Abstract
Recent studies have demonstrated the vital role of P2X4 receptors (a family of ATP-gated non-selective cation channels) in the transmission of neuropathic and inflammatory pain. In this study, we investigated the role of spinal P2X4 receptors in chronic functional visceral hypersensitivity of neonatal maternal separation (NMS) rats. A rat model of irritable bowel syndrome was established by neonatal maternal separation. Visceral sensitivity was assessed by recording the response of the external oblique abdominal muscle to colorectal distension. P2X4 receptor antagonist and agonist were administrated intrathecally. The expression of P2X4 receptor was examined by Western Blot and immunofluorescence. The effect of P2X4 receptor antagonist on expression of brain-derived neurotrophic factor (BDNF) was assessed by Western Blot. We found neonatal maternal separation enhanced visceral hypersensitivity and increased the expression of P2X4 receptor in spinal thoracolumbar and lumbosacral segments of rats. Pharmacological results showed that visceral sensitivity was attenuated after intrathecal injection of P2X4 receptor antagonist, 5-BDBD, at doses of 10 nM or 100 nM, while visceral sensitivity was enhanced after intrathecal injection of P2X4 receptor agonist C5-TDS at doses of 10 μM or 15 μM. In addition, the spinal expression of BDNF significantly increased in NMS rats and intrathecal injection of 5-BDBD significantly decreased the expression of BDNF especially in NMS rats. C5-TDS failed to increase EMG amplitude in the presence of ANA-12 in control rats. Our results suggested the spinal P2X4 receptors played an important role in visceral hypersensitivity of NMS rats through BDNF.
Keyword: Visceral hypersensitivity, Irritable bowel syndrome, P2X4 receptors, Neonatal maternal separation, Brain-derived neurotrophic factor
Introduction
Irritable bowel syndrome (IBS) is a common gastrointestinal disorder characterized by recurrent visceral pain and altered bowel habits. Pain associated with IBS represents chronic functional visceral hypersensitivity [1]. Many symptoms of IBS are attributable to visceral hypersensitivity. There is a general lack of effective treatment modalities for IBS; therefore, understanding the pathogenesis of IBS is a key imperative.
Studies have shown that adenosine triphosphate binds to P2X receptors and plays an important role in nociceptive deficits caused by nerve injury and peripheral lesions [2, 3]. P2X is a subtype of the purine receptor, mainly expressed in the neurons that transmit nociceptive information [4]. Different types of purinergic P2X receptors bind to ATP and open different ion channels, producing different sensory effects [5]. There are seven subtypes of P2X receptors, ranging from P2X1 to P2X7. Tsuda M et al. found that P2X4 receptors in spinal microglia caused tactile hypersensitivity after nerve injury [6] and was involved in the development of nociceptive sensitization [7]. P2X subunits such as P2X4/6 and/or P2X4/7 might be involved in visceral pain [8]. Upregulation of brain-derived neurotrophic factor (BDNF) in DRG and spinal cord contributes to chronic nociceptive sensitization [9]. BDNF is synthesized in the dorsal root ganglion and is released into the dorsal horn of the spinal cord, where it binds to TrkB receptors on secondary sensory neurons [10–12]. P2X4 receptor activation-mediated BDNF release has been shown to induce neuropathic pain [7, 13]. Although chronic visceral pain is different from neuropathic pain, there may be some similarities in the pathogenesis of these two types of pain. Studies suggest that P2X4 may participate in the development of visceral hypersensitivity in some visceral pain models [8]. However, it is unclear whether the P2X4 receptors play a role in mediating chronic functional visceral pain induced by neonatal maternal separation (NMS).
The objective of this research was to explore the role of the spinal P2X4 receptor in chronic visceral pain induced by NMS and to understand its mechanism. First, a rat model of IBS was established by NMS and visceral hypersensitivity was assessed by recording electromyography (EMG) of the external oblique muscle of abdomen in response to colorectal distension. Second, the expressions of P2X4 receptor and BDNF were examined by Western Blot and immunofluorescence. Finally, EMG and the expression of BDNF were examined after intrathecal injection of P2X4 agonists and antagonists.
Methods
Animals
Neonatal male Sprague–Dawley rats were provided by the Department of Experimental Animal Center, Fujian Medical University (Fujian Province, China). The neonatal rats were reared with their mothers until they were 21 days old. After separation from the mother rats, the weaned rats were raised for 8 weeks with ad libitum access to food and water. After intrathecal intubation, the rats were housed individually. All animal experiments were performed according to the guidelines of the International Association for the Study of Pain [14]. All animal experiments were approved by the Animal Care and Use Committee, and the study protocol was approved by the Fujian Medical University.
NMS procedure
A modified version of the previously described NMS procedure was employed [15, 16]. In brief, the litters were randomly assigned to NMS and control groups. In the NMS group, litters were taken away from the home cage and placed in a separate, clean cage for 3 h. The cages were placed on a heating pad (30–33 °C) to keep the litters at a temperature similar to the external body temperature of adult female rats. Then, the litters were returned to their mothers. This procedure was repeated on postnatal days 3–21. The control litters were kept undisturbed in the home cage with their mothers.
Assessment of visceral hypersensitivity
Visceral hypersensitivity was assessed by EMG of the external oblique muscle of abdomen in response to colorectal distension (CRD) (40 and 60 mmHg) as described in previous studies by an experimenter blinded to the treatment [17]. EMG was performed 30 min after intrathecal injection. Briefly, animals were lightly anesthetized with isoflurane using an anesthesia machine (VMR, Matrix, USA). An inflatable balloon (constructed from No. 7 latex, 6 cm in length) was used to apply the CRD stimulus. After the animal was anesthetized, two silver bipolar electrodes were inserted into the external oblique muscle of the abdomen. A collapsed balloon was inserted into the colon lubricated with glycerol. The balloon was inflated to each CRD pressure for 10 s and then rested for 4 min. For each CRD, EMG was recorded three times. The average magnitude of the three recordings was used as the magnitude of EMG activity. The magnitude of EMG activity was measured by a RM6240BD multi-channel physiological signal acquisition and processing system (Chengdu Instrument Factory, Chengdu, China). EMG data recorded for 10 s before CRD (baseline) and 10 s during CRD (response) were used for calculation. The responses were normalized to the percent increase in EMG amplitude over baseline during CRD. To avoid interference from other factors, a single blind operation was adopted in the experiment. Rats were housed in a quiet environment with relative humidity of 40–70% and room temperature of 23-27ºC.
Spinal intrathecal injection
Lumbosacral intrathecal catheters were implanted using the approach described earlier [18]. A spinal cord puncture was made between L5–L6 spinal segments using a 12G needle. A polyethylene (PE10) catheter was implanted into the intrathecal space of the spinal cord. The catheter was filled with sterile phosphate buffered saline. Using the implanted catheter, the drugs or vehicle was intrathecally injected into the cerebrospinal fluid space surrounding the lumbosacral spinal cord. Other tests were performed 30 min after intrathecal injection. Spinal intrathecal injection experiments and EMG experiments were performed by different experimentalists in assessor-blinded manner.
Immunofluorescence
The NMS rats were administered deep anesthesia and then fixed by transcardial perfusion with phosphate-buffered saline (PBS) and 4% paraformaldehyde in PBS. After the perfusion fixation, thoracolumbar (T13–L2) and lumbosacral (L6–S2) spinal cord segments were removed and post-fixed in 4% paraformaldehyde overnight, then replaced with 30% sucrose. Transverse spinal Sects. (20 μm) were cut with a freezing microtome and processed for immunofluorescence. All sections were blocked with 5% donkey serum in 0.3% Triton for 60 min at room temperature. For double immunofluorescence, sections were incubated overnight at 4 °C with primary antibody (anti-P2X4, 1:200, Invitrogen; NEUN, 1: 300, Millipore/GFAP (1: 300,CST) / anti-iba-1, 1:10,000, Woko). Then, the sections were incubated for 1 h with second antibody (Goat anti-Rabbit 1:300, Abcam; Goat anti-Mouse 1:300, Abcam). The images of stained sections were captured with fluorescence microscope (TCS SP8, Germany).
Western Blotting
Western Blotting was performed after 8 weeks of rat age and 2 h after intrathecal administration. Equal amounts of protein (50 μg) from the spinal thoracolumbar and lumbosacral segments of rats were submitted to the gel for separation. The proteins were transferred to polyvinylidene difluoride membranes (Invitrogen, Carlsbad, CA). Membranes were incubated with the following primary antibodies: rabbit anti-P2X4 (1:50, Santa Cruz Biotechnology Inc) and rabbit anti-GAPDH (1:3,000; Bioworld Technology, St. Louis Park, MN). Then the membranes were washed and incubated with peroxidase-conjugated anti-rabbit IgG (1:1,000; Abcam, Cambridge, MA). Bands were visualized using an ECL system. This experiment was conducted using an assessor-blinded method.
Statistical analysis
All data are expressed as mean ± SEM. The two independent samples t-test was used to determine whether there was a significant difference in the response to CRD pressure between NMS and control rats. CRD data, after intrathecal injection of compounds, were analyzed by one-way repeated-measures ANOVA, followed by Bonferroni post hoc test. CRD data, before and after injection, were compared and analyzed using the paired t-test. Western Blot results were analyzed by the two independent samples t-test. For all analyses, significance was assumed at P < 0.05.
Results
Neonatal maternal separation results in visceral hypersensitivity and increased expression of spinal P2X4 receptor
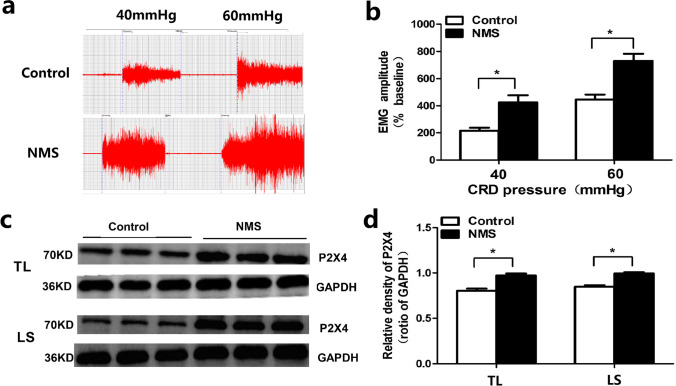
We established the chronic visceral hyperalgesia of rat model by NMS for 3 h daily during post-natal days 3–21. After about 8 weeks, we assessed visceral hypersensitivity by recording the amplitude of EMG under 40 and 60 mmHg CRD pressure. The magnitude of EMG to graded CRD pressure was significantly higher in NMS rats as compared to that in control rats (P < 0.05; Fig. 1). The magnitude of EMG in NMS rates significantly increased by 107% under 40 mmHg CRD and by 72% under 60 mmHg CRD as compared to control rats. These results suggested that NMS significantly increased the visceromotor response to CRD and induced visceral hypersensitivity in rats.
Fig. 1.
NMS results in visceral hypersensitivity and increased expression of spinal P2X4 receptors. (a) & (b) The original graph and statistical chart of EMG amplitude in rats (n=12, *P<0.05, vs control rats.) (c) & (d) The original graph and statistical chart of P2X4 expression at the spinal TL and LS segments in rats (n=3. *P<0.05, vs control rats. EMG: electromyography; CRD: colorectal distension; NMS: neonatal maternal separation; TL: thoracolumbar. LS: lumbosacral)
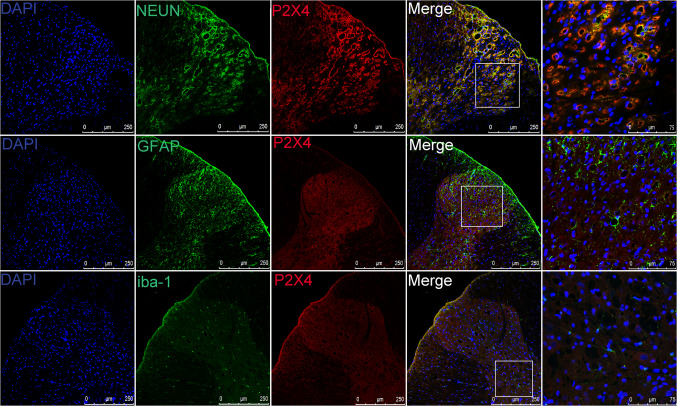
To illustrate the role of P2X4 receptor in NMS-induced visceral hypersensitivity, the expression of P2X4 receptor was examined by Western Blotting. The result showed a significant increase in the expressions of P2X4 receptor in the spinal thoracolumbar (TL) and lumbosacral (LS) segments of NMS rats (Fig. 1). The relative density of P2X4 receptor in spinal TL segments of control rats was 0.80 as against 0.97 in NMS rats (P < 0.05). The relative density of P2X4 receptor in spinal LS segments of control rats was 0.85 as against 0.99 in NMS rats (P < 0.05). These results suggest that NMS increases the expression of spinal P2X4 receptor. Immunofluorescence results showed co-expression of P2X4 receptors and neurons in spinal cord of NMS rats (Fig. 2). However, in NMS rats, P2X4 did not co-localize with astrocytes and microglia.
Fig. 2.
Double immunofluorescence labeling of P2X4R (red) and neurons/astrocytes/Iba-1 (green) in NMS groups. Most P2X4R-positive cells are double-labeled (yellow) with neurons
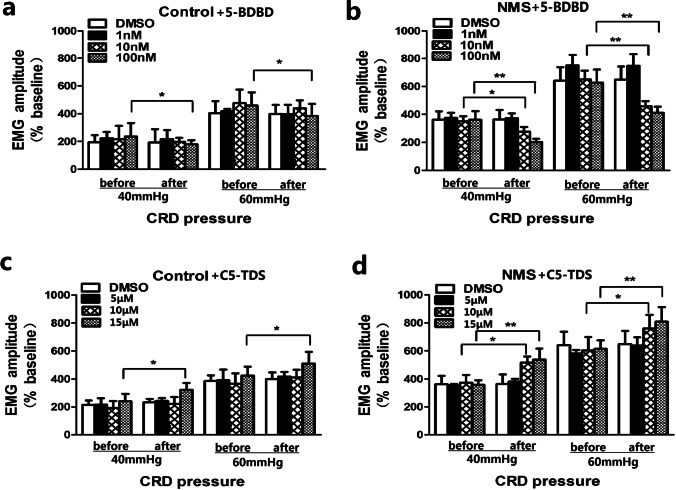
P2X4 receptor antagonist attenuates visceral hypersensitivity
In order to demonstrate the role of P2X4 receptor in visceral hypersensitivity, the EMG response to CRD was examined 30 min after intrathecal injection of P2X4 antagonist 5-(3-bromophenyl)-1,3-dihydro-2H-benzofuro[3,2-e]-1,4-diazepin-2-one (5-BDBD). The results showed that only 100 nM 5-BDBD attenuated EMG in control rats (Fig. 3). However, 10 nM and 100 nM 5-BDBD significantly attenuated EMG in NMS rats (Fig. 3). After intrathecal injection of 10 nM 5-BDBD, the magnitude of EMG significantly decreased by 21% under 40 mmHg CRD and by 29% under 60 mm Hg CRD in NMS rats. After injection of 100 nM 5-BDBD, the magnitude of EMG was significantly decreased by 44% under 40 mmHg CRD and by 34% under 60 mmHg CRD in NMS rats. These results suggest that P2X4 receptor antagonist attenuates visceral hypersensitivity in NMS rats.
Fig. 3.
The roles of P2X4R’ antagonist and agonist in rats.(a) & (b) The effects of 5-BDBD on EMG amplitude in control and NMS rats. (c) & (d) The effects of C5-TDS on EMG amplitude in control and NMS rats (n=6, *P<0.05, **P<0.01 vs before. CRD: colorectal distension; NMS: neonatal maternal separation)
P2X4 receptor agonist enhances visceral hypersensitivity
Furthermore, we also verified the effect of P2X4 receptor agonist on visceral hypersensitivity. C5-TDS, the P2X4 receptor agonist, was intrathecally injected into the control and NMS rats, and visceromotor response to CRD was tested 30 min later. The results showed that only 15 μM C5-TDS enhanced visceral sensitivity in the control rats (Fig. 3). However, 10 μM and 15 μM C5-TDS both significantly enhanced visceral sensitivity in the NMS rats (Fig. 3). After intrathecal injection of 15 μMC5-TDS in control rats, the magnitude of EMG was significantly increased by 34% under 40 mmHg CRD and by 20% under 60 mmHg CRD (P < 0.05, Fig. 3). After intrathecal injection of 10 μM C5-TDS in NMS rats, the magnitude of EMG was significantly increased by 38% under 40 mmHg CRD and by 25% under 60 mmHg CRD (P < 0.05, Fig. 3). After intrathecal injection of 15 μM C5-TDS, the magnitude of EMG significantly increased by 50% under 40 mmHg CRD and by 31% under 60 mmHg CRD in NMS rats (P < 0.05, Fig. 3). These results suggest that P2X4 receptor agonist enhances visceral hypersensitivity.
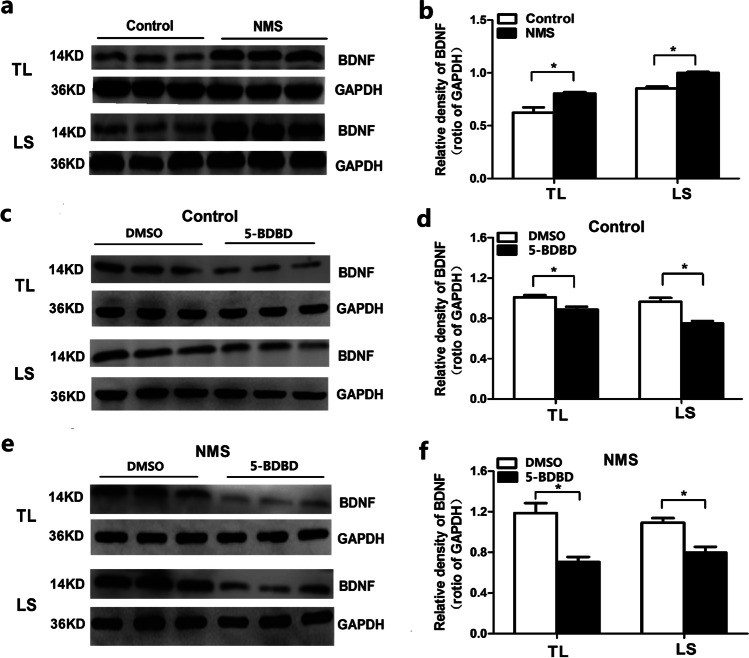
NMS upregulates the expression of BDNF via spinal P2X4 receptor activation
To explore the effect of BDNF on visceral hypersensitivity, the expression of BDNF was detected in NMS rats. Results showed that BDNF was obviously increased in spinal TL and LS segments of NMS rats (Fig. 4). In TL segments, the relative density of BDNF was 0.62 in control rats, while it was 0.80 in NMS rats; the between-group difference was statistically significant (P < 0.05). In LS segments, the relative density of BDNF was 0.85 in control rats, while it was 0.99 in NMS rats; the between-group difference was statistically significant (P < 0.05).
Fig. 4.
The expression of spinal BDNF in rats. (a) & (b) The original graph and statistical chart of BDNF expression at the spinal TL and LS segments in rats. (c) & (d) The original graph and statistical chart of BDNF expression at TL and LS segments after intrathecal injection of 100 nM 5-BDBD in control rats. (e) & (f) The original graph and statistical chart of BDNF expression at TL and LS segments after intrathecal injection of 100 nM 5-BDBD in NMS rats ( n=3. *P< 0.05, vs control rats. NMS: neonatal maternal separation. TL: thoracolumbar. LS: lumbosacral )
To investigate the relationship between BDNF and P2X4 receptor in visceral hypersensitivity, the effect of P2X4 receptor antagonist 5-BDBD on the expression of spinal BDNF was tested in control and NMS rats (Fig. 4).
After intrathecal injection of 100 nM 5-BDBD in control rats, the relative density of BDNF decreased from 1.01 to 0.89 in the TL and from 0.96 to 0.75 in the LS region (Fig. 4). The respective percentage decrease was 11.8% in TL and 21.7% in LS (P < 0.05), as compared with that in the vehicle group.
After intrathecal injection of 100 nM 5-BDBD in NMS rats, the relative density of BDNF decreased from 1.19 to 0.71 in spinal TL region and from 1.09 to 0.79 in spinal LS region (Fig. 4). The respective percentage decrease was 39.9% in TL and 27.5% in LS region (P < 0.05), as compared to that in the vehicle group.
We also found that 5-BDBD decreased BDNF more significantly in TL (39.9 ± 8.1) and LS (27.5 ± 5.5) region of NMS rats, as compared to that in the control rats (Table. 1). In control rats, 5-BDBD decreased BDNF more significantly in LS (21.7 ± 6.8) as compared to that in TL (11.8 ± 1.4). However, in NMS rats, 5-BDBD decreased BDNF more significantly in TL as compared to that in LS. In addition, in TL but not in LS segment, the decrease in percentage of BDNF was significantly higher in NMS rats as compared to that in the control rats (Table. 1).
Table 1.
Western blot analysis of the reduction percentage of BDNF density after intrathecal administration of 5-BDBD in rats
| The reduction percentage of BDNF density | |||
|---|---|---|---|
| Groups | n | TL (∆%) | LS (∆%) |
| Control | 3 | 11.8 ± 1.4 | 21.7 ± 6.8# |
| NMS | 3 | 39.9 ± 8.1* | 27.5 ± 5.5*# |
*P < 0.05 vs. control. #P < 0.05 vs. TL. NMS: neonatal maternal separation
TL: thoracolumbar. LS: lumbosacral
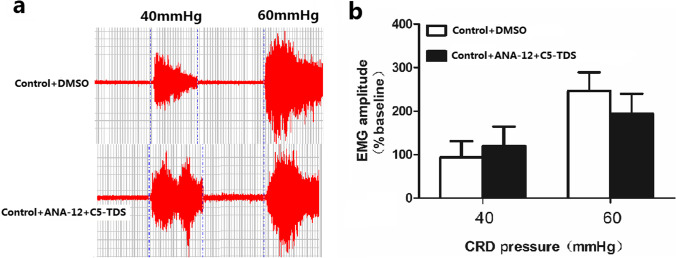
For further verification that spinal P2X4 receptor promotes NMS-induced hypersensitivity via BDNF, C5-TDS was intrathecal injected after ANA-12 (an antagonist of TrkB receptor). EMG was measured first, then 10 μL ANA-12 (20 mmol) was administered, 3 min later, 15 μL 5-BDBD (15 μmol) was intrathecally injected, and EMG was measured again after 30 min interval. C5-TDS failed to increase EMG amplitude by pretreatment with ANA-12 in control rats (Fig. 5). Collectively, these results suggest that spinal P2X4 receptor induced visceral hypersensitivity through the release of BDNF.
Fig. 5.
C5-TDS failed to increase EMG amplitude by pretreatment with ANA-12 in control rats. The original graph (a) and statistical chart (b) of the effect of intrathecal injection of C5-TDS on EMG amplitude by pretreatment with ANA-12 in control rats ( n=3. )
Discussion
In this study, our results revealed that the P2X4 receptor may play an important role in NMS-induced hypersensitivity. Firstly, NMS induced visceral hypersensitivity and increased the expression of P2X4 receptors on spinal neuronal cells. Secondly, P2X4 receptor antagonist attenuated visceral hypersensitivity, while P2X4 receptor agonist enhanced visceral hypersensitivity. Thirdly, the expression of spinal BDNF was significantly enhanced in NMS rats when compared with that in control rats. The expression of BDNF was decreased by P2X4 receptor antagonist in both NMS and control rats, and the percentage decrease in NMS rats was significantly higher than that in controls. Finally, the enhanced effect of P2X4 receptor agonist on EMG was blocked by TrkB receptor antagonist ANA-12.
Purine receptors are widely distributed in various tissues in the body. These combine with ATP to provide energy to the body directly, and these also participate in the body's physiological response as a signal molecule receptor [19]. Numerous studies have shown that ATP plays an important role in the pain associated with peripheral nerve injury by binding to the P2X receptor [19, 20]. When the noxious stimulation acts on the nerve endings, the cells release ATP to activate different P2X receptors, which results in different sensory reactions [20–22]. The effect of resveratrol on gp120-induced neuropathic pain was mediated by the P2X7 receptor in the DRG of rats [23]. P2X3Rs were interconnected at the presynaptic level to control synaptic activities in the right insular cortex, thus contributing to visceral pain of neonatal maternal deprivation rats [24]. P2X4 is likely to participate in the development of neuropathic pain [25–27]. Other research suggested that acetic acid induced visceral pain could increase the expression of P2X4 receptors in tractus solitarius and the dorsal horn of the thoracic cord, which peaked at 60 min [28]. In the present study, the expression of spinal P2X4 receptor in NMS rats was significantly higher than that in control rats. Therefore, we speculate that P2X4 may also be involved in the development of functional visceral hypersensitivity of NMS.
Our results demonstrated an increase in the expression of P2X4 on spinal cord. Further experiments showed that the antagonist of P2X4 receptor attenuated visceral hypersensitivity, while agonists of P2X4 receptor aggravated visceral hypersensitivity. These results suggest that the spinal P2X4 receptor is involved in visceral hypersensitivity. The results of this study are consistent with allodynia after nerve injury [29]. The location of P2X4 receptor expression induced by injurious stimuli is controversial. Some studies showed that hyperalgesia in IBS and inflammatory pain were associated with neuronal activity [30, 31]. But other results suggested that harmful stimuli might activate small glial cells to express large amounts of P2X4 receptors, which induces chronic or neuropathic pain [25, 29, 32]. Our study confirms that P2X4 receptors in NMS rats are predominantly expressed on neurons, but not on astrocytes or microglia. Nerve injury induces expression of transcription factor IRF5, which in turn upregulates spinal microglial P2X4 receptors expression [6, 33, 34]. In contrast, we did not detect an increase in P2X4 receptors in microglia of NMS rats, but found abundant expression of P2X4 receptors on spinal cord neuronal cells, demonstrating a different mechanism for visceral pain and neuralgia. In addition, there is also a study that shows the expression of P2X4 receptors on L4–S4 rat DRG neurons increased obviously in visceral pain model [6]. However, we did not detect P2X4 receptors on DRG neurons in this experiment.
Activation of P2X4 receptor induces the release of some biological factors and inward flow of sodium or calcium ions, which in turn results in central pain hypersensitivity [35]. It was reported that the biological factor that promotes dysmenorrhea might be BDNF [9]. Research confirmed that microglia P2X4R regulated EAAT3 expression through the BDNF-TrkB signaling pathway and played a role in IS-induced trigeminal neuralgia model [13].
BDNF acts on certain neurons of the central nervous system and the peripheral nervous system, which supports the survival of existing neurons and promotes the growth and differentiation of new neurons and synapses [36, 37]. Furthermore, BDNF is important for long-term memory [38]. As a signal molecule between microglia and neurons, BDNF can induce hyperalgesia [39]. Our results showed that the spinal expression of BDNF in NMS rats was significantly greater than that in the control rats. After injection of P2X4 receptor antagonist, the BDNF expression decreased more significantly in NMS rats as compared to that in the control rats. ANA-12 has been proven to be a selective TrkB antagonist, and it is used in the study of BDNF/TrkB signal transduction [40, 41]. We found activation of spinal P2X4 receptor by C5-TDS failed to induce visceral sensitivity in the presence of ANA-12 in control rats, indicating P2X4 receptor plays a role through BDNF. Therefore, we inferred that P2X4 receptor could induce visceral pain in NMS rats through BDNF. Studies have shown that there are gender differences in P2X4 receptor-BDNF signal-driven pain hypersensitivity, this signaling drives pain hypersensitivity in males, but not in females [42]. Therefore, we selected only male rats as the subjects in this experiment.
The afferent nerve of colorectum projects to the spinal cord thoracolumbar (T13-L2) and lumbosacral (L6-S2) segments [43]. In control rats, the colorectal sensation is mainly transmitted through the pelvic nerve to the LS segment, but rarely transmitted through the hypogastric nerve fibers to the TL segment. Our previous results showed that the spikes of afferent nerve fibers in the TL and LS segments were significantly increased in the IBS rats [44]. In this study, P2X4 receptor and BDNF expression were increased in both TL and LS. It was interesting that the inhibitive percentage of 5-BDBD for BDNF was significantly higher in TL of NMS rats as compared to that in controls the inhibitive percentage of 5-BDBD for BDNF was significantly higher in TL of NMS rats as compared to that in controls. Moreover, in NMS rats, the BDNF expression was decreased more significantly by P2X4 antagonist in TL as compared to that in LS. However, in control rats, the BDNF expression was decreased more significantly by P2X4 antagonist in LS as compared to that in TL. We speculate the underlying reason could be that P2X4 receptor is activated more in TL as compared to that in LS of NMS rats. These results indicate that the P2X4 receptor activation-mediated release of BDNF in TL plays a more important role in visceral hypersensitivity of NMS rats as compared to that in LS.
Conclusions
In summary, spinal P2X4 receptors play a key role in visceral hypersensitivity through BDNF in NMS-induced IBS, which provides a potential target for treatment of IBS.
Tang Ying
Associate Professor, received her Bachelor of Medicine degree from Anhui Medical University in 2002 and her Master of Medicine degree from Fujian Medical University in 2008, specializing in physiology. Her research area is the study of the central mechanisms of visceral pain.
Authors' contributions
CL and AC designed the study. BL, PS, ZC, YC, LC, and YT collected and analyzed the data. YH advised on histological staining and analysis. YT and LC drafted and wrote the manuscript. CL and AC revised the manuscript critically for intellectual content. All authors gave intellectual input to the study and approved the final version of the manuscript.
Funding
This work was supported by grants from Fujian Science and Technology Innovation Joint Fund (2016Y9036, 2018Y9069), Natural Science Foundation of Fujian Province (2020J01608), Financial Special Fund of Fujian Province (2019B028), and Educational Research Projects for Young and Middle-aged Teachers of Fujian Provincial Education Department (JAT190172).
Data availability
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethical approval
All animal experiments were approved by the Animal Care and Use Committee, and the study protocol was approved by the Fujian Medical University. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
Conflict of interest
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ying Tang, Email: candy090911@126.com.
Li Chen, Email: lichen@fjmu.edu.cn.
Bin Liu, Email: 1184305090@qq.com.
Pei Sun, Email: 395179713@qq.com.
Zhong Chen, Email: zhongchen1108@foxmail.com.
Yang Huang, Email: huangyangfj@163.com.
Chen Ai-qin, Email: jesseaqc@163.com.
Yu Chen, Email: Yuchen861613@126.com.
Chun Lin, Email: Chunlin77550@126.com.
References
- 1.Taft TH, Keszthelyi D, Van Oudenhove L. A Review of Irritable Bowel Syndrome. Jama-J Am Med Assoc. 2021;326:189–189. doi: 10.1001/jama.2021.6755. [DOI] [PubMed] [Google Scholar]
- 2.Tsuda M, Tozaki-Saitoh H, Inoue K (2010) Pain and purinergic signaling. Brain research reviews 63: 222–32. http://www.ncbi.nlm.nih.gov/pubmed/19931560, https://www.sciencedirect.com/science/article/abs/pii/S0165017309001222?via%3Dihub [DOI] [PubMed]
- 3.Tsuda M, Tozaki-Saitoh H, Inoue K (2012) Purinergic system, microglia and neuropathic pain. Current opinion in pharmacology 12: 74–9. http://www.ncbi.nlm.nih.gov/pubmed/22036170 [DOI] [PubMed]
- 4.Tsuda M, Shigemoto-Mogami Y, Koizumi S, Mizokoshi A, Kohsaka S, et al. (2003) P2X4 receptors induced in spinal microglia gate tactile allodynia after nerve injury. Nature 424: 778–83. http://www.ncbi.nlm.nih.gov/pubmed/12917686 [DOI] [PubMed]
- 5.Inoue K, Tsuda M (2012) P2X4 receptors of microglia in neuropathic pain. CNS & neurological disorders drug targets 11: 699–704. http://www.ncbi.nlm.nih.gov/pubmed/22963435 [DOI] [PubMed]
- 6.Chen L, Liu YW, Yue K, Ru Q, Xiong Q, et al. (2016) Differential expression of ATP-gated P2X receptors in DRG between chronic neuropathic pain and visceralgia rat models. Purinergic signalling 12: 79–87. http://www.ncbi.nlm.nih.gov/pubmed/26531254 [DOI] [PMC free article] [PubMed]
- 7.Obata K, Noguchi K (2006) BDNF in sensory neurons and chronic pain. Neuroscience research 55: 1–10. http://www.ncbi.nlm.nih.gov/pubmed/16516994 [DOI] [PubMed]
- 8.Lever IJ, Bradbury EJ, Cunningham JR, Adelson DW, Jones MG, et al. (2001) Brain-derived neurotrophic factor is released in the dorsal horn by distinctive patterns of afferent fiber stimulation. The Journal of neuroscience : the official journal of the Society for Neuroscience 21: 4469–77. http://www.ncbi.nlm.nih.gov/pubmed/11404434 [DOI] [PMC free article] [PubMed]
- 9.Pezet S, Malcangio M, McMahon SB (2002) BDNF: a neuromodulator in nociceptive pathways? Brain research Brain research reviews 40: 240–9. http://www.ncbi.nlm.nih.gov/pubmed/12589922 [DOI] [PubMed]
- 10.Garraway SM, Petruska JC, Mendell LM (2003) BDNF sensitizes the response of lamina II neurons to high threshold primary afferent inputs. The European journal of neuroscience 18: 2467–76. http://www.ncbi.nlm.nih.gov/pubmed/14622147 [DOI] [PubMed]
- 11.Zimmermann M (1983) Ethical guidelines for investigations of experimental pain in conscious animals. Pain 16: 109–110. http://www.ncbi.nlm.nih.gov/pubmed/6877845 [DOI] [PubMed]
- 12.Kannampalli P, Sengupta JN (2015) Role of principal ionotropic and metabotropic receptors in visceral pain. Journal of neurogastroenterology and motility 21: 147–58. http://www.ncbi.nlm.nih.gov/pubmed/25843070 [DOI] [PMC free article] [PubMed]
- 13.Tang Y, Chen A, Chen Y, Guo L, Dai H, et al. (2016) Zeta Inhibitory Peptide as a Novel Therapy to Control Chronic Visceral Hypersensitivity in a Rat Model. PloS one 11: e0163324. http://www.ncbi.nlm.nih.gov/pubmed/27776136 [DOI] [PMC free article] [PubMed]
- 14.Sawynok J (2007) Adenosine and ATP receptors. Handbook of experimental pharmacology: 309–28. http://www.ncbi.nlm.nih.gov/pubmed/17087128 [DOI] [PubMed]
- 15.Burnstock G (2006) Pathophysiology and therapeutic potential of purinergic signaling. Pharmacological reviews 58: 58–86. http://www.ncbi.nlm.nih.gov/pubmed/16507883 [DOI] [PubMed]
- 16.Hu J, Qin X, Song ZY, Yang PP, Feng Y, et al. (2017) Alpha-lipoic Acid suppresses P2X receptor activities and visceral hypersensitivity to colorectal distention in diabetic rats. Scientific reports 7: 3928. http://www.ncbi.nlm.nih.gov/pubmed/28659591 [DOI] [PMC free article] [PubMed]
- 17.Xu GY, Li G, Liu N, Huang LY (2011) Mechanisms underlying purinergic P2X3 receptor-mediated mechanical allodynia induced in diabetic rats. Molecular pain 7: 60. http://www.ncbi.nlm.nih.gov/pubmed/21851615 [DOI] [PMC free article] [PubMed]
- 18.Wu B, Ma Y, Yi Z, Liu S, Rao S, et al. (2017) Resveratrol-decreased hyperalgesia mediated by the P2X7 receptor in gp120-treated rats. Molecular pain 13: 1744806917707667. http://www.ncbi.nlm.nih.gov/pubmed/28554250 [DOI] [PMC free article] [PubMed]
- 19.Zhang PA, Zhu HY, Xu QY, Du WJ, Hu S, et al. (2018) Sensitization of P2X3 receptors in insular cortex contributes to visceral pain of adult rats with neonatal maternal deprivation. Molecular pain 14: 1744806918764731. http://www.ncbi.nlm.nih.gov/pubmed/29560791 [DOI] [PMC free article] [PubMed]
- 20.Inoue K (2017) Purinergic signaling in microglia in the pathogenesis of neuropathic pain. Proceedings of the Japan Academy Series B, Physical and biological sciences 93: 174–182. http://www.ncbi.nlm.nih.gov/pubmed/28413195 [DOI] [PMC free article] [PubMed]
- 21.Ji RR, Berta T, Nedergaard M (2013) Glia and pain: is chronic pain a gliopathy? Pain 154 Suppl 1: S10-S28. http://www.ncbi.nlm.nih.gov/pubmed/23792284 [DOI] [PMC free article] [PubMed]
- 22.Tsuda M, Masuda T, Tozaki-Saitoh H, Inoue K (2013) P2X4 receptors and neuropathic pain. Frontiers in cellular neuroscience 7: 191. http://www.ncbi.nlm.nih.gov/pubmed/24191146 [DOI] [PMC free article] [PubMed]
- 23.Ming Q, Wang J, Li D, Rong C, Rao Z. The changes of p2x4 receptor expression in nucleus of tractus solitarius and the dorsal horn of thoracic cord after visceral pain induced by acetic acid (in Chinese) J Neuroanatomy. 2006;05:551–554. [Google Scholar]
- 24.Trang T, Salter MW (2012) P2X4 purinoceptor signaling in chronic pain. Purinergic signalling 8: 621–8. http://www.ncbi.nlm.nih.gov/pubmed/22528681 [DOI] [PMC free article] [PubMed]
- 25.Kuan YH, Shyu BC (2016) Nociceptive transmission and modulation via P2X receptors in central pain syndrome. Molecular brain 9: 58. http://www.ncbi.nlm.nih.gov/pubmed/27230068 [DOI] [PMC free article] [PubMed]
- 26.Liu C, Zhang Y, Liu Q, Jiang L, Li M, et al. (2018) P2X4-receptor participates in EAAT3 regulation via BDNF-TrkB signaling in a model of trigeminal allodynia. Molecular pain 14: 1744806918795930. http://www.ncbi.nlm.nih.gov/pubmed/30146940 [DOI] [PMC free article] [PubMed]
- 27.Bekinschtein P, Cammarota M, Katche C, Slipczuk L, Rossato JI, et al. (2008) BDNF is essential to promote persistence of long-term memory storage. Proceedings of the National Academy of Sciences of the United States of America 105: 2711–6. http://www.ncbi.nlm.nih.gov/pubmed/18263738 [DOI] [PMC free article] [PubMed]
- 28.Coull JA, Beggs S, Boudreau D, Boivin D, Tsuda M, et al. (2005) BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature 438: 1017–21. http://www.ncbi.nlm.nih.gov/pubmed/16355225 [DOI] [PubMed]
- 29.Sorge RE, Mapplebeck JC, Rosen S, Beggs S, Taves S, et al. (2015) Different immune cells mediate mechanical pain hypersensitivity in male and female mice. Nature neuroscience 18: 1081–3. http://www.ncbi.nlm.nih.gov/pubmed/26120961 [DOI] [PMC free article] [PubMed]
- 30.Yuan B, Tang WH, Lu LJ, Zhou Y, Zhu HY, et al. (2015) TLR4 upregulates CBS expression through NF-kappaB activation in a rat model of irritable bowel syndrome with chronic visceral hypersensitivity. World journal of gastroenterology 21: 8615–28. http://www.ncbi.nlm.nih.gov/pubmed/26229403 [DOI] [PMC free article] [PubMed]
- 31.Lin C, Al-Chaer ED (2003) Long-term sensitization of primary afferents in adult rats exposed to neonatal colon pain. Brain research 971: 73–82. http://www.ncbi.nlm.nih.gov/pubmed/12691839 [DOI] [PubMed]
- 32.Toyomitsu E, Tsuda M, Yamashita T, Tozaki-Saitoh H, Tanaka Y, et al. (2012) CCL2 promotes P2X4 receptor trafficking to the cell surface of microglia. Purinergic signalling 8: 301–10. http://www.ncbi.nlm.nih.gov/pubmed/22222817 [DOI] [PMC free article] [PubMed]
- 33.Yamashita T, Yamamoto S, Zhang J, Kometani M, Tomiyama D, et al. (2016) Duloxetine Inhibits Microglial P2X4 Receptor Function and Alleviates Neuropathic Pain after Peripheral Nerve Injury. PloS one 11: e0165189. http://www.ncbi.nlm.nih.gov/pubmed/27768754 [DOI] [PMC free article] [PubMed]
- 34.Chen A, Chen Y, Tang Y, Bao C, Cui Z, et al. (2017) Hippocampal AMPARs involve the central sensitization of rats with irritable bowel syndrome. Brain and behavior 7: e00650. http://www.ncbi.nlm.nih.gov/pubmed/28293483 [DOI] [PMC free article] [PubMed]
- 35.Xiao Y, Chen X, Zhang PA, Xu Q, Zheng H, et al. (2016) TRPV1-mediated presynaptic transmission in basolateral amygdala contributes to visceral hypersensitivity in adult rats with neonatal maternal deprivation. Scientific reports 6: 29026. http://www.ncbi.nlm.nih.gov/pubmed/27364923 [DOI] [PMC free article] [PubMed]
- 36.Lalisse S, Hua J, Lenoir M, Linck N, Rassendren F, et al. (2018) Sensory neuronal P2RX4 receptors controls BDNF signaling in inflammatory pain. Scientific reports 8: 964. http://www.ncbi.nlm.nih.gov/pubmed/29343707 [DOI] [PMC free article] [PubMed]
- 37.de Rivero Vaccari JP, Bastien D, Yurcisin G, Pineau I, Dietrich WD, et al. (2012) P2X4 receptors influence inflammasome activation after spinal cord injury. The Journal of neuroscience : the official journal of the Society for Neuroscience 32: 3058–66. http://www.ncbi.nlm.nih.gov/pubmed/22378878 [DOI] [PMC free article] [PubMed]
- 38.Trang T, Beggs S, Salter MW (2012) ATP receptors gate microglia signaling in neuropathic pain. Experimental neurology 234: 354–61. http://www.ncbi.nlm.nih.gov/pubmed/22116040 [DOI] [PMC free article] [PubMed]
- 39.Masuda T, Iwamoto S, Yoshinaga R, Tozaki-Saitoh H, Nishiyama A, et al. (2014) Transcription factor IRF5 drives P2X4R+-reactive microglia gating neuropathic pain. Nature communications 5: 3771. http://www.ncbi.nlm.nih.gov/pubmed/24818655 [DOI] [PMC free article] [PubMed]
- 40.Masuda T, Tsuda M, Yoshinaga R, Tozaki-Saitoh H, Ozato K, et al. (2012) IRF8 is a critical transcription factor for transforming microglia into a reactive phenotype. Cell reports 1: 334–340. http://www.ncbi.nlm.nih.gov/pubmed/22832225 [DOI] [PMC free article] [PubMed]
- 41.Bathina S, Das UN (2015) Brain-derived neurotrophic factor and its clinical implications. Archives of medical science : AMS 11: 1164–78. http://www.ncbi.nlm.nih.gov/pubmed/26788077 [DOI] [PMC free article] [PubMed]
- 42.Saito A, Cai L, Matsuhisa K, Ohtake Y, Kaneko M, et al. (2018) Neuronal activity-dependent local activation of dendritic unfolded protein response promotes expression of brain-derived neurotrophic factor in cell soma. Journal of neurochemistry 144: 35–49. http://www.ncbi.nlm.nih.gov/pubmed/28921568 [DOI] [PMC free article] [PubMed]
- 43.Azogu I, Plamondon H. Inhibition of TrkB at the nucleus accumbens, using ANA-12, regulates basal and stress-induced orexin A expression within the mesolimbic system and affects anxiety, sociability and motivation. Neuropharmacology. 2017;125:129–145. doi: 10.1016/j.neuropharm.2017.07.008. [DOI] [PubMed] [Google Scholar]
- 44.Barnes AK, Koul-Tiwari R, Garner JM, Geist PA, Datta S. Activation of brain-derived neurotrophic factor-tropomyosin receptor kinase B signaling in the pedunculopontine tegmental nucleus: a novel mechanism for the homeostatic regulation of rapid eye movement sleep. J Neurochem. 2017;141:111–123. doi: 10.1111/jnc.13938. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.