematoxylin. The image was obtained with Nikon SCLIPSS TE2000-S microscope (Nikon) with ACT-1 software. Original magnification was ×200.
Cytotoxic killing assay
Splenocytes were cultured in RPMI 1640 medium containing IL-2 (50 U/mL) and hGPC3 protein (10 μg/mL) for 7 days at 37°C with a 5% CO2 atmosphere. The primed cells were washed and resuspended in medium as effector cells. Then, effector cells (5 ×105/well) and target cells (1 ×104/well) were co-cultured totally for 3 days in a 96-well plate at the ratio of 50:1 with 5% CO2 at 37 °C. Afterward, the cells were collected, stained with anti-mouse CD8α and anti-human GPC3 Ab, and then analyzed by flow cytometry analysis. The specific killing rate of CTL was calculated to determine the killing effect on tumor cells.
IFN-γ ELISPOT
Mouse IFN-γ precoated ELISPOT kit (Dakewe, 2210002) was used for the ELISPOT assay according to the manufacturer’s instructions. Briefly, the plates were blocked with 1640 medium supplemented with 10% FBS for 2 h at 37 °C, splenocytes were seeded at a density of 1 ×106/well and stimulated with GPC3 protein (10 μg/mL) for 72 hours. Then, cells were thoroughly washed and incubated with a biotinylated IFN-γ antibody for 1 hour at room temperature. After incubating with streptavidin-HRP solution, the plates were finally developed by AEC substrate. The spot-forming cells (SFC) were developed and photographed via an immunospot analyzer (AID).
Quantification and statistical analysis
GraphPad Prism version 8.0 was used for statistical analysis. The difference between the groups was analyzed by a two-tailed independent Student’s t test or one-way analysis of variance (ANOVA). Data were presented as mean and standard deviations (mean ± SD). The values of significant levels were set as ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, and ∗∗∗∗p < 0.001.
Acknowledgments
This project is supported by grants from the National Natural Science Foundation of China (82072814), Natural Science Foundation of the Jiangsu Higher Education Institutions of China (22KJA320004), Research Foundation of Xuzhou Medical University (RC20552214), Qing Lan Project of Jiangsu Province, Youth Technology Innovation Team of Xuzhou Medical University (TD202003).
Author contributions
D.C., G.W., and J.Z.: conceived and designed the project; X.S., J.D., Y.Y., J.W., and D.C.: performed the project and analyzed the data; D.C. and G.W.: contributed reagents/materials/analysis tools; X.S., J.D, N.S., and P.N.: writing, review, and editing the article. All authors read and approved the final article.
Declaration of interests
The authors declare no competing interests.
Inclusion and diversity
We support inclusive, diverse, and equitable conduct of research.
Published: February 4, 2023
Contributor Information
Gang Wang, Email: wangg@xzhmu.edu.cn.
Junnian Zheng, Email: jnzheng@xzhmu.edu.cn.
Dafei Chai, Email: chaidafei@xzhmu.edu.cn.
Data and code availability
-
•
The data presented in this study are available on request from the lead contact upon request.
-
•
This paper does not report original code.
-
•
Any additional data supporting findings on this study are available from the lead contact upon request.
References
- 1.Jemal A., Bray F., Center M.M., Ferlay J., Ward E., Forman D. Global cancer statistics. CA A Cancer J. Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Korean Liver Cancer Study Group. National Cancer Center Korea [Practice guidelines for management of hepatocellular carcinoma 2009] Korean J. Hepatol. 2009;15:391–423. doi: 10.3350/kjhep.2009.15.3.391. [DOI] [PubMed] [Google Scholar]
- 3.Marrero J.A., Kulik L.M., Sirlin C.B., Zhu A.X., Finn R.S., Abecassis M.M., Roberts L.R., Heimbach J.K. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American association for the study of liver diseases. Hepatology. 2018;68:723–750. doi: 10.1002/hep.29913. [DOI] [PubMed] [Google Scholar]
- 4.Zhan H., Zhao X., Lu Z., Yao Y., Zhang X. Correlation and survival analysis of distant metastasis site and prognosis in patients with hepatocellular carcinoma. Front. Oncol. 2021;11:652768. doi: 10.3389/fonc.2021.652768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen Z., Xie H., Hu M., Huang T., Hu Y., Sang N., Zhao Y. Recent progress in treatment of hepatocellular carcinoma. Am. J. Cancer Res. 2020;10:2993–3036. [PMC free article] [PubMed] [Google Scholar]
- 6.Torimura T., Iwamoto H. Treatment and the prognosis of hepatocellular carcinoma in Asia. Liver Int. 2022;42:2042–2054. doi: 10.1111/liv.15130. [DOI] [PubMed] [Google Scholar]
- 7.Duperret E.K., Trautz A., Ammons D., Perales-Puchalt A., Wise M.C., Yan J., Reed C., Weiner D.B. Alteration of the tumor stroma using a consensus DNA vaccine targeting fibroblast activation protein (FAP) synergizes with antitumor vaccine therapy in mice. Clin. Cancer Res. 2018;24:1190–1201. doi: 10.1158/1078-0432.CCR-17-2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peng M., Mo Y., Wang Y., Wu P., Zhang Y., Xiong F., Guo C., Wu X., Li Y., Li X., et al. Neoantigen vaccine: an emerging tumor immunotherapy. Mol. Cancer. 2019;18:128. doi: 10.1186/s12943-019-1055-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bąbała N., Bovens A., de Vries E., Iglesias-Guimarais V., Ahrends T., Krummel M.F., Borst J., Bins A.D. Subcellular localization of antigen in keratinocytes dictates delivery of CD4(+) T-cell help for the CTL response upon therapeutic DNA vaccination into the skin. Cancer Immunol. Res. 2018;6:835–847. doi: 10.1158/2326-6066.CIR-17-0408. [DOI] [PubMed] [Google Scholar]
- 10.Miao L., Li L., Huang Y., Delcassian D., Chahal J., Han J., Shi Y., Sadtler K., Gao W., Lin J., et al. Delivery of mRNA vaccines with heterocyclic lipids increases anti-tumor efficacy by STING-mediated immune cell activation. Nat. Biotechnol. 2019;37:1174–1185. doi: 10.1038/s41587-019-0247-3. [DOI] [PubMed] [Google Scholar]
- 11.Liao C.W., Chen C.A., Lee C.N., Su Y.N., Chang M.C., Syu M.H., Hsieh C.Y., Cheng W.F. Fusion protein vaccine by domains of bacterial exotoxin linked with a tumor antigen generates potent immunologic responses and antitumor effects. Cancer Res. 2005;65:9089–9098. doi: 10.1158/0008-5472.CAN-05-0958. [DOI] [PubMed] [Google Scholar]
- 12.Kalli K.R., Block M.S., Kasi P.M., Erskine C.L., Hobday T.J., Dietz A., Padley D., Gustafson M.P., Shreeder B., Puglisi-Knutson D., et al. Folate receptor alpha peptide vaccine generates immunity in breast and ovarian cancer patients. Clin. Cancer Res. 2018;24:3014–3025. doi: 10.1158/1078-0432.CCR-17-2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang B., Jeang J., Yang A., Wu T.C., Hung C.F. DNA vaccine for cancer immunotherapy. Hum. Vaccines Immunother. 2014;10:3153–3164. doi: 10.4161/21645515.2014.980686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Capurro M., Wanless I.R., Sherman M., Deboer G., Shi W., Miyoshi E., Filmus J. Glypican-3: a novel serum and histochemical marker for hepatocellular carcinoma. Gastroenterology. 2003;125:89–97. doi: 10.1016/s0016-5085(03)00689-9. [DOI] [PubMed] [Google Scholar]
- 15.Sung Y.K., Hwang S.Y., Park M.K., Farooq M., Han I.S., Bae H.I., Kim J.C., Kim M. Glypican-3 is overexpressed in human hepatocellular carcinoma. Cancer Sci. 2003;94:259–262. doi: 10.1111/j.1349-7006.2003.tb01430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ho M., Kim H. Glypican-3: a new target for cancer immunotherapy. Eur. J. Cancer. 2011;47:333–338. doi: 10.1016/j.ejca.2010.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ikeda M., Okusaka T., Ohno I., Mitsunaga S., Kondo S., Ueno H., Morizane C., Gemmoto K., Suna H., Ushida Y., Furuse J. Phase I studies of peptide vaccine cocktails derived from GPC3, WDRPUH and NEIL3 for advanced hepatocellular carcinoma. Immunotherapy. 2021;13:371–385. doi: 10.2217/imt-2020-0278. [DOI] [PubMed] [Google Scholar]
- 18.Tsuchiya N., Yoshikawa T., Fujinami N., Saito K., Mizuno S., Sawada Y., Endo I., Nakatsura T. Immunological efficacy of glypican-3 peptide vaccine in patients with advanced hepatocellular carcinoma. OncoImmunology. 2017;6:e1346764. doi: 10.1080/2162402X.2017.1346764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buonaguro L., HEPAVAC Consortium Developments in cancer vaccines for hepatocellular carcinoma. Cancer Immunol. Immunother. 2016;65:93–99. doi: 10.1007/s00262-015-1728-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xue J., Suarez J.S., Minaai M., Li S., Gaudino G., Pass H.I., Carbone M., Yang H. HMGB1 as a therapeutic target in disease. J. Cell. Physiol. 2021;236:3406–3419. doi: 10.1002/jcp.30125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qie G.Q., Wang C.T., Chu Y.F., Wang R. Expression of HMGB1/RAGE protein in renal carcinoma and its clinical significance. Int. J. Clin. Exp. Pathol. 2015;8:6262–6268. [PMC free article] [PubMed] [Google Scholar]
- 22.Dong Y.D., Cui L., Peng C.H., Cheng D.F., Han B.S., Huang F. Expression and clinical significance of HMGB1 in human liver cancer: knockdown inhibits tumor growth and metastasis in vitro and in vivo. Oncol. Rep. 2013;29:87–94. doi: 10.3892/or.2012.2070. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Z., Wang M., Zhou L., Feng X., Cheng J., Yu Y., Gong Y., Zhu Y., Li C., Tian L., Huang Q. Increased HMGB1 and cleaved caspase-3 stimulate the proliferation of tumor cells and are correlated with the poor prognosis in colorectal cancer. J. Exp. Clin. Cancer Res. 2015;34:51. doi: 10.1186/s13046-015-0166-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kostova N., Zlateva S., Ugrinova I., Pasheva E. The expression of HMGB1 protein and its receptor RAGE in human malignant tumors. Mol. Cell. Biochem. 2010;337:251–258. doi: 10.1007/s11010-009-0305-0. [DOI] [PubMed] [Google Scholar]
- 25.Hubert P., Roncarati P., Demoulin S., Pilard C., Ancion M., Reynders C., Lerho T., Bruyere D., Lebeau A., Radermecker C., et al. Extracellular HMGB1 blockade inhibits tumor growth through profoundly remodeling immune microenvironment and enhances checkpoint inhibitor-based immunotherapy. J. Immunother. Cancer. 2021;9:e001966. doi: 10.1136/jitc-2020-001966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tripathi A., Shrinet K., Kumar A. HMGB1 protein as a novel target for cancer. Toxicol Rep. 2019;6:253–261. doi: 10.1016/j.toxrep.2019.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu Y., Yan W., Tohme S., Chen M., Fu Y., Tian D., Lotze M., Tang D., Tsung A. Hypoxia induced HMGB1 and mitochondrial DNA interactions mediate tumor growth in hepatocellular carcinoma through Toll-like receptor 9. J. Hepatol. 2015;63:114–121. doi: 10.1016/j.jhep.2015.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bianchi M.E., Manfredi A.A. High-mobility group box 1 (HMGB1) protein at the crossroads between innate and adaptive immunity. Immunol. Rev. 2007;220:35–46. doi: 10.1111/j.1600-065X.2007.00574.x. [DOI] [PubMed] [Google Scholar]
- 29.Dumitriu I.E., Bianchi M.E., Bacci M., Manfredi A.A., Rovere-Querini P. The secretion of HMGB1 is required for the migration of maturing dendritic cells. J. Leukoc. Biol. 2007;81:84–91. doi: 10.1189/jlb.0306171. [DOI] [PubMed] [Google Scholar]
- 30.Rovere-Querini P., Capobianco A., Scaffidi P., Valentinis B., Catalanotti F., Giazzon M., Dumitriu I.E., Müller S., Iannacone M., Traversari C., et al. HMGB1 is an endogenous immune adjuvant released by necrotic cells. EMBO Rep. 2004;5:825–830. doi: 10.1038/sj.embor.7400205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chai D., Qiu D., Shi X., Ding J., Jiang N., Zhang Z., Wang J., Yang J., Xiao P., Wang G., Zheng J. Dual-targeting vaccine of FGL1/CAIX exhibits potent anti-tumor activity by activating DC-mediated multi-functional CD8 T cell immunity. Mol. Ther. Oncolytics. 2022;24:1–13. doi: 10.1016/j.omto.2021.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zheng Y., Lu Z., Ding J., Jiang N., Wang J., Yang J., Song J., Chen H., Fang L., Li H., et al. Therapeutic adenovirus vaccine combined immunization with IL-12 induces potent CD8(+) T cell anti-tumor immunity in hepatocellular carcinoma. Cancers. 2022;14:4512. doi: 10.3390/cancers14184512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kyriakopoulos C.E., Eickhoff J.C., Ferrari A.C., Schweizer M.T., Wargowski E., Olson B.M., McNeel D.G. Multicenter phase I trial of a DNA vaccine encoding the androgen receptor ligand-binding domain (pTVG-AR, MVI-118) in patients with metastatic prostate cancer. Clin. Cancer Res. 2020;26:5162–5171. doi: 10.1158/1078-0432.CCR-20-0945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bernelin-Cottet C., Urien C., McCaffrey J., Collins D., Donadei A., McDaid D., Jakob V., Barnier-Quer C., Collin N., Bouguyon E., et al. Electroporation of a nanoparticle-associated DNA vaccine induces higher inflammation and immunity compared to its delivery with microneedle patches in pigs. J. Contr. Release. 2019;308:14–28. doi: 10.1016/j.jconrel.2019.06.041. [DOI] [PubMed] [Google Scholar]
- 35.Lopes A., Vandermeulen G., Préat V. Cancer DNA vaccines: current preclinical and clinical developments and future perspectives. J. Exp. Clin. Cancer Res. 2019;38:146. doi: 10.1186/s13046-019-1154-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shah M.A.A., He N., Li Z., Ali Z., Zhang L. Nanoparticles for DNA vaccine delivery. J. Biomed. Nanotechnol. 2014;10:2332–2349. doi: 10.1166/jbn.2014.1981. [DOI] [PubMed] [Google Scholar]
- 37.Mir M., Ahmed N., Rehman A.U. Recent applications of PLGA based nanostructures in drug delivery. Colloids Surf. B Biointerfaces. 2017;159:217–231. doi: 10.1016/j.colsurfb.2017.07.038. [DOI] [PubMed] [Google Scholar]
- 38.Zou S.M., Erbacher P., Remy J.S., Behr J.P. Systemic linear polyethylenimine (L-PEI)-mediated gene delivery in the mouse. J. Gene Med. 2000;2:128–134. doi: 10.1002/(SICI)1521-2254(200003/04)2:2<128::AID-JGM95>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 39.Mann J.F.S., McKay P.F., Arokiasamy S., Patel R.K., Klein K., Shattock R.J. Pulmonary delivery of DNA vaccine constructs using deacylated PEI elicits immune responses and protects against viral challenge infection. J. Contr. Release. 2013;170:452–459. doi: 10.1016/j.jconrel.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wculek S.K., Cueto F.J., Mujal A.M., Melero I., Krummel M.F., Sancho D. Dendritic cells in cancer immunology and immunotherapy. Nat. Rev. Immunol. 2020;20:7–24. doi: 10.1038/s41577-019-0210-z. [DOI] [PubMed] [Google Scholar]
- 41.Moku G., Vangala S., Gulla S.K., Yakati V. In vivo targeting of DNA vaccines to dendritic cells via the mannose receptor induces long-lasting immunity against melanoma. Chembiochem. 2021;22:523–531. doi: 10.1002/cbic.202000364. [DOI] [PubMed] [Google Scholar]
- 42.Raskov H., Orhan A., Christensen J.P., Gögenur I. Cytotoxic CD8(+) T cells in cancer and cancer immunotherapy. Br. J. Cancer. 2021;124:359–367. doi: 10.1038/s41416-020-01048-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reina-Campos M., Scharping N.E., Goldrath A.W. CD8+ T cell metabolism in infection and cancer. Nat. Rev. Immunol. 2021;21:718–738. doi: 10.1038/s41577-021-00537-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Park J.S., Yang H.N., Woo D.G., Jeon S.Y., Do H.J., Lim H.Y., Kim J.H., Park K.H. Chondrogenesis of human mesenchymal stem cells mediated by the combination of SOX trio SOX5, 6, and 9 genes complexed with PEI-modified PLGA nanoparticles. Biomaterials. 2011;32:3679–3688. doi: 10.1016/j.biomaterials.2011.01.063. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
-
•
The data presented in this study are available on request from the lead contact upon request.
-
•
This paper does not report original code.
-
•
Any additional data supporting findings on this study are available from the lead contact upon request.